User login
Narrowband UVB Treatment Increases Serum 25-Hydroxyvitamin D Levels in Patients With Chronic Plaque Psoriasis
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
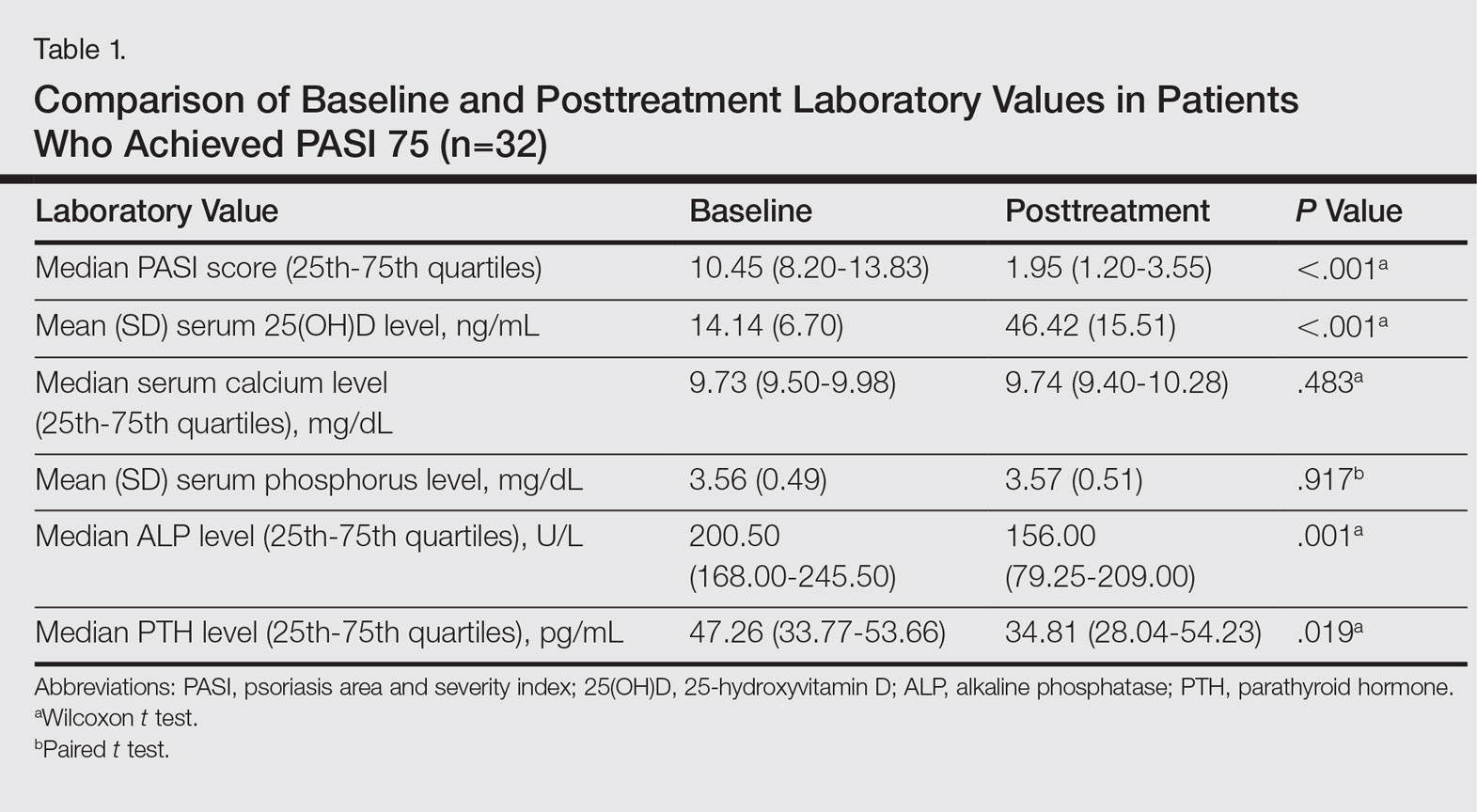
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
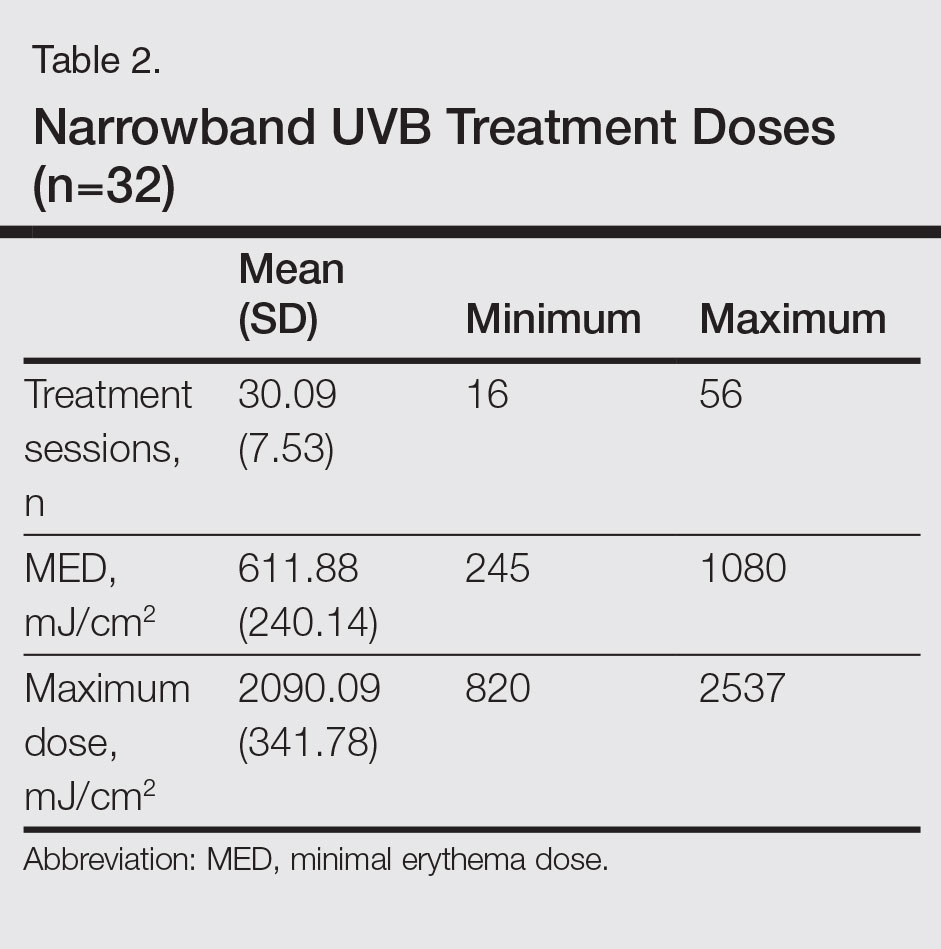
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).
Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).
Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
Psoriasis is a chronic, inflammatory, T-cell–mediated skin disease. Phototherapy, which consists of light used at various wavelengths, is a well-established treatment method for psoriasis vulgaris. Although successful results have been obtained with phototherapy in psoriasis, its mechanism of action is not fully understood. UV light has been shown to have an effect on T-lymphocyte function as well as various components of the natural and acquired immune response. It also has a suppressive effect on the immune system caused by many independent effects.1 Phototherapy currently is available using broadband UVB (290–320 nm), narrowband UVB (NB-UVB)(311–313 nm), 308-nm excimer laser, UVA1 (340–400 nm), psoralen plus UVA, and photopheresis.2 Narrowband UVB treatment with light sources that peak at 311 to 313 nm have been used with high efficacy and a low side-effect profile, becoming the standard phototherapy method for chronic plaque-type psoriasis.3
More than 90% of vitamin D synthesis is formed in the skin following UV exposure, and the wavelengths and the solar spectrum that stimulate vitamin D synthesis have been a focus of research.4 7-Dehydrocholesterol (provitamin D3) is first converted to previtamin D3. Although the necessary UV wavelength for previtamin D3 synthesis is 295 to 300 nm, it is known that production stops below 260 nm and above 315 nm.4-6 Previtamin D3 is unstable and is quickly converted to vitamin D3 in the skinand then to the biologically active form of 1,25-dihydroxyvitamin D3 (calcitriol) following hydroxylation in the liver and kidneys. Calcitriol shows its effect by binding to the special nuclear receptor for vitamin D.7 Many tissues including the keratinocytes, dendritic cells, melanocytes, and sebocytes in the skin have been shown to possess the enzymatic mechanism necessary for 1,25-dihydroxyvitamin D3 production. Vitamin D also is known to have paracrine, autocrine, and intracrine effects on immunomodulation, cell proliferation, differentiation, and apoptosis, in addition to its role in calcium metabolism.5-9 Topical vitamin D and its analogues are used effectively and safely in psoriasis treatment with these effects.10 A correlation between low serum vitamin D levels and chronic inflammation severity has been shown in psoriasis patients in some studies.11,12
In this study, we sought to evaluate the effect of NB-UVB on vitamin D status and related metabolic markers in patients with psoriasis.
Methods
This prospective, single-center study included patients living in or around Eskisehir, Turkey, who were 18 years of age or older and had been diagnosed with chronic plaque psoriasis with a psoriasis area and severity index (PASI) score of 5 or higher. Permission was granted by the local ethics committee. Patients provided written informed consent prior to enrollment. Patients were excluded if they were younger than 18 years; were pregnant or breastfeeding; stayed in open environments for more than 2 hours per day during the summer months (May through September); used drugs affecting calcium metabolism in the last 8 weeks (eg, barbiturates, anticonvulsants, corticosteroids, vitamin D supplements, bisphosphonates); used systemic treatment for psoriasis in the last 8 weeks; used phototherapy or sunbathing in the last 8 weeks; used topical vitamin D analogues in the last 4 weeks; or had a history of psoriatic arthritis and other inflammatory disorders, renal disease, known calcium metabolism disorders, granulomatous disorders, thyroid disease, diabetes mellitus, skin cancer, or abnormal photosensitivity and known lack of response or hypersensitivity to phototherapy.
Clinical Evaluation and Laboratory Studies
The participants’ age, gender, Fitzpatrick skin type, disease duration, dairy intake and vitamin supplement levels, hours of sun exposure per week, detailed medical history, and medications were obtained and documented in the medical records.
Serum 25(OH)D levels were measured using high-performance liquid chromatography/mass spectrometry, serum calcium and phosphorus levels using colorimetric analysis, serum alkaline phosphatase (ALP) levels using the enzymatic colorimetric method, and serum parathyroid hormone (PTH) levels using electrochemiluminescence at baseline and after PASI 75 was achieved with treatment. Vitamin D levels were classified in 3 groups: (1) deficient (<20 ng/mL); (2) inadequate (20–30 ng/mL); and (3) adequate (>30 ng/mL). The PASI scores at baseline and posttreatment were calculated by the same dermatologist (S.S.).
Treatment Protocol and Patient Follow-up
Narrowband UVB treatment was started at 70% of the minimal erythema dose (MED). Phototherapy was administered 3 times weekly for 6 months or until PASI 75 response was achieved. An increase of 20% to 30% from the prior dose was made according to the participants’ clinical status at each treatment session, and the dose was stabilized once the maximum dose was achieved according to skin type—up to 2000 mJ/cm2 for Fitzpatrick skin types I and II, 3000 mJ/cm2 for skin types III and IV, and 5000 mJ/cm2 for skin types V and VI. Participants were allowed to use low- and moderate-potency topical corticosteroids and moisturizers containing urea during the course of treatment. The study physician (S.S.) clinically evaluated participants every 4 weeks for 6 months or until PASI 75 was achieved, and the clinical improvement was calculated as the percentage decrease in PASI score.
Statistical Analysis
The Shapiro-Wilk normalcy test was used for the continuous variables in the study. Variables with a normal distribution were analyzed with the paired t test and 1-way analysis of variance test and presented as mean (SD). Variables without a normal distribution were analyzed with the Wilcoxon t test and the Kruskal-Wallis test and presented as the median and 25th and 75th quartiles. The serum 25(OH)D levels were evaluated according to the seasons with the Kruskal-Wallis test. Categorical variables were expressed as frequency and percentages. The Pearson and Spearman correlation analysis and regression analysis were used to show the relationship between the variables (ie, age, Fitzpatrick skin type, PASI score, maximum NB-UVB dose, and number of sessions). The statistical significance level was set at P≤.05. Statistical analyses were performed using SPSS software version 21.
Results
A total of 49 participants (30 [61.22%] males; 19 [38.78%] females) were included in the study. The mean age (SD) was 40.27 (14.62) years (range, 19–74 years). Three (6.12%) participants were Fitzpatrick skin type I, 15 (30.61%) were skin type II, and 31 (63.27%) were skin type III.
The baseline median PASI score for the 49 participants was 10.20 (7.85–13.65). Baseline serum 25(OH)D levels were noted to be deficient in 40 participants (81.63%) and inadequate in 9 participants (18.37%). The distribution of the serum 25(OH)D levels of the participants according to the season was evaluated with the Kruskal-Wallis test and no association was found between serum 25(OH)D levels and seasonal changes (P=.685). Comparison of 25(OH)D basal values with Fitzpatrick skin type revealed a statistically significant relationship between skin type and vitamin D level (P=.024). The basal serum 25(OH)D levels were significantly lower in Fitzpatrick skin type II versus skin type I (P=.039).
Thirty-two (65.31%) participants achieved PASI 75 by the end of treatment. The baseline median PASI score (25th-75th quartiles) for the 32 patients was 10.45 (8.20-13.83) and the posttreatment PASI score was 1.95 (1.20-3.55), a statistically significant decrease following treatment (P<.001)(Table 1). Mean (SD) baseline serum 25(OH)D levels were 14.14 (6.70) ng/mL and posttreatment levels were 46.42 (15.51) ng/mL in these participants, which demonstrated a statistically significant increase during NB-UVB treatment (P<.001). None of the participants reached the toxicity levels (>80 ng/mL) for serum 25(OH)D. There were no significant changes in serum calcium or phosphorus levels posttreatment (Table 1), but statistically significant decreases in serum ALP and PTH levels were noted (P=.001 and P=.019, respectively)(Table 1).
Participants who completed the study (n=32) received an average (SD) of 30.09 (7.53) sessions of NB-UVB treatment and the mean (SD) MED was 611.88 (240.14) mJ/cm2. The mean (SD) maximum dose was 2090.09 (341.78) mJ/cm2 (Table 2).
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and the maximum dose values. We found that the posttreatment serum 25(OH)D levels correlated with the number of sessions (P=.031) but not with the maximum dose (P=.498).
Using regression analysis, we also evaluated the effect of the increase in vitamin D levels—posttreatment serum 25(OH)D level minus baseline serum 25(OH)D levels—on the decrease in PASI scores—baseline PASI score minus posttreatment PASI score—and found no effect of serum 25(OH)D level increase on PASI decrease (P=.530). There was no correlation between increased serum 25(OH)D levels and age, Fitzpatrick skin type, or baseline PASI score.
Comment
The most effective UV wavelength for vitamin D synthesis is 295 to 300 nm, and therefore broadband UVB is frequently studied when determining the relationship between phototherapy and serum vitamin D levels.4 The current study demonstrated a statistically significant increase in serum 25(OH)D levels following NB-UVB treatment in patients with moderate to severe chronic plaque psoriasis (P<.001). This result supports other studies reporting that NB-UVB treatment in psoriasis patients increases serum 25(OH)D levels.13-18
The main factor in the effective UVB level for vitamin D synthesis is the angle at which solar radiation reaches the earth, which is affected by the longitude, latitude, and time of day.19 For this reason, we planned to perform our study at a single center. Patients who stayed in open areas for more than 2 hours per day during the summer months (May through September) were excluded from the study to decrease the effect of seasonal changes on vitamin D levels. We evaluated the seasonal variation of vitamin D levels and found no relationship between seasonal changes and serum 25(OH)D levels. Therefore, the potential effect of seasonal changes on the vitamin D levels of study participants was excluded from the study.
The response to UV radiation changes according to age and Fitzpatrick skin type because 7-dehydrocholesterol levels decrease with age and melanin prevents the access of UVB photons to 7-dehydrocholesterol.20 The basal serum 25(OH)D levels were deficient in 81.63% of participants and inadequate in 18.37%. In this study, we also observed that the basal serum 25(OH)D levels were significantly lower in patients with Fitzpatrick skin type II than in Fitzpatrick skin type I (P=.039). The mean (SD) serum 25(OH)D level at baseline was 14.14 (6.70) ng/mL and posttreatment was 46.42 (15.51) ng/mL in the 32 patients who completed the study. Serum 25(OH)D levels showed a statistically significant increase after NB-UVB treatment (P<.001). The increased serum 25(OH)D levels after NB-UVB phototherapy were not associated with Fitzpatrick skin type, which was consistent with the results of Osmancevic et al.17 The adjusted NB-UVB doses according to the different skin types might be responsible for this result in our study.
Participant age did not have a significant effect on serum 25(OH)D levels, similar to other studies in the literature.13,17 We believe that artificial UVB radiation at high doses can compensate for the 7-dehydrocholesterol that decreases in the skin with aging.
We observed no significant change in the serum calcium and phosphorus levels with NB-UVB treatment in our study. None of the participants had a metabolic disorder related to increased 25(OH)D levels. The serum ALP and PTH levels decreased significantly following treatment (P=.001 and P=.019, respectively), which may have been secondary to increased serum 25(OH)D levels.
Posttreatment serum 25(OH)D levels were compared with the number of NB-UVB phototherapy sessions and maximum dose values. The posttreatment serum 25(OH)D levels were found to be related to the number of sessions received, but this value was not correlated with the maximum dose received. The MED and maximum dose were determined according to the Fitzpatrick skin type of the participants. Therefore, increased serum 25(OH)D levels with an increased number of sessions was an expected result. Our observation is in accordance with the finding described by Ryan et al.14 On the other hand, an in vitro study conducted by Olds et al21 reported that the relationship between UV light and cholecalciferol synthesis was not linear.
We found that increased serum 25(OH)D levels after treatment were not correlated with the decrease in PASI score, similar to studies by Romaní et al18 and Ryan et al.14 These results suggest that the clinical improvement following NB-UVB treatment is independent of the increased serum 25(OH)D levels in psoriasis patients.
Conclusion
In conclusion, we found that the serum 25(OH)D levels that increase as a result of NB-UVB therapy for the treatment of chronic plaque psoriasis has no statistically significant relationship with the age, Fitzpatrick skin type, baseline PASI score, changes in PASI, or maximum dose, while a positive relationship is present between the serum 25(OH)D levels and the number of sessions of NB-UVB.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
- Şavk E. Immunology of Photo(chemo)therapy. Turkderm. 2010;44(suppl 2):62-66.
- Ferahbaş A. Phototherapy modalities and protocols. Turkderm. 2010;44(suppl 2):67-72.
- Ibbotson SH, Bilsland D, Cox NH, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop report. Br J Dermatol. 2004;151:283-297.
- Norval M, Björn LO, de Gruijl FR. Is the action spectrum for the UV-induced production of previtamin D3 in human skin correct? Photochem Photobiol Sci. 2010;9:11-17.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281.
- McKenzie RL, Liley JB, Björn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85:88-98.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353-373.
- May E, Asadullah K, Zügel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377-393.
- Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618-625.
- Fu LW, Vender R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol Res Pract. 2011;2011:276079.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67:931-938.
- Osmancevic A, Landin-Wilhelmsen K, Larkö O, et al. UVB therapy increases 25 (OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed. 2007;23:172-178.
- Ryan C, Moran B, McKenna MJ, et al. The effect of narrowband UV-B treatment for psoriasis on vitamin D status during wintertime in Ireland. Arch Dermatol. 2010;146:836-842.
- Vahavihu K, Ala-Houhala M, Peric M, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321-328.
- Lesiak A, Narbutt J, Pawlaczyk M, et al. Vitamin D serum level changes in psoriatic patients treated with narrowband ultraviolet B phototherapy are related to the season of the irradiation. Photodermatol Photoimmunol Photomed. 2011;27:304-310.
- Osmancevic A, Landin-Wilhelmsen K, Larko O, et al.Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2009;25:119-123.
- Romaní J, Caixàs A, Carrascosa JM, et al. Effect of narrowband ultraviolet B therapy on inflammatory markers and body fat composition in moderate to severe psoriasis. Br J Dermatol. 2012;166:1237-1244.
- Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther. 2010;23:48-60.
- Armas LA, Dowell S, Akhter M, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588-593.
- Olds WJ, McKinley AR, Moore MR, et al. In vitro model of vitamin D3 (cholecalciferol) synthesis by UV radiation: dose-response relationships. J Photochem Photobiol B. 2008;93:88-93.
Practice Points
- The 25-hydroxyvitamin D (25[OH]D) levels are increased by narrowband UVB (NB-UVB) treatment in psoriasis patients.
- The number of sessions of NB-UVB is associated with increased 25(OH)D levels.
BSA75, BSA90, and BSA100: New Clinical Tools for Measuring Improvement in Psoriasis
Currently, there is no widely accepted tool for assessing the severity of psoriasis in the clinical setting.1-5 Moreover, there is still a need for a simple assessment tool to assist in evaluating a patient’s response to therapy in clinical practice.6
The body surface area (BSA) is a familiar and widely used measurement by clinicians. It is easily calculated by the rule of nines or with the patient’s open palm and thumb approximating 1% of the BSA.7 Body surface area is an uncomplicated concept for patients to understand and interpret. It also promotes patient empowerment and self-care by allowing patients to monitor short-term and long-term response to therapy.
The National Psoriasis Foundation Medical Board published treatment targets for plaque psoriasis. One of the conclusions states, “The acceptable response at 3 months postinitiation was either BSA 3% or less or BSA improvement 75% or more from baseline.”8
We propose a new nomenclature that a 75% improvement in BSA be recognized as BSA75, a 90% improvement in BSA as BSA90, and a 100% improvement in BSA as BSA100. These classifications would be analogous to corresponding improvements in the following psoriasis area and severity index (PASI) scores: PASI 75, PASI 90, PASI 100.9 A loss of BSA goals/milestones (ie, BSA75) could encourage and facilitate physician-patient conversations and further direct modifications to disease management and treatment therapy.
A potential drawback to the implementation of this novel categorization system is that other notable aspects of psoriasis would not be assessed, such as erythema, induration, or scale; subjective measurements; patient quality of life; patient symptoms; areas of involvement (eg, palms, soles of feet); and disease course. Nevertheless, the BSA75, BSA90, and BSA100 classifications can serve as practical, objective, and straightforward tools to monitor disease progression and treatment response in psoriasis patients, which may potentially promote improved patient outcomes in clinical practice.
- van de Kerkhof PC. The Psoriasis Area and Severity Index and alternative approaches for the assessment of severity: persisting areas of confusion. Br J Dermatol. 1997;137:661-662.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Ashcroft DM, Wan Po AL, Williams HC, et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141:185-191.
- Gottlieb AB, Chaudhari U, Baker DG, et al. The National Psoriasis Foundation Psoriasis Score (NPF-PS) system versus the Psoriasis Area Severity Index (PASI) and Physician’s Global Assessment (PGA): a comparison. J Drugs Dermatol. 2003;2:260-266.
- Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157:238-244.
- Sheridan RL, Petras L, Basha G, et al. Planimetry study of the percent of body surface represented by the hand and palm: sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995;16:605-606.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to Psoriasis Area Severity Index 90 and 100. J Drugs Dermatol. 2015;14:1086-1088.
Currently, there is no widely accepted tool for assessing the severity of psoriasis in the clinical setting.1-5 Moreover, there is still a need for a simple assessment tool to assist in evaluating a patient’s response to therapy in clinical practice.6
The body surface area (BSA) is a familiar and widely used measurement by clinicians. It is easily calculated by the rule of nines or with the patient’s open palm and thumb approximating 1% of the BSA.7 Body surface area is an uncomplicated concept for patients to understand and interpret. It also promotes patient empowerment and self-care by allowing patients to monitor short-term and long-term response to therapy.
The National Psoriasis Foundation Medical Board published treatment targets for plaque psoriasis. One of the conclusions states, “The acceptable response at 3 months postinitiation was either BSA 3% or less or BSA improvement 75% or more from baseline.”8
We propose a new nomenclature that a 75% improvement in BSA be recognized as BSA75, a 90% improvement in BSA as BSA90, and a 100% improvement in BSA as BSA100. These classifications would be analogous to corresponding improvements in the following psoriasis area and severity index (PASI) scores: PASI 75, PASI 90, PASI 100.9 A loss of BSA goals/milestones (ie, BSA75) could encourage and facilitate physician-patient conversations and further direct modifications to disease management and treatment therapy.
A potential drawback to the implementation of this novel categorization system is that other notable aspects of psoriasis would not be assessed, such as erythema, induration, or scale; subjective measurements; patient quality of life; patient symptoms; areas of involvement (eg, palms, soles of feet); and disease course. Nevertheless, the BSA75, BSA90, and BSA100 classifications can serve as practical, objective, and straightforward tools to monitor disease progression and treatment response in psoriasis patients, which may potentially promote improved patient outcomes in clinical practice.
Currently, there is no widely accepted tool for assessing the severity of psoriasis in the clinical setting.1-5 Moreover, there is still a need for a simple assessment tool to assist in evaluating a patient’s response to therapy in clinical practice.6
The body surface area (BSA) is a familiar and widely used measurement by clinicians. It is easily calculated by the rule of nines or with the patient’s open palm and thumb approximating 1% of the BSA.7 Body surface area is an uncomplicated concept for patients to understand and interpret. It also promotes patient empowerment and self-care by allowing patients to monitor short-term and long-term response to therapy.
The National Psoriasis Foundation Medical Board published treatment targets for plaque psoriasis. One of the conclusions states, “The acceptable response at 3 months postinitiation was either BSA 3% or less or BSA improvement 75% or more from baseline.”8
We propose a new nomenclature that a 75% improvement in BSA be recognized as BSA75, a 90% improvement in BSA as BSA90, and a 100% improvement in BSA as BSA100. These classifications would be analogous to corresponding improvements in the following psoriasis area and severity index (PASI) scores: PASI 75, PASI 90, PASI 100.9 A loss of BSA goals/milestones (ie, BSA75) could encourage and facilitate physician-patient conversations and further direct modifications to disease management and treatment therapy.
A potential drawback to the implementation of this novel categorization system is that other notable aspects of psoriasis would not be assessed, such as erythema, induration, or scale; subjective measurements; patient quality of life; patient symptoms; areas of involvement (eg, palms, soles of feet); and disease course. Nevertheless, the BSA75, BSA90, and BSA100 classifications can serve as practical, objective, and straightforward tools to monitor disease progression and treatment response in psoriasis patients, which may potentially promote improved patient outcomes in clinical practice.
- van de Kerkhof PC. The Psoriasis Area and Severity Index and alternative approaches for the assessment of severity: persisting areas of confusion. Br J Dermatol. 1997;137:661-662.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Ashcroft DM, Wan Po AL, Williams HC, et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141:185-191.
- Gottlieb AB, Chaudhari U, Baker DG, et al. The National Psoriasis Foundation Psoriasis Score (NPF-PS) system versus the Psoriasis Area Severity Index (PASI) and Physician’s Global Assessment (PGA): a comparison. J Drugs Dermatol. 2003;2:260-266.
- Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157:238-244.
- Sheridan RL, Petras L, Basha G, et al. Planimetry study of the percent of body surface represented by the hand and palm: sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995;16:605-606.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to Psoriasis Area Severity Index 90 and 100. J Drugs Dermatol. 2015;14:1086-1088.
- van de Kerkhof PC. The Psoriasis Area and Severity Index and alternative approaches for the assessment of severity: persisting areas of confusion. Br J Dermatol. 1997;137:661-662.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51:563-569.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Ashcroft DM, Wan Po AL, Williams HC, et al. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141:185-191.
- Gottlieb AB, Chaudhari U, Baker DG, et al. The National Psoriasis Foundation Psoriasis Score (NPF-PS) system versus the Psoriasis Area Severity Index (PASI) and Physician’s Global Assessment (PGA): a comparison. J Drugs Dermatol. 2003;2:260-266.
- Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157:238-244.
- Sheridan RL, Petras L, Basha G, et al. Planimetry study of the percent of body surface represented by the hand and palm: sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995;16:605-606.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to Psoriasis Area Severity Index 90 and 100. J Drugs Dermatol. 2015;14:1086-1088.
Experts endorse routine screening for pediatric psoriasis comorbidities
Pediatric psoriasis patients should be screened regularly to identify risk factors for comorbidities including depression, gastrointestinal problems, diabetes, and dyslipidemia, according to the debut guidelines issued by an expert panel.
The National Psoriasis Foundation and the Pediatric Dermatology Research Alliance joined forces to assess the literature and develop recommendations for screening comorbidities for children with psoriasis. The National Psoriasis Foundation has guidelines for comorbidity screening in adults with psoriasis, but no guidelines previously existed for children, wrote Emily Osier, MD, of Eastern Virginia Medical School, Norfolk, and her colleagues (JAMA Dermatol 2017 May 17. doi: 10.1001/jamadermatol.2017.0499).
The panelists reviewed the literature on psoriasis and comorbidities published between 1999 and 2015 and identified 153 studies, 26 of which involved children.
“The screening recommendations derived are largely consistent with those endorsed by the AAP for the general pediatric patient,” the researchers noted.
Although many young children are screened for a range of comorbid conditions at annual checkups, preteens and teenagers may be less likely to receive preventive services in primary care, they said. “Thus, all health care providers caring for patients with pediatric psoriasis should help assess and ensure that appropriate screening has been performed,” they emphasized.
Some notable recommendations include the following:
• Screen children with psoriasis for overweight and obesity annually using body mass index percentiles.
• Screen for diabetes every 3 years starting at age 10 years.
• Perform universal lipid screening at ages 9-11 years and 17-21 years.
• Screen for nonalcoholic fatty liver disease every 2-3 years starting at age 9-11 years.
• Screen for hypertension annually starting at age 3 years.
• Screen for arthritis at the time of psoriasis diagnosis and periodically.
• Screen yearly for depression and anxiety at all ages, with yearly screening for substance abuse starting at age 11 years.
Uveitis screening is recommended only for children with psoriatic arthritis, the researchers said.
In addition, clinicians “should recognize the profound psychosocial ramifications of psoriasis and the potential significant impact on quality of life of patients and caregivers,” the researchers wrote. Clinicians may consider a formal quality of life measurement, such as the Children’s Dermatology Life Quality Index, or at least asking questions about the impact of psoriasis on the child’s life at home, at school, and during other activities.
Awareness of comorbidities also impacts potential psoriasis treatment, the researchers said. “Direct baseline screening and monitoring tests should be performed as indicated by each individual’s therapeutic plan,” they said.
The consensus statement is a starting point for screening that will be refined over time, and may include stratifying patients by age, disease subtype, or disease severity, the researchers noted.
“Communication and collaboration between dermatologists, primary care providers, and other pediatric specialists will be critical to accomplish the recommended screenings and to limit the sequelae of this disorder,” they wrote.
The National Psoriasis Foundation and the University of California, San Diego, Eczema and Inflammatory Skin Disease Center supported the study. Dr. Osier was supported in part by a Medical Dermatology Research Fellowship grant from the National Psoriasis Foundation in 2014-2016, but she had no financial conflicts to disclose.
Pediatric psoriasis patients should be screened regularly to identify risk factors for comorbidities including depression, gastrointestinal problems, diabetes, and dyslipidemia, according to the debut guidelines issued by an expert panel.
The National Psoriasis Foundation and the Pediatric Dermatology Research Alliance joined forces to assess the literature and develop recommendations for screening comorbidities for children with psoriasis. The National Psoriasis Foundation has guidelines for comorbidity screening in adults with psoriasis, but no guidelines previously existed for children, wrote Emily Osier, MD, of Eastern Virginia Medical School, Norfolk, and her colleagues (JAMA Dermatol 2017 May 17. doi: 10.1001/jamadermatol.2017.0499).
The panelists reviewed the literature on psoriasis and comorbidities published between 1999 and 2015 and identified 153 studies, 26 of which involved children.
“The screening recommendations derived are largely consistent with those endorsed by the AAP for the general pediatric patient,” the researchers noted.
Although many young children are screened for a range of comorbid conditions at annual checkups, preteens and teenagers may be less likely to receive preventive services in primary care, they said. “Thus, all health care providers caring for patients with pediatric psoriasis should help assess and ensure that appropriate screening has been performed,” they emphasized.
Some notable recommendations include the following:
• Screen children with psoriasis for overweight and obesity annually using body mass index percentiles.
• Screen for diabetes every 3 years starting at age 10 years.
• Perform universal lipid screening at ages 9-11 years and 17-21 years.
• Screen for nonalcoholic fatty liver disease every 2-3 years starting at age 9-11 years.
• Screen for hypertension annually starting at age 3 years.
• Screen for arthritis at the time of psoriasis diagnosis and periodically.
• Screen yearly for depression and anxiety at all ages, with yearly screening for substance abuse starting at age 11 years.
Uveitis screening is recommended only for children with psoriatic arthritis, the researchers said.
In addition, clinicians “should recognize the profound psychosocial ramifications of psoriasis and the potential significant impact on quality of life of patients and caregivers,” the researchers wrote. Clinicians may consider a formal quality of life measurement, such as the Children’s Dermatology Life Quality Index, or at least asking questions about the impact of psoriasis on the child’s life at home, at school, and during other activities.
Awareness of comorbidities also impacts potential psoriasis treatment, the researchers said. “Direct baseline screening and monitoring tests should be performed as indicated by each individual’s therapeutic plan,” they said.
The consensus statement is a starting point for screening that will be refined over time, and may include stratifying patients by age, disease subtype, or disease severity, the researchers noted.
“Communication and collaboration between dermatologists, primary care providers, and other pediatric specialists will be critical to accomplish the recommended screenings and to limit the sequelae of this disorder,” they wrote.
The National Psoriasis Foundation and the University of California, San Diego, Eczema and Inflammatory Skin Disease Center supported the study. Dr. Osier was supported in part by a Medical Dermatology Research Fellowship grant from the National Psoriasis Foundation in 2014-2016, but she had no financial conflicts to disclose.
Pediatric psoriasis patients should be screened regularly to identify risk factors for comorbidities including depression, gastrointestinal problems, diabetes, and dyslipidemia, according to the debut guidelines issued by an expert panel.
The National Psoriasis Foundation and the Pediatric Dermatology Research Alliance joined forces to assess the literature and develop recommendations for screening comorbidities for children with psoriasis. The National Psoriasis Foundation has guidelines for comorbidity screening in adults with psoriasis, but no guidelines previously existed for children, wrote Emily Osier, MD, of Eastern Virginia Medical School, Norfolk, and her colleagues (JAMA Dermatol 2017 May 17. doi: 10.1001/jamadermatol.2017.0499).
The panelists reviewed the literature on psoriasis and comorbidities published between 1999 and 2015 and identified 153 studies, 26 of which involved children.
“The screening recommendations derived are largely consistent with those endorsed by the AAP for the general pediatric patient,” the researchers noted.
Although many young children are screened for a range of comorbid conditions at annual checkups, preteens and teenagers may be less likely to receive preventive services in primary care, they said. “Thus, all health care providers caring for patients with pediatric psoriasis should help assess and ensure that appropriate screening has been performed,” they emphasized.
Some notable recommendations include the following:
• Screen children with psoriasis for overweight and obesity annually using body mass index percentiles.
• Screen for diabetes every 3 years starting at age 10 years.
• Perform universal lipid screening at ages 9-11 years and 17-21 years.
• Screen for nonalcoholic fatty liver disease every 2-3 years starting at age 9-11 years.
• Screen for hypertension annually starting at age 3 years.
• Screen for arthritis at the time of psoriasis diagnosis and periodically.
• Screen yearly for depression and anxiety at all ages, with yearly screening for substance abuse starting at age 11 years.
Uveitis screening is recommended only for children with psoriatic arthritis, the researchers said.
In addition, clinicians “should recognize the profound psychosocial ramifications of psoriasis and the potential significant impact on quality of life of patients and caregivers,” the researchers wrote. Clinicians may consider a formal quality of life measurement, such as the Children’s Dermatology Life Quality Index, or at least asking questions about the impact of psoriasis on the child’s life at home, at school, and during other activities.
Awareness of comorbidities also impacts potential psoriasis treatment, the researchers said. “Direct baseline screening and monitoring tests should be performed as indicated by each individual’s therapeutic plan,” they said.
The consensus statement is a starting point for screening that will be refined over time, and may include stratifying patients by age, disease subtype, or disease severity, the researchers noted.
“Communication and collaboration between dermatologists, primary care providers, and other pediatric specialists will be critical to accomplish the recommended screenings and to limit the sequelae of this disorder,” they wrote.
The National Psoriasis Foundation and the University of California, San Diego, Eczema and Inflammatory Skin Disease Center supported the study. Dr. Osier was supported in part by a Medical Dermatology Research Fellowship grant from the National Psoriasis Foundation in 2014-2016, but she had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
Infliximab biosimilar noninferior to originator in IBD – NOR-SWITCH
CHICAGO – The biosimilar infliximab CT-P13 is not inferior to the originator infliximab in terms of efficacy, safety, and immunogenicity in the treatment of inflammatory bowel disease (IBD), a phase IV randomized trial showed.
Patient outcomes were not compromised with the use of the biosimilar, and the cost of treatment was much lower, said lead author Kristin K. Jørgensen, MD, PhD, at Digestive Disease Week®.
“Biologics represent a substantial source of IBD expenditure,” said Dr. Jørgensen of Akershus University Hospital, Lørenskog, Norway. “The medication is expensive, patients are treated on a long-term basis, and the incidence of IBD is increasing.”
Biosimilars are biotherapeutic products that are similar in terms of quality, safety, and efficacy to the already licensed reference biologic product. “Use of biosimilars can potentially dramatically decrease costs and may lead to better patient care,” said Dr. Jørgensen. “The patient gets increased access to biologic therapy, and it is easier to intensify dosing if indicated.”
Tumor necrosis factor–inhibitors are commonly used to treat Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, and chronic plaque psoriasis, and, while they have altered the treatment paradigm, they are expensive products.
The goal of the NOR-SWITCH was to evaluate switching from originator infliximab to CT-P13, in terms of efficacy, safety, and immunogenicity.
Dr. Jørgensen and her colleagues conducted a randomized phase IV trial that enrolled 482 patients who were randomly assigned to either infliximab originator (n = 241) or CT-P13 (n = 241). The primary endpoint was disease worsening during follow-up.
Of the group, 155 patients (32%) had Crohn’s disease, 93 (19%) had ulcerative colitis, 91 (19%) had spondyloarthritis, 77 (16%) had rheumatoid arthritis, 30 (6%) had psoriatic arthritis, and 35 (7%) had chronic plaque psoriasis.
Disease worsening occurred at a similar rate in both groups. In the infliximab originator group, 53 patients (26%) experienced a worsening of their symptoms, compared with 61 patients (30%) in the CT-P13 group. The 95% confidence interval of the adjusted risk difference (−4.4%) was −12.7% to 3.9%, which fell within the prespecified noninferiority margin of 15%.
Therefore, the findings demonstrated that CT-P13 is not inferior to infliximab originator, and the adjusted relative risk of disease worsening for CT-P13 patients was 1.17 (95% CI, 0.82-1.52), compared with the infliximab originator group.
An almost equal number of patients achieved disease remission, 123 patients (61%) in the infliximab originator group and 126 patients (61%) in the CT-P13 group, and the adjusted rate difference was 0.6% (95% CI, –7.5%-8.8%; per-protocol set).
An explorative subgroup analysis that looked at patients with Crohn’s disease and ulcerative colitis showed similar findings between patients treated with either agent.
“Our results support switching from the originator to a biosimilar for nonmedical reasons,” concluded Dr. Jørgensen.
However, she urged caution in generalizing these findings to other biologic agents.
The study was funded by the Norwegian Ministry of Health and Care Services. Dr. Jorgensen reported receiving personal fees from Tillotts, Intercept, and Celltrion. Several coauthors also reported relationships with industry.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – The biosimilar infliximab CT-P13 is not inferior to the originator infliximab in terms of efficacy, safety, and immunogenicity in the treatment of inflammatory bowel disease (IBD), a phase IV randomized trial showed.
Patient outcomes were not compromised with the use of the biosimilar, and the cost of treatment was much lower, said lead author Kristin K. Jørgensen, MD, PhD, at Digestive Disease Week®.
“Biologics represent a substantial source of IBD expenditure,” said Dr. Jørgensen of Akershus University Hospital, Lørenskog, Norway. “The medication is expensive, patients are treated on a long-term basis, and the incidence of IBD is increasing.”
Biosimilars are biotherapeutic products that are similar in terms of quality, safety, and efficacy to the already licensed reference biologic product. “Use of biosimilars can potentially dramatically decrease costs and may lead to better patient care,” said Dr. Jørgensen. “The patient gets increased access to biologic therapy, and it is easier to intensify dosing if indicated.”
Tumor necrosis factor–inhibitors are commonly used to treat Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, and chronic plaque psoriasis, and, while they have altered the treatment paradigm, they are expensive products.
The goal of the NOR-SWITCH was to evaluate switching from originator infliximab to CT-P13, in terms of efficacy, safety, and immunogenicity.
Dr. Jørgensen and her colleagues conducted a randomized phase IV trial that enrolled 482 patients who were randomly assigned to either infliximab originator (n = 241) or CT-P13 (n = 241). The primary endpoint was disease worsening during follow-up.
Of the group, 155 patients (32%) had Crohn’s disease, 93 (19%) had ulcerative colitis, 91 (19%) had spondyloarthritis, 77 (16%) had rheumatoid arthritis, 30 (6%) had psoriatic arthritis, and 35 (7%) had chronic plaque psoriasis.
Disease worsening occurred at a similar rate in both groups. In the infliximab originator group, 53 patients (26%) experienced a worsening of their symptoms, compared with 61 patients (30%) in the CT-P13 group. The 95% confidence interval of the adjusted risk difference (−4.4%) was −12.7% to 3.9%, which fell within the prespecified noninferiority margin of 15%.
Therefore, the findings demonstrated that CT-P13 is not inferior to infliximab originator, and the adjusted relative risk of disease worsening for CT-P13 patients was 1.17 (95% CI, 0.82-1.52), compared with the infliximab originator group.
An almost equal number of patients achieved disease remission, 123 patients (61%) in the infliximab originator group and 126 patients (61%) in the CT-P13 group, and the adjusted rate difference was 0.6% (95% CI, –7.5%-8.8%; per-protocol set).
An explorative subgroup analysis that looked at patients with Crohn’s disease and ulcerative colitis showed similar findings between patients treated with either agent.
“Our results support switching from the originator to a biosimilar for nonmedical reasons,” concluded Dr. Jørgensen.
However, she urged caution in generalizing these findings to other biologic agents.
The study was funded by the Norwegian Ministry of Health and Care Services. Dr. Jorgensen reported receiving personal fees from Tillotts, Intercept, and Celltrion. Several coauthors also reported relationships with industry.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – The biosimilar infliximab CT-P13 is not inferior to the originator infliximab in terms of efficacy, safety, and immunogenicity in the treatment of inflammatory bowel disease (IBD), a phase IV randomized trial showed.
Patient outcomes were not compromised with the use of the biosimilar, and the cost of treatment was much lower, said lead author Kristin K. Jørgensen, MD, PhD, at Digestive Disease Week®.
“Biologics represent a substantial source of IBD expenditure,” said Dr. Jørgensen of Akershus University Hospital, Lørenskog, Norway. “The medication is expensive, patients are treated on a long-term basis, and the incidence of IBD is increasing.”
Biosimilars are biotherapeutic products that are similar in terms of quality, safety, and efficacy to the already licensed reference biologic product. “Use of biosimilars can potentially dramatically decrease costs and may lead to better patient care,” said Dr. Jørgensen. “The patient gets increased access to biologic therapy, and it is easier to intensify dosing if indicated.”
Tumor necrosis factor–inhibitors are commonly used to treat Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, and chronic plaque psoriasis, and, while they have altered the treatment paradigm, they are expensive products.
The goal of the NOR-SWITCH was to evaluate switching from originator infliximab to CT-P13, in terms of efficacy, safety, and immunogenicity.
Dr. Jørgensen and her colleagues conducted a randomized phase IV trial that enrolled 482 patients who were randomly assigned to either infliximab originator (n = 241) or CT-P13 (n = 241). The primary endpoint was disease worsening during follow-up.
Of the group, 155 patients (32%) had Crohn’s disease, 93 (19%) had ulcerative colitis, 91 (19%) had spondyloarthritis, 77 (16%) had rheumatoid arthritis, 30 (6%) had psoriatic arthritis, and 35 (7%) had chronic plaque psoriasis.
Disease worsening occurred at a similar rate in both groups. In the infliximab originator group, 53 patients (26%) experienced a worsening of their symptoms, compared with 61 patients (30%) in the CT-P13 group. The 95% confidence interval of the adjusted risk difference (−4.4%) was −12.7% to 3.9%, which fell within the prespecified noninferiority margin of 15%.
Therefore, the findings demonstrated that CT-P13 is not inferior to infliximab originator, and the adjusted relative risk of disease worsening for CT-P13 patients was 1.17 (95% CI, 0.82-1.52), compared with the infliximab originator group.
An almost equal number of patients achieved disease remission, 123 patients (61%) in the infliximab originator group and 126 patients (61%) in the CT-P13 group, and the adjusted rate difference was 0.6% (95% CI, –7.5%-8.8%; per-protocol set).
An explorative subgroup analysis that looked at patients with Crohn’s disease and ulcerative colitis showed similar findings between patients treated with either agent.
“Our results support switching from the originator to a biosimilar for nonmedical reasons,” concluded Dr. Jørgensen.
However, she urged caution in generalizing these findings to other biologic agents.
The study was funded by the Norwegian Ministry of Health and Care Services. Dr. Jorgensen reported receiving personal fees from Tillotts, Intercept, and Celltrion. Several coauthors also reported relationships with industry.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
AT DDW
Key clinical point: An infliximab biosimilar was not inferior to the originator in terms of efficacy, safety, and immunogenicity in the treatment of inflammatory bowel disease (IBD).
Major finding: In the infliximab originator group, 53 patients (26%) experienced disease worsening, vs. 61 patients (30%) in the CT-P13 group, which fell within the prespecified noninferiority margin of 15%.
Data source: A phase IV randomized trial that included 482 patients with inflammatory bowel disease.
Disclosures: The study was funded by the Norwegian Ministry of Health and Care Services. Dr. Jorgensen reported receiving personal fees from Tillotts, Intercept, and Celltrion. Several coauthors also reported relationships with industry.
Adalimumab outperforms methotrexate in treating severe pediatric plaque psoriasis
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
The initial treatment response to adalimumab was quick with “significant clinical difference compared with methotrexate seen as early as 4 weeks.” Improvement in the pediatric quality of life inventory (PedsQL) score from baseline “was significantly greater” in children in the adalimumab 0.8-mg/kg group than in children in the methotrexate group. Adalimumab provided “several clinically and statistically significant improvements” in children with severe plaque psoriasis, compared with methotrexate.
However, further studies are needed to determine both the short-term and long-term effectiveness and the safety of systemic treatments in children and adolescents with psoriasis.
Dario Kivelevitch, MD, is a third year dermatology resident, and Alan Menter, MD, is chief of dermatology at Baylor University Medical Center, Dallas. These comments are from an editorial that accompanied the study (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31190-X). Dr. Kivelevitch said he had no relevant financial disclosures. Dr. Menter disclosed grants and personal fees from AbbVie and other pharmaceutical companies, all outside the submitted work.
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
Adalimumab appears to be a safe and effective treatment option for severe plaque psoriasis in children, outperforming methotrexate, based on the results of a phase III study, said Kim Papp, MD, PhD, of Probity Medical Research, Waterloo, Ont., and his associates.
“To our knowledge, this is the first randomized controlled study of either adalimumab or methotrexate in children and adolescents with psoriasis,” the researchers said, noting that the study did not include a placebo group because of ethical issues related to treating children with a severe chronic disorder.
At week 16 of the initial treatment period, an improvement of at least 75% in the Psoriasis Area and Severity Index (PASI75) score was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group – 22 (58%) – than in the methotrexate group – 12 (32%). In the 0.4-mg/kg adalimumab group, 17 (44%) of patients reached a PASI75. The PASI75 response was rapid in the 0.8 mg/kg adalimumab group, a significant difference, compared with the methotrexate group. It was reached by week 4 (P = .002).
“At week 16, the 26% difference between adalimumab 0.8 mg/kg and methotrexate in the proportion of patients who achieved PASI75 was significant and clinically relevant,” Dr. Papp and his associates concluded.
At week 16 of treatment, the proportion of patients who achieved a physician global assessment (PGA) score of 0 or 1 (clear or minimal) was higher in the adalimumab 0.8 mg/kg group (23 of 38 [61%]) than in the methotrexate group (15 of 37 [41%]) or in the adalimumab 0.4-mg/kg group (16 of 39 [41%]) (P = .083). At week 16, the difference between the adalimumab 0.8-mg/kg and methotrexate groups was not significant, the investigators said (Lancet. 2017. doi: 10.1016/ S0140-6736[17]31189-3).
After the withdrawal period, PASI75 was achieved in 15 of 19 (79%) patients who were initial responders to adalimumab 0.8 mg/kg and 6 of 11 (55%) patients who were initial responders to adalimumab 0.4 mg/kg. PASI75 was achieved in six of eight (75%) patients who had responded to methotrexate treatment in the initial treatment period and who had loss of disease control in the withdrawal period.
During the initial treatment period, adverse events were reported by 26 of 38 (68%) in the adalimumab 0.8-mg/kg group, 30 of 39 (77%) in the adalimumab 0.4-mg/kg group, and 28 of 37 (76%) in the methotrexate group. Infections were the most frequently reported adverse events. Serious adverse events were infrequent, reported by three patients in the adalimumab 0.4-mg/kg group, and were not considered to be related to the study drug, the researchers said. Eleven severe adverse events were reported by 8 of the 114 (7%) children. Of these, headache was the most common. A case of urticaria during retreatment that led to discontinuation of adalimumab in the patient (who had received methotrexate in the first treatment period), was considered by the investigator as “probably related” to adalimumab.
“No new safety risks were identified in our study; however, longer-term data are needed to fully assess the safety profile of adalimumab in the pediatric population,” Dr. Papp and his associates commented.
“Our results showed better quality of life outcomes in children and adolescents treated with adalimumab compared with methotrexate. The mean 10.8-point change in PedsQL [pediatric quality of life inventory] from baseline to week 16 in the adalimumab 0.8-mg/kg group exceeded the minimal clinically important difference of 4.36, whereas the 1.9-point change in the methotrexate group did not,” they noted.
The study was funded by AbbVie, the manufacturer of adalimumab (Humira). Dr. Papp has served as a consultant for AbbVie and a number of other pharmaceutical companies, for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
FROM THE LANCET
Key clinical point:
Major finding: At week 16 of the initial treatment period, Psoriasis Area and Severity Index (PASI)175 was reached by significantly more of the patients in the 0.8 mg/kg adalimumab group (22 of 38 [58%]) than in the methotrexate group (12 of 37 [32%]).
Data source: A double-blind, phase III trial was done at 38 clinics in 13 countries with 114 children aged 4-17 years, with severe plaque psoriasis that had not responded to topical therapy.
Disclosures: The study was funded by AbbVie. Dr. Papp has served as a consultant for adalimumab manufacturer AbbVie and a number of other pharmaceutical companies for which he has served as consultant or speaker or on advisory boards. His associates listed numerous similar disclosures. Two authors were AbbVie employees.
Living With Psoriasis: How the Disease Impacts the Daily Activities of Patients
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Some data support botulinum toxin for psoriasis and rosacea
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Pediatric psoriasis may have a distinct presentation
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Children may have a distinctive presentation of psoriasis, compared with adults, Dr. Wynnis Tom said at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and UC San Diego School of Medicine.
Infants may present with diaper involvement, also known as “napkin psoriasis,” which may be confused with irritant dermatitis or perineal infections. Moreover, guttate psoriasis, which can be preceded by infectious triggers such as Streptococcus and appears as small, salmon-pink bumps on the skin, is more frequent in children than adults, said Dr. Tom, associate professor of dermatology and pediatrics at the university.
Patients with psoriasis are at higher risk for psychiatric disorders, especially depression and anxiety. A study by Varni et al. discussed QOL ratings by 208 children aged 4-17 years with moderate to severe plaque disease. The study demonstrated a significant negative QOL impact in patients with plaque psoriasis, comparable to the impairment of QOL from arthritis or asthma (Eur J Pediatr. 2011 Sep 30;171[3]485-92).
Dr. Tom talked about other comorbidities associated with psoriasis, including psoriatic arthritis, and encouraged physicians to inquire about morning stiffness, joint pains, swelling, and gait abnormalities. “Psoriatic arthritis occurs in about 10% of children, and it is essential to detect early to prevent permanent joint damage,” she said. “Over the past decade, psoriasis has resurfaced as a systemic disorder as it may be associated with obesity, metabolic syndrome, and inflammatory bowel disease.” Psoriasis also entails an increased risk for cardiovascular disease, myocardial infarction, and stroke.
Dr. Tom emphasized, “because of these risks, we need to extend comorbidity screening to the pediatric population.”
Management of pediatric psoriasis has focused on topical and systemic therapies, in addition to phototherapies. Most systemic agents are used off-label on the basis of experience rather than evidence. Clinical trials are currently underway to extend indications for systemic therapy to the pediatric age group, she said.
Dr. Tom disclosed she is an investigator for Promius Pharma, Celgene, and Janssen.
Study underscores antipsoriatic effect of gastric bypass surgery
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Key clinical point: Gastric bypass, but not gastric banding, was associated with significant drops in rates of psoriasis and psoriatic arthritis.
Major finding: In an adjusted model, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis, with a 56% drop in the rate of severe psoriasis, and with a 71% drop in the rate of psoriatic arthritis.
Data source: A population-based cohort study of 12,364 gastric bypass patients and 1,071 gastric banding patients.
Disclosures: An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies. The other coinvestigators reported having no ties to industry.
Renflexis approved as second infliximab biosimilar
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
[email protected]
On Twitter @denisefulton
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
[email protected]
On Twitter @denisefulton
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
[email protected]
On Twitter @denisefulton