User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Topical Imiquimod Clears Invasive Melanoma
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
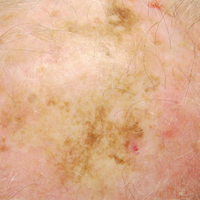
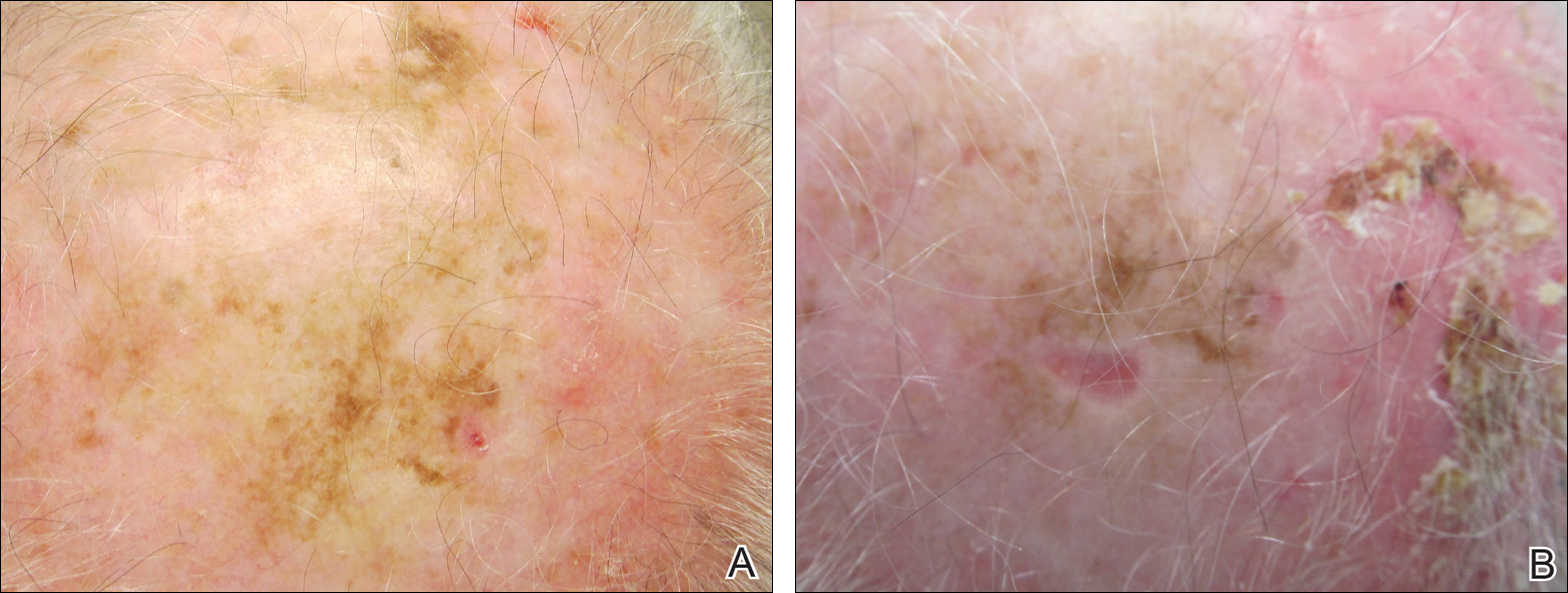
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.
Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
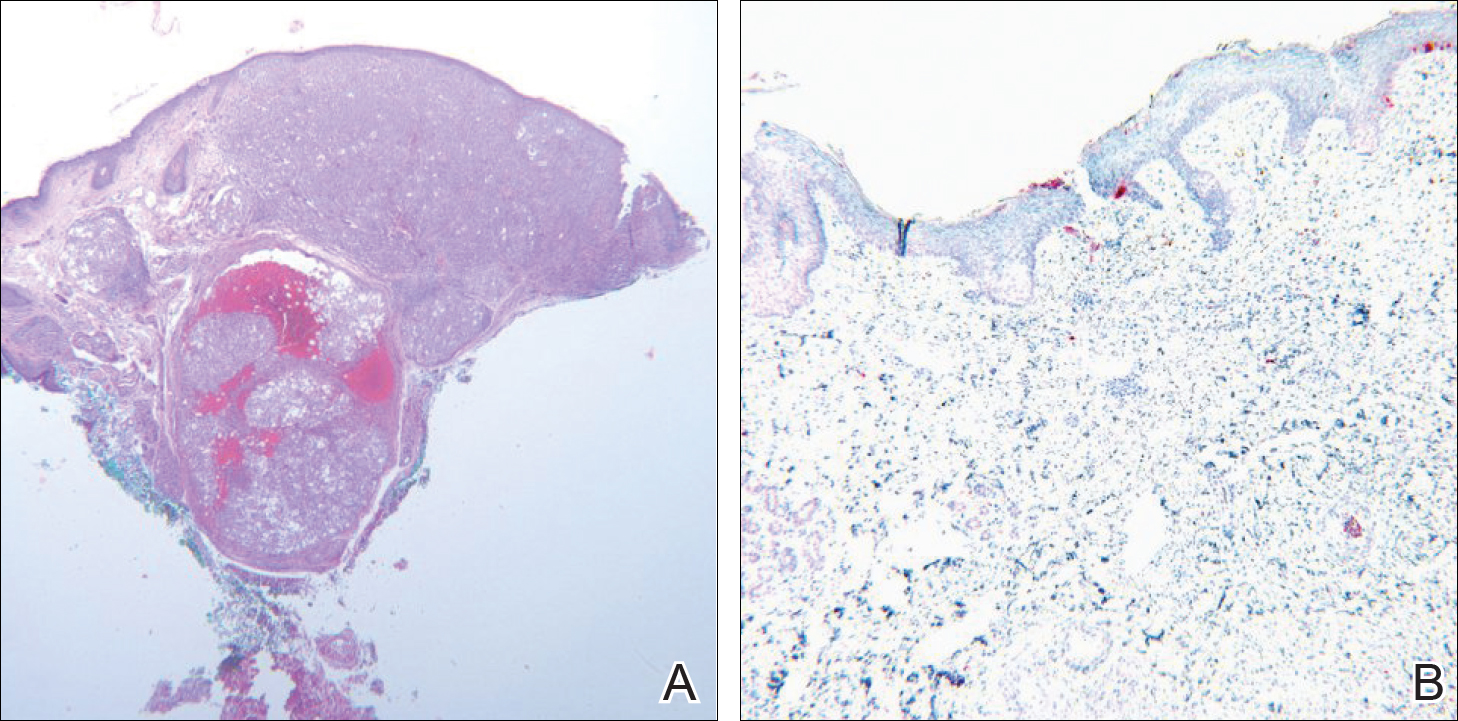
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7
Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.

Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7

Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.

Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7

Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
Practice Points
- Topical imiquimod may clear invasive melanoma as well as melanoma in situ.
- Further study is required to confirm the role of topical imiquimod in melanoma treatment.
Primary Cutaneous Mycobacterium avium Complex Infection Following Squamous Cell Carcinoma Excision
Case Report
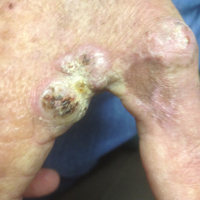
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
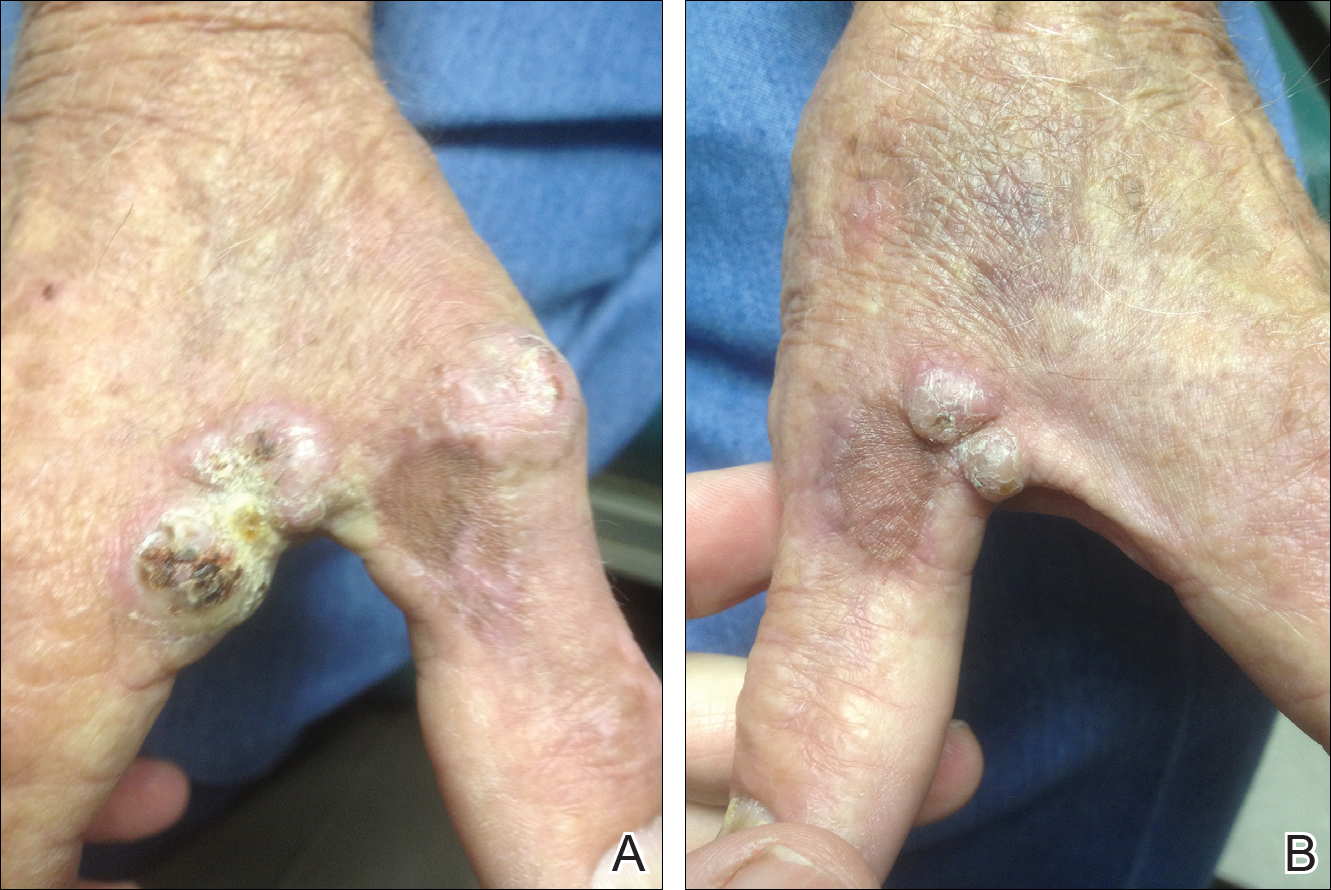
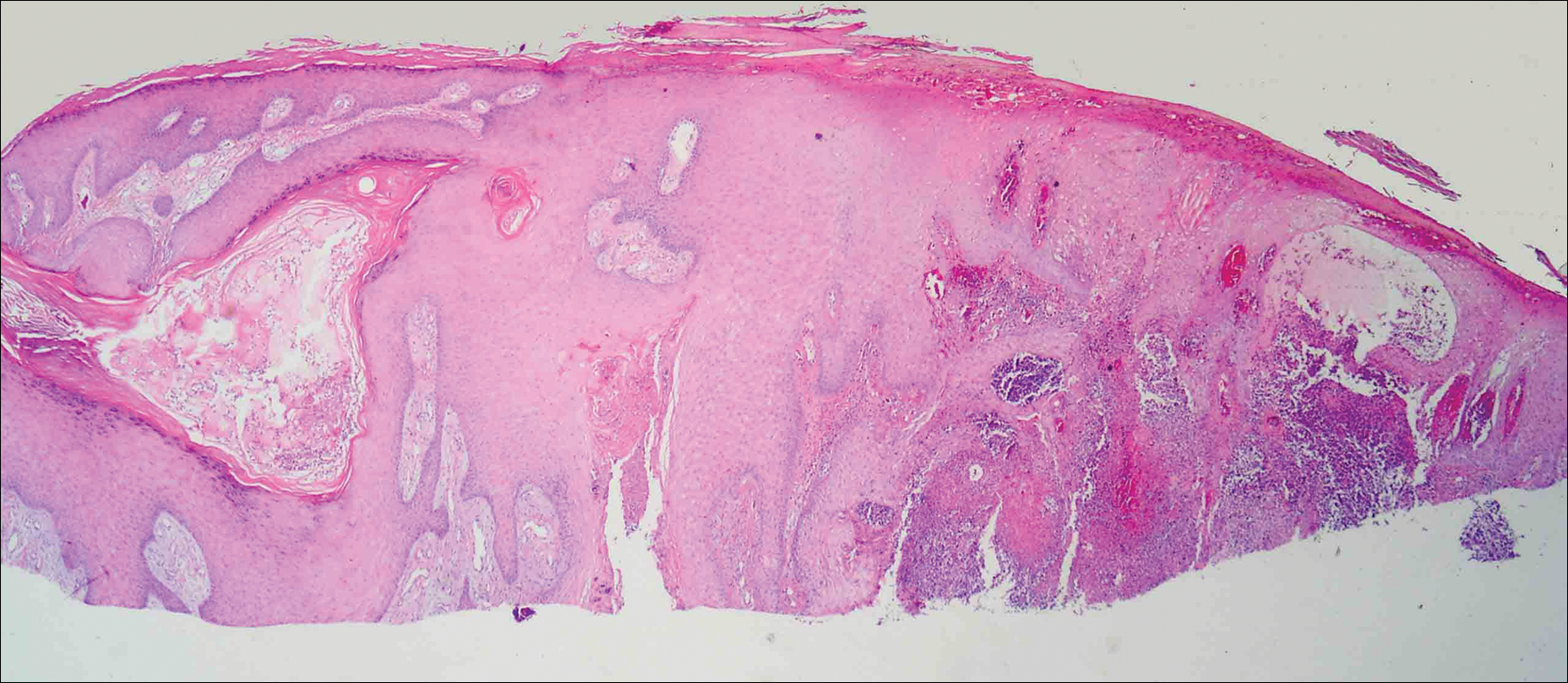
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.
Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.


Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.


Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
Practice Points
- Mycobacterium avium complex (MAC) is a ubiquitous bacterium that commonly infects the lungs and less commonly infects the skin.
- Clinically, cutaneous MAC infection is polymorphous and may present as a nodule, plaque, or ulcer.
- Standard treatment of primary cutaneous MAC includes systemic antibiotics with or without surgical excision.
Purple Curvilinear Papules on the Back
The Diagnosis: Blaschkoid Graft-vs-host Disease
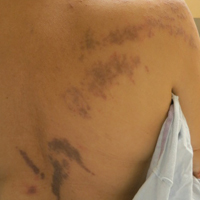
The patient had a history of myelodysplastic syndrome and underwent a bone marrow transplant 1 year prior to presentation. She had acute graft-vs-host disease (GVHD) 6 weeks following the transplant, which resolved with high-dose prednisone followed by UVB phototherapy. Skin biopsy demonstrated lichenoid dermatitis with vacuolar degeneration, dyskeratosis, and prominent pigment incontinence (Figure). Based on these findings and her clinical presentation, a diagnosis of blaschkoid GVHD was made.

Although acute GVHD is the result of immunocompetent donor T cells recognizing host tissues as foreign and initiating an immune response, the pathophysiology of chronic GVHD is not well understood.1,2 Theories for disease pathogenesis in chronic GVHD suggest an underlying autoimmune and/or alloreactive process.2-5 The skin often is the first organ affected in acute GVHD, and patients generally present with a pruritic morbilliform eruption that begins on the trunk and spreads to the rest of the body.1,2 Cutaneous manifestations of chronic GVHD may be protean. Lesions can resemble systemic sclerosis or morphea, lichen planus, psoriasis, ichthyosis, and many other conditions.2
The differential diagnosis of linear dermatoses includes herpes zoster, contact dermatitis, lichen striatus (blaschkitis), nevus unius lateris, inflammatory linear verrucous epidermal nevus, and incontinentia pigmenti.6,7 Lichen planus-like chronic GVHD occurring in a linear distribution has been described.6-14 Distinction between dermatomal and blaschkoid processes is diagnostically important. In the case of GVHD, dermatomal distribution may suggest an association between GVHD and prior herpes simplex virus or varicella-zoster virus infection.6,8 Herpesvirus may alter surface antigens of keratinocytes, rendering them targets of donor lymphocytes, and antibodies to viral particles may cross-react with host keratinocyte HLA antigens. It also is possible that dermatomal GVHD may simply be a type of isomorphic response (Köbner phenomenon).8
When cutaneous GVHD follows Blaschko lines, other mechanisms appear to be at play.9-14 It is plausible that these patients have an underlying genetic mosaicism, perhaps the result of a postzygotic mutation, that results in a daughter cell population that expresses surface antigens different from those of the primary cell population found elsewhere in the skin. Donor lymphocytes may selectively react to this mosaic population, leading to the clinical picture of chronic GVHD oriented along Blaschko lines.10,11,13,14
In conclusion, lichenoid linear GVHD following Blaschko lines is an uncommon presentation of chronic GVHD that highlights the heterogeneity of this disease and should be considered in the appropriate clinical setting.
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550-1561.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-515.e18; quiz 533-534.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
- Shimada M, Onizuka M, Machida S, et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol. 2007;139:458-463.
- Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993-3001.
- Freemer CS, Farmer ER, Corio RL, et al. Lichenoid chronic graft-vs-host disease occurring in a dermatomal distribution. Arch Dermatol. 1994;130:70-72.
- Kikuchi A, Okamoto S, Takahashi S, et al. Linear chronic cutaneous graft-versus-host disease. J Am Acad Dermatol. 1997;37:1004-1006.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Kennedy FE, Hilari H, Ferrer B, et al. Lichenoid chronic graft-vs-host disease following Blaschko lines. ActasDermosifiliogr. 2014;105:89-92.
- Lee SW, Kim YC, Lee E, et al. Linear lichenoid graft versus host disease: an unusual configuration following Blaschko's lines. J Dermatol. 2006;33:583-584.
- Beers B, Kalish RS, Kaye VN, et al. Unilateral linear lichenoid eruption after bone marrow transplantation: an unmasking of tolerance to an abnormal keratinocyte clone? J Am Acad Dermatol. 1993;28(5, pt 2):888-892.
- Wilson B, Lockman D. Linear lichenoid graft-vs-host disease. Arch Dermatol. 1994;130(9):1206-1208.
- Reisfeld PL. Lichenoid chronic graft-vs-host disease. Arch Dermatol. 1994;130:1207-1208.
- Vassallo C, Derlino F, Ripamonti F, et al. Lichenoid cutaneous chronic GvHD following Blaschko lines. Int J Dermatol. 2014;53:473-475.
The Diagnosis: Blaschkoid Graft-vs-host Disease
The patient had a history of myelodysplastic syndrome and underwent a bone marrow transplant 1 year prior to presentation. She had acute graft-vs-host disease (GVHD) 6 weeks following the transplant, which resolved with high-dose prednisone followed by UVB phototherapy. Skin biopsy demonstrated lichenoid dermatitis with vacuolar degeneration, dyskeratosis, and prominent pigment incontinence (Figure). Based on these findings and her clinical presentation, a diagnosis of blaschkoid GVHD was made.

Although acute GVHD is the result of immunocompetent donor T cells recognizing host tissues as foreign and initiating an immune response, the pathophysiology of chronic GVHD is not well understood.1,2 Theories for disease pathogenesis in chronic GVHD suggest an underlying autoimmune and/or alloreactive process.2-5 The skin often is the first organ affected in acute GVHD, and patients generally present with a pruritic morbilliform eruption that begins on the trunk and spreads to the rest of the body.1,2 Cutaneous manifestations of chronic GVHD may be protean. Lesions can resemble systemic sclerosis or morphea, lichen planus, psoriasis, ichthyosis, and many other conditions.2
The differential diagnosis of linear dermatoses includes herpes zoster, contact dermatitis, lichen striatus (blaschkitis), nevus unius lateris, inflammatory linear verrucous epidermal nevus, and incontinentia pigmenti.6,7 Lichen planus-like chronic GVHD occurring in a linear distribution has been described.6-14 Distinction between dermatomal and blaschkoid processes is diagnostically important. In the case of GVHD, dermatomal distribution may suggest an association between GVHD and prior herpes simplex virus or varicella-zoster virus infection.6,8 Herpesvirus may alter surface antigens of keratinocytes, rendering them targets of donor lymphocytes, and antibodies to viral particles may cross-react with host keratinocyte HLA antigens. It also is possible that dermatomal GVHD may simply be a type of isomorphic response (Köbner phenomenon).8
When cutaneous GVHD follows Blaschko lines, other mechanisms appear to be at play.9-14 It is plausible that these patients have an underlying genetic mosaicism, perhaps the result of a postzygotic mutation, that results in a daughter cell population that expresses surface antigens different from those of the primary cell population found elsewhere in the skin. Donor lymphocytes may selectively react to this mosaic population, leading to the clinical picture of chronic GVHD oriented along Blaschko lines.10,11,13,14
In conclusion, lichenoid linear GVHD following Blaschko lines is an uncommon presentation of chronic GVHD that highlights the heterogeneity of this disease and should be considered in the appropriate clinical setting.
The Diagnosis: Blaschkoid Graft-vs-host Disease
The patient had a history of myelodysplastic syndrome and underwent a bone marrow transplant 1 year prior to presentation. She had acute graft-vs-host disease (GVHD) 6 weeks following the transplant, which resolved with high-dose prednisone followed by UVB phototherapy. Skin biopsy demonstrated lichenoid dermatitis with vacuolar degeneration, dyskeratosis, and prominent pigment incontinence (Figure). Based on these findings and her clinical presentation, a diagnosis of blaschkoid GVHD was made.

Although acute GVHD is the result of immunocompetent donor T cells recognizing host tissues as foreign and initiating an immune response, the pathophysiology of chronic GVHD is not well understood.1,2 Theories for disease pathogenesis in chronic GVHD suggest an underlying autoimmune and/or alloreactive process.2-5 The skin often is the first organ affected in acute GVHD, and patients generally present with a pruritic morbilliform eruption that begins on the trunk and spreads to the rest of the body.1,2 Cutaneous manifestations of chronic GVHD may be protean. Lesions can resemble systemic sclerosis or morphea, lichen planus, psoriasis, ichthyosis, and many other conditions.2
The differential diagnosis of linear dermatoses includes herpes zoster, contact dermatitis, lichen striatus (blaschkitis), nevus unius lateris, inflammatory linear verrucous epidermal nevus, and incontinentia pigmenti.6,7 Lichen planus-like chronic GVHD occurring in a linear distribution has been described.6-14 Distinction between dermatomal and blaschkoid processes is diagnostically important. In the case of GVHD, dermatomal distribution may suggest an association between GVHD and prior herpes simplex virus or varicella-zoster virus infection.6,8 Herpesvirus may alter surface antigens of keratinocytes, rendering them targets of donor lymphocytes, and antibodies to viral particles may cross-react with host keratinocyte HLA antigens. It also is possible that dermatomal GVHD may simply be a type of isomorphic response (Köbner phenomenon).8
When cutaneous GVHD follows Blaschko lines, other mechanisms appear to be at play.9-14 It is plausible that these patients have an underlying genetic mosaicism, perhaps the result of a postzygotic mutation, that results in a daughter cell population that expresses surface antigens different from those of the primary cell population found elsewhere in the skin. Donor lymphocytes may selectively react to this mosaic population, leading to the clinical picture of chronic GVHD oriented along Blaschko lines.10,11,13,14
In conclusion, lichenoid linear GVHD following Blaschko lines is an uncommon presentation of chronic GVHD that highlights the heterogeneity of this disease and should be considered in the appropriate clinical setting.
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550-1561.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-515.e18; quiz 533-534.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
- Shimada M, Onizuka M, Machida S, et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol. 2007;139:458-463.
- Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993-3001.
- Freemer CS, Farmer ER, Corio RL, et al. Lichenoid chronic graft-vs-host disease occurring in a dermatomal distribution. Arch Dermatol. 1994;130:70-72.
- Kikuchi A, Okamoto S, Takahashi S, et al. Linear chronic cutaneous graft-versus-host disease. J Am Acad Dermatol. 1997;37:1004-1006.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Kennedy FE, Hilari H, Ferrer B, et al. Lichenoid chronic graft-vs-host disease following Blaschko lines. ActasDermosifiliogr. 2014;105:89-92.
- Lee SW, Kim YC, Lee E, et al. Linear lichenoid graft versus host disease: an unusual configuration following Blaschko's lines. J Dermatol. 2006;33:583-584.
- Beers B, Kalish RS, Kaye VN, et al. Unilateral linear lichenoid eruption after bone marrow transplantation: an unmasking of tolerance to an abnormal keratinocyte clone? J Am Acad Dermatol. 1993;28(5, pt 2):888-892.
- Wilson B, Lockman D. Linear lichenoid graft-vs-host disease. Arch Dermatol. 1994;130(9):1206-1208.
- Reisfeld PL. Lichenoid chronic graft-vs-host disease. Arch Dermatol. 1994;130:1207-1208.
- Vassallo C, Derlino F, Ripamonti F, et al. Lichenoid cutaneous chronic GvHD following Blaschko lines. Int J Dermatol. 2014;53:473-475.
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550-1561.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-515.e18; quiz 533-534.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
- Shimada M, Onizuka M, Machida S, et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol. 2007;139:458-463.
- Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993-3001.
- Freemer CS, Farmer ER, Corio RL, et al. Lichenoid chronic graft-vs-host disease occurring in a dermatomal distribution. Arch Dermatol. 1994;130:70-72.
- Kikuchi A, Okamoto S, Takahashi S, et al. Linear chronic cutaneous graft-versus-host disease. J Am Acad Dermatol. 1997;37:1004-1006.
- Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42:562-564.
- Kennedy FE, Hilari H, Ferrer B, et al. Lichenoid chronic graft-vs-host disease following Blaschko lines. ActasDermosifiliogr. 2014;105:89-92.
- Lee SW, Kim YC, Lee E, et al. Linear lichenoid graft versus host disease: an unusual configuration following Blaschko's lines. J Dermatol. 2006;33:583-584.
- Beers B, Kalish RS, Kaye VN, et al. Unilateral linear lichenoid eruption after bone marrow transplantation: an unmasking of tolerance to an abnormal keratinocyte clone? J Am Acad Dermatol. 1993;28(5, pt 2):888-892.
- Wilson B, Lockman D. Linear lichenoid graft-vs-host disease. Arch Dermatol. 1994;130(9):1206-1208.
- Reisfeld PL. Lichenoid chronic graft-vs-host disease. Arch Dermatol. 1994;130:1207-1208.
- Vassallo C, Derlino F, Ripamonti F, et al. Lichenoid cutaneous chronic GvHD following Blaschko lines. Int J Dermatol. 2014;53:473-475.
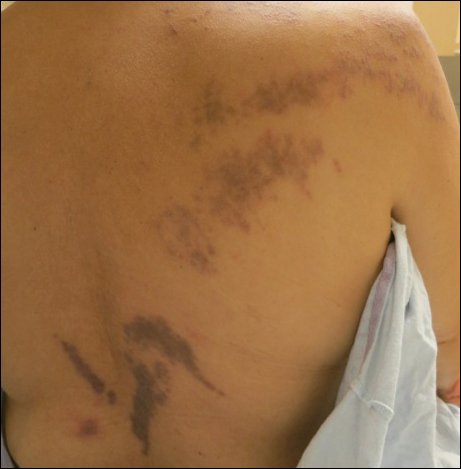
A 56-year-old woman with a history of bone marrow transplant presented for evaluation of a nonpruritic rash of 3 months' duration. Physical examination revealed confluent purple-colored and hyperpigmented papules localized to the back and right arm in a curvilinear pattern. Laboratory results were notable for mildly elevated aspartate transaminase and alanine transaminase levels.
Pruritic and Painful Nodules on the Tongue
The Diagnosis: Chronic Hyperplastic Candidiasis (Nodular Form)
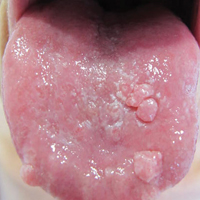
Chronic hyperplastic candidiasis (CHC) is a rare form of oropharyngeal candidiasis. The most frequent clinical presentation is a white plaque that cannot be detached (also known as candidal leukoplakia). It usually involves the anterior buccal mucosa, mainly the commissural area, though the palate and tongue also can be affected. The nodular type of CHC is even less common. Our patient exhibited the typical clinical presentation of the nodular type of CHC.1-3 The differential diagnosis includes leukoplakia, premalignant and malignant epithelial lesions, granular cell tumor, and florid oral papillomatosis.1,3 A biopsy usually is required for diagnostic confirmation. Histologically, CHC is characterized by parakeratosis and a hyperplastic epithelium invaded by Candida hyphae.4 Because Candida species are commensal in up to 50% of the healthy population, superficial colonization of tissues is not enough to indicate notable disease.1 In our patient, the histopathology revealed a hyperplastic mucosa without atypia and numerous hyphae (Figure). Both lingual swab and tissue cultures revealed high growth of Candida albicans.
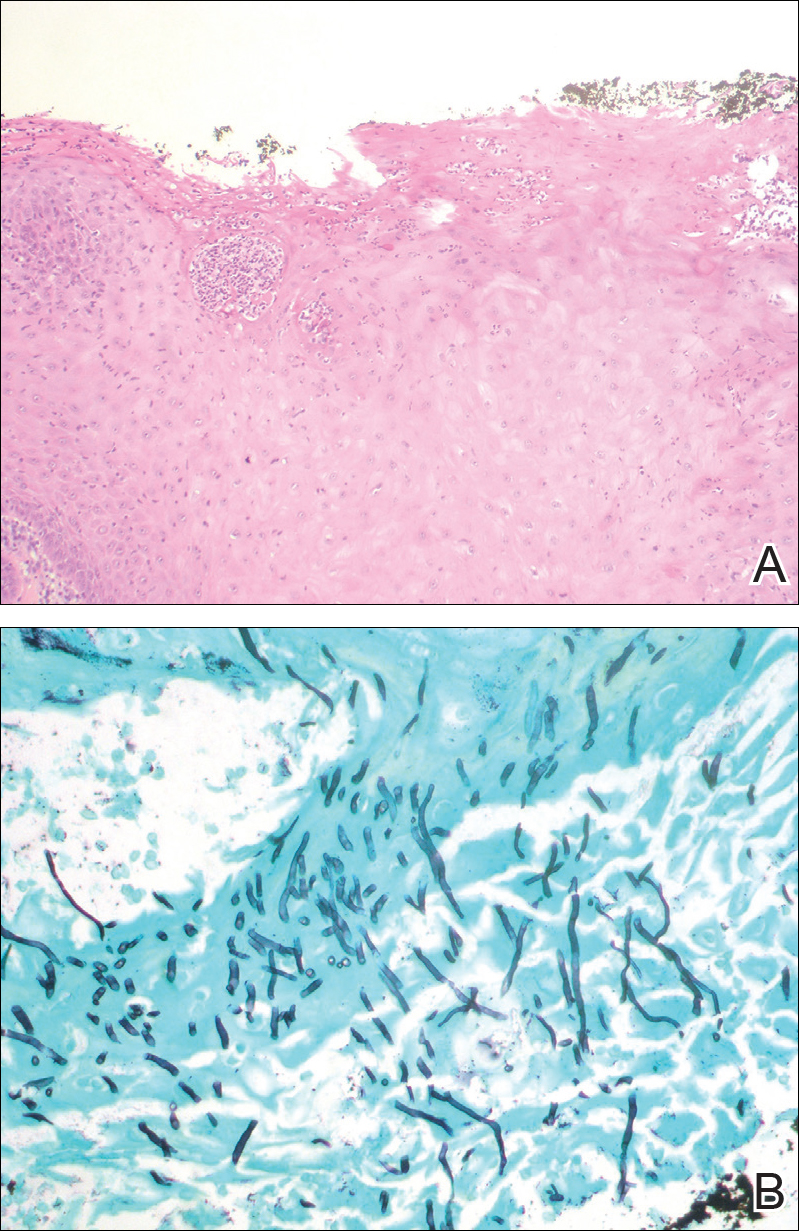
Infection by C albicans depends on pathogen virulence and host factors such as wearing dentures, reduced salivary production, smoking habit, or immunosuppression.1,4 Apart from wearing dentures, our patient did not present with other predisposing factors. It is possible that the immunosuppressive status related to old age and associated oral changes contributed to Candida infection in this case.
Topical or systemic antifungal agents together with the elimination of predisposing factors are usual first-line treatments. Because of the relationship with atypia and the possibility of evolving into carcinoma in untreated or persistent lesions, follow-up is necessary to verify complete resolution after treatment.1,3,4 In the case reported herein, the lesions disappeared after 15 days of oral fluconazole treatment.
- Shibata T, Yamashita D, Hasegawa S, et al. Oral candidiasis mimicking tongue cancer [published online January 12, 2011]. Auris Nasus Larynx. 2011;38:418-420.
- Scardina GA, Ruggieri A, Messina P. Chronic hyperplastic candidosis: a pilot study of the efficacy of 0.18% isotretinoin. J Oral Sci. 2009;51:407-410.
- Sitheeque MA, Samaranayake LP. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia). Crit Rev Oral Biol Med. 2003;14:253-267.
- Williams DW, Bartie KL, Potts AJ, et al. Strain persistence of invasive Candida albicans in chronic hyperplastic candidosis that underwent malignant change. Gerodontology. 2001;18:73-78.
The Diagnosis: Chronic Hyperplastic Candidiasis (Nodular Form)
Chronic hyperplastic candidiasis (CHC) is a rare form of oropharyngeal candidiasis. The most frequent clinical presentation is a white plaque that cannot be detached (also known as candidal leukoplakia). It usually involves the anterior buccal mucosa, mainly the commissural area, though the palate and tongue also can be affected. The nodular type of CHC is even less common. Our patient exhibited the typical clinical presentation of the nodular type of CHC.1-3 The differential diagnosis includes leukoplakia, premalignant and malignant epithelial lesions, granular cell tumor, and florid oral papillomatosis.1,3 A biopsy usually is required for diagnostic confirmation. Histologically, CHC is characterized by parakeratosis and a hyperplastic epithelium invaded by Candida hyphae.4 Because Candida species are commensal in up to 50% of the healthy population, superficial colonization of tissues is not enough to indicate notable disease.1 In our patient, the histopathology revealed a hyperplastic mucosa without atypia and numerous hyphae (Figure). Both lingual swab and tissue cultures revealed high growth of Candida albicans.

Infection by C albicans depends on pathogen virulence and host factors such as wearing dentures, reduced salivary production, smoking habit, or immunosuppression.1,4 Apart from wearing dentures, our patient did not present with other predisposing factors. It is possible that the immunosuppressive status related to old age and associated oral changes contributed to Candida infection in this case.
Topical or systemic antifungal agents together with the elimination of predisposing factors are usual first-line treatments. Because of the relationship with atypia and the possibility of evolving into carcinoma in untreated or persistent lesions, follow-up is necessary to verify complete resolution after treatment.1,3,4 In the case reported herein, the lesions disappeared after 15 days of oral fluconazole treatment.
The Diagnosis: Chronic Hyperplastic Candidiasis (Nodular Form)
Chronic hyperplastic candidiasis (CHC) is a rare form of oropharyngeal candidiasis. The most frequent clinical presentation is a white plaque that cannot be detached (also known as candidal leukoplakia). It usually involves the anterior buccal mucosa, mainly the commissural area, though the palate and tongue also can be affected. The nodular type of CHC is even less common. Our patient exhibited the typical clinical presentation of the nodular type of CHC.1-3 The differential diagnosis includes leukoplakia, premalignant and malignant epithelial lesions, granular cell tumor, and florid oral papillomatosis.1,3 A biopsy usually is required for diagnostic confirmation. Histologically, CHC is characterized by parakeratosis and a hyperplastic epithelium invaded by Candida hyphae.4 Because Candida species are commensal in up to 50% of the healthy population, superficial colonization of tissues is not enough to indicate notable disease.1 In our patient, the histopathology revealed a hyperplastic mucosa without atypia and numerous hyphae (Figure). Both lingual swab and tissue cultures revealed high growth of Candida albicans.

Infection by C albicans depends on pathogen virulence and host factors such as wearing dentures, reduced salivary production, smoking habit, or immunosuppression.1,4 Apart from wearing dentures, our patient did not present with other predisposing factors. It is possible that the immunosuppressive status related to old age and associated oral changes contributed to Candida infection in this case.
Topical or systemic antifungal agents together with the elimination of predisposing factors are usual first-line treatments. Because of the relationship with atypia and the possibility of evolving into carcinoma in untreated or persistent lesions, follow-up is necessary to verify complete resolution after treatment.1,3,4 In the case reported herein, the lesions disappeared after 15 days of oral fluconazole treatment.
- Shibata T, Yamashita D, Hasegawa S, et al. Oral candidiasis mimicking tongue cancer [published online January 12, 2011]. Auris Nasus Larynx. 2011;38:418-420.
- Scardina GA, Ruggieri A, Messina P. Chronic hyperplastic candidosis: a pilot study of the efficacy of 0.18% isotretinoin. J Oral Sci. 2009;51:407-410.
- Sitheeque MA, Samaranayake LP. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia). Crit Rev Oral Biol Med. 2003;14:253-267.
- Williams DW, Bartie KL, Potts AJ, et al. Strain persistence of invasive Candida albicans in chronic hyperplastic candidosis that underwent malignant change. Gerodontology. 2001;18:73-78.
- Shibata T, Yamashita D, Hasegawa S, et al. Oral candidiasis mimicking tongue cancer [published online January 12, 2011]. Auris Nasus Larynx. 2011;38:418-420.
- Scardina GA, Ruggieri A, Messina P. Chronic hyperplastic candidosis: a pilot study of the efficacy of 0.18% isotretinoin. J Oral Sci. 2009;51:407-410.
- Sitheeque MA, Samaranayake LP. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia). Crit Rev Oral Biol Med. 2003;14:253-267.
- Williams DW, Bartie KL, Potts AJ, et al. Strain persistence of invasive Candida albicans in chronic hyperplastic candidosis that underwent malignant change. Gerodontology. 2001;18:73-78.

An 82-year-old woman with atrial fibrillation and chronic obstructive pulmonary disease presented with pruritic and painful lesions on the tongue of 10 years' duration. She had not undergone treatment with systemic or inhaled corticosteroids during the course of the pulmonary disease. On physical examination, several fleshy and well-defined erythematous papules speckled with whitish areas were observed on the dorsal aspect and anterior border of the tongue. Superficial whitish areas could not be removed by scraping.
Common Hair Disorders
Review the PDF of the fact sheet on common hair disorders with board-relevant, easy-to-review material. This fact sheet reviews information about the most common hair disorders, including clinical and histopathological features, trichoscopy, and management of these diseases.
Practice Questions
1. A 40-year-old woman presents to the clinic with a burning sensation and tenderness on the scalp. At physical examination you notice erythematous papules and pustules on the vertex scalp. The most likely diagnosis is:
a. alopecia areata
b. CCSA
c. folliculitis decalvans
d. lichen planopilaris
e. traction alopecia
2. A 60-year-old woman presents with receding hair loss on the frontal and bitemporal scalp. She has noticed hair loss on her eyebrows. She has a history of oral ulcers. On physical examination there is mild erythema and perifollicular scales on the frontal hairline. A hair pull test is positive in this area. The most likely diagnosis is:
a. androgenetic alopecia
b. chronic cutaneous lupus erythematosus
c. frontal fibrosing alopecia
d. telogen effluvium
e. trichotillomania
3. A 5-year-old girl with a history of seasonal allergies and eczema presents with recurrent patchy hair loss on the scalp of 6 months’ duration. Her mother has noticed rapidly progressive hair loss affecting the whole scalp. On trichoscopy, you find yellow dots, broken hairs, and tapering hairs. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. telogen effluvium
d. traction alopecia
e. trichotillomania
4. A 30-year-old white woman with history of obsessive-compulsive disorder presents to the clinic with hair loss for the last 3 years. She says she has noticed worsening of the hair loss when she is under stress. She also bites her nails. On physical examination you identify an irregular patch of alopecia with broken hairs on the occipital scalp. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. lichen planopilaris
d. traction alopecia
e. trichotillomania
5. A 45-year-old black woman who has a family history of hair loss in her mother presents with tenderness and burning sensation on the vertex scalp. She reports the hair loss was worse after she got a hair relaxer 6 months prior. She uses braids on her scalp and she has not had a relaxer since then. The most likely diagnosis is:
a. CCSA
b. chronic cutaneous lupus erythematosus
c. folliculitis decalvans
d. lichen planopilaris
e. trichotillomania
Answers to practice questions provided on next page
Practice Question Answers
1. A 40-year-old woman presents to the clinic with a burning sensation and tenderness on the scalp. At physical examination you notice erythematous papules and pustules on the vertex scalp. The most likely diagnosis is:
a. alopecia areata
b. CCSA
c. folliculitis decalvans
d. lichen planopilaris
e. traction alopecia
2. A 60-year-old woman presents with receding hair loss on the frontal and bitemporal scalp. She has noticed hair loss on her eyebrows. She has a history of oral ulcers. On physical examination there is mild erythema and perifollicular scales on the frontal hairline. A hair pull test is positive in this area. The most likely diagnosis is:
a. androgenetic alopecia
b. chronic cutaneous lupus erythematosus
c. frontal fibrosing alopecia
d. telogen effluvium
e. trichotillomania
3. A 5-year-old girl with a history of seasonal allergies and eczema presents with recurrent patchy hair loss on the scalp of 6 months’ duration. Her mother has noticed rapidly progressive hair loss affecting the whole scalp. On trichoscopy, you find yellow dots, broken hairs, and tapering hairs. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. telogen effluvium
d. traction alopecia
e. trichotillomania
4. A 30-year-old white woman with history of obsessive-compulsive disorder presents to the clinic with hair loss for the last 3 years. She says she has noticed worsening of the hair loss when she is under stress. She also bites her nails. On physical examination you identify an irregular patch of alopecia with broken hairs on the occipital scalp. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. lichen planopilaris
d. traction alopecia
e. trichotillomania
5. A 45-year-old black woman who has a family history of hair loss in her mother presents with tenderness and burning sensation on the vertex scalp. She reports the hair loss was worse after she got a hair relaxer 6 months prior. She uses braids on her scalp and she has not had a relaxer since then. The most likely diagnosis is:
a. CCSA
b. chronic cutaneous lupus erythematosus
c. folliculitis decalvans
d. lichen planopilaris
e. trichotillomania
Review the PDF of the fact sheet on common hair disorders with board-relevant, easy-to-review material. This fact sheet reviews information about the most common hair disorders, including clinical and histopathological features, trichoscopy, and management of these diseases.
Practice Questions
1. A 40-year-old woman presents to the clinic with a burning sensation and tenderness on the scalp. At physical examination you notice erythematous papules and pustules on the vertex scalp. The most likely diagnosis is:
a. alopecia areata
b. CCSA
c. folliculitis decalvans
d. lichen planopilaris
e. traction alopecia
2. A 60-year-old woman presents with receding hair loss on the frontal and bitemporal scalp. She has noticed hair loss on her eyebrows. She has a history of oral ulcers. On physical examination there is mild erythema and perifollicular scales on the frontal hairline. A hair pull test is positive in this area. The most likely diagnosis is:
a. androgenetic alopecia
b. chronic cutaneous lupus erythematosus
c. frontal fibrosing alopecia
d. telogen effluvium
e. trichotillomania
3. A 5-year-old girl with a history of seasonal allergies and eczema presents with recurrent patchy hair loss on the scalp of 6 months’ duration. Her mother has noticed rapidly progressive hair loss affecting the whole scalp. On trichoscopy, you find yellow dots, broken hairs, and tapering hairs. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. telogen effluvium
d. traction alopecia
e. trichotillomania
4. A 30-year-old white woman with history of obsessive-compulsive disorder presents to the clinic with hair loss for the last 3 years. She says she has noticed worsening of the hair loss when she is under stress. She also bites her nails. On physical examination you identify an irregular patch of alopecia with broken hairs on the occipital scalp. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. lichen planopilaris
d. traction alopecia
e. trichotillomania
5. A 45-year-old black woman who has a family history of hair loss in her mother presents with tenderness and burning sensation on the vertex scalp. She reports the hair loss was worse after she got a hair relaxer 6 months prior. She uses braids on her scalp and she has not had a relaxer since then. The most likely diagnosis is:
a. CCSA
b. chronic cutaneous lupus erythematosus
c. folliculitis decalvans
d. lichen planopilaris
e. trichotillomania
Answers to practice questions provided on next page
Practice Question Answers
1. A 40-year-old woman presents to the clinic with a burning sensation and tenderness on the scalp. At physical examination you notice erythematous papules and pustules on the vertex scalp. The most likely diagnosis is:
a. alopecia areata
b. CCSA
c. folliculitis decalvans
d. lichen planopilaris
e. traction alopecia
2. A 60-year-old woman presents with receding hair loss on the frontal and bitemporal scalp. She has noticed hair loss on her eyebrows. She has a history of oral ulcers. On physical examination there is mild erythema and perifollicular scales on the frontal hairline. A hair pull test is positive in this area. The most likely diagnosis is:
a. androgenetic alopecia
b. chronic cutaneous lupus erythematosus
c. frontal fibrosing alopecia
d. telogen effluvium
e. trichotillomania
3. A 5-year-old girl with a history of seasonal allergies and eczema presents with recurrent patchy hair loss on the scalp of 6 months’ duration. Her mother has noticed rapidly progressive hair loss affecting the whole scalp. On trichoscopy, you find yellow dots, broken hairs, and tapering hairs. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. telogen effluvium
d. traction alopecia
e. trichotillomania
4. A 30-year-old white woman with history of obsessive-compulsive disorder presents to the clinic with hair loss for the last 3 years. She says she has noticed worsening of the hair loss when she is under stress. She also bites her nails. On physical examination you identify an irregular patch of alopecia with broken hairs on the occipital scalp. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. lichen planopilaris
d. traction alopecia
e. trichotillomania
5. A 45-year-old black woman who has a family history of hair loss in her mother presents with tenderness and burning sensation on the vertex scalp. She reports the hair loss was worse after she got a hair relaxer 6 months prior. She uses braids on her scalp and she has not had a relaxer since then. The most likely diagnosis is:
a. CCSA
b. chronic cutaneous lupus erythematosus
c. folliculitis decalvans
d. lichen planopilaris
e. trichotillomania
Review the PDF of the fact sheet on common hair disorders with board-relevant, easy-to-review material. This fact sheet reviews information about the most common hair disorders, including clinical and histopathological features, trichoscopy, and management of these diseases.
Practice Questions
1. A 40-year-old woman presents to the clinic with a burning sensation and tenderness on the scalp. At physical examination you notice erythematous papules and pustules on the vertex scalp. The most likely diagnosis is:
a. alopecia areata
b. CCSA
c. folliculitis decalvans
d. lichen planopilaris
e. traction alopecia
2. A 60-year-old woman presents with receding hair loss on the frontal and bitemporal scalp. She has noticed hair loss on her eyebrows. She has a history of oral ulcers. On physical examination there is mild erythema and perifollicular scales on the frontal hairline. A hair pull test is positive in this area. The most likely diagnosis is:
a. androgenetic alopecia
b. chronic cutaneous lupus erythematosus
c. frontal fibrosing alopecia
d. telogen effluvium
e. trichotillomania
3. A 5-year-old girl with a history of seasonal allergies and eczema presents with recurrent patchy hair loss on the scalp of 6 months’ duration. Her mother has noticed rapidly progressive hair loss affecting the whole scalp. On trichoscopy, you find yellow dots, broken hairs, and tapering hairs. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. telogen effluvium
d. traction alopecia
e. trichotillomania
4. A 30-year-old white woman with history of obsessive-compulsive disorder presents to the clinic with hair loss for the last 3 years. She says she has noticed worsening of the hair loss when she is under stress. She also bites her nails. On physical examination you identify an irregular patch of alopecia with broken hairs on the occipital scalp. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. lichen planopilaris
d. traction alopecia
e. trichotillomania
5. A 45-year-old black woman who has a family history of hair loss in her mother presents with tenderness and burning sensation on the vertex scalp. She reports the hair loss was worse after she got a hair relaxer 6 months prior. She uses braids on her scalp and she has not had a relaxer since then. The most likely diagnosis is:
a. CCSA
b. chronic cutaneous lupus erythematosus
c. folliculitis decalvans
d. lichen planopilaris
e. trichotillomania
Answers to practice questions provided on next page
Practice Question Answers
1. A 40-year-old woman presents to the clinic with a burning sensation and tenderness on the scalp. At physical examination you notice erythematous papules and pustules on the vertex scalp. The most likely diagnosis is:
a. alopecia areata
b. CCSA
c. folliculitis decalvans
d. lichen planopilaris
e. traction alopecia
2. A 60-year-old woman presents with receding hair loss on the frontal and bitemporal scalp. She has noticed hair loss on her eyebrows. She has a history of oral ulcers. On physical examination there is mild erythema and perifollicular scales on the frontal hairline. A hair pull test is positive in this area. The most likely diagnosis is:
a. androgenetic alopecia
b. chronic cutaneous lupus erythematosus
c. frontal fibrosing alopecia
d. telogen effluvium
e. trichotillomania
3. A 5-year-old girl with a history of seasonal allergies and eczema presents with recurrent patchy hair loss on the scalp of 6 months’ duration. Her mother has noticed rapidly progressive hair loss affecting the whole scalp. On trichoscopy, you find yellow dots, broken hairs, and tapering hairs. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. telogen effluvium
d. traction alopecia
e. trichotillomania
4. A 30-year-old white woman with history of obsessive-compulsive disorder presents to the clinic with hair loss for the last 3 years. She says she has noticed worsening of the hair loss when she is under stress. She also bites her nails. On physical examination you identify an irregular patch of alopecia with broken hairs on the occipital scalp. The most likely diagnosis is:
a. alopecia areata
b. androgenetic alopecia
c. lichen planopilaris
d. traction alopecia
e. trichotillomania
5. A 45-year-old black woman who has a family history of hair loss in her mother presents with tenderness and burning sensation on the vertex scalp. She reports the hair loss was worse after she got a hair relaxer 6 months prior. She uses braids on her scalp and she has not had a relaxer since then. The most likely diagnosis is:
a. CCSA
b. chronic cutaneous lupus erythematosus
c. folliculitis decalvans
d. lichen planopilaris
e. trichotillomania
Dermoscopy Update and Noninvasive Imaging Devices for Skin Cancer: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dermoscopy Pearls: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Timed Sequential Therapy for Actinic Keratosis: Report From the Mount Sinai Winter Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Nonsurgical Treatment of Basal Cell Carcinoma: Report From the Mount Sinai Winter Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Psoriasis and Internal Disease: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.