User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


I’m a physician battling long COVID. I can assure you it’s real
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
Would a national provider directory save docs’ time, help patients?
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
When a consumer uses a health plan provider directory to look up a physician, there’s a high probability that the entry for that doctor is incomplete or inaccurate. The Centers for Medicare & Medicaid Services would like to change that by creating a National Directory of Healthcare Providers and Services, which the agency believes would be more valuable to consumers.
In asking for public comments on whether and how it should establish the directory, CMS argues that this data repository would help patients locate physicians and could help with care coordination, health information exchange, and public health data reporting.
However, it’s not clear that such a directory would be any better than current insurance company listings or that people would use it. But a national directory could benefit physician practices by reducing their administrative work, according to observers.
In requesting public comment on the proposed national directory, CMS explains that provider organizations face “redundant and burdensome reporting requirements to multiple databases.” The directory could greatly reduce this challenge by requiring health care organizations to report provider information to a single database. Currently, physician practices have to submit these data to an average of 20 payers each, according to CMS.
“Right now, [physicians are] inundated with requests, and it takes a lot of time to update this stuff,” said David Zetter, a practice management consultant in Mechanicsburg, Pa.. “If there were one national repository of this information, that would be a good move.”
CMS envisions the National Directory as a central hub from which payers could obtain the latest provider data, which would be updated through a standardized application programming interface (API). Consequently, the insurers would no longer need to have providers submit this information to them separately.
CMS is soliciting input on what should be included in the directory. It notes that in addition to contact information, insurer directories also include a physicians’ specialties, health plan affiliations, and whether they accept new patients.
CMS’ 60-day public comment period ends Dec. 6. After that, the agency will decide what steps to take if it is decided that CMS has the legal authority to create the directory.
Terrible track record
In its annual reviews of health plan directories, CMS found that, from 2017 to 2022, only 47% of provider entries were complete. Only 73% of the providers could be matched to published directories. And only 28% of the provider names, addresses, and specialties in the directories matched those in the National Provider Identifier (NPI) registry.
Many of the mistakes in provider directories stem from errors made by practice staff, who have many other duties besides updating directory data. Yet an astonishing amount of time and effort is devoted to this task. A 2019 survey found that physician practices spend $2.76 billion annually on directory maintenance, or nearly $1000 per month per practice, on average.
The Council for Affordable Quality Healthcare, which conducted the survey, estimated that placing all directory data collection on a single platform could save the average practice $4,746 per year. For all practices in the United States, that works out to about $1.1 billion annually, CAQH said.
Pros and cons of national directory
For all the money spent on maintaining provider directories, consumers don’t use them very much. According to a 2021 Press Ganey survey, fewer than 5% of consumers seeking a primary care doctor get their information from an insurer or a benefits manager. About half search the internet first, and 24% seek a referral from a physician.
A national provider directory would be useful only if it were done right, Mr. Zetter said. Citing the inaccuracy and incompleteness of health plan directories, he said it was likely that a national directory would have similar problems. Data entered by practice staff would have to be automatically validated, perhaps through use of some kind of AI algorithm.
Effect on coordination of care
Mr. Zetter doubts the directory could improve care coordination, because primary care doctors usually refer patients to specialists they already know.
But Julia Adler-Milstein, PhD, professor of medicine and director of the Center for Clinical Informatics at the University of California, San Francisco, said that a national directory could improve communications among providers when patients select specialists outside of their primary care physician’s referral network.
“Especially if it’s not an established referral relationship, that’s where a national directory would be helpful, not only to locate the physicians but also to understand their preferences in how they’d like to receive information,” she said in an interview.
Dr. Adler-Milstein worries less than Mr. Zetter does about the challenge of ensuring the accuracy of data in the directory. She pointed out that the National Plan and Provider Enumeration System, which includes the NPI registry, has done a good job of validating provider name, address, and specialty information.
Dr. Adler-Milstein is more concerned about whether the proposed directory would address physician preferences as to how they wish to receive information. For example, while some physicians may prefer to be contacted directly, others may prefer or are required to communicate through their practices or health systems.
Efficiency in data exchange
The API used by the proposed directory would be based on the Fast Health Interoperability Resources standard that all electronic health record vendors must now include in their products. That raises the question of whether communications using contact information from the directory would be sent through a secure email system or through integrated EHR systems, Dr. Adler-Milstein said.
“I’m not sure whether the directory could support that [integration],” she said. “If it focuses on the concept of secure email exchange, that’s a relatively inefficient way of doing it,” because providers want clinical messages to pop up in their EHR workflow rather than their inboxes.
Nevertheless, Dr. Milstein-Adler added, the directory “would clearly take a lot of today’s manual work out of the system. I think organizations like UCSF would be very motivated to support the directory, knowing that people were going to a single source to find the updated information, including preferences in how we’d like people to communicate with us. There would be a lot of efficiency reasons for organizations to use this national directory.”
A version of this article first appeared on Medscape.com.
Finerenone: ‘Striking’ cut in pneumonia, COVID-19 risks
The nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) unexpectedly showed that it might protect against incident infective pneumonia and COVID-19. The finding was based on secondary analyses run on more than 13,000 people enrolled in the two pivotal trials for finerenone.
Finerenone was approved by the Food and Drug Administration in 2021 for slowing progressive renal dysfunction and preventing cardiovascular events in adults with type 2 diabetes and chronic kidney disease (CKD).
‘Striking reduction in the risk of pneumonia’
The “striking reduction in risk of pneumonia” in a new analysis suggests that “the propagation of pulmonary infection into lobar or bronchial consolidation may be reduced by finerenone,” write Bertram Pitt, MD, and coauthors in a report published on October 26 in JAMA Network Open.
They also suggest that if further studies confirm that finerenone treatment reduces complications from pneumonia and COVID-19, it would have “significant medical implications,” especially because of the limited treatment options now available for complications from COVID-19.
The new analyses used the FIDELITY dataset, a prespecified merging of results from the FIDELIO-DKD and FIGARO-DKD trials, which together enrolled 13,026 people with type 2 diabetes and CKD, as determined on the basis of the patients’ having a urine albumin-to-creatinine ratio of at least 30 mg/g.
The primary outcomes of these trials showed that treatment with finerenone led to significant slowing of the progression of CKD and a significant reduction in the incidence of cardiovascular events, compared with placebo during median follow-up of 3 years.
The new, secondary analyses focused on the 6.0% of participants in whom there was evidence of pneumonia and the 1.6% in whom there was evidence of having COVID-19. Pneumonia was the most common serious adverse event in the two trials, a finding consistent with the documented risk for pneumonia faced by people with CKD.
Finerenone linked with a 29% relative reduction in pneumonia
When analyzed by treatment, the incidence of pneumonia was 4.7% among those who received finerenone and 6.7% among those who received placebo. This translated into a significant relative risk reduction of 29% associated with finerenone treatment.
Analysis of COVID-19 adverse events showed a 1.3% incidence among those who received finerenone and a 1.8% incidence among those in the placebo group, which translated into a significant 27% relative risk reduction linked with finerenone treatment.
In contrast, the data showed no reduced incidence of several other respiratory infections among the finerenone recipients, including nasopharyngitis, bronchitis, and influenza. The data also showed no signal that pneumonia or COVID-19 was more severe among the people who did not receive finerenone, nor did finerenone treatment appear to affect pneumonia recovery.
Analysis based on adverse events reports
These secondary analyses are far from definitive. The authors relied on pneumonia and COVID-19 being reported as adverse events. Each investigator diagnosed pneumonia at their discretion, and the trials did not specify diagnostic criteria. The authors also acknowledge that testing for COVID-19 was “not widespread” and that one of the two pivotal trials largely ran prior to the onset of the COVID-19 pandemic so that only 6 participants developed COVID-19 symptoms out of more than 5,700 enrolled.
The authors hypothesize that several actions of finerenone might potentially help mediate an effect on pneumonia and COVID-19: improvements in pulmonary inflammation and fibrosis, upregulation of expression of angiotensin converting enzyme 2, and amelioration of right heart pressure and pulmonary congestion. Also, antagonizing the mineralocorticoid receptor on monocytes and macrophages may block macrophage infiltration and accumulation of active macrophages, which can mediate the pulmonary tissue damage caused by COVID-19.
The FIDELIO-DKD and FIGARO-DKD trials and the FIDELITY combined database were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Pitt has received personal fees from Bayer and personal fees and stock options from numerous other companies. Several coauthors reported having a financial relationship with Bayer, as well as with other companies.
A version of this article first appeared on Medscape.com.
The nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) unexpectedly showed that it might protect against incident infective pneumonia and COVID-19. The finding was based on secondary analyses run on more than 13,000 people enrolled in the two pivotal trials for finerenone.
Finerenone was approved by the Food and Drug Administration in 2021 for slowing progressive renal dysfunction and preventing cardiovascular events in adults with type 2 diabetes and chronic kidney disease (CKD).
‘Striking reduction in the risk of pneumonia’
The “striking reduction in risk of pneumonia” in a new analysis suggests that “the propagation of pulmonary infection into lobar or bronchial consolidation may be reduced by finerenone,” write Bertram Pitt, MD, and coauthors in a report published on October 26 in JAMA Network Open.
They also suggest that if further studies confirm that finerenone treatment reduces complications from pneumonia and COVID-19, it would have “significant medical implications,” especially because of the limited treatment options now available for complications from COVID-19.
The new analyses used the FIDELITY dataset, a prespecified merging of results from the FIDELIO-DKD and FIGARO-DKD trials, which together enrolled 13,026 people with type 2 diabetes and CKD, as determined on the basis of the patients’ having a urine albumin-to-creatinine ratio of at least 30 mg/g.
The primary outcomes of these trials showed that treatment with finerenone led to significant slowing of the progression of CKD and a significant reduction in the incidence of cardiovascular events, compared with placebo during median follow-up of 3 years.
The new, secondary analyses focused on the 6.0% of participants in whom there was evidence of pneumonia and the 1.6% in whom there was evidence of having COVID-19. Pneumonia was the most common serious adverse event in the two trials, a finding consistent with the documented risk for pneumonia faced by people with CKD.
Finerenone linked with a 29% relative reduction in pneumonia
When analyzed by treatment, the incidence of pneumonia was 4.7% among those who received finerenone and 6.7% among those who received placebo. This translated into a significant relative risk reduction of 29% associated with finerenone treatment.
Analysis of COVID-19 adverse events showed a 1.3% incidence among those who received finerenone and a 1.8% incidence among those in the placebo group, which translated into a significant 27% relative risk reduction linked with finerenone treatment.
In contrast, the data showed no reduced incidence of several other respiratory infections among the finerenone recipients, including nasopharyngitis, bronchitis, and influenza. The data also showed no signal that pneumonia or COVID-19 was more severe among the people who did not receive finerenone, nor did finerenone treatment appear to affect pneumonia recovery.
Analysis based on adverse events reports
These secondary analyses are far from definitive. The authors relied on pneumonia and COVID-19 being reported as adverse events. Each investigator diagnosed pneumonia at their discretion, and the trials did not specify diagnostic criteria. The authors also acknowledge that testing for COVID-19 was “not widespread” and that one of the two pivotal trials largely ran prior to the onset of the COVID-19 pandemic so that only 6 participants developed COVID-19 symptoms out of more than 5,700 enrolled.
The authors hypothesize that several actions of finerenone might potentially help mediate an effect on pneumonia and COVID-19: improvements in pulmonary inflammation and fibrosis, upregulation of expression of angiotensin converting enzyme 2, and amelioration of right heart pressure and pulmonary congestion. Also, antagonizing the mineralocorticoid receptor on monocytes and macrophages may block macrophage infiltration and accumulation of active macrophages, which can mediate the pulmonary tissue damage caused by COVID-19.
The FIDELIO-DKD and FIGARO-DKD trials and the FIDELITY combined database were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Pitt has received personal fees from Bayer and personal fees and stock options from numerous other companies. Several coauthors reported having a financial relationship with Bayer, as well as with other companies.
A version of this article first appeared on Medscape.com.
The nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) unexpectedly showed that it might protect against incident infective pneumonia and COVID-19. The finding was based on secondary analyses run on more than 13,000 people enrolled in the two pivotal trials for finerenone.
Finerenone was approved by the Food and Drug Administration in 2021 for slowing progressive renal dysfunction and preventing cardiovascular events in adults with type 2 diabetes and chronic kidney disease (CKD).
‘Striking reduction in the risk of pneumonia’
The “striking reduction in risk of pneumonia” in a new analysis suggests that “the propagation of pulmonary infection into lobar or bronchial consolidation may be reduced by finerenone,” write Bertram Pitt, MD, and coauthors in a report published on October 26 in JAMA Network Open.
They also suggest that if further studies confirm that finerenone treatment reduces complications from pneumonia and COVID-19, it would have “significant medical implications,” especially because of the limited treatment options now available for complications from COVID-19.
The new analyses used the FIDELITY dataset, a prespecified merging of results from the FIDELIO-DKD and FIGARO-DKD trials, which together enrolled 13,026 people with type 2 diabetes and CKD, as determined on the basis of the patients’ having a urine albumin-to-creatinine ratio of at least 30 mg/g.
The primary outcomes of these trials showed that treatment with finerenone led to significant slowing of the progression of CKD and a significant reduction in the incidence of cardiovascular events, compared with placebo during median follow-up of 3 years.
The new, secondary analyses focused on the 6.0% of participants in whom there was evidence of pneumonia and the 1.6% in whom there was evidence of having COVID-19. Pneumonia was the most common serious adverse event in the two trials, a finding consistent with the documented risk for pneumonia faced by people with CKD.
Finerenone linked with a 29% relative reduction in pneumonia
When analyzed by treatment, the incidence of pneumonia was 4.7% among those who received finerenone and 6.7% among those who received placebo. This translated into a significant relative risk reduction of 29% associated with finerenone treatment.
Analysis of COVID-19 adverse events showed a 1.3% incidence among those who received finerenone and a 1.8% incidence among those in the placebo group, which translated into a significant 27% relative risk reduction linked with finerenone treatment.
In contrast, the data showed no reduced incidence of several other respiratory infections among the finerenone recipients, including nasopharyngitis, bronchitis, and influenza. The data also showed no signal that pneumonia or COVID-19 was more severe among the people who did not receive finerenone, nor did finerenone treatment appear to affect pneumonia recovery.
Analysis based on adverse events reports
These secondary analyses are far from definitive. The authors relied on pneumonia and COVID-19 being reported as adverse events. Each investigator diagnosed pneumonia at their discretion, and the trials did not specify diagnostic criteria. The authors also acknowledge that testing for COVID-19 was “not widespread” and that one of the two pivotal trials largely ran prior to the onset of the COVID-19 pandemic so that only 6 participants developed COVID-19 symptoms out of more than 5,700 enrolled.
The authors hypothesize that several actions of finerenone might potentially help mediate an effect on pneumonia and COVID-19: improvements in pulmonary inflammation and fibrosis, upregulation of expression of angiotensin converting enzyme 2, and amelioration of right heart pressure and pulmonary congestion. Also, antagonizing the mineralocorticoid receptor on monocytes and macrophages may block macrophage infiltration and accumulation of active macrophages, which can mediate the pulmonary tissue damage caused by COVID-19.
The FIDELIO-DKD and FIGARO-DKD trials and the FIDELITY combined database were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Pitt has received personal fees from Bayer and personal fees and stock options from numerous other companies. Several coauthors reported having a financial relationship with Bayer, as well as with other companies.
A version of this article first appeared on Medscape.com.
What’s the best age to stop smoking? Study offers clue
Researchers also quantified the benefit of quitting for those older than 35. The added risk of death associated with smoking was reduced by 90% for those who quit before age 45 and 66% for those who quit at ages 45 to 64.
“The distal nature of the health consequences for young smokers is a challenge for professionals trying to motivate quitting in younger age groups. Without a proximal goal, it is tempting for smokers to abandon a quit attempt with cognitions such as ‘I don’t really need to do it just now,’ ” John P. Pierce, PhD, director for Population Sciences at UC-San Diego’s Moores Cancer Center, wrote in a commentary.
Current smokers were twice as likely to die from any cause during the study, compared with the group researchers called “never smokers,” who were defined as smoking fewer than 100 lifetime cigarettes.
Published in JAMA Network Open, the study involved 551,388 U.S. participants using information collected by the CDC from 1997 to 2018. Researchers collected data for specific causes of death of participants through the end of 2019.
The results echo past findings but also established whether demographic factors such as a smoker’s race and gender impact the benefits of quitting. (In many areas of health research, a person’s race or gender is associated with varying risks.)
The researchers found that the benefits of quitting smoking in reducing risk of death are comparable across demographic groups.
“Among former smokers in each racial and ethnic group, whether male or female, quitting was associated with reductions of approximately 80% of the excess mortality associated with continued smoking,” the authors stated. “These associations were generally consistent for deaths from cancer, cardiovascular disease, and lower respiratory disease.”
The findings are also important for guiding stop-smoking efforts because while smoking nationwide has decreased, the reduction has varied across demographic groups.
“Monitoring the association of smoking with mortality by race, ethnicity, and sex is critical to understanding how the U.S. tobacco epidemic continues to evolve over time and who is most affected by the changes,” the authors stated. “Despite continued decreases in U.S. smoking prevalence in recent decades, progress has not been equal across demographic groups. Recent progress in raising the quit ratio among U.S. ever-smokers overall has been modest, and the quit ratio has been consistently lower among Black and Hispanic ever-smokers than among non-Hispanic White ever-smokers.”
A version of this article first appeared on WebMD.com.
This article was updated 10/27/22.
Researchers also quantified the benefit of quitting for those older than 35. The added risk of death associated with smoking was reduced by 90% for those who quit before age 45 and 66% for those who quit at ages 45 to 64.
“The distal nature of the health consequences for young smokers is a challenge for professionals trying to motivate quitting in younger age groups. Without a proximal goal, it is tempting for smokers to abandon a quit attempt with cognitions such as ‘I don’t really need to do it just now,’ ” John P. Pierce, PhD, director for Population Sciences at UC-San Diego’s Moores Cancer Center, wrote in a commentary.
Current smokers were twice as likely to die from any cause during the study, compared with the group researchers called “never smokers,” who were defined as smoking fewer than 100 lifetime cigarettes.
Published in JAMA Network Open, the study involved 551,388 U.S. participants using information collected by the CDC from 1997 to 2018. Researchers collected data for specific causes of death of participants through the end of 2019.
The results echo past findings but also established whether demographic factors such as a smoker’s race and gender impact the benefits of quitting. (In many areas of health research, a person’s race or gender is associated with varying risks.)
The researchers found that the benefits of quitting smoking in reducing risk of death are comparable across demographic groups.
“Among former smokers in each racial and ethnic group, whether male or female, quitting was associated with reductions of approximately 80% of the excess mortality associated with continued smoking,” the authors stated. “These associations were generally consistent for deaths from cancer, cardiovascular disease, and lower respiratory disease.”
The findings are also important for guiding stop-smoking efforts because while smoking nationwide has decreased, the reduction has varied across demographic groups.
“Monitoring the association of smoking with mortality by race, ethnicity, and sex is critical to understanding how the U.S. tobacco epidemic continues to evolve over time and who is most affected by the changes,” the authors stated. “Despite continued decreases in U.S. smoking prevalence in recent decades, progress has not been equal across demographic groups. Recent progress in raising the quit ratio among U.S. ever-smokers overall has been modest, and the quit ratio has been consistently lower among Black and Hispanic ever-smokers than among non-Hispanic White ever-smokers.”
A version of this article first appeared on WebMD.com.
This article was updated 10/27/22.
Researchers also quantified the benefit of quitting for those older than 35. The added risk of death associated with smoking was reduced by 90% for those who quit before age 45 and 66% for those who quit at ages 45 to 64.
“The distal nature of the health consequences for young smokers is a challenge for professionals trying to motivate quitting in younger age groups. Without a proximal goal, it is tempting for smokers to abandon a quit attempt with cognitions such as ‘I don’t really need to do it just now,’ ” John P. Pierce, PhD, director for Population Sciences at UC-San Diego’s Moores Cancer Center, wrote in a commentary.
Current smokers were twice as likely to die from any cause during the study, compared with the group researchers called “never smokers,” who were defined as smoking fewer than 100 lifetime cigarettes.
Published in JAMA Network Open, the study involved 551,388 U.S. participants using information collected by the CDC from 1997 to 2018. Researchers collected data for specific causes of death of participants through the end of 2019.
The results echo past findings but also established whether demographic factors such as a smoker’s race and gender impact the benefits of quitting. (In many areas of health research, a person’s race or gender is associated with varying risks.)
The researchers found that the benefits of quitting smoking in reducing risk of death are comparable across demographic groups.
“Among former smokers in each racial and ethnic group, whether male or female, quitting was associated with reductions of approximately 80% of the excess mortality associated with continued smoking,” the authors stated. “These associations were generally consistent for deaths from cancer, cardiovascular disease, and lower respiratory disease.”
The findings are also important for guiding stop-smoking efforts because while smoking nationwide has decreased, the reduction has varied across demographic groups.
“Monitoring the association of smoking with mortality by race, ethnicity, and sex is critical to understanding how the U.S. tobacco epidemic continues to evolve over time and who is most affected by the changes,” the authors stated. “Despite continued decreases in U.S. smoking prevalence in recent decades, progress has not been equal across demographic groups. Recent progress in raising the quit ratio among U.S. ever-smokers overall has been modest, and the quit ratio has been consistently lower among Black and Hispanic ever-smokers than among non-Hispanic White ever-smokers.”
A version of this article first appeared on WebMD.com.
This article was updated 10/27/22.
FROM JAMA NETWORK OPEN
Droplet dispersal in sterile processing units far exceeds guideline limit
In the era of Ebola, COVID-19, and even Legionnaires, technicians and other staff working behind the scenes to ensure provider and patient safety continue to face a long-recognized but under addressed challenge: splashes and airborne droplets.
Granted, National Institute for Occupational Safety and Health (NIOSH) standards, industry standards, and professional guidelines are all in place to prevent unintentional exposure to pathogens. However, findings from a newly published study in the American Journal of Infection Control suggest they fall short.
In the study, researchers found that simulated manual cleaning of medical devices generated a drenching splash throughout the process with droplet dispersal exceeding 7 feet (2.1 meters).
Cori L. Ofstead, MSPH, lead author and president/CEO of Ofstead & Associates, Bloomington, Minn., told this news organization. “That’s the problem with having standards and guidelines that are not based on relevant evidence, [which] in this case, is a single study that was done in an intensive care area where they had an infection outbreak.”
Ms. Ofstead was referring to a report in the journal Infection Control and Hospital Epidemiology, detailing a Canadian investigation involving a multidrug-resistant Pseudomonas aeruginosa outbreak in an ICU. The report implicated the faucets over the hand hygiene sinks, with fluorescent dye showing droplet dispersal roughly 3 feet away from the sinks.
“Somehow it [the 3-feet rule] got implemented in guidelines in sterile processing decontamination areas, which are not the same as hand hygiene,’’ Ms. Ofstead explained.
With a goal of providing more current evidence on droplet generation and dispersal, as well as personal protection equipment (PPE) exposure/effectiveness, she and her colleagues simulated manual cleaning of a decommissioned colonoscope and transvaginal ultrasound probe, using for the study location a new academic sterile processing unit.
To detect droplet generation and dispersal as well as splash following common technician activities (for example, colonoscope brushing, scrubbing, rinsing and transport to an automated endoscope reprocessor [AER] for sterilization), the researchers affixed blue moisture-detection paper to environmental surfaces, on carts positioned 4 feet (1.2 meters) from the sink (to simulate observers), and along a 15-foot pathway between the sink and AER.
They observed droplets everywhere.
Technician activities such as running the faucet and rinsing the probe under running water generated substantial splashing overall. Instrument rinsing in particular produced small and large droplets and confluent puddles of water around the sink and in the broad area surrounding the workspace. Droplets were also dispersed on the floor 7.25 feet (2.2 meters) away and along the entire 15-foot path from the sink to the AER.
At the sink, the technician risked drenching exposure from head to toe during most activities, and even observers positioned 3-4 feet away were found to have droplets on their gowns. In addition, saturated shoe covers reportedly tracked moisture away from the sink to the unit door – a distance of 13 feet (4 meters) – and 2 feet (0.6 meters) farther out into the PPE foyer for donning and doffing.
Although PPE gowns effectively repelled moisture during cleaning of a single device, Ms. Ofstead emphasized that technicians typically handle up to 10 instruments during a normal, 2-hour shift, further increasing exposure risk with each subsequent cleaning.
However, perhaps one of the most surprising findings was that despite an optimal unit design, including physical separation of clean and dirty activities and pressurized air flow to protect workers, droplets were still broadly dispersed.
Current efforts, however well-intentioned, might not be offering the degree of protection (and consideration) that sterile processing technicians need.
“The study was conducted in a new sterile processing area that had an extra excellent kind of distancing and three separate rooms, something that I think most of our hospitals are working toward,” Stella Hines, MD, associate professor at the University of Maryland School of Medicine, Baltimore, explained. Dr. Hines was not directly involved in the study.
“But it also really kind of highlighted what’s happening to workers potentially,” she added. “For example, we want to know if that spray or splatter has a live microbe it in that could cause a problem or ... in a highly wet environment, if that water has some kind of chemical in it that could pose an occupational hazard to the worker based on skin or mucous membrane exposure.”
Ms. Ofstead agreed. “We need to be thinking about the exposure of critically important workers and the environment in an era where we are worried about aerosol-generating procedures and superbugs,” she explained.
Dr. Hines and Ms. Ofstead also noted that the majority of staff involved in front-line patient care have never actually ventured into the sterile processing units nor do they recognize the risks that technicians working in these units face on a daily, or even hourly, basis.
“The people who run these operations are very well trained and knowledgeable. I think that it would be helpful for them to know that they’re appreciated and for the people upstairs on the front lines using the equipment to see what goes on downstairs and all of the painstaking steps that need to be in place for the equipment to come out of sterile processing and be ready to go,” said Dr. Hines.
In the meantime, hospital leaders need to address the challenges and danger posed by migrating infectious droplets, especially for workers involved in processes that stir them up in the first place – workers who by the end of their shifts are unavoidably drenched with infectious blood and tissue secretions.
“I think that it’s going to take a much bigger kind of worldview from hospital leadership,” Dr. Hines said.
The study was supported in part by a grant from Healthmark Industries. Ms. Ofstead reports research grants or consulting fees through her organization with 3M Company, Ambu, Boston Scientific, Cleanis, Fortive/Advanced Sterilization Products, Healthmark Industries, Pentax, and Steris/Cantel/Medviators. Dr. Hines reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the era of Ebola, COVID-19, and even Legionnaires, technicians and other staff working behind the scenes to ensure provider and patient safety continue to face a long-recognized but under addressed challenge: splashes and airborne droplets.
Granted, National Institute for Occupational Safety and Health (NIOSH) standards, industry standards, and professional guidelines are all in place to prevent unintentional exposure to pathogens. However, findings from a newly published study in the American Journal of Infection Control suggest they fall short.
In the study, researchers found that simulated manual cleaning of medical devices generated a drenching splash throughout the process with droplet dispersal exceeding 7 feet (2.1 meters).
Cori L. Ofstead, MSPH, lead author and president/CEO of Ofstead & Associates, Bloomington, Minn., told this news organization. “That’s the problem with having standards and guidelines that are not based on relevant evidence, [which] in this case, is a single study that was done in an intensive care area where they had an infection outbreak.”
Ms. Ofstead was referring to a report in the journal Infection Control and Hospital Epidemiology, detailing a Canadian investigation involving a multidrug-resistant Pseudomonas aeruginosa outbreak in an ICU. The report implicated the faucets over the hand hygiene sinks, with fluorescent dye showing droplet dispersal roughly 3 feet away from the sinks.
“Somehow it [the 3-feet rule] got implemented in guidelines in sterile processing decontamination areas, which are not the same as hand hygiene,’’ Ms. Ofstead explained.
With a goal of providing more current evidence on droplet generation and dispersal, as well as personal protection equipment (PPE) exposure/effectiveness, she and her colleagues simulated manual cleaning of a decommissioned colonoscope and transvaginal ultrasound probe, using for the study location a new academic sterile processing unit.
To detect droplet generation and dispersal as well as splash following common technician activities (for example, colonoscope brushing, scrubbing, rinsing and transport to an automated endoscope reprocessor [AER] for sterilization), the researchers affixed blue moisture-detection paper to environmental surfaces, on carts positioned 4 feet (1.2 meters) from the sink (to simulate observers), and along a 15-foot pathway between the sink and AER.
They observed droplets everywhere.
Technician activities such as running the faucet and rinsing the probe under running water generated substantial splashing overall. Instrument rinsing in particular produced small and large droplets and confluent puddles of water around the sink and in the broad area surrounding the workspace. Droplets were also dispersed on the floor 7.25 feet (2.2 meters) away and along the entire 15-foot path from the sink to the AER.
At the sink, the technician risked drenching exposure from head to toe during most activities, and even observers positioned 3-4 feet away were found to have droplets on their gowns. In addition, saturated shoe covers reportedly tracked moisture away from the sink to the unit door – a distance of 13 feet (4 meters) – and 2 feet (0.6 meters) farther out into the PPE foyer for donning and doffing.
Although PPE gowns effectively repelled moisture during cleaning of a single device, Ms. Ofstead emphasized that technicians typically handle up to 10 instruments during a normal, 2-hour shift, further increasing exposure risk with each subsequent cleaning.
However, perhaps one of the most surprising findings was that despite an optimal unit design, including physical separation of clean and dirty activities and pressurized air flow to protect workers, droplets were still broadly dispersed.
Current efforts, however well-intentioned, might not be offering the degree of protection (and consideration) that sterile processing technicians need.
“The study was conducted in a new sterile processing area that had an extra excellent kind of distancing and three separate rooms, something that I think most of our hospitals are working toward,” Stella Hines, MD, associate professor at the University of Maryland School of Medicine, Baltimore, explained. Dr. Hines was not directly involved in the study.
“But it also really kind of highlighted what’s happening to workers potentially,” she added. “For example, we want to know if that spray or splatter has a live microbe it in that could cause a problem or ... in a highly wet environment, if that water has some kind of chemical in it that could pose an occupational hazard to the worker based on skin or mucous membrane exposure.”
Ms. Ofstead agreed. “We need to be thinking about the exposure of critically important workers and the environment in an era where we are worried about aerosol-generating procedures and superbugs,” she explained.
Dr. Hines and Ms. Ofstead also noted that the majority of staff involved in front-line patient care have never actually ventured into the sterile processing units nor do they recognize the risks that technicians working in these units face on a daily, or even hourly, basis.
“The people who run these operations are very well trained and knowledgeable. I think that it would be helpful for them to know that they’re appreciated and for the people upstairs on the front lines using the equipment to see what goes on downstairs and all of the painstaking steps that need to be in place for the equipment to come out of sterile processing and be ready to go,” said Dr. Hines.
In the meantime, hospital leaders need to address the challenges and danger posed by migrating infectious droplets, especially for workers involved in processes that stir them up in the first place – workers who by the end of their shifts are unavoidably drenched with infectious blood and tissue secretions.
“I think that it’s going to take a much bigger kind of worldview from hospital leadership,” Dr. Hines said.
The study was supported in part by a grant from Healthmark Industries. Ms. Ofstead reports research grants or consulting fees through her organization with 3M Company, Ambu, Boston Scientific, Cleanis, Fortive/Advanced Sterilization Products, Healthmark Industries, Pentax, and Steris/Cantel/Medviators. Dr. Hines reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the era of Ebola, COVID-19, and even Legionnaires, technicians and other staff working behind the scenes to ensure provider and patient safety continue to face a long-recognized but under addressed challenge: splashes and airborne droplets.
Granted, National Institute for Occupational Safety and Health (NIOSH) standards, industry standards, and professional guidelines are all in place to prevent unintentional exposure to pathogens. However, findings from a newly published study in the American Journal of Infection Control suggest they fall short.
In the study, researchers found that simulated manual cleaning of medical devices generated a drenching splash throughout the process with droplet dispersal exceeding 7 feet (2.1 meters).
Cori L. Ofstead, MSPH, lead author and president/CEO of Ofstead & Associates, Bloomington, Minn., told this news organization. “That’s the problem with having standards and guidelines that are not based on relevant evidence, [which] in this case, is a single study that was done in an intensive care area where they had an infection outbreak.”
Ms. Ofstead was referring to a report in the journal Infection Control and Hospital Epidemiology, detailing a Canadian investigation involving a multidrug-resistant Pseudomonas aeruginosa outbreak in an ICU. The report implicated the faucets over the hand hygiene sinks, with fluorescent dye showing droplet dispersal roughly 3 feet away from the sinks.
“Somehow it [the 3-feet rule] got implemented in guidelines in sterile processing decontamination areas, which are not the same as hand hygiene,’’ Ms. Ofstead explained.
With a goal of providing more current evidence on droplet generation and dispersal, as well as personal protection equipment (PPE) exposure/effectiveness, she and her colleagues simulated manual cleaning of a decommissioned colonoscope and transvaginal ultrasound probe, using for the study location a new academic sterile processing unit.
To detect droplet generation and dispersal as well as splash following common technician activities (for example, colonoscope brushing, scrubbing, rinsing and transport to an automated endoscope reprocessor [AER] for sterilization), the researchers affixed blue moisture-detection paper to environmental surfaces, on carts positioned 4 feet (1.2 meters) from the sink (to simulate observers), and along a 15-foot pathway between the sink and AER.
They observed droplets everywhere.
Technician activities such as running the faucet and rinsing the probe under running water generated substantial splashing overall. Instrument rinsing in particular produced small and large droplets and confluent puddles of water around the sink and in the broad area surrounding the workspace. Droplets were also dispersed on the floor 7.25 feet (2.2 meters) away and along the entire 15-foot path from the sink to the AER.
At the sink, the technician risked drenching exposure from head to toe during most activities, and even observers positioned 3-4 feet away were found to have droplets on their gowns. In addition, saturated shoe covers reportedly tracked moisture away from the sink to the unit door – a distance of 13 feet (4 meters) – and 2 feet (0.6 meters) farther out into the PPE foyer for donning and doffing.
Although PPE gowns effectively repelled moisture during cleaning of a single device, Ms. Ofstead emphasized that technicians typically handle up to 10 instruments during a normal, 2-hour shift, further increasing exposure risk with each subsequent cleaning.
However, perhaps one of the most surprising findings was that despite an optimal unit design, including physical separation of clean and dirty activities and pressurized air flow to protect workers, droplets were still broadly dispersed.
Current efforts, however well-intentioned, might not be offering the degree of protection (and consideration) that sterile processing technicians need.
“The study was conducted in a new sterile processing area that had an extra excellent kind of distancing and three separate rooms, something that I think most of our hospitals are working toward,” Stella Hines, MD, associate professor at the University of Maryland School of Medicine, Baltimore, explained. Dr. Hines was not directly involved in the study.
“But it also really kind of highlighted what’s happening to workers potentially,” she added. “For example, we want to know if that spray or splatter has a live microbe it in that could cause a problem or ... in a highly wet environment, if that water has some kind of chemical in it that could pose an occupational hazard to the worker based on skin or mucous membrane exposure.”
Ms. Ofstead agreed. “We need to be thinking about the exposure of critically important workers and the environment in an era where we are worried about aerosol-generating procedures and superbugs,” she explained.
Dr. Hines and Ms. Ofstead also noted that the majority of staff involved in front-line patient care have never actually ventured into the sterile processing units nor do they recognize the risks that technicians working in these units face on a daily, or even hourly, basis.
“The people who run these operations are very well trained and knowledgeable. I think that it would be helpful for them to know that they’re appreciated and for the people upstairs on the front lines using the equipment to see what goes on downstairs and all of the painstaking steps that need to be in place for the equipment to come out of sterile processing and be ready to go,” said Dr. Hines.
In the meantime, hospital leaders need to address the challenges and danger posed by migrating infectious droplets, especially for workers involved in processes that stir them up in the first place – workers who by the end of their shifts are unavoidably drenched with infectious blood and tissue secretions.
“I think that it’s going to take a much bigger kind of worldview from hospital leadership,” Dr. Hines said.
The study was supported in part by a grant from Healthmark Industries. Ms. Ofstead reports research grants or consulting fees through her organization with 3M Company, Ambu, Boston Scientific, Cleanis, Fortive/Advanced Sterilization Products, Healthmark Industries, Pentax, and Steris/Cantel/Medviators. Dr. Hines reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
Is it flu, RSV, or COVID? Experts fear the ‘tripledemic’
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Many specialists are on the wrong side of the patient-jargon relationship
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Doctor, doctor, gimme the news. I got a bad case of misidentifying you
There are a lot of medical specialties out there. A lot. Everything from allergists to urologists, with something like 150 subspecialties grouped in among the larger specialties. Can you name every one? Do you know what they do?
The point is, telling a patient or anyone in the general public that you’re an ophthalmologist may not be as helpful as you might think, if a recent study is to be believed. In a survey of 204 adults, conducted at the Minnesota State Fair of all places, researchers asked volunteers to define 14 different specialties, as well as five medical seniority titles.
The results were less than stellar. While more than 90% of people correctly defined what cardiologists and dermatologists do, 6 of the other 12 specialists were correctly identified by less than half of those surveyed. Nephrology was at the bottom, correctly identified by just 20% of the fair-attending public, followed by internists (21%), intensivists (29%), hospitalists (31%), pulmonologists (43%), and neonatologists at 48%. The hospitalists are particularly concerning. They’re doctors, but in hospitals. How hard is that? (Yes, it’s obviously more complicated than that, but still.)
The general public didn’t fare much better when it came to correctly lining up the order of progression from medical student to attending. Just 12% managed to place all five in the correct order of med student, intern, senior resident, fellow, then attending, with senior resident proving especially troublesome. More than 40% put senior resident at the end, compared with 27% for attending. Which does make a certain amount of sense, since it has senior in the name.
While the results speak for themselves – maybe elaborate on what the heck your fancy title actually means – it’s too bad the researchers didn’t throw in something really tricky. If two-thirds of the population can’t identify a hospitalist, just imagine how many people would misidentify an otolaryngologist.
Beach-to-table sand could fight obesity
People are always looking for the new weight loss solution. Whether it’s to just look good in a new pair of jeans or reduce the risk of cardiovascular disease, there are millions of diets and exercise routines out here. We’re here to tell you that the next new therapy to reduce fat comes from a very unsuspecting place: Sand.
Like sand from the beach and desert, sand? Well, yes and no.
The research involved engineered porous silica particles made from sand that are designed to have a high surface area. Investigators used a two-step GI model in which gastric digestion was modeled for 30 minutes, followed by a 60-minute intestinal phase, to show that the porous silica particles helped prevent fat and sugar adsorption within the GI tract.
By mimicking the gastrointestinal environment during digestion of a high-fat, high-carb meal, the researchers found that the porous silica created an “anti-obesity effect” by restricting the adsorption of those fats and carbohydrates.
Okay, but how is that on the tummy? Much gentler on the stomach than a drug such as orlistat, said senior researcher Paul Joyce, PhD, of the University of South Australia, Adelaide, who noted the lack of effective therapies without side effects, such as bloating, diarrhea, and abdominal pain, that deter people from treatment.
Obesity affects over 1.9 billion people worldwide, so the researchers think this could be a breakthrough. Reducing obesity may be one of the most preventable ways to reduce the risk of type 2 diabetes, heart disease, and other weight-related chronic conditions. A treatment solution this simple could be the answer to this global health crisis.
Who would have thought the solution would be as simple as sand? But how would the sand get in our stomachs? Do we sprinkle it on our food? Mix it in during cooking? Or will the sand come in pill form? We sure hope it’s that third one.
I am Reliebo. I am here to help you
Halloween is almost here, and the LOTME staff has been trying to make the office look as scary as possible: Headless vampires, ghost clowns, Ted Cruz, gray tombstones, pink hearts, green clovers, red balloons. Wait a second, those last three are Lucky Charms marshmallows, aren’t they? We’ll use those some other time.
What are we not using to decorate? Well, besides marshmallows from cereal, we’re not using Reliebo. That’s what we’re not using. Reliebo is a cute little fuzzy robot, and is not at all scary. Reliebo was designed to be the opposite of scary. Reliebo “may reduce fear as well as alleviate the perception of pain during medical treatments, including vaccinations,” senior author Fumihide Tanaka, PhD, of the University of Tsukuba (Japan) said in a written statement.
The soft, fur-covered robot contains small airbags that can inflate in response to hand movements. When study participants were subjected to a moderate heat stimulus on one arm, those who held the robot with the other arm experienced less pain than those who did not have a Reliebo.
The results also were encouraging when Dr. Tanaka and associates measured the levels of oxytocin and cortisol (biomarkers for stress) from the subjects’ saliva samples and evaluated their fear of injections and their psychological state before and after the experiments.
After looking at that photo of Reliebo for a while, though, we have to admit that we’re having a bit of a rethink about its cuteness. Is it cute, or weird-looking? An office full of fuzzy little inflating robots just could be seriously creepy. Please don’t tell the rest of the staff about this. We want to surprise them on Monday.
Ivermectin for COVID-19: Final nail in the coffin
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.
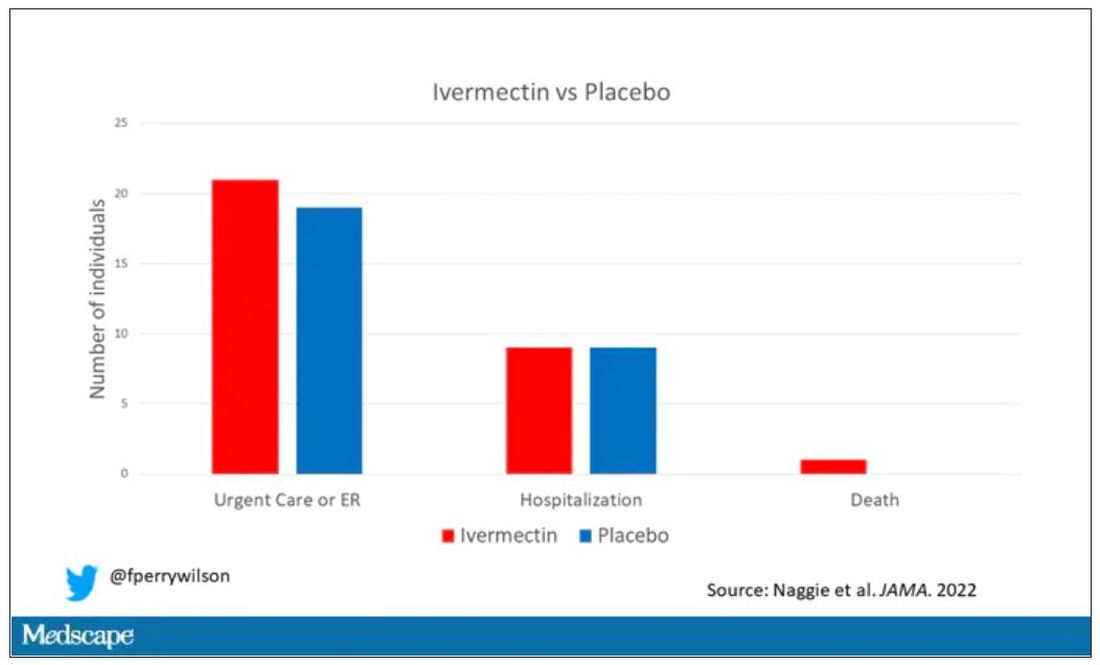
But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases fall to lowest level in over a year
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.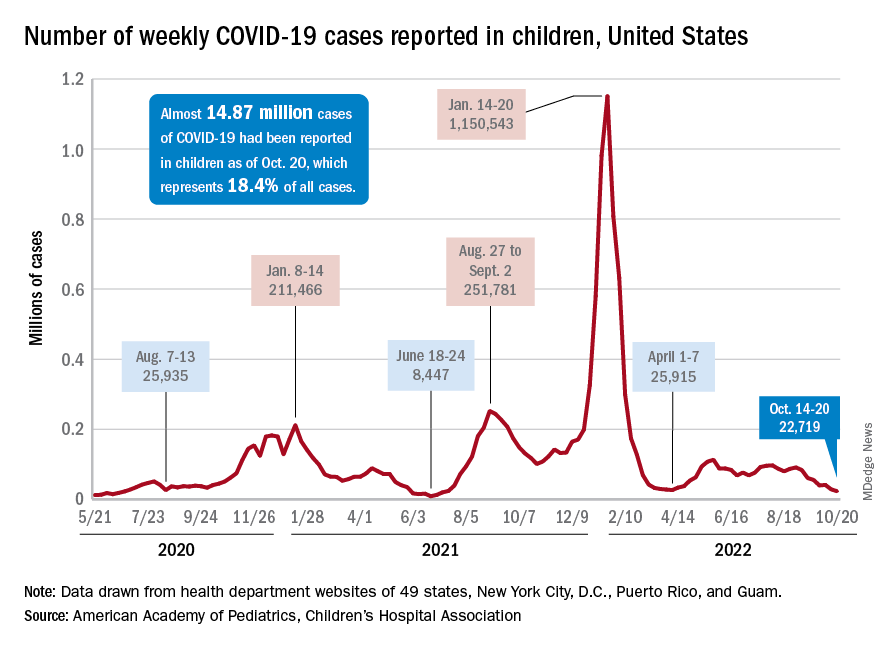
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.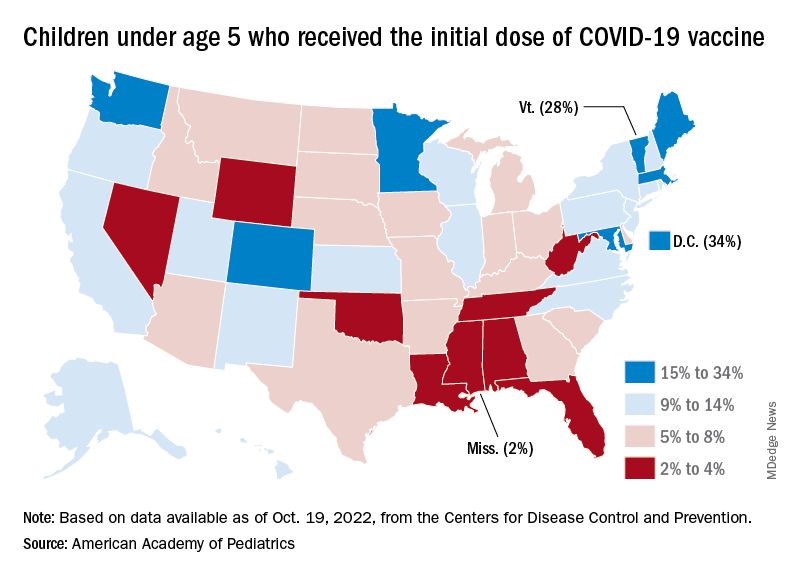
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
Durvalumab combinations show tentative promise in NSCLC
in patients with unresectable stage 3 non–small-cell lung cancer.
Combinations of the PD-L1 inhibitor durvalumab (Imfinzi, AstraZeneca) with the anti-CD73 monoclonal antibody oleclumab or the anti-NKG2A monoclonal antibody monalizumab led to improved overall response rate and progression-free survival compared to durvalumab alone.
The findings support further study in a phase 3 clinical trial, according to the authors of the study recently published in the Journal of Clinical Oncology.
Durvalumab is the standard treatment following consolidation therapy of chemoradiotherapy in unresectable stage 3 non–small-cell lung cancer (NSCLC). Although it extended progression-free survival (PFS) and overall survival in the PACIFIC phase 3 study, some patients experience a recurrence, which has led to exploration of immunotherapy combinations.
Oleclumab inhibits the enzyme CD73, found on the surfaces of both tumor and immune cells. Its activity leads to an immunosuppressive effect in the tumor microenvironment, and preclinical studies have shown that it can have an additive antitumor effect when combined with PD-1 or PD-L1 inhibitors. A phase 1 study also suggested efficacy. Monalizumab blocks interactions between major histocompatibility complex-E (HLA-E) and an inhibitor receptor. A number of tumors overexpress HLA-E, triggering inhibitor signals that inhibit natural killer and CD8+ T cells.
“COAST was an interesting study that, although not definitive, suggested that the combination of durvalumab with oleclumab or with monalizumab was more effective than durvalumab alone in the consolidation setting after definitive concurrent chemoradiation for patients with stage 3 unresectable NSCLC,” said Nathan Pennell, MD, PhD, who wrote an accompanying editorial.
Despite the positive signal, Dr. Pennell expressed some skepticism that the combinations would pass a phase 3 test. He questioned the choice of response rate as the primary endpoint of the phase 2 study, and noted that the durvalumab arm had worse progression-free survival (PFS) than the previous PACIFIC trial. It could be that the clinical characteristics of the study population differed between the two trials, in which case the improved objective response rate (ORR) and PFS results should be encouraging. It’s also possible the COAST trial’s small sample size led to a mismatch between the control and treatment group despite randomization, in which case the findings may not be valid.
“These are the kinds of issues that keep drug developers up at night. There really is no way to know which scenario is correct without doing the larger trial. I do hope though that the phase 3 trials have robust biomarker analysis including PDL1 to make sure the arms are as well matched for known prognostic and predictive markers as possible,” said Dr. Pennell, who is vice chair of clinical research at Taussig Cancer Institute.
The study details
The researchers randomized 189 patients to durvalumab, durvalumab plus oleclumab, or durvalumab plus monalizumab between January 2019 and July 2020. After a median follow-up of 11.5 months, there was a higher confirmed objective response rate in the durvalumab plus oleclumab group (30.0%; 95% confidence interval, 18.8%-43.2%) and the durvalumab plus monalizumab group (35.5%; 95% CI, 23.7%-48.7%) versus durvalumab alone (17.9%; 95% CI, 9.6%-29.2%).
Compared to durvalumab alone, there was improved PFS in both durvalumab plus oleclumab (stratified hazard ratio, 0.44; 95% CI, 0.26-0.75) and durvalumab plus monalizumab (HR, 0.42; 95% CI, 0.24-0.72). At 12 months, PFS was 62.6% (95% CI, 48.1-74.2%) for durvalumab plus oleclumab, 72.7% (95% CI, 58.8-82.6%) for durvalumab plus monalizumab, and 33.9% (95% CI, 21.2-47.1%) for durvalumab alone.
The study was funded by AstraZeneca.
in patients with unresectable stage 3 non–small-cell lung cancer.
Combinations of the PD-L1 inhibitor durvalumab (Imfinzi, AstraZeneca) with the anti-CD73 monoclonal antibody oleclumab or the anti-NKG2A monoclonal antibody monalizumab led to improved overall response rate and progression-free survival compared to durvalumab alone.
The findings support further study in a phase 3 clinical trial, according to the authors of the study recently published in the Journal of Clinical Oncology.
Durvalumab is the standard treatment following consolidation therapy of chemoradiotherapy in unresectable stage 3 non–small-cell lung cancer (NSCLC). Although it extended progression-free survival (PFS) and overall survival in the PACIFIC phase 3 study, some patients experience a recurrence, which has led to exploration of immunotherapy combinations.
Oleclumab inhibits the enzyme CD73, found on the surfaces of both tumor and immune cells. Its activity leads to an immunosuppressive effect in the tumor microenvironment, and preclinical studies have shown that it can have an additive antitumor effect when combined with PD-1 or PD-L1 inhibitors. A phase 1 study also suggested efficacy. Monalizumab blocks interactions between major histocompatibility complex-E (HLA-E) and an inhibitor receptor. A number of tumors overexpress HLA-E, triggering inhibitor signals that inhibit natural killer and CD8+ T cells.
“COAST was an interesting study that, although not definitive, suggested that the combination of durvalumab with oleclumab or with monalizumab was more effective than durvalumab alone in the consolidation setting after definitive concurrent chemoradiation for patients with stage 3 unresectable NSCLC,” said Nathan Pennell, MD, PhD, who wrote an accompanying editorial.
Despite the positive signal, Dr. Pennell expressed some skepticism that the combinations would pass a phase 3 test. He questioned the choice of response rate as the primary endpoint of the phase 2 study, and noted that the durvalumab arm had worse progression-free survival (PFS) than the previous PACIFIC trial. It could be that the clinical characteristics of the study population differed between the two trials, in which case the improved objective response rate (ORR) and PFS results should be encouraging. It’s also possible the COAST trial’s small sample size led to a mismatch between the control and treatment group despite randomization, in which case the findings may not be valid.
“These are the kinds of issues that keep drug developers up at night. There really is no way to know which scenario is correct without doing the larger trial. I do hope though that the phase 3 trials have robust biomarker analysis including PDL1 to make sure the arms are as well matched for known prognostic and predictive markers as possible,” said Dr. Pennell, who is vice chair of clinical research at Taussig Cancer Institute.
The study details
The researchers randomized 189 patients to durvalumab, durvalumab plus oleclumab, or durvalumab plus monalizumab between January 2019 and July 2020. After a median follow-up of 11.5 months, there was a higher confirmed objective response rate in the durvalumab plus oleclumab group (30.0%; 95% confidence interval, 18.8%-43.2%) and the durvalumab plus monalizumab group (35.5%; 95% CI, 23.7%-48.7%) versus durvalumab alone (17.9%; 95% CI, 9.6%-29.2%).
Compared to durvalumab alone, there was improved PFS in both durvalumab plus oleclumab (stratified hazard ratio, 0.44; 95% CI, 0.26-0.75) and durvalumab plus monalizumab (HR, 0.42; 95% CI, 0.24-0.72). At 12 months, PFS was 62.6% (95% CI, 48.1-74.2%) for durvalumab plus oleclumab, 72.7% (95% CI, 58.8-82.6%) for durvalumab plus monalizumab, and 33.9% (95% CI, 21.2-47.1%) for durvalumab alone.
The study was funded by AstraZeneca.
in patients with unresectable stage 3 non–small-cell lung cancer.
Combinations of the PD-L1 inhibitor durvalumab (Imfinzi, AstraZeneca) with the anti-CD73 monoclonal antibody oleclumab or the anti-NKG2A monoclonal antibody monalizumab led to improved overall response rate and progression-free survival compared to durvalumab alone.
The findings support further study in a phase 3 clinical trial, according to the authors of the study recently published in the Journal of Clinical Oncology.
Durvalumab is the standard treatment following consolidation therapy of chemoradiotherapy in unresectable stage 3 non–small-cell lung cancer (NSCLC). Although it extended progression-free survival (PFS) and overall survival in the PACIFIC phase 3 study, some patients experience a recurrence, which has led to exploration of immunotherapy combinations.
Oleclumab inhibits the enzyme CD73, found on the surfaces of both tumor and immune cells. Its activity leads to an immunosuppressive effect in the tumor microenvironment, and preclinical studies have shown that it can have an additive antitumor effect when combined with PD-1 or PD-L1 inhibitors. A phase 1 study also suggested efficacy. Monalizumab blocks interactions between major histocompatibility complex-E (HLA-E) and an inhibitor receptor. A number of tumors overexpress HLA-E, triggering inhibitor signals that inhibit natural killer and CD8+ T cells.
“COAST was an interesting study that, although not definitive, suggested that the combination of durvalumab with oleclumab or with monalizumab was more effective than durvalumab alone in the consolidation setting after definitive concurrent chemoradiation for patients with stage 3 unresectable NSCLC,” said Nathan Pennell, MD, PhD, who wrote an accompanying editorial.
Despite the positive signal, Dr. Pennell expressed some skepticism that the combinations would pass a phase 3 test. He questioned the choice of response rate as the primary endpoint of the phase 2 study, and noted that the durvalumab arm had worse progression-free survival (PFS) than the previous PACIFIC trial. It could be that the clinical characteristics of the study population differed between the two trials, in which case the improved objective response rate (ORR) and PFS results should be encouraging. It’s also possible the COAST trial’s small sample size led to a mismatch between the control and treatment group despite randomization, in which case the findings may not be valid.
“These are the kinds of issues that keep drug developers up at night. There really is no way to know which scenario is correct without doing the larger trial. I do hope though that the phase 3 trials have robust biomarker analysis including PDL1 to make sure the arms are as well matched for known prognostic and predictive markers as possible,” said Dr. Pennell, who is vice chair of clinical research at Taussig Cancer Institute.
The study details
The researchers randomized 189 patients to durvalumab, durvalumab plus oleclumab, or durvalumab plus monalizumab between January 2019 and July 2020. After a median follow-up of 11.5 months, there was a higher confirmed objective response rate in the durvalumab plus oleclumab group (30.0%; 95% confidence interval, 18.8%-43.2%) and the durvalumab plus monalizumab group (35.5%; 95% CI, 23.7%-48.7%) versus durvalumab alone (17.9%; 95% CI, 9.6%-29.2%).
Compared to durvalumab alone, there was improved PFS in both durvalumab plus oleclumab (stratified hazard ratio, 0.44; 95% CI, 0.26-0.75) and durvalumab plus monalizumab (HR, 0.42; 95% CI, 0.24-0.72). At 12 months, PFS was 62.6% (95% CI, 48.1-74.2%) for durvalumab plus oleclumab, 72.7% (95% CI, 58.8-82.6%) for durvalumab plus monalizumab, and 33.9% (95% CI, 21.2-47.1%) for durvalumab alone.
The study was funded by AstraZeneca.
FROM JOURNAL OF CLINICAL ONCOLOGY



