User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


HIV does not appear to worsen COVID-19 outcomes
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
People living with HIV who are admitted to the hospital with COVID-19 are no more likely to die than those without HIV, an analysis conducted in New York City shows. This is despite the fact that comorbidities associated with worse COVID-19 outcomes were more common in the HIV group.
“We don’t see any signs that people with HIV should take extra precautions” to protect themselves from COVID-19, said Keith Sigel, MD, associate professor of medicine and infectious diseases at the Icahn School of Medicine at Mount Sinai, New York, and the lead researcher on the study, published online June 28 in Clinical Infectious Diseases.
“We still don’t have a great explanation for why we’re seeing what we’re seeing,” he added. “But we’re glad we’re seeing it.”
The findings have changed how Dr. Sigel talks to his patients with HIV about protecting themselves from COVID-19. Some patients have so curtailed their behavior for fear of acquiring COVID-19 that they aren’t buying groceries or attending needed medical appointments. With these data, Dr. Sigel said he’s comfortable telling his patients, “COVID-19 is bad all by itself, but you don’t need to go crazy. Wear a mask, practice appropriate social distancing and hygiene, but your risk doesn’t appear to be greater.”
The findings conform with those on the lack of association between HIV and COVID-19 severity seen in a cohort study from Spain, a case study from China, and case series from New Jersey, New York City, and Spain.
One of the only regions reporting something different so far is South Africa. There, HIV is the third most common comorbidity associated with death from COVID-19, according to a cohort analysis conducted in the province of Western Cape.
Along with data from HIV prevention and treatment trials, the conference will feature updates on where the world stands in the control of HIV during the COVID-19 pandemic. And for an even more focused look, the IAS COVID-19 Conference will immediately follow that meeting.
The New York City cohort
For their study, Dr. Sigel and colleagues examined the 4402 COVID-19 cases at the Mount Sinai Health System’s five hospitals between March 12 and April 23.
They found 88 people with COVID-19 whose charts showed codes indicating they were living with HIV. All 88 were receiving treatment, and 81% of them had undetectable viral loads documented at COVID admission or in the 12 months prior to admission.
The median age was 61 years, and 40% of the cohort was black and 30% was Hispanic.
Patients in the comparison group – 405 people without HIV from the Veterans Aging Cohort Study who had been admitted to the hospital for COVID-19 – were matched in terms of age, race, and stage of COVID-19.
The study had an 80% power to detect a 15% increase in the absolute risk for death in people with COVID-19, with or without HIV.
Patients with HIV were almost three times as likely to have smoked and were more likely to have chronic obstructive pulmonary disease, cirrhosis, and a history of cancer.
“This was a group of patients that one might suspect would do worse,” Dr. Sigel said. And yet, “we didn’t see any difference in deaths. We didn’t see any difference in respiratory failure.”
In fact, people with HIV required mechanical ventilation less often than those without HIV (18% vs. 23%). And when it came to mortality, one in five people died from COVID-19 during follow-up whether they had HIV or not (21% vs. 20%).
The only factor associated with significantly worse outcomes was a history of organ transplantation, “suggesting that non-HIV causes of immunodeficiency may be more prominent risks for severe outcomes,” Dr. Sigel and colleagues explained.
A surprise association
What’s more, the researchers found a slight association between the use of nucleoside reverse-transcriptase inhibitors (NRTI) by people with HIV and better outcomes in COVID-19. That echoes findings published June 26 in Annals of Internal Medicine, which showed that people with HIV taking the combination of tenofovir disoproxil fumarate plus emtricitabine (Truvada, Gilead Sciences) were less likely to be diagnosed with COVID-19, less likely to be hospitalized, and less likely to die.
This has led some to wonder whether NRTIs have some effect on SARS-CoV-2, the virus that causes COVID-19. Dr. Sigel said he wonders that too, but right now, it’s just musings.
“These studies are not even remotely designed” to show that NRTIs are protective against COVID-19, he explained. “Ours was extremely underpowered to detect that and there was a high potential for confounding.”
“I’d be wary of any study in a subpopulation – which is what we’re dealing with here – that is looking for signals of protection with certain medications,” he added.
A “modest” increase
Using the South African data, released on June 22, public health officials estimate that people with HIV are 2.75 times more likely to die from COVID-19 than those without HIV, making it the third most common comorbidity in people who died from COVID-19, behind diabetes and hypertension. This held true regardless of whether the people with HIV were on treatment.
But when they looked at COVID-19 deaths in the sickest of the sick – those hospitalized with COVID-19 symptoms – HIV was associated with just a 28% increase in the risk for death. The South African researchers called this risk “modest.”
“While these findings may overestimate the effect of HIV on COVID-19 death due to the presence of residual confounding, people living with HIV should be considered a high-risk group for COVID-19 management, with modestly elevated risk of poor outcomes, irrespective of viral suppression,” they wrote.
Epidemiologist Gregorio Millett, MPH, has been tracking the effect of HIV on COVID-19 outcomes since the start of the pandemic in his role as vice president and head of policy at the American Foundation for AIDS Research (amFAR).
Back in April, he and his colleagues looked at rates of COVID-19 deaths and hospitalizations in counties with disproportionate levels of black residents. These areas often overlapped with the communities selected for the Ending the HIV Epidemic plan to control HIV by 2030. What they found was that there was more HIV and COVID-19 in those communities.
What they didn’t find was that people with HIV in those communities had worse outcomes with COVID-19. This remained true even when they reran the analysis after the number of cases of COVID-19 in the United States surpassed 100,000. Those data have yet to be published, Mr. Millett reported.
“HIV does not pop out,” he said. “It’s still social determinants of health. It’s still underlying conditions. It’s still age as a primary factor.”
“People living with HIV are mainly dying of underlying conditions – so all the things associated with COVID-19 – rather than the association being with HIV itself,” he added.
Although he’s not ruling out the possibility that an association like the one in South Africa could emerge, Mr. Millett, who will present a plenary on the context of the HIV epidemic at the IAS conference, said he suspects we won’t see one.
“If we didn’t see an association with the counties that are disproportionately African American, in the black belt where we see high rates of HIV, particularly where we see the social determinants of health that definitely make a difference – if we’re not seeing that association there, where we have a high proportion of African Americans who are at risk both for HIV and COVID-19 – I just don’t think it’s going to emerge,” he said.
This article first appeared on Medscape.com.
FROM AIDS 2020
Inhaled treprostinil improves walk distance in patients with ILD-associated pulmonary hypertension
over 16 weeks, compared with patients who used a placebo inhaler, results of a phase 3 trial showed.
Among 326 patients with pulmonary hypertension (PH) associated with interstitial lung disease (ILD), those who were randomly assigned to treatment with treprostinil had a placebo-corrected median difference from baseline in 6-minute walk distance of 21 m (P = .004), reported Steven D. Nathan, MD, from Inova Fairfax Hospital in Falls Church, Va., on behalf of coinvestigators in the INCREASE study (NCT02630316).
“These results support an additional treatment avenue, and might herald a shift in the clinical management of patients with interstitial lung disease,” he said in the American Thoracic Society’s virtual clinical trial session.
“This was an outstanding presentation and outstanding results. I personally am very excited, because this is a field where I work,” commented Martin Kolb, MD, PhD, from McMaster University, Hamilton, Ont., the facilitator for the online presentation.
The INCREASE trial compared inhaled treprostinil dose four times daily with placebo in patients with a CT scan–confirmed diagnosis of World Health Organization group 3 PH within 6 months before randomization who had evidence of diffuse parenchymal lung disease. Eligible patients could have any form of ILD or combined pulmonary fibrosis and emphysema.
Key inclusion criteria included right-heart catheterization within the previous year with documented pulmonary vascular resistance greater than 3 Wood units, pulmonary capillary wedge pressure 15 mm Hg or less, and mean pulmonary arterial pressure 25 mm Hg or higher.
Patients also had to have a 6-minute walk distance of at least 100 m and have stable disease while on an optimized dose of medications for underlying lung disease. Patients with group 3 connective tissue disease had to have baseline forced vital capacity of less than 70%.
The final study cohorts included patients with idiopathic interstitial pneumonias, chronic hypersensitivity pneumonitis, connective tissue disease, combined pulmonary fibrosis and emphysema, and occupational lung disease.
The patients were randomized to receive either inhaled treprostinil at a starting dose of 6 mcg/breath four times daily or to placebo (163 patients in each arm). All patients started the study drug at a dose of three breaths four times daily during waking hours. Dose escalations – adding 1 additional breath four times daily – were allowed every 3 days, up to a target dose of 9 breaths (54 mcg) four times daily, and a maximum of 12 breaths (72 mcg) four times daily as clinically tolerated.
A total of 130 patients assigned to treprostinil and 128 assigned to placebo completed 16 weeks of therapy and assessment.
As noted before, patients assigned to treprostinil had a placebo-corrected median difference from baseline in peak 6-minute walk distance, as measured by Hodges-Lehmann estimation, of 21 m (P = .004). An analysis of the same parameter using mixed model repeated measurement showed a placebo-corrected difference from baseline in peak 6-minute walk distance of 31.12 m (P < .001).
Secondary endpoints that were significantly better with treprostinil, compared with placebo, included improvements in N-terminal of the prohormone brain natriuretic peptide, a longer time to clinical worsening, and improvements in peak 6-minute walk distance week 12, and trough 6-minute walk distance at week 15.
Treprostinil was associated with a 39% reduction in risk of clinical worsening (P = .04). In all, 37 patients on treprostinil (22.7%) and 54 on placebo (33.1%) experienced clinical worsening.
For the exploratory endpoints of change in patient reported quality of life as measured by the St. George’s Respiratory Questionnaire, or in peak distance saturation product, however, there were no significant differences between the groups.
In addition, treprostinil was associated with a 34% reduction the risk of exacerbation of underlying lung disease, compared with placebo (P = .03).
The safety profile of treprostinil was similar to that seen in other studies of the drug, and most treatment-related adverse events were mild or moderate in severity. Adverse events led to discontinuation in 10% of patients on treprostinil and 8% on placebo.
Serious adverse events were seen in 23.3% and 25.8%, respectively. The most frequently occurring adverse events of any grade included cough, headache, dyspnea, dizziness, nausea, fatigue, diarrhea, throat irritation, and oropharyngeal pain.
There was no evidence of worsened oxygenation or lung function “allaying V/Q mismatch concerns,” Dr. Nathan said, and there was evidence for an improvement in forced vital capacity with treprostinil.
In the question-and-answer portion of the presentation, Dr. Kolb commented that many clinicians, particularly those who treated patients with ILD, question whether a 21-m difference in walk distance makes much of a difference in patient lives. He relayed a question from a viewer asking how Dr. Nathan and associates reconciled their primary endpoint with the finding that there was no difference in patient-reported quality of life.
“I think that the difference in the 6-minute walk test was both statistically significant and clinically meaningful,” Dr. Nathan replied.
He noted that the primary endpoint used a stringent measure, and that less conservative methods of analysis showed a larger difference in benefit favoring treprostinil. He also pointed out that the original study of inhaled treprostinil added to oral therapy for pulmonary arterial hypertension showed a 20-m improvement in walk distance, and that these results were sufficient to get the inhaled formulation approved in the United States (J Am Coll Cardiol. 2010 May. doi: 10.1016/j.jacc.2010.01.027).
Regarding the failure to detect a difference in quality of life, he said that the study was only 16 weeks in length, and that the St. George’s Respiratory Questionnaire was developed for evaluation of patients with chronic obstructive pulmonary disease, “perhaps not the best instrument to use in an ILD PH study.”
The study was funded by United Therapeutics. Dr. Nathan disclosed advisory committee activity/consulting, research support, and speaker fees from the company. Dr. Kolb has previously disclosed financial relationships with various companies, not including United Therapeutics.
over 16 weeks, compared with patients who used a placebo inhaler, results of a phase 3 trial showed.
Among 326 patients with pulmonary hypertension (PH) associated with interstitial lung disease (ILD), those who were randomly assigned to treatment with treprostinil had a placebo-corrected median difference from baseline in 6-minute walk distance of 21 m (P = .004), reported Steven D. Nathan, MD, from Inova Fairfax Hospital in Falls Church, Va., on behalf of coinvestigators in the INCREASE study (NCT02630316).
“These results support an additional treatment avenue, and might herald a shift in the clinical management of patients with interstitial lung disease,” he said in the American Thoracic Society’s virtual clinical trial session.
“This was an outstanding presentation and outstanding results. I personally am very excited, because this is a field where I work,” commented Martin Kolb, MD, PhD, from McMaster University, Hamilton, Ont., the facilitator for the online presentation.
The INCREASE trial compared inhaled treprostinil dose four times daily with placebo in patients with a CT scan–confirmed diagnosis of World Health Organization group 3 PH within 6 months before randomization who had evidence of diffuse parenchymal lung disease. Eligible patients could have any form of ILD or combined pulmonary fibrosis and emphysema.
Key inclusion criteria included right-heart catheterization within the previous year with documented pulmonary vascular resistance greater than 3 Wood units, pulmonary capillary wedge pressure 15 mm Hg or less, and mean pulmonary arterial pressure 25 mm Hg or higher.
Patients also had to have a 6-minute walk distance of at least 100 m and have stable disease while on an optimized dose of medications for underlying lung disease. Patients with group 3 connective tissue disease had to have baseline forced vital capacity of less than 70%.
The final study cohorts included patients with idiopathic interstitial pneumonias, chronic hypersensitivity pneumonitis, connective tissue disease, combined pulmonary fibrosis and emphysema, and occupational lung disease.
The patients were randomized to receive either inhaled treprostinil at a starting dose of 6 mcg/breath four times daily or to placebo (163 patients in each arm). All patients started the study drug at a dose of three breaths four times daily during waking hours. Dose escalations – adding 1 additional breath four times daily – were allowed every 3 days, up to a target dose of 9 breaths (54 mcg) four times daily, and a maximum of 12 breaths (72 mcg) four times daily as clinically tolerated.
A total of 130 patients assigned to treprostinil and 128 assigned to placebo completed 16 weeks of therapy and assessment.
As noted before, patients assigned to treprostinil had a placebo-corrected median difference from baseline in peak 6-minute walk distance, as measured by Hodges-Lehmann estimation, of 21 m (P = .004). An analysis of the same parameter using mixed model repeated measurement showed a placebo-corrected difference from baseline in peak 6-minute walk distance of 31.12 m (P < .001).
Secondary endpoints that were significantly better with treprostinil, compared with placebo, included improvements in N-terminal of the prohormone brain natriuretic peptide, a longer time to clinical worsening, and improvements in peak 6-minute walk distance week 12, and trough 6-minute walk distance at week 15.
Treprostinil was associated with a 39% reduction in risk of clinical worsening (P = .04). In all, 37 patients on treprostinil (22.7%) and 54 on placebo (33.1%) experienced clinical worsening.
For the exploratory endpoints of change in patient reported quality of life as measured by the St. George’s Respiratory Questionnaire, or in peak distance saturation product, however, there were no significant differences between the groups.
In addition, treprostinil was associated with a 34% reduction the risk of exacerbation of underlying lung disease, compared with placebo (P = .03).
The safety profile of treprostinil was similar to that seen in other studies of the drug, and most treatment-related adverse events were mild or moderate in severity. Adverse events led to discontinuation in 10% of patients on treprostinil and 8% on placebo.
Serious adverse events were seen in 23.3% and 25.8%, respectively. The most frequently occurring adverse events of any grade included cough, headache, dyspnea, dizziness, nausea, fatigue, diarrhea, throat irritation, and oropharyngeal pain.
There was no evidence of worsened oxygenation or lung function “allaying V/Q mismatch concerns,” Dr. Nathan said, and there was evidence for an improvement in forced vital capacity with treprostinil.
In the question-and-answer portion of the presentation, Dr. Kolb commented that many clinicians, particularly those who treated patients with ILD, question whether a 21-m difference in walk distance makes much of a difference in patient lives. He relayed a question from a viewer asking how Dr. Nathan and associates reconciled their primary endpoint with the finding that there was no difference in patient-reported quality of life.
“I think that the difference in the 6-minute walk test was both statistically significant and clinically meaningful,” Dr. Nathan replied.
He noted that the primary endpoint used a stringent measure, and that less conservative methods of analysis showed a larger difference in benefit favoring treprostinil. He also pointed out that the original study of inhaled treprostinil added to oral therapy for pulmonary arterial hypertension showed a 20-m improvement in walk distance, and that these results were sufficient to get the inhaled formulation approved in the United States (J Am Coll Cardiol. 2010 May. doi: 10.1016/j.jacc.2010.01.027).
Regarding the failure to detect a difference in quality of life, he said that the study was only 16 weeks in length, and that the St. George’s Respiratory Questionnaire was developed for evaluation of patients with chronic obstructive pulmonary disease, “perhaps not the best instrument to use in an ILD PH study.”
The study was funded by United Therapeutics. Dr. Nathan disclosed advisory committee activity/consulting, research support, and speaker fees from the company. Dr. Kolb has previously disclosed financial relationships with various companies, not including United Therapeutics.
over 16 weeks, compared with patients who used a placebo inhaler, results of a phase 3 trial showed.
Among 326 patients with pulmonary hypertension (PH) associated with interstitial lung disease (ILD), those who were randomly assigned to treatment with treprostinil had a placebo-corrected median difference from baseline in 6-minute walk distance of 21 m (P = .004), reported Steven D. Nathan, MD, from Inova Fairfax Hospital in Falls Church, Va., on behalf of coinvestigators in the INCREASE study (NCT02630316).
“These results support an additional treatment avenue, and might herald a shift in the clinical management of patients with interstitial lung disease,” he said in the American Thoracic Society’s virtual clinical trial session.
“This was an outstanding presentation and outstanding results. I personally am very excited, because this is a field where I work,” commented Martin Kolb, MD, PhD, from McMaster University, Hamilton, Ont., the facilitator for the online presentation.
The INCREASE trial compared inhaled treprostinil dose four times daily with placebo in patients with a CT scan–confirmed diagnosis of World Health Organization group 3 PH within 6 months before randomization who had evidence of diffuse parenchymal lung disease. Eligible patients could have any form of ILD or combined pulmonary fibrosis and emphysema.
Key inclusion criteria included right-heart catheterization within the previous year with documented pulmonary vascular resistance greater than 3 Wood units, pulmonary capillary wedge pressure 15 mm Hg or less, and mean pulmonary arterial pressure 25 mm Hg or higher.
Patients also had to have a 6-minute walk distance of at least 100 m and have stable disease while on an optimized dose of medications for underlying lung disease. Patients with group 3 connective tissue disease had to have baseline forced vital capacity of less than 70%.
The final study cohorts included patients with idiopathic interstitial pneumonias, chronic hypersensitivity pneumonitis, connective tissue disease, combined pulmonary fibrosis and emphysema, and occupational lung disease.
The patients were randomized to receive either inhaled treprostinil at a starting dose of 6 mcg/breath four times daily or to placebo (163 patients in each arm). All patients started the study drug at a dose of three breaths four times daily during waking hours. Dose escalations – adding 1 additional breath four times daily – were allowed every 3 days, up to a target dose of 9 breaths (54 mcg) four times daily, and a maximum of 12 breaths (72 mcg) four times daily as clinically tolerated.
A total of 130 patients assigned to treprostinil and 128 assigned to placebo completed 16 weeks of therapy and assessment.
As noted before, patients assigned to treprostinil had a placebo-corrected median difference from baseline in peak 6-minute walk distance, as measured by Hodges-Lehmann estimation, of 21 m (P = .004). An analysis of the same parameter using mixed model repeated measurement showed a placebo-corrected difference from baseline in peak 6-minute walk distance of 31.12 m (P < .001).
Secondary endpoints that were significantly better with treprostinil, compared with placebo, included improvements in N-terminal of the prohormone brain natriuretic peptide, a longer time to clinical worsening, and improvements in peak 6-minute walk distance week 12, and trough 6-minute walk distance at week 15.
Treprostinil was associated with a 39% reduction in risk of clinical worsening (P = .04). In all, 37 patients on treprostinil (22.7%) and 54 on placebo (33.1%) experienced clinical worsening.
For the exploratory endpoints of change in patient reported quality of life as measured by the St. George’s Respiratory Questionnaire, or in peak distance saturation product, however, there were no significant differences between the groups.
In addition, treprostinil was associated with a 34% reduction the risk of exacerbation of underlying lung disease, compared with placebo (P = .03).
The safety profile of treprostinil was similar to that seen in other studies of the drug, and most treatment-related adverse events were mild or moderate in severity. Adverse events led to discontinuation in 10% of patients on treprostinil and 8% on placebo.
Serious adverse events were seen in 23.3% and 25.8%, respectively. The most frequently occurring adverse events of any grade included cough, headache, dyspnea, dizziness, nausea, fatigue, diarrhea, throat irritation, and oropharyngeal pain.
There was no evidence of worsened oxygenation or lung function “allaying V/Q mismatch concerns,” Dr. Nathan said, and there was evidence for an improvement in forced vital capacity with treprostinil.
In the question-and-answer portion of the presentation, Dr. Kolb commented that many clinicians, particularly those who treated patients with ILD, question whether a 21-m difference in walk distance makes much of a difference in patient lives. He relayed a question from a viewer asking how Dr. Nathan and associates reconciled their primary endpoint with the finding that there was no difference in patient-reported quality of life.
“I think that the difference in the 6-minute walk test was both statistically significant and clinically meaningful,” Dr. Nathan replied.
He noted that the primary endpoint used a stringent measure, and that less conservative methods of analysis showed a larger difference in benefit favoring treprostinil. He also pointed out that the original study of inhaled treprostinil added to oral therapy for pulmonary arterial hypertension showed a 20-m improvement in walk distance, and that these results were sufficient to get the inhaled formulation approved in the United States (J Am Coll Cardiol. 2010 May. doi: 10.1016/j.jacc.2010.01.027).
Regarding the failure to detect a difference in quality of life, he said that the study was only 16 weeks in length, and that the St. George’s Respiratory Questionnaire was developed for evaluation of patients with chronic obstructive pulmonary disease, “perhaps not the best instrument to use in an ILD PH study.”
The study was funded by United Therapeutics. Dr. Nathan disclosed advisory committee activity/consulting, research support, and speaker fees from the company. Dr. Kolb has previously disclosed financial relationships with various companies, not including United Therapeutics.
FROM ATS 2020
Pulmonary function tests can’t substitute for high-resolution CT in early systemic sclerosis ILD screening
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
Clinicians shouldn’t rely on pulmonary function tests (PFTs) alone to screen for interstitial lung disease (ILD). The tests performed poorly in a retrospective study of 212 patients with systemic sclerosis, reinforcing the findings of previous studies.
Any screening algorithm should include high-resolution CT (HRCT), which is good at prognosticating disease, the investigators wrote in Arthritis & Rheumatology. “I think all newly diagnosed systemic sclerosis patients should have a full set of PFTs (spirometry, lung volumes, and diffusion capacity) and an HRCT at baseline to evaluate for ILD,” the study’s lead author, Elana J. Bernstein, MD, said in an interview.
ILD is a leading cause of death in systemic sclerosis (SSc) patients, affecting 40%-60% of those with the disease. HRCT is currently the preferred option for detection of ILD. PFTs are commonly used to screen for ILD but haven’t performed well in previous studies. “Someone can have abnormalities on HRCT that are consistent with ILD but still have PFTs that are in the ‘normal’ range,” explained Dr. Bernstein of Columbia University, New York. One cross-sectional study of 102 SSc patients found that the test’s sensitivity for the detection of ILD on HRCT was just 37.5% when forced vital capacity (FVC) <80% predicted.
Investigators sought to assess performance characteristics of PFTs in patients with early diffuse cutaneous SSc, a cohort at high risk of developing ILD. The study enlisted patients from the Prospective Registry of Early Systemic Sclerosis (PRESS), a multicenter, prospective cohort study of adults with early diffuse cutaneous SSc. Overall, 212 patients at 11 U.S. academic medical centers participated in the study from April 2012 to January 2019.
All patients had spirometry (PFT) and HRCT chest scans. PFTs were conducted per American Thoracic Society/European Respiratory Society guidelines. The investigators calculated test characteristics for single PFT and combinations of PFT parameters for the detection of ILD on HRCT. The HRCTs were ordered at the discretion of treating physicians, and scrutinized for ILD features such as reticular changes, honeycombing, traction bronchiectasis, and ground-glass opacities. The investigators defined the lower limit of normal for FVC, total lung capacity, and diffusion capacity for carbon monoxide (DLCO) as 80% predicted.
Overall, Dr. Bernstein and her colleagues found that PFTs lacked sufficient sensitivity and negative predictive value for the detection of ILD on HRCT in these patients.
An FVC <80% predicted performed at only 63% sensitivity and an false negative rate of 37%. Total lung capacity or DLCO <80% predicted had a sensitivity of 46% and 80%, respectively. The combination of FVC or DLCO <80% predicted raised sensitivity to 85%. However, the addition of total lung capacity to this combination did not improve results.
Overall, PFTs had a positive predictive value of 64%-74% and an negative predictive value of 61%-70%. “This means that PFT alone will not accurately predict the presence of ILD in about 35%, and not be correctly negative in about 35%,” observed Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, and professor of rheumatology at the University of California, Los Angeles.
While the combination of FVC <80% predicted or DLCO <80% predicted performed better than the other parameters, the sensitivity “is inadequate for an ILD screening test as it results in an false negative rate of 15%, thereby falsely reassuring 15% of patients that they do not have ILD when in fact they do,” the investigators observed.
“This study reinforces the notion that PFTs alone are ineffective screening tools for ILD in the presence of systemic sclerosis, particularly for patients with early systemic sclerosis,” said Elizabeth Volkmann, MD, MS, assistant professor and codirector of the CTD-ILD program in the division of rheumatology at the University of California, Los Angeles.
The study’s scope was relatively small, yet the results provide further evidence to show that HRCT should be performed in all SSc patients to screen for the presence of ILD, Dr. Volkmann said in an interview.
Other research has demonstrated the value of baseline HRCT as a prognosticator of ILD outcomes. The method provides useful information about the degree of fibrosis and degree of damage in early-stage disease, said Dr. Furst, also an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). “If there’s honeycombing, that’s a bad prognosis. If it’s ground glass or reticular changes, the prognosis is better.
“Once there’s a lot of damage, it’s much harder to interpret disease with HRCT,” he added.
HRCT and PFT work well together to assess what’s happening in patients, Dr. Furst explained. HRCT provides an idea of anatomic changes, whereas PFT outlines aspects of functional change to diagnose early ILD in early diffuse SSc. The study results should not apply to patients with later disease who have more developed ILD, he noted.
The investigators acknowledged that they weren’t able to categorize and analyze patients according to disease extent because they didn’t quantify the extent of ILD. Another limitation was that the HRCTs and PFTs were ordered at the discretion of individual physicians, which means that not all participants received the tests.
“Although the tests were done in 90% of the population, there is still a probability of a significant selection bias,” Dr. Furst said.
Dr. Bernstein and several other coauthors in the study received grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to support their work. Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech. Dr. Volkmann disclosed consulting for and/or receiving grant support from Boehringer Ingelheim, Corbus, and Forbius.
SOURCE: Bernstein EJ et al. Arthritis Rheumatol. 2020 Jun 25. doi: 10.1002/art.41415.
FROM ARTHRITIS & RHEUMATOLOGY
Despite guidelines, children receive opioids and steroids for pneumonia and sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
FROM PEDIATRICS
Primary care practices may lose about $68k per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
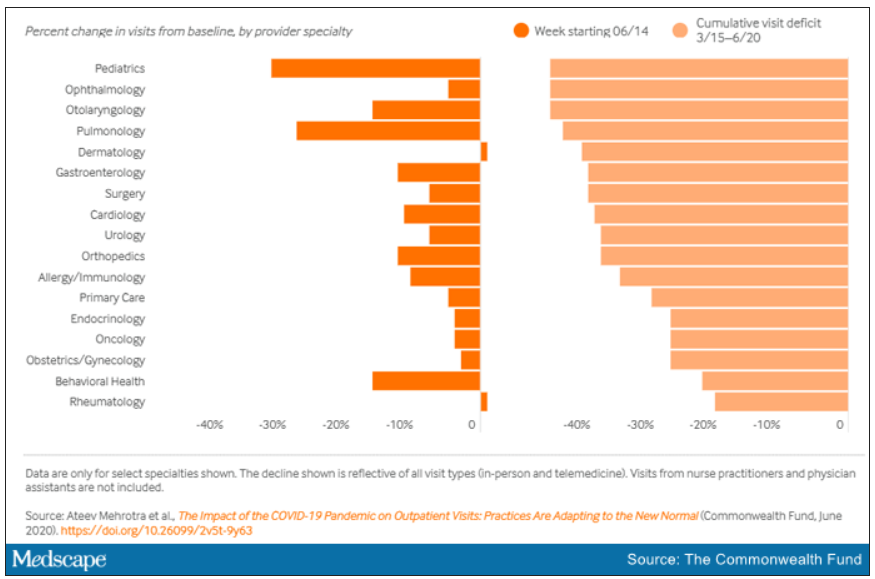
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.

For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.

For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Physician shortage grows in latest projections
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Diagnostic criteria may miss some MIS-C cases, experts say
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
Captopril questioned for diabetes patients in COVID-19 setting
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Lawmakers question mental health disclosure rules
State medical licensing queries criticized
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
State medical licensing queries criticized
State medical licensing queries criticized
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
FROM A HOUSE ENERGY AND COMMERCE’S HEALTH SUBCOMMITTEE HEARING
Sotatercept reversed vascular remodeling in patients with PAH
for patients with pulmonary arterial hypertension (PAH) in a phase 2 trial.
The mean decline change in pulmonary vascular resistance (PVR) after 24 weeks of treatment was significantly greater for patients treated with sotatercept at either of two doses, compared with placebo, David B. Badesch, MD, FCCP, of the University of Colorado, Aurora, reported on behalf of coinvestigators in the PULSAR trial.
“Sotatercept has a novel mechanism of action, rebalancing pro- and antiproliferative signaling through a pathway distinct from the previously approved pulmonary arterial hypertension therapies,” he said in the American Thoracic Society’s virtual clinical trial session.
The drug is a ligand trap with high selectivity for proteins within the tumor growth factor-beta superfamily signaling pathway. Investigators propose a mechanism of action whereby sotatercept promotes a rebalancing of bone morphogenetic protein receptor–II (BMPR-II) signaling to restore vascular homeostasis.
In a preclinical study, sotatercept reduced pulmonary artery pressure, pulmonary arteriolar muscularization and occlusion, right ventricular hypertrophy, and cell proliferation in the lungs of rodent models of pulmonary hypertension (Sci Transl Med. 2020 May 13. doi: 10.1126/scitranslmed.aaz5660).
Two dose levels
The PULSAR trial (NCT03496207) was a phase 2 randomized, double-blind study conducted in the United States, Brazil, Western Europe, and Australia comparing the efficacy and safety of sotatercept vs. placebo added to the standard of care in patients with PAH.
A total of 106 patients with World Health Organization (WHO) group 1 PAH or WHO functional class II or III disease were enrolled. All patients had baseline right-heart e4rcatheterization with PVR of 5 Wood units or more, had baseline 6-minute walk distance from 150 to 550 m, and were on stable treatment with standard-of-care mono, double, or triple therapies, including an endothelin-receptor antagonist, phosphodiesterase 5 inhibitor, soluble guanylate cyclase stimulator, and/or a prostacyclin, including intravenous formulations.
The median patient age was 46 years among 32 patients in the placebo group, and 48.5 years in each sotatercept dose group: 0.3 mg/kg (32 patients) and 0.7 mg/kg (42 patients).
The primary endpoint of PVR change from baseline to week 24, the end of the placebo-controlled treatment period, showed a mean decrease of 162 dynes/cm2 in the 0.3-mg/kg sotatercept group (–20.5%), and a mean decrease of 256 dynes/cm2 in the 0.7-mg/kg group (–33.9%), compared with a mean 16 dyne/cm2 decline in the placebo group (–2,1%). Both doses were associated with significantly larger decreases, compared with placebo (P = .0027 for the 0.3-mg/kg dose, and P < .0001 for the 0.7-mg/kg dose).
Six-minute walk distance, a key secondary endpoint, improved over baseline in each active-drug arm, with a least square (LS) mean improvement of 58 m in the 0.7-mg/kg group, and 50 m in the 0.7-mg/kg group. The prespecified analysis of pooled data from the two sotatercept cohorts showed an LS-mean change of 54 m over baseline, compared with 25 m for the placebo group (nominal P = .03).
Exploratory endpoints also favoring sotatercept over placebo included a 51% reduction in amino-terminal brain natriuretic propeptide (NT-proBNP), and a 20% reduction in mean pulmonary arterial pressure.
There was no significant difference between the study arms in change in cardiac output, however.
Improvements in WHO functional class were seen in 12.5% of patients on placebo, compared with 23% of patients on sotatercept, a difference that was not statistically significant.
Two patients (6%) in the 0.3-mg/kg arm and 10 (24%) in the 0.7-mg/kg arm had a serious treatment-emergent adverse event (TEAE), as did three patients (9%) in the placebo arm. Serious TEAEs included leukopenia, neutropenia, pericardial infusion, tachycardia, chorioretinopathy, peripheral edema, pyrexia, bronchitis, influenza, respiratory tract infection, femur fracture, hypotension, device breakage, syncope, and red blood cell increase.
One patient in the 0.7-mg/kg arm died from cardiac arrest deemed unrelated to study treatment.
TEAEs of special interest included thrombocytopenia in two patients in 0.3– and five patients in the 0.7–mg/kg groups, vs. no patients in the placebo groups. Most patients had existing thrombocytopenia at baseline and all were on concomitant prostacyclin infusions. No patients had grade 3 thrombocytopenia or associated bleeding events.
One patient in the 0.3-mg/kg group and six patients in the 0.7-mg/kg group had an increase in hemoglobin.
“This was not a surprise,” Dr. Badesch said. “We were prepared to manage increases in hemoglobin with dose interruption or dose reduction if necessary, and phlebotomy was also an option if needed.”
One patient in the placebo arm, two in the 0.3-mg/kg and three patients in the 0.7-mg/kg arms had TEAEs leading to discontinuation.
TEAEs occurring in 10% or more of all patients in any arm and of any grade were headache, diarrhea, peripheral edema, dizziness, fatigue, hypokalemia, and nausea.
Why no cardiac improvement?
In the question-and-answer session following the online presentation, facilitator Steven M. Kawut MD, MS, of the University of Pennsylvania and Pennsylvania Hospital in Philadelphia, remarked on the surprising lack of an apparent cardiac benefit in the study.
“You showed pretty robust decreases in NT-proBNP, decreases in pulmonary vascular resistance and right atrial pressure, and increases in 6-minute walk distance, so it’s a bit surprising that cardiac output didn’t change,” he said.
“Unlike other medications that have been tried in this field and have had a significant pulmonary vasodilatory effect, this drug is acting largely on the structure of pulmonary blood vessels,” Dr. Badesch replied. “We have thought that its primary effect is likely remodeling of the pulmonary arteries and arterioles, decreasing pulmonary vascular resistance. Unlike other drugs that have been tested in the field, it probably has no direct inotropic effect, and that may explain why cardiac output didn’t improve.”
He said that there is some echocardiographic evidence that suggests a change in right ventricular function over time. Those data are currently being analyzed, and “it’s possible that we’ll see an effect on cardiac output later.”
As of June 22, 2020, 94 of 97 patients who opted to participate in an 18-month extension period of the trial were still enrolled, and 64 patients have now been treated with sotatercept for at least 12 months.
A phase 3 trial is in the works.
The study was supported by Acceleron Pharma. Badesch disclosed research support from and consulting/advising for the company and others. Dr. Kawut has disclosed grants from several companies and travel support from ATS and the Pulmonary Hypertension Association.
for patients with pulmonary arterial hypertension (PAH) in a phase 2 trial.
The mean decline change in pulmonary vascular resistance (PVR) after 24 weeks of treatment was significantly greater for patients treated with sotatercept at either of two doses, compared with placebo, David B. Badesch, MD, FCCP, of the University of Colorado, Aurora, reported on behalf of coinvestigators in the PULSAR trial.
“Sotatercept has a novel mechanism of action, rebalancing pro- and antiproliferative signaling through a pathway distinct from the previously approved pulmonary arterial hypertension therapies,” he said in the American Thoracic Society’s virtual clinical trial session.
The drug is a ligand trap with high selectivity for proteins within the tumor growth factor-beta superfamily signaling pathway. Investigators propose a mechanism of action whereby sotatercept promotes a rebalancing of bone morphogenetic protein receptor–II (BMPR-II) signaling to restore vascular homeostasis.
In a preclinical study, sotatercept reduced pulmonary artery pressure, pulmonary arteriolar muscularization and occlusion, right ventricular hypertrophy, and cell proliferation in the lungs of rodent models of pulmonary hypertension (Sci Transl Med. 2020 May 13. doi: 10.1126/scitranslmed.aaz5660).
Two dose levels
The PULSAR trial (NCT03496207) was a phase 2 randomized, double-blind study conducted in the United States, Brazil, Western Europe, and Australia comparing the efficacy and safety of sotatercept vs. placebo added to the standard of care in patients with PAH.
A total of 106 patients with World Health Organization (WHO) group 1 PAH or WHO functional class II or III disease were enrolled. All patients had baseline right-heart e4rcatheterization with PVR of 5 Wood units or more, had baseline 6-minute walk distance from 150 to 550 m, and were on stable treatment with standard-of-care mono, double, or triple therapies, including an endothelin-receptor antagonist, phosphodiesterase 5 inhibitor, soluble guanylate cyclase stimulator, and/or a prostacyclin, including intravenous formulations.
The median patient age was 46 years among 32 patients in the placebo group, and 48.5 years in each sotatercept dose group: 0.3 mg/kg (32 patients) and 0.7 mg/kg (42 patients).
The primary endpoint of PVR change from baseline to week 24, the end of the placebo-controlled treatment period, showed a mean decrease of 162 dynes/cm2 in the 0.3-mg/kg sotatercept group (–20.5%), and a mean decrease of 256 dynes/cm2 in the 0.7-mg/kg group (–33.9%), compared with a mean 16 dyne/cm2 decline in the placebo group (–2,1%). Both doses were associated with significantly larger decreases, compared with placebo (P = .0027 for the 0.3-mg/kg dose, and P < .0001 for the 0.7-mg/kg dose).
Six-minute walk distance, a key secondary endpoint, improved over baseline in each active-drug arm, with a least square (LS) mean improvement of 58 m in the 0.7-mg/kg group, and 50 m in the 0.7-mg/kg group. The prespecified analysis of pooled data from the two sotatercept cohorts showed an LS-mean change of 54 m over baseline, compared with 25 m for the placebo group (nominal P = .03).
Exploratory endpoints also favoring sotatercept over placebo included a 51% reduction in amino-terminal brain natriuretic propeptide (NT-proBNP), and a 20% reduction in mean pulmonary arterial pressure.
There was no significant difference between the study arms in change in cardiac output, however.
Improvements in WHO functional class were seen in 12.5% of patients on placebo, compared with 23% of patients on sotatercept, a difference that was not statistically significant.
Two patients (6%) in the 0.3-mg/kg arm and 10 (24%) in the 0.7-mg/kg arm had a serious treatment-emergent adverse event (TEAE), as did three patients (9%) in the placebo arm. Serious TEAEs included leukopenia, neutropenia, pericardial infusion, tachycardia, chorioretinopathy, peripheral edema, pyrexia, bronchitis, influenza, respiratory tract infection, femur fracture, hypotension, device breakage, syncope, and red blood cell increase.
One patient in the 0.7-mg/kg arm died from cardiac arrest deemed unrelated to study treatment.
TEAEs of special interest included thrombocytopenia in two patients in 0.3– and five patients in the 0.7–mg/kg groups, vs. no patients in the placebo groups. Most patients had existing thrombocytopenia at baseline and all were on concomitant prostacyclin infusions. No patients had grade 3 thrombocytopenia or associated bleeding events.
One patient in the 0.3-mg/kg group and six patients in the 0.7-mg/kg group had an increase in hemoglobin.
“This was not a surprise,” Dr. Badesch said. “We were prepared to manage increases in hemoglobin with dose interruption or dose reduction if necessary, and phlebotomy was also an option if needed.”
One patient in the placebo arm, two in the 0.3-mg/kg and three patients in the 0.7-mg/kg arms had TEAEs leading to discontinuation.
TEAEs occurring in 10% or more of all patients in any arm and of any grade were headache, diarrhea, peripheral edema, dizziness, fatigue, hypokalemia, and nausea.
Why no cardiac improvement?
In the question-and-answer session following the online presentation, facilitator Steven M. Kawut MD, MS, of the University of Pennsylvania and Pennsylvania Hospital in Philadelphia, remarked on the surprising lack of an apparent cardiac benefit in the study.
“You showed pretty robust decreases in NT-proBNP, decreases in pulmonary vascular resistance and right atrial pressure, and increases in 6-minute walk distance, so it’s a bit surprising that cardiac output didn’t change,” he said.
“Unlike other medications that have been tried in this field and have had a significant pulmonary vasodilatory effect, this drug is acting largely on the structure of pulmonary blood vessels,” Dr. Badesch replied. “We have thought that its primary effect is likely remodeling of the pulmonary arteries and arterioles, decreasing pulmonary vascular resistance. Unlike other drugs that have been tested in the field, it probably has no direct inotropic effect, and that may explain why cardiac output didn’t improve.”
He said that there is some echocardiographic evidence that suggests a change in right ventricular function over time. Those data are currently being analyzed, and “it’s possible that we’ll see an effect on cardiac output later.”
As of June 22, 2020, 94 of 97 patients who opted to participate in an 18-month extension period of the trial were still enrolled, and 64 patients have now been treated with sotatercept for at least 12 months.
A phase 3 trial is in the works.
The study was supported by Acceleron Pharma. Badesch disclosed research support from and consulting/advising for the company and others. Dr. Kawut has disclosed grants from several companies and travel support from ATS and the Pulmonary Hypertension Association.
for patients with pulmonary arterial hypertension (PAH) in a phase 2 trial.
The mean decline change in pulmonary vascular resistance (PVR) after 24 weeks of treatment was significantly greater for patients treated with sotatercept at either of two doses, compared with placebo, David B. Badesch, MD, FCCP, of the University of Colorado, Aurora, reported on behalf of coinvestigators in the PULSAR trial.
“Sotatercept has a novel mechanism of action, rebalancing pro- and antiproliferative signaling through a pathway distinct from the previously approved pulmonary arterial hypertension therapies,” he said in the American Thoracic Society’s virtual clinical trial session.
The drug is a ligand trap with high selectivity for proteins within the tumor growth factor-beta superfamily signaling pathway. Investigators propose a mechanism of action whereby sotatercept promotes a rebalancing of bone morphogenetic protein receptor–II (BMPR-II) signaling to restore vascular homeostasis.
In a preclinical study, sotatercept reduced pulmonary artery pressure, pulmonary arteriolar muscularization and occlusion, right ventricular hypertrophy, and cell proliferation in the lungs of rodent models of pulmonary hypertension (Sci Transl Med. 2020 May 13. doi: 10.1126/scitranslmed.aaz5660).
Two dose levels
The PULSAR trial (NCT03496207) was a phase 2 randomized, double-blind study conducted in the United States, Brazil, Western Europe, and Australia comparing the efficacy and safety of sotatercept vs. placebo added to the standard of care in patients with PAH.
A total of 106 patients with World Health Organization (WHO) group 1 PAH or WHO functional class II or III disease were enrolled. All patients had baseline right-heart e4rcatheterization with PVR of 5 Wood units or more, had baseline 6-minute walk distance from 150 to 550 m, and were on stable treatment with standard-of-care mono, double, or triple therapies, including an endothelin-receptor antagonist, phosphodiesterase 5 inhibitor, soluble guanylate cyclase stimulator, and/or a prostacyclin, including intravenous formulations.
The median patient age was 46 years among 32 patients in the placebo group, and 48.5 years in each sotatercept dose group: 0.3 mg/kg (32 patients) and 0.7 mg/kg (42 patients).
The primary endpoint of PVR change from baseline to week 24, the end of the placebo-controlled treatment period, showed a mean decrease of 162 dynes/cm2 in the 0.3-mg/kg sotatercept group (–20.5%), and a mean decrease of 256 dynes/cm2 in the 0.7-mg/kg group (–33.9%), compared with a mean 16 dyne/cm2 decline in the placebo group (–2,1%). Both doses were associated with significantly larger decreases, compared with placebo (P = .0027 for the 0.3-mg/kg dose, and P < .0001 for the 0.7-mg/kg dose).
Six-minute walk distance, a key secondary endpoint, improved over baseline in each active-drug arm, with a least square (LS) mean improvement of 58 m in the 0.7-mg/kg group, and 50 m in the 0.7-mg/kg group. The prespecified analysis of pooled data from the two sotatercept cohorts showed an LS-mean change of 54 m over baseline, compared with 25 m for the placebo group (nominal P = .03).
Exploratory endpoints also favoring sotatercept over placebo included a 51% reduction in amino-terminal brain natriuretic propeptide (NT-proBNP), and a 20% reduction in mean pulmonary arterial pressure.
There was no significant difference between the study arms in change in cardiac output, however.
Improvements in WHO functional class were seen in 12.5% of patients on placebo, compared with 23% of patients on sotatercept, a difference that was not statistically significant.
Two patients (6%) in the 0.3-mg/kg arm and 10 (24%) in the 0.7-mg/kg arm had a serious treatment-emergent adverse event (TEAE), as did three patients (9%) in the placebo arm. Serious TEAEs included leukopenia, neutropenia, pericardial infusion, tachycardia, chorioretinopathy, peripheral edema, pyrexia, bronchitis, influenza, respiratory tract infection, femur fracture, hypotension, device breakage, syncope, and red blood cell increase.
One patient in the 0.7-mg/kg arm died from cardiac arrest deemed unrelated to study treatment.
TEAEs of special interest included thrombocytopenia in two patients in 0.3– and five patients in the 0.7–mg/kg groups, vs. no patients in the placebo groups. Most patients had existing thrombocytopenia at baseline and all were on concomitant prostacyclin infusions. No patients had grade 3 thrombocytopenia or associated bleeding events.
One patient in the 0.3-mg/kg group and six patients in the 0.7-mg/kg group had an increase in hemoglobin.
“This was not a surprise,” Dr. Badesch said. “We were prepared to manage increases in hemoglobin with dose interruption or dose reduction if necessary, and phlebotomy was also an option if needed.”
One patient in the placebo arm, two in the 0.3-mg/kg and three patients in the 0.7-mg/kg arms had TEAEs leading to discontinuation.
TEAEs occurring in 10% or more of all patients in any arm and of any grade were headache, diarrhea, peripheral edema, dizziness, fatigue, hypokalemia, and nausea.
Why no cardiac improvement?
In the question-and-answer session following the online presentation, facilitator Steven M. Kawut MD, MS, of the University of Pennsylvania and Pennsylvania Hospital in Philadelphia, remarked on the surprising lack of an apparent cardiac benefit in the study.
“You showed pretty robust decreases in NT-proBNP, decreases in pulmonary vascular resistance and right atrial pressure, and increases in 6-minute walk distance, so it’s a bit surprising that cardiac output didn’t change,” he said.
“Unlike other medications that have been tried in this field and have had a significant pulmonary vasodilatory effect, this drug is acting largely on the structure of pulmonary blood vessels,” Dr. Badesch replied. “We have thought that its primary effect is likely remodeling of the pulmonary arteries and arterioles, decreasing pulmonary vascular resistance. Unlike other drugs that have been tested in the field, it probably has no direct inotropic effect, and that may explain why cardiac output didn’t improve.”
He said that there is some echocardiographic evidence that suggests a change in right ventricular function over time. Those data are currently being analyzed, and “it’s possible that we’ll see an effect on cardiac output later.”
As of June 22, 2020, 94 of 97 patients who opted to participate in an 18-month extension period of the trial were still enrolled, and 64 patients have now been treated with sotatercept for at least 12 months.
A phase 3 trial is in the works.
The study was supported by Acceleron Pharma. Badesch disclosed research support from and consulting/advising for the company and others. Dr. Kawut has disclosed grants from several companies and travel support from ATS and the Pulmonary Hypertension Association.
FROM ATS 2020








