User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Psychiatric patients and pandemics
What can psychiatric clinicians do to keep their patients healthy in this coronavirus time?
In the 3 days between starting this column and finishing it, the world has gone into a tailspin. Perhaps what I write is no longer relevant. But hopefully it is.
I have no right or wrong answers here but thoughts about factors to consider.
- On inpatient psychiatry wards, the emphasis is on communal living. On our ward, bedrooms and bathrooms are shared. Patients eat together. There are numerous group therapies.
- We have decided to restrict visitors out of the concern that one may infect a ward of patients and staff. We are hoping to do video visitation, but that may take a while to implement.
- An open question is how we are going to provide our involuntary patients with access to the public defense attorneys. Public defenders still have the ability to come onto the inpatient ward, but we will start screening them first.
- In terms of sanitation, wall sanitizers are forbidden, since sanitizers may be drank or made into a firebomb. So we are incessantly wiping down the shared phones and game board pieces.
- Looking at the outpatient arena, we have moved our chairs around, so that there are 3 feet between chairs. We have opened up another waiting room to provide more distance.
- We are trying to decide whether to cancel groups. We did cancel our senior group, and I think I will cancel the rest of them shortly.
- We are seriously looking at telepsychiatry.
- Schools are closed. Many of my clinicians have young children, so they may be out. We are expecting many patients to cancel and will see how that plays out. Others of us have elderly parents. My mother’s assisted-living facility is on lockdown. So, having been locked out after a visit, she is with me tonight.
- Psychiatrists are expected to keep up their relative value unit count. Can they meet their targets? Probably not. Will it matter?
- And what about all our homeless patients, who cannot disinfect their tents or shelters?
- Conferences no longer seem so important. I am less worried about coverage for the American Psychiatric Association meeting, since the 2020 conference has been canceled.
On the rosy side, maybe this will be a wake-up call about climate change. So we live in interesting times.
Take care of your patients and each other.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures.
What can psychiatric clinicians do to keep their patients healthy in this coronavirus time?
In the 3 days between starting this column and finishing it, the world has gone into a tailspin. Perhaps what I write is no longer relevant. But hopefully it is.
I have no right or wrong answers here but thoughts about factors to consider.
- On inpatient psychiatry wards, the emphasis is on communal living. On our ward, bedrooms and bathrooms are shared. Patients eat together. There are numerous group therapies.
- We have decided to restrict visitors out of the concern that one may infect a ward of patients and staff. We are hoping to do video visitation, but that may take a while to implement.
- An open question is how we are going to provide our involuntary patients with access to the public defense attorneys. Public defenders still have the ability to come onto the inpatient ward, but we will start screening them first.
- In terms of sanitation, wall sanitizers are forbidden, since sanitizers may be drank or made into a firebomb. So we are incessantly wiping down the shared phones and game board pieces.
- Looking at the outpatient arena, we have moved our chairs around, so that there are 3 feet between chairs. We have opened up another waiting room to provide more distance.
- We are trying to decide whether to cancel groups. We did cancel our senior group, and I think I will cancel the rest of them shortly.
- We are seriously looking at telepsychiatry.
- Schools are closed. Many of my clinicians have young children, so they may be out. We are expecting many patients to cancel and will see how that plays out. Others of us have elderly parents. My mother’s assisted-living facility is on lockdown. So, having been locked out after a visit, she is with me tonight.
- Psychiatrists are expected to keep up their relative value unit count. Can they meet their targets? Probably not. Will it matter?
- And what about all our homeless patients, who cannot disinfect their tents or shelters?
- Conferences no longer seem so important. I am less worried about coverage for the American Psychiatric Association meeting, since the 2020 conference has been canceled.
On the rosy side, maybe this will be a wake-up call about climate change. So we live in interesting times.
Take care of your patients and each other.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures.
What can psychiatric clinicians do to keep their patients healthy in this coronavirus time?
In the 3 days between starting this column and finishing it, the world has gone into a tailspin. Perhaps what I write is no longer relevant. But hopefully it is.
I have no right or wrong answers here but thoughts about factors to consider.
- On inpatient psychiatry wards, the emphasis is on communal living. On our ward, bedrooms and bathrooms are shared. Patients eat together. There are numerous group therapies.
- We have decided to restrict visitors out of the concern that one may infect a ward of patients and staff. We are hoping to do video visitation, but that may take a while to implement.
- An open question is how we are going to provide our involuntary patients with access to the public defense attorneys. Public defenders still have the ability to come onto the inpatient ward, but we will start screening them first.
- In terms of sanitation, wall sanitizers are forbidden, since sanitizers may be drank or made into a firebomb. So we are incessantly wiping down the shared phones and game board pieces.
- Looking at the outpatient arena, we have moved our chairs around, so that there are 3 feet between chairs. We have opened up another waiting room to provide more distance.
- We are trying to decide whether to cancel groups. We did cancel our senior group, and I think I will cancel the rest of them shortly.
- We are seriously looking at telepsychiatry.
- Schools are closed. Many of my clinicians have young children, so they may be out. We are expecting many patients to cancel and will see how that plays out. Others of us have elderly parents. My mother’s assisted-living facility is on lockdown. So, having been locked out after a visit, she is with me tonight.
- Psychiatrists are expected to keep up their relative value unit count. Can they meet their targets? Probably not. Will it matter?
- And what about all our homeless patients, who cannot disinfect their tents or shelters?
- Conferences no longer seem so important. I am less worried about coverage for the American Psychiatric Association meeting, since the 2020 conference has been canceled.
On the rosy side, maybe this will be a wake-up call about climate change. So we live in interesting times.
Take care of your patients and each other.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures.
Here’s what ICUs are putting up against COVID-19
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.
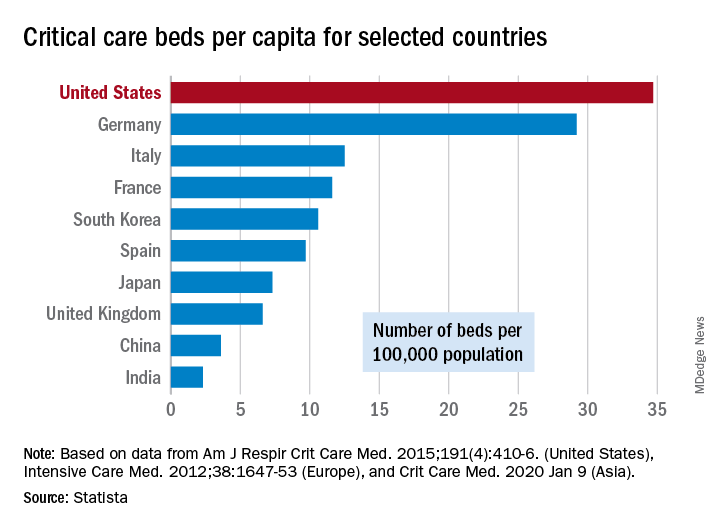
That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
COVID-19 in children, pregnant women: What do we know?
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
Flattening the curve: Viral graphic shows COVID-19 containment needs
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.
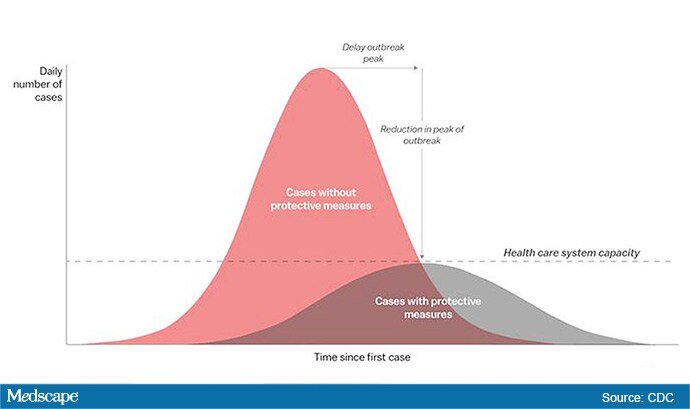
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.

Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.

Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
So you have a COVID-19 patient: How do you treat them?
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
President declares national emergency for COVID-19, ramps up testing capability
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
After weeks of decline, influenza activity increases slightly
The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.

The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.

The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.
Lombardy ICU capacity stressed to breaking point by COVID-19 outbreak
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.
Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
REPORTING FROM JAMA LIVE STREAM
Internist reports from COVID-19 front lines near Seattle
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.
Your medical conference is canceled. Now what?
Khadija Hafidh, MD, was already booked on a 14-hour, direct flight from Dubai to Los Angeles, when the American College of Physicians (ACP) announced it was canceling its internal medicine meeting scheduled for April.
Canceling her hotel reservation was not a problem, and she was assured a refund for the conference fee, but her airline ticket was another matter, said Dr. Hafidh, an internist and diabetologist with the Dubai Health Authority.
“The airline I booked my ticket with is willing to waive the change fees, but will deduct a cancellation fee if I choose not to take the trip,” Dr. Hafidh said in an interview. “The cancellation fees is $300. A bit steep I must admit.”
Dr. Hafidh now faces a dilemma: Lose the $300 and cancel, or change her flight dates to June for the American Diabetes Association meeting in Chicago.
“But then again, we aren’t sure if that meeting will take place,” Dr. Hafidh said. “A few weeks ago I thought this whole thing was just a storm in a tea cup. However when it was declared a pandemic yesterday, it brought about another dimension.”
More than 25 medical meetings and conferences across the globe have been canceled or postponed because of COVID-19 concerns. The sudden cancellations have caused reservation woes and travel headaches for thousands of physicians who planned to attend the meetings. Some societies are considering the idea of virtual conferences, while other associations have scrapped their meetings until next year.
For physicians facing a canceled conference, the most likely question is, what now? Read on for tips and suggestions.
Reservation refunds vary
Refunds on airfare because of conference cancellations differ, depending on the airline and where you were traveling. Some airlines, such as United Airlines, have waived all change fees for tickets issued March 3, 2020, through March 31, 2020, and passengers can change their dates for up to 12 months after the ticket was issued.
Full refunds often depend on whether your ticket was nonrefundable when purchased. Many airlines, such as Delta, are providing full refunds if the airline canceled your flight. JetBlue is waiving all change and cancellation fees for customers scheduled to travel March 10, 2020, through April 30, 2020.
Las Vegas–based dermatologist H.L. Greenberg, MD, was satisfied with the credit he received from Southwest Airlines after the American Academy of Dermatology (AAD) canceled its Denver meeting. He and his staff were looking forward to the gathering, but he noted that the meeting would likely have been limited, even if it had take place as scheduled.
“I am disappointed that I won’t be able to meet with colleagues and industry to explore what the latest advances and interests are in dermatology,” he said. “Because many academic institutions were forbidding their faculty from traveling, the content of the meeting was going to be severely diminished. It’s just a rough time for everyone.”
Meanwhile, Asa Radix, MD, PhD, a New York–based internist, received a full refund for his Amtrak ticket to Boston when the Conference on Retroviruses and Opportunistic Infections (CROI) scheduled for early March was converted to a virtual meeting. Dr. Radix, senior director of research and education at the Callen-Lorde Community Health Center in New York, left another meeting in Brazil early to get to the Boston conference, he said.
“I was packed, but really that was a minor inconvenience,” he said in an interview. “I appreciate that they prioritized health concerns and changed to a virtual meeting. I received full refunds, no issues whatsoever. [It was] really great since I had no travel insurance.”
Check with your individual airline or train line for information about ticket refunds and credits. Many airlines are currently making special accommodations because of COVID-19. If your flight was covered by trip insurance, also called travel assistance, you are generally protected against unforeseen financial losses such as cancellations. The U.S. Department of Transportation provides this general online resource about airline refunds.
Hotel refunds probable
Most meeting organizations who have made the decision to cancel or postpone a conference also have canceled block hotel reservations reserved for the meeting. Medical associations are not directly refunding the hotel costs, but the majority of hotels are refunding reservations with no questions asked. Physicians interviewed for this story all reported no trouble getting refunds for their hotel reservations. However, attendees who did not book a hotel in official housing blocks should contact the hotel directly to cancel.
What about registration fees?
In response to COVID-19 cancellations, most conference leaders are refunding registration fees in full for both attendees and exhibitors. The refund may not be automatic, some associations such as ACP and the American College of Obstetricians and Gynecologists state it may take up to 45 days for the funds to be credited, depending on the payment used.
If the conference you planned to attend was postponed, the registration fee may be assigned to the new meeting dates and the money may not be refunded. Registration fees for the Minimally Invasive Surgery Symposium, for example, delayed until an unconfirmed date, and for the European Association of Urology (EAU) meeting, postponed until July, will be automatically credited to the rescheduled meeting, according to the websites. If attendees cannot attend the rescheduled EAU meeting, the association will not provide a refund and the registration will not apply to the 2021 meeting, according to its website. However, the group is providing registrants with a free access code for the EAU20 Resource Centre, which contains websites of sessions and scientific content.
A number of physicians have expressed disappointment with the EAU’s postponement on social media. On Twitter, some doctors wrote that the rescheduled dates were bad timing, while others lamented the refund refusal.
The EAU said it regrets that some delegates will experience financial losses, but that the organization has already experienced a significant outlay that cannot be recovered including venue, logistics, travel, and accommodation costs.
"We are doing what we can to absorb costs, but we need to be realistic about what is affordable; should the organization have to refund all or even most registrations, it would significantly jeopardize the viability of the organization," the EAU said in a statement. "These are difficult times, not only for the EAU, but on a global scale. Where there are specific cases of hardship or very extenuating financial circumstances, we will be willing to review individual cases. So far, we believe that we have done what we can do to meet the conflicting demands presented by the postponement of the congress, but this is a situation which changes from day to day, and we need to continuously evaluate what might be the best course of action." *
Contact your medical association directly for details on postponements.
What if I’m a presenter?
In an attempt to save the hard work and time that planners and presenters have invested into now-canceled meetings, some conferences are moving to a digital format. The Conference on Retroviruses and Opportunistic Infections (CROI) was the first to convert its in-person conference to a virtual meeting, held from March 8 to 11, 2020. At-home attendees logged onto CROI’s digital platform to hear plenaries, oral abstracts, themed discussion sessions, and symposia.
Dr. Radix was one of many CROI speakers who changed his presentation on HIV prevalence among transgender men to a virtual format.
“We were provided with detailed instructions from CROI about how to do this,” said Dr. Radix, who tweeted about the experience. “For my presentation, I used the video option in PowerPoint; it seemed the most straightforward and didn’t require buying additional software. It was fairly easy to follow the instructions to create the video but it was disappointing to present to an empty room.”
Matthew Spinelli, MD, an HIV researcher with the University of California, San Francisco, who also presented virtually, said it was remarkable that CROI leaders were able to put together the virtual program in such a short time. He delivered his presentation on the accuracy of a real-time urine tenofovir test using PowerPoint and a podcast microphone.
“It seemed to work pretty well,” he said in an interview. “It’s not the same as being there in person, there’s a lot of networking and chance conversations that happen when you’re all in the same place, but it was the right decision to cancel. If I have to be at home or at work doing social distancing, this was the best possible way of doing it.”
Following in CROI’s footsteps, the National Kidney Foundation’s spring conference has moved to a live virtual conference. The 2020 Healthcare Information and Management Systems Society (HIMSS) global health conference also will move to a digital format. Other societies are considering similar virtual options. Check with your meeting website for more details on digital options and attendee access.
*The article was updated on 03/16/2020.
Khadija Hafidh, MD, was already booked on a 14-hour, direct flight from Dubai to Los Angeles, when the American College of Physicians (ACP) announced it was canceling its internal medicine meeting scheduled for April.
Canceling her hotel reservation was not a problem, and she was assured a refund for the conference fee, but her airline ticket was another matter, said Dr. Hafidh, an internist and diabetologist with the Dubai Health Authority.
“The airline I booked my ticket with is willing to waive the change fees, but will deduct a cancellation fee if I choose not to take the trip,” Dr. Hafidh said in an interview. “The cancellation fees is $300. A bit steep I must admit.”
Dr. Hafidh now faces a dilemma: Lose the $300 and cancel, or change her flight dates to June for the American Diabetes Association meeting in Chicago.
“But then again, we aren’t sure if that meeting will take place,” Dr. Hafidh said. “A few weeks ago I thought this whole thing was just a storm in a tea cup. However when it was declared a pandemic yesterday, it brought about another dimension.”
More than 25 medical meetings and conferences across the globe have been canceled or postponed because of COVID-19 concerns. The sudden cancellations have caused reservation woes and travel headaches for thousands of physicians who planned to attend the meetings. Some societies are considering the idea of virtual conferences, while other associations have scrapped their meetings until next year.
For physicians facing a canceled conference, the most likely question is, what now? Read on for tips and suggestions.
Reservation refunds vary
Refunds on airfare because of conference cancellations differ, depending on the airline and where you were traveling. Some airlines, such as United Airlines, have waived all change fees for tickets issued March 3, 2020, through March 31, 2020, and passengers can change their dates for up to 12 months after the ticket was issued.
Full refunds often depend on whether your ticket was nonrefundable when purchased. Many airlines, such as Delta, are providing full refunds if the airline canceled your flight. JetBlue is waiving all change and cancellation fees for customers scheduled to travel March 10, 2020, through April 30, 2020.
Las Vegas–based dermatologist H.L. Greenberg, MD, was satisfied with the credit he received from Southwest Airlines after the American Academy of Dermatology (AAD) canceled its Denver meeting. He and his staff were looking forward to the gathering, but he noted that the meeting would likely have been limited, even if it had take place as scheduled.
“I am disappointed that I won’t be able to meet with colleagues and industry to explore what the latest advances and interests are in dermatology,” he said. “Because many academic institutions were forbidding their faculty from traveling, the content of the meeting was going to be severely diminished. It’s just a rough time for everyone.”
Meanwhile, Asa Radix, MD, PhD, a New York–based internist, received a full refund for his Amtrak ticket to Boston when the Conference on Retroviruses and Opportunistic Infections (CROI) scheduled for early March was converted to a virtual meeting. Dr. Radix, senior director of research and education at the Callen-Lorde Community Health Center in New York, left another meeting in Brazil early to get to the Boston conference, he said.
“I was packed, but really that was a minor inconvenience,” he said in an interview. “I appreciate that they prioritized health concerns and changed to a virtual meeting. I received full refunds, no issues whatsoever. [It was] really great since I had no travel insurance.”
Check with your individual airline or train line for information about ticket refunds and credits. Many airlines are currently making special accommodations because of COVID-19. If your flight was covered by trip insurance, also called travel assistance, you are generally protected against unforeseen financial losses such as cancellations. The U.S. Department of Transportation provides this general online resource about airline refunds.
Hotel refunds probable
Most meeting organizations who have made the decision to cancel or postpone a conference also have canceled block hotel reservations reserved for the meeting. Medical associations are not directly refunding the hotel costs, but the majority of hotels are refunding reservations with no questions asked. Physicians interviewed for this story all reported no trouble getting refunds for their hotel reservations. However, attendees who did not book a hotel in official housing blocks should contact the hotel directly to cancel.
What about registration fees?
In response to COVID-19 cancellations, most conference leaders are refunding registration fees in full for both attendees and exhibitors. The refund may not be automatic, some associations such as ACP and the American College of Obstetricians and Gynecologists state it may take up to 45 days for the funds to be credited, depending on the payment used.
If the conference you planned to attend was postponed, the registration fee may be assigned to the new meeting dates and the money may not be refunded. Registration fees for the Minimally Invasive Surgery Symposium, for example, delayed until an unconfirmed date, and for the European Association of Urology (EAU) meeting, postponed until July, will be automatically credited to the rescheduled meeting, according to the websites. If attendees cannot attend the rescheduled EAU meeting, the association will not provide a refund and the registration will not apply to the 2021 meeting, according to its website. However, the group is providing registrants with a free access code for the EAU20 Resource Centre, which contains websites of sessions and scientific content.
A number of physicians have expressed disappointment with the EAU’s postponement on social media. On Twitter, some doctors wrote that the rescheduled dates were bad timing, while others lamented the refund refusal.
The EAU said it regrets that some delegates will experience financial losses, but that the organization has already experienced a significant outlay that cannot be recovered including venue, logistics, travel, and accommodation costs.
"We are doing what we can to absorb costs, but we need to be realistic about what is affordable; should the organization have to refund all or even most registrations, it would significantly jeopardize the viability of the organization," the EAU said in a statement. "These are difficult times, not only for the EAU, but on a global scale. Where there are specific cases of hardship or very extenuating financial circumstances, we will be willing to review individual cases. So far, we believe that we have done what we can do to meet the conflicting demands presented by the postponement of the congress, but this is a situation which changes from day to day, and we need to continuously evaluate what might be the best course of action." *
Contact your medical association directly for details on postponements.
What if I’m a presenter?
In an attempt to save the hard work and time that planners and presenters have invested into now-canceled meetings, some conferences are moving to a digital format. The Conference on Retroviruses and Opportunistic Infections (CROI) was the first to convert its in-person conference to a virtual meeting, held from March 8 to 11, 2020. At-home attendees logged onto CROI’s digital platform to hear plenaries, oral abstracts, themed discussion sessions, and symposia.
Dr. Radix was one of many CROI speakers who changed his presentation on HIV prevalence among transgender men to a virtual format.
“We were provided with detailed instructions from CROI about how to do this,” said Dr. Radix, who tweeted about the experience. “For my presentation, I used the video option in PowerPoint; it seemed the most straightforward and didn’t require buying additional software. It was fairly easy to follow the instructions to create the video but it was disappointing to present to an empty room.”
Matthew Spinelli, MD, an HIV researcher with the University of California, San Francisco, who also presented virtually, said it was remarkable that CROI leaders were able to put together the virtual program in such a short time. He delivered his presentation on the accuracy of a real-time urine tenofovir test using PowerPoint and a podcast microphone.
“It seemed to work pretty well,” he said in an interview. “It’s not the same as being there in person, there’s a lot of networking and chance conversations that happen when you’re all in the same place, but it was the right decision to cancel. If I have to be at home or at work doing social distancing, this was the best possible way of doing it.”
Following in CROI’s footsteps, the National Kidney Foundation’s spring conference has moved to a live virtual conference. The 2020 Healthcare Information and Management Systems Society (HIMSS) global health conference also will move to a digital format. Other societies are considering similar virtual options. Check with your meeting website for more details on digital options and attendee access.
*The article was updated on 03/16/2020.
Khadija Hafidh, MD, was already booked on a 14-hour, direct flight from Dubai to Los Angeles, when the American College of Physicians (ACP) announced it was canceling its internal medicine meeting scheduled for April.
Canceling her hotel reservation was not a problem, and she was assured a refund for the conference fee, but her airline ticket was another matter, said Dr. Hafidh, an internist and diabetologist with the Dubai Health Authority.
“The airline I booked my ticket with is willing to waive the change fees, but will deduct a cancellation fee if I choose not to take the trip,” Dr. Hafidh said in an interview. “The cancellation fees is $300. A bit steep I must admit.”
Dr. Hafidh now faces a dilemma: Lose the $300 and cancel, or change her flight dates to June for the American Diabetes Association meeting in Chicago.
“But then again, we aren’t sure if that meeting will take place,” Dr. Hafidh said. “A few weeks ago I thought this whole thing was just a storm in a tea cup. However when it was declared a pandemic yesterday, it brought about another dimension.”
More than 25 medical meetings and conferences across the globe have been canceled or postponed because of COVID-19 concerns. The sudden cancellations have caused reservation woes and travel headaches for thousands of physicians who planned to attend the meetings. Some societies are considering the idea of virtual conferences, while other associations have scrapped their meetings until next year.
For physicians facing a canceled conference, the most likely question is, what now? Read on for tips and suggestions.
Reservation refunds vary
Refunds on airfare because of conference cancellations differ, depending on the airline and where you were traveling. Some airlines, such as United Airlines, have waived all change fees for tickets issued March 3, 2020, through March 31, 2020, and passengers can change their dates for up to 12 months after the ticket was issued.
Full refunds often depend on whether your ticket was nonrefundable when purchased. Many airlines, such as Delta, are providing full refunds if the airline canceled your flight. JetBlue is waiving all change and cancellation fees for customers scheduled to travel March 10, 2020, through April 30, 2020.
Las Vegas–based dermatologist H.L. Greenberg, MD, was satisfied with the credit he received from Southwest Airlines after the American Academy of Dermatology (AAD) canceled its Denver meeting. He and his staff were looking forward to the gathering, but he noted that the meeting would likely have been limited, even if it had take place as scheduled.
“I am disappointed that I won’t be able to meet with colleagues and industry to explore what the latest advances and interests are in dermatology,” he said. “Because many academic institutions were forbidding their faculty from traveling, the content of the meeting was going to be severely diminished. It’s just a rough time for everyone.”
Meanwhile, Asa Radix, MD, PhD, a New York–based internist, received a full refund for his Amtrak ticket to Boston when the Conference on Retroviruses and Opportunistic Infections (CROI) scheduled for early March was converted to a virtual meeting. Dr. Radix, senior director of research and education at the Callen-Lorde Community Health Center in New York, left another meeting in Brazil early to get to the Boston conference, he said.
“I was packed, but really that was a minor inconvenience,” he said in an interview. “I appreciate that they prioritized health concerns and changed to a virtual meeting. I received full refunds, no issues whatsoever. [It was] really great since I had no travel insurance.”
Check with your individual airline or train line for information about ticket refunds and credits. Many airlines are currently making special accommodations because of COVID-19. If your flight was covered by trip insurance, also called travel assistance, you are generally protected against unforeseen financial losses such as cancellations. The U.S. Department of Transportation provides this general online resource about airline refunds.
Hotel refunds probable
Most meeting organizations who have made the decision to cancel or postpone a conference also have canceled block hotel reservations reserved for the meeting. Medical associations are not directly refunding the hotel costs, but the majority of hotels are refunding reservations with no questions asked. Physicians interviewed for this story all reported no trouble getting refunds for their hotel reservations. However, attendees who did not book a hotel in official housing blocks should contact the hotel directly to cancel.
What about registration fees?
In response to COVID-19 cancellations, most conference leaders are refunding registration fees in full for both attendees and exhibitors. The refund may not be automatic, some associations such as ACP and the American College of Obstetricians and Gynecologists state it may take up to 45 days for the funds to be credited, depending on the payment used.
If the conference you planned to attend was postponed, the registration fee may be assigned to the new meeting dates and the money may not be refunded. Registration fees for the Minimally Invasive Surgery Symposium, for example, delayed until an unconfirmed date, and for the European Association of Urology (EAU) meeting, postponed until July, will be automatically credited to the rescheduled meeting, according to the websites. If attendees cannot attend the rescheduled EAU meeting, the association will not provide a refund and the registration will not apply to the 2021 meeting, according to its website. However, the group is providing registrants with a free access code for the EAU20 Resource Centre, which contains websites of sessions and scientific content.
A number of physicians have expressed disappointment with the EAU’s postponement on social media. On Twitter, some doctors wrote that the rescheduled dates were bad timing, while others lamented the refund refusal.
The EAU said it regrets that some delegates will experience financial losses, but that the organization has already experienced a significant outlay that cannot be recovered including venue, logistics, travel, and accommodation costs.
"We are doing what we can to absorb costs, but we need to be realistic about what is affordable; should the organization have to refund all or even most registrations, it would significantly jeopardize the viability of the organization," the EAU said in a statement. "These are difficult times, not only for the EAU, but on a global scale. Where there are specific cases of hardship or very extenuating financial circumstances, we will be willing to review individual cases. So far, we believe that we have done what we can do to meet the conflicting demands presented by the postponement of the congress, but this is a situation which changes from day to day, and we need to continuously evaluate what might be the best course of action." *
Contact your medical association directly for details on postponements.
What if I’m a presenter?
In an attempt to save the hard work and time that planners and presenters have invested into now-canceled meetings, some conferences are moving to a digital format. The Conference on Retroviruses and Opportunistic Infections (CROI) was the first to convert its in-person conference to a virtual meeting, held from March 8 to 11, 2020. At-home attendees logged onto CROI’s digital platform to hear plenaries, oral abstracts, themed discussion sessions, and symposia.
Dr. Radix was one of many CROI speakers who changed his presentation on HIV prevalence among transgender men to a virtual format.
“We were provided with detailed instructions from CROI about how to do this,” said Dr. Radix, who tweeted about the experience. “For my presentation, I used the video option in PowerPoint; it seemed the most straightforward and didn’t require buying additional software. It was fairly easy to follow the instructions to create the video but it was disappointing to present to an empty room.”
Matthew Spinelli, MD, an HIV researcher with the University of California, San Francisco, who also presented virtually, said it was remarkable that CROI leaders were able to put together the virtual program in such a short time. He delivered his presentation on the accuracy of a real-time urine tenofovir test using PowerPoint and a podcast microphone.
“It seemed to work pretty well,” he said in an interview. “It’s not the same as being there in person, there’s a lot of networking and chance conversations that happen when you’re all in the same place, but it was the right decision to cancel. If I have to be at home or at work doing social distancing, this was the best possible way of doing it.”
Following in CROI’s footsteps, the National Kidney Foundation’s spring conference has moved to a live virtual conference. The 2020 Healthcare Information and Management Systems Society (HIMSS) global health conference also will move to a digital format. Other societies are considering similar virtual options. Check with your meeting website for more details on digital options and attendee access.
*The article was updated on 03/16/2020.










