User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Sleep complaints in major depression flag risk for other psychiatric disorders
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.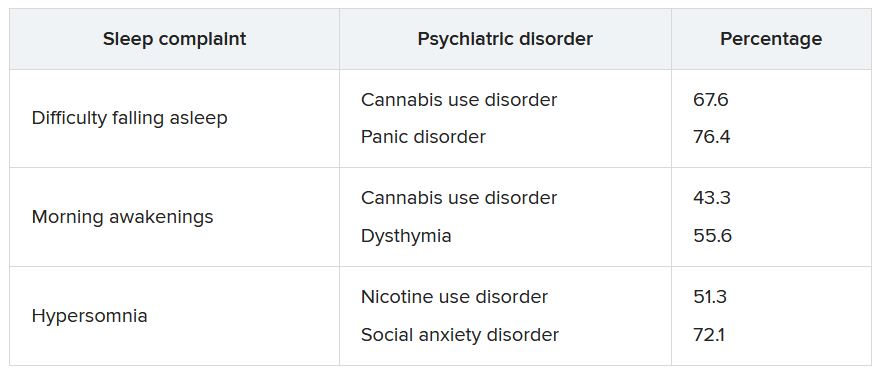
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
A remote mountain bike crash forces a doctor to take knife in hand
It started as a mountain biking excursion with two friends. When we drove into the trailhead parking lot, we saw several emergency vehicles. Then a helicopter passed overhead.
Half a mile down the trail, we encountered another police officer. He asked if we would be willing to go back to get an oxygen tank from the ambulance and carry it out to the scene. The three of us turned around, went back to the parking lot and were able to snag a tank of oxygen. We put it in a backpack and biked out again.
We found the scene about a mile down the trail. An adult male was lying on his back in the dirt after a crash. His eyes were closed and he wasn’t moving except for occasional breaths. Six emergency medical personnel huddled around him, one assisting breaths with a bag mask. I didn’t introduce myself initially. I just listened to hear what was happening.
They were debating the dose of medication to give him in order to intubate. I knew the answer to that question, so I introduced myself. They were happy to have somebody else to assist.
They already had an IV in place and quite a lot of supplies. They administered the meds and the paramedic attempted to intubate through the mouth. Within a few seconds, she pulled the intubating blade out and said, “I’m not going to be able to get this. His tongue is too big.”
I took the blade myself and kneeled at the head of the victim. I made three attempts at intubating, and each time couldn’t view the landmarks. I wasn’t sure if his tongue was too large or if there was some traumatic injury. To make it more difficult, a lot of secretions clogged the airway. The paramedics had a portable suction, which was somewhat functional, but I still couldn’t visualize the landmarks.
I started asking about alternative methods of establishing an airway. They had an i-gel, which is a supraglottic device that goes into the back of the mouth. So, we placed it. But when we attached the bag, air still wasn’t getting into the lungs.
We removed it and put the bag mask back on. Now I was worried. We were having difficulty keeping his oxygen above 90%. I examined the chest and abdomen again. I was wondering if perhaps he was having some gastric distention, which can result from prolonged bagging, but that didn’t seem to be the case.
Bagging became progressively more difficult, and the oxygen slowly trended down through the 80s. Then the 70s. Heart rate dropped below 60 beats per minute. The trajectory was obvious.
That’s when I asked if they had the tools for a surgical airway.
No one thought the question was crazy. In fact, they pulled out a scalpel from an equipment bag.
But now I had to actually do it. I knelt next to the patient, trying to palpate the front of the neck to identify the correct location to cut. I had difficulty finding the appropriate landmarks there as well. Frustrating.
I glanced at the monitor. O2 was now in the 60s. Later the paramedic told me the heart rate was down to 30.
One of the medics looked me in the eye and said, “We’ve got to do something. The time is now.” That helped me snap out of it and act. I made my large vertical incision on the front of the victim’s neck, which of course resulted in quite a bit of bleeding.
My two friends, who were watching, later told me this was the moment the intensity of the scene really increased (it was already pretty intense for me, thanks).
Next, I made the horizontal stab incision. Then I probed with my finger, but it seems the incision hadn’t reached the trachea. I had to make the stab much deeper than I would’ve thought.
And then air bubbled out through the blood. A paramedic was ready with the ET tube in hand and she put it through the incision. We attached the bag. We had air movement into the lungs, and within minutes the oxygen came up.
Not long after, the flight paramedics from the helicopter showed up, having jogged a mile through the woods. They seemed rather surprised to find a patient with a cricothyrotomy. We filled them in on the situation. Now we had to get the patient out of the woods (literally and figuratively).
The emergency responders had a really great transport device: A litter with one big wheel underneath in the middle so we could roll the patient down the mountain bike trail over rocks relatively safely. One person’s job was to hold the tube as we went since we didn’t have suture to hold it in place.
We got back to the parking lot and loaded him into the ambulance, which drove another mile to the helicopter, which then had to take him a hundred miles to the hospital.
To be honest, I thought the prognosis was poor. I suspected he had an intercranial bleed slowly squeezing his brain (that later turned out to not be the case). Even though we had established an airway, it took us so long to get him to the ambulance.
The director of the local EMS called me that evening and said the patient had made it to the hospital. I had never been a part of anything with this intensity. I definitely lost sleep over it. Partly just from the uncertainty of not knowing what the outcome would be. But also second-guessing if I had done everything that I could have.
The story doesn’t quite end there, however.
A week later, a friend of the patient called me. He had recovered well and was going to be discharged from the hospital. He’d chosen to share the story with the media, and the local TV station was going to interview him. They had asked if I would agree to be interviewed.
After the local news story ran, it was kind of a media blitz. In came numerous media requests. But honestly, the portrayal of the story made me feel really weird. It was overly dramatized and not entirely accurate. It really didn’t sit well with me.
Friends all over the country saw the story, and here’s what they got from the coverage:
I was biking behind the patient when he crashed.
I had my own tools. Even the patient himself was told I used my own blade to make the incision.
The true story is what I just told you: A half-dozen emergency medical personnel were already there when I arrived. It was a combination of all of us – together – in the right place at the right time.
A month later, the patient and his family drove to the city where I live to take me out to lunch. It was emotional. There were plenty of tears. His wife and daughter were expressing a lot of gratitude and had some gifts for me. I was able to get his version of the story and learned some details. He had facial trauma in the past with some reconstruction. I realized that perhaps those anatomical changes affected my ability to do the intubation.
I hope to never again have to do this outside of the hospital. But I suppose I’m more prepared than ever now. I’ve reviewed my cricothyrotomy technique many times since then.
I was trained as a family doctor and did clinic and hospital medicine for several years. It was only in 2020 that I transitioned to doing emergency medicine work in a rural hospital. So, 2 years earlier, I’m not sure I would’ve been able to do what I did that day. To me, it was almost symbolic of the transition of my practice to emergency medicine.
I’m still in touch with the patient. We’ve talked about biking together. That hasn’t happened yet, but it may very well happen someday.
Jesse Coenen, MD, is an emergency medicine physician at Hayward Area Memorial Hospital in Hayward, Wisc.
A version of this article first appeared on Medscape.com.
It started as a mountain biking excursion with two friends. When we drove into the trailhead parking lot, we saw several emergency vehicles. Then a helicopter passed overhead.
Half a mile down the trail, we encountered another police officer. He asked if we would be willing to go back to get an oxygen tank from the ambulance and carry it out to the scene. The three of us turned around, went back to the parking lot and were able to snag a tank of oxygen. We put it in a backpack and biked out again.
We found the scene about a mile down the trail. An adult male was lying on his back in the dirt after a crash. His eyes were closed and he wasn’t moving except for occasional breaths. Six emergency medical personnel huddled around him, one assisting breaths with a bag mask. I didn’t introduce myself initially. I just listened to hear what was happening.
They were debating the dose of medication to give him in order to intubate. I knew the answer to that question, so I introduced myself. They were happy to have somebody else to assist.
They already had an IV in place and quite a lot of supplies. They administered the meds and the paramedic attempted to intubate through the mouth. Within a few seconds, she pulled the intubating blade out and said, “I’m not going to be able to get this. His tongue is too big.”
I took the blade myself and kneeled at the head of the victim. I made three attempts at intubating, and each time couldn’t view the landmarks. I wasn’t sure if his tongue was too large or if there was some traumatic injury. To make it more difficult, a lot of secretions clogged the airway. The paramedics had a portable suction, which was somewhat functional, but I still couldn’t visualize the landmarks.
I started asking about alternative methods of establishing an airway. They had an i-gel, which is a supraglottic device that goes into the back of the mouth. So, we placed it. But when we attached the bag, air still wasn’t getting into the lungs.
We removed it and put the bag mask back on. Now I was worried. We were having difficulty keeping his oxygen above 90%. I examined the chest and abdomen again. I was wondering if perhaps he was having some gastric distention, which can result from prolonged bagging, but that didn’t seem to be the case.
Bagging became progressively more difficult, and the oxygen slowly trended down through the 80s. Then the 70s. Heart rate dropped below 60 beats per minute. The trajectory was obvious.
That’s when I asked if they had the tools for a surgical airway.
No one thought the question was crazy. In fact, they pulled out a scalpel from an equipment bag.
But now I had to actually do it. I knelt next to the patient, trying to palpate the front of the neck to identify the correct location to cut. I had difficulty finding the appropriate landmarks there as well. Frustrating.
I glanced at the monitor. O2 was now in the 60s. Later the paramedic told me the heart rate was down to 30.
One of the medics looked me in the eye and said, “We’ve got to do something. The time is now.” That helped me snap out of it and act. I made my large vertical incision on the front of the victim’s neck, which of course resulted in quite a bit of bleeding.
My two friends, who were watching, later told me this was the moment the intensity of the scene really increased (it was already pretty intense for me, thanks).
Next, I made the horizontal stab incision. Then I probed with my finger, but it seems the incision hadn’t reached the trachea. I had to make the stab much deeper than I would’ve thought.
And then air bubbled out through the blood. A paramedic was ready with the ET tube in hand and she put it through the incision. We attached the bag. We had air movement into the lungs, and within minutes the oxygen came up.
Not long after, the flight paramedics from the helicopter showed up, having jogged a mile through the woods. They seemed rather surprised to find a patient with a cricothyrotomy. We filled them in on the situation. Now we had to get the patient out of the woods (literally and figuratively).
The emergency responders had a really great transport device: A litter with one big wheel underneath in the middle so we could roll the patient down the mountain bike trail over rocks relatively safely. One person’s job was to hold the tube as we went since we didn’t have suture to hold it in place.
We got back to the parking lot and loaded him into the ambulance, which drove another mile to the helicopter, which then had to take him a hundred miles to the hospital.
To be honest, I thought the prognosis was poor. I suspected he had an intercranial bleed slowly squeezing his brain (that later turned out to not be the case). Even though we had established an airway, it took us so long to get him to the ambulance.
The director of the local EMS called me that evening and said the patient had made it to the hospital. I had never been a part of anything with this intensity. I definitely lost sleep over it. Partly just from the uncertainty of not knowing what the outcome would be. But also second-guessing if I had done everything that I could have.
The story doesn’t quite end there, however.
A week later, a friend of the patient called me. He had recovered well and was going to be discharged from the hospital. He’d chosen to share the story with the media, and the local TV station was going to interview him. They had asked if I would agree to be interviewed.
After the local news story ran, it was kind of a media blitz. In came numerous media requests. But honestly, the portrayal of the story made me feel really weird. It was overly dramatized and not entirely accurate. It really didn’t sit well with me.
Friends all over the country saw the story, and here’s what they got from the coverage:
I was biking behind the patient when he crashed.
I had my own tools. Even the patient himself was told I used my own blade to make the incision.
The true story is what I just told you: A half-dozen emergency medical personnel were already there when I arrived. It was a combination of all of us – together – in the right place at the right time.
A month later, the patient and his family drove to the city where I live to take me out to lunch. It was emotional. There were plenty of tears. His wife and daughter were expressing a lot of gratitude and had some gifts for me. I was able to get his version of the story and learned some details. He had facial trauma in the past with some reconstruction. I realized that perhaps those anatomical changes affected my ability to do the intubation.
I hope to never again have to do this outside of the hospital. But I suppose I’m more prepared than ever now. I’ve reviewed my cricothyrotomy technique many times since then.
I was trained as a family doctor and did clinic and hospital medicine for several years. It was only in 2020 that I transitioned to doing emergency medicine work in a rural hospital. So, 2 years earlier, I’m not sure I would’ve been able to do what I did that day. To me, it was almost symbolic of the transition of my practice to emergency medicine.
I’m still in touch with the patient. We’ve talked about biking together. That hasn’t happened yet, but it may very well happen someday.
Jesse Coenen, MD, is an emergency medicine physician at Hayward Area Memorial Hospital in Hayward, Wisc.
A version of this article first appeared on Medscape.com.
It started as a mountain biking excursion with two friends. When we drove into the trailhead parking lot, we saw several emergency vehicles. Then a helicopter passed overhead.
Half a mile down the trail, we encountered another police officer. He asked if we would be willing to go back to get an oxygen tank from the ambulance and carry it out to the scene. The three of us turned around, went back to the parking lot and were able to snag a tank of oxygen. We put it in a backpack and biked out again.
We found the scene about a mile down the trail. An adult male was lying on his back in the dirt after a crash. His eyes were closed and he wasn’t moving except for occasional breaths. Six emergency medical personnel huddled around him, one assisting breaths with a bag mask. I didn’t introduce myself initially. I just listened to hear what was happening.
They were debating the dose of medication to give him in order to intubate. I knew the answer to that question, so I introduced myself. They were happy to have somebody else to assist.
They already had an IV in place and quite a lot of supplies. They administered the meds and the paramedic attempted to intubate through the mouth. Within a few seconds, she pulled the intubating blade out and said, “I’m not going to be able to get this. His tongue is too big.”
I took the blade myself and kneeled at the head of the victim. I made three attempts at intubating, and each time couldn’t view the landmarks. I wasn’t sure if his tongue was too large or if there was some traumatic injury. To make it more difficult, a lot of secretions clogged the airway. The paramedics had a portable suction, which was somewhat functional, but I still couldn’t visualize the landmarks.
I started asking about alternative methods of establishing an airway. They had an i-gel, which is a supraglottic device that goes into the back of the mouth. So, we placed it. But when we attached the bag, air still wasn’t getting into the lungs.
We removed it and put the bag mask back on. Now I was worried. We were having difficulty keeping his oxygen above 90%. I examined the chest and abdomen again. I was wondering if perhaps he was having some gastric distention, which can result from prolonged bagging, but that didn’t seem to be the case.
Bagging became progressively more difficult, and the oxygen slowly trended down through the 80s. Then the 70s. Heart rate dropped below 60 beats per minute. The trajectory was obvious.
That’s when I asked if they had the tools for a surgical airway.
No one thought the question was crazy. In fact, they pulled out a scalpel from an equipment bag.
But now I had to actually do it. I knelt next to the patient, trying to palpate the front of the neck to identify the correct location to cut. I had difficulty finding the appropriate landmarks there as well. Frustrating.
I glanced at the monitor. O2 was now in the 60s. Later the paramedic told me the heart rate was down to 30.
One of the medics looked me in the eye and said, “We’ve got to do something. The time is now.” That helped me snap out of it and act. I made my large vertical incision on the front of the victim’s neck, which of course resulted in quite a bit of bleeding.
My two friends, who were watching, later told me this was the moment the intensity of the scene really increased (it was already pretty intense for me, thanks).
Next, I made the horizontal stab incision. Then I probed with my finger, but it seems the incision hadn’t reached the trachea. I had to make the stab much deeper than I would’ve thought.
And then air bubbled out through the blood. A paramedic was ready with the ET tube in hand and she put it through the incision. We attached the bag. We had air movement into the lungs, and within minutes the oxygen came up.
Not long after, the flight paramedics from the helicopter showed up, having jogged a mile through the woods. They seemed rather surprised to find a patient with a cricothyrotomy. We filled them in on the situation. Now we had to get the patient out of the woods (literally and figuratively).
The emergency responders had a really great transport device: A litter with one big wheel underneath in the middle so we could roll the patient down the mountain bike trail over rocks relatively safely. One person’s job was to hold the tube as we went since we didn’t have suture to hold it in place.
We got back to the parking lot and loaded him into the ambulance, which drove another mile to the helicopter, which then had to take him a hundred miles to the hospital.
To be honest, I thought the prognosis was poor. I suspected he had an intercranial bleed slowly squeezing his brain (that later turned out to not be the case). Even though we had established an airway, it took us so long to get him to the ambulance.
The director of the local EMS called me that evening and said the patient had made it to the hospital. I had never been a part of anything with this intensity. I definitely lost sleep over it. Partly just from the uncertainty of not knowing what the outcome would be. But also second-guessing if I had done everything that I could have.
The story doesn’t quite end there, however.
A week later, a friend of the patient called me. He had recovered well and was going to be discharged from the hospital. He’d chosen to share the story with the media, and the local TV station was going to interview him. They had asked if I would agree to be interviewed.
After the local news story ran, it was kind of a media blitz. In came numerous media requests. But honestly, the portrayal of the story made me feel really weird. It was overly dramatized and not entirely accurate. It really didn’t sit well with me.
Friends all over the country saw the story, and here’s what they got from the coverage:
I was biking behind the patient when he crashed.
I had my own tools. Even the patient himself was told I used my own blade to make the incision.
The true story is what I just told you: A half-dozen emergency medical personnel were already there when I arrived. It was a combination of all of us – together – in the right place at the right time.
A month later, the patient and his family drove to the city where I live to take me out to lunch. It was emotional. There were plenty of tears. His wife and daughter were expressing a lot of gratitude and had some gifts for me. I was able to get his version of the story and learned some details. He had facial trauma in the past with some reconstruction. I realized that perhaps those anatomical changes affected my ability to do the intubation.
I hope to never again have to do this outside of the hospital. But I suppose I’m more prepared than ever now. I’ve reviewed my cricothyrotomy technique many times since then.
I was trained as a family doctor and did clinic and hospital medicine for several years. It was only in 2020 that I transitioned to doing emergency medicine work in a rural hospital. So, 2 years earlier, I’m not sure I would’ve been able to do what I did that day. To me, it was almost symbolic of the transition of my practice to emergency medicine.
I’m still in touch with the patient. We’ve talked about biking together. That hasn’t happened yet, but it may very well happen someday.
Jesse Coenen, MD, is an emergency medicine physician at Hayward Area Memorial Hospital in Hayward, Wisc.
A version of this article first appeared on Medscape.com.
Long COVID comes into focus, showing older patients fare worse
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
FROM THE BMJ
Early retirement and the terrible, horrible, no good, very bad cognitive decline
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
The ‘scheme’ in the name should have been a clue
Retirement. The shiny reward to a lifetime’s worth of working and saving. We’re all literally working to get there, some of us more to get there early, but current research reveals that early retirement isn’t the relaxing finish line we dream about, cognitively speaking.
Researchers at Binghamton (N.Y.) University set out to examine just how retirement plans affect cognitive performance. They started off with China’s New Rural Pension Scheme (scheme probably has a less negative connotation in Chinese), a plan that financially aids the growing rural retirement-age population in the country. Then they looked at data from the Chinese Health and Retirement Longitudinal Survey, which tests cognition with a focus on episodic memory and parts of intact mental status.
What they found was the opposite of what you would expect out of retirees with nothing but time on their hands.
The pension program, which had been in place for almost a decade, led to delayed recall, especially among women, supporting “the mental retirement hypothesis that decreased mental activity results in worsening cognitive skills,” the investigators said in a written statement.
There also was a drop in social engagement, with lower rates of volunteering and social interaction than people who didn’t receive the pension. Some behaviors, like regular alcohol consumption, did improve over the previous year, as did total health in general, but “the adverse effects of early retirement on mental and social engagement significantly outweigh the program’s protective effect on various health behaviors,” Plamen Nikolov, PhD, said about his research.
So if you’re looking to retire early, don’t skimp on the crosswords and the bingo nights. Stay busy in a good way. Your brain will thank you.
Indiana Jones and the First Smallpox Ancestor
Smallpox was, not that long ago, one of the most devastating diseases known to humanity, killing 300 million people in the 20th century alone. Eradicating it has to be one of medicine’s crowning achievements. Now it can only be found in museums, which is where it belongs.
Here’s the thing with smallpox though: For all it did to us, we know frustratingly little about where it came from. Until very recently, the best available genetic evidence placed its emergence in the 17th century, which clashes with historical data. You know what that means, right? It’s time to dig out the fedora and whip, cue the music, and dig into a recently published study spanning continents in search of the mythical smallpox origin story.
We pick up in 2020, when genetic evidence definitively showed smallpox in a Viking burial site, moving the disease’s emergence a thousand years earlier. Which is all well and good, but there’s solid visual evidence that Egyptian pharaohs were dying of smallpox, as their bodies show the signature scarring. Historians were pretty sure smallpox went back about 4,000 years, but there was no genetic material to prove it.
Since there aren’t any 4,000-year-old smallpox germs laying around, the researchers chose to attack the problem another way – by burning down a Venetian catacomb, er, conducting a analysis of historical smallpox genetics to find the virus’s origin. By analyzing the genomes of various strains at different periods of time, they were able to determine that the variola virus had a definitive common ancestor. Some of the genetic components in the Viking-age sample, for example, persisted until the 18th century.
Armed with this information, the scientists determined that the first smallpox ancestor emerged about 3,800 years ago. That’s very close to the historians’ estimate for the disease’s emergence. Proof at last of smallpox’s truly ancient origin. One might even say the researchers chose wisely.
The only hall of fame that really matters
LOTME loves the holiday season – the food, the gifts, the radio stations that play nothing but Christmas music – but for us the most wonderful time of the year comes just a bit later. No, it’s not our annual Golden Globes slap bet. Nope, not even the “excitement” of the College Football Playoff National Championship. It’s time for the National Inventors Hall of Fame to announce its latest inductees, and we could hardly sleep last night after putting cookies out for Thomas Edison. Fasten your seatbelts!
- Robert G. Bryant is a NASA chemist who developed Langley Research Center-Soluble Imide (yes, that’s the actual name) a polymer used as an insulation material for leads in implantable cardiac resynchronization therapy devices.
- Rory Cooper is a biomedical engineer who was paralyzed in a bicycle accident. His work has improved manual and electric wheelchairs and advanced the health, mobility, and social inclusion of people with disabilities and older adults. He is also the first NIHF inductee named Rory.
- Katalin Karikó, a biochemist, and Drew Weissman, an immunologist, “discovered how to enable messenger ribonucleic acid (mRNA) to enter cells without triggering the body’s immune system,” NIHF said, and that laid the foundation for the mRNA COVID-19 vaccines developed by Pfizer-BioNTech and Moderna. That, of course, led to the antivax movement, which has provided so much LOTME fodder over the years.
- Angela Hartley Brodie was a biochemist who discovered and developed a class of drugs called aromatase inhibitors, which can stop the production of hormones that fuel cancer cell growth and are used to treat breast cancer in 500,000 women worldwide each year.
We can’t mention all of the inductees for 2023 (our editor made that very clear), but we would like to offer a special shout-out to brothers Cyril (the first Cyril in the NIHF, by the way) and Louis Keller, who invented the world’s first compact loader, which eventually became the Bobcat skid-steer loader. Not really medical, you’re probably thinking, but we’re sure that someone, somewhere, at some time, used one to build a hospital, landscape a hospital, or clean up after the demolition of a hospital.
What to do when patients don’t listen
The term “nonadherent” has gradually replaced “noncompliant” in the physician lexicon as a nod to the evolving doctor-patient relationship. Noncompliance implies that a patient isn’t following their doctor’s orders. Adherence, on the other hand, is a measure of how closely your patient’s behavior matches the recommendations you’ve made. It’s a subtle difference but an important distinction in approaching care.
“Noncompliance is inherently negative feedback to the patient, whereas there’s a reason for nonadherence, and it’s usually external,” said Sharon Rabinovitz, MD, president of the Georgia Academy of Family Physicians.
Why won’t patients listen?
The reasons behind a patient’s nonadherence are multifaceted, but they are often driven by social determinants of health, such as transportation, poor health literacy, finances, and lack of access to pharmacies.
Other times, patients don’t want to take medicine, don’t prioritize their health, or they find the dietary and lifestyle modifications doctors suggest too hard to make or they struggle at losing weight, eating more healthfully, or cutting back on alcohol, for instance.
“When you come down to it, the big hindrance of it all is cost and the ability for the patient to be able to afford some of the things that we think they should be able to do,” said Teresa Lovins, MD, a physician in private practice Columbus, Ind., and a member of the board of directors of the American Academy of Family Physicians.
Another common deterrent to treatment is undesired side effects that a patient may not want to mention.
“For example, a lot of patients who are taking antidepressants have sexual dysfunction associated with those medications,” said Dr. Rabinovitz. “If you don’t ask the right questions, you’re not going to be able to fully assess the experience the patient is having and a reason why they might not take it [the medication].”
Much nonadherence is intentional and is based on experience, belief systems, and knowledge. For example, the American Medical Association finds that patients may not understand why they need a certain treatment (and therefore dismiss it), or they may be overloaded with multiple medications, fear dependency on a drug, have a mistrust of pharmaceutical companies or the medical system as a whole, or have symptoms of depression that make taking healthy actions more difficult. In addition, patients may be unable to afford their medication, or their lack of symptoms may lead them to believe they don’t really need the prescription, as occurs with disorders such as hypertension or high cholesterol.
“In my training, we did something called Balint training, where we would get together as a group with attendings and discuss cases that were difficult from a biopsychosocial perspective and consider all the factors in the patient perspective, including family dynamics, social systems, and economic realities,” said Russell Blackwelder, MD, director of geriatric education and associate professor of family medicine at the Medical University of South Carolina, Charleston.
“That training was, for me, very helpful for opening up and being more empathetic and really examining the patient’s point of view and everything that impacts them.”
Dr. Lovins agreed that it’s crucial to establish a good rapport and build mutual trust.
“If you don’t know the patient, you have a harder time asking the right questions to get to the meat of why they’re not taking their medicine or what they’re not doing to help their health,” she said. “It takes a little bit of trust on both parts to get to that question that really gets to the heart of why they’re not doing what you’re asking them to do.”
How to encourage adherence
Although there may not be a one-size-fits-all approach for achieving general adherence or adherence to a medication regimen, some methods may increase success.
Kenneth Zweig, MD, an internist at Northern Virginia Family Practice Associates, Alexandria, said that convincing patients to make one small change that they can sustain can get the ball rolling.
“I had one patient who was very overweight and had high blood pressure, high cholesterol, back pain, insomnia, and depression, who was also drinking three to four beers a night,” Dr. Zweig said. “After a long discussion, I challenged him to stop all alcohol for 1 week. At the end of the week, he noticed that he slept better, lost some weight, had lower blood pressure, and had more energy. Once he saw the benefits of this one change, he was motivated to improve other aspects of his health as well. He improved his diet, started exercising, and lost over 50 pounds. He has persisted with these lifestyle changes ever since.”
A team-based approach may also increase treatment understanding and adherence. In one older study, patients who were assigned to team-based care, including care by pharmacists, were significantly more adherent to medication regimens. Patients were more comfortable asking questions and raising concerns when they felt their treatment plan was a collaboration between several providers and themselves.
Dr. Lovins said to always approach the patient with a positive. “Say, what can we do together to make this work? What are your questions about this medication? And try and focus on the positive things that you can change instead of leaving the patient with a negative feeling or that you’re angry with them or that you’re unhappy with their choices. Patients respond better when they are treated as part of the team.”
Fear of judgment can also be a barrier to honesty between patients and their doctors. Shame creates a reluctance to admit nonadherence. Dr. Lovins said in an interview that it’s the physician’s responsibility to create a blame-free space for patients to speak openly about their struggles with treatment and reasons for nonadherence.
When should you redirect care?
Ultimately, the goal is good care and treatment of disease. However, if you and your patient are at an impasse and progress is stalling or failing, it may be appropriate to encourage the patient to seek care elsewhere.
“Just like any relationship, some physician-patient relationships are just not a good fit,” said Dr. Blackwelder. And this may be the reason why the patient is nonadherent — something between the two of you doesn’t click.
While there are ethical considerations for this decision, most medical boards have guidelines on how to go about it, Dr. Blackwelder said in an interview. “In the state of South Carolina, we have to be available to provide urgent coverage for at least 30 days and notify the patient in writing that they need to find somebody else and to help them find somebody else if we can.”
Just as with care, a clear conversation is the best practice if you’re proposing a potential shift away from a physician-patient relationship. You might say: We’re not making the kind of progress I’d like to see, and I’m wondering if you think working with another doctor may help you.
“The most important thing is being very honest and transparent with the patient that you’re concerned you’re not making the appropriate strides forward,” said Dr. Rabinovitz. Then you can ask, ‘Am I the right doctor to help you reach your goals? And if not, how can I help you get to where you need to be?’ ”
A version of this article first appeared on Medscape.com.
The term “nonadherent” has gradually replaced “noncompliant” in the physician lexicon as a nod to the evolving doctor-patient relationship. Noncompliance implies that a patient isn’t following their doctor’s orders. Adherence, on the other hand, is a measure of how closely your patient’s behavior matches the recommendations you’ve made. It’s a subtle difference but an important distinction in approaching care.
“Noncompliance is inherently negative feedback to the patient, whereas there’s a reason for nonadherence, and it’s usually external,” said Sharon Rabinovitz, MD, president of the Georgia Academy of Family Physicians.
Why won’t patients listen?
The reasons behind a patient’s nonadherence are multifaceted, but they are often driven by social determinants of health, such as transportation, poor health literacy, finances, and lack of access to pharmacies.
Other times, patients don’t want to take medicine, don’t prioritize their health, or they find the dietary and lifestyle modifications doctors suggest too hard to make or they struggle at losing weight, eating more healthfully, or cutting back on alcohol, for instance.
“When you come down to it, the big hindrance of it all is cost and the ability for the patient to be able to afford some of the things that we think they should be able to do,” said Teresa Lovins, MD, a physician in private practice Columbus, Ind., and a member of the board of directors of the American Academy of Family Physicians.
Another common deterrent to treatment is undesired side effects that a patient may not want to mention.
“For example, a lot of patients who are taking antidepressants have sexual dysfunction associated with those medications,” said Dr. Rabinovitz. “If you don’t ask the right questions, you’re not going to be able to fully assess the experience the patient is having and a reason why they might not take it [the medication].”
Much nonadherence is intentional and is based on experience, belief systems, and knowledge. For example, the American Medical Association finds that patients may not understand why they need a certain treatment (and therefore dismiss it), or they may be overloaded with multiple medications, fear dependency on a drug, have a mistrust of pharmaceutical companies or the medical system as a whole, or have symptoms of depression that make taking healthy actions more difficult. In addition, patients may be unable to afford their medication, or their lack of symptoms may lead them to believe they don’t really need the prescription, as occurs with disorders such as hypertension or high cholesterol.
“In my training, we did something called Balint training, where we would get together as a group with attendings and discuss cases that were difficult from a biopsychosocial perspective and consider all the factors in the patient perspective, including family dynamics, social systems, and economic realities,” said Russell Blackwelder, MD, director of geriatric education and associate professor of family medicine at the Medical University of South Carolina, Charleston.
“That training was, for me, very helpful for opening up and being more empathetic and really examining the patient’s point of view and everything that impacts them.”
Dr. Lovins agreed that it’s crucial to establish a good rapport and build mutual trust.
“If you don’t know the patient, you have a harder time asking the right questions to get to the meat of why they’re not taking their medicine or what they’re not doing to help their health,” she said. “It takes a little bit of trust on both parts to get to that question that really gets to the heart of why they’re not doing what you’re asking them to do.”
How to encourage adherence
Although there may not be a one-size-fits-all approach for achieving general adherence or adherence to a medication regimen, some methods may increase success.
Kenneth Zweig, MD, an internist at Northern Virginia Family Practice Associates, Alexandria, said that convincing patients to make one small change that they can sustain can get the ball rolling.
“I had one patient who was very overweight and had high blood pressure, high cholesterol, back pain, insomnia, and depression, who was also drinking three to four beers a night,” Dr. Zweig said. “After a long discussion, I challenged him to stop all alcohol for 1 week. At the end of the week, he noticed that he slept better, lost some weight, had lower blood pressure, and had more energy. Once he saw the benefits of this one change, he was motivated to improve other aspects of his health as well. He improved his diet, started exercising, and lost over 50 pounds. He has persisted with these lifestyle changes ever since.”
A team-based approach may also increase treatment understanding and adherence. In one older study, patients who were assigned to team-based care, including care by pharmacists, were significantly more adherent to medication regimens. Patients were more comfortable asking questions and raising concerns when they felt their treatment plan was a collaboration between several providers and themselves.
Dr. Lovins said to always approach the patient with a positive. “Say, what can we do together to make this work? What are your questions about this medication? And try and focus on the positive things that you can change instead of leaving the patient with a negative feeling or that you’re angry with them or that you’re unhappy with their choices. Patients respond better when they are treated as part of the team.”
Fear of judgment can also be a barrier to honesty between patients and their doctors. Shame creates a reluctance to admit nonadherence. Dr. Lovins said in an interview that it’s the physician’s responsibility to create a blame-free space for patients to speak openly about their struggles with treatment and reasons for nonadherence.
When should you redirect care?
Ultimately, the goal is good care and treatment of disease. However, if you and your patient are at an impasse and progress is stalling or failing, it may be appropriate to encourage the patient to seek care elsewhere.
“Just like any relationship, some physician-patient relationships are just not a good fit,” said Dr. Blackwelder. And this may be the reason why the patient is nonadherent — something between the two of you doesn’t click.
While there are ethical considerations for this decision, most medical boards have guidelines on how to go about it, Dr. Blackwelder said in an interview. “In the state of South Carolina, we have to be available to provide urgent coverage for at least 30 days and notify the patient in writing that they need to find somebody else and to help them find somebody else if we can.”
Just as with care, a clear conversation is the best practice if you’re proposing a potential shift away from a physician-patient relationship. You might say: We’re not making the kind of progress I’d like to see, and I’m wondering if you think working with another doctor may help you.
“The most important thing is being very honest and transparent with the patient that you’re concerned you’re not making the appropriate strides forward,” said Dr. Rabinovitz. Then you can ask, ‘Am I the right doctor to help you reach your goals? And if not, how can I help you get to where you need to be?’ ”
A version of this article first appeared on Medscape.com.
The term “nonadherent” has gradually replaced “noncompliant” in the physician lexicon as a nod to the evolving doctor-patient relationship. Noncompliance implies that a patient isn’t following their doctor’s orders. Adherence, on the other hand, is a measure of how closely your patient’s behavior matches the recommendations you’ve made. It’s a subtle difference but an important distinction in approaching care.
“Noncompliance is inherently negative feedback to the patient, whereas there’s a reason for nonadherence, and it’s usually external,” said Sharon Rabinovitz, MD, president of the Georgia Academy of Family Physicians.
Why won’t patients listen?
The reasons behind a patient’s nonadherence are multifaceted, but they are often driven by social determinants of health, such as transportation, poor health literacy, finances, and lack of access to pharmacies.
Other times, patients don’t want to take medicine, don’t prioritize their health, or they find the dietary and lifestyle modifications doctors suggest too hard to make or they struggle at losing weight, eating more healthfully, or cutting back on alcohol, for instance.
“When you come down to it, the big hindrance of it all is cost and the ability for the patient to be able to afford some of the things that we think they should be able to do,” said Teresa Lovins, MD, a physician in private practice Columbus, Ind., and a member of the board of directors of the American Academy of Family Physicians.
Another common deterrent to treatment is undesired side effects that a patient may not want to mention.
“For example, a lot of patients who are taking antidepressants have sexual dysfunction associated with those medications,” said Dr. Rabinovitz. “If you don’t ask the right questions, you’re not going to be able to fully assess the experience the patient is having and a reason why they might not take it [the medication].”
Much nonadherence is intentional and is based on experience, belief systems, and knowledge. For example, the American Medical Association finds that patients may not understand why they need a certain treatment (and therefore dismiss it), or they may be overloaded with multiple medications, fear dependency on a drug, have a mistrust of pharmaceutical companies or the medical system as a whole, or have symptoms of depression that make taking healthy actions more difficult. In addition, patients may be unable to afford their medication, or their lack of symptoms may lead them to believe they don’t really need the prescription, as occurs with disorders such as hypertension or high cholesterol.
“In my training, we did something called Balint training, where we would get together as a group with attendings and discuss cases that were difficult from a biopsychosocial perspective and consider all the factors in the patient perspective, including family dynamics, social systems, and economic realities,” said Russell Blackwelder, MD, director of geriatric education and associate professor of family medicine at the Medical University of South Carolina, Charleston.
“That training was, for me, very helpful for opening up and being more empathetic and really examining the patient’s point of view and everything that impacts them.”
Dr. Lovins agreed that it’s crucial to establish a good rapport and build mutual trust.
“If you don’t know the patient, you have a harder time asking the right questions to get to the meat of why they’re not taking their medicine or what they’re not doing to help their health,” she said. “It takes a little bit of trust on both parts to get to that question that really gets to the heart of why they’re not doing what you’re asking them to do.”
How to encourage adherence
Although there may not be a one-size-fits-all approach for achieving general adherence or adherence to a medication regimen, some methods may increase success.
Kenneth Zweig, MD, an internist at Northern Virginia Family Practice Associates, Alexandria, said that convincing patients to make one small change that they can sustain can get the ball rolling.
“I had one patient who was very overweight and had high blood pressure, high cholesterol, back pain, insomnia, and depression, who was also drinking three to four beers a night,” Dr. Zweig said. “After a long discussion, I challenged him to stop all alcohol for 1 week. At the end of the week, he noticed that he slept better, lost some weight, had lower blood pressure, and had more energy. Once he saw the benefits of this one change, he was motivated to improve other aspects of his health as well. He improved his diet, started exercising, and lost over 50 pounds. He has persisted with these lifestyle changes ever since.”
A team-based approach may also increase treatment understanding and adherence. In one older study, patients who were assigned to team-based care, including care by pharmacists, were significantly more adherent to medication regimens. Patients were more comfortable asking questions and raising concerns when they felt their treatment plan was a collaboration between several providers and themselves.
Dr. Lovins said to always approach the patient with a positive. “Say, what can we do together to make this work? What are your questions about this medication? And try and focus on the positive things that you can change instead of leaving the patient with a negative feeling or that you’re angry with them or that you’re unhappy with their choices. Patients respond better when they are treated as part of the team.”
Fear of judgment can also be a barrier to honesty between patients and their doctors. Shame creates a reluctance to admit nonadherence. Dr. Lovins said in an interview that it’s the physician’s responsibility to create a blame-free space for patients to speak openly about their struggles with treatment and reasons for nonadherence.
When should you redirect care?
Ultimately, the goal is good care and treatment of disease. However, if you and your patient are at an impasse and progress is stalling or failing, it may be appropriate to encourage the patient to seek care elsewhere.
“Just like any relationship, some physician-patient relationships are just not a good fit,” said Dr. Blackwelder. And this may be the reason why the patient is nonadherent — something between the two of you doesn’t click.
While there are ethical considerations for this decision, most medical boards have guidelines on how to go about it, Dr. Blackwelder said in an interview. “In the state of South Carolina, we have to be available to provide urgent coverage for at least 30 days and notify the patient in writing that they need to find somebody else and to help them find somebody else if we can.”
Just as with care, a clear conversation is the best practice if you’re proposing a potential shift away from a physician-patient relationship. You might say: We’re not making the kind of progress I’d like to see, and I’m wondering if you think working with another doctor may help you.
“The most important thing is being very honest and transparent with the patient that you’re concerned you’re not making the appropriate strides forward,” said Dr. Rabinovitz. Then you can ask, ‘Am I the right doctor to help you reach your goals? And if not, how can I help you get to where you need to be?’ ”
A version of this article first appeared on Medscape.com.
Age competency exams for physicians – yes or no?
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Joining me today is Sandeep Jauhar, a practicing cardiologist and professor of medicine at Northwell Health, a frequent New York Times op-ed contributor, and highly regarded author of the upcoming book “My Father’s Brain: Life in the Shadow of Alzheimer’s.”
Sandeep Jauhar, MD: Thanks for having me.
Dr. Glatter: Your recent op-ed piece in the New York Times caught my eye. In your piece, you refer to a 2020 survey in which almost one-third of licensed doctors in the United States were 60 years of age or older, up from a quarter in 2010. You also state that, due to a 20% prevalence of mild cognitive impairment in persons older than 65, practicing physicians above this age should probably be screened by a battery of tests to ensure that their reasoning and cognitive abilities are intact. The title of the article is “How Would You Feel About a 100-Year-Old Doctor?”
How would you envision such a process? What aspects of day-to-day functioning would the exams truly be evaluating?
Dr. Jauhar: A significant number of people over 65 have measurable cognitive impairment. By cognitive impairment, we’re not talking about dementia. The best estimates are that 1 in 10 people over age 65 have dementia, and roughly 1 in 5 have what’s called MCI, or mild cognitive impairment, which is cognitive impairment out of proportion to what you’d expect from normal aging. It’s a significant issue.
The argument that I made in the op-ed is that neurocognitive assessment is important. That’s not to say that everyone over age 65 has significant cognitive impairment or that older doctors can’t practice medicine safely and effectively. They absolutely can. The question is, do we leave neurocognitive assessment to physicians who may possibly be suffering from impairment?
In dementia, people very often have impaired self-awareness, a condition called anosognosia, which is a neurological term for not being aware of your own impairment because of your impairment.
I would argue that, instead of having voluntary neurocognitive screening, it should be mandated. The question is how to do that effectively, fairly, and transparently.
One could argue a gerontocracy in medicine today, where there are so many older physicians. What do we do about that? That really is something that I think needs to be debated.
Dr. Glatter: The question I have is, if we (that is, physicians and the health care profession) don’t take care of this, someone’s going to do it for us. We need to jump on this now while we have the opportunity. The AMA has been opposed to this, except when you have reason to suspect cognitive decline or are concerned about patient safety. A mandatory age of retirement is certainly something they’re not for, and we know this.
Your argument in your op-ed piece is very well thought out, and you lay the groundwork for testing (looking at someone’s memory, coordination, processing speed, and other executive functions). Certainly, for a psychiatrist, hearing is important, and for a dermatologist, vision is important. For a surgeon, there are other issues. Based on the specialty, we must be careful to see the important aspects of functioning. I am sure you would agree with this.
Dr. Jauhar: Obviously, the hand skills that are important for ophthalmological surgery certainly aren’t required for office-based psychological counseling, for example. We have to be smart about how we assess impairment.
You describe the spectrum of actions. On the one hand, there’s mandatory retirement at the age of 65 or 70 years. We know that commercial pilots are mandated to essentially retire at 65, and air-traffic controllers must retire in their late 50s.
We know that there’s a large amount of variability in competence. There are internists in their 80s with whom I’ve worked, and I’m absolutely wowed by their experience and judgment. There are new medical resident graduates who don’t really seem to have the requisite level of competence that would make me feel comfortable to have them as my doctor or a doctor for a member of my family.
To mandate retirement, I think the AMA is absolutely right. To not call for any kind of competency testing, to me, seems equally unwise. Because at the end of the day, you have to balance individual physician needs or wants to continue practicing with patient safety. I haven’t really come across too many physicians who say, “There’s absolutely no need for a competency testing.”
We have to meet somewhere in the middle. The middle is either voluntary cognitive competency testing or mandatory. I would argue that, because we know that as the brain changes we have cognitive impairment, but we’re not always aware that we need help, mandatory testing is the way.
One other thing that you mentioned was about having the solution imposed on us. You and I are doctors. We deal with bureaucracy. We deal with poorly thought-out solutions to issues in health care that make our lives that much more difficult. I don’t want that solution imposed on us by some outside agency. I think we need to figure this out within medicine and figure out the right way of doing it.
The AMA is on board with this. They haven’t called for mandatory testing, but they have said that if testing were to occur, these are the guidelines. The guidelines are fair and equitable, not too time-consuming, transparent, and not punitive. If someone comes out and doesn’t test well, we shouldn’t force them out of the profession. We can find ways to use their experience to help train younger doctors, for example.
Dr. Glatter: I wanted to segue to an area where there has been some challenge to the legality of these mandatory types of age restrictions and imposing the exams as well. There’s been a lawsuit as well by the EEOC [Equal Employment Opportunity Commission], on behalf of Yale. Basically, there’s been a concern that ageism is part of what’s going on. Yale now screens their providers beginning at age 70, and they have a program. UCSD [University of California, San Diego] has a program in place. Obviously, these institutions are looking at it. This is a very small part of the overall picture.
Health care systems overall, we’re talking about a fraction of them in the country are really addressing the issue of competency exams. The question is, where do we go from here? How do we get engagement or adoption and get physicians as a whole to embrace this concept?
Dr. Jauhar: The EEOC filed a lawsuit on behalf of the Yale medical staff that argued that Yale’s plan to do vision testing and neurocognitive screening – there may be a physical exam also – constitutes age discrimination because it’s reserved for doctors over the age of 70. Those are the physicians who are most likely to have cognitive impairment.
We have rules already for impaired physicians who are, for example, addicted to illicit drugs or have alcohol abuse. We already have some of those measures in place. This is focused on cognitive impairment in aging physicians because cognitive impairment is an issue that arises with aging. We have to be clear about that.
Most younger physicians will not have measurable cognitive impairment that would impair their ability to practice. To force young physicians (for example, physicians in their forties) to undergo such screening, all in the name of preventing age discrimination, doesn’t strike me as being a good use of resources. They’re more likely to be false positives, as you know from Bayesian statistics. When you have low pretest probability, you’re more likely to get false positives.
How are we going to screen hundreds of thousands of physicians? We have to make a choice about the group that really is more likely to benefit from such screening. Very few hospitals are addressing this issue and it’s going to become more important.
Dr. Glatter: Surgeons have been particularly active in pushing for age-based screening. In 2016, the American College of Surgeons started making surgeons at age 65-70 undergo voluntary health and neurocognitive assessments, and encouraged physicians to disclose any concerning findings as part of their professional obligation, which is pretty impressive in my mind.
Surgeons’ skill set is quite demanding physically and technically. That the Society of Surgical Chairs took it upon themselves to institute this is pretty telling.
Dr. Jauhar: The overall society called for screening, but then in a separate survey of surgical chairs, the idea was advanced that we should have mandatory retirement. Now, I don’t particularly agree with that.
I’ve seen it, where you have the aging surgeon who was a star in their day, and no one wants to say anything when their skills have visibly degraded, and no one wants to carry that torch and tell them that they need to retire. What happens is people whisper, and unfortunately, bad outcomes have to occur before people tend to get involved, and that’s what I’m trying to prevent.
Dr. Glatter: The question is whether older physicians have worse patient outcomes. The evidence is inconclusive, but studies have shown higher mortality rates for cardiovascular surgeons in terms of the procedures that they do. On the flip side, there are also higher mortality rates for GI surgery performed by younger surgeons. It’s a mixed bag.
Dr. Jauhar: For specialized surgery, you need the accrual of a certain amount of experience. The optimal age is about 60, because they’ve seen many things and they’ve seen complications. They don’t have a hand tremor yet so they’re still functioning well, and they’ve accrued a lot of experience. We have to be smart about who we screen.
There’s a learning curve in surgery. By no means am I arguing that younger surgeons are better surgeons. I would say that there’s probably a tipping point where once you get past a certain age and physical deterioration starts to take effect, that can overshadow the accrual of cognitive and surgical experience. We have to balance those things.
I would say neurocognitive screening and vision testing are important, but exactly what do you measure? How much of a hand tremor would constitute a risk? These things have to be figured out. I just want doctors to be leading the charge here and not have this imposed by bureaucrats.
Dr. Glatter: I was reading that some doctors have had these exams administered and they can really pass cognitive aspects of the exam, but there have been nuances in the actual practicing of medicine, day-to-day functioning, which they’re not good at.
Someone made a comment that the only way to know if a doctor can do well in practice is to observe their practice and observe them taking care of patients. In other words, you can game the system and pass the cognitive exam in some form but then have a problem practicing medicine.
Dr. Jauhar: Ultimately, outcomes have to be measured. We can’t adopt such a granular approach for every aging physician. There has to be some sort of screening that maybe raises a red flag and then hospitals and department chairs need to investigate further. What are the outcomes? What are people saying in the operating room? I think the screening is just that; it’s a way of opening the door to further investigation, but it’s not a witch hunt.
I have the highest respect for older physicians, and I learn from them every day, honestly, especially in my field (cardiology), because some of the older physicians can hear and see things on physical exam that I didn’t even know existed. There’s much to be learned from them.
This is not intended to be a witch hunt or to try to get rid of older physicians – by any means. We want to avoid some of the outcomes that I read about in the New York Times comments section. It’s not fair to our patients not to do at least some sort of screening to prevent those kinds of mistakes.
Dr. Glatter: I wanted to go back to data from Yale between October 2016 and January 2019, where 141 Yale clinicians who ranged in age from 69 to 92 years completed cognitive assessments. Of those, 18 clinicians, or about 13% of those tested, demonstrated cognitive deficits that were “deemed likely to impair their ability to practice medicine independently.” That’s telling. These are subtleties, but they’re important to identify. I would love to get your comment on that.
Dr. Jauhar: It’s in keeping with what we know about the proportion of our older citizens who have cognitive impairment. About 10% have dementia and about 20% have at least mild cognitive impairment. That’s in keeping with what we know, and this was a general screening.
There are certain programs, like in San Diego, for example, where physicians are referred, and so there’s a selection bias. But this was just general screening. It’s worrisome. I’m an aging physician myself. I want fairness in this process because I’m going to be assessed as well.
I just don’t really understand yet why there’s so much circling of the wagons and so much resistance. It seems like it would be good for physicians also to be removed from situations where they might get into potential litigation because of mistakes and physical or visual impairment. It seems like it’d be good for patients and physicians alike.
Dr. Glatter: It’s difficult to give up your profession, change fields, or become administrative at some point, and [decide] when to make that transition. As we all get older, we’re not going to have the ability to do what we did in our 20s, 30s, and so forth.
Dr. Jauhar: Much of the resistance is coming from doctors who are used to high levels of autonomy. I’m certainly sympathetic to that because I don’t want anyone telling me how to practice. The reason this is coming up and hasn’t come up in the past is not because of loss of autonomy but because of an actual demographic change. Many physicians were trained in the 1960s, ’70s, or ’80s. They’re getting to retirement age but they’re not retiring, and we can speculate as to why that is.
In America’s educational system, doctors incur a huge amount of debt. I know physicians who are still paying off their debt and they’re in their 50s and 60s, so I’m very sympathetic to that. I’m not trying to force doctors out of practicing. I just want whoever is practicing to be competent and to practice safely. We have to figure out how to do that.
Dr. Glatter: The fact that there is a shortage of physicians forecast in the next 10-15 years makes many physicians reluctant to retire. They feel like they want to be part of that support network and we don’t want to have a dire situation, especially in the rural areas. We’re not immune from aging. We’re human beings. We all have to realize that.
Dr. Jauhar: I know that the ACC is starting to debate this issue, in part because of my op-ed. My hope is that it will start a conversation and we will institute a plan that comes from physicians and serves our patients, and doesn’t serve some cottage industry of testing or serve the needs of insurers or bureaucrats. It has to serve the doctor-patient relationship.
Dr. Glatter: In some random surveys that I’ve read, up to 30%-40% of physicians do support some type of age-based screening or competency assessment. The needle’s moving. It’s just not there yet. I think that wider adoption is coming.
Dr. Jauhar: Data are coming as more hospitals start to adopt these late practitioner programs. Some of the data that came out of Yale, for example, are very important. We’re going to see more published data in this area, and it will clarify what we need to do and how big the problem is.
Dr. Glatter: I want to thank you again for your time and for writing the op-ed because it certainly was well read and opened the eyes of not only physicians, but also the public at large. It’s a conversation that has to be had. Thank you for doing this.
Dr. Jauhar: Thanks for inviting me, Robert. It was a pleasure to talk to you.
Dr. Glatter is assistant professor of emergency medicine, department of emergency medicine, at Hofstra University, Hempstead, N.Y. Dr. Jauhar is director of the heart failure program, Long Island Jewish Medical Center, New Hyde Park, N.Y. Neither Dr. Glatter nor Dr. Jauhar reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Spikes out: A COVID mystery
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
New Omicron subvariant is ‘crazy infectious,’ COVID expert warns
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
Autopsies show COVID virus invades entire body
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
FROM NATURE
New study offers details on post-COVID pediatric illness
Multisystem inflammatory syndrome in children (MIS-C) is more common than previously thought. This pediatric illness occurs 2-6 weeks after being infected with COVID-19.
a new study found. The illness is rare, but it causes dangerous multiorgan dysfunction and frequently requires a stay in the ICU. According to the Centers for Disease Control and Prevention, there have been at least 9,333 cases nationwide and 76 deaths from MIS-C.
Researchers said their findings were in such contrast to previous MIS-C research that it may render the old research “misleading.”
The analysis was powered by improved data extracted from hospital billing systems. Previous analyses of MIS-C were limited to voluntarily reported cases, which is likely the reason for the undercount.
The study reported a mortality rate for people with the most severe cases (affecting six to eight organs) of 5.8%. The authors of a companion editorial to the study said the mortality rate was low when considering the widespread impacts, “reflecting the rapid reversibility of MIS-C” with treatment.
Differences in MIS-C cases were also found based on children’s race and ethnicity. Black patients were more likely to have severe cases affecting more organs, compared to white patients.
The study included 4,107 MIS-C cases, using data from 2021 for patients younger than 21 years old. The median age was 9 years old.
The findings provide direction for further research, the editorial writers suggested.
Questions that need to be answered include asking why Black children with MIS-C are more likely to have a higher number of organ systems affected.
“Identifying patient biological or socioeconomic factors that can be targeted for treatment or prevention should be pursued,” they wrote.
The CDC says symptoms of MIS-C are an ongoing fever plus more than one of the following: stomach pain, bloodshot eyes, diarrhea, dizziness or lightheadedness (signs of low blood pressure), skin rash, or vomiting.
A version of this article first appeared on WebMD.com.
Multisystem inflammatory syndrome in children (MIS-C) is more common than previously thought. This pediatric illness occurs 2-6 weeks after being infected with COVID-19.
a new study found. The illness is rare, but it causes dangerous multiorgan dysfunction and frequently requires a stay in the ICU. According to the Centers for Disease Control and Prevention, there have been at least 9,333 cases nationwide and 76 deaths from MIS-C.
Researchers said their findings were in such contrast to previous MIS-C research that it may render the old research “misleading.”
The analysis was powered by improved data extracted from hospital billing systems. Previous analyses of MIS-C were limited to voluntarily reported cases, which is likely the reason for the undercount.
The study reported a mortality rate for people with the most severe cases (affecting six to eight organs) of 5.8%. The authors of a companion editorial to the study said the mortality rate was low when considering the widespread impacts, “reflecting the rapid reversibility of MIS-C” with treatment.
Differences in MIS-C cases were also found based on children’s race and ethnicity. Black patients were more likely to have severe cases affecting more organs, compared to white patients.
The study included 4,107 MIS-C cases, using data from 2021 for patients younger than 21 years old. The median age was 9 years old.
The findings provide direction for further research, the editorial writers suggested.
Questions that need to be answered include asking why Black children with MIS-C are more likely to have a higher number of organ systems affected.
“Identifying patient biological or socioeconomic factors that can be targeted for treatment or prevention should be pursued,” they wrote.
The CDC says symptoms of MIS-C are an ongoing fever plus more than one of the following: stomach pain, bloodshot eyes, diarrhea, dizziness or lightheadedness (signs of low blood pressure), skin rash, or vomiting.
A version of this article first appeared on WebMD.com.
Multisystem inflammatory syndrome in children (MIS-C) is more common than previously thought. This pediatric illness occurs 2-6 weeks after being infected with COVID-19.
a new study found. The illness is rare, but it causes dangerous multiorgan dysfunction and frequently requires a stay in the ICU. According to the Centers for Disease Control and Prevention, there have been at least 9,333 cases nationwide and 76 deaths from MIS-C.
Researchers said their findings were in such contrast to previous MIS-C research that it may render the old research “misleading.”
The analysis was powered by improved data extracted from hospital billing systems. Previous analyses of MIS-C were limited to voluntarily reported cases, which is likely the reason for the undercount.
The study reported a mortality rate for people with the most severe cases (affecting six to eight organs) of 5.8%. The authors of a companion editorial to the study said the mortality rate was low when considering the widespread impacts, “reflecting the rapid reversibility of MIS-C” with treatment.
Differences in MIS-C cases were also found based on children’s race and ethnicity. Black patients were more likely to have severe cases affecting more organs, compared to white patients.
The study included 4,107 MIS-C cases, using data from 2021 for patients younger than 21 years old. The median age was 9 years old.
The findings provide direction for further research, the editorial writers suggested.
Questions that need to be answered include asking why Black children with MIS-C are more likely to have a higher number of organ systems affected.
“Identifying patient biological or socioeconomic factors that can be targeted for treatment or prevention should be pursued,” they wrote.
The CDC says symptoms of MIS-C are an ongoing fever plus more than one of the following: stomach pain, bloodshot eyes, diarrhea, dizziness or lightheadedness (signs of low blood pressure), skin rash, or vomiting.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN