User login
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
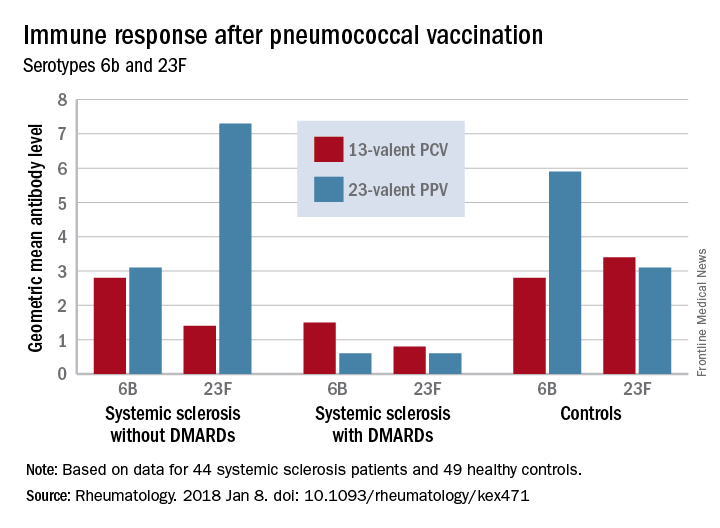
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.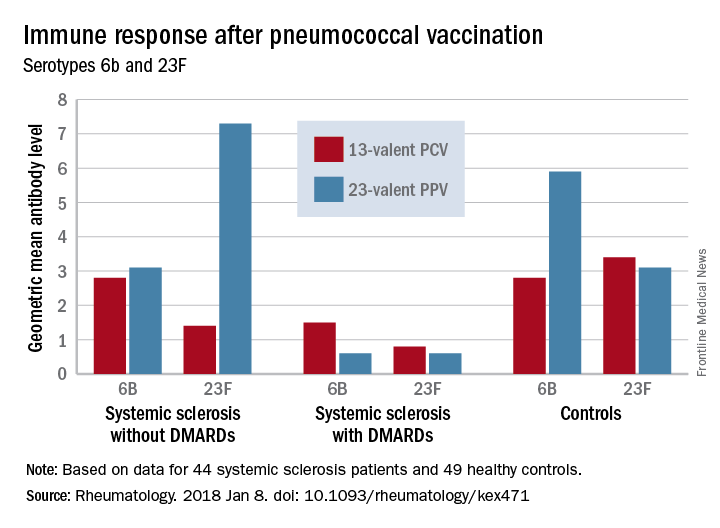
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
Patients taking disease-modifying antirheumatic medications for systemic sclerosis appear to have a decreased response to pneumococcal vaccines, a Swedish study has determined.
Those not taking disease-modifying antirheumatic medications (DMARDs), however, had a normal immune response, suggesting that it’s the immunomodulating medications, not the disease itself, that is affecting antibody levels, Roger Hesselstrand, MD, of Lund (Sweden) University and his colleagues reported online in Rheumatology.
“The currently recommended prime-boost vaccination strategy using a dose of PCV13 [13-valent pneumococcal conjugate vaccine] followed by a dose of PPV23 [23-valent pneumococcal polysaccharide vaccine] might be a possible way of enhancing the vaccine immunogenicity in immunosuppressed patients,” Dr. Hesselstrand and his coauthors wrote.
The study comprised 44 subjects with systemic sclerosis, 12 of whom were taking a DMARD (mycophenolate mofetil, azathioprine, or hydroxychloroquine), and 49 healthy controls; all underwent pneumococcal vaccination. The first 13 got a single dose of PPV23 intramuscularly. PCV13 was then licensed for adults in Sweden, and the remaining 31 patients received this vaccine. The primary outcome was 6-week change from baseline in the level of pneumococcal IgG to Streptococcus pneumoniae serotypes 23F and 6B.
Both vaccines were safe and well-tolerated by all patients, including those taking a DMARD.
Before vaccination, antibody levels to both serotypes were similar between the groups. After vaccination, antibody levels for both serotypes increased significantly in systemic sclerosis patients not taking a DMARD and in controls. However, patients taking a DMARD mounted only an adequate response to serotype 6B.
There were fewer responders among those taking DMARDs, whether they received the PCV13 or the PPV23 vaccine. An increase from prevaccination antibody levels of at least twofold occurred in fewer patients taking DMARDs than did in patients not taking DMARDs and in controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
“We demonstrated that the antibody response ... as well as functionality of antibodies in [systemic sclerosis] patients not receiving DMARDs was as good as in controls regardless of vaccine type,” the investigators concluded. “Systemic sclerosis patients treated with DMARDs, however, had lower proportion of patients with positive antibody response, although the functionality of the antibodies was preserved. These results suggest that immunomodulating drugs but not systemic sclerosis itself and/or immunological disturbance as a part of this disease affect the ability to produce a sufficient amount of vaccine-specific antibodies, but not their function.”
None of the authors had conflicts of interest to disclose.
SOURCE: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471.
FROM RHEUMATOLOGY
Key clinical point:
Major finding: An increase in prevaccination antibody levels of at least twofold occurred in significantly fewer patients taking DMARDs than in patients not taking DMARDs and controls, regardless of vaccine type (PPV23, 50% vs. about 55% and 50%, respectively; PCV13, about 17% vs. 57% and 100%, respectively).
Study details: The prospective study comprised 44 systemic sclerosis patients and 49 healthy controls.
Disclosures: None of the authors had conflicts of interest to disclose.
Source: Hesselstrand R et al. Rheumatology [Oxford]. 2018 Jan 8. doi: 10.1093/rheumatology/kex471