User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Capmatinib shows impressive results in METex14-mutated NSCLC
according to a presentation at the AACR virtual meeting I.
The duration of response was impressive in both treatment-naive and previously treated patients, according to presenter Edward B. Garon, MD, of the University of California, Los Angeles.
In view of these responses, Dr. Garon urged early molecular testing in NSCLC.
He also noted that capmatinib produced responses in patients with brain metastases. However, because of small patient numbers, additional study is needed to validate the intracranial efficacy of capmatinib and ascertain mechanisms of resistance.
Study rationale and details
METex14 mutations are reported in up to 4% of patients with NSCLC and portend poor outcomes with chemotherapy and immune checkpoint inhibitors (PLoS One 2014; 9:e107677; Ann Oncol 2018;29:2085-91).
Capmatinib is a highly selective, reversible, and potent inhibitor of MET tyrosine kinase that crosses the blood-brain barrier.
In the phase 2 GEOMETRY mono-1 study, Dr. Garon and colleagues tested capmatinib, given at 400 mg orally twice a day, in patients with METex14-mutated, ALK and EGFR wild-type, stage IIIB/IV NSCLC. Patients with neurologically stable or asymptomatic brain metastases were eligible.
Dr. Garon presented safety data for all patients enrolled in this study and efficacy data for patients in cohorts 4 and 5b. Cohort 4 enrolled patients who received prior systemic therapy for advanced disease, and cohort 5b enrolled treatment-naive patients. Both cohorts had METex14 gene mutations but not amplification.
Efficacy
There were 97 patients evaluable for efficacy – 69 previously treated and 28 treatment naive. The median age in both cohorts was 71 years, most patients were female (58% of previously treated and 64.3% of treatment-naive patients), and most were never-smokers (58% and 64.3%, respectively). Adenocarcinoma was the predominant histology.
The overall response rate, per an independent review committee, was 40.6% in previously treated patients and 67.9% in treatment-naive patients.
Waterfall plots showed deep responses, with only four cases of disease progression in the previously treated cohort and none in the treatment-naive cohort.
Responses occurred rapidly. Many responses exceeded 1 year and were ongoing at the data cut-off. The median response duration was 9.72 months in previously treated patients and 11.14 months in treatment-naive patients.
There were 13 patients with evaluable baseline brain metastases (3.3 brain lesions per patient [range, 1-8]). Twelve patients had intracranial disease control, and seven patients (54%) had intracranial response. Four patients had complete resolution of all brain lesions.
Intracranial responses were generally seen by the first radiologic evaluation and occurred as rapidly as systemic responses.
Safety
With safety data on all 334 patients in the trial, the GEOMETRY mono-1 study is the largest reported experience with capmatinib in NSCLC patients. The median treatment exposure time was 14.9 weeks.
Overall, 35.6% of patients experienced a grade 3/4 adverse event (AE). Grade 4 AEs were observed in 4.5% of patients, and there were no treatment-related deaths.
Peripheral edema (41.6%), nausea (33.2%), increased blood creatinine (19.5%), and vomiting (18.9%) were the most frequent AEs of any grade.
In all, 21.9% of patients required dose adjustments due to treatment-related AEs, and 11.1% of patients stopped treatment because of an AE.
This study was sponsored by Novartis. Dr. Garon disclosed relationships with Novartis, AstraZeneca, Bristol-Myers Squibb, Dracen, Dynavax, Eli Lilly, EMD Serono, Genentech, GSK, Iovance, Merck, Mirati, and Neon.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Garon EB et al. AACR 2020, Abstract CT082.
according to a presentation at the AACR virtual meeting I.
The duration of response was impressive in both treatment-naive and previously treated patients, according to presenter Edward B. Garon, MD, of the University of California, Los Angeles.
In view of these responses, Dr. Garon urged early molecular testing in NSCLC.
He also noted that capmatinib produced responses in patients with brain metastases. However, because of small patient numbers, additional study is needed to validate the intracranial efficacy of capmatinib and ascertain mechanisms of resistance.
Study rationale and details
METex14 mutations are reported in up to 4% of patients with NSCLC and portend poor outcomes with chemotherapy and immune checkpoint inhibitors (PLoS One 2014; 9:e107677; Ann Oncol 2018;29:2085-91).
Capmatinib is a highly selective, reversible, and potent inhibitor of MET tyrosine kinase that crosses the blood-brain barrier.
In the phase 2 GEOMETRY mono-1 study, Dr. Garon and colleagues tested capmatinib, given at 400 mg orally twice a day, in patients with METex14-mutated, ALK and EGFR wild-type, stage IIIB/IV NSCLC. Patients with neurologically stable or asymptomatic brain metastases were eligible.
Dr. Garon presented safety data for all patients enrolled in this study and efficacy data for patients in cohorts 4 and 5b. Cohort 4 enrolled patients who received prior systemic therapy for advanced disease, and cohort 5b enrolled treatment-naive patients. Both cohorts had METex14 gene mutations but not amplification.
Efficacy
There were 97 patients evaluable for efficacy – 69 previously treated and 28 treatment naive. The median age in both cohorts was 71 years, most patients were female (58% of previously treated and 64.3% of treatment-naive patients), and most were never-smokers (58% and 64.3%, respectively). Adenocarcinoma was the predominant histology.
The overall response rate, per an independent review committee, was 40.6% in previously treated patients and 67.9% in treatment-naive patients.
Waterfall plots showed deep responses, with only four cases of disease progression in the previously treated cohort and none in the treatment-naive cohort.
Responses occurred rapidly. Many responses exceeded 1 year and were ongoing at the data cut-off. The median response duration was 9.72 months in previously treated patients and 11.14 months in treatment-naive patients.
There were 13 patients with evaluable baseline brain metastases (3.3 brain lesions per patient [range, 1-8]). Twelve patients had intracranial disease control, and seven patients (54%) had intracranial response. Four patients had complete resolution of all brain lesions.
Intracranial responses were generally seen by the first radiologic evaluation and occurred as rapidly as systemic responses.
Safety
With safety data on all 334 patients in the trial, the GEOMETRY mono-1 study is the largest reported experience with capmatinib in NSCLC patients. The median treatment exposure time was 14.9 weeks.
Overall, 35.6% of patients experienced a grade 3/4 adverse event (AE). Grade 4 AEs were observed in 4.5% of patients, and there were no treatment-related deaths.
Peripheral edema (41.6%), nausea (33.2%), increased blood creatinine (19.5%), and vomiting (18.9%) were the most frequent AEs of any grade.
In all, 21.9% of patients required dose adjustments due to treatment-related AEs, and 11.1% of patients stopped treatment because of an AE.
This study was sponsored by Novartis. Dr. Garon disclosed relationships with Novartis, AstraZeneca, Bristol-Myers Squibb, Dracen, Dynavax, Eli Lilly, EMD Serono, Genentech, GSK, Iovance, Merck, Mirati, and Neon.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Garon EB et al. AACR 2020, Abstract CT082.
according to a presentation at the AACR virtual meeting I.
The duration of response was impressive in both treatment-naive and previously treated patients, according to presenter Edward B. Garon, MD, of the University of California, Los Angeles.
In view of these responses, Dr. Garon urged early molecular testing in NSCLC.
He also noted that capmatinib produced responses in patients with brain metastases. However, because of small patient numbers, additional study is needed to validate the intracranial efficacy of capmatinib and ascertain mechanisms of resistance.
Study rationale and details
METex14 mutations are reported in up to 4% of patients with NSCLC and portend poor outcomes with chemotherapy and immune checkpoint inhibitors (PLoS One 2014; 9:e107677; Ann Oncol 2018;29:2085-91).
Capmatinib is a highly selective, reversible, and potent inhibitor of MET tyrosine kinase that crosses the blood-brain barrier.
In the phase 2 GEOMETRY mono-1 study, Dr. Garon and colleagues tested capmatinib, given at 400 mg orally twice a day, in patients with METex14-mutated, ALK and EGFR wild-type, stage IIIB/IV NSCLC. Patients with neurologically stable or asymptomatic brain metastases were eligible.
Dr. Garon presented safety data for all patients enrolled in this study and efficacy data for patients in cohorts 4 and 5b. Cohort 4 enrolled patients who received prior systemic therapy for advanced disease, and cohort 5b enrolled treatment-naive patients. Both cohorts had METex14 gene mutations but not amplification.
Efficacy
There were 97 patients evaluable for efficacy – 69 previously treated and 28 treatment naive. The median age in both cohorts was 71 years, most patients were female (58% of previously treated and 64.3% of treatment-naive patients), and most were never-smokers (58% and 64.3%, respectively). Adenocarcinoma was the predominant histology.
The overall response rate, per an independent review committee, was 40.6% in previously treated patients and 67.9% in treatment-naive patients.
Waterfall plots showed deep responses, with only four cases of disease progression in the previously treated cohort and none in the treatment-naive cohort.
Responses occurred rapidly. Many responses exceeded 1 year and were ongoing at the data cut-off. The median response duration was 9.72 months in previously treated patients and 11.14 months in treatment-naive patients.
There were 13 patients with evaluable baseline brain metastases (3.3 brain lesions per patient [range, 1-8]). Twelve patients had intracranial disease control, and seven patients (54%) had intracranial response. Four patients had complete resolution of all brain lesions.
Intracranial responses were generally seen by the first radiologic evaluation and occurred as rapidly as systemic responses.
Safety
With safety data on all 334 patients in the trial, the GEOMETRY mono-1 study is the largest reported experience with capmatinib in NSCLC patients. The median treatment exposure time was 14.9 weeks.
Overall, 35.6% of patients experienced a grade 3/4 adverse event (AE). Grade 4 AEs were observed in 4.5% of patients, and there were no treatment-related deaths.
Peripheral edema (41.6%), nausea (33.2%), increased blood creatinine (19.5%), and vomiting (18.9%) were the most frequent AEs of any grade.
In all, 21.9% of patients required dose adjustments due to treatment-related AEs, and 11.1% of patients stopped treatment because of an AE.
This study was sponsored by Novartis. Dr. Garon disclosed relationships with Novartis, AstraZeneca, Bristol-Myers Squibb, Dracen, Dynavax, Eli Lilly, EMD Serono, Genentech, GSK, Iovance, Merck, Mirati, and Neon.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Garon EB et al. AACR 2020, Abstract CT082.
FROM AACR 2020
COVID-19 pulmonary severity ascribed to coagulation differences
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
Differences in COVID-19-related death rates between people of white and Asian ancestry may be partly explained by documented ethnic/racial differences in risk for blood clotting and pulmonary thrombotic events, investigators propose.
“Our novel findings demonstrate that COVID-19 is associated with a unique type of blood clotting disorder that is primarily focused within the lungs and which undoubtedly contributes to the high levels of mortality being seen in patients with COVID-19,” said James O’Donnell, MB, PhD, director of the Irish Centre for Vascular Biology at the Royal College of Surgeons in Ireland.
Dr. O’Donnell and colleagues studied pulmonary effects and outcomes of 83 patients admitted to St. James Hospital in Dublin, and found evidence to suggest that the diffuse, bilateral pulmonary inflammation seen in many patients with severe COVID-19 infections may be caused by a pulmonary-specific vasculopathy they label “pulmonary intravascular coagulopathy” (PIC), an entity distinct from disseminated intravascular coagulopathy (DIC).
“Given that thrombotic risk is significantly impacted by race, coupled with the accumulating evidence that coagulopathy is important in COVID-19 pathogenesis, our findings raise the intriguing possibility that pulmonary vasculopathy may contribute to the unexplained differences that are beginning to emerge highlighting racial susceptibility to COVID-19 mortality,” they wrote in a study published online in the British Journal of Haematology.
Study flaws harm conclusions
But critical care specialists who agreed to review and comment on the study for MDedge News said that it has significant flaws that affect the ability to interpret the findings and “undermine the conclusions reached by the authors.”
“The underlying premise of the study is that there are racial and ethnic differences in the development of venous thromboembolism that may explain the racial and ethnic differences in outcomes from COVID-19,” J. Daryl Thornton, MD, MPH, a fellow of the American Thoracic Society and associate professor of pulmonary, critical care, and sleep medicine at Case Western Reserve University, Cleveland, said in an interview. “This is an interesting hypothesis and one that could be easily tested in a well-designed study with sufficient representation from the relevant racial and ethnic groups. However, this study is neither well designed nor does it have sufficient racial and ethnic representation.”
Elliott R. Haut, MD, PhD, associate professor of surgery, anesthesiology and critical care medicine at Johns Hopkins Medicine, Baltimore, said in an interview that the study is “mediocre” and has the feel of a paper rushed to press.
“It talks about their theory that race, ethnicity, have an effect on venous thromboembolism, and that’s a pretty well-known fact. No one’s a hundred percent sure why that is, but certainly there are tons and tons of papers that show that there are groups that are at higher risk than others,” he said. “Their idea that this is caused by this pulmonary inflammation, that is totally a guess; there is no data in this paper to support that.”
Dr. Thornton and Dr. Haut both noted that the authors don’t define how race and ethnicity were determined and whether patients were asked to provide it, and although they mention the racial/ethnic breakdown once, subsequent references are to entire cohort are as “Caucasian.”
They also called into question the value of comparing laboratory data across continents in centers with different testing methods and parameters, especially in a time when the clinical picture changes so rapidly.
Coagulation differences
Dr. O’Donnell and colleagues noted that most studies of COVID-19-associated coagulopathy published to date have been with Chinese patients.
“This is important because race and ethnicity have major effects upon thrombotic risk. In particular, epidemiological studies have shown that the incidence of venous thromboembolism (VTE) is approximately three to fourfold lower in Chinese compared to Caucasian individuals. Conversely, VTE risk is significantly higher in African-Americans compared to Caucasians,” they wrote.
Because of the lower risk of VTE in the Chinese population, thromboprophylaxis with low-molecular-weight heparin (LMWH) or other agents is less frequently used in Chinese hospitals than in hospitals with predominantly non-Asian patients, they noted.
To see whether the were differences in coagulopathy between Chinese and white patients, the researchers enrolled 55 men and 28 women, median age 64, who were admitted to St. James Hospital with COVID-19 infections from March 13 through April 10, 2020. The cohort included 67 patients of white background, 10 of Asian ancestry, 5 of African ethnicity, and 1 of Latino/Hispanic ancestry.
Of the 83 patients, 67 had comorbidities at admission. At the time of the report, 50 patients had fully recovered and were discharged, 20 remained in the hospital, and 13 had died. In all, 50 patients were discharged without needing ICU care, 23 were admitted to the ICU, and 10 required ICU but were deemed “clinically unsuitable” for ICU admission.
Although the patients had normal prothrombin time (PT) and normal activated partial thromboplastin time (APTT), plasma d-dimer levels were significantly elevated and were above the range of normal in two-thirds of patients on admission.
Despite the increased d-dimer levels, however, there was no evidence of DIC as defined by the International Society of Thrombosis and Hemostasis Scientific and Standardization committee (ISTH SSC) guidelines. Platelet counts were in the normal range in 83.1% of patients, and only five had counts less than 100 x 109/L at admission. Fibrinogen levels were also elevated, as were C-reactive protein levels, both likely indicating an acute phase response.
“Thus, despite the fact that thrombotic risk is much higher in Caucasian patients and the significant elevated levels of d-dimers observed, overt DIC as defined according to the ISTH SSC DIC score was present in none of our COVID-19 patients at time of admission. Nevertheless, our data confirm that severe COVID-19 infection is associated with a significant coagulopathy in Caucasian patients that appears to be similar in magnitude to that previously reported in the original Chinese cohorts,” they wrote.
When they compared patients who required ICU admission for ventilator support and those who died with patients who were discharged without needing ICU support, they found that survivors were younger (median age 60.2 vs. 75.2 years), and that more critically ill patients were more likely to have comorbidities.
They also found that patients with abnormal coagulation parameters on admission were significantly more likely to have poor prognosis (P = .018), and that patients in the adverse outcomes group had significantly higher fibrinogen and CRP levels (P = .045 and .0005, respectively).
There was no significant difference in PT between the prognosis groups at admission, but by day 4 and beyond PT was a median of 13.1 vs. 12.5 seconds in the favorable outcomes groups (P = .007), and patients with poor prognosis continued to have significantly higher d-dimer levels. (P = .003)
“Cumulatively, these data support the hypothesis that COVID-19–associated coagulopathy probably contributes to the underlying pulmonary pathogenesis,” the researchers wrote.
They noted that the angiotensin converting enzyme 2 (ACE-2) receptor that COVID-19 uses to enter cells is expressed on both type II pneumocytes and vascular endothelial cells within the lung, suggesting that the coagulopathy may be related to direct pulmonary endothelial cell infection , activation, and/or damage, and to the documented cytokine storm that can affect thrombin generation and fibrin deposition within the lungs.
“In the context of this lung-centric vasculopathy, we hypothesize that the refractory acute respiratory distress syndrome phenotype observed in severe COVID-19 is due to concurrent ‘double-hit’ pathologies targeting both ventilation (V) and perfusion (Q) within the lungs where alveoli and pulmonary microvasculature exist in close anatomical juxtaposition,” they wrote.
The investigators noted that larger randomized trials will be needed to determine whether more aggressive anti-coagulation and/or targeted anti-inflammatory therapies could effectively treated PIC in patients with severe COVID-19.
The study was supported by the Wellcome Trust and the Health Research Board Health Service and the Research and Development Division, Northern Ireland. Dr. O’Donnell disclosed speakers bureau activities, advisory board participation, and research grants from multiple companies. The other doctors had no relevant conflicts of interest to disclose.
SOURCE: Fogarty H et al. Br J Haematol. 2020 Apr 24. doi: 10.1111/bjh.16749.
FROM THE BRITISH JOURNAL OF HEMATOLOGY
New angiotensin studies in COVID-19 give more reassurance
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Four more studies of the relationship of angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) with COVID-19 have been published in the past few days in top-tier peer-reviewed journals, and on the whole, the data are reassuring.
Three of the new studies were published in the New England Journal of Medicine on May 1, and one study was published in JAMA Cardiology on May 5.
Although all the studies are observational in design and have some confounding factors, overall, However, there are some contradictory findings in secondary analyses regarding possible differences in the effects of the two drug classes.
Providing commentary, John McMurray, MD, professor of medical cardiology at the University of Glasgow, said: “The overall picture seems to suggest no increase in risk of adverse outcomes in patients taking renin-angiotensin system [RAS] blockers ― but with lots of caveats: These are all observational rather than randomized studies, and there may be residual or unmeasured confounding.”
Was it ‘Much ado about nothing’?
Franz Messerli, MD, professor of medicine at the University of Bern (Switzerland), added: “Given this state of the art, I am inclined to consider RAS blockade and COVID-19 – despite all the hype in the news media – as much ado about nothing.”
But both Dr. McMurray and Dr. Messerli said they were intrigued about possible differences in the effects of ACE inhibitors and ARBs that some of the new results suggest.
In one study, a team led by Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center, Boston, analyzed data from 8,910 patients with COVID-19 admitted to 169 hospitals in Asia, Europe, and North America who had either died in the hospital (5.8%) or survived to hospital discharge (94.2%).
In multivariate logistic-regression analysis, age greater than 65 years, coronary artery disease, congestive heart failure, history of cardiac arrhythmia, chronic obstructive pulmonary disease, and current smoking were associated with an increased risk for in-hospital death. Female sex was associated with a decreased risk. Neither ACE inhibitors nor ARBs were associated with an increased risk for in-hospital death.
In fact, ACE inhibitors were associated with a significant reduction in mortality (odds ratio, 0.33), as were statins (OR, 0.35).
The authors, however, stressed that these observations about reduced mortality with ACE inhibitors and statins “should be considered with extreme caution.”
“Because our study was not a randomized, controlled trial, we cannot exclude the possibility of confounding. In addition, we examined relationships between many variables and in-hospital death, and no primary hypothesis was prespecified; these factors increased the probability of chance associations being found. Therefore, a cause-and-effect relationship between drug therapy and survival should not be inferred,” they wrote.
A secondary analysis that was restricted to patients with hypertension (those for whom an ACE inhibitor or an ARB would be indicated) also did not show harm.
A second study published in the New England Journal of Medicine had a case-control design. The authors, led by Giuseppe Mancia, MD, of the University of Milano-Bicocca (Italy), compared 6,272 patients with confirmed COVID-19 (case patients) with 30,759 control persons who were matched according to age, sex, and municipality of residence.
In a conditional logistic-regression multivariate analysis, neither ACE inhibitors nor ARBs were associated with the likelihood of SARS-CoV-2 infection.
“Thus, our results do not provide evidence of an independent relationship between renin angiotensin aldosterone blockers and the susceptibility to COVID-19 in humans,” the authors concluded.
In addition, a second analysis that compared patients who had severe or fatal infections with matched control persons did not show an association between ACE inhibitors or ARBs and severe disease.
In the third study published in the New England Journal of Medicine, a group led by Harmony R. Reynolds, MD, of New York University, analyzed data from the health records of 12,594 patients in the NYU Langone Health system who had been tested for COVID-19. They found 5,894 patients whose test results were positive. Of these patients, 1,002 had severe illness, which was defined as illness requiring admission to the ICU, need for mechanical ventilation, or death.
Using Bayesian analysis and propensity score matching, the researchers assessed the relation between previous treatment with five different classes of antihypertensive drugs (ACE inhibitors, ARBs, beta blockers, calcium blockers, and thiazide diuretics) and the likelihood of a positive or negative result on COVID-19 testing, as well as the likelihood of severe illness among patients who tested positive.
Results showed no positive association between any of the analyzed drug classes and either a positive test result or severe illness.
In an accompanying editorial, a group led by John A. Jarcho, MD, of Harvard Medical School, Boston, and deputy editor of the New England Journal of Medicine, wrote: “Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test.
“Each of these studies has weaknesses inherent in observational data, but we find it reassuring that three studies in different populations and with different designs arrive at the consistent message that the continued use of ACE inhibitors and ARBs is unlikely to be harmful in patients with COVID-19. Several other smaller studies from China and the United Kingdom have come to the same conclusion,” the authors of the editorial stated.
In the study published in JAMA Cardiology, a group led by Neil Mehta, MBBS, of the Cleveland Clinic, Ohio, analyzed data on 18,472 patients who had been tested for COVID-19 between March 8 and April 12 in the Cleveland Clinic Health System in Ohio and Florida. Of these patients, 9.4% tested positive.
After overlap propensity score weighting for both ACE inhibitors and ARBs to take into account relevant comorbidities, there was no difference in risk for testing positive among patients taking an ACE inhibitor or an ARB in comparison with those not taking such medication.
Are there different effects between ACE inhibitors and ARBs?
A secondary exploratory analysis showed a higher likelihood of hospital admission among patients who tested positive and who were taking either ACE inhibitors (OR, 1.84) or ARBs (OR, 1.61), and there was a higher likelihood of ICU admission among patients who tested positive and who were taking an ACE inhibitor (OR 1.77), but no such difference was observed among those taking ARBs.
Coauthor Ankur Kalra, MD, of the Cleveland Clinic, said in an interview that results of the exploratory analysis fit with the hypothesis that the two drugs classes may have different effects in patients with COVID-19.
“Angiotensin II promotes vasoconstriction, inflammation, and fibrosis in the lungs, and ARBs block the effects of angiotensin II more effectively than ACE inhibitors. In addition, ACE inhibitors (but not ARBs) increase levels of bradykinin, which may be one factor leading to acute respiratory distress syndrome,” he noted.
“However, these results should only be considered exploratory, as there is inherent bias in observational data,” Dr. Kalra stressed.
In an accompanying editorial in JAMA Cardiology, a group led by Laine E. Thomas, PhD, of Duke Clinical Research Institute, Durham, North Carolina, said that the results of this secondary exploratory analysis are limited by a small number of patients and “are likely explained by confounding and should not be inferred as causal.”
The New England Journal of Medicine editorialists reached a similar conclusion regarding the lower mortality in COVID-19 patients who took ACE inhibitors in the study by Dr. Mehra and colleagues. They say this unexpected result “may be due to unmeasured confounding and, in the absence of a randomized trial, should not be regarded as evidence to prescribe these drugs in patients with COVID-19.”
Providing further comment, Dr. McMurray said: “Normally, I would not read too much into the different effects of ACE inhibitors and ARBs suggested in the Cleveland study because of the small numbers (about 28 ACE inhibitor–treated patients admitted to ICU) and the limited information about matching and/or adjustment for potential differences between groups.
“I could also argue that the comparison that would best answer the question about risk related to type of RAS blocker would be the direct comparison of people taking an ACE inhibitor with those taking an ARB (and that doesn’t look very different). The only thing that makes me a little cautious about completely dismissing the possibility of a difference between ACE inhibitor and ARB here is the suggestion of a similar trend in another large study from the VA [Veterans Affairs] system,” he added.
He also noted that speculation about there being mechanisms that involve different effects of the two drug classes on bradykinin and angiotensin II was “plausible but unproven.”
Dr. Messerli added: “Before turning the page, I would like to see an analysis comparing ACE inhibitors and ARBs, since experimentally, their effect on ACE2 (the receptor to which the virus binds) seems to differ. The study of Mehta et al in JAMA Cardiology may be the first clinical hint indicating that ARBs are more protective than ACEIs. However even here, the looming possibility of confounding cannot be excluded.”
Dr. Messerli also pointed to a hypothesis that suggests that direct viral infection of endothelial cells expressing ACE2 receptors may explain worse outcomes in patients with cardiovascular comorbidities, which provides a rationale for therapies to stabilize the endothelium, particularly with anti-inflammatory anticytokine drugs, ACE inhibitors, and statins.
A version of this article originally appeared on Medscape.com.
Renal function data improve risk stratification in patients with PAH
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
FROM THE JOURNAL OF HEART AND LUNG TRANSPLANTATION
Multisociety roadmap eyes restarting elective cardiac cases
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.
As COVID-19 case levels plateau in some regions, 16 North American cardiovascular societies have released a framework for reintroducing cardiovascular services disrupted by the pandemic.
The consensus document outlines a phased approach to restarting invasive cardiovascular (CV) procedures and diagnostic tests that aims to reduce patient and health care provider exposure to the coronavirus and still provide essential care. It also emphasizes some of the ethical considerations in patient selection and the need for a collaborative approach.
“The key message in our document is we need a new unprecedented collaboration with public health officials so that we can carefully monitor the situation and we’re aware of what’s happening with the penetrance of the pandemic in the community, but they’re aware of the morbidity and mortality that’s occurring on our ever-growing waiting list,” lead author David A. Wood, MD, told theheart.org | Medscape Cardiology.
The recommendations were jointly published May 4 in the Canadian Journal of Cardiology , the Journal of the American College of Cardiology, and The Annals of Thoracic Surgery, and are endorsed by, among others, the American Heart Association, American College of Cardiology (ACC), and Canadian Cardiovascular Society.
The guidance comes as hospitals are facing revenue shortfalls because of canceled elective procedures and resource-intensive COVID-19 cases, prompting some healthcare systems to furlough, lay off, or even fire staff.
“It’s obvious that volumes are down between 40% and 60%,” said Wood, director of the cardiac catheterization laboratory at Vancouver General Hospital and professor of medicine at the University of British Columbia, Canada. “Part of that is that some areas have restricted case volumes totally appropriately and it’s partly because patients are very afraid of coming to the hospital and, unfortunately, are having bad events at home. And some are dying.”
The new report features a detailed table outlining three different response levels: reintroduction of some services (level 2); reintroduction of most services (level 1); and regular services (level 0). It covers a range of services from transthoracic echocardiography and exercise testing with imaging to care for acute coronary syndrome and ST-segment elevation myocardial infarction.
“We’ve learned that we can very quickly turn off the tap and go to doing only 10% of our normal volumes, whether that’s surgery, cath lab, EP, diagnostic tests,” Wood said. “It’s much more difficult to thoughtfully turn the tap part way back on or restart the engine … you don’t just go from 0 to 100 [mph]. You go from 0 to 30 to 60 then maybe to 80 [mph].”
The document also includes eight guiding principles such as:
- The expectation that response levels will be different between regions, and even within a given region.
- A “transparent collaborative plan” for COVID-19 testing and personal protective equipment (PPE) must be in place before restarting cases.
- A less invasive test or alternate imaging modality should be considered, if both tests have similar efficacy.
- In general, a minimally invasive procedure with a shorter length of stay is preferable, if both strategies have similar efficacy and safety.
Although previous reports on cath lab considerations during the pandemic or restarting elective surgeries peg various actions to specific thresholds or time intervals, the language here is noticeably and intentionally broad.
Instead of stating when cardiovascular services should resume, for example, the experts say it’s appropriate to put the guidance document into place if there’s a “sustained reduction” in the rate of new COVID-19 admissions and deaths in the relevant geographic region for a “prespecified time interval.”
As for when or how frequently patients and healthcare providers should be tested for COVID-19, the document encourages “routine screening of all patients prior to any cardiovascular procedure or test.”
Overly prescriptive language in previous documents wasn’t felt to be that helpful, whereas language like “selective” cases and “some” or “most” cardiovascular procedures gives clinicians, health systems, and policy makers flexibility when moving between response levels, Wood explained.
“Different regions might be at different levels based on principles of public health as far as the penetrance of the pandemic in that community, as well as how can you actually do the physical distancing in your hospital or ambulatory clinic. Because, I tell you, that is the Achilles heel,” he said. “Our run rates are going to be determined by testing, the availability of PPE, but also how we’re going to use our existing infrastructure and maintain physical distancing.”
That may mean using telehealth for initial visits, having clinics open earlier in the morning or on weekends, or doing partial volumes for surgery or in the cath lab so patients can be staggered and recover at different times and in different areas of the hospital. “These are very granular, specific infrastructure things that we’ve never really had to consider before,” Wood observed.
The document also had to be flexible and nimble enough to respond to a potential rebound of COVID-19 cases, which in newly released models are projected to rise sharply to 200,000 cases a day and be accompanied by some 3,000 deaths each day by June 1.
“This is my own personal opinion but I think it’s foolish to think that we are going to be able to come back to 100% of the cases we were doing before, even with testing, PPE, and all of that until we have a vaccine,” he said.
Similar to decisions made in preparation for the initial COVID-19 surge, the consensus document outlines the need for ethical considerations when turning the tap back on. This means prioritizing procedures and tests that are likely to benefit more people and to a greater degree, and ensuring that patients are treated fairly and consistently, regardless of their ethnicity, perceived social worth, or ability to pay, said coauthor and ACC President Athena Poppas, MD, Brown University School of Medicine, Providence, Rhode Island.
“It’s an ethical tenet that exists in a lot of places but it’s usually not overtly called out,” Poppas told theheart.org | Medscape Cardiology. “It’s not rationing care; I think people jump to that but it’s actually the opposite of rationing care. It’s about being thoughtful about prioritizing patients.”
“There’s a variety of data that should help in the prioritization, not only how much hospital resources are utilized, that’s on one side, but there’s also the patient risk of delaying or doing a procedure, and then the societal risk,” she said.
Susheel Kodali, MD, of New York–Presbyterian Hospital/Columbia University Irving Medical Center, who recently published recommendations on restructuring structural heart disease practice during the pandemic, said the document is timely as centers, including his own, are trying to restart some outpatient visits, as early as next week.
“They made a point about talking about cohesive partnerships with regional public health officials and I think that’s great. The question is how does that happen,” he told theheart.org | Medscape Cardiology. “In New York, we’re not allowed to do elective cases but what’s considered elective is not so clearly defined. An AS [aortic stenosis] patient that had a syncopal episode 2 weeks ago, is that considered elective or is that semi-urgent? I think that’s one of the challenges and that’s where these partnerships would be useful.”
Other challenges include the need for regional partnerships to better align hospitals, which in the New York area means half a dozen large healthcare systems, and to coordinate care between hospital departments – all of which will be scheduling imaging and OR time for their own backlog of hernia, knee, or hip surgeries.
Finally, there’s the need for a lot of conversation with the patient and their family about returning to a hospital amid a deadly pandemic.
“I had a patient today and the daughter was very concerned about bringing her in,” Kodali said. “She’s in class IV heart failure but her [daughter’s] big concern was: who is she going to be exposed to when she gets the echo? What kind of protection is there for her? Is the tech wearing a mask?
“It’s not just the health care providers that have to have the comfort, but it’s the patients and their families who have to feel comfortable bringing their loved ones here for treatment,” he said. “Because everyone is concerned about the environment.”
Wood reports receiving unrestricted grant support from Edwards Lifesciences and Abbott Vascular and serving as a consultant for Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific. Poppas reports no relevant conflicts of interest. Kodali reports consultant (honoraria) from Admedus, Meril Life Sciences, JenaValve, and Abbott Vascular; SAB (equity) from Dura Biotech, MicroInterventional Devices, Thubrikar Aortic Valve, Supira, and Admedus; and institutional funding from Edwards Lifesciences, Medtronic, Abbott Vascular, Boston Scientific, and JenaValve.
This article first appeared on Medscape.com.
Triage, L&D, postpartum care during the COVID-19 pandemic
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
Obstetrics during the COVID-19 pandemic
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Expert discusses red flags for interstitial lung disease in pediatric rheumatology
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
REPORTING FROM RWCS 2020
Pandemic effect: All other health care visits can wait
according to survey conducted at the end of April.
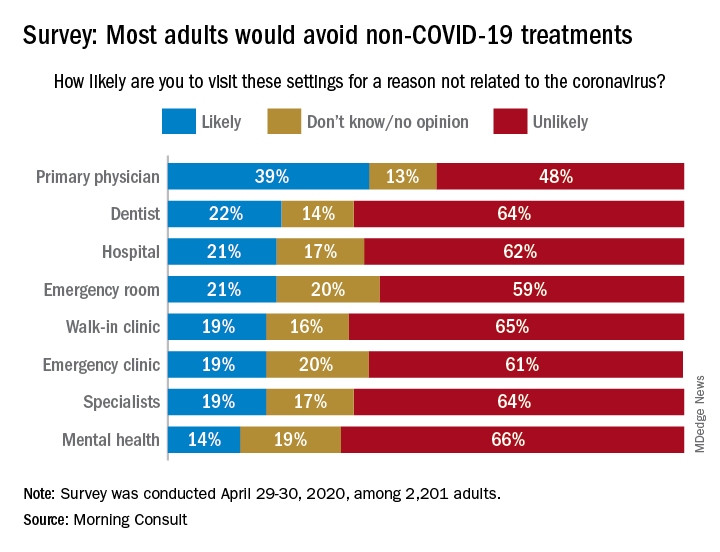
When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
FDA grants EUA to muscle stimulator to reduce mechanical ventilator usage
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.








