User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Metastatic Meningioma of the Scalp
Meningiomas generally present as slow-growing, expanding intracranial lesions and are the most common benign intracranial tumor in adults.1 Rarely, meningioma exhibits malignant potential and presents as an extracranial soft-tissue mass through extension or as a primary extracranial cutaneous neoplasm. The differential diagnosis of scalp neoplasms must be broadened to include uncommon tumors such as meningioma. We present a rare case of a 68-year-old woman with scalp metastasis of meningioma 11 years after initial resection of the primary tumor.
Case Report
A 68-year-old woman presented for evaluation of an asymptomatic nodule on the left parietal scalp of 2 years’ duration. She denied any headaches, difficulty with balance, vision changes, or changes in mentation. Her medical history was remarkable for a benign meningioma removed from the right parietal scalp 11 years prior without radiation therapy, as well as type 2 diabetes mellitus and arthritis. The patient’s son died from a brain tumor, but the exact tumor type and age at the time of death were unknown. Her current medications included metformin, insulin glargine, aspirin, and a daily multivitamin. She denied any allergies or history of smoking.
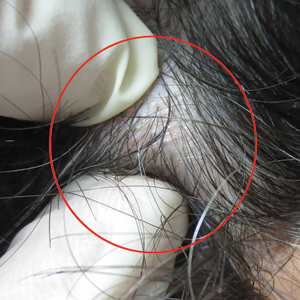
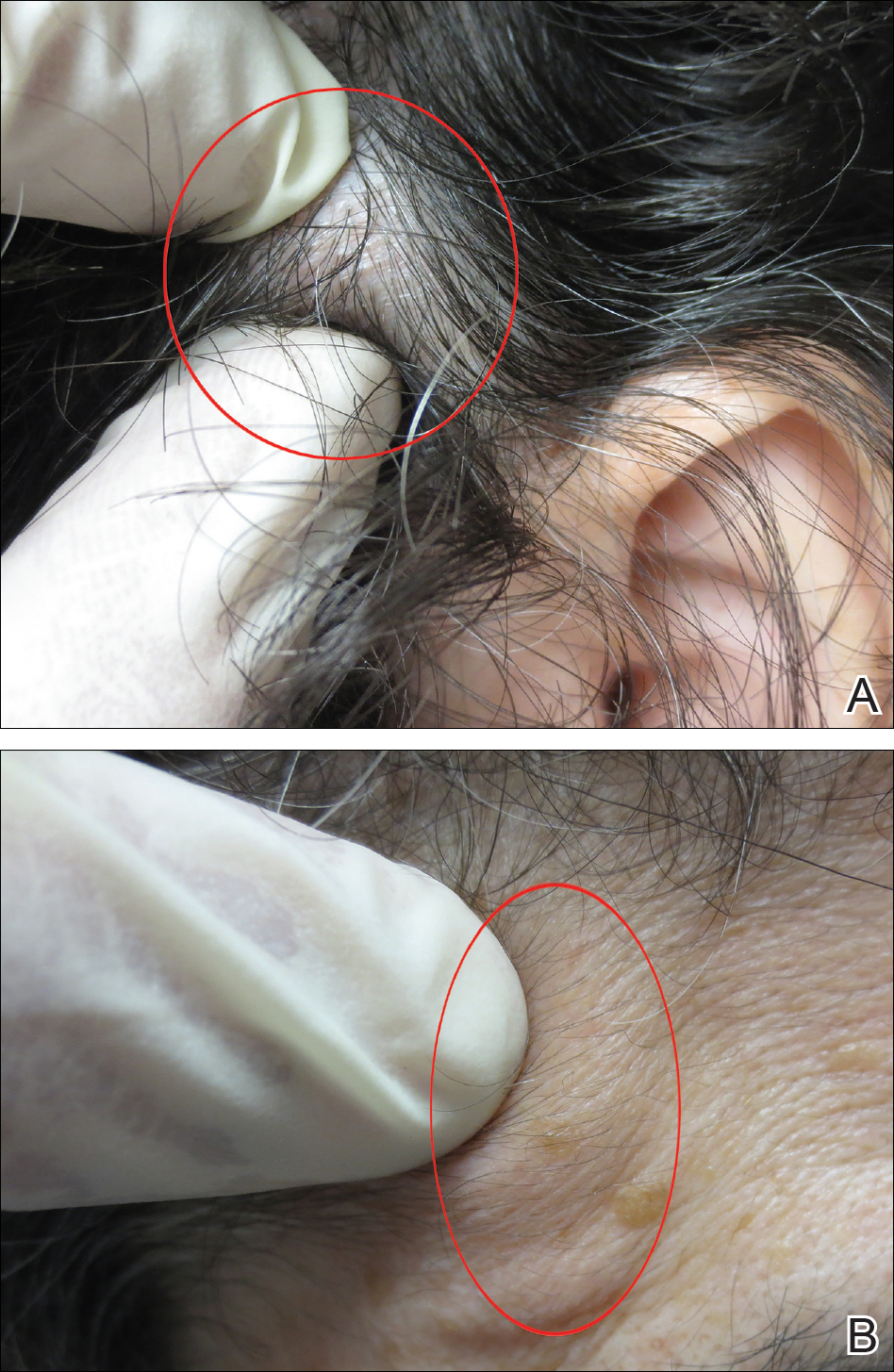
Physical examination of the scalp revealed 4 fixed, nontender, flesh-colored nodules: 2 on the left parietal scalp measuring 3.0 cm and 0.8 cm, respectively (Figure 1A); a 0.4-cm nodule on the right posterior occipital scalp; and a 1.6-cm sausage-shaped nodule on the right temple (Figure 1B). No positive lymph nodes were appreciated, and no additional lesions were noted. No additional atypical lesions were noted on full cutaneous examination.
A diagnostic 6-mm punch biopsy of the largest nodule was performed. Intraoperatively, there was no apparent cyst wall, but coiled, loose, stringlike, pink-yellow tissue was removed from the base of the wound before closing with sutures.
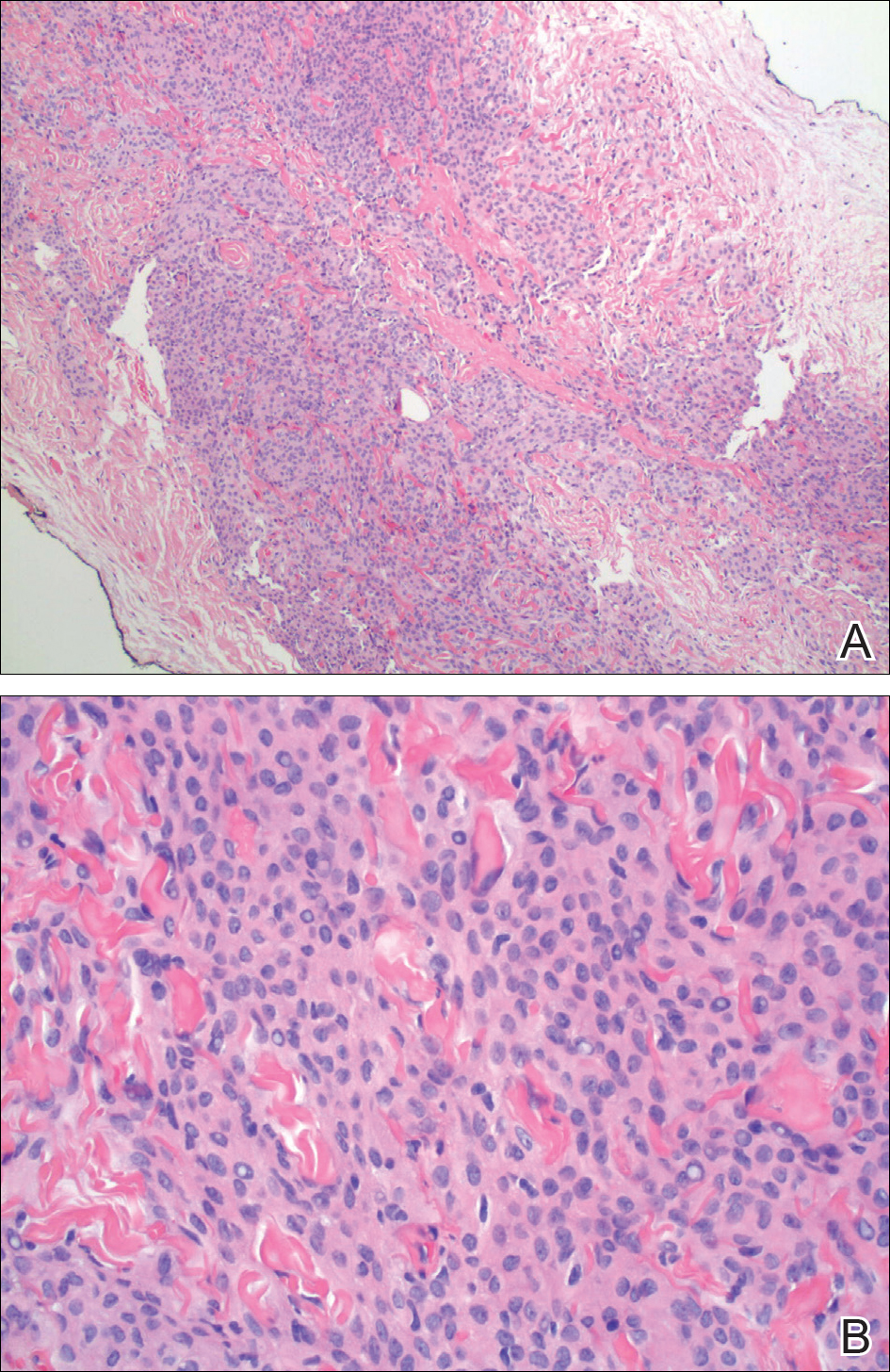
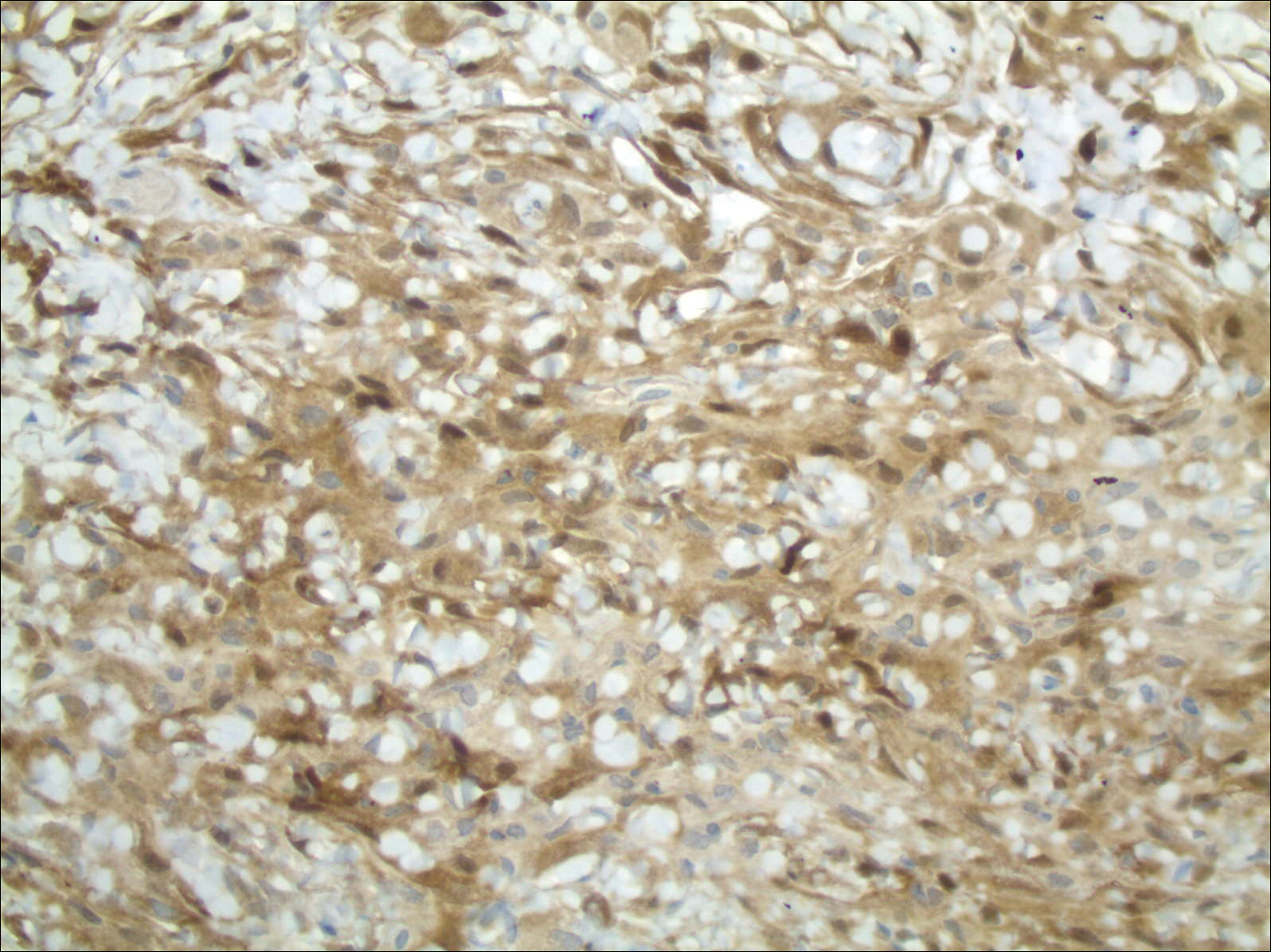
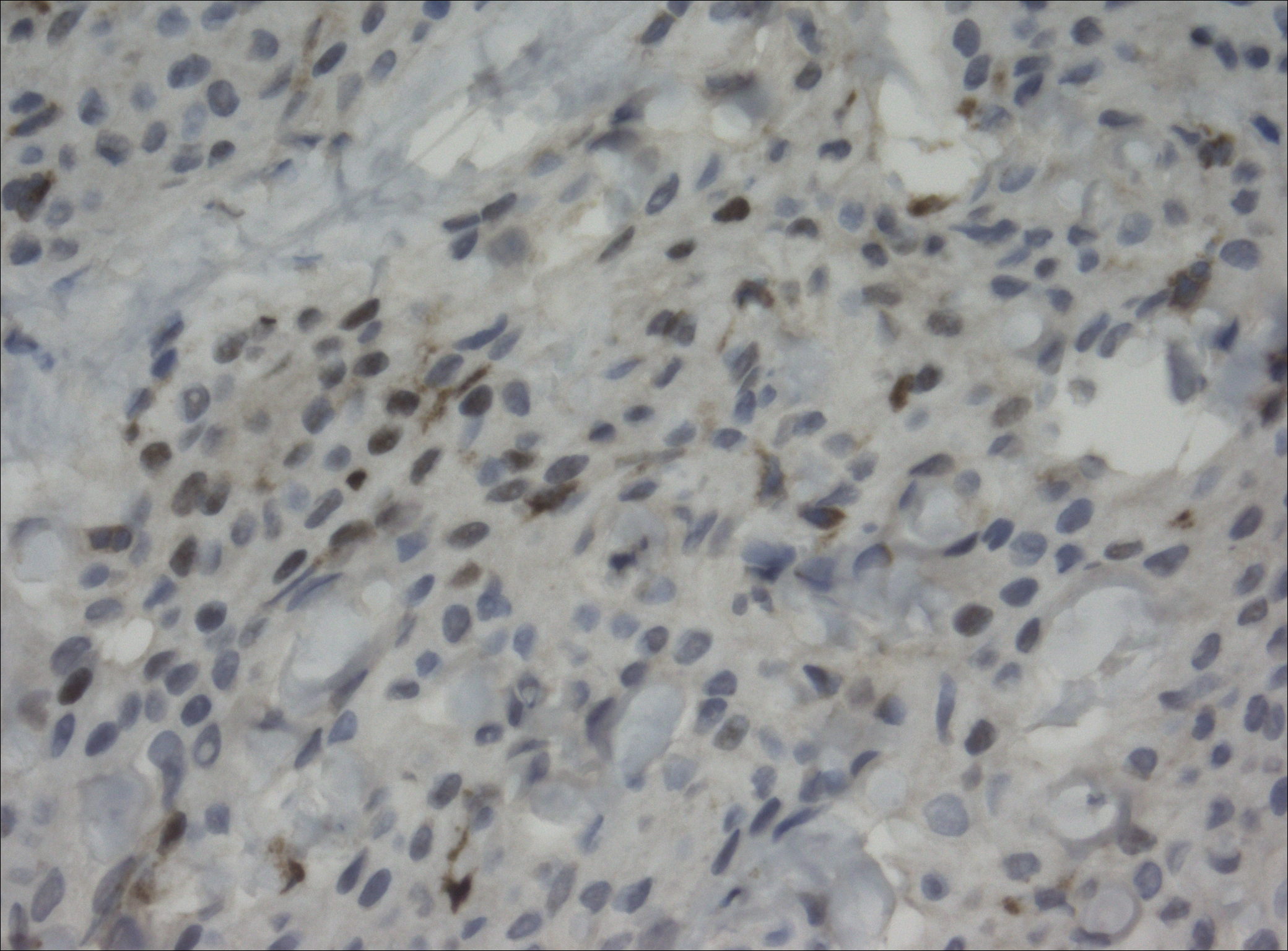
The primary histologic finding was cells within fibrous tissue containing delicate round-oval nuclei, inconspicuous nucleoli, and lightly eosinophilic cytoplasm with an indistinct border (Figure 2). Immunohistochemical studies for S100 protein were focal and limited to the cytoplasm of a subset of neoplastic cells (Figure 3). Tumor cells stained positive for epithelial membrane antigen (EMA) and were focally positive for progesterone receptor (Figure 4). Tumor cells were negative for CD31 and CD34. Based on the clinical and histologic findings, a diagnosis of metastatic meningioma of the scalp was made.
Magnetic resonance imaging and positron emission tomography of the head, neck, and chest demonstrated 3 residual subcutaneous nodules on the scalp and an indeterminate subcentimeter nodule in the right lung. The 0.4-cm nodule on the right posterior occipital scalp was removed without complication, and no radiation therapy was administered. The rest of the lesions were monitored. She remained under the close observation of a neurosurgeon and underwent repeat imaging of the scalp nodules and lungs, initially at 3 months and then routinely at the patient’s comfort. The patient currently denies any neurologic symptoms.
Comment
Meningiomas are derived from meningothelial cells found in the leptomeninges and in the choroid plexus of the ventricles of the brain.2 They are common intracranial neoplasms that generally are associated with a benign course and present during the fourth to sixth decades of life. Meningiomas constitute 13% to 30% of intracranial neoplasms and usually are female predominant (3:1).3,4 Rarely, malignant transformation can lead to local and distant metastasis to the lungs,5,6 liver,7 and skeletal system.8 In cases of metastatic spread, there is an increased incidence in males versus females.9-11
Risk Factors
Although many meningiomas are sporadic, numerous risk factors have been associated with the disease development. One study showed a link between exposure to ionizing radiation and subsequent development of meningioma.12 Another study found a population link between a higher incidence of meningioma and nuclear exposure in Hiroshima, Japan, after the atomic bomb blast in 1980.13 There is an increased incidence of meningioma in patients exposed to radiography from frequent dental imaging, particularly when older machines with higher levels of radiation exposure are used.14Another study demonstrated a correlation between meningioma and hormonal factors (eg, estrogen for hormone therapy) and exacerbation of symptoms during pregnancy.15 There also is an increased incidence of meningioma in breast cancer patients.4 Genetic alterations also have been implicated in the development of meningioma. It was found that 50% of patients with a mutation in the neurofibromatosis 2 gene (which codes for the merlin protein) had associated meningiomas.16,17 Scalp nodules in patients with neurofibromatosis type 2 increases suspicion of a scalp meningioma and necessitates biopsy.
Clinical Presentation
Cutaneous meningiomas typically present as firm, subcutaneous nodules. Scalp nodules ranging from alopecia18,19 to hypertrichosis20 have been reported. These neoplasms can be painless or painful, depending on mass effect and location.
Classification
The primary clinical classification system of metastatic meningioma was first described in 1974.21 Type 1 meningioma refers to congenital lesions that tend to cluster closer to the midline. Type 2 refers to ectopic soft-tissue lesions that extend to the skin from likely remnants of arachnoid cells. These lesions are more likely to be found around the eyes, ears, nose, and mouth. Type 3 meningiomas extend from intracranial tumors that secondarily involve the skin through proliferation through bone or anatomic defects. Type 3 is the result of direct extension and the location of the cutaneous presentation depends on the location of the intracranial lesion.4,22,23
Pathology
Meningiomas exhibit a range of morphologic appearances on histopathology. In almost all meningiomas, tumor cells are concentrically wrapped in tight whorls with round-oval nuclei and delicate chromatin, central clearing, and pale pseudonuclear inclusions. Lamellate calcifications known as psammoma bodies are a common finding. Immunohistochemical studies show that most meningiomas are positive for EMA, vimentin, and progesterone receptor. S100 protein expression, if present, usually is focal.
Differential Diagnosis
Asymptomatic nodules on the scalp may present a diagnostic challenge to physicians. Most common scalp lesions tend to be cystic or lipomatous. In children, a broad differential diagnosis should be considered, including dermoid and epidermoid tumors, dermal sinus tumors, hemangiomas, metastasis of another tumor, aplasia cutis congenita, pilomatricoma, and lipoma. In adults, the differential should focus on epidermoid cysts, lipomas, metastasis of other tumors, osteomas, arteriovenous fistulae, and heterotopic brain tissue. Often, microscopic examination is necessary, along with additional immunohistochemical staining (eg, EMA, vimentin).
Treatment
Treatment options for meningioma include observation, surgical resection, radiotherapy, and systemic therapy, as well as a combination of these modalities. The choice of therapy depends on such variables as patient age; performance status; comorbidities; presence or absence of symptoms (including focal neurologic deficits); and tumor location, size, and grade. It is important to note that there is limited knowledge looking at the results of various treatment modalities, and no consensus approach has been established.
Conclusion
Our patient’s medical history was remarkable for an intracranial meningioma 11 years prior to the current presentation, and she was found to have biopsy-proven metastatic meningioma without recurrence of the initial tumor. Patients presenting with a scalp nodule warrant a thorough medical history and consideration beyond common cysts and lipomas.
- Mackay B, Bruner JM, Luna MA. Malignant meningioma of the scalp. Ultrastruc Pathol. 1994;18:235-240.
- Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet. 2004;363:1535-1543.
- Bauman G, Fisher B, Schild S, et al. Meningioma, ependymoma, and other adult brain tumors. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:539-566.
- Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095.
- Tworek JA, Mikhail AA, Blaivas M. Meningioma: local recurrence and pulmonary metastasis diagnosed by fine needle aspiration. Acta Cytol. 1997;41:946-947.
- Shin MS, Holman WL, Herrera GA, et al. Extensive pulmonary metastasis of an intracranial meningioma with repeated recurrence: radiographic and pathologic features. South Med J. 1996;89:313-318.
- Ferguson JM, Flinn J. Intracranial meningioma with hepatic metastases and hypoglycaemia treated by selective hepatic arterial chemo-embolization. Australas Radiol.1995;39:97-99.
- Palmer JD, Cook PL, Ellison DW. Extracranial osseous metastases from intracranial meningioma. Br J Neurosurg. 1994;8:215-218.
- Glasauer FE, Yuan RH. Intracranial tumours with extracranial metastases. case report and review of the literature. J Neurosurg. 1963;20:474-493.
- Shuangshoti S, Hongsaprabhas C, Netsky MG. Metastasizing meningioma. Cancer. 1970;26:832-841.
- Ohta M, Iwaki T, Kitamoto T, et al. MIB-1 staining index and scoring of histological features in meningioma. Cancer. 1994;74:3176-3189.
- Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278-299.
- Shintani T, Hayakawa N, Hoshi M, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Rad Res. 1999;40:49-57.
- Claus EB, Calvocoressi L, Bondy ML, et al. Dental x-rays and risk of meningioma. Cancer. 2012;118:4530-4537.
- Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26:279-282.
- Fontaine B, Rouleau GA, Seizinger BR, et al. Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuromas and meningioma). Ann N Y Acad Sci. 1991;615:338-343.
- Rabin BM, Meyer JR, Berlin JW, et al. Radiation-induced changes of the central nervous system and head and neck. Radiographics. 1996;16:1055-1072.
- Tanaka S, Okazaki M, Egusa G, et al. A case of pheochromocytoma associated with meningioma. J Intern Med. 1991;229:371-373.
- Zeikus P, Robinson-Bostom L, Stopa E. Primary cutaneous meningioma in association with a sinus pericranii. J Am Acad Dermatol. 2006;54(2 suppl):S49-S50.
- Junaid TA, Nkposong EO, Kolawole TM. Cutaneous meningiomas and an ovarian fibroma in a three-year-old girl. J Pathol. 1972;108:165-167.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningioma—a clinicopathologic study. Cancer. 1974;34:728-744.
- Shuangshoti S, Boonjunwetwat D, Kaoroptham S. Association of primary intraspinal meningiomas and subcutaneous meningioma of the cervical region: case report and review of literature. Surg Neurol. 1992;38:129-134.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol. 2012;136:208-211.
Meningiomas generally present as slow-growing, expanding intracranial lesions and are the most common benign intracranial tumor in adults.1 Rarely, meningioma exhibits malignant potential and presents as an extracranial soft-tissue mass through extension or as a primary extracranial cutaneous neoplasm. The differential diagnosis of scalp neoplasms must be broadened to include uncommon tumors such as meningioma. We present a rare case of a 68-year-old woman with scalp metastasis of meningioma 11 years after initial resection of the primary tumor.
Case Report
A 68-year-old woman presented for evaluation of an asymptomatic nodule on the left parietal scalp of 2 years’ duration. She denied any headaches, difficulty with balance, vision changes, or changes in mentation. Her medical history was remarkable for a benign meningioma removed from the right parietal scalp 11 years prior without radiation therapy, as well as type 2 diabetes mellitus and arthritis. The patient’s son died from a brain tumor, but the exact tumor type and age at the time of death were unknown. Her current medications included metformin, insulin glargine, aspirin, and a daily multivitamin. She denied any allergies or history of smoking.
Physical examination of the scalp revealed 4 fixed, nontender, flesh-colored nodules: 2 on the left parietal scalp measuring 3.0 cm and 0.8 cm, respectively (Figure 1A); a 0.4-cm nodule on the right posterior occipital scalp; and a 1.6-cm sausage-shaped nodule on the right temple (Figure 1B). No positive lymph nodes were appreciated, and no additional lesions were noted. No additional atypical lesions were noted on full cutaneous examination.

A diagnostic 6-mm punch biopsy of the largest nodule was performed. Intraoperatively, there was no apparent cyst wall, but coiled, loose, stringlike, pink-yellow tissue was removed from the base of the wound before closing with sutures.
The primary histologic finding was cells within fibrous tissue containing delicate round-oval nuclei, inconspicuous nucleoli, and lightly eosinophilic cytoplasm with an indistinct border (Figure 2). Immunohistochemical studies for S100 protein were focal and limited to the cytoplasm of a subset of neoplastic cells (Figure 3). Tumor cells stained positive for epithelial membrane antigen (EMA) and were focally positive for progesterone receptor (Figure 4). Tumor cells were negative for CD31 and CD34. Based on the clinical and histologic findings, a diagnosis of metastatic meningioma of the scalp was made.



Magnetic resonance imaging and positron emission tomography of the head, neck, and chest demonstrated 3 residual subcutaneous nodules on the scalp and an indeterminate subcentimeter nodule in the right lung. The 0.4-cm nodule on the right posterior occipital scalp was removed without complication, and no radiation therapy was administered. The rest of the lesions were monitored. She remained under the close observation of a neurosurgeon and underwent repeat imaging of the scalp nodules and lungs, initially at 3 months and then routinely at the patient’s comfort. The patient currently denies any neurologic symptoms.
Comment
Meningiomas are derived from meningothelial cells found in the leptomeninges and in the choroid plexus of the ventricles of the brain.2 They are common intracranial neoplasms that generally are associated with a benign course and present during the fourth to sixth decades of life. Meningiomas constitute 13% to 30% of intracranial neoplasms and usually are female predominant (3:1).3,4 Rarely, malignant transformation can lead to local and distant metastasis to the lungs,5,6 liver,7 and skeletal system.8 In cases of metastatic spread, there is an increased incidence in males versus females.9-11
Risk Factors
Although many meningiomas are sporadic, numerous risk factors have been associated with the disease development. One study showed a link between exposure to ionizing radiation and subsequent development of meningioma.12 Another study found a population link between a higher incidence of meningioma and nuclear exposure in Hiroshima, Japan, after the atomic bomb blast in 1980.13 There is an increased incidence of meningioma in patients exposed to radiography from frequent dental imaging, particularly when older machines with higher levels of radiation exposure are used.14Another study demonstrated a correlation between meningioma and hormonal factors (eg, estrogen for hormone therapy) and exacerbation of symptoms during pregnancy.15 There also is an increased incidence of meningioma in breast cancer patients.4 Genetic alterations also have been implicated in the development of meningioma. It was found that 50% of patients with a mutation in the neurofibromatosis 2 gene (which codes for the merlin protein) had associated meningiomas.16,17 Scalp nodules in patients with neurofibromatosis type 2 increases suspicion of a scalp meningioma and necessitates biopsy.
Clinical Presentation
Cutaneous meningiomas typically present as firm, subcutaneous nodules. Scalp nodules ranging from alopecia18,19 to hypertrichosis20 have been reported. These neoplasms can be painless or painful, depending on mass effect and location.
Classification
The primary clinical classification system of metastatic meningioma was first described in 1974.21 Type 1 meningioma refers to congenital lesions that tend to cluster closer to the midline. Type 2 refers to ectopic soft-tissue lesions that extend to the skin from likely remnants of arachnoid cells. These lesions are more likely to be found around the eyes, ears, nose, and mouth. Type 3 meningiomas extend from intracranial tumors that secondarily involve the skin through proliferation through bone or anatomic defects. Type 3 is the result of direct extension and the location of the cutaneous presentation depends on the location of the intracranial lesion.4,22,23
Pathology
Meningiomas exhibit a range of morphologic appearances on histopathology. In almost all meningiomas, tumor cells are concentrically wrapped in tight whorls with round-oval nuclei and delicate chromatin, central clearing, and pale pseudonuclear inclusions. Lamellate calcifications known as psammoma bodies are a common finding. Immunohistochemical studies show that most meningiomas are positive for EMA, vimentin, and progesterone receptor. S100 protein expression, if present, usually is focal.
Differential Diagnosis
Asymptomatic nodules on the scalp may present a diagnostic challenge to physicians. Most common scalp lesions tend to be cystic or lipomatous. In children, a broad differential diagnosis should be considered, including dermoid and epidermoid tumors, dermal sinus tumors, hemangiomas, metastasis of another tumor, aplasia cutis congenita, pilomatricoma, and lipoma. In adults, the differential should focus on epidermoid cysts, lipomas, metastasis of other tumors, osteomas, arteriovenous fistulae, and heterotopic brain tissue. Often, microscopic examination is necessary, along with additional immunohistochemical staining (eg, EMA, vimentin).
Treatment
Treatment options for meningioma include observation, surgical resection, radiotherapy, and systemic therapy, as well as a combination of these modalities. The choice of therapy depends on such variables as patient age; performance status; comorbidities; presence or absence of symptoms (including focal neurologic deficits); and tumor location, size, and grade. It is important to note that there is limited knowledge looking at the results of various treatment modalities, and no consensus approach has been established.
Conclusion
Our patient’s medical history was remarkable for an intracranial meningioma 11 years prior to the current presentation, and she was found to have biopsy-proven metastatic meningioma without recurrence of the initial tumor. Patients presenting with a scalp nodule warrant a thorough medical history and consideration beyond common cysts and lipomas.
Meningiomas generally present as slow-growing, expanding intracranial lesions and are the most common benign intracranial tumor in adults.1 Rarely, meningioma exhibits malignant potential and presents as an extracranial soft-tissue mass through extension or as a primary extracranial cutaneous neoplasm. The differential diagnosis of scalp neoplasms must be broadened to include uncommon tumors such as meningioma. We present a rare case of a 68-year-old woman with scalp metastasis of meningioma 11 years after initial resection of the primary tumor.
Case Report
A 68-year-old woman presented for evaluation of an asymptomatic nodule on the left parietal scalp of 2 years’ duration. She denied any headaches, difficulty with balance, vision changes, or changes in mentation. Her medical history was remarkable for a benign meningioma removed from the right parietal scalp 11 years prior without radiation therapy, as well as type 2 diabetes mellitus and arthritis. The patient’s son died from a brain tumor, but the exact tumor type and age at the time of death were unknown. Her current medications included metformin, insulin glargine, aspirin, and a daily multivitamin. She denied any allergies or history of smoking.
Physical examination of the scalp revealed 4 fixed, nontender, flesh-colored nodules: 2 on the left parietal scalp measuring 3.0 cm and 0.8 cm, respectively (Figure 1A); a 0.4-cm nodule on the right posterior occipital scalp; and a 1.6-cm sausage-shaped nodule on the right temple (Figure 1B). No positive lymph nodes were appreciated, and no additional lesions were noted. No additional atypical lesions were noted on full cutaneous examination.

A diagnostic 6-mm punch biopsy of the largest nodule was performed. Intraoperatively, there was no apparent cyst wall, but coiled, loose, stringlike, pink-yellow tissue was removed from the base of the wound before closing with sutures.
The primary histologic finding was cells within fibrous tissue containing delicate round-oval nuclei, inconspicuous nucleoli, and lightly eosinophilic cytoplasm with an indistinct border (Figure 2). Immunohistochemical studies for S100 protein were focal and limited to the cytoplasm of a subset of neoplastic cells (Figure 3). Tumor cells stained positive for epithelial membrane antigen (EMA) and were focally positive for progesterone receptor (Figure 4). Tumor cells were negative for CD31 and CD34. Based on the clinical and histologic findings, a diagnosis of metastatic meningioma of the scalp was made.



Magnetic resonance imaging and positron emission tomography of the head, neck, and chest demonstrated 3 residual subcutaneous nodules on the scalp and an indeterminate subcentimeter nodule in the right lung. The 0.4-cm nodule on the right posterior occipital scalp was removed without complication, and no radiation therapy was administered. The rest of the lesions were monitored. She remained under the close observation of a neurosurgeon and underwent repeat imaging of the scalp nodules and lungs, initially at 3 months and then routinely at the patient’s comfort. The patient currently denies any neurologic symptoms.
Comment
Meningiomas are derived from meningothelial cells found in the leptomeninges and in the choroid plexus of the ventricles of the brain.2 They are common intracranial neoplasms that generally are associated with a benign course and present during the fourth to sixth decades of life. Meningiomas constitute 13% to 30% of intracranial neoplasms and usually are female predominant (3:1).3,4 Rarely, malignant transformation can lead to local and distant metastasis to the lungs,5,6 liver,7 and skeletal system.8 In cases of metastatic spread, there is an increased incidence in males versus females.9-11
Risk Factors
Although many meningiomas are sporadic, numerous risk factors have been associated with the disease development. One study showed a link between exposure to ionizing radiation and subsequent development of meningioma.12 Another study found a population link between a higher incidence of meningioma and nuclear exposure in Hiroshima, Japan, after the atomic bomb blast in 1980.13 There is an increased incidence of meningioma in patients exposed to radiography from frequent dental imaging, particularly when older machines with higher levels of radiation exposure are used.14Another study demonstrated a correlation between meningioma and hormonal factors (eg, estrogen for hormone therapy) and exacerbation of symptoms during pregnancy.15 There also is an increased incidence of meningioma in breast cancer patients.4 Genetic alterations also have been implicated in the development of meningioma. It was found that 50% of patients with a mutation in the neurofibromatosis 2 gene (which codes for the merlin protein) had associated meningiomas.16,17 Scalp nodules in patients with neurofibromatosis type 2 increases suspicion of a scalp meningioma and necessitates biopsy.
Clinical Presentation
Cutaneous meningiomas typically present as firm, subcutaneous nodules. Scalp nodules ranging from alopecia18,19 to hypertrichosis20 have been reported. These neoplasms can be painless or painful, depending on mass effect and location.
Classification
The primary clinical classification system of metastatic meningioma was first described in 1974.21 Type 1 meningioma refers to congenital lesions that tend to cluster closer to the midline. Type 2 refers to ectopic soft-tissue lesions that extend to the skin from likely remnants of arachnoid cells. These lesions are more likely to be found around the eyes, ears, nose, and mouth. Type 3 meningiomas extend from intracranial tumors that secondarily involve the skin through proliferation through bone or anatomic defects. Type 3 is the result of direct extension and the location of the cutaneous presentation depends on the location of the intracranial lesion.4,22,23
Pathology
Meningiomas exhibit a range of morphologic appearances on histopathology. In almost all meningiomas, tumor cells are concentrically wrapped in tight whorls with round-oval nuclei and delicate chromatin, central clearing, and pale pseudonuclear inclusions. Lamellate calcifications known as psammoma bodies are a common finding. Immunohistochemical studies show that most meningiomas are positive for EMA, vimentin, and progesterone receptor. S100 protein expression, if present, usually is focal.
Differential Diagnosis
Asymptomatic nodules on the scalp may present a diagnostic challenge to physicians. Most common scalp lesions tend to be cystic or lipomatous. In children, a broad differential diagnosis should be considered, including dermoid and epidermoid tumors, dermal sinus tumors, hemangiomas, metastasis of another tumor, aplasia cutis congenita, pilomatricoma, and lipoma. In adults, the differential should focus on epidermoid cysts, lipomas, metastasis of other tumors, osteomas, arteriovenous fistulae, and heterotopic brain tissue. Often, microscopic examination is necessary, along with additional immunohistochemical staining (eg, EMA, vimentin).
Treatment
Treatment options for meningioma include observation, surgical resection, radiotherapy, and systemic therapy, as well as a combination of these modalities. The choice of therapy depends on such variables as patient age; performance status; comorbidities; presence or absence of symptoms (including focal neurologic deficits); and tumor location, size, and grade. It is important to note that there is limited knowledge looking at the results of various treatment modalities, and no consensus approach has been established.
Conclusion
Our patient’s medical history was remarkable for an intracranial meningioma 11 years prior to the current presentation, and she was found to have biopsy-proven metastatic meningioma without recurrence of the initial tumor. Patients presenting with a scalp nodule warrant a thorough medical history and consideration beyond common cysts and lipomas.
- Mackay B, Bruner JM, Luna MA. Malignant meningioma of the scalp. Ultrastruc Pathol. 1994;18:235-240.
- Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet. 2004;363:1535-1543.
- Bauman G, Fisher B, Schild S, et al. Meningioma, ependymoma, and other adult brain tumors. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:539-566.
- Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095.
- Tworek JA, Mikhail AA, Blaivas M. Meningioma: local recurrence and pulmonary metastasis diagnosed by fine needle aspiration. Acta Cytol. 1997;41:946-947.
- Shin MS, Holman WL, Herrera GA, et al. Extensive pulmonary metastasis of an intracranial meningioma with repeated recurrence: radiographic and pathologic features. South Med J. 1996;89:313-318.
- Ferguson JM, Flinn J. Intracranial meningioma with hepatic metastases and hypoglycaemia treated by selective hepatic arterial chemo-embolization. Australas Radiol.1995;39:97-99.
- Palmer JD, Cook PL, Ellison DW. Extracranial osseous metastases from intracranial meningioma. Br J Neurosurg. 1994;8:215-218.
- Glasauer FE, Yuan RH. Intracranial tumours with extracranial metastases. case report and review of the literature. J Neurosurg. 1963;20:474-493.
- Shuangshoti S, Hongsaprabhas C, Netsky MG. Metastasizing meningioma. Cancer. 1970;26:832-841.
- Ohta M, Iwaki T, Kitamoto T, et al. MIB-1 staining index and scoring of histological features in meningioma. Cancer. 1994;74:3176-3189.
- Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278-299.
- Shintani T, Hayakawa N, Hoshi M, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Rad Res. 1999;40:49-57.
- Claus EB, Calvocoressi L, Bondy ML, et al. Dental x-rays and risk of meningioma. Cancer. 2012;118:4530-4537.
- Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26:279-282.
- Fontaine B, Rouleau GA, Seizinger BR, et al. Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuromas and meningioma). Ann N Y Acad Sci. 1991;615:338-343.
- Rabin BM, Meyer JR, Berlin JW, et al. Radiation-induced changes of the central nervous system and head and neck. Radiographics. 1996;16:1055-1072.
- Tanaka S, Okazaki M, Egusa G, et al. A case of pheochromocytoma associated with meningioma. J Intern Med. 1991;229:371-373.
- Zeikus P, Robinson-Bostom L, Stopa E. Primary cutaneous meningioma in association with a sinus pericranii. J Am Acad Dermatol. 2006;54(2 suppl):S49-S50.
- Junaid TA, Nkposong EO, Kolawole TM. Cutaneous meningiomas and an ovarian fibroma in a three-year-old girl. J Pathol. 1972;108:165-167.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningioma—a clinicopathologic study. Cancer. 1974;34:728-744.
- Shuangshoti S, Boonjunwetwat D, Kaoroptham S. Association of primary intraspinal meningiomas and subcutaneous meningioma of the cervical region: case report and review of literature. Surg Neurol. 1992;38:129-134.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol. 2012;136:208-211.
- Mackay B, Bruner JM, Luna MA. Malignant meningioma of the scalp. Ultrastruc Pathol. 1994;18:235-240.
- Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet. 2004;363:1535-1543.
- Bauman G, Fisher B, Schild S, et al. Meningioma, ependymoma, and other adult brain tumors. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:539-566.
- Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095.
- Tworek JA, Mikhail AA, Blaivas M. Meningioma: local recurrence and pulmonary metastasis diagnosed by fine needle aspiration. Acta Cytol. 1997;41:946-947.
- Shin MS, Holman WL, Herrera GA, et al. Extensive pulmonary metastasis of an intracranial meningioma with repeated recurrence: radiographic and pathologic features. South Med J. 1996;89:313-318.
- Ferguson JM, Flinn J. Intracranial meningioma with hepatic metastases and hypoglycaemia treated by selective hepatic arterial chemo-embolization. Australas Radiol.1995;39:97-99.
- Palmer JD, Cook PL, Ellison DW. Extracranial osseous metastases from intracranial meningioma. Br J Neurosurg. 1994;8:215-218.
- Glasauer FE, Yuan RH. Intracranial tumours with extracranial metastases. case report and review of the literature. J Neurosurg. 1963;20:474-493.
- Shuangshoti S, Hongsaprabhas C, Netsky MG. Metastasizing meningioma. Cancer. 1970;26:832-841.
- Ohta M, Iwaki T, Kitamoto T, et al. MIB-1 staining index and scoring of histological features in meningioma. Cancer. 1994;74:3176-3189.
- Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278-299.
- Shintani T, Hayakawa N, Hoshi M, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Rad Res. 1999;40:49-57.
- Claus EB, Calvocoressi L, Bondy ML, et al. Dental x-rays and risk of meningioma. Cancer. 2012;118:4530-4537.
- Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26:279-282.
- Fontaine B, Rouleau GA, Seizinger BR, et al. Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuromas and meningioma). Ann N Y Acad Sci. 1991;615:338-343.
- Rabin BM, Meyer JR, Berlin JW, et al. Radiation-induced changes of the central nervous system and head and neck. Radiographics. 1996;16:1055-1072.
- Tanaka S, Okazaki M, Egusa G, et al. A case of pheochromocytoma associated with meningioma. J Intern Med. 1991;229:371-373.
- Zeikus P, Robinson-Bostom L, Stopa E. Primary cutaneous meningioma in association with a sinus pericranii. J Am Acad Dermatol. 2006;54(2 suppl):S49-S50.
- Junaid TA, Nkposong EO, Kolawole TM. Cutaneous meningiomas and an ovarian fibroma in a three-year-old girl. J Pathol. 1972;108:165-167.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningioma—a clinicopathologic study. Cancer. 1974;34:728-744.
- Shuangshoti S, Boonjunwetwat D, Kaoroptham S. Association of primary intraspinal meningiomas and subcutaneous meningioma of the cervical region: case report and review of literature. Surg Neurol. 1992;38:129-134.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol. 2012;136:208-211.
Squamoid Eccrine Ductal Carcinoma
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
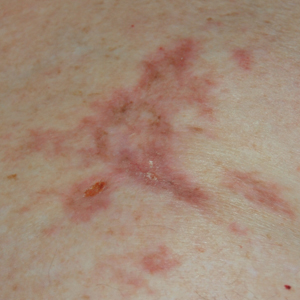
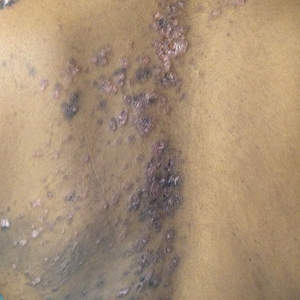
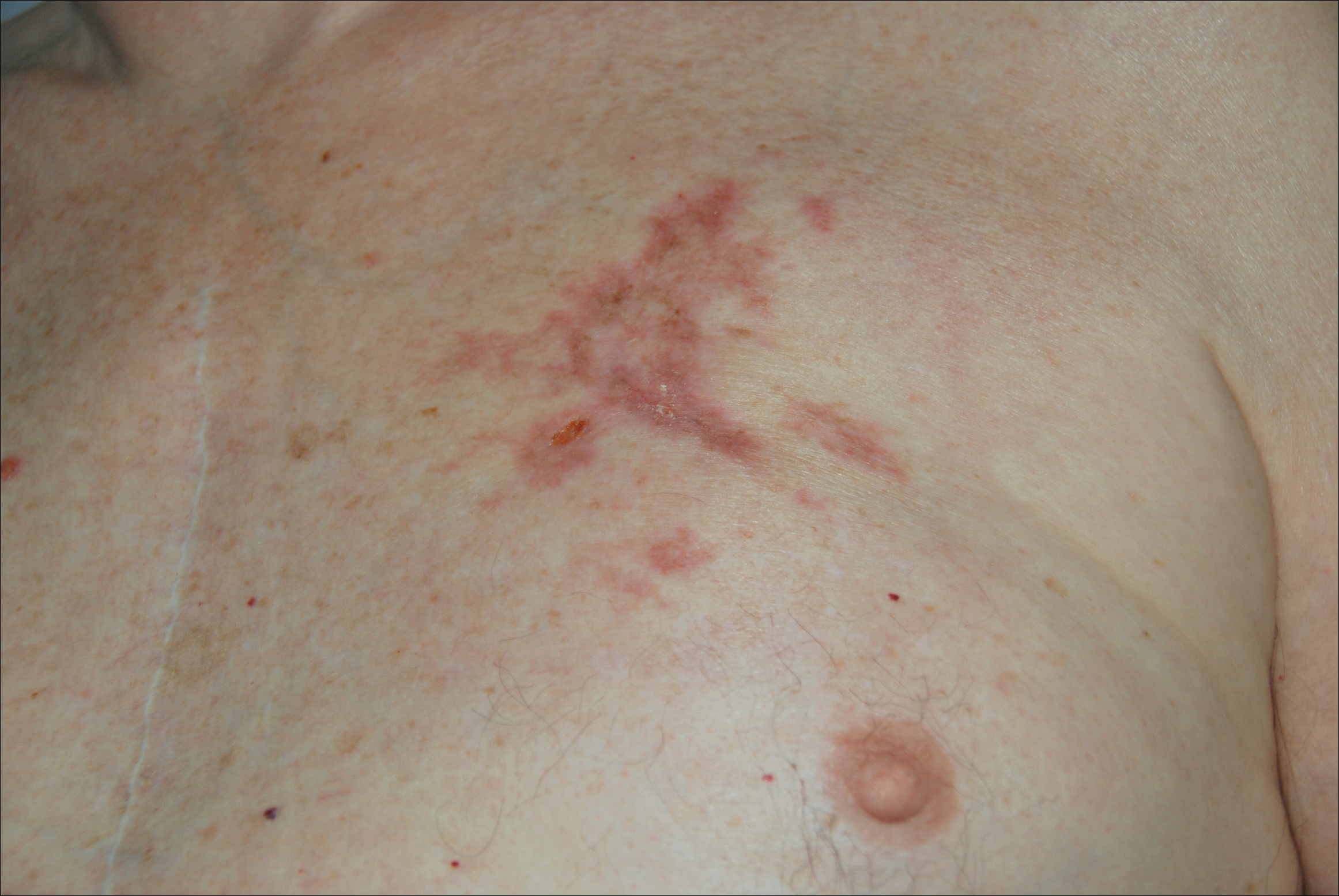
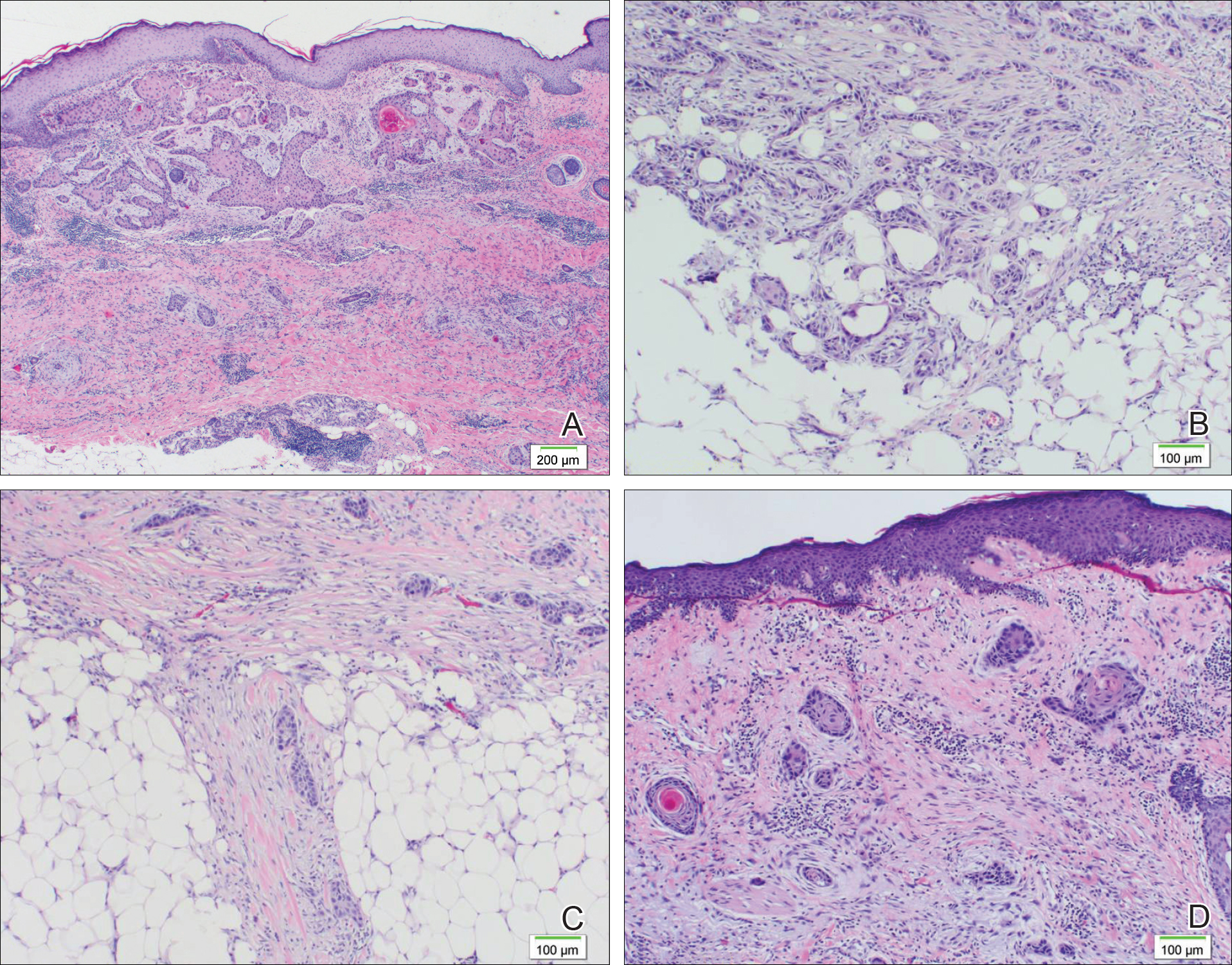
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.
Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.


Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.


Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
Practice Points
- Squamoid eccrine ductal carcinoma (SEDC) is an extremely rare cutaneous tumor of unknown etiology.
- A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Development of a second or even a third primary malignancy in patients with SEDC is increasingly being reported in CLL patients.
Pigmented Squamous Cell Carcinoma Presenting as Longitudinal Melanonychia in a Transplant Recipient
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
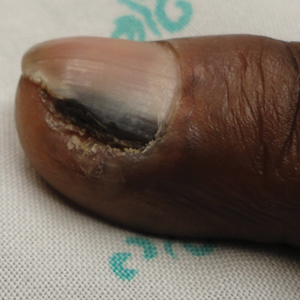
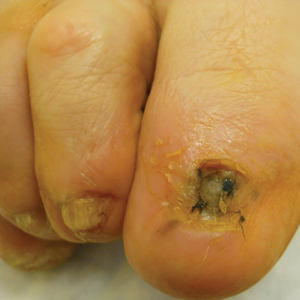
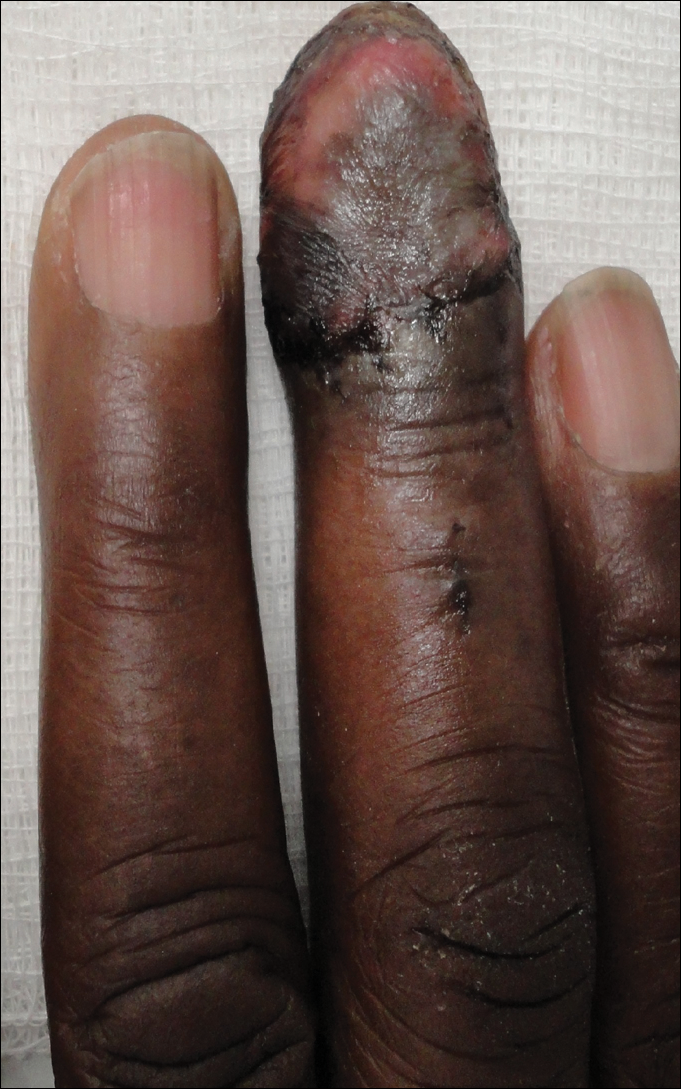
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).
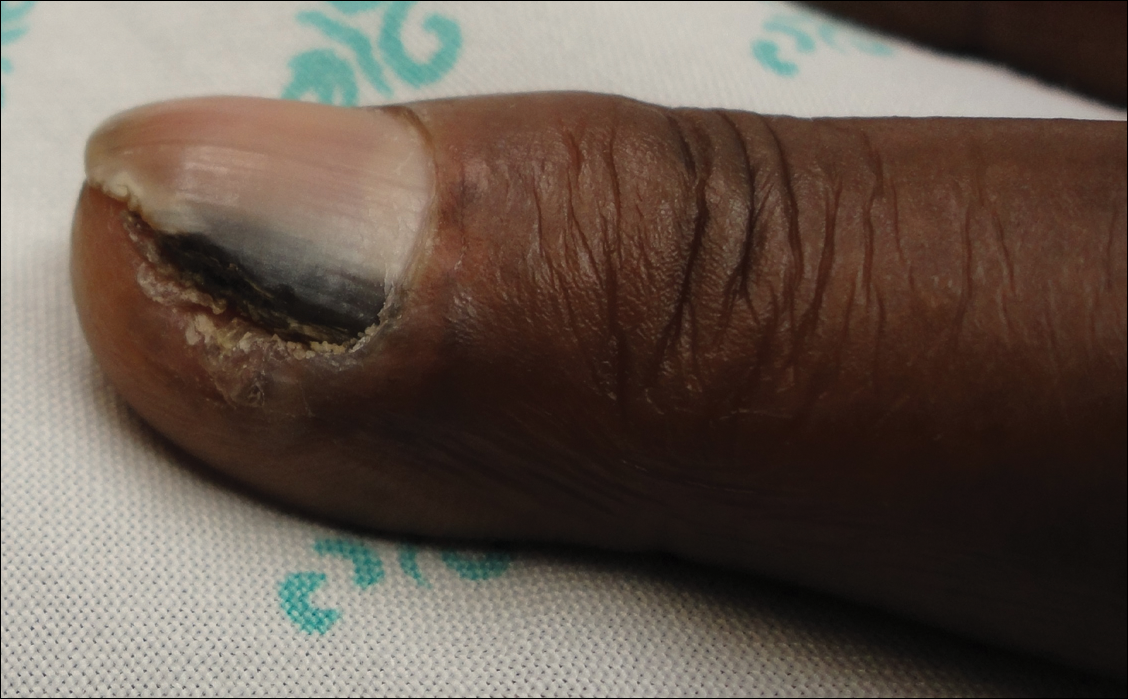
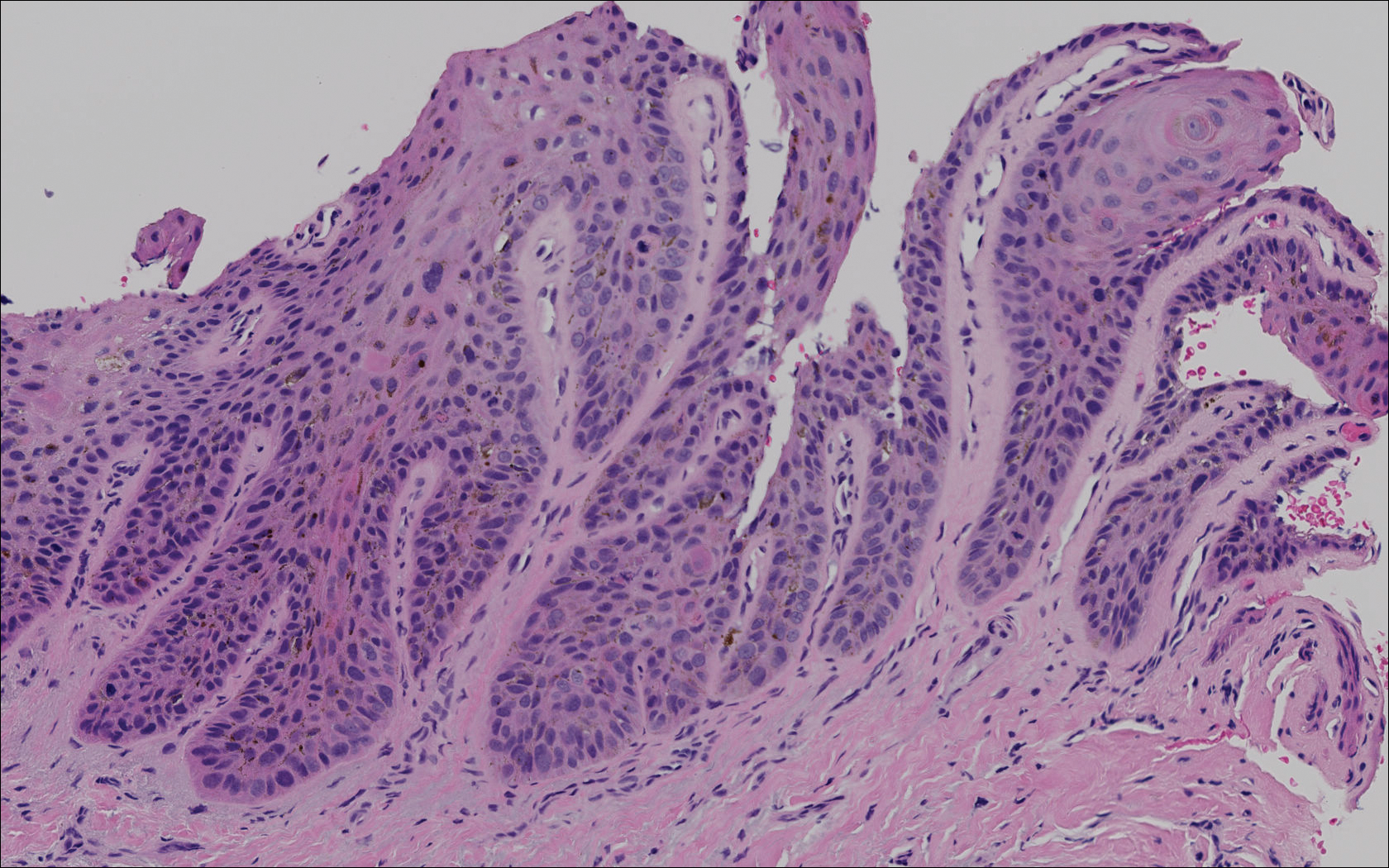
Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).
Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).

Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).


Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).

Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).


Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
Practice Points
- Risk factors for the development of pigmented squamous cell carcinoma (pSCC) include older age, male sex, and use of immunosuppressant medications.
- Subungual pSCC can present as longitudinal melanonychia and should be considered in the differential diagnosis for melanonychia in patients with skin of color or those who are immunosuppressed.
Discoid Lupus Erythematosus Following Herpes Zoster
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.
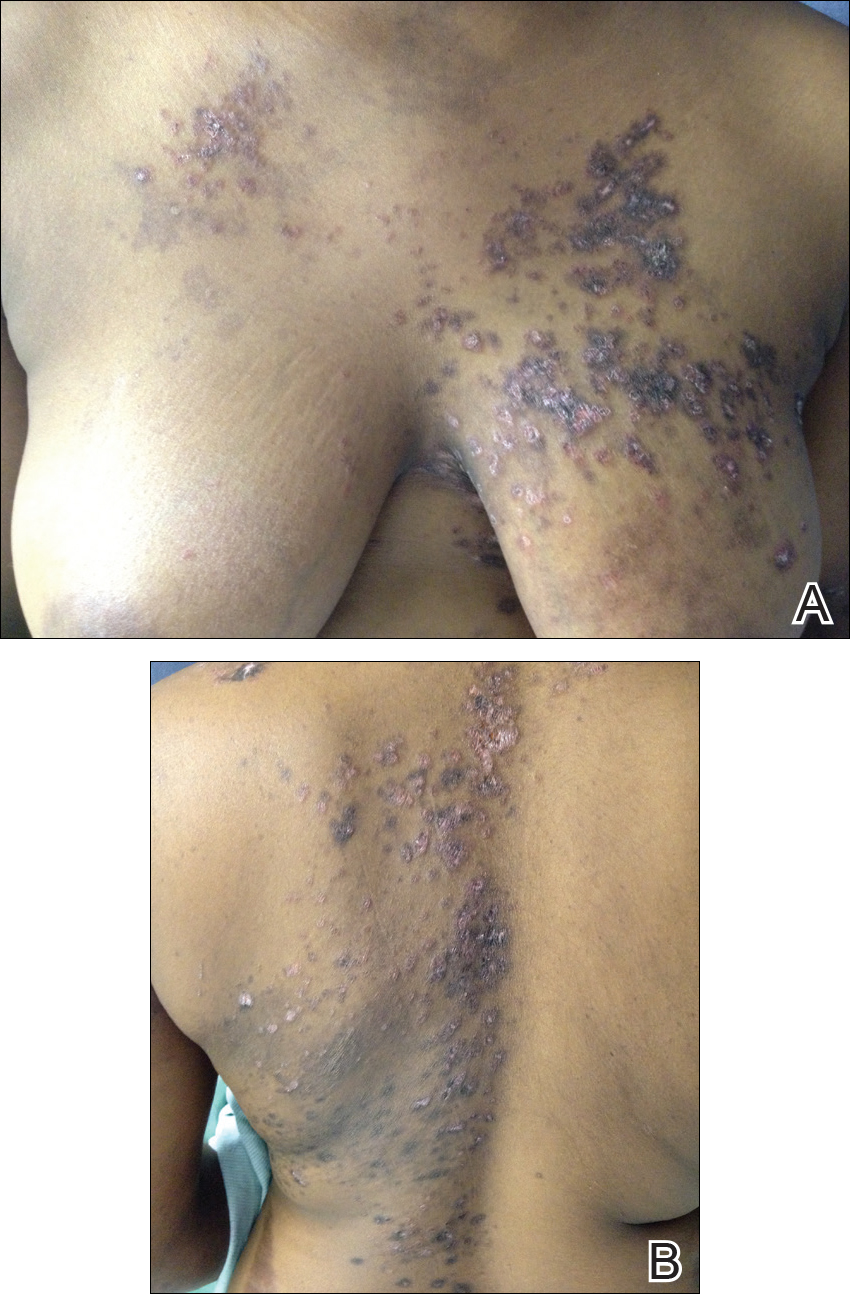
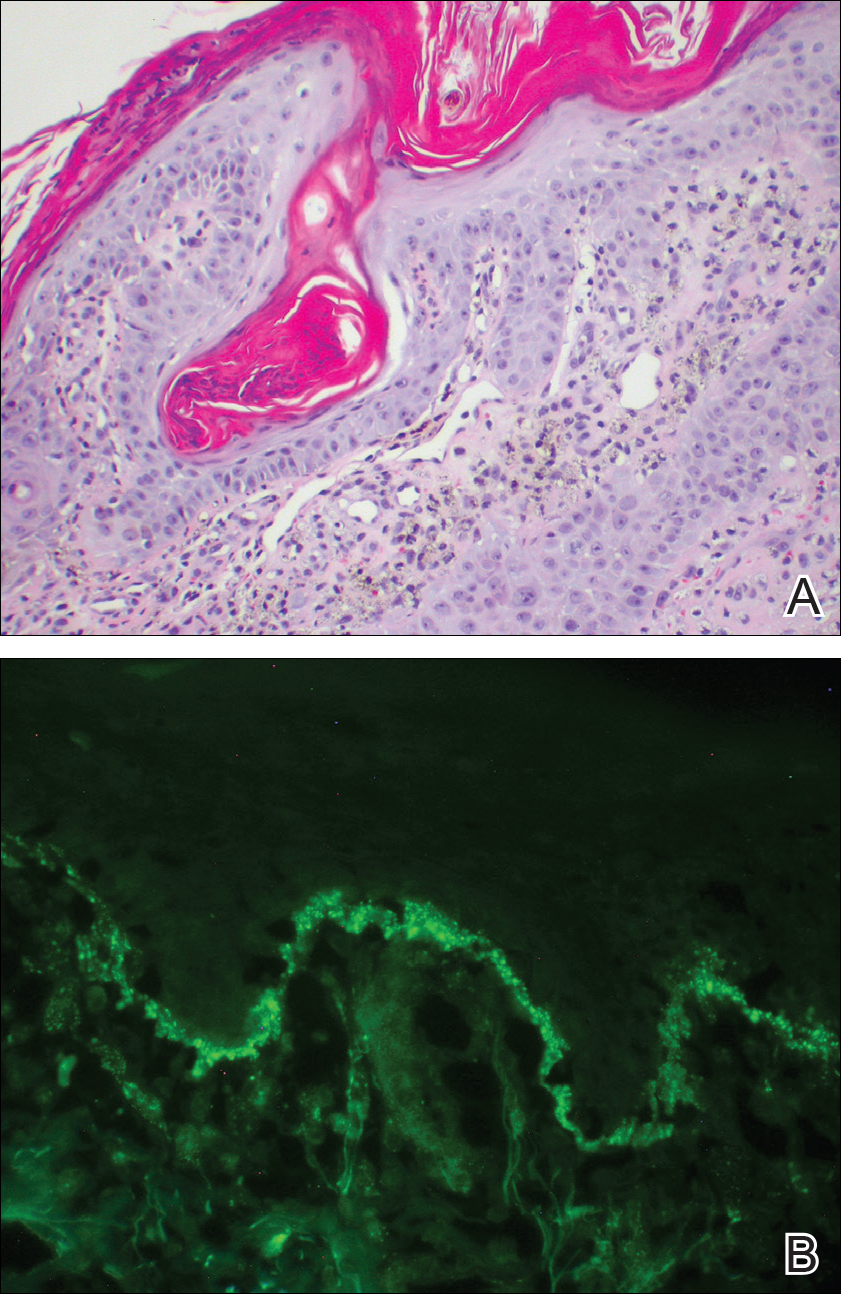
Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.


Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.


Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
Practice Points
- Discoid lupus erythematosus (DLE) most commonly presents as scaling and crusted plaques in sun-exposed areas of the face and arms. It also may present in skin traumatized by tattoos, scratches, scars, prolonged heat exposure, andherpes zoster (HZ).
- Patients with a history of DLE who subsequently develop HZ should be followed closely for the development of DLE in HZ-affected dermatomes.
- Following resolution of HZ, topical corticosteroids may have a role in prevention of DLE in HZ-affected dermatomes.
Secukinumab Emerges as a Rapidly Effective Therapy for Pityriasis Rubra Pilaris
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.
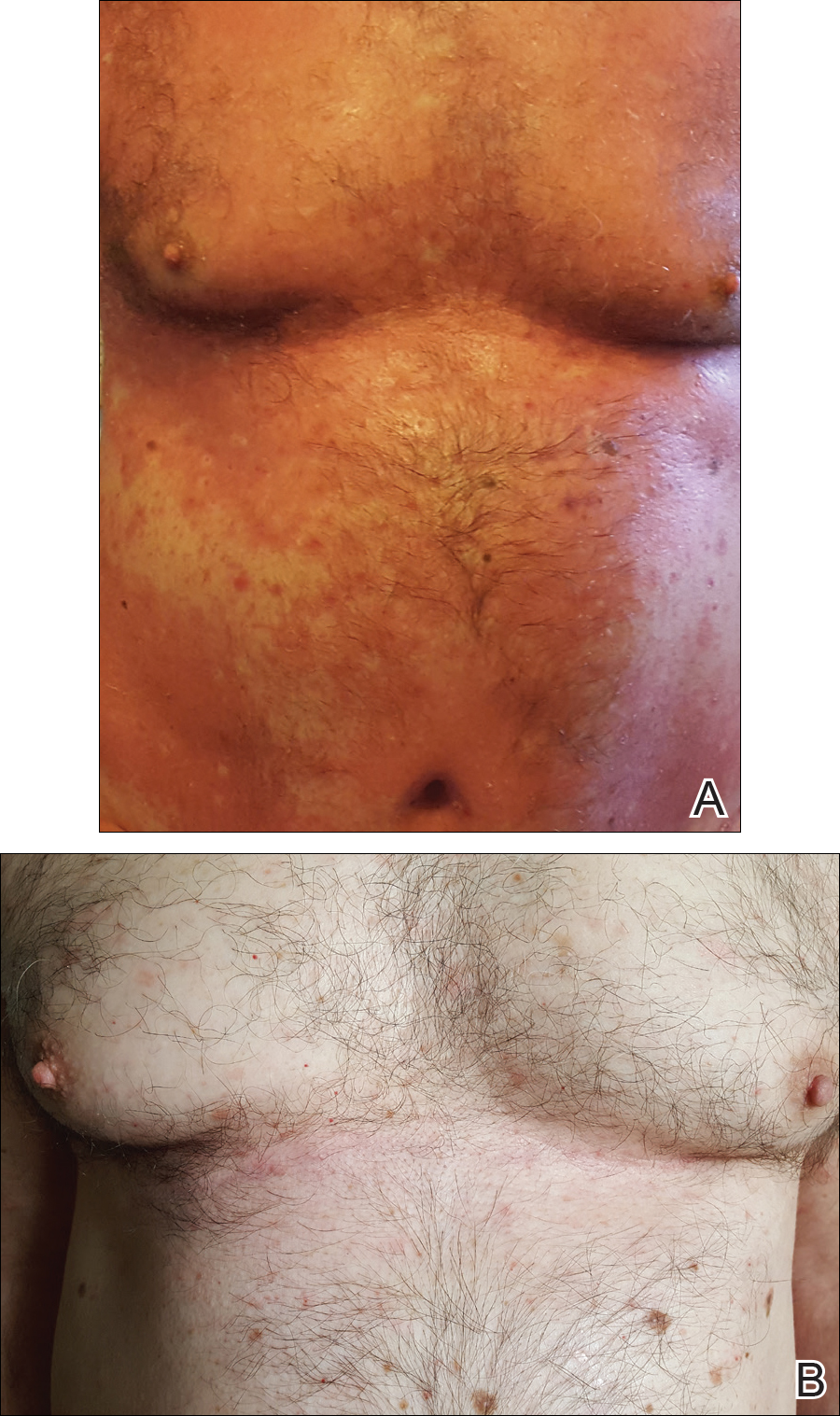
Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.
By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Practice Points
- In patients with pityriasis rubra pilaris (PRP) who have not responded to topical treatments, off-label treatment with systemic therapies approved for plaque psoriasis can be considered.
- Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP.
Perianal Basal Cell Carcinoma Treated With Mohs Micrographic Surgery
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.
A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).

Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.

A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).


Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.

A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).


Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
Practice Points
- Basal cell carcinoma is less common in non–sun-exposed areas of the body and is exceptionally rare in the perineal and perianal regions.
- Thorough total-body skin examinations may lead to early detection of asymptomatic skin lesions, allowing for earlier and less invasive treatment.
- Appointment attendance and patient compliance are common challenges that dermatologists face. Patient reminders via their preferred method of communication may help reduce missed dermatology appointments.
Energy-Based Devices for Actinic Keratosis Field Therapy
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
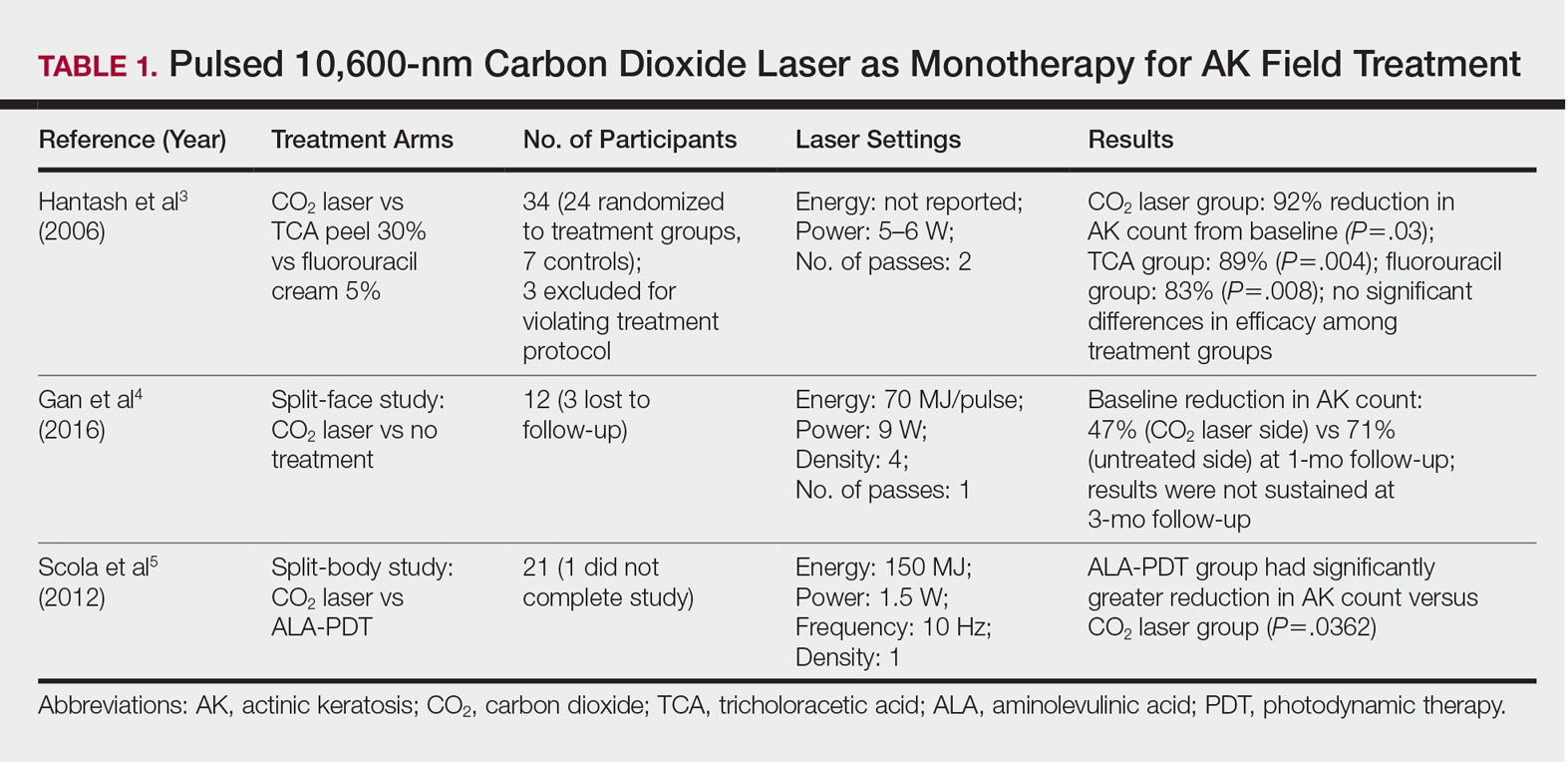
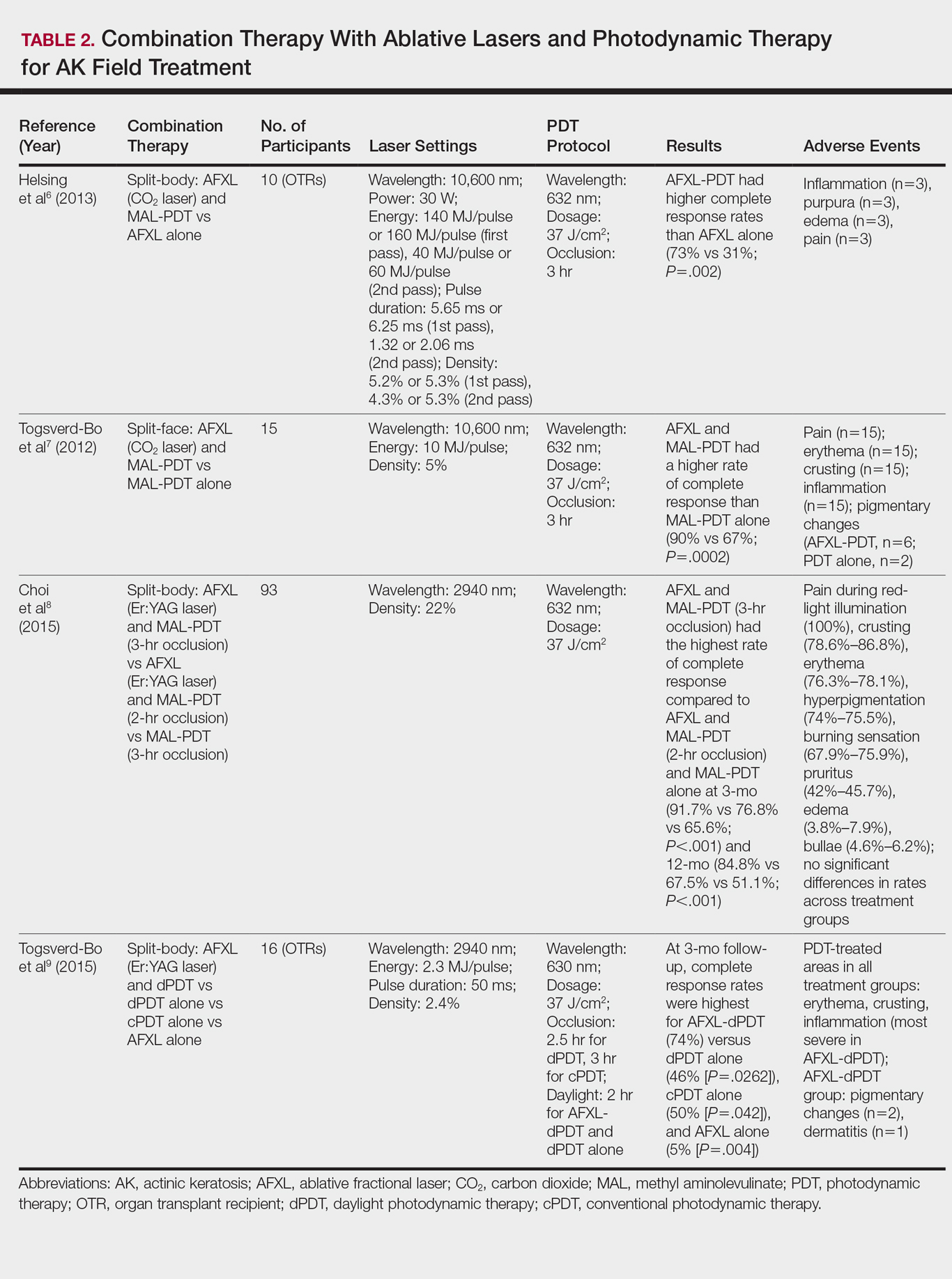
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
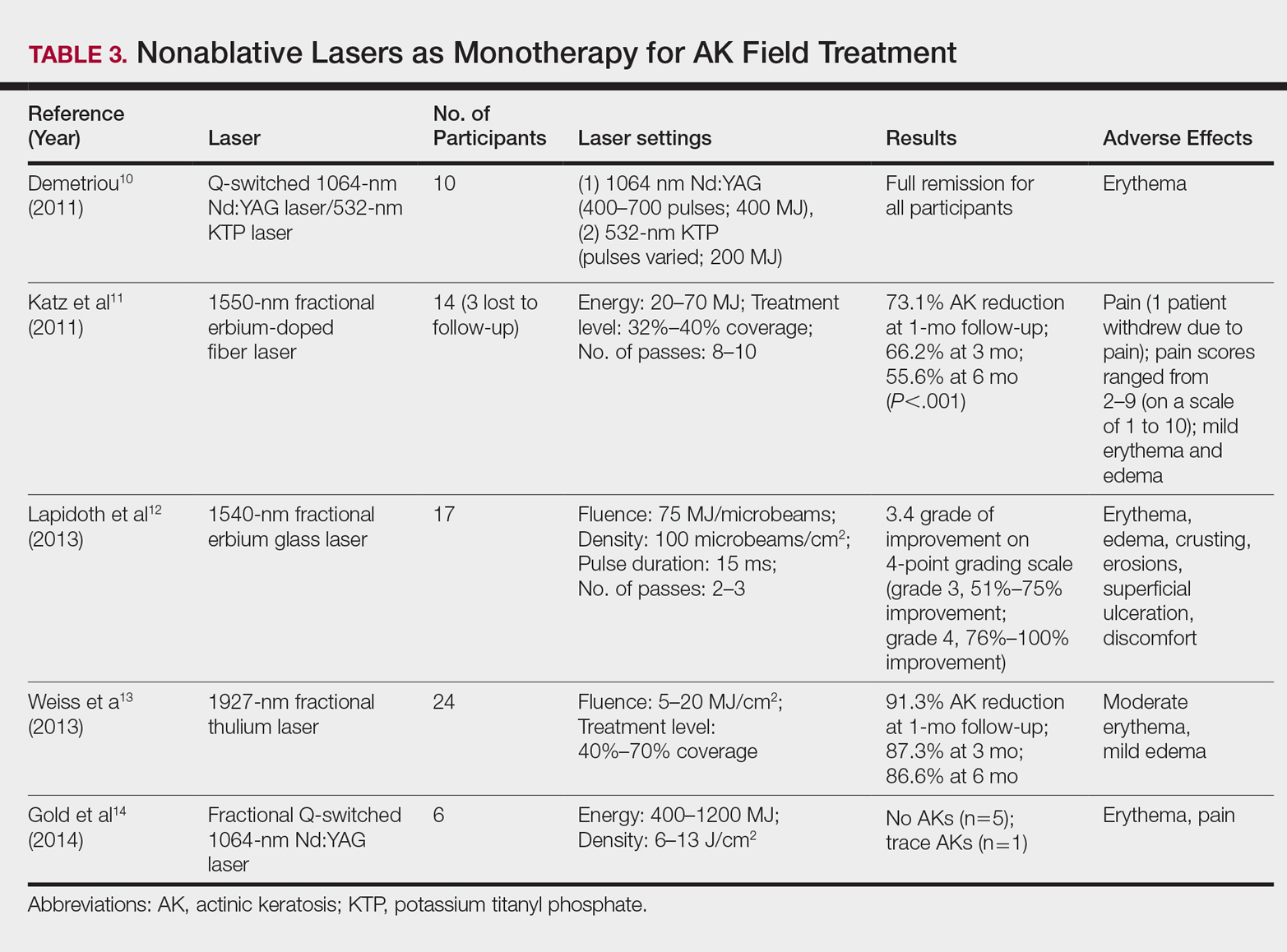
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).


Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).

In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).


Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).

In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
Practice Points
- Ablative fractional laser therapy in combination with photodynamic therapy has demonstrated increased efficacy in treating field actinic keratoses (AKs) for up to 12 months of follow-up over either modality alone.
- Ablative and nonablative lasers as monotherapy in treating field AKs require further studies with larger sample sizes to determine efficacy and safety.
Enhanced Melanoma Diagnosis With Multispectral Digital Skin Lesion Analysis
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
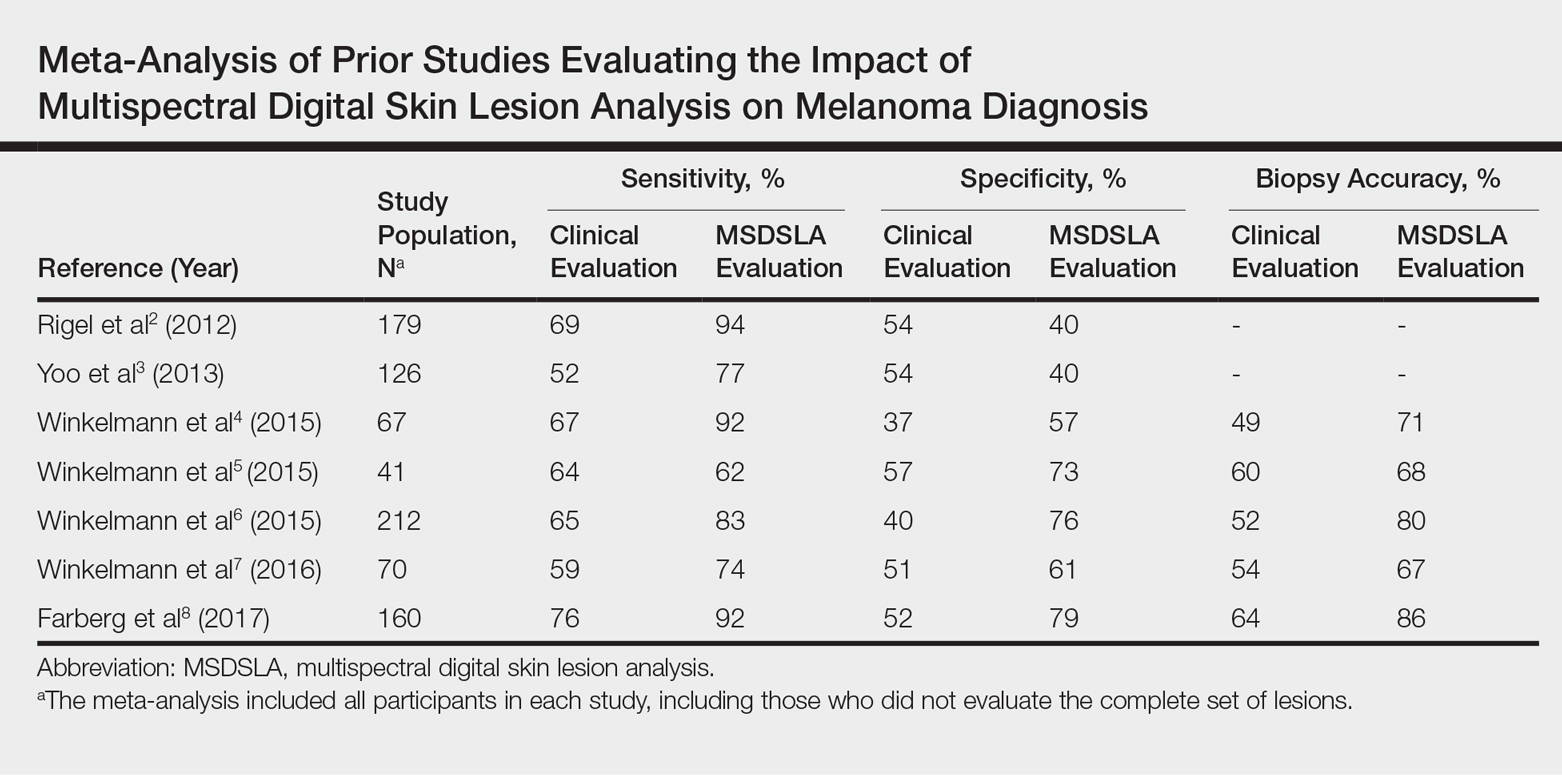
Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
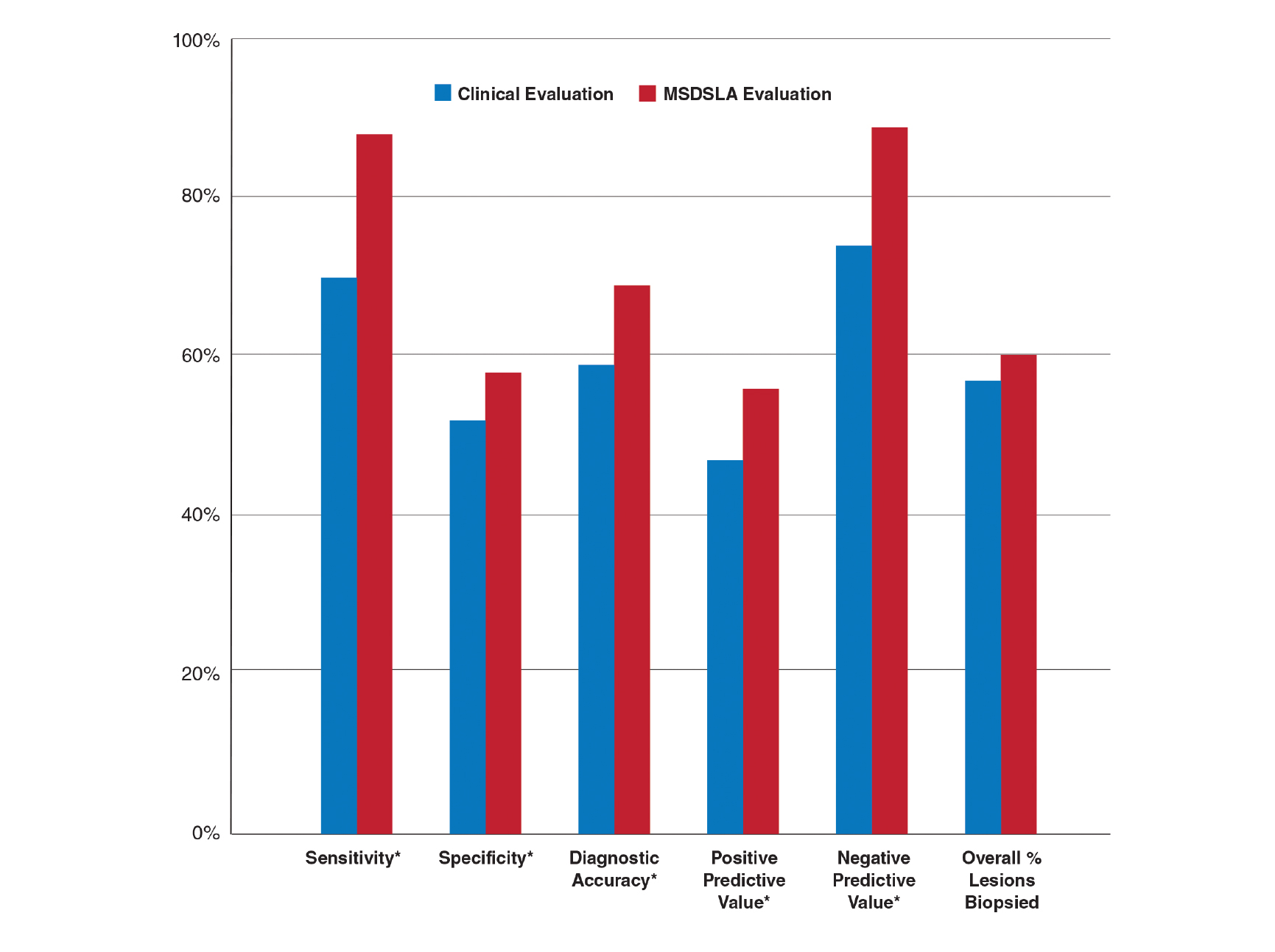
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods

Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.

Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods

Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.

Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Practice Points
- Multispectral digital skin lesion analysis (MSDSLA) can be a valuable tool in the evaluation of pigmented skin lesions (PSLs).
- MSDSLA may help to better identify high-risk PSLs and improve cost of care.
Teledermatology in the US Military: A Historic Foundation for Current and Future Applications
Telemedicine arose from the need to provide critical and timely advice directly to health care providers and patients in remote or resource-scarce settings. Whether by radio, telephone, or other means of telecommunication technology, the US military has long utilized telemedicine. What started as a way to expedite the delivery of emergency consultations and medical expertise to remote populations in need has since evolved into a billion-dollar innovation industry that is poised to improve health care efficiency and access to specialist care as well as to lower health care costs for all patients.
Teledermatology in the Military
A primary mission of military medicine is to keep service members anywhere in the world in good health on the job during training, combat, and humanitarian operations.1 Telemedicine greatly supports this mission by bringing the expertise of medical specialists to service members in the field without the cost or risks of travel for physicians. Telemedicine also is effective in promoting timely triage of patients and administration of the most appropriate levels of care. With the advent and globalization of high-speed wireless networks, advancements in telemedicine continue to develop and are becoming increasingly useful in military medicine.
As a specialty, dermatology is heavily reliant on visual information and therefore is particularly amenable to telemedicine applications. The rising popularity of such services has led to the development of the term teledermatology. While early teledermatology services were provided using radio, telephone, fax, and videoconferencing,2 three distinct visual methods typically are used today, including (1) store-and-forward (S&F), (2) live-interactive, and (3) a hybrid of the two.3 Military dermatology predominantly utilizes an S&F system, as still photographs of lesions generally are preferred over video for more focused visualization.
In 2004, the US Army Medical Department established a centralized telemedicine program using Army Knowledge Online,1 an S&F system that allows providers in remote locations to store and forward information about a patient’s clinical history along with digital photographs of the patient’s condition to a military dermatologist to review and make a diagnosis or suggest a treatment from a different location at a later time. Using this platform to provide asynchronous teledermatology services avoids the logistics required to schedule appointments and promotes convenience and more efficient use of physicians’ time and resources.
Given the ease of use of S&F systems among military practitioners, dermatology became one of the most heavily utilized teleconsultation specialties within the Army Knowledge Online system, accounting for 40% of the 10,817 consultations initiated from April 2004 to December 2012.5 It also is important to note that skin conditions historically account for 15% to 75% of outpatient visits during wartime; therefore, there is a need for dermatologic consultations, as primary care providers typically are responsible for providing dermatologic care to these patients.6 Because of the high demand for and low volume of US military dermatologists, the use of teledermatology (ie, Amy Knowledge Online) in the US military became a helpful educational tool and specialist extender for many primary care providers in the military.
Teledermatology in the military has evolved to not only provide timely and efficient care but also to reduce health care costs.
Advances in Teledermatology
While the military continues to use S&F teleconsultations—a model in which a deployed referring clinician sends information to a military dermatologist for diagnosis and/or management recommendations—a number of teledermatology programs have been developed for civilians that provide additional advantages over standard face-to-face dermatology care. The advantages of S&F teledermatology applications are many, including faster communication with dermatology providers, diagnostic concordance comparable to face-to-face appointments, cost-effective care for patients, the ability to educate providers remotely,8 and similar outcomes to in-person care.9 However, as to be expected, in-person care remains the gold standard, especially when diagnostic accuracy depends on biopsy findings.
The development of the smartphone along with advances in digital photography and consumer-friendly mobile applications has allowed for the emergence of direct-to-consumer (DTC) teledermatology applications. Regardless of the user’s ability, the quality of photographs taken with smartphones has improved, as standard features such as high-resolution cameras with image stabilization, automatic focus, and lighting have become commonplace. The popularity of smartphone technology also has increased, with nearly 75% of all adults and more than 90% of adults younger than 35 years of age owning a smartphone according to a 2016 survey.11
In 2015, there were at least 29 DTC teledermatology applications available on various mobile platforms,12 accounting for an estimated 1.25 million teleconsultations with providers.13 Teledermatology platforms such as DermatologistOnCall and Spruce Health have made accessing dermatologic care convenient, timely, and affordable for patients via patient-friendly mobile applications.
Regular access to dermatologic care is especially important for patients who have chronic skin conditions. Several unique practice models have emerged as innovative solutions to providing more convenient and timely care. For example, Curology (https://curology.com) is an online teledermatology practice specializing in acne treatment.
Although DTC teledermatology practices are convenient for many patients and providers, some have been criticized for providing poor quality of care12 or facilitating fragmented care by not integrating with established electronic health record (EHR) systems.15 As a result, recommended practice guidelines for DTC teledermatology have been developed by the American Academy of Dermatology and some state medical boards.16 Moreover, several EHR systems, such as Epic (www.epic.com) and Modernizing Medicine’s EMA (www.modmed.com), have developed fully integrated S&F teledermatology platforms to be incorporated with established brick-and-mortar care.17
The Future of Teledermatology in the Military
The Army Knowledge Online telemedicine platform used by the US military has continued to be useful, particularly when treating patients in remote locations, and shows promise for improving routine domestic dermatology care. It has reduced the number of medical evacuations and improved care for those who do not have access to a dermatologist.4 Furthermore, one study noted that most consultations submitted via teledermatology applications from a combat zone received a diagnosis and treatment recommendation from a military dermatologist faster than they would have stateside, where the wait often is 4 to 8 weeks. On average, a teledermatology consultation from Afghanistan was answered in less than 6 hours.4 Although this response time might not be realistic for all dermatology practices, there clearly is potential in certain situations and utilizing certain models of care to diagnose and treat more patients more efficiently utilizing teledermatology applications than in an in-person office visit. A review of 658 teledermatology consultations in the US military from January 2011 to December 2012 revealed that the leading diagnoses were eczematous dermatitis (14%), contact dermatitis (9%), nonmelanoma skin cancer (5%), psoriasis (4%), and urticaria (4%).4 Increased use of teledermatology evaluation of these conditions in routine US-based military practice could help expedite care, decrease patient travel time, and utilize in-clinic dermatologist time more efficiently. Teledermatology visits for postoperative concerns also have demonstrated utility and convenience for triage and management of patients in the civilian setting and may be an additional novel use of teledermatology in the military setting.18 With the use of an integrated S&F teledermatology platform within an existing EHR system that is paired with a secure patient mobile application that allows easy upload of photos, medical history, and messaging, it can be argued that quality of life could greatly be enhanced for both military patients and providers.
Limitations of Teledermatology
Certainly, there are and will always be limitations to teledermatology. Even as digital photography improves, the quality and context of clinical images are user dependent, and key associated skin findings in other locations of the body can be missed. The ability to palpate the skin also is lacking in virtual encounters. Therefore, teledermatology might be considered most appropriate for specific diseases and conditions (eg, acne, psoriasis, eczema). Embracing teledermatology does not mean replacing in-person care; rather, it should be seen as an adjunct used to manage the high demand for dermatology expertise in military and civilian practice. For the US military, the promise and potential to embrace innovation in providing dermatologic care is there, as long as there are leaders to continue to champion it. In the current state of health care, many of the perceived barriers of teledermatology applications have already been overcome, including lack of training, lack of reimbursement, and perceived medicolegal risks.19
The US Federal Government is a large entity, and it will undoubtedly take time and effort to implement new and innovative programs such as the ones described here in the military. The first step in implementation is awareness that the possibilities exist; then, with the cooperation of dermatologists and support from the chain of command, it will be possible to incorporate advances in teledermatology and cultivate new ones.
Final Thoughts
The S&F teledermatology method used in the military setting has become commonplace in both military and civilian settings alike. Newer innovations in telemedicine, particularly in teledermatology, will continue to shape the future of military and civilian medicine for years to come.
- Vidmar DA. The history of teledermatology in the Department of Defense. Dermatol Clin. 1999;17:113-124.
- McManus J, Salinas J, Morton M, et al. Teleconsultation program for deployed soldiers and healthcare professionals in remote and austere environments. Prehosp Disaster Med. 2008;23:210-216.
- Tensen E, Van Der Heijden JP, Jaspers MW, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353.
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Shissel DJ, Wilde J. Operational dermatology. Mil Med. 2004;169:444-447.
- Henning JS, Wohltmann W, Hivnor C. Teledermatology from a combat zone. Arch Dermatol. 2010;146:676-677.
- Whited JD, Hall RP, Simel DL, et al. Reliability and accuracy of dermatologists’ clinic-based and digital image consultations. J Am Acad Dermatol. 1999;41:693-702.
- Pak H, Triplett CA, Lindquist JH, et al. Store-and-forward teledermatology results in similar clinical outcomes to conventional clinic-based care. J Telemed Telecare. 2007;13:26-30.
- Finnane A, Dallest K, Janda M, et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327.
- Poushter J. Smartphone ownership and internet usage continues to climb in emerging economies. Washington, DC: Pew Research Center. www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climbin-emerging-economies/. Published February 22, 2016. Accessed February 2, 2018.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Huff C. Medical diagnosis by webcam? Washington, DC: American Association of Retired Persons. www.aarp.org/health/conditions-treatments/info-2015/telemedicine-health-symptoms-diagnosis.html. Published December 2015. Accessed February 2, 2018.
- Mehrotra A. The convenience revolution for the treatment of low-acuity conditions. JAMA. 2013;310:35-36.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Teledermatology toolkit. American Academy of Dermatology website. https://www.aad.org/practicecenter/managing-a-practice/teledermatology. Accessed April 24, 2018.
- Carter ZA, Goldman S, Anderson K, et al. Creation of an internalteledermatology store-and-forward system in an existing electronic health record: a pilot study in a safety-net public health and hospital system. JAMA Dermatol. 2017;153:644-650.
- Jeyamohan SR, Moye MS, Srivastava D, et al. Patient-acquired photographs for the management of postoperative concerns. JAMA Dermatol. 2017;153:226-227.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. Arch Dermatol. 2012;148:650-651.
Telemedicine arose from the need to provide critical and timely advice directly to health care providers and patients in remote or resource-scarce settings. Whether by radio, telephone, or other means of telecommunication technology, the US military has long utilized telemedicine. What started as a way to expedite the delivery of emergency consultations and medical expertise to remote populations in need has since evolved into a billion-dollar innovation industry that is poised to improve health care efficiency and access to specialist care as well as to lower health care costs for all patients.
Teledermatology in the Military
A primary mission of military medicine is to keep service members anywhere in the world in good health on the job during training, combat, and humanitarian operations.1 Telemedicine greatly supports this mission by bringing the expertise of medical specialists to service members in the field without the cost or risks of travel for physicians. Telemedicine also is effective in promoting timely triage of patients and administration of the most appropriate levels of care. With the advent and globalization of high-speed wireless networks, advancements in telemedicine continue to develop and are becoming increasingly useful in military medicine.
As a specialty, dermatology is heavily reliant on visual information and therefore is particularly amenable to telemedicine applications. The rising popularity of such services has led to the development of the term teledermatology. While early teledermatology services were provided using radio, telephone, fax, and videoconferencing,2 three distinct visual methods typically are used today, including (1) store-and-forward (S&F), (2) live-interactive, and (3) a hybrid of the two.3 Military dermatology predominantly utilizes an S&F system, as still photographs of lesions generally are preferred over video for more focused visualization.
In 2004, the US Army Medical Department established a centralized telemedicine program using Army Knowledge Online,1 an S&F system that allows providers in remote locations to store and forward information about a patient’s clinical history along with digital photographs of the patient’s condition to a military dermatologist to review and make a diagnosis or suggest a treatment from a different location at a later time. Using this platform to provide asynchronous teledermatology services avoids the logistics required to schedule appointments and promotes convenience and more efficient use of physicians’ time and resources.
Given the ease of use of S&F systems among military practitioners, dermatology became one of the most heavily utilized teleconsultation specialties within the Army Knowledge Online system, accounting for 40% of the 10,817 consultations initiated from April 2004 to December 2012.5 It also is important to note that skin conditions historically account for 15% to 75% of outpatient visits during wartime; therefore, there is a need for dermatologic consultations, as primary care providers typically are responsible for providing dermatologic care to these patients.6 Because of the high demand for and low volume of US military dermatologists, the use of teledermatology (ie, Amy Knowledge Online) in the US military became a helpful educational tool and specialist extender for many primary care providers in the military.
Teledermatology in the military has evolved to not only provide timely and efficient care but also to reduce health care costs.
Advances in Teledermatology
While the military continues to use S&F teleconsultations—a model in which a deployed referring clinician sends information to a military dermatologist for diagnosis and/or management recommendations—a number of teledermatology programs have been developed for civilians that provide additional advantages over standard face-to-face dermatology care. The advantages of S&F teledermatology applications are many, including faster communication with dermatology providers, diagnostic concordance comparable to face-to-face appointments, cost-effective care for patients, the ability to educate providers remotely,8 and similar outcomes to in-person care.9 However, as to be expected, in-person care remains the gold standard, especially when diagnostic accuracy depends on biopsy findings.
The development of the smartphone along with advances in digital photography and consumer-friendly mobile applications has allowed for the emergence of direct-to-consumer (DTC) teledermatology applications. Regardless of the user’s ability, the quality of photographs taken with smartphones has improved, as standard features such as high-resolution cameras with image stabilization, automatic focus, and lighting have become commonplace. The popularity of smartphone technology also has increased, with nearly 75% of all adults and more than 90% of adults younger than 35 years of age owning a smartphone according to a 2016 survey.11
In 2015, there were at least 29 DTC teledermatology applications available on various mobile platforms,12 accounting for an estimated 1.25 million teleconsultations with providers.13 Teledermatology platforms such as DermatologistOnCall and Spruce Health have made accessing dermatologic care convenient, timely, and affordable for patients via patient-friendly mobile applications.
Regular access to dermatologic care is especially important for patients who have chronic skin conditions. Several unique practice models have emerged as innovative solutions to providing more convenient and timely care. For example, Curology (https://curology.com) is an online teledermatology practice specializing in acne treatment.
Although DTC teledermatology practices are convenient for many patients and providers, some have been criticized for providing poor quality of care12 or facilitating fragmented care by not integrating with established electronic health record (EHR) systems.15 As a result, recommended practice guidelines for DTC teledermatology have been developed by the American Academy of Dermatology and some state medical boards.16 Moreover, several EHR systems, such as Epic (www.epic.com) and Modernizing Medicine’s EMA (www.modmed.com), have developed fully integrated S&F teledermatology platforms to be incorporated with established brick-and-mortar care.17
The Future of Teledermatology in the Military
The Army Knowledge Online telemedicine platform used by the US military has continued to be useful, particularly when treating patients in remote locations, and shows promise for improving routine domestic dermatology care. It has reduced the number of medical evacuations and improved care for those who do not have access to a dermatologist.4 Furthermore, one study noted that most consultations submitted via teledermatology applications from a combat zone received a diagnosis and treatment recommendation from a military dermatologist faster than they would have stateside, where the wait often is 4 to 8 weeks. On average, a teledermatology consultation from Afghanistan was answered in less than 6 hours.4 Although this response time might not be realistic for all dermatology practices, there clearly is potential in certain situations and utilizing certain models of care to diagnose and treat more patients more efficiently utilizing teledermatology applications than in an in-person office visit. A review of 658 teledermatology consultations in the US military from January 2011 to December 2012 revealed that the leading diagnoses were eczematous dermatitis (14%), contact dermatitis (9%), nonmelanoma skin cancer (5%), psoriasis (4%), and urticaria (4%).4 Increased use of teledermatology evaluation of these conditions in routine US-based military practice could help expedite care, decrease patient travel time, and utilize in-clinic dermatologist time more efficiently. Teledermatology visits for postoperative concerns also have demonstrated utility and convenience for triage and management of patients in the civilian setting and may be an additional novel use of teledermatology in the military setting.18 With the use of an integrated S&F teledermatology platform within an existing EHR system that is paired with a secure patient mobile application that allows easy upload of photos, medical history, and messaging, it can be argued that quality of life could greatly be enhanced for both military patients and providers.
Limitations of Teledermatology
Certainly, there are and will always be limitations to teledermatology. Even as digital photography improves, the quality and context of clinical images are user dependent, and key associated skin findings in other locations of the body can be missed. The ability to palpate the skin also is lacking in virtual encounters. Therefore, teledermatology might be considered most appropriate for specific diseases and conditions (eg, acne, psoriasis, eczema). Embracing teledermatology does not mean replacing in-person care; rather, it should be seen as an adjunct used to manage the high demand for dermatology expertise in military and civilian practice. For the US military, the promise and potential to embrace innovation in providing dermatologic care is there, as long as there are leaders to continue to champion it. In the current state of health care, many of the perceived barriers of teledermatology applications have already been overcome, including lack of training, lack of reimbursement, and perceived medicolegal risks.19
The US Federal Government is a large entity, and it will undoubtedly take time and effort to implement new and innovative programs such as the ones described here in the military. The first step in implementation is awareness that the possibilities exist; then, with the cooperation of dermatologists and support from the chain of command, it will be possible to incorporate advances in teledermatology and cultivate new ones.
Final Thoughts
The S&F teledermatology method used in the military setting has become commonplace in both military and civilian settings alike. Newer innovations in telemedicine, particularly in teledermatology, will continue to shape the future of military and civilian medicine for years to come.
Telemedicine arose from the need to provide critical and timely advice directly to health care providers and patients in remote or resource-scarce settings. Whether by radio, telephone, or other means of telecommunication technology, the US military has long utilized telemedicine. What started as a way to expedite the delivery of emergency consultations and medical expertise to remote populations in need has since evolved into a billion-dollar innovation industry that is poised to improve health care efficiency and access to specialist care as well as to lower health care costs for all patients.
Teledermatology in the Military
A primary mission of military medicine is to keep service members anywhere in the world in good health on the job during training, combat, and humanitarian operations.1 Telemedicine greatly supports this mission by bringing the expertise of medical specialists to service members in the field without the cost or risks of travel for physicians. Telemedicine also is effective in promoting timely triage of patients and administration of the most appropriate levels of care. With the advent and globalization of high-speed wireless networks, advancements in telemedicine continue to develop and are becoming increasingly useful in military medicine.
As a specialty, dermatology is heavily reliant on visual information and therefore is particularly amenable to telemedicine applications. The rising popularity of such services has led to the development of the term teledermatology. While early teledermatology services were provided using radio, telephone, fax, and videoconferencing,2 three distinct visual methods typically are used today, including (1) store-and-forward (S&F), (2) live-interactive, and (3) a hybrid of the two.3 Military dermatology predominantly utilizes an S&F system, as still photographs of lesions generally are preferred over video for more focused visualization.
In 2004, the US Army Medical Department established a centralized telemedicine program using Army Knowledge Online,1 an S&F system that allows providers in remote locations to store and forward information about a patient’s clinical history along with digital photographs of the patient’s condition to a military dermatologist to review and make a diagnosis or suggest a treatment from a different location at a later time. Using this platform to provide asynchronous teledermatology services avoids the logistics required to schedule appointments and promotes convenience and more efficient use of physicians’ time and resources.
Given the ease of use of S&F systems among military practitioners, dermatology became one of the most heavily utilized teleconsultation specialties within the Army Knowledge Online system, accounting for 40% of the 10,817 consultations initiated from April 2004 to December 2012.5 It also is important to note that skin conditions historically account for 15% to 75% of outpatient visits during wartime; therefore, there is a need for dermatologic consultations, as primary care providers typically are responsible for providing dermatologic care to these patients.6 Because of the high demand for and low volume of US military dermatologists, the use of teledermatology (ie, Amy Knowledge Online) in the US military became a helpful educational tool and specialist extender for many primary care providers in the military.
Teledermatology in the military has evolved to not only provide timely and efficient care but also to reduce health care costs.
Advances in Teledermatology
While the military continues to use S&F teleconsultations—a model in which a deployed referring clinician sends information to a military dermatologist for diagnosis and/or management recommendations—a number of teledermatology programs have been developed for civilians that provide additional advantages over standard face-to-face dermatology care. The advantages of S&F teledermatology applications are many, including faster communication with dermatology providers, diagnostic concordance comparable to face-to-face appointments, cost-effective care for patients, the ability to educate providers remotely,8 and similar outcomes to in-person care.9 However, as to be expected, in-person care remains the gold standard, especially when diagnostic accuracy depends on biopsy findings.
The development of the smartphone along with advances in digital photography and consumer-friendly mobile applications has allowed for the emergence of direct-to-consumer (DTC) teledermatology applications. Regardless of the user’s ability, the quality of photographs taken with smartphones has improved, as standard features such as high-resolution cameras with image stabilization, automatic focus, and lighting have become commonplace. The popularity of smartphone technology also has increased, with nearly 75% of all adults and more than 90% of adults younger than 35 years of age owning a smartphone according to a 2016 survey.11
In 2015, there were at least 29 DTC teledermatology applications available on various mobile platforms,12 accounting for an estimated 1.25 million teleconsultations with providers.13 Teledermatology platforms such as DermatologistOnCall and Spruce Health have made accessing dermatologic care convenient, timely, and affordable for patients via patient-friendly mobile applications.
Regular access to dermatologic care is especially important for patients who have chronic skin conditions. Several unique practice models have emerged as innovative solutions to providing more convenient and timely care. For example, Curology (https://curology.com) is an online teledermatology practice specializing in acne treatment.
Although DTC teledermatology practices are convenient for many patients and providers, some have been criticized for providing poor quality of care12 or facilitating fragmented care by not integrating with established electronic health record (EHR) systems.15 As a result, recommended practice guidelines for DTC teledermatology have been developed by the American Academy of Dermatology and some state medical boards.16 Moreover, several EHR systems, such as Epic (www.epic.com) and Modernizing Medicine’s EMA (www.modmed.com), have developed fully integrated S&F teledermatology platforms to be incorporated with established brick-and-mortar care.17
The Future of Teledermatology in the Military
The Army Knowledge Online telemedicine platform used by the US military has continued to be useful, particularly when treating patients in remote locations, and shows promise for improving routine domestic dermatology care. It has reduced the number of medical evacuations and improved care for those who do not have access to a dermatologist.4 Furthermore, one study noted that most consultations submitted via teledermatology applications from a combat zone received a diagnosis and treatment recommendation from a military dermatologist faster than they would have stateside, where the wait often is 4 to 8 weeks. On average, a teledermatology consultation from Afghanistan was answered in less than 6 hours.4 Although this response time might not be realistic for all dermatology practices, there clearly is potential in certain situations and utilizing certain models of care to diagnose and treat more patients more efficiently utilizing teledermatology applications than in an in-person office visit. A review of 658 teledermatology consultations in the US military from January 2011 to December 2012 revealed that the leading diagnoses were eczematous dermatitis (14%), contact dermatitis (9%), nonmelanoma skin cancer (5%), psoriasis (4%), and urticaria (4%).4 Increased use of teledermatology evaluation of these conditions in routine US-based military practice could help expedite care, decrease patient travel time, and utilize in-clinic dermatologist time more efficiently. Teledermatology visits for postoperative concerns also have demonstrated utility and convenience for triage and management of patients in the civilian setting and may be an additional novel use of teledermatology in the military setting.18 With the use of an integrated S&F teledermatology platform within an existing EHR system that is paired with a secure patient mobile application that allows easy upload of photos, medical history, and messaging, it can be argued that quality of life could greatly be enhanced for both military patients and providers.
Limitations of Teledermatology
Certainly, there are and will always be limitations to teledermatology. Even as digital photography improves, the quality and context of clinical images are user dependent, and key associated skin findings in other locations of the body can be missed. The ability to palpate the skin also is lacking in virtual encounters. Therefore, teledermatology might be considered most appropriate for specific diseases and conditions (eg, acne, psoriasis, eczema). Embracing teledermatology does not mean replacing in-person care; rather, it should be seen as an adjunct used to manage the high demand for dermatology expertise in military and civilian practice. For the US military, the promise and potential to embrace innovation in providing dermatologic care is there, as long as there are leaders to continue to champion it. In the current state of health care, many of the perceived barriers of teledermatology applications have already been overcome, including lack of training, lack of reimbursement, and perceived medicolegal risks.19
The US Federal Government is a large entity, and it will undoubtedly take time and effort to implement new and innovative programs such as the ones described here in the military. The first step in implementation is awareness that the possibilities exist; then, with the cooperation of dermatologists and support from the chain of command, it will be possible to incorporate advances in teledermatology and cultivate new ones.
Final Thoughts
The S&F teledermatology method used in the military setting has become commonplace in both military and civilian settings alike. Newer innovations in telemedicine, particularly in teledermatology, will continue to shape the future of military and civilian medicine for years to come.
- Vidmar DA. The history of teledermatology in the Department of Defense. Dermatol Clin. 1999;17:113-124.
- McManus J, Salinas J, Morton M, et al. Teleconsultation program for deployed soldiers and healthcare professionals in remote and austere environments. Prehosp Disaster Med. 2008;23:210-216.
- Tensen E, Van Der Heijden JP, Jaspers MW, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353.
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Shissel DJ, Wilde J. Operational dermatology. Mil Med. 2004;169:444-447.
- Henning JS, Wohltmann W, Hivnor C. Teledermatology from a combat zone. Arch Dermatol. 2010;146:676-677.
- Whited JD, Hall RP, Simel DL, et al. Reliability and accuracy of dermatologists’ clinic-based and digital image consultations. J Am Acad Dermatol. 1999;41:693-702.
- Pak H, Triplett CA, Lindquist JH, et al. Store-and-forward teledermatology results in similar clinical outcomes to conventional clinic-based care. J Telemed Telecare. 2007;13:26-30.
- Finnane A, Dallest K, Janda M, et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327.
- Poushter J. Smartphone ownership and internet usage continues to climb in emerging economies. Washington, DC: Pew Research Center. www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climbin-emerging-economies/. Published February 22, 2016. Accessed February 2, 2018.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Huff C. Medical diagnosis by webcam? Washington, DC: American Association of Retired Persons. www.aarp.org/health/conditions-treatments/info-2015/telemedicine-health-symptoms-diagnosis.html. Published December 2015. Accessed February 2, 2018.
- Mehrotra A. The convenience revolution for the treatment of low-acuity conditions. JAMA. 2013;310:35-36.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Teledermatology toolkit. American Academy of Dermatology website. https://www.aad.org/practicecenter/managing-a-practice/teledermatology. Accessed April 24, 2018.
- Carter ZA, Goldman S, Anderson K, et al. Creation of an internalteledermatology store-and-forward system in an existing electronic health record: a pilot study in a safety-net public health and hospital system. JAMA Dermatol. 2017;153:644-650.
- Jeyamohan SR, Moye MS, Srivastava D, et al. Patient-acquired photographs for the management of postoperative concerns. JAMA Dermatol. 2017;153:226-227.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. Arch Dermatol. 2012;148:650-651.
- Vidmar DA. The history of teledermatology in the Department of Defense. Dermatol Clin. 1999;17:113-124.
- McManus J, Salinas J, Morton M, et al. Teleconsultation program for deployed soldiers and healthcare professionals in remote and austere environments. Prehosp Disaster Med. 2008;23:210-216.
- Tensen E, Van Der Heijden JP, Jaspers MW, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353.
- McGraw TA, Norton SA. Military aeromedical evacuations from central and southwest Asia for ill-defined dermatologic diseases. Arch Dermatol. 2009;145:165-170.
- Shissel DJ, Wilde J. Operational dermatology. Mil Med. 2004;169:444-447.
- Henning JS, Wohltmann W, Hivnor C. Teledermatology from a combat zone. Arch Dermatol. 2010;146:676-677.
- Whited JD, Hall RP, Simel DL, et al. Reliability and accuracy of dermatologists’ clinic-based and digital image consultations. J Am Acad Dermatol. 1999;41:693-702.
- Pak H, Triplett CA, Lindquist JH, et al. Store-and-forward teledermatology results in similar clinical outcomes to conventional clinic-based care. J Telemed Telecare. 2007;13:26-30.
- Finnane A, Dallest K, Janda M, et al. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319-327.
- Poushter J. Smartphone ownership and internet usage continues to climb in emerging economies. Washington, DC: Pew Research Center. www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climbin-emerging-economies/. Published February 22, 2016. Accessed February 2, 2018.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Huff C. Medical diagnosis by webcam? Washington, DC: American Association of Retired Persons. www.aarp.org/health/conditions-treatments/info-2015/telemedicine-health-symptoms-diagnosis.html. Published December 2015. Accessed February 2, 2018.
- Mehrotra A. The convenience revolution for the treatment of low-acuity conditions. JAMA. 2013;310:35-36.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Teledermatology toolkit. American Academy of Dermatology website. https://www.aad.org/practicecenter/managing-a-practice/teledermatology. Accessed April 24, 2018.
- Carter ZA, Goldman S, Anderson K, et al. Creation of an internalteledermatology store-and-forward system in an existing electronic health record: a pilot study in a safety-net public health and hospital system. JAMA Dermatol. 2017;153:644-650.
- Jeyamohan SR, Moye MS, Srivastava D, et al. Patient-acquired photographs for the management of postoperative concerns. JAMA Dermatol. 2017;153:226-227.
- Edison KE, Dyer JA, Whited JD, et al. Practice gaps. the barriers and the promise of teledermatology. Arch Dermatol. 2012;148:650-651.
Practice Points
- Teledermatology is increasing in its use and applications in both military and civilian medicine.
- The increased availability of high-quality digital photography as a result of smartphone technology lends itself well to store-and-forward (S&F) teledermatology applications.
- In the civilian community, new methods and platforms for teledermatology have been created based largely on those used by the military to maximize access to and efficiency of health care, including secure direct-to-consumer (DTC) mobile applications, live interactive methods, and integrated S&F platforms within electronic health record (EHR) systems.
Mohs Micrographic Surgery for Digital Melanoma and Nonmelanoma Skin Cancers
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.
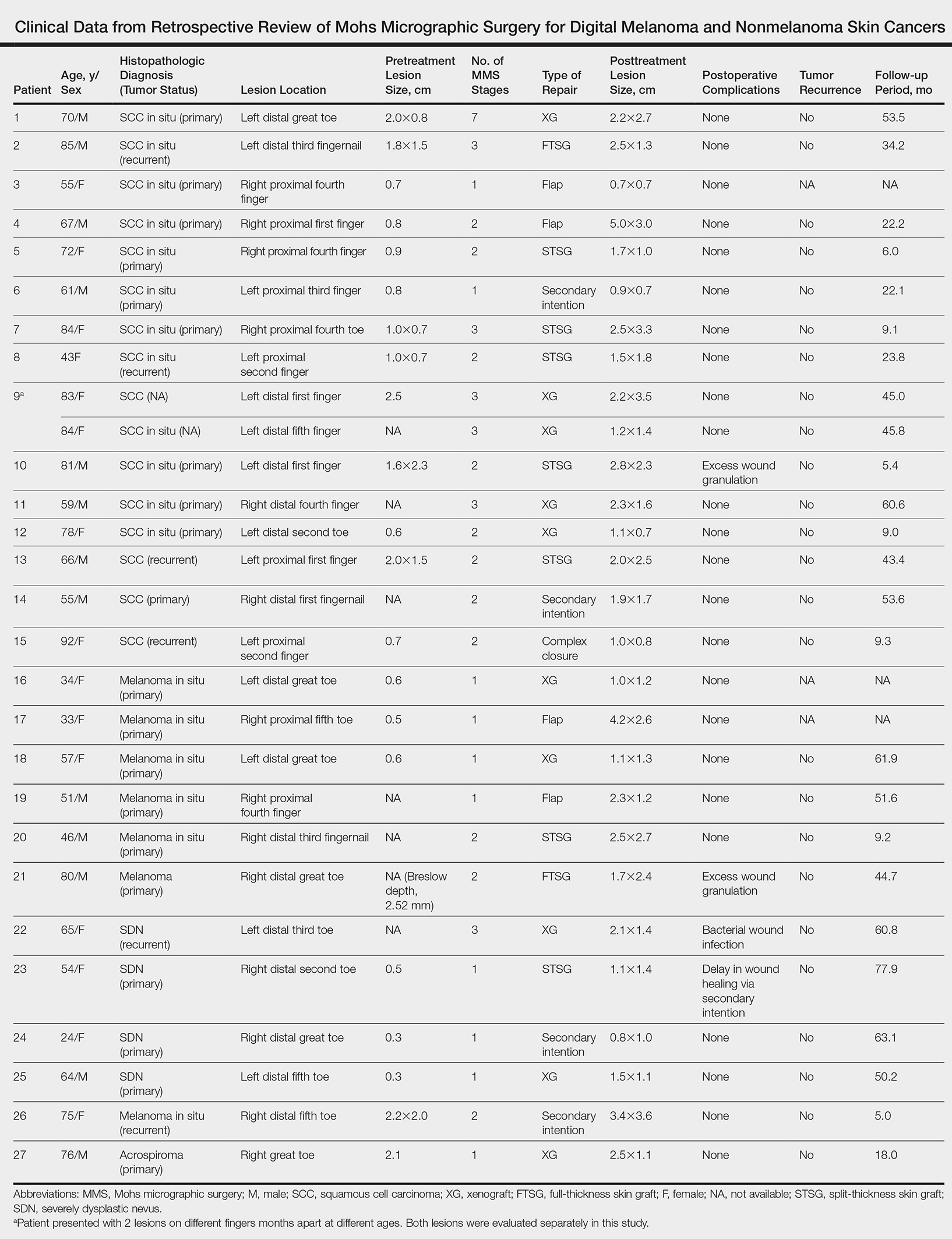
Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.
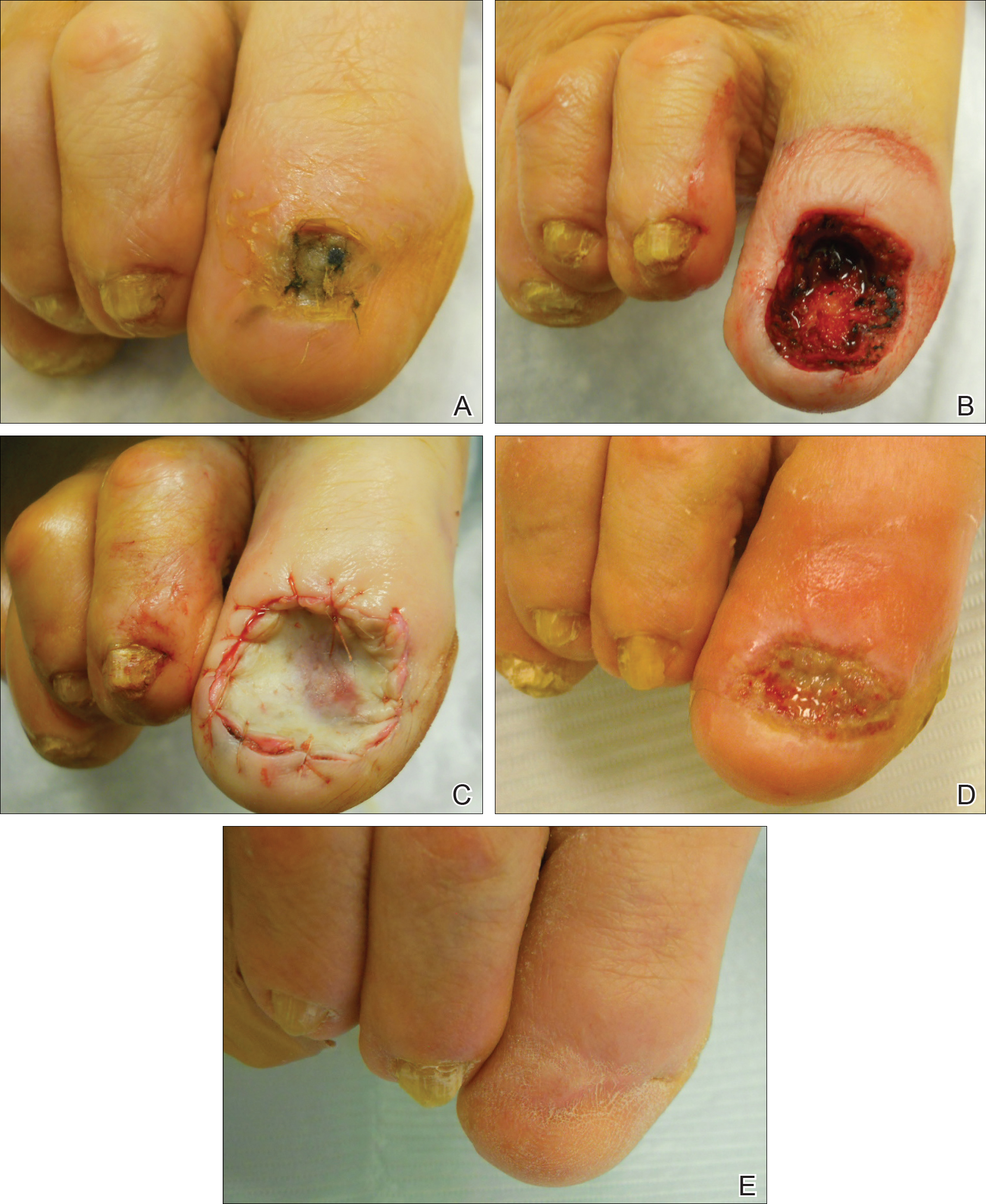
Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.

Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.

Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.

Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.

Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
Practice Points
- Melanoma and nonmelanoma skin cancers of the digits traditionally have been treated with wide local surgical excision and even amputation.
- Conservative tissue sparing techniques such as Mohs micrographic surgery can be used to treat digital skin cancers with high cure rates and improved functional and cosmetic results.