User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
12 state boards have disciplined docs for COVID misinformation
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
Major COVID-19 case growth expected in coming weeks
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
Women struggle with benzodiazepine addiction post chemotherapy treatment
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
SAN ANTONIO – shows a new study.
While benzodiazepines and nonbenzodiazepine sedative-hypnotics are effective for these indications, misuse and increased health care utilization can ensue from their prolonged use, said Jacob C. Cogan, MD, a fellow in oncology/hematology at the Herbert Irving Comprehensive Cancer Center, Columbia University, New York. Dr. Cogan recently presented the results of the study at the San Antonio Breast Cancer Symposium.
The study included patients with breast cancer who received adjuvant chemotherapy between 2008 and 2017. Prescriptions for sedatives were divided into three periods: 365 days prior to chemotherapy to the start of chemotherapy (period one); start of chemotherapy to 90 days after the end of chemotherapy (period two); and 90-365 days after chemotherapy (period three). Patients who filled at least one benzodiazepine prescription in period two and patients who filled at least two benzodiazepine in period three were classified as new persistent benzodiazepine users. The same definitions were then used for nonbenzodiazepine sedative-hypnotics.
Among 17,532 benzodiazepine-naive patients (mean age, 57 years) and 21,863 nonbenzodiazepine sedative-hypnotic drug–naive patients (mean age, 56 years) who received adjuvant chemotherapy for breast cancer, lumpectomies were performed for a small majority (56.6% benzodiazepine naive, 55.1% nonbenzodiazepine sedative-hypnotics naive) versus mastectomy, and about half of patients received less than 4 months of chemotherapy (48.0% benzodiazepine naive, 48.6% nonbenzodiazepine sedative-hypnotics naive). Among benzodiazepine-naive patients, 4,447 (25.4%) filled at least one benzodiazepine prescription during chemotherapy, and 2,160 (9.9%) filled at least one nonbenzodiazepine sedative-hypnotic prescription during chemotherapy. The rate of new persistent benzodiazepine use after initial exposure during chemotherapy was 26.8% (n = 1,192). Similarly, 33.8% (n = 730) of nonbenzodiazepine sedative-hypnotics users became new persistent users. In addition, 115 patients became new persistent users of both types of sedative-hypnotics.
New persistent benzodiazepine use was associated with several characteristics: age 50-65 (odds ratio, 1.23; P = .01) and age greater than 65 (OR, 1.38, P = .005) relative to age less than 49; as well as Medicaid insurance, relative to commercial and Medicare insurance (OR, 1.68; P < .0001). Both new persistent benzodiazepine and nonbenzodiazepine sedative-hypnotics use was associated with chemotherapy duration of less than 4 months relative to 4 or more months of chemotherapy (OR, 1.17; P = .03 for benzodiazepines; OR, 1.58; P < .0001 for nonbenzodiazepine sedative-hypnotics).
It is not clear why shorter chemotherapy duration is associated with more new persistent use, Dr. Cogan said. “It may be that, paradoxically, a shorter duration of treatment could lead to more anxiety about recurrence. These patients may need closer monitoring of mental health symptoms and earlier referral for psychological services.”
Dr. Cogan said that providers should take steps to ensure that benzodiazepines and nonbenzodiazepine sedatives are used appropriately, which includes tapering dosages and, when appropriate, encouraging nonpharmacologic strategies.
There were no funding or other conflicts of interest associated with this study.
AT SABCS 2021
Omicron may require fourth vaccine dose, Pfizer says
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
Medical board stops warning docs against giving false COVID information
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
AMA, hospital group sue federal government over surprise billing law
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
Risk for severe COVID-19 and death plummets with Pfizer booster
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Spam filter failure: Selling physician emails equals big $$
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.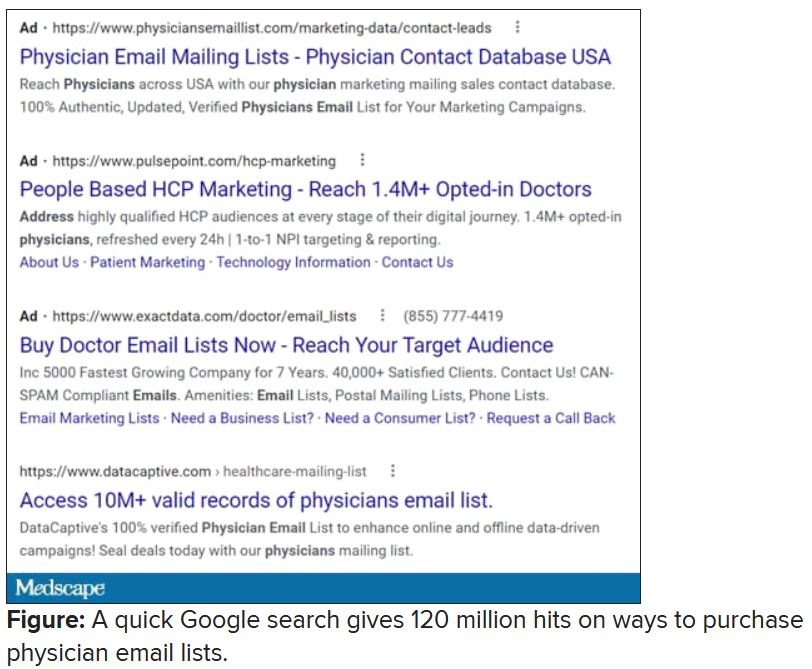
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
New insights into psychogenic seizures in teens
, results of a small study suggest.
The school experience of teens with PNES is overwhelmingly negative, study investigator Andrea Tanner, PhD, a postdoctoral fellow at Indiana University School of Nursing, Indianapolis.
She hopes this research will spur a collaborative effort between students, schools, families, and health care providers “to develop an effective plan to help these adolescents cope, to manage this condition, and hopefully reach seizure freedom.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Anxiety, perfectionism
Although psychogenic seizures resemble epileptic seizures, they have a psychological basis and, unlike epilepsy, are not caused by abnormal electrical brain activity.
While the school experience has previously been identified as a source of predisposing, precipitating, and perpetuating factors for PNES, little is known about the school experience of adolescents with the disorder and the role it may play in PNES management, the investigators noted.
During her 20 years as a school nurse, Dr. Tanner saw firsthand how school staff struggled with responding appropriately to teens with PNES. “They wanted to call 911 every time; they wanted to respond as if it [were] an epileptic seizure.”
For the study, she interviewed 10 teens with PNES, aged 12 to 19 years, whom she found mostly through Facebook support groups but also through flyers. All participants had undergone video EEG and been diagnosed with PNES.
From the interviews, Dr. Tanner and colleagues conducted a qualitative content analysis and uncovered “overarching” themes.
A main theme was stress, some of which focused on bullying by peers or harassment by school personnel, much of which was related to accusations of the children “faking” seizures to get attention, said Dr. Tanner.
Some teens reported being banned from school events, such as field trips, out of concern they would be a “distraction,” which led to feelings of isolation and exclusion, said Dr. Tanner.
Research points to a growing incidence of PNES among adolescents. This may be because it is now better recognized, or it may stem from the unique stressors today’s teens face, said Dr. Tanner.
Adolescents discussed the pressures they feel to be the best at everything. “They wanted to be good in athletics; they wanted to be good in academics; they wanted to get into a good college,” said Dr. Tanner.
Some study participants had undergone psychotherapy, including cognitive-behavioral therapy, and others had investigated mindfulness-based therapy. However, not all were receiving treatment. For some, such care was inaccessible, while others had tried a mental health care intervention but had abandoned it.
Although all the study participants were female, Dr. Tanner has interviewed males outside this study and found their experiences are similar.
Her next research step is to try to quantify the findings. “I would like to begin to look at what would be the appropriate outcomes if I were to do an intervention to improve the school experience.”
Her message for doctors is to see school nurses as a “partner” or “liaison” who “can bridge the world of health care and education.”
Important, novel research
Commenting on the research, Barbara Dworetzky, MD, Chief, Epilepsy, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, said it’s “important and novel.”
The study focuses on the main factors – or themes – that lead to increased stress, such as bullying, isolation, and “not being believed,” that are likely triggers for PNES, said Dr. Dworetzky.
The study is also important because it focuses on factors that help make the girls “feel supported and protected” – for example, having staff “take the episodes seriously,” she said.
The study’s qualitative measures “are a valid way of understanding these girls and giving them a voice,” said Dr. Dworetzky. She added the study provides “practical information” that could help target treatments to improve outcomes in this group.
A limitation of the study was that the very small cohort of teenage girls was selected only through families in Facebook support groups or flyers to school nurses, said Dr. Dworetzky.
“There are likely many other groups who don’t even have families trying to help them. Larger cohorts without this type of bias may be next steps.”
A version of this article first appeared on Medscape.com.
, results of a small study suggest.
The school experience of teens with PNES is overwhelmingly negative, study investigator Andrea Tanner, PhD, a postdoctoral fellow at Indiana University School of Nursing, Indianapolis.
She hopes this research will spur a collaborative effort between students, schools, families, and health care providers “to develop an effective plan to help these adolescents cope, to manage this condition, and hopefully reach seizure freedom.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Anxiety, perfectionism
Although psychogenic seizures resemble epileptic seizures, they have a psychological basis and, unlike epilepsy, are not caused by abnormal electrical brain activity.
While the school experience has previously been identified as a source of predisposing, precipitating, and perpetuating factors for PNES, little is known about the school experience of adolescents with the disorder and the role it may play in PNES management, the investigators noted.
During her 20 years as a school nurse, Dr. Tanner saw firsthand how school staff struggled with responding appropriately to teens with PNES. “They wanted to call 911 every time; they wanted to respond as if it [were] an epileptic seizure.”
For the study, she interviewed 10 teens with PNES, aged 12 to 19 years, whom she found mostly through Facebook support groups but also through flyers. All participants had undergone video EEG and been diagnosed with PNES.
From the interviews, Dr. Tanner and colleagues conducted a qualitative content analysis and uncovered “overarching” themes.
A main theme was stress, some of which focused on bullying by peers or harassment by school personnel, much of which was related to accusations of the children “faking” seizures to get attention, said Dr. Tanner.
Some teens reported being banned from school events, such as field trips, out of concern they would be a “distraction,” which led to feelings of isolation and exclusion, said Dr. Tanner.
Research points to a growing incidence of PNES among adolescents. This may be because it is now better recognized, or it may stem from the unique stressors today’s teens face, said Dr. Tanner.
Adolescents discussed the pressures they feel to be the best at everything. “They wanted to be good in athletics; they wanted to be good in academics; they wanted to get into a good college,” said Dr. Tanner.
Some study participants had undergone psychotherapy, including cognitive-behavioral therapy, and others had investigated mindfulness-based therapy. However, not all were receiving treatment. For some, such care was inaccessible, while others had tried a mental health care intervention but had abandoned it.
Although all the study participants were female, Dr. Tanner has interviewed males outside this study and found their experiences are similar.
Her next research step is to try to quantify the findings. “I would like to begin to look at what would be the appropriate outcomes if I were to do an intervention to improve the school experience.”
Her message for doctors is to see school nurses as a “partner” or “liaison” who “can bridge the world of health care and education.”
Important, novel research
Commenting on the research, Barbara Dworetzky, MD, Chief, Epilepsy, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, said it’s “important and novel.”
The study focuses on the main factors – or themes – that lead to increased stress, such as bullying, isolation, and “not being believed,” that are likely triggers for PNES, said Dr. Dworetzky.
The study is also important because it focuses on factors that help make the girls “feel supported and protected” – for example, having staff “take the episodes seriously,” she said.
The study’s qualitative measures “are a valid way of understanding these girls and giving them a voice,” said Dr. Dworetzky. She added the study provides “practical information” that could help target treatments to improve outcomes in this group.
A limitation of the study was that the very small cohort of teenage girls was selected only through families in Facebook support groups or flyers to school nurses, said Dr. Dworetzky.
“There are likely many other groups who don’t even have families trying to help them. Larger cohorts without this type of bias may be next steps.”
A version of this article first appeared on Medscape.com.
, results of a small study suggest.
The school experience of teens with PNES is overwhelmingly negative, study investigator Andrea Tanner, PhD, a postdoctoral fellow at Indiana University School of Nursing, Indianapolis.
She hopes this research will spur a collaborative effort between students, schools, families, and health care providers “to develop an effective plan to help these adolescents cope, to manage this condition, and hopefully reach seizure freedom.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Anxiety, perfectionism
Although psychogenic seizures resemble epileptic seizures, they have a psychological basis and, unlike epilepsy, are not caused by abnormal electrical brain activity.
While the school experience has previously been identified as a source of predisposing, precipitating, and perpetuating factors for PNES, little is known about the school experience of adolescents with the disorder and the role it may play in PNES management, the investigators noted.
During her 20 years as a school nurse, Dr. Tanner saw firsthand how school staff struggled with responding appropriately to teens with PNES. “They wanted to call 911 every time; they wanted to respond as if it [were] an epileptic seizure.”
For the study, she interviewed 10 teens with PNES, aged 12 to 19 years, whom she found mostly through Facebook support groups but also through flyers. All participants had undergone video EEG and been diagnosed with PNES.
From the interviews, Dr. Tanner and colleagues conducted a qualitative content analysis and uncovered “overarching” themes.
A main theme was stress, some of which focused on bullying by peers or harassment by school personnel, much of which was related to accusations of the children “faking” seizures to get attention, said Dr. Tanner.
Some teens reported being banned from school events, such as field trips, out of concern they would be a “distraction,” which led to feelings of isolation and exclusion, said Dr. Tanner.
Research points to a growing incidence of PNES among adolescents. This may be because it is now better recognized, or it may stem from the unique stressors today’s teens face, said Dr. Tanner.
Adolescents discussed the pressures they feel to be the best at everything. “They wanted to be good in athletics; they wanted to be good in academics; they wanted to get into a good college,” said Dr. Tanner.
Some study participants had undergone psychotherapy, including cognitive-behavioral therapy, and others had investigated mindfulness-based therapy. However, not all were receiving treatment. For some, such care was inaccessible, while others had tried a mental health care intervention but had abandoned it.
Although all the study participants were female, Dr. Tanner has interviewed males outside this study and found their experiences are similar.
Her next research step is to try to quantify the findings. “I would like to begin to look at what would be the appropriate outcomes if I were to do an intervention to improve the school experience.”
Her message for doctors is to see school nurses as a “partner” or “liaison” who “can bridge the world of health care and education.”
Important, novel research
Commenting on the research, Barbara Dworetzky, MD, Chief, Epilepsy, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, said it’s “important and novel.”
The study focuses on the main factors – or themes – that lead to increased stress, such as bullying, isolation, and “not being believed,” that are likely triggers for PNES, said Dr. Dworetzky.
The study is also important because it focuses on factors that help make the girls “feel supported and protected” – for example, having staff “take the episodes seriously,” she said.
The study’s qualitative measures “are a valid way of understanding these girls and giving them a voice,” said Dr. Dworetzky. She added the study provides “practical information” that could help target treatments to improve outcomes in this group.
A limitation of the study was that the very small cohort of teenage girls was selected only through families in Facebook support groups or flyers to school nurses, said Dr. Dworetzky.
“There are likely many other groups who don’t even have families trying to help them. Larger cohorts without this type of bias may be next steps.”
A version of this article first appeared on Medscape.com.
From AES 2021
‘Alarming’ rate of abuse in pregnant women with epilepsy
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
From AES 2021

