User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


CDC endorses Pfizer’s COVID-19 vaccine for young kids
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
Children and COVID: A look at the pace of vaccination
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?
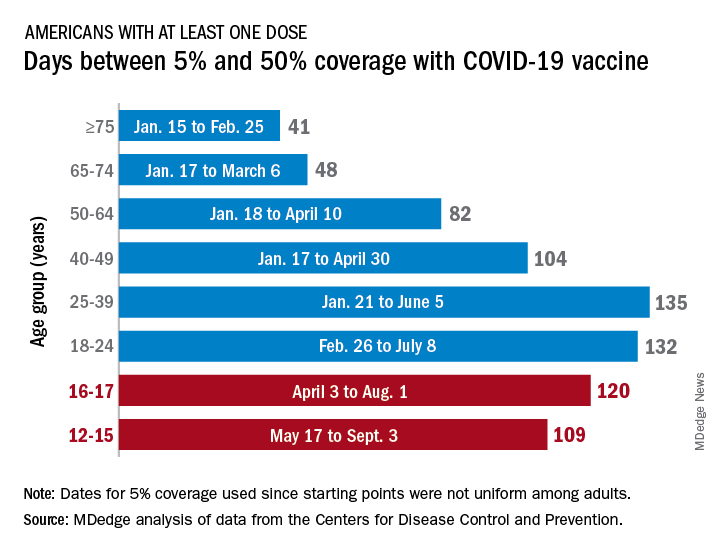
Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?

Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?

Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
ASNC rejects new chest pain guideline it helped create
It was Oct. 28 when the two big North American cardiology societies issued a joint practice guideline on evaluating and managing chest pain that was endorsed by five other subspecialty groups. The next day, another group that had taken part in the document’s genesis explained why it wasn’t one of those five.
Although the American Society of Nuclear Cardiology (ASNC) was “actively engaged at every stage of the guideline-writing and review process,” the society “could not endorse the guideline,” the society announced in a statement released to clinicians and the media. The most prominent cited reason: It doesn’t adequately “support the principle of Patient First Imaging.”
The guideline was published in Circulation and the Journal of the American College of Cardiology, flagship journals of the American Heart Association and American College of Cardiology, respectively.
The document notes at least two clinicians represented ASNC as peer reviewers, and another was on the writing committee, but the organization does not appear in the list of societies endorsing the document.
“We believe that the document fails to provide unbiased guidance to health care professionals on the optimal evaluation of patients with chest pain,” contends an editorial ASNC board members have scheduled for the Jan. 10 issue of the Journal of Nuclear Medicine but is available now on an open-access preprint server.
“Despite the many important and helpful recommendations in the new guideline, there are several recommendations that we could not support,” it states.
“The ASNC board of directors reviewed the document twice during the endorsement process,” and the society “offered substantive comments after the first endorsement review, several of which were addressed,” Randall C. Thompson, MD, St. Luke’s Mid America Heart Institute and University of Missouri–Kansas City, said in an interview.
“However, some of the board’s concerns went unresolved. It was after the board’s second review, when the document had been declared finalized, that they voted not to endorse,” said Dr. Thompson, who is ASNC president.
“When we gather multiple organizations together to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of peer-reviewed, published literature and create clinical practice recommendations,” Guideline Writing Committee Chair Martha Gulati, MD, University of Arizona, Phoenix, told this news organization in a prepared statement.
“The ASNC had a representative on the writing committee who is a coauthor on the paper and actively participated throughout the writing process the past 4 years,” she said. “The final guideline reflects the latest evidence-based recommendations for the evaluation and diagnosis of chest pain, as agreed by the seven endorsing organizations.”
The document does not clearly note that an ASNC representative was on the writing committee. However, ASNC confirmed that Renee Bullock-Palmer, MD, Deborah Heart and Lung Center, Browns Mills, N.J., is a fellow of the ASNC and had represented the group as one of the coauthors. Two “official reviewers” of the document, however, are listed as ASNC representatives.
Points of contention
“The decision about which test to order can be a nuanced one, and cardiac imaging tests tend to be complementary,” elaborates the editorial on the issue of patient-centered management.
Careful patient selection for different tests is important, “and physician and technical local expertise, availability, quality of equipment, and patient preference are extremely important factors to consider. There is not enough emphasis on this important point,” contend the authors. “This is an important limitation of the guideline.”
Other issues of concern include “lack of balance in the document’s presentation of the science on FFR-CT [fractional flow reserve assessment with computed tomography] and its inappropriately prominent endorsement,” the editorial states.
The U.S. Food and Drug Administration–recognized “limitations and contraindications” to FFR-CT tend to be glossed over in the document, Dr. Thompson said. And most ASNC board members were “concerned with the prominent location of the recommendations for FFR-CT in various tables – especially since there was minimal-to-no discussion of the fact that it is currently provided by only one company, that it is not widely available nor covered routinely by health insurance carriers, and [that] the accuracy in the most relevant population is disputed.”
In other concerns, the document “inadequately discusses the benefit” of combining coronary artery calcium (CAC) scores with functional testing, which ASNC said it supports. For example, adding CAC scores to myocardial perfusion imaging improves its diagnostic accuracy and prognostic power.
Functional vs. anatomic testing?
Moreover, “it is no longer appropriate to bundle all types of stress testing together. All stress imaging tests have their unique advantages and limitations.” Yet, “the concept of the dichotomy of functional testing versus anatomic testing is a common theme in the guideline in many important patient groups,” the editorial states. That could overemphasize CT angiography and thus “blur distinction between different types of functional tests.”
Such concerns about “imbalance” in the portrayals of the two kinds of tests were “amplified by the problem of health insurance companies and radiologic benefits managers inappropriately substituting a test that was ordered by a physician with a different test,” Dr. Thompson elaborated. “There is the impression that some of them ‘cherry-pick’ certain guidelines and that this practice is harmful to patients.”
The ASNC currently does not plan its own corresponding guideline, he said. But the editorial says that “over the coming weeks and months ASNC will offer a series of webinars and other programs that address specific patient populations and dilemmas.” Also, “we will enhance our focus on programs to address quality and efficiency to support a patient-first approach to imaging.”
The five subspecialty groups that have endorsed the document are the American Society of Echocardiography, American College of Chest Physicians, Society for Academic Emergency Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance.
Dr. Thompson has reported no relevant financial relationships. Statements of disclosure for the other editorial writers are listed in the publication.
A version of this article first appeared on Medscape.com.
It was Oct. 28 when the two big North American cardiology societies issued a joint practice guideline on evaluating and managing chest pain that was endorsed by five other subspecialty groups. The next day, another group that had taken part in the document’s genesis explained why it wasn’t one of those five.
Although the American Society of Nuclear Cardiology (ASNC) was “actively engaged at every stage of the guideline-writing and review process,” the society “could not endorse the guideline,” the society announced in a statement released to clinicians and the media. The most prominent cited reason: It doesn’t adequately “support the principle of Patient First Imaging.”
The guideline was published in Circulation and the Journal of the American College of Cardiology, flagship journals of the American Heart Association and American College of Cardiology, respectively.
The document notes at least two clinicians represented ASNC as peer reviewers, and another was on the writing committee, but the organization does not appear in the list of societies endorsing the document.
“We believe that the document fails to provide unbiased guidance to health care professionals on the optimal evaluation of patients with chest pain,” contends an editorial ASNC board members have scheduled for the Jan. 10 issue of the Journal of Nuclear Medicine but is available now on an open-access preprint server.
“Despite the many important and helpful recommendations in the new guideline, there are several recommendations that we could not support,” it states.
“The ASNC board of directors reviewed the document twice during the endorsement process,” and the society “offered substantive comments after the first endorsement review, several of which were addressed,” Randall C. Thompson, MD, St. Luke’s Mid America Heart Institute and University of Missouri–Kansas City, said in an interview.
“However, some of the board’s concerns went unresolved. It was after the board’s second review, when the document had been declared finalized, that they voted not to endorse,” said Dr. Thompson, who is ASNC president.
“When we gather multiple organizations together to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of peer-reviewed, published literature and create clinical practice recommendations,” Guideline Writing Committee Chair Martha Gulati, MD, University of Arizona, Phoenix, told this news organization in a prepared statement.
“The ASNC had a representative on the writing committee who is a coauthor on the paper and actively participated throughout the writing process the past 4 years,” she said. “The final guideline reflects the latest evidence-based recommendations for the evaluation and diagnosis of chest pain, as agreed by the seven endorsing organizations.”
The document does not clearly note that an ASNC representative was on the writing committee. However, ASNC confirmed that Renee Bullock-Palmer, MD, Deborah Heart and Lung Center, Browns Mills, N.J., is a fellow of the ASNC and had represented the group as one of the coauthors. Two “official reviewers” of the document, however, are listed as ASNC representatives.
Points of contention
“The decision about which test to order can be a nuanced one, and cardiac imaging tests tend to be complementary,” elaborates the editorial on the issue of patient-centered management.
Careful patient selection for different tests is important, “and physician and technical local expertise, availability, quality of equipment, and patient preference are extremely important factors to consider. There is not enough emphasis on this important point,” contend the authors. “This is an important limitation of the guideline.”
Other issues of concern include “lack of balance in the document’s presentation of the science on FFR-CT [fractional flow reserve assessment with computed tomography] and its inappropriately prominent endorsement,” the editorial states.
The U.S. Food and Drug Administration–recognized “limitations and contraindications” to FFR-CT tend to be glossed over in the document, Dr. Thompson said. And most ASNC board members were “concerned with the prominent location of the recommendations for FFR-CT in various tables – especially since there was minimal-to-no discussion of the fact that it is currently provided by only one company, that it is not widely available nor covered routinely by health insurance carriers, and [that] the accuracy in the most relevant population is disputed.”
In other concerns, the document “inadequately discusses the benefit” of combining coronary artery calcium (CAC) scores with functional testing, which ASNC said it supports. For example, adding CAC scores to myocardial perfusion imaging improves its diagnostic accuracy and prognostic power.
Functional vs. anatomic testing?
Moreover, “it is no longer appropriate to bundle all types of stress testing together. All stress imaging tests have their unique advantages and limitations.” Yet, “the concept of the dichotomy of functional testing versus anatomic testing is a common theme in the guideline in many important patient groups,” the editorial states. That could overemphasize CT angiography and thus “blur distinction between different types of functional tests.”
Such concerns about “imbalance” in the portrayals of the two kinds of tests were “amplified by the problem of health insurance companies and radiologic benefits managers inappropriately substituting a test that was ordered by a physician with a different test,” Dr. Thompson elaborated. “There is the impression that some of them ‘cherry-pick’ certain guidelines and that this practice is harmful to patients.”
The ASNC currently does not plan its own corresponding guideline, he said. But the editorial says that “over the coming weeks and months ASNC will offer a series of webinars and other programs that address specific patient populations and dilemmas.” Also, “we will enhance our focus on programs to address quality and efficiency to support a patient-first approach to imaging.”
The five subspecialty groups that have endorsed the document are the American Society of Echocardiography, American College of Chest Physicians, Society for Academic Emergency Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance.
Dr. Thompson has reported no relevant financial relationships. Statements of disclosure for the other editorial writers are listed in the publication.
A version of this article first appeared on Medscape.com.
It was Oct. 28 when the two big North American cardiology societies issued a joint practice guideline on evaluating and managing chest pain that was endorsed by five other subspecialty groups. The next day, another group that had taken part in the document’s genesis explained why it wasn’t one of those five.
Although the American Society of Nuclear Cardiology (ASNC) was “actively engaged at every stage of the guideline-writing and review process,” the society “could not endorse the guideline,” the society announced in a statement released to clinicians and the media. The most prominent cited reason: It doesn’t adequately “support the principle of Patient First Imaging.”
The guideline was published in Circulation and the Journal of the American College of Cardiology, flagship journals of the American Heart Association and American College of Cardiology, respectively.
The document notes at least two clinicians represented ASNC as peer reviewers, and another was on the writing committee, but the organization does not appear in the list of societies endorsing the document.
“We believe that the document fails to provide unbiased guidance to health care professionals on the optimal evaluation of patients with chest pain,” contends an editorial ASNC board members have scheduled for the Jan. 10 issue of the Journal of Nuclear Medicine but is available now on an open-access preprint server.
“Despite the many important and helpful recommendations in the new guideline, there are several recommendations that we could not support,” it states.
“The ASNC board of directors reviewed the document twice during the endorsement process,” and the society “offered substantive comments after the first endorsement review, several of which were addressed,” Randall C. Thompson, MD, St. Luke’s Mid America Heart Institute and University of Missouri–Kansas City, said in an interview.
“However, some of the board’s concerns went unresolved. It was after the board’s second review, when the document had been declared finalized, that they voted not to endorse,” said Dr. Thompson, who is ASNC president.
“When we gather multiple organizations together to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of peer-reviewed, published literature and create clinical practice recommendations,” Guideline Writing Committee Chair Martha Gulati, MD, University of Arizona, Phoenix, told this news organization in a prepared statement.
“The ASNC had a representative on the writing committee who is a coauthor on the paper and actively participated throughout the writing process the past 4 years,” she said. “The final guideline reflects the latest evidence-based recommendations for the evaluation and diagnosis of chest pain, as agreed by the seven endorsing organizations.”
The document does not clearly note that an ASNC representative was on the writing committee. However, ASNC confirmed that Renee Bullock-Palmer, MD, Deborah Heart and Lung Center, Browns Mills, N.J., is a fellow of the ASNC and had represented the group as one of the coauthors. Two “official reviewers” of the document, however, are listed as ASNC representatives.
Points of contention
“The decision about which test to order can be a nuanced one, and cardiac imaging tests tend to be complementary,” elaborates the editorial on the issue of patient-centered management.
Careful patient selection for different tests is important, “and physician and technical local expertise, availability, quality of equipment, and patient preference are extremely important factors to consider. There is not enough emphasis on this important point,” contend the authors. “This is an important limitation of the guideline.”
Other issues of concern include “lack of balance in the document’s presentation of the science on FFR-CT [fractional flow reserve assessment with computed tomography] and its inappropriately prominent endorsement,” the editorial states.
The U.S. Food and Drug Administration–recognized “limitations and contraindications” to FFR-CT tend to be glossed over in the document, Dr. Thompson said. And most ASNC board members were “concerned with the prominent location of the recommendations for FFR-CT in various tables – especially since there was minimal-to-no discussion of the fact that it is currently provided by only one company, that it is not widely available nor covered routinely by health insurance carriers, and [that] the accuracy in the most relevant population is disputed.”
In other concerns, the document “inadequately discusses the benefit” of combining coronary artery calcium (CAC) scores with functional testing, which ASNC said it supports. For example, adding CAC scores to myocardial perfusion imaging improves its diagnostic accuracy and prognostic power.
Functional vs. anatomic testing?
Moreover, “it is no longer appropriate to bundle all types of stress testing together. All stress imaging tests have their unique advantages and limitations.” Yet, “the concept of the dichotomy of functional testing versus anatomic testing is a common theme in the guideline in many important patient groups,” the editorial states. That could overemphasize CT angiography and thus “blur distinction between different types of functional tests.”
Such concerns about “imbalance” in the portrayals of the two kinds of tests were “amplified by the problem of health insurance companies and radiologic benefits managers inappropriately substituting a test that was ordered by a physician with a different test,” Dr. Thompson elaborated. “There is the impression that some of them ‘cherry-pick’ certain guidelines and that this practice is harmful to patients.”
The ASNC currently does not plan its own corresponding guideline, he said. But the editorial says that “over the coming weeks and months ASNC will offer a series of webinars and other programs that address specific patient populations and dilemmas.” Also, “we will enhance our focus on programs to address quality and efficiency to support a patient-first approach to imaging.”
The five subspecialty groups that have endorsed the document are the American Society of Echocardiography, American College of Chest Physicians, Society for Academic Emergency Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance.
Dr. Thompson has reported no relevant financial relationships. Statements of disclosure for the other editorial writers are listed in the publication.
A version of this article first appeared on Medscape.com.
COVID-19 vaccines provide 5 times the protection of natural immunity, CDC study says
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.
Influenza tied to long-term increased risk for Parkinson’s disease
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
ERs are swamped with seriously ill patients, although many don’t have COVID
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.
Inside the emergency department at Sparrow Hospital in Lansing, Mich., staff members are struggling to care for patients showing up much sicker than they’ve ever seen.
Tiffani Dusang, the ER’s nursing director, practically vibrates with pent-up anxiety, looking at patients lying on a long line of stretchers pushed up against the beige walls of the hospital hallways. “It’s hard to watch,” she said in a warm Texas twang.
But there’s nothing she can do. The ER’s 72 rooms are already filled.
“I always feel very, very bad when I walk down the hallway and see that people are in pain, or needing to sleep, or needing quiet. But they have to be in the hallway with, as you can see, 10 or 15 people walking by every minute,” Ms. Dusang said.
The scene is a stark contrast to where this emergency department — and thousands of others — were at the start of the pandemic. Except for initial hot spots like New York City, in spring 2020 many ERs across the country were often eerily empty. Terrified of contracting COVID-19, people who were sick with other things did their best to stay away from hospitals. Visits to emergency rooms dropped to half their typical levels, according to the Epic Health Research Network, and didn’t fully rebound until this summer.
But now, they’re too full.
Months of treatment delays have exacerbated chronic conditions and worsened symptoms. Doctors and nurses say the severity of illness ranges widely and includes abdominal pain, respiratory problems, blood clots, heart conditions and suicide attempts, among other conditions.
But they can hardly be accommodated. Emergency departments, ideally, are meant to be brief ports in a storm, with patients staying just long enough to be sent home with instructions to follow up with primary care physicians, or sufficiently stabilized to be transferred “upstairs” to inpatient or intensive care units.
Except now those long-term care floors are full too, with a mix of covid and non-covid patients. People coming to the ER get warehoused for hours, even days, forcing ER staffers to perform long-term care roles they weren’t trained to do.
At Sparrow, space is a valuable commodity in the ER: A separate section of the hospital was turned into an overflow unit. Stretchers stack up in halls. A row of brown reclining chairs lines a wall, intended for patients who aren’t sick enough for a stretcher but are too sick to stay in the main waiting room.
Forget privacy, Alejos Perrientoz learned when he arrived. He came to the ER because his arm had been tingling and painful for over a week. He couldn’t hold a cup of coffee. A nurse gave him a full physical exam in a brown recliner, which made him self-conscious about having his shirt lifted in front of strangers. “I felt a little uncomfortable,” he whispered. “But I have no choice, you know? I’m in the hallway. There’s no rooms.
“We could have done the physical in the parking lot,” he added, managing a laugh.
Even patients who arrive by ambulance are not guaranteed a room: One nurse runs triage, screening those who absolutely need a bed, and those who can be put in the waiting area.
“I hate that we even have to make that determination,” MS. Dusang said. Lately, staff members have been pulling out some patients already in the ER’s rooms when others arrive who are more critically ill. “No one likes to take someone out of the privacy of their room and say, ‘We’re going to put you in a hallway because we need to get care to someone else.’”
ER patients have grown sicker
“We are hearing from members in every part of the country,” said Dr. Lisa Moreno, president of the American Academy of Emergency Medicine. “The Midwest, the South, the Northeast, the West … they are seeing this exact same phenomenon.”
Although the number of ER visits returned to pre-COVID levels this summer, admission rates, from the ER to the hospital’s inpatient floors, are still almost 20% higher. That’s according to the most recent analysis by the Epic Health Research Network, which pulls data from more than 120 million patients across the country.
“It’s an early indicator that what’s happening in the ED is that we’re seeing more acute cases than we were pre-pandemic,” said Caleb Cox, a data scientist at Epic.
Less acute cases, such as people with health issues like rashes or conjunctivitis, still aren’t going to the ER as much as they used to. Instead, they may be opting for an urgent care center or their primary care doctor, Mr. Cox explained. Meanwhile, there has been an increase in people coming to the ER with more serious conditions, like strokes and heart attacks.
So, even though the total number of patients coming to ERs is about the same as before the pandemic, “that’s absolutely going to feel like [if I’m an ER doctor or nurse] I’m seeing more patients and I’m seeing more acute patients,” Mr. Cox said.
Dr. Moreno, the AAEM president, works at an emergency department in New Orleans. She said the level of illness, and the inability to admit patients quickly and move them to beds upstairs, has created a level of chaos she described as “not even humane.”
At the beginning of a recent shift, she heard a patient crying nearby and went to investigate. It was a paraplegic man who’d recently had surgery for colon cancer. His large post-operative wound was sealed with a device called a wound vac, which pulls fluid from the wound into a drainage tube attached to a portable vacuum pump.
But the wound vac had malfunctioned, which is why he had come to the ER. Staffers were so busy, however, that by the time Dr. Moreno came in, the fluid from his wound was leaking everywhere.
“When I went in, the bed was covered,” she recalled. “I mean, he was lying in a puddle of secretions from this wound. And he was crying, because he said to me, ‘I’m paralyzed. I can’t move to get away from all these secretions, and I know I’m going to end up getting an infection. I know I’m going to end up getting an ulcer. I’ve been laying in this for, like, eight or nine hours.’”
The nurse in charge of his care told Dr. Moreno she simply hadn’t had time to help this patient yet. “She said, ‘I’ve had so many patients to take care of, and so many critical patients. I started [an IV] drip on this person. This person is on a cardiac monitor. I just didn’t have time to get in there.’”
“This is not humane care,” Dr. Moreno said. “This is horrible care.”
But it’s what can happen when emergency department staffers don’t have the resources they need to deal with the onslaught of competing demands.
“All the nurses and doctors had the highest level of intent to do the right thing for the person,” Dr. Moreno said. “But because of the high acuity of … a large number of patients, the staffing ratio of nurse to patient, even the staffing ratio of doctor to patient, this guy did not get the care that he deserved to get, just as a human being.”
The instance of unintended neglect that Dr. Moreno saw is extreme, and not the experience of most patients who arrive at ERs these days. But the problem is not new: Even before the pandemic, ER overcrowding had been a “widespread problem and a source of patient harm, according to a recent commentary in NEJM Catalyst Innovations in Care Delivery.
“ED crowding is not an issue of inconvenience,” the authors wrote. “There is incontrovertible evidence that ED crowding leads to significant patient harm, including morbidity and mortality related to consequential delays of treatment for both high- and low-acuity patients.”
And already-overwhelmed staffers are burning out.
Burnout feeds staffing shortages, and vice versa
Every morning, Tiffani Dusang wakes up and checks her Sparrow email with one singular hope: that she will not see yet another nurse resignation letter in her inbox.
“I cannot tell you how many of them [the nurses] tell me they went home crying” after their shifts, she said.
Despite Ms. Dusang’s best efforts to support her staffers, they’re leaving too fast to be replaced, either to take higher-paying gigs as a travel nurse, to try a less-stressful type of nursing, or simply walking away from the profession entirely.
Kelly Spitz has been an emergency department nurse at Sparrow for 10 years. But, lately, she has also fantasized about leaving. “It has crossed my mind several times,” she said, and yet she continues to come back. “Because I have a team here. And I love what I do.” But then she started to cry. The issue is not the hard work, or even the stress. She struggles with not being able to give her patients the kind of care and attention she wants to give them, and that they need and deserve, she said.
She often thinks about a patient whose test results revealed terminal cancer, she said. Ms. Spitz spent all day working the phones, hustling case managers, trying to get hospice care set up in the man’s home. He was going to die, and she just didn’t want him to have to die in the hospital, where only one visitor was allowed. She wanted to get him home, and back with his family.
Finally, after many hours, they found an ambulance to take him home.
Three days later, the man’s family members called Ms. Spitz: He had died surrounded by family. They were calling to thank her.
“I felt like I did my job there, because I got him home,” she said. But that’s a rare feeling these days. “I just hope it gets better. I hope it gets better soon.”
Around 4 p.m. at Sparrow Hospital as one shift approached its end, Ms. Dusang faced a new crisis: The overnight shift was more short-staffed than usual.
“Can we get two inpatient nurses?” she asked, hoping to borrow two nurses from one of the hospital floors upstairs.
“Already tried,” replied nurse Troy Latunski.
Without more staff, it’s going to be hard to care for new patients who come in overnight — from car crashes to seizures or other emergencies.
But Mr. Latunski had a plan: He would go home, snatch a few hours of sleep and return at 11 p.m. to work the overnight shift in the ER’s overflow unit. That meant he would be largely caring for eight patients, alone. On just a few short hours of sleep. But lately that seemed to be their only, and best, option.
Ms. Dusang considered for a moment, took a deep breath and nodded. “OK,” she said.
“Go home. Get some sleep. Thank you,” she added, shooting Mr. Latunski a grateful smile. And then she pivoted, because another nurse was approaching with an urgent question. On to the next crisis.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Michigan Radio, NPR and KHN.
Boxed warnings: Legal risks that many physicians never see coming
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
80% of Americans research recommendations post-visit
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
FDA authorizes Pfizer’s COVID-19 vaccine for kids
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
Antidepressant may cut COVID-19–related hospitalization, mortality: TOGETHER
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.



