User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Yoga and other mind-body work good for diabetes control
Mind and body practices, especially yoga, improve glycemic control in type 2 diabetes to a similar extent as medications such as metformin, new research shows.
“To our knowledge, this is the first study that has looked across different modalities of mind-body interventions and the first to show that there is a very consistent effect on A1c regardless of which modality you use,” senior author, Richard Watanabe, PhD, professor of biostatistics, Keck School of Medicine of the University of Southern California, Los Angeles, told this news organization.
“[Because] our study showed that it doesn’t matter which type of intervention patients do, it’s really up to the physician to work with their patients and help them pick something that works for them,” he added.
“Thus, this really is a much more flexible tool than having to tell a patient they should do yoga if their schedule doesn’t allow them to do yoga. There are other options available, so if you are a busy person and getting yourself to a yoga session is not doable, take a little time to learn about meditation and you can do it anywhere,” he said.
The study was published online, in the Journal of Integrative and Complementary Medicine, by Fatimata Sanogo, PhD candidate, also of Keck School of Medicine, USC, and colleagues.
Regularity of yoga practice makes the difference
A total of 28 studies of patients with type 2 diabetes published between 1993 and 2022 were included in the meta-analysis. In all studies, patients who were taking insulin or had any medical complications of diabetes were excluded.
A significant mean reduction in A1c of 0.84% was observed across the board for all types of mindfulness interventions (P < .0001).
For mindfulness-based stress reduction, A1c was reduced by 0.48% (P = 0.03), while the practice of qigong – a coordinated body-posture movement – was associated with a 0.66% drop in A1c (P = .01). For meditation, A1c dropped by 0.50% (P = .64).
However, the largest drop in A1c was seen with yoga, where it fell by 1.00% (P < .0001) – about the same degree of glycemic control achieved with metformin, the authors point out.
Indeed, for every additional day of yoga practiced per week, mean A1c differed by –0.22% (P = .46) between those who engaged in mind-body interventions and those who did not.
There was also a reduction in fasting blood glucose (FBG) with yoga and other practices. “The mean change in FBG was consistent with the mean change in A1c at –22.81 mg/dL (P < .0001),” the authors continue.
The researchers found that the duration of yoga didn’t matter but the frequency did, so it’s the regularity “with which you do yoga that makes the difference,” Dr. Watanabe said.
Dr. Watanabe and his coauthors also point out that because most patients were actively receiving metformin before and throughout the studies, the observed effect of mind and body practices on A1c represents an additional reduction beyond that of medication.
“This raises the question [as to] whether mind and body practices could be useful when initiated early in the course of diabetes therapy along with conventional lifestyle treatments,” they suggest.
While more research is needed to study this specifically, “our results suggest that these mind-body practices might be a good preventative measure,” Dr. Watanabe noted. Mind-body practices may also effectively prevent type 2 diabetes in at-risk patients, the authors propose.
Does meditation help alleviate psychological distress?
How mind-body practices work to improve glycemic control isn’t clear, but one possible theory is that patients experience a decrease in psychological distress when they undertake such practices and in so doing, may be more compliant with their prescribed treatment regimen.
A few of the studies analyzed showed that mind-body work resulted in a significant decrease in serum cortisol, the stress hormone that could plausibly mediate the benefit of mind and body practices through reduced inflammation.
In addition, “people with diabetes live with what we call ‘diabetes distress,’ ” Dr. Watanabe explained.
“Management of blood glucose is very stressful. You have to watch what you eat, you have to measure your glucose, and for the average person, that gets stressful. And that stress just contributes to the difficulty of controlling blood glucose,” he noted.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mind and body practices, especially yoga, improve glycemic control in type 2 diabetes to a similar extent as medications such as metformin, new research shows.
“To our knowledge, this is the first study that has looked across different modalities of mind-body interventions and the first to show that there is a very consistent effect on A1c regardless of which modality you use,” senior author, Richard Watanabe, PhD, professor of biostatistics, Keck School of Medicine of the University of Southern California, Los Angeles, told this news organization.
“[Because] our study showed that it doesn’t matter which type of intervention patients do, it’s really up to the physician to work with their patients and help them pick something that works for them,” he added.
“Thus, this really is a much more flexible tool than having to tell a patient they should do yoga if their schedule doesn’t allow them to do yoga. There are other options available, so if you are a busy person and getting yourself to a yoga session is not doable, take a little time to learn about meditation and you can do it anywhere,” he said.
The study was published online, in the Journal of Integrative and Complementary Medicine, by Fatimata Sanogo, PhD candidate, also of Keck School of Medicine, USC, and colleagues.
Regularity of yoga practice makes the difference
A total of 28 studies of patients with type 2 diabetes published between 1993 and 2022 were included in the meta-analysis. In all studies, patients who were taking insulin or had any medical complications of diabetes were excluded.
A significant mean reduction in A1c of 0.84% was observed across the board for all types of mindfulness interventions (P < .0001).
For mindfulness-based stress reduction, A1c was reduced by 0.48% (P = 0.03), while the practice of qigong – a coordinated body-posture movement – was associated with a 0.66% drop in A1c (P = .01). For meditation, A1c dropped by 0.50% (P = .64).
However, the largest drop in A1c was seen with yoga, where it fell by 1.00% (P < .0001) – about the same degree of glycemic control achieved with metformin, the authors point out.
Indeed, for every additional day of yoga practiced per week, mean A1c differed by –0.22% (P = .46) between those who engaged in mind-body interventions and those who did not.
There was also a reduction in fasting blood glucose (FBG) with yoga and other practices. “The mean change in FBG was consistent with the mean change in A1c at –22.81 mg/dL (P < .0001),” the authors continue.
The researchers found that the duration of yoga didn’t matter but the frequency did, so it’s the regularity “with which you do yoga that makes the difference,” Dr. Watanabe said.
Dr. Watanabe and his coauthors also point out that because most patients were actively receiving metformin before and throughout the studies, the observed effect of mind and body practices on A1c represents an additional reduction beyond that of medication.
“This raises the question [as to] whether mind and body practices could be useful when initiated early in the course of diabetes therapy along with conventional lifestyle treatments,” they suggest.
While more research is needed to study this specifically, “our results suggest that these mind-body practices might be a good preventative measure,” Dr. Watanabe noted. Mind-body practices may also effectively prevent type 2 diabetes in at-risk patients, the authors propose.
Does meditation help alleviate psychological distress?
How mind-body practices work to improve glycemic control isn’t clear, but one possible theory is that patients experience a decrease in psychological distress when they undertake such practices and in so doing, may be more compliant with their prescribed treatment regimen.
A few of the studies analyzed showed that mind-body work resulted in a significant decrease in serum cortisol, the stress hormone that could plausibly mediate the benefit of mind and body practices through reduced inflammation.
In addition, “people with diabetes live with what we call ‘diabetes distress,’ ” Dr. Watanabe explained.
“Management of blood glucose is very stressful. You have to watch what you eat, you have to measure your glucose, and for the average person, that gets stressful. And that stress just contributes to the difficulty of controlling blood glucose,” he noted.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mind and body practices, especially yoga, improve glycemic control in type 2 diabetes to a similar extent as medications such as metformin, new research shows.
“To our knowledge, this is the first study that has looked across different modalities of mind-body interventions and the first to show that there is a very consistent effect on A1c regardless of which modality you use,” senior author, Richard Watanabe, PhD, professor of biostatistics, Keck School of Medicine of the University of Southern California, Los Angeles, told this news organization.
“[Because] our study showed that it doesn’t matter which type of intervention patients do, it’s really up to the physician to work with their patients and help them pick something that works for them,” he added.
“Thus, this really is a much more flexible tool than having to tell a patient they should do yoga if their schedule doesn’t allow them to do yoga. There are other options available, so if you are a busy person and getting yourself to a yoga session is not doable, take a little time to learn about meditation and you can do it anywhere,” he said.
The study was published online, in the Journal of Integrative and Complementary Medicine, by Fatimata Sanogo, PhD candidate, also of Keck School of Medicine, USC, and colleagues.
Regularity of yoga practice makes the difference
A total of 28 studies of patients with type 2 diabetes published between 1993 and 2022 were included in the meta-analysis. In all studies, patients who were taking insulin or had any medical complications of diabetes were excluded.
A significant mean reduction in A1c of 0.84% was observed across the board for all types of mindfulness interventions (P < .0001).
For mindfulness-based stress reduction, A1c was reduced by 0.48% (P = 0.03), while the practice of qigong – a coordinated body-posture movement – was associated with a 0.66% drop in A1c (P = .01). For meditation, A1c dropped by 0.50% (P = .64).
However, the largest drop in A1c was seen with yoga, where it fell by 1.00% (P < .0001) – about the same degree of glycemic control achieved with metformin, the authors point out.
Indeed, for every additional day of yoga practiced per week, mean A1c differed by –0.22% (P = .46) between those who engaged in mind-body interventions and those who did not.
There was also a reduction in fasting blood glucose (FBG) with yoga and other practices. “The mean change in FBG was consistent with the mean change in A1c at –22.81 mg/dL (P < .0001),” the authors continue.
The researchers found that the duration of yoga didn’t matter but the frequency did, so it’s the regularity “with which you do yoga that makes the difference,” Dr. Watanabe said.
Dr. Watanabe and his coauthors also point out that because most patients were actively receiving metformin before and throughout the studies, the observed effect of mind and body practices on A1c represents an additional reduction beyond that of medication.
“This raises the question [as to] whether mind and body practices could be useful when initiated early in the course of diabetes therapy along with conventional lifestyle treatments,” they suggest.
While more research is needed to study this specifically, “our results suggest that these mind-body practices might be a good preventative measure,” Dr. Watanabe noted. Mind-body practices may also effectively prevent type 2 diabetes in at-risk patients, the authors propose.
Does meditation help alleviate psychological distress?
How mind-body practices work to improve glycemic control isn’t clear, but one possible theory is that patients experience a decrease in psychological distress when they undertake such practices and in so doing, may be more compliant with their prescribed treatment regimen.
A few of the studies analyzed showed that mind-body work resulted in a significant decrease in serum cortisol, the stress hormone that could plausibly mediate the benefit of mind and body practices through reduced inflammation.
In addition, “people with diabetes live with what we call ‘diabetes distress,’ ” Dr. Watanabe explained.
“Management of blood glucose is very stressful. You have to watch what you eat, you have to measure your glucose, and for the average person, that gets stressful. And that stress just contributes to the difficulty of controlling blood glucose,” he noted.
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drug repurposing ‘fast track’ to new medicines for obesity, diabetes
for these two conditions.
The scientists identified four pathways with known drug targets for type 2 diabetes and five with known drug targets for obesity.
Their findings suggest that:
- Palbociclib (used to treat breast cancer) and cardiac glycosides (used to treat heart failure and heart rhythm disorders) might be repurposed to treat type 2 diabetes.
- Baclofen (a muscle relaxant) and carfilzomib (a chemotherapy) could potentially be used to treat obesity.
- Fostamatinib (used to treat thrombocytopenia), sucralfate (used to treat stomach ulcers), and regorafenib (used to treat cancer) might be used to treat type 2 diabetes and obesity.
- Baclofen and sucralfate would have favorable safety profiles as repurposed treatments.
Sahar El Shair, a PhD student at the Faculty of Health and Medicine, University of Newcastle, New South Wales, Australia, presented the research during an oral session at the International Congress on Obesity, the biennial congress of the World Obesity Federation, in Melbourne.
“New treatments with higher activity and specificity are urgently needed to tackle a pandemic of chronic illness associated with type 2 diabetes and obesity,” senior coauthor Murray Cairns, PhD, said in a press release from the ICO.
“Our technology harnesses genetically informed precision medicine to identify and target new treatments for these complex disorders,” said Dr. Cairns, from the school of biomedical sciences and pharmacy at the University of Newcastle.
Matchmaking between individual, their genetic traits, and drugs
Dr. Cairns and senior coauthor William Reay, PhD, also from the school of biomedical sciences and pharmacy, have cofounded a company called PolygenRx.
The company website explains that they have developed a propriety platform termed the pharmagenic enrichment score (PES), which is “essentially a matchmaking service between patients and drugs, allowing treatment to be optimized for each individual using their unique combination of genetic risk factors.”
It is important to note the genetic risk from complex traits, such as type 2 diabetes and obesity, “are quite different [from] the rare genetic disorders caused mostly by a devastating mutation in a single gene,” Dr. Cairns explained in an email.
“Complex traits,” he noted, “are associated with thousands of [genetic] variants that are common in people and have a cumulative effect.”
For this specific research, the investigators obtained genetic data from genome-wide association studies of obesity and type 2 diabetes.
“By using very large cohorts (hundreds of thousands of individuals) and comparing the frequency of millions of genetic variants in subjects with these conditions with controls, these studies have revealed regions of the genome and genes associated with the condition,” Dr. Cairns noted.
The pharmagenic enrichment score integrates a person’s genetics with drug pharmacology to determine if a person would respond more readily to a certain drug.
“We are investigating the potential of thousands of drugs across a broad spectrum of complex traits (the list is almost endless),” Dr. Cairns explained.
From the PES score, “we have an estimate of each individual’s likelihood of a positive response to said drug,” he noted. “We all have variants that increase (and decrease) the risk of these conditions to various degrees as they are common (high frequency) genetic variants.”
With this research, “we can implement this precision medicine strategy to match the right [repurposed] drugs for individuals based on their specific burden of genetic risk” for obesity and type 2 diabetes.
“Drug repurposing can be a fast track to new medicines because there is existing knowledge about their safety and activity in humans,” he said.
Next steps: Raising funds for clinical trials
“We would like to progress some of these compounds to preclinical and clinical trials,” Dr. Cairns said, “but need to raise the funds for this expensive research. With limited government research funding opportunities, we have recently spun out a startup company to attract commercial investment in our platform and the development of new drug candidates.”
The authors have reported no relevant financial relationships. Dr. Reay and Dr. Cairns are cofounders of PolygenRx.
A version of this article first appeared on Medscape.com.
for these two conditions.
The scientists identified four pathways with known drug targets for type 2 diabetes and five with known drug targets for obesity.
Their findings suggest that:
- Palbociclib (used to treat breast cancer) and cardiac glycosides (used to treat heart failure and heart rhythm disorders) might be repurposed to treat type 2 diabetes.
- Baclofen (a muscle relaxant) and carfilzomib (a chemotherapy) could potentially be used to treat obesity.
- Fostamatinib (used to treat thrombocytopenia), sucralfate (used to treat stomach ulcers), and regorafenib (used to treat cancer) might be used to treat type 2 diabetes and obesity.
- Baclofen and sucralfate would have favorable safety profiles as repurposed treatments.
Sahar El Shair, a PhD student at the Faculty of Health and Medicine, University of Newcastle, New South Wales, Australia, presented the research during an oral session at the International Congress on Obesity, the biennial congress of the World Obesity Federation, in Melbourne.
“New treatments with higher activity and specificity are urgently needed to tackle a pandemic of chronic illness associated with type 2 diabetes and obesity,” senior coauthor Murray Cairns, PhD, said in a press release from the ICO.
“Our technology harnesses genetically informed precision medicine to identify and target new treatments for these complex disorders,” said Dr. Cairns, from the school of biomedical sciences and pharmacy at the University of Newcastle.
Matchmaking between individual, their genetic traits, and drugs
Dr. Cairns and senior coauthor William Reay, PhD, also from the school of biomedical sciences and pharmacy, have cofounded a company called PolygenRx.
The company website explains that they have developed a propriety platform termed the pharmagenic enrichment score (PES), which is “essentially a matchmaking service between patients and drugs, allowing treatment to be optimized for each individual using their unique combination of genetic risk factors.”
It is important to note the genetic risk from complex traits, such as type 2 diabetes and obesity, “are quite different [from] the rare genetic disorders caused mostly by a devastating mutation in a single gene,” Dr. Cairns explained in an email.
“Complex traits,” he noted, “are associated with thousands of [genetic] variants that are common in people and have a cumulative effect.”
For this specific research, the investigators obtained genetic data from genome-wide association studies of obesity and type 2 diabetes.
“By using very large cohorts (hundreds of thousands of individuals) and comparing the frequency of millions of genetic variants in subjects with these conditions with controls, these studies have revealed regions of the genome and genes associated with the condition,” Dr. Cairns noted.
The pharmagenic enrichment score integrates a person’s genetics with drug pharmacology to determine if a person would respond more readily to a certain drug.
“We are investigating the potential of thousands of drugs across a broad spectrum of complex traits (the list is almost endless),” Dr. Cairns explained.
From the PES score, “we have an estimate of each individual’s likelihood of a positive response to said drug,” he noted. “We all have variants that increase (and decrease) the risk of these conditions to various degrees as they are common (high frequency) genetic variants.”
With this research, “we can implement this precision medicine strategy to match the right [repurposed] drugs for individuals based on their specific burden of genetic risk” for obesity and type 2 diabetes.
“Drug repurposing can be a fast track to new medicines because there is existing knowledge about their safety and activity in humans,” he said.
Next steps: Raising funds for clinical trials
“We would like to progress some of these compounds to preclinical and clinical trials,” Dr. Cairns said, “but need to raise the funds for this expensive research. With limited government research funding opportunities, we have recently spun out a startup company to attract commercial investment in our platform and the development of new drug candidates.”
The authors have reported no relevant financial relationships. Dr. Reay and Dr. Cairns are cofounders of PolygenRx.
A version of this article first appeared on Medscape.com.
for these two conditions.
The scientists identified four pathways with known drug targets for type 2 diabetes and five with known drug targets for obesity.
Their findings suggest that:
- Palbociclib (used to treat breast cancer) and cardiac glycosides (used to treat heart failure and heart rhythm disorders) might be repurposed to treat type 2 diabetes.
- Baclofen (a muscle relaxant) and carfilzomib (a chemotherapy) could potentially be used to treat obesity.
- Fostamatinib (used to treat thrombocytopenia), sucralfate (used to treat stomach ulcers), and regorafenib (used to treat cancer) might be used to treat type 2 diabetes and obesity.
- Baclofen and sucralfate would have favorable safety profiles as repurposed treatments.
Sahar El Shair, a PhD student at the Faculty of Health and Medicine, University of Newcastle, New South Wales, Australia, presented the research during an oral session at the International Congress on Obesity, the biennial congress of the World Obesity Federation, in Melbourne.
“New treatments with higher activity and specificity are urgently needed to tackle a pandemic of chronic illness associated with type 2 diabetes and obesity,” senior coauthor Murray Cairns, PhD, said in a press release from the ICO.
“Our technology harnesses genetically informed precision medicine to identify and target new treatments for these complex disorders,” said Dr. Cairns, from the school of biomedical sciences and pharmacy at the University of Newcastle.
Matchmaking between individual, their genetic traits, and drugs
Dr. Cairns and senior coauthor William Reay, PhD, also from the school of biomedical sciences and pharmacy, have cofounded a company called PolygenRx.
The company website explains that they have developed a propriety platform termed the pharmagenic enrichment score (PES), which is “essentially a matchmaking service between patients and drugs, allowing treatment to be optimized for each individual using their unique combination of genetic risk factors.”
It is important to note the genetic risk from complex traits, such as type 2 diabetes and obesity, “are quite different [from] the rare genetic disorders caused mostly by a devastating mutation in a single gene,” Dr. Cairns explained in an email.
“Complex traits,” he noted, “are associated with thousands of [genetic] variants that are common in people and have a cumulative effect.”
For this specific research, the investigators obtained genetic data from genome-wide association studies of obesity and type 2 diabetes.
“By using very large cohorts (hundreds of thousands of individuals) and comparing the frequency of millions of genetic variants in subjects with these conditions with controls, these studies have revealed regions of the genome and genes associated with the condition,” Dr. Cairns noted.
The pharmagenic enrichment score integrates a person’s genetics with drug pharmacology to determine if a person would respond more readily to a certain drug.
“We are investigating the potential of thousands of drugs across a broad spectrum of complex traits (the list is almost endless),” Dr. Cairns explained.
From the PES score, “we have an estimate of each individual’s likelihood of a positive response to said drug,” he noted. “We all have variants that increase (and decrease) the risk of these conditions to various degrees as they are common (high frequency) genetic variants.”
With this research, “we can implement this precision medicine strategy to match the right [repurposed] drugs for individuals based on their specific burden of genetic risk” for obesity and type 2 diabetes.
“Drug repurposing can be a fast track to new medicines because there is existing knowledge about their safety and activity in humans,” he said.
Next steps: Raising funds for clinical trials
“We would like to progress some of these compounds to preclinical and clinical trials,” Dr. Cairns said, “but need to raise the funds for this expensive research. With limited government research funding opportunities, we have recently spun out a startup company to attract commercial investment in our platform and the development of new drug candidates.”
The authors have reported no relevant financial relationships. Dr. Reay and Dr. Cairns are cofounders of PolygenRx.
A version of this article first appeared on Medscape.com.
FROM ICO 2022
This brain surgery was BYOS: Bring your own saxophone
Tumor vs. saxophone: The surgical grudge match
Brain surgery is a notoriously difficult task. There’s a reason we say, “Well, at least it’s not brain surgery” when we’re trying to convince someone that a task isn’t that tough. Make one wrong incision, cut the wrong neuron, and it’s goodbye higher cognitive function. And most people appreciate thinking. Crazy, right?
One would imagine that the act of brain surgery would become even more difficult when the patient brings his saxophone and plays it randomly throughout the operation. It’s a hospital, after all, not a jazz club. Patients don’t get to play musical instruments during other surgeries. Why should brain surgery patients get special treatment?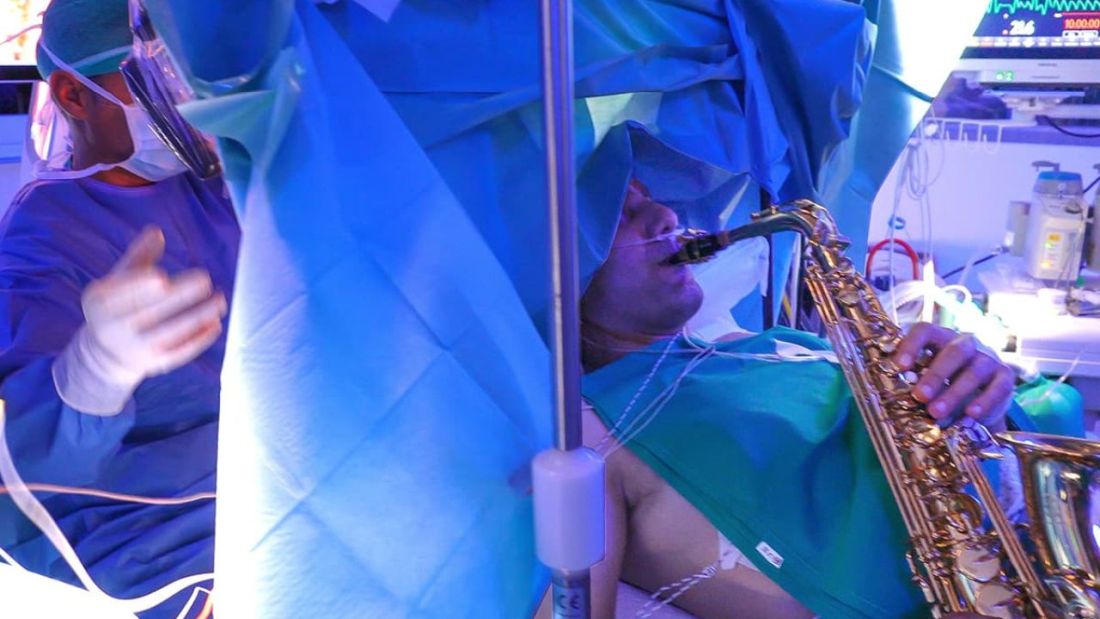
As it turns out, the musical performance was actually quite helpful. A man in Italy had a brain tumor in a particularly complex area, and he’s left-handed, which apparently makes the brain’s neural pathways much more complicated. Plus, he insisted that he retain his musical ability after the surgery. So he and his medical team had a crazy thought: Why not play the saxophone throughout the surgery? After all, according to head surgeon Christian Brogna, MD, playing an instrument means you understand music, which tests many higher cognitive functions such as coordination, mathematics, and memory.
And so, at various points throughout the 9-hour surgery, the patient played his saxophone for his doctors. Doing so allowed the surgeons to map the patient’s brain in a more complete and personalized fashion. With that extra knowledge, they were able to successfully remove the tumor while maintaining the patient’s musical ability, and the patient was discharged on Oct. 13, just 3 days after his operation.
While we’re happy the patient recovered, we do have to question his choice of music. During the surgery, he played the theme to the 1970 movie “Love Story” and the Italian national anthem. Perfectly fine pieces, no doubt, but the saxophone solo in “Jungleland” exists. And we could listen to that for 9 hours straight. In fact, we do that every Friday in the LOTME office.
Basketball has the Big Dance. Mosquitoes get the Big Sniff
In this week’s installment of our seemingly never-ending series, “Mosquitoes and the scientists who love them,” we visit The Rockefeller University in New York, where the olfactory capabilities of Aedes Aegypti – the primary vector species for Zika, dengue, yellow fever, and chikungunya – became the subject of a round robin–style tournament.
First things first, though. If you’re going to test mosquito noses, you have to give them something to smell. The researchers enrolled eight humans who were willing to wear nylon stockings on their forearms for 6 hours a day for multiple days. “Over the next few years, the researchers tested the nylons against each other in all possible pairings,” Leslie B. Vosshall, PhD, and associates said in a statement from the university. In other words, mosquito March Madness.
Nylons from different participants were hooked up in pairs to an olfactometer assay consisting of a plexiglass chamber divided into two tubes, each ending in a box that held a stocking. The mosquitoes were placed in the main chamber and observed as they flew down the tubes toward one stocking or the other.
Eventually, the “winner” of the “tournament” was Subject 33. And no, we don’t know why there was a Subject 33 since the study involved only eight participants. We do know that the nylons worn by Subject 33 were “four times more attractive to the mosquitoes than the next most-attractive study participant, and an astonishing 100 times more appealing than the least attractive, Subject 19,” according to the written statement.
Chemical analysis identified 50 molecular compounds that were elevated in the sebum of the high-attracting participants, and eventually the investigators discovered that mosquito magnets produced carboxylic acids at much higher levels than the less-attractive volunteers.
We could go on about the research team genetically engineering mosquitoes without odor receptors, but we have to save something for later. Tune in again next week for another exciting episode of “Mosquitoes and the scientists who love them.”
Are women better with words?
Men vs. Women is probably the oldest argument in the book, but there may now be movement. Researchers have been able not only to shift the advantage toward women, but also to use that knowledge to medical advantage.
When it comes to the matter of words and remembering them, women apparently have men beat. The margin is small, said lead author Marco Hirnstein, PhD, of the University of Bergen, Norway, but, after performing a meta-analysis of 168 published studies and PhD theses involving more than 350,000 participants, it’s pretty clear. The research supports women’s advantage over men in recall, verbal fluency (categorical and phonemic), and recognition.
So how is this information useful from a medical standpoint?
Dr. Hirnstein and colleagues suggested that this information can help in interpreting diagnostic assessment results. The example given was dementia diagnosis. Since women are underdiagnosed because their baseline exceeds average while men are overdiagnosed, taking gender and performance into account could clear up or catch cases that might otherwise slip through the cracks.
Now, let’s just put this part of the debate to rest and take this not only as a win for women but for science as well.
Tumor vs. saxophone: The surgical grudge match
Brain surgery is a notoriously difficult task. There’s a reason we say, “Well, at least it’s not brain surgery” when we’re trying to convince someone that a task isn’t that tough. Make one wrong incision, cut the wrong neuron, and it’s goodbye higher cognitive function. And most people appreciate thinking. Crazy, right?
One would imagine that the act of brain surgery would become even more difficult when the patient brings his saxophone and plays it randomly throughout the operation. It’s a hospital, after all, not a jazz club. Patients don’t get to play musical instruments during other surgeries. Why should brain surgery patients get special treatment?
As it turns out, the musical performance was actually quite helpful. A man in Italy had a brain tumor in a particularly complex area, and he’s left-handed, which apparently makes the brain’s neural pathways much more complicated. Plus, he insisted that he retain his musical ability after the surgery. So he and his medical team had a crazy thought: Why not play the saxophone throughout the surgery? After all, according to head surgeon Christian Brogna, MD, playing an instrument means you understand music, which tests many higher cognitive functions such as coordination, mathematics, and memory.
And so, at various points throughout the 9-hour surgery, the patient played his saxophone for his doctors. Doing so allowed the surgeons to map the patient’s brain in a more complete and personalized fashion. With that extra knowledge, they were able to successfully remove the tumor while maintaining the patient’s musical ability, and the patient was discharged on Oct. 13, just 3 days after his operation.
While we’re happy the patient recovered, we do have to question his choice of music. During the surgery, he played the theme to the 1970 movie “Love Story” and the Italian national anthem. Perfectly fine pieces, no doubt, but the saxophone solo in “Jungleland” exists. And we could listen to that for 9 hours straight. In fact, we do that every Friday in the LOTME office.
Basketball has the Big Dance. Mosquitoes get the Big Sniff
In this week’s installment of our seemingly never-ending series, “Mosquitoes and the scientists who love them,” we visit The Rockefeller University in New York, where the olfactory capabilities of Aedes Aegypti – the primary vector species for Zika, dengue, yellow fever, and chikungunya – became the subject of a round robin–style tournament.
First things first, though. If you’re going to test mosquito noses, you have to give them something to smell. The researchers enrolled eight humans who were willing to wear nylon stockings on their forearms for 6 hours a day for multiple days. “Over the next few years, the researchers tested the nylons against each other in all possible pairings,” Leslie B. Vosshall, PhD, and associates said in a statement from the university. In other words, mosquito March Madness.
Nylons from different participants were hooked up in pairs to an olfactometer assay consisting of a plexiglass chamber divided into two tubes, each ending in a box that held a stocking. The mosquitoes were placed in the main chamber and observed as they flew down the tubes toward one stocking or the other.
Eventually, the “winner” of the “tournament” was Subject 33. And no, we don’t know why there was a Subject 33 since the study involved only eight participants. We do know that the nylons worn by Subject 33 were “four times more attractive to the mosquitoes than the next most-attractive study participant, and an astonishing 100 times more appealing than the least attractive, Subject 19,” according to the written statement.
Chemical analysis identified 50 molecular compounds that were elevated in the sebum of the high-attracting participants, and eventually the investigators discovered that mosquito magnets produced carboxylic acids at much higher levels than the less-attractive volunteers.
We could go on about the research team genetically engineering mosquitoes without odor receptors, but we have to save something for later. Tune in again next week for another exciting episode of “Mosquitoes and the scientists who love them.”
Are women better with words?
Men vs. Women is probably the oldest argument in the book, but there may now be movement. Researchers have been able not only to shift the advantage toward women, but also to use that knowledge to medical advantage.
When it comes to the matter of words and remembering them, women apparently have men beat. The margin is small, said lead author Marco Hirnstein, PhD, of the University of Bergen, Norway, but, after performing a meta-analysis of 168 published studies and PhD theses involving more than 350,000 participants, it’s pretty clear. The research supports women’s advantage over men in recall, verbal fluency (categorical and phonemic), and recognition.
So how is this information useful from a medical standpoint?
Dr. Hirnstein and colleagues suggested that this information can help in interpreting diagnostic assessment results. The example given was dementia diagnosis. Since women are underdiagnosed because their baseline exceeds average while men are overdiagnosed, taking gender and performance into account could clear up or catch cases that might otherwise slip through the cracks.
Now, let’s just put this part of the debate to rest and take this not only as a win for women but for science as well.
Tumor vs. saxophone: The surgical grudge match
Brain surgery is a notoriously difficult task. There’s a reason we say, “Well, at least it’s not brain surgery” when we’re trying to convince someone that a task isn’t that tough. Make one wrong incision, cut the wrong neuron, and it’s goodbye higher cognitive function. And most people appreciate thinking. Crazy, right?
One would imagine that the act of brain surgery would become even more difficult when the patient brings his saxophone and plays it randomly throughout the operation. It’s a hospital, after all, not a jazz club. Patients don’t get to play musical instruments during other surgeries. Why should brain surgery patients get special treatment?
As it turns out, the musical performance was actually quite helpful. A man in Italy had a brain tumor in a particularly complex area, and he’s left-handed, which apparently makes the brain’s neural pathways much more complicated. Plus, he insisted that he retain his musical ability after the surgery. So he and his medical team had a crazy thought: Why not play the saxophone throughout the surgery? After all, according to head surgeon Christian Brogna, MD, playing an instrument means you understand music, which tests many higher cognitive functions such as coordination, mathematics, and memory.
And so, at various points throughout the 9-hour surgery, the patient played his saxophone for his doctors. Doing so allowed the surgeons to map the patient’s brain in a more complete and personalized fashion. With that extra knowledge, they were able to successfully remove the tumor while maintaining the patient’s musical ability, and the patient was discharged on Oct. 13, just 3 days after his operation.
While we’re happy the patient recovered, we do have to question his choice of music. During the surgery, he played the theme to the 1970 movie “Love Story” and the Italian national anthem. Perfectly fine pieces, no doubt, but the saxophone solo in “Jungleland” exists. And we could listen to that for 9 hours straight. In fact, we do that every Friday in the LOTME office.
Basketball has the Big Dance. Mosquitoes get the Big Sniff
In this week’s installment of our seemingly never-ending series, “Mosquitoes and the scientists who love them,” we visit The Rockefeller University in New York, where the olfactory capabilities of Aedes Aegypti – the primary vector species for Zika, dengue, yellow fever, and chikungunya – became the subject of a round robin–style tournament.
First things first, though. If you’re going to test mosquito noses, you have to give them something to smell. The researchers enrolled eight humans who were willing to wear nylon stockings on their forearms for 6 hours a day for multiple days. “Over the next few years, the researchers tested the nylons against each other in all possible pairings,” Leslie B. Vosshall, PhD, and associates said in a statement from the university. In other words, mosquito March Madness.
Nylons from different participants were hooked up in pairs to an olfactometer assay consisting of a plexiglass chamber divided into two tubes, each ending in a box that held a stocking. The mosquitoes were placed in the main chamber and observed as they flew down the tubes toward one stocking or the other.
Eventually, the “winner” of the “tournament” was Subject 33. And no, we don’t know why there was a Subject 33 since the study involved only eight participants. We do know that the nylons worn by Subject 33 were “four times more attractive to the mosquitoes than the next most-attractive study participant, and an astonishing 100 times more appealing than the least attractive, Subject 19,” according to the written statement.
Chemical analysis identified 50 molecular compounds that were elevated in the sebum of the high-attracting participants, and eventually the investigators discovered that mosquito magnets produced carboxylic acids at much higher levels than the less-attractive volunteers.
We could go on about the research team genetically engineering mosquitoes without odor receptors, but we have to save something for later. Tune in again next week for another exciting episode of “Mosquitoes and the scientists who love them.”
Are women better with words?
Men vs. Women is probably the oldest argument in the book, but there may now be movement. Researchers have been able not only to shift the advantage toward women, but also to use that knowledge to medical advantage.
When it comes to the matter of words and remembering them, women apparently have men beat. The margin is small, said lead author Marco Hirnstein, PhD, of the University of Bergen, Norway, but, after performing a meta-analysis of 168 published studies and PhD theses involving more than 350,000 participants, it’s pretty clear. The research supports women’s advantage over men in recall, verbal fluency (categorical and phonemic), and recognition.
So how is this information useful from a medical standpoint?
Dr. Hirnstein and colleagues suggested that this information can help in interpreting diagnostic assessment results. The example given was dementia diagnosis. Since women are underdiagnosed because their baseline exceeds average while men are overdiagnosed, taking gender and performance into account could clear up or catch cases that might otherwise slip through the cracks.
Now, let’s just put this part of the debate to rest and take this not only as a win for women but for science as well.
New AGA guidelines advise use of antiobesity medications for weight management
Adults with obesity who do not respond adequately to lifestyle interventions alone should be offered one of four suggested medications to treat obesity, with preference for semaglutide before others, according to new guidelines published by the American Gastroenterological Association in Gastroenterology.
Recommended first-line medications include semaglutide, liraglutide, phentermine-topiramate extended-release (ER), and naltrexone-buproprion ER, based on moderate-certainty evidence. Also recommended, albeit based on lower-certainty evidence, are phentermine and diethylpropion. The guidelines suggest avoiding use of orlistat. Evidence was insufficient for Gelesis100 superabsorbent hydrogel.
The substantial increase in obesity prevalence in the United States – from 30.5% to 41.9% in just the 2 decades from 2000 to 2020 – has likely contributed to increases in various obesity-related complications, wrote Eduardo Grunvald, MD, of the University of California San Diego, and colleagues. These include cardiovascular disease, stroke, type 2 diabetes mellitus, nonalcoholic steatohepatitis, obstructive sleep apnea, osteoarthritis, and certain types of cancer, such as colorectal cancer.
“Lifestyle intervention is the foundation in the management of obesity, but it has limited effectiveness and durability for most individuals,” the authors wrote. Despite a range of highly effective pharmacological therapies developed for long-term management of obesity, these agents are not widely used in routine clinical care, and practice variability is wide. There is a “small number of providers responsible for more than 90% of the prescriptions, partly due to lack of familiarity and limited access and insurance coverage,” the authors wrote.
A multidisciplinary panel of 10 experts and one patient representative, therefore, developed the guidelines by first prioritizing key clinical questions, identifying patient-centered outcomes, and conducting an evidence review of the following interventions: semaglutide 2.4 mg, liraglutide 3.0 mg, phentermine-topiramate extended-release (ER), naltrexone-bupropion ER, orlistat, phentermine, diethylpropion, and Gelesis100 superabsorbent hydrogel. The guideline panel then developed management recommendations and provided clinical practice considerations regarding each of the pharmacologic interventions.
The authors focused on adults, noting that pharmacologic treatment of childhood obesity is beyond the scope of these guidelines. The evidence synthesis yielded nine recommendations for the pharmacological management of obesity by gastroenterologists, primary care clinicians, endocrinologists, and other providers caring for patients with overweight or obesity. The target audience of the guidelines, however, includes patients and policymakers, the authors wrote.
“These guidelines are not intended to impose a standard of care, but rather, they provide the basis for rational, informed decisions for patients and health care professionals,” the authors wrote. “No recommendation can include all the unique individual circumstances that must be considered when making recommendations for individual patients. However, discussions around benefits and harms can be used for shared decision-making, especially for conditional recommendations where patients’ values and preferences are important to consider.”
The panel conducted a systematic review and meta-analysis of randomized controlled trials of Food and Drug Administration–approved obesity medications through Jan. 1, 2022. Though they primarily included studies with at least 48 weeks follow-up, they included studies with a follow-up of less than a year if one with 48 weeks’ outcomes did not exist.
The first of the nine recommendations was to add pharmacological agents to lifestyle interventions in treating adults with obesity or overweight and weight-related complications who have not adequately responded to lifestyle interventions alone. This strong recommendation was based on moderate-certainty evidence.
“Antiobesity medications generally need to be used chronically, and the selection of the medication or intervention should be based on the clinical profile and needs of the patient, including, but not limited to, comorbidities, patients’ preferences, costs, and access to the therapy,” the authors wrote. Average difference in total body weight loss with the addition of medication to lifestyle interventions was 3%-10.8%, depending on the drug. Treatment discontinuation ranged from 34 to 219 per 1,000 people in treatment groups, but adverse event rates were low.
The panel’s second recommendation suggested prioritizing of semaglutide along with lifestyle interventions based on the large magnitude of its net benefit. The remaining recommendations describes the use of each of the other medications based on their respective magnitude of effect and risk for adverse events.
Important considerations
“These medications treat a biological disease, not a lifestyle problem,” Dr. Grunvald said in a prepared statement. “Obesity is a disease that often does not respond to lifestyle interventions alone in the long term. Using medications as an option to assist with weight loss can improve weight-related complications like joint pain, diabetes, fatty liver, and hypertension.”
The authors acknowledged that cost remains a concern for the use of these therapies, especially among vulnerable populations. They also noted that the medications should not be used in pregnant individuals or those with bulimia nervosa, and they should be used with caution in people with other eating disorders. Patients with type 2 diabetes taking insulin or sulfonylureas and patients taking antihypertensives may require dosage adjustments since these obesity medications may increase risk of hypoglycemia for the former and decrease blood pressure for the latter.
The panel advised against orlistat, although it added that ”patients who place a high value on the potential small weight loss benefit and low value on gastrointestinal side effects may reasonably choose treatment with orlistat.” Those patients should take a multivitamin daily that contains vitamins A, D, E, and K at least 2 hours apart from orlistat.
The lack of available evidence for Gelesis100 oral superabsorbent hydrogel led the panel to suggest its use only in the context of a clinical trial.
The AGA will update these guidelines no later than 2025 and may issue rapid guidance updates until then as new evidence comes to light.
The guidelines did not receive any external funding, being fully funded by the AGA. The guideline chair and guideline methodologists had no relevant or direct conflicts of interest. All conflict of interest disclosures are maintained by the AGA office.
Adults with obesity who do not respond adequately to lifestyle interventions alone should be offered one of four suggested medications to treat obesity, with preference for semaglutide before others, according to new guidelines published by the American Gastroenterological Association in Gastroenterology.
Recommended first-line medications include semaglutide, liraglutide, phentermine-topiramate extended-release (ER), and naltrexone-buproprion ER, based on moderate-certainty evidence. Also recommended, albeit based on lower-certainty evidence, are phentermine and diethylpropion. The guidelines suggest avoiding use of orlistat. Evidence was insufficient for Gelesis100 superabsorbent hydrogel.
The substantial increase in obesity prevalence in the United States – from 30.5% to 41.9% in just the 2 decades from 2000 to 2020 – has likely contributed to increases in various obesity-related complications, wrote Eduardo Grunvald, MD, of the University of California San Diego, and colleagues. These include cardiovascular disease, stroke, type 2 diabetes mellitus, nonalcoholic steatohepatitis, obstructive sleep apnea, osteoarthritis, and certain types of cancer, such as colorectal cancer.
“Lifestyle intervention is the foundation in the management of obesity, but it has limited effectiveness and durability for most individuals,” the authors wrote. Despite a range of highly effective pharmacological therapies developed for long-term management of obesity, these agents are not widely used in routine clinical care, and practice variability is wide. There is a “small number of providers responsible for more than 90% of the prescriptions, partly due to lack of familiarity and limited access and insurance coverage,” the authors wrote.
A multidisciplinary panel of 10 experts and one patient representative, therefore, developed the guidelines by first prioritizing key clinical questions, identifying patient-centered outcomes, and conducting an evidence review of the following interventions: semaglutide 2.4 mg, liraglutide 3.0 mg, phentermine-topiramate extended-release (ER), naltrexone-bupropion ER, orlistat, phentermine, diethylpropion, and Gelesis100 superabsorbent hydrogel. The guideline panel then developed management recommendations and provided clinical practice considerations regarding each of the pharmacologic interventions.
The authors focused on adults, noting that pharmacologic treatment of childhood obesity is beyond the scope of these guidelines. The evidence synthesis yielded nine recommendations for the pharmacological management of obesity by gastroenterologists, primary care clinicians, endocrinologists, and other providers caring for patients with overweight or obesity. The target audience of the guidelines, however, includes patients and policymakers, the authors wrote.
“These guidelines are not intended to impose a standard of care, but rather, they provide the basis for rational, informed decisions for patients and health care professionals,” the authors wrote. “No recommendation can include all the unique individual circumstances that must be considered when making recommendations for individual patients. However, discussions around benefits and harms can be used for shared decision-making, especially for conditional recommendations where patients’ values and preferences are important to consider.”
The panel conducted a systematic review and meta-analysis of randomized controlled trials of Food and Drug Administration–approved obesity medications through Jan. 1, 2022. Though they primarily included studies with at least 48 weeks follow-up, they included studies with a follow-up of less than a year if one with 48 weeks’ outcomes did not exist.
The first of the nine recommendations was to add pharmacological agents to lifestyle interventions in treating adults with obesity or overweight and weight-related complications who have not adequately responded to lifestyle interventions alone. This strong recommendation was based on moderate-certainty evidence.
“Antiobesity medications generally need to be used chronically, and the selection of the medication or intervention should be based on the clinical profile and needs of the patient, including, but not limited to, comorbidities, patients’ preferences, costs, and access to the therapy,” the authors wrote. Average difference in total body weight loss with the addition of medication to lifestyle interventions was 3%-10.8%, depending on the drug. Treatment discontinuation ranged from 34 to 219 per 1,000 people in treatment groups, but adverse event rates were low.
The panel’s second recommendation suggested prioritizing of semaglutide along with lifestyle interventions based on the large magnitude of its net benefit. The remaining recommendations describes the use of each of the other medications based on their respective magnitude of effect and risk for adverse events.
Important considerations
“These medications treat a biological disease, not a lifestyle problem,” Dr. Grunvald said in a prepared statement. “Obesity is a disease that often does not respond to lifestyle interventions alone in the long term. Using medications as an option to assist with weight loss can improve weight-related complications like joint pain, diabetes, fatty liver, and hypertension.”
The authors acknowledged that cost remains a concern for the use of these therapies, especially among vulnerable populations. They also noted that the medications should not be used in pregnant individuals or those with bulimia nervosa, and they should be used with caution in people with other eating disorders. Patients with type 2 diabetes taking insulin or sulfonylureas and patients taking antihypertensives may require dosage adjustments since these obesity medications may increase risk of hypoglycemia for the former and decrease blood pressure for the latter.
The panel advised against orlistat, although it added that ”patients who place a high value on the potential small weight loss benefit and low value on gastrointestinal side effects may reasonably choose treatment with orlistat.” Those patients should take a multivitamin daily that contains vitamins A, D, E, and K at least 2 hours apart from orlistat.
The lack of available evidence for Gelesis100 oral superabsorbent hydrogel led the panel to suggest its use only in the context of a clinical trial.
The AGA will update these guidelines no later than 2025 and may issue rapid guidance updates until then as new evidence comes to light.
The guidelines did not receive any external funding, being fully funded by the AGA. The guideline chair and guideline methodologists had no relevant or direct conflicts of interest. All conflict of interest disclosures are maintained by the AGA office.
Adults with obesity who do not respond adequately to lifestyle interventions alone should be offered one of four suggested medications to treat obesity, with preference for semaglutide before others, according to new guidelines published by the American Gastroenterological Association in Gastroenterology.
Recommended first-line medications include semaglutide, liraglutide, phentermine-topiramate extended-release (ER), and naltrexone-buproprion ER, based on moderate-certainty evidence. Also recommended, albeit based on lower-certainty evidence, are phentermine and diethylpropion. The guidelines suggest avoiding use of orlistat. Evidence was insufficient for Gelesis100 superabsorbent hydrogel.
The substantial increase in obesity prevalence in the United States – from 30.5% to 41.9% in just the 2 decades from 2000 to 2020 – has likely contributed to increases in various obesity-related complications, wrote Eduardo Grunvald, MD, of the University of California San Diego, and colleagues. These include cardiovascular disease, stroke, type 2 diabetes mellitus, nonalcoholic steatohepatitis, obstructive sleep apnea, osteoarthritis, and certain types of cancer, such as colorectal cancer.
“Lifestyle intervention is the foundation in the management of obesity, but it has limited effectiveness and durability for most individuals,” the authors wrote. Despite a range of highly effective pharmacological therapies developed for long-term management of obesity, these agents are not widely used in routine clinical care, and practice variability is wide. There is a “small number of providers responsible for more than 90% of the prescriptions, partly due to lack of familiarity and limited access and insurance coverage,” the authors wrote.
A multidisciplinary panel of 10 experts and one patient representative, therefore, developed the guidelines by first prioritizing key clinical questions, identifying patient-centered outcomes, and conducting an evidence review of the following interventions: semaglutide 2.4 mg, liraglutide 3.0 mg, phentermine-topiramate extended-release (ER), naltrexone-bupropion ER, orlistat, phentermine, diethylpropion, and Gelesis100 superabsorbent hydrogel. The guideline panel then developed management recommendations and provided clinical practice considerations regarding each of the pharmacologic interventions.
The authors focused on adults, noting that pharmacologic treatment of childhood obesity is beyond the scope of these guidelines. The evidence synthesis yielded nine recommendations for the pharmacological management of obesity by gastroenterologists, primary care clinicians, endocrinologists, and other providers caring for patients with overweight or obesity. The target audience of the guidelines, however, includes patients and policymakers, the authors wrote.
“These guidelines are not intended to impose a standard of care, but rather, they provide the basis for rational, informed decisions for patients and health care professionals,” the authors wrote. “No recommendation can include all the unique individual circumstances that must be considered when making recommendations for individual patients. However, discussions around benefits and harms can be used for shared decision-making, especially for conditional recommendations where patients’ values and preferences are important to consider.”
The panel conducted a systematic review and meta-analysis of randomized controlled trials of Food and Drug Administration–approved obesity medications through Jan. 1, 2022. Though they primarily included studies with at least 48 weeks follow-up, they included studies with a follow-up of less than a year if one with 48 weeks’ outcomes did not exist.
The first of the nine recommendations was to add pharmacological agents to lifestyle interventions in treating adults with obesity or overweight and weight-related complications who have not adequately responded to lifestyle interventions alone. This strong recommendation was based on moderate-certainty evidence.
“Antiobesity medications generally need to be used chronically, and the selection of the medication or intervention should be based on the clinical profile and needs of the patient, including, but not limited to, comorbidities, patients’ preferences, costs, and access to the therapy,” the authors wrote. Average difference in total body weight loss with the addition of medication to lifestyle interventions was 3%-10.8%, depending on the drug. Treatment discontinuation ranged from 34 to 219 per 1,000 people in treatment groups, but adverse event rates were low.
The panel’s second recommendation suggested prioritizing of semaglutide along with lifestyle interventions based on the large magnitude of its net benefit. The remaining recommendations describes the use of each of the other medications based on their respective magnitude of effect and risk for adverse events.
Important considerations
“These medications treat a biological disease, not a lifestyle problem,” Dr. Grunvald said in a prepared statement. “Obesity is a disease that often does not respond to lifestyle interventions alone in the long term. Using medications as an option to assist with weight loss can improve weight-related complications like joint pain, diabetes, fatty liver, and hypertension.”
The authors acknowledged that cost remains a concern for the use of these therapies, especially among vulnerable populations. They also noted that the medications should not be used in pregnant individuals or those with bulimia nervosa, and they should be used with caution in people with other eating disorders. Patients with type 2 diabetes taking insulin or sulfonylureas and patients taking antihypertensives may require dosage adjustments since these obesity medications may increase risk of hypoglycemia for the former and decrease blood pressure for the latter.
The panel advised against orlistat, although it added that ”patients who place a high value on the potential small weight loss benefit and low value on gastrointestinal side effects may reasonably choose treatment with orlistat.” Those patients should take a multivitamin daily that contains vitamins A, D, E, and K at least 2 hours apart from orlistat.
The lack of available evidence for Gelesis100 oral superabsorbent hydrogel led the panel to suggest its use only in the context of a clinical trial.
The AGA will update these guidelines no later than 2025 and may issue rapid guidance updates until then as new evidence comes to light.
The guidelines did not receive any external funding, being fully funded by the AGA. The guideline chair and guideline methodologists had no relevant or direct conflicts of interest. All conflict of interest disclosures are maintained by the AGA office.
Nonhormonal drug fezolinetant found safe for hot flashes in yearlong study
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
The drug fezolinetant, a selective neurokinin-3 receptor antagonist under investigation for treatment of menopausal vasomotor symptoms, showed acceptable long-term safety and tolerability during a 1-year phase 3 randomized controlled trial, according to data presented at the annual meeting of the North American Menopause Society. The study, called SKYLIGHT 4, examined fezolinetant treatment, especially in terms of endometrial health.
The findings mean that fezolinetant “may help bridge a gap in the management of vasomotor symptoms,” according to lead author Genevieve Neal-Perry, MD, PhD, chair of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
This study was an important step in fezolinetant’s path toward potential approval by the Food and Drug Administration for vasomotor symptoms.
”Moderate and severe vasomotor symptoms can adversely affect quality of life of those affected and result in sleep disruption as well as increased risk for heart disease and other high-risk medical problems,” Dr. Neal-Perry said. “Although menopausal hormone therapy significantly improves vasomotor symptoms, it may not be desired or it may not be safe for some women,” resulting in gaps in care and a need for targeted, nonhormonal therapies for hot flashes. A planned study will also assess the safety of the drug in patients with a diagnosis of hormone-sensitive cancer and disorders that increase the risk for blood clots.
”Fezolinetant has a low side effect profile, it is a nonhormonal option, and it is selective for the neurons that trigger and mediate hot flashes,” Dr. Neal-Perry said.
Hot flashes are caused by kisspeptin, neurokinin B, and dynorphin neurons located in the hypothalamus. Fezolinetant works by selectively blocking the neurokinin 3 receptor (NK3R), which regulates a person’s sense of temperature, Dr. Neal-Perry explained. Overactivation of NK3R, resulting from low estrogen levels, plays a role in the hot flashes and cold sweats women experience during menopause.
Drug development for hot flashes ”has been hampered by a lack of knowledge regarding the biological cause,” Dr. Neal-Perry said. “Now that we have a robust understanding of the basic biology of hot flashes, we can develop novel, highly effective, and targeted therapy.”
This safety study involved 1,830 women, ages 40-65, who were experiencing menopausal vasomotor symptoms and were randomly assigned to one of three arms for 52 weeks: 45 mg of fezolinetant, 30 mg of fezolinetant, or a placebo once daily.
The primary endpoints included the percentage of women with endometrial hyperplasia, the percentage of women with endometrial cancer, and the frequency and severity of treatment-emergent adverse events (TEAEs). To meet the primary safety endpoint, no more than 1% of participants could have hyperplasia or malignancy, with an upper confidence interval boundary not greater than 4%. Women who met prespecified criteria for their endometrial health to be assessed, underwent endometrial biopsies at baseline and at the end of the study. Three independent pathologists analyzed the tissue without knowledge of which study arm each sample came from. Among the 599 endometrial biopsy samples, 0.5% of the 203 participants taking 45 mg fezolinetant had hyperplasia while none of the women in the other two arms did. Among the 210 women taking 30 mg of fezolinetant, 0.5% had a malignancy; no malignancies occurred in the other two arms.
Overall adverse events were similar across all three arms, including rates of adverse events leading to discontinuation. The most common adverse events were headache and COVID-19. TEAEs related to the drug were 18.1% in the 45-mg arm, 15.4% in the 30-mg arm, and 17.4% in the placebo arm. Serious adverse events were similar across all three arms, and only 0.5% of participants in the 45-mg arm experienced drug-related serious adverse events, compared with none of the women in the 30-mg arm and 0.2% of women in the placebo group.
”The frequency of transaminase elevations was low, and these TEAEs were generally isolated, transient, and resolved on treatment or with discontinuation,” the authors reported.
The next steps for fezolinetant will be to assess its effect on mood and quality of life measures related to vasomotor symptoms, Dr. Neal-Perry said.
Samantha Dunham, MD, a NAMS-certified menopause practitioner and an associate professor of obstetrics and gynecology at New York University, suggested the drug’s safety in the study is encouraging.
”As a medication that treats menopausal symptoms, the study confirmed there are no issues with the endometrium, or lining of the uterus, not that one would expect issues given the mechanism of action,” Dr. Dunham, also codirector of NYU Langone’s Center for Midlife Health and Menopause, said in an interview. Dr. Dunham was not involved in the study.
”Earlier versions of medication in this class have caused liver enzyme elevation.” The trial of this medication showed that there were only transient elevations in liver enzymes, which resolved upon cessation of the medication. Dr. Dunham said. ”If the medicine proves to be safe over long periods of time in different populations, this will be a very significant medication for treating menopausal vasomotor symptoms.”
The research was funded by Astellas Pharma. Dr. Dunham had no disclosures. Dr. Neal-Perry is a scientific advisory board member for Astellas and Ferring Pharmaceuticals, and has received research funding from Merck and Overa.
FROM NAMS 2022
Islet transplants in type 1 diabetes durable up to 8 years
Transplantation of cadaveric pancreatic islet cells resulted in graft survival and function with acceptable safety for up to 8 years in selected individuals with type 1 diabetes, new research finds.
The study is a long-term follow-up of two phase 3 pivotal trials from the Clinical Islet Transplantation Consortium of a purified human pancreatic islet cell product for treating people with type 1 diabetes.
One trial involved islet transplantation in 48 people who experienced severe hypoglycemia and hypoglycemic unawareness, and the other trial included 24 people who also experienced those complications and were already receiving immunosuppression following kidney transplant. The trials, both registered with the U.S. Food and Drug Administration (FDA), met their primary efficacy and safety endpoints at 2- and 3-year timepoints.
The follow-up data have now been published in Diabetes Care by Michael Rickels, MD, and colleagues.
The procedure involved infusion through the hepatic portal vein of one or more purified human pancreatic islet products under standardized immunosuppression using methods that Dr. Rickels and colleagues have been developing since 2004. The approach involves multiple modalities to protect the islets prior to transplantation.
Among the 34 islet-alone and eight islet-after–kidney transplant recipients who entered the extended follow-up, durable graft survival allowing for achievement of glycemic targets occurred without severe hypoglycemia or adverse effects from immunosuppression.
The primary outcome, actuarial survival of graft islet function, was 56% at the maximum follow-up of 8.3 years for the islet-only transplantation group and 49% at 7.3 years for the islet-after–kidney transplantation group (P = .004).
The findings suggest that “in the long run, islet transplantation has efficacy, including among those who have had kidney transplants ... Most type 1 diabetes patients are improved tremendously with current insulin delivery systems ... but for those having the most difficulty controlling their blood sugar – and those whose diabetes has already been complicated by needing a kidney transplant – the outcomes we saw in this study are what we’ve been hoping to achieve for more than 20 years,” said Dr. Rickels in a statement from his institution, the University of Pennsylvania, Philadelphia.
In the initial trials at day 75 after the initial transplant, 87.5% of the islet-alone and 71% of the islet-after–kidney transplant group achieved hemoglobin A1c under 7%, and 85% and 54%, respectively, achieved A1c at or under 6.5%. At the end of maximal follow-up, 49% of islet-only transplant recipients maintained A1c under 7%, although none had A1c at or under 6.5%. For the islet-after–kidney transplant group, these proportions were 35% and 17%, respectively (P = .0017 for A1c under 7.0% and P < .0001 for A1c ≤ 6.5%, respectively, between the groups).
There were 12 severe hypoglycemic episodes in five patients (three islet-alone and two islet-after–kidney transplant group) during the initial trials, but no additional episodes occurred in either group during long-term follow-up.
Overall, 53 individuals – 37 in the islet-alone and 16 in the islet-after–kidney transplant group – or 74% of the total, achieved a period of insulin independence with A1c under 7%, ranging from 36 to 481 days. The range of time to achieving insulin independence reflects individuals who received one, two, or three islet infusions.
The fact that most patients achieved insulin independence following just one (n = 20) or two (n = 30) infusions and only three patients required three infusions was notable, Dr. Rickels said.
“Currently, around the world, there’s an expectation of two to three donor pancreases being needed. Here, it’s one, maybe two. It’s a much more efficient protocol and opens up access for more islet transplantation as a hoped-for alternative to pancreas transplants.”
Of those who achieved insulin independence, 30 (57%) remained insulin-independent throughout follow-up (20 of 37 islet-alone and 10 of 16 islet-after–kidney transplant patients), with no difference in duration of insulin independence between the groups.
There were no deaths during post-transplant follow-up. Rates of serious adverse events were 0.31 and 0.43 per patient-year for the islet-after–kidney and islet-alone transplant groups, respectively. Of a total of 104 serious adverse events, 65 occurred during the initial trials and had been previously reported. Of the additional 39 serious adverse events that occurred during long-term follow-up, 11 were possibly due to immunosuppression and 27 were deemed unrelated to the procedures.
According to Dr. Rickels, “These are the most seriously affected patients, and you’d be expecting to see some hospitalizations in a population managed on immunosuppression therapy ... It’s important to note that none of the adverse events were related to the actual islet product. Also, kidney function remained stable during long-term follow-up in both cohorts, in fact, improving in those who had kidney transplants.”
Overall, he said, “This is a much less invasive procedure that opens itself up to significantly fewer complications than what many of these patients would otherwise require, a pancreas transplant, which involves major abdominal surgery.”
The investigators plan to submit these data as part of a biologic license application (BLA) to the FDA.
The research was supported by grants from JDRF, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Allergy and Infectious Diseases. Dr. Rickels has reported receiving consulting fees from Sernova and Vertex Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Transplantation of cadaveric pancreatic islet cells resulted in graft survival and function with acceptable safety for up to 8 years in selected individuals with type 1 diabetes, new research finds.
The study is a long-term follow-up of two phase 3 pivotal trials from the Clinical Islet Transplantation Consortium of a purified human pancreatic islet cell product for treating people with type 1 diabetes.
One trial involved islet transplantation in 48 people who experienced severe hypoglycemia and hypoglycemic unawareness, and the other trial included 24 people who also experienced those complications and were already receiving immunosuppression following kidney transplant. The trials, both registered with the U.S. Food and Drug Administration (FDA), met their primary efficacy and safety endpoints at 2- and 3-year timepoints.
The follow-up data have now been published in Diabetes Care by Michael Rickels, MD, and colleagues.
The procedure involved infusion through the hepatic portal vein of one or more purified human pancreatic islet products under standardized immunosuppression using methods that Dr. Rickels and colleagues have been developing since 2004. The approach involves multiple modalities to protect the islets prior to transplantation.
Among the 34 islet-alone and eight islet-after–kidney transplant recipients who entered the extended follow-up, durable graft survival allowing for achievement of glycemic targets occurred without severe hypoglycemia or adverse effects from immunosuppression.
The primary outcome, actuarial survival of graft islet function, was 56% at the maximum follow-up of 8.3 years for the islet-only transplantation group and 49% at 7.3 years for the islet-after–kidney transplantation group (P = .004).
The findings suggest that “in the long run, islet transplantation has efficacy, including among those who have had kidney transplants ... Most type 1 diabetes patients are improved tremendously with current insulin delivery systems ... but for those having the most difficulty controlling their blood sugar – and those whose diabetes has already been complicated by needing a kidney transplant – the outcomes we saw in this study are what we’ve been hoping to achieve for more than 20 years,” said Dr. Rickels in a statement from his institution, the University of Pennsylvania, Philadelphia.
In the initial trials at day 75 after the initial transplant, 87.5% of the islet-alone and 71% of the islet-after–kidney transplant group achieved hemoglobin A1c under 7%, and 85% and 54%, respectively, achieved A1c at or under 6.5%. At the end of maximal follow-up, 49% of islet-only transplant recipients maintained A1c under 7%, although none had A1c at or under 6.5%. For the islet-after–kidney transplant group, these proportions were 35% and 17%, respectively (P = .0017 for A1c under 7.0% and P < .0001 for A1c ≤ 6.5%, respectively, between the groups).
There were 12 severe hypoglycemic episodes in five patients (three islet-alone and two islet-after–kidney transplant group) during the initial trials, but no additional episodes occurred in either group during long-term follow-up.
Overall, 53 individuals – 37 in the islet-alone and 16 in the islet-after–kidney transplant group – or 74% of the total, achieved a period of insulin independence with A1c under 7%, ranging from 36 to 481 days. The range of time to achieving insulin independence reflects individuals who received one, two, or three islet infusions.
The fact that most patients achieved insulin independence following just one (n = 20) or two (n = 30) infusions and only three patients required three infusions was notable, Dr. Rickels said.
“Currently, around the world, there’s an expectation of two to three donor pancreases being needed. Here, it’s one, maybe two. It’s a much more efficient protocol and opens up access for more islet transplantation as a hoped-for alternative to pancreas transplants.”
Of those who achieved insulin independence, 30 (57%) remained insulin-independent throughout follow-up (20 of 37 islet-alone and 10 of 16 islet-after–kidney transplant patients), with no difference in duration of insulin independence between the groups.
There were no deaths during post-transplant follow-up. Rates of serious adverse events were 0.31 and 0.43 per patient-year for the islet-after–kidney and islet-alone transplant groups, respectively. Of a total of 104 serious adverse events, 65 occurred during the initial trials and had been previously reported. Of the additional 39 serious adverse events that occurred during long-term follow-up, 11 were possibly due to immunosuppression and 27 were deemed unrelated to the procedures.
According to Dr. Rickels, “These are the most seriously affected patients, and you’d be expecting to see some hospitalizations in a population managed on immunosuppression therapy ... It’s important to note that none of the adverse events were related to the actual islet product. Also, kidney function remained stable during long-term follow-up in both cohorts, in fact, improving in those who had kidney transplants.”
Overall, he said, “This is a much less invasive procedure that opens itself up to significantly fewer complications than what many of these patients would otherwise require, a pancreas transplant, which involves major abdominal surgery.”
The investigators plan to submit these data as part of a biologic license application (BLA) to the FDA.
The research was supported by grants from JDRF, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Allergy and Infectious Diseases. Dr. Rickels has reported receiving consulting fees from Sernova and Vertex Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Transplantation of cadaveric pancreatic islet cells resulted in graft survival and function with acceptable safety for up to 8 years in selected individuals with type 1 diabetes, new research finds.
The study is a long-term follow-up of two phase 3 pivotal trials from the Clinical Islet Transplantation Consortium of a purified human pancreatic islet cell product for treating people with type 1 diabetes.
One trial involved islet transplantation in 48 people who experienced severe hypoglycemia and hypoglycemic unawareness, and the other trial included 24 people who also experienced those complications and were already receiving immunosuppression following kidney transplant. The trials, both registered with the U.S. Food and Drug Administration (FDA), met their primary efficacy and safety endpoints at 2- and 3-year timepoints.
The follow-up data have now been published in Diabetes Care by Michael Rickels, MD, and colleagues.
The procedure involved infusion through the hepatic portal vein of one or more purified human pancreatic islet products under standardized immunosuppression using methods that Dr. Rickels and colleagues have been developing since 2004. The approach involves multiple modalities to protect the islets prior to transplantation.
Among the 34 islet-alone and eight islet-after–kidney transplant recipients who entered the extended follow-up, durable graft survival allowing for achievement of glycemic targets occurred without severe hypoglycemia or adverse effects from immunosuppression.
The primary outcome, actuarial survival of graft islet function, was 56% at the maximum follow-up of 8.3 years for the islet-only transplantation group and 49% at 7.3 years for the islet-after–kidney transplantation group (P = .004).
The findings suggest that “in the long run, islet transplantation has efficacy, including among those who have had kidney transplants ... Most type 1 diabetes patients are improved tremendously with current insulin delivery systems ... but for those having the most difficulty controlling their blood sugar – and those whose diabetes has already been complicated by needing a kidney transplant – the outcomes we saw in this study are what we’ve been hoping to achieve for more than 20 years,” said Dr. Rickels in a statement from his institution, the University of Pennsylvania, Philadelphia.
In the initial trials at day 75 after the initial transplant, 87.5% of the islet-alone and 71% of the islet-after–kidney transplant group achieved hemoglobin A1c under 7%, and 85% and 54%, respectively, achieved A1c at or under 6.5%. At the end of maximal follow-up, 49% of islet-only transplant recipients maintained A1c under 7%, although none had A1c at or under 6.5%. For the islet-after–kidney transplant group, these proportions were 35% and 17%, respectively (P = .0017 for A1c under 7.0% and P < .0001 for A1c ≤ 6.5%, respectively, between the groups).
There were 12 severe hypoglycemic episodes in five patients (three islet-alone and two islet-after–kidney transplant group) during the initial trials, but no additional episodes occurred in either group during long-term follow-up.
Overall, 53 individuals – 37 in the islet-alone and 16 in the islet-after–kidney transplant group – or 74% of the total, achieved a period of insulin independence with A1c under 7%, ranging from 36 to 481 days. The range of time to achieving insulin independence reflects individuals who received one, two, or three islet infusions.
The fact that most patients achieved insulin independence following just one (n = 20) or two (n = 30) infusions and only three patients required three infusions was notable, Dr. Rickels said.
“Currently, around the world, there’s an expectation of two to three donor pancreases being needed. Here, it’s one, maybe two. It’s a much more efficient protocol and opens up access for more islet transplantation as a hoped-for alternative to pancreas transplants.”
Of those who achieved insulin independence, 30 (57%) remained insulin-independent throughout follow-up (20 of 37 islet-alone and 10 of 16 islet-after–kidney transplant patients), with no difference in duration of insulin independence between the groups.
There were no deaths during post-transplant follow-up. Rates of serious adverse events were 0.31 and 0.43 per patient-year for the islet-after–kidney and islet-alone transplant groups, respectively. Of a total of 104 serious adverse events, 65 occurred during the initial trials and had been previously reported. Of the additional 39 serious adverse events that occurred during long-term follow-up, 11 were possibly due to immunosuppression and 27 were deemed unrelated to the procedures.
According to Dr. Rickels, “These are the most seriously affected patients, and you’d be expecting to see some hospitalizations in a population managed on immunosuppression therapy ... It’s important to note that none of the adverse events were related to the actual islet product. Also, kidney function remained stable during long-term follow-up in both cohorts, in fact, improving in those who had kidney transplants.”
Overall, he said, “This is a much less invasive procedure that opens itself up to significantly fewer complications than what many of these patients would otherwise require, a pancreas transplant, which involves major abdominal surgery.”
The investigators plan to submit these data as part of a biologic license application (BLA) to the FDA.
The research was supported by grants from JDRF, the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Allergy and Infectious Diseases. Dr. Rickels has reported receiving consulting fees from Sernova and Vertex Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Menopause symptoms negatively affect women’s work
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
Symptoms of menopause can significantly disrupt a woman’s ability to work, according to a cross-sectional study presented at the annual meeting of the North American Menopause Society.
The study, by researchers at the Mayo Clinic, found that roughly one in eight women said issues stemming from menopause caused them to miss multiple days of work; reduce hours on the job; and even quit, retire, or be laid off.
“We were shocked to see the significant impact of menopause symptoms in the workplace,” Ekta Kapoor, MD, an associate professor of medicine at the Mayo Clinic in Rochester, Minn. said in an interview. “The potential economic impact of untreated menopause symptoms at the workplace is mind-boggling.”
The findings represent an opportunity to improve the treatment of menopause symptoms in working women and “draw attention to the need for creation of workplace policies that include education of employers, managers, and supervisors in order to support midlife women during this universal life stage transition,” Dr. Kapoor added.
Laurie Jeffers, DNP, certified menopause practitioner and codirector of the Center for Midlife Health and Menopause within the department of obstetrics & gynecology at New York University Langone Health, said the findings agree with the results of previous studies from the Netherlands and elsewhere.
“We know that across different studies up to 80% of women during the menopause transition and early post menopause will have high symptom burden, with vasomotor symptoms being the most common,” Dr. Jeffers said. “Psychological symptoms were notably significant in this study, which is also not surprising given that there can be an exacerbation of anxiety or depression during the menopausal transition due to the variability of hormonal activity during this time.”
4,400 women surveyed
Dr. Kapoor and colleagues analyzed data from 4,440 currently employed women, ages 45-60, who were enrolled in the Mayo Clinic Registry of Midlife Women and completed an online questionnaire between March and June 2021 about their menopause symptoms and the symptoms’ effects on their work. The participants all receive their primary care at one of four Mayo Clinic sites in Rochester; Scottsdale, Ariz.; Jacksonville, Fla.; and northwest Wisconsin.
The researchers defined an adverse outcome from a menopausal symptom as one that directly caused women to miss a day from work in the past year or, within the past 6 months, to cut back on work hours, to experience a layoff or job termination, or to quit, retire or change jobs.
Most of the respondents were White (95%), married (77%), and had at least a college degree (59%), and their average age was 54. Their overall average Menopause Rating Scale (MRS) score – including somatic, psychological, and urogenital domains – was 23.1, which indicated a severe level of menopause symptoms.
More than one in eight women (13%) reported having at least one adverse outcome because of menopause symptoms, most commonly missing work (11%).
The women reported missing an average 3 days of work because of menopause symptoms. About half as many (6%) reported cutting back on hours at work in the past 6 months. A small percentage reported being laid off in the past 6 months (0.3%), or quitting, retiring, or changing jobs in the past 6 months (1%) because of menopause symptoms.
Menopause symptoms may well be contributing to the gender wage gap, Dr. Kapoor said, in the same way that other factors affect women’s overall earnings, such as taking time off for having or raising a family, being responsible for a large share of housework, and taking on more mentoring or teaching roles that aren’t as highly valued at work.
“Women going through the menopause transition, and those who are postmenopausal, are at important stages of their careers,” Dr. Kapoor said. “They are often seeking, or already in leadership positions. Any impediments at this important stage in their professional lives can prove to be very costly, resulting in missed opportunities for promotion and leadership roles.”
Unsurprisingly, the higher a woman’s MRS score, the more likely she was to report an adverse work outcome, regardless of the symptom. For example, women whose symptom severity ranked in the top 25% overall were 15.6 times more likely to have an adverse work experience than those with the lowest level of symptoms (P < .001). Psychological symptoms had the greatest effect on work. Women whose psychological symptoms ranked in the top 25% in terms of severity were 21 times more likely to have an adverse work effect, compared with those with the lowest level of severity, according to the researchers.
The results echo findings from a recent survey from Carrot Fertility of 1,000 women, ages 40-55, about the effects of menopause on their careers. In that survey, 79% of respondents described working during menopause as more challenging than other common life stages and life experiences, including starting a new job, starting a family or getting a promotion.
Yet 77% of women felt uncomfortable talking with executives about the problem, and 63% didn’t feel comfortable talking to human resources about the issue. More than half (58%) didn’t want to discuss it with their immediate supervisor. Only 8% said their employer has offered significant support for menopause.
“Menopause symptoms continue to be undertreated for a variety of reasons [and] impact multiple aspects of a woman’s life, including her performance in the workplace,” Dr. Kapoor said. “In addition to focusing our attention on adequate treatment of menopause symptoms, we need advocacy for creation of workplace policies that can help women navigate this important and universal stage of their lives.”
Those policies might include education about menopause to increase knowledge and awareness among employers and managers, Dr. Kapoor said. She also noted the need to improve communication with women in discussing appropriate support and work adjustments during menopause.
"There is also evidence that less than 20%-30% of women seek help for their symptoms,” Dr. Jeffers said. “There are a variety of evidence-based hormonal and nonhormonal options available to ease these symptoms, and knowledgeable clinical management of these symptoms can favorably impact this transition. This study is interesting in that the population of women surveyed presumably had access to high-quality health resources and yet still had a high symptom burden.”
Dr. Kapoor cautioned that the data collection occurred in the midst of the COVID-19 pandemic, “which may have heightened the adverse experiences of women at the workplace. On the other hand, many of these women may have been working from home, which may have made their menopause experience more favorable than it would have been had they been working in actual offices,” thereby again underrepresenting the problem.
Dr. Kapoor added that the study population may not be representative since they all received treatment at a tertiary health care center and were almost all White women.
“Perhaps the impact of menopause symptoms in the minority populations and the community is even greater,” Dr. Kapoor said. “Our data might be underrepresenting the extent of the problem.”
The research did not use external funding. Dr. Kapoor has received grant support from Mithra Pharmaceuticals and consulted for Astellas, Mithra Pharmaceuticals, Scynexis, and Womaness. Dr. Jeffers had no disclosures.
*This story was updated on Nov. 28, 2022.
FROM NAMS 2022
Chest reconstruction surgeries up nearly fourfold among adolescents
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
The number of chest reconstruction surgeries performed for adolescents rose nearly fourfold between 2016 and 2019, researchers report in a study published in JAMA Pediatrics.
“To our knowledge, this study is the largest investigation to date of gender-affirming chest reconstruction in a pediatric population. The results demonstrate substantial increases in gender-affirming chest reconstruction for adolescents,” the authors report.
The researchers, from Vanderbilt University School of Medicine, Nashville, Tenn., used the Nationwide Ambulatory Surgery Sample to identify youth with gender dysphoria who underwent top surgery to remove, or, in rare cases, to add breasts.
The authors identified 829 chest surgeries. They adjusted the number to a weighted figure of 1,130 patients who underwent chest reconstruction during the study period. Of those, 98.6% underwent masculinizing surgery to remove breasts, and 1.4% underwent feminizing surgery. Roughly 100 individuals received gender-affirming chest surgeries in 2016. In 2019, the number had risen to 489 – a 389% increase, the authors reported.
Approximately 44% of the patients in the study were aged 17 years at the time of surgery, while 5.5% were younger than 14.
Around 78% of the individuals who underwent chest surgeries in 2019 were White, 2.7% were Black, 12.2% were Hispanic, and 2.5% were Asian or Pacific Islander. Half of the patients who underwent surgery had a household income of $82,000 or more, according to the researchers.
“Most transgender adolescents had either public or private health insurance coverage for these procedures, contrasting with the predominance of self-payers reported in earlier studies on transgender adults,” write the researchers, citing a 2018 study of trends in transgender surgery.
Masculinizing chest reconstruction, such as mastectomy, and feminizing chest reconstruction, such as augmentation mammaplasty, can be performed as outpatient procedures or as ambulatory surgeries, according to another study .
The study was supported by a grant from the National Center for Advancing Translational Sciences Clinical and Translational Science Awards Program. One author has reported receiving grant funding from Merck.
A version of this article first appeared on Medscape.com.
FROM JAMA PEDIATRICS
Insulin rationing common, ‘surprising’ even among privately insured
Insulin rationing due to cost in the United States is common even among people with diabetes who have private health insurance, new data show.
The findings from the 2021 National Health Interview Survey (NHIS) suggest that about one in six people with insulin-treated diabetes in the United States practice insulin rationing – skipping doses, taking less insulin than needed, or delaying the purchase of insulin – because of the price.
Not surprisingly, those without insurance had the highest rationing rate, at nearly a third. However, those with private insurance also had higher rates, at nearly one in five, than those of the overall diabetes population. And those with public insurance – Medicare and Medicaid – had lower rates.
The finding regarding privately insured individuals was “somewhat surprising,” lead author Adam Gaffney, MD, told this news organization. But he noted that the finding likely reflects issues such as copays and deductibles, along with other barriers patients experience within the private health insurance system.
The authors pointed out that the $35 copay cap on insulin included in the Inflation Reduction Act of 2022 might improve insulin access for Medicare beneficiaries but a similar cap for privately insured people was removed from the bill. Moreover, copay caps don’t help people who are uninsured.
And, although some states have also passed insulin copay caps that apply to privately insured people, “even a monthly cost of $35 can be a lot of money for people with low incomes. That isn’t negligible. It’s important to keep that in mind,” said Dr. Gaffney, a pulmonary and critical care physician at Harvard Medical School, Boston, and Cambridge (Mass.) Health Alliance.
“Insulin rationing is frequently harmful and sometimes deadly. In the ICU, I have cared for patients who have life-threatening complications of diabetes because they couldn’t afford this life-saving drug. Universal access to insulin, without cost barriers, is urgently needed,” Dr. Gaffney said in a Public Citizen statement.
Senior author Steffie Woolhandler, MD, agrees. “Drug companies have ramped up prices on insulin year after year, even for products that remain completely unchanged,” she noted.
“Drug firms are making vast profits at the expense of the health, and even the lives, of patients,” noted Dr. Woolhandler, a distinguished professor at Hunter College, City University of New York, a lecturer in medicine at Harvard, and a research associate at Public Citizen.
Uninsured, privately insured, and younger people more likely to ration
Dr. Gaffney and colleagues’ findings were published online in Annals of Internal Medicine.
The study is the first to examine insulin rationing across the United States among people with all diabetes types treated with insulin using the nationally representative NHIS data.
The results are consistent with those of previous studies, which have found similar rates of insulin rationing at a single U.S. institution and internationally among just those with type 1 diabetes, Dr. Gaffney noted.
In 2021, questions about insulin rationing were added to the NHIS for the first time.
The sample included 982 insulin users with diabetes, representing about 1.4 million U.S. adults with type 1 diabetes, 5.8 million with type 2 diabetes, and 0.4 million with other/unknown types.
Overall, 16.5% of participants – 1.3 million nationwide – reported skipping or reducing insulin doses or delaying the purchase of it in the past year. Delaying purchase was the most common type of rationing, reported by 14.2%, while taking less than needed was the most common practice among those with type 1 diabetes (16.5%).
Age made a difference, with 11.2% of adults aged 65 or older versus 20.4% of younger people reporting rationing. And by income level, even among those at the top level examined – 400% or higher of the federal poverty line – 10.8% reported rationing.
“The high-income group is not necessarily rich. Many would be considered middle-income,” Dr. Gaffney pointed out.
By race, 23.2% of Black participants reported rationing compared with 16.0% of White and Hispanic individuals.
People without insurance had the highest rationing rate (29.2%), followed by those with private insurance (18.8%), other coverage (16.1%), Medicare (13.5%), and Medicaid (11.6%).
‘It’s a complicated system’
Dr. Gaffney noted that even when the patient has private insurance, it’s challenging for the clinician to know in advance whether there are formulary restrictions on what type of insulin can be prescribed or what the patient’s copay or deductible will be.
“Often the prescription gets written without clear knowledge of coverage beforehand ... Coverage differs from patient to patient, from insurance to insurance. It’s a complicated system.”
He added, though, that some electronic health records (EHRs) incorporate this information. “Currently, some EHRs give real-time feedback. I see no reason why, for all the money we plug into these EHRs, there couldn’t be real-time feedback for every patient so you know what the copay is and whether it’s covered at the time you’re prescribing it. To me that’s a very straightforward technological fix that we could achieve. We have the information, but it’s hard to act on it.”
But beyond the EHR, “there are also problems when the patient’s insurance changes or their network changes, and what insulin is covered changes. And they don’t necessarily get that new prescription in time. And suddenly they have a gap. Gaps can be dangerous.”
What’s more, Dr. Gaffney noted: “The study raises concerning questions about what happens when the public health emergency ends and millions of people with Medicaid lose their coverage. Where are they going to get insulin? That’s another population we have to be worried about.”
All of this puts clinicians in a difficult spot, he said.
“They want the best for their patients but they’re working in a system that’s not letting them focus on practicing medicine and instead is forcing them to think about these economic issues that are in large part out of their control.”
Dr. Gaffney is a member of Physicians for a National Health Program, which advocates for a single-payer health system in the United States.
A version of this article first appeared on Medscape.com.
Insulin rationing due to cost in the United States is common even among people with diabetes who have private health insurance, new data show.
The findings from the 2021 National Health Interview Survey (NHIS) suggest that about one in six people with insulin-treated diabetes in the United States practice insulin rationing – skipping doses, taking less insulin than needed, or delaying the purchase of insulin – because of the price.
Not surprisingly, those without insurance had the highest rationing rate, at nearly a third. However, those with private insurance also had higher rates, at nearly one in five, than those of the overall diabetes population. And those with public insurance – Medicare and Medicaid – had lower rates.
The finding regarding privately insured individuals was “somewhat surprising,” lead author Adam Gaffney, MD, told this news organization. But he noted that the finding likely reflects issues such as copays and deductibles, along with other barriers patients experience within the private health insurance system.
The authors pointed out that the $35 copay cap on insulin included in the Inflation Reduction Act of 2022 might improve insulin access for Medicare beneficiaries but a similar cap for privately insured people was removed from the bill. Moreover, copay caps don’t help people who are uninsured.
And, although some states have also passed insulin copay caps that apply to privately insured people, “even a monthly cost of $35 can be a lot of money for people with low incomes. That isn’t negligible. It’s important to keep that in mind,” said Dr. Gaffney, a pulmonary and critical care physician at Harvard Medical School, Boston, and Cambridge (Mass.) Health Alliance.
“Insulin rationing is frequently harmful and sometimes deadly. In the ICU, I have cared for patients who have life-threatening complications of diabetes because they couldn’t afford this life-saving drug. Universal access to insulin, without cost barriers, is urgently needed,” Dr. Gaffney said in a Public Citizen statement.
Senior author Steffie Woolhandler, MD, agrees. “Drug companies have ramped up prices on insulin year after year, even for products that remain completely unchanged,” she noted.
“Drug firms are making vast profits at the expense of the health, and even the lives, of patients,” noted Dr. Woolhandler, a distinguished professor at Hunter College, City University of New York, a lecturer in medicine at Harvard, and a research associate at Public Citizen.
Uninsured, privately insured, and younger people more likely to ration
Dr. Gaffney and colleagues’ findings were published online in Annals of Internal Medicine.
The study is the first to examine insulin rationing across the United States among people with all diabetes types treated with insulin using the nationally representative NHIS data.
The results are consistent with those of previous studies, which have found similar rates of insulin rationing at a single U.S. institution and internationally among just those with type 1 diabetes, Dr. Gaffney noted.
In 2021, questions about insulin rationing were added to the NHIS for the first time.
The sample included 982 insulin users with diabetes, representing about 1.4 million U.S. adults with type 1 diabetes, 5.8 million with type 2 diabetes, and 0.4 million with other/unknown types.
Overall, 16.5% of participants – 1.3 million nationwide – reported skipping or reducing insulin doses or delaying the purchase of it in the past year. Delaying purchase was the most common type of rationing, reported by 14.2%, while taking less than needed was the most common practice among those with type 1 diabetes (16.5%).
Age made a difference, with 11.2% of adults aged 65 or older versus 20.4% of younger people reporting rationing. And by income level, even among those at the top level examined – 400% or higher of the federal poverty line – 10.8% reported rationing.
“The high-income group is not necessarily rich. Many would be considered middle-income,” Dr. Gaffney pointed out.
By race, 23.2% of Black participants reported rationing compared with 16.0% of White and Hispanic individuals.
People without insurance had the highest rationing rate (29.2%), followed by those with private insurance (18.8%), other coverage (16.1%), Medicare (13.5%), and Medicaid (11.6%).
‘It’s a complicated system’
Dr. Gaffney noted that even when the patient has private insurance, it’s challenging for the clinician to know in advance whether there are formulary restrictions on what type of insulin can be prescribed or what the patient’s copay or deductible will be.
“Often the prescription gets written without clear knowledge of coverage beforehand ... Coverage differs from patient to patient, from insurance to insurance. It’s a complicated system.”
He added, though, that some electronic health records (EHRs) incorporate this information. “Currently, some EHRs give real-time feedback. I see no reason why, for all the money we plug into these EHRs, there couldn’t be real-time feedback for every patient so you know what the copay is and whether it’s covered at the time you’re prescribing it. To me that’s a very straightforward technological fix that we could achieve. We have the information, but it’s hard to act on it.”
But beyond the EHR, “there are also problems when the patient’s insurance changes or their network changes, and what insulin is covered changes. And they don’t necessarily get that new prescription in time. And suddenly they have a gap. Gaps can be dangerous.”
What’s more, Dr. Gaffney noted: “The study raises concerning questions about what happens when the public health emergency ends and millions of people with Medicaid lose their coverage. Where are they going to get insulin? That’s another population we have to be worried about.”
All of this puts clinicians in a difficult spot, he said.
“They want the best for their patients but they’re working in a system that’s not letting them focus on practicing medicine and instead is forcing them to think about these economic issues that are in large part out of their control.”
Dr. Gaffney is a member of Physicians for a National Health Program, which advocates for a single-payer health system in the United States.
A version of this article first appeared on Medscape.com.
Insulin rationing due to cost in the United States is common even among people with diabetes who have private health insurance, new data show.
The findings from the 2021 National Health Interview Survey (NHIS) suggest that about one in six people with insulin-treated diabetes in the United States practice insulin rationing – skipping doses, taking less insulin than needed, or delaying the purchase of insulin – because of the price.
Not surprisingly, those without insurance had the highest rationing rate, at nearly a third. However, those with private insurance also had higher rates, at nearly one in five, than those of the overall diabetes population. And those with public insurance – Medicare and Medicaid – had lower rates.
The finding regarding privately insured individuals was “somewhat surprising,” lead author Adam Gaffney, MD, told this news organization. But he noted that the finding likely reflects issues such as copays and deductibles, along with other barriers patients experience within the private health insurance system.
The authors pointed out that the $35 copay cap on insulin included in the Inflation Reduction Act of 2022 might improve insulin access for Medicare beneficiaries but a similar cap for privately insured people was removed from the bill. Moreover, copay caps don’t help people who are uninsured.
And, although some states have also passed insulin copay caps that apply to privately insured people, “even a monthly cost of $35 can be a lot of money for people with low incomes. That isn’t negligible. It’s important to keep that in mind,” said Dr. Gaffney, a pulmonary and critical care physician at Harvard Medical School, Boston, and Cambridge (Mass.) Health Alliance.
“Insulin rationing is frequently harmful and sometimes deadly. In the ICU, I have cared for patients who have life-threatening complications of diabetes because they couldn’t afford this life-saving drug. Universal access to insulin, without cost barriers, is urgently needed,” Dr. Gaffney said in a Public Citizen statement.
Senior author Steffie Woolhandler, MD, agrees. “Drug companies have ramped up prices on insulin year after year, even for products that remain completely unchanged,” she noted.
“Drug firms are making vast profits at the expense of the health, and even the lives, of patients,” noted Dr. Woolhandler, a distinguished professor at Hunter College, City University of New York, a lecturer in medicine at Harvard, and a research associate at Public Citizen.
Uninsured, privately insured, and younger people more likely to ration
Dr. Gaffney and colleagues’ findings were published online in Annals of Internal Medicine.
The study is the first to examine insulin rationing across the United States among people with all diabetes types treated with insulin using the nationally representative NHIS data.
The results are consistent with those of previous studies, which have found similar rates of insulin rationing at a single U.S. institution and internationally among just those with type 1 diabetes, Dr. Gaffney noted.
In 2021, questions about insulin rationing were added to the NHIS for the first time.
The sample included 982 insulin users with diabetes, representing about 1.4 million U.S. adults with type 1 diabetes, 5.8 million with type 2 diabetes, and 0.4 million with other/unknown types.
Overall, 16.5% of participants – 1.3 million nationwide – reported skipping or reducing insulin doses or delaying the purchase of it in the past year. Delaying purchase was the most common type of rationing, reported by 14.2%, while taking less than needed was the most common practice among those with type 1 diabetes (16.5%).
Age made a difference, with 11.2% of adults aged 65 or older versus 20.4% of younger people reporting rationing. And by income level, even among those at the top level examined – 400% or higher of the federal poverty line – 10.8% reported rationing.
“The high-income group is not necessarily rich. Many would be considered middle-income,” Dr. Gaffney pointed out.
By race, 23.2% of Black participants reported rationing compared with 16.0% of White and Hispanic individuals.
People without insurance had the highest rationing rate (29.2%), followed by those with private insurance (18.8%), other coverage (16.1%), Medicare (13.5%), and Medicaid (11.6%).
‘It’s a complicated system’
Dr. Gaffney noted that even when the patient has private insurance, it’s challenging for the clinician to know in advance whether there are formulary restrictions on what type of insulin can be prescribed or what the patient’s copay or deductible will be.
“Often the prescription gets written without clear knowledge of coverage beforehand ... Coverage differs from patient to patient, from insurance to insurance. It’s a complicated system.”
He added, though, that some electronic health records (EHRs) incorporate this information. “Currently, some EHRs give real-time feedback. I see no reason why, for all the money we plug into these EHRs, there couldn’t be real-time feedback for every patient so you know what the copay is and whether it’s covered at the time you’re prescribing it. To me that’s a very straightforward technological fix that we could achieve. We have the information, but it’s hard to act on it.”
But beyond the EHR, “there are also problems when the patient’s insurance changes or their network changes, and what insulin is covered changes. And they don’t necessarily get that new prescription in time. And suddenly they have a gap. Gaps can be dangerous.”
What’s more, Dr. Gaffney noted: “The study raises concerning questions about what happens when the public health emergency ends and millions of people with Medicaid lose their coverage. Where are they going to get insulin? That’s another population we have to be worried about.”
All of this puts clinicians in a difficult spot, he said.
“They want the best for their patients but they’re working in a system that’s not letting them focus on practicing medicine and instead is forcing them to think about these economic issues that are in large part out of their control.”
Dr. Gaffney is a member of Physicians for a National Health Program, which advocates for a single-payer health system in the United States.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Diabetes becoming less potent risk factor for CVD events
Diabetes persists as a risk factor for cardiovascular events, but where it once meant the same risk of heart attack or stroke as cardiovascular disease itself, a large Canadian population study reports that’s no longer the case. Thanks to advances in diabetes management over the past quarter century, diabetes is no longer considered equivalent to CVD as a risk factor for cardiovascular events, researchers from the University of Toronto reported.
The retrospective, population-based study used administrative data from Ontario’s provincial universal health care system. The researchers created five population-based cohorts of adults at 5-year intervals from 1994 to 2014, consisting of 1.87 million adults in the first cohort and 1.5 million in the last. In that 20-year span, the prevalence of diabetes in this population tripled, from 3.1% to 9%.
“In the last 25 years we’ve seen wholesale changes in the way people approach diabetes,” lead study author Calvin Ke, MD, PhD, an endocrinologist and assistant professor at the University of Toronto, said in an interview. “Part of the findings show that diabetes and cardiovascular disease were equivalent for risk of cardiovascular events in 1994, but by 2014 that was not the case.”
However, Dr. Ke added, “Diabetes is still a very strong cardiovascular risk factor.”
The investigators for the study, reported as a research letter in JAMA, analyzed the risk of cardiovascular events in four subgroups: those who had both diabetes and CVD, CVD only, diabetes only, and no CVD or diabetes.
Between 1994 and 2014, the cardiovascular event rates declined significantly among people with diabetes alone, compared with people with no disease: from 28.4 to 12.7 per 1,000 person-years, or an absolute risk increase (ARI) of 4.4% and a relative risk (RR) more than double (2.06), in 1994 to 14 vs. 8 per 1,000 person-years, and an ARI of 2% and RR less than double (1.58) 20 years later.
Among people with CVD only, those values shifted from 36.1 per 1,000 person-years, ARI of 5.1% and RR of 2.16 in 1994 to 23.9, ARI of 3.7% and RR still more than double (2.06) in 2014.
People with both CVD and diabetes had the highest CVD event rates across all 5-year cohorts: 74 per 1,000 person-years, ARI of 12% and RR almost four times greater (3.81) in 1994 than people with no disease. By 2014, the ARI in this group was 7.6% and the RR 3.10.
The investigators calculated that event rates from 1994 to 2014 declined across all four subgroups, with rate ratios of 0.49 for diabetes only, 0.66 for CVD only, 0.60 for both diabetes and CVD, and 0.63 for neither disease.
Shift in practice
The study noted that the shift in diabetes as a risk factor for heart attack and stroke is “a change that likely reflects the use of modern, multifactorial approaches to diabetes.”
“A number of changes have occurred in practice that really focus on this idea of a multifactorial approach to diabetes: more aggressive management of blood sugar, blood pressure, and lipids,” Dr. Ke said. “We know from the statin trials that statins can reduce the risk of heart disease significantly, and the use of statins increased from 28.4% in 1999 to 56.3% in 2018 in the United States,” Dr. Ke said. He added that statin use in Canada in adults ages 40 and older went from 1.2% in 1994 to 58.4% in 2010-2015. Use of ACE inhibitors and angiotensin receptor blockers for hypertension followed similar trends, contributing further to reducing risks for heart attack and stroke, Dr. Ke said.
Dr. Ke also noted that the evolution of guidelines and advances in treatments for both CVD and diabetes since 1994 have contributed to improving risks for people with diabetes. SGLT2 inhibitors have been linked to a 2%-6% reduction in hemoglobin A1c, he said. “All of these factors combined have had a major effect on the reduced risk of cardiovascular events.”
Prakash Deedwania, MD, professor at the University of California, San Francisco, Fresno, said that this study confirms a trend that others have reported regarding the risk of CVD in diabetes. The large database covering millions of adults is a study strength, he said.
And the findings, Dr. Deedwania added, underscore what’s been published in clinical guidelines, notably the American Heart Association scientific statement for managing CVD risk in patients with diabetes. “This means that, from observations made 20-plus years ago, when most people were not being treated for diabetes or heart disease, the pendulum has swung,” he said.
However, he added, “The authors state clearly that it does not mean that diabetes is not associated with a higher risk of cardiovascular events; it just means it is no longer equivalent to CVD.”
Managing diabetes continues to be “particularly important,” Dr. Deedwania said, because the prevalence of diabetes continues to rise. “This is a phenomenal risk, and it emphasizes that, to really conquer or control diabetes, we should make every effort to prevent diabetes,” he said.
Dr. Ke and Dr. Deedwania have no relevant financial relationships to disclose.
Diabetes persists as a risk factor for cardiovascular events, but where it once meant the same risk of heart attack or stroke as cardiovascular disease itself, a large Canadian population study reports that’s no longer the case. Thanks to advances in diabetes management over the past quarter century, diabetes is no longer considered equivalent to CVD as a risk factor for cardiovascular events, researchers from the University of Toronto reported.
The retrospective, population-based study used administrative data from Ontario’s provincial universal health care system. The researchers created five population-based cohorts of adults at 5-year intervals from 1994 to 2014, consisting of 1.87 million adults in the first cohort and 1.5 million in the last. In that 20-year span, the prevalence of diabetes in this population tripled, from 3.1% to 9%.
“In the last 25 years we’ve seen wholesale changes in the way people approach diabetes,” lead study author Calvin Ke, MD, PhD, an endocrinologist and assistant professor at the University of Toronto, said in an interview. “Part of the findings show that diabetes and cardiovascular disease were equivalent for risk of cardiovascular events in 1994, but by 2014 that was not the case.”
However, Dr. Ke added, “Diabetes is still a very strong cardiovascular risk factor.”
The investigators for the study, reported as a research letter in JAMA, analyzed the risk of cardiovascular events in four subgroups: those who had both diabetes and CVD, CVD only, diabetes only, and no CVD or diabetes.
Between 1994 and 2014, the cardiovascular event rates declined significantly among people with diabetes alone, compared with people with no disease: from 28.4 to 12.7 per 1,000 person-years, or an absolute risk increase (ARI) of 4.4% and a relative risk (RR) more than double (2.06), in 1994 to 14 vs. 8 per 1,000 person-years, and an ARI of 2% and RR less than double (1.58) 20 years later.
Among people with CVD only, those values shifted from 36.1 per 1,000 person-years, ARI of 5.1% and RR of 2.16 in 1994 to 23.9, ARI of 3.7% and RR still more than double (2.06) in 2014.
People with both CVD and diabetes had the highest CVD event rates across all 5-year cohorts: 74 per 1,000 person-years, ARI of 12% and RR almost four times greater (3.81) in 1994 than people with no disease. By 2014, the ARI in this group was 7.6% and the RR 3.10.
The investigators calculated that event rates from 1994 to 2014 declined across all four subgroups, with rate ratios of 0.49 for diabetes only, 0.66 for CVD only, 0.60 for both diabetes and CVD, and 0.63 for neither disease.
Shift in practice
The study noted that the shift in diabetes as a risk factor for heart attack and stroke is “a change that likely reflects the use of modern, multifactorial approaches to diabetes.”
“A number of changes have occurred in practice that really focus on this idea of a multifactorial approach to diabetes: more aggressive management of blood sugar, blood pressure, and lipids,” Dr. Ke said. “We know from the statin trials that statins can reduce the risk of heart disease significantly, and the use of statins increased from 28.4% in 1999 to 56.3% in 2018 in the United States,” Dr. Ke said. He added that statin use in Canada in adults ages 40 and older went from 1.2% in 1994 to 58.4% in 2010-2015. Use of ACE inhibitors and angiotensin receptor blockers for hypertension followed similar trends, contributing further to reducing risks for heart attack and stroke, Dr. Ke said.
Dr. Ke also noted that the evolution of guidelines and advances in treatments for both CVD and diabetes since 1994 have contributed to improving risks for people with diabetes. SGLT2 inhibitors have been linked to a 2%-6% reduction in hemoglobin A1c, he said. “All of these factors combined have had a major effect on the reduced risk of cardiovascular events.”
Prakash Deedwania, MD, professor at the University of California, San Francisco, Fresno, said that this study confirms a trend that others have reported regarding the risk of CVD in diabetes. The large database covering millions of adults is a study strength, he said.
And the findings, Dr. Deedwania added, underscore what’s been published in clinical guidelines, notably the American Heart Association scientific statement for managing CVD risk in patients with diabetes. “This means that, from observations made 20-plus years ago, when most people were not being treated for diabetes or heart disease, the pendulum has swung,” he said.
However, he added, “The authors state clearly that it does not mean that diabetes is not associated with a higher risk of cardiovascular events; it just means it is no longer equivalent to CVD.”
Managing diabetes continues to be “particularly important,” Dr. Deedwania said, because the prevalence of diabetes continues to rise. “This is a phenomenal risk, and it emphasizes that, to really conquer or control diabetes, we should make every effort to prevent diabetes,” he said.
Dr. Ke and Dr. Deedwania have no relevant financial relationships to disclose.
Diabetes persists as a risk factor for cardiovascular events, but where it once meant the same risk of heart attack or stroke as cardiovascular disease itself, a large Canadian population study reports that’s no longer the case. Thanks to advances in diabetes management over the past quarter century, diabetes is no longer considered equivalent to CVD as a risk factor for cardiovascular events, researchers from the University of Toronto reported.
The retrospective, population-based study used administrative data from Ontario’s provincial universal health care system. The researchers created five population-based cohorts of adults at 5-year intervals from 1994 to 2014, consisting of 1.87 million adults in the first cohort and 1.5 million in the last. In that 20-year span, the prevalence of diabetes in this population tripled, from 3.1% to 9%.
“In the last 25 years we’ve seen wholesale changes in the way people approach diabetes,” lead study author Calvin Ke, MD, PhD, an endocrinologist and assistant professor at the University of Toronto, said in an interview. “Part of the findings show that diabetes and cardiovascular disease were equivalent for risk of cardiovascular events in 1994, but by 2014 that was not the case.”
However, Dr. Ke added, “Diabetes is still a very strong cardiovascular risk factor.”
The investigators for the study, reported as a research letter in JAMA, analyzed the risk of cardiovascular events in four subgroups: those who had both diabetes and CVD, CVD only, diabetes only, and no CVD or diabetes.
Between 1994 and 2014, the cardiovascular event rates declined significantly among people with diabetes alone, compared with people with no disease: from 28.4 to 12.7 per 1,000 person-years, or an absolute risk increase (ARI) of 4.4% and a relative risk (RR) more than double (2.06), in 1994 to 14 vs. 8 per 1,000 person-years, and an ARI of 2% and RR less than double (1.58) 20 years later.
Among people with CVD only, those values shifted from 36.1 per 1,000 person-years, ARI of 5.1% and RR of 2.16 in 1994 to 23.9, ARI of 3.7% and RR still more than double (2.06) in 2014.
People with both CVD and diabetes had the highest CVD event rates across all 5-year cohorts: 74 per 1,000 person-years, ARI of 12% and RR almost four times greater (3.81) in 1994 than people with no disease. By 2014, the ARI in this group was 7.6% and the RR 3.10.
The investigators calculated that event rates from 1994 to 2014 declined across all four subgroups, with rate ratios of 0.49 for diabetes only, 0.66 for CVD only, 0.60 for both diabetes and CVD, and 0.63 for neither disease.
Shift in practice
The study noted that the shift in diabetes as a risk factor for heart attack and stroke is “a change that likely reflects the use of modern, multifactorial approaches to diabetes.”
“A number of changes have occurred in practice that really focus on this idea of a multifactorial approach to diabetes: more aggressive management of blood sugar, blood pressure, and lipids,” Dr. Ke said. “We know from the statin trials that statins can reduce the risk of heart disease significantly, and the use of statins increased from 28.4% in 1999 to 56.3% in 2018 in the United States,” Dr. Ke said. He added that statin use in Canada in adults ages 40 and older went from 1.2% in 1994 to 58.4% in 2010-2015. Use of ACE inhibitors and angiotensin receptor blockers for hypertension followed similar trends, contributing further to reducing risks for heart attack and stroke, Dr. Ke said.
Dr. Ke also noted that the evolution of guidelines and advances in treatments for both CVD and diabetes since 1994 have contributed to improving risks for people with diabetes. SGLT2 inhibitors have been linked to a 2%-6% reduction in hemoglobin A1c, he said. “All of these factors combined have had a major effect on the reduced risk of cardiovascular events.”
Prakash Deedwania, MD, professor at the University of California, San Francisco, Fresno, said that this study confirms a trend that others have reported regarding the risk of CVD in diabetes. The large database covering millions of adults is a study strength, he said.
And the findings, Dr. Deedwania added, underscore what’s been published in clinical guidelines, notably the American Heart Association scientific statement for managing CVD risk in patients with diabetes. “This means that, from observations made 20-plus years ago, when most people were not being treated for diabetes or heart disease, the pendulum has swung,” he said.
However, he added, “The authors state clearly that it does not mean that diabetes is not associated with a higher risk of cardiovascular events; it just means it is no longer equivalent to CVD.”
Managing diabetes continues to be “particularly important,” Dr. Deedwania said, because the prevalence of diabetes continues to rise. “This is a phenomenal risk, and it emphasizes that, to really conquer or control diabetes, we should make every effort to prevent diabetes,” he said.
Dr. Ke and Dr. Deedwania have no relevant financial relationships to disclose.
FROM JAMA














