User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


First report of MM patient successfully treated for COVID-19 with tocilizumab
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
FROM BLOOD ADVANCES
FDA grants emergency authorization for first rapid antibody test for COVID-19
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
Survey: COVID-19 is getting in our heads
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
First presumptive case of encephalitis linked to COVID-19 reported
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Survey shows just how dire PPE shortages are at many hospitals
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
iPLEDGE allows at-home pregnancy tests during pandemic
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
Case study shows CLL may mask COVID-19 infection
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
FROM THE LANCET HAEMATOLOGY
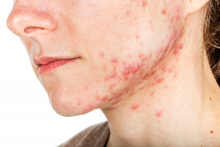
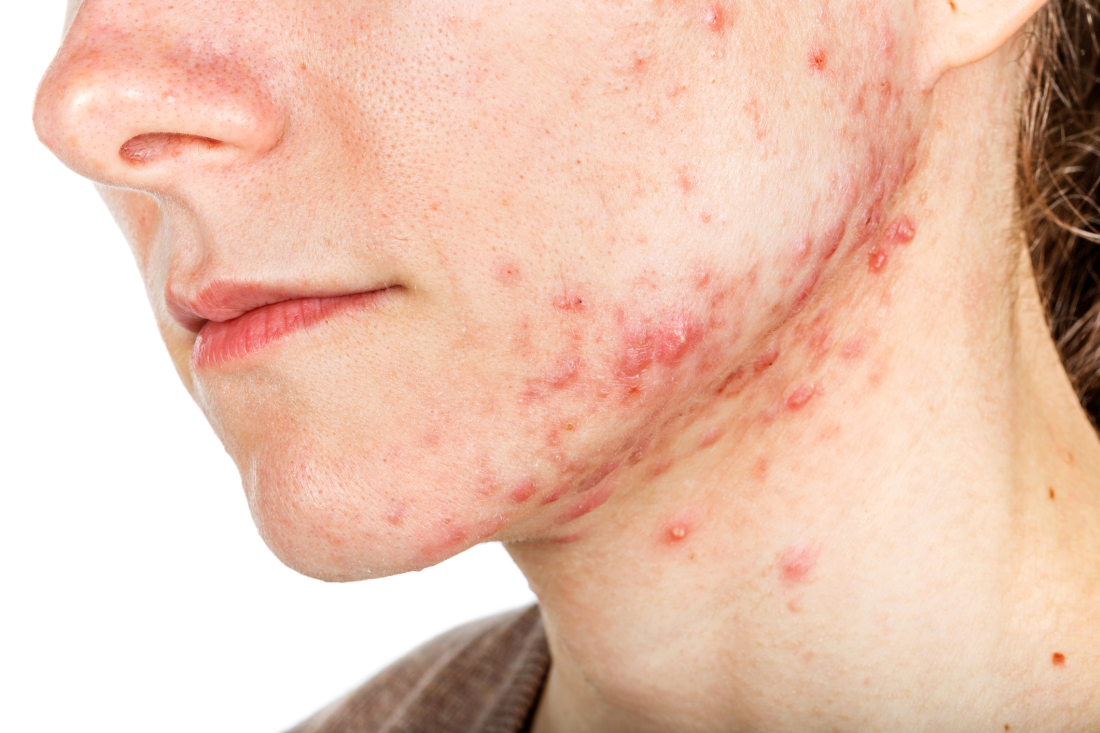
Skin manifestations are emerging in the coronavirus pandemic
Dermatologists there were pulled from their usual duty to help with the pandemic and looked at what was going on with the skin in 148 COVID-19 inpatients. They excluded 60 who had started new drugs within 15 days to rule out acute drug reactions, then reported what they saw (J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387).
Of the 88 COVID-19 patients, 20.5% developed skin manifestations. Eight of the 18 (44%) had skin eruptions at symptom onset, and the rest after hospitalization. Fourteen (78%) had red rashes, three had widespread urticaria, and one had chickenpox-like vesicles. The most commonly affected area was the trunk. Itching was mild or absent, and lesions usually healed up in a few days. Most importantly, skin manifestations did not correlate with disease severity.
These skin manifestations “are similar to cutaneous involvement occurring during common viral infections,” said the author of the report, Sebastiano Recalcati, MD, a dermatologist at Alessandro Manzoni Hospital.
COVID-19 skin manifestations can cloud the diagnosis, according to the authors of another report from Thailand, where the first case of COVID-19 outside of China was reported.
They described a case of a COVID-19 infection in a Bangkok hospital that masqueraded as dengue fever. A person there presented with only a skin rash, petechiae, and a low platelet count, and was diagnosed with Dengue because that’s exactly what it looked like, the authors wrote (J Am Acad Dermatol. 2020 Mar 22. pii: S0190-9622[20]30454-0. doi: 10.1016/j.jaad.2020.03.036).
The correct diagnosis, COVID-19, was made at a tertiary care center after the patient was admitted with respiratory problems.
“There is a possibility that a COVID-19 patient might initially present with a skin rash that can be misdiagnosed as another common disease. ... The practitioner should recognize the possibility that the patient might have only a skin rash” at first, said the lead author of that report, Beuy Joob, PhD, of the Sanitation1 Medical Academic Center, Bangkok, and a coauthor.
There are similar reports in the United States, too. “Many have wondered if COVID-19 presents with any particular skin changes. The answer is yes,” said Randy Jacobs, MD, an assistant clinical professor of dermatology at the University of California, Riverside, who also has a private practice in southern California.
“COVID-19 can feature signs of small blood vessel occlusion. These can be petechiae or tiny bruises, and transient livedoid eruptions,” he said in an interview.
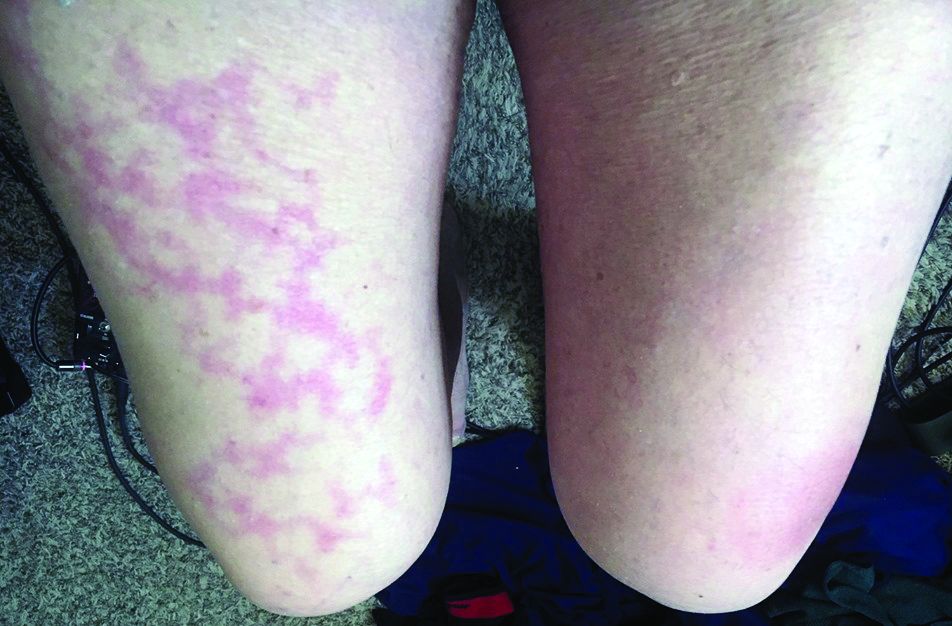
Dr. Jacobs had a 67-year-old patient who presented with a low fever, nasal congestion, postnasal drip, and a wet cough but no shortness of breath. It looked like a common cold. But a week later, the man had a nonpruritic blanching livedoid vascular eruption on his right anterior thigh, and blood in his urine, and he felt weak. The vascular eruption and bloody urine resolved in 24 hours, but the COVID-19 test came back positive and his cough became dry and hacking, and the weakness persisted. He’s in a hospital now and on oxygen, but not ventilated so far.
“Another dermatologist friend of mine also reported a similar transient COVID-19 unilateral livedoid eruption,” Dr. Jacobs said.
It suggests vaso-occlusion. Whether it’s neurogenic, microthrombotic, or immune complex mediated is unknown, but it’s “a skin finding that can help clinicians as they work up their patients with COVID-19 symptoms,” he noted.
Dr. Jacobs and the authors of the studies had no disclosures.
Dermatologists there were pulled from their usual duty to help with the pandemic and looked at what was going on with the skin in 148 COVID-19 inpatients. They excluded 60 who had started new drugs within 15 days to rule out acute drug reactions, then reported what they saw (J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387).
Of the 88 COVID-19 patients, 20.5% developed skin manifestations. Eight of the 18 (44%) had skin eruptions at symptom onset, and the rest after hospitalization. Fourteen (78%) had red rashes, three had widespread urticaria, and one had chickenpox-like vesicles. The most commonly affected area was the trunk. Itching was mild or absent, and lesions usually healed up in a few days. Most importantly, skin manifestations did not correlate with disease severity.
These skin manifestations “are similar to cutaneous involvement occurring during common viral infections,” said the author of the report, Sebastiano Recalcati, MD, a dermatologist at Alessandro Manzoni Hospital.
COVID-19 skin manifestations can cloud the diagnosis, according to the authors of another report from Thailand, where the first case of COVID-19 outside of China was reported.
They described a case of a COVID-19 infection in a Bangkok hospital that masqueraded as dengue fever. A person there presented with only a skin rash, petechiae, and a low platelet count, and was diagnosed with Dengue because that’s exactly what it looked like, the authors wrote (J Am Acad Dermatol. 2020 Mar 22. pii: S0190-9622[20]30454-0. doi: 10.1016/j.jaad.2020.03.036).
The correct diagnosis, COVID-19, was made at a tertiary care center after the patient was admitted with respiratory problems.
“There is a possibility that a COVID-19 patient might initially present with a skin rash that can be misdiagnosed as another common disease. ... The practitioner should recognize the possibility that the patient might have only a skin rash” at first, said the lead author of that report, Beuy Joob, PhD, of the Sanitation1 Medical Academic Center, Bangkok, and a coauthor.
There are similar reports in the United States, too. “Many have wondered if COVID-19 presents with any particular skin changes. The answer is yes,” said Randy Jacobs, MD, an assistant clinical professor of dermatology at the University of California, Riverside, who also has a private practice in southern California.
“COVID-19 can feature signs of small blood vessel occlusion. These can be petechiae or tiny bruises, and transient livedoid eruptions,” he said in an interview.
Dr. Jacobs had a 67-year-old patient who presented with a low fever, nasal congestion, postnasal drip, and a wet cough but no shortness of breath. It looked like a common cold. But a week later, the man had a nonpruritic blanching livedoid vascular eruption on his right anterior thigh, and blood in his urine, and he felt weak. The vascular eruption and bloody urine resolved in 24 hours, but the COVID-19 test came back positive and his cough became dry and hacking, and the weakness persisted. He’s in a hospital now and on oxygen, but not ventilated so far.
“Another dermatologist friend of mine also reported a similar transient COVID-19 unilateral livedoid eruption,” Dr. Jacobs said.
It suggests vaso-occlusion. Whether it’s neurogenic, microthrombotic, or immune complex mediated is unknown, but it’s “a skin finding that can help clinicians as they work up their patients with COVID-19 symptoms,” he noted.
Dr. Jacobs and the authors of the studies had no disclosures.
Dermatologists there were pulled from their usual duty to help with the pandemic and looked at what was going on with the skin in 148 COVID-19 inpatients. They excluded 60 who had started new drugs within 15 days to rule out acute drug reactions, then reported what they saw (J Eur Acad Dermatol Venereol. 2020 Mar 26. doi: 10.1111/jdv.16387).
Of the 88 COVID-19 patients, 20.5% developed skin manifestations. Eight of the 18 (44%) had skin eruptions at symptom onset, and the rest after hospitalization. Fourteen (78%) had red rashes, three had widespread urticaria, and one had chickenpox-like vesicles. The most commonly affected area was the trunk. Itching was mild or absent, and lesions usually healed up in a few days. Most importantly, skin manifestations did not correlate with disease severity.
These skin manifestations “are similar to cutaneous involvement occurring during common viral infections,” said the author of the report, Sebastiano Recalcati, MD, a dermatologist at Alessandro Manzoni Hospital.
COVID-19 skin manifestations can cloud the diagnosis, according to the authors of another report from Thailand, where the first case of COVID-19 outside of China was reported.
They described a case of a COVID-19 infection in a Bangkok hospital that masqueraded as dengue fever. A person there presented with only a skin rash, petechiae, and a low platelet count, and was diagnosed with Dengue because that’s exactly what it looked like, the authors wrote (J Am Acad Dermatol. 2020 Mar 22. pii: S0190-9622[20]30454-0. doi: 10.1016/j.jaad.2020.03.036).
The correct diagnosis, COVID-19, was made at a tertiary care center after the patient was admitted with respiratory problems.
“There is a possibility that a COVID-19 patient might initially present with a skin rash that can be misdiagnosed as another common disease. ... The practitioner should recognize the possibility that the patient might have only a skin rash” at first, said the lead author of that report, Beuy Joob, PhD, of the Sanitation1 Medical Academic Center, Bangkok, and a coauthor.
There are similar reports in the United States, too. “Many have wondered if COVID-19 presents with any particular skin changes. The answer is yes,” said Randy Jacobs, MD, an assistant clinical professor of dermatology at the University of California, Riverside, who also has a private practice in southern California.
“COVID-19 can feature signs of small blood vessel occlusion. These can be petechiae or tiny bruises, and transient livedoid eruptions,” he said in an interview.
Dr. Jacobs had a 67-year-old patient who presented with a low fever, nasal congestion, postnasal drip, and a wet cough but no shortness of breath. It looked like a common cold. But a week later, the man had a nonpruritic blanching livedoid vascular eruption on his right anterior thigh, and blood in his urine, and he felt weak. The vascular eruption and bloody urine resolved in 24 hours, but the COVID-19 test came back positive and his cough became dry and hacking, and the weakness persisted. He’s in a hospital now and on oxygen, but not ventilated so far.
“Another dermatologist friend of mine also reported a similar transient COVID-19 unilateral livedoid eruption,” Dr. Jacobs said.
It suggests vaso-occlusion. Whether it’s neurogenic, microthrombotic, or immune complex mediated is unknown, but it’s “a skin finding that can help clinicians as they work up their patients with COVID-19 symptoms,” he noted.
Dr. Jacobs and the authors of the studies had no disclosures.
Vascular biomarkers predict pulmonary hypertension in systemic sclerosis
Levels of three vascular biomarkers – hepatocyte growth factor, soluble Flt-1, and platelet-derived growth factor – were elevated a mean of 3 years before systemic sclerosis (SSc) patients developed pulmonary hypertension (PH) in a prospective cohort of 300 subjects.
However, the associations with PH were not very robust. For instance, above an optimal cut point of 9.89 pg/mL for platelet-derived growth factor (PlGF), the sensitivity for future PH was 82%, specificity 56%, and area under the curve (AUC) 0.69. An elevation above the optimal cut point for soluble Flt-1 (sFlt1) – 93.8 pg/mL – was 71% specific and 51% sensitive, with an AUC of 0.61.
Adding PlGF and sFlt1 elevations to carbon monoxide diffusing capacity, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) level, and percent forced vital capacity to predict PH increased the AUC modestly, from 0.72 to 0.77.
The data suggest, perhaps, an early warning system for PH. “Once vascular biomarkers are observed to be elevated, the frequency of other screening tests (e.g., NT-proBNP, DLCO) may be increased in a more cost-effective approach,” wrote investigators led by rheumatologist Christopher Mecoli, MD, an assistant professor at Johns Hopkins University, Baltimore, in Arthritis & Rheumatology.
“In the end, the authors did not overstate the case and cautiously recommended that using biomarkers might be useful in the future. The finding that when there are increased numbers of abnormalities of vascular markers, there would be an increased probability of pulmonary hypertension, makes sense.” However, “this was a major fishing expedition, and the data are certainly not sufficient to suggest anything clinical but are of some interest with respect to the general hypothesis,” said rheumatologist Daniel Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, when asked for comment.
The subjects were followed for at least 5 years and had no evidence of PH at study entry. Levels of P1GF, sFlt-1, hepatocyte growth factor (HGF), soluble endoglin, and endostatin were assessed at baseline and at regular intervals thereafter. A total of 46 patients (15%) developed PH after a mean of 3 years.
Risk of PH was associated with baseline elevations of HGF (hazard ratio, 1.99; 95% CI, 1.24-3.17; P = .004); sFlt1 (HR, 3.04; 95% CI, 1.29-7.14; P = .011); and PlGF (HR, 2.74; 95% CI, 1.32-5.69; P = .007).
Just 2 of 25 patients (8%) with no biomarkers elevated at baseline developed PH versus 12 of 29 (42%) with all five elevated. That translated to a dose-response relationship, with each additional elevated biomarker increasing the risk of PH by 78% (95% CI, 1.2-2.6; P = .004).
“There [was] no consistent trend of increasing biomarker levels over time as patients approach[ed] a diagnosis of [PH]. ... Serial testing may have value in patients with early disease to first detect elevations in biomarkers,” but “once elevated, the utility of serially monitoring appears low,” the investigators wrote.
It’s not surprising that “a higher number of elevated biomarkers relating to vascular dysfunction would correspond to a higher risk of PH,” the team wrote. However, “while these biomarkers hold promise in the risk stratification of SSc patients, many more vascular molecules exist which may have similar or greater value.”
There was no substantial correlation between any biomarker and disease duration, age at enrollment, or age at diagnosis, and no significant difference in biomarker level based on patient comorbidities. No biomarker was significantly associated with medication use at cohort entry, and none were significantly associated with the risk of ischemic digital lesions.
The majority of patients were white women. At enrollment, the average age was 52 years, and subjects had SSc for a mean of 10 years.
The work was funded by the National Institutes of Health, among others. Investigator disclosures were not reported.
SOURCE: Mecoli C et al. Arthritis Rheumatol. 2020 Mar 21. doi: 10.1002/art.41265.
Levels of three vascular biomarkers – hepatocyte growth factor, soluble Flt-1, and platelet-derived growth factor – were elevated a mean of 3 years before systemic sclerosis (SSc) patients developed pulmonary hypertension (PH) in a prospective cohort of 300 subjects.
However, the associations with PH were not very robust. For instance, above an optimal cut point of 9.89 pg/mL for platelet-derived growth factor (PlGF), the sensitivity for future PH was 82%, specificity 56%, and area under the curve (AUC) 0.69. An elevation above the optimal cut point for soluble Flt-1 (sFlt1) – 93.8 pg/mL – was 71% specific and 51% sensitive, with an AUC of 0.61.
Adding PlGF and sFlt1 elevations to carbon monoxide diffusing capacity, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) level, and percent forced vital capacity to predict PH increased the AUC modestly, from 0.72 to 0.77.
The data suggest, perhaps, an early warning system for PH. “Once vascular biomarkers are observed to be elevated, the frequency of other screening tests (e.g., NT-proBNP, DLCO) may be increased in a more cost-effective approach,” wrote investigators led by rheumatologist Christopher Mecoli, MD, an assistant professor at Johns Hopkins University, Baltimore, in Arthritis & Rheumatology.
“In the end, the authors did not overstate the case and cautiously recommended that using biomarkers might be useful in the future. The finding that when there are increased numbers of abnormalities of vascular markers, there would be an increased probability of pulmonary hypertension, makes sense.” However, “this was a major fishing expedition, and the data are certainly not sufficient to suggest anything clinical but are of some interest with respect to the general hypothesis,” said rheumatologist Daniel Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, when asked for comment.
The subjects were followed for at least 5 years and had no evidence of PH at study entry. Levels of P1GF, sFlt-1, hepatocyte growth factor (HGF), soluble endoglin, and endostatin were assessed at baseline and at regular intervals thereafter. A total of 46 patients (15%) developed PH after a mean of 3 years.
Risk of PH was associated with baseline elevations of HGF (hazard ratio, 1.99; 95% CI, 1.24-3.17; P = .004); sFlt1 (HR, 3.04; 95% CI, 1.29-7.14; P = .011); and PlGF (HR, 2.74; 95% CI, 1.32-5.69; P = .007).
Just 2 of 25 patients (8%) with no biomarkers elevated at baseline developed PH versus 12 of 29 (42%) with all five elevated. That translated to a dose-response relationship, with each additional elevated biomarker increasing the risk of PH by 78% (95% CI, 1.2-2.6; P = .004).
“There [was] no consistent trend of increasing biomarker levels over time as patients approach[ed] a diagnosis of [PH]. ... Serial testing may have value in patients with early disease to first detect elevations in biomarkers,” but “once elevated, the utility of serially monitoring appears low,” the investigators wrote.
It’s not surprising that “a higher number of elevated biomarkers relating to vascular dysfunction would correspond to a higher risk of PH,” the team wrote. However, “while these biomarkers hold promise in the risk stratification of SSc patients, many more vascular molecules exist which may have similar or greater value.”
There was no substantial correlation between any biomarker and disease duration, age at enrollment, or age at diagnosis, and no significant difference in biomarker level based on patient comorbidities. No biomarker was significantly associated with medication use at cohort entry, and none were significantly associated with the risk of ischemic digital lesions.
The majority of patients were white women. At enrollment, the average age was 52 years, and subjects had SSc for a mean of 10 years.
The work was funded by the National Institutes of Health, among others. Investigator disclosures were not reported.
SOURCE: Mecoli C et al. Arthritis Rheumatol. 2020 Mar 21. doi: 10.1002/art.41265.
Levels of three vascular biomarkers – hepatocyte growth factor, soluble Flt-1, and platelet-derived growth factor – were elevated a mean of 3 years before systemic sclerosis (SSc) patients developed pulmonary hypertension (PH) in a prospective cohort of 300 subjects.
However, the associations with PH were not very robust. For instance, above an optimal cut point of 9.89 pg/mL for platelet-derived growth factor (PlGF), the sensitivity for future PH was 82%, specificity 56%, and area under the curve (AUC) 0.69. An elevation above the optimal cut point for soluble Flt-1 (sFlt1) – 93.8 pg/mL – was 71% specific and 51% sensitive, with an AUC of 0.61.
Adding PlGF and sFlt1 elevations to carbon monoxide diffusing capacity, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) level, and percent forced vital capacity to predict PH increased the AUC modestly, from 0.72 to 0.77.
The data suggest, perhaps, an early warning system for PH. “Once vascular biomarkers are observed to be elevated, the frequency of other screening tests (e.g., NT-proBNP, DLCO) may be increased in a more cost-effective approach,” wrote investigators led by rheumatologist Christopher Mecoli, MD, an assistant professor at Johns Hopkins University, Baltimore, in Arthritis & Rheumatology.
“In the end, the authors did not overstate the case and cautiously recommended that using biomarkers might be useful in the future. The finding that when there are increased numbers of abnormalities of vascular markers, there would be an increased probability of pulmonary hypertension, makes sense.” However, “this was a major fishing expedition, and the data are certainly not sufficient to suggest anything clinical but are of some interest with respect to the general hypothesis,” said rheumatologist Daniel Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, when asked for comment.
The subjects were followed for at least 5 years and had no evidence of PH at study entry. Levels of P1GF, sFlt-1, hepatocyte growth factor (HGF), soluble endoglin, and endostatin were assessed at baseline and at regular intervals thereafter. A total of 46 patients (15%) developed PH after a mean of 3 years.
Risk of PH was associated with baseline elevations of HGF (hazard ratio, 1.99; 95% CI, 1.24-3.17; P = .004); sFlt1 (HR, 3.04; 95% CI, 1.29-7.14; P = .011); and PlGF (HR, 2.74; 95% CI, 1.32-5.69; P = .007).
Just 2 of 25 patients (8%) with no biomarkers elevated at baseline developed PH versus 12 of 29 (42%) with all five elevated. That translated to a dose-response relationship, with each additional elevated biomarker increasing the risk of PH by 78% (95% CI, 1.2-2.6; P = .004).
“There [was] no consistent trend of increasing biomarker levels over time as patients approach[ed] a diagnosis of [PH]. ... Serial testing may have value in patients with early disease to first detect elevations in biomarkers,” but “once elevated, the utility of serially monitoring appears low,” the investigators wrote.
It’s not surprising that “a higher number of elevated biomarkers relating to vascular dysfunction would correspond to a higher risk of PH,” the team wrote. However, “while these biomarkers hold promise in the risk stratification of SSc patients, many more vascular molecules exist which may have similar or greater value.”
There was no substantial correlation between any biomarker and disease duration, age at enrollment, or age at diagnosis, and no significant difference in biomarker level based on patient comorbidities. No biomarker was significantly associated with medication use at cohort entry, and none were significantly associated with the risk of ischemic digital lesions.
The majority of patients were white women. At enrollment, the average age was 52 years, and subjects had SSc for a mean of 10 years.
The work was funded by the National Institutes of Health, among others. Investigator disclosures were not reported.
SOURCE: Mecoli C et al. Arthritis Rheumatol. 2020 Mar 21. doi: 10.1002/art.41265.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Levels of three vascular biomarkers – hepatocyte growth factor, soluble Flt-1, and platelet-derived growth factor – were elevated a mean of 3 years before systemic sclerosis patients developed pulmonary hypertension.
Major finding: The associations with pulmonary hypertension were not very robust. For instance, above an optimal cut point of 9.89 pg/mL for platelet-derived growth factor, the sensitivity for future pulmonary hypertension was 82%, specificity 56%, and area under the curve 0.69. An elevation above the optimal cut point for soluble Flt-1 – 93.8 pg/mL – was 71% specific and 51% sensitive, with an area under the curve of 0.61.
Study details: A prospective cohort of 300 patients
Disclosures: The work was funded by the National Institutes of Health, among others. Investigator disclosures weren’t reported.
Source: Mecoli C et al. Arthritis Rheumatol. 2020 Mar 21. doi: 10.1002/art.41265.
COVID-19: Mental health pros come to the aid of frontline comrades
Frontline COVID-19 healthcare workers across North America are dealing with unprecedented stress, but mental health therapists in both Canada and the US are doing their part to ensure the psychological well-being of their colleagues on the frontlines of the pandemic.
Over the past few weeks, thousands of licensed psychologists, psychotherapists, and social workers have signed up to offer free therapy sessions to healthcare professionals who find themselves psychologically overwhelmed by the pandemic’s economic, social, and financial fallout.
In Canada, the movement was started by Toronto psychotherapist Karen Dougherty, MA, who saw a social media post from someone in New York asking mental health workers to volunteer their time.
Inspired by this, Dougherty reached out to some of her close colleagues with a social media post of her own. A few days later, 450 people had signed up to volunteer and Ontario COVID-19 Therapists was born.
The sessions are provided by licensed Canadian psychotherapists and are free of charge to healthcare workers providing frontline COVID-19 care. After signing up online, users can choose from one of three therapists who will provide up to five free phone sessions.
In New York state, a similar initiative — which is not limited to healthcare workers — has gained incredible momentum. On March 21, Gov. Andrew Cuomo announced the creation of a statewide hotline [844-863-9314] to provide free mental health services to individuals sheltering at home who may be experiencing stress and anxiety as a result of COVID-19.
The governor called on mental-health professionals to volunteer their time and provide telephone and/or telehealth counseling. The New York State Psychiatric Association quickly got on board and encouraged its members to participate.
Just four days later, more than 6,000 mental health workers had volunteered their services, making New York the first state to address the mental health consequences of the pandemic in this way.
Self-care is vital for healthcare workers during the COVID-19 pandemic, particularly as stress mounts and workdays become longer and grimmer. Dougherty recommended that frontline workers manage overwhelming thoughts by limiting their intake of information about the virus.
Self-Care a “Selfless Act”
Clinicians need to balance the need to stay informed with the potential for information overload, which can contribute to anxiety, she said.
She also recommended that individuals continue to connect with loved ones while practicing social distancing. Equally important is talking to someone about the struggles people may be facing at work.
For Amin Azzam MD, MA, the benefits of these initiatives are obvious.
“There is always value in providing additional mental health services and tending to psychological well-being,” Azzam, adjunct professor of psychiatry, University of California, San Francisco and UC Berkeley, told Medscape Medical News.
“If there ever were a time when we can use all the emotional support possible, then it would be during a global pandemic,” added Azzam, who is also director of Open Learning Initiatives at Osmosis, a nonprofit health education company.
Azzam urged healthcare professionals to avail themselves of such resources as often as necessary.
“Taking care of ourselves is not a selfish act. When the oxygen masks come down on airplanes we are always instructed to put our own masks on first before helping those in need. It’s a sign of strength, not weakness, to seek emotional support,” he said.
However, it isn’t always easy. The longstanding stigma associated with seeking help for mental health issues has not stopped for COVID-19. Even workers who are in close daily contact with people infected with the virus are finding they’re not immune to the stigma associated with seeking mental health treatment, Azzam added.
“Nevertheless, the burden these frontline workers are facing is real…and often crushing. Some Ontario doctors have reported pretraumatic stress disorder, which they attribute to having watched the virus wreak havoc in other countries, and knowing that similar difficulties are headed their way,” he said.
A Growing Movement
Doris Grinspun, PhD, MSN, the CEO of Registered Nurses’ Association of Ontario (RNAO), said the province’s nurses are under intense pressure at work, then fear infecting family members once they come home. Some are even staying at hotels to ensure they don’t infect others, as reported by CBC News.
However, she added, most recognize the important role that psychotherapy can play, especially since many frontline healthcare workers find it difficult to speak with their families about the issues they face at work, for fear of adding stress to their family life as well.
“None of us are superhuman and immune to stress. When healthcare workers are facing workplace challenges never before seen in their lifetimes, they need opportunities to decompress to maintain their own health and well-being. This will help them pace themselves for the marathon — not sprint — to continue doing the important work of helping others,” said Azzam.
Given the attention it has garnered in such a short time, Azzam is hopeful that the free therapy movement will spread.
In Canada, mental health professionals in other provinces have already reached out to Dougherty, lending credence to the notion of a pan-Canadian network of therapists offering free services to healthcare workers during the outbreak.
In the US, other local initiatives are already underway.
“The one that I’m personally aware of is at my home institution at the University of California, San Francisco,” Azzam said. “We have a Care for the Caregiver program that is being greatly expanded at this time. As part of that initiative, the institution’s psychiatry department has solicited licensed mental health care providers to volunteer their time to provide those additional services.”
Azzam has also worked with colleagues developing a series of mental health tools that Osmosis has made available free of charge.
These include a central site with educational material about COVID-19, a video about supporting educators’ mental health during high-stress periods; a video about managing students’ mental health during public health emergencies; a summary of recommended resources for psychological health in distressing times; and a YouTube Live event he held regarding tips for maximizing psychological health during stressful times.
This article first appeared on Medscape.com.
Frontline COVID-19 healthcare workers across North America are dealing with unprecedented stress, but mental health therapists in both Canada and the US are doing their part to ensure the psychological well-being of their colleagues on the frontlines of the pandemic.
Over the past few weeks, thousands of licensed psychologists, psychotherapists, and social workers have signed up to offer free therapy sessions to healthcare professionals who find themselves psychologically overwhelmed by the pandemic’s economic, social, and financial fallout.
In Canada, the movement was started by Toronto psychotherapist Karen Dougherty, MA, who saw a social media post from someone in New York asking mental health workers to volunteer their time.
Inspired by this, Dougherty reached out to some of her close colleagues with a social media post of her own. A few days later, 450 people had signed up to volunteer and Ontario COVID-19 Therapists was born.
The sessions are provided by licensed Canadian psychotherapists and are free of charge to healthcare workers providing frontline COVID-19 care. After signing up online, users can choose from one of three therapists who will provide up to five free phone sessions.
In New York state, a similar initiative — which is not limited to healthcare workers — has gained incredible momentum. On March 21, Gov. Andrew Cuomo announced the creation of a statewide hotline [844-863-9314] to provide free mental health services to individuals sheltering at home who may be experiencing stress and anxiety as a result of COVID-19.
The governor called on mental-health professionals to volunteer their time and provide telephone and/or telehealth counseling. The New York State Psychiatric Association quickly got on board and encouraged its members to participate.
Just four days later, more than 6,000 mental health workers had volunteered their services, making New York the first state to address the mental health consequences of the pandemic in this way.
Self-care is vital for healthcare workers during the COVID-19 pandemic, particularly as stress mounts and workdays become longer and grimmer. Dougherty recommended that frontline workers manage overwhelming thoughts by limiting their intake of information about the virus.
Self-Care a “Selfless Act”
Clinicians need to balance the need to stay informed with the potential for information overload, which can contribute to anxiety, she said.
She also recommended that individuals continue to connect with loved ones while practicing social distancing. Equally important is talking to someone about the struggles people may be facing at work.
For Amin Azzam MD, MA, the benefits of these initiatives are obvious.
“There is always value in providing additional mental health services and tending to psychological well-being,” Azzam, adjunct professor of psychiatry, University of California, San Francisco and UC Berkeley, told Medscape Medical News.
“If there ever were a time when we can use all the emotional support possible, then it would be during a global pandemic,” added Azzam, who is also director of Open Learning Initiatives at Osmosis, a nonprofit health education company.
Azzam urged healthcare professionals to avail themselves of such resources as often as necessary.
“Taking care of ourselves is not a selfish act. When the oxygen masks come down on airplanes we are always instructed to put our own masks on first before helping those in need. It’s a sign of strength, not weakness, to seek emotional support,” he said.
However, it isn’t always easy. The longstanding stigma associated with seeking help for mental health issues has not stopped for COVID-19. Even workers who are in close daily contact with people infected with the virus are finding they’re not immune to the stigma associated with seeking mental health treatment, Azzam added.
“Nevertheless, the burden these frontline workers are facing is real…and often crushing. Some Ontario doctors have reported pretraumatic stress disorder, which they attribute to having watched the virus wreak havoc in other countries, and knowing that similar difficulties are headed their way,” he said.
A Growing Movement
Doris Grinspun, PhD, MSN, the CEO of Registered Nurses’ Association of Ontario (RNAO), said the province’s nurses are under intense pressure at work, then fear infecting family members once they come home. Some are even staying at hotels to ensure they don’t infect others, as reported by CBC News.
However, she added, most recognize the important role that psychotherapy can play, especially since many frontline healthcare workers find it difficult to speak with their families about the issues they face at work, for fear of adding stress to their family life as well.
“None of us are superhuman and immune to stress. When healthcare workers are facing workplace challenges never before seen in their lifetimes, they need opportunities to decompress to maintain their own health and well-being. This will help them pace themselves for the marathon — not sprint — to continue doing the important work of helping others,” said Azzam.
Given the attention it has garnered in such a short time, Azzam is hopeful that the free therapy movement will spread.
In Canada, mental health professionals in other provinces have already reached out to Dougherty, lending credence to the notion of a pan-Canadian network of therapists offering free services to healthcare workers during the outbreak.
In the US, other local initiatives are already underway.
“The one that I’m personally aware of is at my home institution at the University of California, San Francisco,” Azzam said. “We have a Care for the Caregiver program that is being greatly expanded at this time. As part of that initiative, the institution’s psychiatry department has solicited licensed mental health care providers to volunteer their time to provide those additional services.”
Azzam has also worked with colleagues developing a series of mental health tools that Osmosis has made available free of charge.
These include a central site with educational material about COVID-19, a video about supporting educators’ mental health during high-stress periods; a video about managing students’ mental health during public health emergencies; a summary of recommended resources for psychological health in distressing times; and a YouTube Live event he held regarding tips for maximizing psychological health during stressful times.
This article first appeared on Medscape.com.
Frontline COVID-19 healthcare workers across North America are dealing with unprecedented stress, but mental health therapists in both Canada and the US are doing their part to ensure the psychological well-being of their colleagues on the frontlines of the pandemic.
Over the past few weeks, thousands of licensed psychologists, psychotherapists, and social workers have signed up to offer free therapy sessions to healthcare professionals who find themselves psychologically overwhelmed by the pandemic’s economic, social, and financial fallout.
In Canada, the movement was started by Toronto psychotherapist Karen Dougherty, MA, who saw a social media post from someone in New York asking mental health workers to volunteer their time.
Inspired by this, Dougherty reached out to some of her close colleagues with a social media post of her own. A few days later, 450 people had signed up to volunteer and Ontario COVID-19 Therapists was born.
The sessions are provided by licensed Canadian psychotherapists and are free of charge to healthcare workers providing frontline COVID-19 care. After signing up online, users can choose from one of three therapists who will provide up to five free phone sessions.
In New York state, a similar initiative — which is not limited to healthcare workers — has gained incredible momentum. On March 21, Gov. Andrew Cuomo announced the creation of a statewide hotline [844-863-9314] to provide free mental health services to individuals sheltering at home who may be experiencing stress and anxiety as a result of COVID-19.
The governor called on mental-health professionals to volunteer their time and provide telephone and/or telehealth counseling. The New York State Psychiatric Association quickly got on board and encouraged its members to participate.
Just four days later, more than 6,000 mental health workers had volunteered their services, making New York the first state to address the mental health consequences of the pandemic in this way.
Self-care is vital for healthcare workers during the COVID-19 pandemic, particularly as stress mounts and workdays become longer and grimmer. Dougherty recommended that frontline workers manage overwhelming thoughts by limiting their intake of information about the virus.
Self-Care a “Selfless Act”
Clinicians need to balance the need to stay informed with the potential for information overload, which can contribute to anxiety, she said.
She also recommended that individuals continue to connect with loved ones while practicing social distancing. Equally important is talking to someone about the struggles people may be facing at work.
For Amin Azzam MD, MA, the benefits of these initiatives are obvious.
“There is always value in providing additional mental health services and tending to psychological well-being,” Azzam, adjunct professor of psychiatry, University of California, San Francisco and UC Berkeley, told Medscape Medical News.
“If there ever were a time when we can use all the emotional support possible, then it would be during a global pandemic,” added Azzam, who is also director of Open Learning Initiatives at Osmosis, a nonprofit health education company.
Azzam urged healthcare professionals to avail themselves of such resources as often as necessary.
“Taking care of ourselves is not a selfish act. When the oxygen masks come down on airplanes we are always instructed to put our own masks on first before helping those in need. It’s a sign of strength, not weakness, to seek emotional support,” he said.
However, it isn’t always easy. The longstanding stigma associated with seeking help for mental health issues has not stopped for COVID-19. Even workers who are in close daily contact with people infected with the virus are finding they’re not immune to the stigma associated with seeking mental health treatment, Azzam added.
“Nevertheless, the burden these frontline workers are facing is real…and often crushing. Some Ontario doctors have reported pretraumatic stress disorder, which they attribute to having watched the virus wreak havoc in other countries, and knowing that similar difficulties are headed their way,” he said.
A Growing Movement
Doris Grinspun, PhD, MSN, the CEO of Registered Nurses’ Association of Ontario (RNAO), said the province’s nurses are under intense pressure at work, then fear infecting family members once they come home. Some are even staying at hotels to ensure they don’t infect others, as reported by CBC News.
However, she added, most recognize the important role that psychotherapy can play, especially since many frontline healthcare workers find it difficult to speak with their families about the issues they face at work, for fear of adding stress to their family life as well.
“None of us are superhuman and immune to stress. When healthcare workers are facing workplace challenges never before seen in their lifetimes, they need opportunities to decompress to maintain their own health and well-being. This will help them pace themselves for the marathon — not sprint — to continue doing the important work of helping others,” said Azzam.
Given the attention it has garnered in such a short time, Azzam is hopeful that the free therapy movement will spread.
In Canada, mental health professionals in other provinces have already reached out to Dougherty, lending credence to the notion of a pan-Canadian network of therapists offering free services to healthcare workers during the outbreak.
In the US, other local initiatives are already underway.
“The one that I’m personally aware of is at my home institution at the University of California, San Francisco,” Azzam said. “We have a Care for the Caregiver program that is being greatly expanded at this time. As part of that initiative, the institution’s psychiatry department has solicited licensed mental health care providers to volunteer their time to provide those additional services.”
Azzam has also worked with colleagues developing a series of mental health tools that Osmosis has made available free of charge.
These include a central site with educational material about COVID-19, a video about supporting educators’ mental health during high-stress periods; a video about managing students’ mental health during public health emergencies; a summary of recommended resources for psychological health in distressing times; and a YouTube Live event he held regarding tips for maximizing psychological health during stressful times.
This article first appeared on Medscape.com.