User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Direct Care Dermatology: Weighing the Pros and Cons for the Early-Career Physician
Direct Care Dermatology: Weighing the Pros and Cons for the Early-Career Physician
As the health care landscape continues to shift, direct care (also known as direct pay) models have emerged as attractive alternatives to traditional insurance-based practice. For dermatology residents poised to enter the workforce, the direct care model offers potential advantages in autonomy, patient relationships, and work-life balance, but not without considerable risks and operational challenges. This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
The transition from dermatology residency to clinical practice allows for a variety of paths, from large academic institutions to private practice to corporate entities (private equity–owned groups). In recent years, the direct care model has gained traction, particularly among physicians seeking greater autonomy and a more sustainable pace of practice.
Direct care dermatology practices operate outside the constraints of third-party payers, offering patients transparent pricing and direct access to care in exchange for fees paid out of pocket. By eliminating insurance companies as the middleman, it allows for less overhead, longer visits with patients, and increased access to care; however, though this model may seem appealing, direct care practices are not without their own set of challenges, especially amid rising concerns over physician burnout and administrative burden.
This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
Despite its appeal, starting a direct care practice is not without substantial risks and hurdles—particularly for residents just out of training. These challenges include financial risks and startup costs, market uncertainty, lack of mentorship or support, and limitations in treating complex dermatologic conditions.
Before committing to practicing via a direct care model, dermatology residents should reflect on the following:
- Risk tolerance: Are you comfortable navigating the business and financial risk?
- Location: Does your target community have patients willing and able to pay out of pocket?
- Scope of interest: Will a direct care practice align with your clinical passions?
- Support systems: Do you have access to mentors, legal and financial advisors, and operational support?
- Long-term goals: Are you building a lifestyle practice, a scalable business, or a stepping stone to a future opportunity?
Ultimately, the decision to pursue a direct care model requires careful reflection on personal values, financial preparedness, and the unique needs of the community one intends to serve.
The direct care dermatology model offers an appealing alternative to traditional practice, especially for those prioritizing autonomy, patient connection, and work-life balance; however, it demands an entrepreneurial spirit as well as careful planning and an acceptance of financial uncertainty—factors that may pose challenges for new graduates. For dermatology residents, the decision to pursue direct care should be grounded in personal values, practical considerations, and a clear understanding of both the opportunities and limitations of this evolving practice model.
- Sinsky CA, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med.
- Dorrell DN, Feldman S, Wei-ting Huang W. The most common causes of burnout among US academic dermatologists based on a survey study. J Am Acad of Dermatol. 2019;81:269-270.
- Carlasare LE. Defining the place of direct primary care in a value-based care system. WMJ. 2018;117:106-110.
As the health care landscape continues to shift, direct care (also known as direct pay) models have emerged as attractive alternatives to traditional insurance-based practice. For dermatology residents poised to enter the workforce, the direct care model offers potential advantages in autonomy, patient relationships, and work-life balance, but not without considerable risks and operational challenges. This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
The transition from dermatology residency to clinical practice allows for a variety of paths, from large academic institutions to private practice to corporate entities (private equity–owned groups). In recent years, the direct care model has gained traction, particularly among physicians seeking greater autonomy and a more sustainable pace of practice.
Direct care dermatology practices operate outside the constraints of third-party payers, offering patients transparent pricing and direct access to care in exchange for fees paid out of pocket. By eliminating insurance companies as the middleman, it allows for less overhead, longer visits with patients, and increased access to care; however, though this model may seem appealing, direct care practices are not without their own set of challenges, especially amid rising concerns over physician burnout and administrative burden.
This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
Despite its appeal, starting a direct care practice is not without substantial risks and hurdles—particularly for residents just out of training. These challenges include financial risks and startup costs, market uncertainty, lack of mentorship or support, and limitations in treating complex dermatologic conditions.
Before committing to practicing via a direct care model, dermatology residents should reflect on the following:
- Risk tolerance: Are you comfortable navigating the business and financial risk?
- Location: Does your target community have patients willing and able to pay out of pocket?
- Scope of interest: Will a direct care practice align with your clinical passions?
- Support systems: Do you have access to mentors, legal and financial advisors, and operational support?
- Long-term goals: Are you building a lifestyle practice, a scalable business, or a stepping stone to a future opportunity?
Ultimately, the decision to pursue a direct care model requires careful reflection on personal values, financial preparedness, and the unique needs of the community one intends to serve.
The direct care dermatology model offers an appealing alternative to traditional practice, especially for those prioritizing autonomy, patient connection, and work-life balance; however, it demands an entrepreneurial spirit as well as careful planning and an acceptance of financial uncertainty—factors that may pose challenges for new graduates. For dermatology residents, the decision to pursue direct care should be grounded in personal values, practical considerations, and a clear understanding of both the opportunities and limitations of this evolving practice model.
As the health care landscape continues to shift, direct care (also known as direct pay) models have emerged as attractive alternatives to traditional insurance-based practice. For dermatology residents poised to enter the workforce, the direct care model offers potential advantages in autonomy, patient relationships, and work-life balance, but not without considerable risks and operational challenges. This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
The transition from dermatology residency to clinical practice allows for a variety of paths, from large academic institutions to private practice to corporate entities (private equity–owned groups). In recent years, the direct care model has gained traction, particularly among physicians seeking greater autonomy and a more sustainable pace of practice.
Direct care dermatology practices operate outside the constraints of third-party payers, offering patients transparent pricing and direct access to care in exchange for fees paid out of pocket. By eliminating insurance companies as the middleman, it allows for less overhead, longer visits with patients, and increased access to care; however, though this model may seem appealing, direct care practices are not without their own set of challenges, especially amid rising concerns over physician burnout and administrative burden.
This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
Despite its appeal, starting a direct care practice is not without substantial risks and hurdles—particularly for residents just out of training. These challenges include financial risks and startup costs, market uncertainty, lack of mentorship or support, and limitations in treating complex dermatologic conditions.
Before committing to practicing via a direct care model, dermatology residents should reflect on the following:
- Risk tolerance: Are you comfortable navigating the business and financial risk?
- Location: Does your target community have patients willing and able to pay out of pocket?
- Scope of interest: Will a direct care practice align with your clinical passions?
- Support systems: Do you have access to mentors, legal and financial advisors, and operational support?
- Long-term goals: Are you building a lifestyle practice, a scalable business, or a stepping stone to a future opportunity?
Ultimately, the decision to pursue a direct care model requires careful reflection on personal values, financial preparedness, and the unique needs of the community one intends to serve.
The direct care dermatology model offers an appealing alternative to traditional practice, especially for those prioritizing autonomy, patient connection, and work-life balance; however, it demands an entrepreneurial spirit as well as careful planning and an acceptance of financial uncertainty—factors that may pose challenges for new graduates. For dermatology residents, the decision to pursue direct care should be grounded in personal values, practical considerations, and a clear understanding of both the opportunities and limitations of this evolving practice model.
- Sinsky CA, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med.
- Dorrell DN, Feldman S, Wei-ting Huang W. The most common causes of burnout among US academic dermatologists based on a survey study. J Am Acad of Dermatol. 2019;81:269-270.
- Carlasare LE. Defining the place of direct primary care in a value-based care system. WMJ. 2018;117:106-110.
- Sinsky CA, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med.
- Dorrell DN, Feldman S, Wei-ting Huang W. The most common causes of burnout among US academic dermatologists based on a survey study. J Am Acad of Dermatol. 2019;81:269-270.
- Carlasare LE. Defining the place of direct primary care in a value-based care system. WMJ. 2018;117:106-110.
Direct Care Dermatology: Weighing the Pros and Cons for the Early-Career Physician
Direct Care Dermatology: Weighing the Pros and Cons for the Early-Career Physician
PRACTICE POINTS
- Direct care practices may be the new horizon of health care.
- Starting a direct care practice offers autonomy but demands entrepreneurial readiness.
- New dermatologists can enjoy control over scheduling, pricing, and patient care, but success requires business acumen, financial planning, and comfort with risk.
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
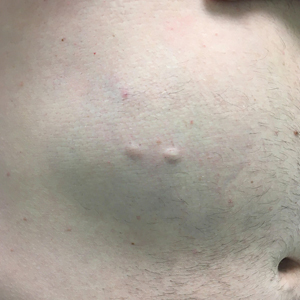
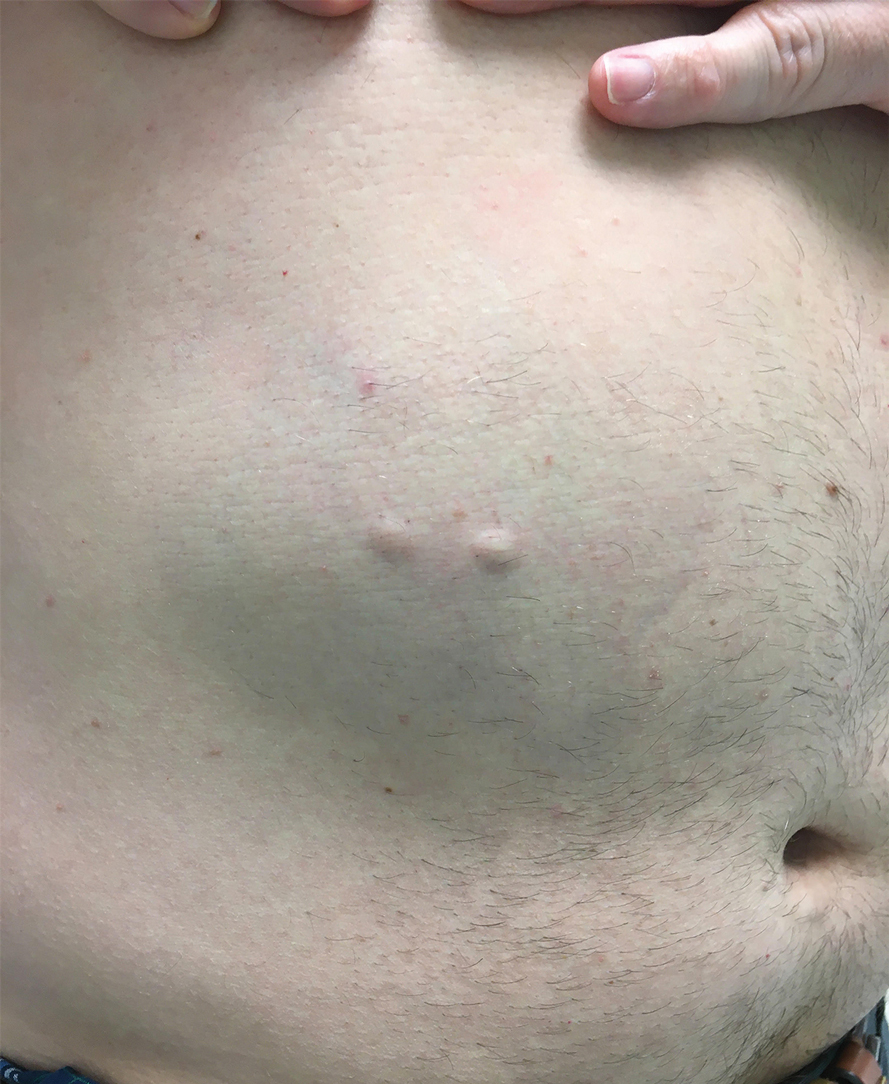
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.
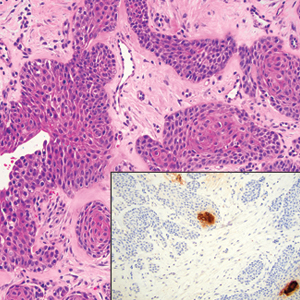
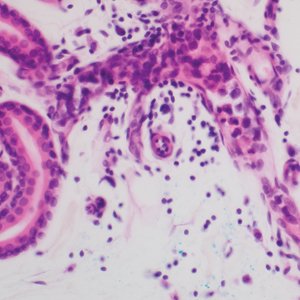
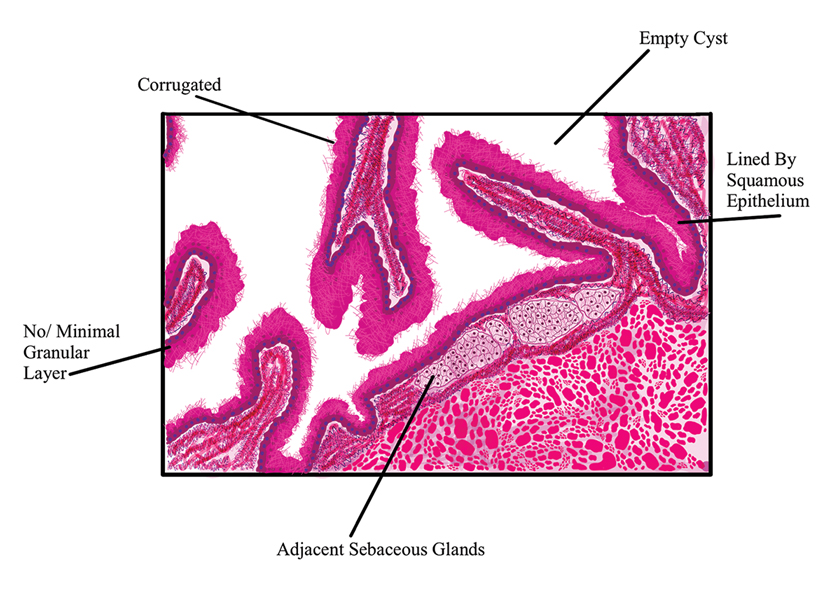
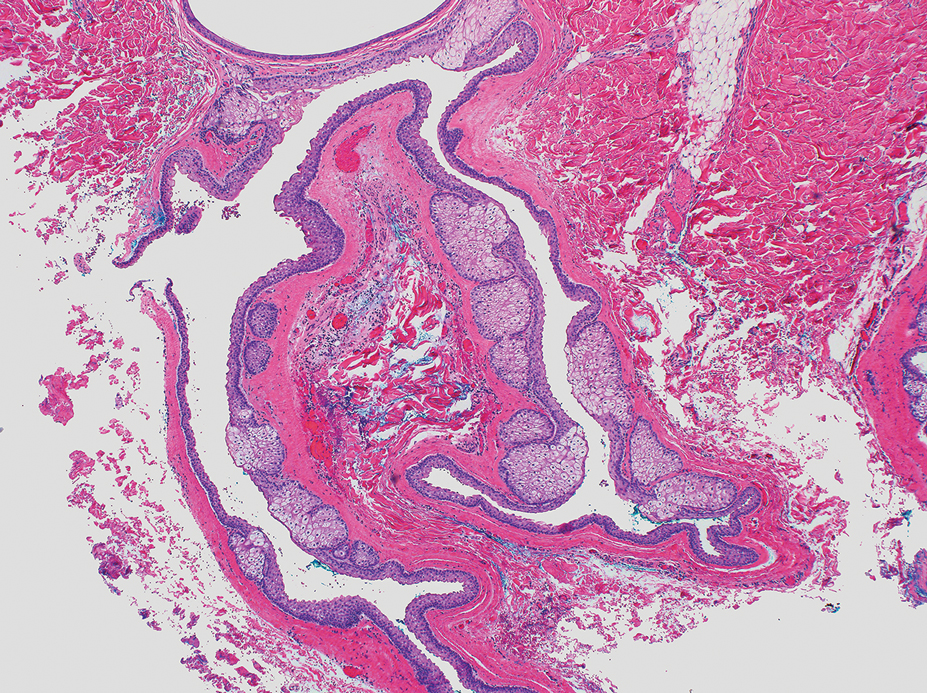
Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23
Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
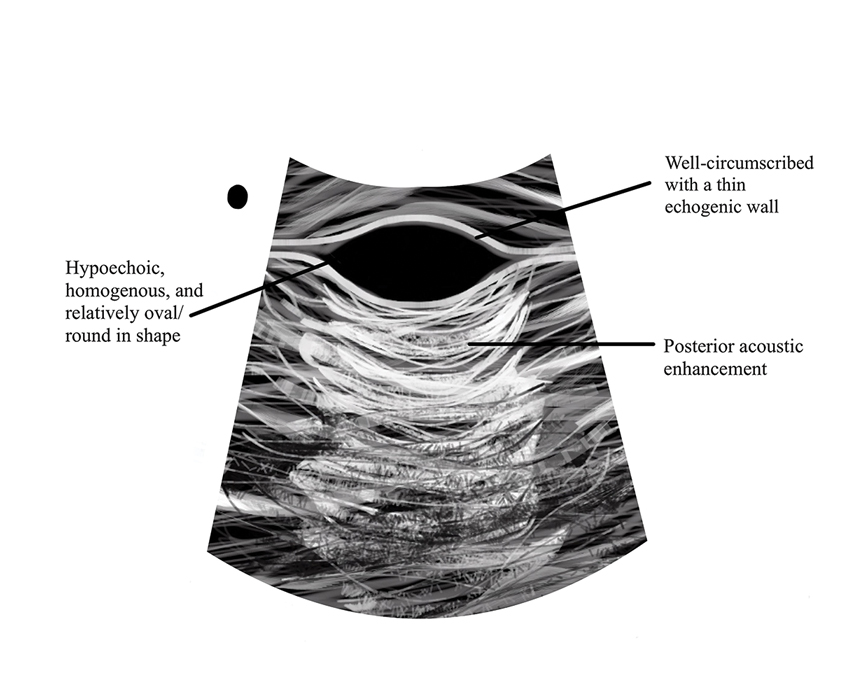
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33
Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
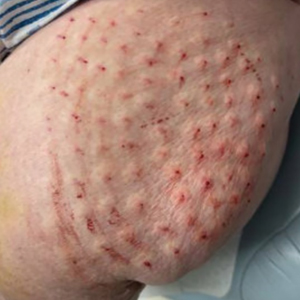
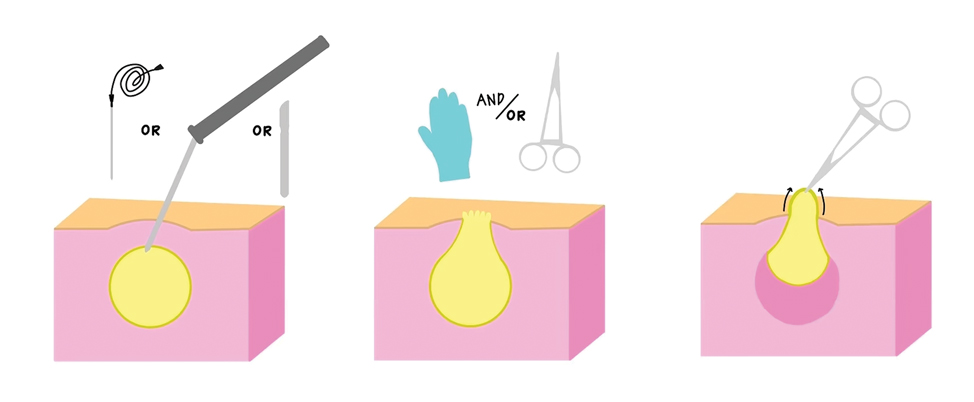
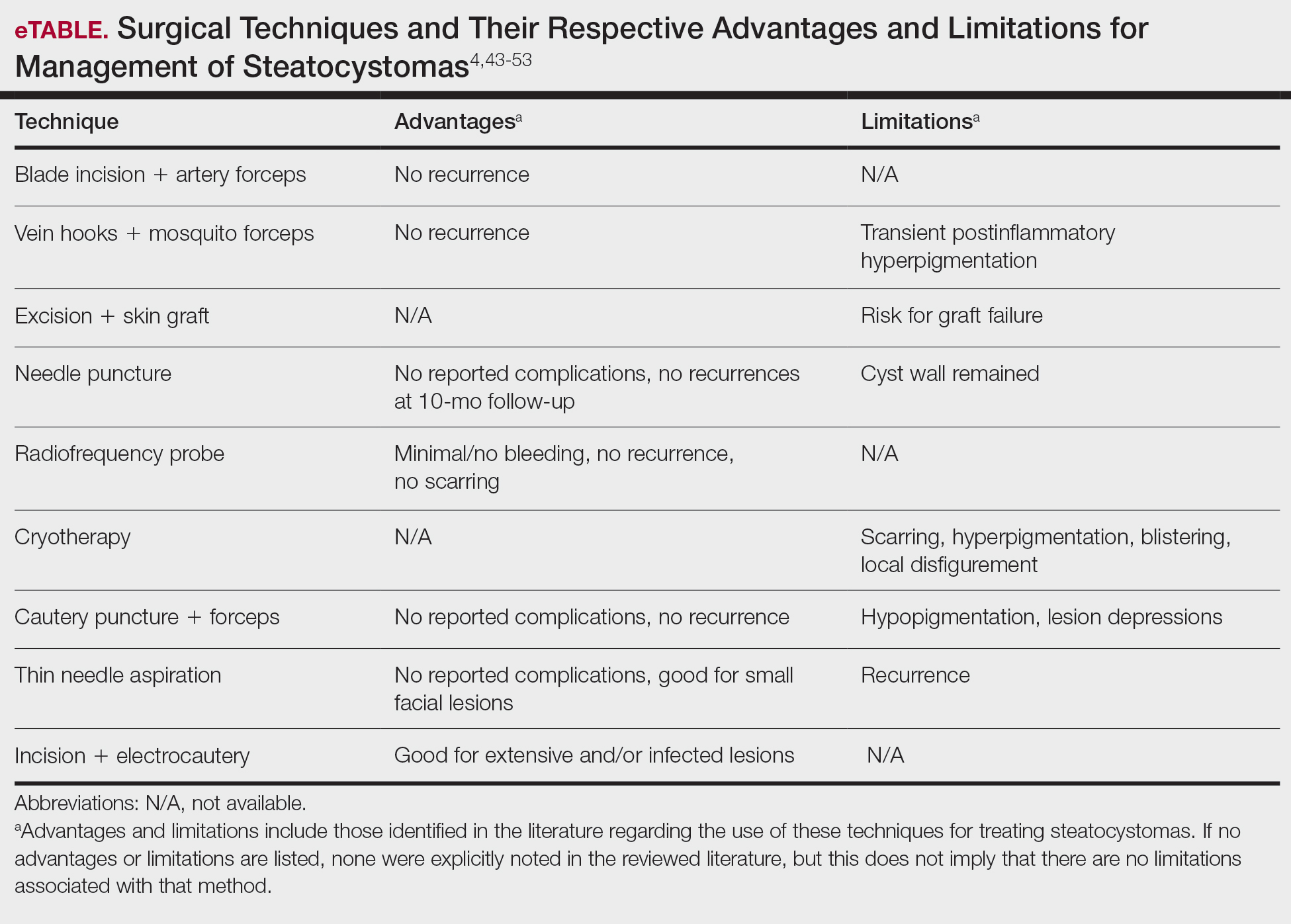
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.
Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.

Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23


Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33

Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.


Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
Steatocystomas are small sebum-filled cysts that typically manifest in the dermis and originate from sebaceous follicles. Although commonly asymptomatic, these lesions can manifest with pruritus or become infected, predisposing patients to further complications.1 Steatocystomas can manifest as single (steatocystoma simplex [SS]) or numerous (steatocystoma multiplex [SM]) lesions; the lesions also can spontaneously rupture with characteristics that resemble hidradenitis suppurativa (HS)(steatocystoma multiplex suppurativa [SMS]).1,2
Steatocystomas are relatively rare, and there is limited consensus in the published literature on the etiology and management of this condition. In this article, we present a comprehensive review of steatocystomas in the current literature. We highlight important features to consider when making the diagnosis and also offer recommendations for best-practice treatment.
Historical Background
Although not explicitly identified by name, the first documentation of steatocystomas is a case report published in 1873. In this account, the author described a patient who presented with approximately 250 flesh-colored dermal cysts across the body that varied in size.3 In 1899, the term steatocystoma multiple—derived from Greek roots meaning “fatty bag”—was first used.4
In 1982, almost a century later, Brownstein5 reported some of the earliest cases of SS. This solitary subtype is identical to SM on a microscopic level; however, unlike SM, this variant occurs as a single lesion that typically forms in adulthood and in the absence of family history. Other benign adnexal tumors (eg, pilomatricomas, pilar cysts, and sebaceous hyperplasias) also can manifest as either solitary or multiple lesions.
In 1976, McDonald and Reed6 reported the first known cases of patients with both SM and HS. At the time, the co-occurrence of these conditions was viewed as coincidental, but there were postulations of a shared inflammatory process and hereditary link6; it was not until 1982 that the term steatocystoma multiplex suppurativum was coined to describe this variant.7 Although rare, there have been multiple documented instances of SMS since. It has been suggested that the convergence of these conditions may indicate a shared follicular proliferation defect.8 Ongoing investigation is warranted to explain the underlying pathogenesis of this unique variant.
Epidemiology
The available epidemiologic data primarily relate to SM, the most common steatocystoma variant. Nevertheless, SM is a relatively rare condition, and the exact incidence and prevalence remain unknown.8,9 Steatocystomas typically manifest in the first and second decades of life and have been observed in patients of both sexes, with studies demonstrating no notable sex bias.4,9
Etiology and Pathophysiology
Steatocystomas can occur sporadically or may be inherited as an autosomal-dominant condition.4 Typically, SS tends to manifest as an isolated occurrence without any inherent genetic predisposition.5 Alternatively, SM may develop sporadically or be associated with a mutation in the keratin 17 gene (KRT17).4 Steatocystoma multiplex also has been associated with at least 4 different missense mutations, including N92H, R94H, and R94C, located on the long (q) arm of chromosome 17.4,10-12
The keratin 17 gene is responsible for encoding the keratin 17 protein, a type I intermediate filament predominantly synthesized in the basal cells of epithelial tissue. This fibrous structural protein can regulate many processes, including inflammation and cell proliferation, and is found in regions such as the sebaceous glands, hair follicles, and eccrine sweat glands. Overexpression of KRT17 has been suggested in other cutaneous conditions, most notably psoriasis.12 Despite KRT17’s many roles, it remains unclear why SM typically manifests with a myriad of sebum-containing cysts as the primary symptom.12 Continued investigation into the genetic underpinnings of SM and the keratin 17 protein is necessary to further elucidate a more comprehensive understanding of this condition.
Hormonal influences have been suggested as a potential trigger for steatocystoma growth.4,13 This condition is associated with dysfunction of the sebaceous glands, and, correspondingly, the incidence of disease is highest in pubertal patients, in whom androgen levels and sebum production are elevated.4,13,14 Two cases of transgender men taking testosterone therapy presenting with steatocystomas provide additional clinical support for this association.15
Additionally, the use of immunomodulatory agents, such as ustekinumab (anti–interleukin 12/interleukin 23), has been shown to trigger SM. It is predicted that the reduced expression of certain interferons and interleukins may lead to downstream consequences in the keratin 17 pathway and lead to SM lesion formation in genetically susceptible individuals.16 Targeting these potential causes in the future may prove efficacious in the secondary prevention of familial SM manifestation or exacerbations.
Mutations in the KRT17 gene also have been implicated in pachyonychia congenita type 2 (PC-2).4 Marked by extensive systemic hyperkeratosis, PC-2 has been observed to coincide with SM in certain patients.4,5 Interestingly, the location of the KRT17 mutations are identical in both PC-2 and SM.4 Although most individuals with hereditary SM do not exhibit the characteristic features of PC-2, mild nail and dental abnormalities have been observed in some SM cases.4,10 This relationship suggests that SM may be a less severe variant of PC-2 or part of a complex polygenetic spectrum of disease.10 Further research is imperative to determine the exact nature and extent of the relationship between these conditions.
Clinical Manifestations
Steatocystomas are flesh-colored subcutaneous cysts that range in size from less than 3 mm to larger than 3 cm in diameter (Figure). They form within a single pilosebaceous unit and typically display firm attachment due to their origination in the dermis.2,7,17 Steatocystomas generally contain lipid material, and less frequently, keratin and hair shafts, distinguishing them as the only “true” sebaceous cysts.18 Their color can range from flesh-toned to yellow, with reports of occasional dark-blue shades and calcifications.19,20 Steatocystomas can persist indefinitely, and they usually are asymptomatic.

Diagnosis of steatocystoma is confirmed by biopsy.4 Steatocystomas are characterized by a dermal cyst lined by stratified squamous cell epithelium (eFigures 1 and 2).21 Classically they feature flattened sebaceous lobules, multinucleated giant cells, and abortive hair follicles. The lining of these cysts is marked by lymphocytic infiltrate and a dense, wrinkled, eosinophilic keratin cuticle that replaces the granular layer.22 The cyst maintains an epidermal connection through a follicular infundibulum characterized by clumps of keratinocytes, sebocytes, corneocytes, and/or hair follicles.7 Aspirated contents reveal crystalline structures and anucleate squamous cells upon microscopic analysis. That being said, variable histologic findings of steatocystomas have been described.23


Steatocystoma simplex, as the name implies, classifies a single isolated steatocystoma. This subtype exhibits similar histopathologic and clinical features to the other subtypes of steatocystomas. Notably, SS is not associated with a genetic mutation and is not an inherited condition within families.5 Steatocystoma multiplex manifests with many steatocystomas, often distributed widely across the body.3,4 The chest, axillae, and groin are the most common locations; however, these cysts can manifest on the face, back, abdomen, and extremities.4,18-22 Rare occurrences of SM limited to the face, scalp, and distal extremities have been documented.18,21,24,25 Due to the possibility of an autosomal-dominant inheritance, it is advisable to take a comprehensive family history in patients for whom SM is in the differential.17
Steatocystoma multiplex—especially familial variants—has been shown to develop in conjunction with other dermatologic conditions, including eruptive vellus hair (EVH) cysts, persistent infantile milia, and epidermoid/dermoid cysts.26 While some investigators regard these as separate entities due to their varied genetic etiology, it has been suggested that these conditions may be related and that the diagnosis is determined by the location of cyst origin along the sebaceous ducts.26,27 Other dermatologic conditions and lesions that frequently manifest comorbidly with SM include hidrocystomas, syringomas, pilonidal cysts, lichen planus, nodulocystic acne, trichotillomania, trichoblastomas, trichoepithelioma, HS, keratoacanthomas, acrokeratosis verruciformis of Hopf, and embryonal hair formation. Steatocystoma multiplex, manifesting comorbidly with dental and orofacial malformations (eg, partial noneruption of secondary teeth, natal and defective teeth, and bilateral preauricular sinuses) has been classified as SM natal teeth syndrome.6
Steatocystoma multiplex suppurativa is a rare and serious variant of SM characterized by inflammation, cyst rupture, sinus tract formation, and scarring.24 Patients with SMS typically have multiple intact SM cysts, which can aid in differentiation from HS.2,24 Steatocystoma multiplex suppurativa is associated with more complications than SS and SM, including cyst perforation, development of purulent and/or foul-smelling discharge, infection, scarring, pain, and overall discomfort.2
Given its rarity and the potential manifestations that overlap with other conditions, steatocystomas easily can be misdiagnosed. In some clinical instances, EVHs may share similar characteristics with SM; however, certain distinguishing features exist, including a central tuft of protruding hairs and different expressed contents, such as the vellus hair shafts, from the cyst’s lumen.28 Furthermore, histologic examination of EVHs reveals epidermoid keratinization of the lining as well as a lack of sebaceous glands within the wall.28,29 Other similar conditions include epidermoid cysts, pilar cysts, lipomas, epidermal inclusion cysts, dermoid cysts, sebaceous hyperplasia, folliculitis, xanthomas, neurofibromatosis, and syringomas.30 Occasionally, SMS can be mistaken for HS or acne conglobata, and SM lesions with a facial distribution can mimic acne vulgaris.1,31 These conditions should be excluded before a diagnosis of SS, SM, or SMS is made.
Importantly, SM is visually indistinguishable from subcutaneous metastasis on physical examination, and there are reports of oncologic conditions (eg, pulmonary adenocarcinoma metastasized to the skin) being mistaken for SS or SM.32 Therefore, a thorough clinical examination, histopathologic analysis, and potential use of other imaging modalities such as ultrasonography (US) are needed to ensure an accurate diagnosis.
Ultrasonography has demonstrated utility in diagnosing steatocystomas.33-35 Steatocystomas have incidentally been found on routine mammograms and can demonstrate well-defined circular nodules with radiolucent characteristics and a thin radiodense outline.33,36 Homogeneous hypoechoic nodules within the dermis without posterior acoustic features generally are observed (eFigure 3).33,37 In patients declining biopsy, US may be useful in further characterization of an unknown lesion. Color Doppler US can be used to distinguish SMS from HS. Specifically, SM typically exhibits an absence of Doppler signaling due to a lack of vascularity, providing a helpful diagnostic clue for the SMS variant.33

Management and Treatment Options
There is no established standard treatment for steatocystomas; therefore, the approach to management is contingent on clinical presentation and patient preferences. Various medical, surgical, and laser management options are available, each with its own advantages and limitations. Treatment of SM is difficult due to the large number of lesions.38 In many cases, continued observation is a viable treatment option, as most SS and SM lesions are asymptomatic; however, cosmetic concerns can be debilitating for patients with SM and may warrant intervention.39 More extensive medical and surgical management often are necessary in SMS due to associated morbidity. Discussing options and goals as well as setting realistic expectations with the patient are essential in determining the optimal approach.
Medical Management—In medical literature, oral isotretinoin (13-cis-retinoic acid) has been the mainstay of therapy for steatocystoma, as its effect on the size and activity of sebaceous glands is hypothesized to decrease disease activity.38,40 Interventional studies and case reports have exhibited varying degrees of effectiveness.1,38-41 Some reports depict a reduction in the formation of new lesions and a decrease in the size of pre-existing lesions, some show mild delayed therapeutic efficacy, and others suggest exacerbation of the condition.1,38-41 This outcome variability is attributed to isotretinoin’s preferential efficacy in treating inflammatory lesions.40,42
Tetracycline derivatives and intralesional steroid injections also have been employed with some efficacy in patients with focal inflammatory SM and SMS.43 There is limited evidence on the long-term outcomes of these interventions, and intralesional injections often are not recommended in conditions such as SM, in which there are many lesions present.
Surgical Management—Minimally invasive surgical procedures including drainage and resections have been used with varying efficacy in SS and SM. Typically, a 2- to 3-mm incision or sharp-tipped cautery is employed to puncture the cyst. Alternatively, radiofrequency probes with a 2.4-MHz frequency setting have been used to minimize incision size.44 The contents then are expressed with manual pressure or forceps, and the cyst sac is extracted using forceps and/or a vein hook (eFigure 4).44,45 The specific surgical techniques and their respective advantages and limitations are summarized in the eTable. Reported advantages and limitations of surgical techniques are derived from information provided by the authors of steatocystoma case reports, which are based on observations of a very limited sample size.


Laser Treatment—Various laser modalities have been used in the management of steatocystomas, including carbon dioxide lasers, erbium-doped yttrium aluminum garnet lasers, 1450-nm diode plus 1550-nm fractionated erbium-doped fiber lasers, and 1927-nm diode lasers.54,55-57 These lasers are used to perforate the cyst before extirpation and have displayed advantages in minimizing scar length.58 The super-pulse mode of carbon dioxide lasers demonstrates efficacy with minimal scarring and recurrence, and this mode is preferred to minimize thermal damage.54,59 Furthermore, this modality can be especially useful in patients whose condition is refractory to other noninvasive options.59 Similarly, the erbium-doped yttrium aluminum garnet laser was well tolerated with no complications noted.55 The 1927-nm diode laser also displayed good outcomes as well as no recurrence.57 With laser use, it is important to note that multiple treatments are needed to see optimal outcomes.54 Moreover, laser settings must be carefully considered, especially in patients with Fitzpatrick skin type III or higher, and topical anti-inflammatory agents should be considered posttreatment to minimize complications.54,59,60
Recommendations
For management of SS, we recommend conservative therapy of watchful observation, as scarring or postinflammatory pigment change may be brought on by medical or surgical therapy; however, if SS is cosmetically bothersome, laser or surgical excision can be done (eFigure 4).4,43-53 It is important to counsel the patient on risks/benefits. For SM, watchful observation also is indicated; however, systemic therapies aimed at prevention may be the most efficacious by limiting disease progression, and oral tetracycline or isotretinoin may be tried.4 Tetracyclines have the risk for photosensitivity and are teratogenic, while isotretinoin is extremely teratogenic, requires laboratory monitoring and regular pregnancy tests in women, and often causes substantial mucosal dryness. If lesions are bothersome or refractory to these therapies, intralesional steroids or surgical/laser procedures can be tried throughout multiple visits.43-53 For SMS, systemic therapies frequently are recommended. The risks of systemic tetracycline and isotretinoin therapies must be discussed. Patients with treatment-refractory SMS may require surgical excision or deroofing of sinus tracts.43-53 This management is similar to that of HS and must be tailored to the patient.
Conclusion
Overall, steatocystomas are a relatively rare pathology, with a limited consensus on their etiology and management. This review summarizes the current knowledge on the condition to support clinicians in diagnosis and management, ranging from watchful waiting to surgical removal. By individualizing treatment plans, clinicians ultimately can optimize outcomes in patients with steatocystomas.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
- Santana CN, Pereira DD, Lisboa AP, et al. Steatocystoma multiplex suppurativa: case report of a rare condition. An Bras Dermatol. 2016;91(5 suppl 1):51-53.
- Atzori L, Zanniello R, Pilloni L, et al. Steatocystoma multiplex suppurativa associated with hidradenitis suppurativa successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):42-44.
- Jamieson WA. Case of numerous cutaneous cysts scattered over the body. Edinb Med J. 1873;19:223-225.
- Kamra HT, Gadgil PA, Ovhal AG, et al. Steatocystoma multiplex-a rare genetic disorder: a case report and review of the literature. J Clin Diagn Res. 2013;7:166-168.
- Brownstein MH. Steatocystoma simplex. A solitary steatocystoma. Arch Dermatol. 1982;118:409-411.
- McDonald RM, Reed WB. Natal teeth and steatocystoma multiplex complicated by hidradenitis suppurativa. A new syndrome. Arch Dermatol. 1976;112:1132-1134.
- Plewig G, Wolff HH, Braun-Falco O. Steatocystoma multiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol. 1982;272:363-380.
- Fletcher J, Posso-De Los Rios C, Jambrosic J, A, et al. Coexistence of hidradenitis suppurativa and steatocystoma multiplex: is it a new variant of hidradenitis suppurativa? J Cutan Med Surg. 2021;25:586-590.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Yang L, Zhang S, Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. J Pathol. 2019;247:158-165.
- Shamloul G, Khachemoune A. An updated review of the sebaceous gland and its role in health and diseases Part 1: embryology, evolution, structure, and function of sebaceous glands. Dermatol Ther. 2021;34:e14695.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19:30-35.
- Porras Fimbres DC, Wolfe SA, Kelley CE. Proliferation of steatocystomas in 2 transgender men. JAAD Case Rep. 2022;26:70-72.
- Marasca C, Megna M, Donnarumma M, et al. A case of steatocystoma multiplex in a psoriatic patient during treatment with anti-IL-12/23. Skin Appendage Disord. 2020;6:309-311.
- Gordon Spratt EA, Kaplan J, Patel RR, et al. Steatocystoma. Dermatol Online J. 2013;19:20721.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Rahman MH, Islam MS, Ansari NP. Atypical steatocystoma multiplex with calcification. ISRN Dermatol. 2011;2011:381901.
- Beyer AV, Vossmann D. Steatocystoma multiplex. Article in German. Hautarzt. 1996;47:469-471.
- Yanagi T, Matsumura T. Steatocystoma multiplex presenting as acral subcutaneous nodules. Acta Derm Venereol. 2006;86:374-375.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Ferrandiz C, Peyri J. Steatocystoma multiplex. Article in Spanish. Med Cutan Ibero Lat Am. 1984;12:173-176.
- Alotaibi L, Alsaif M, Alhumidi A, et al. Steatocystoma multiplex suppurativa: a case with unusual giant cysts over the scalp and neck. Case Rep Dermatol. 2019;11:71-76.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to scalp. Ann Dermatol. 2009;21:106-109.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Tomková H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Patokar AS, Holani AR, Khandait GH, et al. Eruptive vellus hair cysts: an underdiagnosed entity. Int J Trichology. 2022;14:31-33.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5 Pt 2):876-878.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Varshney M, Aziz M, Maheshwari V, et al. Steatocystoma multiplex. BMJ Case Rep. 2011;2011:bcr0420114165.
- Tsai MH, Hsiao YP, Lin WL, et al. Steatocystoma multiplex as initial impression of non-small cell lung cancer with complete response to gefitinib. Chin J Cancer Res. 2014;26:E5-E9.
- Zussino M, Nazzaro G, Moltrasio C, et al. Coexistence of steatocystoma multiplex and hidradenitis suppurativa: assessment of this unique association by means of ultrasonography and color Doppler. Skin Res Technol. 2019;25:877-880.
- Whittle C, Silva-Hirschberg C, Loyola K, et al. Ultrasonographic spectrum of cutaneous cysts with stratified squamous epithelium in pediatric dermatology: pictorial essay. J Ultrasound Med. 2023;42:923-930.
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260.
- Reick-Mitrisin V, Reddy A, Shah BA. A breast imaging case of steatocystoma multiplex: a rare condition involving multiple anatomic regions. Cureus. 2022;14:E27756.
- Yoon H, Kang Y, Park H, et al. Sonographic appearance of steatocystoma: an analysis of 14 pathologically confirmed lesions. Taehan Yongsang Uihakhoe Chi. 2021;82:382-392.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sharma A, Agrawal S, Dhurat R, et al. An unusual case of facial steatocystoma multiplex: a clinicopathologic and dermoscopic report. Dermatopathology (Basel). 2018;5:58-63.
- Moritz DL, Silverman RA. Steatocystoma multiplex treated with isotretinoin: a delayed response. Cutis. 1988;42:437-439.
- Schwartz JL, Goldsmith LA. Steatocystoma multiplex suppurativum: treatment with isotretinoin. Cutis. 1984;34:149-153.
- Kim SJ, Park HJ, Oh ST, et al. A case of steatocystoma multiplex limited to the scalp. Ann Dermatol. 2009;21:106-109.
- Fekete GL, Fekete JE. Steatocystoma multiplex generalisata partially suppurativa--case report. Acta Dermatovenerol Croat. 2010;18:114-119.
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Oertel YC, Scott DM. Cytologic-pathologic correlations: fine needle aspiration of three cases of steatocystoma multiplex. Ann Diagn Pathol. 1998;2:318-320.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Lee SJ, Choe YS, Park BC, et al. The vein hook successfully used for eradication of steatocystoma multiplex. Dermatol Surg. 2007;33:82-84.
- Bettes PSL, Lopes SL, Prestes MA, et al. Treatment of a facial variant of the multiple steatocystoma with skin graft: case report. Rev Bras Cir Plást. 1998;13:31-36
- Düzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. Int J Dermatol. 2004;43:60-62. doi:10.1111/j.1365-4632.2004.02068.x
- Choudhary S, Koley S, Salodkar A. A modified surgical technique for steatocystoma multiplex. J Cutan Aesthet Surg. 2010;3:25-28.
- Kaya TI, Ikizoglu G, Kokturk A, et al. A simple surgical technique for the treatment of steatocystoma multiplex. Int J Dermatol. 2001;40:785-788.
- Bakkour W, Madan V. Carbon dioxide laser perforation and extirpation of steatocystoma multiplex. Dermatol Surg. 2014;40:658-662.
- Mumcuog?lu CT, Gurel MS, Kiremitci U, et al. Er: yag laser therapy for steatocystoma multiplex. Indian J Dermatol. 2010;55:300-301.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7 Pt 1):1104-1106.
- Cheon DU, Ko JY. 1927-nm fiber-optic diode laser: a novel therapeutic option for facial steatocystoma multiplex. J Cosmet Dermatol. 2019;18:1326-1329.
- Kim KT, Sun H, Chung EH. Comparison of complete surgical excision and minimally invasive excision using CO2 laser for removal of epidermal cysts on the face. Arch Craniofac Surg. 2019;20:84-88.
- Kassira S, Korta DZ, de Feraudy S, et al. Fractionated ablative carbon dioxide laser treatment of steatocystoma multiplex. J Cosmet Laser Ther. 2016;18:364-366.
- Dixit N, Sardana K, Paliwal P. The rationale of ideal pulse duration and pulse interval in the treatment of steatocystoma multiplex using the carbon dioxide laser in a super-pulse mode as opposedto the ultra-pulse mode. Indian J Dermatol Venereol Leprol. 2020;86:454-456.
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Steatocystomas: Update on Clinical Manifestations, Diagnosis, and Management
Practice Points
- Steatocystomas, which manifest as single or multiple flesh-colored subcutaneous cysts ranging from less than 3 mm to more than 3 cm, typically are asymptomatic and can persist indefinitely.
- Treatment options for steatocystomas include oral isotretinoin, tetracycline derivatives, and intralesional steroid injections. Minimally invasive procedures such as drainage and resection also are available, employing techniques such as blade incision, radiofrequency probes, and laser treatments to minimize scarring and recurrence.
- Conservative therapies such as watchful waiting are recommended for the simplex and multiplex variants, while more aggressive management such as surgical removal is recommended for the multiplex suppurativa variant due to its elevated risk for complications.
Operational Risk Management in Dermatologic Procedures
Operational Risk Management in Dermatologic Procedures
Operational risk management (ORM) refers to the systematic identification and assessment of daily operational risks within an organization designed to mitigate negative financial, reputational, and safety outcomes while maximizing efficiency and achievement of objectives.1 Operational risk management is indispensable to modern military operations, optimizing mission readiness while minimizing complications and personnel morbidity. Application of ORM in medicine holds considerable promise due to the emphasis on precise and efficient decision-making in high-stakes environments, where the margin for error is minimal. In this article, we propose integrating ORM principles into dermatologic surgery to enhance patient-centered care through improved counseling, risk assessment, and procedural outcomes.
Principles and Processes of ORM
The ORM framework is built on 4 fundamental principles: accept risk when benefits outweigh the cost, accept no unnecessary risk, anticipate and manage risk by planning, and make risk decisions at the right level.2 These principles form the foundation of the ORM’s systematic 5-step approach to identify hazards, assess hazards, make risk decisions, implement controls, and supervise. Key to the ORM process is the use of risk assessment codes and the risk assessment matrix to quantify and prioritize risks. Risk assessment codes are numerical values assigned to hazards based on their assessed severity and probability. The risk assessment matrix is a tool that plots the severity of a hazard against its probability. By locating a hazard on the matrix, users can visualize its risk level in terms of severity and probability. Building and using the risk assessment matrix begins with determining severity by assessing the potential impact of a hazard and categorizing it into levels (catastrophic, critical, moderate, or negligible). Next, probability is determined by evaluating the likelihood of occurrence (frequent, likely, occasional, seldom, or unlikely). Finally, the severity and probability are combined to assign a risk assessment code, which indicates the risk level and helps visualize criticality. Systematically applying these principles and processes enables users to make informed decisions that balance mission objectives with safety.
Proposed Framework for ORM in Dermatology Surgery
Current risk mitigation in dermatologic surgery includes strict medication oversight, sterilization protocols, and photography to prevent wrong-site surgeries. Preoperative risk assessment through conducting a thorough patient history is vital, considering factors such as pregnancy, allergies, bleeding history, cardiac devices, and keloid propensity, all of which impact surgical outcomes.3-5 After gathering the patient’s history, dermatologists determine appropriateness for surgery and its inherent risks, typically via an informed consent process outlining the diagnosis and procedure purpose as well as a list of risks, benefits, and alternatives, including forgoing treatment.
Importantly, the standard process for dermatologic risk evaluation often lacks a comprehensive systematic approach seen in other higher-risk surgical fields. For example, general surgeons frequently utilize risk assessment calculators such as the one developed by the American College of Surgeons’ National Surgical Quality Improvement Program to estimate surgical complications.6 While specific guidelines exist for evaluating factors such as hypertension or anticoagulant use, no single tool synthesizes all patient risk factors for a unified assessment. Therefore, we propose integrating ORM as a structured decision-making process that offers a more consistent means for dermatologists to evaluate, synthesize, categorize, and present risks to patients. Our proposed process includes translating military mishap severity into a framework that helps patients better understand decisions about their health care when using ORM (eTable 1). The proposed process also provides dermatologists with a systematic, proactive, and iterative approach to assessing risks that allows them to consistently qualify medical decisions (eTable 2).
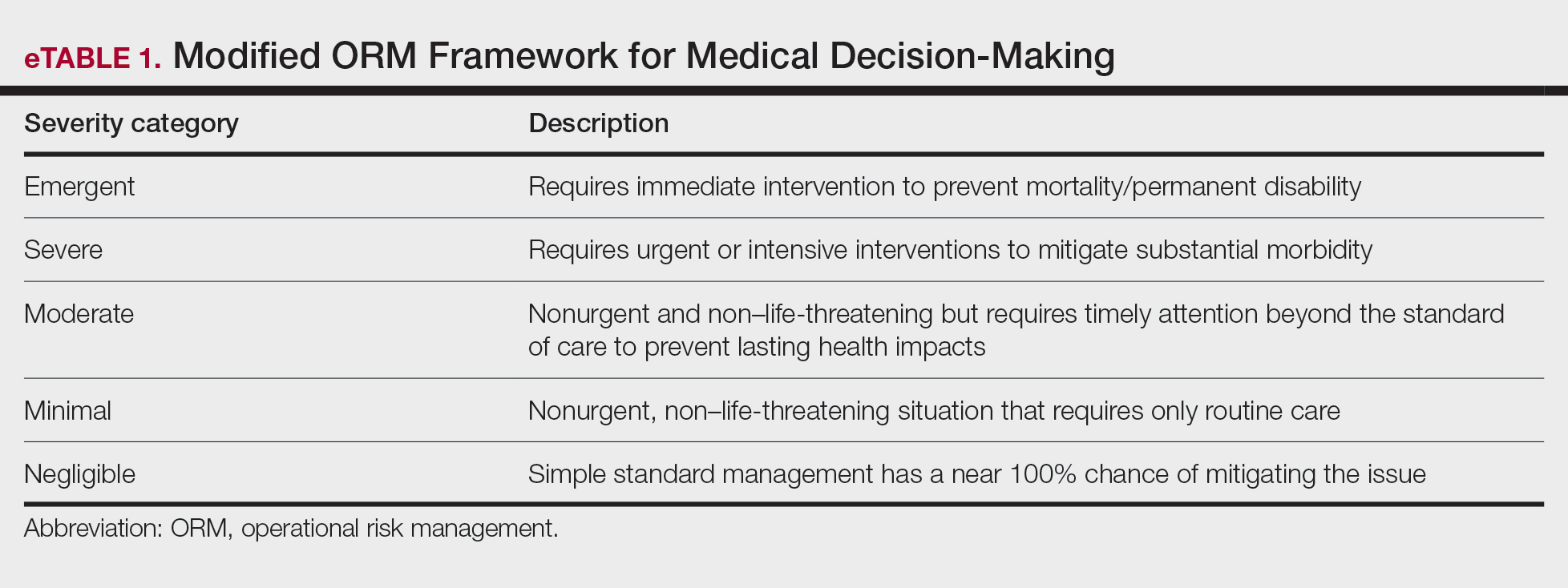
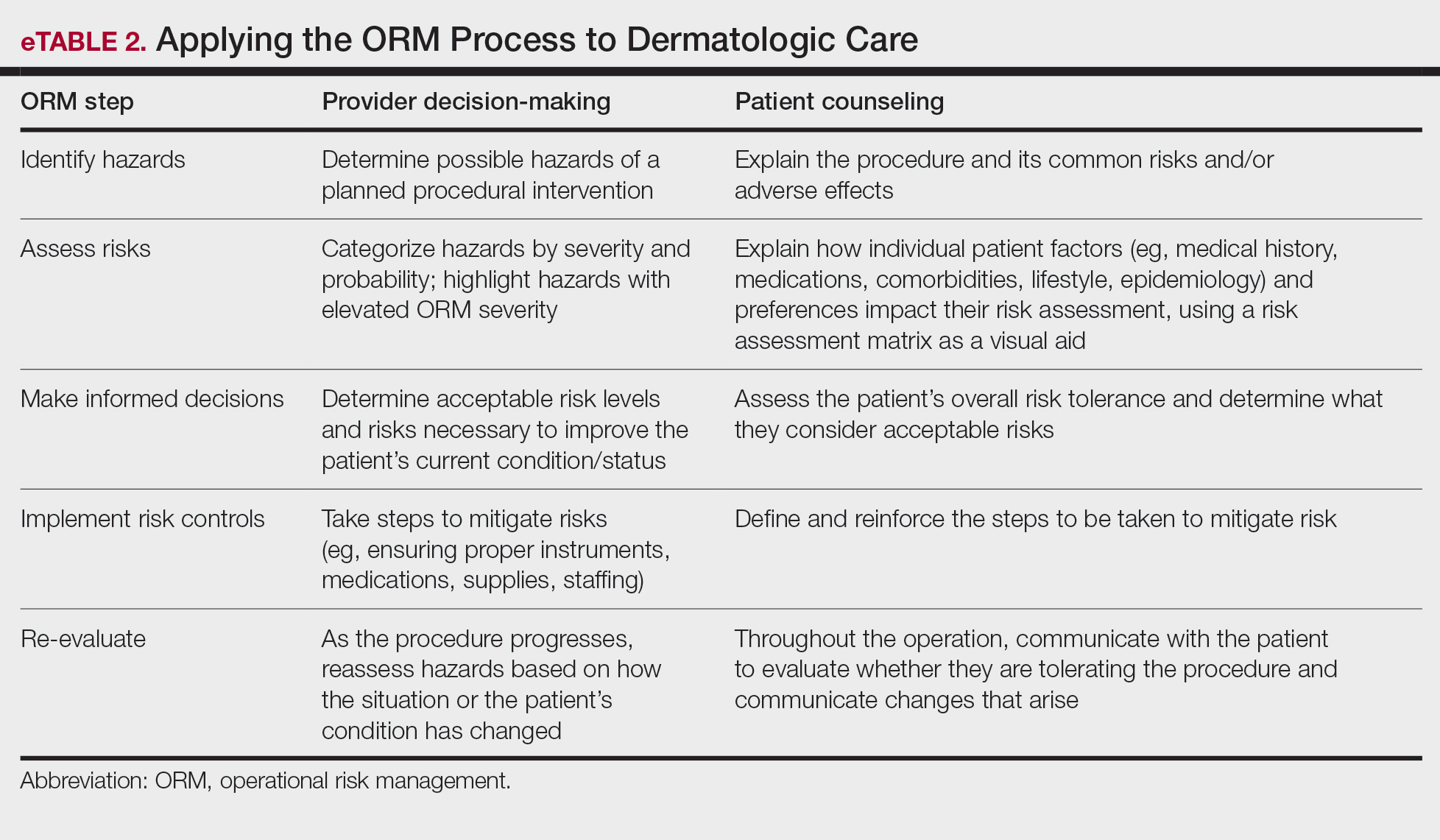
Patients often struggle to understand surgical risk severity, including overestimating the risks of routine minor procedures or underestimating the risks of more intensive procedures.7,8 Incorporating ORM into patient communication mirrors the provider’s process but uses patient-friendly terminology—it is discussion based and integrates patient preferences and tolerances (eTable 2). These steps often occur informally in dermatologic counseling; however, an organized structured approach, especially using a visual aid such as a risk assessment matrix, enhances patient comprehension, recall, and satisfaction.9
Practical Scenarios
Integrating ORM into dermatologic surgery is a proactive iterative process for both provider decision-making and patient communication. Leveraging a risk assessment matrix as a visual aid allows for clear identification, evaluation, and mitigation of hazards, fostering collaborative choices with regard to the treatment approach. Here we provide 2 case scenarios highlighting how ORM and the risk assessment matrix can be used in the management of a complex patient with a lesion in a high-risk location as well as to address patient anxiety and comorbidities. It is important to note that the way the matrices are completed in the examples provided may differ compared to other providers. The purpose of ORM is not to dictate risk categories but to serve as a tool for providers to take their own experiences and knowledge of the patient to guide their decision-making and counseling processes.
Case Scenario 1—An elderly man with a history of diabetes, cardiovascular accident, coronary artery bypass grafting, and multiple squamous cell carcinoma excisions presents for evaluation of a 1-cm squamous cell carcinoma in situ on the left leg. His current medications include an anticoagulant and antihypertensives.
In this scenario, the provider would apply ORM by identifying and assessing hazards, making risk decisions, implementing controls, and supervising care.
General hazards for excision on the leg include bleeding, infection, scarring, pain, delayed healing, activity limitations, and possible further procedures. Before the visit, the provider should prepare baseline risk matrices for 2 potential treatment options: wide local excision and electrodessication and curettage. For example, surgical bleeding may be assessed as negligible severity and almost certain probability for a general excision.
Next, the provider would incorporate the patient’s unique history in the risk matrices (eFigures 1 and 2). The patient’s use of an anticoagulant indicates a bleeding risk; therefore, the provider may shift the severity to minimal clinical concern, understanding the need for enhanced perioperative management. The history of diabetes also has a considerable impact on wound healing, so the provider might elevate the probability of delayed wound healing from rare to unlikely and the severity from moderate to severe. The prior cardiovascular accident also raises concerns about mobility and activity limitations during recovery, which could be escalated from minimal to moderate clinical concern if postoperative limitations on ambulation increase the risk for new clots. Based on this internal assessment, the provider identifies which risks are elevated and require further attention and discussion with the patient, helping tailor the counseling approach and potential treatment plan. The provider should begin to consider initial control measures such as coordinating anticoagulant management, ensuring diabetes is well controlled, and planning for postoperative ambulation support.
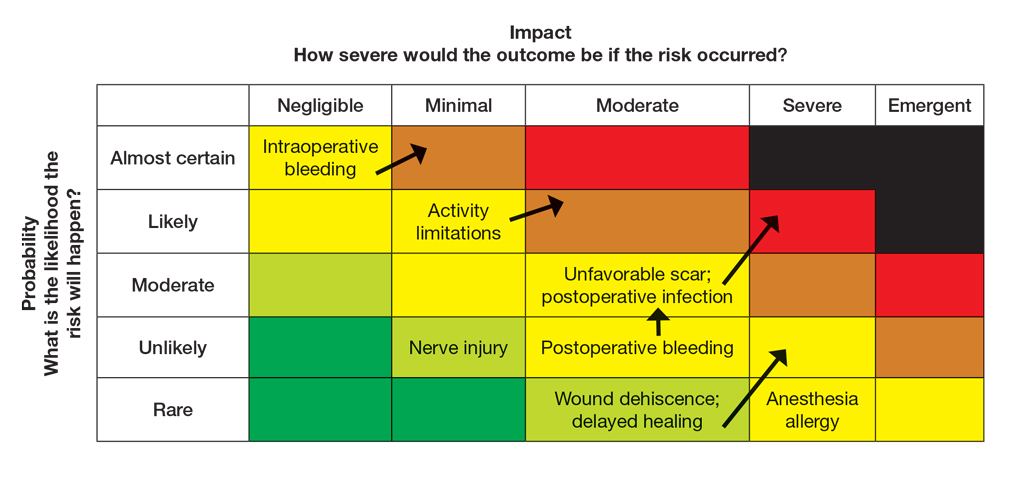
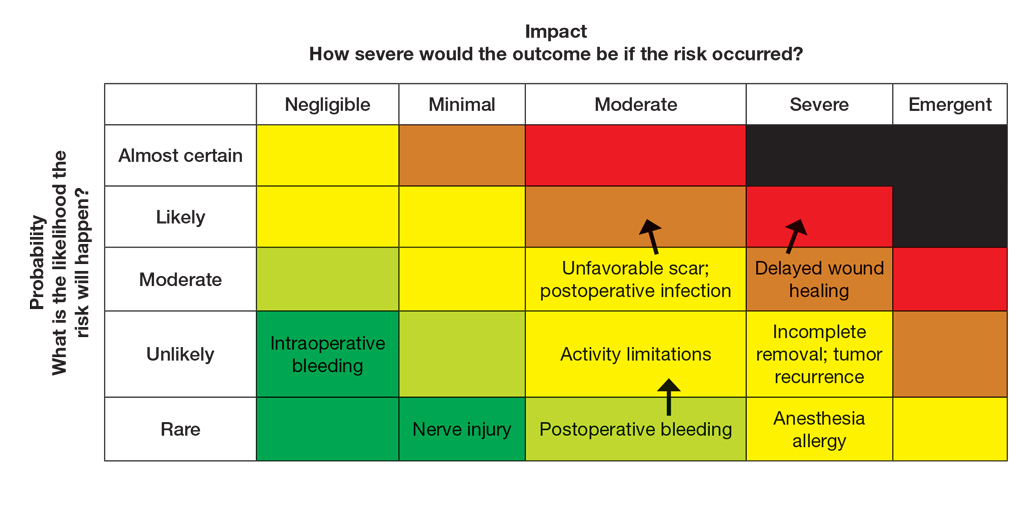
Once the provider has conducted the internal assessment, the ORM matrices become powerful tools for shared decision-making with the patient. The provider can walk the patient through the procedures and their common risks and then explain how their individual situation modifies the risks. The visual and explicit upgrade on the matrices allows the patient to clearly see how unique factors influence their personal risk profile, moving beyond a generic list of complications. The provider then should engage the patient in a discussion about their risk tolerance, which is crucial for mutual agreement on whether to proceed with treatment and, if so, which procedure is most appropriate given the patient’s comfort level with their individualized risk profile. Then the provider should reinforce the proactive steps planned to mitigate the identified risks to provide assurance and reinforce the collaborative approach to safety.
Finally, throughout the preoperative and postoperative phases, the provider should continuously monitor the patient’s condition and the effectiveness of the control measures, adjusting the plan as needed.
In this scenario, both the provider and the patient participated in the risk assessment, with the provider completing the assessment before the visit and presenting it to the patient or performing the assessment in real time with the patient present to explain the reasoning behind assignment of risk based on each procedure and the patient’s unique risk factors.
Case Scenario 2—A 38-year-old woman with a history of hypertension and procedural anxiety presents for evaluation of a biopsy-proven basal cell carcinoma on the nasal ala. The patient is taking diltiazem for hypertension and is compliant with her medication. Her blood pressure at the current visit is 148/96 mm Hg, which she attributes to white coat syndrome. Mohs micrographic surgery generally is the gold standard treatment for this case.
The provider’s ORM process, conducted either before or in real time during the visit, would begin with identification and assessment of the hazards. For Mohs surgery on the nasal ala, common hazards would include scarring, pain, infection, bleeding, and potential cosmetic distortion. Unique to this patient are the procedural anxiety and hypertension.
To populate the risk assessment matrix (eFigure 3), the provider would first map the baseline risks of Mohs surgery, which include considerable scarring as a moderate clinical concern but a seldom probability. Because the patient’s procedural anxiety directly increases the probability of intraoperative distress or elevated blood pressure during the procedure, the provider might assess patient distress/anxiety as a moderate clinical concern with a likely probability. While the patient’s blood pressure is controlled, the white coat syndrome raises the probability of hypertensive urgency/emergency during surgery; this might be elevated from unlikely to occasional or likely probability, and severity might increase from minimal to moderate due to its potential impact on procedural safety. The provider should consider strategies to address these elevated risks during the consultation. Then, as part of preprocedure planning, the provider should consider discussing anxiolytics, emphasizing medication compliance, and ensuring a calm environment for the patient’s surgery.
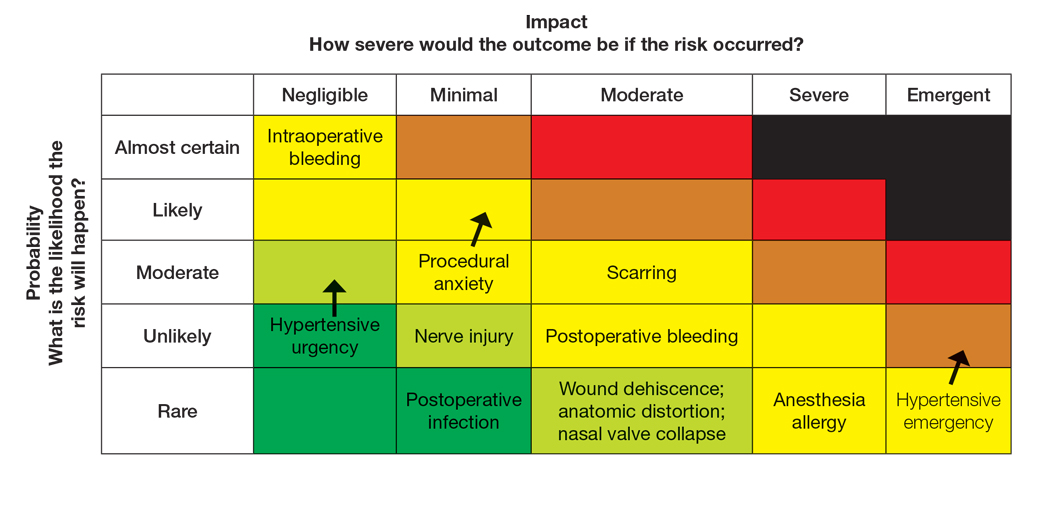
For this patient, the risk assessment matrix becomes a powerful tool to address fears and proactively manage her unique risk factors. To start the counseling process, the provider should explain the procedure, its benefits, and potential adverse effects. Then, the patient’s individualized risks can be visualized using the matrix, which also is an opportunity for reassurance, as it can alleviate patient fears by contextualizing rare but impactful outcomes.9
Now the provider can assess the patient’s risk tolerance. This discussion ensures that the patient’s comfort level and preferences are central to the treatment decision, even for a gold-standard procedure such as Mohs surgery. By listening and responding to the patient’s input, the provider can build trust and discuss strategies that can help control for some risk factors.
Finally, the provider would re-evaluate throughout the procedure by continuously monitoring the patient’s anxiety and vital signs. The provider should also be ready to adjust pain management or employ anxiety-reduction techniques.
Final Thoughts
Reviewing the risk assessment matrix can be an effective way to nonjudgmentally discuss a patient’s unique risk factors and provide a complete understanding of the planned treatment or procedure. It conveys to the patient that, as the provider, you are taking their health seriously when considering treatment options and can be a means to build patient rapport and trust. This approach mirrors risk communication strategies long employed in military operational planning, where transparency and structured risk evaluation are essential to maintaining mission readiness and unit cohesion.
- The OR Society. The history of OR. The OR Society. Published 2023.
- Naval Postgraduate School. ORM: operational risk management. Accessed September 12, 2025. https://nps.edu/web/safety/orm
- Smith C, Srivastava D, Nijhawan RI. Optimizing patient safety in dermatologic surgery. Dermatol Clin. 2019;37:319-328.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Pomerantz RG, Lee DA, Siegel DM. Risk assessment in surgical patients: balancing iatrogenic risks and benefits. Clin Dermatol. 2011;29:669-677.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surgeons. 2013;217:833-842.
- Lloyd AJ. The extent of patients’ understanding of the risk of treatments. BMJ Qual Saf. 2001;10:i14-i18.
- Falagas ME, Korbila IP, Giannopoulou KP, et al. Informed consent: how much and what do patients understand? Am J Surg. 2009;198:420-435.
- Cohen SM, Baimas-George M, Ponce C, et al. Is a picture worth a thousand words? a scoping review of the impact of visual aids on patients undergoing surgery. J Surg Educ. 2024;81:1276-1292.
Operational risk management (ORM) refers to the systematic identification and assessment of daily operational risks within an organization designed to mitigate negative financial, reputational, and safety outcomes while maximizing efficiency and achievement of objectives.1 Operational risk management is indispensable to modern military operations, optimizing mission readiness while minimizing complications and personnel morbidity. Application of ORM in medicine holds considerable promise due to the emphasis on precise and efficient decision-making in high-stakes environments, where the margin for error is minimal. In this article, we propose integrating ORM principles into dermatologic surgery to enhance patient-centered care through improved counseling, risk assessment, and procedural outcomes.
Principles and Processes of ORM
The ORM framework is built on 4 fundamental principles: accept risk when benefits outweigh the cost, accept no unnecessary risk, anticipate and manage risk by planning, and make risk decisions at the right level.2 These principles form the foundation of the ORM’s systematic 5-step approach to identify hazards, assess hazards, make risk decisions, implement controls, and supervise. Key to the ORM process is the use of risk assessment codes and the risk assessment matrix to quantify and prioritize risks. Risk assessment codes are numerical values assigned to hazards based on their assessed severity and probability. The risk assessment matrix is a tool that plots the severity of a hazard against its probability. By locating a hazard on the matrix, users can visualize its risk level in terms of severity and probability. Building and using the risk assessment matrix begins with determining severity by assessing the potential impact of a hazard and categorizing it into levels (catastrophic, critical, moderate, or negligible). Next, probability is determined by evaluating the likelihood of occurrence (frequent, likely, occasional, seldom, or unlikely). Finally, the severity and probability are combined to assign a risk assessment code, which indicates the risk level and helps visualize criticality. Systematically applying these principles and processes enables users to make informed decisions that balance mission objectives with safety.
Proposed Framework for ORM in Dermatology Surgery
Current risk mitigation in dermatologic surgery includes strict medication oversight, sterilization protocols, and photography to prevent wrong-site surgeries. Preoperative risk assessment through conducting a thorough patient history is vital, considering factors such as pregnancy, allergies, bleeding history, cardiac devices, and keloid propensity, all of which impact surgical outcomes.3-5 After gathering the patient’s history, dermatologists determine appropriateness for surgery and its inherent risks, typically via an informed consent process outlining the diagnosis and procedure purpose as well as a list of risks, benefits, and alternatives, including forgoing treatment.
Importantly, the standard process for dermatologic risk evaluation often lacks a comprehensive systematic approach seen in other higher-risk surgical fields. For example, general surgeons frequently utilize risk assessment calculators such as the one developed by the American College of Surgeons’ National Surgical Quality Improvement Program to estimate surgical complications.6 While specific guidelines exist for evaluating factors such as hypertension or anticoagulant use, no single tool synthesizes all patient risk factors for a unified assessment. Therefore, we propose integrating ORM as a structured decision-making process that offers a more consistent means for dermatologists to evaluate, synthesize, categorize, and present risks to patients. Our proposed process includes translating military mishap severity into a framework that helps patients better understand decisions about their health care when using ORM (eTable 1). The proposed process also provides dermatologists with a systematic, proactive, and iterative approach to assessing risks that allows them to consistently qualify medical decisions (eTable 2).


Patients often struggle to understand surgical risk severity, including overestimating the risks of routine minor procedures or underestimating the risks of more intensive procedures.7,8 Incorporating ORM into patient communication mirrors the provider’s process but uses patient-friendly terminology—it is discussion based and integrates patient preferences and tolerances (eTable 2). These steps often occur informally in dermatologic counseling; however, an organized structured approach, especially using a visual aid such as a risk assessment matrix, enhances patient comprehension, recall, and satisfaction.9
Practical Scenarios
Integrating ORM into dermatologic surgery is a proactive iterative process for both provider decision-making and patient communication. Leveraging a risk assessment matrix as a visual aid allows for clear identification, evaluation, and mitigation of hazards, fostering collaborative choices with regard to the treatment approach. Here we provide 2 case scenarios highlighting how ORM and the risk assessment matrix can be used in the management of a complex patient with a lesion in a high-risk location as well as to address patient anxiety and comorbidities. It is important to note that the way the matrices are completed in the examples provided may differ compared to other providers. The purpose of ORM is not to dictate risk categories but to serve as a tool for providers to take their own experiences and knowledge of the patient to guide their decision-making and counseling processes.
Case Scenario 1—An elderly man with a history of diabetes, cardiovascular accident, coronary artery bypass grafting, and multiple squamous cell carcinoma excisions presents for evaluation of a 1-cm squamous cell carcinoma in situ on the left leg. His current medications include an anticoagulant and antihypertensives.
In this scenario, the provider would apply ORM by identifying and assessing hazards, making risk decisions, implementing controls, and supervising care.
General hazards for excision on the leg include bleeding, infection, scarring, pain, delayed healing, activity limitations, and possible further procedures. Before the visit, the provider should prepare baseline risk matrices for 2 potential treatment options: wide local excision and electrodessication and curettage. For example, surgical bleeding may be assessed as negligible severity and almost certain probability for a general excision.
Next, the provider would incorporate the patient’s unique history in the risk matrices (eFigures 1 and 2). The patient’s use of an anticoagulant indicates a bleeding risk; therefore, the provider may shift the severity to minimal clinical concern, understanding the need for enhanced perioperative management. The history of diabetes also has a considerable impact on wound healing, so the provider might elevate the probability of delayed wound healing from rare to unlikely and the severity from moderate to severe. The prior cardiovascular accident also raises concerns about mobility and activity limitations during recovery, which could be escalated from minimal to moderate clinical concern if postoperative limitations on ambulation increase the risk for new clots. Based on this internal assessment, the provider identifies which risks are elevated and require further attention and discussion with the patient, helping tailor the counseling approach and potential treatment plan. The provider should begin to consider initial control measures such as coordinating anticoagulant management, ensuring diabetes is well controlled, and planning for postoperative ambulation support.


Once the provider has conducted the internal assessment, the ORM matrices become powerful tools for shared decision-making with the patient. The provider can walk the patient through the procedures and their common risks and then explain how their individual situation modifies the risks. The visual and explicit upgrade on the matrices allows the patient to clearly see how unique factors influence their personal risk profile, moving beyond a generic list of complications. The provider then should engage the patient in a discussion about their risk tolerance, which is crucial for mutual agreement on whether to proceed with treatment and, if so, which procedure is most appropriate given the patient’s comfort level with their individualized risk profile. Then the provider should reinforce the proactive steps planned to mitigate the identified risks to provide assurance and reinforce the collaborative approach to safety.
Finally, throughout the preoperative and postoperative phases, the provider should continuously monitor the patient’s condition and the effectiveness of the control measures, adjusting the plan as needed.
In this scenario, both the provider and the patient participated in the risk assessment, with the provider completing the assessment before the visit and presenting it to the patient or performing the assessment in real time with the patient present to explain the reasoning behind assignment of risk based on each procedure and the patient’s unique risk factors.
Case Scenario 2—A 38-year-old woman with a history of hypertension and procedural anxiety presents for evaluation of a biopsy-proven basal cell carcinoma on the nasal ala. The patient is taking diltiazem for hypertension and is compliant with her medication. Her blood pressure at the current visit is 148/96 mm Hg, which she attributes to white coat syndrome. Mohs micrographic surgery generally is the gold standard treatment for this case.
The provider’s ORM process, conducted either before or in real time during the visit, would begin with identification and assessment of the hazards. For Mohs surgery on the nasal ala, common hazards would include scarring, pain, infection, bleeding, and potential cosmetic distortion. Unique to this patient are the procedural anxiety and hypertension.
To populate the risk assessment matrix (eFigure 3), the provider would first map the baseline risks of Mohs surgery, which include considerable scarring as a moderate clinical concern but a seldom probability. Because the patient’s procedural anxiety directly increases the probability of intraoperative distress or elevated blood pressure during the procedure, the provider might assess patient distress/anxiety as a moderate clinical concern with a likely probability. While the patient’s blood pressure is controlled, the white coat syndrome raises the probability of hypertensive urgency/emergency during surgery; this might be elevated from unlikely to occasional or likely probability, and severity might increase from minimal to moderate due to its potential impact on procedural safety. The provider should consider strategies to address these elevated risks during the consultation. Then, as part of preprocedure planning, the provider should consider discussing anxiolytics, emphasizing medication compliance, and ensuring a calm environment for the patient’s surgery.

For this patient, the risk assessment matrix becomes a powerful tool to address fears and proactively manage her unique risk factors. To start the counseling process, the provider should explain the procedure, its benefits, and potential adverse effects. Then, the patient’s individualized risks can be visualized using the matrix, which also is an opportunity for reassurance, as it can alleviate patient fears by contextualizing rare but impactful outcomes.9
Now the provider can assess the patient’s risk tolerance. This discussion ensures that the patient’s comfort level and preferences are central to the treatment decision, even for a gold-standard procedure such as Mohs surgery. By listening and responding to the patient’s input, the provider can build trust and discuss strategies that can help control for some risk factors.
Finally, the provider would re-evaluate throughout the procedure by continuously monitoring the patient’s anxiety and vital signs. The provider should also be ready to adjust pain management or employ anxiety-reduction techniques.
Final Thoughts
Reviewing the risk assessment matrix can be an effective way to nonjudgmentally discuss a patient’s unique risk factors and provide a complete understanding of the planned treatment or procedure. It conveys to the patient that, as the provider, you are taking their health seriously when considering treatment options and can be a means to build patient rapport and trust. This approach mirrors risk communication strategies long employed in military operational planning, where transparency and structured risk evaluation are essential to maintaining mission readiness and unit cohesion.
Operational risk management (ORM) refers to the systematic identification and assessment of daily operational risks within an organization designed to mitigate negative financial, reputational, and safety outcomes while maximizing efficiency and achievement of objectives.1 Operational risk management is indispensable to modern military operations, optimizing mission readiness while minimizing complications and personnel morbidity. Application of ORM in medicine holds considerable promise due to the emphasis on precise and efficient decision-making in high-stakes environments, where the margin for error is minimal. In this article, we propose integrating ORM principles into dermatologic surgery to enhance patient-centered care through improved counseling, risk assessment, and procedural outcomes.
Principles and Processes of ORM
The ORM framework is built on 4 fundamental principles: accept risk when benefits outweigh the cost, accept no unnecessary risk, anticipate and manage risk by planning, and make risk decisions at the right level.2 These principles form the foundation of the ORM’s systematic 5-step approach to identify hazards, assess hazards, make risk decisions, implement controls, and supervise. Key to the ORM process is the use of risk assessment codes and the risk assessment matrix to quantify and prioritize risks. Risk assessment codes are numerical values assigned to hazards based on their assessed severity and probability. The risk assessment matrix is a tool that plots the severity of a hazard against its probability. By locating a hazard on the matrix, users can visualize its risk level in terms of severity and probability. Building and using the risk assessment matrix begins with determining severity by assessing the potential impact of a hazard and categorizing it into levels (catastrophic, critical, moderate, or negligible). Next, probability is determined by evaluating the likelihood of occurrence (frequent, likely, occasional, seldom, or unlikely). Finally, the severity and probability are combined to assign a risk assessment code, which indicates the risk level and helps visualize criticality. Systematically applying these principles and processes enables users to make informed decisions that balance mission objectives with safety.
Proposed Framework for ORM in Dermatology Surgery
Current risk mitigation in dermatologic surgery includes strict medication oversight, sterilization protocols, and photography to prevent wrong-site surgeries. Preoperative risk assessment through conducting a thorough patient history is vital, considering factors such as pregnancy, allergies, bleeding history, cardiac devices, and keloid propensity, all of which impact surgical outcomes.3-5 After gathering the patient’s history, dermatologists determine appropriateness for surgery and its inherent risks, typically via an informed consent process outlining the diagnosis and procedure purpose as well as a list of risks, benefits, and alternatives, including forgoing treatment.
Importantly, the standard process for dermatologic risk evaluation often lacks a comprehensive systematic approach seen in other higher-risk surgical fields. For example, general surgeons frequently utilize risk assessment calculators such as the one developed by the American College of Surgeons’ National Surgical Quality Improvement Program to estimate surgical complications.6 While specific guidelines exist for evaluating factors such as hypertension or anticoagulant use, no single tool synthesizes all patient risk factors for a unified assessment. Therefore, we propose integrating ORM as a structured decision-making process that offers a more consistent means for dermatologists to evaluate, synthesize, categorize, and present risks to patients. Our proposed process includes translating military mishap severity into a framework that helps patients better understand decisions about their health care when using ORM (eTable 1). The proposed process also provides dermatologists with a systematic, proactive, and iterative approach to assessing risks that allows them to consistently qualify medical decisions (eTable 2).


Patients often struggle to understand surgical risk severity, including overestimating the risks of routine minor procedures or underestimating the risks of more intensive procedures.7,8 Incorporating ORM into patient communication mirrors the provider’s process but uses patient-friendly terminology—it is discussion based and integrates patient preferences and tolerances (eTable 2). These steps often occur informally in dermatologic counseling; however, an organized structured approach, especially using a visual aid such as a risk assessment matrix, enhances patient comprehension, recall, and satisfaction.9
Practical Scenarios
Integrating ORM into dermatologic surgery is a proactive iterative process for both provider decision-making and patient communication. Leveraging a risk assessment matrix as a visual aid allows for clear identification, evaluation, and mitigation of hazards, fostering collaborative choices with regard to the treatment approach. Here we provide 2 case scenarios highlighting how ORM and the risk assessment matrix can be used in the management of a complex patient with a lesion in a high-risk location as well as to address patient anxiety and comorbidities. It is important to note that the way the matrices are completed in the examples provided may differ compared to other providers. The purpose of ORM is not to dictate risk categories but to serve as a tool for providers to take their own experiences and knowledge of the patient to guide their decision-making and counseling processes.
Case Scenario 1—An elderly man with a history of diabetes, cardiovascular accident, coronary artery bypass grafting, and multiple squamous cell carcinoma excisions presents for evaluation of a 1-cm squamous cell carcinoma in situ on the left leg. His current medications include an anticoagulant and antihypertensives.
In this scenario, the provider would apply ORM by identifying and assessing hazards, making risk decisions, implementing controls, and supervising care.
General hazards for excision on the leg include bleeding, infection, scarring, pain, delayed healing, activity limitations, and possible further procedures. Before the visit, the provider should prepare baseline risk matrices for 2 potential treatment options: wide local excision and electrodessication and curettage. For example, surgical bleeding may be assessed as negligible severity and almost certain probability for a general excision.
Next, the provider would incorporate the patient’s unique history in the risk matrices (eFigures 1 and 2). The patient’s use of an anticoagulant indicates a bleeding risk; therefore, the provider may shift the severity to minimal clinical concern, understanding the need for enhanced perioperative management. The history of diabetes also has a considerable impact on wound healing, so the provider might elevate the probability of delayed wound healing from rare to unlikely and the severity from moderate to severe. The prior cardiovascular accident also raises concerns about mobility and activity limitations during recovery, which could be escalated from minimal to moderate clinical concern if postoperative limitations on ambulation increase the risk for new clots. Based on this internal assessment, the provider identifies which risks are elevated and require further attention and discussion with the patient, helping tailor the counseling approach and potential treatment plan. The provider should begin to consider initial control measures such as coordinating anticoagulant management, ensuring diabetes is well controlled, and planning for postoperative ambulation support.


Once the provider has conducted the internal assessment, the ORM matrices become powerful tools for shared decision-making with the patient. The provider can walk the patient through the procedures and their common risks and then explain how their individual situation modifies the risks. The visual and explicit upgrade on the matrices allows the patient to clearly see how unique factors influence their personal risk profile, moving beyond a generic list of complications. The provider then should engage the patient in a discussion about their risk tolerance, which is crucial for mutual agreement on whether to proceed with treatment and, if so, which procedure is most appropriate given the patient’s comfort level with their individualized risk profile. Then the provider should reinforce the proactive steps planned to mitigate the identified risks to provide assurance and reinforce the collaborative approach to safety.
Finally, throughout the preoperative and postoperative phases, the provider should continuously monitor the patient’s condition and the effectiveness of the control measures, adjusting the plan as needed.
In this scenario, both the provider and the patient participated in the risk assessment, with the provider completing the assessment before the visit and presenting it to the patient or performing the assessment in real time with the patient present to explain the reasoning behind assignment of risk based on each procedure and the patient’s unique risk factors.
Case Scenario 2—A 38-year-old woman with a history of hypertension and procedural anxiety presents for evaluation of a biopsy-proven basal cell carcinoma on the nasal ala. The patient is taking diltiazem for hypertension and is compliant with her medication. Her blood pressure at the current visit is 148/96 mm Hg, which she attributes to white coat syndrome. Mohs micrographic surgery generally is the gold standard treatment for this case.
The provider’s ORM process, conducted either before or in real time during the visit, would begin with identification and assessment of the hazards. For Mohs surgery on the nasal ala, common hazards would include scarring, pain, infection, bleeding, and potential cosmetic distortion. Unique to this patient are the procedural anxiety and hypertension.
To populate the risk assessment matrix (eFigure 3), the provider would first map the baseline risks of Mohs surgery, which include considerable scarring as a moderate clinical concern but a seldom probability. Because the patient’s procedural anxiety directly increases the probability of intraoperative distress or elevated blood pressure during the procedure, the provider might assess patient distress/anxiety as a moderate clinical concern with a likely probability. While the patient’s blood pressure is controlled, the white coat syndrome raises the probability of hypertensive urgency/emergency during surgery; this might be elevated from unlikely to occasional or likely probability, and severity might increase from minimal to moderate due to its potential impact on procedural safety. The provider should consider strategies to address these elevated risks during the consultation. Then, as part of preprocedure planning, the provider should consider discussing anxiolytics, emphasizing medication compliance, and ensuring a calm environment for the patient’s surgery.

For this patient, the risk assessment matrix becomes a powerful tool to address fears and proactively manage her unique risk factors. To start the counseling process, the provider should explain the procedure, its benefits, and potential adverse effects. Then, the patient’s individualized risks can be visualized using the matrix, which also is an opportunity for reassurance, as it can alleviate patient fears by contextualizing rare but impactful outcomes.9
Now the provider can assess the patient’s risk tolerance. This discussion ensures that the patient’s comfort level and preferences are central to the treatment decision, even for a gold-standard procedure such as Mohs surgery. By listening and responding to the patient’s input, the provider can build trust and discuss strategies that can help control for some risk factors.
Finally, the provider would re-evaluate throughout the procedure by continuously monitoring the patient’s anxiety and vital signs. The provider should also be ready to adjust pain management or employ anxiety-reduction techniques.
Final Thoughts
Reviewing the risk assessment matrix can be an effective way to nonjudgmentally discuss a patient’s unique risk factors and provide a complete understanding of the planned treatment or procedure. It conveys to the patient that, as the provider, you are taking their health seriously when considering treatment options and can be a means to build patient rapport and trust. This approach mirrors risk communication strategies long employed in military operational planning, where transparency and structured risk evaluation are essential to maintaining mission readiness and unit cohesion.
- The OR Society. The history of OR. The OR Society. Published 2023.
- Naval Postgraduate School. ORM: operational risk management. Accessed September 12, 2025. https://nps.edu/web/safety/orm
- Smith C, Srivastava D, Nijhawan RI. Optimizing patient safety in dermatologic surgery. Dermatol Clin. 2019;37:319-328.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Pomerantz RG, Lee DA, Siegel DM. Risk assessment in surgical patients: balancing iatrogenic risks and benefits. Clin Dermatol. 2011;29:669-677.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surgeons. 2013;217:833-842.
- Lloyd AJ. The extent of patients’ understanding of the risk of treatments. BMJ Qual Saf. 2001;10:i14-i18.
- Falagas ME, Korbila IP, Giannopoulou KP, et al. Informed consent: how much and what do patients understand? Am J Surg. 2009;198:420-435.
- Cohen SM, Baimas-George M, Ponce C, et al. Is a picture worth a thousand words? a scoping review of the impact of visual aids on patients undergoing surgery. J Surg Educ. 2024;81:1276-1292.
- The OR Society. The history of OR. The OR Society. Published 2023.
- Naval Postgraduate School. ORM: operational risk management. Accessed September 12, 2025. https://nps.edu/web/safety/orm
- Smith C, Srivastava D, Nijhawan RI. Optimizing patient safety in dermatologic surgery. Dermatol Clin. 2019;37:319-328.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Pomerantz RG, Lee DA, Siegel DM. Risk assessment in surgical patients: balancing iatrogenic risks and benefits. Clin Dermatol. 2011;29:669-677.
- Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surgeons. 2013;217:833-842.
- Lloyd AJ. The extent of patients’ understanding of the risk of treatments. BMJ Qual Saf. 2001;10:i14-i18.
- Falagas ME, Korbila IP, Giannopoulou KP, et al. Informed consent: how much and what do patients understand? Am J Surg. 2009;198:420-435.
- Cohen SM, Baimas-George M, Ponce C, et al. Is a picture worth a thousand words? a scoping review of the impact of visual aids on patients undergoing surgery. J Surg Educ. 2024;81:1276-1292.
Operational Risk Management in Dermatologic Procedures
Operational Risk Management in Dermatologic Procedures
Dermoscopic Documentation of a No-see-um Bite
Dermoscopic Documentation of a No-see-um Bite
Biting midges, commonly known as no-see-ums, are true flies (order Diptera) and members of the Ceratopogonidae family. Regionally, they are known as punkies in the Northeast, pinyon gnats in the Southwest, moose flies in Canada, and sand gnats in Georgia, among other names.1 There are 6206 species found worldwide except for Antarctica.2 The 3 genera of greatest importance to human and livestock health in the United States are Culicoides, Leptoconops, and Forcipomyia.1 Forty-seven species of the genus Culicoides are known to be present in Florida.3 Species belonging to the genus Leptoconops also are present in coastal areas of southeast Florida as well as in the tropics, subtropics, and Caribbean.3 In the United States, biting midges primarily are a nuisance; the major medical issue associated with Culicoides insects are allergic reactions to their bites. Even though no-see-ums are not known to transmit disease in humans, they have an impact on other animal species in the United States as biting pests and vectors of disease-causing pathogens.1 Biting midges pose quite a nuisance for the proper enjoyment of outdoor spaces in the southeastern United States.
Characteristics
Morphologically, no-see-ums are gray flies measuring 1 to 3 mm in length (eFigure 1). Adults have 2 wings with distinctive patterns, large compound eyes, a thorax that extends slightly over the head, an abdomen with 9 segments, and antennae with 15 segments (eFigure 2).1,3,4 Females have modified mouth parts including mandibles that lacerate the skin during feeding, which is mainly on blood from vertebrate hosts (primarily mammals but also birds, reptiles, and amphibians).1,4 They also can feed on invertebrate hosts. Both male and female no-see-ums feed on nectar, but adult females require a blood meal to develop their eggs.2 Their life cycle progresses in stages from egg to larva to pupa to adult. Larval habitats include salt marshes, swamps, shores of streams and ponds, water-holding plants, rotting fruit, and saturated wood- and manure-enriched soil. Adults can live 2 to 7 weeks. They are weak fliers, particularly in windy conditions.1


In Florida, no-see-ums are more active during the rainy months of May to October but are active year-round in the southeastern United States and the Gulf Coast from Florida to West Texas. They are active throughout the United States in the warmer months of June and July.5 Their peak feeding activity occurs at dawn and dusk, but different species of biting midges such as Leptoconops and Culicoides also can feed during daylight hours and at night, respectively.1,6,7
Case Report
One of the authors (M.J.S.), a healthy 54-year-old man with no remarkable medical history or current use of medications, documented the natural progression of a no-see-um bite by sitting in an outdoor Florida space at 8:00

Clinical Manifestations
Although no-see-ums are not known to transmit disease in the United States, they are important biting pests that can affect tourism and prevent enjoyment of outdoor spaces and activities.2 The bite reactions on the host can range from wheal-like lesions to papules measuring 2 to 3 mm (at times with overlying vesicles) to nodules up to 1 cm in diameter.8 In our reported case, the small wheals disappeared within hours, but pruritic papules have been described to last from weeks to months. Published histopathologic correlation of biopsied indurated papules within 3 days of bite occurrence have revealed a superficial infiltrate composed of lymphocytes and histiocytes, while eosinophils were found in the deeper dermis and subcutaneous fat. Within 2 weeks, as the lesions aged, the infiltrate contained a smaller percentage of eosinophils and predominantly was present in only the superficial dermis.8 Delayed-type hypersensitivity reactions including pustules and bullous lesions also have been described.9,10 Host immune reaction to the saliva introduced during the bite dictates the severity of the response, and lesions may become secondarily infected due to scratching.11
Management Recommendations
Management consists of cleaning the bite site with soap and water to prevent infection, applying cold compresses or ice packs to relieve the intense itch, and avoiding scratching.11 Application of over-the-counter calamine lotion or hydrocortisone cream can relieve itch, and mid- to high-potency topical corticosteroids also can be prescribed for 1 to 2 weeks for more intense bite reactions in conjunction with oral antihistamines. Topical or oral antibiotics may be indicated if redness and swelling progress at the bite site or if breaks in the skin become secondarily infected.
Final Thoughts
Because of the wide-ranging habitats of no-see-ums, eradication programs using insecticides have been inefficient or environmentally suboptimal. Emptying all standing water in outdoor spaces will reduce the number of no-see-ums. Avoidance of the outdoors at dawn and dusk when no-see-ums are most active is helpful, as well as protecting exposed skin by wearing long-sleeved shirts and long pants when outside. Insect repellents containing DEET (N-N-diethyl-meta-toluamide) or picaridin can offer additional protection on the remaining exposed skin. Oil of lemon eucalyptus, or active compound p-menthane-3,8-diol, has been shown to be effective against no-see-ums. Use of DEET should be avoided in children younger than 2 years and p-menthane-3,8-diol in those younger than 3 years. Picaridin is safe for use in children.12 Citronella oil is ineffective. Installing window and patio screens with a mesh size less than 16 can prevent no-see-ums from passing through the netting but will restrict air flow.3 Turning off porch lights also is helpful, as no-see-ums are attracted to light sources.6 Since no-see-ums are weak flyers, setting ceiling or window fans at high speeds can minimize exposure; similarly, being outdoors on a windy day may decrease the likelihood of being bitten. Ultimately, the best remedy for a bite is to prevent them from happening.
- Hill CA, MacDonald JF. Biting midges: biology and public health risk. Purdue University. Published July 2013. Accessed September 3, 2025. http://extension.entm.purdue.edu/publichealth/insects/bitingmidge.html
- Borkent A, Dominiak P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa. 2020;4787:1-377.
- Connelly CR. Biting midges, no-see-ums Culicoides spp. (Insecta: Diptera: Ceratopogonidae). University of Florida publication #EENY 349. Published August 2, 2022. Accessed September 3, 2025. https://edis.ifas.ufl.edu/publication/IN626
- Mullen GR, Murphree CS. Biting midges (Ceratopogonidae). In: Mullen GR, Durden LA, eds. Medical and Veterinary Entomology. 3rd ed. Academic Press; 2019:213-236.
- Best Bee Brothers. No-see-um seasonality range map & season information. Published March 4, 2022. Accessed September 3, 2025. https://bestbeebrothers.com/blogs/blog/no-see-um-season
- Biology Insights. Is there a season for no see ums in Florida? Published August 28, 2025. Accessed September 16, 2025. https://biologyinsights.com/is-there-a-season-for-no-see-ums-in-florida/
- Burris S. Florida no see ums: how to navigate the woes of no see ums in Florida. The Bug Agenda. Published February 2, 2022. Accessed September 3, 2025. https://thebugagenda.com/no-see-ums-in-florida/
- Steffen C. Clinical and histopathologic correlation of midge bites. Arch Dermatol. 1981;117:785-787.
- Krakowski AC, Ho B. Arthropod assault from biting midges. J Pediatr. 2013;163:298.
- Maves RC, Reaves EJ, Martin GJ. Images in clinical tropical medicine: bullous leg lesions caused by Culicoides midges after travel in the Amazon basin. Am J Trop Med Hyg. 2010;83:447.
- Swank B. How long do no-see-ums live? Pest Source. Updated March 17, 2025. Accessed September 3, 2025. https://pestsource.com/no-see-um/lifespan/
- Nguyen QD, Vu MN, Herbert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2023;88:123-130.
Biting midges, commonly known as no-see-ums, are true flies (order Diptera) and members of the Ceratopogonidae family. Regionally, they are known as punkies in the Northeast, pinyon gnats in the Southwest, moose flies in Canada, and sand gnats in Georgia, among other names.1 There are 6206 species found worldwide except for Antarctica.2 The 3 genera of greatest importance to human and livestock health in the United States are Culicoides, Leptoconops, and Forcipomyia.1 Forty-seven species of the genus Culicoides are known to be present in Florida.3 Species belonging to the genus Leptoconops also are present in coastal areas of southeast Florida as well as in the tropics, subtropics, and Caribbean.3 In the United States, biting midges primarily are a nuisance; the major medical issue associated with Culicoides insects are allergic reactions to their bites. Even though no-see-ums are not known to transmit disease in humans, they have an impact on other animal species in the United States as biting pests and vectors of disease-causing pathogens.1 Biting midges pose quite a nuisance for the proper enjoyment of outdoor spaces in the southeastern United States.
Characteristics
Morphologically, no-see-ums are gray flies measuring 1 to 3 mm in length (eFigure 1). Adults have 2 wings with distinctive patterns, large compound eyes, a thorax that extends slightly over the head, an abdomen with 9 segments, and antennae with 15 segments (eFigure 2).1,3,4 Females have modified mouth parts including mandibles that lacerate the skin during feeding, which is mainly on blood from vertebrate hosts (primarily mammals but also birds, reptiles, and amphibians).1,4 They also can feed on invertebrate hosts. Both male and female no-see-ums feed on nectar, but adult females require a blood meal to develop their eggs.2 Their life cycle progresses in stages from egg to larva to pupa to adult. Larval habitats include salt marshes, swamps, shores of streams and ponds, water-holding plants, rotting fruit, and saturated wood- and manure-enriched soil. Adults can live 2 to 7 weeks. They are weak fliers, particularly in windy conditions.1


In Florida, no-see-ums are more active during the rainy months of May to October but are active year-round in the southeastern United States and the Gulf Coast from Florida to West Texas. They are active throughout the United States in the warmer months of June and July.5 Their peak feeding activity occurs at dawn and dusk, but different species of biting midges such as Leptoconops and Culicoides also can feed during daylight hours and at night, respectively.1,6,7
Case Report
One of the authors (M.J.S.), a healthy 54-year-old man with no remarkable medical history or current use of medications, documented the natural progression of a no-see-um bite by sitting in an outdoor Florida space at 8:00



Clinical Manifestations
Although no-see-ums are not known to transmit disease in the United States, they are important biting pests that can affect tourism and prevent enjoyment of outdoor spaces and activities.2 The bite reactions on the host can range from wheal-like lesions to papules measuring 2 to 3 mm (at times with overlying vesicles) to nodules up to 1 cm in diameter.8 In our reported case, the small wheals disappeared within hours, but pruritic papules have been described to last from weeks to months. Published histopathologic correlation of biopsied indurated papules within 3 days of bite occurrence have revealed a superficial infiltrate composed of lymphocytes and histiocytes, while eosinophils were found in the deeper dermis and subcutaneous fat. Within 2 weeks, as the lesions aged, the infiltrate contained a smaller percentage of eosinophils and predominantly was present in only the superficial dermis.8 Delayed-type hypersensitivity reactions including pustules and bullous lesions also have been described.9,10 Host immune reaction to the saliva introduced during the bite dictates the severity of the response, and lesions may become secondarily infected due to scratching.11
Management Recommendations
Management consists of cleaning the bite site with soap and water to prevent infection, applying cold compresses or ice packs to relieve the intense itch, and avoiding scratching.11 Application of over-the-counter calamine lotion or hydrocortisone cream can relieve itch, and mid- to high-potency topical corticosteroids also can be prescribed for 1 to 2 weeks for more intense bite reactions in conjunction with oral antihistamines. Topical or oral antibiotics may be indicated if redness and swelling progress at the bite site or if breaks in the skin become secondarily infected.
Final Thoughts
Because of the wide-ranging habitats of no-see-ums, eradication programs using insecticides have been inefficient or environmentally suboptimal. Emptying all standing water in outdoor spaces will reduce the number of no-see-ums. Avoidance of the outdoors at dawn and dusk when no-see-ums are most active is helpful, as well as protecting exposed skin by wearing long-sleeved shirts and long pants when outside. Insect repellents containing DEET (N-N-diethyl-meta-toluamide) or picaridin can offer additional protection on the remaining exposed skin. Oil of lemon eucalyptus, or active compound p-menthane-3,8-diol, has been shown to be effective against no-see-ums. Use of DEET should be avoided in children younger than 2 years and p-menthane-3,8-diol in those younger than 3 years. Picaridin is safe for use in children.12 Citronella oil is ineffective. Installing window and patio screens with a mesh size less than 16 can prevent no-see-ums from passing through the netting but will restrict air flow.3 Turning off porch lights also is helpful, as no-see-ums are attracted to light sources.6 Since no-see-ums are weak flyers, setting ceiling or window fans at high speeds can minimize exposure; similarly, being outdoors on a windy day may decrease the likelihood of being bitten. Ultimately, the best remedy for a bite is to prevent them from happening.
Biting midges, commonly known as no-see-ums, are true flies (order Diptera) and members of the Ceratopogonidae family. Regionally, they are known as punkies in the Northeast, pinyon gnats in the Southwest, moose flies in Canada, and sand gnats in Georgia, among other names.1 There are 6206 species found worldwide except for Antarctica.2 The 3 genera of greatest importance to human and livestock health in the United States are Culicoides, Leptoconops, and Forcipomyia.1 Forty-seven species of the genus Culicoides are known to be present in Florida.3 Species belonging to the genus Leptoconops also are present in coastal areas of southeast Florida as well as in the tropics, subtropics, and Caribbean.3 In the United States, biting midges primarily are a nuisance; the major medical issue associated with Culicoides insects are allergic reactions to their bites. Even though no-see-ums are not known to transmit disease in humans, they have an impact on other animal species in the United States as biting pests and vectors of disease-causing pathogens.1 Biting midges pose quite a nuisance for the proper enjoyment of outdoor spaces in the southeastern United States.
Characteristics
Morphologically, no-see-ums are gray flies measuring 1 to 3 mm in length (eFigure 1). Adults have 2 wings with distinctive patterns, large compound eyes, a thorax that extends slightly over the head, an abdomen with 9 segments, and antennae with 15 segments (eFigure 2).1,3,4 Females have modified mouth parts including mandibles that lacerate the skin during feeding, which is mainly on blood from vertebrate hosts (primarily mammals but also birds, reptiles, and amphibians).1,4 They also can feed on invertebrate hosts. Both male and female no-see-ums feed on nectar, but adult females require a blood meal to develop their eggs.2 Their life cycle progresses in stages from egg to larva to pupa to adult. Larval habitats include salt marshes, swamps, shores of streams and ponds, water-holding plants, rotting fruit, and saturated wood- and manure-enriched soil. Adults can live 2 to 7 weeks. They are weak fliers, particularly in windy conditions.1


In Florida, no-see-ums are more active during the rainy months of May to October but are active year-round in the southeastern United States and the Gulf Coast from Florida to West Texas. They are active throughout the United States in the warmer months of June and July.5 Their peak feeding activity occurs at dawn and dusk, but different species of biting midges such as Leptoconops and Culicoides also can feed during daylight hours and at night, respectively.1,6,7
Case Report
One of the authors (M.J.S.), a healthy 54-year-old man with no remarkable medical history or current use of medications, documented the natural progression of a no-see-um bite by sitting in an outdoor Florida space at 8:00



Clinical Manifestations
Although no-see-ums are not known to transmit disease in the United States, they are important biting pests that can affect tourism and prevent enjoyment of outdoor spaces and activities.2 The bite reactions on the host can range from wheal-like lesions to papules measuring 2 to 3 mm (at times with overlying vesicles) to nodules up to 1 cm in diameter.8 In our reported case, the small wheals disappeared within hours, but pruritic papules have been described to last from weeks to months. Published histopathologic correlation of biopsied indurated papules within 3 days of bite occurrence have revealed a superficial infiltrate composed of lymphocytes and histiocytes, while eosinophils were found in the deeper dermis and subcutaneous fat. Within 2 weeks, as the lesions aged, the infiltrate contained a smaller percentage of eosinophils and predominantly was present in only the superficial dermis.8 Delayed-type hypersensitivity reactions including pustules and bullous lesions also have been described.9,10 Host immune reaction to the saliva introduced during the bite dictates the severity of the response, and lesions may become secondarily infected due to scratching.11
Management Recommendations
Management consists of cleaning the bite site with soap and water to prevent infection, applying cold compresses or ice packs to relieve the intense itch, and avoiding scratching.11 Application of over-the-counter calamine lotion or hydrocortisone cream can relieve itch, and mid- to high-potency topical corticosteroids also can be prescribed for 1 to 2 weeks for more intense bite reactions in conjunction with oral antihistamines. Topical or oral antibiotics may be indicated if redness and swelling progress at the bite site or if breaks in the skin become secondarily infected.
Final Thoughts
Because of the wide-ranging habitats of no-see-ums, eradication programs using insecticides have been inefficient or environmentally suboptimal. Emptying all standing water in outdoor spaces will reduce the number of no-see-ums. Avoidance of the outdoors at dawn and dusk when no-see-ums are most active is helpful, as well as protecting exposed skin by wearing long-sleeved shirts and long pants when outside. Insect repellents containing DEET (N-N-diethyl-meta-toluamide) or picaridin can offer additional protection on the remaining exposed skin. Oil of lemon eucalyptus, or active compound p-menthane-3,8-diol, has been shown to be effective against no-see-ums. Use of DEET should be avoided in children younger than 2 years and p-menthane-3,8-diol in those younger than 3 years. Picaridin is safe for use in children.12 Citronella oil is ineffective. Installing window and patio screens with a mesh size less than 16 can prevent no-see-ums from passing through the netting but will restrict air flow.3 Turning off porch lights also is helpful, as no-see-ums are attracted to light sources.6 Since no-see-ums are weak flyers, setting ceiling or window fans at high speeds can minimize exposure; similarly, being outdoors on a windy day may decrease the likelihood of being bitten. Ultimately, the best remedy for a bite is to prevent them from happening.
- Hill CA, MacDonald JF. Biting midges: biology and public health risk. Purdue University. Published July 2013. Accessed September 3, 2025. http://extension.entm.purdue.edu/publichealth/insects/bitingmidge.html
- Borkent A, Dominiak P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa. 2020;4787:1-377.
- Connelly CR. Biting midges, no-see-ums Culicoides spp. (Insecta: Diptera: Ceratopogonidae). University of Florida publication #EENY 349. Published August 2, 2022. Accessed September 3, 2025. https://edis.ifas.ufl.edu/publication/IN626
- Mullen GR, Murphree CS. Biting midges (Ceratopogonidae). In: Mullen GR, Durden LA, eds. Medical and Veterinary Entomology. 3rd ed. Academic Press; 2019:213-236.
- Best Bee Brothers. No-see-um seasonality range map & season information. Published March 4, 2022. Accessed September 3, 2025. https://bestbeebrothers.com/blogs/blog/no-see-um-season
- Biology Insights. Is there a season for no see ums in Florida? Published August 28, 2025. Accessed September 16, 2025. https://biologyinsights.com/is-there-a-season-for-no-see-ums-in-florida/
- Burris S. Florida no see ums: how to navigate the woes of no see ums in Florida. The Bug Agenda. Published February 2, 2022. Accessed September 3, 2025. https://thebugagenda.com/no-see-ums-in-florida/
- Steffen C. Clinical and histopathologic correlation of midge bites. Arch Dermatol. 1981;117:785-787.
- Krakowski AC, Ho B. Arthropod assault from biting midges. J Pediatr. 2013;163:298.
- Maves RC, Reaves EJ, Martin GJ. Images in clinical tropical medicine: bullous leg lesions caused by Culicoides midges after travel in the Amazon basin. Am J Trop Med Hyg. 2010;83:447.
- Swank B. How long do no-see-ums live? Pest Source. Updated March 17, 2025. Accessed September 3, 2025. https://pestsource.com/no-see-um/lifespan/
- Nguyen QD, Vu MN, Herbert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2023;88:123-130.
- Hill CA, MacDonald JF. Biting midges: biology and public health risk. Purdue University. Published July 2013. Accessed September 3, 2025. http://extension.entm.purdue.edu/publichealth/insects/bitingmidge.html
- Borkent A, Dominiak P. Catalog of the biting midges of the world (Diptera: Ceratopogonidae). Zootaxa. 2020;4787:1-377.
- Connelly CR. Biting midges, no-see-ums Culicoides spp. (Insecta: Diptera: Ceratopogonidae). University of Florida publication #EENY 349. Published August 2, 2022. Accessed September 3, 2025. https://edis.ifas.ufl.edu/publication/IN626
- Mullen GR, Murphree CS. Biting midges (Ceratopogonidae). In: Mullen GR, Durden LA, eds. Medical and Veterinary Entomology. 3rd ed. Academic Press; 2019:213-236.
- Best Bee Brothers. No-see-um seasonality range map & season information. Published March 4, 2022. Accessed September 3, 2025. https://bestbeebrothers.com/blogs/blog/no-see-um-season
- Biology Insights. Is there a season for no see ums in Florida? Published August 28, 2025. Accessed September 16, 2025. https://biologyinsights.com/is-there-a-season-for-no-see-ums-in-florida/
- Burris S. Florida no see ums: how to navigate the woes of no see ums in Florida. The Bug Agenda. Published February 2, 2022. Accessed September 3, 2025. https://thebugagenda.com/no-see-ums-in-florida/
- Steffen C. Clinical and histopathologic correlation of midge bites. Arch Dermatol. 1981;117:785-787.
- Krakowski AC, Ho B. Arthropod assault from biting midges. J Pediatr. 2013;163:298.
- Maves RC, Reaves EJ, Martin GJ. Images in clinical tropical medicine: bullous leg lesions caused by Culicoides midges after travel in the Amazon basin. Am J Trop Med Hyg. 2010;83:447.
- Swank B. How long do no-see-ums live? Pest Source. Updated March 17, 2025. Accessed September 3, 2025. https://pestsource.com/no-see-um/lifespan/
- Nguyen QD, Vu MN, Herbert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2023;88:123-130.
Dermoscopic Documentation of a No-see-um Bite
Dermoscopic Documentation of a No-see-um Bite
Practice Points
- Biting midges, commonly known as no-see-ums, are extremely small flies whose bites can cause a burning sensation, mild pain, and reactions ranging from small wheals to intensely pruritic papules.
- Medical management of no-see-um bites is based on the severity of the skin reaction.
Nonhealing Friable Nodule on the Distal Edge of the Toe
Nonhealing Friable Nodule on the Distal Edge of the Toe
THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1
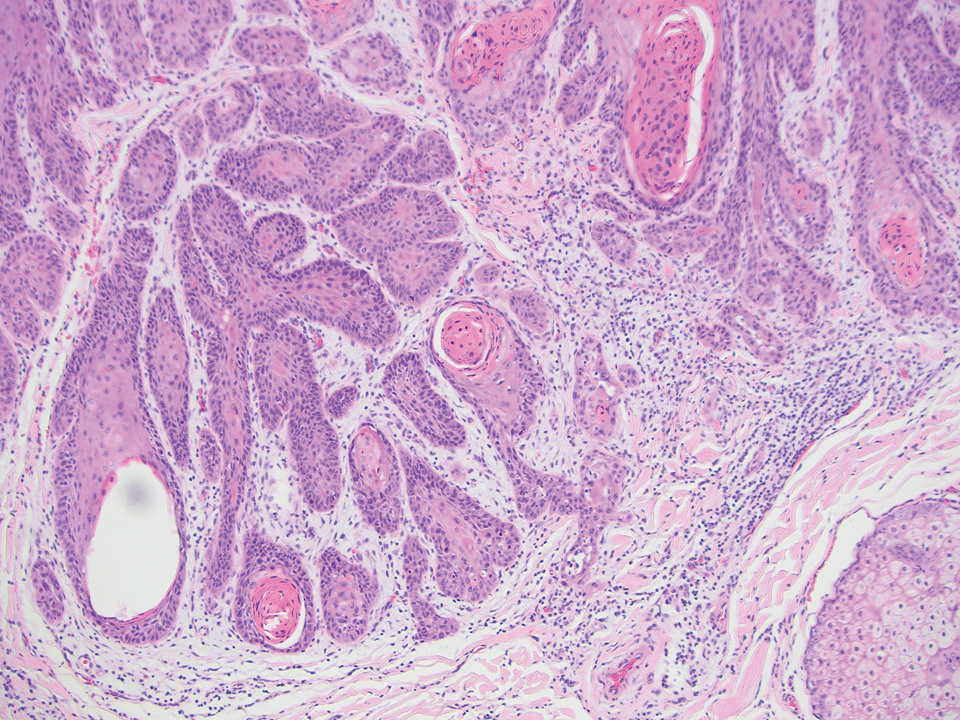
The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4
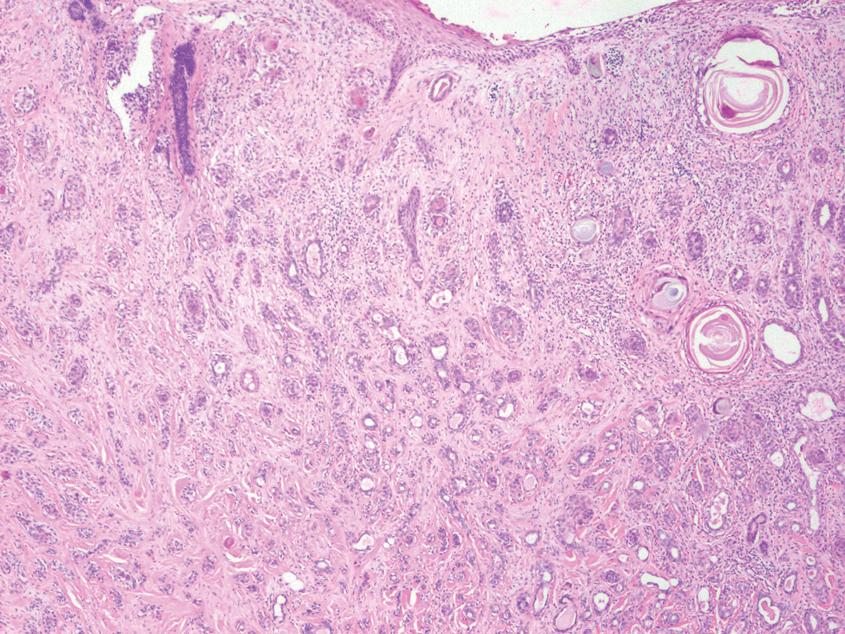
Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5
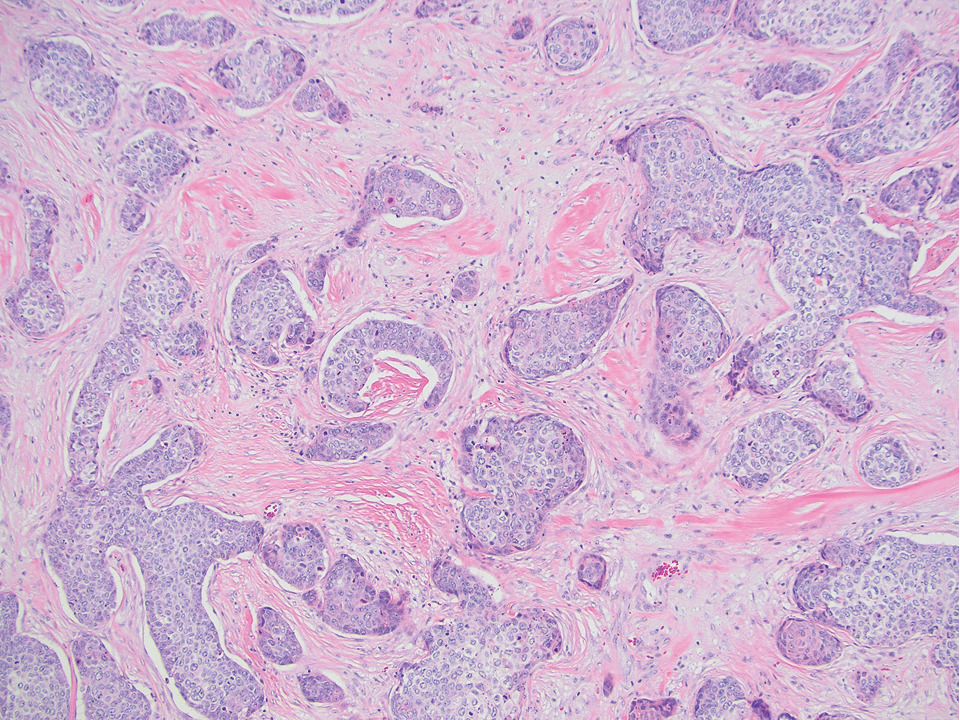
Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.
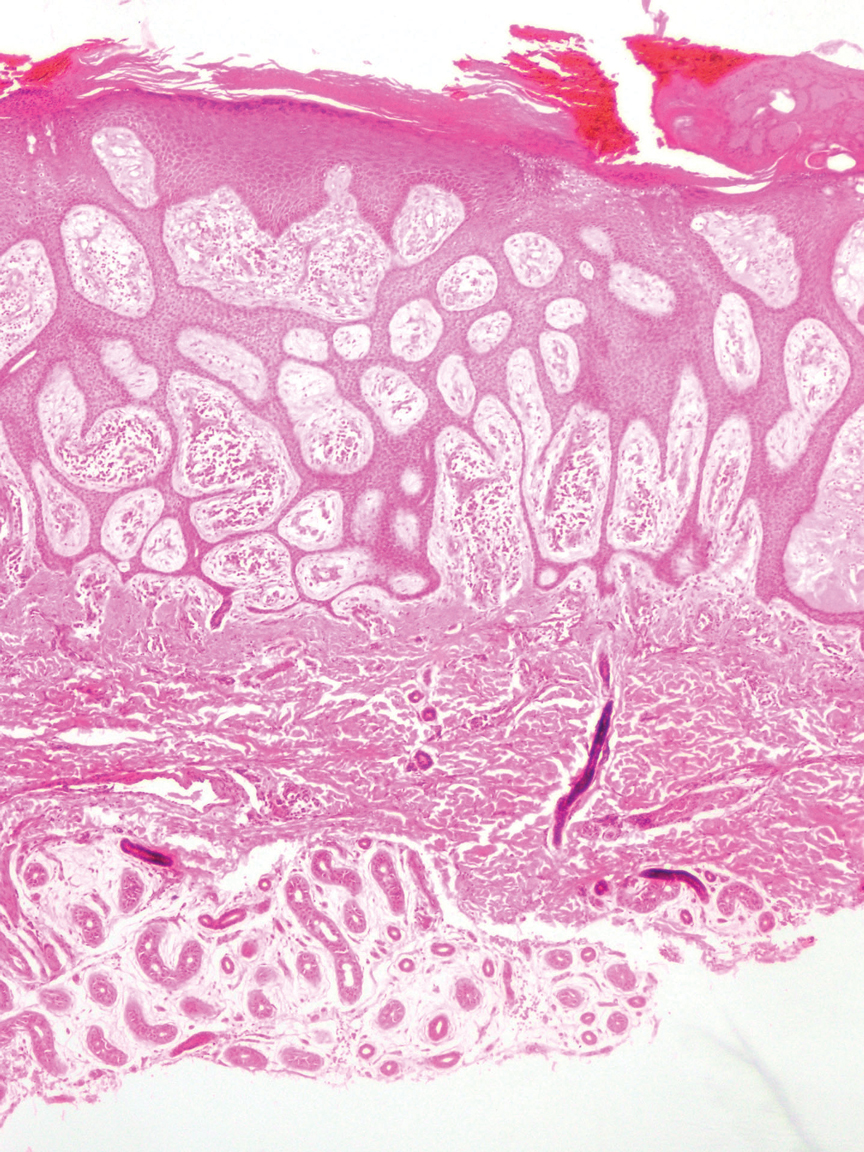
- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1

The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4

Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5

Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.

THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1

The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4

Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5

Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.

- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
Nonhealing Friable Nodule on the Distal Edge of the Toe
Nonhealing Friable Nodule on the Distal Edge of the Toe
A 37-year-old woman with no notable medical history presented to the dermatology clinic with a nonhealing wound on the left fifth toe of 10 month’s duration. The patient reported that the wound developed after burning the toe on an indoor space heater. Physical examination revealed a friable pink papule with a hemorrhagic crust. A biopsy of the lesion was performed.
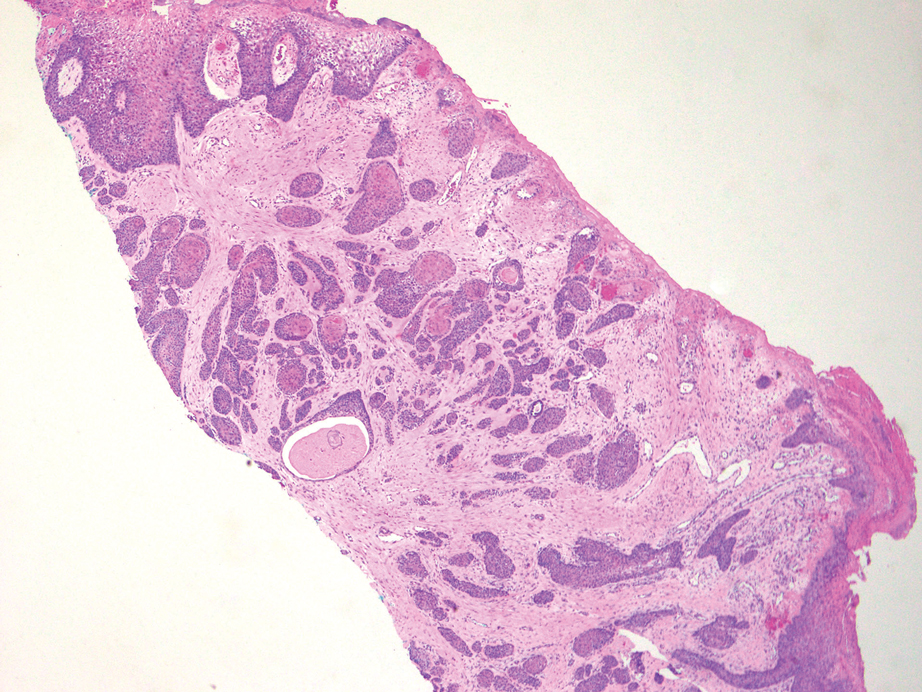
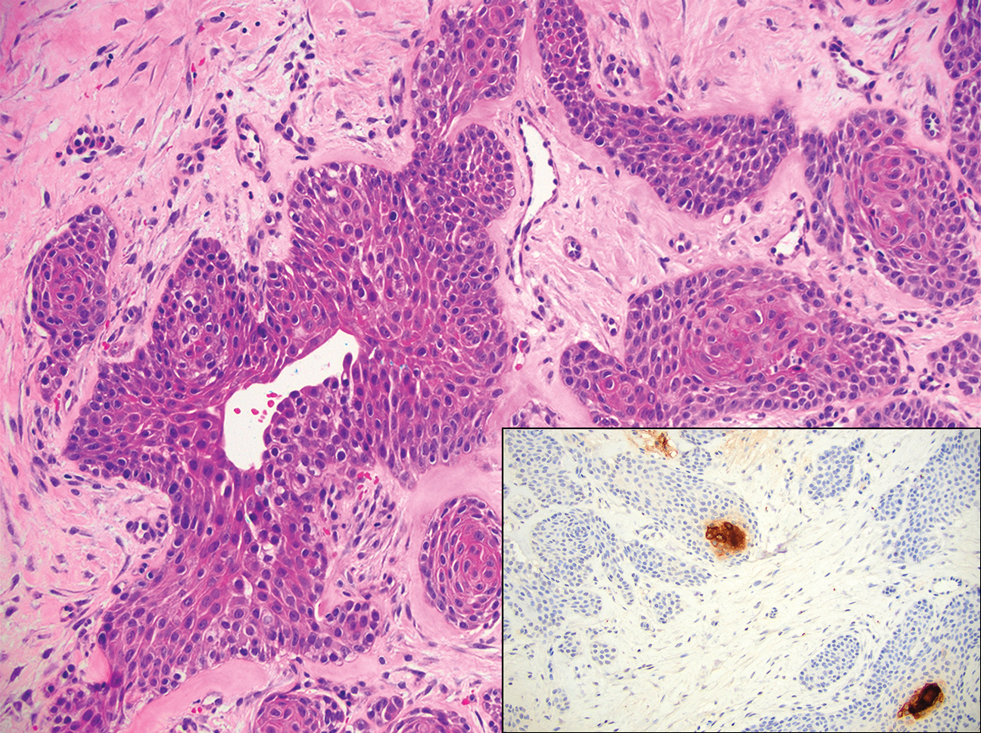
Shaping the Future of Dermatology Practice: Leadership Insight From Susan C. Taylor, MD
Shaping the Future of Dermatology Practice: Leadership Insight From Susan C. Taylor, MD
What are the American Academy of Dermatology’s (AAD’s) top advocacy priorities related to Medicare physician reimbursement?
Dr. Taylor: Medicare physician payment has failed to keep up with inflation, threatening the viability of medical practices. The AAD urges Congress to stabilize the Medicare payment system to ensure continued patient access to essential health care by
What is the AAD’s stance on transitioning from traditional fee-for-service to value-based care models in dermatology under Medicare?
Dr. Taylor: Current value-based programs are extremely burdensome, have not demonstrated improved patient care, and are not clinically relevant to physicians or patients. The AAD has serious concerns about the viability and effectiveness of the Quality Payment Program (QPP), especially the Merit-Based Incentive Payment System (MIPS). Numerous studies have highlighted persistent challenges associated with MIPS, including practices serving high-risk patients and those that are small or in rural areas. For instance, researchers examined whether MIPS disproportionately penalized surgeons who care for these patients and found a connection between caring for these patients, lower MIPS scores, and a higher likelihood of facing negative payment adjustments.
Additionally, the US Government Accountability Office was tasked with reviewing several aspects concerning small and rural practices in relation to Medicare payment incentive programs, including MIPS. Findings indicated that physician practices with 15 or fewer providers, whether located in rural or nonrural areas, had a higher likelihood of receiving negative payment adjustments in Medicare incentive programs compared to larger practices. To maximize participation and facilitate the best possible outcomes for dermatologists within the MIPS program, the AAD maintains that we must continue to develop and advocate that the Centers for Medicare and Medicaid Services approve dermatology-specific measures for MIPS reporting.
Does the AAD have plans to develop or expand dermatology-specific quality measures that are more clinically relevant and less administratively taxing?
Dr. Taylor: The AAD is committed to ensuring that dermatologists can be successful in the QPP and its MIPS Value Pathways and Advanced Alternative Payment Model programs. These payment pathways for QPP-eligible participants allow physicians to increase their future Medicare reimbursements but also penalize those who do not meet performance objectives. The AAD is constantly reviewing and proposing new dermatology-specific quality measures to the Centers for Medicare and Medicaid Services based on member feedback to reduce administrative burdens of MIPS reporting. All of our quality measures are developed by dermatologists for dermatologists.
How is the AAD supporting practices dealing with insurer-mandated switch policies that disrupt continuity of care and increase documentation burden?
Dr. Taylor: The AAD works with private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for services provided, and ensure patients can access covered quality care by building and maintaining relationships with public and private payers. This critical collaboration addresses immediate needs affecting our members’ ability to deliver care, such as when policy changes affect claims and formulary coverage or payment. Our coordinated strategy ensures payer policies align with everyday practice for dermatologists so they can focus on treating patients. The AAD has resources and tools to guide dermatology practices in appropriate documentation and coding.
What initiatives is the AAD pursuing to specifically support independent or small dermatology practices in coping with administrative overload?
Dr. Taylor: The AAD is continuously advocating for our small and independent dermatology practices. In every comment letter we submit on proposed medical practice reporting regulation, we demand small practice exemptions. Moreover, the AAD has resources and practical tools for all types of practices to cope with administrative burdens, including MIPS reporting requirements. These resources and tools were created by dermatologists for dermatologists to take the guesswork out of administrative compliance. DataDerm is the AAD’s clinical data registry used for MIPS reporting. Since its launch in 2016, DataDerm has become dermatology’s largest clinical data registry, capturing information on more than 16 million unique patients and 69 million encounters. It supports the advancement of skin disease diagnosis and treatment, informs clinical practice, streamlines MIPS reporting, and drives clinically relevant research using real-world data.
What are the biggest contributors to physician burnout right now? What resources does AAD offer to support dermatologists in managing burnout?
Dr. Taylor: The biggest contributors to burnout that dermatologists are facing are demanding workloads, administrative burdens, and loss of autonomy. Dermatologists welcome medical challenges, but they face growing administrative and regulatory burdens that take time away from patient care and contribute to burnout. Taking a wellness-centered approach can help, which is why the AAD includes both practical tools to reduce burdens and strategies to sustain your practice in its online resources. The burnout and wellness section of the AAD website can help with administrative burdens, building a supportive work culture, recognizing drivers of burnout, reconnecting with your purpose, and more.
How is the AAD working to ensure that the expanding scope of practice does not compromise patient safety, particularly when it comes to diagnosis and treatment of complex skin cancers or prescribing systemic medications?
Dr. Taylor: The AAD advocates to ensure that each member of the care team is practicing at a level consistent with their training and education and opposes scope-of-practice expansions for physician assistants, nurse practitioners, and other nonphysician clinicians that threaten patient safety by allowing them to practice independently and advertise as skin experts. Each state has its own scope-of-practice laws governing what nonphysicians can do, whether supervision is required, and how they can represent their training, both in advertising and in a medical practice. The AAD supports appropriate safeguards to ensure patient safety and a focus on the highest-quality appropriate care as the nonphysician workforce expands. The AAD encourages patients to report adverse outcomes to the appropriate state licensing boards.
Is the AAD developing or recommending best practices for dermatologists who supervise NPs or PAs, especially in large practices or retail clinics where oversight can be inconsistent?
Dr. Taylor: The AAD firmly believes that the optimal quality of medical care is delivered when a qualified and licensed physician provides direct on-site supervision to all qualified nonphysician personnel. A medical director of a medical spa facility should be a physician licensed in the state where the facility is located and also should be clearly identified by state licensure and any state-recognized board certification as well as by medical specialty, training, and education. The individual also should be identified as the medical director in all marketing materials and on websites and social media accounts related to the medical spa facility. The AAD would like to see policies that would provide increased transparency in state licensure and specialty board certification including requiring disclosure that a physician is certified or eligible for certification by a private or public board, parent association, or multidisciplinary board or association; requiring disclosure of the certifying board or association with one’s field of study or specialty; requiring display of visible identification—including one’s state licensure, professional degree, field of study, and the use of clarifying titles—in facilities, in marketing materials, and on websites and social media; and requiring all personnel in private medical practices, hospitals, clinics, or other settings employing physicians and/or other personnel that offer medical, surgical, or aesthetic procedures to wear a photo identification name tag during all patient encounters.
What are the American Academy of Dermatology’s (AAD’s) top advocacy priorities related to Medicare physician reimbursement?
Dr. Taylor: Medicare physician payment has failed to keep up with inflation, threatening the viability of medical practices. The AAD urges Congress to stabilize the Medicare payment system to ensure continued patient access to essential health care by
What is the AAD’s stance on transitioning from traditional fee-for-service to value-based care models in dermatology under Medicare?
Dr. Taylor: Current value-based programs are extremely burdensome, have not demonstrated improved patient care, and are not clinically relevant to physicians or patients. The AAD has serious concerns about the viability and effectiveness of the Quality Payment Program (QPP), especially the Merit-Based Incentive Payment System (MIPS). Numerous studies have highlighted persistent challenges associated with MIPS, including practices serving high-risk patients and those that are small or in rural areas. For instance, researchers examined whether MIPS disproportionately penalized surgeons who care for these patients and found a connection between caring for these patients, lower MIPS scores, and a higher likelihood of facing negative payment adjustments.
Additionally, the US Government Accountability Office was tasked with reviewing several aspects concerning small and rural practices in relation to Medicare payment incentive programs, including MIPS. Findings indicated that physician practices with 15 or fewer providers, whether located in rural or nonrural areas, had a higher likelihood of receiving negative payment adjustments in Medicare incentive programs compared to larger practices. To maximize participation and facilitate the best possible outcomes for dermatologists within the MIPS program, the AAD maintains that we must continue to develop and advocate that the Centers for Medicare and Medicaid Services approve dermatology-specific measures for MIPS reporting.
Does the AAD have plans to develop or expand dermatology-specific quality measures that are more clinically relevant and less administratively taxing?
Dr. Taylor: The AAD is committed to ensuring that dermatologists can be successful in the QPP and its MIPS Value Pathways and Advanced Alternative Payment Model programs. These payment pathways for QPP-eligible participants allow physicians to increase their future Medicare reimbursements but also penalize those who do not meet performance objectives. The AAD is constantly reviewing and proposing new dermatology-specific quality measures to the Centers for Medicare and Medicaid Services based on member feedback to reduce administrative burdens of MIPS reporting. All of our quality measures are developed by dermatologists for dermatologists.
How is the AAD supporting practices dealing with insurer-mandated switch policies that disrupt continuity of care and increase documentation burden?
Dr. Taylor: The AAD works with private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for services provided, and ensure patients can access covered quality care by building and maintaining relationships with public and private payers. This critical collaboration addresses immediate needs affecting our members’ ability to deliver care, such as when policy changes affect claims and formulary coverage or payment. Our coordinated strategy ensures payer policies align with everyday practice for dermatologists so they can focus on treating patients. The AAD has resources and tools to guide dermatology practices in appropriate documentation and coding.
What initiatives is the AAD pursuing to specifically support independent or small dermatology practices in coping with administrative overload?
Dr. Taylor: The AAD is continuously advocating for our small and independent dermatology practices. In every comment letter we submit on proposed medical practice reporting regulation, we demand small practice exemptions. Moreover, the AAD has resources and practical tools for all types of practices to cope with administrative burdens, including MIPS reporting requirements. These resources and tools were created by dermatologists for dermatologists to take the guesswork out of administrative compliance. DataDerm is the AAD’s clinical data registry used for MIPS reporting. Since its launch in 2016, DataDerm has become dermatology’s largest clinical data registry, capturing information on more than 16 million unique patients and 69 million encounters. It supports the advancement of skin disease diagnosis and treatment, informs clinical practice, streamlines MIPS reporting, and drives clinically relevant research using real-world data.
What are the biggest contributors to physician burnout right now? What resources does AAD offer to support dermatologists in managing burnout?
Dr. Taylor: The biggest contributors to burnout that dermatologists are facing are demanding workloads, administrative burdens, and loss of autonomy. Dermatologists welcome medical challenges, but they face growing administrative and regulatory burdens that take time away from patient care and contribute to burnout. Taking a wellness-centered approach can help, which is why the AAD includes both practical tools to reduce burdens and strategies to sustain your practice in its online resources. The burnout and wellness section of the AAD website can help with administrative burdens, building a supportive work culture, recognizing drivers of burnout, reconnecting with your purpose, and more.
How is the AAD working to ensure that the expanding scope of practice does not compromise patient safety, particularly when it comes to diagnosis and treatment of complex skin cancers or prescribing systemic medications?
Dr. Taylor: The AAD advocates to ensure that each member of the care team is practicing at a level consistent with their training and education and opposes scope-of-practice expansions for physician assistants, nurse practitioners, and other nonphysician clinicians that threaten patient safety by allowing them to practice independently and advertise as skin experts. Each state has its own scope-of-practice laws governing what nonphysicians can do, whether supervision is required, and how they can represent their training, both in advertising and in a medical practice. The AAD supports appropriate safeguards to ensure patient safety and a focus on the highest-quality appropriate care as the nonphysician workforce expands. The AAD encourages patients to report adverse outcomes to the appropriate state licensing boards.
Is the AAD developing or recommending best practices for dermatologists who supervise NPs or PAs, especially in large practices or retail clinics where oversight can be inconsistent?
Dr. Taylor: The AAD firmly believes that the optimal quality of medical care is delivered when a qualified and licensed physician provides direct on-site supervision to all qualified nonphysician personnel. A medical director of a medical spa facility should be a physician licensed in the state where the facility is located and also should be clearly identified by state licensure and any state-recognized board certification as well as by medical specialty, training, and education. The individual also should be identified as the medical director in all marketing materials and on websites and social media accounts related to the medical spa facility. The AAD would like to see policies that would provide increased transparency in state licensure and specialty board certification including requiring disclosure that a physician is certified or eligible for certification by a private or public board, parent association, or multidisciplinary board or association; requiring disclosure of the certifying board or association with one’s field of study or specialty; requiring display of visible identification—including one’s state licensure, professional degree, field of study, and the use of clarifying titles—in facilities, in marketing materials, and on websites and social media; and requiring all personnel in private medical practices, hospitals, clinics, or other settings employing physicians and/or other personnel that offer medical, surgical, or aesthetic procedures to wear a photo identification name tag during all patient encounters.
What are the American Academy of Dermatology’s (AAD’s) top advocacy priorities related to Medicare physician reimbursement?
Dr. Taylor: Medicare physician payment has failed to keep up with inflation, threatening the viability of medical practices. The AAD urges Congress to stabilize the Medicare payment system to ensure continued patient access to essential health care by
What is the AAD’s stance on transitioning from traditional fee-for-service to value-based care models in dermatology under Medicare?
Dr. Taylor: Current value-based programs are extremely burdensome, have not demonstrated improved patient care, and are not clinically relevant to physicians or patients. The AAD has serious concerns about the viability and effectiveness of the Quality Payment Program (QPP), especially the Merit-Based Incentive Payment System (MIPS). Numerous studies have highlighted persistent challenges associated with MIPS, including practices serving high-risk patients and those that are small or in rural areas. For instance, researchers examined whether MIPS disproportionately penalized surgeons who care for these patients and found a connection between caring for these patients, lower MIPS scores, and a higher likelihood of facing negative payment adjustments.
Additionally, the US Government Accountability Office was tasked with reviewing several aspects concerning small and rural practices in relation to Medicare payment incentive programs, including MIPS. Findings indicated that physician practices with 15 or fewer providers, whether located in rural or nonrural areas, had a higher likelihood of receiving negative payment adjustments in Medicare incentive programs compared to larger practices. To maximize participation and facilitate the best possible outcomes for dermatologists within the MIPS program, the AAD maintains that we must continue to develop and advocate that the Centers for Medicare and Medicaid Services approve dermatology-specific measures for MIPS reporting.
Does the AAD have plans to develop or expand dermatology-specific quality measures that are more clinically relevant and less administratively taxing?
Dr. Taylor: The AAD is committed to ensuring that dermatologists can be successful in the QPP and its MIPS Value Pathways and Advanced Alternative Payment Model programs. These payment pathways for QPP-eligible participants allow physicians to increase their future Medicare reimbursements but also penalize those who do not meet performance objectives. The AAD is constantly reviewing and proposing new dermatology-specific quality measures to the Centers for Medicare and Medicaid Services based on member feedback to reduce administrative burdens of MIPS reporting. All of our quality measures are developed by dermatologists for dermatologists.
How is the AAD supporting practices dealing with insurer-mandated switch policies that disrupt continuity of care and increase documentation burden?
Dr. Taylor: The AAD works with private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for services provided, and ensure patients can access covered quality care by building and maintaining relationships with public and private payers. This critical collaboration addresses immediate needs affecting our members’ ability to deliver care, such as when policy changes affect claims and formulary coverage or payment. Our coordinated strategy ensures payer policies align with everyday practice for dermatologists so they can focus on treating patients. The AAD has resources and tools to guide dermatology practices in appropriate documentation and coding.
What initiatives is the AAD pursuing to specifically support independent or small dermatology practices in coping with administrative overload?
Dr. Taylor: The AAD is continuously advocating for our small and independent dermatology practices. In every comment letter we submit on proposed medical practice reporting regulation, we demand small practice exemptions. Moreover, the AAD has resources and practical tools for all types of practices to cope with administrative burdens, including MIPS reporting requirements. These resources and tools were created by dermatologists for dermatologists to take the guesswork out of administrative compliance. DataDerm is the AAD’s clinical data registry used for MIPS reporting. Since its launch in 2016, DataDerm has become dermatology’s largest clinical data registry, capturing information on more than 16 million unique patients and 69 million encounters. It supports the advancement of skin disease diagnosis and treatment, informs clinical practice, streamlines MIPS reporting, and drives clinically relevant research using real-world data.
What are the biggest contributors to physician burnout right now? What resources does AAD offer to support dermatologists in managing burnout?
Dr. Taylor: The biggest contributors to burnout that dermatologists are facing are demanding workloads, administrative burdens, and loss of autonomy. Dermatologists welcome medical challenges, but they face growing administrative and regulatory burdens that take time away from patient care and contribute to burnout. Taking a wellness-centered approach can help, which is why the AAD includes both practical tools to reduce burdens and strategies to sustain your practice in its online resources. The burnout and wellness section of the AAD website can help with administrative burdens, building a supportive work culture, recognizing drivers of burnout, reconnecting with your purpose, and more.
How is the AAD working to ensure that the expanding scope of practice does not compromise patient safety, particularly when it comes to diagnosis and treatment of complex skin cancers or prescribing systemic medications?
Dr. Taylor: The AAD advocates to ensure that each member of the care team is practicing at a level consistent with their training and education and opposes scope-of-practice expansions for physician assistants, nurse practitioners, and other nonphysician clinicians that threaten patient safety by allowing them to practice independently and advertise as skin experts. Each state has its own scope-of-practice laws governing what nonphysicians can do, whether supervision is required, and how they can represent their training, both in advertising and in a medical practice. The AAD supports appropriate safeguards to ensure patient safety and a focus on the highest-quality appropriate care as the nonphysician workforce expands. The AAD encourages patients to report adverse outcomes to the appropriate state licensing boards.
Is the AAD developing or recommending best practices for dermatologists who supervise NPs or PAs, especially in large practices or retail clinics where oversight can be inconsistent?
Dr. Taylor: The AAD firmly believes that the optimal quality of medical care is delivered when a qualified and licensed physician provides direct on-site supervision to all qualified nonphysician personnel. A medical director of a medical spa facility should be a physician licensed in the state where the facility is located and also should be clearly identified by state licensure and any state-recognized board certification as well as by medical specialty, training, and education. The individual also should be identified as the medical director in all marketing materials and on websites and social media accounts related to the medical spa facility. The AAD would like to see policies that would provide increased transparency in state licensure and specialty board certification including requiring disclosure that a physician is certified or eligible for certification by a private or public board, parent association, or multidisciplinary board or association; requiring disclosure of the certifying board or association with one’s field of study or specialty; requiring display of visible identification—including one’s state licensure, professional degree, field of study, and the use of clarifying titles—in facilities, in marketing materials, and on websites and social media; and requiring all personnel in private medical practices, hospitals, clinics, or other settings employing physicians and/or other personnel that offer medical, surgical, or aesthetic procedures to wear a photo identification name tag during all patient encounters.
Shaping the Future of Dermatology Practice: Leadership Insight From Susan C. Taylor, MD
Shaping the Future of Dermatology Practice: Leadership Insight From Susan C. Taylor, MD
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.
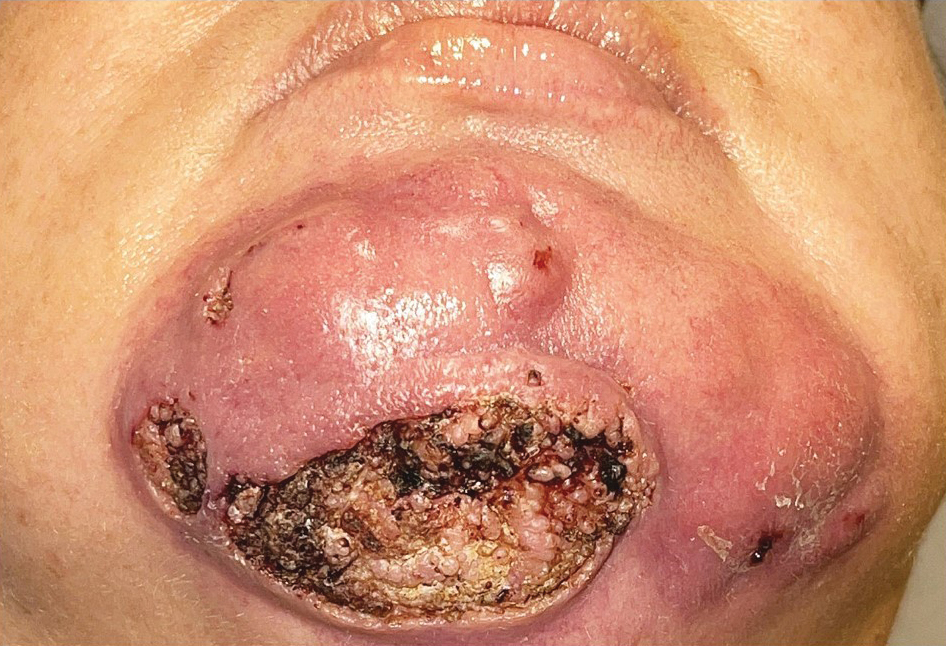
The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.
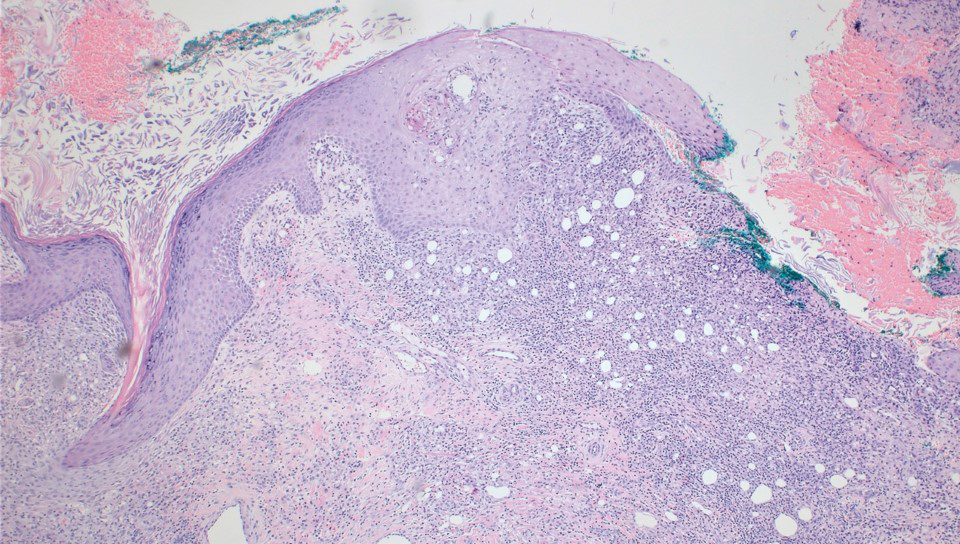
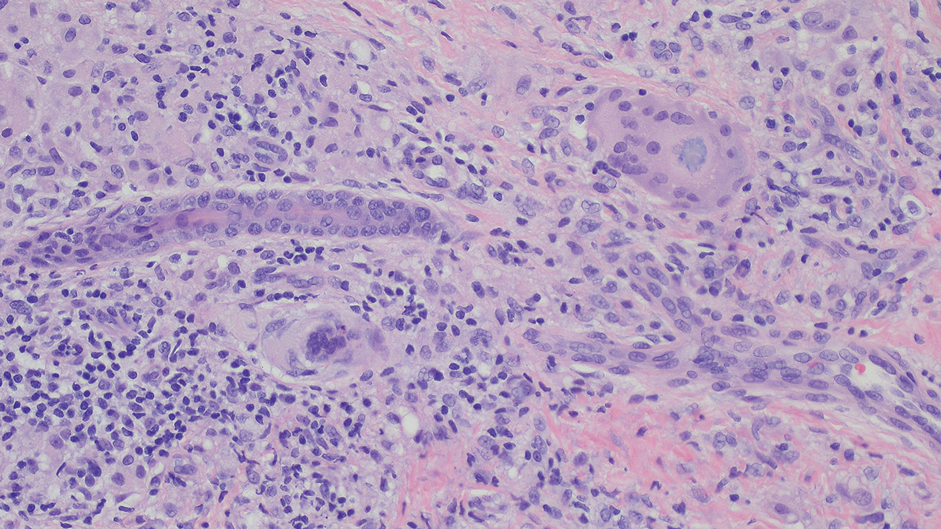
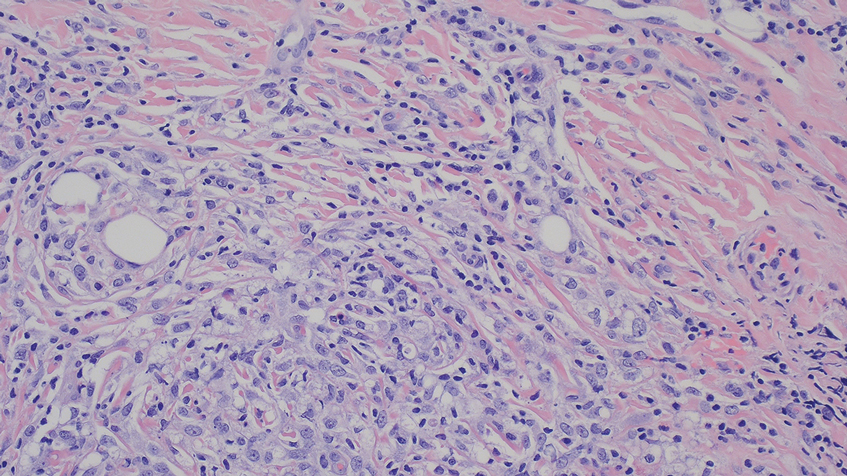
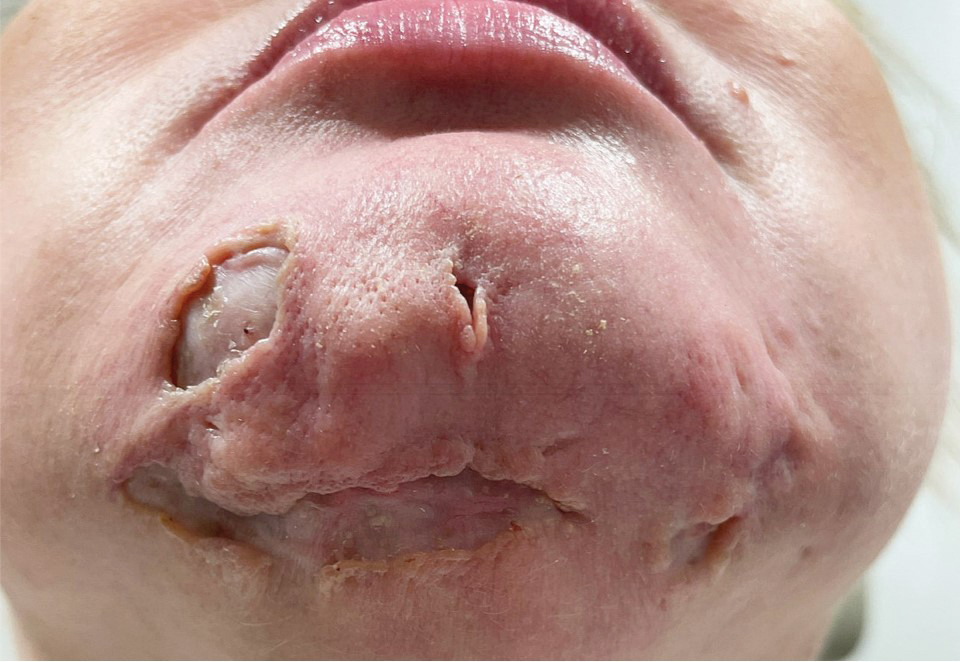
Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.

The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.




Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.

The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.




Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Practice Points
- Severe complications can potentially arise from at-home microneedling procedures when combined with cosmeceuticals such as vitamin E oil.
- Clinicopathologic correlation with cosmetic procedures is imperative to prompt diagnosis and treatment of these skin reactions.
- Microneedling procedures should be performed under the supervision of a board-certified dermatologist to avoid complications, and clinicians should inquire specifically about skin care routines and cosmetic procedures when patients present with unusual lesions on the face.
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.
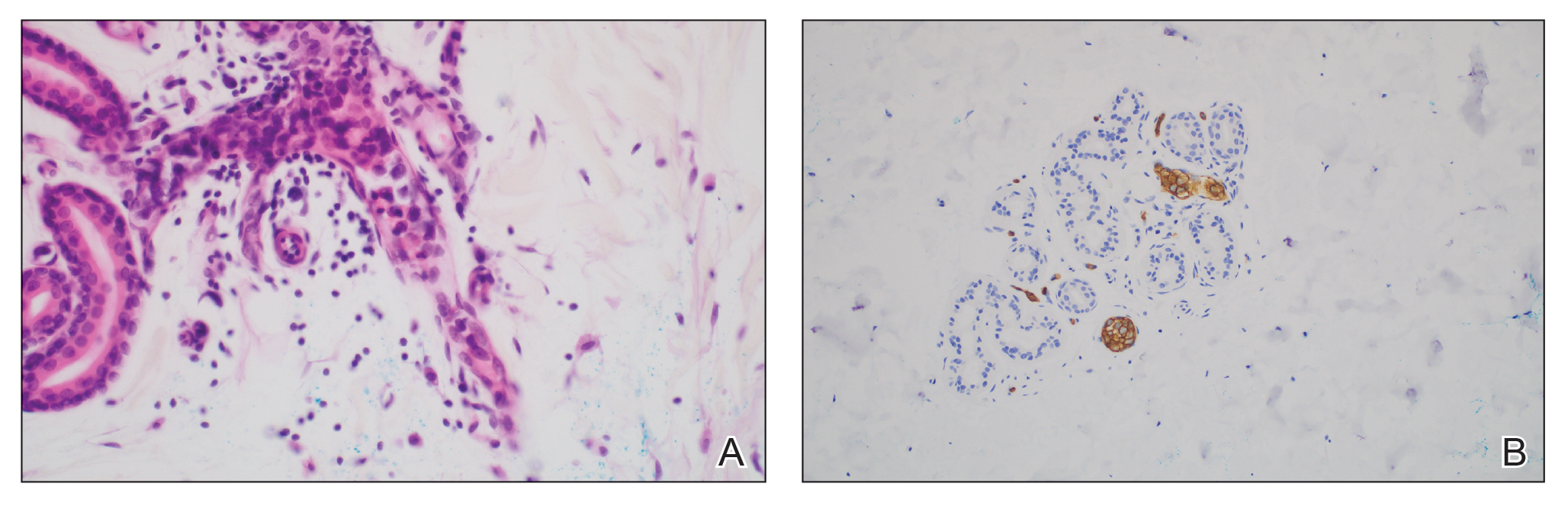
Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).
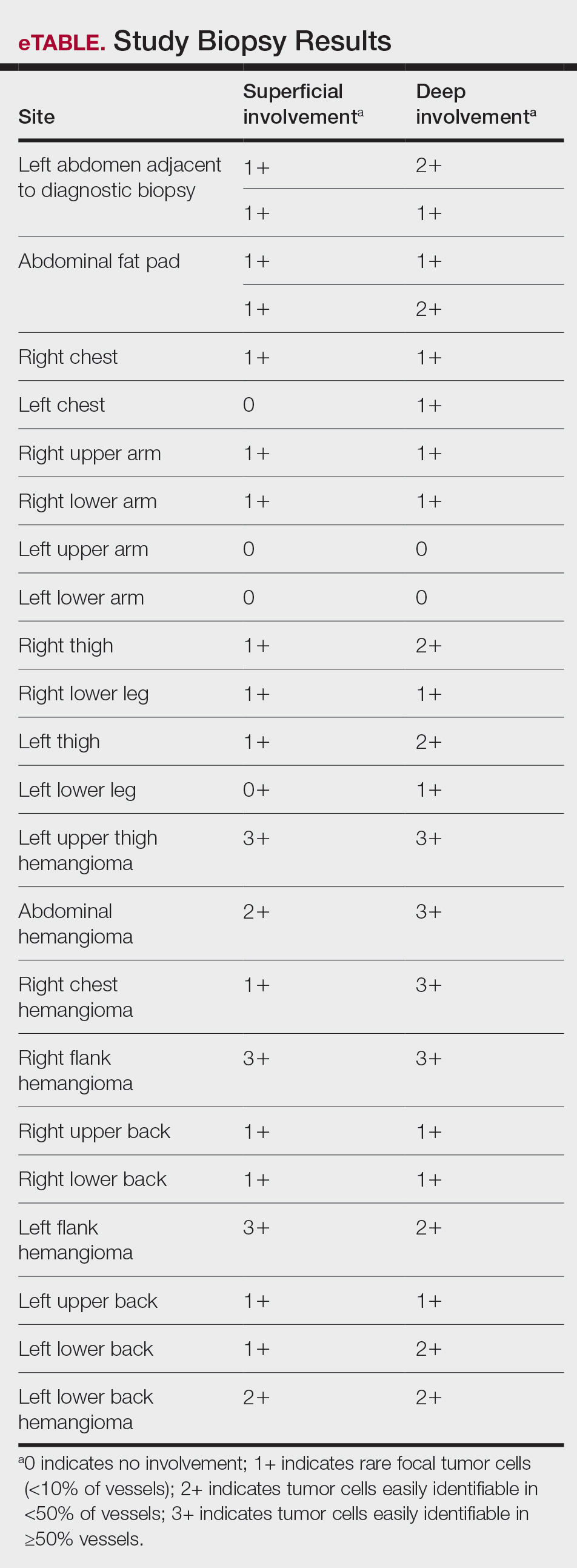
Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).
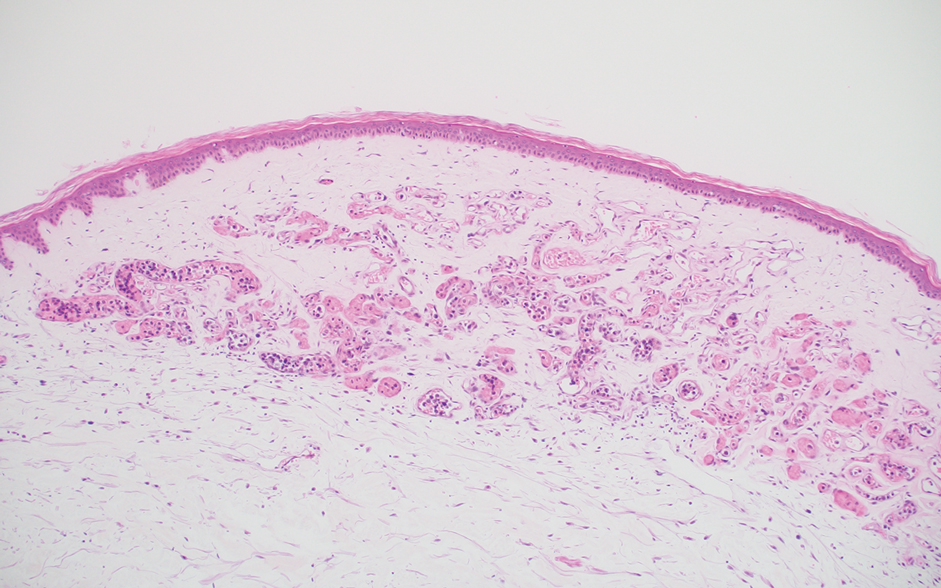
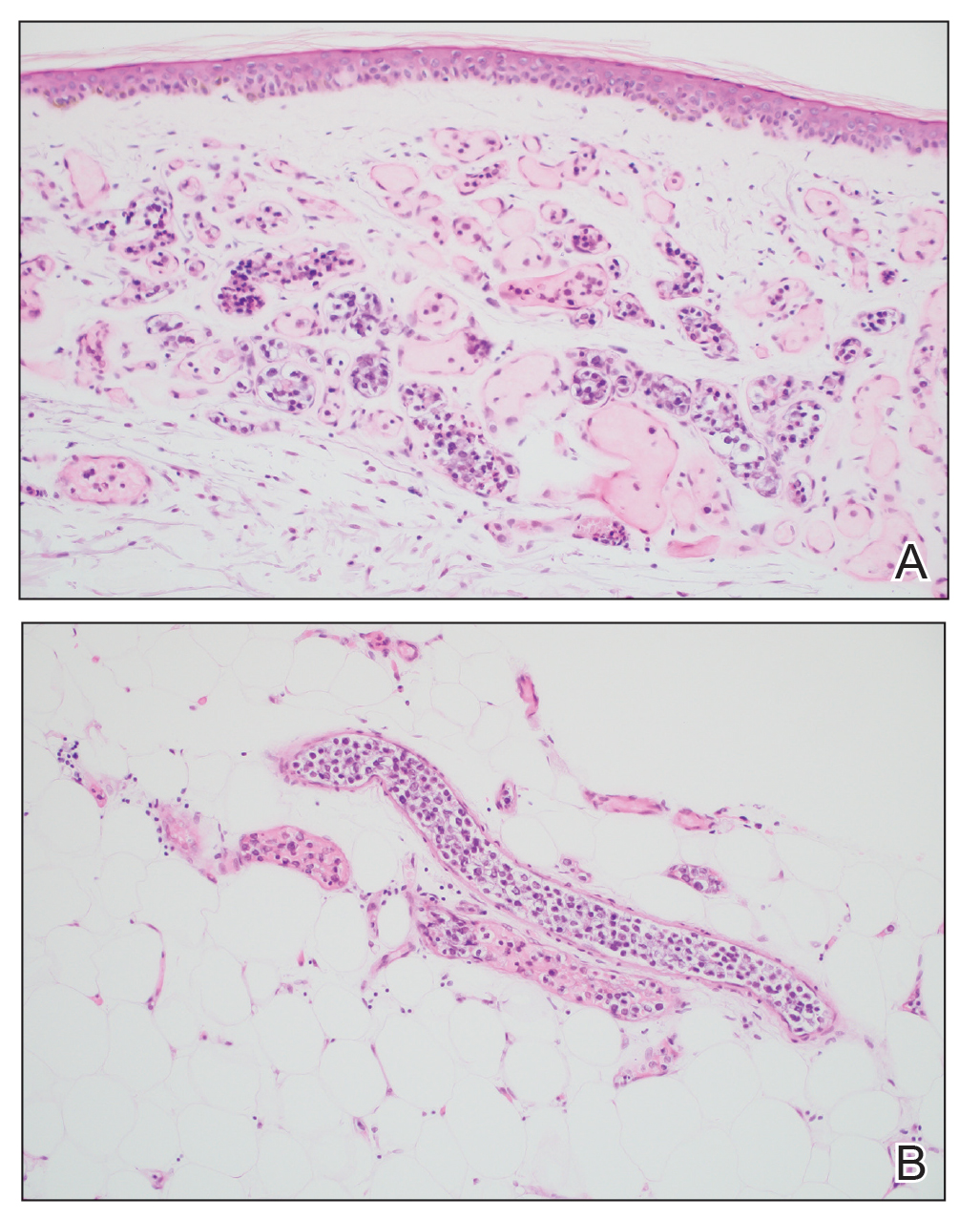
Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.

Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).

Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).


Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.

Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).

Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).


Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Practice Points
- Skin biopsy is an effective method for identifying intravascular large B-cell lymphoma (IVBCL).
- Deep punch biopsies of sites involving hemangiomas may further heighten sensitivity for detection of IVBCL, as these lesions may harbor increased numbers of intravascular lymphoma cells.
- Deep and strategically placed skin biopsies offer potential improvements in timely diagnosis and outcomes of patients with IVBCL.
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).
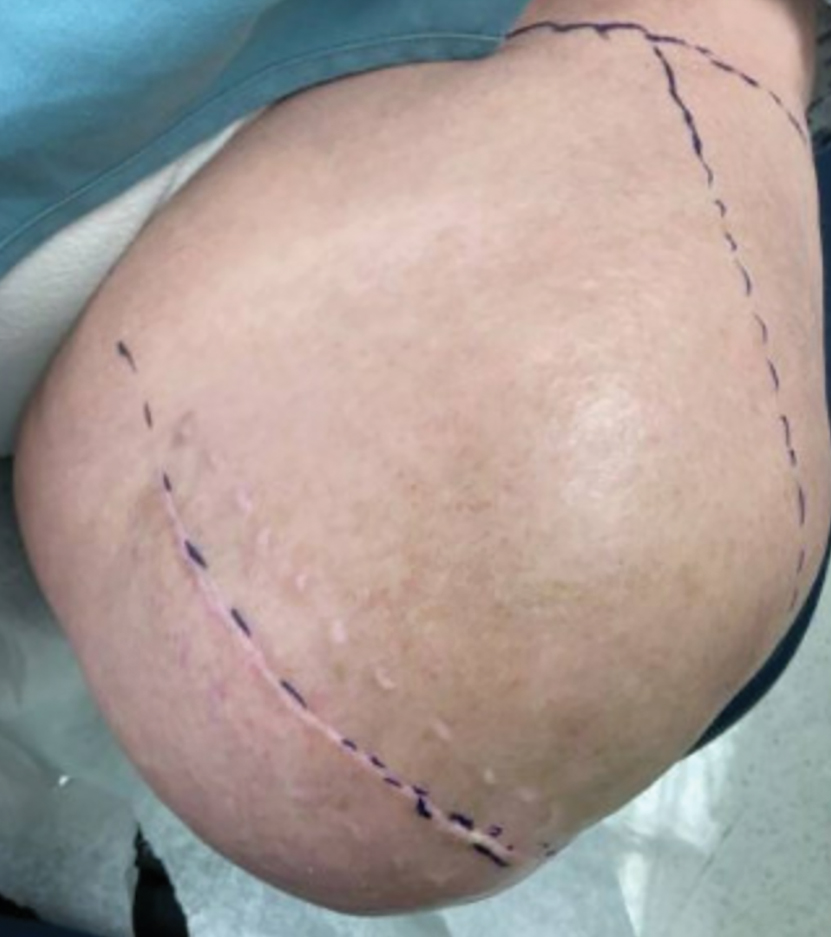
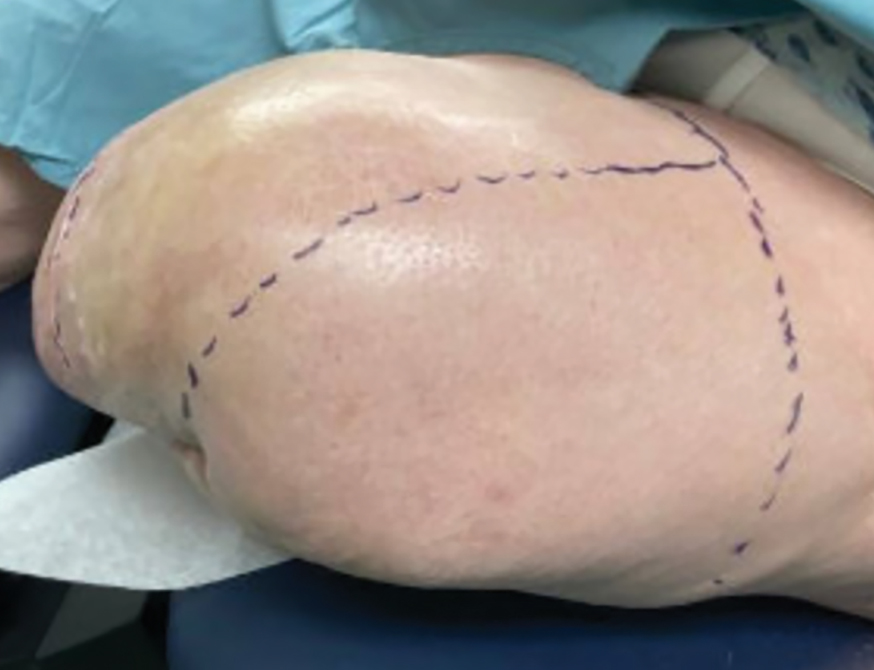
The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.
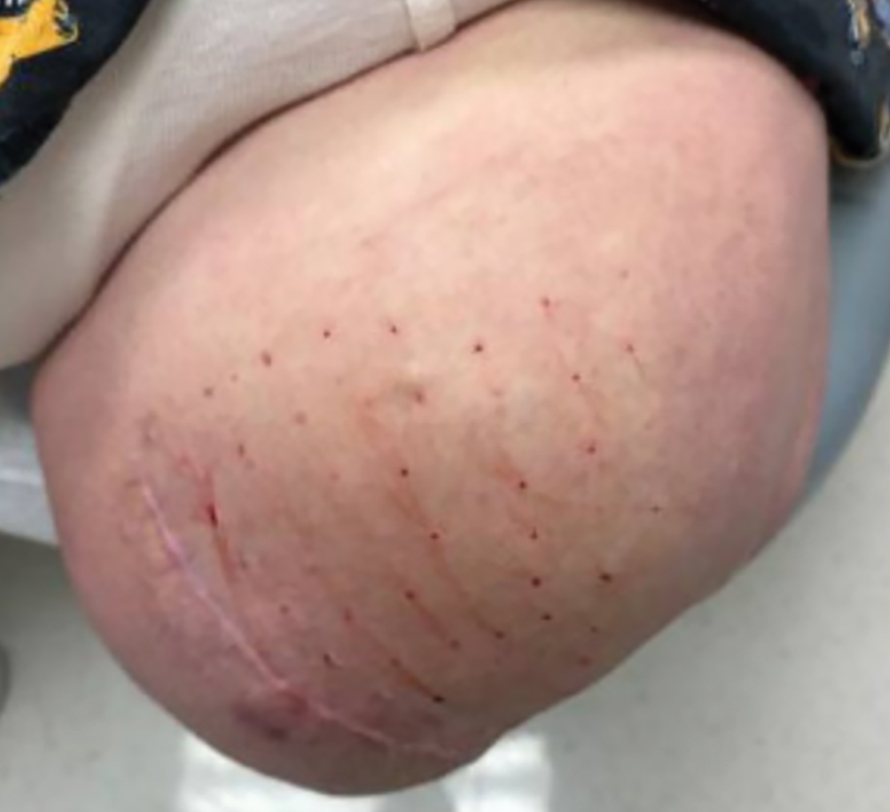
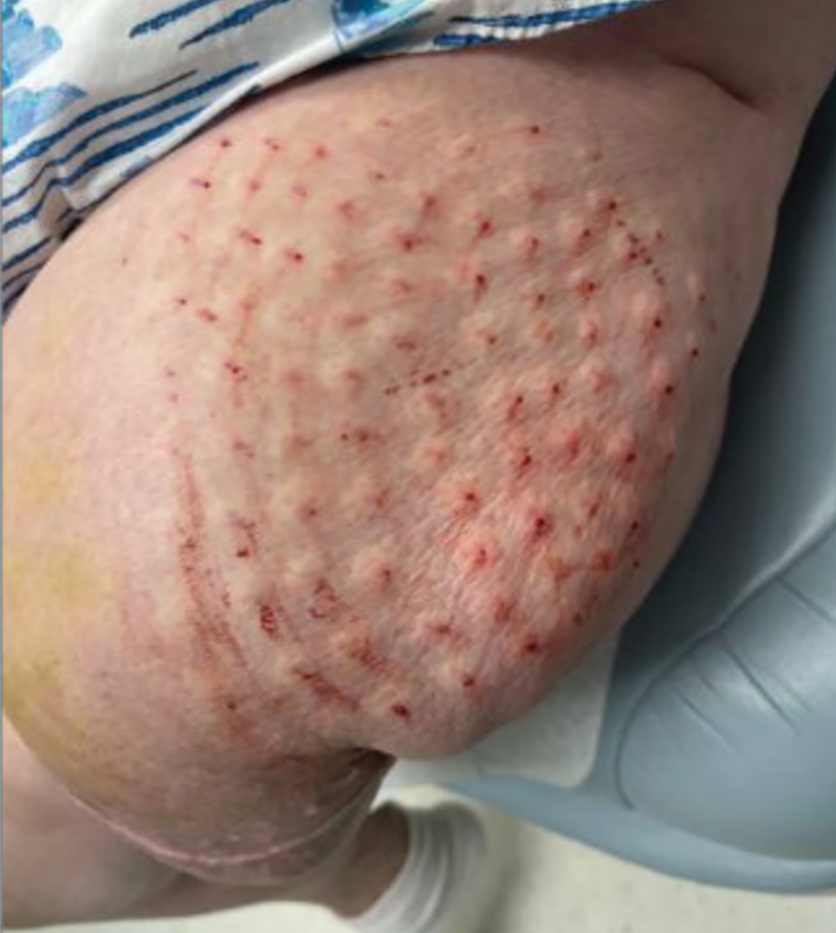
Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).


The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.


Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
Practice Gap
Hyperhidrosis poses a considerable challenge for many amputees who use prosthetic devices, particularly at the interface between the residual limb and the prosthetic socket. The enclosed environment of the socket often leads to excessive sweating, which can compromise suction fit and increase the risk for skin chafing, irritation, and slippage. Persistent moisture also promotes bacterial and fungal growth, raising the likelihood of infections and foul odors within the socket. Research has shown that skin complications are highly prevalent among amputees, affecting up to 73.9% of this population in the United States.1 Commonly reported complications include wounds, abscesses, and blisters, many of which can be triggered or worsened by hyperhidrosis.2 Current treatment options for residual limb sweating include topical antiperspirants, botulinum toxin (BTX) injections, iontophoresis, and liner-liner socks.
While BTX commonly is used to treat hyperhidrosis in areas such as the palms and axillae, it typically is not considered as a first-line therapy for residual limb sweating; however, both BTX type A and type B have shown safety and effectiveness in managing hyperhidrosis in amputees, enhancing prosthetic use, and improving overall quality of life.3 Despite these benefits, BTX remains relatively underutilized for
Tools and Techniques
A 64-year-old man initially presented to our dermatology clinic after undergoing an above-the-knee amputation of the left leg 1 year prior. The amputation had been performed due to chronic prosthetic joint infections with Escherichia coli. He reported persistent sweating of the residual limb, which severely limited his use of a prosthesis and led to frequent falls.
During the initial visit, treatment options for primary hyperhidrosis including topical and injectable therapies were discussed. Due to a fear of needles, the patient chose topical treatment, with the option to pursue BTX injections later if better control was needed. An aluminum chloride hexahydrate prosthetic antiperspirant was prescribed for nightly application on the anterior and posterior
Botulinum toxin injections were administered in a grid-like pattern across the surface area where the residual limb made contact with the prosthetic. Using a surgical marker, the patient assisted the medical team in identifying the areas where sweating occurred most frequently. The area was divided into 4 equal sections, with each section treated per weekly interval sequentially over 4 weeks. The targeted areas included the left anterior (extending from the anterior tensor fasciae latae band to the lateral thigh) and left posterior residual limb (Figure 1 and eFigure 1, respectively).


The treated section was cleaned with an alcohol wipe prior to each injection, and 50 units of BTX (diluted to 2.5 units per 0.1 mL in bacteriostatic saline) were injected intradermally into each section (Figure 2 and eFigure 2). The injections were administered in rows, with the needle inserted at evenly spaced intervals approximately 1 inch apart. A total of 100 units were administered per section at each weekly appointment. The patient tolerated the procedure well, and no complications were observed.


Practice Implications
This staged approach to administering BTX ensures even distribution of the injections, optimizes hyperhidrosis control, minimizes the risk for complications, and allows for precise targeting of the affected areas to maximize therapeutic benefit. Following the initial procedure, our patient was scheduled for follow-ups approximately every 3 to 4 months starting from the first set of injections for each area. Over 9 months, the patient successfully completed 3 treatment sessions using this method. The patient reported improved quality of life after starting the BTX injections.
After evaluating the initial treatment outcomes with 100 units per section, the dosage was increased to 200 units per section to reduce the number of visits from 4 every 3 months to cover the entire area to 2 visits every 3 months. This adjustment aimed to optimize results and better manage the patient’s ongoing symptoms. At about 1 to 2 weeks after beginning treatment, the patient noticed decreased sweating and discomfort during his daily activities and reduced friction with his prosthetic leg. No adverse effects were noted with the increased dosage during a clinical visit.
Our case highlights the importance of ensuring equitable access to hyperhidrosis treatment. Dermatologists should prioritize patient-centered care by factoring in financial constraints when recommending therapies. In this patient’s case, offering a range of options including over-the-counter antiperspirants and prescription treatments allowed for a management plan tailored to his individual needs and circumstances.
DaxibotulinumtoxinA, known for its longer duration of action compared to other BTX formulations, presents a promising alternative for treating hyperhidrosis.4 However, a gap in care emerged for our patient when prescription antiperspirant was not covered by his insurance, and daxibotulinumtoxinA, which could have offered a more durable solution, was not yet available at our clinic for hyperhidrosis management. Expanding insurance coverage for effective prescription treatments and improving access to newer treatment options are crucial for enhancing patient outcomes and ensuring more equitable care.
Focusing dermatologic care on amputees presents distinct challenges and opportunities for improving their care and decreasing discomfort. Amputees, particularly those with residual limb hyperhidrosis, often experience additional discomfort and difficulty while using prosthetics, as excessive sweating can interfere with fit and function.5,6 Dermatologists should proactively address these specific needs by tailoring treatment accordingly. Incorporating targeted therapies, such as BTX injections, in addition to education on lifestyle modifications and managing treatment expectations, ensures comprehensive care that enhances both quality of life and functional outcomes. Engaging patients in discussions about all available options, including emerging therapies, is essential for improving care for this underserved population.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Koc E, Tunca M, Akar A, et al. Skin problems in amputees: a descriptive study. Int J Dermatol. 2008;47:463–466. doi:10.1111/j.1365-4632.2008.03604.x
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090. doi:10.1682/jrrd.2009.04.0052
- Rocha Melo J, Rodrigues MA, Caetano M, et al. Botulinum toxin in the treatment of residual limb hyperhidrosis: a systematic review. Rehabilitacion (Madr). 2023;57:100754. doi:10.1016/j.rh.2022.07.003
- Hansen C, Godfrey B, Wixom J, et al. Incidence, severity, and impact of hyperhidrosis in people with lower-limb amputation. J Rehabil Res Dev. 2015;52:31-40. doi:10.1682/JRRD.2014.04.0108
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486. doi:10.1097/PXR.0000000000000040
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Botulinum Toxin as a Tool to Reduce Hyperhidrosis in Amputees
Dermatology Immediate Care: A Game Changer for the Health Care System?
Dermatology Immediate Care: A Game Changer for the Health Care System?
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
Dermatology Immediate Care: A Game Changer for the Health Care System?
Dermatology Immediate Care: A Game Changer for the Health Care System?
Practice Points
- Emergency departments and most immediate care (IC) centers often lack prompt access to board-certified dermatologists.
- A dermatology urgent care/IC model may shorten wait times, improve access for vulnerable patients and pediatric populations, and reduce unnecessary hospital admissions and costs.
- Increased access to dermatology benefits other specialties by enabling multidisciplinary care leading to faster diagnosis and treatment.
- A staged referral-first dermatology IC pilot with defined staffing and triage rules is a practical path to demonstrate value and scale the service.