User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


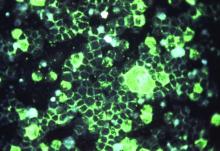
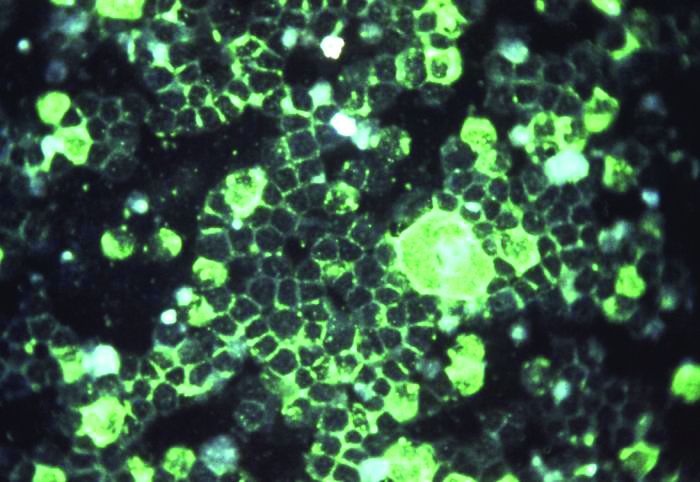
Getting closer to a lifesaving RSV vaccine
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
FROM ESPID 2020
Two different radiation boost strategies reduce local failures in NSCLC
The European PET-Boost trial finds that both of two strategies for delivering a radiation boost to locally advanced non–small cell lung cancer (NSCLC) tumors improve local control relative to that seen historically. Results were reported at the European Society for Radiology and Oncology 2020 Online Congress.
“From previous studies, we know that local recurrences have an important negative impact on survival,” said presenting author Saskia A. Cooke, an MD, PhD candidate in the department of Radiation Oncology Research, the Netherlands Cancer Institute, Amsterdam.
In addition, research shows that, despite advances in drug therapy, the most common site of progression in this population is intrathoracic.
“These results further underline the need to develop treatment strategies which effectively prevent intrathoracic and local recurrences,” Ms. Cooke said.
PET-Boost is a multicenter, randomized trial that enrolled patients with inoperable stage II or III NSCLC and a primary tumor measuring 4 cm or greater.
“The study was a phase 2 ‘pick the winner’ trial, which, by design, does not compare the two arms to one another but to a historic rate of outcome,” Ms. Cooke explained.
The patients were randomized evenly to receive the standard 66 Gy of radiotherapy given in 24 fractions of 2.75 Gy with one of two dose-escalation strategies: a boost to the whole primary tumor or a boost to only the tumor area having high metabolic activity, with a maximum standard uptake value (SUVmax) of at least 50% on the pretreatment FDG-PET scan.
For each patient, both plans were created before randomization, with the dose escalated as high as possible up to an organ-at-risk constraint, Ms. Cooke noted.
“A key element is that the two plans were made isotoxic by equaling the mean lung dose, and in both arms, the dose was delivered integrated into the 24 fractions, so without prolongation of the overall treatment time,” she said.
The trial’s goal was to improve the 1-year rate of freedom from local failure from the 70% seen historically with conventional chemoradiotherapy to 85%.
The trial was stopped early because of slow accrual, after enrollment of 107 patients, Ms. Cooke reported. The large majority received concurrent or sequential chemotherapy with their radiotherapy.
With a median follow-up of 12.6 months for the endpoint, the 1-year rate of freedom from local failure as determined on centrally reviewed CT scans was 97% with the whole-tumor boost and 91% with the PET-directed boost. The 2-year rates were 89% and 82%, respectively.
With a median follow-up of 61 months for the endpoint, the 1-year rate of overall survival was 77% with the whole-tumor boost and 62% with the PET-directed boost. The 2-year rates were 46% and 43%, respectively.
The two boost strategies increased acute and late toxicity over that seen historically, but not to unacceptable levels, as reported previously (Radiother Oncol. 2019;131:166-73).
“In this PET-Boost trial, using hypofractionated personalized dose escalation led to a very good local control rate, which, in both arms, was more than 90% at 1 year,” Ms. Cooke summarized.
In fact, values compare favorably with those seen in the phase 3 RTOG 0617 trial using conventional chemoradiotherapy and dose escalation, even though patients in that trial had smaller tumors.
“Survival, especially in the group treated with the homogeneous boost, was actually similar to the RTOG 0617 high-dose arm and also quite similar to the 1-year survival in the placebo arm of the PACIFIC trial,” she added. The somewhat poorer survival at 2 years in PET-Boost was likely related, in part, to the large tumor volumes and the mediastinal radiation dose, she speculated.
The investigators are now evaluating specific sites of failure and extrathoracic recurrences, as well as assessing associations of toxicity with organ-at-risk doses and quality of life.
“While further results of the trial are awaited, so far, we do believe that in selected patients with locally advanced NSCLC, hypofractionated dose escalation to the tumor is a very important subject for future research,” Ms. Cooke said.
The investigators plan to carry the whole-tumor boost strategy forward because it yields similar efficacy but is easier to plan.
Not ready for prime time
“Overall, this study conceptually is well designed as it is forward thinking and uses imaging to personalize radiation treatment, going to higher doses to active areas of disease based on FDG-PET imaging,” Arya Amini, MD, assistant clinical professor in the department of radiation oncology, City of Hope Comprehensive Cancer Center, Duarte, Calif., said in an interview.
However, he cautioned, local failure is challenging to assess at 1 year because of radiation-induced changes. In fact, more than a quarter of study patients had scans that were not evaluable for this reason. Furthermore, rates of late cardiac toxicity and esophageal stenosis are unknown.
“Longer-term follow-up is needed as the current data does not support dose escalation in unresectable lung cancer, specifically stage III NSCLC, based on RTOG 0617,” Dr. Amini said. “However, the overall survival detriment from dose escalation in RTOG 0617 could have been due to poor radiation techniques and toxicities including cardiac side effects, which we now better understand. The PET-Boost trial focuses on delivering higher doses of hypofractionated radiation based on PET, which essentially leads to a smaller area getting a radiation boost, which, in turn, should have less side effects.”
“This area of work will continue to be more exciting as more tumor-targeting radiotracers can be utilized with PET,” he predicted. “One of the future avenues in radiation oncology is incorporating novel imaging modalities including tumor-specific radiotracers with PET scans, for example, to dose-paint disease, delivering higher doses to more active parts of the primary and lymph nodes, while reducing doses to less active areas, which potentially could lead to higher rates of local control with minimal side effects.”
The trial was sponsored by The Netherlands Cancer Institute. Ms. Cooke and Dr. Amini disclosed no conflicts of interest.
SOURCE: Lalezari F et al. ESTRO 2020. Abstract OC-0609.
The European PET-Boost trial finds that both of two strategies for delivering a radiation boost to locally advanced non–small cell lung cancer (NSCLC) tumors improve local control relative to that seen historically. Results were reported at the European Society for Radiology and Oncology 2020 Online Congress.
“From previous studies, we know that local recurrences have an important negative impact on survival,” said presenting author Saskia A. Cooke, an MD, PhD candidate in the department of Radiation Oncology Research, the Netherlands Cancer Institute, Amsterdam.
In addition, research shows that, despite advances in drug therapy, the most common site of progression in this population is intrathoracic.
“These results further underline the need to develop treatment strategies which effectively prevent intrathoracic and local recurrences,” Ms. Cooke said.
PET-Boost is a multicenter, randomized trial that enrolled patients with inoperable stage II or III NSCLC and a primary tumor measuring 4 cm or greater.
“The study was a phase 2 ‘pick the winner’ trial, which, by design, does not compare the two arms to one another but to a historic rate of outcome,” Ms. Cooke explained.
The patients were randomized evenly to receive the standard 66 Gy of radiotherapy given in 24 fractions of 2.75 Gy with one of two dose-escalation strategies: a boost to the whole primary tumor or a boost to only the tumor area having high metabolic activity, with a maximum standard uptake value (SUVmax) of at least 50% on the pretreatment FDG-PET scan.
For each patient, both plans were created before randomization, with the dose escalated as high as possible up to an organ-at-risk constraint, Ms. Cooke noted.
“A key element is that the two plans were made isotoxic by equaling the mean lung dose, and in both arms, the dose was delivered integrated into the 24 fractions, so without prolongation of the overall treatment time,” she said.
The trial’s goal was to improve the 1-year rate of freedom from local failure from the 70% seen historically with conventional chemoradiotherapy to 85%.
The trial was stopped early because of slow accrual, after enrollment of 107 patients, Ms. Cooke reported. The large majority received concurrent or sequential chemotherapy with their radiotherapy.
With a median follow-up of 12.6 months for the endpoint, the 1-year rate of freedom from local failure as determined on centrally reviewed CT scans was 97% with the whole-tumor boost and 91% with the PET-directed boost. The 2-year rates were 89% and 82%, respectively.
With a median follow-up of 61 months for the endpoint, the 1-year rate of overall survival was 77% with the whole-tumor boost and 62% with the PET-directed boost. The 2-year rates were 46% and 43%, respectively.
The two boost strategies increased acute and late toxicity over that seen historically, but not to unacceptable levels, as reported previously (Radiother Oncol. 2019;131:166-73).
“In this PET-Boost trial, using hypofractionated personalized dose escalation led to a very good local control rate, which, in both arms, was more than 90% at 1 year,” Ms. Cooke summarized.
In fact, values compare favorably with those seen in the phase 3 RTOG 0617 trial using conventional chemoradiotherapy and dose escalation, even though patients in that trial had smaller tumors.
“Survival, especially in the group treated with the homogeneous boost, was actually similar to the RTOG 0617 high-dose arm and also quite similar to the 1-year survival in the placebo arm of the PACIFIC trial,” she added. The somewhat poorer survival at 2 years in PET-Boost was likely related, in part, to the large tumor volumes and the mediastinal radiation dose, she speculated.
The investigators are now evaluating specific sites of failure and extrathoracic recurrences, as well as assessing associations of toxicity with organ-at-risk doses and quality of life.
“While further results of the trial are awaited, so far, we do believe that in selected patients with locally advanced NSCLC, hypofractionated dose escalation to the tumor is a very important subject for future research,” Ms. Cooke said.
The investigators plan to carry the whole-tumor boost strategy forward because it yields similar efficacy but is easier to plan.
Not ready for prime time
“Overall, this study conceptually is well designed as it is forward thinking and uses imaging to personalize radiation treatment, going to higher doses to active areas of disease based on FDG-PET imaging,” Arya Amini, MD, assistant clinical professor in the department of radiation oncology, City of Hope Comprehensive Cancer Center, Duarte, Calif., said in an interview.
However, he cautioned, local failure is challenging to assess at 1 year because of radiation-induced changes. In fact, more than a quarter of study patients had scans that were not evaluable for this reason. Furthermore, rates of late cardiac toxicity and esophageal stenosis are unknown.
“Longer-term follow-up is needed as the current data does not support dose escalation in unresectable lung cancer, specifically stage III NSCLC, based on RTOG 0617,” Dr. Amini said. “However, the overall survival detriment from dose escalation in RTOG 0617 could have been due to poor radiation techniques and toxicities including cardiac side effects, which we now better understand. The PET-Boost trial focuses on delivering higher doses of hypofractionated radiation based on PET, which essentially leads to a smaller area getting a radiation boost, which, in turn, should have less side effects.”
“This area of work will continue to be more exciting as more tumor-targeting radiotracers can be utilized with PET,” he predicted. “One of the future avenues in radiation oncology is incorporating novel imaging modalities including tumor-specific radiotracers with PET scans, for example, to dose-paint disease, delivering higher doses to more active parts of the primary and lymph nodes, while reducing doses to less active areas, which potentially could lead to higher rates of local control with minimal side effects.”
The trial was sponsored by The Netherlands Cancer Institute. Ms. Cooke and Dr. Amini disclosed no conflicts of interest.
SOURCE: Lalezari F et al. ESTRO 2020. Abstract OC-0609.
The European PET-Boost trial finds that both of two strategies for delivering a radiation boost to locally advanced non–small cell lung cancer (NSCLC) tumors improve local control relative to that seen historically. Results were reported at the European Society for Radiology and Oncology 2020 Online Congress.
“From previous studies, we know that local recurrences have an important negative impact on survival,” said presenting author Saskia A. Cooke, an MD, PhD candidate in the department of Radiation Oncology Research, the Netherlands Cancer Institute, Amsterdam.
In addition, research shows that, despite advances in drug therapy, the most common site of progression in this population is intrathoracic.
“These results further underline the need to develop treatment strategies which effectively prevent intrathoracic and local recurrences,” Ms. Cooke said.
PET-Boost is a multicenter, randomized trial that enrolled patients with inoperable stage II or III NSCLC and a primary tumor measuring 4 cm or greater.
“The study was a phase 2 ‘pick the winner’ trial, which, by design, does not compare the two arms to one another but to a historic rate of outcome,” Ms. Cooke explained.
The patients were randomized evenly to receive the standard 66 Gy of radiotherapy given in 24 fractions of 2.75 Gy with one of two dose-escalation strategies: a boost to the whole primary tumor or a boost to only the tumor area having high metabolic activity, with a maximum standard uptake value (SUVmax) of at least 50% on the pretreatment FDG-PET scan.
For each patient, both plans were created before randomization, with the dose escalated as high as possible up to an organ-at-risk constraint, Ms. Cooke noted.
“A key element is that the two plans were made isotoxic by equaling the mean lung dose, and in both arms, the dose was delivered integrated into the 24 fractions, so without prolongation of the overall treatment time,” she said.
The trial’s goal was to improve the 1-year rate of freedom from local failure from the 70% seen historically with conventional chemoradiotherapy to 85%.
The trial was stopped early because of slow accrual, after enrollment of 107 patients, Ms. Cooke reported. The large majority received concurrent or sequential chemotherapy with their radiotherapy.
With a median follow-up of 12.6 months for the endpoint, the 1-year rate of freedom from local failure as determined on centrally reviewed CT scans was 97% with the whole-tumor boost and 91% with the PET-directed boost. The 2-year rates were 89% and 82%, respectively.
With a median follow-up of 61 months for the endpoint, the 1-year rate of overall survival was 77% with the whole-tumor boost and 62% with the PET-directed boost. The 2-year rates were 46% and 43%, respectively.
The two boost strategies increased acute and late toxicity over that seen historically, but not to unacceptable levels, as reported previously (Radiother Oncol. 2019;131:166-73).
“In this PET-Boost trial, using hypofractionated personalized dose escalation led to a very good local control rate, which, in both arms, was more than 90% at 1 year,” Ms. Cooke summarized.
In fact, values compare favorably with those seen in the phase 3 RTOG 0617 trial using conventional chemoradiotherapy and dose escalation, even though patients in that trial had smaller tumors.
“Survival, especially in the group treated with the homogeneous boost, was actually similar to the RTOG 0617 high-dose arm and also quite similar to the 1-year survival in the placebo arm of the PACIFIC trial,” she added. The somewhat poorer survival at 2 years in PET-Boost was likely related, in part, to the large tumor volumes and the mediastinal radiation dose, she speculated.
The investigators are now evaluating specific sites of failure and extrathoracic recurrences, as well as assessing associations of toxicity with organ-at-risk doses and quality of life.
“While further results of the trial are awaited, so far, we do believe that in selected patients with locally advanced NSCLC, hypofractionated dose escalation to the tumor is a very important subject for future research,” Ms. Cooke said.
The investigators plan to carry the whole-tumor boost strategy forward because it yields similar efficacy but is easier to plan.
Not ready for prime time
“Overall, this study conceptually is well designed as it is forward thinking and uses imaging to personalize radiation treatment, going to higher doses to active areas of disease based on FDG-PET imaging,” Arya Amini, MD, assistant clinical professor in the department of radiation oncology, City of Hope Comprehensive Cancer Center, Duarte, Calif., said in an interview.
However, he cautioned, local failure is challenging to assess at 1 year because of radiation-induced changes. In fact, more than a quarter of study patients had scans that were not evaluable for this reason. Furthermore, rates of late cardiac toxicity and esophageal stenosis are unknown.
“Longer-term follow-up is needed as the current data does not support dose escalation in unresectable lung cancer, specifically stage III NSCLC, based on RTOG 0617,” Dr. Amini said. “However, the overall survival detriment from dose escalation in RTOG 0617 could have been due to poor radiation techniques and toxicities including cardiac side effects, which we now better understand. The PET-Boost trial focuses on delivering higher doses of hypofractionated radiation based on PET, which essentially leads to a smaller area getting a radiation boost, which, in turn, should have less side effects.”
“This area of work will continue to be more exciting as more tumor-targeting radiotracers can be utilized with PET,” he predicted. “One of the future avenues in radiation oncology is incorporating novel imaging modalities including tumor-specific radiotracers with PET scans, for example, to dose-paint disease, delivering higher doses to more active parts of the primary and lymph nodes, while reducing doses to less active areas, which potentially could lead to higher rates of local control with minimal side effects.”
The trial was sponsored by The Netherlands Cancer Institute. Ms. Cooke and Dr. Amini disclosed no conflicts of interest.
SOURCE: Lalezari F et al. ESTRO 2020. Abstract OC-0609.
FROM ESTRO 2020
More severe AD correlates with worse sleep health and attention problems in children
, results from a national survey demonstrated.
“We think it’s important for dermatologists and pediatricians to be monitoring children with AD for sleep and attention dysregulation,” Nina Y. Zhou said during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium. “It’s also important to highlight sleep hygiene habits to improve sleep health overall.”
In an effort to determine the impact of AD severity on these symptoms in young children with AD and characterize sleep health and attention regulation behaviors, Ms. Zhou, a medical student at Northwestern University, Chicago, and colleagues drew from a national survey distributed via panel company OP4G and the National Eczema Association that was conducted with parents of 60 children with AD aged 1-5 years. Questionnaires included the Patient Reported Outcomes Measurement Information System (PROMIS) Early Childhood Sleep Health Measures to assess sleep health, the Peak Pruritus NRS to measure itch severity, and the Multidimensional Assessment Profile of Attention Regulation (MAPS-AR) to measure attention dysregulation related to inattention and hyperactivity. The researchers performed linear regression to determine the predictors of sleep health and attention dysregulation.
The mean age of 60 children was 3 years, 55% were male, 32% were black, 42% had severe disease, 42% had moderate disease, and 16% had mild disease. Children with more extensive AD were significantly more likely to report worse sleep disturbance. The proportion of children who reported sleep disturbance on at least 5 nights per week was 67% among those with severe AD, 24% among those with moderate AD, and 0% among those with mild AD.
In addition, 72% of parents of children with severe AD reported trouble paying attention at least 3 times per week “no matter what was going on,” compared with 24% of those with moderate AD and none of those with mild AD.
Parents of children with more severe AD reported more itch-related burden and significantly decreased quality of life for their children. For example, 76% of parents with children who had severe AD reported “because of itch, their child was frustrated,” compared to 44% of those with moderate AD and 10% with mild AD.
In fully adjusted linear regression analysis, the strongest predictors of sleep disturbance were AD severity (unstandardized beta value = 0.79, P less than .01) and being Black (unstandardized beta value = 3.89, P = .03). AD severity (unstandardized beta value = 1.22, P less than .01) and being Black (unstandardized beta value = 7.79, P less than .01) also predicted more attention dysregulation.
Household income appeared to differ significantly based on AD severity groups. “If you have mild AD, you are more likely to come from a higher income household,” Ms. Zhou said.
She concluded her presentation by calling for future studies with larger samples sizes to establish causality and directional effects between AD severity, itch, sleep, race, and attention.
The study was funded by the Agency for Healthcare Research and Quality. Ms. Zhou reported having no financial disclosures.
, results from a national survey demonstrated.
“We think it’s important for dermatologists and pediatricians to be monitoring children with AD for sleep and attention dysregulation,” Nina Y. Zhou said during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium. “It’s also important to highlight sleep hygiene habits to improve sleep health overall.”
In an effort to determine the impact of AD severity on these symptoms in young children with AD and characterize sleep health and attention regulation behaviors, Ms. Zhou, a medical student at Northwestern University, Chicago, and colleagues drew from a national survey distributed via panel company OP4G and the National Eczema Association that was conducted with parents of 60 children with AD aged 1-5 years. Questionnaires included the Patient Reported Outcomes Measurement Information System (PROMIS) Early Childhood Sleep Health Measures to assess sleep health, the Peak Pruritus NRS to measure itch severity, and the Multidimensional Assessment Profile of Attention Regulation (MAPS-AR) to measure attention dysregulation related to inattention and hyperactivity. The researchers performed linear regression to determine the predictors of sleep health and attention dysregulation.
The mean age of 60 children was 3 years, 55% were male, 32% were black, 42% had severe disease, 42% had moderate disease, and 16% had mild disease. Children with more extensive AD were significantly more likely to report worse sleep disturbance. The proportion of children who reported sleep disturbance on at least 5 nights per week was 67% among those with severe AD, 24% among those with moderate AD, and 0% among those with mild AD.
In addition, 72% of parents of children with severe AD reported trouble paying attention at least 3 times per week “no matter what was going on,” compared with 24% of those with moderate AD and none of those with mild AD.
Parents of children with more severe AD reported more itch-related burden and significantly decreased quality of life for their children. For example, 76% of parents with children who had severe AD reported “because of itch, their child was frustrated,” compared to 44% of those with moderate AD and 10% with mild AD.
In fully adjusted linear regression analysis, the strongest predictors of sleep disturbance were AD severity (unstandardized beta value = 0.79, P less than .01) and being Black (unstandardized beta value = 3.89, P = .03). AD severity (unstandardized beta value = 1.22, P less than .01) and being Black (unstandardized beta value = 7.79, P less than .01) also predicted more attention dysregulation.
Household income appeared to differ significantly based on AD severity groups. “If you have mild AD, you are more likely to come from a higher income household,” Ms. Zhou said.
She concluded her presentation by calling for future studies with larger samples sizes to establish causality and directional effects between AD severity, itch, sleep, race, and attention.
The study was funded by the Agency for Healthcare Research and Quality. Ms. Zhou reported having no financial disclosures.
, results from a national survey demonstrated.
“We think it’s important for dermatologists and pediatricians to be monitoring children with AD for sleep and attention dysregulation,” Nina Y. Zhou said during a late-breaking research session at the Revolutionizing Atopic Dermatitis virtual symposium. “It’s also important to highlight sleep hygiene habits to improve sleep health overall.”
In an effort to determine the impact of AD severity on these symptoms in young children with AD and characterize sleep health and attention regulation behaviors, Ms. Zhou, a medical student at Northwestern University, Chicago, and colleagues drew from a national survey distributed via panel company OP4G and the National Eczema Association that was conducted with parents of 60 children with AD aged 1-5 years. Questionnaires included the Patient Reported Outcomes Measurement Information System (PROMIS) Early Childhood Sleep Health Measures to assess sleep health, the Peak Pruritus NRS to measure itch severity, and the Multidimensional Assessment Profile of Attention Regulation (MAPS-AR) to measure attention dysregulation related to inattention and hyperactivity. The researchers performed linear regression to determine the predictors of sleep health and attention dysregulation.
The mean age of 60 children was 3 years, 55% were male, 32% were black, 42% had severe disease, 42% had moderate disease, and 16% had mild disease. Children with more extensive AD were significantly more likely to report worse sleep disturbance. The proportion of children who reported sleep disturbance on at least 5 nights per week was 67% among those with severe AD, 24% among those with moderate AD, and 0% among those with mild AD.
In addition, 72% of parents of children with severe AD reported trouble paying attention at least 3 times per week “no matter what was going on,” compared with 24% of those with moderate AD and none of those with mild AD.
Parents of children with more severe AD reported more itch-related burden and significantly decreased quality of life for their children. For example, 76% of parents with children who had severe AD reported “because of itch, their child was frustrated,” compared to 44% of those with moderate AD and 10% with mild AD.
In fully adjusted linear regression analysis, the strongest predictors of sleep disturbance were AD severity (unstandardized beta value = 0.79, P less than .01) and being Black (unstandardized beta value = 3.89, P = .03). AD severity (unstandardized beta value = 1.22, P less than .01) and being Black (unstandardized beta value = 7.79, P less than .01) also predicted more attention dysregulation.
Household income appeared to differ significantly based on AD severity groups. “If you have mild AD, you are more likely to come from a higher income household,” Ms. Zhou said.
She concluded her presentation by calling for future studies with larger samples sizes to establish causality and directional effects between AD severity, itch, sleep, race, and attention.
The study was funded by the Agency for Healthcare Research and Quality. Ms. Zhou reported having no financial disclosures.
FROM REVOLUTIONIZING AD 2020
COVID-19 mortality rates declined, but vary by hospital
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
After COVID-19 infection, antibodies highly protective for months, prospective study shows
results of the first prospective study of the subject revealed.
The main message for health care workers is, “if you’ve had COVID, at least in the short term, you are unlikely to get it again,” David Eyre, DPhil, senior author, associate professor at the Big Data Institute and infectious diseases clinician at the University of Oxford (England), said in an interview.
Dr. Eyre and colleagues assessed for the presence of two antibodies to SARS-CoV-2 among 12,541 health care workers in the United Kingdom, including about 10% who had a history of polymerase chain reaction (PCR)–confirmed infection. Of those, 223 who did not have antibodies tested positive on PCR for the virus during 31 weeks of follow-up; two participants who did not have antibodies at baseline tested positive.
The study was published online Dec. 23 in The New England Journal of Medicine.
“It’s great news because there have been so many questions regarding whether or not you can be protected against reinfection, and this health care worker study is really an elegant way to address that question,” Mark Slifka, PhD, said in an interview when asked to comment on the findings.
Although “there are millions of people in the U.S. who have been infected with COVID, we don’t know how common reinfection is,” said Dr. Slifka, a researcher at the Oregon National Primate Research Center and professor at Oregon Health & Science University, Portland.
The likelihood of a subsequent positive PCR test result was 1.09 per 10,000 days at risk among those without antibodies, compared with 0.13 per 10,000 days among those with anti-spike antibodies.
The investigators also assessed for the presence of anti–nucleocapsid IgG antibody titers. They found a significant trend for increasing PCR-positive test results with increasing antibody levels. As with the anti-spike antibody findings, 226 of 11,543 health care providers who did not have anti–nucleocapsid IgG antibodies subsequently tested positive on PCR; by contrast, two of 1,172 participants who did not have antibodies tested positive. Adjusted for age, sex, and calendar time, this finding translates to a 0.11 incidence rate ratio (0.13 per 10,000 days at risk; 95% confidence interval, 0.03-0.45; P = .002).
“This is a study a number of us have been trying to do,” said Christopher L. King, MD, PhD, professor of pathology and associate professor of medicine at Case Western Reserve University, Cleveland.
“To really follow a group like this longitudinally like they’ve done, with a large population, and to see such a big difference – it really confirms our suspicion that those who do become infected and develop an antibody response are significantly protected from reinfection.
“What’s great about this study is it’s nearly a 10-fold reduction in risk if you’ve recovered from COVID and have antibodies,” said Dr. King, who was not involved with the research. “That’s what a lot of us have been wanting to know.”
Unanswered questions remain
“How long this immunity lasts, we don’t know,” Dr. King said. He predicted that antibody protection could last a year to a year and a half. The duration of protection could vary. “We know some people lose their antibodies pretty quickly, and other people don’t,” he said.
Dr. Slifka said the suggestion of “a substantially reduced risk for at least 6 months ... is great news, and the timing couldn’t be better, because we’re rolling out the vaccines.”
Not all antibody responses are alike. For example, data indicate that antibody levels following immunization with the Pfizer/BioNTech or Moderna vaccines are higher on average than those of people who’ve had a natural infection, Dr. King said. He added that initial data on the AstraZeneca COVID-19 vaccine in development showed lower antibody levels compared with natural immunity.
The Centers for Disease Control and Prevention recommends immunization for those with a history of infection. “People who have gotten sick with COVID-19 may still benefit from getting vaccinated,” the CDC notes on its Facts About COVID-19 Vaccines website. “Due to the severe health risks associated with COVID-19 and the fact that re-infection with COVID-19 is possible, people may be advised to get a COVID-19 vaccine even if they have been sick with COVID-19 before,” the CDC stated.
The agency also notes that people appear to become susceptible to reinfection approximately 90 days after onset of infection. However, the new evidence from the UK study that persons have up to 6 months of immune protection might lead to a modification of recommendations, especially at a time when vaccine supplies are limited, Dr. Slifka said.
Another unanswered question is why the two study participants with antibodies subsequently tested positive for reinfection. “There are a lot of things that could have made these people more susceptible,” Dr. King said. For example, they could have been heavily exposed to SARS-CoV-2 or been immunocompromised for another reason.
Furthermore, the immune response involves more than antibody levels, Dr. King noted. Research in rhesus monkeys suggests that T cells play a role, but not as prominent a part as antibodies. “What I think is protecting us from infection is primarily the antibodies, although the T cells are probably important. Once you get infected, the T cells are probably playing a more important role in terms of whether you get very sick or not,” he said.
Multiplication + addition = more protected?
The 90% natural immunity protection in the study approaches the 95% efficacy associated with the Pfizer and Moderna vaccines, Dr. Slifka noted. Even without immunization, this could mean a portion of the U.S. population is already protected against future infection.
Furthermore, the CDC estimates that there are about 7.7 cases of COVID-19 for every case reported.
As of Sept. 30, the CDC reported that there were 6,891,764 confirmed cases. The agency estimated that overall, approximately 53 million people in the United States have been infected. More recent numbers from Johns Hopkins University’s Coronavirus Resource Center indicate that there were 18.2 million cases in the United States as of Dec. 22. If that tally is multiplied by 7.7, the total number protected could approach 140 million, Dr. Slifka said.
“That could really be a boost in terms of knocking this pandemic down in the next couple of months,” Dr. Slifka said.
“Now, if we were to modify the current recommendations and briefly defer vaccination of people with confirmed cases of COVID-19 until later on, we could start reaching herd immunity pretty quickly,” he added.
Real-life implications
“There is no such thing as 100% protection, even from the infection itself. So when you’re dealing with someone with possible exposure to COVID-19, you still need to follow the proper precautions,” Dr. Slifka said.
Nonetheless, he said, “This is great news for those on the front lines who are wondering whether or not they would have any protection if they had COVID-19 before. And the answer is yes – there is a very good chance they will have protection, based on this quite large study.”
One limitation of the study is that the population consisted predominantly of healthy adult health care workers aged 65 years or younger. “Further studies are needed to assess postinfection immunity in other populations, including children, older adults, and persons with coexisting conditions, including immunosuppression,” the researchers noted.
Dr. Eyre plans to continue following the health care workers in the study, some of whom have been vaccinated for COVID-19. This ongoing research will allow him and coinvestigators to “confirm the protection offered by vaccination and investigate how postvaccine antibody responses vary by whether you have had COVID-19 before or not. We also want to understand more about how long postinfection immunity lasts.”
Dr. Eyre has received grants as a Robinson Foundation Fellow and NIHR Oxford BRC senior fellow during the conduct of the study. Dr. Slifka and Dr. King report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of the first prospective study of the subject revealed.
The main message for health care workers is, “if you’ve had COVID, at least in the short term, you are unlikely to get it again,” David Eyre, DPhil, senior author, associate professor at the Big Data Institute and infectious diseases clinician at the University of Oxford (England), said in an interview.
Dr. Eyre and colleagues assessed for the presence of two antibodies to SARS-CoV-2 among 12,541 health care workers in the United Kingdom, including about 10% who had a history of polymerase chain reaction (PCR)–confirmed infection. Of those, 223 who did not have antibodies tested positive on PCR for the virus during 31 weeks of follow-up; two participants who did not have antibodies at baseline tested positive.
The study was published online Dec. 23 in The New England Journal of Medicine.
“It’s great news because there have been so many questions regarding whether or not you can be protected against reinfection, and this health care worker study is really an elegant way to address that question,” Mark Slifka, PhD, said in an interview when asked to comment on the findings.
Although “there are millions of people in the U.S. who have been infected with COVID, we don’t know how common reinfection is,” said Dr. Slifka, a researcher at the Oregon National Primate Research Center and professor at Oregon Health & Science University, Portland.
The likelihood of a subsequent positive PCR test result was 1.09 per 10,000 days at risk among those without antibodies, compared with 0.13 per 10,000 days among those with anti-spike antibodies.
The investigators also assessed for the presence of anti–nucleocapsid IgG antibody titers. They found a significant trend for increasing PCR-positive test results with increasing antibody levels. As with the anti-spike antibody findings, 226 of 11,543 health care providers who did not have anti–nucleocapsid IgG antibodies subsequently tested positive on PCR; by contrast, two of 1,172 participants who did not have antibodies tested positive. Adjusted for age, sex, and calendar time, this finding translates to a 0.11 incidence rate ratio (0.13 per 10,000 days at risk; 95% confidence interval, 0.03-0.45; P = .002).
“This is a study a number of us have been trying to do,” said Christopher L. King, MD, PhD, professor of pathology and associate professor of medicine at Case Western Reserve University, Cleveland.
“To really follow a group like this longitudinally like they’ve done, with a large population, and to see such a big difference – it really confirms our suspicion that those who do become infected and develop an antibody response are significantly protected from reinfection.
“What’s great about this study is it’s nearly a 10-fold reduction in risk if you’ve recovered from COVID and have antibodies,” said Dr. King, who was not involved with the research. “That’s what a lot of us have been wanting to know.”
Unanswered questions remain
“How long this immunity lasts, we don’t know,” Dr. King said. He predicted that antibody protection could last a year to a year and a half. The duration of protection could vary. “We know some people lose their antibodies pretty quickly, and other people don’t,” he said.
Dr. Slifka said the suggestion of “a substantially reduced risk for at least 6 months ... is great news, and the timing couldn’t be better, because we’re rolling out the vaccines.”
Not all antibody responses are alike. For example, data indicate that antibody levels following immunization with the Pfizer/BioNTech or Moderna vaccines are higher on average than those of people who’ve had a natural infection, Dr. King said. He added that initial data on the AstraZeneca COVID-19 vaccine in development showed lower antibody levels compared with natural immunity.
The Centers for Disease Control and Prevention recommends immunization for those with a history of infection. “People who have gotten sick with COVID-19 may still benefit from getting vaccinated,” the CDC notes on its Facts About COVID-19 Vaccines website. “Due to the severe health risks associated with COVID-19 and the fact that re-infection with COVID-19 is possible, people may be advised to get a COVID-19 vaccine even if they have been sick with COVID-19 before,” the CDC stated.
The agency also notes that people appear to become susceptible to reinfection approximately 90 days after onset of infection. However, the new evidence from the UK study that persons have up to 6 months of immune protection might lead to a modification of recommendations, especially at a time when vaccine supplies are limited, Dr. Slifka said.
Another unanswered question is why the two study participants with antibodies subsequently tested positive for reinfection. “There are a lot of things that could have made these people more susceptible,” Dr. King said. For example, they could have been heavily exposed to SARS-CoV-2 or been immunocompromised for another reason.
Furthermore, the immune response involves more than antibody levels, Dr. King noted. Research in rhesus monkeys suggests that T cells play a role, but not as prominent a part as antibodies. “What I think is protecting us from infection is primarily the antibodies, although the T cells are probably important. Once you get infected, the T cells are probably playing a more important role in terms of whether you get very sick or not,” he said.
Multiplication + addition = more protected?
The 90% natural immunity protection in the study approaches the 95% efficacy associated with the Pfizer and Moderna vaccines, Dr. Slifka noted. Even without immunization, this could mean a portion of the U.S. population is already protected against future infection.
Furthermore, the CDC estimates that there are about 7.7 cases of COVID-19 for every case reported.
As of Sept. 30, the CDC reported that there were 6,891,764 confirmed cases. The agency estimated that overall, approximately 53 million people in the United States have been infected. More recent numbers from Johns Hopkins University’s Coronavirus Resource Center indicate that there were 18.2 million cases in the United States as of Dec. 22. If that tally is multiplied by 7.7, the total number protected could approach 140 million, Dr. Slifka said.
“That could really be a boost in terms of knocking this pandemic down in the next couple of months,” Dr. Slifka said.
“Now, if we were to modify the current recommendations and briefly defer vaccination of people with confirmed cases of COVID-19 until later on, we could start reaching herd immunity pretty quickly,” he added.
Real-life implications
“There is no such thing as 100% protection, even from the infection itself. So when you’re dealing with someone with possible exposure to COVID-19, you still need to follow the proper precautions,” Dr. Slifka said.
Nonetheless, he said, “This is great news for those on the front lines who are wondering whether or not they would have any protection if they had COVID-19 before. And the answer is yes – there is a very good chance they will have protection, based on this quite large study.”
One limitation of the study is that the population consisted predominantly of healthy adult health care workers aged 65 years or younger. “Further studies are needed to assess postinfection immunity in other populations, including children, older adults, and persons with coexisting conditions, including immunosuppression,” the researchers noted.
Dr. Eyre plans to continue following the health care workers in the study, some of whom have been vaccinated for COVID-19. This ongoing research will allow him and coinvestigators to “confirm the protection offered by vaccination and investigate how postvaccine antibody responses vary by whether you have had COVID-19 before or not. We also want to understand more about how long postinfection immunity lasts.”
Dr. Eyre has received grants as a Robinson Foundation Fellow and NIHR Oxford BRC senior fellow during the conduct of the study. Dr. Slifka and Dr. King report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of the first prospective study of the subject revealed.
The main message for health care workers is, “if you’ve had COVID, at least in the short term, you are unlikely to get it again,” David Eyre, DPhil, senior author, associate professor at the Big Data Institute and infectious diseases clinician at the University of Oxford (England), said in an interview.
Dr. Eyre and colleagues assessed for the presence of two antibodies to SARS-CoV-2 among 12,541 health care workers in the United Kingdom, including about 10% who had a history of polymerase chain reaction (PCR)–confirmed infection. Of those, 223 who did not have antibodies tested positive on PCR for the virus during 31 weeks of follow-up; two participants who did not have antibodies at baseline tested positive.
The study was published online Dec. 23 in The New England Journal of Medicine.
“It’s great news because there have been so many questions regarding whether or not you can be protected against reinfection, and this health care worker study is really an elegant way to address that question,” Mark Slifka, PhD, said in an interview when asked to comment on the findings.
Although “there are millions of people in the U.S. who have been infected with COVID, we don’t know how common reinfection is,” said Dr. Slifka, a researcher at the Oregon National Primate Research Center and professor at Oregon Health & Science University, Portland.
The likelihood of a subsequent positive PCR test result was 1.09 per 10,000 days at risk among those without antibodies, compared with 0.13 per 10,000 days among those with anti-spike antibodies.
The investigators also assessed for the presence of anti–nucleocapsid IgG antibody titers. They found a significant trend for increasing PCR-positive test results with increasing antibody levels. As with the anti-spike antibody findings, 226 of 11,543 health care providers who did not have anti–nucleocapsid IgG antibodies subsequently tested positive on PCR; by contrast, two of 1,172 participants who did not have antibodies tested positive. Adjusted for age, sex, and calendar time, this finding translates to a 0.11 incidence rate ratio (0.13 per 10,000 days at risk; 95% confidence interval, 0.03-0.45; P = .002).
“This is a study a number of us have been trying to do,” said Christopher L. King, MD, PhD, professor of pathology and associate professor of medicine at Case Western Reserve University, Cleveland.
“To really follow a group like this longitudinally like they’ve done, with a large population, and to see such a big difference – it really confirms our suspicion that those who do become infected and develop an antibody response are significantly protected from reinfection.
“What’s great about this study is it’s nearly a 10-fold reduction in risk if you’ve recovered from COVID and have antibodies,” said Dr. King, who was not involved with the research. “That’s what a lot of us have been wanting to know.”
Unanswered questions remain
“How long this immunity lasts, we don’t know,” Dr. King said. He predicted that antibody protection could last a year to a year and a half. The duration of protection could vary. “We know some people lose their antibodies pretty quickly, and other people don’t,” he said.
Dr. Slifka said the suggestion of “a substantially reduced risk for at least 6 months ... is great news, and the timing couldn’t be better, because we’re rolling out the vaccines.”
Not all antibody responses are alike. For example, data indicate that antibody levels following immunization with the Pfizer/BioNTech or Moderna vaccines are higher on average than those of people who’ve had a natural infection, Dr. King said. He added that initial data on the AstraZeneca COVID-19 vaccine in development showed lower antibody levels compared with natural immunity.
The Centers for Disease Control and Prevention recommends immunization for those with a history of infection. “People who have gotten sick with COVID-19 may still benefit from getting vaccinated,” the CDC notes on its Facts About COVID-19 Vaccines website. “Due to the severe health risks associated with COVID-19 and the fact that re-infection with COVID-19 is possible, people may be advised to get a COVID-19 vaccine even if they have been sick with COVID-19 before,” the CDC stated.
The agency also notes that people appear to become susceptible to reinfection approximately 90 days after onset of infection. However, the new evidence from the UK study that persons have up to 6 months of immune protection might lead to a modification of recommendations, especially at a time when vaccine supplies are limited, Dr. Slifka said.
Another unanswered question is why the two study participants with antibodies subsequently tested positive for reinfection. “There are a lot of things that could have made these people more susceptible,” Dr. King said. For example, they could have been heavily exposed to SARS-CoV-2 or been immunocompromised for another reason.
Furthermore, the immune response involves more than antibody levels, Dr. King noted. Research in rhesus monkeys suggests that T cells play a role, but not as prominent a part as antibodies. “What I think is protecting us from infection is primarily the antibodies, although the T cells are probably important. Once you get infected, the T cells are probably playing a more important role in terms of whether you get very sick or not,” he said.
Multiplication + addition = more protected?
The 90% natural immunity protection in the study approaches the 95% efficacy associated with the Pfizer and Moderna vaccines, Dr. Slifka noted. Even without immunization, this could mean a portion of the U.S. population is already protected against future infection.
Furthermore, the CDC estimates that there are about 7.7 cases of COVID-19 for every case reported.
As of Sept. 30, the CDC reported that there were 6,891,764 confirmed cases. The agency estimated that overall, approximately 53 million people in the United States have been infected. More recent numbers from Johns Hopkins University’s Coronavirus Resource Center indicate that there were 18.2 million cases in the United States as of Dec. 22. If that tally is multiplied by 7.7, the total number protected could approach 140 million, Dr. Slifka said.
“That could really be a boost in terms of knocking this pandemic down in the next couple of months,” Dr. Slifka said.
“Now, if we were to modify the current recommendations and briefly defer vaccination of people with confirmed cases of COVID-19 until later on, we could start reaching herd immunity pretty quickly,” he added.
Real-life implications
“There is no such thing as 100% protection, even from the infection itself. So when you’re dealing with someone with possible exposure to COVID-19, you still need to follow the proper precautions,” Dr. Slifka said.
Nonetheless, he said, “This is great news for those on the front lines who are wondering whether or not they would have any protection if they had COVID-19 before. And the answer is yes – there is a very good chance they will have protection, based on this quite large study.”
One limitation of the study is that the population consisted predominantly of healthy adult health care workers aged 65 years or younger. “Further studies are needed to assess postinfection immunity in other populations, including children, older adults, and persons with coexisting conditions, including immunosuppression,” the researchers noted.
Dr. Eyre plans to continue following the health care workers in the study, some of whom have been vaccinated for COVID-19. This ongoing research will allow him and coinvestigators to “confirm the protection offered by vaccination and investigate how postvaccine antibody responses vary by whether you have had COVID-19 before or not. We also want to understand more about how long postinfection immunity lasts.”
Dr. Eyre has received grants as a Robinson Foundation Fellow and NIHR Oxford BRC senior fellow during the conduct of the study. Dr. Slifka and Dr. King report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seeking new vaccines against whooping cough: The PERISCOPE project
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
FROM ESPID 2020
Moderna’s COVID-19 vaccine deemed ‘highly effective,’ but further studies needed
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
Key clinical point: The FDA’s Vaccines and Related Biological Products Advisory Committee regarded Moderna’s COVID-19 vaccine as highly effective with a favorable safety profile, based on interim phase 3 results.
Major finding: The two-dose vaccine regimen had a low frequency of serious adverse events (1.0% each in the mRNA-1273 and placebo arms, respectively) and demonstrated 94.1% (95% CI, 89.3%-96.8%) vaccine efficacy.
Study details: A briefing document summarized interim data and recommendations from the FDA’s VRBPAC on Moderna’s mRNA-1273 COVID-19 vaccine.
Disclosures: The associated phase 3 study was sponsored by ModernaTX.
Source: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
Current PERISCOPE vaccine studies: Toward better pertussis prevention?
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
FROM ESPID 2020
Call to arms: vaccinating the health workforce of 21 million strong
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
COVID-19 anticoagulation trials ‘paused’ for futility, safety
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.