User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Children and COVID: Weekly cases down by more than half
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.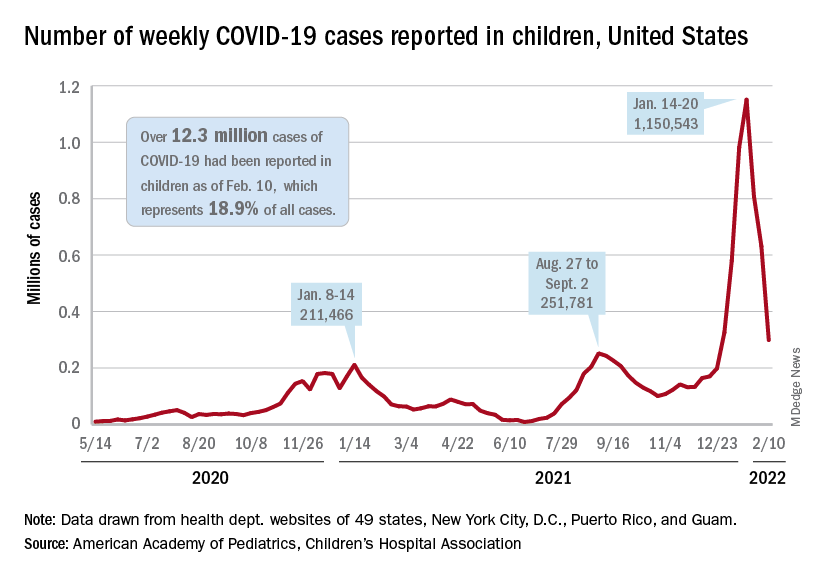
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
Long COVID symptoms linked to effects on vagus nerve
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Optimal NIV Medicare access promotion – a hopeful way forward for users of NIV
Use of positive airway pressure (PAP) devices for treatment of sleep apnea was first described in 1981. Subsequent use of PAP devices expanded to treat patients with respiratory failure. While the treatment in this population has rapidly gained widespread use and undoubtedly has reduced morbidity and mortality in these populations, policies governing these prescriptions have not really kept up with the burgeoning need.
In 2020, Drs. Peter Gay and Robert Owens brought together a technical expert panel (TEP) to systematically review the CMS policies with an eye to remove “regulatory barriers” to improve access for these patients with the mantra: “the right device gets to the right patient at the right time.”
The panel focused on “Optimal NIV Medicare Access Promotion (ONMAP),” and members with specific expertise were recruited for five patient groups: Thoracic Restrictive Disorders (TRD), COPD, Central Sleep Apnea (CSA), Hypoventilation Syndromes (HVS), and Obstructive Sleep Apnea (OSA). Each group reviewed the current coverage, outlined the deficiencies, and suggested revisions. Herein, I will briefly highlight each group’s most important points.
TRD: The goal for this group was to bring the US standards of care closer to the rest of the world. This group advocates that the start of noninvasive ventilation (NIV) should be substantially earlier, to provide the largest improvement in disease outcome and stability. Other prominent features submitted included arterial blood gases (ABG) to not be the only form of CO2 measurement allowed; paying for a second device if patients are using NIV continuously; qualification for a BiPAP to include if vital capacity is ≤ 80%; and, to obtain a home mechanical ventilator, a patient must either fail BiPAP or have extreme loss of function, high pressure requirements, or need mouthpiece ventilation.
CSA: The big challenges with this diagnosis related to qualifying coverage language in the current policies, which are confusing for many providers. Additionally, these policies often deny certain PAP devices and/or oxygen therapy. The group proposed: a single definition of CSA; eliminate discussion of hypoventilation; mirror qualifying symptoms, and, continuing coverage, to the same as that for OSA treatment; and remove need for a prior failure of BiPAP without a backup rate (BUR). The group also had specific recommendations for when oxygen therapy should be covered in patients with CSA.
COPD: This group also focused on the oxygen therapy and promoting use of devices with a BUR. Two problematic areas included the requirement that nocturnal oxygen saturation must drop to ≤ 88% for at least 5 cumulative minutes, and, that patients must begin with an S mode device (no BUR) for at least 2 months and can only then be prescribed a device with a BUR if CO2 fails to drop. The group advocates for the removal of both, the need for a nocturnal oximetry test, and, to “try” an S mode device. The panel advocated giving the prescribing physician discretion in making this determination. The panel also provided recommendations on when a home mechanical ventilator (HMV) should be considered instead of BiPAP therapy.
HVS: Hypoventilation syndromes are a heterogeneous group of disorders with hypercapnia, defined as a Paco2 ≥45 mm Hg. This panel noted that the current coverage criteria are outdated and fail to recognize the spectrum of disease severity and advances in technology, which often leads to circumvention by prescribing more costly home mechanical ventilators (HMV). Consistent with the TRD group, this panel recommended acceptance of surrogate noninvasive end tidal and transcutaneous Pco2 and venous blood gases in lieu of arterial blood gases. Additionally, they suggested no longer requiring CO2 measures while using prescribed oxygen; eliminating the need for a sleep study to avoid delays in care for patients being discharged from the hospital; removing spirometry as a requirement; and no longer a failure of BiPAP without a BUR.
OSA: The initial purpose of examining OSA in this process was to examine when BiPAP should be utilized for treatment; however, it necessitated examination of the entire policy for PAP. The areas that were identified as needing revision included: expansion of the symptom list for patients with OSA; revising the “4 hour rule,” suggesting that 2 hours has been proven to provide benefit; eliminating the need for another sleep study to re-qualify for PAP or supplemental oxygen; and embracing telehealth as a way to improve accessibility for follow-up visits.
For details, please review the papers published in the November 2021 issue of the journal CHEST® (2021; 160[5]:1579-1990, e377-e543).
We now await what CMS will do with our recommendations and work for “the right device to the right patient at the right time.”
Acknowledgment: Drs. Gerald Criner, Nicholas Hill, Babak Mohklesi, Timothy Morgenthaler, and Lisa Wolfe assisted with the content.
Use of positive airway pressure (PAP) devices for treatment of sleep apnea was first described in 1981. Subsequent use of PAP devices expanded to treat patients with respiratory failure. While the treatment in this population has rapidly gained widespread use and undoubtedly has reduced morbidity and mortality in these populations, policies governing these prescriptions have not really kept up with the burgeoning need.
In 2020, Drs. Peter Gay and Robert Owens brought together a technical expert panel (TEP) to systematically review the CMS policies with an eye to remove “regulatory barriers” to improve access for these patients with the mantra: “the right device gets to the right patient at the right time.”
The panel focused on “Optimal NIV Medicare Access Promotion (ONMAP),” and members with specific expertise were recruited for five patient groups: Thoracic Restrictive Disorders (TRD), COPD, Central Sleep Apnea (CSA), Hypoventilation Syndromes (HVS), and Obstructive Sleep Apnea (OSA). Each group reviewed the current coverage, outlined the deficiencies, and suggested revisions. Herein, I will briefly highlight each group’s most important points.
TRD: The goal for this group was to bring the US standards of care closer to the rest of the world. This group advocates that the start of noninvasive ventilation (NIV) should be substantially earlier, to provide the largest improvement in disease outcome and stability. Other prominent features submitted included arterial blood gases (ABG) to not be the only form of CO2 measurement allowed; paying for a second device if patients are using NIV continuously; qualification for a BiPAP to include if vital capacity is ≤ 80%; and, to obtain a home mechanical ventilator, a patient must either fail BiPAP or have extreme loss of function, high pressure requirements, or need mouthpiece ventilation.
CSA: The big challenges with this diagnosis related to qualifying coverage language in the current policies, which are confusing for many providers. Additionally, these policies often deny certain PAP devices and/or oxygen therapy. The group proposed: a single definition of CSA; eliminate discussion of hypoventilation; mirror qualifying symptoms, and, continuing coverage, to the same as that for OSA treatment; and remove need for a prior failure of BiPAP without a backup rate (BUR). The group also had specific recommendations for when oxygen therapy should be covered in patients with CSA.
COPD: This group also focused on the oxygen therapy and promoting use of devices with a BUR. Two problematic areas included the requirement that nocturnal oxygen saturation must drop to ≤ 88% for at least 5 cumulative minutes, and, that patients must begin with an S mode device (no BUR) for at least 2 months and can only then be prescribed a device with a BUR if CO2 fails to drop. The group advocates for the removal of both, the need for a nocturnal oximetry test, and, to “try” an S mode device. The panel advocated giving the prescribing physician discretion in making this determination. The panel also provided recommendations on when a home mechanical ventilator (HMV) should be considered instead of BiPAP therapy.
HVS: Hypoventilation syndromes are a heterogeneous group of disorders with hypercapnia, defined as a Paco2 ≥45 mm Hg. This panel noted that the current coverage criteria are outdated and fail to recognize the spectrum of disease severity and advances in technology, which often leads to circumvention by prescribing more costly home mechanical ventilators (HMV). Consistent with the TRD group, this panel recommended acceptance of surrogate noninvasive end tidal and transcutaneous Pco2 and venous blood gases in lieu of arterial blood gases. Additionally, they suggested no longer requiring CO2 measures while using prescribed oxygen; eliminating the need for a sleep study to avoid delays in care for patients being discharged from the hospital; removing spirometry as a requirement; and no longer a failure of BiPAP without a BUR.
OSA: The initial purpose of examining OSA in this process was to examine when BiPAP should be utilized for treatment; however, it necessitated examination of the entire policy for PAP. The areas that were identified as needing revision included: expansion of the symptom list for patients with OSA; revising the “4 hour rule,” suggesting that 2 hours has been proven to provide benefit; eliminating the need for another sleep study to re-qualify for PAP or supplemental oxygen; and embracing telehealth as a way to improve accessibility for follow-up visits.
For details, please review the papers published in the November 2021 issue of the journal CHEST® (2021; 160[5]:1579-1990, e377-e543).
We now await what CMS will do with our recommendations and work for “the right device to the right patient at the right time.”
Acknowledgment: Drs. Gerald Criner, Nicholas Hill, Babak Mohklesi, Timothy Morgenthaler, and Lisa Wolfe assisted with the content.
Use of positive airway pressure (PAP) devices for treatment of sleep apnea was first described in 1981. Subsequent use of PAP devices expanded to treat patients with respiratory failure. While the treatment in this population has rapidly gained widespread use and undoubtedly has reduced morbidity and mortality in these populations, policies governing these prescriptions have not really kept up with the burgeoning need.
In 2020, Drs. Peter Gay and Robert Owens brought together a technical expert panel (TEP) to systematically review the CMS policies with an eye to remove “regulatory barriers” to improve access for these patients with the mantra: “the right device gets to the right patient at the right time.”
The panel focused on “Optimal NIV Medicare Access Promotion (ONMAP),” and members with specific expertise were recruited for five patient groups: Thoracic Restrictive Disorders (TRD), COPD, Central Sleep Apnea (CSA), Hypoventilation Syndromes (HVS), and Obstructive Sleep Apnea (OSA). Each group reviewed the current coverage, outlined the deficiencies, and suggested revisions. Herein, I will briefly highlight each group’s most important points.
TRD: The goal for this group was to bring the US standards of care closer to the rest of the world. This group advocates that the start of noninvasive ventilation (NIV) should be substantially earlier, to provide the largest improvement in disease outcome and stability. Other prominent features submitted included arterial blood gases (ABG) to not be the only form of CO2 measurement allowed; paying for a second device if patients are using NIV continuously; qualification for a BiPAP to include if vital capacity is ≤ 80%; and, to obtain a home mechanical ventilator, a patient must either fail BiPAP or have extreme loss of function, high pressure requirements, or need mouthpiece ventilation.
CSA: The big challenges with this diagnosis related to qualifying coverage language in the current policies, which are confusing for many providers. Additionally, these policies often deny certain PAP devices and/or oxygen therapy. The group proposed: a single definition of CSA; eliminate discussion of hypoventilation; mirror qualifying symptoms, and, continuing coverage, to the same as that for OSA treatment; and remove need for a prior failure of BiPAP without a backup rate (BUR). The group also had specific recommendations for when oxygen therapy should be covered in patients with CSA.
COPD: This group also focused on the oxygen therapy and promoting use of devices with a BUR. Two problematic areas included the requirement that nocturnal oxygen saturation must drop to ≤ 88% for at least 5 cumulative minutes, and, that patients must begin with an S mode device (no BUR) for at least 2 months and can only then be prescribed a device with a BUR if CO2 fails to drop. The group advocates for the removal of both, the need for a nocturnal oximetry test, and, to “try” an S mode device. The panel advocated giving the prescribing physician discretion in making this determination. The panel also provided recommendations on when a home mechanical ventilator (HMV) should be considered instead of BiPAP therapy.
HVS: Hypoventilation syndromes are a heterogeneous group of disorders with hypercapnia, defined as a Paco2 ≥45 mm Hg. This panel noted that the current coverage criteria are outdated and fail to recognize the spectrum of disease severity and advances in technology, which often leads to circumvention by prescribing more costly home mechanical ventilators (HMV). Consistent with the TRD group, this panel recommended acceptance of surrogate noninvasive end tidal and transcutaneous Pco2 and venous blood gases in lieu of arterial blood gases. Additionally, they suggested no longer requiring CO2 measures while using prescribed oxygen; eliminating the need for a sleep study to avoid delays in care for patients being discharged from the hospital; removing spirometry as a requirement; and no longer a failure of BiPAP without a BUR.
OSA: The initial purpose of examining OSA in this process was to examine when BiPAP should be utilized for treatment; however, it necessitated examination of the entire policy for PAP. The areas that were identified as needing revision included: expansion of the symptom list for patients with OSA; revising the “4 hour rule,” suggesting that 2 hours has been proven to provide benefit; eliminating the need for another sleep study to re-qualify for PAP or supplemental oxygen; and embracing telehealth as a way to improve accessibility for follow-up visits.
For details, please review the papers published in the November 2021 issue of the journal CHEST® (2021; 160[5]:1579-1990, e377-e543).
We now await what CMS will do with our recommendations and work for “the right device to the right patient at the right time.”
Acknowledgment: Drs. Gerald Criner, Nicholas Hill, Babak Mohklesi, Timothy Morgenthaler, and Lisa Wolfe assisted with the content.
Inhaled corticosteroids for COVID-19
Since the onset of the pandemic, the role for corticosteroids (CS) as a therapy for COVID-19 has evolved. Initially, there was reluctance to use oral corticosteroids (OCS) outside of COVID-19-related sepsis or acute respiratory distress syndrome (ARDS). This was in keeping with community-acquired pneumonia (CAP) guidelines (Metlay JP, et al.Am J Respir Crit Care Med. 2019; 200:e45-e67) and reflected concerns that OCS might worsen outcomes in viral pneumonias. At my hospital, the reluctance to use OCS was extended to inhaled corticosteroids (ICS), with early protocols advising cessation in patients with COVID-19.
In fairness, the hesitation to use ICS was short-lived and reflected attempts to provide reasonable guidance during the early pandemic data vacuum. Over time, OCS therapy has gained acceptance as a treatment for moderate-to-severe COVID-19. On top of this, the relationship between COVID-19 and asthma has proved to be complicated. It seemed intuitive that asthmatics would fair worse in the face of a highly transmissible respiratory pathogen. Data on COVID-19 and asthma provide a mixed picture, though. It also appears that the interaction varies by phenotype (Zhu Z, et al. J Allergy Clin Immunol. 2020;146:327-329).
Improvements with OCS and the complicated interaction between COVID-19 and asthma led some to speculate that ICS, the primary treatment for asthma, may actually be protective. There is biologic plausibility to support this concept. Generally, we’ve seen a variety of immunomodulators show efficacy against moderate or severe disease. Specific to ICS, data have shown a down-regulation in COVID-19 gene expression and reduction in proteins required by the virus for cell entry. This includes a reduction in the evil, much maligned ACE-2 receptor (Peters M, et al. Am J Respir Crit Care Med. 2020;202:83-90).
Like much with COVID-19, the initial asthma phenotype and ICS data were observational and hypothesis- generating, at best. More recently, a series of randomized trials has tested the effects of ICS in patients with milder forms of COVID-19. The data are promising and are worth a thorough review by all physicians caring for COVID-19 outside of the hospital.
The STOIC trial (Ramakrishnan S, et al. Lancet Respir Med. 2021;9:763–772) randomized 146 patients to budesonide via dry powder inhaler (DPI), 800 ug twice per day (BID), versus usual care. The primary outcome was clinical deterioration, defined as presentation to acute or emergency care or need for hospitalization. There was a number of secondary outcomes designed to assess time-to-recovery, predominantly by self-report via questionnaires. The results were nothing short of spectacular. There was a significant difference in the primary outcome with a number-needed to treat (NNT) of only 8 to prevent one instance of COVID-19 deterioration. A number of the secondary outcomes reached significance, as well.
The PRINCIPLE trial, only available in preprint form (https://tinyurl.com/mr4cah7j), also randomized patients to budesonide via ICS vs usual care. PRINCIPLE is one of those cool, adaptive platform trials designed to evaluate multiple therapies simultaneously that have gained popularity in the pandemic era. These trials include predefined criteria for success and futility that allow treatments to be added and others to be dropped. The dosage of budesonide was identical to that in STOIC, and, again, it was delivered via DPI. By design, patients were older with co-morbidities, and there were two primary outcomes. The first was a composite of hospitalization and death, and the second was time to recovery.
The PRINCIPLE preprint is only an interim analysis. There were 751 and 1,028 patients who received budesonide and usual care, respectively. Time to recovery was significantly shorter in the budesonide group, but budesonide failed to meet their prespecified criteria for reducing hospitalization/death. The authors noted that the composite outcome of hospitalization or death did not occur at the rates originally anticipated, presumably due to high vaccination rates. This may have led to type II error.
In a third trial published online in November (Clemency BM, et al. JAMA Intern Med. 2021;10.1001/jamainternmed.2021.6759), patients were randomized to 640 micrograms per day of the ICS ciclesonide. Delivery was via metered-dose inhaler (MDI) for a total duration of 30 days. Unlike the STOIC and PRINCIPLE trials, this one wasn’t open label. It was blinded and placebo-controlled. The investigators found no difference in their primary outcome, time to resolution of symptoms. Ciclesonide did reduce the composite secondary outcome of ED visits or hospital admissions. The number needed to treat was 23.
Please indulge me while I overreact. It seems we’ve got a positive signal in all three. In the era of the Omnicron variant and limited health resources, a widely available therapy that curtails symptoms and prevents acute care visits and hospitalizations could have a tremendous impact. It doesn’t require administration in a clinic and, in theory, efficacy shouldn’t be affected by future mutations of the virus.
A more sober look mutes my enthusiasm. First, as the authors of the ciclesonide article note, open-label trials tracking subjective outcomes via self-assessment can be prone to bias. The ciclesonide trial was double-blinded and didn’t find a difference in time to symptom resolution, only the two open-label trials did. Second, the largest study (PRINCIPLE) didn’t show a difference in escalation of care.
Given, they defined “escalation” as hospitalization or death, and vaccines and patient selection (enrolled only outpatients with mild disease) made proving a statistical reduction difficult. However, in the text they state there wasn’t an improvement in “health care services use” either. In essence, the largest trial showed no change in escalation of care, and the trial with the best design did not show reduction in symptoms.
Although three randomized trials are enough for the inevitable meta-analysis that’ll be published soon; don’t expect it to shed much light. Combining data won’t be particularly helpful because the PRINCIPLE trial is larger than the other two combined, so its results will dominate any statistical analysis of combined data. Not to worry though – there are several more ICS COVID-19 trials underway (NCT04355637, NCT04331054, NCT04193878, NCT04330586, NCT04331054, NCT04331470, NCT04355637, NCT04356495, and NCT04381364). Providers will have to decide for themselves whether what we have so far is sufficient to change practice.
Dr. Holley is Program Director, Pulmonary and Critical Care Medicine Fellowship; and Associate Professor of Medicine USU, Walter Reed National Military Medical Center, Bethesda, Maryland. He also serves as Section Editor for Pulmonary Perspectives®.
Since the onset of the pandemic, the role for corticosteroids (CS) as a therapy for COVID-19 has evolved. Initially, there was reluctance to use oral corticosteroids (OCS) outside of COVID-19-related sepsis or acute respiratory distress syndrome (ARDS). This was in keeping with community-acquired pneumonia (CAP) guidelines (Metlay JP, et al.Am J Respir Crit Care Med. 2019; 200:e45-e67) and reflected concerns that OCS might worsen outcomes in viral pneumonias. At my hospital, the reluctance to use OCS was extended to inhaled corticosteroids (ICS), with early protocols advising cessation in patients with COVID-19.
In fairness, the hesitation to use ICS was short-lived and reflected attempts to provide reasonable guidance during the early pandemic data vacuum. Over time, OCS therapy has gained acceptance as a treatment for moderate-to-severe COVID-19. On top of this, the relationship between COVID-19 and asthma has proved to be complicated. It seemed intuitive that asthmatics would fair worse in the face of a highly transmissible respiratory pathogen. Data on COVID-19 and asthma provide a mixed picture, though. It also appears that the interaction varies by phenotype (Zhu Z, et al. J Allergy Clin Immunol. 2020;146:327-329).
Improvements with OCS and the complicated interaction between COVID-19 and asthma led some to speculate that ICS, the primary treatment for asthma, may actually be protective. There is biologic plausibility to support this concept. Generally, we’ve seen a variety of immunomodulators show efficacy against moderate or severe disease. Specific to ICS, data have shown a down-regulation in COVID-19 gene expression and reduction in proteins required by the virus for cell entry. This includes a reduction in the evil, much maligned ACE-2 receptor (Peters M, et al. Am J Respir Crit Care Med. 2020;202:83-90).
Like much with COVID-19, the initial asthma phenotype and ICS data were observational and hypothesis- generating, at best. More recently, a series of randomized trials has tested the effects of ICS in patients with milder forms of COVID-19. The data are promising and are worth a thorough review by all physicians caring for COVID-19 outside of the hospital.
The STOIC trial (Ramakrishnan S, et al. Lancet Respir Med. 2021;9:763–772) randomized 146 patients to budesonide via dry powder inhaler (DPI), 800 ug twice per day (BID), versus usual care. The primary outcome was clinical deterioration, defined as presentation to acute or emergency care or need for hospitalization. There was a number of secondary outcomes designed to assess time-to-recovery, predominantly by self-report via questionnaires. The results were nothing short of spectacular. There was a significant difference in the primary outcome with a number-needed to treat (NNT) of only 8 to prevent one instance of COVID-19 deterioration. A number of the secondary outcomes reached significance, as well.
The PRINCIPLE trial, only available in preprint form (https://tinyurl.com/mr4cah7j), also randomized patients to budesonide via ICS vs usual care. PRINCIPLE is one of those cool, adaptive platform trials designed to evaluate multiple therapies simultaneously that have gained popularity in the pandemic era. These trials include predefined criteria for success and futility that allow treatments to be added and others to be dropped. The dosage of budesonide was identical to that in STOIC, and, again, it was delivered via DPI. By design, patients were older with co-morbidities, and there were two primary outcomes. The first was a composite of hospitalization and death, and the second was time to recovery.
The PRINCIPLE preprint is only an interim analysis. There were 751 and 1,028 patients who received budesonide and usual care, respectively. Time to recovery was significantly shorter in the budesonide group, but budesonide failed to meet their prespecified criteria for reducing hospitalization/death. The authors noted that the composite outcome of hospitalization or death did not occur at the rates originally anticipated, presumably due to high vaccination rates. This may have led to type II error.
In a third trial published online in November (Clemency BM, et al. JAMA Intern Med. 2021;10.1001/jamainternmed.2021.6759), patients were randomized to 640 micrograms per day of the ICS ciclesonide. Delivery was via metered-dose inhaler (MDI) for a total duration of 30 days. Unlike the STOIC and PRINCIPLE trials, this one wasn’t open label. It was blinded and placebo-controlled. The investigators found no difference in their primary outcome, time to resolution of symptoms. Ciclesonide did reduce the composite secondary outcome of ED visits or hospital admissions. The number needed to treat was 23.
Please indulge me while I overreact. It seems we’ve got a positive signal in all three. In the era of the Omnicron variant and limited health resources, a widely available therapy that curtails symptoms and prevents acute care visits and hospitalizations could have a tremendous impact. It doesn’t require administration in a clinic and, in theory, efficacy shouldn’t be affected by future mutations of the virus.
A more sober look mutes my enthusiasm. First, as the authors of the ciclesonide article note, open-label trials tracking subjective outcomes via self-assessment can be prone to bias. The ciclesonide trial was double-blinded and didn’t find a difference in time to symptom resolution, only the two open-label trials did. Second, the largest study (PRINCIPLE) didn’t show a difference in escalation of care.
Given, they defined “escalation” as hospitalization or death, and vaccines and patient selection (enrolled only outpatients with mild disease) made proving a statistical reduction difficult. However, in the text they state there wasn’t an improvement in “health care services use” either. In essence, the largest trial showed no change in escalation of care, and the trial with the best design did not show reduction in symptoms.
Although three randomized trials are enough for the inevitable meta-analysis that’ll be published soon; don’t expect it to shed much light. Combining data won’t be particularly helpful because the PRINCIPLE trial is larger than the other two combined, so its results will dominate any statistical analysis of combined data. Not to worry though – there are several more ICS COVID-19 trials underway (NCT04355637, NCT04331054, NCT04193878, NCT04330586, NCT04331054, NCT04331470, NCT04355637, NCT04356495, and NCT04381364). Providers will have to decide for themselves whether what we have so far is sufficient to change practice.
Dr. Holley is Program Director, Pulmonary and Critical Care Medicine Fellowship; and Associate Professor of Medicine USU, Walter Reed National Military Medical Center, Bethesda, Maryland. He also serves as Section Editor for Pulmonary Perspectives®.
Since the onset of the pandemic, the role for corticosteroids (CS) as a therapy for COVID-19 has evolved. Initially, there was reluctance to use oral corticosteroids (OCS) outside of COVID-19-related sepsis or acute respiratory distress syndrome (ARDS). This was in keeping with community-acquired pneumonia (CAP) guidelines (Metlay JP, et al.Am J Respir Crit Care Med. 2019; 200:e45-e67) and reflected concerns that OCS might worsen outcomes in viral pneumonias. At my hospital, the reluctance to use OCS was extended to inhaled corticosteroids (ICS), with early protocols advising cessation in patients with COVID-19.
In fairness, the hesitation to use ICS was short-lived and reflected attempts to provide reasonable guidance during the early pandemic data vacuum. Over time, OCS therapy has gained acceptance as a treatment for moderate-to-severe COVID-19. On top of this, the relationship between COVID-19 and asthma has proved to be complicated. It seemed intuitive that asthmatics would fair worse in the face of a highly transmissible respiratory pathogen. Data on COVID-19 and asthma provide a mixed picture, though. It also appears that the interaction varies by phenotype (Zhu Z, et al. J Allergy Clin Immunol. 2020;146:327-329).
Improvements with OCS and the complicated interaction between COVID-19 and asthma led some to speculate that ICS, the primary treatment for asthma, may actually be protective. There is biologic plausibility to support this concept. Generally, we’ve seen a variety of immunomodulators show efficacy against moderate or severe disease. Specific to ICS, data have shown a down-regulation in COVID-19 gene expression and reduction in proteins required by the virus for cell entry. This includes a reduction in the evil, much maligned ACE-2 receptor (Peters M, et al. Am J Respir Crit Care Med. 2020;202:83-90).
Like much with COVID-19, the initial asthma phenotype and ICS data were observational and hypothesis- generating, at best. More recently, a series of randomized trials has tested the effects of ICS in patients with milder forms of COVID-19. The data are promising and are worth a thorough review by all physicians caring for COVID-19 outside of the hospital.
The STOIC trial (Ramakrishnan S, et al. Lancet Respir Med. 2021;9:763–772) randomized 146 patients to budesonide via dry powder inhaler (DPI), 800 ug twice per day (BID), versus usual care. The primary outcome was clinical deterioration, defined as presentation to acute or emergency care or need for hospitalization. There was a number of secondary outcomes designed to assess time-to-recovery, predominantly by self-report via questionnaires. The results were nothing short of spectacular. There was a significant difference in the primary outcome with a number-needed to treat (NNT) of only 8 to prevent one instance of COVID-19 deterioration. A number of the secondary outcomes reached significance, as well.
The PRINCIPLE trial, only available in preprint form (https://tinyurl.com/mr4cah7j), also randomized patients to budesonide via ICS vs usual care. PRINCIPLE is one of those cool, adaptive platform trials designed to evaluate multiple therapies simultaneously that have gained popularity in the pandemic era. These trials include predefined criteria for success and futility that allow treatments to be added and others to be dropped. The dosage of budesonide was identical to that in STOIC, and, again, it was delivered via DPI. By design, patients were older with co-morbidities, and there were two primary outcomes. The first was a composite of hospitalization and death, and the second was time to recovery.
The PRINCIPLE preprint is only an interim analysis. There were 751 and 1,028 patients who received budesonide and usual care, respectively. Time to recovery was significantly shorter in the budesonide group, but budesonide failed to meet their prespecified criteria for reducing hospitalization/death. The authors noted that the composite outcome of hospitalization or death did not occur at the rates originally anticipated, presumably due to high vaccination rates. This may have led to type II error.
In a third trial published online in November (Clemency BM, et al. JAMA Intern Med. 2021;10.1001/jamainternmed.2021.6759), patients were randomized to 640 micrograms per day of the ICS ciclesonide. Delivery was via metered-dose inhaler (MDI) for a total duration of 30 days. Unlike the STOIC and PRINCIPLE trials, this one wasn’t open label. It was blinded and placebo-controlled. The investigators found no difference in their primary outcome, time to resolution of symptoms. Ciclesonide did reduce the composite secondary outcome of ED visits or hospital admissions. The number needed to treat was 23.
Please indulge me while I overreact. It seems we’ve got a positive signal in all three. In the era of the Omnicron variant and limited health resources, a widely available therapy that curtails symptoms and prevents acute care visits and hospitalizations could have a tremendous impact. It doesn’t require administration in a clinic and, in theory, efficacy shouldn’t be affected by future mutations of the virus.
A more sober look mutes my enthusiasm. First, as the authors of the ciclesonide article note, open-label trials tracking subjective outcomes via self-assessment can be prone to bias. The ciclesonide trial was double-blinded and didn’t find a difference in time to symptom resolution, only the two open-label trials did. Second, the largest study (PRINCIPLE) didn’t show a difference in escalation of care.
Given, they defined “escalation” as hospitalization or death, and vaccines and patient selection (enrolled only outpatients with mild disease) made proving a statistical reduction difficult. However, in the text they state there wasn’t an improvement in “health care services use” either. In essence, the largest trial showed no change in escalation of care, and the trial with the best design did not show reduction in symptoms.
Although three randomized trials are enough for the inevitable meta-analysis that’ll be published soon; don’t expect it to shed much light. Combining data won’t be particularly helpful because the PRINCIPLE trial is larger than the other two combined, so its results will dominate any statistical analysis of combined data. Not to worry though – there are several more ICS COVID-19 trials underway (NCT04355637, NCT04331054, NCT04193878, NCT04330586, NCT04331054, NCT04331470, NCT04355637, NCT04356495, and NCT04381364). Providers will have to decide for themselves whether what we have so far is sufficient to change practice.
Dr. Holley is Program Director, Pulmonary and Critical Care Medicine Fellowship; and Associate Professor of Medicine USU, Walter Reed National Military Medical Center, Bethesda, Maryland. He also serves as Section Editor for Pulmonary Perspectives®.
Early-onset severe COPD: Similar physical symptoms, but higher depression rates
Younger and older patients with severe chronic obstructive pulmonary disease have similar pulmonary and physical health limitations, based on data from 1,058 adults.
Although chronic obstructive pulmonary disease (COPD) generally appears in older patients, the prevalence among adults aged 45-55 years was 6.5% in 2014-2015, wrote Rosanne J.H.C.G. Beijers, PhD, of Maastricht (the Netherlands) University Medical Center, and colleagues. However, data on the early-onset COPD phenotype are limited. In particular, the extent to which younger patients with early-onset severe COPD experienced the same physical and mental health problems as older patients with similar degree of airflow limitation has not been examined, they said.
In a study published in Clinical Nutrition, the researchers analyzed data from adults with COPD who were referred for pulmonary rehabilitation at a single center between July 2013 and August 2018. Severe disease was defined as FEV1< 50%, and early onset was defined as younger than 55 years. The mean age difference between older and younger patient groups was 15.8 years.
The study population included 79 individuals with early-onset severe disease, 54 with early-onset mild to moderate disease, 158 older adults with severe disease, and 103 older adults with mild to moderate disease. The researchers compared disease markers including body composition, physical performance, and mental health between the groups. A significantly greater proportion of the early-onset group were women, compared to the older group (64% vs. 44%).
In comparing early-onset and older patients with severe COPD, the researchers found that clinical characteristics were similar for body composition, skeletal muscle index, fat percentage, and bone mineral content, and for physical performance factors including the percent predicted maximal work capacity (Wmax), 6-minute walk test, and isokinetic strength. However, a higher prevalence of depression appeared in the early-onset severe-disease patients, compared with the older severe-disease patients (51.9% vs. 32.7%; P = .029).
Although the prevalence of depression was not based on a clinical diagnosis, this finding should prompt health care professionals to pay more attention to psychosocial and emotional well-being in early-onset severe COPD patients, the researchers noted.
In comparing early-onset severe-disease patients and early-onset patients with mild to moderate disease, patients with early-onset severe COPD had significantly lower exercise performance, based on a 6-minute walk test and percent predicted Wmax. However, body composition and isokinetic muscle strength were not significantly different between both early-onset groups.
The findings were limited by several factors including the relatively small number of early-onset patients and the lack of data on whether older patients were diagnosed with severe COPD at a younger age, and more research using age and lung function at the time of diagnosis is needed, the researchers noted. However, the results highlight the importance of early identification of patients at risk for early-onset severe COPD, they said. “Within these individuals at risk, special attention should also be paid to the development of extrapulmonary disease manifestations such as exercise limitations, impaired body composition, and psychological and emotional problems,” the researchers said. “Subsequently, intervention strategies need to be applied that not only focus on the regular advice of quitting smoking but also include decreasing the exposure to air pollutants and promoting a healthy lifestyle including physical activity and a healthy diet,” they added.
The study received no outside funding. Lead author Dr. Beijers had no financial conflicts to disclose.
Younger and older patients with severe chronic obstructive pulmonary disease have similar pulmonary and physical health limitations, based on data from 1,058 adults.
Although chronic obstructive pulmonary disease (COPD) generally appears in older patients, the prevalence among adults aged 45-55 years was 6.5% in 2014-2015, wrote Rosanne J.H.C.G. Beijers, PhD, of Maastricht (the Netherlands) University Medical Center, and colleagues. However, data on the early-onset COPD phenotype are limited. In particular, the extent to which younger patients with early-onset severe COPD experienced the same physical and mental health problems as older patients with similar degree of airflow limitation has not been examined, they said.
In a study published in Clinical Nutrition, the researchers analyzed data from adults with COPD who were referred for pulmonary rehabilitation at a single center between July 2013 and August 2018. Severe disease was defined as FEV1< 50%, and early onset was defined as younger than 55 years. The mean age difference between older and younger patient groups was 15.8 years.
The study population included 79 individuals with early-onset severe disease, 54 with early-onset mild to moderate disease, 158 older adults with severe disease, and 103 older adults with mild to moderate disease. The researchers compared disease markers including body composition, physical performance, and mental health between the groups. A significantly greater proportion of the early-onset group were women, compared to the older group (64% vs. 44%).
In comparing early-onset and older patients with severe COPD, the researchers found that clinical characteristics were similar for body composition, skeletal muscle index, fat percentage, and bone mineral content, and for physical performance factors including the percent predicted maximal work capacity (Wmax), 6-minute walk test, and isokinetic strength. However, a higher prevalence of depression appeared in the early-onset severe-disease patients, compared with the older severe-disease patients (51.9% vs. 32.7%; P = .029).
Although the prevalence of depression was not based on a clinical diagnosis, this finding should prompt health care professionals to pay more attention to psychosocial and emotional well-being in early-onset severe COPD patients, the researchers noted.
In comparing early-onset severe-disease patients and early-onset patients with mild to moderate disease, patients with early-onset severe COPD had significantly lower exercise performance, based on a 6-minute walk test and percent predicted Wmax. However, body composition and isokinetic muscle strength were not significantly different between both early-onset groups.
The findings were limited by several factors including the relatively small number of early-onset patients and the lack of data on whether older patients were diagnosed with severe COPD at a younger age, and more research using age and lung function at the time of diagnosis is needed, the researchers noted. However, the results highlight the importance of early identification of patients at risk for early-onset severe COPD, they said. “Within these individuals at risk, special attention should also be paid to the development of extrapulmonary disease manifestations such as exercise limitations, impaired body composition, and psychological and emotional problems,” the researchers said. “Subsequently, intervention strategies need to be applied that not only focus on the regular advice of quitting smoking but also include decreasing the exposure to air pollutants and promoting a healthy lifestyle including physical activity and a healthy diet,” they added.
The study received no outside funding. Lead author Dr. Beijers had no financial conflicts to disclose.
Younger and older patients with severe chronic obstructive pulmonary disease have similar pulmonary and physical health limitations, based on data from 1,058 adults.
Although chronic obstructive pulmonary disease (COPD) generally appears in older patients, the prevalence among adults aged 45-55 years was 6.5% in 2014-2015, wrote Rosanne J.H.C.G. Beijers, PhD, of Maastricht (the Netherlands) University Medical Center, and colleagues. However, data on the early-onset COPD phenotype are limited. In particular, the extent to which younger patients with early-onset severe COPD experienced the same physical and mental health problems as older patients with similar degree of airflow limitation has not been examined, they said.
In a study published in Clinical Nutrition, the researchers analyzed data from adults with COPD who were referred for pulmonary rehabilitation at a single center between July 2013 and August 2018. Severe disease was defined as FEV1< 50%, and early onset was defined as younger than 55 years. The mean age difference between older and younger patient groups was 15.8 years.
The study population included 79 individuals with early-onset severe disease, 54 with early-onset mild to moderate disease, 158 older adults with severe disease, and 103 older adults with mild to moderate disease. The researchers compared disease markers including body composition, physical performance, and mental health between the groups. A significantly greater proportion of the early-onset group were women, compared to the older group (64% vs. 44%).
In comparing early-onset and older patients with severe COPD, the researchers found that clinical characteristics were similar for body composition, skeletal muscle index, fat percentage, and bone mineral content, and for physical performance factors including the percent predicted maximal work capacity (Wmax), 6-minute walk test, and isokinetic strength. However, a higher prevalence of depression appeared in the early-onset severe-disease patients, compared with the older severe-disease patients (51.9% vs. 32.7%; P = .029).
Although the prevalence of depression was not based on a clinical diagnosis, this finding should prompt health care professionals to pay more attention to psychosocial and emotional well-being in early-onset severe COPD patients, the researchers noted.
In comparing early-onset severe-disease patients and early-onset patients with mild to moderate disease, patients with early-onset severe COPD had significantly lower exercise performance, based on a 6-minute walk test and percent predicted Wmax. However, body composition and isokinetic muscle strength were not significantly different between both early-onset groups.
The findings were limited by several factors including the relatively small number of early-onset patients and the lack of data on whether older patients were diagnosed with severe COPD at a younger age, and more research using age and lung function at the time of diagnosis is needed, the researchers noted. However, the results highlight the importance of early identification of patients at risk for early-onset severe COPD, they said. “Within these individuals at risk, special attention should also be paid to the development of extrapulmonary disease manifestations such as exercise limitations, impaired body composition, and psychological and emotional problems,” the researchers said. “Subsequently, intervention strategies need to be applied that not only focus on the regular advice of quitting smoking but also include decreasing the exposure to air pollutants and promoting a healthy lifestyle including physical activity and a healthy diet,” they added.
The study received no outside funding. Lead author Dr. Beijers had no financial conflicts to disclose.
FROM CLINICAL NUTRITION
Clinical data affirm dupilumab for chronic nasal polyps
In a specialty clinic, dupilumab (Dupixent) injections significantly improved symptoms for patients with chronic rhinosinusitis with nasal polyps, based on provisional data from more than 100 adults.
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a significant burden among working-age adults. Symptom control remains a challenge for many of these patients, and the cost in lost productivity and health care consumption can be substantial, write Rik J.L. van der Lans, MD, of the University of Amsterdam, and colleagues.
Dupilumab, a biologic that targets components of the type 2 inflammatory pathway, represents a new option that has shown effectiveness in clinical trials for regulatory approval, they said.
A new observational study tests dupilumab in patients who met criteria for biological treatment proposed in a recent major systematic review. The findings were published in the journal Allergy.
In the study, the researchers identified 131 adults older than 18 years (mean age 51.7) with CRSwNP treated at a single tertiary care center. Participants received 300 mg of dupilumab subcutaneous injection every 2 weeks for at least 12 weeks.
The primary outcomes were scores on several measures, including the SinoNasal Outcome Test-22 (SNOT-22, scale of 0-110), the bilateral Nasal Polyp Score (NPS, scale of 0-8), and the Sniffin’ Sticks-12 identification test (SSIT-12, scale of 0-6 anosmia, 7-10 hyposmia, 11-12 normosmia).
The mean scores on all three outcomes improved significantly from baseline to both 24 weeks and 48 weeks. Scores on the SNOT-22 improved from 52.4 at baseline to 18.5 and 16.8 at weeks 24 and 48, respectively. NPS improved from 5.4 at baseline to 1.6 and 1.0, respectively. SSIT-12 scores improved from 3.6 at baseline to 7.3 and 8.3, respectively.
At baseline, 95.8% of the patients had uncontrolled chronic rhinosinusitis, but at 24 and 48 weeks, respectively, 24.3% and 6.2% were uncontrolled.
Approximately half of the patients experienced treatment-emergent adverse events, but these were “mild and decreased in occurrence and intensity throughout treatment,” the researchers say.
For patients with a strong response, the researchers also tested an extension of the interval between doses to 4 weeks and 6 weeks, in a provisional indication of continued established control at these timepoints.
The study findings were limited by several factors, including the potential for selection bias, and data from only the first patient cohort, the researchers noted. However, the results were strengthened by the real-life context, standardized indications, and long-term follow up for almost a year, they said.
More research is needed on nonacademic patient cohorts, but the current data confirm the effectiveness of dupilumab as an add-on for difficult-to-treat CRSwNP, they concluded. The findings also validate the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS2020) inclusion criteria for biologic treatment, they said.
The new study is important because of the need for verification of results from randomized controlled trials using real-world data, Dr. Van der Lans told this news organization.
“For example, differences in treatment efficacy might result from differing indication criteria, and the inclusion/exclusion criteria in the RCTs might have excluded patients one would encounter in daily practice,” he said. “With our prospective observational cohort, we are seeking to verify efficacy, monitor pharmacovigilance, and evaluate and advance the indication criteria and positioning of biologicals registered for CRSwNP, such as dupilumab.”
These cross-sectional results suggest dupilumab is more effective in preventing possibly harmful escape treatments, such as oral corticosteroids and/or surgery, than reported by the registration trials.
“Additionally, it appears possible to maintain established CRS-control during response-dependent, stepwise, interdose interval prolongation of up to 6 weeks, which is officially an off-label dosing interval,” he said. “This would greatly benefit patients’ treatment burden and direct costs,” he said. However, both findings require corroboration by anticipated longitudinal results in 2022, he noted.
The key message for clinicians in practice: “Biologicals like dupilumab are a potent and promising treatment for severe CRSwNP when conventional medical and surgical therapy fails,” he emphasized.
Looking ahead, important research objectives include head-on comparison studies of the diverse biological agents, establishing biomarkers to guide preferential therapy, and evaluating the economics of biologics compared with conventional therapy, Dr. Van der Lans added. Such research is vital not only for improving patient-centered care but to sustain the use of biologicals in a health-economic perspective.
One of the greatest criticisms of biologic therapy for CRSwNP is cost, particularly in a setting of ever-increasing health care costs. A recent review noted the average cost per year is greater than $30,000.
Real-life study verifies effectiveness
“As the authors pointed out, this is a real-life, prospective observational cohort with a decently large size, evaluating the therapeutic efficacy of add-on dupilumab,” said Seong H. Cho, MD, of the University of South Florida, Tampa, in an interview.
“Dupilumab is the first FDA-approved biologic to treat severe chronic rhinosinusitis with nasal polyps based on two phase 3 clinical trials,” said Dr. Cho, who was not involved with the study. “It has been more than 2 years since dupilumab was approved for severe CRSwNP by the FDA and EMA. This real-life, prospective, observational study with a decent size verified the efficacy of dupilumab as an add-on treatment when used with a proper indication such as EPOS2020 indication criteria.
“I am not surprised by the efficacy of dupilumab on severe CRSwNP, based on my clinical experience. My clinical observation is similar to the results of this study. This study verifies that dupilumab is highly efficacious in treating refractory and severe CRSwNP in a real-life setting by improving all subjective and objective clinical outcomes such as SNOT-22, NPS, and smell test score,” he said. The study also confirms that a stepwise, interdose interval prolongation from every 2-4 weeks for CRSwNP patients with good response should be a consideration for clinical practice, he added.
The cost-effectiveness of dupilumab is the main barrier to more consistent use, Dr. Cho said. “There is no evidence that dupilumab can change the course of the disease, and we don’t know how long patients need to be on this drug. Therefore, nasal polyps need to be refractory and severe enough to use dupilumab and other biologics,” he explained.
Consequently, proper indication criteria, such as the EPOS2020 indication criteria for biologics, should be established before initiating dupilumab, Dr. Cho noted.
“Generally, endoscopic sinus surgery would be preferred in sinus-surgery naive CRSwNP patients, unless surgery is contraindicated or refused by patients because of cost-effectiveness rather than the superior efficacy,” he said. “If surgery fails, then dupilumab can be considered. In addition, proper evaluation of nasal polyp severity would be important.”
“One should establish an objective NPS by endoscopic exam before initiation of dupilumab. This baseline score would be an important marker to assess the efficacy of dupilumab in the course of treatment.”
Monitoring of the NPS together with the patient’s symptom improvement would be essential to implementing a stepwise, interdose interval prolongation to reduce the cost, he emphasized.
“The most crucial additional research is establishing suitable biomarkers for the response of dupilumab and other biologics,” said Dr. Cho. “Overall, the performance of dupilumab seems to be good. But there are patients unresponsive to dupilumab, even more to other recently FDA-approved biologics for CRSwNP.”
Blood eosinophils and exhaled nitric oxide can be a good biomarker for type 2 asthma, Dr. Cho added. “Still, there is no evidence that these biomarkers are decent for CRSwNP, even though CRSwNP is mostly considered as type 2 disease. Therefore, it would be essential to find promising biomarkers for severe CRSwNP.”
Dr. Van der Lans disclosed serving as a consultant for GlaxoSmithKline, and several coauthors disclosed relationships with companies including Sanofi and Novartis. The patient registry from which the study population was drawn is cofunded by Sanofi and Novartis. Dr. Cho has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a specialty clinic, dupilumab (Dupixent) injections significantly improved symptoms for patients with chronic rhinosinusitis with nasal polyps, based on provisional data from more than 100 adults.
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a significant burden among working-age adults. Symptom control remains a challenge for many of these patients, and the cost in lost productivity and health care consumption can be substantial, write Rik J.L. van der Lans, MD, of the University of Amsterdam, and colleagues.
Dupilumab, a biologic that targets components of the type 2 inflammatory pathway, represents a new option that has shown effectiveness in clinical trials for regulatory approval, they said.
A new observational study tests dupilumab in patients who met criteria for biological treatment proposed in a recent major systematic review. The findings were published in the journal Allergy.
In the study, the researchers identified 131 adults older than 18 years (mean age 51.7) with CRSwNP treated at a single tertiary care center. Participants received 300 mg of dupilumab subcutaneous injection every 2 weeks for at least 12 weeks.
The primary outcomes were scores on several measures, including the SinoNasal Outcome Test-22 (SNOT-22, scale of 0-110), the bilateral Nasal Polyp Score (NPS, scale of 0-8), and the Sniffin’ Sticks-12 identification test (SSIT-12, scale of 0-6 anosmia, 7-10 hyposmia, 11-12 normosmia).
The mean scores on all three outcomes improved significantly from baseline to both 24 weeks and 48 weeks. Scores on the SNOT-22 improved from 52.4 at baseline to 18.5 and 16.8 at weeks 24 and 48, respectively. NPS improved from 5.4 at baseline to 1.6 and 1.0, respectively. SSIT-12 scores improved from 3.6 at baseline to 7.3 and 8.3, respectively.
At baseline, 95.8% of the patients had uncontrolled chronic rhinosinusitis, but at 24 and 48 weeks, respectively, 24.3% and 6.2% were uncontrolled.
Approximately half of the patients experienced treatment-emergent adverse events, but these were “mild and decreased in occurrence and intensity throughout treatment,” the researchers say.
For patients with a strong response, the researchers also tested an extension of the interval between doses to 4 weeks and 6 weeks, in a provisional indication of continued established control at these timepoints.
The study findings were limited by several factors, including the potential for selection bias, and data from only the first patient cohort, the researchers noted. However, the results were strengthened by the real-life context, standardized indications, and long-term follow up for almost a year, they said.
More research is needed on nonacademic patient cohorts, but the current data confirm the effectiveness of dupilumab as an add-on for difficult-to-treat CRSwNP, they concluded. The findings also validate the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS2020) inclusion criteria for biologic treatment, they said.
The new study is important because of the need for verification of results from randomized controlled trials using real-world data, Dr. Van der Lans told this news organization.
“For example, differences in treatment efficacy might result from differing indication criteria, and the inclusion/exclusion criteria in the RCTs might have excluded patients one would encounter in daily practice,” he said. “With our prospective observational cohort, we are seeking to verify efficacy, monitor pharmacovigilance, and evaluate and advance the indication criteria and positioning of biologicals registered for CRSwNP, such as dupilumab.”
These cross-sectional results suggest dupilumab is more effective in preventing possibly harmful escape treatments, such as oral corticosteroids and/or surgery, than reported by the registration trials.
“Additionally, it appears possible to maintain established CRS-control during response-dependent, stepwise, interdose interval prolongation of up to 6 weeks, which is officially an off-label dosing interval,” he said. “This would greatly benefit patients’ treatment burden and direct costs,” he said. However, both findings require corroboration by anticipated longitudinal results in 2022, he noted.
The key message for clinicians in practice: “Biologicals like dupilumab are a potent and promising treatment for severe CRSwNP when conventional medical and surgical therapy fails,” he emphasized.
Looking ahead, important research objectives include head-on comparison studies of the diverse biological agents, establishing biomarkers to guide preferential therapy, and evaluating the economics of biologics compared with conventional therapy, Dr. Van der Lans added. Such research is vital not only for improving patient-centered care but to sustain the use of biologicals in a health-economic perspective.
One of the greatest criticisms of biologic therapy for CRSwNP is cost, particularly in a setting of ever-increasing health care costs. A recent review noted the average cost per year is greater than $30,000.
Real-life study verifies effectiveness
“As the authors pointed out, this is a real-life, prospective observational cohort with a decently large size, evaluating the therapeutic efficacy of add-on dupilumab,” said Seong H. Cho, MD, of the University of South Florida, Tampa, in an interview.
“Dupilumab is the first FDA-approved biologic to treat severe chronic rhinosinusitis with nasal polyps based on two phase 3 clinical trials,” said Dr. Cho, who was not involved with the study. “It has been more than 2 years since dupilumab was approved for severe CRSwNP by the FDA and EMA. This real-life, prospective, observational study with a decent size verified the efficacy of dupilumab as an add-on treatment when used with a proper indication such as EPOS2020 indication criteria.
“I am not surprised by the efficacy of dupilumab on severe CRSwNP, based on my clinical experience. My clinical observation is similar to the results of this study. This study verifies that dupilumab is highly efficacious in treating refractory and severe CRSwNP in a real-life setting by improving all subjective and objective clinical outcomes such as SNOT-22, NPS, and smell test score,” he said. The study also confirms that a stepwise, interdose interval prolongation from every 2-4 weeks for CRSwNP patients with good response should be a consideration for clinical practice, he added.
The cost-effectiveness of dupilumab is the main barrier to more consistent use, Dr. Cho said. “There is no evidence that dupilumab can change the course of the disease, and we don’t know how long patients need to be on this drug. Therefore, nasal polyps need to be refractory and severe enough to use dupilumab and other biologics,” he explained.
Consequently, proper indication criteria, such as the EPOS2020 indication criteria for biologics, should be established before initiating dupilumab, Dr. Cho noted.
“Generally, endoscopic sinus surgery would be preferred in sinus-surgery naive CRSwNP patients, unless surgery is contraindicated or refused by patients because of cost-effectiveness rather than the superior efficacy,” he said. “If surgery fails, then dupilumab can be considered. In addition, proper evaluation of nasal polyp severity would be important.”
“One should establish an objective NPS by endoscopic exam before initiation of dupilumab. This baseline score would be an important marker to assess the efficacy of dupilumab in the course of treatment.”
Monitoring of the NPS together with the patient’s symptom improvement would be essential to implementing a stepwise, interdose interval prolongation to reduce the cost, he emphasized.
“The most crucial additional research is establishing suitable biomarkers for the response of dupilumab and other biologics,” said Dr. Cho. “Overall, the performance of dupilumab seems to be good. But there are patients unresponsive to dupilumab, even more to other recently FDA-approved biologics for CRSwNP.”
Blood eosinophils and exhaled nitric oxide can be a good biomarker for type 2 asthma, Dr. Cho added. “Still, there is no evidence that these biomarkers are decent for CRSwNP, even though CRSwNP is mostly considered as type 2 disease. Therefore, it would be essential to find promising biomarkers for severe CRSwNP.”
Dr. Van der Lans disclosed serving as a consultant for GlaxoSmithKline, and several coauthors disclosed relationships with companies including Sanofi and Novartis. The patient registry from which the study population was drawn is cofunded by Sanofi and Novartis. Dr. Cho has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a specialty clinic, dupilumab (Dupixent) injections significantly improved symptoms for patients with chronic rhinosinusitis with nasal polyps, based on provisional data from more than 100 adults.
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a significant burden among working-age adults. Symptom control remains a challenge for many of these patients, and the cost in lost productivity and health care consumption can be substantial, write Rik J.L. van der Lans, MD, of the University of Amsterdam, and colleagues.
Dupilumab, a biologic that targets components of the type 2 inflammatory pathway, represents a new option that has shown effectiveness in clinical trials for regulatory approval, they said.
A new observational study tests dupilumab in patients who met criteria for biological treatment proposed in a recent major systematic review. The findings were published in the journal Allergy.
In the study, the researchers identified 131 adults older than 18 years (mean age 51.7) with CRSwNP treated at a single tertiary care center. Participants received 300 mg of dupilumab subcutaneous injection every 2 weeks for at least 12 weeks.
The primary outcomes were scores on several measures, including the SinoNasal Outcome Test-22 (SNOT-22, scale of 0-110), the bilateral Nasal Polyp Score (NPS, scale of 0-8), and the Sniffin’ Sticks-12 identification test (SSIT-12, scale of 0-6 anosmia, 7-10 hyposmia, 11-12 normosmia).
The mean scores on all three outcomes improved significantly from baseline to both 24 weeks and 48 weeks. Scores on the SNOT-22 improved from 52.4 at baseline to 18.5 and 16.8 at weeks 24 and 48, respectively. NPS improved from 5.4 at baseline to 1.6 and 1.0, respectively. SSIT-12 scores improved from 3.6 at baseline to 7.3 and 8.3, respectively.
At baseline, 95.8% of the patients had uncontrolled chronic rhinosinusitis, but at 24 and 48 weeks, respectively, 24.3% and 6.2% were uncontrolled.
Approximately half of the patients experienced treatment-emergent adverse events, but these were “mild and decreased in occurrence and intensity throughout treatment,” the researchers say.
For patients with a strong response, the researchers also tested an extension of the interval between doses to 4 weeks and 6 weeks, in a provisional indication of continued established control at these timepoints.
The study findings were limited by several factors, including the potential for selection bias, and data from only the first patient cohort, the researchers noted. However, the results were strengthened by the real-life context, standardized indications, and long-term follow up for almost a year, they said.
More research is needed on nonacademic patient cohorts, but the current data confirm the effectiveness of dupilumab as an add-on for difficult-to-treat CRSwNP, they concluded. The findings also validate the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS2020) inclusion criteria for biologic treatment, they said.
The new study is important because of the need for verification of results from randomized controlled trials using real-world data, Dr. Van der Lans told this news organization.
“For example, differences in treatment efficacy might result from differing indication criteria, and the inclusion/exclusion criteria in the RCTs might have excluded patients one would encounter in daily practice,” he said. “With our prospective observational cohort, we are seeking to verify efficacy, monitor pharmacovigilance, and evaluate and advance the indication criteria and positioning of biologicals registered for CRSwNP, such as dupilumab.”
These cross-sectional results suggest dupilumab is more effective in preventing possibly harmful escape treatments, such as oral corticosteroids and/or surgery, than reported by the registration trials.
“Additionally, it appears possible to maintain established CRS-control during response-dependent, stepwise, interdose interval prolongation of up to 6 weeks, which is officially an off-label dosing interval,” he said. “This would greatly benefit patients’ treatment burden and direct costs,” he said. However, both findings require corroboration by anticipated longitudinal results in 2022, he noted.
The key message for clinicians in practice: “Biologicals like dupilumab are a potent and promising treatment for severe CRSwNP when conventional medical and surgical therapy fails,” he emphasized.
Looking ahead, important research objectives include head-on comparison studies of the diverse biological agents, establishing biomarkers to guide preferential therapy, and evaluating the economics of biologics compared with conventional therapy, Dr. Van der Lans added. Such research is vital not only for improving patient-centered care but to sustain the use of biologicals in a health-economic perspective.
One of the greatest criticisms of biologic therapy for CRSwNP is cost, particularly in a setting of ever-increasing health care costs. A recent review noted the average cost per year is greater than $30,000.
Real-life study verifies effectiveness
“As the authors pointed out, this is a real-life, prospective observational cohort with a decently large size, evaluating the therapeutic efficacy of add-on dupilumab,” said Seong H. Cho, MD, of the University of South Florida, Tampa, in an interview.
“Dupilumab is the first FDA-approved biologic to treat severe chronic rhinosinusitis with nasal polyps based on two phase 3 clinical trials,” said Dr. Cho, who was not involved with the study. “It has been more than 2 years since dupilumab was approved for severe CRSwNP by the FDA and EMA. This real-life, prospective, observational study with a decent size verified the efficacy of dupilumab as an add-on treatment when used with a proper indication such as EPOS2020 indication criteria.
“I am not surprised by the efficacy of dupilumab on severe CRSwNP, based on my clinical experience. My clinical observation is similar to the results of this study. This study verifies that dupilumab is highly efficacious in treating refractory and severe CRSwNP in a real-life setting by improving all subjective and objective clinical outcomes such as SNOT-22, NPS, and smell test score,” he said. The study also confirms that a stepwise, interdose interval prolongation from every 2-4 weeks for CRSwNP patients with good response should be a consideration for clinical practice, he added.
The cost-effectiveness of dupilumab is the main barrier to more consistent use, Dr. Cho said. “There is no evidence that dupilumab can change the course of the disease, and we don’t know how long patients need to be on this drug. Therefore, nasal polyps need to be refractory and severe enough to use dupilumab and other biologics,” he explained.
Consequently, proper indication criteria, such as the EPOS2020 indication criteria for biologics, should be established before initiating dupilumab, Dr. Cho noted.
“Generally, endoscopic sinus surgery would be preferred in sinus-surgery naive CRSwNP patients, unless surgery is contraindicated or refused by patients because of cost-effectiveness rather than the superior efficacy,” he said. “If surgery fails, then dupilumab can be considered. In addition, proper evaluation of nasal polyp severity would be important.”
“One should establish an objective NPS by endoscopic exam before initiation of dupilumab. This baseline score would be an important marker to assess the efficacy of dupilumab in the course of treatment.”
Monitoring of the NPS together with the patient’s symptom improvement would be essential to implementing a stepwise, interdose interval prolongation to reduce the cost, he emphasized.
“The most crucial additional research is establishing suitable biomarkers for the response of dupilumab and other biologics,” said Dr. Cho. “Overall, the performance of dupilumab seems to be good. But there are patients unresponsive to dupilumab, even more to other recently FDA-approved biologics for CRSwNP.”
Blood eosinophils and exhaled nitric oxide can be a good biomarker for type 2 asthma, Dr. Cho added. “Still, there is no evidence that these biomarkers are decent for CRSwNP, even though CRSwNP is mostly considered as type 2 disease. Therefore, it would be essential to find promising biomarkers for severe CRSwNP.”
Dr. Van der Lans disclosed serving as a consultant for GlaxoSmithKline, and several coauthors disclosed relationships with companies including Sanofi and Novartis. The patient registry from which the study population was drawn is cofunded by Sanofi and Novartis. Dr. Cho has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY
Sepsis common cause of ICU admissions in patients with MS
Sepsis is an alarmingly common cause behind ICU admissions in patients with multiple sclerosis (MS), a retrospective, population-based cohort study indicates.
Furthermore, it contributes to a disproportionately high percentage of the short-term mortality risk among patients with MS admitted to the ICU, findings also show. Short-term mortality risk was defined in the study as a combination of in-hospital death or discharge to hospice.
“We found that the risk of short-term mortality in critically ill patients with MS is four times higher among those with sepsis ... so sepsis appears to be comparatively more lethal among patients with MS than in the general population,” Lavi Oud, MD, professor of medicine, Texas Tech University HSC at the Permian Basin, Odessa, said in an email.
“[Although] the specific mechanisms underlying the markedly higher risk of sepsis among patients with MS compared to the general population remain to be fully elucidated ... it’s thought that the risk may stem from the dysfunction of the immune system in these patients related to MS itself and to the potentially adverse effect of the immunomodulating therapy we use in these patients,” he added.
The study was published online Jan. 11 in the Journal of Critical Care.
Sepsis rates
The Texas Inpatient Public Use Data File was used to identify adults with a diagnosis of MS admitted to the hospital between 2010 and 2017. Among the 19,837 patients with MS admitted to the ICU during the study interval, almost one-third (31.5%) had sepsis, investigators report. “The rate of sepsis among ICU admissions increased with age, ranging from 20.8% among those aged 18-44 to 39.4% among those aged 65 years or older,” investigators note.
The most common site of infection among MS patients admitted to the ICU were urinary in nature (65.2%), followed by respiratory (36.1%). A smaller proportion of infections (7.6%) involved the skin and soft tissues, researchers note. A full one-quarter of patients developed septic shock in response to their infection while the length of stay among patients with sepsis (mean of 10.9 days) was substantially longer than it was for those without sepsis (mean of 5.6 days), they observe.
At a mean total hospital cost of $121,797 for each ICU patient with sepsis, the cost of caring for each patient was nearly twofold higher than the mean total cost of taking care of ICU patients without sepsis (mean total cost, $65,179). On adjusted analysis, sepsis was associated with a 42.7% (95% confidence interval, 38.9-46.5; P < .0001) longer length of hospital stay and a 26.2% (95% CI, 23.1-29.1; P < .0001) higher total hospital cost compared with patients without sepsis, the authors point out.
Indeed, ICU admissions with sepsis accounted for 47.3% of all hospital days and for 46.1% of the aggregate hospital charges among all MS patients admitted to the ICU.
“The adjusted probability of short-term mortality was 13.4% (95% CI, 13.0-13.7) among ICU admissions with sepsis and 3.3% (95% CI, 3.2-3.4) among ICU admissions without sepsis,” the authors report.
This translated into a 44% higher risk of short-term mortality at an adjusted odds ratio of 1.44 (95% CI, 1.23-1.69; P < .0001) for those with sepsis, compared with those without, they add. Among all ICU admissions, sepsis was reported in over two-thirds of documented short-term mortality events. The risk of short-term mortality was also almost threefold higher among patients with sepsis who were age 65 years and older compared with patients aged 18-44.
As Dr. Oud noted, there is no specific test for sepsis, and it can initially present in an atypical manner, especially in older, frailer, chronically ill patients as well as in patients with immune dysfunction. “Thus, considering sepsis as a possible cause of new deterioration in a patient’s condition is essential, along with the timely start of sepsis-related care,” Dr. Oud observed.
A limitation of the study was that the dataset did not include information on the type of MS a patient had, the duration of their illness, the treatment received, the level of disease activity, or the level of disability.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sepsis is an alarmingly common cause behind ICU admissions in patients with multiple sclerosis (MS), a retrospective, population-based cohort study indicates.
Furthermore, it contributes to a disproportionately high percentage of the short-term mortality risk among patients with MS admitted to the ICU, findings also show. Short-term mortality risk was defined in the study as a combination of in-hospital death or discharge to hospice.
“We found that the risk of short-term mortality in critically ill patients with MS is four times higher among those with sepsis ... so sepsis appears to be comparatively more lethal among patients with MS than in the general population,” Lavi Oud, MD, professor of medicine, Texas Tech University HSC at the Permian Basin, Odessa, said in an email.
“[Although] the specific mechanisms underlying the markedly higher risk of sepsis among patients with MS compared to the general population remain to be fully elucidated ... it’s thought that the risk may stem from the dysfunction of the immune system in these patients related to MS itself and to the potentially adverse effect of the immunomodulating therapy we use in these patients,” he added.
The study was published online Jan. 11 in the Journal of Critical Care.
Sepsis rates
The Texas Inpatient Public Use Data File was used to identify adults with a diagnosis of MS admitted to the hospital between 2010 and 2017. Among the 19,837 patients with MS admitted to the ICU during the study interval, almost one-third (31.5%) had sepsis, investigators report. “The rate of sepsis among ICU admissions increased with age, ranging from 20.8% among those aged 18-44 to 39.4% among those aged 65 years or older,” investigators note.
The most common site of infection among MS patients admitted to the ICU were urinary in nature (65.2%), followed by respiratory (36.1%). A smaller proportion of infections (7.6%) involved the skin and soft tissues, researchers note. A full one-quarter of patients developed septic shock in response to their infection while the length of stay among patients with sepsis (mean of 10.9 days) was substantially longer than it was for those without sepsis (mean of 5.6 days), they observe.
At a mean total hospital cost of $121,797 for each ICU patient with sepsis, the cost of caring for each patient was nearly twofold higher than the mean total cost of taking care of ICU patients without sepsis (mean total cost, $65,179). On adjusted analysis, sepsis was associated with a 42.7% (95% confidence interval, 38.9-46.5; P < .0001) longer length of hospital stay and a 26.2% (95% CI, 23.1-29.1; P < .0001) higher total hospital cost compared with patients without sepsis, the authors point out.
Indeed, ICU admissions with sepsis accounted for 47.3% of all hospital days and for 46.1% of the aggregate hospital charges among all MS patients admitted to the ICU.
“The adjusted probability of short-term mortality was 13.4% (95% CI, 13.0-13.7) among ICU admissions with sepsis and 3.3% (95% CI, 3.2-3.4) among ICU admissions without sepsis,” the authors report.
This translated into a 44% higher risk of short-term mortality at an adjusted odds ratio of 1.44 (95% CI, 1.23-1.69; P < .0001) for those with sepsis, compared with those without, they add. Among all ICU admissions, sepsis was reported in over two-thirds of documented short-term mortality events. The risk of short-term mortality was also almost threefold higher among patients with sepsis who were age 65 years and older compared with patients aged 18-44.
As Dr. Oud noted, there is no specific test for sepsis, and it can initially present in an atypical manner, especially in older, frailer, chronically ill patients as well as in patients with immune dysfunction. “Thus, considering sepsis as a possible cause of new deterioration in a patient’s condition is essential, along with the timely start of sepsis-related care,” Dr. Oud observed.
A limitation of the study was that the dataset did not include information on the type of MS a patient had, the duration of their illness, the treatment received, the level of disease activity, or the level of disability.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sepsis is an alarmingly common cause behind ICU admissions in patients with multiple sclerosis (MS), a retrospective, population-based cohort study indicates.
Furthermore, it contributes to a disproportionately high percentage of the short-term mortality risk among patients with MS admitted to the ICU, findings also show. Short-term mortality risk was defined in the study as a combination of in-hospital death or discharge to hospice.
“We found that the risk of short-term mortality in critically ill patients with MS is four times higher among those with sepsis ... so sepsis appears to be comparatively more lethal among patients with MS than in the general population,” Lavi Oud, MD, professor of medicine, Texas Tech University HSC at the Permian Basin, Odessa, said in an email.
“[Although] the specific mechanisms underlying the markedly higher risk of sepsis among patients with MS compared to the general population remain to be fully elucidated ... it’s thought that the risk may stem from the dysfunction of the immune system in these patients related to MS itself and to the potentially adverse effect of the immunomodulating therapy we use in these patients,” he added.
The study was published online Jan. 11 in the Journal of Critical Care.
Sepsis rates
The Texas Inpatient Public Use Data File was used to identify adults with a diagnosis of MS admitted to the hospital between 2010 and 2017. Among the 19,837 patients with MS admitted to the ICU during the study interval, almost one-third (31.5%) had sepsis, investigators report. “The rate of sepsis among ICU admissions increased with age, ranging from 20.8% among those aged 18-44 to 39.4% among those aged 65 years or older,” investigators note.
The most common site of infection among MS patients admitted to the ICU were urinary in nature (65.2%), followed by respiratory (36.1%). A smaller proportion of infections (7.6%) involved the skin and soft tissues, researchers note. A full one-quarter of patients developed septic shock in response to their infection while the length of stay among patients with sepsis (mean of 10.9 days) was substantially longer than it was for those without sepsis (mean of 5.6 days), they observe.
At a mean total hospital cost of $121,797 for each ICU patient with sepsis, the cost of caring for each patient was nearly twofold higher than the mean total cost of taking care of ICU patients without sepsis (mean total cost, $65,179). On adjusted analysis, sepsis was associated with a 42.7% (95% confidence interval, 38.9-46.5; P < .0001) longer length of hospital stay and a 26.2% (95% CI, 23.1-29.1; P < .0001) higher total hospital cost compared with patients without sepsis, the authors point out.
Indeed, ICU admissions with sepsis accounted for 47.3% of all hospital days and for 46.1% of the aggregate hospital charges among all MS patients admitted to the ICU.
“The adjusted probability of short-term mortality was 13.4% (95% CI, 13.0-13.7) among ICU admissions with sepsis and 3.3% (95% CI, 3.2-3.4) among ICU admissions without sepsis,” the authors report.
This translated into a 44% higher risk of short-term mortality at an adjusted odds ratio of 1.44 (95% CI, 1.23-1.69; P < .0001) for those with sepsis, compared with those without, they add. Among all ICU admissions, sepsis was reported in over two-thirds of documented short-term mortality events. The risk of short-term mortality was also almost threefold higher among patients with sepsis who were age 65 years and older compared with patients aged 18-44.
As Dr. Oud noted, there is no specific test for sepsis, and it can initially present in an atypical manner, especially in older, frailer, chronically ill patients as well as in patients with immune dysfunction. “Thus, considering sepsis as a possible cause of new deterioration in a patient’s condition is essential, along with the timely start of sepsis-related care,” Dr. Oud observed.
A limitation of the study was that the dataset did not include information on the type of MS a patient had, the duration of their illness, the treatment received, the level of disease activity, or the level of disability.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CRITICAL CARE
ctDNA shows promise for assessing lung cancer treatment response
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
New stroke risk score developed for COVID patients
Researchers have developed a quick and easy scoring system to predict which hospitalized COVID-19 patients are more at risk for stroke.
“The system is simple. You can calculate the points in 5 seconds and then predict the chances the patient will have a stroke,” Alexander E. Merkler, MD, assistant professor of neurology at Weill Cornell Medical College/NewYork-Presbyterian Hospital, and lead author of a study of the system, told this news organization.
The new system will allow clinicians to stratify patients and lead to closer monitoring of those at highest risk for stroke, said Dr. Merkler.
The study was presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Some, but not all, studies suggest COVID-19 increases the risk of stroke and worsens stroke outcomes, and the association isn’t clear, investigators note.
Researchers used the American Heart Association Get With the Guidelines COVID-19 cardiovascular disease registry for this analysis. They evaluated 21,420 adult patients (mean age 61 years, 54% men), who were hospitalized with COVID-19 at 122 centers from March 2020 to March 2021.
Investigators tapped into the vast amounts of data in this registry on different variables, including demographics, comorbidities, and lab values.
The outcome was a cerebrovascular event, defined as any ischemic or hemorrhagic stroke, transient ischemic attack (TIA), or cerebral vein thrombosis. Of the total hospitalized COVID-19 population, 312 (1.5%) had a cerebrovascular event.
Researchers first used standard statistical models to determine which risk factors are most associated with the development of stroke. They identified six such factors:
- history of stroke
- no fever at the time of hospital admission
- no history of pulmonary disease
- high white blood cell count
- history of hypertension
- high systolic blood pressure at the time of hospital admission
That the list of risk factors included absence of fever and no history of pulmonary disease was somewhat surprising, said Dr. Merkler, but there may be possible explanations, he added.
A high fever is an inflammatory response, and perhaps patients who aren’t responding appropriately “could be sicker in general and have a poor immune system, and thereby be at increased risk for stroke,” said Dr. Merkler.
In the case of pulmonary disease, patients without a history who are admitted for COVID “may have an extremely high burden of COVID, or are extremely sick, and that’s why they’re at higher risk for stroke.”
The scoring system assigns points for each variable, with more points conferring a higher risk of stroke. For example, someone who has 0-1 points has 0.2% risk of having a stroke, and someone with 4-6 points has 2% to 3% risk, said Dr. Merkler.
“So, we’re talking about a 10- to 15-fold increased risk of having a stroke with 4 to 6 versus 0 to 1 variables.”
The accuracy of the risk stratification score (C-statistic of 0.66; 95% confidence interval, 0.60-0.72) is “fairly good or modestly good,” said Dr. Merkler.
A patient with a score of 5 or 6 may need more vigilant monitoring to make sure symptoms are caught early and therapies such as thrombolytics and thrombectomy are readily available, he added.
Researchers also used a sophisticated machine-learning approach where a computer takes all the variables and identifies the best algorithm to predict stroke.
“The machine-learning algorithm was basically just as good as our standard model; it was almost identical,” said Dr. Merkler.
Outside of COVID, other scoring systems are used to predict stroke. For example, the ABCD2 score uses various factors to predict risk of recurrent stroke.
Philip B. Gorelick, MD, adjunct professor, Northwestern University Feinberg School of Medicine, Chicago, said the results are promising, as they may lead to identifying modifiable factors to prevent stroke.
Dr. Gorelick noted that the authors identified risk factors to predict risk of stroke “after an extensive analysis of baseline factors that included an internal validation process.”
The finding that no fever and no history of pulmonary disease were included in those risk factors was “unexpected,” said Dr. Gorelick, who is also medical director of the Hauenstein Neuroscience Center in Grand Rapids, Michigan. “This may reflect the baseline timing of data collection.”
He added further validation of the results in other data sets “will be useful to determine the consistency of the predictive model and its potential value in general practice.”
Louise D. McCullough, MD, PhD, professor and chair of neurology, McGovern Medical School, The University of Texas Health Science Center, Houston, said the association between stroke risk and COVID exposure “has been very unclear.”
“Some people find a very strong association between stroke and COVID, some do not,” said Dr. McCullough, who served as the chair of the ISC 2022 meeting.
This new study looking at a risk stratification model for COVID patients was “very nicely done,” she added.
“They used the American Heart Association Get With The Guidelines COVID registry, which was an amazing feat that was done very quickly by the AHA to establish COVID reporting in the Get With The Guidelines data, allowing us to really look at other factors related to stroke that are in this unique database.”
The study received funding support from the American Stroke Association. Dr. Merkler has received funding from the American Heart Association and the Leon Levy Foundation. Dr. Gorelick was not involved in the study and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have developed a quick and easy scoring system to predict which hospitalized COVID-19 patients are more at risk for stroke.
“The system is simple. You can calculate the points in 5 seconds and then predict the chances the patient will have a stroke,” Alexander E. Merkler, MD, assistant professor of neurology at Weill Cornell Medical College/NewYork-Presbyterian Hospital, and lead author of a study of the system, told this news organization.
The new system will allow clinicians to stratify patients and lead to closer monitoring of those at highest risk for stroke, said Dr. Merkler.
The study was presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Some, but not all, studies suggest COVID-19 increases the risk of stroke and worsens stroke outcomes, and the association isn’t clear, investigators note.
Researchers used the American Heart Association Get With the Guidelines COVID-19 cardiovascular disease registry for this analysis. They evaluated 21,420 adult patients (mean age 61 years, 54% men), who were hospitalized with COVID-19 at 122 centers from March 2020 to March 2021.
Investigators tapped into the vast amounts of data in this registry on different variables, including demographics, comorbidities, and lab values.
The outcome was a cerebrovascular event, defined as any ischemic or hemorrhagic stroke, transient ischemic attack (TIA), or cerebral vein thrombosis. Of the total hospitalized COVID-19 population, 312 (1.5%) had a cerebrovascular event.
Researchers first used standard statistical models to determine which risk factors are most associated with the development of stroke. They identified six such factors:
- history of stroke
- no fever at the time of hospital admission
- no history of pulmonary disease
- high white blood cell count
- history of hypertension
- high systolic blood pressure at the time of hospital admission
That the list of risk factors included absence of fever and no history of pulmonary disease was somewhat surprising, said Dr. Merkler, but there may be possible explanations, he added.
A high fever is an inflammatory response, and perhaps patients who aren’t responding appropriately “could be sicker in general and have a poor immune system, and thereby be at increased risk for stroke,” said Dr. Merkler.
In the case of pulmonary disease, patients without a history who are admitted for COVID “may have an extremely high burden of COVID, or are extremely sick, and that’s why they’re at higher risk for stroke.”
The scoring system assigns points for each variable, with more points conferring a higher risk of stroke. For example, someone who has 0-1 points has 0.2% risk of having a stroke, and someone with 4-6 points has 2% to 3% risk, said Dr. Merkler.
“So, we’re talking about a 10- to 15-fold increased risk of having a stroke with 4 to 6 versus 0 to 1 variables.”
The accuracy of the risk stratification score (C-statistic of 0.66; 95% confidence interval, 0.60-0.72) is “fairly good or modestly good,” said Dr. Merkler.
A patient with a score of 5 or 6 may need more vigilant monitoring to make sure symptoms are caught early and therapies such as thrombolytics and thrombectomy are readily available, he added.
Researchers also used a sophisticated machine-learning approach where a computer takes all the variables and identifies the best algorithm to predict stroke.
“The machine-learning algorithm was basically just as good as our standard model; it was almost identical,” said Dr. Merkler.
Outside of COVID, other scoring systems are used to predict stroke. For example, the ABCD2 score uses various factors to predict risk of recurrent stroke.
Philip B. Gorelick, MD, adjunct professor, Northwestern University Feinberg School of Medicine, Chicago, said the results are promising, as they may lead to identifying modifiable factors to prevent stroke.
Dr. Gorelick noted that the authors identified risk factors to predict risk of stroke “after an extensive analysis of baseline factors that included an internal validation process.”
The finding that no fever and no history of pulmonary disease were included in those risk factors was “unexpected,” said Dr. Gorelick, who is also medical director of the Hauenstein Neuroscience Center in Grand Rapids, Michigan. “This may reflect the baseline timing of data collection.”
He added further validation of the results in other data sets “will be useful to determine the consistency of the predictive model and its potential value in general practice.”
Louise D. McCullough, MD, PhD, professor and chair of neurology, McGovern Medical School, The University of Texas Health Science Center, Houston, said the association between stroke risk and COVID exposure “has been very unclear.”
“Some people find a very strong association between stroke and COVID, some do not,” said Dr. McCullough, who served as the chair of the ISC 2022 meeting.
This new study looking at a risk stratification model for COVID patients was “very nicely done,” she added.
“They used the American Heart Association Get With The Guidelines COVID registry, which was an amazing feat that was done very quickly by the AHA to establish COVID reporting in the Get With The Guidelines data, allowing us to really look at other factors related to stroke that are in this unique database.”
The study received funding support from the American Stroke Association. Dr. Merkler has received funding from the American Heart Association and the Leon Levy Foundation. Dr. Gorelick was not involved in the study and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have developed a quick and easy scoring system to predict which hospitalized COVID-19 patients are more at risk for stroke.
“The system is simple. You can calculate the points in 5 seconds and then predict the chances the patient will have a stroke,” Alexander E. Merkler, MD, assistant professor of neurology at Weill Cornell Medical College/NewYork-Presbyterian Hospital, and lead author of a study of the system, told this news organization.
The new system will allow clinicians to stratify patients and lead to closer monitoring of those at highest risk for stroke, said Dr. Merkler.
The study was presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Some, but not all, studies suggest COVID-19 increases the risk of stroke and worsens stroke outcomes, and the association isn’t clear, investigators note.
Researchers used the American Heart Association Get With the Guidelines COVID-19 cardiovascular disease registry for this analysis. They evaluated 21,420 adult patients (mean age 61 years, 54% men), who were hospitalized with COVID-19 at 122 centers from March 2020 to March 2021.
Investigators tapped into the vast amounts of data in this registry on different variables, including demographics, comorbidities, and lab values.
The outcome was a cerebrovascular event, defined as any ischemic or hemorrhagic stroke, transient ischemic attack (TIA), or cerebral vein thrombosis. Of the total hospitalized COVID-19 population, 312 (1.5%) had a cerebrovascular event.
Researchers first used standard statistical models to determine which risk factors are most associated with the development of stroke. They identified six such factors:
- history of stroke
- no fever at the time of hospital admission
- no history of pulmonary disease
- high white blood cell count
- history of hypertension
- high systolic blood pressure at the time of hospital admission
That the list of risk factors included absence of fever and no history of pulmonary disease was somewhat surprising, said Dr. Merkler, but there may be possible explanations, he added.
A high fever is an inflammatory response, and perhaps patients who aren’t responding appropriately “could be sicker in general and have a poor immune system, and thereby be at increased risk for stroke,” said Dr. Merkler.
In the case of pulmonary disease, patients without a history who are admitted for COVID “may have an extremely high burden of COVID, or are extremely sick, and that’s why they’re at higher risk for stroke.”
The scoring system assigns points for each variable, with more points conferring a higher risk of stroke. For example, someone who has 0-1 points has 0.2% risk of having a stroke, and someone with 4-6 points has 2% to 3% risk, said Dr. Merkler.
“So, we’re talking about a 10- to 15-fold increased risk of having a stroke with 4 to 6 versus 0 to 1 variables.”
The accuracy of the risk stratification score (C-statistic of 0.66; 95% confidence interval, 0.60-0.72) is “fairly good or modestly good,” said Dr. Merkler.
A patient with a score of 5 or 6 may need more vigilant monitoring to make sure symptoms are caught early and therapies such as thrombolytics and thrombectomy are readily available, he added.
Researchers also used a sophisticated machine-learning approach where a computer takes all the variables and identifies the best algorithm to predict stroke.
“The machine-learning algorithm was basically just as good as our standard model; it was almost identical,” said Dr. Merkler.
Outside of COVID, other scoring systems are used to predict stroke. For example, the ABCD2 score uses various factors to predict risk of recurrent stroke.
Philip B. Gorelick, MD, adjunct professor, Northwestern University Feinberg School of Medicine, Chicago, said the results are promising, as they may lead to identifying modifiable factors to prevent stroke.
Dr. Gorelick noted that the authors identified risk factors to predict risk of stroke “after an extensive analysis of baseline factors that included an internal validation process.”
The finding that no fever and no history of pulmonary disease were included in those risk factors was “unexpected,” said Dr. Gorelick, who is also medical director of the Hauenstein Neuroscience Center in Grand Rapids, Michigan. “This may reflect the baseline timing of data collection.”
He added further validation of the results in other data sets “will be useful to determine the consistency of the predictive model and its potential value in general practice.”
Louise D. McCullough, MD, PhD, professor and chair of neurology, McGovern Medical School, The University of Texas Health Science Center, Houston, said the association between stroke risk and COVID exposure “has been very unclear.”
“Some people find a very strong association between stroke and COVID, some do not,” said Dr. McCullough, who served as the chair of the ISC 2022 meeting.
This new study looking at a risk stratification model for COVID patients was “very nicely done,” she added.
“They used the American Heart Association Get With The Guidelines COVID registry, which was an amazing feat that was done very quickly by the AHA to establish COVID reporting in the Get With The Guidelines data, allowing us to really look at other factors related to stroke that are in this unique database.”
The study received funding support from the American Stroke Association. Dr. Merkler has received funding from the American Heart Association and the Leon Levy Foundation. Dr. Gorelick was not involved in the study and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
FDA delays action on Pfizer vaccine for kids under 5
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.