User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Could the Surgisphere Lancet and NEJM retractions debacle happen again?
In May 2020, two major scientific journals published and subsequently retracted studies that relied on data provided by the now-disgraced data analytics company Surgisphere.
One of the studies, published in The Lancet, reported an association between the antimalarial drugs hydroxychloroquine and chloroquine and increased in-hospital mortality and cardiac arrhythmias in patients with COVID-19. The second study, which appeared in the New England Journal of Medicine, described an association between underlying cardiovascular disease, but not related drug therapy, with increased mortality in COVID-19 patients.
The retractions in June 2020 followed an open letter to each publication penned by scientists, ethicists, and clinicians who flagged serious methodological and ethical anomalies in the data used in the studies.
On the 1-year anniversary, researchers and journal editors spoke about what was learned to reduce the risk of something like this happening again.
“The Surgisphere incident served as a wake-up call for everyone involved with scientific research to make sure that data have integrity and are robust,” Sunil Rao, MD, professor of medicine, Duke University Health System, Durham, N.C., and editor-in-chief of Circulation: Cardiovascular Interventions, said in an interview.
“I’m sure this isn’t going to be the last incident of this nature, and we have to be vigilant about new datasets or datasets that we haven’t heard of as having a track record of publication,” Dr. Rao said.
Spotlight on authors
The editors of the Lancet Group responded to the “wake-up call” with a statement, Learning From a Retraction, which announced changes to reduce the risks of research and publication misconduct.
The changes affect multiple phases of the publication process. For example, the declaration form that authors must sign “will require that more than one author has directly accessed and verified the data reported in the manuscript.” Additionally, when a research article is the result of an academic and commercial partnership – as was the case in the two retracted studies – “one of the authors named as having accessed and verified data must be from the academic team.”
This was particularly important because it appears that the academic coauthors of the retracted studies did not have access to the data provided by Surgisphere, a private commercial entity.
Mandeep R. Mehra, MD, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, who was the lead author of both studies, declined to be interviewed for this article. In a letter to the New England Journal of Medicine editors requesting that the article be retracted, he wrote: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article.”
In a similar communication with The Lancet, Dr. Mehra wrote even more pointedly that, in light of the refusal of Surgisphere to make the data available to the third-party auditor, “we can no longer vouch for the veracity of the primary data sources.”
“It is very disturbing that the authors were willing to put their names on a paper without ever seeing and verifying the data,” Mario Malički, MD, PhD, a postdoctoral researcher at METRICS at Stanford (Calif.) University, said in an interview. “Saying that they could ‘no longer vouch’ suggests that at one point they could vouch for it. Most likely they took its existence and veracity entirely on trust.”
Dr. Malički pointed out that one of the four criteria of the International Committee of Medical Journal Editors for being an author on a study is the “agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.”
The new policies put forth by The Lancet are “encouraging,” but perhaps do not go far enough. “Every author, not only one or two authors, should personally take responsibility for the integrity of data,” he stated.
Many journals “adhere to ICMJE rules in principle and have checkboxes for authors to confirm that they guarantee the veracity of the data.” However, they “do not have the resources to verify the authors’ statements.”
Ideally, “it is the institutions where the researchers work that should guarantee the veracity of the raw data – but I do not know any university or institute that does this,” he said.
No ‘good-housekeeping’ seal
For articles based on large, real-world datasets, the Lancet Group will now require that editors ensure that at least one peer reviewer is “knowledgeable about the details of the dataset being reported and can understand its strengths and limitations in relation to the question being addressed.”
For studies that use “very large datasets,” the editors are now required to ensure that, in addition to a statistical peer review, a review from an “expert in data science” is obtained. Reviewers will also be explicitly asked if they have “concerns about research integrity or publication ethics regarding the manuscript they are reviewing.”
Although these changes are encouraging, Harlan Krumholz, MD, professor of medicine (cardiology), Yale University, New Haven, Conn., is not convinced that they are realistic.
Dr. Krumholz, who is also the founder and director of the Yale New Haven Hospital Center for Outcome Research and Evaluation, said in an interview that “large, real-world datasets” are of two varieties. Datasets drawn from publicly available sources, such as Medicare or Medicaid health records, are utterly transparent.
By contrast, Surgisphere was a privately owned database, and “it is not unusual for privately owned databases to have proprietary data from multiple sources that the company may choose to keep confidential,” Dr. Krumholz said.
He noted that several large datasets are widely used for research purposes, such as IBM, Optum, and Komodo – a data analytics company that recently entered into partnership with a fourth company, PicnicHealth.
These companies receive deidentified electronic health records from health systems and insurers nationwide. Komodo boasts “real-time and longitudinal data on more than 325 million patients, representing more than 65 billion clinical encounters with 15 million new encounters added daily.”
“One has to raise an eyebrow – how were these data acquired? And, given that the U.S. has a population of around 328 million people, is it really plausible that a single company has health records of almost the entire U.S. population?” Dr. Krumholz commented. (A spokesperson for Komodo said in an interview that the company has records on 325 million U.S. patients.)
This is “an issue across the board with ‘real-world evidence,’ which is that it’s like the ‘Wild West’ – the transparencies of private databases are less than optimal and there are no common standards to help us move forward,” Dr. Krumholz said, noting that there is “no external authority overseeing, validating, or auditing these databases. In the end, we are trusting the companies.”
Although the Food and Drug Administration has laid out a framework for how real-world data and real-world evidence can be used to advance scientific research, the FDA does not oversee the databases.
“Thus, there is no ‘good housekeeping seal’ that a peer reviewer or author would be in a position to evaluate,” Dr. Krumholz said. “No journal can do an audit of these types of private databases, so ultimately, it boils down to trust.”
Nevertheless, there were red flags with Surgisphere, Dr. Rao pointed out. Unlike more established and widely used databases, the Surgisphere database had been catapulted from relative obscurity onto center stage, which should have given researchers pause.
AI-assisted peer review
A series of investigative reports by The Guardian raised questions about Sapan Desai, the CEO of Surgisphere, including the fact that hospitals purporting to have contributed data to Surgisphere had never heard of the company.
However, peer reviewers are not expected to be investigative reporters, explained Dr. Malički.
“In an ideal world, editors and peer reviewers would have a chance to look at raw data or would have a certificate from the academic institution the authors are affiliated with that the data have been inspected by the institution, but in the real world, of course, this does not happen,” he said.
Artificial intelligence software is being developed and deployed to assist in the peer review process, Dr. Malički noted. In July 2020, Frontiers Science News debuted its Artificial Intelligence Review Assistant to help editors, reviewers, and authors evaluate the quality of a manuscript. The program can make up to 20 recommendations, including “the assessment of language quality, the detection of plagiarism, and identification of potential conflicts of interest.” The program is now in use in all 103 journals published by Frontiers. Preliminary software is also available to detect statistical errors.
Another system under development is FAIRware, an initiative of the Research on Research Institute in partnership with the Stanford Center for Biomedical Informatics Research. The partnership’s goal is to “develop an automated online tool (or suite of tools) to help researchers ensure that the datasets they produce are ‘FAIR’ at the point of creation,” said Dr. Malički, referring to the findability, accessibility, interoperability, and reusability (FAIR) guiding principles for data management. The principles aim to increase the ability of machines to automatically find and use the data, as well as to support its reuse by individuals.
He added that these advanced tools cannot replace human reviewers, who will “likely always be a necessary quality check in the process.”
Greater transparency needed
Another limitation of peer review is the reviewers themselves, according to Dr. Malički. “It’s a step in the right direction that The Lancet is now requesting a peer reviewer with expertise in big datasets, but it does not go far enough to increase accountability of peer reviewers,” he said.
Dr. Malički is the co–editor-in-chief of the journal Research Integrity and Peer Review , which has “an open and transparent review process – meaning that we reveal the names of the reviewers to the public and we publish the full review report alongside the paper.” The publication also allows the authors to make public the original version they sent.
Dr. Malički cited several advantages to transparent peer review, particularly the increased accountability that results from placing potential conflicts of interest under the microscope.
As for the concern that identifying the reviewers might soften the review process, “there is little evidence to substantiate that concern,” he added.
Dr. Malički emphasized that making reviews public “is not a problem – people voice strong opinions at conferences and elsewhere. The question remains, who gets to decide if the criticism has been adequately addressed, so that the findings of the study still stand?”
He acknowledged that, “as in politics and on many social platforms, rage, hatred, and personal attacks divert the discussion from the topic at hand, which is why a good moderator is needed.”
A journal editor or a moderator at a scientific conference may be tasked with “stopping all talk not directly related to the topic.”
Widening the circle of scrutiny
Dr. Malički added: “A published paper should not be considered the ‘final word,’ even if it has gone through peer review and is published in a reputable journal. The peer-review process means that a limited number of people have seen the study.”
Once the study is published, “the whole world gets to see it and criticize it, and that widens the circle of scrutiny.”
One classic way to raise concerns about a study post publication is to write a letter to the journal editor. But there is no guarantee that the letter will be published or the authors notified of the feedback.
Dr. Malički encourages readers to use PubPeer, an online forum in which members of the public can post comments on scientific studies and articles.
Once a comment is posted, the authors are alerted. “There is no ‘police department’ that forces authors to acknowledge comments or forces journal editors to take action, but at least PubPeer guarantees that readers’ messages will reach the authors and – depending on how many people raise similar issues – the comments can lead to errata or even full retractions,” he said.
PubPeer was key in pointing out errors in a suspect study from France (which did not involve Surgisphere) that supported the use of hydroxychloroquine in COVID-19.
A message to policy makers
High stakes are involved in ensuring the integrity of scientific publications: The French government revoked a decree that allowed hospitals to prescribe hydroxychloroquine for certain COVID-19 patients.
After the Surgisphere Lancet article, the World Health Organization temporarily halted enrollment in the hydroxychloroquine component of the Solidarity international randomized trial of medications to treat COVID-19.
Similarly, the U.K. Medicines and Healthcare Products Regulatory Agency instructed the organizers of COPCOV, an international trial of the use of hydroxychloroquine as prophylaxis against COVID-19, to suspend recruitment of patients. The SOLIDARITY trial briefly resumed, but that arm of the trial was ultimately suspended after a preliminary analysis suggested that hydroxychloroquine provided no benefit for patients with COVID-19.
Dr. Malički emphasized that governments and organizations should not “blindly trust journal articles” and make policy decisions based exclusively on study findings in published journals – even with the current improvements in the peer review process – without having their own experts conduct a thorough review of the data.
“If you are not willing to do your own due diligence, then at least be brave enough and say transparently why you are making this policy, or any other changes, and clearly state if your decision is based primarily or solely on the fact that ‘X’ study was published in ‘Y’ journal,” he stated.
Dr. Rao believes that the most important take-home message of the Surgisphere scandal is “that we should be skeptical and do our own due diligence about the kinds of data published – a responsibility that applies to all of us, whether we are investigators, editors at journals, the press, scientists, and readers.”
Dr. Rao reported being on the steering committee of the National Heart, Lung, and Blood Institute–sponsored MINT trial and the Bayer-sponsored PACIFIC AMI trial. Dr. Malički reports being a postdoc at METRICS Stanford in the past 3 years. Dr. Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing. He is an owner of Refactor Health and HugoHealth and had grants and/or contracts from the Centers for Medicare & Medicaid Services, the FDA, Johnson & Johnson, and the Shenzhen Center for Health Information.
A version of this article first appeared on Medscape.com.
In May 2020, two major scientific journals published and subsequently retracted studies that relied on data provided by the now-disgraced data analytics company Surgisphere.
One of the studies, published in The Lancet, reported an association between the antimalarial drugs hydroxychloroquine and chloroquine and increased in-hospital mortality and cardiac arrhythmias in patients with COVID-19. The second study, which appeared in the New England Journal of Medicine, described an association between underlying cardiovascular disease, but not related drug therapy, with increased mortality in COVID-19 patients.
The retractions in June 2020 followed an open letter to each publication penned by scientists, ethicists, and clinicians who flagged serious methodological and ethical anomalies in the data used in the studies.
On the 1-year anniversary, researchers and journal editors spoke about what was learned to reduce the risk of something like this happening again.
“The Surgisphere incident served as a wake-up call for everyone involved with scientific research to make sure that data have integrity and are robust,” Sunil Rao, MD, professor of medicine, Duke University Health System, Durham, N.C., and editor-in-chief of Circulation: Cardiovascular Interventions, said in an interview.
“I’m sure this isn’t going to be the last incident of this nature, and we have to be vigilant about new datasets or datasets that we haven’t heard of as having a track record of publication,” Dr. Rao said.
Spotlight on authors
The editors of the Lancet Group responded to the “wake-up call” with a statement, Learning From a Retraction, which announced changes to reduce the risks of research and publication misconduct.
The changes affect multiple phases of the publication process. For example, the declaration form that authors must sign “will require that more than one author has directly accessed and verified the data reported in the manuscript.” Additionally, when a research article is the result of an academic and commercial partnership – as was the case in the two retracted studies – “one of the authors named as having accessed and verified data must be from the academic team.”
This was particularly important because it appears that the academic coauthors of the retracted studies did not have access to the data provided by Surgisphere, a private commercial entity.
Mandeep R. Mehra, MD, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, who was the lead author of both studies, declined to be interviewed for this article. In a letter to the New England Journal of Medicine editors requesting that the article be retracted, he wrote: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article.”
In a similar communication with The Lancet, Dr. Mehra wrote even more pointedly that, in light of the refusal of Surgisphere to make the data available to the third-party auditor, “we can no longer vouch for the veracity of the primary data sources.”
“It is very disturbing that the authors were willing to put their names on a paper without ever seeing and verifying the data,” Mario Malički, MD, PhD, a postdoctoral researcher at METRICS at Stanford (Calif.) University, said in an interview. “Saying that they could ‘no longer vouch’ suggests that at one point they could vouch for it. Most likely they took its existence and veracity entirely on trust.”
Dr. Malički pointed out that one of the four criteria of the International Committee of Medical Journal Editors for being an author on a study is the “agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.”
The new policies put forth by The Lancet are “encouraging,” but perhaps do not go far enough. “Every author, not only one or two authors, should personally take responsibility for the integrity of data,” he stated.
Many journals “adhere to ICMJE rules in principle and have checkboxes for authors to confirm that they guarantee the veracity of the data.” However, they “do not have the resources to verify the authors’ statements.”
Ideally, “it is the institutions where the researchers work that should guarantee the veracity of the raw data – but I do not know any university or institute that does this,” he said.
No ‘good-housekeeping’ seal
For articles based on large, real-world datasets, the Lancet Group will now require that editors ensure that at least one peer reviewer is “knowledgeable about the details of the dataset being reported and can understand its strengths and limitations in relation to the question being addressed.”
For studies that use “very large datasets,” the editors are now required to ensure that, in addition to a statistical peer review, a review from an “expert in data science” is obtained. Reviewers will also be explicitly asked if they have “concerns about research integrity or publication ethics regarding the manuscript they are reviewing.”
Although these changes are encouraging, Harlan Krumholz, MD, professor of medicine (cardiology), Yale University, New Haven, Conn., is not convinced that they are realistic.
Dr. Krumholz, who is also the founder and director of the Yale New Haven Hospital Center for Outcome Research and Evaluation, said in an interview that “large, real-world datasets” are of two varieties. Datasets drawn from publicly available sources, such as Medicare or Medicaid health records, are utterly transparent.
By contrast, Surgisphere was a privately owned database, and “it is not unusual for privately owned databases to have proprietary data from multiple sources that the company may choose to keep confidential,” Dr. Krumholz said.
He noted that several large datasets are widely used for research purposes, such as IBM, Optum, and Komodo – a data analytics company that recently entered into partnership with a fourth company, PicnicHealth.
These companies receive deidentified electronic health records from health systems and insurers nationwide. Komodo boasts “real-time and longitudinal data on more than 325 million patients, representing more than 65 billion clinical encounters with 15 million new encounters added daily.”
“One has to raise an eyebrow – how were these data acquired? And, given that the U.S. has a population of around 328 million people, is it really plausible that a single company has health records of almost the entire U.S. population?” Dr. Krumholz commented. (A spokesperson for Komodo said in an interview that the company has records on 325 million U.S. patients.)
This is “an issue across the board with ‘real-world evidence,’ which is that it’s like the ‘Wild West’ – the transparencies of private databases are less than optimal and there are no common standards to help us move forward,” Dr. Krumholz said, noting that there is “no external authority overseeing, validating, or auditing these databases. In the end, we are trusting the companies.”
Although the Food and Drug Administration has laid out a framework for how real-world data and real-world evidence can be used to advance scientific research, the FDA does not oversee the databases.
“Thus, there is no ‘good housekeeping seal’ that a peer reviewer or author would be in a position to evaluate,” Dr. Krumholz said. “No journal can do an audit of these types of private databases, so ultimately, it boils down to trust.”
Nevertheless, there were red flags with Surgisphere, Dr. Rao pointed out. Unlike more established and widely used databases, the Surgisphere database had been catapulted from relative obscurity onto center stage, which should have given researchers pause.
AI-assisted peer review
A series of investigative reports by The Guardian raised questions about Sapan Desai, the CEO of Surgisphere, including the fact that hospitals purporting to have contributed data to Surgisphere had never heard of the company.
However, peer reviewers are not expected to be investigative reporters, explained Dr. Malički.
“In an ideal world, editors and peer reviewers would have a chance to look at raw data or would have a certificate from the academic institution the authors are affiliated with that the data have been inspected by the institution, but in the real world, of course, this does not happen,” he said.
Artificial intelligence software is being developed and deployed to assist in the peer review process, Dr. Malički noted. In July 2020, Frontiers Science News debuted its Artificial Intelligence Review Assistant to help editors, reviewers, and authors evaluate the quality of a manuscript. The program can make up to 20 recommendations, including “the assessment of language quality, the detection of plagiarism, and identification of potential conflicts of interest.” The program is now in use in all 103 journals published by Frontiers. Preliminary software is also available to detect statistical errors.
Another system under development is FAIRware, an initiative of the Research on Research Institute in partnership with the Stanford Center for Biomedical Informatics Research. The partnership’s goal is to “develop an automated online tool (or suite of tools) to help researchers ensure that the datasets they produce are ‘FAIR’ at the point of creation,” said Dr. Malički, referring to the findability, accessibility, interoperability, and reusability (FAIR) guiding principles for data management. The principles aim to increase the ability of machines to automatically find and use the data, as well as to support its reuse by individuals.
He added that these advanced tools cannot replace human reviewers, who will “likely always be a necessary quality check in the process.”
Greater transparency needed
Another limitation of peer review is the reviewers themselves, according to Dr. Malički. “It’s a step in the right direction that The Lancet is now requesting a peer reviewer with expertise in big datasets, but it does not go far enough to increase accountability of peer reviewers,” he said.
Dr. Malički is the co–editor-in-chief of the journal Research Integrity and Peer Review , which has “an open and transparent review process – meaning that we reveal the names of the reviewers to the public and we publish the full review report alongside the paper.” The publication also allows the authors to make public the original version they sent.
Dr. Malički cited several advantages to transparent peer review, particularly the increased accountability that results from placing potential conflicts of interest under the microscope.
As for the concern that identifying the reviewers might soften the review process, “there is little evidence to substantiate that concern,” he added.
Dr. Malički emphasized that making reviews public “is not a problem – people voice strong opinions at conferences and elsewhere. The question remains, who gets to decide if the criticism has been adequately addressed, so that the findings of the study still stand?”
He acknowledged that, “as in politics and on many social platforms, rage, hatred, and personal attacks divert the discussion from the topic at hand, which is why a good moderator is needed.”
A journal editor or a moderator at a scientific conference may be tasked with “stopping all talk not directly related to the topic.”
Widening the circle of scrutiny
Dr. Malički added: “A published paper should not be considered the ‘final word,’ even if it has gone through peer review and is published in a reputable journal. The peer-review process means that a limited number of people have seen the study.”
Once the study is published, “the whole world gets to see it and criticize it, and that widens the circle of scrutiny.”
One classic way to raise concerns about a study post publication is to write a letter to the journal editor. But there is no guarantee that the letter will be published or the authors notified of the feedback.
Dr. Malički encourages readers to use PubPeer, an online forum in which members of the public can post comments on scientific studies and articles.
Once a comment is posted, the authors are alerted. “There is no ‘police department’ that forces authors to acknowledge comments or forces journal editors to take action, but at least PubPeer guarantees that readers’ messages will reach the authors and – depending on how many people raise similar issues – the comments can lead to errata or even full retractions,” he said.
PubPeer was key in pointing out errors in a suspect study from France (which did not involve Surgisphere) that supported the use of hydroxychloroquine in COVID-19.
A message to policy makers
High stakes are involved in ensuring the integrity of scientific publications: The French government revoked a decree that allowed hospitals to prescribe hydroxychloroquine for certain COVID-19 patients.
After the Surgisphere Lancet article, the World Health Organization temporarily halted enrollment in the hydroxychloroquine component of the Solidarity international randomized trial of medications to treat COVID-19.
Similarly, the U.K. Medicines and Healthcare Products Regulatory Agency instructed the organizers of COPCOV, an international trial of the use of hydroxychloroquine as prophylaxis against COVID-19, to suspend recruitment of patients. The SOLIDARITY trial briefly resumed, but that arm of the trial was ultimately suspended after a preliminary analysis suggested that hydroxychloroquine provided no benefit for patients with COVID-19.
Dr. Malički emphasized that governments and organizations should not “blindly trust journal articles” and make policy decisions based exclusively on study findings in published journals – even with the current improvements in the peer review process – without having their own experts conduct a thorough review of the data.
“If you are not willing to do your own due diligence, then at least be brave enough and say transparently why you are making this policy, or any other changes, and clearly state if your decision is based primarily or solely on the fact that ‘X’ study was published in ‘Y’ journal,” he stated.
Dr. Rao believes that the most important take-home message of the Surgisphere scandal is “that we should be skeptical and do our own due diligence about the kinds of data published – a responsibility that applies to all of us, whether we are investigators, editors at journals, the press, scientists, and readers.”
Dr. Rao reported being on the steering committee of the National Heart, Lung, and Blood Institute–sponsored MINT trial and the Bayer-sponsored PACIFIC AMI trial. Dr. Malički reports being a postdoc at METRICS Stanford in the past 3 years. Dr. Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing. He is an owner of Refactor Health and HugoHealth and had grants and/or contracts from the Centers for Medicare & Medicaid Services, the FDA, Johnson & Johnson, and the Shenzhen Center for Health Information.
A version of this article first appeared on Medscape.com.
In May 2020, two major scientific journals published and subsequently retracted studies that relied on data provided by the now-disgraced data analytics company Surgisphere.
One of the studies, published in The Lancet, reported an association between the antimalarial drugs hydroxychloroquine and chloroquine and increased in-hospital mortality and cardiac arrhythmias in patients with COVID-19. The second study, which appeared in the New England Journal of Medicine, described an association between underlying cardiovascular disease, but not related drug therapy, with increased mortality in COVID-19 patients.
The retractions in June 2020 followed an open letter to each publication penned by scientists, ethicists, and clinicians who flagged serious methodological and ethical anomalies in the data used in the studies.
On the 1-year anniversary, researchers and journal editors spoke about what was learned to reduce the risk of something like this happening again.
“The Surgisphere incident served as a wake-up call for everyone involved with scientific research to make sure that data have integrity and are robust,” Sunil Rao, MD, professor of medicine, Duke University Health System, Durham, N.C., and editor-in-chief of Circulation: Cardiovascular Interventions, said in an interview.
“I’m sure this isn’t going to be the last incident of this nature, and we have to be vigilant about new datasets or datasets that we haven’t heard of as having a track record of publication,” Dr. Rao said.
Spotlight on authors
The editors of the Lancet Group responded to the “wake-up call” with a statement, Learning From a Retraction, which announced changes to reduce the risks of research and publication misconduct.
The changes affect multiple phases of the publication process. For example, the declaration form that authors must sign “will require that more than one author has directly accessed and verified the data reported in the manuscript.” Additionally, when a research article is the result of an academic and commercial partnership – as was the case in the two retracted studies – “one of the authors named as having accessed and verified data must be from the academic team.”
This was particularly important because it appears that the academic coauthors of the retracted studies did not have access to the data provided by Surgisphere, a private commercial entity.
Mandeep R. Mehra, MD, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, who was the lead author of both studies, declined to be interviewed for this article. In a letter to the New England Journal of Medicine editors requesting that the article be retracted, he wrote: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article.”
In a similar communication with The Lancet, Dr. Mehra wrote even more pointedly that, in light of the refusal of Surgisphere to make the data available to the third-party auditor, “we can no longer vouch for the veracity of the primary data sources.”
“It is very disturbing that the authors were willing to put their names on a paper without ever seeing and verifying the data,” Mario Malički, MD, PhD, a postdoctoral researcher at METRICS at Stanford (Calif.) University, said in an interview. “Saying that they could ‘no longer vouch’ suggests that at one point they could vouch for it. Most likely they took its existence and veracity entirely on trust.”
Dr. Malički pointed out that one of the four criteria of the International Committee of Medical Journal Editors for being an author on a study is the “agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.”
The new policies put forth by The Lancet are “encouraging,” but perhaps do not go far enough. “Every author, not only one or two authors, should personally take responsibility for the integrity of data,” he stated.
Many journals “adhere to ICMJE rules in principle and have checkboxes for authors to confirm that they guarantee the veracity of the data.” However, they “do not have the resources to verify the authors’ statements.”
Ideally, “it is the institutions where the researchers work that should guarantee the veracity of the raw data – but I do not know any university or institute that does this,” he said.
No ‘good-housekeeping’ seal
For articles based on large, real-world datasets, the Lancet Group will now require that editors ensure that at least one peer reviewer is “knowledgeable about the details of the dataset being reported and can understand its strengths and limitations in relation to the question being addressed.”
For studies that use “very large datasets,” the editors are now required to ensure that, in addition to a statistical peer review, a review from an “expert in data science” is obtained. Reviewers will also be explicitly asked if they have “concerns about research integrity or publication ethics regarding the manuscript they are reviewing.”
Although these changes are encouraging, Harlan Krumholz, MD, professor of medicine (cardiology), Yale University, New Haven, Conn., is not convinced that they are realistic.
Dr. Krumholz, who is also the founder and director of the Yale New Haven Hospital Center for Outcome Research and Evaluation, said in an interview that “large, real-world datasets” are of two varieties. Datasets drawn from publicly available sources, such as Medicare or Medicaid health records, are utterly transparent.
By contrast, Surgisphere was a privately owned database, and “it is not unusual for privately owned databases to have proprietary data from multiple sources that the company may choose to keep confidential,” Dr. Krumholz said.
He noted that several large datasets are widely used for research purposes, such as IBM, Optum, and Komodo – a data analytics company that recently entered into partnership with a fourth company, PicnicHealth.
These companies receive deidentified electronic health records from health systems and insurers nationwide. Komodo boasts “real-time and longitudinal data on more than 325 million patients, representing more than 65 billion clinical encounters with 15 million new encounters added daily.”
“One has to raise an eyebrow – how were these data acquired? And, given that the U.S. has a population of around 328 million people, is it really plausible that a single company has health records of almost the entire U.S. population?” Dr. Krumholz commented. (A spokesperson for Komodo said in an interview that the company has records on 325 million U.S. patients.)
This is “an issue across the board with ‘real-world evidence,’ which is that it’s like the ‘Wild West’ – the transparencies of private databases are less than optimal and there are no common standards to help us move forward,” Dr. Krumholz said, noting that there is “no external authority overseeing, validating, or auditing these databases. In the end, we are trusting the companies.”
Although the Food and Drug Administration has laid out a framework for how real-world data and real-world evidence can be used to advance scientific research, the FDA does not oversee the databases.
“Thus, there is no ‘good housekeeping seal’ that a peer reviewer or author would be in a position to evaluate,” Dr. Krumholz said. “No journal can do an audit of these types of private databases, so ultimately, it boils down to trust.”
Nevertheless, there were red flags with Surgisphere, Dr. Rao pointed out. Unlike more established and widely used databases, the Surgisphere database had been catapulted from relative obscurity onto center stage, which should have given researchers pause.
AI-assisted peer review
A series of investigative reports by The Guardian raised questions about Sapan Desai, the CEO of Surgisphere, including the fact that hospitals purporting to have contributed data to Surgisphere had never heard of the company.
However, peer reviewers are not expected to be investigative reporters, explained Dr. Malički.
“In an ideal world, editors and peer reviewers would have a chance to look at raw data or would have a certificate from the academic institution the authors are affiliated with that the data have been inspected by the institution, but in the real world, of course, this does not happen,” he said.
Artificial intelligence software is being developed and deployed to assist in the peer review process, Dr. Malički noted. In July 2020, Frontiers Science News debuted its Artificial Intelligence Review Assistant to help editors, reviewers, and authors evaluate the quality of a manuscript. The program can make up to 20 recommendations, including “the assessment of language quality, the detection of plagiarism, and identification of potential conflicts of interest.” The program is now in use in all 103 journals published by Frontiers. Preliminary software is also available to detect statistical errors.
Another system under development is FAIRware, an initiative of the Research on Research Institute in partnership with the Stanford Center for Biomedical Informatics Research. The partnership’s goal is to “develop an automated online tool (or suite of tools) to help researchers ensure that the datasets they produce are ‘FAIR’ at the point of creation,” said Dr. Malički, referring to the findability, accessibility, interoperability, and reusability (FAIR) guiding principles for data management. The principles aim to increase the ability of machines to automatically find and use the data, as well as to support its reuse by individuals.
He added that these advanced tools cannot replace human reviewers, who will “likely always be a necessary quality check in the process.”
Greater transparency needed
Another limitation of peer review is the reviewers themselves, according to Dr. Malički. “It’s a step in the right direction that The Lancet is now requesting a peer reviewer with expertise in big datasets, but it does not go far enough to increase accountability of peer reviewers,” he said.
Dr. Malički is the co–editor-in-chief of the journal Research Integrity and Peer Review , which has “an open and transparent review process – meaning that we reveal the names of the reviewers to the public and we publish the full review report alongside the paper.” The publication also allows the authors to make public the original version they sent.
Dr. Malički cited several advantages to transparent peer review, particularly the increased accountability that results from placing potential conflicts of interest under the microscope.
As for the concern that identifying the reviewers might soften the review process, “there is little evidence to substantiate that concern,” he added.
Dr. Malički emphasized that making reviews public “is not a problem – people voice strong opinions at conferences and elsewhere. The question remains, who gets to decide if the criticism has been adequately addressed, so that the findings of the study still stand?”
He acknowledged that, “as in politics and on many social platforms, rage, hatred, and personal attacks divert the discussion from the topic at hand, which is why a good moderator is needed.”
A journal editor or a moderator at a scientific conference may be tasked with “stopping all talk not directly related to the topic.”
Widening the circle of scrutiny
Dr. Malički added: “A published paper should not be considered the ‘final word,’ even if it has gone through peer review and is published in a reputable journal. The peer-review process means that a limited number of people have seen the study.”
Once the study is published, “the whole world gets to see it and criticize it, and that widens the circle of scrutiny.”
One classic way to raise concerns about a study post publication is to write a letter to the journal editor. But there is no guarantee that the letter will be published or the authors notified of the feedback.
Dr. Malički encourages readers to use PubPeer, an online forum in which members of the public can post comments on scientific studies and articles.
Once a comment is posted, the authors are alerted. “There is no ‘police department’ that forces authors to acknowledge comments or forces journal editors to take action, but at least PubPeer guarantees that readers’ messages will reach the authors and – depending on how many people raise similar issues – the comments can lead to errata or even full retractions,” he said.
PubPeer was key in pointing out errors in a suspect study from France (which did not involve Surgisphere) that supported the use of hydroxychloroquine in COVID-19.
A message to policy makers
High stakes are involved in ensuring the integrity of scientific publications: The French government revoked a decree that allowed hospitals to prescribe hydroxychloroquine for certain COVID-19 patients.
After the Surgisphere Lancet article, the World Health Organization temporarily halted enrollment in the hydroxychloroquine component of the Solidarity international randomized trial of medications to treat COVID-19.
Similarly, the U.K. Medicines and Healthcare Products Regulatory Agency instructed the organizers of COPCOV, an international trial of the use of hydroxychloroquine as prophylaxis against COVID-19, to suspend recruitment of patients. The SOLIDARITY trial briefly resumed, but that arm of the trial was ultimately suspended after a preliminary analysis suggested that hydroxychloroquine provided no benefit for patients with COVID-19.
Dr. Malički emphasized that governments and organizations should not “blindly trust journal articles” and make policy decisions based exclusively on study findings in published journals – even with the current improvements in the peer review process – without having their own experts conduct a thorough review of the data.
“If you are not willing to do your own due diligence, then at least be brave enough and say transparently why you are making this policy, or any other changes, and clearly state if your decision is based primarily or solely on the fact that ‘X’ study was published in ‘Y’ journal,” he stated.
Dr. Rao believes that the most important take-home message of the Surgisphere scandal is “that we should be skeptical and do our own due diligence about the kinds of data published – a responsibility that applies to all of us, whether we are investigators, editors at journals, the press, scientists, and readers.”
Dr. Rao reported being on the steering committee of the National Heart, Lung, and Blood Institute–sponsored MINT trial and the Bayer-sponsored PACIFIC AMI trial. Dr. Malički reports being a postdoc at METRICS Stanford in the past 3 years. Dr. Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing. He is an owner of Refactor Health and HugoHealth and had grants and/or contracts from the Centers for Medicare & Medicaid Services, the FDA, Johnson & Johnson, and the Shenzhen Center for Health Information.
A version of this article first appeared on Medscape.com.
Few clinical guidelines exist for treating post-COVID symptoms
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
FDA to add myocarditis warning to mRNA COVID-19 vaccines
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
‘Dreck’ to drama: How the media handled, and got handled by, COVID
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
Tofacitinib shows mortality benefit in patients with COVID-19 pneumonia
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Children and COVID: Vaccination trends beginning to diverge
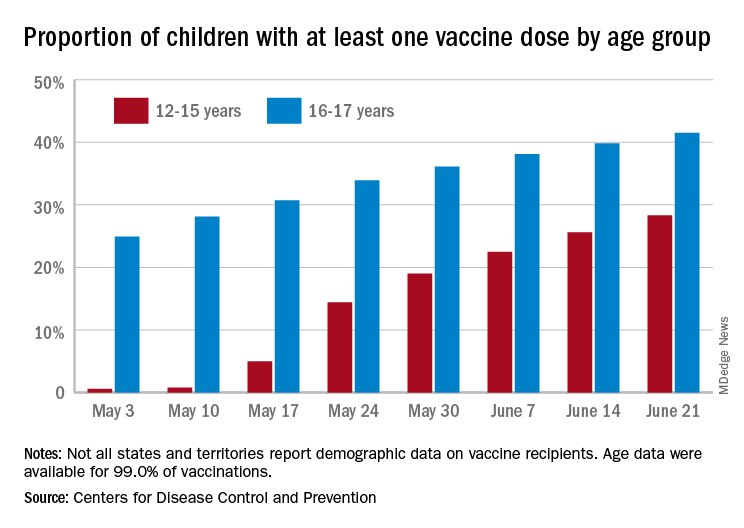
As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.
New data on COVID-19’s cognitive fallout
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
Investigators found cognitive changes, depression, and PTSD in infected patients, both in the subacute phase and 10 months after hospital discharge.
“We showed that cognitive and behavioral alterations are associated with COVID-19 infection within 2 months from hospital discharge and that they partially persist in the post-COVID phase,” study investigator Elisa Canu, PhD, neuroimaging research unit, division of neuroscience, IRCCS San Raffaele Scientific Institute, Milan, told a press briefing.
The findings were presented at the annual congress of the European Academy of Neurology.
Executive dysfunction
Previous research suggests about 30% of COVID-19 survivors have cognitive disturbances and 30%-40% have psychopathological disorders including anxiety and depression, said Dr. Canu.
These disturbances have been associated with the severity of acute-phase respiratory symptoms, infection-triggered neuroinflammation, cerebrovascular alterations, and/or neurodegeneration.
However, it’s unclear whether these disturbances persist in the post-COVID phase.
To investigate, the researchers explored cognitive and psychopathological features in 49 patients with confirmed COVID-19 admitted to a hospital ED. They examined these factors at 2 months (subacute phase) and at 10 months (post-COVID phase).
Participants had an average age of 61 years (age range, 40-75 years) and 73% were men. Most had at least one cardiovascular risk factor such as hypertension (55%), smoking (22%), and dyslipidemia (18%).
At hospital admission, 71% had an abnormal neurologic exam, 59% had hypogeusia (reduced sense of taste), 45% hyposmia (reduced sense of smell), 39% headache, and 20% confusion or drowsiness. During hospitalization, 27% had noninvasive ventilation.
In addition to cognitive and neurologic assessments, participants underwent MRI 2 months after hospital discharge. Researchers obtained data on gray matter, white matter, and total brain volume.
At 2 months post discharge, 53% of patients presented with at least one cognitive deficit. Many deficits related to executive function including difficulty planning, attention, and problem solving (16%).
However, some participants had memory issues (6%) or visuospatial disturbances (6%). Almost a quarter (23%) presented with a combination of symptoms related to executive dysfunction.
Low oxygen tied to more cognitive deficits
More than one-third of patients experienced symptoms of depression (16%) or PTSD (18%).
Patients younger than 50 years had more executive dysfunction, with these symptoms affecting 75% of younger patients. “Our explanation for that is that younger people had a milder clinical profile regarding COVID, so they were cared for at home,” said Dr. Canu.
While in hospital, patients may be on “continued alert” and receive structured interventions for cognitive and behavioral issues, she said.
More severe respiratory symptoms at hospital admission were significantly associated with deficits during the subacute phase (P = .002 for information processing).
“Low levels of oxygen in the brain could lead to confusion, headache, and brain fog, and cause the cognitive disturbances that we see,” said Dr. Canu.
White-matter hyperintensities were linked to cognitive deficits during this phase (P < .001 for verbal memory and delayed recall).
“These white-matter lesions are probably preexisting due to cardiovascular risk factors that were present in our population and may have amplified the memory disturbances we saw,” commented Dr. Canu.
The investigators did not find a significant relationship between cognitive performance and brain volume. Dr. Canu noted that cognitive and psychopathological disturbances are linked. For instance, she said, a patient with PTSD or depression may also have problems with attention or memory.
In the post-COVID phase, cognitive symptoms were reduced from 53% to 36%; again, the most common deficit was combined executive dysfunction symptoms. Depression persisted in 15% of patients and PTSD in 18%.
“We still don’t know if these alterations are a consequence of the infection,” said Dr. Canu. “And we don’t know whether the deficits are reversible or are part of a neurodegenerative process.”
The researchers plan to follow these patients further. “We definitely need longer follow-up and bigger populations, if possible, to see if these cognitive and psychopathological disturbances can improve in some way,” said Dr. Canu.
The study results underline the need for neuropsychological and neurologic monitoring in COVID patients. Cognitive stimulation training and physical activity, preferably outdoors, could be beneficial, Dr. Canu added.
A version of this article first appeared on Medscape.com.
FDA approves OTC antihistamine nasal spray
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ten killer steps to writing a great medical thriller
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
Treating sleep apnea lowers MI and stroke risk
particularly for patients with moderate to severe OSA and those who are more adherent to CPAP therapy, a new study suggests.
“Most clinical trials on the effect of CPAP on CV diseases to date have focused on secondary CV prevention. This study contributes another piece of evidence about the role of CPAP therapy to prevent CV diseases,” said Diego R. Mazzotti, PhD, an assistant professor at the University of Kansas Medical Center, Kansas City.
“Our study, while observational, suggests that clinical trials focused on understanding how to sustain long-term CPAP adherence in obstructive sleep apnea patients are necessary and could be critical for optimizing comorbidity risk reduction,” Dr. Mazzotti said.
The study was presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Good adherence important
The researchers analyzed the electronic health records of adults referred for a sleep study through the Kaiser Permanente Southern California health system. The sample included 11,145 adults without OSA, 13,898 with OSA who used CPAP, and 20,884 adults with OSA who did not use CPAP. None of them had CV disease at baseline. Median follow-up was 262 days.
The primary outcome was first occurrence of myocardial infarction, stroke, unstable angina, heart failure, or death caused by CV disease.
In adjusted models, adults with moderate to severe OSA (apnea-hypopnea index ≥15) who did not use CPAP were 71% more likely than those without OSA to have a first CV event (hazard ratio, 1.71; 95% CI, 1.11-2.64). However, the risk for a CV event during follow-up was 32% lower among OSA patients with any CPAP use (HR, 0.68; 95% CI, 0.50-0.93; P = .016).
The effect was mostly driven by those who used CPAP for at least 4 hours per night (HR, 0.60; 95% CI, 0.39-0.95). This association was stronger for those with moderate to severe OSA (HR, 0.56; 95% CI, 0.39-0.81).
“This study highlights the importance of long-term management of CPAP therapy in patients with moderate-severe OSA,” Dr. Mazzotti said in an interview.
“It suggests that maintaining good CPAP adherence might be beneficial for cardiovascular health, besides the already established benefits on quality of life, sleepiness, and other cardiometabolic functions,” he said.
Dr. Mazzotti said several mechanisms might explain the association between CPAP use and lower risk for CV events. “CPAP treats OSA by preventing respiratory pauses that occur during sleep, therefore preventing arousals, sleep fragmentation, and decreases in blood oxygen. These improved cardiorespiratory functions can be beneficial to avoid certain molecular changes that are known to contribute to cardiovascular risk, such as oxidative stress and inflammation,” he explained.
“However, specific studies fully understanding these mechanisms are necessary,” Dr. Mazzotti added.
In a comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said that “the frequent decreases in oxygen levels and fragmented sleep from apnea are associated with cardiovascular disorders. We know this from multiple studies. This, however, was a large study and strengthens the association between improving apnea and reduced serious cardiovascular events.”
Funding for the study was provided by the American Academy of Sleep Medicine Foundation and the American Heart Association. Dr. Mazzotti and Dr. Verma disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
particularly for patients with moderate to severe OSA and those who are more adherent to CPAP therapy, a new study suggests.
“Most clinical trials on the effect of CPAP on CV diseases to date have focused on secondary CV prevention. This study contributes another piece of evidence about the role of CPAP therapy to prevent CV diseases,” said Diego R. Mazzotti, PhD, an assistant professor at the University of Kansas Medical Center, Kansas City.
“Our study, while observational, suggests that clinical trials focused on understanding how to sustain long-term CPAP adherence in obstructive sleep apnea patients are necessary and could be critical for optimizing comorbidity risk reduction,” Dr. Mazzotti said.
The study was presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Good adherence important
The researchers analyzed the electronic health records of adults referred for a sleep study through the Kaiser Permanente Southern California health system. The sample included 11,145 adults without OSA, 13,898 with OSA who used CPAP, and 20,884 adults with OSA who did not use CPAP. None of them had CV disease at baseline. Median follow-up was 262 days.
The primary outcome was first occurrence of myocardial infarction, stroke, unstable angina, heart failure, or death caused by CV disease.
In adjusted models, adults with moderate to severe OSA (apnea-hypopnea index ≥15) who did not use CPAP were 71% more likely than those without OSA to have a first CV event (hazard ratio, 1.71; 95% CI, 1.11-2.64). However, the risk for a CV event during follow-up was 32% lower among OSA patients with any CPAP use (HR, 0.68; 95% CI, 0.50-0.93; P = .016).
The effect was mostly driven by those who used CPAP for at least 4 hours per night (HR, 0.60; 95% CI, 0.39-0.95). This association was stronger for those with moderate to severe OSA (HR, 0.56; 95% CI, 0.39-0.81).
“This study highlights the importance of long-term management of CPAP therapy in patients with moderate-severe OSA,” Dr. Mazzotti said in an interview.
“It suggests that maintaining good CPAP adherence might be beneficial for cardiovascular health, besides the already established benefits on quality of life, sleepiness, and other cardiometabolic functions,” he said.
Dr. Mazzotti said several mechanisms might explain the association between CPAP use and lower risk for CV events. “CPAP treats OSA by preventing respiratory pauses that occur during sleep, therefore preventing arousals, sleep fragmentation, and decreases in blood oxygen. These improved cardiorespiratory functions can be beneficial to avoid certain molecular changes that are known to contribute to cardiovascular risk, such as oxidative stress and inflammation,” he explained.
“However, specific studies fully understanding these mechanisms are necessary,” Dr. Mazzotti added.
In a comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said that “the frequent decreases in oxygen levels and fragmented sleep from apnea are associated with cardiovascular disorders. We know this from multiple studies. This, however, was a large study and strengthens the association between improving apnea and reduced serious cardiovascular events.”
Funding for the study was provided by the American Academy of Sleep Medicine Foundation and the American Heart Association. Dr. Mazzotti and Dr. Verma disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
particularly for patients with moderate to severe OSA and those who are more adherent to CPAP therapy, a new study suggests.
“Most clinical trials on the effect of CPAP on CV diseases to date have focused on secondary CV prevention. This study contributes another piece of evidence about the role of CPAP therapy to prevent CV diseases,” said Diego R. Mazzotti, PhD, an assistant professor at the University of Kansas Medical Center, Kansas City.
“Our study, while observational, suggests that clinical trials focused on understanding how to sustain long-term CPAP adherence in obstructive sleep apnea patients are necessary and could be critical for optimizing comorbidity risk reduction,” Dr. Mazzotti said.
The study was presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Good adherence important
The researchers analyzed the electronic health records of adults referred for a sleep study through the Kaiser Permanente Southern California health system. The sample included 11,145 adults without OSA, 13,898 with OSA who used CPAP, and 20,884 adults with OSA who did not use CPAP. None of them had CV disease at baseline. Median follow-up was 262 days.
The primary outcome was first occurrence of myocardial infarction, stroke, unstable angina, heart failure, or death caused by CV disease.
In adjusted models, adults with moderate to severe OSA (apnea-hypopnea index ≥15) who did not use CPAP were 71% more likely than those without OSA to have a first CV event (hazard ratio, 1.71; 95% CI, 1.11-2.64). However, the risk for a CV event during follow-up was 32% lower among OSA patients with any CPAP use (HR, 0.68; 95% CI, 0.50-0.93; P = .016).
The effect was mostly driven by those who used CPAP for at least 4 hours per night (HR, 0.60; 95% CI, 0.39-0.95). This association was stronger for those with moderate to severe OSA (HR, 0.56; 95% CI, 0.39-0.81).
“This study highlights the importance of long-term management of CPAP therapy in patients with moderate-severe OSA,” Dr. Mazzotti said in an interview.
“It suggests that maintaining good CPAP adherence might be beneficial for cardiovascular health, besides the already established benefits on quality of life, sleepiness, and other cardiometabolic functions,” he said.
Dr. Mazzotti said several mechanisms might explain the association between CPAP use and lower risk for CV events. “CPAP treats OSA by preventing respiratory pauses that occur during sleep, therefore preventing arousals, sleep fragmentation, and decreases in blood oxygen. These improved cardiorespiratory functions can be beneficial to avoid certain molecular changes that are known to contribute to cardiovascular risk, such as oxidative stress and inflammation,” he explained.
“However, specific studies fully understanding these mechanisms are necessary,” Dr. Mazzotti added.
In a comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said that “the frequent decreases in oxygen levels and fragmented sleep from apnea are associated with cardiovascular disorders. We know this from multiple studies. This, however, was a large study and strengthens the association between improving apnea and reduced serious cardiovascular events.”
Funding for the study was provided by the American Academy of Sleep Medicine Foundation and the American Heart Association. Dr. Mazzotti and Dr. Verma disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2021





