User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


CHEST 2021 transitions from hybrid meeting to fully online
After an extensive review of the present and potential conditions affecting in-person participation, CHEST 2021 will be fully online again this year. Our goal is to make attending CHEST 2021 as accessible as possible for the entire chest medicine community. Make sure to join your colleagues online for the most exciting event in chest medicine, October 17-20.
After an extensive review of the present and potential conditions affecting in-person participation, CHEST 2021 will be fully online again this year. Our goal is to make attending CHEST 2021 as accessible as possible for the entire chest medicine community. Make sure to join your colleagues online for the most exciting event in chest medicine, October 17-20.
After an extensive review of the present and potential conditions affecting in-person participation, CHEST 2021 will be fully online again this year. Our goal is to make attending CHEST 2021 as accessible as possible for the entire chest medicine community. Make sure to join your colleagues online for the most exciting event in chest medicine, October 17-20.
Genetic link may tie cannabis use disorder to severe COVID-19
The same genetic variations may boost susceptibility to both severe COVID-19 and cannabis use disorder (CUD), a new study suggests. The research does not confirm a genetic link, but the lead author said the signs of an association are still “troubling.”
“Reducing cannabis use among heavy users may potentially provide protection against severe COVID-19 presentations,” Alexander S. Hatoum, PhD, a postdoctoral scholar at Washington University, St. Louis, said in an interview. “Outside of individual risk, these data also have important implications for policy regarding vaccination as well as treatment prioritization in an overly taxed medical system.”
The study was published in the journal Biological Psychiatry Global Open Science.
Dr. Hatoum and colleagues launched the study to gain insight into whether CUD might be a risk factor for severe COVID-19 presentations.
As defined by the DSM-5, people with CUD suffer from impairment or distress because of their cannabis use and meet at least 2 of 11 criteria over a 12-month period, such as cravings, cannabis tolerance, and withdrawal symptoms. According to a 2020 study that examined 2008-2016 data, 2.72% of children aged 12-17 showed signs of CUD, as did 1.23% of those aged over 26.
The primary reasons for hospitalization and death related to COVID-19 are respiratory symptoms. “And we have observed that genetic vulnerability to CUD is shared with respiratory disease, even after tobacco use is considered,” Dr. Hatoum said.
He and his colleagues examined data from genomewide association studies and searched for genetic correlations between CUD (14,080 cases, 343,726 controls) and COVID-19 hospitalization (9,373 cases, 1,197,256 controls). “Genetic vulnerability to COVID-19 was correlated with genetic liability to CUD (P = 1.33e–6),” the researchers wrote. “This association remained when accounting for genetic liability to related risk factors and covariates (P = .012-.049).”
According to Dr. Hatoum, the researchers found inconclusive evidence that CUD might worsen COVID-19 cases. “We applied statistical causal models, which found an effect consistent with causality, but it was nonsignificant,” he said.
Despite the absence of causality, the study findings could prove useful for clinicians and policy makers.
“Those struggling with CUD may be prioritized for vaccination and vaccination boosters to mitigate their higher likelihood of a severe COVID-19 presentation,” Dr. Hatoum said. “When testing positive for COVID-19, they may also be prioritized for earlier treatment.”
The study authors also added that the findings “urge caution” in regard to the wave of U.S. states legalizing cannabis. “Our data suggest that heavy cannabis use, but not lifetime cannabis use, represents a risk factor for severe COVID-19 presentations,” Dr. Hatoum said.
In an interview, Danielle Dick, PhD, who was not involved with the study, said it applies “cutting-edge methods to an important research question” and offers a “hint” of a genetic risk factor that makes some people more likely to be hospitalized for COVID-19. However, “the study does not tell us what those underlying genetically influenced processes might be,” added Dr. Dick, professor of psychology, and human and molecular genetics at Virginia Commonwealth University, Richmond. “And it’s an important caveat to point out that the results from this study are limited in that they are based on data from people from European descent – so they can’t necessarily be applied to address the harm experienced by so many people of color from the COVID pandemic. That’s an unfortunate limitation.”
As for the idea that the study findings should prompt caution about marijuana legalization, Dr. Dick said it’s true that increased acceptability of drug use “increases the likelihood that individuals who are genetically vulnerable will develop problems. There is robust evidence of this.”
However, Dr. Dick said, “the legalization of marijuana is a complex topic because the health consequences aren’t the only consideration when it comes to legalization. The other side of the coin is the huge harm that has been caused to communities of color through marijuana criminalization. Legalization will hopefully lead to decreased harm on that front. So it’s a double-edged sword.”
Dr. Hatoum, his colleagues, and Dr. Dick reported no relevant disclosures.
The same genetic variations may boost susceptibility to both severe COVID-19 and cannabis use disorder (CUD), a new study suggests. The research does not confirm a genetic link, but the lead author said the signs of an association are still “troubling.”
“Reducing cannabis use among heavy users may potentially provide protection against severe COVID-19 presentations,” Alexander S. Hatoum, PhD, a postdoctoral scholar at Washington University, St. Louis, said in an interview. “Outside of individual risk, these data also have important implications for policy regarding vaccination as well as treatment prioritization in an overly taxed medical system.”
The study was published in the journal Biological Psychiatry Global Open Science.
Dr. Hatoum and colleagues launched the study to gain insight into whether CUD might be a risk factor for severe COVID-19 presentations.
As defined by the DSM-5, people with CUD suffer from impairment or distress because of their cannabis use and meet at least 2 of 11 criteria over a 12-month period, such as cravings, cannabis tolerance, and withdrawal symptoms. According to a 2020 study that examined 2008-2016 data, 2.72% of children aged 12-17 showed signs of CUD, as did 1.23% of those aged over 26.
The primary reasons for hospitalization and death related to COVID-19 are respiratory symptoms. “And we have observed that genetic vulnerability to CUD is shared with respiratory disease, even after tobacco use is considered,” Dr. Hatoum said.
He and his colleagues examined data from genomewide association studies and searched for genetic correlations between CUD (14,080 cases, 343,726 controls) and COVID-19 hospitalization (9,373 cases, 1,197,256 controls). “Genetic vulnerability to COVID-19 was correlated with genetic liability to CUD (P = 1.33e–6),” the researchers wrote. “This association remained when accounting for genetic liability to related risk factors and covariates (P = .012-.049).”
According to Dr. Hatoum, the researchers found inconclusive evidence that CUD might worsen COVID-19 cases. “We applied statistical causal models, which found an effect consistent with causality, but it was nonsignificant,” he said.
Despite the absence of causality, the study findings could prove useful for clinicians and policy makers.
“Those struggling with CUD may be prioritized for vaccination and vaccination boosters to mitigate their higher likelihood of a severe COVID-19 presentation,” Dr. Hatoum said. “When testing positive for COVID-19, they may also be prioritized for earlier treatment.”
The study authors also added that the findings “urge caution” in regard to the wave of U.S. states legalizing cannabis. “Our data suggest that heavy cannabis use, but not lifetime cannabis use, represents a risk factor for severe COVID-19 presentations,” Dr. Hatoum said.
In an interview, Danielle Dick, PhD, who was not involved with the study, said it applies “cutting-edge methods to an important research question” and offers a “hint” of a genetic risk factor that makes some people more likely to be hospitalized for COVID-19. However, “the study does not tell us what those underlying genetically influenced processes might be,” added Dr. Dick, professor of psychology, and human and molecular genetics at Virginia Commonwealth University, Richmond. “And it’s an important caveat to point out that the results from this study are limited in that they are based on data from people from European descent – so they can’t necessarily be applied to address the harm experienced by so many people of color from the COVID pandemic. That’s an unfortunate limitation.”
As for the idea that the study findings should prompt caution about marijuana legalization, Dr. Dick said it’s true that increased acceptability of drug use “increases the likelihood that individuals who are genetically vulnerable will develop problems. There is robust evidence of this.”
However, Dr. Dick said, “the legalization of marijuana is a complex topic because the health consequences aren’t the only consideration when it comes to legalization. The other side of the coin is the huge harm that has been caused to communities of color through marijuana criminalization. Legalization will hopefully lead to decreased harm on that front. So it’s a double-edged sword.”
Dr. Hatoum, his colleagues, and Dr. Dick reported no relevant disclosures.
The same genetic variations may boost susceptibility to both severe COVID-19 and cannabis use disorder (CUD), a new study suggests. The research does not confirm a genetic link, but the lead author said the signs of an association are still “troubling.”
“Reducing cannabis use among heavy users may potentially provide protection against severe COVID-19 presentations,” Alexander S. Hatoum, PhD, a postdoctoral scholar at Washington University, St. Louis, said in an interview. “Outside of individual risk, these data also have important implications for policy regarding vaccination as well as treatment prioritization in an overly taxed medical system.”
The study was published in the journal Biological Psychiatry Global Open Science.
Dr. Hatoum and colleagues launched the study to gain insight into whether CUD might be a risk factor for severe COVID-19 presentations.
As defined by the DSM-5, people with CUD suffer from impairment or distress because of their cannabis use and meet at least 2 of 11 criteria over a 12-month period, such as cravings, cannabis tolerance, and withdrawal symptoms. According to a 2020 study that examined 2008-2016 data, 2.72% of children aged 12-17 showed signs of CUD, as did 1.23% of those aged over 26.
The primary reasons for hospitalization and death related to COVID-19 are respiratory symptoms. “And we have observed that genetic vulnerability to CUD is shared with respiratory disease, even after tobacco use is considered,” Dr. Hatoum said.
He and his colleagues examined data from genomewide association studies and searched for genetic correlations between CUD (14,080 cases, 343,726 controls) and COVID-19 hospitalization (9,373 cases, 1,197,256 controls). “Genetic vulnerability to COVID-19 was correlated with genetic liability to CUD (P = 1.33e–6),” the researchers wrote. “This association remained when accounting for genetic liability to related risk factors and covariates (P = .012-.049).”
According to Dr. Hatoum, the researchers found inconclusive evidence that CUD might worsen COVID-19 cases. “We applied statistical causal models, which found an effect consistent with causality, but it was nonsignificant,” he said.
Despite the absence of causality, the study findings could prove useful for clinicians and policy makers.
“Those struggling with CUD may be prioritized for vaccination and vaccination boosters to mitigate their higher likelihood of a severe COVID-19 presentation,” Dr. Hatoum said. “When testing positive for COVID-19, they may also be prioritized for earlier treatment.”
The study authors also added that the findings “urge caution” in regard to the wave of U.S. states legalizing cannabis. “Our data suggest that heavy cannabis use, but not lifetime cannabis use, represents a risk factor for severe COVID-19 presentations,” Dr. Hatoum said.
In an interview, Danielle Dick, PhD, who was not involved with the study, said it applies “cutting-edge methods to an important research question” and offers a “hint” of a genetic risk factor that makes some people more likely to be hospitalized for COVID-19. However, “the study does not tell us what those underlying genetically influenced processes might be,” added Dr. Dick, professor of psychology, and human and molecular genetics at Virginia Commonwealth University, Richmond. “And it’s an important caveat to point out that the results from this study are limited in that they are based on data from people from European descent – so they can’t necessarily be applied to address the harm experienced by so many people of color from the COVID pandemic. That’s an unfortunate limitation.”
As for the idea that the study findings should prompt caution about marijuana legalization, Dr. Dick said it’s true that increased acceptability of drug use “increases the likelihood that individuals who are genetically vulnerable will develop problems. There is robust evidence of this.”
However, Dr. Dick said, “the legalization of marijuana is a complex topic because the health consequences aren’t the only consideration when it comes to legalization. The other side of the coin is the huge harm that has been caused to communities of color through marijuana criminalization. Legalization will hopefully lead to decreased harm on that front. So it’s a double-edged sword.”
Dr. Hatoum, his colleagues, and Dr. Dick reported no relevant disclosures.
FROM BIOLOGICAL PSYCHIATRY GLOBAL OPEN SCIENCE
Peanut allergy patients reap continuing benefits past first year, Palforzia study shows
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A recent analysis of 142 peanut-allergic children treated for 1.5 to 2 years with a licensed oral immunotherapy (OIT) product confirms what various smaller studies have shown: Maintaining treatment for longer periods improves protection and reduces adverse effects. The findings offer some reassurance regarding the controversial approach, which has become available at a small number of clinics yet faces an uncertain future.
The new study, published July 28 in Allergy, included a subset of patients who chose to complete an extension of the phase 3 PALISADE trial of Palforzia, a proprietary set of premeasured peanut flour capsules developed by Aimmune Therapeutics.
Palforzia was approved last year for children aged 4 to 17 years with peanut allergy – one of the most common food allergies, affecting around 2% of children in the United States and Europe. The treatment is not a cure – patients must still watch what they eat and carry epinephrine for emergency reactions – but it helps build protection through daily ingestion of gradually increasing amounts of the allergen over a period of months.
In the 1-year PALISADE trial, which enrolled 496 peanut-allergic children at 66 sites in North America and Europe, participants received daily doses of study drug or placebo. The dose of the drug was escalated from 3 mg to 300 mg over 6 months; the 300-mg dose was then maintained for another 6 months. By the end of the study, about two-thirds of the children who underwent treatment could safely consume at least 600 mg of peanut protein, about the equivalent of two peanuts.
Could protection be increased with further treatment, and what would be required to sustain it? To address these questions, PALISADE patients who successfully reached the 600-mg threshold, along with those from the placebo group, were invited to participate in Aimmune’s open-label follow-on study. The extension study also explored whether protection could be maintained with less frequent dosing.
Among the 358 eligible participants who opted into the 1-year extension study, 256 came from the PALISADE treatment arm. These children were assigned to five cohorts to continue for 6 months or 12 months with daily or less frequent doses. Within the 6-month group, all started with the 300-mg daily dose. A subset received two doses a week. Within the 12-month group, some patients maintained daily dosing throughout; others received doses every other day, twice weekly, or once every 2 weeks.
The children who continued daily maintenance dosing the longest gained the most protection. Those in less-frequent dosing groups experienced more adverse events than those who received doses every day, the company reported last December in The Journal of Allergy and Clinical Immunology: In Practice.
More than a quarter (97 of 358, or 27.1%) of participants failed to complete the extension. Families could withdraw any time for any reason. Participating in an OIT trial is demanding – it requires office visits for dosing adjustments and blood tests, rest periods, keeping symptom logs in which daily doses are recorded, and possible allergic reactions from the treatment itself. “A common reason for ‘withdrawal of consent’ in clinical studies is the inconvenience of remaining in a long-term study,” Mohamed Yassine, MD, Aimmune’s senior vice present of medical affairs, said via email.
Attrition was concentrated within certain subgroups. Most participants in (88.7%; 102 of 115) PALISADE who received placebo elected to enter the open-label extension; nearly half did not finish. Dropout rates were also high (29.2%) for non-daily dosing participants who had come from the PALISADE treatment arm.
The authors did not report on those high-dropout groups. Instead, they focused their analysis on the 142 treated PALISADE participants who continued daily dosing through the extension – 110 patients for a total of about 1.5 years and 32 patients for about 2 years. In a subgroup analysis, 48.1% of children in the 1.5-year group upped their tolerance to 2,000 mg peanut protein, and even more (80.8%) in the 2-year group reached that threshold – all while taking a 300-mg maintenance dose.
Those who remained on treatment longer also had fewer adverse events. At the exit food challenge, 24% of the 1.5-year participants had reactions that required epinephrine, but among 2-year participants, only 3.8% needed the rescue medication.
Continuing therapy past the first year seemed to have additional benefits, Sandra Hong, MD, director of the Cleveland Clinic Food Allergy Center of Excellence, said in an interview. Dr. Hong was not involved in the new research and has no financial ties with Aimmune or other food allergy companies. “Not only can you ingest more, but your reaction when you do react is going to be less,” she says.
Palforzia is only available through a risk evaluation and mitigation strategy (REMS) program, which educates patients, health care professionals, and pharmacies about immunotherapy risks and precautionary measures. As of last summer, before Aimmune was acquired by Nestlé Health Science, about 100 allergists in the United States had enrolled patients in the REMS program. Families can find allergists who are certified to prescribe Palforzia using the website’s Certified Participant Locator.
Although the field at large remains apprehensive about OIT and other forms of immunotherapy, an estimated 200 or more U.S. clinics are administering home-grown OIT using commercial food products, says Richard Wasserman, an OIT pioneer whose clinic in Dallas has treated allergies to about 20 foods since the practice started offering the therapy in 2008. OIT practitioners have treated more than 15,000 food allergy patients nationwide, Dr. Wasserman said via email, yet they make up just a tiny fraction of the more than 6,000 board-certified allergists in the United States.
Whether using Palforzia or nonproprietary food products, oral immunotherapy requires a lot of time and effort – not just for patients but also practitioners. “You need more space. You need more staffing. Patients doing oral challenges stay in your office for 4 to 5 hours, and we have one-to-one nursing care for them,” said Dr. Hong. “So it’s a lot of resources.”
Her team has treated about 20 children with Palforzia since the Cleveland Clinic began offering the therapy last summer. Dr. Hong and coworkers have administered OIT using commercial peanut flour and peanut butter to some 80 peanut-allergic toddlers younger than 4 years who are too young to receive for the U.S. Food and Drug Administration–approved treatment. Their early data, which were presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology in February, suggest that toddlers get complete OIT more quickly with fewer side effects than older children, Dr. Hong says. A recent study of preschoolers in Canada also found that nonproprietary OIT is very safe and effective in this younger set and could be cost-saving in the long run.
By comparison, Palforzia, which has a list price of $890 per month, was judged to be less cost-effective in analyses by academic allergists and by the Institute for Clinical and Economic Review. But through a copay savings program, depending on their insurance coverage, some eligible families can pay as little as $20 per month for the FDA-approved treatment.
Because the therapy is time consuming for families and is resource intensive for practices, questions remain as to how long and how frequently patients need to remain on treatment to sustain protection. Do they need to keep taking Palforzia, or “can we switch them to an equivalent amount of food and not bother with the study drug?” said Edwin Kim, director of the UNC Food Allergy Initiative, Chapel Hill, North Carolina, and study investigator for several Palforzia trials, in an interview.
The Food Allergy Support Team, a nonprofit group started by Dr. Wasserman and colleagues, publishes best practices and meets annually to discuss research and protocols. However, the best maintenance dose, the best dosing frequency, and the duration of daily dosing that yields the best outcomes are not known, Dr. Wasserman says.
“We think the best way to answer that question is with a regulated, pharmaceutical-grade form of peanut protein,” Dr. Yassine said.
The field’s experience with Palforzia raises a dilemma: Does its approval legitimize oral immunotherapy in general, or will rigorous, multi-million dollar trials be needed to approve products for each food or combination of foods? About 32 million people in the United States have food allergies – about 1 in 10 adults and 1 in 13 children.
“I think the field has always grappled with that, honestly,” said Stacie Jones, MD, professor of pediatrics and chief of allergy and immunology at the University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, in an interview. Home-grown OIT is “easier to do when you have high control of your small patient volumes or you’re in a clinical trial,” said Dr. Jones, who has served as an investigator on Palforzia trials and last year received more than $30,000 in consulting fees from Aimmune. “It becomes a very different situation when it becomes a national or an international recommended therapy.”
The Canadian Society of Allergy and Clinical Immunology has published clinical practice guidelines and provides practical information on its website on how to implement OIT – including protocols for dozens of foods and diary sheets for patients to log doses and symptoms.
However, U.S. professional societies still consider OIT investigational and suggest that it will not be approved by the FDA. “As a field, are we willing to wait 4 to 5 more years for an egg product? Should we? Are we willing?” said Dr. Kim. “These are tough questions.”
Stacie M. Jones reports advisory board fees, Aimmune Therapeutics, FARE; personal fees, DBV Technologies; clinical trials grants, Aimmune Therapeutics, DBV Technologies, Astellas, Sanofi, Regeneron, FARE, Genentech, and NIH-NIAID. Edwin Kim reports consultancy with Aimmune Therapeutics, Allako, AllerGenis, Belhaven Pharma, DBV Technologies, Duke Clinical Research Institute, and Nutricia; advisory board membership with ALK, DBV Technologies, Kenota Health, and Ukko; grant support from the NIH’s National Institute of Allergy and Infectious Diseases, National Center for Complementary and Integrative Health and Immune Tolerance Network; Food Allergy Research and Education, and the Wallace Research Foundation. Richard Wasserman receives consulting fees from Aimmune Therapeutics and DBV Technologies. Mohamed Yassine is employed by Aimmune Therapeutics. Sandra Hong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One-third in U.S. had been infected by SARS-CoV-2 through 2020: Study
, according to a modeling study published Aug. 26 online in Nature.
Jeffrey Shaman, PhD, professor in the department of environmental health sciences and director of the climate and health program at the Columbia University Mailman School of Public Health, New York City, and colleagues developed a model to simulate how SARS-CoV-2 was transmitted within and between all 3,142 counties in the United States.
In their model, the researchers considered migration data between counties, the observed case numbers, and estimates of infections based on the number of people who test positive for SARS-CoV-2 antibodies.
The United States had the highest number of confirmed COVID-19 cases and deaths in the world during 2020. More than 19.6 million cases were reported by the end of the year.
But the authors point out that “69% of the population remained susceptible to viral infection.”
The researchers also studied the ascertainment rate, or the ratio of detected cases to the number of confirmed cases. Nationally, that value increased from 11.3% in March 2020 to 24.5% in December 2020.
That’s one of the biggest pandemic lessons from the data, Dr. Shaman said: “It is vitally important when there is an outbreak and you’re counting cases that there are many more people infected in your community who are contagious than reported cases. Each individual is infectious for multiple days, and there are many more unreported cases.”
That applies now with the Delta variant, he said.
“Vaccinated people who get infected with the Delta variant are part of the transmission chain,” he said.
Fatality rates dropped
Some of the data were very positive, Dr. Shaman told this news organization. The infection fatality rate fell from 0.77% in April to 0.31% in December. The authors suggest that that may be because of improvements in diagnosis and treatment, patient care, and reduced disease severity.
However, the fatality rate was still nearly four times as high as the estimated fatality rate for seasonal influenza (0.08%) and the 2009 influenza pandemic (0.0076%), the authors point out.
Joe K. Gerald, MD, PhD, associate professor and program director of public health policy and management at the University of Arizona, Tucson, told this news organization that this article helps confirm that COVID is much deadlier than the flu and that the intensity of the response has been appropriate.
“We should be willing to invest a lot more in mitigating COVID-19 than seasonal influenza because it has much greater consequences,” he said.
The numbers emphasize that testing must improve.
“We didn’t have enough tests available, and they weren’t easily accessible. For much of the year we were flying in the dark,” Dr. Gerald said.
The number of tests has increased this year, he acknowledged, but testing still lags. “We just can’t miss this many infections or diagnoses and hope to gain control,” he said.
The study also points out the huge variation by state and by county in infections and deaths, and that variation continues. Gerald noted that the varied numbers make it difficult for some regions to accept broader mandates, because the threat from COVID-19 appears very different where they are.
“We have to think about regions, how many people are susceptible, and what the testing capacity is,” he said. “States and even counties should have some leeway to make some important public health decisions, because local conditions are going to differ at different points in time.”
‘We have not turned the corner’
Jill Foster, MD, a pediatric infectious disease physician at the University of Minnesota Medical School, Minneapolis, said in an interview that the study adds evidence: “We have not turned the corner on COVID-19 and are nowhere near herd immunity – if it exists for SARS-CoV-2.”
She said the numbers presented are particularly concerning in regard to how many people were susceptible and were actively able to infect others: “Much higher than most people imagined and very much higher than their comparison, influenza.
“There are still more people susceptible than we had believed,” Dr. Foster added. “If the pattern continues where the Delta variant infects a significant portion of those vaccinated, the number of people susceptible rises even higher than was predicted.”
She said that it is reassuring that the analysis shows a decrease in case fatality and said the finding supports the common opinion that medicine is better able to fight the disease.
“However,” she said, “the optimism is tempered by acknowledging that in order to benefit from these advances, we must not overwhelm the facilities where patients are cared for so that optimal care can be delivered.”
Dr. Foster said these numbers represent a warning that COVID should be treated as a continuing threat.
“We need to acknowledge that there is COVID-19 infection simmering and periodically erupting throughout the country,” she said. “It is not monolithic and varies by geography and seasons in ways that are difficult to predict other than at any given time there is likely more infection present than we are identifying and more people susceptible to infection than we have calculated.”
The authors and Dr. Gerald have disclosed no relevant financial relationships. Dr. Foster has received clinical trials funding from Moderna.
A version of this article first appeared on Medscape.com.
, according to a modeling study published Aug. 26 online in Nature.
Jeffrey Shaman, PhD, professor in the department of environmental health sciences and director of the climate and health program at the Columbia University Mailman School of Public Health, New York City, and colleagues developed a model to simulate how SARS-CoV-2 was transmitted within and between all 3,142 counties in the United States.
In their model, the researchers considered migration data between counties, the observed case numbers, and estimates of infections based on the number of people who test positive for SARS-CoV-2 antibodies.
The United States had the highest number of confirmed COVID-19 cases and deaths in the world during 2020. More than 19.6 million cases were reported by the end of the year.
But the authors point out that “69% of the population remained susceptible to viral infection.”
The researchers also studied the ascertainment rate, or the ratio of detected cases to the number of confirmed cases. Nationally, that value increased from 11.3% in March 2020 to 24.5% in December 2020.
That’s one of the biggest pandemic lessons from the data, Dr. Shaman said: “It is vitally important when there is an outbreak and you’re counting cases that there are many more people infected in your community who are contagious than reported cases. Each individual is infectious for multiple days, and there are many more unreported cases.”
That applies now with the Delta variant, he said.
“Vaccinated people who get infected with the Delta variant are part of the transmission chain,” he said.
Fatality rates dropped
Some of the data were very positive, Dr. Shaman told this news organization. The infection fatality rate fell from 0.77% in April to 0.31% in December. The authors suggest that that may be because of improvements in diagnosis and treatment, patient care, and reduced disease severity.
However, the fatality rate was still nearly four times as high as the estimated fatality rate for seasonal influenza (0.08%) and the 2009 influenza pandemic (0.0076%), the authors point out.
Joe K. Gerald, MD, PhD, associate professor and program director of public health policy and management at the University of Arizona, Tucson, told this news organization that this article helps confirm that COVID is much deadlier than the flu and that the intensity of the response has been appropriate.
“We should be willing to invest a lot more in mitigating COVID-19 than seasonal influenza because it has much greater consequences,” he said.
The numbers emphasize that testing must improve.
“We didn’t have enough tests available, and they weren’t easily accessible. For much of the year we were flying in the dark,” Dr. Gerald said.
The number of tests has increased this year, he acknowledged, but testing still lags. “We just can’t miss this many infections or diagnoses and hope to gain control,” he said.
The study also points out the huge variation by state and by county in infections and deaths, and that variation continues. Gerald noted that the varied numbers make it difficult for some regions to accept broader mandates, because the threat from COVID-19 appears very different where they are.
“We have to think about regions, how many people are susceptible, and what the testing capacity is,” he said. “States and even counties should have some leeway to make some important public health decisions, because local conditions are going to differ at different points in time.”
‘We have not turned the corner’
Jill Foster, MD, a pediatric infectious disease physician at the University of Minnesota Medical School, Minneapolis, said in an interview that the study adds evidence: “We have not turned the corner on COVID-19 and are nowhere near herd immunity – if it exists for SARS-CoV-2.”
She said the numbers presented are particularly concerning in regard to how many people were susceptible and were actively able to infect others: “Much higher than most people imagined and very much higher than their comparison, influenza.
“There are still more people susceptible than we had believed,” Dr. Foster added. “If the pattern continues where the Delta variant infects a significant portion of those vaccinated, the number of people susceptible rises even higher than was predicted.”
She said that it is reassuring that the analysis shows a decrease in case fatality and said the finding supports the common opinion that medicine is better able to fight the disease.
“However,” she said, “the optimism is tempered by acknowledging that in order to benefit from these advances, we must not overwhelm the facilities where patients are cared for so that optimal care can be delivered.”
Dr. Foster said these numbers represent a warning that COVID should be treated as a continuing threat.
“We need to acknowledge that there is COVID-19 infection simmering and periodically erupting throughout the country,” she said. “It is not monolithic and varies by geography and seasons in ways that are difficult to predict other than at any given time there is likely more infection present than we are identifying and more people susceptible to infection than we have calculated.”
The authors and Dr. Gerald have disclosed no relevant financial relationships. Dr. Foster has received clinical trials funding from Moderna.
A version of this article first appeared on Medscape.com.
, according to a modeling study published Aug. 26 online in Nature.
Jeffrey Shaman, PhD, professor in the department of environmental health sciences and director of the climate and health program at the Columbia University Mailman School of Public Health, New York City, and colleagues developed a model to simulate how SARS-CoV-2 was transmitted within and between all 3,142 counties in the United States.
In their model, the researchers considered migration data between counties, the observed case numbers, and estimates of infections based on the number of people who test positive for SARS-CoV-2 antibodies.
The United States had the highest number of confirmed COVID-19 cases and deaths in the world during 2020. More than 19.6 million cases were reported by the end of the year.
But the authors point out that “69% of the population remained susceptible to viral infection.”
The researchers also studied the ascertainment rate, or the ratio of detected cases to the number of confirmed cases. Nationally, that value increased from 11.3% in March 2020 to 24.5% in December 2020.
That’s one of the biggest pandemic lessons from the data, Dr. Shaman said: “It is vitally important when there is an outbreak and you’re counting cases that there are many more people infected in your community who are contagious than reported cases. Each individual is infectious for multiple days, and there are many more unreported cases.”
That applies now with the Delta variant, he said.
“Vaccinated people who get infected with the Delta variant are part of the transmission chain,” he said.
Fatality rates dropped
Some of the data were very positive, Dr. Shaman told this news organization. The infection fatality rate fell from 0.77% in April to 0.31% in December. The authors suggest that that may be because of improvements in diagnosis and treatment, patient care, and reduced disease severity.
However, the fatality rate was still nearly four times as high as the estimated fatality rate for seasonal influenza (0.08%) and the 2009 influenza pandemic (0.0076%), the authors point out.
Joe K. Gerald, MD, PhD, associate professor and program director of public health policy and management at the University of Arizona, Tucson, told this news organization that this article helps confirm that COVID is much deadlier than the flu and that the intensity of the response has been appropriate.
“We should be willing to invest a lot more in mitigating COVID-19 than seasonal influenza because it has much greater consequences,” he said.
The numbers emphasize that testing must improve.
“We didn’t have enough tests available, and they weren’t easily accessible. For much of the year we were flying in the dark,” Dr. Gerald said.
The number of tests has increased this year, he acknowledged, but testing still lags. “We just can’t miss this many infections or diagnoses and hope to gain control,” he said.
The study also points out the huge variation by state and by county in infections and deaths, and that variation continues. Gerald noted that the varied numbers make it difficult for some regions to accept broader mandates, because the threat from COVID-19 appears very different where they are.
“We have to think about regions, how many people are susceptible, and what the testing capacity is,” he said. “States and even counties should have some leeway to make some important public health decisions, because local conditions are going to differ at different points in time.”
‘We have not turned the corner’
Jill Foster, MD, a pediatric infectious disease physician at the University of Minnesota Medical School, Minneapolis, said in an interview that the study adds evidence: “We have not turned the corner on COVID-19 and are nowhere near herd immunity – if it exists for SARS-CoV-2.”
She said the numbers presented are particularly concerning in regard to how many people were susceptible and were actively able to infect others: “Much higher than most people imagined and very much higher than their comparison, influenza.
“There are still more people susceptible than we had believed,” Dr. Foster added. “If the pattern continues where the Delta variant infects a significant portion of those vaccinated, the number of people susceptible rises even higher than was predicted.”
She said that it is reassuring that the analysis shows a decrease in case fatality and said the finding supports the common opinion that medicine is better able to fight the disease.
“However,” she said, “the optimism is tempered by acknowledging that in order to benefit from these advances, we must not overwhelm the facilities where patients are cared for so that optimal care can be delivered.”
Dr. Foster said these numbers represent a warning that COVID should be treated as a continuing threat.
“We need to acknowledge that there is COVID-19 infection simmering and periodically erupting throughout the country,” she said. “It is not monolithic and varies by geography and seasons in ways that are difficult to predict other than at any given time there is likely more infection present than we are identifying and more people susceptible to infection than we have calculated.”
The authors and Dr. Gerald have disclosed no relevant financial relationships. Dr. Foster has received clinical trials funding from Moderna.
A version of this article first appeared on Medscape.com.
Two patients with metastatic lung cancer are disease-free for 1.5 years after TILs
Adoptive cell therapy using tumor-infiltrating lymphocytes (TILs) led to complete and long-lasting responses in 2 of 16 patients with metastatic non-small cell lung cancer (NSCLC) who fully underwent the therapy in a phase 1 study.
The results are “very impressive, with two complete responses that are ongoing 1.5 years later, so it’s durable, and that’s encouraging,” Fred Hirsch, MD, PhD, director of the Center of Excellence for Thoracic Oncology at Mount Sinai’s Tisch Cancer Institute, New York, told this news organization.
Dr. Hirsch, who wasn’t involved in the research, also noted that this study is in immunotherapy-resistant metastatic lung cancer – “a situation where we actually don’t know exactly how best to treat it today.”
The study was published online in Nature Medicine.
A form of immunotherapy, TILs have been studied extensively in melanoma, as reported by this news organization, but this is the first test of the TIL therapy in metastatic NSCLC.
The single-arm, open-label phase 1 trial involved 20 patients who had TILs collected, including 16 who eventually received TILs. Median age was 54 years; all patients had metastatic NSCLC and disease progression after nivolumab monotherapy.
TILs cultured from an individual patient’s tumor were expanded ex vivo from minced tumors cultured with interleukin-2 (IL-2). Via this method, “billions of activated T cells can be produced and infused back into a patient,” explain the authors.
The full treatment regimen comprised cyclophosphamide and fludarabine lymphodepletion, TIL infusion and IL-2, followed by maintenance nivolumab.
“We found that infusion of TILs in combination with lymphodepletion and IL-2 had manageable toxicity and mediated tumor regressions in several patients, including complete responses,” report Benjamin Creelan, MD, of the Moffitt Cancer Center & Research Institute, Tampa, and colleagues.
The endpoint of safety was met according to the prespecified criteria of a rate of severe toxicity of 17% or less.
Among 13 evaluable patients, three had confirmed responses and 11 had reduction in tumor burden. Two patients achieved complete responses that were ongoing 1.5 years following TIL treatment.
One durable complete response occurred in a PD-L1-negative never-smoker, who had a low tumor mutation burden and who was refractory to nivolumab.
“This may be particularly encouraging for the large subset of never-smoker patients, for whom immune-checkpoint inhibitors have historically had limited efficacy,” the investigators say.
This complete responder had “features where you wouldn’t expect to see a response for immunotherapy,” Dr. Hirsch told this news organization.
“Low tumor mutation burden, negative PDL-1, and never-smoker are three factors which indicate some kind of resistance to immunotherapy, and despite that, there was a complete response with this specific therapy. That is fascinating,” he said.
In exploratory analyses, T cells recognizing multiple types of cancer mutations were detected after TIL treatment and were enriched in patients who responded to treatment.
The researchers say these early data indicate that TILs can mediate effective responses in tumor subtypes that are not sensitive to traditional immune-checkpoint-targeted therapy.
“Therefore, therapy with TILs may extend the scope and impact of immunotherapy into wider populations,” they write.
‘Yeoman’s effort’ paving the way forward
Also weighing in on the study, Philip Greenberg, MD, professor and head of immunology, Fred Hutchinson Cancer Center, Seattle, said, “In some respects, it’s quite promising and in other respects, actually more limited than you would hope for.”
“I think it’s a great demonstration that there is activity here, and there’s a world of things that can be done to improve the activity and no doubt that will be done. After this trial, I’m sure we will see next-generation trials,” said Dr. Greenberg.
He said key issues going forward are how cells are selected and manufactured: “That’s going to be a critical piece for making it better.”
“There is now a world of data that says T cells that recognize mutations in cancers can be effective in solid tumors,” Dr. Greenberg said.
“Sustaining that response is still a huge obstacle for achieving the kinds of therapeutic benefits we’d like to be achieved. And having that response be broad enough, particularly in the setting where most of the mutations are just passenger mutations, not driver oncogenes, is going to require a way of generating a large polyspecific population of cells that can persist for a long time,” he further commented.
All in all, this study was a “yeoman’s effort” and the researchers “deserve a lot of credit for pushing it forward,” Dr. Greenberg said.
The study was supported in part by grants from Stand Up to Cancer Foundation, the Barbara Bauer Prelude to a Cure Foundation, Iovance Biotherapeutics, and a Young Investigator award from Adaptive Biotechnologies. Nivolumab was supplied by Bristol-Myers Squibb. Aldesleukin (IL-2) was supplied by Clinigen Group. A complete list of author disclosures is available with the original article. Dr. Hirsch and Dr. Greenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adoptive cell therapy using tumor-infiltrating lymphocytes (TILs) led to complete and long-lasting responses in 2 of 16 patients with metastatic non-small cell lung cancer (NSCLC) who fully underwent the therapy in a phase 1 study.
The results are “very impressive, with two complete responses that are ongoing 1.5 years later, so it’s durable, and that’s encouraging,” Fred Hirsch, MD, PhD, director of the Center of Excellence for Thoracic Oncology at Mount Sinai’s Tisch Cancer Institute, New York, told this news organization.
Dr. Hirsch, who wasn’t involved in the research, also noted that this study is in immunotherapy-resistant metastatic lung cancer – “a situation where we actually don’t know exactly how best to treat it today.”
The study was published online in Nature Medicine.
A form of immunotherapy, TILs have been studied extensively in melanoma, as reported by this news organization, but this is the first test of the TIL therapy in metastatic NSCLC.
The single-arm, open-label phase 1 trial involved 20 patients who had TILs collected, including 16 who eventually received TILs. Median age was 54 years; all patients had metastatic NSCLC and disease progression after nivolumab monotherapy.
TILs cultured from an individual patient’s tumor were expanded ex vivo from minced tumors cultured with interleukin-2 (IL-2). Via this method, “billions of activated T cells can be produced and infused back into a patient,” explain the authors.
The full treatment regimen comprised cyclophosphamide and fludarabine lymphodepletion, TIL infusion and IL-2, followed by maintenance nivolumab.
“We found that infusion of TILs in combination with lymphodepletion and IL-2 had manageable toxicity and mediated tumor regressions in several patients, including complete responses,” report Benjamin Creelan, MD, of the Moffitt Cancer Center & Research Institute, Tampa, and colleagues.
The endpoint of safety was met according to the prespecified criteria of a rate of severe toxicity of 17% or less.
Among 13 evaluable patients, three had confirmed responses and 11 had reduction in tumor burden. Two patients achieved complete responses that were ongoing 1.5 years following TIL treatment.
One durable complete response occurred in a PD-L1-negative never-smoker, who had a low tumor mutation burden and who was refractory to nivolumab.
“This may be particularly encouraging for the large subset of never-smoker patients, for whom immune-checkpoint inhibitors have historically had limited efficacy,” the investigators say.
This complete responder had “features where you wouldn’t expect to see a response for immunotherapy,” Dr. Hirsch told this news organization.
“Low tumor mutation burden, negative PDL-1, and never-smoker are three factors which indicate some kind of resistance to immunotherapy, and despite that, there was a complete response with this specific therapy. That is fascinating,” he said.
In exploratory analyses, T cells recognizing multiple types of cancer mutations were detected after TIL treatment and were enriched in patients who responded to treatment.
The researchers say these early data indicate that TILs can mediate effective responses in tumor subtypes that are not sensitive to traditional immune-checkpoint-targeted therapy.
“Therefore, therapy with TILs may extend the scope and impact of immunotherapy into wider populations,” they write.
‘Yeoman’s effort’ paving the way forward
Also weighing in on the study, Philip Greenberg, MD, professor and head of immunology, Fred Hutchinson Cancer Center, Seattle, said, “In some respects, it’s quite promising and in other respects, actually more limited than you would hope for.”
“I think it’s a great demonstration that there is activity here, and there’s a world of things that can be done to improve the activity and no doubt that will be done. After this trial, I’m sure we will see next-generation trials,” said Dr. Greenberg.
He said key issues going forward are how cells are selected and manufactured: “That’s going to be a critical piece for making it better.”
“There is now a world of data that says T cells that recognize mutations in cancers can be effective in solid tumors,” Dr. Greenberg said.
“Sustaining that response is still a huge obstacle for achieving the kinds of therapeutic benefits we’d like to be achieved. And having that response be broad enough, particularly in the setting where most of the mutations are just passenger mutations, not driver oncogenes, is going to require a way of generating a large polyspecific population of cells that can persist for a long time,” he further commented.
All in all, this study was a “yeoman’s effort” and the researchers “deserve a lot of credit for pushing it forward,” Dr. Greenberg said.
The study was supported in part by grants from Stand Up to Cancer Foundation, the Barbara Bauer Prelude to a Cure Foundation, Iovance Biotherapeutics, and a Young Investigator award from Adaptive Biotechnologies. Nivolumab was supplied by Bristol-Myers Squibb. Aldesleukin (IL-2) was supplied by Clinigen Group. A complete list of author disclosures is available with the original article. Dr. Hirsch and Dr. Greenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adoptive cell therapy using tumor-infiltrating lymphocytes (TILs) led to complete and long-lasting responses in 2 of 16 patients with metastatic non-small cell lung cancer (NSCLC) who fully underwent the therapy in a phase 1 study.
The results are “very impressive, with two complete responses that are ongoing 1.5 years later, so it’s durable, and that’s encouraging,” Fred Hirsch, MD, PhD, director of the Center of Excellence for Thoracic Oncology at Mount Sinai’s Tisch Cancer Institute, New York, told this news organization.
Dr. Hirsch, who wasn’t involved in the research, also noted that this study is in immunotherapy-resistant metastatic lung cancer – “a situation where we actually don’t know exactly how best to treat it today.”
The study was published online in Nature Medicine.
A form of immunotherapy, TILs have been studied extensively in melanoma, as reported by this news organization, but this is the first test of the TIL therapy in metastatic NSCLC.
The single-arm, open-label phase 1 trial involved 20 patients who had TILs collected, including 16 who eventually received TILs. Median age was 54 years; all patients had metastatic NSCLC and disease progression after nivolumab monotherapy.
TILs cultured from an individual patient’s tumor were expanded ex vivo from minced tumors cultured with interleukin-2 (IL-2). Via this method, “billions of activated T cells can be produced and infused back into a patient,” explain the authors.
The full treatment regimen comprised cyclophosphamide and fludarabine lymphodepletion, TIL infusion and IL-2, followed by maintenance nivolumab.
“We found that infusion of TILs in combination with lymphodepletion and IL-2 had manageable toxicity and mediated tumor regressions in several patients, including complete responses,” report Benjamin Creelan, MD, of the Moffitt Cancer Center & Research Institute, Tampa, and colleagues.
The endpoint of safety was met according to the prespecified criteria of a rate of severe toxicity of 17% or less.
Among 13 evaluable patients, three had confirmed responses and 11 had reduction in tumor burden. Two patients achieved complete responses that were ongoing 1.5 years following TIL treatment.
One durable complete response occurred in a PD-L1-negative never-smoker, who had a low tumor mutation burden and who was refractory to nivolumab.
“This may be particularly encouraging for the large subset of never-smoker patients, for whom immune-checkpoint inhibitors have historically had limited efficacy,” the investigators say.
This complete responder had “features where you wouldn’t expect to see a response for immunotherapy,” Dr. Hirsch told this news organization.
“Low tumor mutation burden, negative PDL-1, and never-smoker are three factors which indicate some kind of resistance to immunotherapy, and despite that, there was a complete response with this specific therapy. That is fascinating,” he said.
In exploratory analyses, T cells recognizing multiple types of cancer mutations were detected after TIL treatment and were enriched in patients who responded to treatment.
The researchers say these early data indicate that TILs can mediate effective responses in tumor subtypes that are not sensitive to traditional immune-checkpoint-targeted therapy.
“Therefore, therapy with TILs may extend the scope and impact of immunotherapy into wider populations,” they write.
‘Yeoman’s effort’ paving the way forward
Also weighing in on the study, Philip Greenberg, MD, professor and head of immunology, Fred Hutchinson Cancer Center, Seattle, said, “In some respects, it’s quite promising and in other respects, actually more limited than you would hope for.”
“I think it’s a great demonstration that there is activity here, and there’s a world of things that can be done to improve the activity and no doubt that will be done. After this trial, I’m sure we will see next-generation trials,” said Dr. Greenberg.
He said key issues going forward are how cells are selected and manufactured: “That’s going to be a critical piece for making it better.”
“There is now a world of data that says T cells that recognize mutations in cancers can be effective in solid tumors,” Dr. Greenberg said.
“Sustaining that response is still a huge obstacle for achieving the kinds of therapeutic benefits we’d like to be achieved. And having that response be broad enough, particularly in the setting where most of the mutations are just passenger mutations, not driver oncogenes, is going to require a way of generating a large polyspecific population of cells that can persist for a long time,” he further commented.
All in all, this study was a “yeoman’s effort” and the researchers “deserve a lot of credit for pushing it forward,” Dr. Greenberg said.
The study was supported in part by grants from Stand Up to Cancer Foundation, the Barbara Bauer Prelude to a Cure Foundation, Iovance Biotherapeutics, and a Young Investigator award from Adaptive Biotechnologies. Nivolumab was supplied by Bristol-Myers Squibb. Aldesleukin (IL-2) was supplied by Clinigen Group. A complete list of author disclosures is available with the original article. Dr. Hirsch and Dr. Greenberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A hot dog a day takes 36 minutes away
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
The death ‘dog’
Imagine you’re out in your backyard managing the grill for a big family barbecue. You’ve got a dazzling assortment of meat assorted on your fancy new propane grill, all charring nicely. Naturally, the hot dogs finish first, and as you pull them off, you figure you’ll help yourself to one now. After all, you are the chef, you deserve a reward. But, as you bite into your smoking hot sandwich, a cold, bony finger taps you on the shoulder. You turn and come face to face with the Grim Reaper. “YOU JUST LOST 36 MINUTES,” Death says. “ALSO, MAY I HAVE ONE OF THOSE? THEY LOOK DELICIOUS.”
Nonplussed and moving automatically, you scoop up another hot dog and place it in a bun. “WITH KETCHUP PLEASE,” Death says. “I NEVER CARED FOR MUSTARD.”
“I don’t understand,” you say. “Surely I won’t die at a family barbecue.”
“DO NOT CALL ME SHIRLEY,” Death says. “AND YOU WILL NOT. IT’S PART OF MY NEW CONTRACT.”
A new study, published in Nature Food, found that a person may lose up to 36 minutes for every hot dog consumed. Researchers from the University of Michigan analyzed nearly 6,000 different foods using a new nutritional index to quantify their health effects in minutes of healthy life lost or gained. Eating a serving of nuts adds an extra 26 minutes of life. The researchers determined that replacing just 10% of daily caloric intake from beef and processed foods with fruits, vegetables, and nuts can add 48 minutes per day. It would also reduce the daily carbon footprint by 33%.
“So you go around to everyone eating bad food and tell them how much life they’ve lost?” you ask when the Grim Reaper finishes his story. “Sounds like a drag.”
“IT IS. WE’VE HAD TO HIRE NEW BLOOD.” Death chuckles at its own bad pun. “NOW IF YOU’LL EXCUSE ME, I MUST CHASTISE A MAN IN FLORIDA FOR EATING A WELL-DONE STEAK.”
More stress, less sex
As the world becomes a more stressful place, the human population could face a 50% drop by the end of the century.
Think of stress as a one-two punch to the libido and human fertility. The more people are stressed out, the less likely they are to have quality interactions with others. Many of us would rather be alone with our wine and cheese to watch our favorite show.
Researchers have found that high stress levels have been known to drop sperm count, ovulation, and sexual activity. Guess what? There has been a 50% decrease in sperm counts over the last 50 years. That’s the second punch. But let’s not forget, the times are changing.
“Changes in reproductive behavior that contribute to the population drop include more young couples choosing to be ‘child-free,’ people having fewer children, and couples waiting longer to start families,” said Alexander Suvorov, PhD, of the University of Massachusetts, the paper’s author.
Let’s summarize: The more stress we’re dealing with, the less people want to deal with each other.
Who would have thought the future would be less fun?
‘You are not a horse. You are not a cow. Seriously, y’all. Stop it.’
WARNING: The following descriptions of COVID-19–related insanity may be offensive to some readers.
Greetings, ladies and gentlemen! Welcome to the first round of Pandemic Pandemonium. Let’s get right to the action.
This week’s preshow match-off involves face mask woes. The first comes to us from Alabama, where a woman wore a space helmet to a school board meeting to protest mask mandates. The second comes from Australia, in the form of mischievous magpies. We will explain.
It is not uncommon for magpies to attack those who come too close to their nests in the spring, or “swooping season,” as it’s affectionately called. The magpies are smart enough to recognize the faces of people they see regularly and not attack; however, it’s feared that mask wearing will change this.
While you’re chewing on that exciting appetizer, let’s take a look at our main course, which has a distinct governmental flavor. Jeff Landry is the attorney general of Louisiana, and, like our space-helmet wearer, he’s not a fan of mask mandates. According to Business Insider, Mr. Landry “drafted and distributed sample letters intended to help parents evade mask-wearing ordinances and COVID-19 vaccination requirements for their children in schools.”
Up against him is the Food and Drug Administration’s Twitter account. In an unrelated matter, the agency tweeted, “You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This was in response to people using the nonhuman forms of ivermectin to treat very human COVID-19.
Well, there you have it. Who will win tonight’s exciting edition of Pandemic Pandemonium? The first reader to contact us gets to decide the fate of these worthy contestants.
From venomous poison to heart drug
It’s not likely that anyone who sees a giant, venomous spider is thinking, “Hey! That thing could save my life!” It’s usually quite the opposite. Honestly, we would run away from just about any spider. But what if one of the deadliest spiders in the world could also save you from dying of a heart attack?
You probably don’t believe us, right? That’s fair, but the deadly Fraser Island (K’gari) funnel web spider, might also be the most helpful. Investigators from the University of Queensland in Australia have found a way to extract a molecule from the spider’s venom that might help stop damage from heart attacks and may even preserve hearts being used for transplants. “The Hi1a protein from spider venom blocks acid-sensing ion channels in the heart, so the death message is blocked, cell death is reduced, and we see improved heart cell survival,” Nathan Palpant, PhD, of the university, noted in a written statement.
No one has ever developed a drug to stop the “death signal,” so maybe it’s time to befriend spiders instead of running away from them in horror. Just leave the venom extraction to the professionals.
AHA targets rising prevalence of obstructive sleep apnea in children
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
Obstructive sleep apnea is becoming more common in children and adolescents as the prevalence of obesity increases, but it may also be a preventable risk factor for cardiovascular disease, according to a new scientific statement from the American Heart Association.
The statement focuses on the links between OSA and CVD risk factors in children and adolescents, and reviews diagnostic strategies and treatments. The writing committee reported that 1%-6% of children and adolescents have OSA, as do up to 60% of adolescents considered obese.
The statement was created by the AHA’s Atherosclerosis, Hypertension, and Obesity in the Young subcommittee of the Council on Cardiovascular Disease in the Young and was published online in the Journal of the American Heart Association.
Carissa M. Baker-Smith, MD, chair of the writing group chair and director of pediatric preventive cardiology at Nemours Cardiac Center, Alfred I. duPont Hospital for Children, Wilmington, Del., explained the rationale for issuing the statement at this time, noting that the relationship between OSA and CVD in adults is well documented.
“There has been less focus on the importance of recognizing and treating sleep apnea in youth,” she said in an interview. “Thus, we felt that it was vitally important to get the word out to parents and to providers that paying attention to the quality and duration of your child’s sleep is vitally important to a child’s long-term heart health. Risk factors for heart disease, when present in childhood, can persist into adulthood.”
Clarity on polysomnography
For making the diagnosis of OSA in children, the statement provides clarity on the use of polysomnography and the role of the apnea-hypopnea index, which is lower in children with OSA than in adults. “One controversy, or at least as I saw it, was whether or not polysomnography testing is always required to make the diagnosis of OSA and before proceeding with tonsil and adenoid removal among children for whom enlarged tonsils and adenoids are present,” Dr. Baker-Smith said. “Polysomnography testing is not always needed before an ear, nose, and throat surgeon may recommend surgery.”
The statement also noted that history and physical examination may not yield enough reliable information to distinguish OSA from snoring.
In areas where sleep laboratories that work with children aren’t available, alternative tests such as daytime nap polysomnography, nocturnal oximetry, and nocturnal video recording may be used – with a caveat. “These alternative tests have weaker positive and negative predictive values when compared with polysomnography,” the writing committee noted. Home sleep apnea tests aren’t recommended in children. Questionnaires “are useful as screening, but not as diagnostic tools.”
Pediatric patients being evaluated for OSA should also be screened for hypertension and metabolic syndrome, as well as central nervous system and behavioral disorders. Diagnosing OSA in children and adolescents requires “a high index of suspicion,” the committee wrote.
Pediatricians and pediatric cardiologists should exercise that high index of suspicion when receiving referrals for cardiac evaluations for attention deficit hyperactivity disorder medication, Dr. Baker-Smith said. “Take the time to ask about a child’s sleep – snoring, apnea, etc. – especially if the child has obesity, difficulty focusing during the day, and if there is evidence of systemic hypertension or other signs of metabolic syndrome,” she said.
Risk factors for OSA in children
The statement also reviewed risk factors for OSA, among them obesity, particularly among children younger than 6 years. Other risk factors include upper and lower airway disease, hypotonia, parental history of hyperplasia of the adenoids and tonsils, craniofacial malformations, and neuromuscular disorders. However, the committee cited “limited data” to support that children with congenital heart disease may be at greater risk for OSA and sleep-disordered breathing (SDB).
Black children are at significantly greater risk, and socioeconomic factors “may be potential confounders,” the committee stated. Other risk factors include allergic rhinitis and sickle cell disease.
But the statement underscores that “obesity is the main risk factor” for OSA in children and adolescents, and that the presence of increased inflammation may explain this relationship. Steroids may alleviate these symptoms, even in nonobese children, and removal of the adenoids or tonsils is an option to reduce inflammation in children with OSA.
“Obesity is a significant risk factor for sleep disturbances and obstructive sleep apnea, and the severity of sleep apnea may be improved by weight-loss interventions, which then improves metabolic syndrome factors such as insulin sensitivity,” Dr. Baker-Smith said. “We need to increase awareness about how the rising prevalence of obesity may be impacting sleep quality in kids and recognize sleep-disordered breathing as something that could contribute to risks for hypertension and later cardiovascular disease.”
Children in whom OSA is suspected should also undergo screening for metabolic syndrome, and central nervous system and behavioral disorders.
Cardiovascular risks
The statement explores the connection between cardiovascular complications and SDB and OSA in depth.
“Inadequate sleep duration of < 5 hours per night in children and adolescents has been linked to an increased risk of hypertension and is also associated with an increased prevalence of obesity,” the committee wrote.
However, the statement left one question hanging: whether OSA alone or obesity cause higher BP in younger patients with OSA. But the committee concluded that BP levels increase with the severity of OSA, although the effects can vary with age. OSA in children peaks between ages 2 and 8, corresponding to the peak prevalence of hypertrophy of the tonsils and adenoids. Children aged 10-11 with more severe OSA may have BP dysregulation, while older adolescents develop higher sustained BP. Obesity may be a confounder for daytime BP elevations, while nighttime hypertension depends less on obesity and more on OSA severity.
“OSA is associated with abnormal BP in youth and, in particular, higher nighttime blood pressures and loss of the normal decline in BP that should occur during sleep,” Dr. Baker-Smith said. “Children with OSA appear to have higher BP than controls during both sleep and wake times, and BP levels increase with increasing severity of OSA.”
Nonetheless, children with OSA are at greater risk for other cardiovascular problems. Left ventricular hypertrophy may be a secondary outcome. “The presence of obstructive sleep apnea in children is associated with an 11-fold increased risk for LVH in children, a relationship not seen in the presence of primary snoring alone,” Dr. Baker-Smith said.
Dr. Baker-Smith had no relevant disclosures. Coauthor Amal Isaiah, MD, is coinventor of an imaging system for sleep apnea and receives royalties from the University of Maryland. The other coauthors have no relevant financial relationships to disclose.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Children and COVID: New cases soar to near-record level
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.
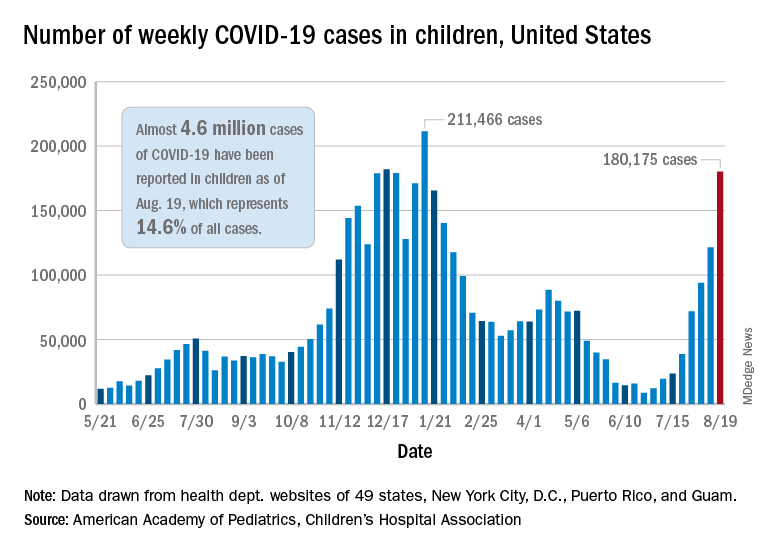
The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.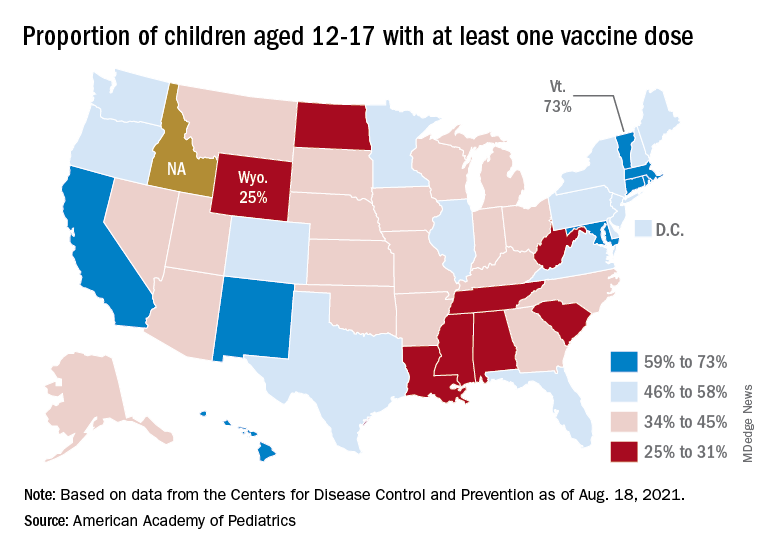
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Health care workers eager for COVID booster shots
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
Q&A: Get flu shot early this year? Same time as COVID vaccine?
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.









