User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Botulinum toxin associated with antidepressant effects across indications, injection sites
, according to the study’s authors.
Their results show that the antidepressant effect of botulinum toxin “administered for various indications goes beyond the control of the intended disease states and does not depend on the location of the injection,” according to Tigran Makunts, PharmD, of the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego, and coauthors.
Previous high-quality studies have found botulinum toxin treatment has been associated with antidepressant effects when administered to the glabellar region of the face, they noted. The study was published in Scientific Reports.
The researchers evaluated adverse events reported to the Food and Drug Administration’s current adverse event reporting system (FAERS) between September 2012 and December 2019, and the FDA’s previous adverse event reporting system between January 2004 and August 2012. Overall, they analyzed 174,243 reports, which were divided into eight treatment-related groups based on the indication for botulinum toxin: Cosmetic use (20,684 patients), migraine (4,180 patients), spasms and spasticity not involving facial muscles (2,335 patients), neurological and urinary bladder disorders (915 patients), torticollis (1,360 patients), hyperhidrosis (601 patients), blepharospasm (487 patients), and sialorrhea (157 patients). Each group was matched to controls from the FAERS database, who had different treatments for the same indications. (Reports in which patients were on an antidepressant or where depression was listed as an indication were not included).
In nearly all treatment groups, reports of depression and depression-related adverse events were significantly lower among those who received botulinum toxin, compared with controls: For those who received botulinum toxin injections in facial muscles for cosmetic uses, the reporting odds ratio was 0.46 (95% confidence interval, 0.27-0.78). Significant effects were also see in the following groups: those who received injections into facial and head muscles for migraine (ROR, 0.60; 95% CI, 0.48-0.74), injections into the upper and lower limbs for spasms and spasticity (ROR, 0.28; 95% CI, 0.18-0.42), injections into neck muscles for torticollis and neck pain (ROR, 0.30; 95% CI, 0.20-0.44), injections into eyelid muscles for blepharospasm (ROR, 0.13; 95% CI, 0.05-0.39), and injections into the axilla and palm for hyperhidrosis (ROR, 0.12; 95% CI, 0.04-0.33).
There were no cases of depression or depression-related adverse event reports among those treated with botulinum toxin for sialorrhea with injections into the parotid and submandibular glands, and there were decreased reports of depression among those who received detrusor muscle injections for neurological and urinary bladder disorders, but the results in both groups were not statistically significant, according to the researchers.
In an interview, Ruben Abagyan, PhD, study coauthor and professor at Skaggs School of Pharmacy and Pharmaceutical Sciences, said the study’s finding go “beyond breaking a positive feedback loop between depression and the ‘frown’ wrinkles in the glabellar region of the forehead.” The data showing efficacy with botulinum toxin injected in other areas of the body can help clinicians “expand their search for the most effective injection location and dose beyond the facial injections to improve the depression-related therapeutic outcomes.”
Another takeaway from the study, he noted, is that botulinum toxin can have effects beyond the local effect seen near an injection site. Administering botulinum toxin for spasms and spasticity, excessive sweating, migraine, urinary bladder disorders, blepharospasm, or excessive salivation/drooling could result in reduced depression and improved systemic neurological effects.
“Severe depression remains a very difficult condition to treat. The existing drugs have dangerous side effects, the onset of the therapeutic action is delayed by at least a month, and the adherence to the medication is suboptimal. Therefore, finding new ways to treat depression is critical,” Dr. Abagyan said. “Botulinum toxin opens up a new physiological mechanism to be tried to reduce depression.”
Michelle Magid, MD, MBA, of the department of psychiatry at the University of Texas at Austin, said in an interview that, although the study was retrospective, “physicians can feel confident that botulinum toxin treatment will not cause depression; it may very well lead to improved mood in some of their patients.” Dr. Magid was not an author of this study, but has studied botulinum toxin as a possible treatment of depression.
“Previous studies have shown that botulinum toxin injected into the forehead region can improve symptoms of depression. The studies were small and confined to treating the glabellar region only,” she added. “This is a large retrospective study showing that botulinum toxin injected into other regions, such as the neck, underarms, bladder, hands, arms, and legs, can also have an antidepressant effect.”
Dr. Magid agreed that the use of botulinum toxin as an antidepressant should be investigated further, and could be a tool for patients who do not respond well to traditional antidepressant medications.
In their paper, the authors offered several plausible mechanisms for the antidepressant effects of botulinum toxin, including transneuronal transport to the parts of the central nervous system that regulate mood and emotion, systemic distribution, distributed muscle stress memory, and efficacy in the primary indication treatment. Although the mechanism of action is not well understood, Dr. Magid noted it could be the removal of somatic symptoms that contribute to an improvement in mood.
“It is possible that alleviating the psychological distress associated with neck spasms, excessive sweating [and so on] can be causing the antidepressive effects,” she said. “However, it is also possible that depression is actualized by a series of somatic symptoms – body aches, insomnia, sweating, for example. By removing somatic symptoms, one may also remove the correlating mood dysregulation.”
The study “certainly raises a lot of questions,” particularly about the “apparent multiple mechanisms of action of BoNT that we don’t understand yet,” Mark Rubin, MD, a cosmetic dermatologist who practices in Beverly Hills, Calif., said in an interview. “I believe it lends great deal of credence to the use of [botulinum toxin] for depression and certainly validates the need for more robust clinical trials for that indication,” he added.
“I think what we all as clinicians need to take away from this paper is that there is a great deal we don’t understand about the global pharmacologic effects of [botulinum toxin] and equally important, that there are apparently other pharmacologic pathways we need to explore in the treatment of depression, said Dr. Rubin, of the department of dermatology at the University of California, San Diego, who was not an investigator in the study.
One author reported being a consultant for Allergan. Dr. Makunts and the other author report no relevant conflicts of interest; Dr. Magid reported being a consultant for Allergan and a speaker for Ipsen. Dr. Rubin had no related disclosures.
SOURCE: Makunts T et al. Sci Rep. 2020 Jul 30;10(1):12851. doi: 10.1038/s41598-020-69773-7.
, according to the study’s authors.
Their results show that the antidepressant effect of botulinum toxin “administered for various indications goes beyond the control of the intended disease states and does not depend on the location of the injection,” according to Tigran Makunts, PharmD, of the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego, and coauthors.
Previous high-quality studies have found botulinum toxin treatment has been associated with antidepressant effects when administered to the glabellar region of the face, they noted. The study was published in Scientific Reports.
The researchers evaluated adverse events reported to the Food and Drug Administration’s current adverse event reporting system (FAERS) between September 2012 and December 2019, and the FDA’s previous adverse event reporting system between January 2004 and August 2012. Overall, they analyzed 174,243 reports, which were divided into eight treatment-related groups based on the indication for botulinum toxin: Cosmetic use (20,684 patients), migraine (4,180 patients), spasms and spasticity not involving facial muscles (2,335 patients), neurological and urinary bladder disorders (915 patients), torticollis (1,360 patients), hyperhidrosis (601 patients), blepharospasm (487 patients), and sialorrhea (157 patients). Each group was matched to controls from the FAERS database, who had different treatments for the same indications. (Reports in which patients were on an antidepressant or where depression was listed as an indication were not included).
In nearly all treatment groups, reports of depression and depression-related adverse events were significantly lower among those who received botulinum toxin, compared with controls: For those who received botulinum toxin injections in facial muscles for cosmetic uses, the reporting odds ratio was 0.46 (95% confidence interval, 0.27-0.78). Significant effects were also see in the following groups: those who received injections into facial and head muscles for migraine (ROR, 0.60; 95% CI, 0.48-0.74), injections into the upper and lower limbs for spasms and spasticity (ROR, 0.28; 95% CI, 0.18-0.42), injections into neck muscles for torticollis and neck pain (ROR, 0.30; 95% CI, 0.20-0.44), injections into eyelid muscles for blepharospasm (ROR, 0.13; 95% CI, 0.05-0.39), and injections into the axilla and palm for hyperhidrosis (ROR, 0.12; 95% CI, 0.04-0.33).
There were no cases of depression or depression-related adverse event reports among those treated with botulinum toxin for sialorrhea with injections into the parotid and submandibular glands, and there were decreased reports of depression among those who received detrusor muscle injections for neurological and urinary bladder disorders, but the results in both groups were not statistically significant, according to the researchers.
In an interview, Ruben Abagyan, PhD, study coauthor and professor at Skaggs School of Pharmacy and Pharmaceutical Sciences, said the study’s finding go “beyond breaking a positive feedback loop between depression and the ‘frown’ wrinkles in the glabellar region of the forehead.” The data showing efficacy with botulinum toxin injected in other areas of the body can help clinicians “expand their search for the most effective injection location and dose beyond the facial injections to improve the depression-related therapeutic outcomes.”
Another takeaway from the study, he noted, is that botulinum toxin can have effects beyond the local effect seen near an injection site. Administering botulinum toxin for spasms and spasticity, excessive sweating, migraine, urinary bladder disorders, blepharospasm, or excessive salivation/drooling could result in reduced depression and improved systemic neurological effects.
“Severe depression remains a very difficult condition to treat. The existing drugs have dangerous side effects, the onset of the therapeutic action is delayed by at least a month, and the adherence to the medication is suboptimal. Therefore, finding new ways to treat depression is critical,” Dr. Abagyan said. “Botulinum toxin opens up a new physiological mechanism to be tried to reduce depression.”
Michelle Magid, MD, MBA, of the department of psychiatry at the University of Texas at Austin, said in an interview that, although the study was retrospective, “physicians can feel confident that botulinum toxin treatment will not cause depression; it may very well lead to improved mood in some of their patients.” Dr. Magid was not an author of this study, but has studied botulinum toxin as a possible treatment of depression.
“Previous studies have shown that botulinum toxin injected into the forehead region can improve symptoms of depression. The studies were small and confined to treating the glabellar region only,” she added. “This is a large retrospective study showing that botulinum toxin injected into other regions, such as the neck, underarms, bladder, hands, arms, and legs, can also have an antidepressant effect.”
Dr. Magid agreed that the use of botulinum toxin as an antidepressant should be investigated further, and could be a tool for patients who do not respond well to traditional antidepressant medications.
In their paper, the authors offered several plausible mechanisms for the antidepressant effects of botulinum toxin, including transneuronal transport to the parts of the central nervous system that regulate mood and emotion, systemic distribution, distributed muscle stress memory, and efficacy in the primary indication treatment. Although the mechanism of action is not well understood, Dr. Magid noted it could be the removal of somatic symptoms that contribute to an improvement in mood.
“It is possible that alleviating the psychological distress associated with neck spasms, excessive sweating [and so on] can be causing the antidepressive effects,” she said. “However, it is also possible that depression is actualized by a series of somatic symptoms – body aches, insomnia, sweating, for example. By removing somatic symptoms, one may also remove the correlating mood dysregulation.”
The study “certainly raises a lot of questions,” particularly about the “apparent multiple mechanisms of action of BoNT that we don’t understand yet,” Mark Rubin, MD, a cosmetic dermatologist who practices in Beverly Hills, Calif., said in an interview. “I believe it lends great deal of credence to the use of [botulinum toxin] for depression and certainly validates the need for more robust clinical trials for that indication,” he added.
“I think what we all as clinicians need to take away from this paper is that there is a great deal we don’t understand about the global pharmacologic effects of [botulinum toxin] and equally important, that there are apparently other pharmacologic pathways we need to explore in the treatment of depression, said Dr. Rubin, of the department of dermatology at the University of California, San Diego, who was not an investigator in the study.
One author reported being a consultant for Allergan. Dr. Makunts and the other author report no relevant conflicts of interest; Dr. Magid reported being a consultant for Allergan and a speaker for Ipsen. Dr. Rubin had no related disclosures.
SOURCE: Makunts T et al. Sci Rep. 2020 Jul 30;10(1):12851. doi: 10.1038/s41598-020-69773-7.
, according to the study’s authors.
Their results show that the antidepressant effect of botulinum toxin “administered for various indications goes beyond the control of the intended disease states and does not depend on the location of the injection,” according to Tigran Makunts, PharmD, of the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego, and coauthors.
Previous high-quality studies have found botulinum toxin treatment has been associated with antidepressant effects when administered to the glabellar region of the face, they noted. The study was published in Scientific Reports.
The researchers evaluated adverse events reported to the Food and Drug Administration’s current adverse event reporting system (FAERS) between September 2012 and December 2019, and the FDA’s previous adverse event reporting system between January 2004 and August 2012. Overall, they analyzed 174,243 reports, which were divided into eight treatment-related groups based on the indication for botulinum toxin: Cosmetic use (20,684 patients), migraine (4,180 patients), spasms and spasticity not involving facial muscles (2,335 patients), neurological and urinary bladder disorders (915 patients), torticollis (1,360 patients), hyperhidrosis (601 patients), blepharospasm (487 patients), and sialorrhea (157 patients). Each group was matched to controls from the FAERS database, who had different treatments for the same indications. (Reports in which patients were on an antidepressant or where depression was listed as an indication were not included).
In nearly all treatment groups, reports of depression and depression-related adverse events were significantly lower among those who received botulinum toxin, compared with controls: For those who received botulinum toxin injections in facial muscles for cosmetic uses, the reporting odds ratio was 0.46 (95% confidence interval, 0.27-0.78). Significant effects were also see in the following groups: those who received injections into facial and head muscles for migraine (ROR, 0.60; 95% CI, 0.48-0.74), injections into the upper and lower limbs for spasms and spasticity (ROR, 0.28; 95% CI, 0.18-0.42), injections into neck muscles for torticollis and neck pain (ROR, 0.30; 95% CI, 0.20-0.44), injections into eyelid muscles for blepharospasm (ROR, 0.13; 95% CI, 0.05-0.39), and injections into the axilla and palm for hyperhidrosis (ROR, 0.12; 95% CI, 0.04-0.33).
There were no cases of depression or depression-related adverse event reports among those treated with botulinum toxin for sialorrhea with injections into the parotid and submandibular glands, and there were decreased reports of depression among those who received detrusor muscle injections for neurological and urinary bladder disorders, but the results in both groups were not statistically significant, according to the researchers.
In an interview, Ruben Abagyan, PhD, study coauthor and professor at Skaggs School of Pharmacy and Pharmaceutical Sciences, said the study’s finding go “beyond breaking a positive feedback loop between depression and the ‘frown’ wrinkles in the glabellar region of the forehead.” The data showing efficacy with botulinum toxin injected in other areas of the body can help clinicians “expand their search for the most effective injection location and dose beyond the facial injections to improve the depression-related therapeutic outcomes.”
Another takeaway from the study, he noted, is that botulinum toxin can have effects beyond the local effect seen near an injection site. Administering botulinum toxin for spasms and spasticity, excessive sweating, migraine, urinary bladder disorders, blepharospasm, or excessive salivation/drooling could result in reduced depression and improved systemic neurological effects.
“Severe depression remains a very difficult condition to treat. The existing drugs have dangerous side effects, the onset of the therapeutic action is delayed by at least a month, and the adherence to the medication is suboptimal. Therefore, finding new ways to treat depression is critical,” Dr. Abagyan said. “Botulinum toxin opens up a new physiological mechanism to be tried to reduce depression.”
Michelle Magid, MD, MBA, of the department of psychiatry at the University of Texas at Austin, said in an interview that, although the study was retrospective, “physicians can feel confident that botulinum toxin treatment will not cause depression; it may very well lead to improved mood in some of their patients.” Dr. Magid was not an author of this study, but has studied botulinum toxin as a possible treatment of depression.
“Previous studies have shown that botulinum toxin injected into the forehead region can improve symptoms of depression. The studies were small and confined to treating the glabellar region only,” she added. “This is a large retrospective study showing that botulinum toxin injected into other regions, such as the neck, underarms, bladder, hands, arms, and legs, can also have an antidepressant effect.”
Dr. Magid agreed that the use of botulinum toxin as an antidepressant should be investigated further, and could be a tool for patients who do not respond well to traditional antidepressant medications.
In their paper, the authors offered several plausible mechanisms for the antidepressant effects of botulinum toxin, including transneuronal transport to the parts of the central nervous system that regulate mood and emotion, systemic distribution, distributed muscle stress memory, and efficacy in the primary indication treatment. Although the mechanism of action is not well understood, Dr. Magid noted it could be the removal of somatic symptoms that contribute to an improvement in mood.
“It is possible that alleviating the psychological distress associated with neck spasms, excessive sweating [and so on] can be causing the antidepressive effects,” she said. “However, it is also possible that depression is actualized by a series of somatic symptoms – body aches, insomnia, sweating, for example. By removing somatic symptoms, one may also remove the correlating mood dysregulation.”
The study “certainly raises a lot of questions,” particularly about the “apparent multiple mechanisms of action of BoNT that we don’t understand yet,” Mark Rubin, MD, a cosmetic dermatologist who practices in Beverly Hills, Calif., said in an interview. “I believe it lends great deal of credence to the use of [botulinum toxin] for depression and certainly validates the need for more robust clinical trials for that indication,” he added.
“I think what we all as clinicians need to take away from this paper is that there is a great deal we don’t understand about the global pharmacologic effects of [botulinum toxin] and equally important, that there are apparently other pharmacologic pathways we need to explore in the treatment of depression, said Dr. Rubin, of the department of dermatology at the University of California, San Diego, who was not an investigator in the study.
One author reported being a consultant for Allergan. Dr. Makunts and the other author report no relevant conflicts of interest; Dr. Magid reported being a consultant for Allergan and a speaker for Ipsen. Dr. Rubin had no related disclosures.
SOURCE: Makunts T et al. Sci Rep. 2020 Jul 30;10(1):12851. doi: 10.1038/s41598-020-69773-7.
FROM SCIENTIFIC REPORTS
Pandemic hampers reopening of joint replacement gold mine
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.
Experimental nonstimulant effective, fast-acting for ADHD
The experimental nonstimulant medication viloxazine extended-release, known as SPN-812, reduced symptoms of attention-deficit/hyperactivity disorder (ADHD) as soon as 1 week after dosing and was well tolerated in a randomized, placebo-controlled phase 3 study that included more than 400 children.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interviews.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The findings were published online July 25 in Clinical Therapeutics.
Novel modulating agent
Viloxazine extended-release is a novel multimodal serotonergic and noradrenergic modulating agent with activity at serotonin receptors and the norepinephrine transporter.
About two-thirds were boys.
All participants had an ADHD-Rating Scale–5 (ADHD-RS-5) score of at least 28 and a Clinical Global Impression–Severity score of at least 4. None had taken ADHD medication for at least 1 week prior to randomization.
The intent-to-treat population included 460 children. Of these, 155 were randomly assigned to receive placebo, 147 to receive viloxazine 100 mg, and 158 to receive viloxazine 200 mg.
The primary efficacy endpoint was change from baseline in ADHD-RS-5 total score at week 6. Score changes for both the 100-mg (P = .0004) and the 200-mg (P < .0001) viloxazine groups met statistical significance compared with the placebo group.
Change from baseline in both the ADHD-RS-5 inattention and hyperactivity/impulsivity subscale scores was also significantly reduced in the 100-mg (P = .0006 and .0026, respectively) and 200-mg (P < .0001 and P < .0001, respectively) treatment groups compared with the placebo group.
Improvements occurred after 1 week of treatment and were maintained throughout the 6-week trial, “indicating an early and sustained effect,” the investigators wrote.
FDA target action date
The Clinical Global Impression–Improvement (CGI-I) score at 6 weeks was significantly improved in those receiving 100 mg (P = .0020) and 200 mg (P < .0001) of the active treatment compared with placebo.
The CGI-I responder rate, the percentage of children with a CGI-I score of 1 (very much improved) or 2 (much improved), was significantly higher at 6 weeks with viloxazine 100 mg and 200 mg vs. placebo (45% and 51% vs. 30%, respectively; P = .0065 and P = .0002).
These standard investigator-rated assessments were supported by two parent self-rated assessments: the Conners 3–Parent Short Form and the Weiss Functional Impairment Rating Scale–Parent Form.
Parents noted improvement not only in their children’s ADHD symptoms but also in ADHD-associated learning problems, executive functioning, defiance/aggression, peer relations, and functioning in different settings.
At both doses, once-daily viloxazine was generally well tolerated, with a low rate of discontinuation because of adverse events (<5%). Most adverse events were characterized as mild or moderate in severity and included somnolence (8.9%), decreased appetite (6.0%), and headache (5.4%).
On the basis of results of this study and others, the Food and Drug Administration accepted the company’s new drug application for viloxazine extended-release for ADHD in children and adolescents. The application has a target action date of Nov. 8, 2020.
Potential advantages
Commenting on the study in an interview, Dean Elbe, PharmD, clinical pharmacy specialist, child and adolescent mental health, BC Children’s Hospital, Vancouver, B.C., said that use of viloxazine to treat ADHD is “interesting.”
Dr. Elbe, who was not involved with the current research, noted that “it is actually an old drug that has been around since the mid-1970s in Europe as an antidepressant. It was removed from the market due to poor sales, not safety issues.”
Overall, on the basis of this study, viloxazine has potential to offer “modest improvements” over atomoxetine (Strattera), and the dosing may be “more straightforward and somewhat less challenging than with atomoxetine, with no taper up and no adjustment for poor 2D6 metabolizers,” Dr. Elbe noted.
“The onset of action appears somewhat quicker than we typically see with atomoxetine, so that is also helpful for parent and clinician acceptance and partially overcomes a perceived barrier with atomoxetine,” he said.
Dr. Elbe said he wonders, however, whether viloxazine will show “real-world clinical utility for both hyperactive-impulsive as well as inattentive symptoms. Although the study shows efficacy in both symptom clusters, so did the atomoxetine RCTs, and this has not been the clinical impression for atomoxetine.”
The study was funded by Supernus Pharmaceuticals. Dr. Cutler is a consultant for Supernus, as well as for Adlon Therapeutics, Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, NLS Pharma, Otsuka, Purdue, Shire, Sunovion, Takeda, and Tris Pharma. He has received speaker/promotional honoraria from Adlon Therapeutics, Arbor Pharmaceuticals, Lundbeck, Neos Therapeutics, Otsuka, Shire, Sunovion, Takeda, and Tris Pharma and has received research grants from Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, Otsuka, Purdue, Shire, Sunovion, Supernus Pharmaceuticals, Takeda, and Tris Pharma. A complete list of disclosures for the other authors is available in the original article. Dr. Elbe has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The experimental nonstimulant medication viloxazine extended-release, known as SPN-812, reduced symptoms of attention-deficit/hyperactivity disorder (ADHD) as soon as 1 week after dosing and was well tolerated in a randomized, placebo-controlled phase 3 study that included more than 400 children.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interviews.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The findings were published online July 25 in Clinical Therapeutics.
Novel modulating agent
Viloxazine extended-release is a novel multimodal serotonergic and noradrenergic modulating agent with activity at serotonin receptors and the norepinephrine transporter.
About two-thirds were boys.
All participants had an ADHD-Rating Scale–5 (ADHD-RS-5) score of at least 28 and a Clinical Global Impression–Severity score of at least 4. None had taken ADHD medication for at least 1 week prior to randomization.
The intent-to-treat population included 460 children. Of these, 155 were randomly assigned to receive placebo, 147 to receive viloxazine 100 mg, and 158 to receive viloxazine 200 mg.
The primary efficacy endpoint was change from baseline in ADHD-RS-5 total score at week 6. Score changes for both the 100-mg (P = .0004) and the 200-mg (P < .0001) viloxazine groups met statistical significance compared with the placebo group.
Change from baseline in both the ADHD-RS-5 inattention and hyperactivity/impulsivity subscale scores was also significantly reduced in the 100-mg (P = .0006 and .0026, respectively) and 200-mg (P < .0001 and P < .0001, respectively) treatment groups compared with the placebo group.
Improvements occurred after 1 week of treatment and were maintained throughout the 6-week trial, “indicating an early and sustained effect,” the investigators wrote.
FDA target action date
The Clinical Global Impression–Improvement (CGI-I) score at 6 weeks was significantly improved in those receiving 100 mg (P = .0020) and 200 mg (P < .0001) of the active treatment compared with placebo.
The CGI-I responder rate, the percentage of children with a CGI-I score of 1 (very much improved) or 2 (much improved), was significantly higher at 6 weeks with viloxazine 100 mg and 200 mg vs. placebo (45% and 51% vs. 30%, respectively; P = .0065 and P = .0002).
These standard investigator-rated assessments were supported by two parent self-rated assessments: the Conners 3–Parent Short Form and the Weiss Functional Impairment Rating Scale–Parent Form.
Parents noted improvement not only in their children’s ADHD symptoms but also in ADHD-associated learning problems, executive functioning, defiance/aggression, peer relations, and functioning in different settings.
At both doses, once-daily viloxazine was generally well tolerated, with a low rate of discontinuation because of adverse events (<5%). Most adverse events were characterized as mild or moderate in severity and included somnolence (8.9%), decreased appetite (6.0%), and headache (5.4%).
On the basis of results of this study and others, the Food and Drug Administration accepted the company’s new drug application for viloxazine extended-release for ADHD in children and adolescents. The application has a target action date of Nov. 8, 2020.
Potential advantages
Commenting on the study in an interview, Dean Elbe, PharmD, clinical pharmacy specialist, child and adolescent mental health, BC Children’s Hospital, Vancouver, B.C., said that use of viloxazine to treat ADHD is “interesting.”
Dr. Elbe, who was not involved with the current research, noted that “it is actually an old drug that has been around since the mid-1970s in Europe as an antidepressant. It was removed from the market due to poor sales, not safety issues.”
Overall, on the basis of this study, viloxazine has potential to offer “modest improvements” over atomoxetine (Strattera), and the dosing may be “more straightforward and somewhat less challenging than with atomoxetine, with no taper up and no adjustment for poor 2D6 metabolizers,” Dr. Elbe noted.
“The onset of action appears somewhat quicker than we typically see with atomoxetine, so that is also helpful for parent and clinician acceptance and partially overcomes a perceived barrier with atomoxetine,” he said.
Dr. Elbe said he wonders, however, whether viloxazine will show “real-world clinical utility for both hyperactive-impulsive as well as inattentive symptoms. Although the study shows efficacy in both symptom clusters, so did the atomoxetine RCTs, and this has not been the clinical impression for atomoxetine.”
The study was funded by Supernus Pharmaceuticals. Dr. Cutler is a consultant for Supernus, as well as for Adlon Therapeutics, Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, NLS Pharma, Otsuka, Purdue, Shire, Sunovion, Takeda, and Tris Pharma. He has received speaker/promotional honoraria from Adlon Therapeutics, Arbor Pharmaceuticals, Lundbeck, Neos Therapeutics, Otsuka, Shire, Sunovion, Takeda, and Tris Pharma and has received research grants from Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, Otsuka, Purdue, Shire, Sunovion, Supernus Pharmaceuticals, Takeda, and Tris Pharma. A complete list of disclosures for the other authors is available in the original article. Dr. Elbe has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The experimental nonstimulant medication viloxazine extended-release, known as SPN-812, reduced symptoms of attention-deficit/hyperactivity disorder (ADHD) as soon as 1 week after dosing and was well tolerated in a randomized, placebo-controlled phase 3 study that included more than 400 children.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interviews.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The findings were published online July 25 in Clinical Therapeutics.
Novel modulating agent
Viloxazine extended-release is a novel multimodal serotonergic and noradrenergic modulating agent with activity at serotonin receptors and the norepinephrine transporter.
About two-thirds were boys.
All participants had an ADHD-Rating Scale–5 (ADHD-RS-5) score of at least 28 and a Clinical Global Impression–Severity score of at least 4. None had taken ADHD medication for at least 1 week prior to randomization.
The intent-to-treat population included 460 children. Of these, 155 were randomly assigned to receive placebo, 147 to receive viloxazine 100 mg, and 158 to receive viloxazine 200 mg.
The primary efficacy endpoint was change from baseline in ADHD-RS-5 total score at week 6. Score changes for both the 100-mg (P = .0004) and the 200-mg (P < .0001) viloxazine groups met statistical significance compared with the placebo group.
Change from baseline in both the ADHD-RS-5 inattention and hyperactivity/impulsivity subscale scores was also significantly reduced in the 100-mg (P = .0006 and .0026, respectively) and 200-mg (P < .0001 and P < .0001, respectively) treatment groups compared with the placebo group.
Improvements occurred after 1 week of treatment and were maintained throughout the 6-week trial, “indicating an early and sustained effect,” the investigators wrote.
FDA target action date
The Clinical Global Impression–Improvement (CGI-I) score at 6 weeks was significantly improved in those receiving 100 mg (P = .0020) and 200 mg (P < .0001) of the active treatment compared with placebo.
The CGI-I responder rate, the percentage of children with a CGI-I score of 1 (very much improved) or 2 (much improved), was significantly higher at 6 weeks with viloxazine 100 mg and 200 mg vs. placebo (45% and 51% vs. 30%, respectively; P = .0065 and P = .0002).
These standard investigator-rated assessments were supported by two parent self-rated assessments: the Conners 3–Parent Short Form and the Weiss Functional Impairment Rating Scale–Parent Form.
Parents noted improvement not only in their children’s ADHD symptoms but also in ADHD-associated learning problems, executive functioning, defiance/aggression, peer relations, and functioning in different settings.
At both doses, once-daily viloxazine was generally well tolerated, with a low rate of discontinuation because of adverse events (<5%). Most adverse events were characterized as mild or moderate in severity and included somnolence (8.9%), decreased appetite (6.0%), and headache (5.4%).
On the basis of results of this study and others, the Food and Drug Administration accepted the company’s new drug application for viloxazine extended-release for ADHD in children and adolescents. The application has a target action date of Nov. 8, 2020.
Potential advantages
Commenting on the study in an interview, Dean Elbe, PharmD, clinical pharmacy specialist, child and adolescent mental health, BC Children’s Hospital, Vancouver, B.C., said that use of viloxazine to treat ADHD is “interesting.”
Dr. Elbe, who was not involved with the current research, noted that “it is actually an old drug that has been around since the mid-1970s in Europe as an antidepressant. It was removed from the market due to poor sales, not safety issues.”
Overall, on the basis of this study, viloxazine has potential to offer “modest improvements” over atomoxetine (Strattera), and the dosing may be “more straightforward and somewhat less challenging than with atomoxetine, with no taper up and no adjustment for poor 2D6 metabolizers,” Dr. Elbe noted.
“The onset of action appears somewhat quicker than we typically see with atomoxetine, so that is also helpful for parent and clinician acceptance and partially overcomes a perceived barrier with atomoxetine,” he said.
Dr. Elbe said he wonders, however, whether viloxazine will show “real-world clinical utility for both hyperactive-impulsive as well as inattentive symptoms. Although the study shows efficacy in both symptom clusters, so did the atomoxetine RCTs, and this has not been the clinical impression for atomoxetine.”
The study was funded by Supernus Pharmaceuticals. Dr. Cutler is a consultant for Supernus, as well as for Adlon Therapeutics, Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, NLS Pharma, Otsuka, Purdue, Shire, Sunovion, Takeda, and Tris Pharma. He has received speaker/promotional honoraria from Adlon Therapeutics, Arbor Pharmaceuticals, Lundbeck, Neos Therapeutics, Otsuka, Shire, Sunovion, Takeda, and Tris Pharma and has received research grants from Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore, KemPharm, Lundbeck, Neos Therapeutics, Otsuka, Purdue, Shire, Sunovion, Supernus Pharmaceuticals, Takeda, and Tris Pharma. A complete list of disclosures for the other authors is available in the original article. Dr. Elbe has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Vitamin D fails to prevent late-life depression, boost mood
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
Findings from a large randomized, controlled trial do not support the use of vitamin D3 supplementation for adults for the sole purpose of preventing depression.
Among adults aged 50 years or older who were without clinically relevant depressive symptoms at baseline, vitamin D3 supplementation taken over 5 years did not reduce the risk for depression or make a difference in the quality of mood.
“The study is among the largest of its kind ever, and it was able to address whether vitamin D3 supplementation is useful for what we call ‘universal prevention’ of depression,” Olivia Okereke, MD, Massachusetts General Hospital, Boston, said in an interview.
“These results tell us that there is no benefit to using vitamin D3 supplements for the sole purpose of preventing depression in the general population of middle-aged and older adults,” said Dr. Okereke.
“Because of the high dose and long duration of treatment and the randomized placebo-controlled design, we can have high confidence in results,” she added.
The study was published online August 4 in JAMA.
The VITAL-DEP trial
The findings are based on 18,353 older adults (mean age, 67.5 years; 49% women) in the VITAL-DEP study; 16,657 were at risk for incident depression (ie, had no history of depression), and 1696 were at risk for recurrent depression (i.e., had a history of depression but had not undergone treatment for depression within the past 2 years).
Roughly half were randomly allocated to receive vitamin D3 (2000 IU/d of cholecalciferol) and half to receive matching placebo for a median of 5.3 years. The participants’ mean level of 25-hydroxyvitamin D was 31.1 ng/mL; for about 12%, levels were lower than 20 ng/mL.
The risk for depression or clinically relevant depressive symptoms (total of incident and recurrent cases) was not significantly different between the vitamin D3 group (609 depression or clinically relevant depressive symptom events; 12.9/1000 person-years) and the placebo group (625 depression or clinically relevant depressive symptom events; 13.3/1000 person-years). The hazard ratio was 0.97 (95% confidence interval, 0.87-1.09; P = .62).
“Cumulative incidence curves showed lack of separation between treatment groups over the entire follow-up,” the researchers report.
There was also no significant between-group difference in the other primary outcome – the mean difference in mood scores on the eight-item Patient Health Questionnaire depression scale (PHQ-8).
The mean difference for change between treatment groups in PHQ-8 scores was not significantly different from 0 over the entire follow-up (0.01 points; 95% CI, −0.04 to 0.05 points) or at any point during follow-up.
To date, 13 randomized clinical trials have examined the effects of vitamin D3 supplementation on depression or mood during middle age or in older adults, and all except one reported null findings, Dr. Okereke and colleagues noted in their article.
The current study is the only one large enough to examine vitamin D3 supplementation for the universal prevention of depression, they point out.
Although the findings do not support vitamin D3 supplementation for depression prevention, Dr. Okereke said, We also know that vitamin D is essential for bone health, and this study does not tell us whether vitamin D3 is useful for prevention of other health outcomes.”
VITAL-DEP was supported by a grant from the National Institute of Mental Health. Pharmavite donated the vitamin D3, matching placebos, and packaging in the form of calendar packs. Dr. Okereke reported receiving royalties from Springer Publishing for a book on the prevention of late-life depression.
This article first appeared on Medscape.com.
Studies gauge role of schools, kids in spread of COVID-19
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
When officials closed U.S. schools in March to limit the spread of COVID-19, they may have prevented more than 1 million cases over a 26-day period, a new estimate published online July 29 in JAMA suggests.
But school closures also left blind spots in understanding how children and schools affect disease transmission.
“School closures early in pandemic responses thwarted larger-scale investigations of schools as a source of community transmission,” researchers noted in a separate study, published online July 30 in JAMA Pediatrics, that examined levels of viral RNA in children and adults with COVID-19.
“Our analyses suggest children younger than 5 years with mild to moderate COVID-19 have high amounts of SARS-CoV-2 viral RNA in their nasopharynx, compared with older children and adults,” reported Taylor Heald-Sargent, MD, PhD, and colleagues. “Thus, young children can potentially be important drivers of SARS-CoV-2 spread in the general population, as has been demonstrated with respiratory syncytial virus, where children with high viral loads are more likely to transmit.”
Although the study “was not designed to prove that younger children spread COVID-19 as much as adults,” it is a possibility, Dr. Heald-Sargent, a pediatric infectious diseases specialist at Ann and Robert H. Lurie Children’s Hospital and assistant professor of pediatrics at Northwestern University, Chicago, said in a related news release. “We need to take that into account in efforts to reduce transmission as we continue to learn more about this virus.”.
The study included 145 patients with mild or moderate illness who were within 1 week of symptom onset. The researchers used reverse transcriptase–polymerase chain reaction (rt-PCR) on nasopharyngeal swabs collected at inpatient, outpatient, emergency department, or drive-through testing sites to measure SARS-CoV-2 levels. The investigators compared PCR amplification cycle threshold (CT) values for children younger than 5 years (n = 46), children aged 5-17 years (n = 51), and adults aged 18-65 years (n = 48); lower CT values indicate higher amounts of viral nucleic acid.
Median CT values for older children and adults were similar (about 11), whereas the median CT value for young children was significantly lower (6.5). The differences between young children and adults “approximate a 10-fold to 100-fold greater amount of SARS-CoV-2 in the upper respiratory tract of young children,” the researchers wrote.
“Behavioral habits of young children and close quarters in school and day care settings raise concern for SARS-CoV-2 amplification in this population as public health restrictions are eased,” they write.
Modeling the impact of school closures
In the JAMA study, Katherine A. Auger, MD, of Cincinnati Children’s Hospital Medical Center, and colleagues examined at the U.S. population level whether closing schools, as all 50 states did in March, was associated with relative decreases in COVID-19 incidence and mortality.
To isolate the effect of school closures, the researchers used an interrupted time series analysis and included other state-level nonpharmaceutical interventions and variables in their regression models.
“Per week, the incidence was estimated to have been 39% of what it would have been had schools remained open,” Dr. Auger and colleagues wrote. “Extrapolating the absolute differences of 423.9 cases and 12.6 deaths per 100,000 to 322.2 million residents nationally suggests that school closure may have been associated with approximately 1.37 million fewer cases of COVID-19 over a 26-day period and 40,600 fewer deaths over a 16-day period; however, these figures do not account for uncertainty in the model assumptions and the resulting estimates.”
Relative reductions in incidence and mortality were largest in states that closed schools when the incidence of COVID-19 was low, the authors found.
Decisions with high stakes
In an accompanying editorial, Julie M. Donohue, PhD, and Elizabeth Miller, MD, PhD, both affiliated with the University of Pittsburgh, emphasized that the results are estimates. “School closures were enacted in close proximity ... to other physical distancing measures, such as nonessential business closures and stay-at-home orders, making it difficult to disentangle the potential effect of each intervention.”
Although the findings “suggest a role for school closures in virus mitigation, school and health officials must balance this with academic, health, and economic consequences,” Dr. Donohue and Dr. Miller added. “Given the strong connection between education, income, and life expectancy, school closures could have long-term deleterious consequences for child health, likely reaching into adulthood.” Schools provide “meals and nutrition, health care including behavioral health supports, physical activity, social interaction, supports for students with special education needs and disabilities, and other vital resources for healthy development.”
In a viewpoint article also published in JAMA, authors involved in the creation of a National Academies of Sciences, Engineering, and Medicine reported on the reopening of schools recommend that districts “make every effort to prioritize reopening with an emphasis on providing in-person instruction for students in kindergarten through grade 5 as well as those students with special needs who might be best served by in-person instruction.
“To reopen safely, school districts are encouraged to ensure ventilation and air filtration, clean surfaces frequently, provide facilities for regular handwashing, and provide space for physical distancing,” write Kenne A. Dibner, PhD, of the NASEM in Washington, D.C., and coauthors.
Furthermore, districts “need to consider transparent communication of the reality that while measures can be implemented to lower the risk of transmitting COVID-19 when schools reopen, there is no way to eliminate that risk entirely. It is critical to share both the risks and benefits of different scenarios,” they wrote.
The JAMA modeling study received funding from the Agency for Healthcare Research and Quality and the National Institutes of Health. The NASEM report was funded by the Brady Education Foundation and the Spencer Foundation. The authors disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
Telemedicine in primary care
How to effectively utilize this tool
By now it is well known that the COVID-19 pandemic has significantly disrupted primary care. Office visits and revenues have precipitously dropped as physicians and patients alike fear in-person visits may increase their risks of contracting the virus. However, telemedicine has emerged as a lifeline of sorts for many practices, enabling them to conduct visits and maintain contact with patients.
Telemedicine is likely to continue to serve as a tool for primary care providers to improve access to convenient, cost-effective, high-quality care after the pandemic. Another benefit of telemedicine is it can help maintain a portion of a practice’s revenue stream for physicians during uncertain times.
Indeed, the nation has seen recent progress toward telemedicine parity, which refers to the concept of reimbursing providers’ telehealth visits at the same rates as similar in-person visits.
A challenge to adopting telemedicine is that it calls for adjusting established workflows for in-person encounters. A practice cannot simply replicate in-person processes to work for telehealth. While both in-person and virtual visits require adherence to HIPAA, for example, how you actually protect patient privacy will call for different measures. Harking back to the early days of EMR implementation, one does not need to like the telemedicine platform or process, but come to terms with the fact that it is a tool that is here to stay to deliver patient care.
Treat your practice like a laboratory
Adoption may vary between practices depending on many factors, including clinicians’ comfort with technology, clinical tolerance and triage rules for nontouch encounters, state regulations, and more. Every provider group should begin experimenting with telemedicine in specific ways that make sense for them.
One physician may practice telemedicine full-time while the rest abstain, or perhaps the practice prefers to offer telemedicine services during specific hours on specific days. Don’t be afraid to start slowly when you’re trying something new – but do get started with telehealth. It will increasingly be a mainstream medium and more patients will come to expect it.
Train the entire team
Many primary care practices do not enjoy the resources of an information technology team, so all team members essentially need to learn the new skill of telemedicine usage, in addition to assisting patients. That can’t happen without staff buy-in, so it is essential that everyone from the office manager to medical assistants have the training they need to make the technology work. Juggling schedules for telehealth and in-office, activating an account through email, starting and joining a telehealth meeting, and preparing a patient for a visit are just a handful of basic tasks your staff should be trained to do to contribute to the successful integration of telehealth.
Educate and encourage patients to use telehealth
While unfamiliarity with technology may represent a roadblock for some patients, others resist telemedicine simply because no one has explained to them why it’s so important and the benefits it can hold for them. Education and communication are critical, including the sometimes painstaking work of slowly walking patients through the process of performing important functions on the telemedicine app. By providing them with some friendly coaching, patients won’t feel lost or abandoned during what for some may be an unfamiliar and frustrating process.
Manage more behavioral health
Different states and health plans incentivize primary practices for integrating behavioral health into their offerings. Rather than dismiss this addition to your own practice as too cumbersome to take on, I would recommend using telehealth to expand behavioral health care services.
If your practice is working toward a team-based, interdisciplinary approach to care delivery, behavioral health is a critical component. While other elements of this “whole person” health care may be better suited for an office visit, the vast majority of behavioral health services can be delivered virtually.
To decide if your patient may benefit from behavioral health care, the primary care provider (PCP) can conduct a screening via telehealth. Once the screening is complete, the PCP can discuss results and refer the patient to a mental health professional – all via telehealth. While patients may be reluctant to receive behavioral health treatment, perhaps because of stigma or inexperience, they may appreciate the telemedicine option as they can remain in the comfort and familiarity of their homes.
Collaborative Care is both an in-person and virtual model that allows PCP practices to offer behavioral health services in a cost effective way by utilizing a psychiatrist as a “consultant” to the practice as opposed to hiring a full-time psychiatrist. All services within the Collaborative Care Model can be offered via telehealth, and all major insurance providers reimburse primary care providers for delivering Collaborative Care.
When PCPs provide behavioral health treatment as an “extension” of the primary care service offerings, the stigma is reduced and more patients are willing to accept the care they need.
Many areas of the country suffer from a lack of access to behavioral health specialists. In rural counties, for example, the nearest therapist may be located over an hour away. By integrating behavioral telehealth services into your practice’s offerings, you can remove geographic and transportation obstacles to care for your patient population.
Doing this can lead to providing more culturally competent care. It’s important that you’re able to offer mental health services to your patients from a professional with a similar ethnic or racial background. Language barriers and cultural differences may limit a provider’s ability to treat a patient, particularly if the patient faces health disparities related to race or ethnicity. If your practice needs to look outside of your community to tap into a more diverse pool of providers to better meet your patients’ needs, telehealth makes it easier to do that.
Adopting telemedicine for consultative patient visits offers primary care a path toward restoring patient volume and hope for a postpandemic future.
Mark Stephan, MD, is chief medical officer at Equality Health, a whole-health delivery system. He practiced family medicine for 19 years, including hospital medicine and obstetrics in rural and urban settings. Dr. Stephan has no conflicts related to the content of this piece.
How to effectively utilize this tool
How to effectively utilize this tool
By now it is well known that the COVID-19 pandemic has significantly disrupted primary care. Office visits and revenues have precipitously dropped as physicians and patients alike fear in-person visits may increase their risks of contracting the virus. However, telemedicine has emerged as a lifeline of sorts for many practices, enabling them to conduct visits and maintain contact with patients.
Telemedicine is likely to continue to serve as a tool for primary care providers to improve access to convenient, cost-effective, high-quality care after the pandemic. Another benefit of telemedicine is it can help maintain a portion of a practice’s revenue stream for physicians during uncertain times.
Indeed, the nation has seen recent progress toward telemedicine parity, which refers to the concept of reimbursing providers’ telehealth visits at the same rates as similar in-person visits.
A challenge to adopting telemedicine is that it calls for adjusting established workflows for in-person encounters. A practice cannot simply replicate in-person processes to work for telehealth. While both in-person and virtual visits require adherence to HIPAA, for example, how you actually protect patient privacy will call for different measures. Harking back to the early days of EMR implementation, one does not need to like the telemedicine platform or process, but come to terms with the fact that it is a tool that is here to stay to deliver patient care.
Treat your practice like a laboratory
Adoption may vary between practices depending on many factors, including clinicians’ comfort with technology, clinical tolerance and triage rules for nontouch encounters, state regulations, and more. Every provider group should begin experimenting with telemedicine in specific ways that make sense for them.
One physician may practice telemedicine full-time while the rest abstain, or perhaps the practice prefers to offer telemedicine services during specific hours on specific days. Don’t be afraid to start slowly when you’re trying something new – but do get started with telehealth. It will increasingly be a mainstream medium and more patients will come to expect it.
Train the entire team
Many primary care practices do not enjoy the resources of an information technology team, so all team members essentially need to learn the new skill of telemedicine usage, in addition to assisting patients. That can’t happen without staff buy-in, so it is essential that everyone from the office manager to medical assistants have the training they need to make the technology work. Juggling schedules for telehealth and in-office, activating an account through email, starting and joining a telehealth meeting, and preparing a patient for a visit are just a handful of basic tasks your staff should be trained to do to contribute to the successful integration of telehealth.
Educate and encourage patients to use telehealth
While unfamiliarity with technology may represent a roadblock for some patients, others resist telemedicine simply because no one has explained to them why it’s so important and the benefits it can hold for them. Education and communication are critical, including the sometimes painstaking work of slowly walking patients through the process of performing important functions on the telemedicine app. By providing them with some friendly coaching, patients won’t feel lost or abandoned during what for some may be an unfamiliar and frustrating process.
Manage more behavioral health
Different states and health plans incentivize primary practices for integrating behavioral health into their offerings. Rather than dismiss this addition to your own practice as too cumbersome to take on, I would recommend using telehealth to expand behavioral health care services.
If your practice is working toward a team-based, interdisciplinary approach to care delivery, behavioral health is a critical component. While other elements of this “whole person” health care may be better suited for an office visit, the vast majority of behavioral health services can be delivered virtually.
To decide if your patient may benefit from behavioral health care, the primary care provider (PCP) can conduct a screening via telehealth. Once the screening is complete, the PCP can discuss results and refer the patient to a mental health professional – all via telehealth. While patients may be reluctant to receive behavioral health treatment, perhaps because of stigma or inexperience, they may appreciate the telemedicine option as they can remain in the comfort and familiarity of their homes.
Collaborative Care is both an in-person and virtual model that allows PCP practices to offer behavioral health services in a cost effective way by utilizing a psychiatrist as a “consultant” to the practice as opposed to hiring a full-time psychiatrist. All services within the Collaborative Care Model can be offered via telehealth, and all major insurance providers reimburse primary care providers for delivering Collaborative Care.
When PCPs provide behavioral health treatment as an “extension” of the primary care service offerings, the stigma is reduced and more patients are willing to accept the care they need.
Many areas of the country suffer from a lack of access to behavioral health specialists. In rural counties, for example, the nearest therapist may be located over an hour away. By integrating behavioral telehealth services into your practice’s offerings, you can remove geographic and transportation obstacles to care for your patient population.
Doing this can lead to providing more culturally competent care. It’s important that you’re able to offer mental health services to your patients from a professional with a similar ethnic or racial background. Language barriers and cultural differences may limit a provider’s ability to treat a patient, particularly if the patient faces health disparities related to race or ethnicity. If your practice needs to look outside of your community to tap into a more diverse pool of providers to better meet your patients’ needs, telehealth makes it easier to do that.
Adopting telemedicine for consultative patient visits offers primary care a path toward restoring patient volume and hope for a postpandemic future.
Mark Stephan, MD, is chief medical officer at Equality Health, a whole-health delivery system. He practiced family medicine for 19 years, including hospital medicine and obstetrics in rural and urban settings. Dr. Stephan has no conflicts related to the content of this piece.
By now it is well known that the COVID-19 pandemic has significantly disrupted primary care. Office visits and revenues have precipitously dropped as physicians and patients alike fear in-person visits may increase their risks of contracting the virus. However, telemedicine has emerged as a lifeline of sorts for many practices, enabling them to conduct visits and maintain contact with patients.
Telemedicine is likely to continue to serve as a tool for primary care providers to improve access to convenient, cost-effective, high-quality care after the pandemic. Another benefit of telemedicine is it can help maintain a portion of a practice’s revenue stream for physicians during uncertain times.
Indeed, the nation has seen recent progress toward telemedicine parity, which refers to the concept of reimbursing providers’ telehealth visits at the same rates as similar in-person visits.
A challenge to adopting telemedicine is that it calls for adjusting established workflows for in-person encounters. A practice cannot simply replicate in-person processes to work for telehealth. While both in-person and virtual visits require adherence to HIPAA, for example, how you actually protect patient privacy will call for different measures. Harking back to the early days of EMR implementation, one does not need to like the telemedicine platform or process, but come to terms with the fact that it is a tool that is here to stay to deliver patient care.
Treat your practice like a laboratory
Adoption may vary between practices depending on many factors, including clinicians’ comfort with technology, clinical tolerance and triage rules for nontouch encounters, state regulations, and more. Every provider group should begin experimenting with telemedicine in specific ways that make sense for them.
One physician may practice telemedicine full-time while the rest abstain, or perhaps the practice prefers to offer telemedicine services during specific hours on specific days. Don’t be afraid to start slowly when you’re trying something new – but do get started with telehealth. It will increasingly be a mainstream medium and more patients will come to expect it.
Train the entire team
Many primary care practices do not enjoy the resources of an information technology team, so all team members essentially need to learn the new skill of telemedicine usage, in addition to assisting patients. That can’t happen without staff buy-in, so it is essential that everyone from the office manager to medical assistants have the training they need to make the technology work. Juggling schedules for telehealth and in-office, activating an account through email, starting and joining a telehealth meeting, and preparing a patient for a visit are just a handful of basic tasks your staff should be trained to do to contribute to the successful integration of telehealth.
Educate and encourage patients to use telehealth
While unfamiliarity with technology may represent a roadblock for some patients, others resist telemedicine simply because no one has explained to them why it’s so important and the benefits it can hold for them. Education and communication are critical, including the sometimes painstaking work of slowly walking patients through the process of performing important functions on the telemedicine app. By providing them with some friendly coaching, patients won’t feel lost or abandoned during what for some may be an unfamiliar and frustrating process.
Manage more behavioral health
Different states and health plans incentivize primary practices for integrating behavioral health into their offerings. Rather than dismiss this addition to your own practice as too cumbersome to take on, I would recommend using telehealth to expand behavioral health care services.
If your practice is working toward a team-based, interdisciplinary approach to care delivery, behavioral health is a critical component. While other elements of this “whole person” health care may be better suited for an office visit, the vast majority of behavioral health services can be delivered virtually.
To decide if your patient may benefit from behavioral health care, the primary care provider (PCP) can conduct a screening via telehealth. Once the screening is complete, the PCP can discuss results and refer the patient to a mental health professional – all via telehealth. While patients may be reluctant to receive behavioral health treatment, perhaps because of stigma or inexperience, they may appreciate the telemedicine option as they can remain in the comfort and familiarity of their homes.
Collaborative Care is both an in-person and virtual model that allows PCP practices to offer behavioral health services in a cost effective way by utilizing a psychiatrist as a “consultant” to the practice as opposed to hiring a full-time psychiatrist. All services within the Collaborative Care Model can be offered via telehealth, and all major insurance providers reimburse primary care providers for delivering Collaborative Care.
When PCPs provide behavioral health treatment as an “extension” of the primary care service offerings, the stigma is reduced and more patients are willing to accept the care they need.
Many areas of the country suffer from a lack of access to behavioral health specialists. In rural counties, for example, the nearest therapist may be located over an hour away. By integrating behavioral telehealth services into your practice’s offerings, you can remove geographic and transportation obstacles to care for your patient population.
Doing this can lead to providing more culturally competent care. It’s important that you’re able to offer mental health services to your patients from a professional with a similar ethnic or racial background. Language barriers and cultural differences may limit a provider’s ability to treat a patient, particularly if the patient faces health disparities related to race or ethnicity. If your practice needs to look outside of your community to tap into a more diverse pool of providers to better meet your patients’ needs, telehealth makes it easier to do that.
Adopting telemedicine for consultative patient visits offers primary care a path toward restoring patient volume and hope for a postpandemic future.
Mark Stephan, MD, is chief medical officer at Equality Health, a whole-health delivery system. He practiced family medicine for 19 years, including hospital medicine and obstetrics in rural and urban settings. Dr. Stephan has no conflicts related to the content of this piece.
ED visits for mental health, substance use doubled in 1 decade
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).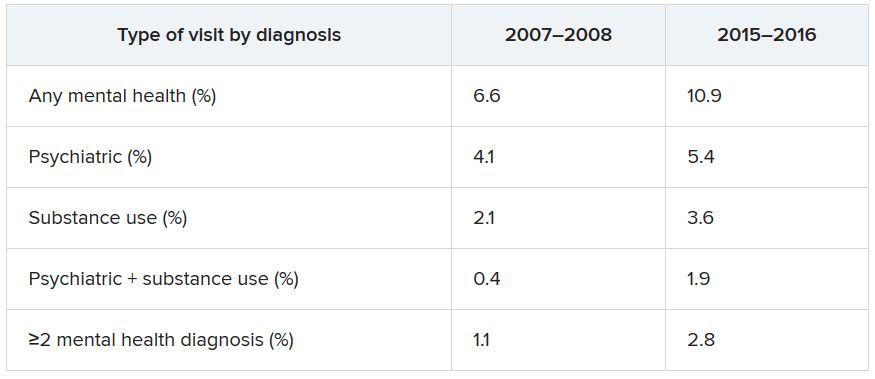
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ED visits related to mental health conditions increased nearly twofold from 2007-2008 to 2015-2016, new research suggests.
Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that, over the 10-year study period, the proportion of ED visits for mental health diagnoses increased from 6.6% to 10.9%, with substance use accounting for much of the increase.
Although there have been policy efforts, such as expanding access to mental health care as part of the Affordable Care Act (ACA) of 2011, the senior author Taeho Greg Rhee, PhD, MSW, said in an interview.
“Treating mental health conditions in EDs is often considered suboptimal” because of limited time for full psychiatric assessment, lack of trained providers, and limited privacy in EDs, said Dr. Rhee of Yale University, New Haven, Conn.
The findings were published online July 28 in The Journal of Clinical Psychiatry.
“Outdated” research
Roughly one-fifth of U.S. adults experience some type of mental, behavioral, or emotional disorder annually. Moreover, the suicide rate has been steadily increasing, and there continues to be a “raging opioid epidemic,” the researchers wrote.
Despite these alarming figures, 57.4% of adults with mental illness reported in 2017 that they had not received any mental health treatment in the past year, reported the investigators.
Previous research has suggested that many adults have difficulty seeking outpatient mental health treatment and may turn to EDs instead. However, most studies of mental health ED use “are by now outdated, as they used data from years prior to the full implementation of the ACA,” the researchers noted.
“More Americans are suffering from mental illness, and given the recent policy efforts of expanding access to mental health care, we were questioning if ED visits due to mental health has changed or not,” Dr. Rhee said.
To investigate the question, the researchers conducted a cross-sectional analysis of data from the NHAMCS, a publicly available dataset provided by the National Center for Health Statistics of the Centers for Disease Control and Prevention.
They grouped psychiatric diagnoses into five categories: mood disorders, anxiety disorders, psychosis or schizophrenia, suicide attempt or ideation, or other/unspecified. Substance use diagnoses were grouped into six categories: alcohol, amphetamine, cannabis, cocaine, opioid, or other/unspecified.
These categories were used to determine the type of disorder a patient had, whether the patient had both psychiatric and substance-related diagnoses, and whether the patient received multiple mental health diagnoses at the time of the ED visit.
Sociodemographic covariates included age, sex, race/ethnicity, and insurance coverage.
Twofold and fourfold increases
Of 100.9 million outpatient ED visits that took place between 2007 and 2016, approximately 8.4 million (8.3%) were for psychiatric or substance use–related diagnoses. Also, the visits were more likely from adults who were younger than 45 years, male, non-Hispanic White, and covered by Medicaid or other public insurance types (58.5%, 52.5%, 65.2%, and 58.6%, respectively).
The overall rate of ED visits for any mental health diagnosis nearly doubled between 2007-2008 and 2015-2016. The rate of visits in which both psychiatric and substance use–related diagnoses increased fourfold during that time span. ED visits involving at least two mental health diagnoses increased twofold.
Additional changes in the number of visits are listed below (for each, P < .001).
When these comparisons were adjusted for age, sex, and race/ethnicity, “linearly increasing trends of mental health–related ED visits were consistently found in all categories,” the authors reported. No trends were found regarding age, sex, or race/ethnicity. By contrast, mental health–related ED visits in which Medicaid was identified as the primary source of insurance nearly doubled between 2007–2008 and 2015–2016 (from 27.2% to 42.8%).
Other/unspecified psychiatric diagnoses, such as adjustment disorder and personality disorders, almost tripled between 2007-2008 and 2015-2016 (from 1,040 to 2,961 per 100,000 ED visits). ED visits for mood disorders and anxiety disorders also increased over time.
Alcohol-related ED visits were the most common substance use visits, increasing from 1,669 in 2007-2008 to 3,007 per 100,000 visits in 2015-2016. Amphetamine- and opioid-related ED visits more than doubled, and other/unspecified–related ED visits more than tripled during that time.
“One explanation why ED visits for mental health conditions have increased is that substance-related problems, which include overdose/self-injury issues, have increased over time,” Dr. Rhee noted, which “makes sense,” inasmuch as opioid, cannabis, and amphetamine use has increased across the country.
Another explanation is that, although mental health care access has been expanded through the ACA, “people, especially those with lower socioeconomic backgrounds, do not know how to get access to care and are still underserved,” he said.
“If mental health–related ED visits continue to increase in the future, there are several steps to be made. ED providers need to be better equipped with mental health care, and behavioral health should be better integrated as part of the care coordination,” said Dr. Rhee.
He added that reimbursement models across different insurance types, such as Medicare, Medicaid, and private insurance, “should consider expanding their coverage of mental health treatment in ED settings.”
“Canary in the coal mine”
Commenting on the study in an interview, Benjamin Druss, MD, MPH, professor and Rosalynn Carter Chair in Mental Health, Rollins School of Public Health, Emory University, Atlanta, called EDs the “canaries in the coal mine” for the broader health system.
The growing number of ED visits for behavioral problems “could represent both a rise in acute conditions such as substance use and lack of access to outpatient treatment,” said Dr. Druss, who was not involved with the research.
The findings “suggest the importance of strategies to effectively manage patients with behavioral conditions in ED settings and to effectively link them with high-quality outpatient care,” he noted.
Dr. Rhee has received funding from the National Institute on Aging and the American Foundation for Suicide Prevention. The other study authors and Dr. Druss report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Many children with COVID-19 present without classic symptoms
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
FROM HOSPITAL PEDIATRICS
Twelve risk factors linked to 40% of world’s dementia cases
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
according to an update of the Lancet Commission on Dementia Prevention, Intervention, and Care.
The original report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one-third of dementia cases. The commission has now added three new modifiable risk factors to the list.
“We reconvened the 2017 Lancet Commission on Dementia Prevention, Intervention, and Care to identify the evidence for advances likely to have the greatest impact since our 2017 paper,” the authors wrote.
The 2020 report was presented at the virtual annual meeting of the Alzheimer’s Association International Conference (AAIC) 2020 and also was published online July 30 in the Lancet.
Alcohol, TBI, air pollution
The three new risk factors that have been added in the latest update are excessive alcohol intake, traumatic brain injury (TBI), and air pollution. The original nine risk factors were not completing secondary education; hypertension; obesity; hearing loss; smoking; depression; physical inactivity; social isolation; and diabetes. Together, these 12 risk factors are estimated to account for 40% of the world’s dementia cases.
“We knew in 2017 when we published our first report with the nine risk factors that they would only be part of the story and that several other factors would likely be involved,” said lead author Gill Livingston, MD, professor, University College London (England). “We now have more published data giving enough evidence” to justify adding the three new factors to the list, she said.
The report includes the following nine recommendations for policymakers and individuals to prevent risk for dementia in the general population:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss, and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent , particularly by targeting high-risk occupations and transport.
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking and support individuals to stop smoking, which the authors stress is beneficial at any age.
- Provide all children with primary and secondary education.
- Lead an active life into midlife and possibly later life.
- Reduce obesity and diabetes.
The report also summarizes the evidence supporting the three new risk factors for dementia.
TBI is usually caused by car, motorcycle, and bicycle injuries; military exposures; boxing, horse riding, and other recreational sports; firearms; and falls. The report notes that a single severe TBI is associated in humans and in mouse models with widespread hyperphosphorylated tau pathology. It also cites several nationwide studies that show that TBI is linked with a significantly increased risk for long-term dementia.
“We are not advising against partaking in sports, as playing sports is healthy. But we are urging people to take precautions to protect themselves properly,” Dr. Livingston said.
For excessive alcohol consumption, the report states that an “increasing body of evidence is emerging on alcohol’s complex relationship with cognition and dementia outcomes from a variety of sources including detailed cohorts and large-scale record-based studies.” One French study, which included more than 31 million individuals admitted to the hospital, showed that alcohol use disorders were associated with a threefold increased dementia risk. However, other studies have suggested that moderate drinking may be protective.
“We are not saying it is bad to drink, but we are saying it is bad to drink more than 21 units a week,” Dr. Livingston noted.
On air pollution, the report notes that in animal studies, airborne particulate pollutants have been found to accelerate neurodegenerative processes. Also, high nitrogen dioxide concentrations, fine ambient particulate matter from traffic exhaust, and residential wood burning have been shown in past research to be associated with increased dementia incidence.
“While we need international policy on reducing air pollution, individuals can take some action to reduce their risk,” Dr. Livingston said. For example, she suggested avoiding walking right next to busy roads and instead walking “a few streets back if possible.”
Hearing loss
The researchers assessed how much each risk factor contributes to dementia, expressed as the population-attributable fraction (PAF). Hearing loss had the greatest effect, accounting for an estimated 8.2% of dementia cases. This was followed by lower education levels in young people (7.1%) and smoking (5.2%).
Dr. Livingston noted that the evidence that hearing loss is one of the most important risk factors for dementia is very strong. New studies show that correcting hearing loss with hearing aids negates any increased risk.
Hearing loss “has both a high relative risk for dementia and is a common problem, so it contributes a significant amount to dementia cases. This is really something that we can reduce relatively easily by encouraging use of hearing aids. They need to be made more accessible, more comfortable, and more acceptable,” she said.
“This could make a huge difference in reducing dementia cases in the future,” Dr. Livingston added.
Other risk factors for which the evidence base has strengthened since the 2017 report include systolic blood pressure, social interaction, and early-life education.
Dr. Livingston noted that the SPRINT MIND trial showed that aiming for a target systolic blood pressure of 120 mm Hg reduced risk for future mild cognitive impairment. “Before, we thought under 140 was the target, but now are recommending under 130 to reduce risks of dementia,” she said.
Evidence on social interaction “has been very consistent, and we now have more certainty on this. It is now well established that increased social interaction in midlife reduces dementia in late life,” said Dr. Livingston.
On the benefits of education in the young, she noted that it has been known for some time that education for individuals younger than 11 years is important in reducing later-life dementia. However, it is now thought that education to the age of 20 also makes a difference.
“While keeping the brain active in later years has some positive effects, increasing brain activity in young people seems to be more important. This is probably because of the better plasticity of the brain in the young,” she said.
Sleep and diet
Two risk factors that have not made it onto the list are diet and sleep. “While there has also been a lot more data published on nutrition and sleep with regard to dementia in the last few years, we didn’t think the evidence stacked up enough to include these on the list of modifiable risk factors,” Dr. Livingston said.
The report cites studies that suggest that both more sleep and less sleep are associated with increased risk for dementia, which the authors thought did not make “biological sense.” In addition, other underlying factors involved in sleep, such as depression, apathy, and different sleep patterns, may be symptoms of early dementia.
More data have been published on diet and dementia, “but there isn’t any individual vitamin deficit that is associated with the condition. The evidence is quite clear on that,” Dr. Livingston said. “Global diets, such as the Mediterranean or Nordic diets, can probably make a difference, but there doesn’t seem to be any one particular element that is needed,” she noted.
“We just recommend to eat a healthy diet and stay a healthy weight. Diet is very connected to economic circumstances and so very difficult to separate out as a risk factor. We do think it is linked, but we are not convinced enough to put it in the model,” she added.
Among other key information that has become available since 2017, Dr. Livingston highlighted new data showing that dementia is more common in less privileged populations, including Black and minority ethnic groups and low- and middle-income countries.
Although dementia was traditionally considered a disease of high-income countries, that has now been shown not to be the case. “People in low- and middle-income countries are now living longer and so are developing dementia more, and they have higher rates of many of the risk factors, including smoking and low education levels. There is a huge potential for prevention in these countries,” said Dr. Livingston.
She also highlighted new evidence showing that patients with dementia do not do well when admitted to the hospital. “So we need to do more to keep them well at home,” she said.
COVID-19 advice
The report also has a section on COVID-19. It points out that patients with dementia are particularly vulnerable to the disease because of their age, multimorbidities, and difficulties in maintaining physical distancing. Death certificates from the United Kingdom indicate that dementia and Alzheimer’s disease were the most common underlying conditions (present in 25.6% of all deaths involving COVID-19).
The situation is particularly concerning in care homes. In one U.S. study, nursing home residents living with dementia made up 52% of COVID-19 cases, yet they accounted for 72% of all deaths (increased risk, 1.7), the commission reported.
The authors recommended rigorous public health measures, such as protective equipment and hygiene, not moving staff or residents between care homes, and not admitting new residents when their COVID-19 status is unknown. The report also recommends regular testing of staff in care homes and the provision of oxygen therapy at the home to avoid hospital admission.
It is also important to reduce isolation by providing the necessary equipment to relatives and offering them brief training on how to protect themselves and others from COVID-19 so that they can visit their relatives with dementia in nursing homes safely when it is allowed.
“Most comprehensive overview to date”
Alzheimer’s Research UK welcomed the new report. “This is the most comprehensive overview into dementia risk to date, building on previous work by this commission and moving our understanding forward,” Rosa Sancho, PhD, head of research at the charity, said.
“This report underlines the importance of acting at a personal and policy level to reduce dementia risk. With Alzheimer’s Research UK’s Dementia Attitudes Monitor showing just a third of people think it’s possible to reduce their risk of developing dementia, there’s clearly much to do here to increase people’s awareness of the steps they can take,” Dr. Sancho said.
She added that, although there is “no surefire way of preventing dementia,” the best way to keep a brain healthy as it ages is for an individual to stay physically and mentally active, eat a healthy balanced diet, not smoke, drink only within the recommended limits, and keep weight, cholesterol level, and blood pressure in check. “With no treatments yet able to slow or stop the onset of dementia, taking action to reduce these risks is an important part of our strategy for tackling the condition,” Dr. Sancho said.
The Lancet Commission is partnered by University College London, the Alzheimer’s Society UK, the Economic and Social Research Council, and Alzheimer’s Research UK, which funded fares, accommodation, and food for the commission meeting but had no role in the writing of the manuscript or the decision to submit it for publication.
A version of this article originally appeared on Medscape.com.
From AAIC 2020
Diagnostic testing for COVID-19: A quick summary for PCPs
Information about COVID has evolved so quickly that it can be difficult for clinicians to feel confident that they are staying current. These summaries include links to our reference article on diagnosis of COVID-19, which is constantly updated to make sure you have the latest information.
Diagnostic testing for COVID-19 is critical. No one disputes that. But what is in dispute is whom to test, when to test, how to test, what to do while waiting for results, and how accurate those results are when you finally get them.
Here are the answers to those questions, based on the current information.
Whom to test. This is the (relatively) easy part. The ideal answer is that everyone should be tested. The Infectious Diseases Society of America issued tier-based recommendations way back in March, and they still apply. First priority continues to be patients who are ill, healthcare workers, and those with known exposure. But to truly figure out the amount of community spread in a given area, we need to test people who do not have a clear indication for testing. That is particularly true as more people return to work and the Centers for Disease Control and Prevention (CDC) has issued guidelines for workplaces to establish testing programs. Universal testing is recommended for some high-risk settings, such as nursing homes.
One key change: CDC no longer recommends testing to determine whether someone with a known infection is still infectious.
When to test. People with any symptoms suggestive of COVID should be tested, ideally as soon as feasible. But given the ongoing shortages of tests, that may not be possible, particularly for those requiring only symptomatic care. Rather, these patients should be treated as probable cases, with appropriate instructions regarding quarantine. Testing of those with known exposures ideally should be done about 5 days after exposure.
How to test. Only viral nucleic acid or antigen tests should be used to diagnose acute illness. CDC does not currently recommend using serologic assays, now broadly available, for diagnosis of acute infection, though they obviously play an important role in understanding the transmission dynamic of the virus in the general population.
Testing strategies vary from state to state and even within communities in a single state. It is recommended that clinicians check with their own local or state health department for specifics on tests available, indications for testing, and processing details. While often forgotten, it is worth emphasizing that no diagnostic tests have been approved by the US Food and Drug Administration (FDA). Rather, they are available under emergency use authorization (EUA), meaning that they have not been fully vetted by the FDA.
In late July, the FDA expanded authorization for real-time reverse transcription–polymerase chain reaction (rRT-PCR) molecular assays, utilizing nasal or nasopharyngeal swabs, to permit testing of all persons, regardless of exposure history or symptoms. The FDA maintains a list of all approved diagnostic tests and corresponding labs. Patients will have to get what is available via their health department or insurance plan.
Two point-of-care antigen tests using nasopharyngeal or nasal samples have been issued an EUA. These tests can be used only in settings with a valid CLIA certificate.
Several commercial laboratories have received approval to process diagnostic tests using patients’ self-collected saliva rather than swabs. One lab has now received authorization for in-home testing without any input from a clinician. These testing options can be a boon for patients who have symptoms or exposure and for whatever reason are unable to get to a diagnostic site. These samples are collected at home and mailed to a lab. Note that these tests are not yet widely available.
Waiting for results. If waiting for results meant a day or even a couple of days, the answer to this one would be easier. But if the wait extends to 1 and even sometimes 2 weeks, then the test is not able to meaningfully guide clinical decisions. The latest guidance from the CDC is that individuals with symptoms suggestive of COVID who do not require hospitalization should remain at home in self-quarantine for at least 10 days from symptom onset. Asymptomatic individuals with a known exposure to someone else with COVID, or participation in a high-risk event like an indoor gathering involving more than 10 persons, should self-quarantine either until they receive a negative test result or 14 days after the exposure.
Accuracy of results. A positive rRT-PCR antigen test is highly accurate, indicating presence of SARS-CoV-2 RNA. There appears to be no significant cross-reactivity with other respiratory viruses or even other coronaviruses. A small study conducted in Korea suggests that patients with persistent positive tests who are beyond 10 days from the initial positive test and are now symptom free are no longer infectious.
For patients with a high suspicion of COVID-19, a negative test should not rule out the infection. The number of false-negative results is not well known, though the resultant risk is “substantial.” A number of factors affect the likelihood of a false-negative test, including when the sample was collected relative to the timing of illness and the type of specimen collected; for example, nasopharyngeal swabs are more likely to be accurate vs nasal or throat specimens. Repeat or serial testing increases the sensitivity but may not always be available. Although rRT-PCR is the current criterion standard, more inclusive consensus-based criteria are likely to emerge because of the concern about these false-negative results.
This article first appeared on Medscape.com.
Information about COVID has evolved so quickly that it can be difficult for clinicians to feel confident that they are staying current. These summaries include links to our reference article on diagnosis of COVID-19, which is constantly updated to make sure you have the latest information.
Diagnostic testing for COVID-19 is critical. No one disputes that. But what is in dispute is whom to test, when to test, how to test, what to do while waiting for results, and how accurate those results are when you finally get them.
Here are the answers to those questions, based on the current information.
Whom to test. This is the (relatively) easy part. The ideal answer is that everyone should be tested. The Infectious Diseases Society of America issued tier-based recommendations way back in March, and they still apply. First priority continues to be patients who are ill, healthcare workers, and those with known exposure. But to truly figure out the amount of community spread in a given area, we need to test people who do not have a clear indication for testing. That is particularly true as more people return to work and the Centers for Disease Control and Prevention (CDC) has issued guidelines for workplaces to establish testing programs. Universal testing is recommended for some high-risk settings, such as nursing homes.
One key change: CDC no longer recommends testing to determine whether someone with a known infection is still infectious.
When to test. People with any symptoms suggestive of COVID should be tested, ideally as soon as feasible. But given the ongoing shortages of tests, that may not be possible, particularly for those requiring only symptomatic care. Rather, these patients should be treated as probable cases, with appropriate instructions regarding quarantine. Testing of those with known exposures ideally should be done about 5 days after exposure.
How to test. Only viral nucleic acid or antigen tests should be used to diagnose acute illness. CDC does not currently recommend using serologic assays, now broadly available, for diagnosis of acute infection, though they obviously play an important role in understanding the transmission dynamic of the virus in the general population.
Testing strategies vary from state to state and even within communities in a single state. It is recommended that clinicians check with their own local or state health department for specifics on tests available, indications for testing, and processing details. While often forgotten, it is worth emphasizing that no diagnostic tests have been approved by the US Food and Drug Administration (FDA). Rather, they are available under emergency use authorization (EUA), meaning that they have not been fully vetted by the FDA.
In late July, the FDA expanded authorization for real-time reverse transcription–polymerase chain reaction (rRT-PCR) molecular assays, utilizing nasal or nasopharyngeal swabs, to permit testing of all persons, regardless of exposure history or symptoms. The FDA maintains a list of all approved diagnostic tests and corresponding labs. Patients will have to get what is available via their health department or insurance plan.
Two point-of-care antigen tests using nasopharyngeal or nasal samples have been issued an EUA. These tests can be used only in settings with a valid CLIA certificate.
Several commercial laboratories have received approval to process diagnostic tests using patients’ self-collected saliva rather than swabs. One lab has now received authorization for in-home testing without any input from a clinician. These testing options can be a boon for patients who have symptoms or exposure and for whatever reason are unable to get to a diagnostic site. These samples are collected at home and mailed to a lab. Note that these tests are not yet widely available.
Waiting for results. If waiting for results meant a day or even a couple of days, the answer to this one would be easier. But if the wait extends to 1 and even sometimes 2 weeks, then the test is not able to meaningfully guide clinical decisions. The latest guidance from the CDC is that individuals with symptoms suggestive of COVID who do not require hospitalization should remain at home in self-quarantine for at least 10 days from symptom onset. Asymptomatic individuals with a known exposure to someone else with COVID, or participation in a high-risk event like an indoor gathering involving more than 10 persons, should self-quarantine either until they receive a negative test result or 14 days after the exposure.
Accuracy of results. A positive rRT-PCR antigen test is highly accurate, indicating presence of SARS-CoV-2 RNA. There appears to be no significant cross-reactivity with other respiratory viruses or even other coronaviruses. A small study conducted in Korea suggests that patients with persistent positive tests who are beyond 10 days from the initial positive test and are now symptom free are no longer infectious.
For patients with a high suspicion of COVID-19, a negative test should not rule out the infection. The number of false-negative results is not well known, though the resultant risk is “substantial.” A number of factors affect the likelihood of a false-negative test, including when the sample was collected relative to the timing of illness and the type of specimen collected; for example, nasopharyngeal swabs are more likely to be accurate vs nasal or throat specimens. Repeat or serial testing increases the sensitivity but may not always be available. Although rRT-PCR is the current criterion standard, more inclusive consensus-based criteria are likely to emerge because of the concern about these false-negative results.
This article first appeared on Medscape.com.
Information about COVID has evolved so quickly that it can be difficult for clinicians to feel confident that they are staying current. These summaries include links to our reference article on diagnosis of COVID-19, which is constantly updated to make sure you have the latest information.
Diagnostic testing for COVID-19 is critical. No one disputes that. But what is in dispute is whom to test, when to test, how to test, what to do while waiting for results, and how accurate those results are when you finally get them.
Here are the answers to those questions, based on the current information.
Whom to test. This is the (relatively) easy part. The ideal answer is that everyone should be tested. The Infectious Diseases Society of America issued tier-based recommendations way back in March, and they still apply. First priority continues to be patients who are ill, healthcare workers, and those with known exposure. But to truly figure out the amount of community spread in a given area, we need to test people who do not have a clear indication for testing. That is particularly true as more people return to work and the Centers for Disease Control and Prevention (CDC) has issued guidelines for workplaces to establish testing programs. Universal testing is recommended for some high-risk settings, such as nursing homes.
One key change: CDC no longer recommends testing to determine whether someone with a known infection is still infectious.
When to test. People with any symptoms suggestive of COVID should be tested, ideally as soon as feasible. But given the ongoing shortages of tests, that may not be possible, particularly for those requiring only symptomatic care. Rather, these patients should be treated as probable cases, with appropriate instructions regarding quarantine. Testing of those with known exposures ideally should be done about 5 days after exposure.
How to test. Only viral nucleic acid or antigen tests should be used to diagnose acute illness. CDC does not currently recommend using serologic assays, now broadly available, for diagnosis of acute infection, though they obviously play an important role in understanding the transmission dynamic of the virus in the general population.
Testing strategies vary from state to state and even within communities in a single state. It is recommended that clinicians check with their own local or state health department for specifics on tests available, indications for testing, and processing details. While often forgotten, it is worth emphasizing that no diagnostic tests have been approved by the US Food and Drug Administration (FDA). Rather, they are available under emergency use authorization (EUA), meaning that they have not been fully vetted by the FDA.
In late July, the FDA expanded authorization for real-time reverse transcription–polymerase chain reaction (rRT-PCR) molecular assays, utilizing nasal or nasopharyngeal swabs, to permit testing of all persons, regardless of exposure history or symptoms. The FDA maintains a list of all approved diagnostic tests and corresponding labs. Patients will have to get what is available via their health department or insurance plan.
Two point-of-care antigen tests using nasopharyngeal or nasal samples have been issued an EUA. These tests can be used only in settings with a valid CLIA certificate.
Several commercial laboratories have received approval to process diagnostic tests using patients’ self-collected saliva rather than swabs. One lab has now received authorization for in-home testing without any input from a clinician. These testing options can be a boon for patients who have symptoms or exposure and for whatever reason are unable to get to a diagnostic site. These samples are collected at home and mailed to a lab. Note that these tests are not yet widely available.
Waiting for results. If waiting for results meant a day or even a couple of days, the answer to this one would be easier. But if the wait extends to 1 and even sometimes 2 weeks, then the test is not able to meaningfully guide clinical decisions. The latest guidance from the CDC is that individuals with symptoms suggestive of COVID who do not require hospitalization should remain at home in self-quarantine for at least 10 days from symptom onset. Asymptomatic individuals with a known exposure to someone else with COVID, or participation in a high-risk event like an indoor gathering involving more than 10 persons, should self-quarantine either until they receive a negative test result or 14 days after the exposure.
Accuracy of results. A positive rRT-PCR antigen test is highly accurate, indicating presence of SARS-CoV-2 RNA. There appears to be no significant cross-reactivity with other respiratory viruses or even other coronaviruses. A small study conducted in Korea suggests that patients with persistent positive tests who are beyond 10 days from the initial positive test and are now symptom free are no longer infectious.
For patients with a high suspicion of COVID-19, a negative test should not rule out the infection. The number of false-negative results is not well known, though the resultant risk is “substantial.” A number of factors affect the likelihood of a false-negative test, including when the sample was collected relative to the timing of illness and the type of specimen collected; for example, nasopharyngeal swabs are more likely to be accurate vs nasal or throat specimens. Repeat or serial testing increases the sensitivity but may not always be available. Although rRT-PCR is the current criterion standard, more inclusive consensus-based criteria are likely to emerge because of the concern about these false-negative results.
This article first appeared on Medscape.com.