User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
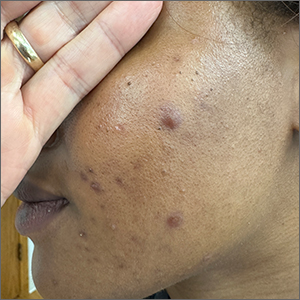
Frustrating facial lesions
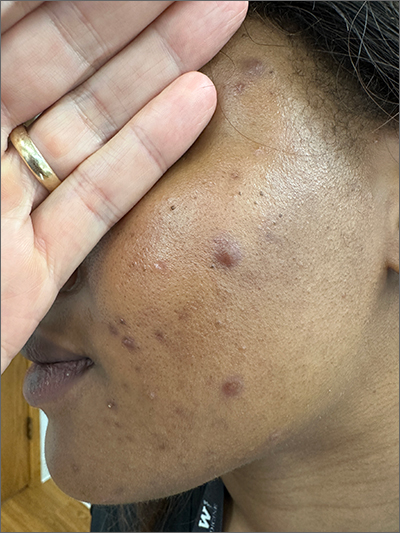
These tender nodules are classic for cystic acne and are common in women older than 20 years. Instead of outgrowing acne in their teenage years, some people (such as this patient) develop frequent tender cystic acne lesions that often heal with hyperpigmented scars.
Acne is the most prevalent chronic skin condition in the United States, affecting up to 50 million people.1 Approximately 12% of adult women are affected.2 The main contributing factors include increased sebum production, follicular hyperkeratinization, microbial follicular colonization with Propionibacterium acnes, and an inflammatory reaction.3
Treatment is available in both topical and oral forms. Topical antibiotics are used predominantly for treating mild-to-moderate inflammatory acne. They are not recommended as monotherapy due to the risk for bacterial resistance; this can be prevented by adding benzoyl peroxide, which exfoliates and acts as an antibacterial agent. Clindamycin 1% solution or gel is the preferred topical antibiotic for treatment of acne.4
Topical retinoids can be used as monotherapy or in combination with antibiotics. They also can be used for maintenance after treatment goals are reached and systemic antibiotics are discontinued. Retinoids generally are applied in the evening because the sun weakens their effect. Patients on retinoids also are more sensitive to the sun and should be counseled to use sunscreen daily. Counseling on pregnancy risks and appropriate use of contraception also should be offered to patients using retinoids. It is advisable to consider the use of combination oral contraceptives, particularly in women who have adult-onset acne or experience flare-ups around the time of their menstrual cycle.3
Azelaic acid has anticomedonal, antibacterial, and anti-inflammatory properties and may be effective in treating mild-to-moderate inflammatory acne and hyperpigmentation. Salicylic acid also has comedolytic properties, although there have been limited studies examining its effectiveness. Both azelaic and salicylic acid are considered safe for use in pregnancy.
Oral antibiotics are recommended in the treatment of moderate-to-severe acne. Both doxycycline and minocycline are more effective than tetracycline for treating acne, with no clear superiority between the two.4 Macrolides also can be effective in treating acne, although their use should be limited to those who cannot tolerate tetracyclines. Systemic antibiotic use should be limited to 3 to 4 months due to decreasing efficacy over time and to minimize the development of bacterial resistance. If treatment goals are attained, the antibiotics can be replaced with retinoids.
Oral isotretinoin is reserved for treatment of severe nodular acne or moderate acne that is treatment resistant. Patients should be counseled on contraceptive methods, as isotretinoin is highly teratogenic and therefore prescribed through the iPLEDGE program.3,4
Given this patient’s persistent symptoms despite use of topical antibiotics and topical tretinoin, she decided to try oral antibiotics (doxycycline 100 mg twice daily) for 3 months and to start long-term oral contraceptives. If her symptoms continue, she will enroll in the iPLEDGE program and start treatment with oral isotretinoin.
Photo courtesy of Ayo Sorunke, MD. Text courtesy of Ayo Sorunke, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.
1. White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi: 10.1016/s0190-9622(98)70442-6
2. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
3. Titus S, Hodge J. Diagnosis and treatment of acne. Am Fam Physician. 2012;86:734-740.
4. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi: 10.1016/j.jaad.2015.12.037

These tender nodules are classic for cystic acne and are common in women older than 20 years. Instead of outgrowing acne in their teenage years, some people (such as this patient) develop frequent tender cystic acne lesions that often heal with hyperpigmented scars.
Acne is the most prevalent chronic skin condition in the United States, affecting up to 50 million people.1 Approximately 12% of adult women are affected.2 The main contributing factors include increased sebum production, follicular hyperkeratinization, microbial follicular colonization with Propionibacterium acnes, and an inflammatory reaction.3
Treatment is available in both topical and oral forms. Topical antibiotics are used predominantly for treating mild-to-moderate inflammatory acne. They are not recommended as monotherapy due to the risk for bacterial resistance; this can be prevented by adding benzoyl peroxide, which exfoliates and acts as an antibacterial agent. Clindamycin 1% solution or gel is the preferred topical antibiotic for treatment of acne.4
Topical retinoids can be used as monotherapy or in combination with antibiotics. They also can be used for maintenance after treatment goals are reached and systemic antibiotics are discontinued. Retinoids generally are applied in the evening because the sun weakens their effect. Patients on retinoids also are more sensitive to the sun and should be counseled to use sunscreen daily. Counseling on pregnancy risks and appropriate use of contraception also should be offered to patients using retinoids. It is advisable to consider the use of combination oral contraceptives, particularly in women who have adult-onset acne or experience flare-ups around the time of their menstrual cycle.3
Azelaic acid has anticomedonal, antibacterial, and anti-inflammatory properties and may be effective in treating mild-to-moderate inflammatory acne and hyperpigmentation. Salicylic acid also has comedolytic properties, although there have been limited studies examining its effectiveness. Both azelaic and salicylic acid are considered safe for use in pregnancy.
Oral antibiotics are recommended in the treatment of moderate-to-severe acne. Both doxycycline and minocycline are more effective than tetracycline for treating acne, with no clear superiority between the two.4 Macrolides also can be effective in treating acne, although their use should be limited to those who cannot tolerate tetracyclines. Systemic antibiotic use should be limited to 3 to 4 months due to decreasing efficacy over time and to minimize the development of bacterial resistance. If treatment goals are attained, the antibiotics can be replaced with retinoids.
Oral isotretinoin is reserved for treatment of severe nodular acne or moderate acne that is treatment resistant. Patients should be counseled on contraceptive methods, as isotretinoin is highly teratogenic and therefore prescribed through the iPLEDGE program.3,4
Given this patient’s persistent symptoms despite use of topical antibiotics and topical tretinoin, she decided to try oral antibiotics (doxycycline 100 mg twice daily) for 3 months and to start long-term oral contraceptives. If her symptoms continue, she will enroll in the iPLEDGE program and start treatment with oral isotretinoin.
Photo courtesy of Ayo Sorunke, MD. Text courtesy of Ayo Sorunke, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.

These tender nodules are classic for cystic acne and are common in women older than 20 years. Instead of outgrowing acne in their teenage years, some people (such as this patient) develop frequent tender cystic acne lesions that often heal with hyperpigmented scars.
Acne is the most prevalent chronic skin condition in the United States, affecting up to 50 million people.1 Approximately 12% of adult women are affected.2 The main contributing factors include increased sebum production, follicular hyperkeratinization, microbial follicular colonization with Propionibacterium acnes, and an inflammatory reaction.3
Treatment is available in both topical and oral forms. Topical antibiotics are used predominantly for treating mild-to-moderate inflammatory acne. They are not recommended as monotherapy due to the risk for bacterial resistance; this can be prevented by adding benzoyl peroxide, which exfoliates and acts as an antibacterial agent. Clindamycin 1% solution or gel is the preferred topical antibiotic for treatment of acne.4
Topical retinoids can be used as monotherapy or in combination with antibiotics. They also can be used for maintenance after treatment goals are reached and systemic antibiotics are discontinued. Retinoids generally are applied in the evening because the sun weakens their effect. Patients on retinoids also are more sensitive to the sun and should be counseled to use sunscreen daily. Counseling on pregnancy risks and appropriate use of contraception also should be offered to patients using retinoids. It is advisable to consider the use of combination oral contraceptives, particularly in women who have adult-onset acne or experience flare-ups around the time of their menstrual cycle.3
Azelaic acid has anticomedonal, antibacterial, and anti-inflammatory properties and may be effective in treating mild-to-moderate inflammatory acne and hyperpigmentation. Salicylic acid also has comedolytic properties, although there have been limited studies examining its effectiveness. Both azelaic and salicylic acid are considered safe for use in pregnancy.
Oral antibiotics are recommended in the treatment of moderate-to-severe acne. Both doxycycline and minocycline are more effective than tetracycline for treating acne, with no clear superiority between the two.4 Macrolides also can be effective in treating acne, although their use should be limited to those who cannot tolerate tetracyclines. Systemic antibiotic use should be limited to 3 to 4 months due to decreasing efficacy over time and to minimize the development of bacterial resistance. If treatment goals are attained, the antibiotics can be replaced with retinoids.
Oral isotretinoin is reserved for treatment of severe nodular acne or moderate acne that is treatment resistant. Patients should be counseled on contraceptive methods, as isotretinoin is highly teratogenic and therefore prescribed through the iPLEDGE program.3,4
Given this patient’s persistent symptoms despite use of topical antibiotics and topical tretinoin, she decided to try oral antibiotics (doxycycline 100 mg twice daily) for 3 months and to start long-term oral contraceptives. If her symptoms continue, she will enroll in the iPLEDGE program and start treatment with oral isotretinoin.
Photo courtesy of Ayo Sorunke, MD. Text courtesy of Ayo Sorunke, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.
1. White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi: 10.1016/s0190-9622(98)70442-6
2. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
3. Titus S, Hodge J. Diagnosis and treatment of acne. Am Fam Physician. 2012;86:734-740.
4. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi: 10.1016/j.jaad.2015.12.037
1. White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi: 10.1016/s0190-9622(98)70442-6
2. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
3. Titus S, Hodge J. Diagnosis and treatment of acne. Am Fam Physician. 2012;86:734-740.
4. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi: 10.1016/j.jaad.2015.12.037
CMS ‘million hearts’ CVD risk reduction model works
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
The Million Hearts Model, a U.S. Centers for Medicare & Medicaid Services (CMS) initiative that encouraged and paid health care organizations to assess and reduce cardiovascular disease (CVD) risk, reduced first-time myocardial infarction (MI) and strokes among Medicare beneficiaries without significant changes in Medicare spending, a randomized trial finds.
METHODOLOGY:
- Researchers assessed the Million Hearts CVD Risk Reduction Model in a pragmatic, cluster-randomized trial among 342 health care organizations – half in the intervention group and half in the standard care control group.
- Among 218,684 medium- or high-risk Medicare beneficiaries (median age, 72 years), 130,578 were in the intervention group in which Medicare paid for guideline-concordant care including routine CVD risk assessment, and 88,286 were in the standard care group.
- Outcomes included first time CVD events (for instance, MI, stroke, transient ischemic attack), combined first-time CVD events and CVD deaths, and Medicare spending.
TAKEAWAY:
- Over a median follow-up of 4.3 years, the intervention group had a 3.3% lower rate of CVD events than the control group (adjusted hazard ratio, 0.97; 90% confidence interval, 0.93-1.00; P = .09) and a 4.2% lower rate of combined first-time CVD events and CVD deaths (HR, 0.96; 90% CI, 0.93-0.99; P = .02).
- These relative effects represent an absolute re.duction of 0.3 percentage points in the probability of a CVD event over 5 years (7.8% intervention vs 8.1%) and 0.4 percentage points in the probability of a CVD event or CVD death over 5 years (9.3% intervention vs. 9.7% control).
- The intervention group also had a 4.3% lower death rate (HR, 0.96; 90% CI, 0.93-0.98; P = .01; absolute reduction of 0.5 percentage points over 5 years).
- Analyses by cause of death showed the largest relative declines (10.6%) among deaths due to coronary heart disease and CVD.
- There was no significant between-group difference in Medicare spending on CVD events or in overall Medicare Parts A and B spending.
IN PRACTICE:
“The model was unique in paying for overall CVD risk reduction, measured by a novel, longitudinal risk calculator, rather than tying performance-based payments to control of individual risk factors,” the authors write.
“The encouraging findings from the Million Hearts Model suggest that modernized payment models may be an affirmative strategy to [incentivize guideline-concordant CVD preventive care and improve outcomes], though further work is needed to ensure that these models are patient-centric, optimally deployed, and equity-enhancing,” add the editorial writers.
SOURCE:
The study, with first author Laura Blue, PhD, Mathematica, Washington, was published online in JAMA, with an accompanying editorial.
LIMITATIONS:
The main limitation is nonparticipation of many of the organizations (516 were randomly assigned to one of the study groups, 342 participated) and incomplete entry of beneficiary data into the registry, which could have led to systematic differences between the two groups. Bias due to the selective participation of organizations and beneficiaries cannot be ruled out.
DISCLOSURES:
Funding for the study was provided by CMS, Department of Health & Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What predicts successful weight loss maintenance in WeightWatchers?
DALLAS – Researchers identified behavioral, psychological, and environmental predictors of continued weight loss maintenance vs. weight regain in a large cohort of members of WeightWatchers who had successful long-term weight loss.
On average, the participants had lost 25.5 kg (56 lb) and kept it off for 3.5 years, when they entered the 1-year study.
At study entry and 1 year later, the participants replied to several questionnaires that asked about predictors of weight loss maintenance.
Compared with people who gained weight over the 1-year study,
They also had reduced disinhibition (tendency to overeat) when faced with food cues, as well as less pain and a more positive body image “at any weight, shape, or size,” Suzanne Phelan, MD, PhD, reported.
Dr. Phelan, from the department of kinesiology and public health, California Polytechnic State University, San Luis Obispo, presented the study in an Obesity symposium at the annual meeting of the Obesity Society, and it was simultaneously published in the journal. The study was selected as one of five top papers submitted to the journal to coincide with the meeting.
Future interventions to prevent weight regain should target overeating in response to internal and external food cues and declines in self-monitoring and body image, Dr. Phelan said.
The study aimed to identify behaviors that might predict who might “beat the odds” and sustain long-term weight loss, she said in an interview.
The findings suggest that the people who maintained their weight loss had developed skills to help them cope with cravings and not respond by eating, she said. Continued self-monitoring and body acceptance and appreciation (all body sizes are beautiful) were key elements of successful weight-loss maintenance.
No antiobesity drugs or surgery; don’t forget behavioral stuff
Importantly, although 43% of the study participants regained more than five pounds during this 1-year study, they still remained at 18% below their starting weight, “indicating that they were largely successful at weight loss,” Dr. Phelan said.
Michael D. Jensen, MD, editor-in-chief of Obesity, echoed this.
The researchers “did find some weak predictors of success,” said Dr. Jensen, from Mayo Clinic, Rochester, Minn. “But perhaps as important,” he said, “was that at the end of the trial, even those who had regained some slight weight still had 18% weight loss – which is not trivial – after, on average, 4.5 years with a standard commercial weight management program.
“At every talk I go to here,” the message is, “Let’s stampede towards use of the drugs and skip diet and exercise and behavioral stuff,” he observed. “I would argue,” he said, “that when it works, it works really well, and it’s free. So this idea that we shouldn’t even try it, because we know it’s going to fail, is wrong.
“If you have the right group, they have a decent chance of having a sufficiently good response that you don’t have to give the medications and you don’t have to send them for bariatric surgery.
“Once you learn from these programs what to do, you’re not paying $1,000 a month for a drug and you haven’t had bariatric surgery,” Dr. Jensen noted. “Their 3 years of follow-up of WeightWatchers cost less than 1 month worth of one of these [antiobesity medications].”
The predictive findings were like ‘”icing on the cake,” he said. Anybody can find five people who’ve done well with therapy, but this study was in more than 2,800 people who did well with a commercial program that is not expensive.
Study design and findings
Between 2019 and 2020, WeightWatchers invited adult members who had maintained weight loss of at least 9.1 kg (20 lb) for at least 1 year to participate in this study.
Of 7,025 participants who entered the study, 4,004 individuals (57%) who did not complete the 1-year questionnaires and others with implausible weight were excluded, leaving a final sample of 2,843 participants.
Most participants were women (92%), non-Hispanic White (95%), married (92%), and college educated. They had a mean age of 56 years.
On average, the participants had a body mass index (BMI) of 35.9 kg/m2 (grade 2 obesity) at the start of the WeightWatchers program and a BMI of 26.7 when they entered the current study.
At the 1-year follow-up, 57% of the participants had maintained the same weight (within 2.3 kg) as when they enrolled in the study, and 43% had gained ≥ 2.3 kg.
On average, the weight-loss maintainers had gained 0.4 kg (0.88 lb). The weight gainers had gained 7.2 kg (15.9 lb) but were still 19.1 kg (42.1 lb) below the weight they had when they started the WeightWatchers program.
At baseline, compared with the weight gainers, the weight-loss maintainers were on average older (58 vs. 52 years), had a lower initial BMI (26 vs. 28), and had longer duration of weight loss maintenance (4 vs. 3 years).
At 1 year, those who had maintained their weight loss had greater self-monitoring, psychological coping, physical activity strategies, dietary choice strategies, and eating and physical activity habits, and they had less eating initiation in the absence of hunger.
They also had less disinhibition, more willingness to ignore cravings and accept food urges, more future orientation, more mindfulness, more positive body image and body satisfaction, better general health and mental health, and less bodily pain.
This research was supported by a grant to Dr. Phelan from WeightWatchers (WW) International, and three study authors are employees and shareholders of the company. Dr. Jensen discloses consulting for Biohaven Pharmaceuticals and for Seattle Gummy Co.
A version of this article first appeared on Medscape.com.
DALLAS – Researchers identified behavioral, psychological, and environmental predictors of continued weight loss maintenance vs. weight regain in a large cohort of members of WeightWatchers who had successful long-term weight loss.
On average, the participants had lost 25.5 kg (56 lb) and kept it off for 3.5 years, when they entered the 1-year study.
At study entry and 1 year later, the participants replied to several questionnaires that asked about predictors of weight loss maintenance.
Compared with people who gained weight over the 1-year study,
They also had reduced disinhibition (tendency to overeat) when faced with food cues, as well as less pain and a more positive body image “at any weight, shape, or size,” Suzanne Phelan, MD, PhD, reported.
Dr. Phelan, from the department of kinesiology and public health, California Polytechnic State University, San Luis Obispo, presented the study in an Obesity symposium at the annual meeting of the Obesity Society, and it was simultaneously published in the journal. The study was selected as one of five top papers submitted to the journal to coincide with the meeting.
Future interventions to prevent weight regain should target overeating in response to internal and external food cues and declines in self-monitoring and body image, Dr. Phelan said.
The study aimed to identify behaviors that might predict who might “beat the odds” and sustain long-term weight loss, she said in an interview.
The findings suggest that the people who maintained their weight loss had developed skills to help them cope with cravings and not respond by eating, she said. Continued self-monitoring and body acceptance and appreciation (all body sizes are beautiful) were key elements of successful weight-loss maintenance.
No antiobesity drugs or surgery; don’t forget behavioral stuff
Importantly, although 43% of the study participants regained more than five pounds during this 1-year study, they still remained at 18% below their starting weight, “indicating that they were largely successful at weight loss,” Dr. Phelan said.
Michael D. Jensen, MD, editor-in-chief of Obesity, echoed this.
The researchers “did find some weak predictors of success,” said Dr. Jensen, from Mayo Clinic, Rochester, Minn. “But perhaps as important,” he said, “was that at the end of the trial, even those who had regained some slight weight still had 18% weight loss – which is not trivial – after, on average, 4.5 years with a standard commercial weight management program.
“At every talk I go to here,” the message is, “Let’s stampede towards use of the drugs and skip diet and exercise and behavioral stuff,” he observed. “I would argue,” he said, “that when it works, it works really well, and it’s free. So this idea that we shouldn’t even try it, because we know it’s going to fail, is wrong.
“If you have the right group, they have a decent chance of having a sufficiently good response that you don’t have to give the medications and you don’t have to send them for bariatric surgery.
“Once you learn from these programs what to do, you’re not paying $1,000 a month for a drug and you haven’t had bariatric surgery,” Dr. Jensen noted. “Their 3 years of follow-up of WeightWatchers cost less than 1 month worth of one of these [antiobesity medications].”
The predictive findings were like ‘”icing on the cake,” he said. Anybody can find five people who’ve done well with therapy, but this study was in more than 2,800 people who did well with a commercial program that is not expensive.
Study design and findings
Between 2019 and 2020, WeightWatchers invited adult members who had maintained weight loss of at least 9.1 kg (20 lb) for at least 1 year to participate in this study.
Of 7,025 participants who entered the study, 4,004 individuals (57%) who did not complete the 1-year questionnaires and others with implausible weight were excluded, leaving a final sample of 2,843 participants.
Most participants were women (92%), non-Hispanic White (95%), married (92%), and college educated. They had a mean age of 56 years.
On average, the participants had a body mass index (BMI) of 35.9 kg/m2 (grade 2 obesity) at the start of the WeightWatchers program and a BMI of 26.7 when they entered the current study.
At the 1-year follow-up, 57% of the participants had maintained the same weight (within 2.3 kg) as when they enrolled in the study, and 43% had gained ≥ 2.3 kg.
On average, the weight-loss maintainers had gained 0.4 kg (0.88 lb). The weight gainers had gained 7.2 kg (15.9 lb) but were still 19.1 kg (42.1 lb) below the weight they had when they started the WeightWatchers program.
At baseline, compared with the weight gainers, the weight-loss maintainers were on average older (58 vs. 52 years), had a lower initial BMI (26 vs. 28), and had longer duration of weight loss maintenance (4 vs. 3 years).
At 1 year, those who had maintained their weight loss had greater self-monitoring, psychological coping, physical activity strategies, dietary choice strategies, and eating and physical activity habits, and they had less eating initiation in the absence of hunger.
They also had less disinhibition, more willingness to ignore cravings and accept food urges, more future orientation, more mindfulness, more positive body image and body satisfaction, better general health and mental health, and less bodily pain.
This research was supported by a grant to Dr. Phelan from WeightWatchers (WW) International, and three study authors are employees and shareholders of the company. Dr. Jensen discloses consulting for Biohaven Pharmaceuticals and for Seattle Gummy Co.
A version of this article first appeared on Medscape.com.
DALLAS – Researchers identified behavioral, psychological, and environmental predictors of continued weight loss maintenance vs. weight regain in a large cohort of members of WeightWatchers who had successful long-term weight loss.
On average, the participants had lost 25.5 kg (56 lb) and kept it off for 3.5 years, when they entered the 1-year study.
At study entry and 1 year later, the participants replied to several questionnaires that asked about predictors of weight loss maintenance.
Compared with people who gained weight over the 1-year study,
They also had reduced disinhibition (tendency to overeat) when faced with food cues, as well as less pain and a more positive body image “at any weight, shape, or size,” Suzanne Phelan, MD, PhD, reported.
Dr. Phelan, from the department of kinesiology and public health, California Polytechnic State University, San Luis Obispo, presented the study in an Obesity symposium at the annual meeting of the Obesity Society, and it was simultaneously published in the journal. The study was selected as one of five top papers submitted to the journal to coincide with the meeting.
Future interventions to prevent weight regain should target overeating in response to internal and external food cues and declines in self-monitoring and body image, Dr. Phelan said.
The study aimed to identify behaviors that might predict who might “beat the odds” and sustain long-term weight loss, she said in an interview.
The findings suggest that the people who maintained their weight loss had developed skills to help them cope with cravings and not respond by eating, she said. Continued self-monitoring and body acceptance and appreciation (all body sizes are beautiful) were key elements of successful weight-loss maintenance.
No antiobesity drugs or surgery; don’t forget behavioral stuff
Importantly, although 43% of the study participants regained more than five pounds during this 1-year study, they still remained at 18% below their starting weight, “indicating that they were largely successful at weight loss,” Dr. Phelan said.
Michael D. Jensen, MD, editor-in-chief of Obesity, echoed this.
The researchers “did find some weak predictors of success,” said Dr. Jensen, from Mayo Clinic, Rochester, Minn. “But perhaps as important,” he said, “was that at the end of the trial, even those who had regained some slight weight still had 18% weight loss – which is not trivial – after, on average, 4.5 years with a standard commercial weight management program.
“At every talk I go to here,” the message is, “Let’s stampede towards use of the drugs and skip diet and exercise and behavioral stuff,” he observed. “I would argue,” he said, “that when it works, it works really well, and it’s free. So this idea that we shouldn’t even try it, because we know it’s going to fail, is wrong.
“If you have the right group, they have a decent chance of having a sufficiently good response that you don’t have to give the medications and you don’t have to send them for bariatric surgery.
“Once you learn from these programs what to do, you’re not paying $1,000 a month for a drug and you haven’t had bariatric surgery,” Dr. Jensen noted. “Their 3 years of follow-up of WeightWatchers cost less than 1 month worth of one of these [antiobesity medications].”
The predictive findings were like ‘”icing on the cake,” he said. Anybody can find five people who’ve done well with therapy, but this study was in more than 2,800 people who did well with a commercial program that is not expensive.
Study design and findings
Between 2019 and 2020, WeightWatchers invited adult members who had maintained weight loss of at least 9.1 kg (20 lb) for at least 1 year to participate in this study.
Of 7,025 participants who entered the study, 4,004 individuals (57%) who did not complete the 1-year questionnaires and others with implausible weight were excluded, leaving a final sample of 2,843 participants.
Most participants were women (92%), non-Hispanic White (95%), married (92%), and college educated. They had a mean age of 56 years.
On average, the participants had a body mass index (BMI) of 35.9 kg/m2 (grade 2 obesity) at the start of the WeightWatchers program and a BMI of 26.7 when they entered the current study.
At the 1-year follow-up, 57% of the participants had maintained the same weight (within 2.3 kg) as when they enrolled in the study, and 43% had gained ≥ 2.3 kg.
On average, the weight-loss maintainers had gained 0.4 kg (0.88 lb). The weight gainers had gained 7.2 kg (15.9 lb) but were still 19.1 kg (42.1 lb) below the weight they had when they started the WeightWatchers program.
At baseline, compared with the weight gainers, the weight-loss maintainers were on average older (58 vs. 52 years), had a lower initial BMI (26 vs. 28), and had longer duration of weight loss maintenance (4 vs. 3 years).
At 1 year, those who had maintained their weight loss had greater self-monitoring, psychological coping, physical activity strategies, dietary choice strategies, and eating and physical activity habits, and they had less eating initiation in the absence of hunger.
They also had less disinhibition, more willingness to ignore cravings and accept food urges, more future orientation, more mindfulness, more positive body image and body satisfaction, better general health and mental health, and less bodily pain.
This research was supported by a grant to Dr. Phelan from WeightWatchers (WW) International, and three study authors are employees and shareholders of the company. Dr. Jensen discloses consulting for Biohaven Pharmaceuticals and for Seattle Gummy Co.
A version of this article first appeared on Medscape.com.
FROM OBESITY WEEK® 2023
When digestive symptoms signal Parkinson’s disease
The enteric nervous system (ENS), which is regarded as our second brain, is the part of the autonomic nervous system that controls the digestive tract. Housed along the entire length of the digestive tract, it is made up of more than 100 million neurons. It plays a central role in controlling the regulation of gastrointestinal motility, absorption of nutrients, and control of the intestinal barrier that protects the body from external pathogens.
Braak’s hypothesis suggests that the digestive tract could be the starting point for Parkinson’s disease. The fact that nearly all patients with Parkinson’s disease experience digestive problems and have neuropathological lesions in intrinsic and extrinsic innervation of the gastrointestinal tract suggests that Parkinson’s disease also has a gastrointestinal component.
Besides the ascending pathway formulated by Braak, a descending etiology in which gastrointestinal symptoms are present in early stages when neurological signposts have not yet been noticed is supported by evidence from trials. These gastrointestinal symptoms then represent a risk factor. Links have also been described between a history of gastrointestinal symptoms and Alzheimer’s disease and cerebrovascular diseases (CVD), thus justifying studies on a larger scale.
Large combined study
The authors have conducted a combined case-control and cohort study using TriNetX, a national network of medical records based in the United States. They identified 24,624 patients with idiopathic Parkinson’s disease in the case-control analysis and compared them with control subjects without neurological disease. They also identified subjects with Alzheimer’s disease and CVD, to study previous gastrointestinal signs. Secondly, 18 cohorts with each exposure (various gastrointestinal symptoms, appendectomy, vagotomy) were compared with their negative controls (NC) for the development of Parkinson’s disease, Alzheimer’s disease, or CVD in 5 years.
Gastroparesis, dysphagia, and irritable bowel syndrome (IBS) without diarrhea or constipation were shown to have specific associations with Parkinson’s disease (vs. NC, Alzheimer’s disease, and CVD) in both case-controls (odds ratios all P < .0001) and cohort analyses (relative risks all P < .05). While functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not specific to Parkinson’s disease, IBS with constipation and intestinal pseudo-obstruction showed specificity to Parkinson’s disease in the case-control (OR, 4.11) and cohort (RR, 1.84) analyses. Appendectomy reduced the risk of Parkinson’s disease in the cohort study (RR, 0.48). Neither inflammatory bowel disease nor vagotomy was associated with Parkinson’s disease.
A ‘second brain’
This broad study attempted to explore the gut-brain axis by looking for associations between neurological diagnoses and prior gastrointestinal symptoms and later development of Parkinson’s disease. After adjustment to account for multiple comparisons and acknowledgment of the initial risk in patients with Alzheimer’s disease and CVD, only dysphagia, gastroparesis, IBS without diarrhea, and isolated constipation were significantly and specifically associated with Parkinson’s disease.
Numerous literature reviews mention that ENS lesions are responsible for gastrointestinal disorders observed in patients with Parkinson’s disease. Tests on gastrointestinal autopsy and biopsy specimens have established that alpha synuclein clusters, which are morphologically similar to Lewy bodies in the CNS, are seen in the vagus nerve and in the ENS in most subjects with Parkinson’s disease. However, these studies have not shown any loss of neurons in the ENS in Parkinson’s disease, and the presence of alpha synuclein deposits in the ENS is not sufficient in itself to explain these gastrointestinal disorders.
It therefore remains to be determined whether vagal nerve damage alone can explain gastrointestinal disorders or whether dysfunction of enteric neurons without neuronal loss is occurring. So, damage to the ENS from alpha synuclein deposits would be early and would precede damage to the CNS, thus affording evidence in support of Braak’s hypothesis, which relies on autopsy data that does not allow for longitudinal monitoring in a single individual.
Appendectomy appeared to be protective, leading to additional speculation about its role in the pathophysiology of Parkinson’s disease. Additional mechanistic studies are therefore needed to establish causality and confirm the gut-brain axis or the role of dysbiosis and of intestinal permeability problems.
In conclusion, this large, first-of-its-kind multicenter study conducted on a national scale shows that Subject to future longitudinal mechanistic studies, early detection of these gastrointestinal disorders could aid in identifying patients at risk of Parkinson’s, and it could then be assumed that disease-modifying treatments could, at this early stage, halt progression of the disease linked to toxic clusters of alpha synuclein.
This article was translated from JIM, which is part of the Medscape professional network.
A version of this article first appeared on Medscape.com.
The enteric nervous system (ENS), which is regarded as our second brain, is the part of the autonomic nervous system that controls the digestive tract. Housed along the entire length of the digestive tract, it is made up of more than 100 million neurons. It plays a central role in controlling the regulation of gastrointestinal motility, absorption of nutrients, and control of the intestinal barrier that protects the body from external pathogens.
Braak’s hypothesis suggests that the digestive tract could be the starting point for Parkinson’s disease. The fact that nearly all patients with Parkinson’s disease experience digestive problems and have neuropathological lesions in intrinsic and extrinsic innervation of the gastrointestinal tract suggests that Parkinson’s disease also has a gastrointestinal component.
Besides the ascending pathway formulated by Braak, a descending etiology in which gastrointestinal symptoms are present in early stages when neurological signposts have not yet been noticed is supported by evidence from trials. These gastrointestinal symptoms then represent a risk factor. Links have also been described between a history of gastrointestinal symptoms and Alzheimer’s disease and cerebrovascular diseases (CVD), thus justifying studies on a larger scale.
Large combined study
The authors have conducted a combined case-control and cohort study using TriNetX, a national network of medical records based in the United States. They identified 24,624 patients with idiopathic Parkinson’s disease in the case-control analysis and compared them with control subjects without neurological disease. They also identified subjects with Alzheimer’s disease and CVD, to study previous gastrointestinal signs. Secondly, 18 cohorts with each exposure (various gastrointestinal symptoms, appendectomy, vagotomy) were compared with their negative controls (NC) for the development of Parkinson’s disease, Alzheimer’s disease, or CVD in 5 years.
Gastroparesis, dysphagia, and irritable bowel syndrome (IBS) without diarrhea or constipation were shown to have specific associations with Parkinson’s disease (vs. NC, Alzheimer’s disease, and CVD) in both case-controls (odds ratios all P < .0001) and cohort analyses (relative risks all P < .05). While functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not specific to Parkinson’s disease, IBS with constipation and intestinal pseudo-obstruction showed specificity to Parkinson’s disease in the case-control (OR, 4.11) and cohort (RR, 1.84) analyses. Appendectomy reduced the risk of Parkinson’s disease in the cohort study (RR, 0.48). Neither inflammatory bowel disease nor vagotomy was associated with Parkinson’s disease.
A ‘second brain’
This broad study attempted to explore the gut-brain axis by looking for associations between neurological diagnoses and prior gastrointestinal symptoms and later development of Parkinson’s disease. After adjustment to account for multiple comparisons and acknowledgment of the initial risk in patients with Alzheimer’s disease and CVD, only dysphagia, gastroparesis, IBS without diarrhea, and isolated constipation were significantly and specifically associated with Parkinson’s disease.
Numerous literature reviews mention that ENS lesions are responsible for gastrointestinal disorders observed in patients with Parkinson’s disease. Tests on gastrointestinal autopsy and biopsy specimens have established that alpha synuclein clusters, which are morphologically similar to Lewy bodies in the CNS, are seen in the vagus nerve and in the ENS in most subjects with Parkinson’s disease. However, these studies have not shown any loss of neurons in the ENS in Parkinson’s disease, and the presence of alpha synuclein deposits in the ENS is not sufficient in itself to explain these gastrointestinal disorders.
It therefore remains to be determined whether vagal nerve damage alone can explain gastrointestinal disorders or whether dysfunction of enteric neurons without neuronal loss is occurring. So, damage to the ENS from alpha synuclein deposits would be early and would precede damage to the CNS, thus affording evidence in support of Braak’s hypothesis, which relies on autopsy data that does not allow for longitudinal monitoring in a single individual.
Appendectomy appeared to be protective, leading to additional speculation about its role in the pathophysiology of Parkinson’s disease. Additional mechanistic studies are therefore needed to establish causality and confirm the gut-brain axis or the role of dysbiosis and of intestinal permeability problems.
In conclusion, this large, first-of-its-kind multicenter study conducted on a national scale shows that Subject to future longitudinal mechanistic studies, early detection of these gastrointestinal disorders could aid in identifying patients at risk of Parkinson’s, and it could then be assumed that disease-modifying treatments could, at this early stage, halt progression of the disease linked to toxic clusters of alpha synuclein.
This article was translated from JIM, which is part of the Medscape professional network.
A version of this article first appeared on Medscape.com.
The enteric nervous system (ENS), which is regarded as our second brain, is the part of the autonomic nervous system that controls the digestive tract. Housed along the entire length of the digestive tract, it is made up of more than 100 million neurons. It plays a central role in controlling the regulation of gastrointestinal motility, absorption of nutrients, and control of the intestinal barrier that protects the body from external pathogens.
Braak’s hypothesis suggests that the digestive tract could be the starting point for Parkinson’s disease. The fact that nearly all patients with Parkinson’s disease experience digestive problems and have neuropathological lesions in intrinsic and extrinsic innervation of the gastrointestinal tract suggests that Parkinson’s disease also has a gastrointestinal component.
Besides the ascending pathway formulated by Braak, a descending etiology in which gastrointestinal symptoms are present in early stages when neurological signposts have not yet been noticed is supported by evidence from trials. These gastrointestinal symptoms then represent a risk factor. Links have also been described between a history of gastrointestinal symptoms and Alzheimer’s disease and cerebrovascular diseases (CVD), thus justifying studies on a larger scale.
Large combined study
The authors have conducted a combined case-control and cohort study using TriNetX, a national network of medical records based in the United States. They identified 24,624 patients with idiopathic Parkinson’s disease in the case-control analysis and compared them with control subjects without neurological disease. They also identified subjects with Alzheimer’s disease and CVD, to study previous gastrointestinal signs. Secondly, 18 cohorts with each exposure (various gastrointestinal symptoms, appendectomy, vagotomy) were compared with their negative controls (NC) for the development of Parkinson’s disease, Alzheimer’s disease, or CVD in 5 years.
Gastroparesis, dysphagia, and irritable bowel syndrome (IBS) without diarrhea or constipation were shown to have specific associations with Parkinson’s disease (vs. NC, Alzheimer’s disease, and CVD) in both case-controls (odds ratios all P < .0001) and cohort analyses (relative risks all P < .05). While functional dyspepsia, IBS with diarrhea, diarrhea, and fecal incontinence were not specific to Parkinson’s disease, IBS with constipation and intestinal pseudo-obstruction showed specificity to Parkinson’s disease in the case-control (OR, 4.11) and cohort (RR, 1.84) analyses. Appendectomy reduced the risk of Parkinson’s disease in the cohort study (RR, 0.48). Neither inflammatory bowel disease nor vagotomy was associated with Parkinson’s disease.
A ‘second brain’
This broad study attempted to explore the gut-brain axis by looking for associations between neurological diagnoses and prior gastrointestinal symptoms and later development of Parkinson’s disease. After adjustment to account for multiple comparisons and acknowledgment of the initial risk in patients with Alzheimer’s disease and CVD, only dysphagia, gastroparesis, IBS without diarrhea, and isolated constipation were significantly and specifically associated with Parkinson’s disease.
Numerous literature reviews mention that ENS lesions are responsible for gastrointestinal disorders observed in patients with Parkinson’s disease. Tests on gastrointestinal autopsy and biopsy specimens have established that alpha synuclein clusters, which are morphologically similar to Lewy bodies in the CNS, are seen in the vagus nerve and in the ENS in most subjects with Parkinson’s disease. However, these studies have not shown any loss of neurons in the ENS in Parkinson’s disease, and the presence of alpha synuclein deposits in the ENS is not sufficient in itself to explain these gastrointestinal disorders.
It therefore remains to be determined whether vagal nerve damage alone can explain gastrointestinal disorders or whether dysfunction of enteric neurons without neuronal loss is occurring. So, damage to the ENS from alpha synuclein deposits would be early and would precede damage to the CNS, thus affording evidence in support of Braak’s hypothesis, which relies on autopsy data that does not allow for longitudinal monitoring in a single individual.
Appendectomy appeared to be protective, leading to additional speculation about its role in the pathophysiology of Parkinson’s disease. Additional mechanistic studies are therefore needed to establish causality and confirm the gut-brain axis or the role of dysbiosis and of intestinal permeability problems.
In conclusion, this large, first-of-its-kind multicenter study conducted on a national scale shows that Subject to future longitudinal mechanistic studies, early detection of these gastrointestinal disorders could aid in identifying patients at risk of Parkinson’s, and it could then be assumed that disease-modifying treatments could, at this early stage, halt progression of the disease linked to toxic clusters of alpha synuclein.
This article was translated from JIM, which is part of the Medscape professional network.
A version of this article first appeared on Medscape.com.
Playing board games may slow cognitive decline, improve QoL
“For patients who are elderly and suffer from social isolation and mild cognitive issues, I would definitely recommend board games,” study investigator Frederico Emanuele Pozzi, MD, a neurology resident at Fondazione IRCCS San Gerardo dei Tintori in Monza, Italy, told this news organization.
The findings were presented at the virtual XXVI World Congress of Neurology (WCN).
After searching the published literature, Dr. Pozzi and his colleagues selected 15 studies for the review. The studies assessed the impact of board games on older individuals at risk of or with cognitive impairment, or those with mild cognitive impairment (MCI) at any age.
The studies included different board games including chess, Mah-jongg, and Go, a two-player game popular in China, Japan, and Korea that involves moving board pieces to surround and capture as much territory as possible.
Most interventions lasted about an hour and were held once or twice per week for 3-4 months.
Which games are best?
Researchers found that board games improved cognitive function, as measured by improved scores on the Montreal Cognitive Assessment (P = .003) and Mini-Mental State Examination (P = .02).
Playing Go was linked with improved working memory, as measured by the Trail Making Test-A. Those who played Mah-jongg reported improved executive functioning and a temporary decrease in depressive symptoms. And chess players reported improved quality of life on the World Health Organization Quality of Life scale from playing chess (P < .00001).
In general, cognition improved across different populations. For example, some studies looked at unimpaired elderly while others looked at MCI, said Dr. Pozzi.
Playing board games in a social context appeared to be especially good at boosting brain power. One Japanese study included a control group that just did tai chi, a group that did Go alone on tablets, and another group that did Go in groups. Both Go groups improved cognitively, but participants who played together improved the most.
The results also seemed to suggest that Go and chess have different biological effects. “For example, Go increased [brain-derived neurotrophic factor] (BDNF) levels and metabolism in areas key for cognition like the middle temporal gyrus,” Dr. Pozzi said.
He noted that the methodology of the studies was generally “not bad,” although in some cases the analyses were per protocol and in others intention-to-treat. Outcomes varied across studies, there were a lot of dropouts, and some were not randomized, meaning reverse causality can’t be ruled out.
Dr. Pozzi has started a randomized controlled trial at a Go and chess club in Italy. He’s enrolling patients aged 60 and over with subjective cognitive decline or MCI and separating participants into a control group, a group that plays chess, another that plays Go, and another that plays both Go and chess.
In addition to the standard cognitive tests, and measures of depression and quality of life, Dr. Pozzi aims to assess cognitive reserve and is in the process of validating a questionnaire that will look at leisure activities and lifestyle.
Social and cognitive value
Commenting on the research for this news organization, Vladimir Hachinski, MD, a professor of clinical neurological sciences at Western University in London, Ont., said the results make sense.
Playing a board game involves concentration, strategy, and intermittent rewards – all of which are good for the brain and may involve the prefrontal cortex, he noted. Board games are also typically timed, which involves brain speed processing, and they have a winner and loser so emotions can run high, which also affects the brain, Dr. Hachinski added.
There may also be social value in playing a board game with someone else, added Dr. Hachinski.
“It’s encouraging that people can improve what they’re doing, and the longer they’re at it, the more of the brain they use,” he said. “There might be a long-term effect because players are building up networks.”
But Dr. Hachinski cautioned that playing a lot of chess does not necessarily make you a better thinker, just as learning to play one instrument doesn’t mean you can automatically play others.
“Learning one skill will translate only partially to another, and only if it’s related,” he said. “It increases cognition in the area you’re practicing in, but it doesn’t spread to other areas.”
Dr. Pozzi and Dr. Hachinski report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“For patients who are elderly and suffer from social isolation and mild cognitive issues, I would definitely recommend board games,” study investigator Frederico Emanuele Pozzi, MD, a neurology resident at Fondazione IRCCS San Gerardo dei Tintori in Monza, Italy, told this news organization.
The findings were presented at the virtual XXVI World Congress of Neurology (WCN).
After searching the published literature, Dr. Pozzi and his colleagues selected 15 studies for the review. The studies assessed the impact of board games on older individuals at risk of or with cognitive impairment, or those with mild cognitive impairment (MCI) at any age.
The studies included different board games including chess, Mah-jongg, and Go, a two-player game popular in China, Japan, and Korea that involves moving board pieces to surround and capture as much territory as possible.
Most interventions lasted about an hour and were held once or twice per week for 3-4 months.
Which games are best?
Researchers found that board games improved cognitive function, as measured by improved scores on the Montreal Cognitive Assessment (P = .003) and Mini-Mental State Examination (P = .02).
Playing Go was linked with improved working memory, as measured by the Trail Making Test-A. Those who played Mah-jongg reported improved executive functioning and a temporary decrease in depressive symptoms. And chess players reported improved quality of life on the World Health Organization Quality of Life scale from playing chess (P < .00001).
In general, cognition improved across different populations. For example, some studies looked at unimpaired elderly while others looked at MCI, said Dr. Pozzi.
Playing board games in a social context appeared to be especially good at boosting brain power. One Japanese study included a control group that just did tai chi, a group that did Go alone on tablets, and another group that did Go in groups. Both Go groups improved cognitively, but participants who played together improved the most.
The results also seemed to suggest that Go and chess have different biological effects. “For example, Go increased [brain-derived neurotrophic factor] (BDNF) levels and metabolism in areas key for cognition like the middle temporal gyrus,” Dr. Pozzi said.
He noted that the methodology of the studies was generally “not bad,” although in some cases the analyses were per protocol and in others intention-to-treat. Outcomes varied across studies, there were a lot of dropouts, and some were not randomized, meaning reverse causality can’t be ruled out.
Dr. Pozzi has started a randomized controlled trial at a Go and chess club in Italy. He’s enrolling patients aged 60 and over with subjective cognitive decline or MCI and separating participants into a control group, a group that plays chess, another that plays Go, and another that plays both Go and chess.
In addition to the standard cognitive tests, and measures of depression and quality of life, Dr. Pozzi aims to assess cognitive reserve and is in the process of validating a questionnaire that will look at leisure activities and lifestyle.
Social and cognitive value
Commenting on the research for this news organization, Vladimir Hachinski, MD, a professor of clinical neurological sciences at Western University in London, Ont., said the results make sense.
Playing a board game involves concentration, strategy, and intermittent rewards – all of which are good for the brain and may involve the prefrontal cortex, he noted. Board games are also typically timed, which involves brain speed processing, and they have a winner and loser so emotions can run high, which also affects the brain, Dr. Hachinski added.
There may also be social value in playing a board game with someone else, added Dr. Hachinski.
“It’s encouraging that people can improve what they’re doing, and the longer they’re at it, the more of the brain they use,” he said. “There might be a long-term effect because players are building up networks.”
But Dr. Hachinski cautioned that playing a lot of chess does not necessarily make you a better thinker, just as learning to play one instrument doesn’t mean you can automatically play others.
“Learning one skill will translate only partially to another, and only if it’s related,” he said. “It increases cognition in the area you’re practicing in, but it doesn’t spread to other areas.”
Dr. Pozzi and Dr. Hachinski report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“For patients who are elderly and suffer from social isolation and mild cognitive issues, I would definitely recommend board games,” study investigator Frederico Emanuele Pozzi, MD, a neurology resident at Fondazione IRCCS San Gerardo dei Tintori in Monza, Italy, told this news organization.
The findings were presented at the virtual XXVI World Congress of Neurology (WCN).
After searching the published literature, Dr. Pozzi and his colleagues selected 15 studies for the review. The studies assessed the impact of board games on older individuals at risk of or with cognitive impairment, or those with mild cognitive impairment (MCI) at any age.
The studies included different board games including chess, Mah-jongg, and Go, a two-player game popular in China, Japan, and Korea that involves moving board pieces to surround and capture as much territory as possible.
Most interventions lasted about an hour and were held once or twice per week for 3-4 months.
Which games are best?
Researchers found that board games improved cognitive function, as measured by improved scores on the Montreal Cognitive Assessment (P = .003) and Mini-Mental State Examination (P = .02).
Playing Go was linked with improved working memory, as measured by the Trail Making Test-A. Those who played Mah-jongg reported improved executive functioning and a temporary decrease in depressive symptoms. And chess players reported improved quality of life on the World Health Organization Quality of Life scale from playing chess (P < .00001).
In general, cognition improved across different populations. For example, some studies looked at unimpaired elderly while others looked at MCI, said Dr. Pozzi.
Playing board games in a social context appeared to be especially good at boosting brain power. One Japanese study included a control group that just did tai chi, a group that did Go alone on tablets, and another group that did Go in groups. Both Go groups improved cognitively, but participants who played together improved the most.
The results also seemed to suggest that Go and chess have different biological effects. “For example, Go increased [brain-derived neurotrophic factor] (BDNF) levels and metabolism in areas key for cognition like the middle temporal gyrus,” Dr. Pozzi said.
He noted that the methodology of the studies was generally “not bad,” although in some cases the analyses were per protocol and in others intention-to-treat. Outcomes varied across studies, there were a lot of dropouts, and some were not randomized, meaning reverse causality can’t be ruled out.
Dr. Pozzi has started a randomized controlled trial at a Go and chess club in Italy. He’s enrolling patients aged 60 and over with subjective cognitive decline or MCI and separating participants into a control group, a group that plays chess, another that plays Go, and another that plays both Go and chess.
In addition to the standard cognitive tests, and measures of depression and quality of life, Dr. Pozzi aims to assess cognitive reserve and is in the process of validating a questionnaire that will look at leisure activities and lifestyle.
Social and cognitive value
Commenting on the research for this news organization, Vladimir Hachinski, MD, a professor of clinical neurological sciences at Western University in London, Ont., said the results make sense.
Playing a board game involves concentration, strategy, and intermittent rewards – all of which are good for the brain and may involve the prefrontal cortex, he noted. Board games are also typically timed, which involves brain speed processing, and they have a winner and loser so emotions can run high, which also affects the brain, Dr. Hachinski added.
There may also be social value in playing a board game with someone else, added Dr. Hachinski.
“It’s encouraging that people can improve what they’re doing, and the longer they’re at it, the more of the brain they use,” he said. “There might be a long-term effect because players are building up networks.”
But Dr. Hachinski cautioned that playing a lot of chess does not necessarily make you a better thinker, just as learning to play one instrument doesn’t mean you can automatically play others.
“Learning one skill will translate only partially to another, and only if it’s related,” he said. “It increases cognition in the area you’re practicing in, but it doesn’t spread to other areas.”
Dr. Pozzi and Dr. Hachinski report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment order evidence comes to light for premenopausal idiopathic osteoporosis: What to do after denosumab
VANCOUVER – With treatment with a bisphosphonate following sequential use of teriparatide (Forteo) and denosumab (Prolia) for premenopausal women with idiopathic osteoporosis, bone mineral density (BMD) was maintained over the first year following denosumab cessation, according to results from a small, nonrandomized extension of a phase 2 study.
Bisphosphonates are recommended for patients after they have completed a course of denosumab because cessation of the bone resorption blocker is known to increase bone turnover markers, decrease BMD, and raise the risk of vertebral fractures. Although there is evidence to support this treatment sequence for postmenopausal women, there was no evidence regarding premenopausal women with idiopathic osteoporosis, said Adi Cohen, MD, who presented the results of the study at the annual meeting of the American Society for Bone and Mineral Research.
In the extension study, neither length of treatment with denosumab nor transition to menopause affected BMD results. Weekly doses of alendronate (ALN) better suppressed C-terminal telopeptide (CTX) than did zoledronic acid (ZOL) and led to better maintenance of BMD than did a single dose of ZOL. The researchers suggested that single-dose ZOL may not prevent bone loss for an entire year.
It is too early to call the results practice changing, said Dr. Cohen, professor of medicine and endocrinology at Columbia University Irving Medical Center, New York, but she noted, “It’s important just to provide information about how sequences of osteoporosis medications might be used in a rare but certainly understudied group of premenopausal women with osteoporosis who need treatment, and these data hopefully will help make some treatment decisions.”
In the early 2000s, researchers initially believed that premenopausal women with low BMD had experienced some kind of temporary event and that they would likely improve on their own over time. “I think we now recognize that whatever it is that causes this is an ongoing issue and that this is a problem they’re going to have to deal with for the rest of their lives. This is something that they have to stay on top of,” said coauthor Elizabeth Shane, MD, who is a professor of medicine at CUIMC.
However, there are no practice guidelines for the management of osteoporosis in premenopausal women, according to Dr. Shane. She noted that there is controversy as to whether to treat women with low bone density who do not have a history of fractures. “I think that there’s pretty much agreement that anybody who has a lot of fractures has an early-onset form of osteoporosis. The controversy is what to do about the person who just has a low bone density and hasn’t yet fractured and what is the utility of trying to treat them at that point and perhaps prevent a fracture. I don’t think we have enough data to address that,” Dr. Shane said.
Still, the research has provided some clarity in her own practice. “I think if somebody would come to my office who had very low bone density, I would probably treat them. If they have fractures, I would definitely treat them. I think that our work has provided a framework for people to approach that,” she said.
The study was an extension of a sequential treatment approach that began with 2 years of teriparatide (20 mcg daily) followed by an extension study of 2–3 years of treatment with denosumab (60 mg every 6 months). Seven months after the last dose of denosumab, patients underwent 1 year of treatment with ALN (70 mg weekly; n = 18) or a single dose of ZOL (5 mg IV; n = 6), according to patient choice.
The original phase 2 study started with 41 women. At 24 months, teriparatide treatment led to BMD increases of 13% in the lumbar spine (LS), 5% in the total hip (TH), and 5% in the femoral neck (FN). There was a 2% decline in BMD in the forearm (distal radius [DR]). A group of 32 of the women participated in an extension study and took denosumab for 12 months. Of those patients, 29 continued to take it for another 12 months. At 12 months, BMD increased 5% in the LS, 3% in the TH, 3% in the FN, and 1% in the DR (P < .05 for all). At 24 months, BMD rose by 22%, 10%, and 10% at the first three of those locations. BMD in the DR remained stable, compared with the baseline after taking teriparatide.
The bisphosphonate phase of the extension study included 24 women (mean age, 43 years). The mean body mass index of the patients was 23.0 kg/m2. The patients had experienced a mean of 3.0 fractures in adulthood, and 38% of patients had a history of vertebral fracture.
Over 12 months of follow-up, the researchers found no statistically significant difference in BMD in the LS, TH, or FN, compared with bisphosphonate extension baseline. There was also no statistically significant change in serum CTX. There was evidence that, among patients with higher rates of bone turnover, there were higher rates of LS and FN bone loss during bisphosphonate treatment.
Among patients taking ZOL, at 12 months there was a statistically significant rise in CTX levels, but not among patients taking ALN. There were no new vertebral fractures among any participants during the bisphosphonate extension period.
The results represent critical data for an understudied population, according to Yumie Rhee, MD, PhD, who was comoderator of the session in which the study was presented. “They are showing that by using a bisphosphonate [patients] have this just slight decrease, but within error, so it’s maintaining the BMD, at least. I think it’s very important. It will be fascinating to see next year’s follow-up,” said Dr. Rhee, a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea. “The problem with premenopausal osteoporosis is that we don’t have good evidence. Even though this study is very small, we’re just following that data, all of us.”
Comoderator Maria Zanchetta, MD, a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires, agreed. “We know what to do when we stop denosumab in postmenopausal women. We didn’t have any work about what to do when we stopped in premenopausal women. You can think that probably it’s going to be the same, but this is the first time you have the evidence that if you give bisphosphonate, you will maintain BMD.”
Limitations to the study include its small size and the lack of a placebo-treated control group. In addition, the bisphosphonate extension was not randomized.
The studies were funded by the U.S. Food and Drug Administration and Amgen. Dr. Cohen and Dr. Shane received research funding from Amgen. Dr. Rhee and Dr. Zanchetta have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
VANCOUVER – With treatment with a bisphosphonate following sequential use of teriparatide (Forteo) and denosumab (Prolia) for premenopausal women with idiopathic osteoporosis, bone mineral density (BMD) was maintained over the first year following denosumab cessation, according to results from a small, nonrandomized extension of a phase 2 study.
Bisphosphonates are recommended for patients after they have completed a course of denosumab because cessation of the bone resorption blocker is known to increase bone turnover markers, decrease BMD, and raise the risk of vertebral fractures. Although there is evidence to support this treatment sequence for postmenopausal women, there was no evidence regarding premenopausal women with idiopathic osteoporosis, said Adi Cohen, MD, who presented the results of the study at the annual meeting of the American Society for Bone and Mineral Research.
In the extension study, neither length of treatment with denosumab nor transition to menopause affected BMD results. Weekly doses of alendronate (ALN) better suppressed C-terminal telopeptide (CTX) than did zoledronic acid (ZOL) and led to better maintenance of BMD than did a single dose of ZOL. The researchers suggested that single-dose ZOL may not prevent bone loss for an entire year.
It is too early to call the results practice changing, said Dr. Cohen, professor of medicine and endocrinology at Columbia University Irving Medical Center, New York, but she noted, “It’s important just to provide information about how sequences of osteoporosis medications might be used in a rare but certainly understudied group of premenopausal women with osteoporosis who need treatment, and these data hopefully will help make some treatment decisions.”
In the early 2000s, researchers initially believed that premenopausal women with low BMD had experienced some kind of temporary event and that they would likely improve on their own over time. “I think we now recognize that whatever it is that causes this is an ongoing issue and that this is a problem they’re going to have to deal with for the rest of their lives. This is something that they have to stay on top of,” said coauthor Elizabeth Shane, MD, who is a professor of medicine at CUIMC.
However, there are no practice guidelines for the management of osteoporosis in premenopausal women, according to Dr. Shane. She noted that there is controversy as to whether to treat women with low bone density who do not have a history of fractures. “I think that there’s pretty much agreement that anybody who has a lot of fractures has an early-onset form of osteoporosis. The controversy is what to do about the person who just has a low bone density and hasn’t yet fractured and what is the utility of trying to treat them at that point and perhaps prevent a fracture. I don’t think we have enough data to address that,” Dr. Shane said.
Still, the research has provided some clarity in her own practice. “I think if somebody would come to my office who had very low bone density, I would probably treat them. If they have fractures, I would definitely treat them. I think that our work has provided a framework for people to approach that,” she said.
The study was an extension of a sequential treatment approach that began with 2 years of teriparatide (20 mcg daily) followed by an extension study of 2–3 years of treatment with denosumab (60 mg every 6 months). Seven months after the last dose of denosumab, patients underwent 1 year of treatment with ALN (70 mg weekly; n = 18) or a single dose of ZOL (5 mg IV; n = 6), according to patient choice.
The original phase 2 study started with 41 women. At 24 months, teriparatide treatment led to BMD increases of 13% in the lumbar spine (LS), 5% in the total hip (TH), and 5% in the femoral neck (FN). There was a 2% decline in BMD in the forearm (distal radius [DR]). A group of 32 of the women participated in an extension study and took denosumab for 12 months. Of those patients, 29 continued to take it for another 12 months. At 12 months, BMD increased 5% in the LS, 3% in the TH, 3% in the FN, and 1% in the DR (P < .05 for all). At 24 months, BMD rose by 22%, 10%, and 10% at the first three of those locations. BMD in the DR remained stable, compared with the baseline after taking teriparatide.
The bisphosphonate phase of the extension study included 24 women (mean age, 43 years). The mean body mass index of the patients was 23.0 kg/m2. The patients had experienced a mean of 3.0 fractures in adulthood, and 38% of patients had a history of vertebral fracture.
Over 12 months of follow-up, the researchers found no statistically significant difference in BMD in the LS, TH, or FN, compared with bisphosphonate extension baseline. There was also no statistically significant change in serum CTX. There was evidence that, among patients with higher rates of bone turnover, there were higher rates of LS and FN bone loss during bisphosphonate treatment.
Among patients taking ZOL, at 12 months there was a statistically significant rise in CTX levels, but not among patients taking ALN. There were no new vertebral fractures among any participants during the bisphosphonate extension period.
The results represent critical data for an understudied population, according to Yumie Rhee, MD, PhD, who was comoderator of the session in which the study was presented. “They are showing that by using a bisphosphonate [patients] have this just slight decrease, but within error, so it’s maintaining the BMD, at least. I think it’s very important. It will be fascinating to see next year’s follow-up,” said Dr. Rhee, a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea. “The problem with premenopausal osteoporosis is that we don’t have good evidence. Even though this study is very small, we’re just following that data, all of us.”
Comoderator Maria Zanchetta, MD, a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires, agreed. “We know what to do when we stop denosumab in postmenopausal women. We didn’t have any work about what to do when we stopped in premenopausal women. You can think that probably it’s going to be the same, but this is the first time you have the evidence that if you give bisphosphonate, you will maintain BMD.”
Limitations to the study include its small size and the lack of a placebo-treated control group. In addition, the bisphosphonate extension was not randomized.
The studies were funded by the U.S. Food and Drug Administration and Amgen. Dr. Cohen and Dr. Shane received research funding from Amgen. Dr. Rhee and Dr. Zanchetta have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
VANCOUVER – With treatment with a bisphosphonate following sequential use of teriparatide (Forteo) and denosumab (Prolia) for premenopausal women with idiopathic osteoporosis, bone mineral density (BMD) was maintained over the first year following denosumab cessation, according to results from a small, nonrandomized extension of a phase 2 study.
Bisphosphonates are recommended for patients after they have completed a course of denosumab because cessation of the bone resorption blocker is known to increase bone turnover markers, decrease BMD, and raise the risk of vertebral fractures. Although there is evidence to support this treatment sequence for postmenopausal women, there was no evidence regarding premenopausal women with idiopathic osteoporosis, said Adi Cohen, MD, who presented the results of the study at the annual meeting of the American Society for Bone and Mineral Research.
In the extension study, neither length of treatment with denosumab nor transition to menopause affected BMD results. Weekly doses of alendronate (ALN) better suppressed C-terminal telopeptide (CTX) than did zoledronic acid (ZOL) and led to better maintenance of BMD than did a single dose of ZOL. The researchers suggested that single-dose ZOL may not prevent bone loss for an entire year.
It is too early to call the results practice changing, said Dr. Cohen, professor of medicine and endocrinology at Columbia University Irving Medical Center, New York, but she noted, “It’s important just to provide information about how sequences of osteoporosis medications might be used in a rare but certainly understudied group of premenopausal women with osteoporosis who need treatment, and these data hopefully will help make some treatment decisions.”
In the early 2000s, researchers initially believed that premenopausal women with low BMD had experienced some kind of temporary event and that they would likely improve on their own over time. “I think we now recognize that whatever it is that causes this is an ongoing issue and that this is a problem they’re going to have to deal with for the rest of their lives. This is something that they have to stay on top of,” said coauthor Elizabeth Shane, MD, who is a professor of medicine at CUIMC.
However, there are no practice guidelines for the management of osteoporosis in premenopausal women, according to Dr. Shane. She noted that there is controversy as to whether to treat women with low bone density who do not have a history of fractures. “I think that there’s pretty much agreement that anybody who has a lot of fractures has an early-onset form of osteoporosis. The controversy is what to do about the person who just has a low bone density and hasn’t yet fractured and what is the utility of trying to treat them at that point and perhaps prevent a fracture. I don’t think we have enough data to address that,” Dr. Shane said.
Still, the research has provided some clarity in her own practice. “I think if somebody would come to my office who had very low bone density, I would probably treat them. If they have fractures, I would definitely treat them. I think that our work has provided a framework for people to approach that,” she said.
The study was an extension of a sequential treatment approach that began with 2 years of teriparatide (20 mcg daily) followed by an extension study of 2–3 years of treatment with denosumab (60 mg every 6 months). Seven months after the last dose of denosumab, patients underwent 1 year of treatment with ALN (70 mg weekly; n = 18) or a single dose of ZOL (5 mg IV; n = 6), according to patient choice.
The original phase 2 study started with 41 women. At 24 months, teriparatide treatment led to BMD increases of 13% in the lumbar spine (LS), 5% in the total hip (TH), and 5% in the femoral neck (FN). There was a 2% decline in BMD in the forearm (distal radius [DR]). A group of 32 of the women participated in an extension study and took denosumab for 12 months. Of those patients, 29 continued to take it for another 12 months. At 12 months, BMD increased 5% in the LS, 3% in the TH, 3% in the FN, and 1% in the DR (P < .05 for all). At 24 months, BMD rose by 22%, 10%, and 10% at the first three of those locations. BMD in the DR remained stable, compared with the baseline after taking teriparatide.
The bisphosphonate phase of the extension study included 24 women (mean age, 43 years). The mean body mass index of the patients was 23.0 kg/m2. The patients had experienced a mean of 3.0 fractures in adulthood, and 38% of patients had a history of vertebral fracture.
Over 12 months of follow-up, the researchers found no statistically significant difference in BMD in the LS, TH, or FN, compared with bisphosphonate extension baseline. There was also no statistically significant change in serum CTX. There was evidence that, among patients with higher rates of bone turnover, there were higher rates of LS and FN bone loss during bisphosphonate treatment.
Among patients taking ZOL, at 12 months there was a statistically significant rise in CTX levels, but not among patients taking ALN. There were no new vertebral fractures among any participants during the bisphosphonate extension period.
The results represent critical data for an understudied population, according to Yumie Rhee, MD, PhD, who was comoderator of the session in which the study was presented. “They are showing that by using a bisphosphonate [patients] have this just slight decrease, but within error, so it’s maintaining the BMD, at least. I think it’s very important. It will be fascinating to see next year’s follow-up,” said Dr. Rhee, a professor of endocrinology at Yonsei University College of Medicine in Seoul, South Korea. “The problem with premenopausal osteoporosis is that we don’t have good evidence. Even though this study is very small, we’re just following that data, all of us.”
Comoderator Maria Zanchetta, MD, a professor of osteology at the Institute of Diagnostics and Metabolic Research, Universidad del Salvador, Buenos Aires, agreed. “We know what to do when we stop denosumab in postmenopausal women. We didn’t have any work about what to do when we stopped in premenopausal women. You can think that probably it’s going to be the same, but this is the first time you have the evidence that if you give bisphosphonate, you will maintain BMD.”
Limitations to the study include its small size and the lack of a placebo-treated control group. In addition, the bisphosphonate extension was not randomized.
The studies were funded by the U.S. Food and Drug Administration and Amgen. Dr. Cohen and Dr. Shane received research funding from Amgen. Dr. Rhee and Dr. Zanchetta have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT ASBMR 2023
ESG yields significant, sustained weight loss across obesity classes
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of 1,506 adults (85% female, 70% White) with severe obesity (501 class I, 546 class II, and 459 class III) who underwent ESG at seven academic and private U.S. centers from 2013 to 2022.
- Average percent total body weight loss (%TBWL) was evaluated at 6, 12, 18, and 24 months after the procedure.
- Weight loss and safety outcomes were evaluated according to obesity class.
TAKEAWAY:
- At 12 months, 83.2% of patients achieved ≥10% TBWL and 60.9% achieved ≥15% TBWL across all obesity classes.
- There was a significant difference in TBWL by baseline obesity class, with average weight loss significantly greater in class III than classes I and II at all time points. At 24 months, class III patients had mean TBWL of 20.4%, compared with 13.3% for class I and 13.6% for class II patients.
- As early as 6 months post-ESG, patients in all BMI classes were able to drop to the next lower BMI class and remained there through 2 years. However, ongoing improvement in BMI until the end of follow-up was seen only in class III patients. Notably, class III patients were significantly younger and taller than class I and class II patients.
- There were no differences in adverse events between obesity classes. Only 2.6% of patients had an adverse event requiring hospitalization. Most of these events (86%) were for symptom management and/or fluid replacement.
IN PRACTICE:
“Traditionally, ESG has been proposed as a treatment choice for patients with class I and II obesity because of its modest weight loss outcomes. However, our data show a %TBWL crossing 20% in patients with class III disease, which may push the envelope of perceived utility of ESG,” the authors write.
SOURCE:
The study, with first author Khushboo Gala, MBBS, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., was published online in Clinical and Translational Gastroenterology.
LIMITATIONS:
Limitations include the retrospective design, with outcomes only out to 2 years, and loss of follow-up, with only 339 of the 1506 patients evaluated at 2 years.
DISCLOSURES:
The study had no financial support. Several study authors reported ties to industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of 1,506 adults (85% female, 70% White) with severe obesity (501 class I, 546 class II, and 459 class III) who underwent ESG at seven academic and private U.S. centers from 2013 to 2022.
- Average percent total body weight loss (%TBWL) was evaluated at 6, 12, 18, and 24 months after the procedure.
- Weight loss and safety outcomes were evaluated according to obesity class.
TAKEAWAY:
- At 12 months, 83.2% of patients achieved ≥10% TBWL and 60.9% achieved ≥15% TBWL across all obesity classes.
- There was a significant difference in TBWL by baseline obesity class, with average weight loss significantly greater in class III than classes I and II at all time points. At 24 months, class III patients had mean TBWL of 20.4%, compared with 13.3% for class I and 13.6% for class II patients.
- As early as 6 months post-ESG, patients in all BMI classes were able to drop to the next lower BMI class and remained there through 2 years. However, ongoing improvement in BMI until the end of follow-up was seen only in class III patients. Notably, class III patients were significantly younger and taller than class I and class II patients.
- There were no differences in adverse events between obesity classes. Only 2.6% of patients had an adverse event requiring hospitalization. Most of these events (86%) were for symptom management and/or fluid replacement.
IN PRACTICE:
“Traditionally, ESG has been proposed as a treatment choice for patients with class I and II obesity because of its modest weight loss outcomes. However, our data show a %TBWL crossing 20% in patients with class III disease, which may push the envelope of perceived utility of ESG,” the authors write.
SOURCE:
The study, with first author Khushboo Gala, MBBS, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., was published online in Clinical and Translational Gastroenterology.
LIMITATIONS:
Limitations include the retrospective design, with outcomes only out to 2 years, and loss of follow-up, with only 339 of the 1506 patients evaluated at 2 years.
DISCLOSURES:
The study had no financial support. Several study authors reported ties to industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective analysis of 1,506 adults (85% female, 70% White) with severe obesity (501 class I, 546 class II, and 459 class III) who underwent ESG at seven academic and private U.S. centers from 2013 to 2022.
- Average percent total body weight loss (%TBWL) was evaluated at 6, 12, 18, and 24 months after the procedure.
- Weight loss and safety outcomes were evaluated according to obesity class.
TAKEAWAY:
- At 12 months, 83.2% of patients achieved ≥10% TBWL and 60.9% achieved ≥15% TBWL across all obesity classes.
- There was a significant difference in TBWL by baseline obesity class, with average weight loss significantly greater in class III than classes I and II at all time points. At 24 months, class III patients had mean TBWL of 20.4%, compared with 13.3% for class I and 13.6% for class II patients.
- As early as 6 months post-ESG, patients in all BMI classes were able to drop to the next lower BMI class and remained there through 2 years. However, ongoing improvement in BMI until the end of follow-up was seen only in class III patients. Notably, class III patients were significantly younger and taller than class I and class II patients.
- There were no differences in adverse events between obesity classes. Only 2.6% of patients had an adverse event requiring hospitalization. Most of these events (86%) were for symptom management and/or fluid replacement.
IN PRACTICE:
“Traditionally, ESG has been proposed as a treatment choice for patients with class I and II obesity because of its modest weight loss outcomes. However, our data show a %TBWL crossing 20% in patients with class III disease, which may push the envelope of perceived utility of ESG,” the authors write.
SOURCE:
The study, with first author Khushboo Gala, MBBS, division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., was published online in Clinical and Translational Gastroenterology.
LIMITATIONS:
Limitations include the retrospective design, with outcomes only out to 2 years, and loss of follow-up, with only 339 of the 1506 patients evaluated at 2 years.
DISCLOSURES:
The study had no financial support. Several study authors reported ties to industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
FDA proposes ban on hair straightener ingredients
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
Amitriptyline use nearly doubles symptom improvement in IBS
GLASGOW – in what the researchers call the largest randomized controlled trial (RCT) of a tricyclic antidepressant in the condition.
Patients who took low-dose amitriptyline were almost twice as likely to report an overall improvement in symptoms as those taking placebo, according to investigators of the Amitriptyline at Low-Dose and Titrated for Irritable Bowel Syndrome as Second-Line Treatment (ATLANTIS) trial. Low-dose amitriptyline appeared safe and well tolerated, they reported.
Hazel Everitt, PhD, professor of primary care research at the University of Southampton, England, presented the findings at the annual conference of the Royal College of General Practitioners.
The data were also published in The Lancet and were presented at the recent United European Gastroenterology Week 2023.
Clinicians “should offer low-dose amitriptyline to patients with IBS whose symptoms do not improve with first-line therapies, with appropriate support to guide patient-led dose titration,” the researchers wrote in the journal article.
Despite first-line treatments such as diet, fiber, and antispasmodics, many patients with IBS continue to have troublesome symptoms, Dr. Everitt said in an interview. “GPs haven’t often prescribed amitriptyline for IBS – probably because of the lack of research evidence for its use in primary care.”
Dr. Everitt added that primary care physicians and patients interviewed for the study welcomed low-dose amitriptyline as a potential additional option, especially with increased patient-led care. “The dose titration document that was developed with patients specifically for the trial enables patients to be more empowered to manage their IBS by helping them to titrate their dose up or down depending on their symptoms and side effects.”
Judith Danby, MBBS, a retired GP who moderated the session at which the ATLANTIS results were presented, said, “Self-titration of the dose equals patient empowerment, and if patients can be helped to manage their own medication, then they will also be more empowered to think about lifestyle change, too.”
RCT across 55 practices
The U.K.’s National Institute for Health and Care Excellence guidance for the management of IBS in primary care says clinicians should “consider” using low-dose tricyclic antidepressants as a second-line treatment but highlight the need for an RCT of these drugs carried out solely in primary care.
The ATLANTIS trial was conducted across 55 general practices in England and included adults with Rome IV IBS of any subtype and ongoing symptoms (IBS Severity Scoring System [IBS-SSS] score ≥ 75 points) despite dietary changes and first-line therapies. Participants had normal full blood counts and C-reactive protein measures, negative celiac serology, and no evidence of suicidal ideation. The mean age was 48.5 years, and 68% were female. The mean IBS-SSS score in all participants was 272.8 at baseline.
Patients were randomly assigned in a 1:1 ratio to receive either low-dose oral amitriptyline (10 mg once daily; n = 232) with dose titration over 3 weeks (up to a maximum dose of 30 mg once daily) as determined by a participant’s symptoms and tolerability; or placebo (n = 231). Both groups participated for 6 months. The primary outcome was the IBS-SSS score at 6 months.
Amitriptyline
Three-quarters of participants adhered to the therapy over the 6 months, which was particularly notable given that the trial was conducted during the COVID-19 pandemic, according to the researchers.
An intent-to-treat analysis found that at 6 months, amitriptyline was superior to placebo, with a significant mean difference in IBS-SSS score between groups of –27.0 (95% confidence interval, –46.9 to –7.1; P = .0079; mean IBS-SSS, 170.4 vs. 200.1 with amitriptyline and placebo). A secondary outcome showed an increased likelihood of relief of IBS symptoms by subjective global assessment (odds ratio, 1.78; 95% CI. 1.19-2.66; P = .0050).
At 3 months, the difference in mean change in IBS-SSS score between groups was also significant, at –23.3 (95% CI, –42.0 to –4.6; P = .014), the researchers reported.
People who took the drug were 70% more likely to report relief of symptoms on SGA than those who took placebo (P = .08), according to the researchers.
The researchers reported no effect of low-dose amitriptyline on psychiatric symptoms, such as distressing thoughts, anxiety, and depression, during the 6-month follow-up, nor was there any effect on ability to work or go about social activities.
“This was a pragmatic trial performed in a large number of participants with IBS, with an average duration of symptoms of 10 years and with 80% having moderate to severe symptoms at baseline,” Alexander Ford, MD, professor of gastroenterology at the University of Leeds, England, and a coinvestigator on the study, told this news organization. “The fact that amitriptyline showed such a strong effect over placebo in this group of patients, with a mean decrease in IBS-SSS of almost 100 points at both 3 and 6 months, is therefore all the more impressive.”
Mild adverse events, such as dry mouth and drowsiness, were more frequent with low-dose amitriptyline than with placebo,
Dr. Everitt said the ATLANTIS findings could change practice. Previous trials of low-dose amitriptyline for IBS had mostly been small and were conducted in secondary care settings such as gastroenterology clinics with relatively short follow-up times.
“This is a problem for a long-term condition that fluctuates over time and is diagnosed and managed mostly in primary care,” she said. “The ATLANTIS trial is the largest trial of low-dose amitriptyline for IBS undertaken worldwide and was rigorously conducted with 6 months follow-up, providing reliable results that can help inform GPs and patients’ treatment decision-making in usual clinical practice.”
On a pragmatic level, the research group developed a dose titration document for use by patients and GPs. “Both the GPs and participants found the ATLANTIS dose titration document acceptable and helpful,” Dr. Everitt pointed out. She noted, “We’ve made the dose titration document freely available to support patients and clinicians to try low-dose amitriptyline for IBS.”
Dr. Ford and Dr. Everitt received grant funding (institutional) from the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
GLASGOW – in what the researchers call the largest randomized controlled trial (RCT) of a tricyclic antidepressant in the condition.
Patients who took low-dose amitriptyline were almost twice as likely to report an overall improvement in symptoms as those taking placebo, according to investigators of the Amitriptyline at Low-Dose and Titrated for Irritable Bowel Syndrome as Second-Line Treatment (ATLANTIS) trial. Low-dose amitriptyline appeared safe and well tolerated, they reported.
Hazel Everitt, PhD, professor of primary care research at the University of Southampton, England, presented the findings at the annual conference of the Royal College of General Practitioners.
The data were also published in The Lancet and were presented at the recent United European Gastroenterology Week 2023.
Clinicians “should offer low-dose amitriptyline to patients with IBS whose symptoms do not improve with first-line therapies, with appropriate support to guide patient-led dose titration,” the researchers wrote in the journal article.
Despite first-line treatments such as diet, fiber, and antispasmodics, many patients with IBS continue to have troublesome symptoms, Dr. Everitt said in an interview. “GPs haven’t often prescribed amitriptyline for IBS – probably because of the lack of research evidence for its use in primary care.”
Dr. Everitt added that primary care physicians and patients interviewed for the study welcomed low-dose amitriptyline as a potential additional option, especially with increased patient-led care. “The dose titration document that was developed with patients specifically for the trial enables patients to be more empowered to manage their IBS by helping them to titrate their dose up or down depending on their symptoms and side effects.”
Judith Danby, MBBS, a retired GP who moderated the session at which the ATLANTIS results were presented, said, “Self-titration of the dose equals patient empowerment, and if patients can be helped to manage their own medication, then they will also be more empowered to think about lifestyle change, too.”
RCT across 55 practices
The U.K.’s National Institute for Health and Care Excellence guidance for the management of IBS in primary care says clinicians should “consider” using low-dose tricyclic antidepressants as a second-line treatment but highlight the need for an RCT of these drugs carried out solely in primary care.
The ATLANTIS trial was conducted across 55 general practices in England and included adults with Rome IV IBS of any subtype and ongoing symptoms (IBS Severity Scoring System [IBS-SSS] score ≥ 75 points) despite dietary changes and first-line therapies. Participants had normal full blood counts and C-reactive protein measures, negative celiac serology, and no evidence of suicidal ideation. The mean age was 48.5 years, and 68% were female. The mean IBS-SSS score in all participants was 272.8 at baseline.
Patients were randomly assigned in a 1:1 ratio to receive either low-dose oral amitriptyline (10 mg once daily; n = 232) with dose titration over 3 weeks (up to a maximum dose of 30 mg once daily) as determined by a participant’s symptoms and tolerability; or placebo (n = 231). Both groups participated for 6 months. The primary outcome was the IBS-SSS score at 6 months.
Amitriptyline
Three-quarters of participants adhered to the therapy over the 6 months, which was particularly notable given that the trial was conducted during the COVID-19 pandemic, according to the researchers.
An intent-to-treat analysis found that at 6 months, amitriptyline was superior to placebo, with a significant mean difference in IBS-SSS score between groups of –27.0 (95% confidence interval, –46.9 to –7.1; P = .0079; mean IBS-SSS, 170.4 vs. 200.1 with amitriptyline and placebo). A secondary outcome showed an increased likelihood of relief of IBS symptoms by subjective global assessment (odds ratio, 1.78; 95% CI. 1.19-2.66; P = .0050).
At 3 months, the difference in mean change in IBS-SSS score between groups was also significant, at –23.3 (95% CI, –42.0 to –4.6; P = .014), the researchers reported.
People who took the drug were 70% more likely to report relief of symptoms on SGA than those who took placebo (P = .08), according to the researchers.
The researchers reported no effect of low-dose amitriptyline on psychiatric symptoms, such as distressing thoughts, anxiety, and depression, during the 6-month follow-up, nor was there any effect on ability to work or go about social activities.
“This was a pragmatic trial performed in a large number of participants with IBS, with an average duration of symptoms of 10 years and with 80% having moderate to severe symptoms at baseline,” Alexander Ford, MD, professor of gastroenterology at the University of Leeds, England, and a coinvestigator on the study, told this news organization. “The fact that amitriptyline showed such a strong effect over placebo in this group of patients, with a mean decrease in IBS-SSS of almost 100 points at both 3 and 6 months, is therefore all the more impressive.”
Mild adverse events, such as dry mouth and drowsiness, were more frequent with low-dose amitriptyline than with placebo,
Dr. Everitt said the ATLANTIS findings could change practice. Previous trials of low-dose amitriptyline for IBS had mostly been small and were conducted in secondary care settings such as gastroenterology clinics with relatively short follow-up times.
“This is a problem for a long-term condition that fluctuates over time and is diagnosed and managed mostly in primary care,” she said. “The ATLANTIS trial is the largest trial of low-dose amitriptyline for IBS undertaken worldwide and was rigorously conducted with 6 months follow-up, providing reliable results that can help inform GPs and patients’ treatment decision-making in usual clinical practice.”
On a pragmatic level, the research group developed a dose titration document for use by patients and GPs. “Both the GPs and participants found the ATLANTIS dose titration document acceptable and helpful,” Dr. Everitt pointed out. She noted, “We’ve made the dose titration document freely available to support patients and clinicians to try low-dose amitriptyline for IBS.”
Dr. Ford and Dr. Everitt received grant funding (institutional) from the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
GLASGOW – in what the researchers call the largest randomized controlled trial (RCT) of a tricyclic antidepressant in the condition.
Patients who took low-dose amitriptyline were almost twice as likely to report an overall improvement in symptoms as those taking placebo, according to investigators of the Amitriptyline at Low-Dose and Titrated for Irritable Bowel Syndrome as Second-Line Treatment (ATLANTIS) trial. Low-dose amitriptyline appeared safe and well tolerated, they reported.
Hazel Everitt, PhD, professor of primary care research at the University of Southampton, England, presented the findings at the annual conference of the Royal College of General Practitioners.
The data were also published in The Lancet and were presented at the recent United European Gastroenterology Week 2023.
Clinicians “should offer low-dose amitriptyline to patients with IBS whose symptoms do not improve with first-line therapies, with appropriate support to guide patient-led dose titration,” the researchers wrote in the journal article.
Despite first-line treatments such as diet, fiber, and antispasmodics, many patients with IBS continue to have troublesome symptoms, Dr. Everitt said in an interview. “GPs haven’t often prescribed amitriptyline for IBS – probably because of the lack of research evidence for its use in primary care.”
Dr. Everitt added that primary care physicians and patients interviewed for the study welcomed low-dose amitriptyline as a potential additional option, especially with increased patient-led care. “The dose titration document that was developed with patients specifically for the trial enables patients to be more empowered to manage their IBS by helping them to titrate their dose up or down depending on their symptoms and side effects.”
Judith Danby, MBBS, a retired GP who moderated the session at which the ATLANTIS results were presented, said, “Self-titration of the dose equals patient empowerment, and if patients can be helped to manage their own medication, then they will also be more empowered to think about lifestyle change, too.”
RCT across 55 practices
The U.K.’s National Institute for Health and Care Excellence guidance for the management of IBS in primary care says clinicians should “consider” using low-dose tricyclic antidepressants as a second-line treatment but highlight the need for an RCT of these drugs carried out solely in primary care.
The ATLANTIS trial was conducted across 55 general practices in England and included adults with Rome IV IBS of any subtype and ongoing symptoms (IBS Severity Scoring System [IBS-SSS] score ≥ 75 points) despite dietary changes and first-line therapies. Participants had normal full blood counts and C-reactive protein measures, negative celiac serology, and no evidence of suicidal ideation. The mean age was 48.5 years, and 68% were female. The mean IBS-SSS score in all participants was 272.8 at baseline.
Patients were randomly assigned in a 1:1 ratio to receive either low-dose oral amitriptyline (10 mg once daily; n = 232) with dose titration over 3 weeks (up to a maximum dose of 30 mg once daily) as determined by a participant’s symptoms and tolerability; or placebo (n = 231). Both groups participated for 6 months. The primary outcome was the IBS-SSS score at 6 months.
Amitriptyline
Three-quarters of participants adhered to the therapy over the 6 months, which was particularly notable given that the trial was conducted during the COVID-19 pandemic, according to the researchers.
An intent-to-treat analysis found that at 6 months, amitriptyline was superior to placebo, with a significant mean difference in IBS-SSS score between groups of –27.0 (95% confidence interval, –46.9 to –7.1; P = .0079; mean IBS-SSS, 170.4 vs. 200.1 with amitriptyline and placebo). A secondary outcome showed an increased likelihood of relief of IBS symptoms by subjective global assessment (odds ratio, 1.78; 95% CI. 1.19-2.66; P = .0050).
At 3 months, the difference in mean change in IBS-SSS score between groups was also significant, at –23.3 (95% CI, –42.0 to –4.6; P = .014), the researchers reported.
People who took the drug were 70% more likely to report relief of symptoms on SGA than those who took placebo (P = .08), according to the researchers.
The researchers reported no effect of low-dose amitriptyline on psychiatric symptoms, such as distressing thoughts, anxiety, and depression, during the 6-month follow-up, nor was there any effect on ability to work or go about social activities.
“This was a pragmatic trial performed in a large number of participants with IBS, with an average duration of symptoms of 10 years and with 80% having moderate to severe symptoms at baseline,” Alexander Ford, MD, professor of gastroenterology at the University of Leeds, England, and a coinvestigator on the study, told this news organization. “The fact that amitriptyline showed such a strong effect over placebo in this group of patients, with a mean decrease in IBS-SSS of almost 100 points at both 3 and 6 months, is therefore all the more impressive.”
Mild adverse events, such as dry mouth and drowsiness, were more frequent with low-dose amitriptyline than with placebo,
Dr. Everitt said the ATLANTIS findings could change practice. Previous trials of low-dose amitriptyline for IBS had mostly been small and were conducted in secondary care settings such as gastroenterology clinics with relatively short follow-up times.
“This is a problem for a long-term condition that fluctuates over time and is diagnosed and managed mostly in primary care,” she said. “The ATLANTIS trial is the largest trial of low-dose amitriptyline for IBS undertaken worldwide and was rigorously conducted with 6 months follow-up, providing reliable results that can help inform GPs and patients’ treatment decision-making in usual clinical practice.”
On a pragmatic level, the research group developed a dose titration document for use by patients and GPs. “Both the GPs and participants found the ATLANTIS dose titration document acceptable and helpful,” Dr. Everitt pointed out. She noted, “We’ve made the dose titration document freely available to support patients and clinicians to try low-dose amitriptyline for IBS.”
Dr. Ford and Dr. Everitt received grant funding (institutional) from the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
AT RCGP 2023
Stair climbing tied to reduced risk for heart disease
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
new observational data suggest.
METHODOLOGY:
- The prospective cohort study used data from 458,860 adults in the UK Biobank cohort who were 38-73 years old at baseline (2006-2010).
- Information about stair climbing, sociodemographic, and lifestyle factors was collected at baseline and 5 years later.
- Cases of ASCVD – defined as coronary artery disease (CAD), ischemic stroke, or acute complications – were identified via hospital records and death registry.
- Associations between stair climbing and ASCVD were examined as hazard ratios from Cox proportional hazards model. Analyses were stratified by susceptibility to ASCVD based on family history, genetic risk, and established risk factors.
TAKEAWAY:
- A total of 39,043 ASCVD, 30,718 CAD, and 10,521 ischemic stroke cases were recorded during a median follow-up of 12.5 years.
- Compared with no-stair climbing, climbing 6-10 flights of stairs daily was associated with a 7% lower ASCVD risk (multivariable-adjusted HR, 0.93; 95% confidence interval, 0.90-0.96) and climbing 16-20 flights daily was associated with a 10% lower risk (HR, 0.90; 95% CI, 0.85-0.94).
- The benefits plateaued at 20 flights daily; comparable results were obtained for CAD and ischemic stroke; the protective effect of stair climbing was attenuated by increasing levels of disease susceptibility.
- Adults who stopped climbing stairs daily during the study had a 32% higher risk of ASCVD (HR, 1.32; 95% CI,1.06-1.65), compared with peers who never reported stair climbing.
IN PRACTICE:
“These findings highlight the potential advantages of stair climbing as a primary preventive measure for ASCVD in the general population. Short bursts of high-intensity stair climbing are a time-efficient way to improve cardiorespiratory fitness and lipid profile, especially among those unable to achieve the current physical activity recommendations,” study author Lu Qi, with Tulane University, New Orleans, said in a news release.
SOURCE:
The study was published online in Atherosclerosis.
LIMITATIONS:
The observational design limits causal inferences. Stair climbing was self-reported via questionnaires and recall bias is a possibility. The UK Biobank participants do not represent the entire population of the country, with a healthy volunteer selection bias previously reported.
DISCLOSURES:
The study was supported by grants from the National Key R&D Program of China. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.