User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Hyaluronic Acid Gel Filler for Nipple Enhancement Following Breast Reconstruction
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
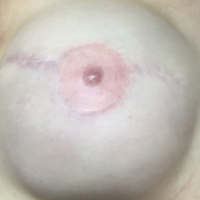
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
The most frequently used surgical techniques in nipple-areola complex (NAC) reconstruction involve the use of local tissue flaps and yield the fewest complications, though these techniques can be associated with up to a 75% loss in nipple projection over time.1 In a best-case scenario for both the surgeon and the patient, the NAC is preserved during mastectomy; however, even when the tissues are spared, an eventual loss of nipple projection is expected due to atrophy and contraction of the healing skin.2 Loss of nipple projection is the most common attribute that patients dislike regarding their NAC reconstruction results.Additional efforts made to restore the natural look and feel of the NAC provides undeniable benefit to the patient in the form of improved body image and psychosocial well-being.3
Augmentation with a grafted material can include cartilage or fat (autologous grafts), calcium hydroxylapatite or polymethyl methacrylate (PMMA)(alloplastic grafts), and acellular dermal matrix or biologic collagen (allografts). Among these options, successive treatment with autologous fat has been shown to provide satisfactory projections over time with minimal complications.4 However, an additional consideration associated with graft augmentation is the need for an additional surgical site (autologous grafts) or the possibility that graft material may not be compatible with subsequent breast examination techniques. For example, calcium hydroxylapatite is a radiopaque material that may interfere with the interpretation of radiography and mammography.5
The use of injectable hyaluronic acid (HA) dermal fillers to enhance nipple projection represents a noninvasive procedure with immediate and adjustable results. A variety of dermal fillers that do not interfere with subsequent breast imaging needs have already been successfully used for nipple reconstruction including HA 60% plus acrylic hydrogel 40%, PMMA microspheres in a bovine collagen 3.5% gel, and poly-L-lactic acid.5-7
The results achieved with HA 60% plus acrylic hydrogel 40% were as much as a 2.5-mm mean increase in nipple projection after 12 months for 70 nipples reconstructed using a small wedge from the labia minora.5 In these treatments, an initial injection of 0.1 to 0.3 mL of filler into each nipple along with a 0.2-mL injection at the base of each nipple was made. Further optional treatments at 2 and 4 months after the initial injection were made using up to 0.3 mL additional volume depending on filler reabsorption.5 Results achieved with PMMA microspheres in a bovine collagen 3.5% gel included a 1.6-mm mean increase in nipple projection at 9 months versus baseline for 33 nipples in 23 patients, which involved up to 2 injections at baseline and again at 3 months.6 Treatment with poly-L-lactic acid provided a 2.3-mm mean increase in nipple projection for 12 patients after 1 year of treatment, which involved 0.5-mL injections every 4 weeks over a series of 4 treatments.7
This report describes the technique and cosmetic outcome using an injectable HA gel to postoperatively restore the 3-dimensional contour of the nipple following surgical breast reconstruction. This chemically cross-linked, stabilized HA gel suspended in phosphate-buffered saline at a pH of 7 and a concentration of 20 mg/mL with lidocaine 0.3% is indicated for mid to deep dermal implantation for the correction of moderate to severe facial wrinkles and folds, such as the nasolabial folds.8
Case Report
A 49-year-old woman with a history of breast cancer with a focal, high-grade ductal carcinoma in situ underwent a complete bilateral mastectomy. The sentinel lymph nodes were negative at the time of mastectomy. One year later, the patient elected to have breast and nipple-areola (flap) reconstruction. Following the reconstructive surgery, her nipples had become visibly atrophic and flat, and she was interested in cosmetic enhancement.
After informed consent had been obtained from the patient, a baseline measurement of each nipple was made while the patient was standing. Each nipple was then injected with up to 0.1 to 0.2 mL of HA gel filler using a 30-gauge needle inserted 2-mm deep (bilaterally) into each nipple. The patient tolerated the procedure well with no pain, bleeding, or bruising. Although HA gel filler contains lidocaine 0.3% and tricaine can further be used to ensure patient comfort, the nipple reconstruction surgery left the patient with little sensation in the treatment area. Rubbing alcohol was used to prepare the skin prior to the procedure, and fractionated coconut oil spray with a nonadherent dressing was used postprocedure.
Following the injection, an immediate increase of 1.6 and 1.5 mm in nipple projection in the right and left breasts, respectively, was achieved with HA gel. The nipple projection of the right breast was 1.7 mm before injection (Figure, A) and 3.3 mm immediately postinjection (Figure, C). The nipple projection of the left breast was 1.8 mm before injection (Figure, B) and 3.3 mm immediately postinjection (Figure, D).

Comment
With a single treatment consisting of 0.2 mL or less of filler volume, the HA gel used in this procedure provided an immediate mean increase in nipple projection of 1.5 mm. Although our assessment involved a single patient evaluated at baseline and immediately post-injection of HA filler only, it is reasonable to assume that subsequent reinjections would provide results comparable to other fillers. Although other fillers that are semipermanent (acrylic hydrogel) and nonbiodegradable (PMMA) make them more durable, these properties also make the augmentation less reversible in the case of overfilling. As with all dermal fillers, rare side effects associated with injection of HA gel filler could potentially include injection-site inflammation, extrusion of filler at the needle insertion site, minimal pain or discomfort during or after injections, bruising, swelling, or delayed-type hypersensitivity reaction. Ideally, HA gel is a soft transparent filler that is reversible with hyaluronidase, an advantage not shared by other filler materials.9
Conclusion
Nipple augmentation with HA gel is a simple noninvasive
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
- Sisti A, Grimaldi L, Tassinari J, et al. Nipple-areola complex reconstruction techniques: a literature review. Eur J Surg Oncol. 2016;42:441-465.
- Murthy V, Chamberlain RS. Defining a place for nipple sparing mastectomy in modern breast care: an evidence based review. Breast J. 2013;19:571-581.
- Jabor MA, Shayani P, Collins DR Jr, et al. Nipple-areola reconstruction: satisfaction and clinical determinants. Plast Reconstr Surg. 2002;110:457-463.
- Kaoutzanis C, Xin M, Ballard TN, et al. Autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76:270-275.
- Panettiere P, Marchetti L, Accorsi D. Filler injection enhances the projection of the reconstructed nipple: an original easy technique. Aesthet Plast Surg. 2005;29:287-294.
- McCarthy CM, Van Laeken N, Lennox P, et al. The efficacy of Artecoll injections for the augmentation of nipple projection in breast reconstruction. Eplasty. 2010;10:E7.
- Dessy LA, Troccola A, Ranno RL, et al. The use of Poly-lactic acid to improve projection of reconstructed nipple. Breast. 2011;20:220-224.
- Restylane L [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2016.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295-316.
Practice Points
- The use of injectable hyaluronic acid (HA) gel to restore 3-dimensional contour of the nipple following nipple-areola complex (NAC) reconstruction is a noninvasive procedure that contributes to patient well-being.
- The use of HA gel for NAC augmentation can be performed in an office setting and may eliminate the need for secondary reconstructive surgeries.
Aggressive Merkel Cell Carcinoma in a Liver Transplant Recipient
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
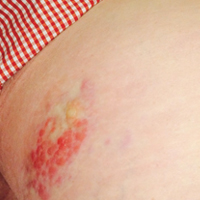
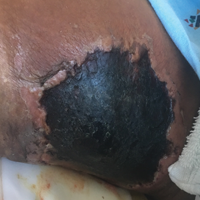
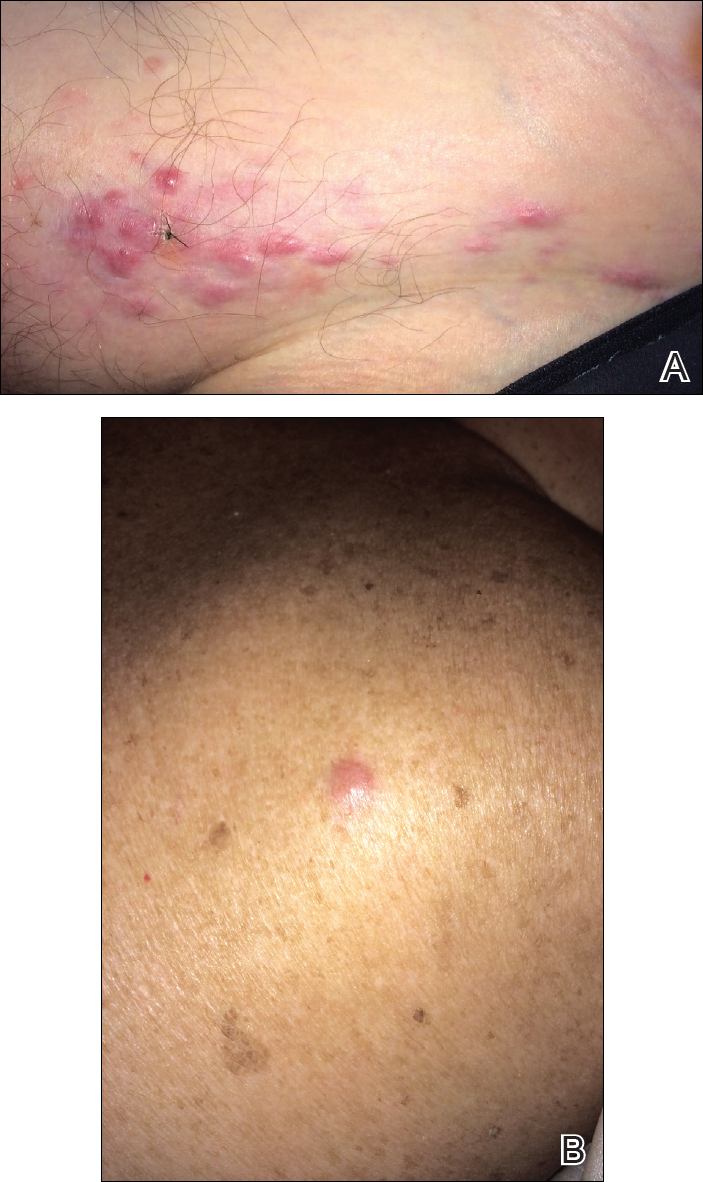
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.
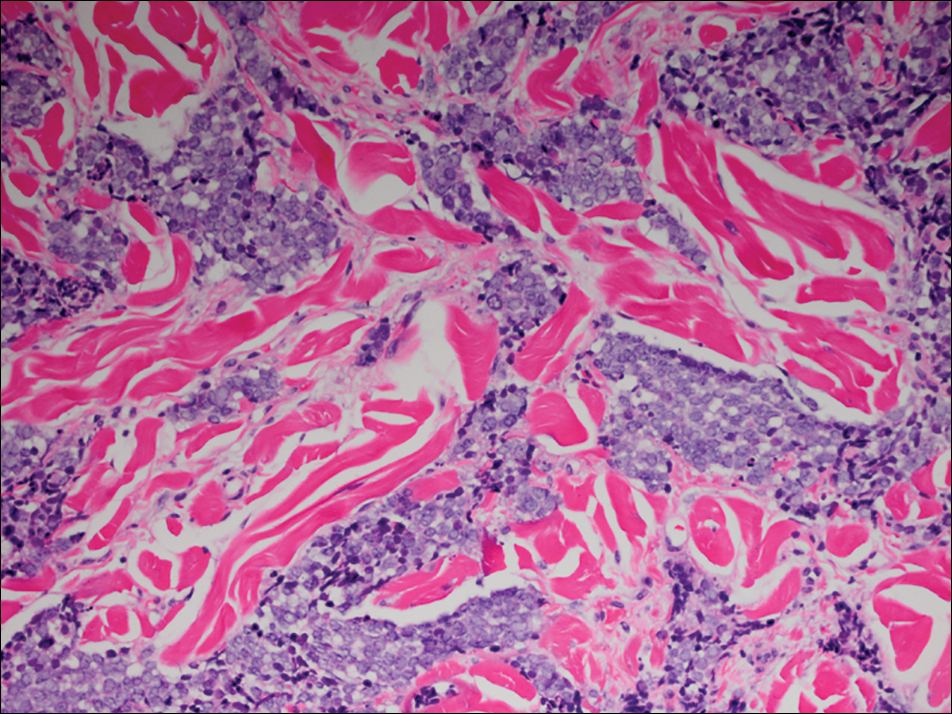
Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.
Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
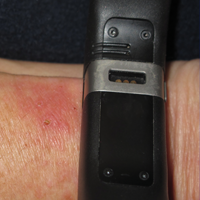
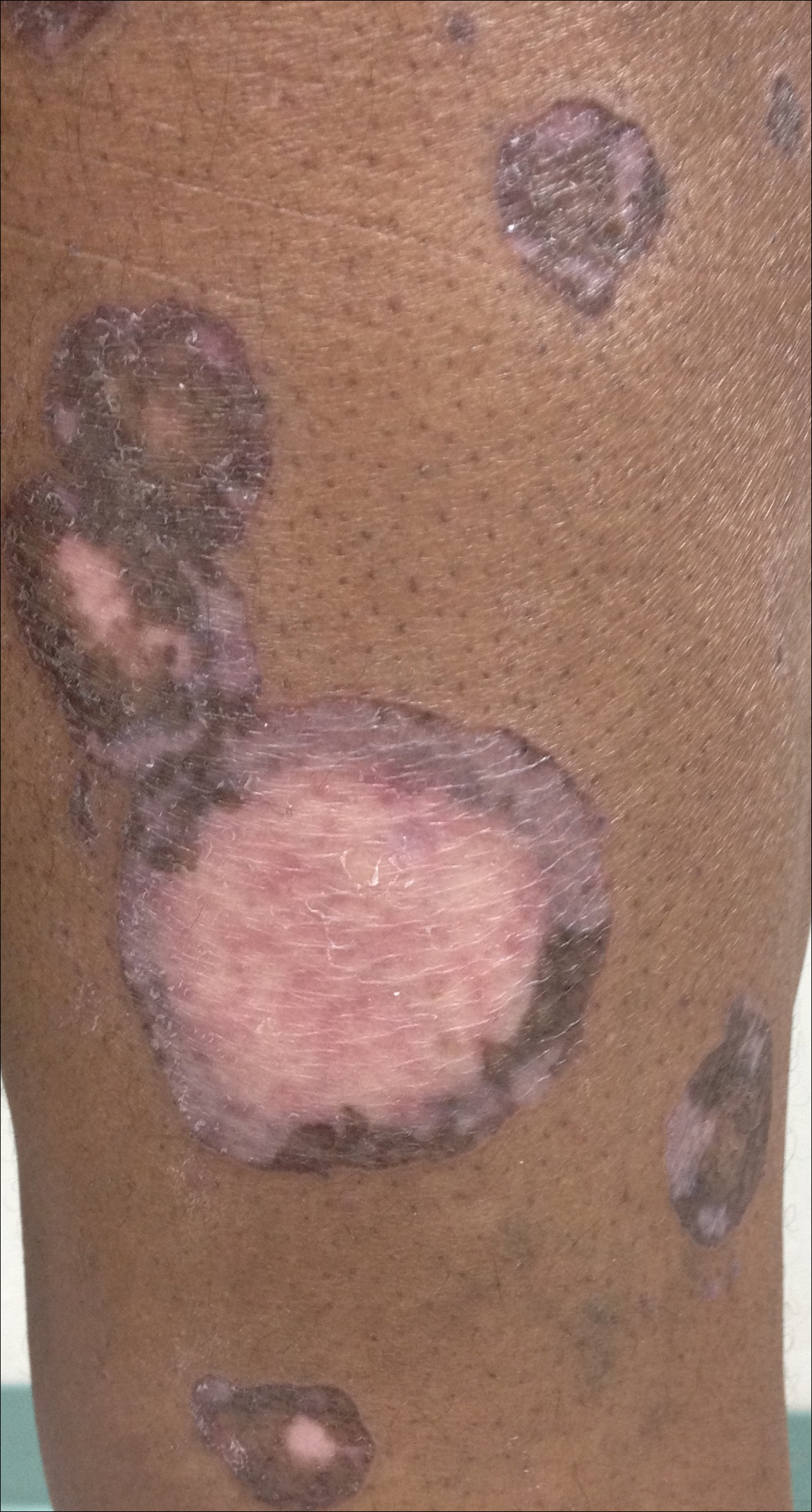
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.
Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.

Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.

Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.

Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
Merkel cell carcinoma (MCC) is a rare cutaneous neuroendocrine tumor derived from the nerve-associated Merkel cell touch receptors.1 It typically presents as a solitary, rapidly growing, red to violaceous, asymptomatic nodule, though ulcerated, acneform, and cystic lesions also have been described.2 Merkel cell carcinoma follows an aggressive clinical course with a tendency for rapid growth, local recurrence (26%–60% of cases), lymph node invasion, and distant metastases (18%–52% of cases).3
Several risk factors contribute to the development of MCC, including chronic immunosuppression, exposure to UV radiation, and infection with the Merkel cell polyomavirus. Immunosuppression has been shown to increase the risk for MCC and is associated with a worse prognosis independent of stage at diagnosis.4 Organ transplant recipients represent a subset of immunosuppressed patients who are at increased risk for the development of MCC. We report a case of metastatic MCC in a 67-year-old woman 6 years after liver transplantation.
Case Report
A 67-year-old woman presented to our clinic with 2 masses—1 on the left buttock and 1 on the left hip—of 4 months’ duration. The patient’s medical history was remarkable for autoimmune hepatitis requiring liver transplantation 6 years prior as well as hypertension and thyroid disorder. Her posttransplantation course was unremarkable, and she was maintained on chronic immunosuppression with tacrolimus and mycophenolate mofetil. Six years after transplantation, the patient was observed to have a 4-cm, red-violaceous, painless, dome-shaped tumor on the left buttock (Figure 1). She also was noted to have pink-red papulonodules forming a painless 8-cm plaque on the left hip that was present for 2 weeks prior to presentation (Figure 1). Both lesions were subsequently biopsied.

Microscopic examination of both lesions was consistent with the diagnosis of MCC. On histopathology, both samples exhibited a dense cellular dermis composed of atypical basophilic tumor cells with extension into superficial dilated lymphatic channels indicating lymphovascular invasion (Figure 2). Tumor cells were positive for the immunohistochemical markers pankeratin AE1/AE3, CAM 5.2, cytokeratin 20, synaptophysin, chromogranin A, and Merkel cell polyomavirus.

Total-body computed tomography and positron emission tomography revealed a hypermetabolic lobular density in the left gluteal region measuring 3.9×1.1 cm. The mass was associated with avid disease involving the left inguinal, bilateral iliac chain, and retroperitoneal lymph nodes. The patient was determined to have stage IV MCC based on the presence of distant lymph node metastases. The mass on the left hip was identified as an in-transit metastasis from the primary tumor on the left buttock.
The patient was referred to surgical and medical oncology. The decision was made to start palliative chemotherapy without surgical intervention given the extent of metastases not amenable for resection. The patient was subsequently initiated on chemotherapy with etoposide and carboplatin. After one cycle of chemotherapy, both tumors initially decreased in size; however, 4 months later, despite multiple cycles of chemotherapy, the patient was noted to have growth of existing tumors and interval development of a new 7×5-cm erythematous plaque in the left groin (Figure 3A) and a 1.1×1.0-cm smooth nodule on the right upper back (Figure 3B), both also found to be consistent with distant skin metastases of MCC upon microscopic examination after biopsy. Despite chemotherapy, the patient’s tumor continued to spread and the patient died within 8 months of diagnosis.

Comment
Transplant recipients represent a well-described cohort of immunosuppressed patients prone to the development of MCC. Merkel cell carcinoma in organ transplant recipients has been most frequently documented to occur after kidney transplantation and less frequently after heart and liver transplantations.5,6 However, the role of organ type and immunosuppressive regimen is not well characterized in the literature. Clarke et al7 investigated the risk for MCC in a large cohort of solid organ transplant recipients based on specific immunosuppression medications. They found a higher risk for MCC in patients who were maintained on cyclosporine, azathioprine, and mTOR (mechanistic target of rapamycin) inhibitors rather than tacrolimus, mycophenolate mofetil, and corticosteroids. In comparison to combination tacrolimus–mycophenolate mofetil, cyclosporine-azathioprine was associated with an increased incidence of MCC; this risk rose remarkably in patients who resided in geographic locations with a higher average of UV exposure. The authors suggested that UV radiation and immunosuppression-induced DNA damage may be synergistic in the development of MCC.7
Merkel cell carcinoma most frequently occurs on sun-exposed sites, including the face, head, and neck (55%); upper and lower extremities (40%); and truncal regions (5%).8 However, case reports highlight MCC arising in atypical locations such as the buttocks and gluteal region in organ transplant recipients.7,9 In the general population, MCC predominantly arises in elderly patients (ie, >70 years), but it is more likely to present at an earlier age in transplant recipients.6,10 In a retrospective analysis of 41 solid organ transplant recipients, 12 were diagnosed before the age of 50 years.6 Data from the US Scientific Registry of Transplant Recipients showed a median age at diagnosis of 62 years, with the highest incidence occurring 10 or more years after transplantation.7
Merkel cell carcinoma behaves aggressively and is the most common cause of skin cancer death after melanoma.11 Organ transplant recipients with MCC have a worse prognosis than MCC patients who are not transplant recipients. In a retrospective registry analysis of 45 de novo cases, Buell at al5 found a 60% mortality rate in transplant recipients, almost double the 33% mortality rate of the general population. Furthermore, Arron et al10 revealed substantially increased rates of disease progression and decreased rates of disease-specific and overall survival in solid organ transplant recipients on immunosuppression compared to immunocompetent controls. The most important factor for poor prognosis is the presence of lymph node invasion, which lowers survival rate.12
Conclusion
Merkel cell carcinoma following liver transplantation is not well described in the literature. We highlight a case of an aggressive MCC arising in a sun-protected site with rapid metastasis 6 years after liver transplantation. This case emphasizes the importance of surveillance for cutaneous malignancy in solid organ transplant recipients.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
- Gould VE, Moll R, Moll I, et al. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985;52:334-353.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29(2, pt 1):143-156.
- Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17:1489-1495.
- Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642-646.
- Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780-1781.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107. pii:dju382. doi:10.1093/jnci/dju382.
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis and clinical management [published online July 13, 2009]. J Clin Oncol. 2009;27:4021-4026.
- Krejčí K, Tichý T, Horák P, et al. Merkel cell carcinoma of the gluteal region with ipsilateral metastasis into the pancreatic graft of a patient after combined kidney-pancreas transplantation [published online September 20, 2010]. Onkologie. 2010;33:520-524.
- Arron ST, Canavan T, Yu SS. Organ transplant recipients with Merkel cell carcinoma have reduced progression-free, overall, and disease-specific survival independent of stage at presentation [published online July 1, 2014]. J Am Acad Dermatol. 2014;71:684-690.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population-based study [published online July 23, 2009]. J Cutan Pathol. 2010;37:20-27.
- Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510-515.
Practice Points
- Organ transplant recipients are at an increased risk for Merkel cell carcinoma (MCC).
- Early recognition and diagnosis of MCC is important to improve morbidity and mortality.
Annular Atrophic Lichen Planus Responds to Hydroxychloroquine and Acitretin
Annular atrophic lichen planus (AALP) is a rare variant of lichen planus that was first described by Friedman and Hashimoto1 in 1991. Clinically, it combines the configuration and morphological features of both annular and atrophic lichen planus. It is a rare entity. We report a case of AALP in a 69-year-old black man. The clinical and histopathological presentation depicted the defining features of this entity with a characteristic loss of elastic fibers corresponding to central atrophy of active lesions.
Case Report
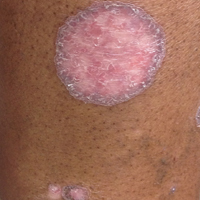
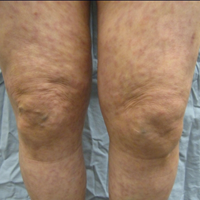
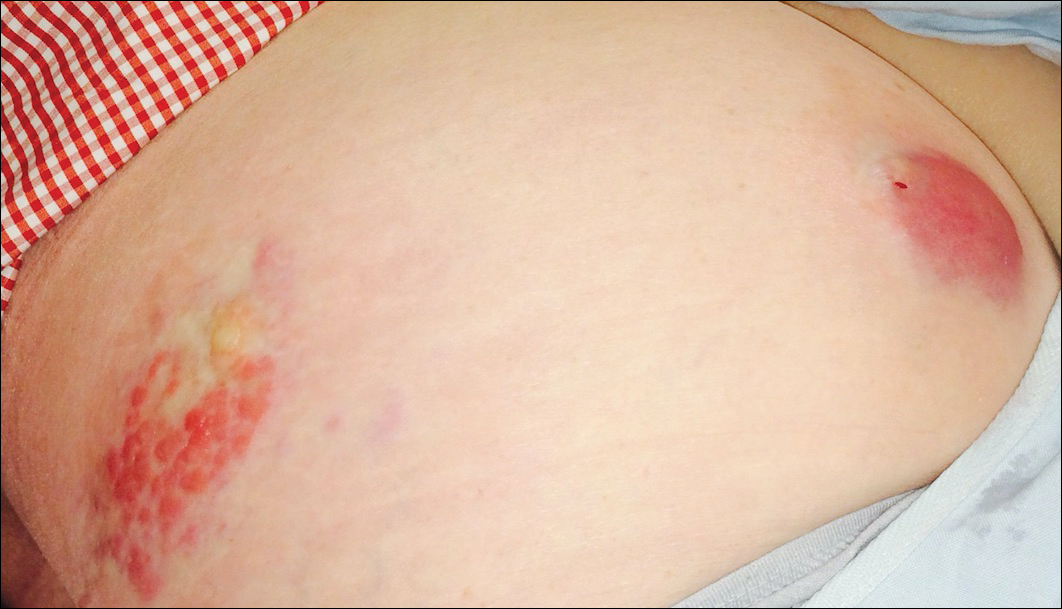
A 69-year-old black man with a history of hepatitis C virus infection and hypothyroidism presented to the dermatology clinic with a pruritic rash on the trunk, extremities, groin, and scalp of 4 months' duration. He denied any new medications, recent illnesses, or sick contacts. Physical examination demonstrated well-demarcated violaceous papules and plaques on the trunk, extensor aspect of the forearms, and thighs involving 10% of the body surface area (Figure 1A). The lesions were annular with raised borders and central depigmented atrophic scarring (Figure 1B). The examination also revealed several large hypopigmented atrophic patches and plaques in the right inguinal region and on the dorsal aspect of the penile shaft and buttocks as well as a single atrophic plaque on the scalp. No oral lesions were seen. An initial punch biopsy was consistent with a nonspecific lichenoid dermatitis (Figure 2), and the patient was prescribed triamcinolone ointment 0.1% for the trunk and extremities and tacrolimus ointment 0.1% for the groin and genital region.


The patient continued to develop new annular atrophic skin lesions over the next several months. Repeat punch biopsies of lesional and uninvolved perilesional skin from the trunk were obtained for histopathologic confirmation and special staining. Lichenoid dermatitis again was noted on the lesional biopsy, and no notable histopathologic changes were observed on the perilesional biopsy. Verhoeff-van Gieson staining for elastic fibers was performed on both biopsies, which revealed destruction of elastic fibers in the central papillary dermis and upper reticular dermis of the lesional biopsy (Figure 3A). The elastic fibers on the perilesional biopsy were preserved (Figure 3B).

The clinical presentation and histopathological findings confirmed a diagnosis of AALP. The patient was prescribed a short taper of oral prednisone, which halted further disease progression. The patient was then started on pentoxifylline and continued on tacrolimus ointment 0.1% with minimal improvement in existing lesions. These medications were discontinued after 3 months. Hydroxychloroquine 400 mg once daily was administered, which initially resulted in some thinning of the plaques on the trunk; however, further progression of the disease was noted after 3 months. Acitretin 25 mg once daily was added to his treatment regimen. Marked thinning of active lesions, hyperpigmentation, and residual scarring was noted after 2 months of combined therapy with acitretin and hydroxychloroquine (Figure 4), with continued improvement appreciable several months later.
Comment
Lichen planus is a common pruritic inflammatory disease of the skin, mucous membranes, hair follicles, and nails with a highly variable clinical pattern and disease course that typically affects the adult population.2 There are many clinical variants of lichen planus, which all demonstrate lichenoid dermatitis on histology. Annular lichen planus is an uncommon variant most commonly seen in men with asymptomatic lesions involving the axillae and groin.2 Atrophic lichen planus is another variant demonstrating atrophic papules and plaques on the trunk and extremities.3 Annular atrophic lichen planus is the rarest variant of lichen planus, incorporating features of both annular and atrophic lichen planus.
The first case of AALP involved a 56-year-old black man with a 25-year history of annular atrophic papules and plaques on the trunk and extremities.1 The second case reported by Requena et al4 in 1994 described a 65-year-old woman with characteristic lesions on the right elbow and left knee. Lipsker et al5 reported a third case in a 41-year-old man with a history of Sneddon syndrome who had lesions typical for AALP for 20 years. In all of these cases, histopathologic examination revealed a lichenoid infiltrate with thinning of the epidermis and loss of elastic fibers in the center of the active lesions.
In more recent cases of AALP, the characteristic findings primarily occurred on the trunk and extremities.6-10 Treatment with topical corticosteroids failed in most cases and some patients noted moderate improvement with tacrolimus ointment 0.1%. Sugashima and Yamamoto11 reported a unique case in 2012 of a 32-year-old woman with AALP on the lower lip. She had notable improvement with tacrolimus ointment 0.1% after 6 months.11
All of the known cases of AALP to date have occurred in adults, both male and female, presenting with a limited number of annular plaques with slightly elevated borders and depressed atrophic centers.1,3-11 Disease duration of AALP has ranged from 2 months to 25 years.11 Histopathologic findings characteristically demonstrate a lichenoid dermatitis of the raised lesional border with a flattened epidermis, loss of rete ridges, and fibrosis of dermal papillae in the lesion center.7 The elastic fibers are destroyed in the papillary dermis of the lesion center, presumably due to elastolytic activity of inflammatory cells.1 Macrophages present in the lichenoid infiltrate of acute lesions release elastases contributing to this destruction.7 Furthermore, elastic fibers appear fragmented on electron microscopy.1
The clinical course of AALP has proven to be chronic in most cases and frequently is resistant to treatment with topical corticosteroids, retinoids, phototherapy, and immunosuppressive agents.3 Treatment administered early in the disease course may provide a more favorable outcome.11 Lesions characteristically heal with scarring and hyperpigmentation. Our case displayed more extensive involvement than has previously been reported. Our patient showed minimal improvement with topical therapy; however, he demonstrated thinning and regression of active lesions after 2 months of combined treatment with hydroxychloroquine and acitretin. Our use of oral pentoxifylline, hydroxychloroquine, and acitretin has not been previously reported in the other cases of AALP we reviewed. Acitretin is the only systemic agent for lichen planus that has achieved level A evidence, as it previously was shown to be highly effective in a placebo-controlled, double-blind study of 65 patients.12
Conclusion
Annular atrophic lichen planus is a known variant of lichen planus characterized by a loss of elastic fibers in the papillary dermis in the center of active lesions. Treatment with topical corticosteroids and phototherapy frequently is ineffective. To our knowledge, there are no studies to date regarding the efficacy of systemic therapy in treatment of AALP. Hydroxychloroquine and acitretin may prove to be beneficial treatment options for resistant AALP. Additional alternative treatments continue to be explored. We encourage reporting additional cases of AALP to further characterize its clinical presentation and response to treatments.
- Friedman DB, Hashimoto K. Annular atrophic lichen planus. J Am Acad Dermatol. 1991;25:392-394.
- James WD, Berger TG, Elston DM. Lichen planus and related conditions. In: James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:213-215.
- Kim BS, Seo SH, Jang BS, et al. A case of annular atrophic lichen planus. J Eur Acad Dermatol Venereol. 2007;21:989-990.
- Requena L, Olivares M, Pique E, et al. Annular atrophic lichen planus. Dermatology. 1994;189:95-98.
- Lipsker D, Piette JC, Laporte JL, et al. Annular atrophic lichen planus and Sneddon's syndrome. Dermatology. 1997;105:402-403.
- Mseddi M, Bouassadi S, Marrakchi S, et al. Annular atrophic lichen planus. Dermatology. 2003;207:208-209.
- Morales-Callaghan A Jr, Martinez G, Aragoneses H, et al. Annular atrophic lichen planus. J Am Acad Dermatol. 2005;52:906-908.
- Ponce-Olivera RM, Tirado-Sánchez A, Montes-de-Oca-Sánchez G, et al. Annular atrophic lichen planus. Int J Dermatol. 2007;46:490-491.
- Kim JS, Kang MS, Sagong C, et al. Annular atrophic lichen planus associated with hypertrophic lichen planus. Clin Exp Dermatol. 2008;33:195-197.
- Li B, Li JH, Xiao T, et al. Annular atrophic lichen planus. Eur J Dermatol. 2010;20:842-843.
- Sugashima Y, Yamamoto T. Annular atrophic lichen planus of the lip. Dermatol Online J. 2012;18:14.
- Manousaridis I, Manousaridis K, Peitsch WK, et al. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. 2013;11:981-991.
Annular atrophic lichen planus (AALP) is a rare variant of lichen planus that was first described by Friedman and Hashimoto1 in 1991. Clinically, it combines the configuration and morphological features of both annular and atrophic lichen planus. It is a rare entity. We report a case of AALP in a 69-year-old black man. The clinical and histopathological presentation depicted the defining features of this entity with a characteristic loss of elastic fibers corresponding to central atrophy of active lesions.
Case Report
A 69-year-old black man with a history of hepatitis C virus infection and hypothyroidism presented to the dermatology clinic with a pruritic rash on the trunk, extremities, groin, and scalp of 4 months' duration. He denied any new medications, recent illnesses, or sick contacts. Physical examination demonstrated well-demarcated violaceous papules and plaques on the trunk, extensor aspect of the forearms, and thighs involving 10% of the body surface area (Figure 1A). The lesions were annular with raised borders and central depigmented atrophic scarring (Figure 1B). The examination also revealed several large hypopigmented atrophic patches and plaques in the right inguinal region and on the dorsal aspect of the penile shaft and buttocks as well as a single atrophic plaque on the scalp. No oral lesions were seen. An initial punch biopsy was consistent with a nonspecific lichenoid dermatitis (Figure 2), and the patient was prescribed triamcinolone ointment 0.1% for the trunk and extremities and tacrolimus ointment 0.1% for the groin and genital region.


The patient continued to develop new annular atrophic skin lesions over the next several months. Repeat punch biopsies of lesional and uninvolved perilesional skin from the trunk were obtained for histopathologic confirmation and special staining. Lichenoid dermatitis again was noted on the lesional biopsy, and no notable histopathologic changes were observed on the perilesional biopsy. Verhoeff-van Gieson staining for elastic fibers was performed on both biopsies, which revealed destruction of elastic fibers in the central papillary dermis and upper reticular dermis of the lesional biopsy (Figure 3A). The elastic fibers on the perilesional biopsy were preserved (Figure 3B).

The clinical presentation and histopathological findings confirmed a diagnosis of AALP. The patient was prescribed a short taper of oral prednisone, which halted further disease progression. The patient was then started on pentoxifylline and continued on tacrolimus ointment 0.1% with minimal improvement in existing lesions. These medications were discontinued after 3 months. Hydroxychloroquine 400 mg once daily was administered, which initially resulted in some thinning of the plaques on the trunk; however, further progression of the disease was noted after 3 months. Acitretin 25 mg once daily was added to his treatment regimen. Marked thinning of active lesions, hyperpigmentation, and residual scarring was noted after 2 months of combined therapy with acitretin and hydroxychloroquine (Figure 4), with continued improvement appreciable several months later.

Comment
Lichen planus is a common pruritic inflammatory disease of the skin, mucous membranes, hair follicles, and nails with a highly variable clinical pattern and disease course that typically affects the adult population.2 There are many clinical variants of lichen planus, which all demonstrate lichenoid dermatitis on histology. Annular lichen planus is an uncommon variant most commonly seen in men with asymptomatic lesions involving the axillae and groin.2 Atrophic lichen planus is another variant demonstrating atrophic papules and plaques on the trunk and extremities.3 Annular atrophic lichen planus is the rarest variant of lichen planus, incorporating features of both annular and atrophic lichen planus.
The first case of AALP involved a 56-year-old black man with a 25-year history of annular atrophic papules and plaques on the trunk and extremities.1 The second case reported by Requena et al4 in 1994 described a 65-year-old woman with characteristic lesions on the right elbow and left knee. Lipsker et al5 reported a third case in a 41-year-old man with a history of Sneddon syndrome who had lesions typical for AALP for 20 years. In all of these cases, histopathologic examination revealed a lichenoid infiltrate with thinning of the epidermis and loss of elastic fibers in the center of the active lesions.
In more recent cases of AALP, the characteristic findings primarily occurred on the trunk and extremities.6-10 Treatment with topical corticosteroids failed in most cases and some patients noted moderate improvement with tacrolimus ointment 0.1%. Sugashima and Yamamoto11 reported a unique case in 2012 of a 32-year-old woman with AALP on the lower lip. She had notable improvement with tacrolimus ointment 0.1% after 6 months.11
All of the known cases of AALP to date have occurred in adults, both male and female, presenting with a limited number of annular plaques with slightly elevated borders and depressed atrophic centers.1,3-11 Disease duration of AALP has ranged from 2 months to 25 years.11 Histopathologic findings characteristically demonstrate a lichenoid dermatitis of the raised lesional border with a flattened epidermis, loss of rete ridges, and fibrosis of dermal papillae in the lesion center.7 The elastic fibers are destroyed in the papillary dermis of the lesion center, presumably due to elastolytic activity of inflammatory cells.1 Macrophages present in the lichenoid infiltrate of acute lesions release elastases contributing to this destruction.7 Furthermore, elastic fibers appear fragmented on electron microscopy.1
The clinical course of AALP has proven to be chronic in most cases and frequently is resistant to treatment with topical corticosteroids, retinoids, phototherapy, and immunosuppressive agents.3 Treatment administered early in the disease course may provide a more favorable outcome.11 Lesions characteristically heal with scarring and hyperpigmentation. Our case displayed more extensive involvement than has previously been reported. Our patient showed minimal improvement with topical therapy; however, he demonstrated thinning and regression of active lesions after 2 months of combined treatment with hydroxychloroquine and acitretin. Our use of oral pentoxifylline, hydroxychloroquine, and acitretin has not been previously reported in the other cases of AALP we reviewed. Acitretin is the only systemic agent for lichen planus that has achieved level A evidence, as it previously was shown to be highly effective in a placebo-controlled, double-blind study of 65 patients.12
Conclusion
Annular atrophic lichen planus is a known variant of lichen planus characterized by a loss of elastic fibers in the papillary dermis in the center of active lesions. Treatment with topical corticosteroids and phototherapy frequently is ineffective. To our knowledge, there are no studies to date regarding the efficacy of systemic therapy in treatment of AALP. Hydroxychloroquine and acitretin may prove to be beneficial treatment options for resistant AALP. Additional alternative treatments continue to be explored. We encourage reporting additional cases of AALP to further characterize its clinical presentation and response to treatments.
Annular atrophic lichen planus (AALP) is a rare variant of lichen planus that was first described by Friedman and Hashimoto1 in 1991. Clinically, it combines the configuration and morphological features of both annular and atrophic lichen planus. It is a rare entity. We report a case of AALP in a 69-year-old black man. The clinical and histopathological presentation depicted the defining features of this entity with a characteristic loss of elastic fibers corresponding to central atrophy of active lesions.
Case Report
A 69-year-old black man with a history of hepatitis C virus infection and hypothyroidism presented to the dermatology clinic with a pruritic rash on the trunk, extremities, groin, and scalp of 4 months' duration. He denied any new medications, recent illnesses, or sick contacts. Physical examination demonstrated well-demarcated violaceous papules and plaques on the trunk, extensor aspect of the forearms, and thighs involving 10% of the body surface area (Figure 1A). The lesions were annular with raised borders and central depigmented atrophic scarring (Figure 1B). The examination also revealed several large hypopigmented atrophic patches and plaques in the right inguinal region and on the dorsal aspect of the penile shaft and buttocks as well as a single atrophic plaque on the scalp. No oral lesions were seen. An initial punch biopsy was consistent with a nonspecific lichenoid dermatitis (Figure 2), and the patient was prescribed triamcinolone ointment 0.1% for the trunk and extremities and tacrolimus ointment 0.1% for the groin and genital region.


The patient continued to develop new annular atrophic skin lesions over the next several months. Repeat punch biopsies of lesional and uninvolved perilesional skin from the trunk were obtained for histopathologic confirmation and special staining. Lichenoid dermatitis again was noted on the lesional biopsy, and no notable histopathologic changes were observed on the perilesional biopsy. Verhoeff-van Gieson staining for elastic fibers was performed on both biopsies, which revealed destruction of elastic fibers in the central papillary dermis and upper reticular dermis of the lesional biopsy (Figure 3A). The elastic fibers on the perilesional biopsy were preserved (Figure 3B).

The clinical presentation and histopathological findings confirmed a diagnosis of AALP. The patient was prescribed a short taper of oral prednisone, which halted further disease progression. The patient was then started on pentoxifylline and continued on tacrolimus ointment 0.1% with minimal improvement in existing lesions. These medications were discontinued after 3 months. Hydroxychloroquine 400 mg once daily was administered, which initially resulted in some thinning of the plaques on the trunk; however, further progression of the disease was noted after 3 months. Acitretin 25 mg once daily was added to his treatment regimen. Marked thinning of active lesions, hyperpigmentation, and residual scarring was noted after 2 months of combined therapy with acitretin and hydroxychloroquine (Figure 4), with continued improvement appreciable several months later.

Comment
Lichen planus is a common pruritic inflammatory disease of the skin, mucous membranes, hair follicles, and nails with a highly variable clinical pattern and disease course that typically affects the adult population.2 There are many clinical variants of lichen planus, which all demonstrate lichenoid dermatitis on histology. Annular lichen planus is an uncommon variant most commonly seen in men with asymptomatic lesions involving the axillae and groin.2 Atrophic lichen planus is another variant demonstrating atrophic papules and plaques on the trunk and extremities.3 Annular atrophic lichen planus is the rarest variant of lichen planus, incorporating features of both annular and atrophic lichen planus.
The first case of AALP involved a 56-year-old black man with a 25-year history of annular atrophic papules and plaques on the trunk and extremities.1 The second case reported by Requena et al4 in 1994 described a 65-year-old woman with characteristic lesions on the right elbow and left knee. Lipsker et al5 reported a third case in a 41-year-old man with a history of Sneddon syndrome who had lesions typical for AALP for 20 years. In all of these cases, histopathologic examination revealed a lichenoid infiltrate with thinning of the epidermis and loss of elastic fibers in the center of the active lesions.
In more recent cases of AALP, the characteristic findings primarily occurred on the trunk and extremities.6-10 Treatment with topical corticosteroids failed in most cases and some patients noted moderate improvement with tacrolimus ointment 0.1%. Sugashima and Yamamoto11 reported a unique case in 2012 of a 32-year-old woman with AALP on the lower lip. She had notable improvement with tacrolimus ointment 0.1% after 6 months.11
All of the known cases of AALP to date have occurred in adults, both male and female, presenting with a limited number of annular plaques with slightly elevated borders and depressed atrophic centers.1,3-11 Disease duration of AALP has ranged from 2 months to 25 years.11 Histopathologic findings characteristically demonstrate a lichenoid dermatitis of the raised lesional border with a flattened epidermis, loss of rete ridges, and fibrosis of dermal papillae in the lesion center.7 The elastic fibers are destroyed in the papillary dermis of the lesion center, presumably due to elastolytic activity of inflammatory cells.1 Macrophages present in the lichenoid infiltrate of acute lesions release elastases contributing to this destruction.7 Furthermore, elastic fibers appear fragmented on electron microscopy.1
The clinical course of AALP has proven to be chronic in most cases and frequently is resistant to treatment with topical corticosteroids, retinoids, phototherapy, and immunosuppressive agents.3 Treatment administered early in the disease course may provide a more favorable outcome.11 Lesions characteristically heal with scarring and hyperpigmentation. Our case displayed more extensive involvement than has previously been reported. Our patient showed minimal improvement with topical therapy; however, he demonstrated thinning and regression of active lesions after 2 months of combined treatment with hydroxychloroquine and acitretin. Our use of oral pentoxifylline, hydroxychloroquine, and acitretin has not been previously reported in the other cases of AALP we reviewed. Acitretin is the only systemic agent for lichen planus that has achieved level A evidence, as it previously was shown to be highly effective in a placebo-controlled, double-blind study of 65 patients.12
Conclusion
Annular atrophic lichen planus is a known variant of lichen planus characterized by a loss of elastic fibers in the papillary dermis in the center of active lesions. Treatment with topical corticosteroids and phototherapy frequently is ineffective. To our knowledge, there are no studies to date regarding the efficacy of systemic therapy in treatment of AALP. Hydroxychloroquine and acitretin may prove to be beneficial treatment options for resistant AALP. Additional alternative treatments continue to be explored. We encourage reporting additional cases of AALP to further characterize its clinical presentation and response to treatments.
- Friedman DB, Hashimoto K. Annular atrophic lichen planus. J Am Acad Dermatol. 1991;25:392-394.
- James WD, Berger TG, Elston DM. Lichen planus and related conditions. In: James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:213-215.
- Kim BS, Seo SH, Jang BS, et al. A case of annular atrophic lichen planus. J Eur Acad Dermatol Venereol. 2007;21:989-990.
- Requena L, Olivares M, Pique E, et al. Annular atrophic lichen planus. Dermatology. 1994;189:95-98.
- Lipsker D, Piette JC, Laporte JL, et al. Annular atrophic lichen planus and Sneddon's syndrome. Dermatology. 1997;105:402-403.
- Mseddi M, Bouassadi S, Marrakchi S, et al. Annular atrophic lichen planus. Dermatology. 2003;207:208-209.
- Morales-Callaghan A Jr, Martinez G, Aragoneses H, et al. Annular atrophic lichen planus. J Am Acad Dermatol. 2005;52:906-908.
- Ponce-Olivera RM, Tirado-Sánchez A, Montes-de-Oca-Sánchez G, et al. Annular atrophic lichen planus. Int J Dermatol. 2007;46:490-491.
- Kim JS, Kang MS, Sagong C, et al. Annular atrophic lichen planus associated with hypertrophic lichen planus. Clin Exp Dermatol. 2008;33:195-197.
- Li B, Li JH, Xiao T, et al. Annular atrophic lichen planus. Eur J Dermatol. 2010;20:842-843.
- Sugashima Y, Yamamoto T. Annular atrophic lichen planus of the lip. Dermatol Online J. 2012;18:14.
- Manousaridis I, Manousaridis K, Peitsch WK, et al. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. 2013;11:981-991.
- Friedman DB, Hashimoto K. Annular atrophic lichen planus. J Am Acad Dermatol. 1991;25:392-394.
- James WD, Berger TG, Elston DM. Lichen planus and related conditions. In: James WD, Berger TG, Elston DM, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 11th ed. China: Saunders Elsevier; 2011:213-215.
- Kim BS, Seo SH, Jang BS, et al. A case of annular atrophic lichen planus. J Eur Acad Dermatol Venereol. 2007;21:989-990.
- Requena L, Olivares M, Pique E, et al. Annular atrophic lichen planus. Dermatology. 1994;189:95-98.
- Lipsker D, Piette JC, Laporte JL, et al. Annular atrophic lichen planus and Sneddon's syndrome. Dermatology. 1997;105:402-403.
- Mseddi M, Bouassadi S, Marrakchi S, et al. Annular atrophic lichen planus. Dermatology. 2003;207:208-209.
- Morales-Callaghan A Jr, Martinez G, Aragoneses H, et al. Annular atrophic lichen planus. J Am Acad Dermatol. 2005;52:906-908.
- Ponce-Olivera RM, Tirado-Sánchez A, Montes-de-Oca-Sánchez G, et al. Annular atrophic lichen planus. Int J Dermatol. 2007;46:490-491.
- Kim JS, Kang MS, Sagong C, et al. Annular atrophic lichen planus associated with hypertrophic lichen planus. Clin Exp Dermatol. 2008;33:195-197.
- Li B, Li JH, Xiao T, et al. Annular atrophic lichen planus. Eur J Dermatol. 2010;20:842-843.
- Sugashima Y, Yamamoto T. Annular atrophic lichen planus of the lip. Dermatol Online J. 2012;18:14.
- Manousaridis I, Manousaridis K, Peitsch WK, et al. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. 2013;11:981-991.
Asymptomatic Cutaneous Polyarteritis Nodosa: Treatment Options and Therapeutic Guidelines
In 1931, Lindberg1 described a cutaneous variant of polyarteritis nodosa, which lacked visceral involvement and possessed a more favorable prognosis.2 Cutaneous polyarteritis nodosa (CPAN) is a localized small- to medium-vessel vasculitis restricted to the skin. Both benign and chronic courses have been described, and systemic involvement does not occur.3 Diagnostic criteria proposed by Nakamura et al3 in 2009 included cutaneous nodules, livedo reticularis, purpura, or ulcers; histopathologic fibrinoid necrotizing vasculitis of small- to medium-sized vessels; and exclusion of systemic symptoms (eg, fever, hypertension, weight loss, renal failure, cerebral hemorrhage, neuropathy, myocardial infarction, ischemic heart disease, pericarditis, pleuritis, arthralgia/myalgia). Nodules occur in 30% to 50% of cases and can remain for years if left untreated. Ulcerations occur in up to 30% of patients. Myositis, arthritis, and weakness also have been reported with this condition.4 Cutaneous polyarteritis nodosa has been associated with abnormal antibody testing with elevations of antiphospholipid cofactor antibody, lupus anticoagulant, anticardiolipin antibody, and anti-β2-glycoprotein I–dependent cardiolipin antibody, as well as elevated anti–phosphatidylserine-prothrombin complex antibody.5 These antibodies suggest increased risk for thrombosis and systemic diseases such as lupus or other autoimmune connective tissue disease. The distinction of this entity from systemic polyartertitis nodosa is key when determining treatment options and monitoring parameters.
Case Report
A 66-year-old woman was referred to our facility by an outside dermatologist with a mildly pruritic, blanchable, reticulated erythema on the chest and bilateral arms and legs of 3 months’ duration consistent with livedo reticularis (Figure 1). Prior systemic therapy included prednisone 10 mg 3 times daily, fexofenadine, loratadine, and hydroxyzine. When the systemic steroid was tapered, the patient developed an asymptomatic flare of her eruption. On presentation, the lesions had waxed and waned, and the patient was taking only vitamin B12 and vitamin C. Her medical history was notable for an unknown-type lymphoma of the chest wall diagnosed at 46 years of age that was treated with an unknown chemotherapeutic agent, chronic pancreatitis that resulted in a duodenectomy at 61 years of age, chronic cholecystitis, and 1 first-trimester miscarriage. Outside laboratory tests, including a comprehensive metabolic panel, complete blood cell count, urinalysis, renal function, and liver function tests were within reference range, except for the finding of mild leukocytosis (11,000/µL)(reference range, 3800–10,800/µL), which resolved after steroids were discontinued, with otherwise normal results. Punch biopsy of a specimen from the right thigh revealed medium-vessel vasculitis consistent with polyarteritis nodosa (Figure 2). Laboratory workup by our facility including hepatitis panel, perinuclear antineutrophil cytoplasmic antibody, cytoplasmic antineutrophil cytoplasmic antibody, factor V Leiden, prothrombin time/international normalized ratio, anticardiolipin antibody, and proteins C and S were all within reference range. Abnormal values included a low positive but nondiagnostic antinuclear antibody screen with negative titers, and the lupus anticoagulant titer was mildly elevated at 44 IgG binding units (reference range, <40 IgG binding units). Serum protein electrophoresis (SPEP) and urine protein electrophoresis also were performed, and SPEP was low positive for elevated κ and γ light chains. The patient was referred to oncology, and further testing revealed no underlying malignancy. The patient was monitored and no treatment was initiated; her rash completely resolved within 3 months. Laboratory monitoring at 6 months including SPEP, urine protein electrophoresis, lupus anticoagulant, and clotting studies all were within reference range.
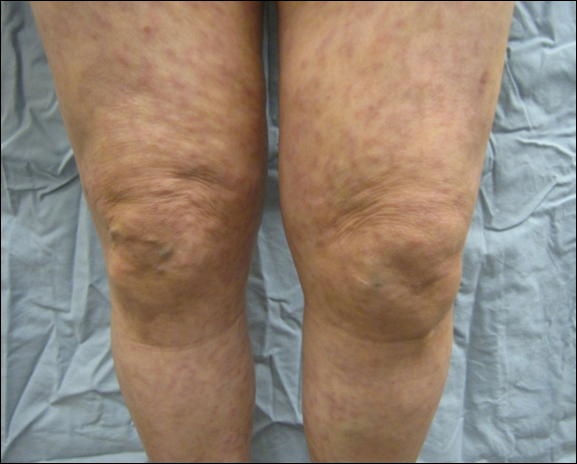
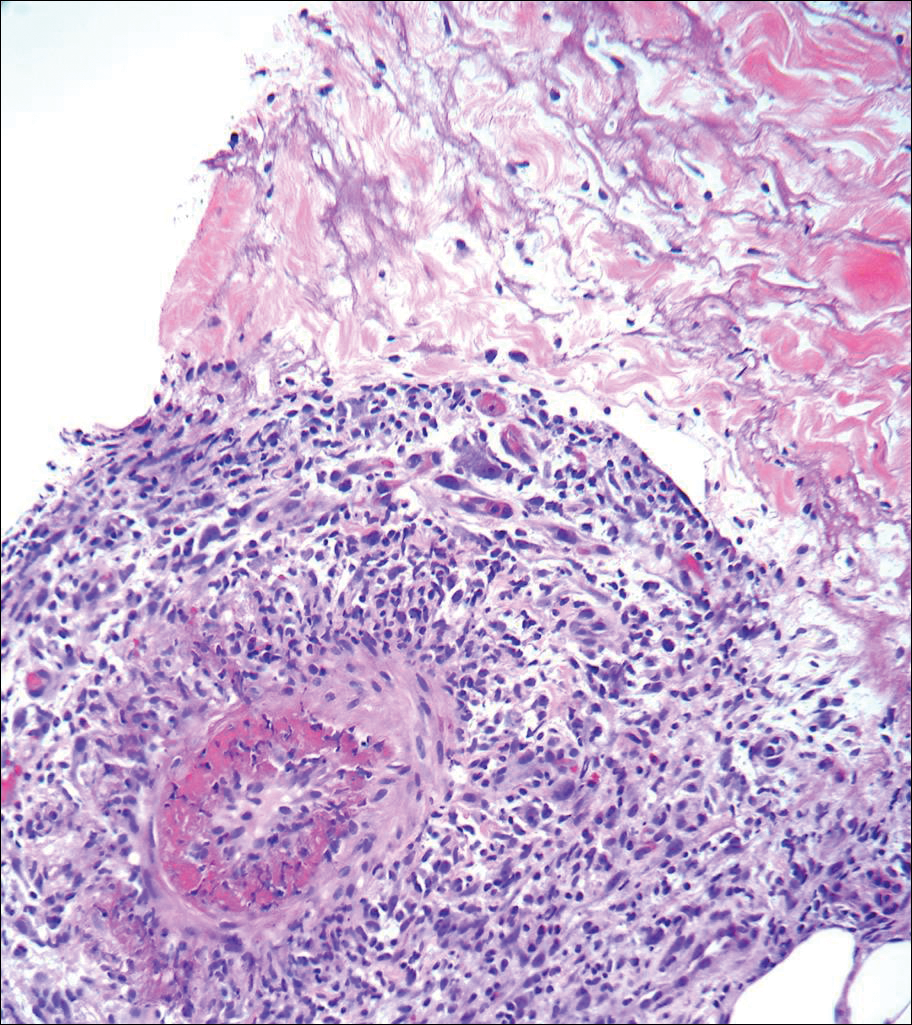
Comment
Although the treatment of systemic polyarteritis nodosa often is necessary and typically involves high-dose corticosteroids and cyclophosphamide, the treatment of CPAN initially is less aggressive. Of the options available for treatment of CPAN, each has associated risks and side effects. Chen6 classified CPAN into 3 groups: 1 (mild), 2 (severe with no systemic involvement), and 3 (severe with progression to systemic disease)(Table). The authors performed a review of all the published treatments and their respective side effects to evaluate if treatment should be instituted for asymptomatic (group 1) disease presenting with abnormal antibody findings as demonstrated in our case.
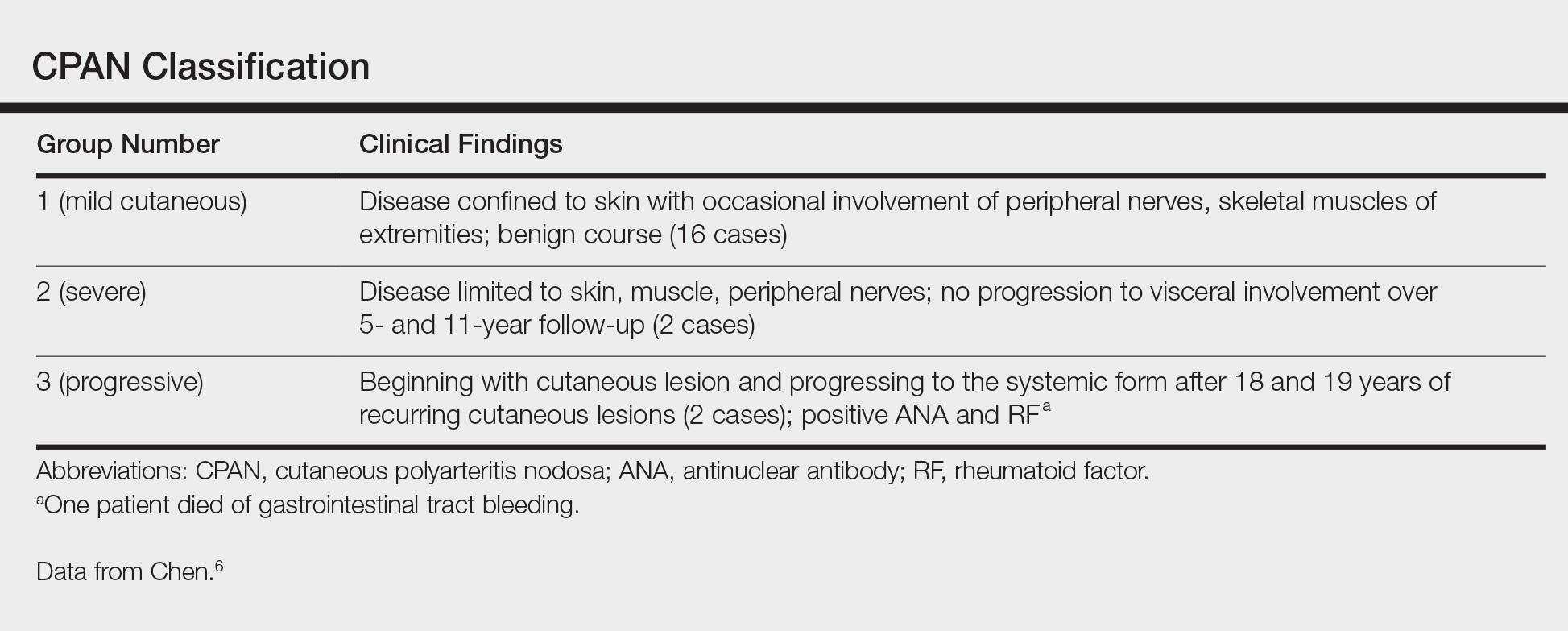
First-line treatment of CPAN includes nonsteroidal anti-inflammatory drugs (NSAIDS) and colchicine.7 Nonsteroidal anti-inflammatory drugs are preferred; however, they also have been associated with gastrointestinal tract upset and increased risk for peptic ulcer disease with long-term use. Although colchicine often is used in conjunction with NSAIDS8 for its anti-inflammatory activity, no studies have been performed on this drug as monotherapy, and the side effect of diarrhea often limits its use.
Other therapies include dapsone, which should be monitored carefully due to the risk for dapsone hypersensitivity syndrome.8,9 Topical corticosteroids have been proven effective for mild cases of confluent erythema with remission occurring as early as 4 weeks.4 Some reports emphasize the role of streptococcal infections in CPAN, especially in children.8,10-12 Consequently it is recommended that anti–streptolysin O titers should be included in the workup for CPAN. Long-term penicillin prophylaxis and tonsillectomy have been used to prevent disease flares with limited success.8,10-12
For more severe disease, especially with neuromuscular involvement, oral methylprednisolone up to 1 mg/kg daily has been used and has proven effective in the control of acute exacerbations.7,13 However, the many adverse effects of systemic steroids limit their use long-term, and taper will often result in flare of disease.4,7 Medications used in conjunction with steroids include hydroxychloroquine, dapsone, azathioprine, cyclophosphamide, methotrexate, sulfapyridine, pentoxifylline, infliximab, etanercept, and intravenous immunoglobulin.4,9,12-17
Low-dose methotrexate has shown some improvement in skin disease with CPAN, but other case reports suggest that complete remission is not achieved with this drug.15,18 More studies are needed to assess the use of methotrexate for CPAN.
Immunomodulators have been used in multiple case reports with varying levels of success. Rogalski and Sticherling4 reported 3 cases that cleared with methylprednisolone plus azathioprine ranging from 4 weeks to 6 months; nausea limited tolerance of azathioprine in 1 case. Mycophenolate mofetil also was successfully used in 2 cases with clearance at 17 weeks and 6 months. In this series of cases, cyclosporine was ineffective for CPAN.4 Two case reports documented cutaneous clearance with cyclophosphamide in conjunction with prednisolone.9,10 No prospective trials have been performed on these medications, and immunosuppressants should only be considered in steroid-resistant cases.
The use of intravenous immunoglobulin has been reported effective in prior cases that showed resistance to more conventional trials of steroids, azathioprine, and/or cyclophosphamide.12,14 Intravenous immunoglobulin may be regarded as a treatment option for severe resistant disease. Several case reports also have documented success using tumor necrosis factor α blockers, particularly infliximab, as an adjunct to steroids and etanercept as both a steroid adjunct and monotherapy.16,17,19 More studies are necessary to evaluate these treatments.
Additionally, single case reports have outlined the use of other therapeutic agents, including tamoxifen (10 mg twice daily increased to 20 mg twice daily during episodes of breakthrough lesions),20 hyperbaric oxygen therapy (100% oxygen for 90 minutes 5 times weekly at 1.5 atm absolute followed by 2 weeks of 2 atm absolute),21 and granulocyte-macrophage colony-stimulating factor (300 µg injection in small portion to ulcer edges twice monthly for 2 months).22 All of these treatments show promise, but data are limited.
Because thrombosis is postulated to be a potential mechanism leading to CPAN, agents such as pentoxifylline, clopidogrel, and warfarin have been examined as treatment options. Pentoxifylline in combination with mycophenolate mofetil has been successful in treating a case that was resistant to other immunosuppressants.23 Clopidogrel blocks the adenosine diphosphate pathway and impairs clot retraction. Clopidogrel was reported effective in an acute flare of CPAN for clearance of skin lesions and normalization of lupus anticoagulant.24 It also was used successfully in recurrent CPAN after steroid treatments in a patient with neuromuscular symptoms. There was no recurrence in either of the patients in this case report series. Warfarin therapy at an international normalized ratio of 3.0 also has demonstrated success in halting disease progression and in facilitating the resolution of skin changes and normalization of anti–phosphatidylserine-prothrombin complex antibodies.24 Our review of the literature did not reveal evidence of a standardized length of treatment following symptom resolution or if treatment is indicated in asymptomatic disease, or as in our case, with only mild elevations of antiphospholipid antibodies.
Conclusion
Multiple treatment options exist for CPAN, but the data on their efficacies is limited and based only on anecdotal evidence, not prospective analysis. We believe that it seems reasonable to initiate treatment only for symptomatic disease or cases in which the antibody titers suggest that the patient may be at high risk for thrombosis. Mild symptoms and mild cutaneous changes would suggest the likely choice of NSAIDs, colchicine, or dapsone as treatment options versus no treatment. In patients with antibody titers, pentoxifylline, clopidogrel, or warfarin may be considered first-line therapies. With severe ulcerative lesions and neuromuscular involvement, steroids, immunosuppressants, and other investigative agents should be contemplated. In our patient, the laboratory studies were repeated and normalized on complete resolution of her livedo eruption. She remained asymptomatic and clear for 8 months without any treatment. The incidence of this presentation of CPAN is unknown and is likely underreported, as we would not expect most patients to present to their physicians for the evaluation of otherwise asymptomatic livedo reticularis. In essence, our case report suggests that it may be prudent to simply monitor patients with asymptomatic CPAN.
- Lindberg K. Ein Beitrag zur Kenntnis der Periarteritis nodosa. Acta Med Scand. 1931;76:183-225.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa [published online August 26, 2005]. J Neurol. 2005;252:1155-1166.
- Nakamura T, Kanazawa N, Ikeda T, et al. Cutaneous polyarteritis nodosa: revisiting its definition and diagnostic criteria. Arch Dermatol Res. 2009;301:117-121.
- Rogalski C, Sticherling M. Panateritis cutanea benigna—an entity limited to the skin or cutaneous presentation of a systemic necrotizing vasculitis? report of seven cases and review of the literature. Int J Dermatol. 2007;46:817-821.
- Kawakami T, Yamazaki M, Mizoguchi M, et al. High titer of anti-phosphatidylserine-prothrombin complex antibodies in patients with cutaneous polyarteritis nodosa. Arthritis Rheum. 2007;57:1507-1513.
- Chen KR. Cutaneous polyarteritis nodosa: a clinical and histopathological study of 20 cases. J Dermatol. 1989;6:429-442.
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756.
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathologic analysis and review of the published work. J Dermatol. 2010;37:85-93.
- Flanagan N, Casey EB, Watson R, et al. Cutaneous polyartertitis nodosa with seronegative arthritis. Rheumatology (Oxford). 1999;38:1161-1162.
- Fathalla B, Miller L, Brady S, et al. Cutaneous polyarteritis nodosa in children. J Am Acad Dermatol. 2005;53:724-728.
- Misago N, Mochizuki Y, Sekiyama-Kodera H, et al. Cutaneous polyarteritis nodosa: therapy and clinical course in four cases. J Dermatol. 2001;28:719-727.
- Breda L, Franchini S, Marzetti V, et al. Intravenous immunoglobulins for cutaneous polyarteritis nodosa resistant to conventional treatment. Scand J Rheumatol. 2016;45:169-170.
- Maillard H, Szczesniak S, Martin L. Cutaneous periarteritis nodosa: diagnostic and therapeutic aspects of 9 cases. Ann Dermatol Venereol. 1999;26:125-129.
- Lobo I, Ferreira M, Silva E. Cutaneous polyarteritis nodosa treated with intravenous immunoglobulin. J Eur Acad Dermatol Venereol. 2007;22:880-882.
- Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000;136:167-169.
- Campanilho-Marques R, Ramos F, Canhão H, et al. Remission induced by infliximab in a childhood polyarteritis nodosa refractory to conventional immunosuppression and rituximab. Joint Bone Spine. 2014;81:277-278.
- Inoue N, Shimizu M, Mizuta M, et al. Refractory cutaneous polyarteritis nodosa: successful treatment with etanercept. Pediatr Int. 2017;59:751-752.
- Schartz NE. Successful treatment in two cases of steroid dependent cutaneous polyarteritis nodosa with low-dose methotrexate. Dermatology. 2001;203:336-338.
- Valor L, Monteagudo I, de la Torre I, et al. Young male patient diagnosed with cutaneous polyarteritis nodosa successfully treated with etanercept. Mod Rheumatol. 2014;24:688-689.
- Cvancara JL, Meffert JJ, Elston DM. Estrogen sensitive cutaneous polyarteritis nodosa: response to tamoxifen. J Am Acad Dermatol. 1998;39:643-646.
- Mazokopakis E, Milkas A, Tsartsalis A, et al. Improvement of cutaneous polyarteritis nodosa with hyperbaric oxygen. Int J Dermatol. 2009;48:1017-1029.
- Tursen U, Api H, Kaya TI, et al. Rapid healing of chronic leg ulcers during perilesional injections of granulocyte-macrophage colony stimulating factor in a patient with cutaneous polyarteritis nodosa. J Eur Acad Dermatol Venereol. 2006;20:1341-1343.
- Kluger N, Guillot B, Bessis D. Ulcerative cutaneous polyarteritis nodosa treated with mycophenolate mofetil and pentoxifylline. J Dermatolog Treat. 2011;22:175-177.
- Kawakami T, Soma Y. Use of warfarin therapy at a target international normalized ratio of 3.0 for cutaneous polyarteritis nodosa. J Am Acad Dermatol. 2010;63:602-606.
In 1931, Lindberg1 described a cutaneous variant of polyarteritis nodosa, which lacked visceral involvement and possessed a more favorable prognosis.2 Cutaneous polyarteritis nodosa (CPAN) is a localized small- to medium-vessel vasculitis restricted to the skin. Both benign and chronic courses have been described, and systemic involvement does not occur.3 Diagnostic criteria proposed by Nakamura et al3 in 2009 included cutaneous nodules, livedo reticularis, purpura, or ulcers; histopathologic fibrinoid necrotizing vasculitis of small- to medium-sized vessels; and exclusion of systemic symptoms (eg, fever, hypertension, weight loss, renal failure, cerebral hemorrhage, neuropathy, myocardial infarction, ischemic heart disease, pericarditis, pleuritis, arthralgia/myalgia). Nodules occur in 30% to 50% of cases and can remain for years if left untreated. Ulcerations occur in up to 30% of patients. Myositis, arthritis, and weakness also have been reported with this condition.4 Cutaneous polyarteritis nodosa has been associated with abnormal antibody testing with elevations of antiphospholipid cofactor antibody, lupus anticoagulant, anticardiolipin antibody, and anti-β2-glycoprotein I–dependent cardiolipin antibody, as well as elevated anti–phosphatidylserine-prothrombin complex antibody.5 These antibodies suggest increased risk for thrombosis and systemic diseases such as lupus or other autoimmune connective tissue disease. The distinction of this entity from systemic polyartertitis nodosa is key when determining treatment options and monitoring parameters.
Case Report
A 66-year-old woman was referred to our facility by an outside dermatologist with a mildly pruritic, blanchable, reticulated erythema on the chest and bilateral arms and legs of 3 months’ duration consistent with livedo reticularis (Figure 1). Prior systemic therapy included prednisone 10 mg 3 times daily, fexofenadine, loratadine, and hydroxyzine. When the systemic steroid was tapered, the patient developed an asymptomatic flare of her eruption. On presentation, the lesions had waxed and waned, and the patient was taking only vitamin B12 and vitamin C. Her medical history was notable for an unknown-type lymphoma of the chest wall diagnosed at 46 years of age that was treated with an unknown chemotherapeutic agent, chronic pancreatitis that resulted in a duodenectomy at 61 years of age, chronic cholecystitis, and 1 first-trimester miscarriage. Outside laboratory tests, including a comprehensive metabolic panel, complete blood cell count, urinalysis, renal function, and liver function tests were within reference range, except for the finding of mild leukocytosis (11,000/µL)(reference range, 3800–10,800/µL), which resolved after steroids were discontinued, with otherwise normal results. Punch biopsy of a specimen from the right thigh revealed medium-vessel vasculitis consistent with polyarteritis nodosa (Figure 2). Laboratory workup by our facility including hepatitis panel, perinuclear antineutrophil cytoplasmic antibody, cytoplasmic antineutrophil cytoplasmic antibody, factor V Leiden, prothrombin time/international normalized ratio, anticardiolipin antibody, and proteins C and S were all within reference range. Abnormal values included a low positive but nondiagnostic antinuclear antibody screen with negative titers, and the lupus anticoagulant titer was mildly elevated at 44 IgG binding units (reference range, <40 IgG binding units). Serum protein electrophoresis (SPEP) and urine protein electrophoresis also were performed, and SPEP was low positive for elevated κ and γ light chains. The patient was referred to oncology, and further testing revealed no underlying malignancy. The patient was monitored and no treatment was initiated; her rash completely resolved within 3 months. Laboratory monitoring at 6 months including SPEP, urine protein electrophoresis, lupus anticoagulant, and clotting studies all were within reference range.


Comment
Although the treatment of systemic polyarteritis nodosa often is necessary and typically involves high-dose corticosteroids and cyclophosphamide, the treatment of CPAN initially is less aggressive. Of the options available for treatment of CPAN, each has associated risks and side effects. Chen6 classified CPAN into 3 groups: 1 (mild), 2 (severe with no systemic involvement), and 3 (severe with progression to systemic disease)(Table). The authors performed a review of all the published treatments and their respective side effects to evaluate if treatment should be instituted for asymptomatic (group 1) disease presenting with abnormal antibody findings as demonstrated in our case.

First-line treatment of CPAN includes nonsteroidal anti-inflammatory drugs (NSAIDS) and colchicine.7 Nonsteroidal anti-inflammatory drugs are preferred; however, they also have been associated with gastrointestinal tract upset and increased risk for peptic ulcer disease with long-term use. Although colchicine often is used in conjunction with NSAIDS8 for its anti-inflammatory activity, no studies have been performed on this drug as monotherapy, and the side effect of diarrhea often limits its use.
Other therapies include dapsone, which should be monitored carefully due to the risk for dapsone hypersensitivity syndrome.8,9 Topical corticosteroids have been proven effective for mild cases of confluent erythema with remission occurring as early as 4 weeks.4 Some reports emphasize the role of streptococcal infections in CPAN, especially in children.8,10-12 Consequently it is recommended that anti–streptolysin O titers should be included in the workup for CPAN. Long-term penicillin prophylaxis and tonsillectomy have been used to prevent disease flares with limited success.8,10-12
For more severe disease, especially with neuromuscular involvement, oral methylprednisolone up to 1 mg/kg daily has been used and has proven effective in the control of acute exacerbations.7,13 However, the many adverse effects of systemic steroids limit their use long-term, and taper will often result in flare of disease.4,7 Medications used in conjunction with steroids include hydroxychloroquine, dapsone, azathioprine, cyclophosphamide, methotrexate, sulfapyridine, pentoxifylline, infliximab, etanercept, and intravenous immunoglobulin.4,9,12-17
Low-dose methotrexate has shown some improvement in skin disease with CPAN, but other case reports suggest that complete remission is not achieved with this drug.15,18 More studies are needed to assess the use of methotrexate for CPAN.
Immunomodulators have been used in multiple case reports with varying levels of success. Rogalski and Sticherling4 reported 3 cases that cleared with methylprednisolone plus azathioprine ranging from 4 weeks to 6 months; nausea limited tolerance of azathioprine in 1 case. Mycophenolate mofetil also was successfully used in 2 cases with clearance at 17 weeks and 6 months. In this series of cases, cyclosporine was ineffective for CPAN.4 Two case reports documented cutaneous clearance with cyclophosphamide in conjunction with prednisolone.9,10 No prospective trials have been performed on these medications, and immunosuppressants should only be considered in steroid-resistant cases.
The use of intravenous immunoglobulin has been reported effective in prior cases that showed resistance to more conventional trials of steroids, azathioprine, and/or cyclophosphamide.12,14 Intravenous immunoglobulin may be regarded as a treatment option for severe resistant disease. Several case reports also have documented success using tumor necrosis factor α blockers, particularly infliximab, as an adjunct to steroids and etanercept as both a steroid adjunct and monotherapy.16,17,19 More studies are necessary to evaluate these treatments.
Additionally, single case reports have outlined the use of other therapeutic agents, including tamoxifen (10 mg twice daily increased to 20 mg twice daily during episodes of breakthrough lesions),20 hyperbaric oxygen therapy (100% oxygen for 90 minutes 5 times weekly at 1.5 atm absolute followed by 2 weeks of 2 atm absolute),21 and granulocyte-macrophage colony-stimulating factor (300 µg injection in small portion to ulcer edges twice monthly for 2 months).22 All of these treatments show promise, but data are limited.
Because thrombosis is postulated to be a potential mechanism leading to CPAN, agents such as pentoxifylline, clopidogrel, and warfarin have been examined as treatment options. Pentoxifylline in combination with mycophenolate mofetil has been successful in treating a case that was resistant to other immunosuppressants.23 Clopidogrel blocks the adenosine diphosphate pathway and impairs clot retraction. Clopidogrel was reported effective in an acute flare of CPAN for clearance of skin lesions and normalization of lupus anticoagulant.24 It also was used successfully in recurrent CPAN after steroid treatments in a patient with neuromuscular symptoms. There was no recurrence in either of the patients in this case report series. Warfarin therapy at an international normalized ratio of 3.0 also has demonstrated success in halting disease progression and in facilitating the resolution of skin changes and normalization of anti–phosphatidylserine-prothrombin complex antibodies.24 Our review of the literature did not reveal evidence of a standardized length of treatment following symptom resolution or if treatment is indicated in asymptomatic disease, or as in our case, with only mild elevations of antiphospholipid antibodies.
Conclusion
Multiple treatment options exist for CPAN, but the data on their efficacies is limited and based only on anecdotal evidence, not prospective analysis. We believe that it seems reasonable to initiate treatment only for symptomatic disease or cases in which the antibody titers suggest that the patient may be at high risk for thrombosis. Mild symptoms and mild cutaneous changes would suggest the likely choice of NSAIDs, colchicine, or dapsone as treatment options versus no treatment. In patients with antibody titers, pentoxifylline, clopidogrel, or warfarin may be considered first-line therapies. With severe ulcerative lesions and neuromuscular involvement, steroids, immunosuppressants, and other investigative agents should be contemplated. In our patient, the laboratory studies were repeated and normalized on complete resolution of her livedo eruption. She remained asymptomatic and clear for 8 months without any treatment. The incidence of this presentation of CPAN is unknown and is likely underreported, as we would not expect most patients to present to their physicians for the evaluation of otherwise asymptomatic livedo reticularis. In essence, our case report suggests that it may be prudent to simply monitor patients with asymptomatic CPAN.
In 1931, Lindberg1 described a cutaneous variant of polyarteritis nodosa, which lacked visceral involvement and possessed a more favorable prognosis.2 Cutaneous polyarteritis nodosa (CPAN) is a localized small- to medium-vessel vasculitis restricted to the skin. Both benign and chronic courses have been described, and systemic involvement does not occur.3 Diagnostic criteria proposed by Nakamura et al3 in 2009 included cutaneous nodules, livedo reticularis, purpura, or ulcers; histopathologic fibrinoid necrotizing vasculitis of small- to medium-sized vessels; and exclusion of systemic symptoms (eg, fever, hypertension, weight loss, renal failure, cerebral hemorrhage, neuropathy, myocardial infarction, ischemic heart disease, pericarditis, pleuritis, arthralgia/myalgia). Nodules occur in 30% to 50% of cases and can remain for years if left untreated. Ulcerations occur in up to 30% of patients. Myositis, arthritis, and weakness also have been reported with this condition.4 Cutaneous polyarteritis nodosa has been associated with abnormal antibody testing with elevations of antiphospholipid cofactor antibody, lupus anticoagulant, anticardiolipin antibody, and anti-β2-glycoprotein I–dependent cardiolipin antibody, as well as elevated anti–phosphatidylserine-prothrombin complex antibody.5 These antibodies suggest increased risk for thrombosis and systemic diseases such as lupus or other autoimmune connective tissue disease. The distinction of this entity from systemic polyartertitis nodosa is key when determining treatment options and monitoring parameters.
Case Report
A 66-year-old woman was referred to our facility by an outside dermatologist with a mildly pruritic, blanchable, reticulated erythema on the chest and bilateral arms and legs of 3 months’ duration consistent with livedo reticularis (Figure 1). Prior systemic therapy included prednisone 10 mg 3 times daily, fexofenadine, loratadine, and hydroxyzine. When the systemic steroid was tapered, the patient developed an asymptomatic flare of her eruption. On presentation, the lesions had waxed and waned, and the patient was taking only vitamin B12 and vitamin C. Her medical history was notable for an unknown-type lymphoma of the chest wall diagnosed at 46 years of age that was treated with an unknown chemotherapeutic agent, chronic pancreatitis that resulted in a duodenectomy at 61 years of age, chronic cholecystitis, and 1 first-trimester miscarriage. Outside laboratory tests, including a comprehensive metabolic panel, complete blood cell count, urinalysis, renal function, and liver function tests were within reference range, except for the finding of mild leukocytosis (11,000/µL)(reference range, 3800–10,800/µL), which resolved after steroids were discontinued, with otherwise normal results. Punch biopsy of a specimen from the right thigh revealed medium-vessel vasculitis consistent with polyarteritis nodosa (Figure 2). Laboratory workup by our facility including hepatitis panel, perinuclear antineutrophil cytoplasmic antibody, cytoplasmic antineutrophil cytoplasmic antibody, factor V Leiden, prothrombin time/international normalized ratio, anticardiolipin antibody, and proteins C and S were all within reference range. Abnormal values included a low positive but nondiagnostic antinuclear antibody screen with negative titers, and the lupus anticoagulant titer was mildly elevated at 44 IgG binding units (reference range, <40 IgG binding units). Serum protein electrophoresis (SPEP) and urine protein electrophoresis also were performed, and SPEP was low positive for elevated κ and γ light chains. The patient was referred to oncology, and further testing revealed no underlying malignancy. The patient was monitored and no treatment was initiated; her rash completely resolved within 3 months. Laboratory monitoring at 6 months including SPEP, urine protein electrophoresis, lupus anticoagulant, and clotting studies all were within reference range.


Comment
Although the treatment of systemic polyarteritis nodosa often is necessary and typically involves high-dose corticosteroids and cyclophosphamide, the treatment of CPAN initially is less aggressive. Of the options available for treatment of CPAN, each has associated risks and side effects. Chen6 classified CPAN into 3 groups: 1 (mild), 2 (severe with no systemic involvement), and 3 (severe with progression to systemic disease)(Table). The authors performed a review of all the published treatments and their respective side effects to evaluate if treatment should be instituted for asymptomatic (group 1) disease presenting with abnormal antibody findings as demonstrated in our case.

First-line treatment of CPAN includes nonsteroidal anti-inflammatory drugs (NSAIDS) and colchicine.7 Nonsteroidal anti-inflammatory drugs are preferred; however, they also have been associated with gastrointestinal tract upset and increased risk for peptic ulcer disease with long-term use. Although colchicine often is used in conjunction with NSAIDS8 for its anti-inflammatory activity, no studies have been performed on this drug as monotherapy, and the side effect of diarrhea often limits its use.
Other therapies include dapsone, which should be monitored carefully due to the risk for dapsone hypersensitivity syndrome.8,9 Topical corticosteroids have been proven effective for mild cases of confluent erythema with remission occurring as early as 4 weeks.4 Some reports emphasize the role of streptococcal infections in CPAN, especially in children.8,10-12 Consequently it is recommended that anti–streptolysin O titers should be included in the workup for CPAN. Long-term penicillin prophylaxis and tonsillectomy have been used to prevent disease flares with limited success.8,10-12
For more severe disease, especially with neuromuscular involvement, oral methylprednisolone up to 1 mg/kg daily has been used and has proven effective in the control of acute exacerbations.7,13 However, the many adverse effects of systemic steroids limit their use long-term, and taper will often result in flare of disease.4,7 Medications used in conjunction with steroids include hydroxychloroquine, dapsone, azathioprine, cyclophosphamide, methotrexate, sulfapyridine, pentoxifylline, infliximab, etanercept, and intravenous immunoglobulin.4,9,12-17
Low-dose methotrexate has shown some improvement in skin disease with CPAN, but other case reports suggest that complete remission is not achieved with this drug.15,18 More studies are needed to assess the use of methotrexate for CPAN.
Immunomodulators have been used in multiple case reports with varying levels of success. Rogalski and Sticherling4 reported 3 cases that cleared with methylprednisolone plus azathioprine ranging from 4 weeks to 6 months; nausea limited tolerance of azathioprine in 1 case. Mycophenolate mofetil also was successfully used in 2 cases with clearance at 17 weeks and 6 months. In this series of cases, cyclosporine was ineffective for CPAN.4 Two case reports documented cutaneous clearance with cyclophosphamide in conjunction with prednisolone.9,10 No prospective trials have been performed on these medications, and immunosuppressants should only be considered in steroid-resistant cases.
The use of intravenous immunoglobulin has been reported effective in prior cases that showed resistance to more conventional trials of steroids, azathioprine, and/or cyclophosphamide.12,14 Intravenous immunoglobulin may be regarded as a treatment option for severe resistant disease. Several case reports also have documented success using tumor necrosis factor α blockers, particularly infliximab, as an adjunct to steroids and etanercept as both a steroid adjunct and monotherapy.16,17,19 More studies are necessary to evaluate these treatments.
Additionally, single case reports have outlined the use of other therapeutic agents, including tamoxifen (10 mg twice daily increased to 20 mg twice daily during episodes of breakthrough lesions),20 hyperbaric oxygen therapy (100% oxygen for 90 minutes 5 times weekly at 1.5 atm absolute followed by 2 weeks of 2 atm absolute),21 and granulocyte-macrophage colony-stimulating factor (300 µg injection in small portion to ulcer edges twice monthly for 2 months).22 All of these treatments show promise, but data are limited.
Because thrombosis is postulated to be a potential mechanism leading to CPAN, agents such as pentoxifylline, clopidogrel, and warfarin have been examined as treatment options. Pentoxifylline in combination with mycophenolate mofetil has been successful in treating a case that was resistant to other immunosuppressants.23 Clopidogrel blocks the adenosine diphosphate pathway and impairs clot retraction. Clopidogrel was reported effective in an acute flare of CPAN for clearance of skin lesions and normalization of lupus anticoagulant.24 It also was used successfully in recurrent CPAN after steroid treatments in a patient with neuromuscular symptoms. There was no recurrence in either of the patients in this case report series. Warfarin therapy at an international normalized ratio of 3.0 also has demonstrated success in halting disease progression and in facilitating the resolution of skin changes and normalization of anti–phosphatidylserine-prothrombin complex antibodies.24 Our review of the literature did not reveal evidence of a standardized length of treatment following symptom resolution or if treatment is indicated in asymptomatic disease, or as in our case, with only mild elevations of antiphospholipid antibodies.
Conclusion
Multiple treatment options exist for CPAN, but the data on their efficacies is limited and based only on anecdotal evidence, not prospective analysis. We believe that it seems reasonable to initiate treatment only for symptomatic disease or cases in which the antibody titers suggest that the patient may be at high risk for thrombosis. Mild symptoms and mild cutaneous changes would suggest the likely choice of NSAIDs, colchicine, or dapsone as treatment options versus no treatment. In patients with antibody titers, pentoxifylline, clopidogrel, or warfarin may be considered first-line therapies. With severe ulcerative lesions and neuromuscular involvement, steroids, immunosuppressants, and other investigative agents should be contemplated. In our patient, the laboratory studies were repeated and normalized on complete resolution of her livedo eruption. She remained asymptomatic and clear for 8 months without any treatment. The incidence of this presentation of CPAN is unknown and is likely underreported, as we would not expect most patients to present to their physicians for the evaluation of otherwise asymptomatic livedo reticularis. In essence, our case report suggests that it may be prudent to simply monitor patients with asymptomatic CPAN.
- Lindberg K. Ein Beitrag zur Kenntnis der Periarteritis nodosa. Acta Med Scand. 1931;76:183-225.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa [published online August 26, 2005]. J Neurol. 2005;252:1155-1166.
- Nakamura T, Kanazawa N, Ikeda T, et al. Cutaneous polyarteritis nodosa: revisiting its definition and diagnostic criteria. Arch Dermatol Res. 2009;301:117-121.
- Rogalski C, Sticherling M. Panateritis cutanea benigna—an entity limited to the skin or cutaneous presentation of a systemic necrotizing vasculitis? report of seven cases and review of the literature. Int J Dermatol. 2007;46:817-821.
- Kawakami T, Yamazaki M, Mizoguchi M, et al. High titer of anti-phosphatidylserine-prothrombin complex antibodies in patients with cutaneous polyarteritis nodosa. Arthritis Rheum. 2007;57:1507-1513.
- Chen KR. Cutaneous polyarteritis nodosa: a clinical and histopathological study of 20 cases. J Dermatol. 1989;6:429-442.
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756.
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathologic analysis and review of the published work. J Dermatol. 2010;37:85-93.
- Flanagan N, Casey EB, Watson R, et al. Cutaneous polyartertitis nodosa with seronegative arthritis. Rheumatology (Oxford). 1999;38:1161-1162.
- Fathalla B, Miller L, Brady S, et al. Cutaneous polyarteritis nodosa in children. J Am Acad Dermatol. 2005;53:724-728.
- Misago N, Mochizuki Y, Sekiyama-Kodera H, et al. Cutaneous polyarteritis nodosa: therapy and clinical course in four cases. J Dermatol. 2001;28:719-727.
- Breda L, Franchini S, Marzetti V, et al. Intravenous immunoglobulins for cutaneous polyarteritis nodosa resistant to conventional treatment. Scand J Rheumatol. 2016;45:169-170.
- Maillard H, Szczesniak S, Martin L. Cutaneous periarteritis nodosa: diagnostic and therapeutic aspects of 9 cases. Ann Dermatol Venereol. 1999;26:125-129.
- Lobo I, Ferreira M, Silva E. Cutaneous polyarteritis nodosa treated with intravenous immunoglobulin. J Eur Acad Dermatol Venereol. 2007;22:880-882.
- Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000;136:167-169.
- Campanilho-Marques R, Ramos F, Canhão H, et al. Remission induced by infliximab in a childhood polyarteritis nodosa refractory to conventional immunosuppression and rituximab. Joint Bone Spine. 2014;81:277-278.
- Inoue N, Shimizu M, Mizuta M, et al. Refractory cutaneous polyarteritis nodosa: successful treatment with etanercept. Pediatr Int. 2017;59:751-752.
- Schartz NE. Successful treatment in two cases of steroid dependent cutaneous polyarteritis nodosa with low-dose methotrexate. Dermatology. 2001;203:336-338.
- Valor L, Monteagudo I, de la Torre I, et al. Young male patient diagnosed with cutaneous polyarteritis nodosa successfully treated with etanercept. Mod Rheumatol. 2014;24:688-689.
- Cvancara JL, Meffert JJ, Elston DM. Estrogen sensitive cutaneous polyarteritis nodosa: response to tamoxifen. J Am Acad Dermatol. 1998;39:643-646.
- Mazokopakis E, Milkas A, Tsartsalis A, et al. Improvement of cutaneous polyarteritis nodosa with hyperbaric oxygen. Int J Dermatol. 2009;48:1017-1029.
- Tursen U, Api H, Kaya TI, et al. Rapid healing of chronic leg ulcers during perilesional injections of granulocyte-macrophage colony stimulating factor in a patient with cutaneous polyarteritis nodosa. J Eur Acad Dermatol Venereol. 2006;20:1341-1343.
- Kluger N, Guillot B, Bessis D. Ulcerative cutaneous polyarteritis nodosa treated with mycophenolate mofetil and pentoxifylline. J Dermatolog Treat. 2011;22:175-177.
- Kawakami T, Soma Y. Use of warfarin therapy at a target international normalized ratio of 3.0 for cutaneous polyarteritis nodosa. J Am Acad Dermatol. 2010;63:602-606.
- Lindberg K. Ein Beitrag zur Kenntnis der Periarteritis nodosa. Acta Med Scand. 1931;76:183-225.
- Kraemer M, Linden D, Berlit P. The spectrum of differential diagnosis in neurological patients with livedo reticularis and livedo racemosa [published online August 26, 2005]. J Neurol. 2005;252:1155-1166.
- Nakamura T, Kanazawa N, Ikeda T, et al. Cutaneous polyarteritis nodosa: revisiting its definition and diagnostic criteria. Arch Dermatol Res. 2009;301:117-121.
- Rogalski C, Sticherling M. Panateritis cutanea benigna—an entity limited to the skin or cutaneous presentation of a systemic necrotizing vasculitis? report of seven cases and review of the literature. Int J Dermatol. 2007;46:817-821.
- Kawakami T, Yamazaki M, Mizoguchi M, et al. High titer of anti-phosphatidylserine-prothrombin complex antibodies in patients with cutaneous polyarteritis nodosa. Arthritis Rheum. 2007;57:1507-1513.
- Chen KR. Cutaneous polyarteritis nodosa: a clinical and histopathological study of 20 cases. J Dermatol. 1989;6:429-442.
- Morgan AJ, Schwartz RA. Cutaneous polyarteritis nodosa: a comprehensive review. Int J Dermatol. 2010;49:750-756.
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathologic analysis and review of the published work. J Dermatol. 2010;37:85-93.
- Flanagan N, Casey EB, Watson R, et al. Cutaneous polyartertitis nodosa with seronegative arthritis. Rheumatology (Oxford). 1999;38:1161-1162.
- Fathalla B, Miller L, Brady S, et al. Cutaneous polyarteritis nodosa in children. J Am Acad Dermatol. 2005;53:724-728.
- Misago N, Mochizuki Y, Sekiyama-Kodera H, et al. Cutaneous polyarteritis nodosa: therapy and clinical course in four cases. J Dermatol. 2001;28:719-727.
- Breda L, Franchini S, Marzetti V, et al. Intravenous immunoglobulins for cutaneous polyarteritis nodosa resistant to conventional treatment. Scand J Rheumatol. 2016;45:169-170.
- Maillard H, Szczesniak S, Martin L. Cutaneous periarteritis nodosa: diagnostic and therapeutic aspects of 9 cases. Ann Dermatol Venereol. 1999;26:125-129.
- Lobo I, Ferreira M, Silva E. Cutaneous polyarteritis nodosa treated with intravenous immunoglobulin. J Eur Acad Dermatol Venereol. 2007;22:880-882.
- Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000;136:167-169.
- Campanilho-Marques R, Ramos F, Canhão H, et al. Remission induced by infliximab in a childhood polyarteritis nodosa refractory to conventional immunosuppression and rituximab. Joint Bone Spine. 2014;81:277-278.
- Inoue N, Shimizu M, Mizuta M, et al. Refractory cutaneous polyarteritis nodosa: successful treatment with etanercept. Pediatr Int. 2017;59:751-752.
- Schartz NE. Successful treatment in two cases of steroid dependent cutaneous polyarteritis nodosa with low-dose methotrexate. Dermatology. 2001;203:336-338.
- Valor L, Monteagudo I, de la Torre I, et al. Young male patient diagnosed with cutaneous polyarteritis nodosa successfully treated with etanercept. Mod Rheumatol. 2014;24:688-689.
- Cvancara JL, Meffert JJ, Elston DM. Estrogen sensitive cutaneous polyarteritis nodosa: response to tamoxifen. J Am Acad Dermatol. 1998;39:643-646.
- Mazokopakis E, Milkas A, Tsartsalis A, et al. Improvement of cutaneous polyarteritis nodosa with hyperbaric oxygen. Int J Dermatol. 2009;48:1017-1029.
- Tursen U, Api H, Kaya TI, et al. Rapid healing of chronic leg ulcers during perilesional injections of granulocyte-macrophage colony stimulating factor in a patient with cutaneous polyarteritis nodosa. J Eur Acad Dermatol Venereol. 2006;20:1341-1343.
- Kluger N, Guillot B, Bessis D. Ulcerative cutaneous polyarteritis nodosa treated with mycophenolate mofetil and pentoxifylline. J Dermatolog Treat. 2011;22:175-177.
- Kawakami T, Soma Y. Use of warfarin therapy at a target international normalized ratio of 3.0 for cutaneous polyarteritis nodosa. J Am Acad Dermatol. 2010;63:602-606.
Practice Points
- Cutaneous polyarteritis nodosa should be in the differential of new-onset livedo reticularis.
- Workup with biopsy and specific blood work is important.
- Treatment options at this time are limited.
Necrotic Ulcer on the Thigh
The Diagnosis: Disseminated Cryptococcosis
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.
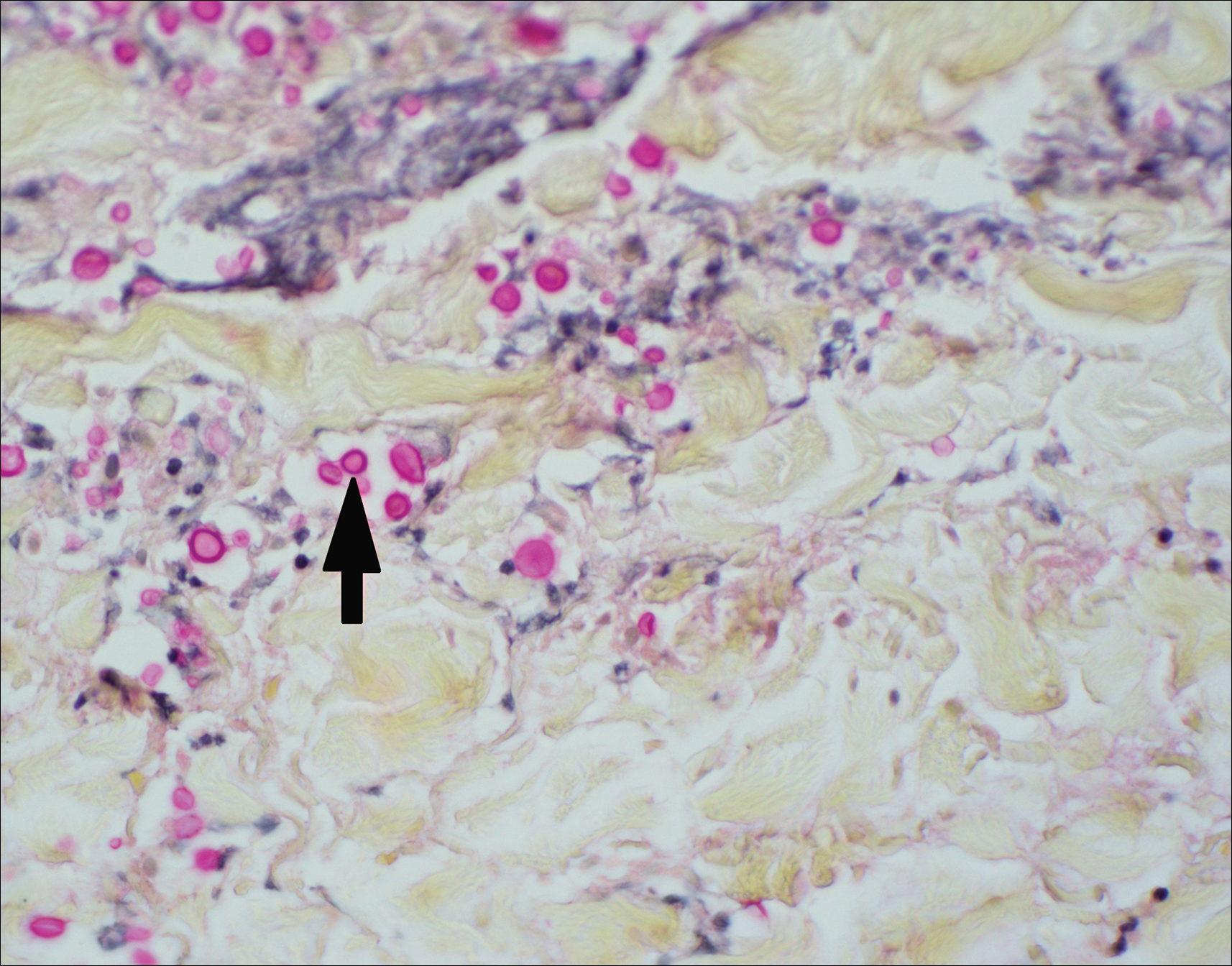
Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
The Diagnosis: Disseminated Cryptococcosis
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.

Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
The Diagnosis: Disseminated Cryptococcosis
Histopathologic examination of a 3-mm punch biopsy showed a diffuse dermal neutrophilic infiltrate with necrosis and subcutaneous tissue with round yeast surrounded by a prominent halo staining bright red with mucicarmine, representing a thick mucinous capsule (Figure). Grocott-Gomori methenamine-silver and periodic acid-Schiff stains also demonstrated fungal spores morphologically. Cerebrospinal fluid culture grew Cryptococcus neoformans, and cryptococcal antigen titers were positive in both serum and cerebrospinal fluid samples (>1:4096). The patient had autolytic debridement of the ulcer after completing a 4-week induction course of intravenous liposomal amphotericin B with oral flucytosine. He was transitioned to oral fluconazole for the consolidation phase of treatment.

Cryptococcus is an opportunistic basidiomycetous yeast with worldwide distribution and 2 primary pathogenic species in humans: C neoformans and Cryptococcus gattii. It is associated with bird feces, composted food, and decayed wood.1,2 A predilection toward an immunosuppressed host is recognized in 70% to 90% of the infections caused by C neoformans; however, C gattii commonly affects individuals with apparently intact immune systems.1,3 Risk factors for infection include advanced human immunodeficiency virus infection, solid organ transplantation, chronic liver disease, autoimmune disease, hematological malignancy, and underlying genetic susceptibility.1,2
Initial exposure is through the respiratory tract with formation of latent reservoirs in the pulmonary lymph nodes with subsequent reactivation that can result in hematogenous dissemination.1,2 Cutaneous involvement was described in 108 patients (5%) in a large review of 1974 cases in France.4 Among those with cutaneous involvement, disseminated disease was diagnosed in 80 cases (74%), and 28 cases (26%) were considered primary cutaneous cryptococcosis. Primary cutaneous cryptococcosis typically presents as a single lesion, predominantly on the hand, with whitlow and more rarely with extensive cellulitis or necrotizing fasciitis.4 In disseminated cutaneous disease, there is no pathognomonic single lesion; however, it is commonly associated with multiple cutaneous lesions predominantly involving the head and neck. Plaques, abscesses, nodules, and pustular or umbilicated papules have been reported.1,5 There are few case reports that describe a single isolated necrotic ulcer with disseminated disease similar to our presented case, and more typically the necrotic ulcer is seen in transplanted patients.6 The differential diagnosis of a necrotic thigh ulcer includes pseudomonal ecthyma gangrenosum, cutaneous anthrax and aspergillosis, fusariosis, and a bite from the brown recluse spider.7 Our patient had an increased susceptibility to infection from his ongoing chemotherapy, a risk previously described in oncology patients with cell-mediated immunosuppression.8
Management for disseminated cryptococcosis is a 3-phase therapy including induction with intravenous amphotericin B and oral flucytosine for a minimum of 2 weeks, with consolidation and maintenance phases both with oral fluconazole for a length depending on underlying immunosuppression.9
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
- Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980-1024.
- Williamson PR, Jarvis JN, Panackal AA, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis, and therapy [published online November 25, 2016]. Nat Rev Neurol. 2017;13:13-24.
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21:28-34.
- Neuville S, Dromer F, Morin O, et al; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity [published online January 17, 2003]. Clin Infect Dis. 2003;36:337-347.
- Murakawa GJ, Kerschmann R, Berger T. Cutaneous cryptococcus infection and AIDS: report of 12 cases and review of the literature. JAMA Dermatol. 1996;132:545-548.
- Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
- Grossman ME, Fox LP, Kovarik C, et al. Cutaneous Manifestations of Infection in the Immunocompromised Host. Baltimore, MD: Williams & Wilkins; 2012.
- Korfel A, Menssen HD, Schwartz S, et al. Cryptococcosis in Hodgkin's disease: description of two cases and review of the literature. Ann Hematol. 1998;76:283-286.
- Perfect JR, Dismukes WE, Dromer F. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
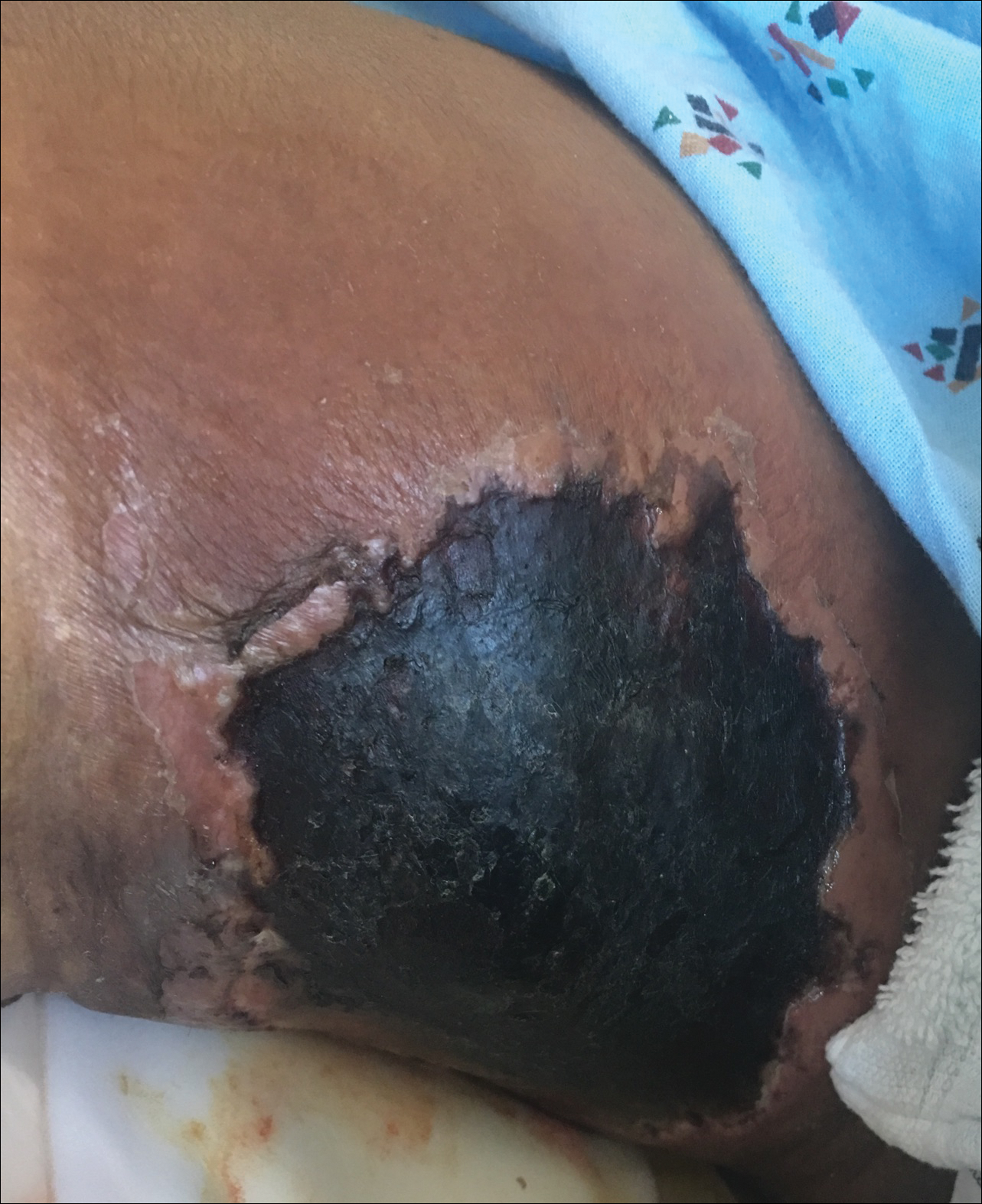
A 29-year-old man with a history of acute lymphoblastic leukemia was admitted for acute encephalopathy and a necrotic ulcer on the right thigh of 2 weeks' duration. He had received chemotherapy with pegaspargase and vincristine 6 weeks prior to admission. He reported headache with nausea and vomiting of 2 weeks' duration and had sustained a fall in the bathtub a week prior that initially resulted in a right thigh abrasion. He denied recent travel, unusual food consumption, animal exposure, exposure to sick persons, and alcohol or other drug use. On examination the patient was alert but was not oriented to person, place, or time. A 10.2 ×10-cm necrotic ulcer with surrounding mild erythema and tenderness was noted on the right inner thigh.
Wearable Health Device Dermatitis: A Case of Acrylate-Related Contact Allergy
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).
Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).



Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Practice Points
- Mobile wearable health devices are likely to become an important potential source of contact sensitization as their use increases given their often prolonged contact time with the skin.
- Mobile wearable health devices may pose a risk for allergic contact dermatitis as a result of a variety of components that come into contact with the skin, including but not limited to metals, rubber components, adhesives, and dyes.
What’s Eating You? Minute Brown Scavenger Beetle
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
Delusional infestation is the fixed false belief of skin infestation with a pathogen. Patients will often bring “proof” of their infestation to their visit to a physician. The presentation of a specimen was previously referred to by several names that reflected the receptacle that the patient utilized to bring the specimen (eg, a baggie or matchbox), but now the more encompassing term specimen sign is employed.1 Establishing rapport with the patient is critically important in the treatment of delusional infestation. Examining the specimen samples brought by the patient is a simple manner of communicating to a patient that the clinician is empathetic to and respectful of his/her concerns.2,3 The specimens often consist of dirt, dust, debris, fibers, and skin flakes and fragments, but they also have been reported to contain flies and insect parts.4,5 In our case, the patient captured a minute brown scavenger beetle with adhesive tape.
Case Report
A woman in her mid-30s with a history of generalized anxiety disorder presented to the dermatology clinic with a concern of bugs infesting her skin. The symptoms occurred just after she moved into a new home with her family approximately 4 months prior to presentation. She felt the home was not cleaned properly, but they could not afford to move. She reported a crawling sensation that she identified as bugs biting her all over her body. Prior to presentation in the dermatology clinic, she and her family were treated by primary care for scabies 3 times with permethrin cream, and she was prescribed 1 course of oral ivermectin. She reported seeing bugs all over her house, which led her to clean her home and clothing many times. She was more concerned now because she thought her 2 children also were starting to be affected.
Physical examination revealed pressured speech, and the patient became tearful several times. The skin demonstrated several excoriations in various stages of healing on the breasts, legs, and upper back, as well as small scars in the same distribution. She brought several specimens stuck to clear tape to the visit. Examination of the specimens revealed fabric fibers; various debris; and a small, brown, 6-legged beetle with punctate indentations in rows along the wing covers (Figure). The head was narrower than the thorax, which was narrower than the abdomen.

We diagnosed the patient with a delusional infestation and discussed the beetle that we saw when examining the specimen the patient brought to the clinic. We provided reassurance that the minute brown scavenger beetle is not pathogenic and was present incidentally. Thus far, the patient has been resistant to initiating specific therapy for the delusional infestation, such as risperidone, olanzapine, or pimozide. We co
Comment
Minute brown scavenger beetles are arthropod members of the family Latridiidae. They also are commonly referred to as plaster or mold beetles. They are small (0.8–3.0 mm) and can be found in moist environments such as dead and rotting foliage, bird’s nests, debris, moist wallpaper/plaster, and stored products. They feed exclusively on fungus, such as mold and mildew, and pose no threat to humans.6 It is important for clinicians to recognize the appearance of the minute brown scavenger beetle so as not to mistake it for a pathogenic arthropod in patients presenting with delusional parasitosis.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
- Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22:690-732.
- Heller MM, Wong JW, Lee ES, et al. Delusional infestations: clinical presentation, diagnosis and treatment. Int J Dermatol. 2013;52:775-783.
- Patel V, Koo JY. Delusions of parasitosis; suggested dialogue between dermatologist and patient. J Dermatolog Treat. 2015;26:456-460.
- Zomer SF, De Wit RF, Van Bronswijk JE, et al. Delusions of parasitosis. a psychiatric disorder to be treated by dermatologists? an analysis of 33 patients. Br J Dermatol. 1998;138:1030-1032.
- Freudenmann RW, Kölle M, Schönfeldt-Lecuona C, et al. Delusional parasitosis and the matchbox sign revisited: the international perspective. Acta Derm Venereol. 2010;90:517-519.
- Bousquet Y. Beetles Associated With Stored Products in Canada: An identification Guide. Ottawa, Canada: Canadian Governement Publishing Centre; 1990.
Practice Points
- Examining the specimens brought by a patient with delusional infestation is important for the therapeutic relationship.
- Clinicians must be able to recognize nonpathogenic insects that may incidentally be present in the specimen such as the minute brown scavenger beetle.
Successive Potassium Hydroxide Testing for Improved Diagnosis of Tinea Pedis
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).
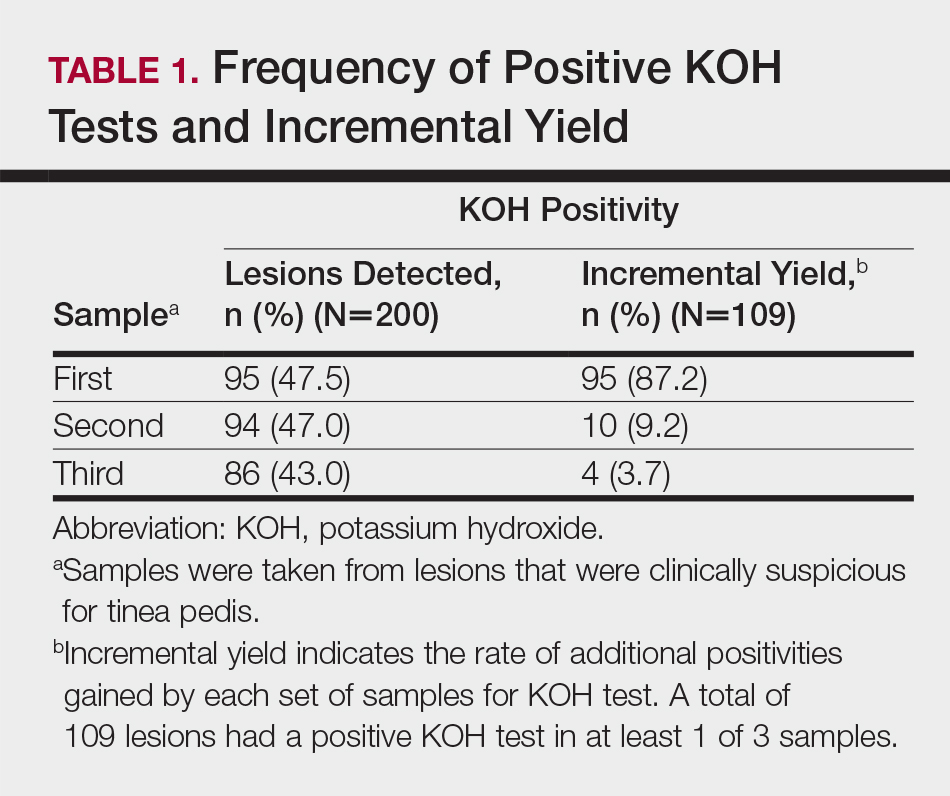
According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).
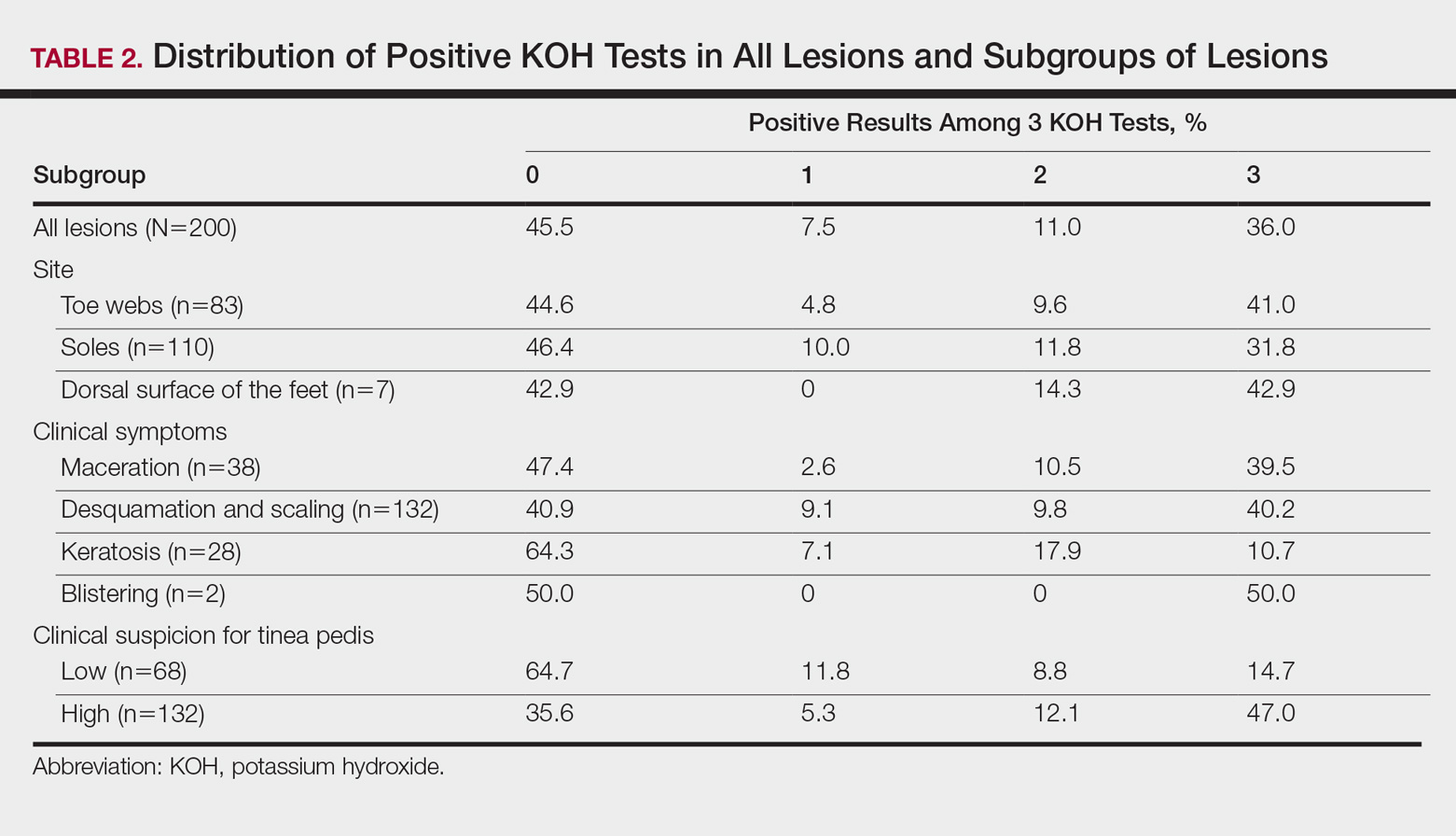
For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.
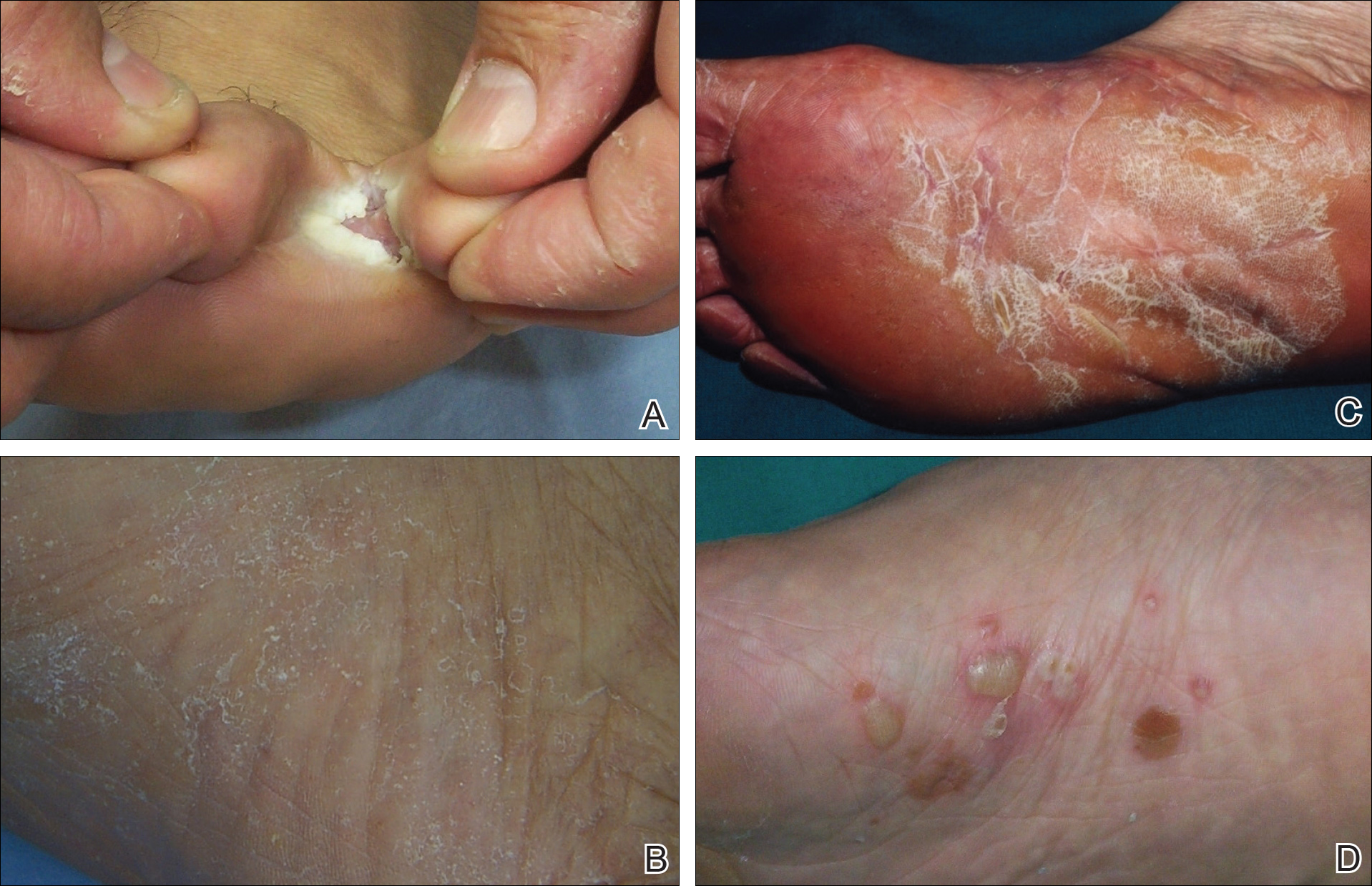
If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).

According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).

For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.

If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).

According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).

For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.

If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
Practice Points
- At least 2 samples should be taken for potassium hydroxide examination when tinea pedis is sus-pected clinically.
- The number of samples should be at least 3 if keratotic lesions are present.
Adalimumab for Hidradenitis Suppurativa
We applaud Kimball et al1 on their report that adalimumab demonstrated clinical improvement in patients with hidradenitis suppurativa (HS) versus placebo in 2 phase 3 trials. Hidradenitis suppurativa is a chronic relapsing condition with painful subcutaneous abscesses, malodorous drainage, sinus tract formation, and scarring that typically occurs in the axillae and anogenital region. It impairs the quality of life for these patients, as evidenced by higher Dermatology Life Quality Index scores compared to psoriasis, pimples, hand rash, atopic eczema, or control.2
The exact pathogenesis of HS is unknown but likely involves a complex interaction of genetic, hormonal, immunologic, and environmental factors.3 The levels of inflammatory cytokines are elevated in HS lesions, specifically IL-1β, tumor necrosis factor α, IL-10, and CXCL9, as well as monokines from IFN-γ, IL-11, and IL-17A. Additionally, the dermis of affected regions contains IL-12– and IL-23–containing macrophages along with IL-17–producing T cells.3 These findings reveal many potential therapeutic targets for the treatment of HS.
PIONEER I and PIONEER II are similarly designed 36-week phase 3 trials of 633 patients with HS who were unresponsive to oral antibiotic treatment.1 By week 12, a significantly greater proportion of patients receiving adalimumab demonstrated clinical improvement (≥50% reduction in total abscess and nodule count) compared to placebo in both trials (PIONEER I: 41.8% vs 26.0%, P=.003; PIONEER II: 58.9% vs 27.6%, P<.001). Secondary end points (inflammatory-nodule count, pain score, and disease severity) were only achieved in PIONEER II. The difference in clinical improvement between the trials is likely due to higher baseline disease severity in the HS patients in PIONEER I versus PIONEER II. No new safety risks were reported and were in accordance with prior adalimumab trials for other diseases. Notably, 10 paradoxical psoriasislike eruptions were reported.
Adalimumab is the first and only US Food and Drug Administration–approved therapy for HS. Further understanding of the pathogenesis of HS may result in additional biologic treatments for HS. We encourage the manufacturers of other biologic therapies, such as infliximab,4 ustekinumab,5 anakinra,6 secukinumab, ixekizumab, and brodalumab, to consider conducting further clinical trials in HS to enhance the therapeutic options available for this debilitating disease.
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Vinding GR, Knudsen KM, Ellervik C, et al. Self-reported skin morbidities and health-related quality of life: a population-based nested case-control study. Dermatology. 2014;228:261-268.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
We applaud Kimball et al1 on their report that adalimumab demonstrated clinical improvement in patients with hidradenitis suppurativa (HS) versus placebo in 2 phase 3 trials. Hidradenitis suppurativa is a chronic relapsing condition with painful subcutaneous abscesses, malodorous drainage, sinus tract formation, and scarring that typically occurs in the axillae and anogenital region. It impairs the quality of life for these patients, as evidenced by higher Dermatology Life Quality Index scores compared to psoriasis, pimples, hand rash, atopic eczema, or control.2
The exact pathogenesis of HS is unknown but likely involves a complex interaction of genetic, hormonal, immunologic, and environmental factors.3 The levels of inflammatory cytokines are elevated in HS lesions, specifically IL-1β, tumor necrosis factor α, IL-10, and CXCL9, as well as monokines from IFN-γ, IL-11, and IL-17A. Additionally, the dermis of affected regions contains IL-12– and IL-23–containing macrophages along with IL-17–producing T cells.3 These findings reveal many potential therapeutic targets for the treatment of HS.
PIONEER I and PIONEER II are similarly designed 36-week phase 3 trials of 633 patients with HS who were unresponsive to oral antibiotic treatment.1 By week 12, a significantly greater proportion of patients receiving adalimumab demonstrated clinical improvement (≥50% reduction in total abscess and nodule count) compared to placebo in both trials (PIONEER I: 41.8% vs 26.0%, P=.003; PIONEER II: 58.9% vs 27.6%, P<.001). Secondary end points (inflammatory-nodule count, pain score, and disease severity) were only achieved in PIONEER II. The difference in clinical improvement between the trials is likely due to higher baseline disease severity in the HS patients in PIONEER I versus PIONEER II. No new safety risks were reported and were in accordance with prior adalimumab trials for other diseases. Notably, 10 paradoxical psoriasislike eruptions were reported.
Adalimumab is the first and only US Food and Drug Administration–approved therapy for HS. Further understanding of the pathogenesis of HS may result in additional biologic treatments for HS. We encourage the manufacturers of other biologic therapies, such as infliximab,4 ustekinumab,5 anakinra,6 secukinumab, ixekizumab, and brodalumab, to consider conducting further clinical trials in HS to enhance the therapeutic options available for this debilitating disease.
We applaud Kimball et al1 on their report that adalimumab demonstrated clinical improvement in patients with hidradenitis suppurativa (HS) versus placebo in 2 phase 3 trials. Hidradenitis suppurativa is a chronic relapsing condition with painful subcutaneous abscesses, malodorous drainage, sinus tract formation, and scarring that typically occurs in the axillae and anogenital region. It impairs the quality of life for these patients, as evidenced by higher Dermatology Life Quality Index scores compared to psoriasis, pimples, hand rash, atopic eczema, or control.2
The exact pathogenesis of HS is unknown but likely involves a complex interaction of genetic, hormonal, immunologic, and environmental factors.3 The levels of inflammatory cytokines are elevated in HS lesions, specifically IL-1β, tumor necrosis factor α, IL-10, and CXCL9, as well as monokines from IFN-γ, IL-11, and IL-17A. Additionally, the dermis of affected regions contains IL-12– and IL-23–containing macrophages along with IL-17–producing T cells.3 These findings reveal many potential therapeutic targets for the treatment of HS.
PIONEER I and PIONEER II are similarly designed 36-week phase 3 trials of 633 patients with HS who were unresponsive to oral antibiotic treatment.1 By week 12, a significantly greater proportion of patients receiving adalimumab demonstrated clinical improvement (≥50% reduction in total abscess and nodule count) compared to placebo in both trials (PIONEER I: 41.8% vs 26.0%, P=.003; PIONEER II: 58.9% vs 27.6%, P<.001). Secondary end points (inflammatory-nodule count, pain score, and disease severity) were only achieved in PIONEER II. The difference in clinical improvement between the trials is likely due to higher baseline disease severity in the HS patients in PIONEER I versus PIONEER II. No new safety risks were reported and were in accordance with prior adalimumab trials for other diseases. Notably, 10 paradoxical psoriasislike eruptions were reported.
Adalimumab is the first and only US Food and Drug Administration–approved therapy for HS. Further understanding of the pathogenesis of HS may result in additional biologic treatments for HS. We encourage the manufacturers of other biologic therapies, such as infliximab,4 ustekinumab,5 anakinra,6 secukinumab, ixekizumab, and brodalumab, to consider conducting further clinical trials in HS to enhance the therapeutic options available for this debilitating disease.
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Vinding GR, Knudsen KM, Ellervik C, et al. Self-reported skin morbidities and health-related quality of life: a population-based nested case-control study. Dermatology. 2014;228:261-268.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Kimball AB, Okun MM, Williams DA, et al. Two Phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Vinding GR, Knudsen KM, Ellervik C, et al. Self-reported skin morbidities and health-related quality of life: a population-based nested case-control study. Dermatology. 2014;228:261-268.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol. 2016;174:970-978.
- Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
Product News: 08 2017
Avène A-OXitive
Pierre Fabre Dermo-Cosmetique introduces Avène A-OXitive Antioxidant Defense Serum and Avène A-OXitive Antioxidant Water-Cream to fight against oxidative stress. Stable forms of time-released vitamin E and vitamin C work synergistically to defend against environmental aggressors without irritation. Both products contain hyaluronic acid to visibly plump and firm the skin and soothing Avène thermal spring water to soften and restore the skin’s balance. To preserve stability, each formula comes in an airless pump bottle. For more information, visit www.aveneusa.com.
Orencia
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Orencia (abatacept) for the treatment of adults with active psoriatic arthritis. Orencia is available in both intravenous and subcutaneous injection formulations. It should not be administered concomitantly with tumor necrosis factor antagonists and is not recommended for use with other biologic rheumatoid arthritis therapy. Orencia also is indicated for the treatment of adult rheumatoid arthritis and juvenile idiopathic arthritis. For more information, visit www.orenciahcp.com.
Tremfya
Janssen Biotech, Inc, announces US Food and Drug Administration approval of Tremfya (guselkumab) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Tremfya is a biologic therapy that selectively blocks only IL-23, a cytokine that plays a key role in plaque psoriasis. Tremfya is administered as a 100-mg subcutaneous injection every 8 weeks, following 2 starter doses at weeks 0 and 4. Clinical studies documented skin clearance at week 16 and up to week 48. For more information, visit www.tremfyahcp.com.
If you would like your product included in Product News, please email a press release to the Edi
Avène A-OXitive
Pierre Fabre Dermo-Cosmetique introduces Avène A-OXitive Antioxidant Defense Serum and Avène A-OXitive Antioxidant Water-Cream to fight against oxidative stress. Stable forms of time-released vitamin E and vitamin C work synergistically to defend against environmental aggressors without irritation. Both products contain hyaluronic acid to visibly plump and firm the skin and soothing Avène thermal spring water to soften and restore the skin’s balance. To preserve stability, each formula comes in an airless pump bottle. For more information, visit www.aveneusa.com.
Orencia
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Orencia (abatacept) for the treatment of adults with active psoriatic arthritis. Orencia is available in both intravenous and subcutaneous injection formulations. It should not be administered concomitantly with tumor necrosis factor antagonists and is not recommended for use with other biologic rheumatoid arthritis therapy. Orencia also is indicated for the treatment of adult rheumatoid arthritis and juvenile idiopathic arthritis. For more information, visit www.orenciahcp.com.
Tremfya
Janssen Biotech, Inc, announces US Food and Drug Administration approval of Tremfya (guselkumab) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Tremfya is a biologic therapy that selectively blocks only IL-23, a cytokine that plays a key role in plaque psoriasis. Tremfya is administered as a 100-mg subcutaneous injection every 8 weeks, following 2 starter doses at weeks 0 and 4. Clinical studies documented skin clearance at week 16 and up to week 48. For more information, visit www.tremfyahcp.com.
If you would like your product included in Product News, please email a press release to the Edi
Avène A-OXitive
Pierre Fabre Dermo-Cosmetique introduces Avène A-OXitive Antioxidant Defense Serum and Avène A-OXitive Antioxidant Water-Cream to fight against oxidative stress. Stable forms of time-released vitamin E and vitamin C work synergistically to defend against environmental aggressors without irritation. Both products contain hyaluronic acid to visibly plump and firm the skin and soothing Avène thermal spring water to soften and restore the skin’s balance. To preserve stability, each formula comes in an airless pump bottle. For more information, visit www.aveneusa.com.
Orencia
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Orencia (abatacept) for the treatment of adults with active psoriatic arthritis. Orencia is available in both intravenous and subcutaneous injection formulations. It should not be administered concomitantly with tumor necrosis factor antagonists and is not recommended for use with other biologic rheumatoid arthritis therapy. Orencia also is indicated for the treatment of adult rheumatoid arthritis and juvenile idiopathic arthritis. For more information, visit www.orenciahcp.com.
Tremfya
Janssen Biotech, Inc, announces US Food and Drug Administration approval of Tremfya (guselkumab) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Tremfya is a biologic therapy that selectively blocks only IL-23, a cytokine that plays a key role in plaque psoriasis. Tremfya is administered as a 100-mg subcutaneous injection every 8 weeks, following 2 starter doses at weeks 0 and 4. Clinical studies documented skin clearance at week 16 and up to week 48. For more information, visit www.tremfyahcp.com.
If you would like your product included in Product News, please email a press release to the Edi