User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


What docs don’t know about the Disabilities Act can hurt them and patients
Lisa Iezzoni, MD, a professor of medicine at Harvard Medical School and a disability researcher at Massachusetts General Hospital, both in Boston, has used a wheelchair for more than 30 years because of multiple sclerosis. When she visits her primary care doctor, she doesn’t get weighed because the scales are not wheelchair accessible.
This failure to weigh her and other patients in wheelchairs could lead to serious medical problems. Weight is used to monitor a person’s overall health and prenatal health and to determine accurate doses for medications such as some chemotherapies, said Dr. Iezzoni.
In another situation, a man who used a wheelchair said that his primary care doctor never got him out of it for a complete physical exam. The patient later developed lymphoma, which first appeared in his groin. The doctor should have accommodated his disability and used a height-adjustable exam table or a portable lift to transfer him onto the table.
When physicians don’t provide access to medical care that patients with disabilities need, they put themselves at greater risk of lawsuits, fines, and settlements.
Yet, a new study in Health Affairs suggests that a large percentage of doctors are not fully aware of what they are legally required to do.
Under federal nondiscrimination laws (Americans With Disabilities Act, American Rehabilitation Act, and ADA Amendments Act), medical practices must provide equal access to people with disabilities, accommodate their disability-related needs, and not refuse them medical services because of their disabilities, say disability experts.
Where doctors go wrong with disability laws
What doctors don’t know about providing reasonable accommodations makes them vulnerable to lawsuits, which worries more than two-thirds of the 714 outpatient doctors surveyed.
Not only are they required to provide reasonable accommodations, but they also have to pay for them, the researchers said. One-fifth of the surveyed doctors said they didn’t know that practice owners have to pay.
More than one practice has made patients pay for services needed for their disability, such as sign language interpreters – the patients later complained this violated the ADA to enforcement agencies.
Doctors also don’t know that they have to collaborate with patients to determine what reasonable accommodations they need – over two-thirds of those surveyed said they didn’t know it was a joint responsibility, the study found.
When doctors fail to accommodate patients’ disability needs, they engage in discrimination and violate the ADA, says Elizabeth Pendo, JD, a coauthor of the study and the Joseph J. Simeone Professor of Law at Saint Louis University.
The Department of Justice has investigated several patient complaints of alleged disability discrimination recently and resolved the disputes with agreements and small fines in some cases. “The goal is not to get large financial settlements but to work with practices to get the correct procedures in place to be compliant,” said Ms. Pendo.
Physicians would be wise to check out whether their practices are as accessible as they think. Even if there’s a ramp to the office building, the parking lot may not have a van-accessible space or enough handicapped parking signs, or the exam room may be too narrow for a wheelchair to navigate.
These practices violated the ADA and agreed to make changes:
- Hamden, Conn., has two buildings that patients with physical disabilities couldn’t easily enter. The physician owners agreed to change the buildings’ entrances and access routes and add features to make it easier to use examination rooms and restrooms and the check-in and check-out areas.
- Seven medical offices in Riverside, Calif., failed to communicate effectively with deaf and hard-of-hearing patients. They should have had a qualified sign language interpreter, an assistive listening device, or another appropriate aid or service available to a deaf patient and her family. Instead, the office relied on a video remote interpretation system that often failed to work. The agreement requires the clinic to provide those aids and services to patients and their companions who are deaf or hard of hearing, advertise their availability, assess each patient who is deaf or hard of hearing to determine the best aids and services for their needs, and pay $5,000 in compensation to the complainant and a $1,000 civil penalty to the United States.
- Springfield, Mass., refused to provide full joint replacements to two patients being treated with buprenorphine, a medication used to treat opioid use disorder. Rather than accommodate the patients, the surgeons referred them elsewhere because they were uncomfortable with the postoperative pain management protocol for patients prescribed buprenorphine. “The Americans With Disabilities Act protects health care access for people under medical treatment for opioid use disorder,” said Acting U.S. Attorney Nathaniel R. Mendell. “Health care providers must comply with the ADA, even when doing so is inconvenient or makes them uncomfortable.” The agreement requires the practice to adopt a nondiscrimination policy, provide training on the ADA and opioid use disorder, and pay two complainants $15,000 each for pain and suffering.
The DOJ has filed civil lawsuits against medical practices when they failed to resolve the allegations. Recent cases include an ophthalmology practice with 24 facilities in Arizona that refused to help transfer patients in wheelchairs to surgery tables for eye surgery and required them to pay for transfer support services and two obstetricians-gynecologists in Bakersfield, Calif., who refused to provide routine medical care to a patient because of her HIV status.
What doctors should know
Many people tend to think of a person with a disability as being in a wheelchair. But the ADA has a very broad definition of disability, which includes any physical or mental impairment that substantially limits any major life activity, said Ms. Pendo.
“It was amended in 2008 to clarify that the definition includes people with chronic diseases such as diabetes and cancer, cognitive and neurological disorders, substance abuse disorders, vision and hearing loss, and learning and other disabilities,” she said.
That means that doctors have to accommodate many types of disabilities, which can be challenging. The ADA only specifies that fixed structures need to be accessible, such as parking lots, driveways, and buildings, said Dr. Iezzoni.
When it comes to “reasonable accommodations,” doctors should decide that on a case-by-case basis, she said.
“We can say based on our study that 71% of doctors don’t know the right way to think about the accommodations – they don’t know they need to talk to patients so they can explain to them exactly what they need to accommodate their disability,” said Dr. Iezzoni.
Doctors are also required to provide effective communication for patients with sensory or cognitive disabilities, which can depend on the severity, said Ms. Pendo. Is the person deaf or hard of hearing, blind or partially sighted – is the dementia mild or severe?
“The requirement is there, but what that looks like will vary by patient. That’s what’s challenging,” said Ms. Pendo.
Dr. Iezzoni recommends that doctor’s offices ask patients whether they need special help or individual assistance when they make appointments and enter their responses in their records. She also suggests that patients be asked at follow-up appointments whether they still need the same help or not.
“Disabilities can change over time – a person with bad arthritis may need help getting onto an exam table, but later get a knee or hip replacement that is effective and no longer need that help,” said Dr. Iezzoni.
Benefits outweigh costs
Physicians have made progress in meeting the ADA’s physical accessibility requirements, said Dr. Iezzoni. “The literature suggests that doctors have done a good job at fixing the structural barriers people with mobility issues face, such as ramps and bathrooms.”
However, there are exceptions in rural older buildings which can be harder to retrofit for wheelchair accessibility, she said. “I recall interviewing a rural doctor several years ago who said that he knew his patients well and when a patient visits with mobility problems, he goes down and carries the patient up the steps to his office. My response was that is not respectful of the patient or safe for the patient or you. That doctor has since changed the location of his practice,” said Dr. Iezzoni.
Some doctors may resist paying for accessible medical equipment because of cost, but she said the benefits are worth it. These include preventing staff injuries when they transfer patients and being used by patients with temporary disabilities and aging people with bad knees, backs, hearing and sight. In addition, businesses may be eligible for federal and state tax credits.
Dr. Iezzoni recently visited her doctor where they finally got height-adjustable exam tables. “I asked the assistant, who really likes these tables? She said it’s the elderly ladies of short stature – the table is lowered and they sit down and get on it.”
But, Dr. Iezonni’s main message to doctors is that patients with disabilities deserve equal quality of care. “Just because we have a disability doesn’t mean we should get worse care than other people. It’s a matter of professionalism that doctors should want to give the same quality care to all their patients.”
A version of this article first appeared on Medscape.com.
Lisa Iezzoni, MD, a professor of medicine at Harvard Medical School and a disability researcher at Massachusetts General Hospital, both in Boston, has used a wheelchair for more than 30 years because of multiple sclerosis. When she visits her primary care doctor, she doesn’t get weighed because the scales are not wheelchair accessible.
This failure to weigh her and other patients in wheelchairs could lead to serious medical problems. Weight is used to monitor a person’s overall health and prenatal health and to determine accurate doses for medications such as some chemotherapies, said Dr. Iezzoni.
In another situation, a man who used a wheelchair said that his primary care doctor never got him out of it for a complete physical exam. The patient later developed lymphoma, which first appeared in his groin. The doctor should have accommodated his disability and used a height-adjustable exam table or a portable lift to transfer him onto the table.
When physicians don’t provide access to medical care that patients with disabilities need, they put themselves at greater risk of lawsuits, fines, and settlements.
Yet, a new study in Health Affairs suggests that a large percentage of doctors are not fully aware of what they are legally required to do.
Under federal nondiscrimination laws (Americans With Disabilities Act, American Rehabilitation Act, and ADA Amendments Act), medical practices must provide equal access to people with disabilities, accommodate their disability-related needs, and not refuse them medical services because of their disabilities, say disability experts.
Where doctors go wrong with disability laws
What doctors don’t know about providing reasonable accommodations makes them vulnerable to lawsuits, which worries more than two-thirds of the 714 outpatient doctors surveyed.
Not only are they required to provide reasonable accommodations, but they also have to pay for them, the researchers said. One-fifth of the surveyed doctors said they didn’t know that practice owners have to pay.
More than one practice has made patients pay for services needed for their disability, such as sign language interpreters – the patients later complained this violated the ADA to enforcement agencies.
Doctors also don’t know that they have to collaborate with patients to determine what reasonable accommodations they need – over two-thirds of those surveyed said they didn’t know it was a joint responsibility, the study found.
When doctors fail to accommodate patients’ disability needs, they engage in discrimination and violate the ADA, says Elizabeth Pendo, JD, a coauthor of the study and the Joseph J. Simeone Professor of Law at Saint Louis University.
The Department of Justice has investigated several patient complaints of alleged disability discrimination recently and resolved the disputes with agreements and small fines in some cases. “The goal is not to get large financial settlements but to work with practices to get the correct procedures in place to be compliant,” said Ms. Pendo.
Physicians would be wise to check out whether their practices are as accessible as they think. Even if there’s a ramp to the office building, the parking lot may not have a van-accessible space or enough handicapped parking signs, or the exam room may be too narrow for a wheelchair to navigate.
These practices violated the ADA and agreed to make changes:
- Hamden, Conn., has two buildings that patients with physical disabilities couldn’t easily enter. The physician owners agreed to change the buildings’ entrances and access routes and add features to make it easier to use examination rooms and restrooms and the check-in and check-out areas.
- Seven medical offices in Riverside, Calif., failed to communicate effectively with deaf and hard-of-hearing patients. They should have had a qualified sign language interpreter, an assistive listening device, or another appropriate aid or service available to a deaf patient and her family. Instead, the office relied on a video remote interpretation system that often failed to work. The agreement requires the clinic to provide those aids and services to patients and their companions who are deaf or hard of hearing, advertise their availability, assess each patient who is deaf or hard of hearing to determine the best aids and services for their needs, and pay $5,000 in compensation to the complainant and a $1,000 civil penalty to the United States.
- Springfield, Mass., refused to provide full joint replacements to two patients being treated with buprenorphine, a medication used to treat opioid use disorder. Rather than accommodate the patients, the surgeons referred them elsewhere because they were uncomfortable with the postoperative pain management protocol for patients prescribed buprenorphine. “The Americans With Disabilities Act protects health care access for people under medical treatment for opioid use disorder,” said Acting U.S. Attorney Nathaniel R. Mendell. “Health care providers must comply with the ADA, even when doing so is inconvenient or makes them uncomfortable.” The agreement requires the practice to adopt a nondiscrimination policy, provide training on the ADA and opioid use disorder, and pay two complainants $15,000 each for pain and suffering.
The DOJ has filed civil lawsuits against medical practices when they failed to resolve the allegations. Recent cases include an ophthalmology practice with 24 facilities in Arizona that refused to help transfer patients in wheelchairs to surgery tables for eye surgery and required them to pay for transfer support services and two obstetricians-gynecologists in Bakersfield, Calif., who refused to provide routine medical care to a patient because of her HIV status.
What doctors should know
Many people tend to think of a person with a disability as being in a wheelchair. But the ADA has a very broad definition of disability, which includes any physical or mental impairment that substantially limits any major life activity, said Ms. Pendo.
“It was amended in 2008 to clarify that the definition includes people with chronic diseases such as diabetes and cancer, cognitive and neurological disorders, substance abuse disorders, vision and hearing loss, and learning and other disabilities,” she said.
That means that doctors have to accommodate many types of disabilities, which can be challenging. The ADA only specifies that fixed structures need to be accessible, such as parking lots, driveways, and buildings, said Dr. Iezzoni.
When it comes to “reasonable accommodations,” doctors should decide that on a case-by-case basis, she said.
“We can say based on our study that 71% of doctors don’t know the right way to think about the accommodations – they don’t know they need to talk to patients so they can explain to them exactly what they need to accommodate their disability,” said Dr. Iezzoni.
Doctors are also required to provide effective communication for patients with sensory or cognitive disabilities, which can depend on the severity, said Ms. Pendo. Is the person deaf or hard of hearing, blind or partially sighted – is the dementia mild or severe?
“The requirement is there, but what that looks like will vary by patient. That’s what’s challenging,” said Ms. Pendo.
Dr. Iezzoni recommends that doctor’s offices ask patients whether they need special help or individual assistance when they make appointments and enter their responses in their records. She also suggests that patients be asked at follow-up appointments whether they still need the same help or not.
“Disabilities can change over time – a person with bad arthritis may need help getting onto an exam table, but later get a knee or hip replacement that is effective and no longer need that help,” said Dr. Iezzoni.
Benefits outweigh costs
Physicians have made progress in meeting the ADA’s physical accessibility requirements, said Dr. Iezzoni. “The literature suggests that doctors have done a good job at fixing the structural barriers people with mobility issues face, such as ramps and bathrooms.”
However, there are exceptions in rural older buildings which can be harder to retrofit for wheelchair accessibility, she said. “I recall interviewing a rural doctor several years ago who said that he knew his patients well and when a patient visits with mobility problems, he goes down and carries the patient up the steps to his office. My response was that is not respectful of the patient or safe for the patient or you. That doctor has since changed the location of his practice,” said Dr. Iezzoni.
Some doctors may resist paying for accessible medical equipment because of cost, but she said the benefits are worth it. These include preventing staff injuries when they transfer patients and being used by patients with temporary disabilities and aging people with bad knees, backs, hearing and sight. In addition, businesses may be eligible for federal and state tax credits.
Dr. Iezzoni recently visited her doctor where they finally got height-adjustable exam tables. “I asked the assistant, who really likes these tables? She said it’s the elderly ladies of short stature – the table is lowered and they sit down and get on it.”
But, Dr. Iezonni’s main message to doctors is that patients with disabilities deserve equal quality of care. “Just because we have a disability doesn’t mean we should get worse care than other people. It’s a matter of professionalism that doctors should want to give the same quality care to all their patients.”
A version of this article first appeared on Medscape.com.
Lisa Iezzoni, MD, a professor of medicine at Harvard Medical School and a disability researcher at Massachusetts General Hospital, both in Boston, has used a wheelchair for more than 30 years because of multiple sclerosis. When she visits her primary care doctor, she doesn’t get weighed because the scales are not wheelchair accessible.
This failure to weigh her and other patients in wheelchairs could lead to serious medical problems. Weight is used to monitor a person’s overall health and prenatal health and to determine accurate doses for medications such as some chemotherapies, said Dr. Iezzoni.
In another situation, a man who used a wheelchair said that his primary care doctor never got him out of it for a complete physical exam. The patient later developed lymphoma, which first appeared in his groin. The doctor should have accommodated his disability and used a height-adjustable exam table or a portable lift to transfer him onto the table.
When physicians don’t provide access to medical care that patients with disabilities need, they put themselves at greater risk of lawsuits, fines, and settlements.
Yet, a new study in Health Affairs suggests that a large percentage of doctors are not fully aware of what they are legally required to do.
Under federal nondiscrimination laws (Americans With Disabilities Act, American Rehabilitation Act, and ADA Amendments Act), medical practices must provide equal access to people with disabilities, accommodate their disability-related needs, and not refuse them medical services because of their disabilities, say disability experts.
Where doctors go wrong with disability laws
What doctors don’t know about providing reasonable accommodations makes them vulnerable to lawsuits, which worries more than two-thirds of the 714 outpatient doctors surveyed.
Not only are they required to provide reasonable accommodations, but they also have to pay for them, the researchers said. One-fifth of the surveyed doctors said they didn’t know that practice owners have to pay.
More than one practice has made patients pay for services needed for their disability, such as sign language interpreters – the patients later complained this violated the ADA to enforcement agencies.
Doctors also don’t know that they have to collaborate with patients to determine what reasonable accommodations they need – over two-thirds of those surveyed said they didn’t know it was a joint responsibility, the study found.
When doctors fail to accommodate patients’ disability needs, they engage in discrimination and violate the ADA, says Elizabeth Pendo, JD, a coauthor of the study and the Joseph J. Simeone Professor of Law at Saint Louis University.
The Department of Justice has investigated several patient complaints of alleged disability discrimination recently and resolved the disputes with agreements and small fines in some cases. “The goal is not to get large financial settlements but to work with practices to get the correct procedures in place to be compliant,” said Ms. Pendo.
Physicians would be wise to check out whether their practices are as accessible as they think. Even if there’s a ramp to the office building, the parking lot may not have a van-accessible space or enough handicapped parking signs, or the exam room may be too narrow for a wheelchair to navigate.
These practices violated the ADA and agreed to make changes:
- Hamden, Conn., has two buildings that patients with physical disabilities couldn’t easily enter. The physician owners agreed to change the buildings’ entrances and access routes and add features to make it easier to use examination rooms and restrooms and the check-in and check-out areas.
- Seven medical offices in Riverside, Calif., failed to communicate effectively with deaf and hard-of-hearing patients. They should have had a qualified sign language interpreter, an assistive listening device, or another appropriate aid or service available to a deaf patient and her family. Instead, the office relied on a video remote interpretation system that often failed to work. The agreement requires the clinic to provide those aids and services to patients and their companions who are deaf or hard of hearing, advertise their availability, assess each patient who is deaf or hard of hearing to determine the best aids and services for their needs, and pay $5,000 in compensation to the complainant and a $1,000 civil penalty to the United States.
- Springfield, Mass., refused to provide full joint replacements to two patients being treated with buprenorphine, a medication used to treat opioid use disorder. Rather than accommodate the patients, the surgeons referred them elsewhere because they were uncomfortable with the postoperative pain management protocol for patients prescribed buprenorphine. “The Americans With Disabilities Act protects health care access for people under medical treatment for opioid use disorder,” said Acting U.S. Attorney Nathaniel R. Mendell. “Health care providers must comply with the ADA, even when doing so is inconvenient or makes them uncomfortable.” The agreement requires the practice to adopt a nondiscrimination policy, provide training on the ADA and opioid use disorder, and pay two complainants $15,000 each for pain and suffering.
The DOJ has filed civil lawsuits against medical practices when they failed to resolve the allegations. Recent cases include an ophthalmology practice with 24 facilities in Arizona that refused to help transfer patients in wheelchairs to surgery tables for eye surgery and required them to pay for transfer support services and two obstetricians-gynecologists in Bakersfield, Calif., who refused to provide routine medical care to a patient because of her HIV status.
What doctors should know
Many people tend to think of a person with a disability as being in a wheelchair. But the ADA has a very broad definition of disability, which includes any physical or mental impairment that substantially limits any major life activity, said Ms. Pendo.
“It was amended in 2008 to clarify that the definition includes people with chronic diseases such as diabetes and cancer, cognitive and neurological disorders, substance abuse disorders, vision and hearing loss, and learning and other disabilities,” she said.
That means that doctors have to accommodate many types of disabilities, which can be challenging. The ADA only specifies that fixed structures need to be accessible, such as parking lots, driveways, and buildings, said Dr. Iezzoni.
When it comes to “reasonable accommodations,” doctors should decide that on a case-by-case basis, she said.
“We can say based on our study that 71% of doctors don’t know the right way to think about the accommodations – they don’t know they need to talk to patients so they can explain to them exactly what they need to accommodate their disability,” said Dr. Iezzoni.
Doctors are also required to provide effective communication for patients with sensory or cognitive disabilities, which can depend on the severity, said Ms. Pendo. Is the person deaf or hard of hearing, blind or partially sighted – is the dementia mild or severe?
“The requirement is there, but what that looks like will vary by patient. That’s what’s challenging,” said Ms. Pendo.
Dr. Iezzoni recommends that doctor’s offices ask patients whether they need special help or individual assistance when they make appointments and enter their responses in their records. She also suggests that patients be asked at follow-up appointments whether they still need the same help or not.
“Disabilities can change over time – a person with bad arthritis may need help getting onto an exam table, but later get a knee or hip replacement that is effective and no longer need that help,” said Dr. Iezzoni.
Benefits outweigh costs
Physicians have made progress in meeting the ADA’s physical accessibility requirements, said Dr. Iezzoni. “The literature suggests that doctors have done a good job at fixing the structural barriers people with mobility issues face, such as ramps and bathrooms.”
However, there are exceptions in rural older buildings which can be harder to retrofit for wheelchair accessibility, she said. “I recall interviewing a rural doctor several years ago who said that he knew his patients well and when a patient visits with mobility problems, he goes down and carries the patient up the steps to his office. My response was that is not respectful of the patient or safe for the patient or you. That doctor has since changed the location of his practice,” said Dr. Iezzoni.
Some doctors may resist paying for accessible medical equipment because of cost, but she said the benefits are worth it. These include preventing staff injuries when they transfer patients and being used by patients with temporary disabilities and aging people with bad knees, backs, hearing and sight. In addition, businesses may be eligible for federal and state tax credits.
Dr. Iezzoni recently visited her doctor where they finally got height-adjustable exam tables. “I asked the assistant, who really likes these tables? She said it’s the elderly ladies of short stature – the table is lowered and they sit down and get on it.”
But, Dr. Iezonni’s main message to doctors is that patients with disabilities deserve equal quality of care. “Just because we have a disability doesn’t mean we should get worse care than other people. It’s a matter of professionalism that doctors should want to give the same quality care to all their patients.”
A version of this article first appeared on Medscape.com.
Children and COVID-19: The Omicron tide may have turned
The Omicron-fueled surge appears to have peaked as new cases of COVID-19 in U.S. children dropped for the first time since late November 2021, dipping back below the 1 million mark for the week, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total number of cases in children was up to 11.4 million as of Jan. 27, with children representing 18.6% of all cases reported since the pandemic started, the AAP and CHA said in their weekly COVID-19 report.
As children remain the largest reservoir of unvaccinated Americans, their share of the COVID case load continues to rise quickly. Just 2 weeks ago, children made up 17.8% of the cumulative number of cases, and at the end of December it was 17.4%, the AAP/CHA data show.
The latest data from the Centers for Disease Control and Prevention show that trends for admissions and emergency department visits reflect the decline in new cases. New admissions of children aged 0-17 years with diagnosed COVID-19 peaked at 1.25 per 100,000 population on Jan. 15 and were down to 0.95 per 100,000 on Jan. 29.
Daily ED visits for COVID-19, measured as a percentage of all ED visits, peaked at 13.9% on Jan. 14 for children aged 0-11 years and on Jan. 9 for both 12- to 15-year-olds (14.1%) and 16- to 17-year-olds (13.8%). By Jan. 28, the rates were down to 5.6% (0-11), 3.1% (12-15), and 3.3% (16-17), the CDC reported based on data from the National Syndromic Surveillance Program.
Trends involving more severe illness support observations that Omicron is milder than earlier variants. Children hospitalized with COVID-19 were less likely to be admitted to an intensive care unit over the last 2 months than during the Delta surge in the late summer and early fall or during the winter of 2020-2021, the CDC said based on data from the BD Insights Research Database, which includes 229,000 patients and 267 hospitals.
Those data show that the highest monthly rate occurred early on, in May of 2020, when 27.8% of children with COVID-19 ended up in the ICU. The rates for December 2021 and January 2022, by comparison, were 11.0% and 11.3%, respectively, the CDC said.
Vaccination lags in younger children
As reports surface about Pfizer-BioNTech filing an emergency use request to extend vaccine coverage to children aged 6 months to 5 years, it does appear that prevention efforts could use the proverbial shot in the arm.
As of Jan. 30, just 30.4% of children aged 5-11 have received at least one dose of the COVID-19 vaccine, and only 21.6% are fully vaccinated. At a comparable point in their timeline – just short of 3 months after approval – the respective numbers for children aged 12-15 were about 42% and 31%, CDC data show.
In the younger group, both initial doses and completions rose slightly in the first 2 weeks of January but then dropped in each of the last 2 weeks. There was a more significant surge in interest among the 12- to 17-year-olds in mid-January, but the last full week of the month brought declines of more than 50% in both measures, according to a separate AAP analysis.
The Omicron-fueled surge appears to have peaked as new cases of COVID-19 in U.S. children dropped for the first time since late November 2021, dipping back below the 1 million mark for the week, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total number of cases in children was up to 11.4 million as of Jan. 27, with children representing 18.6% of all cases reported since the pandemic started, the AAP and CHA said in their weekly COVID-19 report.
As children remain the largest reservoir of unvaccinated Americans, their share of the COVID case load continues to rise quickly. Just 2 weeks ago, children made up 17.8% of the cumulative number of cases, and at the end of December it was 17.4%, the AAP/CHA data show.
The latest data from the Centers for Disease Control and Prevention show that trends for admissions and emergency department visits reflect the decline in new cases. New admissions of children aged 0-17 years with diagnosed COVID-19 peaked at 1.25 per 100,000 population on Jan. 15 and were down to 0.95 per 100,000 on Jan. 29.
Daily ED visits for COVID-19, measured as a percentage of all ED visits, peaked at 13.9% on Jan. 14 for children aged 0-11 years and on Jan. 9 for both 12- to 15-year-olds (14.1%) and 16- to 17-year-olds (13.8%). By Jan. 28, the rates were down to 5.6% (0-11), 3.1% (12-15), and 3.3% (16-17), the CDC reported based on data from the National Syndromic Surveillance Program.
Trends involving more severe illness support observations that Omicron is milder than earlier variants. Children hospitalized with COVID-19 were less likely to be admitted to an intensive care unit over the last 2 months than during the Delta surge in the late summer and early fall or during the winter of 2020-2021, the CDC said based on data from the BD Insights Research Database, which includes 229,000 patients and 267 hospitals.
Those data show that the highest monthly rate occurred early on, in May of 2020, when 27.8% of children with COVID-19 ended up in the ICU. The rates for December 2021 and January 2022, by comparison, were 11.0% and 11.3%, respectively, the CDC said.
Vaccination lags in younger children
As reports surface about Pfizer-BioNTech filing an emergency use request to extend vaccine coverage to children aged 6 months to 5 years, it does appear that prevention efforts could use the proverbial shot in the arm.
As of Jan. 30, just 30.4% of children aged 5-11 have received at least one dose of the COVID-19 vaccine, and only 21.6% are fully vaccinated. At a comparable point in their timeline – just short of 3 months after approval – the respective numbers for children aged 12-15 were about 42% and 31%, CDC data show.
In the younger group, both initial doses and completions rose slightly in the first 2 weeks of January but then dropped in each of the last 2 weeks. There was a more significant surge in interest among the 12- to 17-year-olds in mid-January, but the last full week of the month brought declines of more than 50% in both measures, according to a separate AAP analysis.
The Omicron-fueled surge appears to have peaked as new cases of COVID-19 in U.S. children dropped for the first time since late November 2021, dipping back below the 1 million mark for the week, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The total number of cases in children was up to 11.4 million as of Jan. 27, with children representing 18.6% of all cases reported since the pandemic started, the AAP and CHA said in their weekly COVID-19 report.
As children remain the largest reservoir of unvaccinated Americans, their share of the COVID case load continues to rise quickly. Just 2 weeks ago, children made up 17.8% of the cumulative number of cases, and at the end of December it was 17.4%, the AAP/CHA data show.
The latest data from the Centers for Disease Control and Prevention show that trends for admissions and emergency department visits reflect the decline in new cases. New admissions of children aged 0-17 years with diagnosed COVID-19 peaked at 1.25 per 100,000 population on Jan. 15 and were down to 0.95 per 100,000 on Jan. 29.
Daily ED visits for COVID-19, measured as a percentage of all ED visits, peaked at 13.9% on Jan. 14 for children aged 0-11 years and on Jan. 9 for both 12- to 15-year-olds (14.1%) and 16- to 17-year-olds (13.8%). By Jan. 28, the rates were down to 5.6% (0-11), 3.1% (12-15), and 3.3% (16-17), the CDC reported based on data from the National Syndromic Surveillance Program.
Trends involving more severe illness support observations that Omicron is milder than earlier variants. Children hospitalized with COVID-19 were less likely to be admitted to an intensive care unit over the last 2 months than during the Delta surge in the late summer and early fall or during the winter of 2020-2021, the CDC said based on data from the BD Insights Research Database, which includes 229,000 patients and 267 hospitals.
Those data show that the highest monthly rate occurred early on, in May of 2020, when 27.8% of children with COVID-19 ended up in the ICU. The rates for December 2021 and January 2022, by comparison, were 11.0% and 11.3%, respectively, the CDC said.
Vaccination lags in younger children
As reports surface about Pfizer-BioNTech filing an emergency use request to extend vaccine coverage to children aged 6 months to 5 years, it does appear that prevention efforts could use the proverbial shot in the arm.
As of Jan. 30, just 30.4% of children aged 5-11 have received at least one dose of the COVID-19 vaccine, and only 21.6% are fully vaccinated. At a comparable point in their timeline – just short of 3 months after approval – the respective numbers for children aged 12-15 were about 42% and 31%, CDC data show.
In the younger group, both initial doses and completions rose slightly in the first 2 weeks of January but then dropped in each of the last 2 weeks. There was a more significant surge in interest among the 12- to 17-year-olds in mid-January, but the last full week of the month brought declines of more than 50% in both measures, according to a separate AAP analysis.
CDC issues new pneumococcal vaccine recommendations for adults
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Omicron subvariant 1.5 times more contagious than Omicron
according to CNBC.
The Statens Serum Institut, which monitors infectious diseases in Denmark, said that BA.2 is more contagious, but it doesn’t appear to increase hospitalizations or reduce how well the vaccine works.
BA.2 overtook BA.1 as the primary variant in Denmark within a few weeks, Troels Lillebaek, director of the institute, told CNBC. The subvariant has five unique mutations on a key part of the spike protein, which is what the coronavirus uses to invade human cells. This often means a higher rate of spreading.
The Omicron subvariant has been detected in at least 29 states in the United States and 56 countries, according to the latest update from Outbreak.info. The United States has detected 188 infections, with the worldwide total nearing 25,000.
Denmark has reported the highest number of cases, followed by the United Kingdom and India. Both Denmark and India have reported that BA.2 now accounts for about half of new COVID-19 cases in those countries.
On Jan. 28, the U.K. Health Security Agency said BA.2 has a “substantial” growth advantage over the original Omicron strain. The subvariant has spread faster in all regions of England where there were enough cases to conduct an analysis, the agency said in a report.
A preliminary evaluation found that BA.2 doesn’t appear to change how well the vaccine works compared to the original Omicron strain, the agency said. A booster dose was 70% effective at preventing symptomatic illness for BA.2, compared with 63% for the original Omicron strain.
The Centers for Disease Control and Prevention also said on Jan. 28 that, although the subvariant has become more common in some countries, it is currently at a low level in the United States and doesn’t appear to be more serious.
“Currently there is no evidence that the BA.2 lineage is more severe than the BA.1 lineage,” Kristen Nordlund, a CDC spokesperson, told CNBC.
The World Health Organization hasn’t labeled BA.2 a “variant of concern” so far but will continue to monitor it. WHO officials have said that new variants will arise as Omicron spreads across the world.
“The next variant of concern will be more fit, and what we mean by that is it will be more transmissible because it will have to overtake what is currently circulating,” Maria Van Kerkhove, the WHO’s COVID-19 technical lead, said during a livestream on Jan. 25.
“The big question is whether or not future variants will be more or less severe,” she said.
A version of this article first appeared on WebMD.com.
according to CNBC.
The Statens Serum Institut, which monitors infectious diseases in Denmark, said that BA.2 is more contagious, but it doesn’t appear to increase hospitalizations or reduce how well the vaccine works.
BA.2 overtook BA.1 as the primary variant in Denmark within a few weeks, Troels Lillebaek, director of the institute, told CNBC. The subvariant has five unique mutations on a key part of the spike protein, which is what the coronavirus uses to invade human cells. This often means a higher rate of spreading.
The Omicron subvariant has been detected in at least 29 states in the United States and 56 countries, according to the latest update from Outbreak.info. The United States has detected 188 infections, with the worldwide total nearing 25,000.
Denmark has reported the highest number of cases, followed by the United Kingdom and India. Both Denmark and India have reported that BA.2 now accounts for about half of new COVID-19 cases in those countries.
On Jan. 28, the U.K. Health Security Agency said BA.2 has a “substantial” growth advantage over the original Omicron strain. The subvariant has spread faster in all regions of England where there were enough cases to conduct an analysis, the agency said in a report.
A preliminary evaluation found that BA.2 doesn’t appear to change how well the vaccine works compared to the original Omicron strain, the agency said. A booster dose was 70% effective at preventing symptomatic illness for BA.2, compared with 63% for the original Omicron strain.
The Centers for Disease Control and Prevention also said on Jan. 28 that, although the subvariant has become more common in some countries, it is currently at a low level in the United States and doesn’t appear to be more serious.
“Currently there is no evidence that the BA.2 lineage is more severe than the BA.1 lineage,” Kristen Nordlund, a CDC spokesperson, told CNBC.
The World Health Organization hasn’t labeled BA.2 a “variant of concern” so far but will continue to monitor it. WHO officials have said that new variants will arise as Omicron spreads across the world.
“The next variant of concern will be more fit, and what we mean by that is it will be more transmissible because it will have to overtake what is currently circulating,” Maria Van Kerkhove, the WHO’s COVID-19 technical lead, said during a livestream on Jan. 25.
“The big question is whether or not future variants will be more or less severe,” she said.
A version of this article first appeared on WebMD.com.
according to CNBC.
The Statens Serum Institut, which monitors infectious diseases in Denmark, said that BA.2 is more contagious, but it doesn’t appear to increase hospitalizations or reduce how well the vaccine works.
BA.2 overtook BA.1 as the primary variant in Denmark within a few weeks, Troels Lillebaek, director of the institute, told CNBC. The subvariant has five unique mutations on a key part of the spike protein, which is what the coronavirus uses to invade human cells. This often means a higher rate of spreading.
The Omicron subvariant has been detected in at least 29 states in the United States and 56 countries, according to the latest update from Outbreak.info. The United States has detected 188 infections, with the worldwide total nearing 25,000.
Denmark has reported the highest number of cases, followed by the United Kingdom and India. Both Denmark and India have reported that BA.2 now accounts for about half of new COVID-19 cases in those countries.
On Jan. 28, the U.K. Health Security Agency said BA.2 has a “substantial” growth advantage over the original Omicron strain. The subvariant has spread faster in all regions of England where there were enough cases to conduct an analysis, the agency said in a report.
A preliminary evaluation found that BA.2 doesn’t appear to change how well the vaccine works compared to the original Omicron strain, the agency said. A booster dose was 70% effective at preventing symptomatic illness for BA.2, compared with 63% for the original Omicron strain.
The Centers for Disease Control and Prevention also said on Jan. 28 that, although the subvariant has become more common in some countries, it is currently at a low level in the United States and doesn’t appear to be more serious.
“Currently there is no evidence that the BA.2 lineage is more severe than the BA.1 lineage,” Kristen Nordlund, a CDC spokesperson, told CNBC.
The World Health Organization hasn’t labeled BA.2 a “variant of concern” so far but will continue to monitor it. WHO officials have said that new variants will arise as Omicron spreads across the world.
“The next variant of concern will be more fit, and what we mean by that is it will be more transmissible because it will have to overtake what is currently circulating,” Maria Van Kerkhove, the WHO’s COVID-19 technical lead, said during a livestream on Jan. 25.
“The big question is whether or not future variants will be more or less severe,” she said.
A version of this article first appeared on WebMD.com.
Pandemic pushed death rates to historic highs
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
FROM ANNALS OF INTERNAL MEDICINE
FDA grants full approval to Moderna COVID-19 vaccine
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
ICIs for NSCLC: Patients with ILD show greater risk
, a systematic review and meta-analysis indicated.
“Patients with preexisting ILD, especially symptomatic ILD, are frequently excluded from clinical trials so almost all the patients [we analyzed] were diagnosed with mild preexisting ILD,” said Yuan Cheng, MD, Peking University First Hospital, Beijing, China.
“At this stage, we think that mild ILD is not a contraindication to the use of anti-programmed death-1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) treatment for patients with NSCLC but whether ICIs can be used in patients with moderate to severe ILD needs further study,” she added.
The study was published online Jan. 10 in the journal CHEST.
Ten studies
A total of 179 patients from 10 studies were included in the review and meta-analysis. Among these, six were retrospective case-control studies, one was a retrospective noncontrolled study and three were prospective, noncontrolled clinical trials. “All the included studies were from East Asian countries,” the authors noted.
Preexisting ILD was diagnosed by use of CT or high-resolution CT. The mean age of patients was 71 years (range, 33-85 years), 87% were male and 96% of the cohort had a history of smoking. Approximately one-quarter of patients with ILD had usual interstitial pneumonitis (UIP); about the same percentage had possible UIP; one-third were diagnosed with inconsistent UIP; 14% had nonspecific interstitial pneumonia (NSIP); and 6% had indeterminate UIP.
Patients received ICIs either as first-, second-, or third-line or higher therapy and all were treated with ICI monotherapy by way of either nivolumab (Opdivo), pembrolizumab (Keytruda), or atezolizumab (Tecentriq). About 10% of patients had a PD-L1 tumor proportion score (TPS) of less than 1%, one-quarter had a PD-L1 TPS of 1%-49%, and approximately two-thirds had a TPS of 50% or greater.
Objective response rates
Some 35% of patients with both NSCLC and preexisting ILD achieved an objective response rate (ORR) to ICI therapy and almost two-thirds of patients achieved disease control. However, there was considerable heterogeneity in ORRs between the studies where it ranged from 5.9% to 70%, the authors cautioned.
On meta-analysis, the pooled ORR was 34% (95% confidence interval, 20%-47%) but again, with significant heterogeneity (I2 = 75.9%). However, on meta-analysis of eligible studies, patients with NSCLC who had preexisting ILD were 99% more likely to achieve an ORR compared to those without ILD (odds ratio, 1.99; 95% CI, 1.31-3.00), the investigators pointed out.
The disease control rate (DCR) also varied considerably between studies from a low of 33.3% to a high of 100%, they added. On meta-analysis, the pooled DCR was 66% (95% CI, 56%-75%). “Meanwhile, in patients without preexisting ILD, the crude ORR and pooled ORR were 24.3% and 24% (95% CI, 17%-31%), respectively” – again with significant heterogeneity between studies (I2 = 87.4%).
In contrast to the ORR, there was no difference in the DCR between the two groups, with no evidence of heterogeneity. There were no significant differences between the two groups in either median progression-free survival (PFS) or overall survival (OS). In patients with NSCLC and preexisting ILD, median PFS ranged from 1.4 to 8 months whereas median OS ranged from 15.6 to 27.8 months.
For those without preexisting ILD, the median PFS ranged from 2.3 to 8.1 months while median OS ranged from 17.4 to 25.5 months.
ICI safety
In patients with NSCLC and preexisting ILD, the incidence of immune-related adverse events (irAes) of any grade was 56.7%, whereas the incidence of irAEs grade 3 and higher was 27.7%. “Among the 179 patients included in the studies, 45 developed any grade of CIP, corresponding to a crude incidence of 25.1%,” the authors noted – very similar to the pooled incidence of 27% on meta-analysis.
The pooled incidence of grade 3 and higher CIP in the same group of patients was 15%. The median time from initiation of ICIs to the development of CIP ranged from 31 to 74 days, but 88% of patients who developed CIP improved with appropriate treatment. In patients with NSCLC who did not have ILD, the pooled incidence of CIP was 10% (95% CI, 6%-13%), again with significant heterogeneity between studies (I2 = 78.8%). “Generally, CIP can be managed through ICI discontinuation with or without steroid administration,” the authors noted.
However, even if most CIP can be easily managed, “the incidence of severe CIP is higher [in NSCLC patients with preexisting ILD] than in other populations,” Dr. Chen observed. “So patients with preexisting ILD should be closely monitored during ICI therapy,” she added.
Indeed, compared with patients without preexisting ILD, grade 3 or higher CIP in patients with the dual diagnosis was significantly higher at an OR of 3.23 (95%, 2.06-5.06), the investigators emphasized.
A limitation to the review and systematic meta-analysis included the fact that none of the studies analyzed were randomized clinical trials and most of the studies were retrospective and had several other shortcomings.
Umbrella diagnosis
Asked to comment on the review, Karthik Suresh, MD, associate professor of medicine, Johns Hopkins University, Baltimore, pointed out that ILD is really an “umbrella” diagnosis that a few hundred diseases fit under, so the first question he and members of his multidisciplinary team ask is: What is the nature of the ILD in this patient? What is the actual underlying etiology?
It could, for example, be that the patient has undergone prior chemotherapy or radiation therapy and has developed ILD as a result, as Dr. Suresh and his coauthor, Jarushka Naidoo, MD, Sidney Kimmel Comprehensive Cancer Center, Baltimore, pointed out in their paper on how to approach patients with preexisting lung disease to avoid ICI toxicities. “We’ll go back to their prior CT scans and can see the ILD has been there for years – it’s stable and the patient’s lung function is not changing,” Dr. Suresh related to this news organization.
“That’s a very different story from a [patients] whom there are new interstitial changes, who are progressing and who are symptomatic,” he noted. Essentially, what Dr. Suresh and his team members want to know is: What is the specific subdiagnosis of this disease, how severe is it, and is it progressing? Then they need to take the tumor itself into consideration.
“Some tumors have high PD-L1 expression, others have low PD-L1 expression so response to immunotherapy is usually very different based on tumor histology,” Dr. Suresh pointed out. Thus, the next question that needs to be addressed is: What is the expected response of the tumor to ICI therapy? If a tumor is exquisitely sensitive to immunotherapy, “that changes the game,” Dr. Suresh said, “whereas with other tumors, the oncologist might say there may be some benefit but it won’t be dramatic.”
The third risk factor for ICI toxicity that needs to be evaluated is the patient’s general cardiopulmonary status – for example, if a patient has mild, even moderate, ILD but is still walking 3 miles a day, has no heart problems, and is doing fine. Another patient with the same severity of disease in turn may have mild heart failure, be relatively debilitated, and sedentary: “Performance status also plays a big role in determining treatment,” Dr. Suresh emphasized.
The presence of other pulmonary conditions such as chronic obstructive pulmonary disease – common in patients with NSCLC – has to be taken into account, too. Lastly, clinicians need to ask themselves if there are any alternative therapies that might work just as well if not better than ICI therapy for this particular patient. If the patient has had genomic testing, results might indicate that the tumor has a mutation that may respond well to targeted therapies. “We put all these factors out on the table,” Dr. Suresh said.
“And you obviously have to involve the patient, too, so they understand the risks of ICI therapy and together we decide, ‘Yes, this patient with ILD should get immunotherapy or no, they should not,’ “ he said.
The study had no specific funding. The study authors and Dr. Suresh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a systematic review and meta-analysis indicated.
“Patients with preexisting ILD, especially symptomatic ILD, are frequently excluded from clinical trials so almost all the patients [we analyzed] were diagnosed with mild preexisting ILD,” said Yuan Cheng, MD, Peking University First Hospital, Beijing, China.
“At this stage, we think that mild ILD is not a contraindication to the use of anti-programmed death-1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) treatment for patients with NSCLC but whether ICIs can be used in patients with moderate to severe ILD needs further study,” she added.
The study was published online Jan. 10 in the journal CHEST.
Ten studies
A total of 179 patients from 10 studies were included in the review and meta-analysis. Among these, six were retrospective case-control studies, one was a retrospective noncontrolled study and three were prospective, noncontrolled clinical trials. “All the included studies were from East Asian countries,” the authors noted.
Preexisting ILD was diagnosed by use of CT or high-resolution CT. The mean age of patients was 71 years (range, 33-85 years), 87% were male and 96% of the cohort had a history of smoking. Approximately one-quarter of patients with ILD had usual interstitial pneumonitis (UIP); about the same percentage had possible UIP; one-third were diagnosed with inconsistent UIP; 14% had nonspecific interstitial pneumonia (NSIP); and 6% had indeterminate UIP.
Patients received ICIs either as first-, second-, or third-line or higher therapy and all were treated with ICI monotherapy by way of either nivolumab (Opdivo), pembrolizumab (Keytruda), or atezolizumab (Tecentriq). About 10% of patients had a PD-L1 tumor proportion score (TPS) of less than 1%, one-quarter had a PD-L1 TPS of 1%-49%, and approximately two-thirds had a TPS of 50% or greater.
Objective response rates
Some 35% of patients with both NSCLC and preexisting ILD achieved an objective response rate (ORR) to ICI therapy and almost two-thirds of patients achieved disease control. However, there was considerable heterogeneity in ORRs between the studies where it ranged from 5.9% to 70%, the authors cautioned.
On meta-analysis, the pooled ORR was 34% (95% confidence interval, 20%-47%) but again, with significant heterogeneity (I2 = 75.9%). However, on meta-analysis of eligible studies, patients with NSCLC who had preexisting ILD were 99% more likely to achieve an ORR compared to those without ILD (odds ratio, 1.99; 95% CI, 1.31-3.00), the investigators pointed out.
The disease control rate (DCR) also varied considerably between studies from a low of 33.3% to a high of 100%, they added. On meta-analysis, the pooled DCR was 66% (95% CI, 56%-75%). “Meanwhile, in patients without preexisting ILD, the crude ORR and pooled ORR were 24.3% and 24% (95% CI, 17%-31%), respectively” – again with significant heterogeneity between studies (I2 = 87.4%).
In contrast to the ORR, there was no difference in the DCR between the two groups, with no evidence of heterogeneity. There were no significant differences between the two groups in either median progression-free survival (PFS) or overall survival (OS). In patients with NSCLC and preexisting ILD, median PFS ranged from 1.4 to 8 months whereas median OS ranged from 15.6 to 27.8 months.
For those without preexisting ILD, the median PFS ranged from 2.3 to 8.1 months while median OS ranged from 17.4 to 25.5 months.
ICI safety
In patients with NSCLC and preexisting ILD, the incidence of immune-related adverse events (irAes) of any grade was 56.7%, whereas the incidence of irAEs grade 3 and higher was 27.7%. “Among the 179 patients included in the studies, 45 developed any grade of CIP, corresponding to a crude incidence of 25.1%,” the authors noted – very similar to the pooled incidence of 27% on meta-analysis.
The pooled incidence of grade 3 and higher CIP in the same group of patients was 15%. The median time from initiation of ICIs to the development of CIP ranged from 31 to 74 days, but 88% of patients who developed CIP improved with appropriate treatment. In patients with NSCLC who did not have ILD, the pooled incidence of CIP was 10% (95% CI, 6%-13%), again with significant heterogeneity between studies (I2 = 78.8%). “Generally, CIP can be managed through ICI discontinuation with or without steroid administration,” the authors noted.
However, even if most CIP can be easily managed, “the incidence of severe CIP is higher [in NSCLC patients with preexisting ILD] than in other populations,” Dr. Chen observed. “So patients with preexisting ILD should be closely monitored during ICI therapy,” she added.
Indeed, compared with patients without preexisting ILD, grade 3 or higher CIP in patients with the dual diagnosis was significantly higher at an OR of 3.23 (95%, 2.06-5.06), the investigators emphasized.
A limitation to the review and systematic meta-analysis included the fact that none of the studies analyzed were randomized clinical trials and most of the studies were retrospective and had several other shortcomings.
Umbrella diagnosis
Asked to comment on the review, Karthik Suresh, MD, associate professor of medicine, Johns Hopkins University, Baltimore, pointed out that ILD is really an “umbrella” diagnosis that a few hundred diseases fit under, so the first question he and members of his multidisciplinary team ask is: What is the nature of the ILD in this patient? What is the actual underlying etiology?
It could, for example, be that the patient has undergone prior chemotherapy or radiation therapy and has developed ILD as a result, as Dr. Suresh and his coauthor, Jarushka Naidoo, MD, Sidney Kimmel Comprehensive Cancer Center, Baltimore, pointed out in their paper on how to approach patients with preexisting lung disease to avoid ICI toxicities. “We’ll go back to their prior CT scans and can see the ILD has been there for years – it’s stable and the patient’s lung function is not changing,” Dr. Suresh related to this news organization.
“That’s a very different story from a [patients] whom there are new interstitial changes, who are progressing and who are symptomatic,” he noted. Essentially, what Dr. Suresh and his team members want to know is: What is the specific subdiagnosis of this disease, how severe is it, and is it progressing? Then they need to take the tumor itself into consideration.
“Some tumors have high PD-L1 expression, others have low PD-L1 expression so response to immunotherapy is usually very different based on tumor histology,” Dr. Suresh pointed out. Thus, the next question that needs to be addressed is: What is the expected response of the tumor to ICI therapy? If a tumor is exquisitely sensitive to immunotherapy, “that changes the game,” Dr. Suresh said, “whereas with other tumors, the oncologist might say there may be some benefit but it won’t be dramatic.”
The third risk factor for ICI toxicity that needs to be evaluated is the patient’s general cardiopulmonary status – for example, if a patient has mild, even moderate, ILD but is still walking 3 miles a day, has no heart problems, and is doing fine. Another patient with the same severity of disease in turn may have mild heart failure, be relatively debilitated, and sedentary: “Performance status also plays a big role in determining treatment,” Dr. Suresh emphasized.
The presence of other pulmonary conditions such as chronic obstructive pulmonary disease – common in patients with NSCLC – has to be taken into account, too. Lastly, clinicians need to ask themselves if there are any alternative therapies that might work just as well if not better than ICI therapy for this particular patient. If the patient has had genomic testing, results might indicate that the tumor has a mutation that may respond well to targeted therapies. “We put all these factors out on the table,” Dr. Suresh said.
“And you obviously have to involve the patient, too, so they understand the risks of ICI therapy and together we decide, ‘Yes, this patient with ILD should get immunotherapy or no, they should not,’ “ he said.
The study had no specific funding. The study authors and Dr. Suresh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a systematic review and meta-analysis indicated.
“Patients with preexisting ILD, especially symptomatic ILD, are frequently excluded from clinical trials so almost all the patients [we analyzed] were diagnosed with mild preexisting ILD,” said Yuan Cheng, MD, Peking University First Hospital, Beijing, China.
“At this stage, we think that mild ILD is not a contraindication to the use of anti-programmed death-1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) treatment for patients with NSCLC but whether ICIs can be used in patients with moderate to severe ILD needs further study,” she added.
The study was published online Jan. 10 in the journal CHEST.
Ten studies
A total of 179 patients from 10 studies were included in the review and meta-analysis. Among these, six were retrospective case-control studies, one was a retrospective noncontrolled study and three were prospective, noncontrolled clinical trials. “All the included studies were from East Asian countries,” the authors noted.
Preexisting ILD was diagnosed by use of CT or high-resolution CT. The mean age of patients was 71 years (range, 33-85 years), 87% were male and 96% of the cohort had a history of smoking. Approximately one-quarter of patients with ILD had usual interstitial pneumonitis (UIP); about the same percentage had possible UIP; one-third were diagnosed with inconsistent UIP; 14% had nonspecific interstitial pneumonia (NSIP); and 6% had indeterminate UIP.
Patients received ICIs either as first-, second-, or third-line or higher therapy and all were treated with ICI monotherapy by way of either nivolumab (Opdivo), pembrolizumab (Keytruda), or atezolizumab (Tecentriq). About 10% of patients had a PD-L1 tumor proportion score (TPS) of less than 1%, one-quarter had a PD-L1 TPS of 1%-49%, and approximately two-thirds had a TPS of 50% or greater.
Objective response rates
Some 35% of patients with both NSCLC and preexisting ILD achieved an objective response rate (ORR) to ICI therapy and almost two-thirds of patients achieved disease control. However, there was considerable heterogeneity in ORRs between the studies where it ranged from 5.9% to 70%, the authors cautioned.
On meta-analysis, the pooled ORR was 34% (95% confidence interval, 20%-47%) but again, with significant heterogeneity (I2 = 75.9%). However, on meta-analysis of eligible studies, patients with NSCLC who had preexisting ILD were 99% more likely to achieve an ORR compared to those without ILD (odds ratio, 1.99; 95% CI, 1.31-3.00), the investigators pointed out.
The disease control rate (DCR) also varied considerably between studies from a low of 33.3% to a high of 100%, they added. On meta-analysis, the pooled DCR was 66% (95% CI, 56%-75%). “Meanwhile, in patients without preexisting ILD, the crude ORR and pooled ORR were 24.3% and 24% (95% CI, 17%-31%), respectively” – again with significant heterogeneity between studies (I2 = 87.4%).
In contrast to the ORR, there was no difference in the DCR between the two groups, with no evidence of heterogeneity. There were no significant differences between the two groups in either median progression-free survival (PFS) or overall survival (OS). In patients with NSCLC and preexisting ILD, median PFS ranged from 1.4 to 8 months whereas median OS ranged from 15.6 to 27.8 months.
For those without preexisting ILD, the median PFS ranged from 2.3 to 8.1 months while median OS ranged from 17.4 to 25.5 months.
ICI safety
In patients with NSCLC and preexisting ILD, the incidence of immune-related adverse events (irAes) of any grade was 56.7%, whereas the incidence of irAEs grade 3 and higher was 27.7%. “Among the 179 patients included in the studies, 45 developed any grade of CIP, corresponding to a crude incidence of 25.1%,” the authors noted – very similar to the pooled incidence of 27% on meta-analysis.
The pooled incidence of grade 3 and higher CIP in the same group of patients was 15%. The median time from initiation of ICIs to the development of CIP ranged from 31 to 74 days, but 88% of patients who developed CIP improved with appropriate treatment. In patients with NSCLC who did not have ILD, the pooled incidence of CIP was 10% (95% CI, 6%-13%), again with significant heterogeneity between studies (I2 = 78.8%). “Generally, CIP can be managed through ICI discontinuation with or without steroid administration,” the authors noted.
However, even if most CIP can be easily managed, “the incidence of severe CIP is higher [in NSCLC patients with preexisting ILD] than in other populations,” Dr. Chen observed. “So patients with preexisting ILD should be closely monitored during ICI therapy,” she added.
Indeed, compared with patients without preexisting ILD, grade 3 or higher CIP in patients with the dual diagnosis was significantly higher at an OR of 3.23 (95%, 2.06-5.06), the investigators emphasized.
A limitation to the review and systematic meta-analysis included the fact that none of the studies analyzed were randomized clinical trials and most of the studies were retrospective and had several other shortcomings.
Umbrella diagnosis
Asked to comment on the review, Karthik Suresh, MD, associate professor of medicine, Johns Hopkins University, Baltimore, pointed out that ILD is really an “umbrella” diagnosis that a few hundred diseases fit under, so the first question he and members of his multidisciplinary team ask is: What is the nature of the ILD in this patient? What is the actual underlying etiology?
It could, for example, be that the patient has undergone prior chemotherapy or radiation therapy and has developed ILD as a result, as Dr. Suresh and his coauthor, Jarushka Naidoo, MD, Sidney Kimmel Comprehensive Cancer Center, Baltimore, pointed out in their paper on how to approach patients with preexisting lung disease to avoid ICI toxicities. “We’ll go back to their prior CT scans and can see the ILD has been there for years – it’s stable and the patient’s lung function is not changing,” Dr. Suresh related to this news organization.
“That’s a very different story from a [patients] whom there are new interstitial changes, who are progressing and who are symptomatic,” he noted. Essentially, what Dr. Suresh and his team members want to know is: What is the specific subdiagnosis of this disease, how severe is it, and is it progressing? Then they need to take the tumor itself into consideration.
“Some tumors have high PD-L1 expression, others have low PD-L1 expression so response to immunotherapy is usually very different based on tumor histology,” Dr. Suresh pointed out. Thus, the next question that needs to be addressed is: What is the expected response of the tumor to ICI therapy? If a tumor is exquisitely sensitive to immunotherapy, “that changes the game,” Dr. Suresh said, “whereas with other tumors, the oncologist might say there may be some benefit but it won’t be dramatic.”
The third risk factor for ICI toxicity that needs to be evaluated is the patient’s general cardiopulmonary status – for example, if a patient has mild, even moderate, ILD but is still walking 3 miles a day, has no heart problems, and is doing fine. Another patient with the same severity of disease in turn may have mild heart failure, be relatively debilitated, and sedentary: “Performance status also plays a big role in determining treatment,” Dr. Suresh emphasized.
The presence of other pulmonary conditions such as chronic obstructive pulmonary disease – common in patients with NSCLC – has to be taken into account, too. Lastly, clinicians need to ask themselves if there are any alternative therapies that might work just as well if not better than ICI therapy for this particular patient. If the patient has had genomic testing, results might indicate that the tumor has a mutation that may respond well to targeted therapies. “We put all these factors out on the table,” Dr. Suresh said.
“And you obviously have to involve the patient, too, so they understand the risks of ICI therapy and together we decide, ‘Yes, this patient with ILD should get immunotherapy or no, they should not,’ “ he said.
The study had no specific funding. The study authors and Dr. Suresh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long COVID is real, and many real questions remain
Long story short, we still have a lot to learn about long COVID-19.
But it is a real phenomenon with real long-term health effects for people recovering from coronavirus infections. And diagnosing and managing it can get tricky, as some symptoms of long COVID-19 overlap with those of other conditions – and what many people have as they recover from any challenging stay in the ICU.
Risk factors remain largely unknown as well: What makes one person more likely to have symptoms like fatigue, “brain fog,” or headaches versus someone else? Researchers are just starting to offer some intriguing answers, but the evidence is preliminary at this point, experts said at a media briefing sponsored by the Infectious Diseases Society of America.
Unanswered questions include: Does an autoimmune reaction drive long COVID? Does the coronavirus linger in reservoirs within the body and reactivate later? What protection against long COVID do vaccines and treatments offer, if any?
To get a handle on these and other questions, nailing down a standard definition of long COVID would be a good start.
“Studies so far have used different definitions of long COVID,” Nahid Bhadelia, MD, founding director of the Boston University Center for Emerging Infectious Diseases Policy and Research, said during the briefing.
Fatigue is the most commonly symptom of long COVID in research so far, said Dr. Bhadelia, who is also an associate professor of medicine at Boston University.
“What’s difficult in this situation is it’s been 2 years in a global pandemic. We’re all fatigued. How do you tease this apart?” she asked.
Other common symptoms are a hard time thinking quickly – also known as “brain fog” – and the feeling that, despite normal oxygen levels, breathing is difficult, said Kathleen Bell, MD.
Headache, joint and muscle pain, and persistent loss of smell and taste are also widely reported, said Dr. Bell, a professor and chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center in Dallas.
Not all the symptoms are physical either.
“Pretty prominent things that we’re seeing are very high levels of anxiety, depression, and insomnia,” Dr. Bell said. These “actually seem to be associated independently with the virus as opposed to just being a completely reactive component.”
More research will be needed to distinguish the causes of these conditions.
A difficult diagnosis
the experts said.
“We are starting to see some interesting features of inaccurate attributions to COVID, both on the part of perhaps the person with long COVID symptoms and health care providers,” Dr. Bell said.“It’s sometimes a little difficult to sort it out.”
Dr. Bell said she was not suggesting misdiagnoses are common, “but it is difficult for physicians that don’t see a lot of people with long COVID.”
The advice is to consider other conditions. “You can have both a long COVID syndrome and other syndromes as well,” she said. “As one of my teachers used to say: ‘You can have both ticks and fleas.’ ”
Predicting long COVID
In a study getting attention, researchers identified four early things linked to greater chances that someone with COVID-19 will have long-term effects: type 2 diabetes at the time of diagnosis, the presence of specific autoantibodies, unusual levels of SARS-CoV-2 RNA in the blood, and signs of the Epstein-Barr virus in the blood.
The study, published in Cell, followed 309 people 2-3 months after COVID-19.
“That’s important work, but it’s early work,” Dr. Bhadelia said. “I think we still have a while to go in terms of understanding the mechanism of long COVID.”
Unexpected patients getting long COVID care
“We are seeing different populations than we all expected to see when this pandemic first started,” Dr. Bell said.
Instead of seeing primarily patients who had severe COVID-19, “the preponderance of people that we’re seeing in long COVID clinics are people who are enabled, were never hospitalized, and have what people might call mild to moderate cases of coronavirus infection,” she said.
Also, instead of just older patients, people of all ages are seeking long COVID care.
One thing that appears more certain is a lack of diversity in people seeking care at long COVID clinics nationwide.
“Many of us who have long COVID specialty clinics will tell you that we are tending to see fairly educated, socioeconomically stable population in these clinics,” Dr. Bell said. “We know that based on the early statistics of who’s getting COVID and having significant COVID that we may not be seeing those populations for follow-up.”
Is an autoinflammatory process to blame?
It remains unclear if a hyperinflammatory response is driving persistent post–COVID-19 symptoms. Children and some adults have developed multisystem inflammatory conditions associated with COVID-19, for example.
There is a signal, and “I think there is enough data now to show something does happen,” Dr. Bhadelia said. “The question is, how often does it happen?”
Spending time in critical care, even without COVID-19, can result in persistent symptoms after a hospital stay, such as acute respiratory distress syndrome. Recovery can take time because being in an ICU is “basically the physiologically equivalent of a car crash,” Dr. Bhadelia said. “So you’re recovering from that, too.”
Dr. Bell agreed. “You’re not only recovering from the virus itself, you’re recovering from intubation, secondary infections, secondary lung conditions, perhaps other organ failure, and prolonged bed rest. There are so many things that go into that, that it’s a little bit hard to sort that out from what long COVID is and what the direct effects of the virus are.”
Also a research opportunity
“I hate to call it this, but we’ve never had an opportunity [where] we have so many people in such a short amount of time with the same viral disorder,” Dr. Bell said. “We also have the technology to investigate it. This has never happened.
“SARS-CoV-2 is not the only virus. This is just the only one we’ve gotten whacked with in such a huge quantity at one time,” she said.
What researchers learn now about COVID-19 and long COVID “is a model that’s going to be able to be applied in the future to infectious diseases in general,” Dr. Bell predicted.
How long will long COVID last?
The vast majority of people with long COVID will get better over time, given enough support and relief of their symptoms, Dr. Bell said.
Type 2 diabetes, preexisting pulmonary disease, and other things could affect how long it takes to recover from long COVID, she said, although more evidence is needed.
“I don’t think at this point that anyone can say how long this long COVID will last because there are a variety of factors,” Dr. Bell said.
A version of this article first appeared on WebMD.com.
Long story short, we still have a lot to learn about long COVID-19.
But it is a real phenomenon with real long-term health effects for people recovering from coronavirus infections. And diagnosing and managing it can get tricky, as some symptoms of long COVID-19 overlap with those of other conditions – and what many people have as they recover from any challenging stay in the ICU.
Risk factors remain largely unknown as well: What makes one person more likely to have symptoms like fatigue, “brain fog,” or headaches versus someone else? Researchers are just starting to offer some intriguing answers, but the evidence is preliminary at this point, experts said at a media briefing sponsored by the Infectious Diseases Society of America.
Unanswered questions include: Does an autoimmune reaction drive long COVID? Does the coronavirus linger in reservoirs within the body and reactivate later? What protection against long COVID do vaccines and treatments offer, if any?
To get a handle on these and other questions, nailing down a standard definition of long COVID would be a good start.
“Studies so far have used different definitions of long COVID,” Nahid Bhadelia, MD, founding director of the Boston University Center for Emerging Infectious Diseases Policy and Research, said during the briefing.
Fatigue is the most commonly symptom of long COVID in research so far, said Dr. Bhadelia, who is also an associate professor of medicine at Boston University.
“What’s difficult in this situation is it’s been 2 years in a global pandemic. We’re all fatigued. How do you tease this apart?” she asked.
Other common symptoms are a hard time thinking quickly – also known as “brain fog” – and the feeling that, despite normal oxygen levels, breathing is difficult, said Kathleen Bell, MD.
Headache, joint and muscle pain, and persistent loss of smell and taste are also widely reported, said Dr. Bell, a professor and chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center in Dallas.
Not all the symptoms are physical either.
“Pretty prominent things that we’re seeing are very high levels of anxiety, depression, and insomnia,” Dr. Bell said. These “actually seem to be associated independently with the virus as opposed to just being a completely reactive component.”
More research will be needed to distinguish the causes of these conditions.
A difficult diagnosis
the experts said.
“We are starting to see some interesting features of inaccurate attributions to COVID, both on the part of perhaps the person with long COVID symptoms and health care providers,” Dr. Bell said.“It’s sometimes a little difficult to sort it out.”
Dr. Bell said she was not suggesting misdiagnoses are common, “but it is difficult for physicians that don’t see a lot of people with long COVID.”
The advice is to consider other conditions. “You can have both a long COVID syndrome and other syndromes as well,” she said. “As one of my teachers used to say: ‘You can have both ticks and fleas.’ ”
Predicting long COVID
In a study getting attention, researchers identified four early things linked to greater chances that someone with COVID-19 will have long-term effects: type 2 diabetes at the time of diagnosis, the presence of specific autoantibodies, unusual levels of SARS-CoV-2 RNA in the blood, and signs of the Epstein-Barr virus in the blood.
The study, published in Cell, followed 309 people 2-3 months after COVID-19.
“That’s important work, but it’s early work,” Dr. Bhadelia said. “I think we still have a while to go in terms of understanding the mechanism of long COVID.”
Unexpected patients getting long COVID care
“We are seeing different populations than we all expected to see when this pandemic first started,” Dr. Bell said.
Instead of seeing primarily patients who had severe COVID-19, “the preponderance of people that we’re seeing in long COVID clinics are people who are enabled, were never hospitalized, and have what people might call mild to moderate cases of coronavirus infection,” she said.
Also, instead of just older patients, people of all ages are seeking long COVID care.
One thing that appears more certain is a lack of diversity in people seeking care at long COVID clinics nationwide.
“Many of us who have long COVID specialty clinics will tell you that we are tending to see fairly educated, socioeconomically stable population in these clinics,” Dr. Bell said. “We know that based on the early statistics of who’s getting COVID and having significant COVID that we may not be seeing those populations for follow-up.”
Is an autoinflammatory process to blame?
It remains unclear if a hyperinflammatory response is driving persistent post–COVID-19 symptoms. Children and some adults have developed multisystem inflammatory conditions associated with COVID-19, for example.
There is a signal, and “I think there is enough data now to show something does happen,” Dr. Bhadelia said. “The question is, how often does it happen?”
Spending time in critical care, even without COVID-19, can result in persistent symptoms after a hospital stay, such as acute respiratory distress syndrome. Recovery can take time because being in an ICU is “basically the physiologically equivalent of a car crash,” Dr. Bhadelia said. “So you’re recovering from that, too.”
Dr. Bell agreed. “You’re not only recovering from the virus itself, you’re recovering from intubation, secondary infections, secondary lung conditions, perhaps other organ failure, and prolonged bed rest. There are so many things that go into that, that it’s a little bit hard to sort that out from what long COVID is and what the direct effects of the virus are.”
Also a research opportunity
“I hate to call it this, but we’ve never had an opportunity [where] we have so many people in such a short amount of time with the same viral disorder,” Dr. Bell said. “We also have the technology to investigate it. This has never happened.
“SARS-CoV-2 is not the only virus. This is just the only one we’ve gotten whacked with in such a huge quantity at one time,” she said.
What researchers learn now about COVID-19 and long COVID “is a model that’s going to be able to be applied in the future to infectious diseases in general,” Dr. Bell predicted.
How long will long COVID last?
The vast majority of people with long COVID will get better over time, given enough support and relief of their symptoms, Dr. Bell said.
Type 2 diabetes, preexisting pulmonary disease, and other things could affect how long it takes to recover from long COVID, she said, although more evidence is needed.
“I don’t think at this point that anyone can say how long this long COVID will last because there are a variety of factors,” Dr. Bell said.
A version of this article first appeared on WebMD.com.
Long story short, we still have a lot to learn about long COVID-19.
But it is a real phenomenon with real long-term health effects for people recovering from coronavirus infections. And diagnosing and managing it can get tricky, as some symptoms of long COVID-19 overlap with those of other conditions – and what many people have as they recover from any challenging stay in the ICU.
Risk factors remain largely unknown as well: What makes one person more likely to have symptoms like fatigue, “brain fog,” or headaches versus someone else? Researchers are just starting to offer some intriguing answers, but the evidence is preliminary at this point, experts said at a media briefing sponsored by the Infectious Diseases Society of America.
Unanswered questions include: Does an autoimmune reaction drive long COVID? Does the coronavirus linger in reservoirs within the body and reactivate later? What protection against long COVID do vaccines and treatments offer, if any?
To get a handle on these and other questions, nailing down a standard definition of long COVID would be a good start.
“Studies so far have used different definitions of long COVID,” Nahid Bhadelia, MD, founding director of the Boston University Center for Emerging Infectious Diseases Policy and Research, said during the briefing.
Fatigue is the most commonly symptom of long COVID in research so far, said Dr. Bhadelia, who is also an associate professor of medicine at Boston University.
“What’s difficult in this situation is it’s been 2 years in a global pandemic. We’re all fatigued. How do you tease this apart?” she asked.
Other common symptoms are a hard time thinking quickly – also known as “brain fog” – and the feeling that, despite normal oxygen levels, breathing is difficult, said Kathleen Bell, MD.
Headache, joint and muscle pain, and persistent loss of smell and taste are also widely reported, said Dr. Bell, a professor and chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center in Dallas.
Not all the symptoms are physical either.
“Pretty prominent things that we’re seeing are very high levels of anxiety, depression, and insomnia,” Dr. Bell said. These “actually seem to be associated independently with the virus as opposed to just being a completely reactive component.”
More research will be needed to distinguish the causes of these conditions.
A difficult diagnosis
the experts said.
“We are starting to see some interesting features of inaccurate attributions to COVID, both on the part of perhaps the person with long COVID symptoms and health care providers,” Dr. Bell said.“It’s sometimes a little difficult to sort it out.”
Dr. Bell said she was not suggesting misdiagnoses are common, “but it is difficult for physicians that don’t see a lot of people with long COVID.”
The advice is to consider other conditions. “You can have both a long COVID syndrome and other syndromes as well,” she said. “As one of my teachers used to say: ‘You can have both ticks and fleas.’ ”
Predicting long COVID
In a study getting attention, researchers identified four early things linked to greater chances that someone with COVID-19 will have long-term effects: type 2 diabetes at the time of diagnosis, the presence of specific autoantibodies, unusual levels of SARS-CoV-2 RNA in the blood, and signs of the Epstein-Barr virus in the blood.
The study, published in Cell, followed 309 people 2-3 months after COVID-19.
“That’s important work, but it’s early work,” Dr. Bhadelia said. “I think we still have a while to go in terms of understanding the mechanism of long COVID.”
Unexpected patients getting long COVID care
“We are seeing different populations than we all expected to see when this pandemic first started,” Dr. Bell said.
Instead of seeing primarily patients who had severe COVID-19, “the preponderance of people that we’re seeing in long COVID clinics are people who are enabled, were never hospitalized, and have what people might call mild to moderate cases of coronavirus infection,” she said.
Also, instead of just older patients, people of all ages are seeking long COVID care.
One thing that appears more certain is a lack of diversity in people seeking care at long COVID clinics nationwide.
“Many of us who have long COVID specialty clinics will tell you that we are tending to see fairly educated, socioeconomically stable population in these clinics,” Dr. Bell said. “We know that based on the early statistics of who’s getting COVID and having significant COVID that we may not be seeing those populations for follow-up.”
Is an autoinflammatory process to blame?
It remains unclear if a hyperinflammatory response is driving persistent post–COVID-19 symptoms. Children and some adults have developed multisystem inflammatory conditions associated with COVID-19, for example.
There is a signal, and “I think there is enough data now to show something does happen,” Dr. Bhadelia said. “The question is, how often does it happen?”
Spending time in critical care, even without COVID-19, can result in persistent symptoms after a hospital stay, such as acute respiratory distress syndrome. Recovery can take time because being in an ICU is “basically the physiologically equivalent of a car crash,” Dr. Bhadelia said. “So you’re recovering from that, too.”
Dr. Bell agreed. “You’re not only recovering from the virus itself, you’re recovering from intubation, secondary infections, secondary lung conditions, perhaps other organ failure, and prolonged bed rest. There are so many things that go into that, that it’s a little bit hard to sort that out from what long COVID is and what the direct effects of the virus are.”
Also a research opportunity
“I hate to call it this, but we’ve never had an opportunity [where] we have so many people in such a short amount of time with the same viral disorder,” Dr. Bell said. “We also have the technology to investigate it. This has never happened.
“SARS-CoV-2 is not the only virus. This is just the only one we’ve gotten whacked with in such a huge quantity at one time,” she said.
What researchers learn now about COVID-19 and long COVID “is a model that’s going to be able to be applied in the future to infectious diseases in general,” Dr. Bell predicted.
How long will long COVID last?
The vast majority of people with long COVID will get better over time, given enough support and relief of their symptoms, Dr. Bell said.
Type 2 diabetes, preexisting pulmonary disease, and other things could affect how long it takes to recover from long COVID, she said, although more evidence is needed.
“I don’t think at this point that anyone can say how long this long COVID will last because there are a variety of factors,” Dr. Bell said.
A version of this article first appeared on WebMD.com.
Hong Kong, U.S., Israeli data illuminate COVID vaccine myocarditis
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.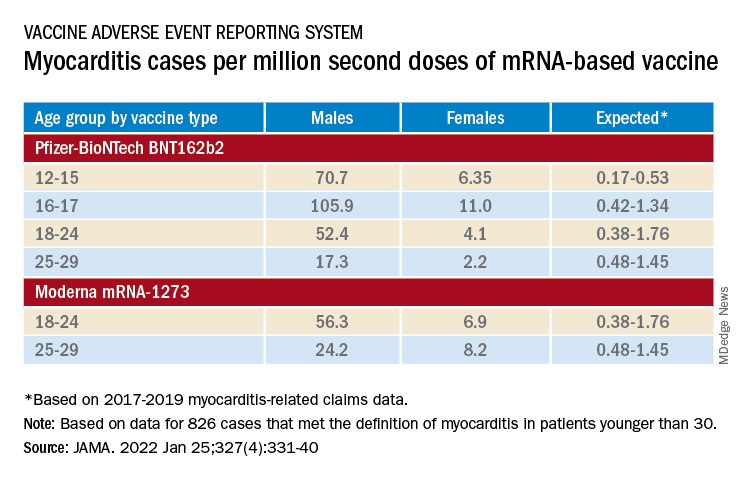
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Doc accused of killing 14 patients in the ICU: Upcoming trial notes patient safety lapses
On Dec. 5, 2017, Danny Mollette, age 74, was brought to the emergency department of Mount Carmel West Medical Center in Columbus, Ohio, in critical condition. Staff inserted a breathing tube and sent him to the intensive care unit.
Mr. Mollette, who had diabetes, previously had been hospitalized for treatment of a gangrenous foot. When he arrived in the ICU, he was suffering from acute renal failure and low blood pressure, and had had two heart stoppages, according to a 2020 Ohio Board of Pharmacy report. He was placed under the care of William Husel, DO, the sole physician on duty in the ICU during the overnight shift.
Around 9:00 p.m., Dr. Husel discussed Mr. Mollette’s “grim prognosis” with family members at the patient’s bedside. He advised them that Mr. Mollette had “minutes to live” and asked, “How would you want him to take his last breath: on the ventilator or without these machines?”
In less than an hour, Mr. Mollette was dead. Some said that what happened in his case was similar to what happened with 34 other ICU patients at Mount Carmel West and Mount Carmel St. Ann’s in Westerville, Ohio, from 2014 through 2018 – all under Dr. Husel’s care.
Like Mr. Mollette, most of these gravely ill patients died minutes after receiving a single, unusually large intravenous dose of the powerful opioid fentanyl – often combined with a dose of one or more other painkillers or sedatives like hydromorphone – and being withdrawn from the ventilator. These deaths all occurred following a procedure called palliative extubation, the removal of the endotracheal tube in patients who are expected to die.
Mount Carmel fired Dr. Husel in December 2018 following an investigation that concluded that the opioid dosages he used were “significantly excessive and potentially fatal,” and “went beyond providing comfort.” His Ohio medical license was suspended. In February 2022, he is scheduled to go on trial in Columbus on 14 counts of murder.*
Hanging over the murder case against Dr. Husel is the question of how Mount Carmel, a 136-year-old Catholic hospital owned by the giant Trinity Health system, allowed this pattern of care to continue for so many patients over 4 years, and why numerous registered nurses and hospital pharmacists went along with Dr. Husel’s actions. Nearly two dozen RNs and two pharmacists involved in these cases have faced disciplinary action, mostly license suspension.
“The first time a patient died on a very high dose, someone should have flagged this,” said Lewis Nelson, MD, chair of emergency medicine at Rutgers New Jersey Medical School, Newark. “As soon as I see it the second time or 27th time, it doesn’t seem okay. There was a breakdown in oversight to allow this to continue. The hospital didn’t have guardrails in place.”
The Franklin County (Ohio) Prosecuting Attorney’s Office faces two big challenges in trying Dr. Husel for murder. The prosecutors must prove that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Federal and state agencies have cited the hospital system for faults in its patient safety systems and culture that were exposed by the Husel cases. An outside medical expert, Robert Powers, MD, a professor of emergency medicine at the University of Virginia, Charlottesville, testified in one of the dozens of wrongful death lawsuits against Mount Carmel and Dr. Husel that there was no record of anyone supervising Dr. Husel or monitoring his care.
There also are questions about why Mount Carmel administrators and physician leaders did not find out about Dr. Husel’s criminal record as a young man before hiring and credentialing him, even though the Ohio Medical Board had obtained that record. As a college freshman in West Virginia in 1994, Dr. Husel and a friend allegedly stole car stereos, and after a classmate reported their behavior, they built a pipe bomb they planned to plant under the classmate’s car, according to court records.
Dr. Husel pleaded guilty in 1996 to a federal misdemeanor for improperly storing explosive materials, and he received a 6-month sentence followed by supervision. He did not disclose that criminal conviction on his application for medical liability insurance as part of his Mount Carmel employment application, attorneys representing the families of his deceased patients say.
A Mount Carmel spokeswoman said the hospital only checks a physician applicant’s background record for the previous 10 years.
“I think [the credentialing process] should have been more careful and more comprehensive than it was,” Robert Powers testified in a September 2020 deposition. “This guy was a bomber and a thief. You don’t hire bombers and thieves to take care of patients.”
Mount Carmel and Trinity leaders say they knew nothing about Dr. Husel’s palliative extubation practices until a staffer reported Dr. Husel’s high-dose fentanyl orders in October 2018. However, three more Husel patients died under similar circumstances before he was removed from patient care in November 2018.
Mount Carmel and Trinity already have settled a number of wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. The Mount Carmel CEO, the chief clinical officer, other physician, nursing, and pharmacy leaders, as well as dozens of nurses and pharmacists have been terminated or entered into retirement.
“What happened is tragic and unacceptable,” the Mount Carmel spokeswoman said in a written statement. “We have made a number of changes designed to prevent this from ever happening again. … Our new hospital leadership team is committed to patient safety and will take immediate action whenever patient safety is at issue.”
In January 2019, Mount Carmel’s then-CEO Ed Lamb acknowledged that “processes in place were not sufficient to prevent these actions from happening.” Mr. Lamb later said Mount Carmel was investigating whether five of the ICU patients who died under Dr. Husel’s care could have been treated and survived. Mr. Lamb stepped down in June 2019.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved one gasping for breath. But most medical experts say the fentanyl doses Dr. Husel ordered – 500-2,000 mcg – were five to 20 times larger than doses normally used in palliative extubation. Such doses, they say, would quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 mcg for relieving pain, and its 2018 guidelines reduced that to 25-50 mcg.
“If I perform a painful procedure, I might give 100 or 150 micrograms of fentanyl, or 500 or 600 for open heart surgery,” said Dr. Nelson of Rutgers, who also practices medical toxicology and addiction medicine. “But you’ll be intubated and monitored carefully. Without having a tube in your airway to help you breathe, those doses will kill you.”**
Mount Carmel West hired Dr. Husel in 2013 to work the late-night shift in its ICU. It was his first job as a full-fledged physician, after completing a residency and fellowship in critical care medicine at Cleveland Clinic. A good-looking and charismatic former high school basketball star, he was a hard worker and was popular with the ICU nurses and staff, who looked to him as a teacher and mentor, according to depositions of nurses and Ohio Board of Nursing reports.
In 2014, Dr. Husel was chosen by his hospital colleagues as physician of the year. He was again nominated in 2018. Before October 2018, there were no complaints about his care, according to the deposition of Larry Swanner, MD, Mount Carmel’s former vice president of medical affairs, who was fired in 2019.
“Dr. Husel is so knowledgeable that we would try to soak up as much knowledge as we could,” said Jason Schulze, RN, in a July 2020 deposition. Mr. Schulze’s license was suspended, however, that suspension was stayed for a minimum period of two years. This was in connection with his care of one of Dr. Husel’s ICU patients, 44-year-old Troy Allison, who died 3 minutes after Mr. Schulze administered a 1,000-microgram dose of fentanyl ordered by Dr. Husel in July 2018.
Dr. Husel’s winning personality and seeming expertise in the use of pain drugs, combined with his training at the prestigious Cleveland Clinic, may have lulled other hospital staff into going along with his decisions.
“They’re thinking, the guy’s likable and he must know what he’s doing,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “But you can’t get fooled by that. You need a policy in place for what to do if pharmacists or nurses disagree with an order, and you need to have practice simulations so people know how to handle these situations.”
Dr. Husel’s criminal defense attorney, Jose Baez, said Dr. Husel’s treatment of all these palliative extubation patients, including his prescribed dosages of fentanyl and other drugs, was completely appropriate. “Dr. Husel practiced medicine with compassion, and never wanted to see any of his patients suffer, nor their family,” Mr. Baez said.
Most medical and pharmacy experts sharply disagree. “I’m a pharmacist, and I’ve never seen anything like those kinds of doses,” Mr. Cohen said. “Something strange was going on there.”
Complicating these issues, eight nurses and a pharmacist have sued Mount Carmel and Trinity for wrongful termination and defamation in connection with the Husel allegations. They strongly defend Dr. Husel’s and their care as compassionate and appropriate. Beyond that, they argue that the changes Mount Carmel and Trinity made to ICU procedures to prevent such situations from happening again are potentially harmful to patient care.
“None of the nurses ever thought that Dr. Husel did anything to harm his patients or do anything other than provide comfort care during a very difficult time,” said Robert Landy, a New York attorney who’s representing the plaintiffs in the federal wrongful termination suit. “The real harm came in January 2019, when there were substantial policy changes that were detrimental to patient care and safety.”
Many of these patient deaths occurred during a period when the Mount Carmel system and Trinity were in the process of closing the old Mount Carmel West hospital, located in the low-income, inner-city neighborhood of Columbus, and opening a new hospital in the affluent suburb of Grove City, Ohio.
“They were done with this old, worn-out, inner-city hospital and its patient base and wanted a brand-new sparkling object in the suburbs,” said Gerry Leeseberg, a Columbus attorney who is representing 17 families of patients who died under Dr. Husel’s care. “They may have directed less energy, attention, and resources to the inner-city hospital.”
The case of Danny Mollette illustrates the multiple issues with Mount Carmel’s patient safety system.
First, there was no evidence in the record that Mr. Mollette was in pain or lacked the ability to breathe on his own prior to Dr. Husel’s palliative extubation. He had received no pain medications in the hospital that day, according to the report of an Ohio Board of Nursing examiner in a licensure discipline action brought against nurse Jacob Deemer for his care of Mr. Mollette and two other ICU patients who died. Mr. Deemer said Dr. Husel told him that the patient had to be in pain given his condition.
After consulting with Mr. Mollette’s family at the bedside, Dr. Husel ordered Mr. Deemer to administer 1,000 mcg of fentanyl, followed by 2 mg of hydromorphone, and 4 mg of midzolam, a sedative. Mr. Deemer withdrew the drugs from the Pyxis dispensing cabinet, overriding the pharmacist preapproval system. He said Dr. Husel told him the pharmacist had said, “It is okay.”
Actually, according to the pharmacy board report, the pharmacist, Gregory White, wrote in the medical record system that he did not agree to the fentanyl order. But his dissent came as the drugs were being administered, the breathing tube was being removed, and the patient was about to die. Mr. White was later disciplined by the Ohio Board of Pharmacy for failing to inform his supervisors about the incident and preventing the use of those high drug dosages in the cases of Mr. Mollette and two subsequent Husel patients.
Then there are questions about whether the families of Mr. Mollette and other Husel patients were fully and accurately informed about their loved ones’ conditions before agreeing to the palliative extubation. Mr. Mollette’s son, Brian, told reporters in July 2019 that Dr. Husel “said my father’s organs were shutting down and he was brain damaged. In hindsight, we felt kind of rushed to make that decision.”
Plaintiff attorneys bringing civil wrongful death cases against Mount Carmel and Dr. Husel must overcome hurdles similar to those faced by prosecutors in the murder case against Dr. Husel. Even if the patients were likely to die from their underlying conditions, did the drugs hasten their deaths, and by how much? In the civil cases, there’s the additional question of how much a few more hours or days or weeks of life are worth in terms of monetary damages.
Another challenge in bringing both the criminal and civil cases is that physicians and other medical providers have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia, and that’s illegal in the United States.
“There is no evidence that medication played any part in the death of any of these patients,” said Mr. Landy, who’s representing the nurses and pharmacists in the wrongful termination suit. “The only evidence we have is that higher dosages of opioids following extubation extend life, not shorten it.”
Dr. Husel, as well as the nurses and pharmacists who have faced licensure actions, claim their actions were legally shielded by the double-effect principle. But the Centers for Medicare & Medicaid Services, the Ohio Board of Nursing, and Ohio Board of Pharmacy haven’t accepted that defense. Instead, they have cited Mount Carmel, Dr. Husel, and the nurses and pharmacists for numerous patient safety violations, including administering excessive dosages of fentanyl and other drugs.
Among those violations is that many of Dr. Husel’s drug orders were given verbally instead of through the standard process of entering the orders into the electronic health record. He and the nurses on duty skipped the standard nonemergency process of getting preapproval from the pharmacist on duty. Instead, they used the override function on Mount Carmel’s automated Pyxis system to withdraw the drugs from the cabinet and avoid pharmacist review. In many cases, there was no retrospective review of the appropriateness of the orders by a pharmacist after the drugs were administered, which is required.
After threatening to cut off Medicare and Medicaid payments to Mount Carmel, CMS in June 2019 accepted the hospital’s correction plan, which restricted use of verbal drug orders and prohibited Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Under the agreement with CMS, Mount Carmel physicians must receive permission from a physician executive to order painkilling drugs that exceed hospital-set dosage parameters for palliative ventilator withdrawal. In addition, pharmacists must immediately report concerns about drug-prescribing safety up the hospital pharmacy chain of command.
“We have trained staff to ensure they feel empowered to speak up when appropriate,” the Mount Carmel spokeswoman said. “Staff members have multiple avenues for elevating a complaint or concern.”
Dr. Husel’s high dosages of fentanyl and other painkillers were well-known among the ICU nurses and pharmacists, who rarely – if ever – questioned those dosages, and went along with his standard use of verbal orders and overrides of the Pyxis system, according to depositions of nurses and pharmacists in the wrongful death lawsuits.
But the Mount Carmel nurses and pharmacists had a professional responsibility to question such dosages and demand evidence from the medical literature to support their use, according to hearing examiners at the nursing and pharmacy boards, who meted out licensure actions to providers working with Dr. Husel.
Nursing board hearing examiner Jack Decker emphasized those responsibilities in his November 30, 2020, report on nurse Deemer’s actions regarding three patients who died under Dr. Husel’s care in 2017 and 2018. Mr. Deemer’s license was suspended, however, that suspension was stayed for a minimum period of three years. Mr. Decker wrote that the ICU nurses had a professional responsibility to question Dr. Husel and, if necessary, refuse to carry out the doctor’s order and report their concerns to managers.
“Challenging a physician’s order is a difficult step even under ideal circumstances,” wrote Mr. Decker, who called Mount Carmel West’s ICU a “dysfunctional” environment. “But,” he noted, “when Mr. Deemer signed on to become a nurse, he enlisted to use his own critical thinking skills to serve as a patient protector and advocate. … Clearly, Mr. Deemer trusted Dr. Husel. But Dr. Husel was not to be trusted.”
While patient safety experts say these cases reveal that Mount Carmel had a flawed system and culture that did not train and empower staff to report safety concerns up the chain of command, they acknowledged that this could have happened at many U.S. hospitals.
“Sadly, I’m not sure it’s all that uncommon,” said Dr. Nelson of Rutgers. “Nurses and pharmacists have historically been afraid to raise concerns about physicians. We’ve been trying to break down barriers, but it’s a natural human instinct to play your role in the hierarchy.”
A version of this article first appeared on Medscape.com.
Corrections 2/1/22: An earlier version of this article misstated (*) the number of murder counts and (**) Dr. Nelson's area of practice.
This article was updated 2/2/22 to reflect the fact that the license suspensions of Mr. Deemer and Mr. Schulze were stayed.
On Dec. 5, 2017, Danny Mollette, age 74, was brought to the emergency department of Mount Carmel West Medical Center in Columbus, Ohio, in critical condition. Staff inserted a breathing tube and sent him to the intensive care unit.
Mr. Mollette, who had diabetes, previously had been hospitalized for treatment of a gangrenous foot. When he arrived in the ICU, he was suffering from acute renal failure and low blood pressure, and had had two heart stoppages, according to a 2020 Ohio Board of Pharmacy report. He was placed under the care of William Husel, DO, the sole physician on duty in the ICU during the overnight shift.
Around 9:00 p.m., Dr. Husel discussed Mr. Mollette’s “grim prognosis” with family members at the patient’s bedside. He advised them that Mr. Mollette had “minutes to live” and asked, “How would you want him to take his last breath: on the ventilator or without these machines?”
In less than an hour, Mr. Mollette was dead. Some said that what happened in his case was similar to what happened with 34 other ICU patients at Mount Carmel West and Mount Carmel St. Ann’s in Westerville, Ohio, from 2014 through 2018 – all under Dr. Husel’s care.
Like Mr. Mollette, most of these gravely ill patients died minutes after receiving a single, unusually large intravenous dose of the powerful opioid fentanyl – often combined with a dose of one or more other painkillers or sedatives like hydromorphone – and being withdrawn from the ventilator. These deaths all occurred following a procedure called palliative extubation, the removal of the endotracheal tube in patients who are expected to die.
Mount Carmel fired Dr. Husel in December 2018 following an investigation that concluded that the opioid dosages he used were “significantly excessive and potentially fatal,” and “went beyond providing comfort.” His Ohio medical license was suspended. In February 2022, he is scheduled to go on trial in Columbus on 14 counts of murder.*
Hanging over the murder case against Dr. Husel is the question of how Mount Carmel, a 136-year-old Catholic hospital owned by the giant Trinity Health system, allowed this pattern of care to continue for so many patients over 4 years, and why numerous registered nurses and hospital pharmacists went along with Dr. Husel’s actions. Nearly two dozen RNs and two pharmacists involved in these cases have faced disciplinary action, mostly license suspension.
“The first time a patient died on a very high dose, someone should have flagged this,” said Lewis Nelson, MD, chair of emergency medicine at Rutgers New Jersey Medical School, Newark. “As soon as I see it the second time or 27th time, it doesn’t seem okay. There was a breakdown in oversight to allow this to continue. The hospital didn’t have guardrails in place.”
The Franklin County (Ohio) Prosecuting Attorney’s Office faces two big challenges in trying Dr. Husel for murder. The prosecutors must prove that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Federal and state agencies have cited the hospital system for faults in its patient safety systems and culture that were exposed by the Husel cases. An outside medical expert, Robert Powers, MD, a professor of emergency medicine at the University of Virginia, Charlottesville, testified in one of the dozens of wrongful death lawsuits against Mount Carmel and Dr. Husel that there was no record of anyone supervising Dr. Husel or monitoring his care.
There also are questions about why Mount Carmel administrators and physician leaders did not find out about Dr. Husel’s criminal record as a young man before hiring and credentialing him, even though the Ohio Medical Board had obtained that record. As a college freshman in West Virginia in 1994, Dr. Husel and a friend allegedly stole car stereos, and after a classmate reported their behavior, they built a pipe bomb they planned to plant under the classmate’s car, according to court records.
Dr. Husel pleaded guilty in 1996 to a federal misdemeanor for improperly storing explosive materials, and he received a 6-month sentence followed by supervision. He did not disclose that criminal conviction on his application for medical liability insurance as part of his Mount Carmel employment application, attorneys representing the families of his deceased patients say.
A Mount Carmel spokeswoman said the hospital only checks a physician applicant’s background record for the previous 10 years.
“I think [the credentialing process] should have been more careful and more comprehensive than it was,” Robert Powers testified in a September 2020 deposition. “This guy was a bomber and a thief. You don’t hire bombers and thieves to take care of patients.”
Mount Carmel and Trinity leaders say they knew nothing about Dr. Husel’s palliative extubation practices until a staffer reported Dr. Husel’s high-dose fentanyl orders in October 2018. However, three more Husel patients died under similar circumstances before he was removed from patient care in November 2018.
Mount Carmel and Trinity already have settled a number of wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. The Mount Carmel CEO, the chief clinical officer, other physician, nursing, and pharmacy leaders, as well as dozens of nurses and pharmacists have been terminated or entered into retirement.
“What happened is tragic and unacceptable,” the Mount Carmel spokeswoman said in a written statement. “We have made a number of changes designed to prevent this from ever happening again. … Our new hospital leadership team is committed to patient safety and will take immediate action whenever patient safety is at issue.”
In January 2019, Mount Carmel’s then-CEO Ed Lamb acknowledged that “processes in place were not sufficient to prevent these actions from happening.” Mr. Lamb later said Mount Carmel was investigating whether five of the ICU patients who died under Dr. Husel’s care could have been treated and survived. Mr. Lamb stepped down in June 2019.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved one gasping for breath. But most medical experts say the fentanyl doses Dr. Husel ordered – 500-2,000 mcg – were five to 20 times larger than doses normally used in palliative extubation. Such doses, they say, would quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 mcg for relieving pain, and its 2018 guidelines reduced that to 25-50 mcg.
“If I perform a painful procedure, I might give 100 or 150 micrograms of fentanyl, or 500 or 600 for open heart surgery,” said Dr. Nelson of Rutgers, who also practices medical toxicology and addiction medicine. “But you’ll be intubated and monitored carefully. Without having a tube in your airway to help you breathe, those doses will kill you.”**
Mount Carmel West hired Dr. Husel in 2013 to work the late-night shift in its ICU. It was his first job as a full-fledged physician, after completing a residency and fellowship in critical care medicine at Cleveland Clinic. A good-looking and charismatic former high school basketball star, he was a hard worker and was popular with the ICU nurses and staff, who looked to him as a teacher and mentor, according to depositions of nurses and Ohio Board of Nursing reports.
In 2014, Dr. Husel was chosen by his hospital colleagues as physician of the year. He was again nominated in 2018. Before October 2018, there were no complaints about his care, according to the deposition of Larry Swanner, MD, Mount Carmel’s former vice president of medical affairs, who was fired in 2019.
“Dr. Husel is so knowledgeable that we would try to soak up as much knowledge as we could,” said Jason Schulze, RN, in a July 2020 deposition. Mr. Schulze’s license was suspended, however, that suspension was stayed for a minimum period of two years. This was in connection with his care of one of Dr. Husel’s ICU patients, 44-year-old Troy Allison, who died 3 minutes after Mr. Schulze administered a 1,000-microgram dose of fentanyl ordered by Dr. Husel in July 2018.
Dr. Husel’s winning personality and seeming expertise in the use of pain drugs, combined with his training at the prestigious Cleveland Clinic, may have lulled other hospital staff into going along with his decisions.
“They’re thinking, the guy’s likable and he must know what he’s doing,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “But you can’t get fooled by that. You need a policy in place for what to do if pharmacists or nurses disagree with an order, and you need to have practice simulations so people know how to handle these situations.”
Dr. Husel’s criminal defense attorney, Jose Baez, said Dr. Husel’s treatment of all these palliative extubation patients, including his prescribed dosages of fentanyl and other drugs, was completely appropriate. “Dr. Husel practiced medicine with compassion, and never wanted to see any of his patients suffer, nor their family,” Mr. Baez said.
Most medical and pharmacy experts sharply disagree. “I’m a pharmacist, and I’ve never seen anything like those kinds of doses,” Mr. Cohen said. “Something strange was going on there.”
Complicating these issues, eight nurses and a pharmacist have sued Mount Carmel and Trinity for wrongful termination and defamation in connection with the Husel allegations. They strongly defend Dr. Husel’s and their care as compassionate and appropriate. Beyond that, they argue that the changes Mount Carmel and Trinity made to ICU procedures to prevent such situations from happening again are potentially harmful to patient care.
“None of the nurses ever thought that Dr. Husel did anything to harm his patients or do anything other than provide comfort care during a very difficult time,” said Robert Landy, a New York attorney who’s representing the plaintiffs in the federal wrongful termination suit. “The real harm came in January 2019, when there were substantial policy changes that were detrimental to patient care and safety.”
Many of these patient deaths occurred during a period when the Mount Carmel system and Trinity were in the process of closing the old Mount Carmel West hospital, located in the low-income, inner-city neighborhood of Columbus, and opening a new hospital in the affluent suburb of Grove City, Ohio.
“They were done with this old, worn-out, inner-city hospital and its patient base and wanted a brand-new sparkling object in the suburbs,” said Gerry Leeseberg, a Columbus attorney who is representing 17 families of patients who died under Dr. Husel’s care. “They may have directed less energy, attention, and resources to the inner-city hospital.”
The case of Danny Mollette illustrates the multiple issues with Mount Carmel’s patient safety system.
First, there was no evidence in the record that Mr. Mollette was in pain or lacked the ability to breathe on his own prior to Dr. Husel’s palliative extubation. He had received no pain medications in the hospital that day, according to the report of an Ohio Board of Nursing examiner in a licensure discipline action brought against nurse Jacob Deemer for his care of Mr. Mollette and two other ICU patients who died. Mr. Deemer said Dr. Husel told him that the patient had to be in pain given his condition.
After consulting with Mr. Mollette’s family at the bedside, Dr. Husel ordered Mr. Deemer to administer 1,000 mcg of fentanyl, followed by 2 mg of hydromorphone, and 4 mg of midzolam, a sedative. Mr. Deemer withdrew the drugs from the Pyxis dispensing cabinet, overriding the pharmacist preapproval system. He said Dr. Husel told him the pharmacist had said, “It is okay.”
Actually, according to the pharmacy board report, the pharmacist, Gregory White, wrote in the medical record system that he did not agree to the fentanyl order. But his dissent came as the drugs were being administered, the breathing tube was being removed, and the patient was about to die. Mr. White was later disciplined by the Ohio Board of Pharmacy for failing to inform his supervisors about the incident and preventing the use of those high drug dosages in the cases of Mr. Mollette and two subsequent Husel patients.
Then there are questions about whether the families of Mr. Mollette and other Husel patients were fully and accurately informed about their loved ones’ conditions before agreeing to the palliative extubation. Mr. Mollette’s son, Brian, told reporters in July 2019 that Dr. Husel “said my father’s organs were shutting down and he was brain damaged. In hindsight, we felt kind of rushed to make that decision.”
Plaintiff attorneys bringing civil wrongful death cases against Mount Carmel and Dr. Husel must overcome hurdles similar to those faced by prosecutors in the murder case against Dr. Husel. Even if the patients were likely to die from their underlying conditions, did the drugs hasten their deaths, and by how much? In the civil cases, there’s the additional question of how much a few more hours or days or weeks of life are worth in terms of monetary damages.
Another challenge in bringing both the criminal and civil cases is that physicians and other medical providers have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia, and that’s illegal in the United States.
“There is no evidence that medication played any part in the death of any of these patients,” said Mr. Landy, who’s representing the nurses and pharmacists in the wrongful termination suit. “The only evidence we have is that higher dosages of opioids following extubation extend life, not shorten it.”
Dr. Husel, as well as the nurses and pharmacists who have faced licensure actions, claim their actions were legally shielded by the double-effect principle. But the Centers for Medicare & Medicaid Services, the Ohio Board of Nursing, and Ohio Board of Pharmacy haven’t accepted that defense. Instead, they have cited Mount Carmel, Dr. Husel, and the nurses and pharmacists for numerous patient safety violations, including administering excessive dosages of fentanyl and other drugs.
Among those violations is that many of Dr. Husel’s drug orders were given verbally instead of through the standard process of entering the orders into the electronic health record. He and the nurses on duty skipped the standard nonemergency process of getting preapproval from the pharmacist on duty. Instead, they used the override function on Mount Carmel’s automated Pyxis system to withdraw the drugs from the cabinet and avoid pharmacist review. In many cases, there was no retrospective review of the appropriateness of the orders by a pharmacist after the drugs were administered, which is required.
After threatening to cut off Medicare and Medicaid payments to Mount Carmel, CMS in June 2019 accepted the hospital’s correction plan, which restricted use of verbal drug orders and prohibited Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Under the agreement with CMS, Mount Carmel physicians must receive permission from a physician executive to order painkilling drugs that exceed hospital-set dosage parameters for palliative ventilator withdrawal. In addition, pharmacists must immediately report concerns about drug-prescribing safety up the hospital pharmacy chain of command.
“We have trained staff to ensure they feel empowered to speak up when appropriate,” the Mount Carmel spokeswoman said. “Staff members have multiple avenues for elevating a complaint or concern.”
Dr. Husel’s high dosages of fentanyl and other painkillers were well-known among the ICU nurses and pharmacists, who rarely – if ever – questioned those dosages, and went along with his standard use of verbal orders and overrides of the Pyxis system, according to depositions of nurses and pharmacists in the wrongful death lawsuits.
But the Mount Carmel nurses and pharmacists had a professional responsibility to question such dosages and demand evidence from the medical literature to support their use, according to hearing examiners at the nursing and pharmacy boards, who meted out licensure actions to providers working with Dr. Husel.
Nursing board hearing examiner Jack Decker emphasized those responsibilities in his November 30, 2020, report on nurse Deemer’s actions regarding three patients who died under Dr. Husel’s care in 2017 and 2018. Mr. Deemer’s license was suspended, however, that suspension was stayed for a minimum period of three years. Mr. Decker wrote that the ICU nurses had a professional responsibility to question Dr. Husel and, if necessary, refuse to carry out the doctor’s order and report their concerns to managers.
“Challenging a physician’s order is a difficult step even under ideal circumstances,” wrote Mr. Decker, who called Mount Carmel West’s ICU a “dysfunctional” environment. “But,” he noted, “when Mr. Deemer signed on to become a nurse, he enlisted to use his own critical thinking skills to serve as a patient protector and advocate. … Clearly, Mr. Deemer trusted Dr. Husel. But Dr. Husel was not to be trusted.”
While patient safety experts say these cases reveal that Mount Carmel had a flawed system and culture that did not train and empower staff to report safety concerns up the chain of command, they acknowledged that this could have happened at many U.S. hospitals.
“Sadly, I’m not sure it’s all that uncommon,” said Dr. Nelson of Rutgers. “Nurses and pharmacists have historically been afraid to raise concerns about physicians. We’ve been trying to break down barriers, but it’s a natural human instinct to play your role in the hierarchy.”
A version of this article first appeared on Medscape.com.
Corrections 2/1/22: An earlier version of this article misstated (*) the number of murder counts and (**) Dr. Nelson's area of practice.
This article was updated 2/2/22 to reflect the fact that the license suspensions of Mr. Deemer and Mr. Schulze were stayed.
On Dec. 5, 2017, Danny Mollette, age 74, was brought to the emergency department of Mount Carmel West Medical Center in Columbus, Ohio, in critical condition. Staff inserted a breathing tube and sent him to the intensive care unit.
Mr. Mollette, who had diabetes, previously had been hospitalized for treatment of a gangrenous foot. When he arrived in the ICU, he was suffering from acute renal failure and low blood pressure, and had had two heart stoppages, according to a 2020 Ohio Board of Pharmacy report. He was placed under the care of William Husel, DO, the sole physician on duty in the ICU during the overnight shift.
Around 9:00 p.m., Dr. Husel discussed Mr. Mollette’s “grim prognosis” with family members at the patient’s bedside. He advised them that Mr. Mollette had “minutes to live” and asked, “How would you want him to take his last breath: on the ventilator or without these machines?”
In less than an hour, Mr. Mollette was dead. Some said that what happened in his case was similar to what happened with 34 other ICU patients at Mount Carmel West and Mount Carmel St. Ann’s in Westerville, Ohio, from 2014 through 2018 – all under Dr. Husel’s care.
Like Mr. Mollette, most of these gravely ill patients died minutes after receiving a single, unusually large intravenous dose of the powerful opioid fentanyl – often combined with a dose of one or more other painkillers or sedatives like hydromorphone – and being withdrawn from the ventilator. These deaths all occurred following a procedure called palliative extubation, the removal of the endotracheal tube in patients who are expected to die.
Mount Carmel fired Dr. Husel in December 2018 following an investigation that concluded that the opioid dosages he used were “significantly excessive and potentially fatal,” and “went beyond providing comfort.” His Ohio medical license was suspended. In February 2022, he is scheduled to go on trial in Columbus on 14 counts of murder.*
Hanging over the murder case against Dr. Husel is the question of how Mount Carmel, a 136-year-old Catholic hospital owned by the giant Trinity Health system, allowed this pattern of care to continue for so many patients over 4 years, and why numerous registered nurses and hospital pharmacists went along with Dr. Husel’s actions. Nearly two dozen RNs and two pharmacists involved in these cases have faced disciplinary action, mostly license suspension.
“The first time a patient died on a very high dose, someone should have flagged this,” said Lewis Nelson, MD, chair of emergency medicine at Rutgers New Jersey Medical School, Newark. “As soon as I see it the second time or 27th time, it doesn’t seem okay. There was a breakdown in oversight to allow this to continue. The hospital didn’t have guardrails in place.”
The Franklin County (Ohio) Prosecuting Attorney’s Office faces two big challenges in trying Dr. Husel for murder. The prosecutors must prove that the drugs Dr. Husel ordered are what directly caused these critically ill patients to die, and that he intended to kill them.
Federal and state agencies have cited the hospital system for faults in its patient safety systems and culture that were exposed by the Husel cases. An outside medical expert, Robert Powers, MD, a professor of emergency medicine at the University of Virginia, Charlottesville, testified in one of the dozens of wrongful death lawsuits against Mount Carmel and Dr. Husel that there was no record of anyone supervising Dr. Husel or monitoring his care.
There also are questions about why Mount Carmel administrators and physician leaders did not find out about Dr. Husel’s criminal record as a young man before hiring and credentialing him, even though the Ohio Medical Board had obtained that record. As a college freshman in West Virginia in 1994, Dr. Husel and a friend allegedly stole car stereos, and after a classmate reported their behavior, they built a pipe bomb they planned to plant under the classmate’s car, according to court records.
Dr. Husel pleaded guilty in 1996 to a federal misdemeanor for improperly storing explosive materials, and he received a 6-month sentence followed by supervision. He did not disclose that criminal conviction on his application for medical liability insurance as part of his Mount Carmel employment application, attorneys representing the families of his deceased patients say.
A Mount Carmel spokeswoman said the hospital only checks a physician applicant’s background record for the previous 10 years.
“I think [the credentialing process] should have been more careful and more comprehensive than it was,” Robert Powers testified in a September 2020 deposition. “This guy was a bomber and a thief. You don’t hire bombers and thieves to take care of patients.”
Mount Carmel and Trinity leaders say they knew nothing about Dr. Husel’s palliative extubation practices until a staffer reported Dr. Husel’s high-dose fentanyl orders in October 2018. However, three more Husel patients died under similar circumstances before he was removed from patient care in November 2018.
Mount Carmel and Trinity already have settled a number of wrongful death lawsuits filed by the families of Dr. Husel’s patients for nearly $20 million, with many more suits pending. The Mount Carmel CEO, the chief clinical officer, other physician, nursing, and pharmacy leaders, as well as dozens of nurses and pharmacists have been terminated or entered into retirement.
“What happened is tragic and unacceptable,” the Mount Carmel spokeswoman said in a written statement. “We have made a number of changes designed to prevent this from ever happening again. … Our new hospital leadership team is committed to patient safety and will take immediate action whenever patient safety is at issue.”
In January 2019, Mount Carmel’s then-CEO Ed Lamb acknowledged that “processes in place were not sufficient to prevent these actions from happening.” Mr. Lamb later said Mount Carmel was investigating whether five of the ICU patients who died under Dr. Husel’s care could have been treated and survived. Mr. Lamb stepped down in June 2019.
Before performing a palliative extubation, physicians commonly administer opioids and/or sedatives to ease pain and discomfort, and spare family members from witnessing their loved one gasping for breath. But most medical experts say the fentanyl doses Dr. Husel ordered – 500-2,000 mcg – were five to 20 times larger than doses normally used in palliative extubation. Such doses, they say, would quickly kill most patients – except those with high opioid tolerance – by stopping their breathing.
Physicians say they typically give much smaller doses of fentanyl or morphine, then administer more as needed if they observe the patient experiencing pain or distress. Mount Carmel’s 2016 guidelines for IV administration of fentanyl specified a dosage range of 50-100 mcg for relieving pain, and its 2018 guidelines reduced that to 25-50 mcg.
“If I perform a painful procedure, I might give 100 or 150 micrograms of fentanyl, or 500 or 600 for open heart surgery,” said Dr. Nelson of Rutgers, who also practices medical toxicology and addiction medicine. “But you’ll be intubated and monitored carefully. Without having a tube in your airway to help you breathe, those doses will kill you.”**
Mount Carmel West hired Dr. Husel in 2013 to work the late-night shift in its ICU. It was his first job as a full-fledged physician, after completing a residency and fellowship in critical care medicine at Cleveland Clinic. A good-looking and charismatic former high school basketball star, he was a hard worker and was popular with the ICU nurses and staff, who looked to him as a teacher and mentor, according to depositions of nurses and Ohio Board of Nursing reports.
In 2014, Dr. Husel was chosen by his hospital colleagues as physician of the year. He was again nominated in 2018. Before October 2018, there were no complaints about his care, according to the deposition of Larry Swanner, MD, Mount Carmel’s former vice president of medical affairs, who was fired in 2019.
“Dr. Husel is so knowledgeable that we would try to soak up as much knowledge as we could,” said Jason Schulze, RN, in a July 2020 deposition. Mr. Schulze’s license was suspended, however, that suspension was stayed for a minimum period of two years. This was in connection with his care of one of Dr. Husel’s ICU patients, 44-year-old Troy Allison, who died 3 minutes after Mr. Schulze administered a 1,000-microgram dose of fentanyl ordered by Dr. Husel in July 2018.
Dr. Husel’s winning personality and seeming expertise in the use of pain drugs, combined with his training at the prestigious Cleveland Clinic, may have lulled other hospital staff into going along with his decisions.
“They’re thinking, the guy’s likable and he must know what he’s doing,” said Michael Cohen, RPh, founder and president emeritus of the Institute for Safe Medication Practices. “But you can’t get fooled by that. You need a policy in place for what to do if pharmacists or nurses disagree with an order, and you need to have practice simulations so people know how to handle these situations.”
Dr. Husel’s criminal defense attorney, Jose Baez, said Dr. Husel’s treatment of all these palliative extubation patients, including his prescribed dosages of fentanyl and other drugs, was completely appropriate. “Dr. Husel practiced medicine with compassion, and never wanted to see any of his patients suffer, nor their family,” Mr. Baez said.
Most medical and pharmacy experts sharply disagree. “I’m a pharmacist, and I’ve never seen anything like those kinds of doses,” Mr. Cohen said. “Something strange was going on there.”
Complicating these issues, eight nurses and a pharmacist have sued Mount Carmel and Trinity for wrongful termination and defamation in connection with the Husel allegations. They strongly defend Dr. Husel’s and their care as compassionate and appropriate. Beyond that, they argue that the changes Mount Carmel and Trinity made to ICU procedures to prevent such situations from happening again are potentially harmful to patient care.
“None of the nurses ever thought that Dr. Husel did anything to harm his patients or do anything other than provide comfort care during a very difficult time,” said Robert Landy, a New York attorney who’s representing the plaintiffs in the federal wrongful termination suit. “The real harm came in January 2019, when there were substantial policy changes that were detrimental to patient care and safety.”
Many of these patient deaths occurred during a period when the Mount Carmel system and Trinity were in the process of closing the old Mount Carmel West hospital, located in the low-income, inner-city neighborhood of Columbus, and opening a new hospital in the affluent suburb of Grove City, Ohio.
“They were done with this old, worn-out, inner-city hospital and its patient base and wanted a brand-new sparkling object in the suburbs,” said Gerry Leeseberg, a Columbus attorney who is representing 17 families of patients who died under Dr. Husel’s care. “They may have directed less energy, attention, and resources to the inner-city hospital.”
The case of Danny Mollette illustrates the multiple issues with Mount Carmel’s patient safety system.
First, there was no evidence in the record that Mr. Mollette was in pain or lacked the ability to breathe on his own prior to Dr. Husel’s palliative extubation. He had received no pain medications in the hospital that day, according to the report of an Ohio Board of Nursing examiner in a licensure discipline action brought against nurse Jacob Deemer for his care of Mr. Mollette and two other ICU patients who died. Mr. Deemer said Dr. Husel told him that the patient had to be in pain given his condition.
After consulting with Mr. Mollette’s family at the bedside, Dr. Husel ordered Mr. Deemer to administer 1,000 mcg of fentanyl, followed by 2 mg of hydromorphone, and 4 mg of midzolam, a sedative. Mr. Deemer withdrew the drugs from the Pyxis dispensing cabinet, overriding the pharmacist preapproval system. He said Dr. Husel told him the pharmacist had said, “It is okay.”
Actually, according to the pharmacy board report, the pharmacist, Gregory White, wrote in the medical record system that he did not agree to the fentanyl order. But his dissent came as the drugs were being administered, the breathing tube was being removed, and the patient was about to die. Mr. White was later disciplined by the Ohio Board of Pharmacy for failing to inform his supervisors about the incident and preventing the use of those high drug dosages in the cases of Mr. Mollette and two subsequent Husel patients.
Then there are questions about whether the families of Mr. Mollette and other Husel patients were fully and accurately informed about their loved ones’ conditions before agreeing to the palliative extubation. Mr. Mollette’s son, Brian, told reporters in July 2019 that Dr. Husel “said my father’s organs were shutting down and he was brain damaged. In hindsight, we felt kind of rushed to make that decision.”
Plaintiff attorneys bringing civil wrongful death cases against Mount Carmel and Dr. Husel must overcome hurdles similar to those faced by prosecutors in the murder case against Dr. Husel. Even if the patients were likely to die from their underlying conditions, did the drugs hasten their deaths, and by how much? In the civil cases, there’s the additional question of how much a few more hours or days or weeks of life are worth in terms of monetary damages.
Another challenge in bringing both the criminal and civil cases is that physicians and other medical providers have certain legal protections for administering drugs to patients for the purpose of relieving pain and suffering, even if the drugs hasten the patients’ deaths – as long the intent was not to cause death and the drugs were properly used. This is known as the double-effect principle. In contrast, intentional killing to relieve pain and suffering is called euthanasia, and that’s illegal in the United States.
“There is no evidence that medication played any part in the death of any of these patients,” said Mr. Landy, who’s representing the nurses and pharmacists in the wrongful termination suit. “The only evidence we have is that higher dosages of opioids following extubation extend life, not shorten it.”
Dr. Husel, as well as the nurses and pharmacists who have faced licensure actions, claim their actions were legally shielded by the double-effect principle. But the Centers for Medicare & Medicaid Services, the Ohio Board of Nursing, and Ohio Board of Pharmacy haven’t accepted that defense. Instead, they have cited Mount Carmel, Dr. Husel, and the nurses and pharmacists for numerous patient safety violations, including administering excessive dosages of fentanyl and other drugs.
Among those violations is that many of Dr. Husel’s drug orders were given verbally instead of through the standard process of entering the orders into the electronic health record. He and the nurses on duty skipped the standard nonemergency process of getting preapproval from the pharmacist on duty. Instead, they used the override function on Mount Carmel’s automated Pyxis system to withdraw the drugs from the cabinet and avoid pharmacist review. In many cases, there was no retrospective review of the appropriateness of the orders by a pharmacist after the drugs were administered, which is required.
After threatening to cut off Medicare and Medicaid payments to Mount Carmel, CMS in June 2019 accepted the hospital’s correction plan, which restricted use of verbal drug orders and prohibited Pyxis system overrides for opioids except in life-threatening emergencies. The Ohio Board of Pharmacy hit Mount Carmel with $477,000 in fines and costs for pharmacy rules violations.
Under the agreement with CMS, Mount Carmel physicians must receive permission from a physician executive to order painkilling drugs that exceed hospital-set dosage parameters for palliative ventilator withdrawal. In addition, pharmacists must immediately report concerns about drug-prescribing safety up the hospital pharmacy chain of command.
“We have trained staff to ensure they feel empowered to speak up when appropriate,” the Mount Carmel spokeswoman said. “Staff members have multiple avenues for elevating a complaint or concern.”
Dr. Husel’s high dosages of fentanyl and other painkillers were well-known among the ICU nurses and pharmacists, who rarely – if ever – questioned those dosages, and went along with his standard use of verbal orders and overrides of the Pyxis system, according to depositions of nurses and pharmacists in the wrongful death lawsuits.
But the Mount Carmel nurses and pharmacists had a professional responsibility to question such dosages and demand evidence from the medical literature to support their use, according to hearing examiners at the nursing and pharmacy boards, who meted out licensure actions to providers working with Dr. Husel.
Nursing board hearing examiner Jack Decker emphasized those responsibilities in his November 30, 2020, report on nurse Deemer’s actions regarding three patients who died under Dr. Husel’s care in 2017 and 2018. Mr. Deemer’s license was suspended, however, that suspension was stayed for a minimum period of three years. Mr. Decker wrote that the ICU nurses had a professional responsibility to question Dr. Husel and, if necessary, refuse to carry out the doctor’s order and report their concerns to managers.
“Challenging a physician’s order is a difficult step even under ideal circumstances,” wrote Mr. Decker, who called Mount Carmel West’s ICU a “dysfunctional” environment. “But,” he noted, “when Mr. Deemer signed on to become a nurse, he enlisted to use his own critical thinking skills to serve as a patient protector and advocate. … Clearly, Mr. Deemer trusted Dr. Husel. But Dr. Husel was not to be trusted.”
While patient safety experts say these cases reveal that Mount Carmel had a flawed system and culture that did not train and empower staff to report safety concerns up the chain of command, they acknowledged that this could have happened at many U.S. hospitals.
“Sadly, I’m not sure it’s all that uncommon,” said Dr. Nelson of Rutgers. “Nurses and pharmacists have historically been afraid to raise concerns about physicians. We’ve been trying to break down barriers, but it’s a natural human instinct to play your role in the hierarchy.”
A version of this article first appeared on Medscape.com.
Corrections 2/1/22: An earlier version of this article misstated (*) the number of murder counts and (**) Dr. Nelson's area of practice.
This article was updated 2/2/22 to reflect the fact that the license suspensions of Mr. Deemer and Mr. Schulze were stayed.

