User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
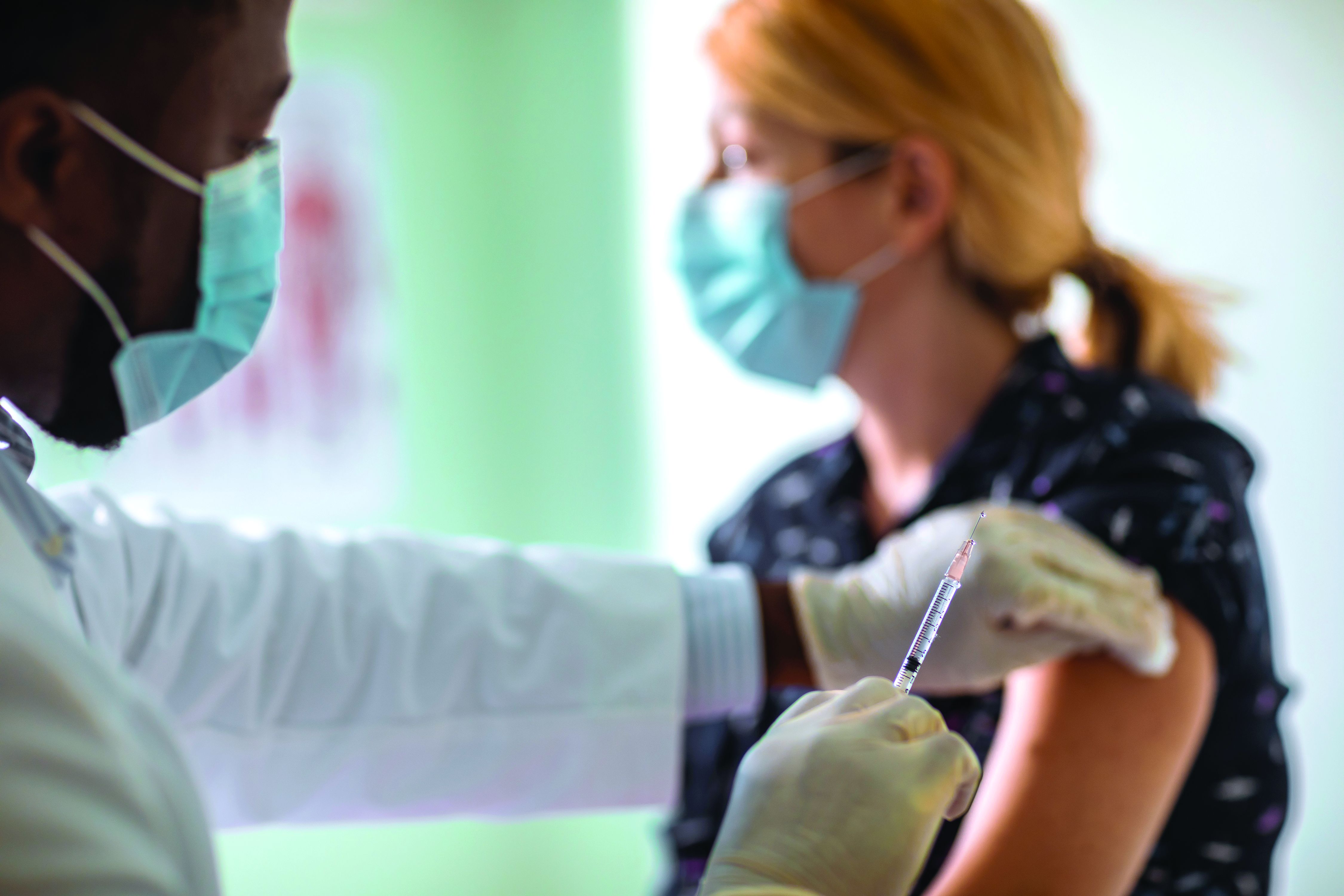
Let me tell you about my vaccine
Welcome to our national obsession: Vaccines! You may have noticed – it’s all talk, all the time, with short breaks to discuss what we’re watching on Netflix.
For months, every session with almost every patient includes a commentary on someone they know who has gotten “the shot.” Before our state expanded eligibility to all adults, the discussion might include thoughts about who deserves to go first, who “cut the line,” how they did it, what vaccine is best, and worries about side effects.
And it’s not just my patients: With every friend, with every acquaintance, and even just walking by strangers who are conversing, the topic of discussion is vaccines. The narratives are similar; people want to talk about who has gotten vaccinated, why they qualified, where they went, which one they got, and what side effects they experienced. This is followed by a discussion about what they are now doing that they weren’t doing before being vaccinated, if anything. Some have returned to indoor restaurant dining, others only dine outdoors, still others continue to avoid public settings. There are the fully vaccinated, the partially vaccinated, and those scheduled for the first shot. In the unvaccinated/unregistered group there are the vaccine-hesitants and vaccine-refusers, with their concerns about everything from the safety of the agent to whether the government is using this as a way to insert tracker chips into all of us. There is enthusiasm, trepidation, anxiety, fear, excitement, relief, and absolute joy.
Recently I opened two emails from old friends I have not communicated with in a long time. Both emails began with, “I am fully vaccinated.” And yes, I heard the status of his wife and two children. In the course of one work day, I received distressed text messages from two patients about vaccines – one was anxious about having received the Janssen vaccine that was paused that morning, another was worried about getting a second dose of the Pfizer vaccine later in the week because he was having a symptom that could be indicative of COVID-19. I suggested that his primary care physician might be a better resource for this, but then added that he should probably get tested and delay having the second dose if positive. It seems I did have thoughts about a course of action after all.
Some psychiatrists have wondered how to handle patient questions about their own vaccination status. I have taken the stance that we are physicians, and that patients who may be seeing us – now or in the future – for in-person appointments are entitled to know if we pose a risk to their health, and so I have chosen to answer, without further exploration, when patients ask if I’ve received that coronavirus vaccine. Some psychiatrists feel it is our responsibility to share this information with our patients as a way of modeling safe behavior, and I have had one patient who said she would not be getting vaccinated until I told her that I thought she should.
“Did you get it?” she asked.
“I did,” I responded.
“Okay, if you got it, I will.” She soon discovered that vaccinations were hard to come by and that in her social group, being vaccinated was something of a status symbol. In addition to the worry about contracting a potentially fatal virus, her hesitancy yielded to “vaccine FOMO” or fear of missing out.
Some psychiatrists have felt uneasy with a question that pertains to their personal health, or have used the question as a springboard for exploration. Nicole Leistikow, MD (fully vaccinated, Moderna), is a psychiatrist in private practice in Baltimore. She notes, “Recently, I was discussing vaccination with a patient who wasn’t sure what information to believe or how much to trust the U.S. government. My careful exploration comparing different risks was not very helpful. I mentioned that I was vaccinated and that if he got vaccinated, he could come for a low-risk, in-person appointment after a year of telephone visits. This proved to be a winning argument and he called back later that day to say he had already had his first shot from leftover vaccine at his pharmacy.”
I grew up in a world that did not question vaccines. You got them and they were good things. No one asked which pharmaceutical company manufactured the vaccine. We trusted the system and our physicians. Schools asked for proof of vaccination, and it never occurred to me not to be vaccinated. Life has grown more complicated in the last 30 years, and the groups of people who are opposed to being vaccinated are more diverse. Those opposed to getting a COVID-19 vaccine are not necessarily the same as the broader group of anti-vaxxers that spawned from the fear that childhood vaccines cause autism. For some, it’s a personal issue related to their own health and risk perception, for others it’s a polarized political issue, and for another group there is the question of where their trust lies.
What lies ahead in our postvaccine world? This will be our next national conversation, and just as we negotiated our own levels of comfort with regard to working and socializing during the pandemic, I imagine the postvaccine world will have the same adjustment. There already are cases of COVID-19 in those who have been fully vaccinated, as well as the rare hospitalizations and deaths – we simply cannot expect a vaccine that did so well in controlled studies of tens of thousands of study subjects to do as well when given to tens of millions of uncontrolled citizens. One of the first deaths in a fully vaccinated person in late March was an older psychologist, and it remains unclear how effective the vaccine is for immunocompromised patients. Some people will play it very safe, eschewing all activities that entail risk, while others will choose to adhere to either their own intuition about what is safe, or to the recommendations of Anthony S. Fauci, MD, and the Centers for Disease Control and Prevention.
I’ll end with a final thought from the Twitter feed of Ashish K. Jha, MD, dean of the School of Public Health at Brown University, Providence, R.I. Dr. Jha tweeted, “Once you get fully vaccinated, it absolutely changes what you can do safely.” It seems our national conversation is not slated to change anytime soon.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Welcome to our national obsession: Vaccines! You may have noticed – it’s all talk, all the time, with short breaks to discuss what we’re watching on Netflix.
For months, every session with almost every patient includes a commentary on someone they know who has gotten “the shot.” Before our state expanded eligibility to all adults, the discussion might include thoughts about who deserves to go first, who “cut the line,” how they did it, what vaccine is best, and worries about side effects.
And it’s not just my patients: With every friend, with every acquaintance, and even just walking by strangers who are conversing, the topic of discussion is vaccines. The narratives are similar; people want to talk about who has gotten vaccinated, why they qualified, where they went, which one they got, and what side effects they experienced. This is followed by a discussion about what they are now doing that they weren’t doing before being vaccinated, if anything. Some have returned to indoor restaurant dining, others only dine outdoors, still others continue to avoid public settings. There are the fully vaccinated, the partially vaccinated, and those scheduled for the first shot. In the unvaccinated/unregistered group there are the vaccine-hesitants and vaccine-refusers, with their concerns about everything from the safety of the agent to whether the government is using this as a way to insert tracker chips into all of us. There is enthusiasm, trepidation, anxiety, fear, excitement, relief, and absolute joy.
Recently I opened two emails from old friends I have not communicated with in a long time. Both emails began with, “I am fully vaccinated.” And yes, I heard the status of his wife and two children. In the course of one work day, I received distressed text messages from two patients about vaccines – one was anxious about having received the Janssen vaccine that was paused that morning, another was worried about getting a second dose of the Pfizer vaccine later in the week because he was having a symptom that could be indicative of COVID-19. I suggested that his primary care physician might be a better resource for this, but then added that he should probably get tested and delay having the second dose if positive. It seems I did have thoughts about a course of action after all.
Some psychiatrists have wondered how to handle patient questions about their own vaccination status. I have taken the stance that we are physicians, and that patients who may be seeing us – now or in the future – for in-person appointments are entitled to know if we pose a risk to their health, and so I have chosen to answer, without further exploration, when patients ask if I’ve received that coronavirus vaccine. Some psychiatrists feel it is our responsibility to share this information with our patients as a way of modeling safe behavior, and I have had one patient who said she would not be getting vaccinated until I told her that I thought she should.
“Did you get it?” she asked.
“I did,” I responded.
“Okay, if you got it, I will.” She soon discovered that vaccinations were hard to come by and that in her social group, being vaccinated was something of a status symbol. In addition to the worry about contracting a potentially fatal virus, her hesitancy yielded to “vaccine FOMO” or fear of missing out.
Some psychiatrists have felt uneasy with a question that pertains to their personal health, or have used the question as a springboard for exploration. Nicole Leistikow, MD (fully vaccinated, Moderna), is a psychiatrist in private practice in Baltimore. She notes, “Recently, I was discussing vaccination with a patient who wasn’t sure what information to believe or how much to trust the U.S. government. My careful exploration comparing different risks was not very helpful. I mentioned that I was vaccinated and that if he got vaccinated, he could come for a low-risk, in-person appointment after a year of telephone visits. This proved to be a winning argument and he called back later that day to say he had already had his first shot from leftover vaccine at his pharmacy.”
I grew up in a world that did not question vaccines. You got them and they were good things. No one asked which pharmaceutical company manufactured the vaccine. We trusted the system and our physicians. Schools asked for proof of vaccination, and it never occurred to me not to be vaccinated. Life has grown more complicated in the last 30 years, and the groups of people who are opposed to being vaccinated are more diverse. Those opposed to getting a COVID-19 vaccine are not necessarily the same as the broader group of anti-vaxxers that spawned from the fear that childhood vaccines cause autism. For some, it’s a personal issue related to their own health and risk perception, for others it’s a polarized political issue, and for another group there is the question of where their trust lies.
What lies ahead in our postvaccine world? This will be our next national conversation, and just as we negotiated our own levels of comfort with regard to working and socializing during the pandemic, I imagine the postvaccine world will have the same adjustment. There already are cases of COVID-19 in those who have been fully vaccinated, as well as the rare hospitalizations and deaths – we simply cannot expect a vaccine that did so well in controlled studies of tens of thousands of study subjects to do as well when given to tens of millions of uncontrolled citizens. One of the first deaths in a fully vaccinated person in late March was an older psychologist, and it remains unclear how effective the vaccine is for immunocompromised patients. Some people will play it very safe, eschewing all activities that entail risk, while others will choose to adhere to either their own intuition about what is safe, or to the recommendations of Anthony S. Fauci, MD, and the Centers for Disease Control and Prevention.
I’ll end with a final thought from the Twitter feed of Ashish K. Jha, MD, dean of the School of Public Health at Brown University, Providence, R.I. Dr. Jha tweeted, “Once you get fully vaccinated, it absolutely changes what you can do safely.” It seems our national conversation is not slated to change anytime soon.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Welcome to our national obsession: Vaccines! You may have noticed – it’s all talk, all the time, with short breaks to discuss what we’re watching on Netflix.
For months, every session with almost every patient includes a commentary on someone they know who has gotten “the shot.” Before our state expanded eligibility to all adults, the discussion might include thoughts about who deserves to go first, who “cut the line,” how they did it, what vaccine is best, and worries about side effects.
And it’s not just my patients: With every friend, with every acquaintance, and even just walking by strangers who are conversing, the topic of discussion is vaccines. The narratives are similar; people want to talk about who has gotten vaccinated, why they qualified, where they went, which one they got, and what side effects they experienced. This is followed by a discussion about what they are now doing that they weren’t doing before being vaccinated, if anything. Some have returned to indoor restaurant dining, others only dine outdoors, still others continue to avoid public settings. There are the fully vaccinated, the partially vaccinated, and those scheduled for the first shot. In the unvaccinated/unregistered group there are the vaccine-hesitants and vaccine-refusers, with their concerns about everything from the safety of the agent to whether the government is using this as a way to insert tracker chips into all of us. There is enthusiasm, trepidation, anxiety, fear, excitement, relief, and absolute joy.
Recently I opened two emails from old friends I have not communicated with in a long time. Both emails began with, “I am fully vaccinated.” And yes, I heard the status of his wife and two children. In the course of one work day, I received distressed text messages from two patients about vaccines – one was anxious about having received the Janssen vaccine that was paused that morning, another was worried about getting a second dose of the Pfizer vaccine later in the week because he was having a symptom that could be indicative of COVID-19. I suggested that his primary care physician might be a better resource for this, but then added that he should probably get tested and delay having the second dose if positive. It seems I did have thoughts about a course of action after all.
Some psychiatrists have wondered how to handle patient questions about their own vaccination status. I have taken the stance that we are physicians, and that patients who may be seeing us – now or in the future – for in-person appointments are entitled to know if we pose a risk to their health, and so I have chosen to answer, without further exploration, when patients ask if I’ve received that coronavirus vaccine. Some psychiatrists feel it is our responsibility to share this information with our patients as a way of modeling safe behavior, and I have had one patient who said she would not be getting vaccinated until I told her that I thought she should.
“Did you get it?” she asked.
“I did,” I responded.
“Okay, if you got it, I will.” She soon discovered that vaccinations were hard to come by and that in her social group, being vaccinated was something of a status symbol. In addition to the worry about contracting a potentially fatal virus, her hesitancy yielded to “vaccine FOMO” or fear of missing out.
Some psychiatrists have felt uneasy with a question that pertains to their personal health, or have used the question as a springboard for exploration. Nicole Leistikow, MD (fully vaccinated, Moderna), is a psychiatrist in private practice in Baltimore. She notes, “Recently, I was discussing vaccination with a patient who wasn’t sure what information to believe or how much to trust the U.S. government. My careful exploration comparing different risks was not very helpful. I mentioned that I was vaccinated and that if he got vaccinated, he could come for a low-risk, in-person appointment after a year of telephone visits. This proved to be a winning argument and he called back later that day to say he had already had his first shot from leftover vaccine at his pharmacy.”
I grew up in a world that did not question vaccines. You got them and they were good things. No one asked which pharmaceutical company manufactured the vaccine. We trusted the system and our physicians. Schools asked for proof of vaccination, and it never occurred to me not to be vaccinated. Life has grown more complicated in the last 30 years, and the groups of people who are opposed to being vaccinated are more diverse. Those opposed to getting a COVID-19 vaccine are not necessarily the same as the broader group of anti-vaxxers that spawned from the fear that childhood vaccines cause autism. For some, it’s a personal issue related to their own health and risk perception, for others it’s a polarized political issue, and for another group there is the question of where their trust lies.
What lies ahead in our postvaccine world? This will be our next national conversation, and just as we negotiated our own levels of comfort with regard to working and socializing during the pandemic, I imagine the postvaccine world will have the same adjustment. There already are cases of COVID-19 in those who have been fully vaccinated, as well as the rare hospitalizations and deaths – we simply cannot expect a vaccine that did so well in controlled studies of tens of thousands of study subjects to do as well when given to tens of millions of uncontrolled citizens. One of the first deaths in a fully vaccinated person in late March was an older psychologist, and it remains unclear how effective the vaccine is for immunocompromised patients. Some people will play it very safe, eschewing all activities that entail risk, while others will choose to adhere to either their own intuition about what is safe, or to the recommendations of Anthony S. Fauci, MD, and the Centers for Disease Control and Prevention.
I’ll end with a final thought from the Twitter feed of Ashish K. Jha, MD, dean of the School of Public Health at Brown University, Providence, R.I. Dr. Jha tweeted, “Once you get fully vaccinated, it absolutely changes what you can do safely.” It seems our national conversation is not slated to change anytime soon.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
COVID vaccine ‘side effect’ or functional neurologic disorder?
The development of unusual movements after COVID-19 vaccination may be a result of functional neurologic disorder rather than being a direct adverse effect of the vaccine, it has been suggested.
Writing in an article published online in JAMA Neurology on April 9, 2021, two neurologists and a psychiatrist report the recent circulation of videos on social media about major neurologic adverse events, including continuous movements of the trunk and limbs or walking difficulties after administration of the COVID-19 vaccine. Some of these videos have been viewed millions of times by the public, they noted.
While these videos may be unsubstantiated, and it is not definitively known if the COVID-19 vaccine was administered in these cases, it was reported in the news that at least one patient was told by their physician that the diagnosis was conversion disorder, also known as functional neurological disorder (FND), the authors noted.
In addition, the Functional Neurological Disorder Society released a statement in January 2021 pointing out that the conditions described in these videos are seemingly consistent with FND, they added.
“We thought it would be useful to explain more about what functional neurological disorder is, as many people are not familiar with it,” lead author David Kim, MD, said in an interview. “We wanted to provide some contextual information about the condition, as these reports may not necessarily mean the vaccine is unsafe.”
Dr. Kim, who is part of the division of cognitive behavioral neurology at Massachusetts General Hospital, Boston, explained that, in FND, physical symptoms can be brought about after events such as head injury, surgery, vaccination, other medical procedures, or life events such as loss of employment.
“Many different factors can bring these symptoms on, and while there are definitely cases associated with stressful events, it is not necessarily stress induced,” he said. “However, the event itself does not cause the condition, rather it is the reaction of the patient to the event.”
FND is now viewed as a true brain-based disorder, Dr. Kim noted. “While in the past it has been described as psychosomatic, we are now moving away from that terminology, toward the idea of a neurological disorder that affects function. It is a neuropsychiatric disorder on the borderline between neurology and psychiatry.”
The authors believed that some of these cases of unusual movements reported after COVID vaccination are likely to be FND.
“In these cases, it is not the substance in the vaccine that is causing the condition, but the common side effects that can occur after vaccination such as aches and chills bring the attention of the patient to their bodily functions and this reaction can become maladaptive, triggering FND,” Dr. Kim said.
“We believe that health care professionals should be more aware of FND at the current time. They need to know that the general public are aware that some people are experiencing movement disorders after COVID vaccination, and that this conversation is happening on social media,” he commented. “If they see patients with these symptoms, they could consider FND to be one possibility.”
The authors emphasized that, because they have not seen the individual patients, they cannot comment on any specific cases.
“But as some of these videos circulating can be consistent with the condition being FND, and especially with news reports indicating that at least one patient was given that diagnosis, we wanted to raise awareness of this condition among health professionals,” Dr. Kim added.
He explained that, in the past, FND has been a diagnosis of exclusion but now it is diagnosed with a clinical history and physical examination, looking for appropriate rule-in signs. Ancillary testing such as neuroimaging, electrophysiological studies, and blood tests are often used to rule out other conditions.
“Neurologists have a lot of training in this condition, as it is the second most common reason for a patient to visit a neurologist after headache,” Dr. Kim noted.
It is managed with education, counseling, physical rehabilitation and cognitive behavioral therapy. “A key part of the therapeutic process is working with the patient to explain the diagnosis. If they understand the condition, they do better. Patients can learn distraction techniques to allow more fluid movements,” he reported.
“As neurologists, and health care professionals more broadly, we must explain transparently and nonjudgmentally the nature of FND, including that these symptoms are real but not the direct result of toxic vaccine effects,” the authors wrote.
“Transparency and effective communication are needed in our society more than ever, and a condition as prevalent and potentially debilitating as FND can no longer remain marginalized and in the shadows. Effective communication will help educate the public and reduce fears so that patients can make informed decisions for themselves on receiving the vaccine to reduce the risk of COVID-19,” they concluded.
A version of this article first appeared on Medscape.com.
The development of unusual movements after COVID-19 vaccination may be a result of functional neurologic disorder rather than being a direct adverse effect of the vaccine, it has been suggested.
Writing in an article published online in JAMA Neurology on April 9, 2021, two neurologists and a psychiatrist report the recent circulation of videos on social media about major neurologic adverse events, including continuous movements of the trunk and limbs or walking difficulties after administration of the COVID-19 vaccine. Some of these videos have been viewed millions of times by the public, they noted.
While these videos may be unsubstantiated, and it is not definitively known if the COVID-19 vaccine was administered in these cases, it was reported in the news that at least one patient was told by their physician that the diagnosis was conversion disorder, also known as functional neurological disorder (FND), the authors noted.
In addition, the Functional Neurological Disorder Society released a statement in January 2021 pointing out that the conditions described in these videos are seemingly consistent with FND, they added.
“We thought it would be useful to explain more about what functional neurological disorder is, as many people are not familiar with it,” lead author David Kim, MD, said in an interview. “We wanted to provide some contextual information about the condition, as these reports may not necessarily mean the vaccine is unsafe.”
Dr. Kim, who is part of the division of cognitive behavioral neurology at Massachusetts General Hospital, Boston, explained that, in FND, physical symptoms can be brought about after events such as head injury, surgery, vaccination, other medical procedures, or life events such as loss of employment.
“Many different factors can bring these symptoms on, and while there are definitely cases associated with stressful events, it is not necessarily stress induced,” he said. “However, the event itself does not cause the condition, rather it is the reaction of the patient to the event.”
FND is now viewed as a true brain-based disorder, Dr. Kim noted. “While in the past it has been described as psychosomatic, we are now moving away from that terminology, toward the idea of a neurological disorder that affects function. It is a neuropsychiatric disorder on the borderline between neurology and psychiatry.”
The authors believed that some of these cases of unusual movements reported after COVID vaccination are likely to be FND.
“In these cases, it is not the substance in the vaccine that is causing the condition, but the common side effects that can occur after vaccination such as aches and chills bring the attention of the patient to their bodily functions and this reaction can become maladaptive, triggering FND,” Dr. Kim said.
“We believe that health care professionals should be more aware of FND at the current time. They need to know that the general public are aware that some people are experiencing movement disorders after COVID vaccination, and that this conversation is happening on social media,” he commented. “If they see patients with these symptoms, they could consider FND to be one possibility.”
The authors emphasized that, because they have not seen the individual patients, they cannot comment on any specific cases.
“But as some of these videos circulating can be consistent with the condition being FND, and especially with news reports indicating that at least one patient was given that diagnosis, we wanted to raise awareness of this condition among health professionals,” Dr. Kim added.
He explained that, in the past, FND has been a diagnosis of exclusion but now it is diagnosed with a clinical history and physical examination, looking for appropriate rule-in signs. Ancillary testing such as neuroimaging, electrophysiological studies, and blood tests are often used to rule out other conditions.
“Neurologists have a lot of training in this condition, as it is the second most common reason for a patient to visit a neurologist after headache,” Dr. Kim noted.
It is managed with education, counseling, physical rehabilitation and cognitive behavioral therapy. “A key part of the therapeutic process is working with the patient to explain the diagnosis. If they understand the condition, they do better. Patients can learn distraction techniques to allow more fluid movements,” he reported.
“As neurologists, and health care professionals more broadly, we must explain transparently and nonjudgmentally the nature of FND, including that these symptoms are real but not the direct result of toxic vaccine effects,” the authors wrote.
“Transparency and effective communication are needed in our society more than ever, and a condition as prevalent and potentially debilitating as FND can no longer remain marginalized and in the shadows. Effective communication will help educate the public and reduce fears so that patients can make informed decisions for themselves on receiving the vaccine to reduce the risk of COVID-19,” they concluded.
A version of this article first appeared on Medscape.com.
The development of unusual movements after COVID-19 vaccination may be a result of functional neurologic disorder rather than being a direct adverse effect of the vaccine, it has been suggested.
Writing in an article published online in JAMA Neurology on April 9, 2021, two neurologists and a psychiatrist report the recent circulation of videos on social media about major neurologic adverse events, including continuous movements of the trunk and limbs or walking difficulties after administration of the COVID-19 vaccine. Some of these videos have been viewed millions of times by the public, they noted.
While these videos may be unsubstantiated, and it is not definitively known if the COVID-19 vaccine was administered in these cases, it was reported in the news that at least one patient was told by their physician that the diagnosis was conversion disorder, also known as functional neurological disorder (FND), the authors noted.
In addition, the Functional Neurological Disorder Society released a statement in January 2021 pointing out that the conditions described in these videos are seemingly consistent with FND, they added.
“We thought it would be useful to explain more about what functional neurological disorder is, as many people are not familiar with it,” lead author David Kim, MD, said in an interview. “We wanted to provide some contextual information about the condition, as these reports may not necessarily mean the vaccine is unsafe.”
Dr. Kim, who is part of the division of cognitive behavioral neurology at Massachusetts General Hospital, Boston, explained that, in FND, physical symptoms can be brought about after events such as head injury, surgery, vaccination, other medical procedures, or life events such as loss of employment.
“Many different factors can bring these symptoms on, and while there are definitely cases associated with stressful events, it is not necessarily stress induced,” he said. “However, the event itself does not cause the condition, rather it is the reaction of the patient to the event.”
FND is now viewed as a true brain-based disorder, Dr. Kim noted. “While in the past it has been described as psychosomatic, we are now moving away from that terminology, toward the idea of a neurological disorder that affects function. It is a neuropsychiatric disorder on the borderline between neurology and psychiatry.”
The authors believed that some of these cases of unusual movements reported after COVID vaccination are likely to be FND.
“In these cases, it is not the substance in the vaccine that is causing the condition, but the common side effects that can occur after vaccination such as aches and chills bring the attention of the patient to their bodily functions and this reaction can become maladaptive, triggering FND,” Dr. Kim said.
“We believe that health care professionals should be more aware of FND at the current time. They need to know that the general public are aware that some people are experiencing movement disorders after COVID vaccination, and that this conversation is happening on social media,” he commented. “If they see patients with these symptoms, they could consider FND to be one possibility.”
The authors emphasized that, because they have not seen the individual patients, they cannot comment on any specific cases.
“But as some of these videos circulating can be consistent with the condition being FND, and especially with news reports indicating that at least one patient was given that diagnosis, we wanted to raise awareness of this condition among health professionals,” Dr. Kim added.
He explained that, in the past, FND has been a diagnosis of exclusion but now it is diagnosed with a clinical history and physical examination, looking for appropriate rule-in signs. Ancillary testing such as neuroimaging, electrophysiological studies, and blood tests are often used to rule out other conditions.
“Neurologists have a lot of training in this condition, as it is the second most common reason for a patient to visit a neurologist after headache,” Dr. Kim noted.
It is managed with education, counseling, physical rehabilitation and cognitive behavioral therapy. “A key part of the therapeutic process is working with the patient to explain the diagnosis. If they understand the condition, they do better. Patients can learn distraction techniques to allow more fluid movements,” he reported.
“As neurologists, and health care professionals more broadly, we must explain transparently and nonjudgmentally the nature of FND, including that these symptoms are real but not the direct result of toxic vaccine effects,” the authors wrote.
“Transparency and effective communication are needed in our society more than ever, and a condition as prevalent and potentially debilitating as FND can no longer remain marginalized and in the shadows. Effective communication will help educate the public and reduce fears so that patients can make informed decisions for themselves on receiving the vaccine to reduce the risk of COVID-19,” they concluded.
A version of this article first appeared on Medscape.com.
Adolescent substance use and the COVID-19 pandemic
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
During the past year, adolescents, families, educators, and health care providers have had to press forward through myriad challenges and stressors with flexibility and adaptability. With appropriate concern, we ask ourselves how children and youth are coping emotionally with the unprecedented changes of the past year.
Adolescent substance use represents an important area of concern. What has happened during the pandemic? Has youth substance use increased or decreased? Has access to substances increased or decreased, has monitoring and support for at-risk youth increased or decreased?
The answers to these questions are mixed. If anything, the pandemic has highlighted the heterogeneity of adolescent substance use. Now is a key time for assessment, support, and conversation with teens and families.
Monitoring the Future (MTF), a nationally representative annual survey, has provided a broad perspective on trends of adolescent substance use for decades.1 The MTF data is usually collected from February to May and was cut short in 2020 because of school closures associated with the pandemic. The sample size, though still nationally representative, was about a quarter of the typical volume. Some of the data are encouraging, including a flattening out of previous years’ stark increase in vaping of both nicotine and cannabis products (though overall numbers remain alarmingly high). Other data are more concerning including a continued increase in misuse of cough medicine, amphetamines, and inhalants among the youngest cohort surveyed (eighth graders). However, these data were largely representative of prepandemic circumstances.
The COVID-19 pandemic has significantly affected risk and protective factors for teen drug and alcohol use. Most notably, it has had a widely observed negative impact on adolescent mental health, across multiple disease categories.2 In addition, the cancellation of in-person academic and extracurricular activities such as arts and athletics markedly increased unstructured time, a known associated factor for higher-risk activities including substance use. This has also led to decreased contact with many supportive adults such as teachers and coaches. On the other hand, some adolescents now have more time with supportive parents and caregivers, more meals together, and more supervision, all of which are associated with decreased likelihood of substance use disorders.
The highly variable reasons for substance use affect highly variable pandemic-related changes in use. Understanding the impetus for use is a good place to start conversation and can help providers assess risk of escalation during the pandemic. Some teens primarily use for social enhancement while others use as a means of coping with stress or to mask or escape negative emotions. Still others continue use because of physiological dependence, craving, and other symptoms consistent with use disorders.
Highlighting the heterogeneity of this issue, one study assessing use early in the pandemic showed a decrease in the percentage of teens who use substances but an increase in frequency of use for those who are using.3 Though expected, an increase in frequency of use by oneself as compared with peers was also notable. Using substances alone is associated with more severe use disorders, carries greater risk of overdose, and can increase shame and secrecy, further fueling use disorders.
The pandemic has thus represented a protective pause for some experimental or socially motivated substance-using teens who have experienced a period of abstinence even if not fully by choice. For others, it has represented an acute amplification of risk factors and use has accelerated. This latter group includes those whose use represents an effort to cope with depression, anxiety, and loneliness or for whom isolation at home represents less monitoring, increased access, and greater exposure to substances.
Over the past year, in the treatment of adolescents struggling with substance use, many clinicians have observed a sifting effect during these unprecedented social changes. Many youth, who no longer have access to substances, have found they can “take it or leave it”. Other youth have been observed engaging in additional risk or going to greater lengths to access substances and continue their use. For both groups and everyone in between, this is an important time for screening, clinical assessment, and support.
While anticipating further research and data regarding broad substance use trends, including MTF data from 2021, recognizing that the impact of the COVID-19 pandemic is individual, with marked differences from adolescent to adolescent, will help us continue to act now to assess this important area of adolescent health. The first step for primary care providers is unchanged: to routinely screen for and discuss substance use in clinical settings.
Two brief, validated, easily accessible screening tools are available for primary care settings. They can both be self-administered and take less than 2 minutes to complete. Screening, Brief Intervention and Referral to Treatment and the Brief Screener for Tobacco, Alcohol and other Drugs can both be used for youth aged 12-17 years.4,5 Both screens are available online at drugabuse.gov.6
Routine screening will normalize conversations about substance use and healthy choices, provide opportunities for positive reinforcement, identify adolescents at risk, increase comfort and competence in providing brief intervention, and expedite referrals for additional support and treatment.
A false assumption that a particular adolescent isn’t using substances creates a missed opportunity to offer guidance and treatment. An oft-overlooked opportunity is that of providing positive reinforcement for an adolescent who isn’t using any substances or experimenting at all. Positive reinforcement is a strong component of reinforcing health maintenance.
Parent guidance and family assessment will also be critical tools. Parents and caregivers play a primary role in substance use treatment for teens and have a contributory impact on risk through both genes and environment. Of note, research suggests a moderate overall increase in adult substance use during the pandemic, particularly substances that are widely available such as alcohol. Adolescents may thus have greater access and exposure to substance use. A remarkably high percentage, 42%, of substance-using teens surveyed early in the pandemic indicated that they were using substances with their parents.3 Parents, who have equally been challenged by the pandemic, may need guidance in balancing compassion and support for struggling youth, while setting appropriate limits and maintaining expectations of healthy activities.
Unprecedented change and uncertainty provide an opportunity to reassess risks and openly discuss substance use with youth and families. Even with much on our minds during the COVID-19 pandemic, we can maintain focus on this significant risk to adolescent health and wellness. Our efforts now, from screening to treatment for adolescent substance use should be reinforced rather than delayed.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington.
References
1. Monitoringthefuture.org
2. Jones EAK et al. Int J Environ Res Public Health, 2021;18(5):2470.
3. Dumas TM et al. J Adolesc Health, 2020;67(3):354-61.
4. Levy S et al. JAMA Pediatr. 2014;168(9):822-8.
5. Kelly SM et al. Pediatrics. 2014;133(5):819-26.
6. National Institute on Drug Abuse. Adolescent Substance Use Screening Tools. 2016 Apr 27. https://www.drugabuse.gov/nidamed-medical-health-professionals/screening-tools-prevention/screening-tools-adolescent-substance-use/adolescent-substance-use-screening-tools
CDC panel: Pause of J&J COVID-19 vaccine to remain for now
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
Adjunctive MDMA safe, effective for severe PTSD
Adding 3,4-methylenedioxymethamphetamine (MDMA) to integrative psychotherapy may significantly improve symptoms and well-being for patients with severe posttraumatic stress disorder, including those with the dissociative subtype, new research suggests.
MAPP1 is the first phase 3 randomized controlled trial of MDMA-assisted therapy in this population. Participants who received the active treatment showed greater improvement in PTSD symptoms, mood, and empathy in comparison with participants who received placebo.
MDMA was “extremely effective, particularly for a subpopulation that ordinarily does not respond well to conventional treatment,” study coinvestigator Bessel van der Kolk, MD, professor of psychiatry at Boston University School of Medicine, told delegates attending the virtual European Psychiatric Association (EPA) 2021 Congress.
Growing interest
, particularly because failure rates with most available evidence-based treatments have been relatively high.
As previously reported by this news organization, in 2017, the U.S. Food and Drug Administration approved the trial design of Dr. van der Kolk’s and colleagues’ MAPP1 study after granting MDMA breakthrough designation.
The MAPP1 investigators assessed 90 patients with PTSD (mean age, 41 years; 77% White; 66% women) from 50 sites. For the majority of patients (84%), trauma history was developmental. “In other words, trauma [occurred] very early in life, usually at the hands of their own caregivers,” Dr. van der Kolk noted.
In addition, 18% of the patients were veterans, and 12% had combat exposure. The average duration of PTSD before enrollment was 18 years. All patients underwent screening and three preparatory psychotherapy sessions at enrollment.
Participants were randomly assigned to receive MDMA 80 mg or 120 mg (n = 46) or placebo (n = 44) followed by three integrative psychotherapy sessions lasting a total of 8 hours. A supplemental dose of 40 or 60 mg of MDMA could be administered from 1.5 to 2 hours after the first dose.
The patients stayed in the laboratory on the evening of the treatment session and attended a debriefing the next morning. The session was repeated a month later and again a month after that. In between, patients had telephone contact with the raters, who were blinded to the treatment received.
Follow-up assessments were conducted 2 months after the third treatment session and again at 12 months. The primary outcome measure was change in Clinician Administered PTSD Scale for DSM 5 (CAPS-5) score from baseline.
‘Dramatic improvement’
Results showed that both the MDMA and placebo groups experienced a statistically significant improvement in PTSD symptoms, “but MDMA had a dramatically significant improvement, with an effect size of over 0.9,” Dr. van der Kolk said.
The MDMA group also reported enhanced mood and well-being, increased responsiveness to emotional and sensory stimuli, a greater sense of closeness to other people, and a greater feeling of empathy.
Patients also reported having heightened openness, “and clearly the issue of empathy for themselves and others was a very large part of the process,” said Dr. van der Kolk.
“But for me, the most interesting part of the study is that the Adverse Childhood Experiences scale had no effect,” he noted. In other words, “the amount of childhood adverse experiences did not predict outcomes, which was very surprising because usually those patients are very treatment resistant.”
Dr. van der Kolk added that the dissociative subtype of PTSD was first described in the DSM-5 and that patients are “notoriously unresponsive to most unconventional treatments.”
In the current study, 13 patients met the criteria for the subtype, and investigators found they “did better than people with classical PTSD,” Dr. van der Kolk said. He added that this is a “very, very important finding.”
Carefully controlled
Overall, 82% of patients reported a significant improvement by the end of the study; 56% reported that they no longer had PTSD.
In addition, 67% of patients no longer met diagnostic criteria for PTSD. These included patients who had crossed over to active treatment from the placebo group.
Eleven patients (12%) experienced relapse by 12 months; in nine of the cases, this was due to the presence of additional stressors.
There were “very few adverse side effects” during the study, Dr. van der Kolk noted. In addition, “there were really no serious mental side effects,” despite the patients’ “opening up so much very painful material,” he added.
The most common adverse events among the MDMA group were muscle tightness (63%), decreased appetite (52%), nausea (30%), hyperhidrosis (20%), and feeling cold (20%). These effects were “quite small [and] the sort of side effects you would expect in response to an amphetamine substance like MDMA,” said Dr. van der Kolk.
“An important reason why we think the side effect profile is so good is because the study was extremely carefully done, very carefully controlled,” he added. “There was a great deal of support, [and] we paid an enormous amount of attention to creating a very safe context in which this drug was being used.”
However, he expressed concern that “as people see the very good results, they may skimp a little bit on the creation of the context and not have as careful a psychotherapy protocol as we had here.”
‘On the right track’
Commenting on the findings for this news organization, David Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, said the results are proof that the investigators’ “earlier smaller trials of MDMA were on the right track.”
“This larger and multicenter trial shows that MDMA therapy can be broadened into newer research groups, which augurs well for the much larger rollout that will be required once it gets a license,” said Dr. Nutt, who was not involved with the research.
He added, “the prior evidence of the safety of MDMA has [now] been confirmed.”
The study represents an “important step in the path to the clinical use of MDMA for PTSD,” Dr. Nutt said.
The study was sponsored by the Multidisciplinary Association for Psychedelic Studies. The investigators and Dr. Nutt have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adding 3,4-methylenedioxymethamphetamine (MDMA) to integrative psychotherapy may significantly improve symptoms and well-being for patients with severe posttraumatic stress disorder, including those with the dissociative subtype, new research suggests.
MAPP1 is the first phase 3 randomized controlled trial of MDMA-assisted therapy in this population. Participants who received the active treatment showed greater improvement in PTSD symptoms, mood, and empathy in comparison with participants who received placebo.
MDMA was “extremely effective, particularly for a subpopulation that ordinarily does not respond well to conventional treatment,” study coinvestigator Bessel van der Kolk, MD, professor of psychiatry at Boston University School of Medicine, told delegates attending the virtual European Psychiatric Association (EPA) 2021 Congress.
Growing interest
, particularly because failure rates with most available evidence-based treatments have been relatively high.
As previously reported by this news organization, in 2017, the U.S. Food and Drug Administration approved the trial design of Dr. van der Kolk’s and colleagues’ MAPP1 study after granting MDMA breakthrough designation.
The MAPP1 investigators assessed 90 patients with PTSD (mean age, 41 years; 77% White; 66% women) from 50 sites. For the majority of patients (84%), trauma history was developmental. “In other words, trauma [occurred] very early in life, usually at the hands of their own caregivers,” Dr. van der Kolk noted.
In addition, 18% of the patients were veterans, and 12% had combat exposure. The average duration of PTSD before enrollment was 18 years. All patients underwent screening and three preparatory psychotherapy sessions at enrollment.
Participants were randomly assigned to receive MDMA 80 mg or 120 mg (n = 46) or placebo (n = 44) followed by three integrative psychotherapy sessions lasting a total of 8 hours. A supplemental dose of 40 or 60 mg of MDMA could be administered from 1.5 to 2 hours after the first dose.
The patients stayed in the laboratory on the evening of the treatment session and attended a debriefing the next morning. The session was repeated a month later and again a month after that. In between, patients had telephone contact with the raters, who were blinded to the treatment received.
Follow-up assessments were conducted 2 months after the third treatment session and again at 12 months. The primary outcome measure was change in Clinician Administered PTSD Scale for DSM 5 (CAPS-5) score from baseline.
‘Dramatic improvement’
Results showed that both the MDMA and placebo groups experienced a statistically significant improvement in PTSD symptoms, “but MDMA had a dramatically significant improvement, with an effect size of over 0.9,” Dr. van der Kolk said.
The MDMA group also reported enhanced mood and well-being, increased responsiveness to emotional and sensory stimuli, a greater sense of closeness to other people, and a greater feeling of empathy.
Patients also reported having heightened openness, “and clearly the issue of empathy for themselves and others was a very large part of the process,” said Dr. van der Kolk.
“But for me, the most interesting part of the study is that the Adverse Childhood Experiences scale had no effect,” he noted. In other words, “the amount of childhood adverse experiences did not predict outcomes, which was very surprising because usually those patients are very treatment resistant.”
Dr. van der Kolk added that the dissociative subtype of PTSD was first described in the DSM-5 and that patients are “notoriously unresponsive to most unconventional treatments.”
In the current study, 13 patients met the criteria for the subtype, and investigators found they “did better than people with classical PTSD,” Dr. van der Kolk said. He added that this is a “very, very important finding.”
Carefully controlled
Overall, 82% of patients reported a significant improvement by the end of the study; 56% reported that they no longer had PTSD.
In addition, 67% of patients no longer met diagnostic criteria for PTSD. These included patients who had crossed over to active treatment from the placebo group.
Eleven patients (12%) experienced relapse by 12 months; in nine of the cases, this was due to the presence of additional stressors.
There were “very few adverse side effects” during the study, Dr. van der Kolk noted. In addition, “there were really no serious mental side effects,” despite the patients’ “opening up so much very painful material,” he added.
The most common adverse events among the MDMA group were muscle tightness (63%), decreased appetite (52%), nausea (30%), hyperhidrosis (20%), and feeling cold (20%). These effects were “quite small [and] the sort of side effects you would expect in response to an amphetamine substance like MDMA,” said Dr. van der Kolk.
“An important reason why we think the side effect profile is so good is because the study was extremely carefully done, very carefully controlled,” he added. “There was a great deal of support, [and] we paid an enormous amount of attention to creating a very safe context in which this drug was being used.”
However, he expressed concern that “as people see the very good results, they may skimp a little bit on the creation of the context and not have as careful a psychotherapy protocol as we had here.”
‘On the right track’
Commenting on the findings for this news organization, David Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, said the results are proof that the investigators’ “earlier smaller trials of MDMA were on the right track.”
“This larger and multicenter trial shows that MDMA therapy can be broadened into newer research groups, which augurs well for the much larger rollout that will be required once it gets a license,” said Dr. Nutt, who was not involved with the research.
He added, “the prior evidence of the safety of MDMA has [now] been confirmed.”
The study represents an “important step in the path to the clinical use of MDMA for PTSD,” Dr. Nutt said.
The study was sponsored by the Multidisciplinary Association for Psychedelic Studies. The investigators and Dr. Nutt have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adding 3,4-methylenedioxymethamphetamine (MDMA) to integrative psychotherapy may significantly improve symptoms and well-being for patients with severe posttraumatic stress disorder, including those with the dissociative subtype, new research suggests.
MAPP1 is the first phase 3 randomized controlled trial of MDMA-assisted therapy in this population. Participants who received the active treatment showed greater improvement in PTSD symptoms, mood, and empathy in comparison with participants who received placebo.
MDMA was “extremely effective, particularly for a subpopulation that ordinarily does not respond well to conventional treatment,” study coinvestigator Bessel van der Kolk, MD, professor of psychiatry at Boston University School of Medicine, told delegates attending the virtual European Psychiatric Association (EPA) 2021 Congress.
Growing interest
, particularly because failure rates with most available evidence-based treatments have been relatively high.
As previously reported by this news organization, in 2017, the U.S. Food and Drug Administration approved the trial design of Dr. van der Kolk’s and colleagues’ MAPP1 study after granting MDMA breakthrough designation.
The MAPP1 investigators assessed 90 patients with PTSD (mean age, 41 years; 77% White; 66% women) from 50 sites. For the majority of patients (84%), trauma history was developmental. “In other words, trauma [occurred] very early in life, usually at the hands of their own caregivers,” Dr. van der Kolk noted.
In addition, 18% of the patients were veterans, and 12% had combat exposure. The average duration of PTSD before enrollment was 18 years. All patients underwent screening and three preparatory psychotherapy sessions at enrollment.
Participants were randomly assigned to receive MDMA 80 mg or 120 mg (n = 46) or placebo (n = 44) followed by three integrative psychotherapy sessions lasting a total of 8 hours. A supplemental dose of 40 or 60 mg of MDMA could be administered from 1.5 to 2 hours after the first dose.
The patients stayed in the laboratory on the evening of the treatment session and attended a debriefing the next morning. The session was repeated a month later and again a month after that. In between, patients had telephone contact with the raters, who were blinded to the treatment received.
Follow-up assessments were conducted 2 months after the third treatment session and again at 12 months. The primary outcome measure was change in Clinician Administered PTSD Scale for DSM 5 (CAPS-5) score from baseline.
‘Dramatic improvement’
Results showed that both the MDMA and placebo groups experienced a statistically significant improvement in PTSD symptoms, “but MDMA had a dramatically significant improvement, with an effect size of over 0.9,” Dr. van der Kolk said.
The MDMA group also reported enhanced mood and well-being, increased responsiveness to emotional and sensory stimuli, a greater sense of closeness to other people, and a greater feeling of empathy.
Patients also reported having heightened openness, “and clearly the issue of empathy for themselves and others was a very large part of the process,” said Dr. van der Kolk.
“But for me, the most interesting part of the study is that the Adverse Childhood Experiences scale had no effect,” he noted. In other words, “the amount of childhood adverse experiences did not predict outcomes, which was very surprising because usually those patients are very treatment resistant.”
Dr. van der Kolk added that the dissociative subtype of PTSD was first described in the DSM-5 and that patients are “notoriously unresponsive to most unconventional treatments.”
In the current study, 13 patients met the criteria for the subtype, and investigators found they “did better than people with classical PTSD,” Dr. van der Kolk said. He added that this is a “very, very important finding.”
Carefully controlled
Overall, 82% of patients reported a significant improvement by the end of the study; 56% reported that they no longer had PTSD.
In addition, 67% of patients no longer met diagnostic criteria for PTSD. These included patients who had crossed over to active treatment from the placebo group.
Eleven patients (12%) experienced relapse by 12 months; in nine of the cases, this was due to the presence of additional stressors.
There were “very few adverse side effects” during the study, Dr. van der Kolk noted. In addition, “there were really no serious mental side effects,” despite the patients’ “opening up so much very painful material,” he added.
The most common adverse events among the MDMA group were muscle tightness (63%), decreased appetite (52%), nausea (30%), hyperhidrosis (20%), and feeling cold (20%). These effects were “quite small [and] the sort of side effects you would expect in response to an amphetamine substance like MDMA,” said Dr. van der Kolk.
“An important reason why we think the side effect profile is so good is because the study was extremely carefully done, very carefully controlled,” he added. “There was a great deal of support, [and] we paid an enormous amount of attention to creating a very safe context in which this drug was being used.”
However, he expressed concern that “as people see the very good results, they may skimp a little bit on the creation of the context and not have as careful a psychotherapy protocol as we had here.”
‘On the right track’
Commenting on the findings for this news organization, David Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, said the results are proof that the investigators’ “earlier smaller trials of MDMA were on the right track.”
“This larger and multicenter trial shows that MDMA therapy can be broadened into newer research groups, which augurs well for the much larger rollout that will be required once it gets a license,” said Dr. Nutt, who was not involved with the research.
He added, “the prior evidence of the safety of MDMA has [now] been confirmed.”
The study represents an “important step in the path to the clinical use of MDMA for PTSD,” Dr. Nutt said.
The study was sponsored by the Multidisciplinary Association for Psychedelic Studies. The investigators and Dr. Nutt have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How to counsel worried patients about the J&J vaccine news
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
Data about COVID-19-related skin manifestations in children continue to emerge
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
New global telepsychiatry guidelines released
The World Psychiatric Association (WPA) has released new global telemedicine guidelines.
Prompted by the worldwide explosion of interest in telepsychiatry driven by the COVID-19 pandemic, the guidelines emphasize the need for international collaboration in psychiatry.
“Global teamwork is the light at the end of the tunnel” of the current crisis, lead author Davor Mucic, MD, The Little Prince Treatment Center, Copenhagen, told meeting attendees.
“Now is the time to build a user-friendly digital health care system that can better meet the inevitable future challenges,” Dr. Mucic said. “The hope is that WPA’s global guidelines for telepsychiatry can help us to move forward.”
The guidelines, which also address concerns over data security and device intercompatibility, were presented at the virtual European Psychiatric Association (EPA) 2021 Congress.
Breaking down barriers
Although telepsychiatry has been around since 1959, only with the rapid technologic advances of the past decade has it become available to the majority of psychiatric patients, Dr. Mucic noted.
“Unfortunately, regulatory constraints, in combination with clinicians’ concerns, kept telepsychiatry from being widely adopted and implemented prior to the current COVID-19 pandemic,” he added.
Concerns have been with regard to data safety, reimbursement for consultations, quality of care, lack of technical experience, and difficulties in changing routines.
For many clinicians, the pandemic was the “first time they used telepsychiatry, and very few have received training in how to do it,” Dr. Mucic said.
He pointed out that , including the 2018 Best Practices in Videoconferencing-Based Telemental Health, released by the American Psychiatric Association and the American Telemedicine Association.
Dr. Mucic noted that because these documents are relevant and useful, clinicians may wonder, “Why do we need another set of guidelines?”
He explained that the current WPA guidelines outline universal recommendations that apply “regardless of local or regional regulations.” Therefore, they can be used just as easily in low- and middle-income countries as in countries where telepsychiatry is already established.
A new paradigm
Similar to other guidelines, the WPA’s guidelines discuss legal and regulatory requirements, informed consent, billing and reimbursement, patient selection, clinician training, the clinical setting, and more.
However, what makes the new document “so new and special” is that it opens the door to “some new and previously undiscussed aspects of telepsychiatry ... that are capable of changing the whole delivery of mental health care,” Dr. Mucic said.
The first of these new aspects is in regard to cross-cultural telepsychiatry. The goal is to eliminate the need for interpreters or competency in a different language for patients who do not speak the host country’s language by connecting them remotely with a bilingual health care professional who shares their cultural or ethnic background.
This “ethnic matching” model may lead to a “more precise and detailed symptomatology,” the authors note. They add that minimizing the risk for misinterpretation and misunderstanding can enable better diagnosis and treatment.
The second area highlighted by Dr. Mucic is in regard to international telepsychiatry; the technology could be used to obtain a second opinion from colleagues who share the relevant cultural and linguistic background.
“Further, international expertise may be brought via [telepsychiatry] to local health workers as a part of education, supervision, and scientific collaboration,” he said.
“The hope is the guidelines will pave the way for improved international collaboration, not only by clinicians but also by policymakers.”
A blended future?
Also at EPA 2021, two experts debated whether the COVID-19 pandemic represented a turning point for e-health in psychiatry.
Taking the pro stance, Heleen Riper, PhD, professor of eMental-Health at the Vrije Universiteit Amsterdam, argued that the future is likely to blend face-to-face interaction with video conferencing.
She believes that to maintain current progress, the focus should be on treatment personalization, engagement, and improvement, rather than cost-effectiveness.
Hans-Jürgen Möller, MD, professor emeritus, department of psychiatry, Ludwig-Maximilians-University, Munich, argued against the idea that e-health represented a turning point in psychiatry. He noted that a survey of German psychotherapists indicated that there have been a number of drawbacks to video sessions during the pandemic.
These included that the technology was not available or could be used by all patients, especially the elderly, and that unstable internet connections have posed a problem. Moreover, video conferencing is considered a “poor substitute” for face-to-face interactions by many patients.
In the subsequent discussion, Dr. Möller told this news organization that he believes guidelines in this area are important, especially to differentiate among various offerings on the internet, some of which are “not very good,” and to help patients identify those that are “very well established.”
Dr. Riper agreed, saying that several initiatives to introduce guidelines at the European level are now underway.
The biggest challenge from a technological standpoint is to offer flexibility to patients while still applying “therapeutic principles,” she noted.
“There is a need for guidelines, but those guidelines need to be open to a certain amount of flexibility if you really want to upscale technology into routine care,” Dr. Riper said.
Cautious optimism
Session chair Judit Simon, MD, DPhil, professor of health economics, Medical University of Vienna, asked the debaters whether video interventions will continue to replace in-person interventions once the pandemic is over or whether things will return to “where we were prepandemic.”
Dr. Riper said she did not believe that clinicians will return completely to in-patient practice. However, she emphasized the need for training and the development of new skills to improve the therapeutic relationship with patients.
Although Dr. Riper believes there is still a need for in-person doctor/patient interactions, “we will never get back to the pre-COVID phase, both in terms of diagnostics and treatment,” she said.
Dr. Möller added that although he has “some reservations” regarding the adoption of technologies by older patients and the lack of long-term data on telepsychiatry, he partially shares Dr. Riper’s optimism.
He suggested that there is an opportunity in psychiatry to use video conferencing for multidisciplinary team meetings similar to those seen in oncology.
This would allow discussion of patient diagnosis and treatment and would enable experts in mental health to help clinicians in other specialties. For example, it could help a general practitioner differentiate between depression and a depressive phase of schizophrenia, Dr. Riper said.
The presenters have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The World Psychiatric Association (WPA) has released new global telemedicine guidelines.
Prompted by the worldwide explosion of interest in telepsychiatry driven by the COVID-19 pandemic, the guidelines emphasize the need for international collaboration in psychiatry.
“Global teamwork is the light at the end of the tunnel” of the current crisis, lead author Davor Mucic, MD, The Little Prince Treatment Center, Copenhagen, told meeting attendees.
“Now is the time to build a user-friendly digital health care system that can better meet the inevitable future challenges,” Dr. Mucic said. “The hope is that WPA’s global guidelines for telepsychiatry can help us to move forward.”
The guidelines, which also address concerns over data security and device intercompatibility, were presented at the virtual European Psychiatric Association (EPA) 2021 Congress.
Breaking down barriers
Although telepsychiatry has been around since 1959, only with the rapid technologic advances of the past decade has it become available to the majority of psychiatric patients, Dr. Mucic noted.
“Unfortunately, regulatory constraints, in combination with clinicians’ concerns, kept telepsychiatry from being widely adopted and implemented prior to the current COVID-19 pandemic,” he added.
Concerns have been with regard to data safety, reimbursement for consultations, quality of care, lack of technical experience, and difficulties in changing routines.
For many clinicians, the pandemic was the “first time they used telepsychiatry, and very few have received training in how to do it,” Dr. Mucic said.
He pointed out that , including the 2018 Best Practices in Videoconferencing-Based Telemental Health, released by the American Psychiatric Association and the American Telemedicine Association.
Dr. Mucic noted that because these documents are relevant and useful, clinicians may wonder, “Why do we need another set of guidelines?”
He explained that the current WPA guidelines outline universal recommendations that apply “regardless of local or regional regulations.” Therefore, they can be used just as easily in low- and middle-income countries as in countries where telepsychiatry is already established.
A new paradigm
Similar to other guidelines, the WPA’s guidelines discuss legal and regulatory requirements, informed consent, billing and reimbursement, patient selection, clinician training, the clinical setting, and more.
However, what makes the new document “so new and special” is that it opens the door to “some new and previously undiscussed aspects of telepsychiatry ... that are capable of changing the whole delivery of mental health care,” Dr. Mucic said.
The first of these new aspects is in regard to cross-cultural telepsychiatry. The goal is to eliminate the need for interpreters or competency in a different language for patients who do not speak the host country’s language by connecting them remotely with a bilingual health care professional who shares their cultural or ethnic background.
This “ethnic matching” model may lead to a “more precise and detailed symptomatology,” the authors note. They add that minimizing the risk for misinterpretation and misunderstanding can enable better diagnosis and treatment.
The second area highlighted by Dr. Mucic is in regard to international telepsychiatry; the technology could be used to obtain a second opinion from colleagues who share the relevant cultural and linguistic background.
“Further, international expertise may be brought via [telepsychiatry] to local health workers as a part of education, supervision, and scientific collaboration,” he said.
“The hope is the guidelines will pave the way for improved international collaboration, not only by clinicians but also by policymakers.”
A blended future?
Also at EPA 2021, two experts debated whether the COVID-19 pandemic represented a turning point for e-health in psychiatry.
Taking the pro stance, Heleen Riper, PhD, professor of eMental-Health at the Vrije Universiteit Amsterdam, argued that the future is likely to blend face-to-face interaction with video conferencing.
She believes that to maintain current progress, the focus should be on treatment personalization, engagement, and improvement, rather than cost-effectiveness.
Hans-Jürgen Möller, MD, professor emeritus, department of psychiatry, Ludwig-Maximilians-University, Munich, argued against the idea that e-health represented a turning point in psychiatry. He noted that a survey of German psychotherapists indicated that there have been a number of drawbacks to video sessions during the pandemic.
These included that the technology was not available or could be used by all patients, especially the elderly, and that unstable internet connections have posed a problem. Moreover, video conferencing is considered a “poor substitute” for face-to-face interactions by many patients.
In the subsequent discussion, Dr. Möller told this news organization that he believes guidelines in this area are important, especially to differentiate among various offerings on the internet, some of which are “not very good,” and to help patients identify those that are “very well established.”
Dr. Riper agreed, saying that several initiatives to introduce guidelines at the European level are now underway.
The biggest challenge from a technological standpoint is to offer flexibility to patients while still applying “therapeutic principles,” she noted.
“There is a need for guidelines, but those guidelines need to be open to a certain amount of flexibility if you really want to upscale technology into routine care,” Dr. Riper said.
Cautious optimism
Session chair Judit Simon, MD, DPhil, professor of health economics, Medical University of Vienna, asked the debaters whether video interventions will continue to replace in-person interventions once the pandemic is over or whether things will return to “where we were prepandemic.”
Dr. Riper said she did not believe that clinicians will return completely to in-patient practice. However, she emphasized the need for training and the development of new skills to improve the therapeutic relationship with patients.
Although Dr. Riper believes there is still a need for in-person doctor/patient interactions, “we will never get back to the pre-COVID phase, both in terms of diagnostics and treatment,” she said.
Dr. Möller added that although he has “some reservations” regarding the adoption of technologies by older patients and the lack of long-term data on telepsychiatry, he partially shares Dr. Riper’s optimism.
He suggested that there is an opportunity in psychiatry to use video conferencing for multidisciplinary team meetings similar to those seen in oncology.
This would allow discussion of patient diagnosis and treatment and would enable experts in mental health to help clinicians in other specialties. For example, it could help a general practitioner differentiate between depression and a depressive phase of schizophrenia, Dr. Riper said.
The presenters have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The World Psychiatric Association (WPA) has released new global telemedicine guidelines.
Prompted by the worldwide explosion of interest in telepsychiatry driven by the COVID-19 pandemic, the guidelines emphasize the need for international collaboration in psychiatry.
“Global teamwork is the light at the end of the tunnel” of the current crisis, lead author Davor Mucic, MD, The Little Prince Treatment Center, Copenhagen, told meeting attendees.
“Now is the time to build a user-friendly digital health care system that can better meet the inevitable future challenges,” Dr. Mucic said. “The hope is that WPA’s global guidelines for telepsychiatry can help us to move forward.”
The guidelines, which also address concerns over data security and device intercompatibility, were presented at the virtual European Psychiatric Association (EPA) 2021 Congress.
Breaking down barriers
Although telepsychiatry has been around since 1959, only with the rapid technologic advances of the past decade has it become available to the majority of psychiatric patients, Dr. Mucic noted.
“Unfortunately, regulatory constraints, in combination with clinicians’ concerns, kept telepsychiatry from being widely adopted and implemented prior to the current COVID-19 pandemic,” he added.
Concerns have been with regard to data safety, reimbursement for consultations, quality of care, lack of technical experience, and difficulties in changing routines.
For many clinicians, the pandemic was the “first time they used telepsychiatry, and very few have received training in how to do it,” Dr. Mucic said.
He pointed out that , including the 2018 Best Practices in Videoconferencing-Based Telemental Health, released by the American Psychiatric Association and the American Telemedicine Association.
Dr. Mucic noted that because these documents are relevant and useful, clinicians may wonder, “Why do we need another set of guidelines?”
He explained that the current WPA guidelines outline universal recommendations that apply “regardless of local or regional regulations.” Therefore, they can be used just as easily in low- and middle-income countries as in countries where telepsychiatry is already established.
A new paradigm
Similar to other guidelines, the WPA’s guidelines discuss legal and regulatory requirements, informed consent, billing and reimbursement, patient selection, clinician training, the clinical setting, and more.
However, what makes the new document “so new and special” is that it opens the door to “some new and previously undiscussed aspects of telepsychiatry ... that are capable of changing the whole delivery of mental health care,” Dr. Mucic said.
The first of these new aspects is in regard to cross-cultural telepsychiatry. The goal is to eliminate the need for interpreters or competency in a different language for patients who do not speak the host country’s language by connecting them remotely with a bilingual health care professional who shares their cultural or ethnic background.
This “ethnic matching” model may lead to a “more precise and detailed symptomatology,” the authors note. They add that minimizing the risk for misinterpretation and misunderstanding can enable better diagnosis and treatment.
The second area highlighted by Dr. Mucic is in regard to international telepsychiatry; the technology could be used to obtain a second opinion from colleagues who share the relevant cultural and linguistic background.
“Further, international expertise may be brought via [telepsychiatry] to local health workers as a part of education, supervision, and scientific collaboration,” he said.
“The hope is the guidelines will pave the way for improved international collaboration, not only by clinicians but also by policymakers.”
A blended future?
Also at EPA 2021, two experts debated whether the COVID-19 pandemic represented a turning point for e-health in psychiatry.
Taking the pro stance, Heleen Riper, PhD, professor of eMental-Health at the Vrije Universiteit Amsterdam, argued that the future is likely to blend face-to-face interaction with video conferencing.
She believes that to maintain current progress, the focus should be on treatment personalization, engagement, and improvement, rather than cost-effectiveness.
Hans-Jürgen Möller, MD, professor emeritus, department of psychiatry, Ludwig-Maximilians-University, Munich, argued against the idea that e-health represented a turning point in psychiatry. He noted that a survey of German psychotherapists indicated that there have been a number of drawbacks to video sessions during the pandemic.
These included that the technology was not available or could be used by all patients, especially the elderly, and that unstable internet connections have posed a problem. Moreover, video conferencing is considered a “poor substitute” for face-to-face interactions by many patients.
In the subsequent discussion, Dr. Möller told this news organization that he believes guidelines in this area are important, especially to differentiate among various offerings on the internet, some of which are “not very good,” and to help patients identify those that are “very well established.”
Dr. Riper agreed, saying that several initiatives to introduce guidelines at the European level are now underway.
The biggest challenge from a technological standpoint is to offer flexibility to patients while still applying “therapeutic principles,” she noted.
“There is a need for guidelines, but those guidelines need to be open to a certain amount of flexibility if you really want to upscale technology into routine care,” Dr. Riper said.
Cautious optimism
Session chair Judit Simon, MD, DPhil, professor of health economics, Medical University of Vienna, asked the debaters whether video interventions will continue to replace in-person interventions once the pandemic is over or whether things will return to “where we were prepandemic.”
Dr. Riper said she did not believe that clinicians will return completely to in-patient practice. However, she emphasized the need for training and the development of new skills to improve the therapeutic relationship with patients.
Although Dr. Riper believes there is still a need for in-person doctor/patient interactions, “we will never get back to the pre-COVID phase, both in terms of diagnostics and treatment,” she said.
Dr. Möller added that although he has “some reservations” regarding the adoption of technologies by older patients and the lack of long-term data on telepsychiatry, he partially shares Dr. Riper’s optimism.
He suggested that there is an opportunity in psychiatry to use video conferencing for multidisciplinary team meetings similar to those seen in oncology.
This would allow discussion of patient diagnosis and treatment and would enable experts in mental health to help clinicians in other specialties. For example, it could help a general practitioner differentiate between depression and a depressive phase of schizophrenia, Dr. Riper said.
The presenters have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Helping psychiatric patients heal holistically
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.