User login
Ixekizumab helps PsA patients who failed a TNFi
MADRID – The anti–interleukin-17 drug ixekizumab, already on the U.S. market for treating psoriasis, showed efficacy and safety for treating psoriatic arthritis in patients who previously failed to respond to or tolerate a tumor necrosis factor inhibitor in a pivotal, phase 3 trial with 363 patients.
Treatment of patients with psoriatic arthritis (PsA) with ixekizumab (Taltz) led to improvements, compared with placebo, in arthritis, physical function, and psoriasis. These patients were unresponsive to or intolerant of a tumor necrosis factor inhibitor (TNFi) at rates similar to previously reported response rates for PsA patients who were TNFi naive, Peter Nash, MD, said at the European Congress of Rheumatology.
A published report with the data presented by Dr. Nash also recently appeared (Lancet. 2017;389[10086]:2317-27).
Based in part on the results from this trial, as well as results from a companion phase 3 trial that enrolled PsA patients naive to a TNFi (Ann Rheum Dis. 2017 Jan; 6[1]:79-87), the company that markets ixekizumab, Eli Lilly, filed an application with the Food and Drug Administration in early 2017 to have a new label indication for PsA, said a company spokeswoman.
“At least half of PsA patients don’t get at least a 20% improvement [an ACR20 response] on a TNFi, and so they are looking for something else,” explained Mark C. Genovese, MD, professor of medicine and director of the Rheumatology Clinic at Stanford (Calif.) University and a coinvestigator on the trial reported by Dr. Nash. “There is pent up demand” for an alternative to a TNFi for treating PsA, Dr. Genovese said in an interview.
The finding also sets ixekizumab apart from secukinumab (Cosentyx), another interleukin-17 inhibitor that already has FDA approval for treating PsA but that has not been specifically tested in PsA patients who failed or didn’t tolerate a TNFi, he noted.
The SPIRIT-P2 results also showed superior outcomes for patients treated with an ixekizumab injection once every 2 or 4 weeks, compared with placebo, by several secondary measures, including ACR50 and ACR70 rates and minimal disease activity. The ACR70 rate after 24 weeks on treatment was 23% with a dose of ixekizumab every 4 weeks and none with placebo. Minimal disease activity was reached by about a quarter of patients on either dosage of the active drug and by 3% of patients on placebo.
Despite the apparent role for ixekizumab when TNFi treatment fails, the TNFi drug class remains the clear first-line choice for PsA patients who are starting a biological drug for the first time. Not only do the TNFis have a much longer and more extensive track record but they also generally receive better insurance coverage that minimizes out-of-pocket expenses for patients, Dr. Genovese said.
SPIRIT-P2 was sponsored by Eli Lilly, the company that markets ixekizumab. Dr. Nash has been a speaker for or consultant to and has received research funding from Eli Lily and for several other companies. Dr. Genovese has been a consultant to and has received research funding from Eli Lilly, AbbVie, Astellas, Galapagos, Pfizer, and Vertex.
[email protected]
On Twitter @mitchelzoler
MADRID – The anti–interleukin-17 drug ixekizumab, already on the U.S. market for treating psoriasis, showed efficacy and safety for treating psoriatic arthritis in patients who previously failed to respond to or tolerate a tumor necrosis factor inhibitor in a pivotal, phase 3 trial with 363 patients.
Treatment of patients with psoriatic arthritis (PsA) with ixekizumab (Taltz) led to improvements, compared with placebo, in arthritis, physical function, and psoriasis. These patients were unresponsive to or intolerant of a tumor necrosis factor inhibitor (TNFi) at rates similar to previously reported response rates for PsA patients who were TNFi naive, Peter Nash, MD, said at the European Congress of Rheumatology.
A published report with the data presented by Dr. Nash also recently appeared (Lancet. 2017;389[10086]:2317-27).
Based in part on the results from this trial, as well as results from a companion phase 3 trial that enrolled PsA patients naive to a TNFi (Ann Rheum Dis. 2017 Jan; 6[1]:79-87), the company that markets ixekizumab, Eli Lilly, filed an application with the Food and Drug Administration in early 2017 to have a new label indication for PsA, said a company spokeswoman.
“At least half of PsA patients don’t get at least a 20% improvement [an ACR20 response] on a TNFi, and so they are looking for something else,” explained Mark C. Genovese, MD, professor of medicine and director of the Rheumatology Clinic at Stanford (Calif.) University and a coinvestigator on the trial reported by Dr. Nash. “There is pent up demand” for an alternative to a TNFi for treating PsA, Dr. Genovese said in an interview.
The finding also sets ixekizumab apart from secukinumab (Cosentyx), another interleukin-17 inhibitor that already has FDA approval for treating PsA but that has not been specifically tested in PsA patients who failed or didn’t tolerate a TNFi, he noted.
The SPIRIT-P2 results also showed superior outcomes for patients treated with an ixekizumab injection once every 2 or 4 weeks, compared with placebo, by several secondary measures, including ACR50 and ACR70 rates and minimal disease activity. The ACR70 rate after 24 weeks on treatment was 23% with a dose of ixekizumab every 4 weeks and none with placebo. Minimal disease activity was reached by about a quarter of patients on either dosage of the active drug and by 3% of patients on placebo.
Despite the apparent role for ixekizumab when TNFi treatment fails, the TNFi drug class remains the clear first-line choice for PsA patients who are starting a biological drug for the first time. Not only do the TNFis have a much longer and more extensive track record but they also generally receive better insurance coverage that minimizes out-of-pocket expenses for patients, Dr. Genovese said.
SPIRIT-P2 was sponsored by Eli Lilly, the company that markets ixekizumab. Dr. Nash has been a speaker for or consultant to and has received research funding from Eli Lily and for several other companies. Dr. Genovese has been a consultant to and has received research funding from Eli Lilly, AbbVie, Astellas, Galapagos, Pfizer, and Vertex.
[email protected]
On Twitter @mitchelzoler
MADRID – The anti–interleukin-17 drug ixekizumab, already on the U.S. market for treating psoriasis, showed efficacy and safety for treating psoriatic arthritis in patients who previously failed to respond to or tolerate a tumor necrosis factor inhibitor in a pivotal, phase 3 trial with 363 patients.
Treatment of patients with psoriatic arthritis (PsA) with ixekizumab (Taltz) led to improvements, compared with placebo, in arthritis, physical function, and psoriasis. These patients were unresponsive to or intolerant of a tumor necrosis factor inhibitor (TNFi) at rates similar to previously reported response rates for PsA patients who were TNFi naive, Peter Nash, MD, said at the European Congress of Rheumatology.
A published report with the data presented by Dr. Nash also recently appeared (Lancet. 2017;389[10086]:2317-27).
Based in part on the results from this trial, as well as results from a companion phase 3 trial that enrolled PsA patients naive to a TNFi (Ann Rheum Dis. 2017 Jan; 6[1]:79-87), the company that markets ixekizumab, Eli Lilly, filed an application with the Food and Drug Administration in early 2017 to have a new label indication for PsA, said a company spokeswoman.
“At least half of PsA patients don’t get at least a 20% improvement [an ACR20 response] on a TNFi, and so they are looking for something else,” explained Mark C. Genovese, MD, professor of medicine and director of the Rheumatology Clinic at Stanford (Calif.) University and a coinvestigator on the trial reported by Dr. Nash. “There is pent up demand” for an alternative to a TNFi for treating PsA, Dr. Genovese said in an interview.
The finding also sets ixekizumab apart from secukinumab (Cosentyx), another interleukin-17 inhibitor that already has FDA approval for treating PsA but that has not been specifically tested in PsA patients who failed or didn’t tolerate a TNFi, he noted.
The SPIRIT-P2 results also showed superior outcomes for patients treated with an ixekizumab injection once every 2 or 4 weeks, compared with placebo, by several secondary measures, including ACR50 and ACR70 rates and minimal disease activity. The ACR70 rate after 24 weeks on treatment was 23% with a dose of ixekizumab every 4 weeks and none with placebo. Minimal disease activity was reached by about a quarter of patients on either dosage of the active drug and by 3% of patients on placebo.
Despite the apparent role for ixekizumab when TNFi treatment fails, the TNFi drug class remains the clear first-line choice for PsA patients who are starting a biological drug for the first time. Not only do the TNFis have a much longer and more extensive track record but they also generally receive better insurance coverage that minimizes out-of-pocket expenses for patients, Dr. Genovese said.
SPIRIT-P2 was sponsored by Eli Lilly, the company that markets ixekizumab. Dr. Nash has been a speaker for or consultant to and has received research funding from Eli Lily and for several other companies. Dr. Genovese has been a consultant to and has received research funding from Eli Lilly, AbbVie, Astellas, Galapagos, Pfizer, and Vertex.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: The ACR20 rate after 24 weeks of treatment was 53% with monthly ixekizumab and 20% on placebo.
Data source: The SPIRIT-P2 trial, a phase 3 multicenter trial with 363 patients.
Disclosures: SPIRIT-P2 was sponsored by Eli Lilly, the company that markets ixekizumab (Taltz). Dr. Nash has been a speaker for or consultant to and has received research funding from Eli Lily and for several other companies. Dr. Genovese has been a consultant to and has received research funding from Eli Lilly, AbbVie, Astellas, Galapagos, Pfizer, and Vertex.
First IL-23 blocker, guselkumab, earns FDA approval for psoriasis
, based on three phase 3 studies of more than 2,000 adults, the manufacturer announced July 13.
The approved indication is for adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, according to a press release issued by Janssen Biotech, which stated that this is the first IL-23 blocker approved for psoriasis.
Results of one of the phase 3 trials, VOYAGE 1, included a significantly greater proportion of patients treated with guselkumab achieving at least a 90% improvement in the Psoriasis Area Severity Index (PASI 90) at 16 weeks, compared with placebo (73.3% vs. 2.9%). At 16 weeks, 85.1% of those treated with guselkumab achieved an Investigator’s Global Assessment (IGA) score of 0 (cleared) or 1 (minimal disease), compared with 6.9% of those on placebo. Superior responses continued through 48 weeks.
In an active comparator arm of the study comparing guselkumab with the TNF blocker adalimumab (Humira), a significantly higher proportion of those treated with guselkumab achieved PASI 90 scores (76.3% vs. 47.9%) and IGA 0/1 scores (80.5% vs. 55.4%) at week 48. The results were published in March (J Am Acad Dermatol. 2017 Mar;76[3]:405-17).
Results of VOYAGE 2 comparing guselkumab with adalimumab included a PASI 90 rate of 66.1% at week 48 among adalimumab nonresponders who switched to guselkumab (J Am Acad Dermatol. 2017 Mar;76[3]:418-31).
The most common serious adverse effects associated with treatment included upper respiratory infections, headache, injection site reactions, arthralgias, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections, according to the company statement.
Phase 3 studies of guselkumab for active psoriatic arthritis and in comparison with secukinumab (Cosentyx) in patients with moderate to severe plaque psoriasis are underway, according to Janssen, which is marketing guselkumab as Tremfya.
, based on three phase 3 studies of more than 2,000 adults, the manufacturer announced July 13.
The approved indication is for adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, according to a press release issued by Janssen Biotech, which stated that this is the first IL-23 blocker approved for psoriasis.
Results of one of the phase 3 trials, VOYAGE 1, included a significantly greater proportion of patients treated with guselkumab achieving at least a 90% improvement in the Psoriasis Area Severity Index (PASI 90) at 16 weeks, compared with placebo (73.3% vs. 2.9%). At 16 weeks, 85.1% of those treated with guselkumab achieved an Investigator’s Global Assessment (IGA) score of 0 (cleared) or 1 (minimal disease), compared with 6.9% of those on placebo. Superior responses continued through 48 weeks.
In an active comparator arm of the study comparing guselkumab with the TNF blocker adalimumab (Humira), a significantly higher proportion of those treated with guselkumab achieved PASI 90 scores (76.3% vs. 47.9%) and IGA 0/1 scores (80.5% vs. 55.4%) at week 48. The results were published in March (J Am Acad Dermatol. 2017 Mar;76[3]:405-17).
Results of VOYAGE 2 comparing guselkumab with adalimumab included a PASI 90 rate of 66.1% at week 48 among adalimumab nonresponders who switched to guselkumab (J Am Acad Dermatol. 2017 Mar;76[3]:418-31).
The most common serious adverse effects associated with treatment included upper respiratory infections, headache, injection site reactions, arthralgias, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections, according to the company statement.
Phase 3 studies of guselkumab for active psoriatic arthritis and in comparison with secukinumab (Cosentyx) in patients with moderate to severe plaque psoriasis are underway, according to Janssen, which is marketing guselkumab as Tremfya.
, based on three phase 3 studies of more than 2,000 adults, the manufacturer announced July 13.
The approved indication is for adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, according to a press release issued by Janssen Biotech, which stated that this is the first IL-23 blocker approved for psoriasis.
Results of one of the phase 3 trials, VOYAGE 1, included a significantly greater proportion of patients treated with guselkumab achieving at least a 90% improvement in the Psoriasis Area Severity Index (PASI 90) at 16 weeks, compared with placebo (73.3% vs. 2.9%). At 16 weeks, 85.1% of those treated with guselkumab achieved an Investigator’s Global Assessment (IGA) score of 0 (cleared) or 1 (minimal disease), compared with 6.9% of those on placebo. Superior responses continued through 48 weeks.
In an active comparator arm of the study comparing guselkumab with the TNF blocker adalimumab (Humira), a significantly higher proportion of those treated with guselkumab achieved PASI 90 scores (76.3% vs. 47.9%) and IGA 0/1 scores (80.5% vs. 55.4%) at week 48. The results were published in March (J Am Acad Dermatol. 2017 Mar;76[3]:405-17).
Results of VOYAGE 2 comparing guselkumab with adalimumab included a PASI 90 rate of 66.1% at week 48 among adalimumab nonresponders who switched to guselkumab (J Am Acad Dermatol. 2017 Mar;76[3]:418-31).
The most common serious adverse effects associated with treatment included upper respiratory infections, headache, injection site reactions, arthralgias, diarrhea, gastroenteritis, fungal skin infections, and herpes simplex infections, according to the company statement.
Phase 3 studies of guselkumab for active psoriatic arthritis and in comparison with secukinumab (Cosentyx) in patients with moderate to severe plaque psoriasis are underway, according to Janssen, which is marketing guselkumab as Tremfya.
Comorbidities in psoriatic arthritis flag worse prognosis
MADRID – Comorbidities are relatively common in psoriatic arthritis patients, and they are more prevalent in patients with a worse disease course while on initial treatment with a tumor necrosis factor inhibitor, based on data from more than 1,700 Danish patients.
The presence of comorbidities in psoriatic arthritis (PsA) patients on initial tumor necrosis factor inhibitor (TNFi) treatment “was associated with higher disease activity, shorter adherence to the first TNFi, and reduced clinical response,” Lars Erik Kristensen, MD, said at the European Congress of Rheumatology.
To better understand the possible impact of comorbidities on PsA, he and his associates reviewed 1,750 Danish patients with PsA enrolled in a national registry at the time they began treatment with a TNFi. At the time they started treatment, 1,066 (61%) had no comorbidities, 493 (28%) had one comorbidity, and 191 (11%) had two or more comorbidities.
A comparison of the subgroups with no comorbidities and those with two or more showed several important and statistically significant differences in their baseline characteristics. Patients with at least two comorbidities had longer disease duration, and they had more active disease as measured by parameters including the Disease Activity Score 28 and the Health Assessment Questionnaire. Patients with two or more comorbidities also were older and had a higher average body mass index.
Further analyses showed that patients with two or more comorbidities were 72% more like to discontinue their TNFi treatment, compared with patients with no comorbidities – a statistically significant difference, Dr. Kristensen reported.
After 6 months of TNFi treatment, patients with two or more comorbidities had lower rates of achieving the American College of Rheumatology 20%, 50%, or 70% improvement criteria compared with patients with no comorbidities. For example, an ACR20 response occurred in 40% of patients with no comorbidities and in 31% of patients with two or more comorbidities after 6 months in an adjusted analysis.
Dr. Kristensen has been a consultant to or a speaker for several drug companies.
[email protected]
On Twitter @mitchelzoler
MADRID – Comorbidities are relatively common in psoriatic arthritis patients, and they are more prevalent in patients with a worse disease course while on initial treatment with a tumor necrosis factor inhibitor, based on data from more than 1,700 Danish patients.
The presence of comorbidities in psoriatic arthritis (PsA) patients on initial tumor necrosis factor inhibitor (TNFi) treatment “was associated with higher disease activity, shorter adherence to the first TNFi, and reduced clinical response,” Lars Erik Kristensen, MD, said at the European Congress of Rheumatology.
To better understand the possible impact of comorbidities on PsA, he and his associates reviewed 1,750 Danish patients with PsA enrolled in a national registry at the time they began treatment with a TNFi. At the time they started treatment, 1,066 (61%) had no comorbidities, 493 (28%) had one comorbidity, and 191 (11%) had two or more comorbidities.
A comparison of the subgroups with no comorbidities and those with two or more showed several important and statistically significant differences in their baseline characteristics. Patients with at least two comorbidities had longer disease duration, and they had more active disease as measured by parameters including the Disease Activity Score 28 and the Health Assessment Questionnaire. Patients with two or more comorbidities also were older and had a higher average body mass index.
Further analyses showed that patients with two or more comorbidities were 72% more like to discontinue their TNFi treatment, compared with patients with no comorbidities – a statistically significant difference, Dr. Kristensen reported.
After 6 months of TNFi treatment, patients with two or more comorbidities had lower rates of achieving the American College of Rheumatology 20%, 50%, or 70% improvement criteria compared with patients with no comorbidities. For example, an ACR20 response occurred in 40% of patients with no comorbidities and in 31% of patients with two or more comorbidities after 6 months in an adjusted analysis.
Dr. Kristensen has been a consultant to or a speaker for several drug companies.
[email protected]
On Twitter @mitchelzoler
MADRID – Comorbidities are relatively common in psoriatic arthritis patients, and they are more prevalent in patients with a worse disease course while on initial treatment with a tumor necrosis factor inhibitor, based on data from more than 1,700 Danish patients.
The presence of comorbidities in psoriatic arthritis (PsA) patients on initial tumor necrosis factor inhibitor (TNFi) treatment “was associated with higher disease activity, shorter adherence to the first TNFi, and reduced clinical response,” Lars Erik Kristensen, MD, said at the European Congress of Rheumatology.
To better understand the possible impact of comorbidities on PsA, he and his associates reviewed 1,750 Danish patients with PsA enrolled in a national registry at the time they began treatment with a TNFi. At the time they started treatment, 1,066 (61%) had no comorbidities, 493 (28%) had one comorbidity, and 191 (11%) had two or more comorbidities.
A comparison of the subgroups with no comorbidities and those with two or more showed several important and statistically significant differences in their baseline characteristics. Patients with at least two comorbidities had longer disease duration, and they had more active disease as measured by parameters including the Disease Activity Score 28 and the Health Assessment Questionnaire. Patients with two or more comorbidities also were older and had a higher average body mass index.
Further analyses showed that patients with two or more comorbidities were 72% more like to discontinue their TNFi treatment, compared with patients with no comorbidities – a statistically significant difference, Dr. Kristensen reported.
After 6 months of TNFi treatment, patients with two or more comorbidities had lower rates of achieving the American College of Rheumatology 20%, 50%, or 70% improvement criteria compared with patients with no comorbidities. For example, an ACR20 response occurred in 40% of patients with no comorbidities and in 31% of patients with two or more comorbidities after 6 months in an adjusted analysis.
Dr. Kristensen has been a consultant to or a speaker for several drug companies.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point: , compared with patients with no comorbidities.
Major finding: An ACR20 response occurred in 40% of patients with no comorbidities but only 31% of those with two or more comorbidities.
Data source: Review of national registry data for 1,750 Danish psoriatic arthritis patients.
Disclosures: Dr. Kristensen has been a consultant to or a speaker for several drug companies.
FDA approves abatacept for adults with psoriatic arthritis
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.
Phototherapy Coding and Documentation in the Time of Biologics
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
In this era of biologics for psoriasis with ever-increasing effectiveness and safety as well as patients who have less and less time to visit the physician's office, it would seem that the days of in-office UV treatments would be numbered. However, rumors of the demise of phototherapy may be greatly exaggerated. Phototherapy is still one of the safest and most cost-effective treatments for psoriasis and other dermatoses.1 Its use often is a prerequisite for biologic therapy, and it may be the only therapeutic option for certain subsets of patients, such as children, pregnant women, and immunosuppressed patients. Moreover, narrowband UVB technology has breathed new life into phototherapy, with better efficacy and less long-term risk. Although the utilization of psoralen plus UVA (PUVA) light therapy has indeed decreased over the last 2 decades, the use of UVB therapies continues to increase dramatically.2
Phototherapy Codes
There are 4 chief Current Procedural Terminology (CPT) codes for reporting phototherapy services: (1) 96900: actinotherapy (UV light treatment); (2) 96910: photochemotherapy, tar, and UVB (Goeckerman treatment) or petrolatum and UVB; (3) 96912: photochemotherapy and PUVA; and (4) 96913: photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician.3
There is lack of specificity of the CPT code descriptions for phototherapy. Moreover, insurer guidance for documentation for phototherapy is vague to nonexistent, and of course whenever the use of any medical service increases, insurer scrutiny is sure to follow. Therefore, it is not surprising that dermatology practices have reported that private insurers as well as Medicare are auditing medical records for phototherapy treatments.4 In fact, recently we have seen a Midwest private insurer demand payment from dermatologists for hundreds of 96910 phototherapy services, which the insurer asserted should have been coded as 96900 because topical therapies were not applied by the dermatology staff. The insurer did not just evaluate medical records but also contacted patients directly and asked how services had been provided. Clearly, more detailed guidance for dermatologists and insurers on documentation and performance standards for each phototherapy service is needed.
Existing coding guidance for phototherapy indicates that actinotherapy (96900) defines the basic service of treating a patient with a UV light unit.5 Actinotherapy does not involve application of topical medications while the patient is in the office.
In contrast, photochemotherapy (96910) implies addition of a chemo agent to phototherapy. Despite the somewhat nonspecific nature of the code descriptor, it is apparent that application of photoenhancing agents such as tar, petrolatum, or distillates of petrolatum meet the requirements of 96910. The Coder's Desk Reference for Procedures 2017 describes 96910 as "the physician uses photosensitizing chemicals and light rays to treat skin ailments."6 Application of light-enhancing topical products should occur within the office by either staff or the patient. In fact, examination of practice expense data from the Centers for Medicare & Medicaid Services indicated that the 96910 code includes payment for clinical staff time to apply topical products as well as the cost of the topical agent(s).7
The PUVA code 96912 is defined by the use of photosensitizing psoralen medication, which can be administered topically or orally, followed by UVA treatment. In my experience, PUVA has similar performance standards with in-office application of psoralen, if applicable. If application of topical photoenhancing products occurs outside the office, the requirements of photochemotherapy are not met, and 96900 should be reported.
The 96913 code defines prolonged phototherapy service with intensive topical therapy requirements and multiple phototherapy sessions per day.3 This code is rarely reported (average of fewer than 100 times in the Medicare population per year), and most insurers do not reimburse this service.
Protecting Yourself From an Audit
In my experience, review of private insurer audits of phototherapy services has yielded important lessons. First, having a written standard operating procedure in place regarding the performance of phototherapy services and how application of topicals will be handled has been helpful in audit defense. The other key to beating audits for phototherapy services is to have detailed documentation or a flowchart in the medical record regarding the topical agent and the light administration. The medical record should include what topical agent was applied, if any; whether the topical agent was applied in the office; where the topical product was applied; and who applied the topical product. Sometimes topical product application by a physician or staff is not feasible because of patient preference or the site of application. If the patient applied the topical, document that assistance was offered and refused, along with what type of UV light was used and the dosage. Inclusion of these elements in the medical record provides a clear picture of the delivery of the phototherapy service and will aid in responding to medical record audit.
Final Thoughts
Phototherapy is a critical treatment modality that continues to be utilized frequently in the expanding armamentarium of treatments for dermatoses. Phototherapy is performed almost exclusively by dermatologists and allows dermatologists to offer a unique level of care and value in the treatment of skin disease. Careful documentation, a written standard operating procedure, and adherence to proper performance standards will allow dermatologists to be compensated fairly for this important treatment modality and pass audits that are likely to occur.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
- Lapolla W, Yentzer BA, Bagel J, et al. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011;64:936-949.
- Simpson GL, Yelverton CB, Rittenberg S, et al. Do utilization management controls for phototherapy increase the prescription of biologics? J Dermatolog Treat. 2006;17:359-361.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- American Academy of Dermatology Association. Insurers review billing for photochemotherapy (CPT 96910). Derm Coding Consult. Spring 2009;13:4.
- American Academy of Dermatology Association. Coding Q&A's. Derm Coding Consult. Spring 2007;11:5, 7, 8.
- Coders' Desk Reference for Procedures 2017. Chicago, IL: Optum360; 2017.
- Relative Value Scale Update Committee Database. Chicago, IL: American Medical Association; 2016.
Topical Cannabinoids in Dermatology
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
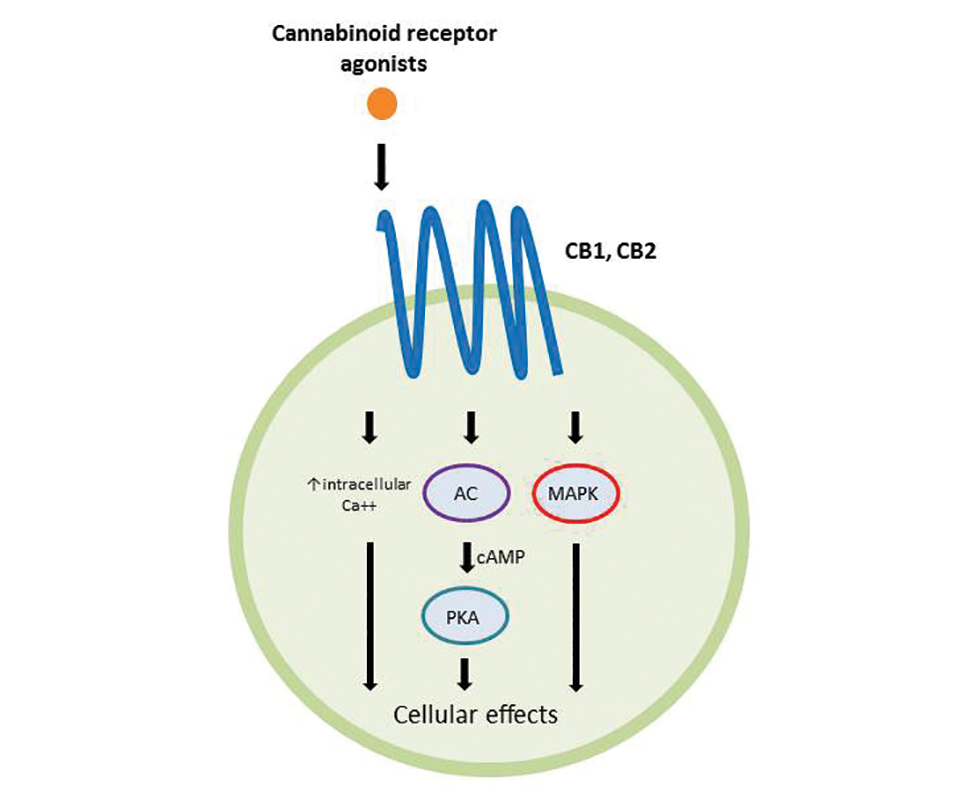
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.
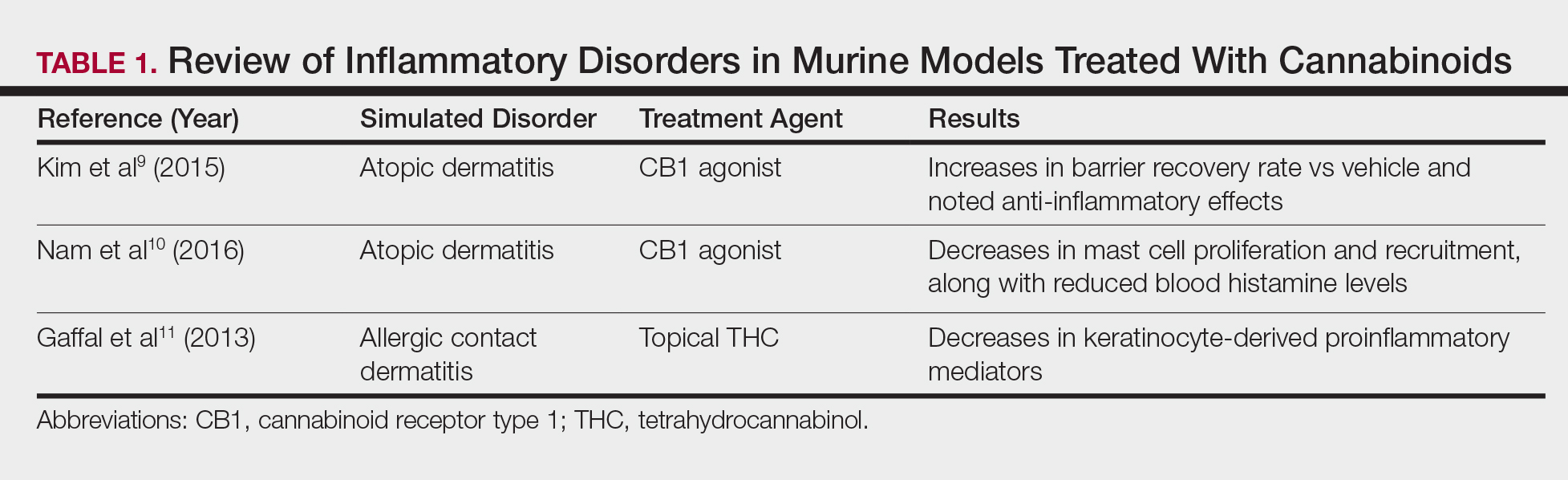
Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
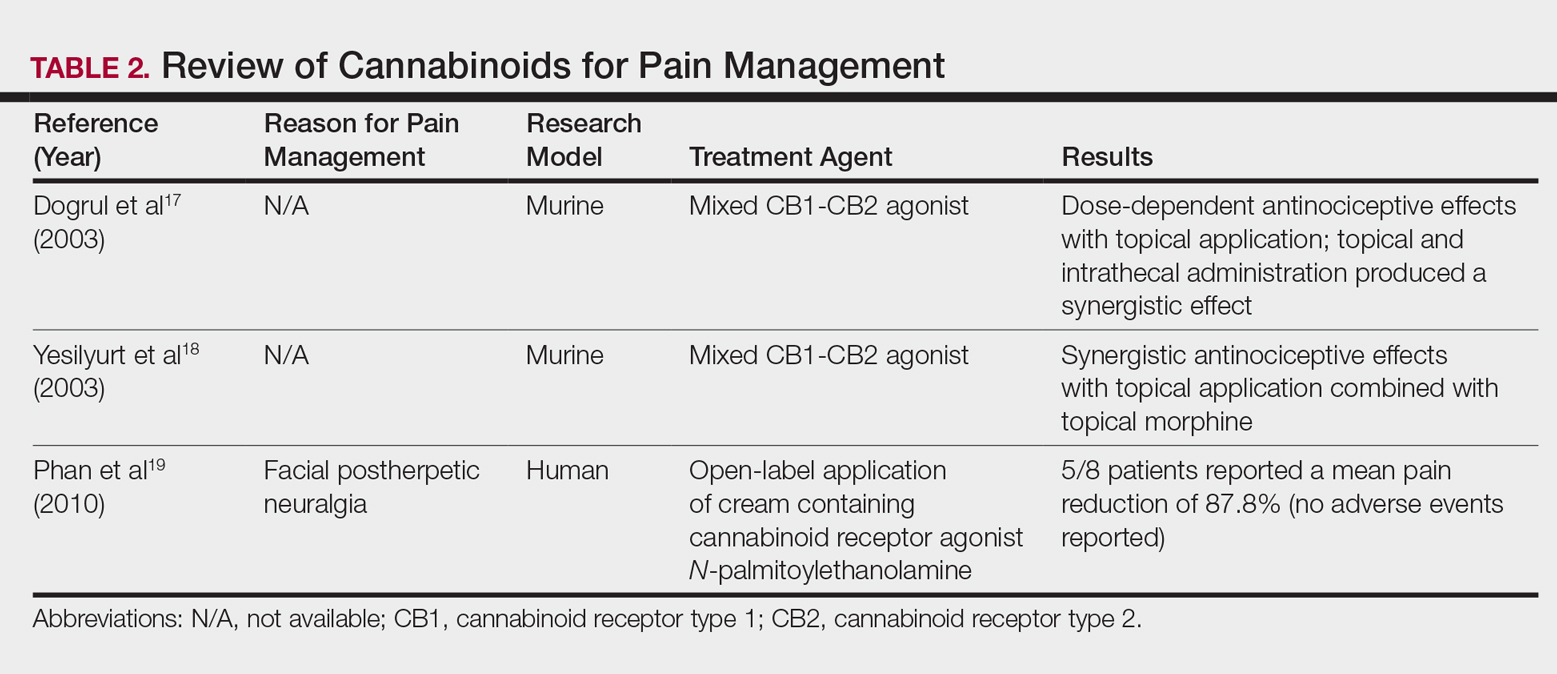
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
Practice Points
- Topical cannabinoids are advertised by companies as treatment options for numerous dermatologic conditions.
- Despite promising data in rodent models, there have been no rigorous studies to date confirming efficacy or safety in humans.
- Dermatologists should therefore inquire with patients about the use of any topical cannabinoid products, especially around the time of planned procedures, as they may affect treatment outcomes.
Early phase III data positive for adalimumab biosimilar, for both psoriasis and PsA
MADRID – To date, an adalimumab biosimilar has proven itself in a large, phase III trial of patients with psoriasis, including a subset with mild to moderate psoriatic arthritis (PsA).
The biosimilar, known as CHS-1420, cleared psoriatic plaques and improved health-related quality of life just as well as adalimumab after 12 weeks of treatment, Barbara Finck, MD, said at the European Congress of Rheumatology. It also suppressed high-sensitivity C-reactive protein (CRP) as well as the originator molecule, she said.
Dr. Finck, the chief medical officer of Coherus Biosciences, the developer of CHS-1420, reported results from the first 16-week phase of the 48-week study. Data are still to come on a 6-week period during which half those taking adalimumab switched to CHS-1420 in a blinded fashion, and 26 weeks of open-label CHS-1420 for all patients.
The study’s primary endpoint was a 75% reduction in the Psoriasis Area and Severity Index (PASI) score (PASI 75) Two additional endpoints were evaluated in patients with PsA: change in the Health Assessment Questionnaire-Disability Index (HAQ-DI) and changes in CRP.
Dr. Finck bemoaned the lack of the clinical rheumatologic endpoint, tender and swollen joint count. “I advocated for this but was unable to convince our dermatology colleagues” to conduct this exam, she said. “I think we have a ways to go to educate our colleagues in this regard.”
The study comprised 545 patients with mild to moderate psoriasis; of these, 127 had PsA. They received subcutaneous injections of either CHS-1420 or adalimumab at identical doses (80 mg at week 1, followed by 40 mg every other week). They were a mean of 44 years o
In the entire study population, treatment with CHS-1420 and adalimumab followed almost identical response curves. By week 4, 22% of the CHS-1420 group and 20% of the adalimumab group had reached a PASI75 response. By week 8, those numbers were 57% and 61%, respectively, and by week 12, they were 69% and 72% – not significantly different.
Response was similar in the subgroup of PsA patients: By week 12, 82% of the CHS-1420 group and 77% of the adalimumab group had reached a PASI 75. PsA patients also responded equally well to both medications on the HAQ-DI by week 12. At baseline, the mean HAQ-DI was about 1 in each group. At 12 weeks, it was reduced by about half a point in both groups. High-sensitivity CRP decreased similarly in the CHS-1420 and adalimumab groups as well (reductions of 8.9 mg/L and 6.3 mg/L, respectively).
Adalimumab, a tumor necrosis factor blocker, is a highly immunogenic molecule, and as such, many patients developed antibodies to both it and to CHS-1420. By week 12, 84% of both treatment groups had developed anti-drug antibodies and 32%, neutralizing antibodies. Among those with PsA, 82% taking CHS-1420 and 88% of those taking adalimumab developed antidrug antibodies. Neutralizing antibodies developed in 33% and 30%, respectively. Neither of these differences was statistically significant.
Other adverse events were similar, Dr. Finck noted. These included nasopharyngitis (9% of both groups), upper respiratory tract infection (6%), injection site reaction (4%), headache (3%), and worsening of psoriasis (1% for CHS-1420, and 3% for adalimumab).
If the switching study data are similarly positive, Coherus expects to file a Biologics License Application with the Food and Drug Administration in early 2018, Dr. Finck said.
[email protected]
On Twitter @Alz_gal
MADRID – To date, an adalimumab biosimilar has proven itself in a large, phase III trial of patients with psoriasis, including a subset with mild to moderate psoriatic arthritis (PsA).
The biosimilar, known as CHS-1420, cleared psoriatic plaques and improved health-related quality of life just as well as adalimumab after 12 weeks of treatment, Barbara Finck, MD, said at the European Congress of Rheumatology. It also suppressed high-sensitivity C-reactive protein (CRP) as well as the originator molecule, she said.
Dr. Finck, the chief medical officer of Coherus Biosciences, the developer of CHS-1420, reported results from the first 16-week phase of the 48-week study. Data are still to come on a 6-week period during which half those taking adalimumab switched to CHS-1420 in a blinded fashion, and 26 weeks of open-label CHS-1420 for all patients.
The study’s primary endpoint was a 75% reduction in the Psoriasis Area and Severity Index (PASI) score (PASI 75) Two additional endpoints were evaluated in patients with PsA: change in the Health Assessment Questionnaire-Disability Index (HAQ-DI) and changes in CRP.
Dr. Finck bemoaned the lack of the clinical rheumatologic endpoint, tender and swollen joint count. “I advocated for this but was unable to convince our dermatology colleagues” to conduct this exam, she said. “I think we have a ways to go to educate our colleagues in this regard.”
The study comprised 545 patients with mild to moderate psoriasis; of these, 127 had PsA. They received subcutaneous injections of either CHS-1420 or adalimumab at identical doses (80 mg at week 1, followed by 40 mg every other week). They were a mean of 44 years o
In the entire study population, treatment with CHS-1420 and adalimumab followed almost identical response curves. By week 4, 22% of the CHS-1420 group and 20% of the adalimumab group had reached a PASI75 response. By week 8, those numbers were 57% and 61%, respectively, and by week 12, they were 69% and 72% – not significantly different.
Response was similar in the subgroup of PsA patients: By week 12, 82% of the CHS-1420 group and 77% of the adalimumab group had reached a PASI 75. PsA patients also responded equally well to both medications on the HAQ-DI by week 12. At baseline, the mean HAQ-DI was about 1 in each group. At 12 weeks, it was reduced by about half a point in both groups. High-sensitivity CRP decreased similarly in the CHS-1420 and adalimumab groups as well (reductions of 8.9 mg/L and 6.3 mg/L, respectively).
Adalimumab, a tumor necrosis factor blocker, is a highly immunogenic molecule, and as such, many patients developed antibodies to both it and to CHS-1420. By week 12, 84% of both treatment groups had developed anti-drug antibodies and 32%, neutralizing antibodies. Among those with PsA, 82% taking CHS-1420 and 88% of those taking adalimumab developed antidrug antibodies. Neutralizing antibodies developed in 33% and 30%, respectively. Neither of these differences was statistically significant.
Other adverse events were similar, Dr. Finck noted. These included nasopharyngitis (9% of both groups), upper respiratory tract infection (6%), injection site reaction (4%), headache (3%), and worsening of psoriasis (1% for CHS-1420, and 3% for adalimumab).
If the switching study data are similarly positive, Coherus expects to file a Biologics License Application with the Food and Drug Administration in early 2018, Dr. Finck said.
[email protected]
On Twitter @Alz_gal
MADRID – To date, an adalimumab biosimilar has proven itself in a large, phase III trial of patients with psoriasis, including a subset with mild to moderate psoriatic arthritis (PsA).
The biosimilar, known as CHS-1420, cleared psoriatic plaques and improved health-related quality of life just as well as adalimumab after 12 weeks of treatment, Barbara Finck, MD, said at the European Congress of Rheumatology. It also suppressed high-sensitivity C-reactive protein (CRP) as well as the originator molecule, she said.
Dr. Finck, the chief medical officer of Coherus Biosciences, the developer of CHS-1420, reported results from the first 16-week phase of the 48-week study. Data are still to come on a 6-week period during which half those taking adalimumab switched to CHS-1420 in a blinded fashion, and 26 weeks of open-label CHS-1420 for all patients.
The study’s primary endpoint was a 75% reduction in the Psoriasis Area and Severity Index (PASI) score (PASI 75) Two additional endpoints were evaluated in patients with PsA: change in the Health Assessment Questionnaire-Disability Index (HAQ-DI) and changes in CRP.
Dr. Finck bemoaned the lack of the clinical rheumatologic endpoint, tender and swollen joint count. “I advocated for this but was unable to convince our dermatology colleagues” to conduct this exam, she said. “I think we have a ways to go to educate our colleagues in this regard.”
The study comprised 545 patients with mild to moderate psoriasis; of these, 127 had PsA. They received subcutaneous injections of either CHS-1420 or adalimumab at identical doses (80 mg at week 1, followed by 40 mg every other week). They were a mean of 44 years o
In the entire study population, treatment with CHS-1420 and adalimumab followed almost identical response curves. By week 4, 22% of the CHS-1420 group and 20% of the adalimumab group had reached a PASI75 response. By week 8, those numbers were 57% and 61%, respectively, and by week 12, they were 69% and 72% – not significantly different.
Response was similar in the subgroup of PsA patients: By week 12, 82% of the CHS-1420 group and 77% of the adalimumab group had reached a PASI 75. PsA patients also responded equally well to both medications on the HAQ-DI by week 12. At baseline, the mean HAQ-DI was about 1 in each group. At 12 weeks, it was reduced by about half a point in both groups. High-sensitivity CRP decreased similarly in the CHS-1420 and adalimumab groups as well (reductions of 8.9 mg/L and 6.3 mg/L, respectively).
Adalimumab, a tumor necrosis factor blocker, is a highly immunogenic molecule, and as such, many patients developed antibodies to both it and to CHS-1420. By week 12, 84% of both treatment groups had developed anti-drug antibodies and 32%, neutralizing antibodies. Among those with PsA, 82% taking CHS-1420 and 88% of those taking adalimumab developed antidrug antibodies. Neutralizing antibodies developed in 33% and 30%, respectively. Neither of these differences was statistically significant.
Other adverse events were similar, Dr. Finck noted. These included nasopharyngitis (9% of both groups), upper respiratory tract infection (6%), injection site reaction (4%), headache (3%), and worsening of psoriasis (1% for CHS-1420, and 3% for adalimumab).
If the switching study data are similarly positive, Coherus expects to file a Biologics License Application with the Food and Drug Administration in early 2018, Dr. Finck said.
[email protected]
On Twitter @Alz_gal
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: By week 12, 69% of those who received CHS-1420 and 72% of those who received adalimumab had reached a PASI75, response rates that were not significantly different.
Data source: The phase III trial randomized 545 patients with psoriasis, including 127 with PsA, to treatment with adalimumab or the biosimilar.
Disclosures: Dr. Finck is chief medical officer of Coherus Biosciences, which is developing CHS-1420.
TWEAKing inflammation: Studies reflect potential treatment target for psoriasis, atopic dermatitis
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
An immunomodulatory pathway that has been linked to cancer, kidney disease, and other disease processes is becoming a focus of dermatologic research.
New evidence suggests that TNF-like weak inducer of apoptosis (TWEAK), a member of the tumor necrosis family (TNF) superfamily, may be involved in both atopic dermatitis (AD) and psoriasis (Nat Commun. 2017 May 22;8:15395. doi: 10.1038/ncomms15395). The research showed that mice engineered to have low TWEAK levels had less severe disease when both AD and psoriasis were induced.
The TWEAK receptor, Fn14, was upregulated in keratinocytes and dermal fibroblasts in mouse disease models of AD and psoriasis, and TWEAK induced production of a range of cytokines associated with both AD and psoriasis. Subcutaneous injection of recombinant TWEAK led to cutaneous inflammation, as well as histological and molecular signals of the two diseases.
The pathophysiology of both AD and psoriasis is nebulously complex, sharing a similar theme of immune dysregulation, but historically polar opposites based on the different branches of the immune response implicated.
The study is not the only recent work tying TWEAK/Fn14 to dermatologic diseases. Other recent papers have shown evidence of their involvement in chronic cutaneous lupus (J Invest Dermatol. 2015;135[8]:1986-95), UVB irradiation-induced cutaneous lupus (Exp Dermatol. 2016 Dec;25[12]:969-76), and bullous pemphigoid (J Invest Dermatol. 2017 Jul;137[7]:1512-22).
The spate of findings hint that TWEAK/Fn14 could be a novel therapeutic pathway to attack inflammatory disease. Many therapies for autoimmune disease focus on immunosuppressive agents, which are associated with an increased risk of infection. But mice engineered to lack either TWEAK or Fn14 appear normal, and a phase I trial of an anti-TWEAK antibody in patients with rheumatoid arthritis did not reveal any worrisome safety concerns. “It doesn’t seem to have the broad immunosuppressive effects which characterize the therapies we currently use,” said Chaim Putterman, MD, chief of the division of rheumatology and professor of medicine and microbiology & immunology at the Albert Einstein College of Medicine, New York.
Instead, TWEAK seems to be regulating inflammation in target organs. It almost certainly plays a role in healthy functions like wound healing and cell survival, but Dr. Putterman believes there are redundant mechanisms that can pick up the slack, as the healthy knockout mice attest. The evidence suggests that the TWEAK pathway may become overactive in some diseases and, if so, a therapeutic antibody might be able to reset it to a more normal balance. “The utopian vision is that you would block this cytokine and bring its downstream effects back to normal levels, rather than totally abrogating its homeostatic functions,” Dr. Putterman noted.
Because blocking TWEAK has no apparent immunosuppressive effects, it might be a candidate for combination therapy with existing cytotoxic drugs. “If you have a disease like psoriasis where some standard of care medications are immunosuppressive, such as methotrexate, you might not get more risk by adding an antibody targeting TWEAK, as opposed to using immunosuppressives in combination. That, I think, has potential,” he said.
Work remains, however. A proof-of-concept study in lupus nephritis, sponsored by Biogen, failed to show a benefit when an anti-TWEAK antibody was combined with the standard of care.
But the potential impact of this approach holds much promise, and the fact that TWEAK has been linked to multiple diseases should make it a more attractive drug target for drug companies. “Now we have a target, that if you knock it out, or its receptor, you can potentially affect both diseases. This may the start of a whole new direction for biologics to treat inflammatory disease, and cancer as well,” Dr. Friedman said.
Dr. Putterman and Dr. Friedman were among the authors of the 2015 JID study on TWEAK/Fn14 signaling in spontaneous lupus and the Experimental Dermatology study. Dr. Putterman has research funding from Biogen Idec. Dr. Friedman had no related disclosures. The authors of the Nature Communications study were from the La Jolla Institute for Allergy and Immunology, and Biogen.
Pain often persists despite biologic treatment in PsA
MADRID – Pain is common in patients with psoriatic arthritis (PsA) and can be disruptive to their lives and jobs, even among those whose inflammatory symptoms have been treated with biologic drugs for 3 months or longer, according to findings from a multinational survey.
At the European Congress of Rheumatology, Dr. Philip G. Conaghan of the University of Leeds (England) presented findings from the survey of 782 consecutive PsA patients from 13 countries in Europe, the Middle East, Asia, and the Americas, as well as Australia. All patients included in the analysis were on biologic agents – mainly tumor necrosis factor inhibitors – for at least 90 days.
In an interview. Dr. Conaghan said that it’s important for clinicians not to assume that pain in a PsA patient on a biologic means that the drug is not working.
“The main limitation of our study is that we haven’t worked out how well-controlled patients’ psoriatic arthritis is, so, although we know they’re on a biologic for more than 3 months, we don’t know if they were responding well to it.” But, even in the absence of systemic inflammation, he said, there are other potential causes for pain that should not be overlooked.
“There’s no reason why PsA patients wouldn’t have pain due to tendinitis, enthesitis, and osteoarthritis – the same mechanical-type joint pain that we see in the whole community of people over 40,” Dr. Conaghan said. “I am concerned that, once we give someone a label of inflammatory arthritis, we stop looking at all the other things that can happen to their musculoskeletal system.”
Moreover, he said, “people who’ve had arthritis severe enough to need a biologic treatment will have muscle deconditioning and weakness. It’s very common that PsA patients have trouble opening jars and getting out of chairs.”
Such weakness “can lead to mechanical joint pain, which fortunately can be improved – along with the pain – through muscle strengthening and rehabilitation.”
For their study, Dr. Conaghan and his colleagues collected information from clinicians on treatment and from patients. The questionnaires incorporated several measures of disability, pain, functional impairment, and health-related quality of life that have been validated for use in PsA patients.
Severe pain was significantly associated with increased use of prescription nonsteroidal anti-inflammatory drugs and opioids, as well as nonprescription pain medication. Patients 65 years and older had a significantly greater likelihood of being unemployed or retired because of PsA if they reported severe pain, compared with those reporting mild or moderate pain.
A number of quality of life and work-related measures were also associated with pain severity. Dr. Conaghan and his colleagues found that the risk of disability increased with bodily pain, and more severe pain was associated with greater activity impairment, worse social functioning, more work impairment, and work time missed, among other measures (P less than .0001 for all).
“What we saw is that, the more pain you have, the more your world shrinks in,” Dr. Conaghan said.
Dr. Conaghan reported financial relationships with AbbVie, Eli Lilly, Novartis, Pfizer, Bristol-Myers Squibb, and Roche. Some of his study coauthors have similar disclosures. Four coauthors are employees of Novartis.
MADRID – Pain is common in patients with psoriatic arthritis (PsA) and can be disruptive to their lives and jobs, even among those whose inflammatory symptoms have been treated with biologic drugs for 3 months or longer, according to findings from a multinational survey.
At the European Congress of Rheumatology, Dr. Philip G. Conaghan of the University of Leeds (England) presented findings from the survey of 782 consecutive PsA patients from 13 countries in Europe, the Middle East, Asia, and the Americas, as well as Australia. All patients included in the analysis were on biologic agents – mainly tumor necrosis factor inhibitors – for at least 90 days.
In an interview. Dr. Conaghan said that it’s important for clinicians not to assume that pain in a PsA patient on a biologic means that the drug is not working.
“The main limitation of our study is that we haven’t worked out how well-controlled patients’ psoriatic arthritis is, so, although we know they’re on a biologic for more than 3 months, we don’t know if they were responding well to it.” But, even in the absence of systemic inflammation, he said, there are other potential causes for pain that should not be overlooked.
“There’s no reason why PsA patients wouldn’t have pain due to tendinitis, enthesitis, and osteoarthritis – the same mechanical-type joint pain that we see in the whole community of people over 40,” Dr. Conaghan said. “I am concerned that, once we give someone a label of inflammatory arthritis, we stop looking at all the other things that can happen to their musculoskeletal system.”
Moreover, he said, “people who’ve had arthritis severe enough to need a biologic treatment will have muscle deconditioning and weakness. It’s very common that PsA patients have trouble opening jars and getting out of chairs.”
Such weakness “can lead to mechanical joint pain, which fortunately can be improved – along with the pain – through muscle strengthening and rehabilitation.”
For their study, Dr. Conaghan and his colleagues collected information from clinicians on treatment and from patients. The questionnaires incorporated several measures of disability, pain, functional impairment, and health-related quality of life that have been validated for use in PsA patients.
Severe pain was significantly associated with increased use of prescription nonsteroidal anti-inflammatory drugs and opioids, as well as nonprescription pain medication. Patients 65 years and older had a significantly greater likelihood of being unemployed or retired because of PsA if they reported severe pain, compared with those reporting mild or moderate pain.
A number of quality of life and work-related measures were also associated with pain severity. Dr. Conaghan and his colleagues found that the risk of disability increased with bodily pain, and more severe pain was associated with greater activity impairment, worse social functioning, more work impairment, and work time missed, among other measures (P less than .0001 for all).
“What we saw is that, the more pain you have, the more your world shrinks in,” Dr. Conaghan said.
Dr. Conaghan reported financial relationships with AbbVie, Eli Lilly, Novartis, Pfizer, Bristol-Myers Squibb, and Roche. Some of his study coauthors have similar disclosures. Four coauthors are employees of Novartis.
MADRID – Pain is common in patients with psoriatic arthritis (PsA) and can be disruptive to their lives and jobs, even among those whose inflammatory symptoms have been treated with biologic drugs for 3 months or longer, according to findings from a multinational survey.
At the European Congress of Rheumatology, Dr. Philip G. Conaghan of the University of Leeds (England) presented findings from the survey of 782 consecutive PsA patients from 13 countries in Europe, the Middle East, Asia, and the Americas, as well as Australia. All patients included in the analysis were on biologic agents – mainly tumor necrosis factor inhibitors – for at least 90 days.
In an interview. Dr. Conaghan said that it’s important for clinicians not to assume that pain in a PsA patient on a biologic means that the drug is not working.
“The main limitation of our study is that we haven’t worked out how well-controlled patients’ psoriatic arthritis is, so, although we know they’re on a biologic for more than 3 months, we don’t know if they were responding well to it.” But, even in the absence of systemic inflammation, he said, there are other potential causes for pain that should not be overlooked.
“There’s no reason why PsA patients wouldn’t have pain due to tendinitis, enthesitis, and osteoarthritis – the same mechanical-type joint pain that we see in the whole community of people over 40,” Dr. Conaghan said. “I am concerned that, once we give someone a label of inflammatory arthritis, we stop looking at all the other things that can happen to their musculoskeletal system.”
Moreover, he said, “people who’ve had arthritis severe enough to need a biologic treatment will have muscle deconditioning and weakness. It’s very common that PsA patients have trouble opening jars and getting out of chairs.”
Such weakness “can lead to mechanical joint pain, which fortunately can be improved – along with the pain – through muscle strengthening and rehabilitation.”
For their study, Dr. Conaghan and his colleagues collected information from clinicians on treatment and from patients. The questionnaires incorporated several measures of disability, pain, functional impairment, and health-related quality of life that have been validated for use in PsA patients.
Severe pain was significantly associated with increased use of prescription nonsteroidal anti-inflammatory drugs and opioids, as well as nonprescription pain medication. Patients 65 years and older had a significantly greater likelihood of being unemployed or retired because of PsA if they reported severe pain, compared with those reporting mild or moderate pain.
A number of quality of life and work-related measures were also associated with pain severity. Dr. Conaghan and his colleagues found that the risk of disability increased with bodily pain, and more severe pain was associated with greater activity impairment, worse social functioning, more work impairment, and work time missed, among other measures (P less than .0001 for all).
“What we saw is that, the more pain you have, the more your world shrinks in,” Dr. Conaghan said.
Dr. Conaghan reported financial relationships with AbbVie, Eli Lilly, Novartis, Pfizer, Bristol-Myers Squibb, and Roche. Some of his study coauthors have similar disclosures. Four coauthors are employees of Novartis.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Overall, 37% of PsA patients reported severe pain despite treatment with biologic agents.
Data source: A multinational survey of 782 consecutive PsA patients on biologic agents.
Disclosures: Dr. Conaghan reported financial relationships with AbbVie, Eli Lilly, Novartis, Pfizer, Bristol-Myers Squibb, and Roche. Some of his study coauthors have similar disclosures. Four coauthors are employees of Novartis.
Phototherapy and Nondrug Therapies for Psoriasis Considered Beneficial by Patients
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Oral or injected medications for psoriasis can be burdensome for patients, making them inclined to use alternative therapies such as phototherapy and other nondrug therapies, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than half of participants indicated that they have used phototherapy. Both positive and negative experiences were reported. One participant reported that a home UVB 3-panel light box "dramatically changed [his/her] life." Other participants indicated phototherapy was less successful for them. Participants also indicated fears about skin cancer.
RELATED ARTICLE: Does UVB phototherapy cause skin cancer?
However, several participants reported that phototherapy was more effective when used in combination with other medical therapies. Similarly, most participants indicated using 1 or more nondrug therapies to manage their psoriatic symptoms. Approximately one-third used over-the-counter products, such as coal tar, salicylic acid, and Epsom salt. Slightly more than one-fourth indicated the importance of complementary or alternative therapy, including exercise and meditation, to manage their psoriasis symptoms. Diet modifications, such as eliminating alcohol, sugar, processed foods, drugs, gluten, and tobacco, also were reported as successful.
RELATED ARTICLE: Yoga for dermatologic conditions
RELATED VIDEO: Answering patient questions about diet
Psoriasis patients emphasized that an effective multimodal approach including drug, phototherapy, and nondrug therapies usually is done through trial and error based on each patient's individual needs. Dermatologists would benefit from knowing that nearly all participants in this public meeting indicated they value the benefits of nondrug therapies, and combination therapies using drug and nondrug therapies should be discussed with patients.
The psoriasis public meeting in March 2016 was the FDA's 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.