User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Diabetes drug class appears to reduce recurrent gout flares
The glucose-lowering drug class sodium-glucose cotransporter 2 (SGLT2) inhibitors appear to reduce the risk for recurrent gout flares in people with gout and type 2 diabetes, and to lessen excess mortality in those individuals, compared with those who initiated other types of glucose-lowering medications, new data suggest.
Among nearly 6,000 adults with both type 2 diabetes and gout from a U.K. primary care database, initiation of SGLT2 inhibitor treatment was associated with 19% fewer recurrent gout flares and 29% lower mortality.
Moreover, unlike other urate-lowering therapies, there were no apparent transient increases in the risk of gout flares after initiating therapy, Jie Wei, PhD, of Health Management Center, Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in JAMA Network Open.
These results are important because current management of gout is suboptimal. Many patients either don’t receive adequate urate-lowering therapies such as allopurinol or stop taking them, Dr. Wei and colleagues said.
In addition to lowering glucose, SGLT2 inhibitors also reduce the risk for major adverse cardiovascular events and all-cause mortality in people regardless of their diabetes status. Previous studies have also found that SGLT2 inhibitors reduce the risk for developing gout and of gout flares.
Asked to comment, gout specialist John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, said in an interview: “I think it’s a well-done paper, with a large dataset. I think it just reinforces the findings from the other papers. Mostly anything that lowers uric acid levels is going to lower recurrent gout attacks, so it all makes sense.”
However, while Dr. FitzGerald thinks the drug class is a good option for people with diabetes or cardiorenal indications for them who also have gout, he doesn’t envision it as first-line for most other patients with gout. “The current treatments are very effective. Allopurinol brings down uric acid levels by 5-7 points. There are patients who fail allopurinol, but those are less than 5%.”
The most common reason patients stop taking allopurinol is the frequent initial gout flare. But that’s preventable, Dr. FitzGerald said, either by titrating up slowly, or by adding colchicine along with it. “By going slowly, you can avoid that flare risk. I think that’s what’s going on with the SGLT2 inhibitor. It’s not a dramatic urate-lowering drug, but it is clinically meaningful. I think that’s what this paper is showing.”
But, he noted, “I think there are so many reasons to start the SGLT2 inhibitors that if somebody also has gout, all the better. And, if somebody is on the margin with diabetes and gout control and can’t go with allopurinol, it would be great to add for both conditions.”
Less gout recurrence, lower mortality
The retrospective study was conducted from Jan. 1, 2013, to March 31, 2022. Among 5,931 patients with both type 2 diabetes and gout, 1,548 (26.1%) initiated an SGLT2 inhibitor (dapagliflozin, empagliflozin, or canagliflozin), while 4,383 (73.9%) initiated treatment with other active comparators, mostly (92.6%) dipeptidyl peptidase–4 inhibitors.
Gout flares were identified in the charts for a total of 86% of the participants. The weighted incidence rates for the first recurrent flare were 32.4 versus 41.2 per 1,000 person-years in the SGLT2 inhibitor versus comparator groups, with a weighted absolute rate difference of –8.8/1,000 and weighted hazard ratio of 0.81, a significant difference.
All-cause mortality was 18.8 versus 24.9 per 1,000 person-years, respectively, giving an HR of 0.71 at 5-year follow-up.
Dr. FitzGerald, who chaired the American College of Rheumatology’s 2020 gout guidelines, said he anticipates that the SGLT2 inhibitors will be mentioned in the next update to the ACR’s now “living” guidelines, although he was not speaking on the organization’s behalf.
“We talk about losartan in the current [ACR guidelines], about its specific uric acid–lowering effect. Drugs can make uric acid worse or better. For example, thiazides make it higher. I think the SGLT2 [inhibitors] are important, but I don’t think they’re huge. The study is great, and I think the drugs are great, but I don’t think they will change the way gout is managed.”
This work was supported by grants from the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and from the Natural Science Foundation of Hunan Province. Dr. Wei reported receiving grant funding from Xiangya Hospital Central South University Project Program of National Clinical Research Center for Geriatric Disorders and the Science and Technology Department of Hunan Province, the Natural Science Foundation of Hunan Province, during the conduct of the study. Dr. FitzGerald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The glucose-lowering drug class sodium-glucose cotransporter 2 (SGLT2) inhibitors appear to reduce the risk for recurrent gout flares in people with gout and type 2 diabetes, and to lessen excess mortality in those individuals, compared with those who initiated other types of glucose-lowering medications, new data suggest.
Among nearly 6,000 adults with both type 2 diabetes and gout from a U.K. primary care database, initiation of SGLT2 inhibitor treatment was associated with 19% fewer recurrent gout flares and 29% lower mortality.
Moreover, unlike other urate-lowering therapies, there were no apparent transient increases in the risk of gout flares after initiating therapy, Jie Wei, PhD, of Health Management Center, Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in JAMA Network Open.
These results are important because current management of gout is suboptimal. Many patients either don’t receive adequate urate-lowering therapies such as allopurinol or stop taking them, Dr. Wei and colleagues said.
In addition to lowering glucose, SGLT2 inhibitors also reduce the risk for major adverse cardiovascular events and all-cause mortality in people regardless of their diabetes status. Previous studies have also found that SGLT2 inhibitors reduce the risk for developing gout and of gout flares.
Asked to comment, gout specialist John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, said in an interview: “I think it’s a well-done paper, with a large dataset. I think it just reinforces the findings from the other papers. Mostly anything that lowers uric acid levels is going to lower recurrent gout attacks, so it all makes sense.”
However, while Dr. FitzGerald thinks the drug class is a good option for people with diabetes or cardiorenal indications for them who also have gout, he doesn’t envision it as first-line for most other patients with gout. “The current treatments are very effective. Allopurinol brings down uric acid levels by 5-7 points. There are patients who fail allopurinol, but those are less than 5%.”
The most common reason patients stop taking allopurinol is the frequent initial gout flare. But that’s preventable, Dr. FitzGerald said, either by titrating up slowly, or by adding colchicine along with it. “By going slowly, you can avoid that flare risk. I think that’s what’s going on with the SGLT2 inhibitor. It’s not a dramatic urate-lowering drug, but it is clinically meaningful. I think that’s what this paper is showing.”
But, he noted, “I think there are so many reasons to start the SGLT2 inhibitors that if somebody also has gout, all the better. And, if somebody is on the margin with diabetes and gout control and can’t go with allopurinol, it would be great to add for both conditions.”
Less gout recurrence, lower mortality
The retrospective study was conducted from Jan. 1, 2013, to March 31, 2022. Among 5,931 patients with both type 2 diabetes and gout, 1,548 (26.1%) initiated an SGLT2 inhibitor (dapagliflozin, empagliflozin, or canagliflozin), while 4,383 (73.9%) initiated treatment with other active comparators, mostly (92.6%) dipeptidyl peptidase–4 inhibitors.
Gout flares were identified in the charts for a total of 86% of the participants. The weighted incidence rates for the first recurrent flare were 32.4 versus 41.2 per 1,000 person-years in the SGLT2 inhibitor versus comparator groups, with a weighted absolute rate difference of –8.8/1,000 and weighted hazard ratio of 0.81, a significant difference.
All-cause mortality was 18.8 versus 24.9 per 1,000 person-years, respectively, giving an HR of 0.71 at 5-year follow-up.
Dr. FitzGerald, who chaired the American College of Rheumatology’s 2020 gout guidelines, said he anticipates that the SGLT2 inhibitors will be mentioned in the next update to the ACR’s now “living” guidelines, although he was not speaking on the organization’s behalf.
“We talk about losartan in the current [ACR guidelines], about its specific uric acid–lowering effect. Drugs can make uric acid worse or better. For example, thiazides make it higher. I think the SGLT2 [inhibitors] are important, but I don’t think they’re huge. The study is great, and I think the drugs are great, but I don’t think they will change the way gout is managed.”
This work was supported by grants from the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and from the Natural Science Foundation of Hunan Province. Dr. Wei reported receiving grant funding from Xiangya Hospital Central South University Project Program of National Clinical Research Center for Geriatric Disorders and the Science and Technology Department of Hunan Province, the Natural Science Foundation of Hunan Province, during the conduct of the study. Dr. FitzGerald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The glucose-lowering drug class sodium-glucose cotransporter 2 (SGLT2) inhibitors appear to reduce the risk for recurrent gout flares in people with gout and type 2 diabetes, and to lessen excess mortality in those individuals, compared with those who initiated other types of glucose-lowering medications, new data suggest.
Among nearly 6,000 adults with both type 2 diabetes and gout from a U.K. primary care database, initiation of SGLT2 inhibitor treatment was associated with 19% fewer recurrent gout flares and 29% lower mortality.
Moreover, unlike other urate-lowering therapies, there were no apparent transient increases in the risk of gout flares after initiating therapy, Jie Wei, PhD, of Health Management Center, Xiangya Hospital, Central South University, Changsha, China, and colleagues reported in JAMA Network Open.
These results are important because current management of gout is suboptimal. Many patients either don’t receive adequate urate-lowering therapies such as allopurinol or stop taking them, Dr. Wei and colleagues said.
In addition to lowering glucose, SGLT2 inhibitors also reduce the risk for major adverse cardiovascular events and all-cause mortality in people regardless of their diabetes status. Previous studies have also found that SGLT2 inhibitors reduce the risk for developing gout and of gout flares.
Asked to comment, gout specialist John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, said in an interview: “I think it’s a well-done paper, with a large dataset. I think it just reinforces the findings from the other papers. Mostly anything that lowers uric acid levels is going to lower recurrent gout attacks, so it all makes sense.”
However, while Dr. FitzGerald thinks the drug class is a good option for people with diabetes or cardiorenal indications for them who also have gout, he doesn’t envision it as first-line for most other patients with gout. “The current treatments are very effective. Allopurinol brings down uric acid levels by 5-7 points. There are patients who fail allopurinol, but those are less than 5%.”
The most common reason patients stop taking allopurinol is the frequent initial gout flare. But that’s preventable, Dr. FitzGerald said, either by titrating up slowly, or by adding colchicine along with it. “By going slowly, you can avoid that flare risk. I think that’s what’s going on with the SGLT2 inhibitor. It’s not a dramatic urate-lowering drug, but it is clinically meaningful. I think that’s what this paper is showing.”
But, he noted, “I think there are so many reasons to start the SGLT2 inhibitors that if somebody also has gout, all the better. And, if somebody is on the margin with diabetes and gout control and can’t go with allopurinol, it would be great to add for both conditions.”
Less gout recurrence, lower mortality
The retrospective study was conducted from Jan. 1, 2013, to March 31, 2022. Among 5,931 patients with both type 2 diabetes and gout, 1,548 (26.1%) initiated an SGLT2 inhibitor (dapagliflozin, empagliflozin, or canagliflozin), while 4,383 (73.9%) initiated treatment with other active comparators, mostly (92.6%) dipeptidyl peptidase–4 inhibitors.
Gout flares were identified in the charts for a total of 86% of the participants. The weighted incidence rates for the first recurrent flare were 32.4 versus 41.2 per 1,000 person-years in the SGLT2 inhibitor versus comparator groups, with a weighted absolute rate difference of –8.8/1,000 and weighted hazard ratio of 0.81, a significant difference.
All-cause mortality was 18.8 versus 24.9 per 1,000 person-years, respectively, giving an HR of 0.71 at 5-year follow-up.
Dr. FitzGerald, who chaired the American College of Rheumatology’s 2020 gout guidelines, said he anticipates that the SGLT2 inhibitors will be mentioned in the next update to the ACR’s now “living” guidelines, although he was not speaking on the organization’s behalf.
“We talk about losartan in the current [ACR guidelines], about its specific uric acid–lowering effect. Drugs can make uric acid worse or better. For example, thiazides make it higher. I think the SGLT2 [inhibitors] are important, but I don’t think they’re huge. The study is great, and I think the drugs are great, but I don’t think they will change the way gout is managed.”
This work was supported by grants from the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, and from the Natural Science Foundation of Hunan Province. Dr. Wei reported receiving grant funding from Xiangya Hospital Central South University Project Program of National Clinical Research Center for Geriatric Disorders and the Science and Technology Department of Hunan Province, the Natural Science Foundation of Hunan Province, during the conduct of the study. Dr. FitzGerald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
NPs, PAs, and physicians hope to join doctors’ union in rare alliance
Advanced practice providers (APPs) such as nurse practitioners (NPs) and physician assistants (PAs) have long been at odds with doctor groups over scope of practice issues. But in a rare alliance, in late September. If successful, the Allina group will join the Doctors Council SEIU, Local 10MD.
The Allina health care providers share concerns about their working conditions, such as understaffing and inadequate resources, limited decision-making authority, and health systems valuing productivity and profit over patient care.
Although doctors and APPs have said that they generally work well together, the relationship has been strained in recent years as APPs argue for greater scope of practice. Meanwhile, physician groups, such as the American Medical Association, believe that APPs need more oversight.
An Allina union organizer, Britta V. Kasmarik, CNP, acknowledges the tension between physicians and APPs. But she said in an interview that the union effort helped bond this group of health care providers. “We share common goals of providing high-quality care for patients in a safe way, and we see the same things day in and day out with our patients.”
Matt Hoffman, MD, a primary care physician at Allina, told this news organization that APPs in his specialty perform the same job as doctors “and the working conditions are really identical. In our view, that means we should be unionizing together.”
The decision to hold a union vote follows similar action by nearly 150 Allina Mercy Hospital physicians in March. Allina Health appealed the vote.
In response to a New York Times investigation, the Minnesota Attorney General’s office began reviewing reports of aggressive billing practices and denied care at Allina Health.
The Allina Health system, which reports $4 billion in annual revenue, cut off nonemergency services to patients, including children, if their medical debt exceeded $4,500, according to the New York Times article. For Allina’s physicians and APPs, that meant leaving patients’ illnesses untreated.
Less than a week after the attorney general announced its investigation, the health system ended this practice.
In a prepared statement to this news organization, Allina Health said that its providers are “critical members of our teams. … We deeply value and share their commitment to providing high-quality care to our patients.”
The health system said it planned to make operational improvements, implement new communication tools, and provide additional well-being resources and enhanced employee benefits “to improve the provider experience.” In addition, it hoped to continue to “foster a culture of collaboration with all our employees.”
Having a union will allow health care providers to advocate for their patients and give health care providers more decision-making power instead of corporate leaders maintaining full authority, Ms. Kasmarik told this news organization.
Union organizers are also concerned with changes to the daily practice of medicine. “We don’t want to be spending our time doing paperwork and calling insurance companies and filling out forms,” said Dr. Hoffman. “We want to be in the exam room with a patient.”
The Allina providers organized after multiple requests to corporate managers failed to address their concerns. Their demands include increased staffing and help with nonclinical work so that clinicians can spend more time with their patients.
“What I’m really excited about is that we will be able to work with the other unionized groups to make change ... by being involved in health care policy at a state or national level,” Dr. Hoffman said. For example, that involvement might include challenging insurance company decisions.
Doctors Council bills itself as the largest union for attending physicians in the country, with 3,500 members, according to Joe Crane, national organizing director.
Despite an increase in union efforts since the pandemic, health care workers – particularly doctors – have been slow to join unions. Mr. Crane estimated that only about 3% of U.S. physicians are currently union members. He cited union campaigns in Massachusetts, New York, and Washington, DC. For comparison, a minority of advanced practice registered nurses (APRNs) (9%) report union membership, according to Medscape’s APRN compensation report last year.
Dr. Hoffman is confident the Allina health care providers will have enough votes to win the election to join the union. “We should have done this years ago.”
A version of this article appeared on Medscape.com.
Advanced practice providers (APPs) such as nurse practitioners (NPs) and physician assistants (PAs) have long been at odds with doctor groups over scope of practice issues. But in a rare alliance, in late September. If successful, the Allina group will join the Doctors Council SEIU, Local 10MD.
The Allina health care providers share concerns about their working conditions, such as understaffing and inadequate resources, limited decision-making authority, and health systems valuing productivity and profit over patient care.
Although doctors and APPs have said that they generally work well together, the relationship has been strained in recent years as APPs argue for greater scope of practice. Meanwhile, physician groups, such as the American Medical Association, believe that APPs need more oversight.
An Allina union organizer, Britta V. Kasmarik, CNP, acknowledges the tension between physicians and APPs. But she said in an interview that the union effort helped bond this group of health care providers. “We share common goals of providing high-quality care for patients in a safe way, and we see the same things day in and day out with our patients.”
Matt Hoffman, MD, a primary care physician at Allina, told this news organization that APPs in his specialty perform the same job as doctors “and the working conditions are really identical. In our view, that means we should be unionizing together.”
The decision to hold a union vote follows similar action by nearly 150 Allina Mercy Hospital physicians in March. Allina Health appealed the vote.
In response to a New York Times investigation, the Minnesota Attorney General’s office began reviewing reports of aggressive billing practices and denied care at Allina Health.
The Allina Health system, which reports $4 billion in annual revenue, cut off nonemergency services to patients, including children, if their medical debt exceeded $4,500, according to the New York Times article. For Allina’s physicians and APPs, that meant leaving patients’ illnesses untreated.
Less than a week after the attorney general announced its investigation, the health system ended this practice.
In a prepared statement to this news organization, Allina Health said that its providers are “critical members of our teams. … We deeply value and share their commitment to providing high-quality care to our patients.”
The health system said it planned to make operational improvements, implement new communication tools, and provide additional well-being resources and enhanced employee benefits “to improve the provider experience.” In addition, it hoped to continue to “foster a culture of collaboration with all our employees.”
Having a union will allow health care providers to advocate for their patients and give health care providers more decision-making power instead of corporate leaders maintaining full authority, Ms. Kasmarik told this news organization.
Union organizers are also concerned with changes to the daily practice of medicine. “We don’t want to be spending our time doing paperwork and calling insurance companies and filling out forms,” said Dr. Hoffman. “We want to be in the exam room with a patient.”
The Allina providers organized after multiple requests to corporate managers failed to address their concerns. Their demands include increased staffing and help with nonclinical work so that clinicians can spend more time with their patients.
“What I’m really excited about is that we will be able to work with the other unionized groups to make change ... by being involved in health care policy at a state or national level,” Dr. Hoffman said. For example, that involvement might include challenging insurance company decisions.
Doctors Council bills itself as the largest union for attending physicians in the country, with 3,500 members, according to Joe Crane, national organizing director.
Despite an increase in union efforts since the pandemic, health care workers – particularly doctors – have been slow to join unions. Mr. Crane estimated that only about 3% of U.S. physicians are currently union members. He cited union campaigns in Massachusetts, New York, and Washington, DC. For comparison, a minority of advanced practice registered nurses (APRNs) (9%) report union membership, according to Medscape’s APRN compensation report last year.
Dr. Hoffman is confident the Allina health care providers will have enough votes to win the election to join the union. “We should have done this years ago.”
A version of this article appeared on Medscape.com.
Advanced practice providers (APPs) such as nurse practitioners (NPs) and physician assistants (PAs) have long been at odds with doctor groups over scope of practice issues. But in a rare alliance, in late September. If successful, the Allina group will join the Doctors Council SEIU, Local 10MD.
The Allina health care providers share concerns about their working conditions, such as understaffing and inadequate resources, limited decision-making authority, and health systems valuing productivity and profit over patient care.
Although doctors and APPs have said that they generally work well together, the relationship has been strained in recent years as APPs argue for greater scope of practice. Meanwhile, physician groups, such as the American Medical Association, believe that APPs need more oversight.
An Allina union organizer, Britta V. Kasmarik, CNP, acknowledges the tension between physicians and APPs. But she said in an interview that the union effort helped bond this group of health care providers. “We share common goals of providing high-quality care for patients in a safe way, and we see the same things day in and day out with our patients.”
Matt Hoffman, MD, a primary care physician at Allina, told this news organization that APPs in his specialty perform the same job as doctors “and the working conditions are really identical. In our view, that means we should be unionizing together.”
The decision to hold a union vote follows similar action by nearly 150 Allina Mercy Hospital physicians in March. Allina Health appealed the vote.
In response to a New York Times investigation, the Minnesota Attorney General’s office began reviewing reports of aggressive billing practices and denied care at Allina Health.
The Allina Health system, which reports $4 billion in annual revenue, cut off nonemergency services to patients, including children, if their medical debt exceeded $4,500, according to the New York Times article. For Allina’s physicians and APPs, that meant leaving patients’ illnesses untreated.
Less than a week after the attorney general announced its investigation, the health system ended this practice.
In a prepared statement to this news organization, Allina Health said that its providers are “critical members of our teams. … We deeply value and share their commitment to providing high-quality care to our patients.”
The health system said it planned to make operational improvements, implement new communication tools, and provide additional well-being resources and enhanced employee benefits “to improve the provider experience.” In addition, it hoped to continue to “foster a culture of collaboration with all our employees.”
Having a union will allow health care providers to advocate for their patients and give health care providers more decision-making power instead of corporate leaders maintaining full authority, Ms. Kasmarik told this news organization.
Union organizers are also concerned with changes to the daily practice of medicine. “We don’t want to be spending our time doing paperwork and calling insurance companies and filling out forms,” said Dr. Hoffman. “We want to be in the exam room with a patient.”
The Allina providers organized after multiple requests to corporate managers failed to address their concerns. Their demands include increased staffing and help with nonclinical work so that clinicians can spend more time with their patients.
“What I’m really excited about is that we will be able to work with the other unionized groups to make change ... by being involved in health care policy at a state or national level,” Dr. Hoffman said. For example, that involvement might include challenging insurance company decisions.
Doctors Council bills itself as the largest union for attending physicians in the country, with 3,500 members, according to Joe Crane, national organizing director.
Despite an increase in union efforts since the pandemic, health care workers – particularly doctors – have been slow to join unions. Mr. Crane estimated that only about 3% of U.S. physicians are currently union members. He cited union campaigns in Massachusetts, New York, and Washington, DC. For comparison, a minority of advanced practice registered nurses (APRNs) (9%) report union membership, according to Medscape’s APRN compensation report last year.
Dr. Hoffman is confident the Allina health care providers will have enough votes to win the election to join the union. “We should have done this years ago.”
A version of this article appeared on Medscape.com.
FDA to step up oversight of cosmetics, assess ‘forever chemicals’
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
They are also preparing to assess potential risks of so-called forever chemicals in these products.
The Food and Drug Administration last year gained new authority over cosmetics when Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) by adding this bill to a December budget package.
“On average, consumers in the U.S. use six to 12 cosmetics products daily. But, until recently the FDA didn’t have the authority to require manufacturers to submit cosmetic product listings, including a list of ingredients used in these products, or register the facilities where they were produced,” Namandjé Bumpus, PhD, FDA’s chief scientist, said in a press release.
In the statement, the FDA announced the release of a draft guidance document that is intended to help companies comply with the transparency requirements slated to kick in this December. The agency is accepting comments on this draft guidance through Sept. 7.
“Later this year, registration and listing of cosmetic product facilities and products will become a requirement, making information about cosmetic products, including the ingredients used in products and the facilities where they are produced, readily available to the agency,” Dr. Bumpus said.
The products, according to the FDA statement, include makeup, nail polishes, shaving creams, other grooming products, perfumes, face and body cleansers, hair products, moisturizers, and other skin care items.
MoCRA “represents a sea change in how FDA regulates the cosmetics industry,” attorneys Frederick R. Ball, Alyson Walker Lotman, and Kelly A. Bonner, wrote in an article for the Food and Drug Law Institute published in spring 2023.
The FDA has called the MoCRA law “the most significant expansion” of its authority to regulate cosmetics since the Federal Food, Drug, and Cosmetic Act was passed in 1938.
The agency is in the process of expanding its staff to carry out newly authorized duties, including the tracking of adverse events. The FDA budget request for fiscal 2024, which begins Oct. 1, seeks $5 million for work needed to implement MoCRA.
PFAS, or ‘forever chemicals’
Some of the requested FDA funding is intended to prepare the agency to assess the use of per-and polyfluoroalkyl substances (PFAS) in cosmetics.
MoCRA sets a 3-year deadline for the FDA to issue an assessment of the use and potential risks of PFAS in cosmetics products. PFAS are sometimes added as ingredients in some cosmetic products, including lotions, cleansers, nail polish, shaving cream, foundation, lipstick, eyeliner, eyeshadow, and mascara, according to the FDA. Sometimes the presence of PFAS in cosmetics is unintentional and is the result of impurities in raw materials or is due to the breakdown of ingredients, the FDA said.
The FDA’s website says that so far, the available research doesn’t allow for “definitive conclusions about the potential health risks of PFAS in cosmetics.”
The Centers for Disease Control and Prevention has stated that research has suggested potential links between high levels of certain PFAS, in general, with increased cholesterol levels, changes in liver enzyme levels, increased risk of hypertension or preeclampsia in pregnant women, and increased risk of kidney or testicular cancer.
PFAS compounds often are used to resist grease, oil, water, and heat in industrial settings. They are used in thousands of products, from nonstick cookware to firefighting foams and protective gear, because they can reduce friction, according to a National Academies of Sciences, Engineering, and Medicine report on PFAS that was issued last year.
PFAS are known as “forever chemicals” because they contain a carbon-fluorine bond, which does not break naturally. Even when PFAS are transformed in the body, they can assume other forms of PFAS that preserve the troublesome carbon-fluorine bond. With PFAS, the human body is confronted with a substance it doesn’t have the tools to process.
This is in contrast to proteins and carbohydrates, which are in a sense prepackaged for relatively easy disassembly in the human body. Many of these compounds have weak links that enzymes and stomach acid can take apart, such as sulfur-to-sulfur (disulfide) bonds. That’s why protein-based biotech drugs are injected instead of administered as pills. The ultimate goal of this digestion is for the body to gain energy from these compounds.
But with PFAS, the body faces the challenge of carbon-fluorine bonds that are very hard to break down, and there is no payoff for these efforts, Graham F. Peaslee, PhD, professor of physics at the University of Notre Dame (Indiana), told this news organization.
“Nothing will naturally eat it because when you break the bond, it’s like eating celery,” he said. “You use more calories to eat the celery than you gain back from it.”
Interest from a U.S. senator
Dr. Peaslee was one of the authors of a 2021 article about PFAS in cosmetics that appeared in the journal Environmental Science and Technology Letters.
In the article, Dr. Peaslee and colleagues reported on their screening of 231 cosmetic products purchased in the United States and Canada using particle-induced gamma-ray emission spectroscopy. They found cases of undisclosed PFAS in cosmetic products. Foundations, mascaras, and lip products were noted as being especially problematic.
Sen. Susan Collins (R-ME) cited Dr. Peaslee’s article in a 2021 floor speech as she argued for having the FDA ban the intentional addition of PFAS to cosmetics.
“The findings of this study are particularly alarming, as many of these products are subject to direct human exposure,” Sen. Collins said. “For example, lipstick is often inadvertently ingested, and mascara is sometimes absorbed through tear ducts.”
In addition, workers at cosmetics plants may be exposed to PFAS and discarded cosmetics that have these compounds, which could potentially contaminate drinking water, Sen. Collins said. In 2021, she introduced legislation seeking a ban on PFAS that are intentionally added to cosmetics. That legislation did not advance through the Senate.
But the Senate Appropriations Committee, on which Sen. Collins is the ranking Republican, wants the FDA to keep a ban on PFAS in mind.
The Senate Agriculture Appropriations subcommittee, which oversees the FDA’s budget, raised the issue of PFAS and cosmetics in a June report. The FDA should develop a plan outlining research needed to inform “regulatory decision making, including potential development of a proposed rule to ban intentionally added PFAS substances in cosmetics,” the subcommittee said.
A version of this article first appeared on Medscape.com.
FDA clears new capabilities for diabetes app BlueStar
The latest clearance, announced on Aug. 23, enables the app-based platform to provide bolus insulin dose recommendations that are based on glucose and trend data from a compatible continuous glucose monitoring (CGM) device. On Aug. 15, the FDA cleared the BlueStar to use connected insulin dosing data in personalized bolus insulin dosing recommendations.
“Welldoc is the first company to receive clearance for a CGM-informed bolus calculator specifically designed for adults who manage their diabetes with multiple daily injections of insulin,” according to a company statement.
“With this clearance, Welldoc is filling a significant gap for people who require complex insulin regimens. By connecting directly with CGM data and using both glucose values and trend arrows, the BlueStar solution will provide precise and in-the-moment insulin dosing guidance directly to individuals, helping them reach their glucose targets,” endocrinologist Grazia Aleppo, MD, of Northwestern University, Chicago, said in the statement.
The new features extend the platform’s existing digital diet and lifestyle coaching capabilities. Previous FDA clearances included expansions to use most types of available insulins, including bolus and premixed insulin titration for patients with type 2 diabetes, in September 2021 and for basal insulin adjustment in June 2020.
Dr. Aleppo was a principal investigator in Welldoc’s clinical validation study for BlueStar.
A version of this article first appeared on Medscape.com.
The latest clearance, announced on Aug. 23, enables the app-based platform to provide bolus insulin dose recommendations that are based on glucose and trend data from a compatible continuous glucose monitoring (CGM) device. On Aug. 15, the FDA cleared the BlueStar to use connected insulin dosing data in personalized bolus insulin dosing recommendations.
“Welldoc is the first company to receive clearance for a CGM-informed bolus calculator specifically designed for adults who manage their diabetes with multiple daily injections of insulin,” according to a company statement.
“With this clearance, Welldoc is filling a significant gap for people who require complex insulin regimens. By connecting directly with CGM data and using both glucose values and trend arrows, the BlueStar solution will provide precise and in-the-moment insulin dosing guidance directly to individuals, helping them reach their glucose targets,” endocrinologist Grazia Aleppo, MD, of Northwestern University, Chicago, said in the statement.
The new features extend the platform’s existing digital diet and lifestyle coaching capabilities. Previous FDA clearances included expansions to use most types of available insulins, including bolus and premixed insulin titration for patients with type 2 diabetes, in September 2021 and for basal insulin adjustment in June 2020.
Dr. Aleppo was a principal investigator in Welldoc’s clinical validation study for BlueStar.
A version of this article first appeared on Medscape.com.
The latest clearance, announced on Aug. 23, enables the app-based platform to provide bolus insulin dose recommendations that are based on glucose and trend data from a compatible continuous glucose monitoring (CGM) device. On Aug. 15, the FDA cleared the BlueStar to use connected insulin dosing data in personalized bolus insulin dosing recommendations.
“Welldoc is the first company to receive clearance for a CGM-informed bolus calculator specifically designed for adults who manage their diabetes with multiple daily injections of insulin,” according to a company statement.
“With this clearance, Welldoc is filling a significant gap for people who require complex insulin regimens. By connecting directly with CGM data and using both glucose values and trend arrows, the BlueStar solution will provide precise and in-the-moment insulin dosing guidance directly to individuals, helping them reach their glucose targets,” endocrinologist Grazia Aleppo, MD, of Northwestern University, Chicago, said in the statement.
The new features extend the platform’s existing digital diet and lifestyle coaching capabilities. Previous FDA clearances included expansions to use most types of available insulins, including bolus and premixed insulin titration for patients with type 2 diabetes, in September 2021 and for basal insulin adjustment in June 2020.
Dr. Aleppo was a principal investigator in Welldoc’s clinical validation study for BlueStar.
A version of this article first appeared on Medscape.com.
Gender-affirming surgeries nearly tripled between 2016 and 2019: Study
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).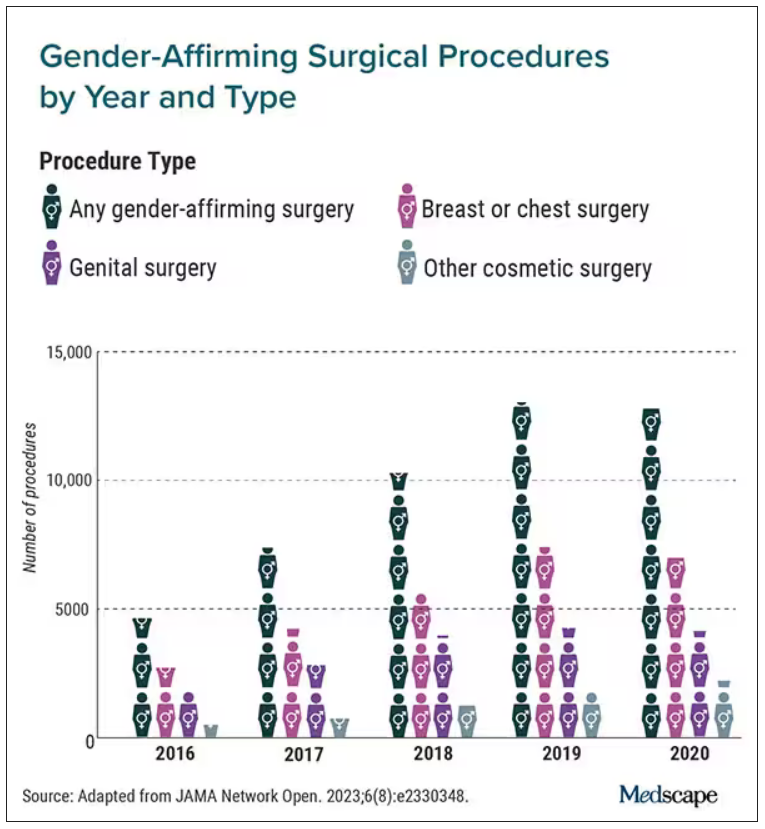
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
a new study published in JAMA Network Open found.
Breast and chest surgeries were the most common procedures performed, and the number of surgical procedures carried out increased with age. The researchers said that, in addition to legal shifts, the established safety of the surgeries and resulting increase in quality of life may also help explain the increase.
“The point of this is to raise awareness and to really document the patterns of care in the United States,” said Jason Wright, MD, an associate professor at Columbia University, New York. “We hope that people understand that these procedures are being performed more commonly and they’re out there.”
A study published in 2022 in JAMA Pediatrics found that the number of chest reconstruction surgeries among U.S. adolescents rose fourfold between 2016 and 2019.
The new study included data from 2016 to 2020 in the Nationwide Ambulatory Surgery Sample and the National Inpatient Sample. More than 48,000 patients with diagnosis codes for gender identity disorder, transsexualism, or a personal history of sex reassignment were identified. Age ranges were grouped as 12-18 (7.7%), 19-30 (52.3%), and 31-40 (21.8%).
The number of gender-affirming procedures rose from 4,552 in 2016 to a peak of 13,011 in 2019. (A slight decline to 12,818 procedures in 2020 was attributed to the COVID-19 pandemic.) The surgeries were grouped into three categories: breast and chest procedures, which occurred in 56.6% of patients; genital reconstructive surgeries (35.1%), and other facial cosmetic procedures (13.9%).
“We really wanted to try to make this as representative as we could,” Dr. Wright said. “I think this is really the best estimates that are available to date.”
Chest and breast procedures made up a higher percentage of surgeries in younger patients, while genital surgical procedures made up a higher percentage in older patients. For example, patients aged 19-30 made up 59.1% of breast or chest surgeries and 44.2% of genital surgeries. However, those aged 31-40 accounted for 26.2% of genital surgeries and 18.1% of breast or chest surgeries. For ages 41-50, the spread was more than double, accounting for 12.8% of genital surgeries and only 6.1% of breast or chest surgeries, according to the researchers.
Undocumented uptick
In addition to more inclusive health insurance, Dr. Wright said the increase in these procedures can also be attributed to studies showing their safety and the long-term association with high patient satisfaction.
Kevin Wang, MD, medical director of Providence–Swedish Health Services’ LGBTQIA+ program in Seattle, agreed that changes in health insurance coverage for gender-affirming surgery likely account in part for their increase. But he added that more clinicians are performing these procedures.
He said gender-affirming surgeries improve quality of life for the people who undergo them. The American Academy of Pediatrics has said it would be conducting a thorough review of the effects of transgender care on youth. A 2018 policy statement from the group said transgender youth should “have access to comprehensive, gender-affirming, and developmentally appropriate health care that is provided in a safe and inclusive clinical space.”
Dr. Wright cited several limitations to his group’s study that may result in the undercapture of transgender individuals and gender-affirming surgery; in particular, while the study captured inpatient and ambulatory surgical procedures in large, nationwide datasets, a small number of the procedures could have been performed in other settings.
Guiding a patient through gender-affirming care and surgical procedures can be an arduous process, including understanding their goals, using hormone therapy, and making referrals to specialists. Dr. Wang said he works to maximize his patients’ physical, mental, and emotional health, and helps them understand the risks.
He cited the double standard of a cisgender woman wanting breast augmentation without justification, but someone who identifies as transgender has many more boxes to check – for example, seeing a behavior health specialist to demonstrate they understand the risks and securing a letter of support from their primary care physician to undergo a similar procedure.
“It’s just interesting how the transgender community has to jump through so many more barriers and hoops for affirming, lifesaving procedures where you have other people who are doing it for aesthetic purposes and do not require any type of authorization,” Dr. Wang said.
Dr. Wright said he hopes the findings call attention to the need for more professionals working in the gender-affirming care field.
“I think for the medical community, it’s important to raise the idea that these procedures are becoming more common,” Dr. Wright said. “We are going to need specialists who have expertise in transgender care and surgeons who have the ability to perform these operations. Hopefully, this sheds light on the resources that are going to be required to care for these patients going forward.”
Dr. Wright reported receiving grants from Merck and personal fees from UpToDate outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Medicare announces 10 drugs targeted for price cuts in 2026
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
Even an hour’s walk a week lowers risk in type 2 diabetes
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
although the impact on retinopathy is weaker, reveals a cohort study of U.K. individuals.
The research, based on data from more than 18,000 participants in the U.K. Biobank, suggests that the minimal level of self-reported activity to reduce the risk for both neuropathy and nephropathy may be the equivalent of less than 1.5 hours of walking per week.
The results are “encouraging and reassuring for both physicians and patients,” lead author Frederik P.B. Kristensen, MSc, PhD student, department of clinical epidemiology, Aarhus (Denmark) University, said in an interview.
“Our findings are particularly promising for neuropathy since, currently, no disease-modifying treatment exists, and there are limited preventive strategies available.”
Mr. Kristensen highlighted that “most diabetes research has focused on all-cause mortality and macrovascular complications. In the current study, we also found the same pattern for microvascular complications: Even small amounts of physical activity will benefit your health status.”
The minimal level of activity they identified, he said, is also an “achievable [goal] for most type 2 diabetes patients.”
Mr. Kristensen added, however, that the study was limited by excluding individuals with limited mobility and those living in temporary accommodation or care homes.
And prospective studies are required to determine the dose-response relationship between total, not just leisure-time, activity – ideally measured objectively – and risk for microvascular complications, he observed.
The research was published recently in Diabetes Care.
Impact of exercise on microvascular complications in T2D has been uncertain
The authors point out that microvascular complications – such as nerve damage (neuropathy), kidney problems (nephropathy), and eye complications (retinopathy) – occur in more than 50% of individuals with type 2 diabetes and have a “substantial impact” on quality of life, on top of the impact of macrovascular complications (such as cardiovascular disease), disability, and mortality.
Although physical activity is seen as a “cornerstone in the multifactorial management of type 2 diabetes because of its beneficial effects on metabolic risk factors,” the impact on microvascular complications is “uncertain” and the evidence is limited and “conflicting.”
The researchers therefore sought to examine the dose-response association, including the minimal effective level, between leisure-time physical activity and neuropathy, nephropathy, and retinopathy.
They conducted a cohort study of individuals aged 37-82 years from the U.K. Biobank who had type 2 diabetes, which was identified using the Eastwood algorithm and/or an A1c greater than or equal to 48 mmol/mol (6.5%).
Individuals with type 1 diabetes or gestational diabetes were excluded, as were those with major disabling somatic disorders, neurodegenerative diseases, and mental disorders, among others.
Leisure-time physical activity was based on the self-reported frequency, duration, and types of physical activities and was combined to calculate the total leisure time activity in MET-hours per week.
Using the American Diabetes Association/World Health Organization recommendations of 150-300 minutes of moderate to vigorous leisure-time physical activity per week, the researchers determined the recommended moderate activity level to be 150 minutes, (equivalent to 2.5 hours, or 7.5 MET-hours per week).
In all, 18,092 individuals with type 2 diabetes were included in the analysis, of whom 37% were women. The mean age was 60 years.
Ten percent of participants performed no leisure-time physical activity, 38% performed activity below the threshold for moderate activity, 20% performed at the recommended level, and 32% were more active.
Those performing no physical activity were more likely to be women, to be younger, to have a higher body mass index, and to have a greater average A1c, as well as have a more unfavorable sociodemographic and behavioral profile.
Over a median follow-up of 12.1 years, 3.7% of the participants were diagnosed with neuropathy, 10.2% with nephropathy, and 11.7% with retinopathy, equating to an incidence per 1,000 person-years of 3.5, 9,8, and 11.4, respectively.
The researchers found that any level of physical activity was associated with an approximate reduction in the risk for neuropathy and nephropathy.
Multivariate analysis indicated that, compared with no physical activity, activity below the recommended level was associated with an adjusted hazard ratio (aHR) for neuropathy of 0.71, whereas the aHR for activity at the recommended level was 0.73 and that for activity above the recommended level was 0.67.
The aHR for nephropathy, compared with no physical activity, was 0.79 for activity below the recommended level, 0.80 for activity at the recommended level, and 0.80 for activity above the recommended level.
The association between physical activity and retinopathy was weaker, however, at aHRs of 0.91, 0.91, and 0.98 for activity below, at, and above the recommended level, respectively.
The researchers suggest that this lower association could be due to differences in the etiology of the different forms of microvascular complications.
Hyperglycemia is the key driver in the development of retinopathy, they note, whereas other metabolic risk factors, such as obesity, insulin resistance, inflammation, dyslipidemia, and hypertension, play a role in neuropathy and nephropathy.
The associations were also less pronounced in women.
Mr. Kristensen said that this is “an important area that needs to be addressed.”
“While different rates between men and women regarding incidence of type 2 diabetes, metabolic risk factors, complications, and the initiation of, and adherence to, therapy have been found,” he continued, “the exact mechanisms remain unclear. We need a further understanding of sex-differences in metabolic regulation, as well as in material living conditions, social and psychological factors, and access to health care, which may influence the risk of complications.”
Mr. Kristensen added, “Sex differences may be present in more areas than we are aware.”
Mr. Kristensen is supported by a PhD grant from Aarhus University. Other authors received funding from the Danish Diabetes Association, the Australian National Health and Medical Research Council, the New South Wales Government, the Spanish Ministry of Universities, the European Union NextGenerationEU/PRTR (Plan de Recuperación) through a Margarita Salas contract of the University of Vigo, and the Government of Andalusia, Research Talent Recruitment Programme. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM DIABETES CARE
More weight loss linked with more benefit in STEP-HFpEF
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , including symptoms and physical limitations, exercise capacity, and inflammation, new analyses from the trial show.
At the annual congress of the European Society of Cardiology where he presented these new findings, Mikhail N. Kosiborod, MD, also posited that weight loss produced by weekly subcutaneous injections of 2.4 mg semaglutide (Wegovy) for 52 weeks in the study does not fully explain the multiple mechanisms that may be involved in producing this intervention’s effects in the STEP-HFpEF trial.
His report earlier at the congress and in a simultaneously published report of the trial’s primary outcomes established a role for medically induced weight loss in managing patients with obesity-phenotype HFpEF in a total of 529 randomized individuals with HFpEF and obesity but without diabetes.
The new analyses showed that for one of the two primary endpoints – the change from baseline in patients’ assessment on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ), the placebo-adjusted average change was a 16.1-point improvement in the 51 people with a 5%-10% weight loss during the 1-year study, and a 21.6-point improvement in the 58 who had at least a 20% weight loss, a between-group average 5.5 point difference that represents a clinically meaningful incremental improvement in this validated metric of symptoms and functional limitations.
Similar weight-related differences in benefit also occurred for the secondary outcomes of changes from baseline in 6-minute walk distance and in levels of C-reactive protein (CRP), a measure of systemic inflammation.
In an adjusted regression model, every 10% drop from baseline body weight was significantly linked with a 6.4-point improvement in KCCQ score, a 14.4 meter improvement in 6-minute walk distance, and a 28% relative reduction from baseline in CRP, reported Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
These new, prespecified analyses also showed that people with obesity and HFpEF responded roughly the same to semaglutide treatment compared with placebo-treated controls regardless of their starting body mass index, including people with class 1 (30-34 kg/m2), class 2 (35-39 kg/m2), and class 3 (≥ 40 kg/m2) obesity.
Simultaneously with Dr. Kosiborod’s report at the congress, these findings appeared in a report posted online in Nature Medicine.
Not every benefit was fully mediated by weight loss
These analyses “do not tell us how much of the benefit was mediated by weight loss, but the data do say that the more weight a person lost, the more benefit they got,” Dr. Kosiborod explained in an interview. “That is not the same as saying that everything is mediated by weight. It doesn’t say that nothing beyond weight loss matters.”
He and his associates are planning a mediation analysis of data from STEP-HFpEF that will more directly address this issue.
“It’s likely that people who lost more weight with semaglutide also had greater benefits from other effects of semaglutide at the same time. Weight loss is a good surrogate marker” for the range of effects that a person receives from treatment with semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, Dr. Kosiborod said.
“GLP-1 receptor agonists may have direct effects on atherosclerosis, as well as other effects that are uncoupled from weight loss,” such as proven anti-inflammatory effects, he added.
Another exploratory effect from semaglutide treatment in the study and reported by Dr. Kosiborod was a significant reduction in serum levels of N-terminal pro brain natriuretic peptide, an association never previously seen with weight loss in people with heart failure.
“The outcomes we’ve already seen in STEP-HFpEF were largely symptomatic, which are extraordinarily important, but there may be a completely different relationship between weight and clinical events,” said John E. Deanfield, PhD, a professor of cardiology at University College Hospital, London, who was not involved in the study.
Dr. Deanfield noted that important prognostic markers such as cholesterol levels and blood pressure reductions are usually not temporally related to weight loss. “The idea that [the benefits seen in STEP-HFpEF] are purely from weight loss is something we need to be careful about,” he said.
“My gut feeling is that at least 75% of the effect [in STEP-HFpEF} was due to weight loss,” said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow, who was not associated with the research.
STEP-HFpEF was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to, and has received honoraria from, Novo Nordisk. He has been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Deanfield has been a consultant to Novo Nordisk as well as to Aegerion, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Sanofi, and Takeda, and has received research funding from Aegerion, Colgate, MSD, Pfizer, and Roche. Dr. Sattar has been a consultant to Novo Nordisk as well as to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Pfizer, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
Both too much and not enough sleep raises T2D risk
TOPLINE:
suggests an analysis of a Dutch study.
METHODOLOGY:
- Data on 5,561 participants aged 40–75 years from The Maastricht Study who completed the baseline survey between November 2010 and January 2018 and had full data available were included.
- Sleep duration was assessed as the in-bed time in minutes, using a median of 7 nights’ data from an activPAL3 (PAL Technologies) accelerometer, which is worn on the thigh.
- Glucose metabolism was determined via an oral glucose tolerance test and categorized as prediabetes or type 2 diabetes in line with World Health Organization diagnostic criteria.
- The association between sleep duration and type 2 diabetes was assessed on multivariate logistic regression analysis, taking into account a range of potential confounding factors.
TAKEAWAY:
- The mean age of the participants was 60.1 years, and there was an even split between men and women. In all, 832 had prediabetes and 1,341 type 2 diabetes, and the mean sleep duration was 8.3 hours.
- The results indicated there was a U-shaped relationship between sleep duration and type 2 diabetes, so that both long and short sleep durations increased the risk.
- In the fully adjusted model, a sleep duration of 5 hours was associated with an odds ratio for type 2 diabetes versus 8 hours sleep of 2.9. For a sleep duration of 12 hours, the odds ratio was 1.8.
- The association between sleep duration and diabetes was not significant.
IN PRACTICE:
The results “support the idea that sleep duration could be a relevant risk factor for type 2 diabetes independent of lifestyle risk factors, including diet, physical activity, smoking behavior, and alcohol consumption,” wrote the authors.
“These findings underpin the importance of promoting healthy sleep habits to avoid sleep deprivation,” they added.
STUDY DETAILS:
The research was led by Jeroen D. Albers, MSc, department of social medicine, Maastricht (the Netherlands) University, and published in Sleep Health. It is an analysis of The Maastricht Study.
LIMITATIONS:
The study is limited by its cross-sectional nature, particularly because there are “plausible causal paths between sleep duration and type 2 in both directions,” the authors note. The accelerometer used in the study also cannot reliably distinguish between waking and sleeping time in bed, with the potential for misclassification. Daytime naps were also not included, and long-term changes sleep patterns were not measured. In addition, it was not possible to control for some potential confounding factors.
DISCLOSURES:
The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs, Stichting De Weijerhorst, the Pearl String Initiative Diabetes, the School for Cardiovascular Diseases, the School for Public Health and Primary Care, the School for Nutrition and Translational Research in Metabolism, Stichting Annadal, Health Foundation Limburg, and unrestricted grants from Janssen-Cilag, Novo Nordisk, and Sanofi Aventis Netherlands. One author declares a relationship with Novo Nordisk outside the submitted work. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
TOPLINE:
suggests an analysis of a Dutch study.
METHODOLOGY:
- Data on 5,561 participants aged 40–75 years from The Maastricht Study who completed the baseline survey between November 2010 and January 2018 and had full data available were included.
- Sleep duration was assessed as the in-bed time in minutes, using a median of 7 nights’ data from an activPAL3 (PAL Technologies) accelerometer, which is worn on the thigh.
- Glucose metabolism was determined via an oral glucose tolerance test and categorized as prediabetes or type 2 diabetes in line with World Health Organization diagnostic criteria.
- The association between sleep duration and type 2 diabetes was assessed on multivariate logistic regression analysis, taking into account a range of potential confounding factors.
TAKEAWAY:
- The mean age of the participants was 60.1 years, and there was an even split between men and women. In all, 832 had prediabetes and 1,341 type 2 diabetes, and the mean sleep duration was 8.3 hours.
- The results indicated there was a U-shaped relationship between sleep duration and type 2 diabetes, so that both long and short sleep durations increased the risk.
- In the fully adjusted model, a sleep duration of 5 hours was associated with an odds ratio for type 2 diabetes versus 8 hours sleep of 2.9. For a sleep duration of 12 hours, the odds ratio was 1.8.
- The association between sleep duration and diabetes was not significant.
IN PRACTICE:
The results “support the idea that sleep duration could be a relevant risk factor for type 2 diabetes independent of lifestyle risk factors, including diet, physical activity, smoking behavior, and alcohol consumption,” wrote the authors.
“These findings underpin the importance of promoting healthy sleep habits to avoid sleep deprivation,” they added.
STUDY DETAILS:
The research was led by Jeroen D. Albers, MSc, department of social medicine, Maastricht (the Netherlands) University, and published in Sleep Health. It is an analysis of The Maastricht Study.
LIMITATIONS:
The study is limited by its cross-sectional nature, particularly because there are “plausible causal paths between sleep duration and type 2 in both directions,” the authors note. The accelerometer used in the study also cannot reliably distinguish between waking and sleeping time in bed, with the potential for misclassification. Daytime naps were also not included, and long-term changes sleep patterns were not measured. In addition, it was not possible to control for some potential confounding factors.
DISCLOSURES:
The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs, Stichting De Weijerhorst, the Pearl String Initiative Diabetes, the School for Cardiovascular Diseases, the School for Public Health and Primary Care, the School for Nutrition and Translational Research in Metabolism, Stichting Annadal, Health Foundation Limburg, and unrestricted grants from Janssen-Cilag, Novo Nordisk, and Sanofi Aventis Netherlands. One author declares a relationship with Novo Nordisk outside the submitted work. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
TOPLINE:
suggests an analysis of a Dutch study.
METHODOLOGY:
- Data on 5,561 participants aged 40–75 years from The Maastricht Study who completed the baseline survey between November 2010 and January 2018 and had full data available were included.
- Sleep duration was assessed as the in-bed time in minutes, using a median of 7 nights’ data from an activPAL3 (PAL Technologies) accelerometer, which is worn on the thigh.
- Glucose metabolism was determined via an oral glucose tolerance test and categorized as prediabetes or type 2 diabetes in line with World Health Organization diagnostic criteria.
- The association between sleep duration and type 2 diabetes was assessed on multivariate logistic regression analysis, taking into account a range of potential confounding factors.
TAKEAWAY:
- The mean age of the participants was 60.1 years, and there was an even split between men and women. In all, 832 had prediabetes and 1,341 type 2 diabetes, and the mean sleep duration was 8.3 hours.
- The results indicated there was a U-shaped relationship between sleep duration and type 2 diabetes, so that both long and short sleep durations increased the risk.
- In the fully adjusted model, a sleep duration of 5 hours was associated with an odds ratio for type 2 diabetes versus 8 hours sleep of 2.9. For a sleep duration of 12 hours, the odds ratio was 1.8.
- The association between sleep duration and diabetes was not significant.
IN PRACTICE:
The results “support the idea that sleep duration could be a relevant risk factor for type 2 diabetes independent of lifestyle risk factors, including diet, physical activity, smoking behavior, and alcohol consumption,” wrote the authors.
“These findings underpin the importance of promoting healthy sleep habits to avoid sleep deprivation,” they added.
STUDY DETAILS:
The research was led by Jeroen D. Albers, MSc, department of social medicine, Maastricht (the Netherlands) University, and published in Sleep Health. It is an analysis of The Maastricht Study.
LIMITATIONS:
The study is limited by its cross-sectional nature, particularly because there are “plausible causal paths between sleep duration and type 2 in both directions,” the authors note. The accelerometer used in the study also cannot reliably distinguish between waking and sleeping time in bed, with the potential for misclassification. Daytime naps were also not included, and long-term changes sleep patterns were not measured. In addition, it was not possible to control for some potential confounding factors.
DISCLOSURES:
The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs, Stichting De Weijerhorst, the Pearl String Initiative Diabetes, the School for Cardiovascular Diseases, the School for Public Health and Primary Care, the School for Nutrition and Translational Research in Metabolism, Stichting Annadal, Health Foundation Limburg, and unrestricted grants from Janssen-Cilag, Novo Nordisk, and Sanofi Aventis Netherlands. One author declares a relationship with Novo Nordisk outside the submitted work. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM SLEEP HEALTH
ESC backs SGLT2 inhibitor plus GLP-1 in diabetes with high CVD risk
AMSTERDAM – The era of guidelines that recommended treatment with either a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or a glucagonlike peptide-1 (GLP-1) receptor agonist in people with type 2 diabetes mellitus and established cardiovascular disease (CVD) ended with new recommendations from the European Society of Cardiology that call for starting both classes simultaneously.
said Darren K. McGuire, MD, at the annual congress of the European Society of Cardiology.
The society’s new guidelines for managing CVD in patients with diabetes, released on Aug. 25 and presented in several sessions at the Congress, also break with the past by calling for starting treatment with both an SGLT-2 inhibitor and a GLP-1 receptor agonist without regard to a person’s existing level of glucose control, including their current and target hemoglobin A1c levels, and regardless of background therapy, added Dr. McGuire, a cardiologist and professor at the UT Southwestern Medical Center in Dallas and a member of the ESC panel that wrote the new guidelines.
Instead, the new guidance calls for starting both drug classes promptly in people diagnosed with type 2 diabetes and established atherosclerotic CVD.
Both the previous ESC guidelines from 2019 as well as the current Standards of Care for 2023 document from the American Diabetes Association call for using one class or the other, but they hedge on combined treatment as discretionary.
Different mechanisms mean additive benefits
“With increasing numbers of patients with type 2 diabetes in trials for SGLT-2 inhibitors or GLP-1 receptor agonists who were also on the other drug class, we’ve done large, stratified analyses that suggest no treatment-effect modification” when people received agents from both drug classes, Dr. McGuire explained in an interview. “While we don’t understand the mechanisms of action of these drugs for CVD, we’ve become very confident that they use different mechanisms” that appear to have at least partially additive effects.
“Their benefits for CVD risk reduction are completely independent of their glucose effects. They are cardiology drugs,” Dr. McGuire added.
The new ESC guidelines highlight two other clinical settings where people with type 2 diabetes should receive an SGLT-2 inhibitor regardless of their existing level of glucose control and any other medical treatment: people with heart failure and people with chronic kidney disease (CKD) based on a depressed estimated glomerular filtration rate and an elevated urine albumin-to-creatinine ratio.
Nephropathy was considered by the ESC’s guideline panel to confer risk that is similar to that of established atherosclerotic CVD, Dr. McGuire said.
The guidelines also, for the first time for ESC recommendations, made treatment with finerenone (Kerendia, Bayer) a class 1 level A recommendation for people with type 2 diabetes and CKD.
SCORE2-Diabetes risk estimator
Another major change in the new ESC guideline revision is introduction of a CVD risk calculator intended to estimate the risk among people with type 2 diabetes but without established CVD, heart failure, or CKD.
Called the SCORE2-Diabetes risk estimator, it calculates a person’s 10-year risk for CVD and includes adjustment based on the European region where a person lives; it also tallies different risk levels for women and for men.
The researchers who developed the SCORE2-Diabetes calculator used data from nearly 230,000 people to devise the tool and then validated it with data from an additional 217,000 Europeans with type 2 diabetes.
Key features of the calculator include its use of routinely collected clinical values, such as age, sex, systolic blood pressure, smoking status, serum cholesterol levels, age at diabetes diagnosis, hemoglobin A1c level, and estimated glomerular filtration rate.
“For the first time we have a clear score to categorize risk” in people with type 2 diabetes and identify who needs more aggressive treatment to prevent CVD development,” said Emanuele Di Angelantonio, MD, PhD, deputy director of the cardiovascular epidemiology unit at the University of Cambridge (England).
The guidelines say that people who have a low (< 5%) or moderate (5%-9%) 10-year risk for CVD are possible candidates for metformin treatment. Those with high (10%-19%) or very high (≥ 20%) risk are possible candidates for treatment with metformin and/or an SGLT-2 inhibitor and/or a GLP-1 receptor agonist, said Dr. Di Angelantonio during his talk at the congress on the new risk score.
“The risk score is a good addition” because it estimates future CVD risk better and more systematically than usual practice, which generally relies on no systematic tool, said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow (Scotland) and also a member of the guideline-writing panel.
The new risk score “is a reasonable way” to identify people without CVD but at elevated risk who might benefit from treatment with a relatively expensive drug, such as an SGLT-2 inhibitor, Dr. Sattar said in an interview. “It doesn’t rely on any fancy biomarkers or imaging, and it takes about 30 seconds to calculate. It’s not perfect, but it gets the job done,” and it will increase the number of people with type 2 diabetes who will receive an SGLT-2 inhibitor, he predicted.
Dr. McGuire has been a consultant to Altimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer-Ingelheim, Intercept, Lexion, Lilly, Merck, New Amsterdam, and Pfizer. Dr. Di Angelantonio had no disclosures. Dr. Sattar has been a consultant to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, Pfizer, and Roche Diagnostics.
A version of this article appeared on Medscape.com.
AMSTERDAM – The era of guidelines that recommended treatment with either a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or a glucagonlike peptide-1 (GLP-1) receptor agonist in people with type 2 diabetes mellitus and established cardiovascular disease (CVD) ended with new recommendations from the European Society of Cardiology that call for starting both classes simultaneously.
said Darren K. McGuire, MD, at the annual congress of the European Society of Cardiology.
The society’s new guidelines for managing CVD in patients with diabetes, released on Aug. 25 and presented in several sessions at the Congress, also break with the past by calling for starting treatment with both an SGLT-2 inhibitor and a GLP-1 receptor agonist without regard to a person’s existing level of glucose control, including their current and target hemoglobin A1c levels, and regardless of background therapy, added Dr. McGuire, a cardiologist and professor at the UT Southwestern Medical Center in Dallas and a member of the ESC panel that wrote the new guidelines.
Instead, the new guidance calls for starting both drug classes promptly in people diagnosed with type 2 diabetes and established atherosclerotic CVD.
Both the previous ESC guidelines from 2019 as well as the current Standards of Care for 2023 document from the American Diabetes Association call for using one class or the other, but they hedge on combined treatment as discretionary.
Different mechanisms mean additive benefits
“With increasing numbers of patients with type 2 diabetes in trials for SGLT-2 inhibitors or GLP-1 receptor agonists who were also on the other drug class, we’ve done large, stratified analyses that suggest no treatment-effect modification” when people received agents from both drug classes, Dr. McGuire explained in an interview. “While we don’t understand the mechanisms of action of these drugs for CVD, we’ve become very confident that they use different mechanisms” that appear to have at least partially additive effects.
“Their benefits for CVD risk reduction are completely independent of their glucose effects. They are cardiology drugs,” Dr. McGuire added.
The new ESC guidelines highlight two other clinical settings where people with type 2 diabetes should receive an SGLT-2 inhibitor regardless of their existing level of glucose control and any other medical treatment: people with heart failure and people with chronic kidney disease (CKD) based on a depressed estimated glomerular filtration rate and an elevated urine albumin-to-creatinine ratio.
Nephropathy was considered by the ESC’s guideline panel to confer risk that is similar to that of established atherosclerotic CVD, Dr. McGuire said.
The guidelines also, for the first time for ESC recommendations, made treatment with finerenone (Kerendia, Bayer) a class 1 level A recommendation for people with type 2 diabetes and CKD.
SCORE2-Diabetes risk estimator
Another major change in the new ESC guideline revision is introduction of a CVD risk calculator intended to estimate the risk among people with type 2 diabetes but without established CVD, heart failure, or CKD.
Called the SCORE2-Diabetes risk estimator, it calculates a person’s 10-year risk for CVD and includes adjustment based on the European region where a person lives; it also tallies different risk levels for women and for men.
The researchers who developed the SCORE2-Diabetes calculator used data from nearly 230,000 people to devise the tool and then validated it with data from an additional 217,000 Europeans with type 2 diabetes.
Key features of the calculator include its use of routinely collected clinical values, such as age, sex, systolic blood pressure, smoking status, serum cholesterol levels, age at diabetes diagnosis, hemoglobin A1c level, and estimated glomerular filtration rate.
“For the first time we have a clear score to categorize risk” in people with type 2 diabetes and identify who needs more aggressive treatment to prevent CVD development,” said Emanuele Di Angelantonio, MD, PhD, deputy director of the cardiovascular epidemiology unit at the University of Cambridge (England).
The guidelines say that people who have a low (< 5%) or moderate (5%-9%) 10-year risk for CVD are possible candidates for metformin treatment. Those with high (10%-19%) or very high (≥ 20%) risk are possible candidates for treatment with metformin and/or an SGLT-2 inhibitor and/or a GLP-1 receptor agonist, said Dr. Di Angelantonio during his talk at the congress on the new risk score.
“The risk score is a good addition” because it estimates future CVD risk better and more systematically than usual practice, which generally relies on no systematic tool, said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow (Scotland) and also a member of the guideline-writing panel.
The new risk score “is a reasonable way” to identify people without CVD but at elevated risk who might benefit from treatment with a relatively expensive drug, such as an SGLT-2 inhibitor, Dr. Sattar said in an interview. “It doesn’t rely on any fancy biomarkers or imaging, and it takes about 30 seconds to calculate. It’s not perfect, but it gets the job done,” and it will increase the number of people with type 2 diabetes who will receive an SGLT-2 inhibitor, he predicted.
Dr. McGuire has been a consultant to Altimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer-Ingelheim, Intercept, Lexion, Lilly, Merck, New Amsterdam, and Pfizer. Dr. Di Angelantonio had no disclosures. Dr. Sattar has been a consultant to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, Pfizer, and Roche Diagnostics.
A version of this article appeared on Medscape.com.
AMSTERDAM – The era of guidelines that recommended treatment with either a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or a glucagonlike peptide-1 (GLP-1) receptor agonist in people with type 2 diabetes mellitus and established cardiovascular disease (CVD) ended with new recommendations from the European Society of Cardiology that call for starting both classes simultaneously.
said Darren K. McGuire, MD, at the annual congress of the European Society of Cardiology.
The society’s new guidelines for managing CVD in patients with diabetes, released on Aug. 25 and presented in several sessions at the Congress, also break with the past by calling for starting treatment with both an SGLT-2 inhibitor and a GLP-1 receptor agonist without regard to a person’s existing level of glucose control, including their current and target hemoglobin A1c levels, and regardless of background therapy, added Dr. McGuire, a cardiologist and professor at the UT Southwestern Medical Center in Dallas and a member of the ESC panel that wrote the new guidelines.
Instead, the new guidance calls for starting both drug classes promptly in people diagnosed with type 2 diabetes and established atherosclerotic CVD.
Both the previous ESC guidelines from 2019 as well as the current Standards of Care for 2023 document from the American Diabetes Association call for using one class or the other, but they hedge on combined treatment as discretionary.
Different mechanisms mean additive benefits
“With increasing numbers of patients with type 2 diabetes in trials for SGLT-2 inhibitors or GLP-1 receptor agonists who were also on the other drug class, we’ve done large, stratified analyses that suggest no treatment-effect modification” when people received agents from both drug classes, Dr. McGuire explained in an interview. “While we don’t understand the mechanisms of action of these drugs for CVD, we’ve become very confident that they use different mechanisms” that appear to have at least partially additive effects.
“Their benefits for CVD risk reduction are completely independent of their glucose effects. They are cardiology drugs,” Dr. McGuire added.
The new ESC guidelines highlight two other clinical settings where people with type 2 diabetes should receive an SGLT-2 inhibitor regardless of their existing level of glucose control and any other medical treatment: people with heart failure and people with chronic kidney disease (CKD) based on a depressed estimated glomerular filtration rate and an elevated urine albumin-to-creatinine ratio.
Nephropathy was considered by the ESC’s guideline panel to confer risk that is similar to that of established atherosclerotic CVD, Dr. McGuire said.
The guidelines also, for the first time for ESC recommendations, made treatment with finerenone (Kerendia, Bayer) a class 1 level A recommendation for people with type 2 diabetes and CKD.
SCORE2-Diabetes risk estimator
Another major change in the new ESC guideline revision is introduction of a CVD risk calculator intended to estimate the risk among people with type 2 diabetes but without established CVD, heart failure, or CKD.
Called the SCORE2-Diabetes risk estimator, it calculates a person’s 10-year risk for CVD and includes adjustment based on the European region where a person lives; it also tallies different risk levels for women and for men.
The researchers who developed the SCORE2-Diabetes calculator used data from nearly 230,000 people to devise the tool and then validated it with data from an additional 217,000 Europeans with type 2 diabetes.
Key features of the calculator include its use of routinely collected clinical values, such as age, sex, systolic blood pressure, smoking status, serum cholesterol levels, age at diabetes diagnosis, hemoglobin A1c level, and estimated glomerular filtration rate.
“For the first time we have a clear score to categorize risk” in people with type 2 diabetes and identify who needs more aggressive treatment to prevent CVD development,” said Emanuele Di Angelantonio, MD, PhD, deputy director of the cardiovascular epidemiology unit at the University of Cambridge (England).
The guidelines say that people who have a low (< 5%) or moderate (5%-9%) 10-year risk for CVD are possible candidates for metformin treatment. Those with high (10%-19%) or very high (≥ 20%) risk are possible candidates for treatment with metformin and/or an SGLT-2 inhibitor and/or a GLP-1 receptor agonist, said Dr. Di Angelantonio during his talk at the congress on the new risk score.
“The risk score is a good addition” because it estimates future CVD risk better and more systematically than usual practice, which generally relies on no systematic tool, said Naveed Sattar, PhD, professor of metabolic medicine at the University of Glasgow (Scotland) and also a member of the guideline-writing panel.
The new risk score “is a reasonable way” to identify people without CVD but at elevated risk who might benefit from treatment with a relatively expensive drug, such as an SGLT-2 inhibitor, Dr. Sattar said in an interview. “It doesn’t rely on any fancy biomarkers or imaging, and it takes about 30 seconds to calculate. It’s not perfect, but it gets the job done,” and it will increase the number of people with type 2 diabetes who will receive an SGLT-2 inhibitor, he predicted.
Dr. McGuire has been a consultant to Altimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer-Ingelheim, Intercept, Lexion, Lilly, Merck, New Amsterdam, and Pfizer. Dr. Di Angelantonio had no disclosures. Dr. Sattar has been a consultant to Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, Pfizer, and Roche Diagnostics.
A version of this article appeared on Medscape.com.
AT ESC CONGRESS 2023









