User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Is it time to stop treating high triglycerides?
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.
Sweaty treatment for social anxiety could pass the sniff test
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
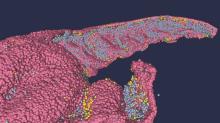
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Getting sweet on sweat
Are you the sort of person who struggles in social situations? Have the past 3 years been a secret respite from the terror and exhaustion of meeting new people? We understand your plight. People kind of suck. And you don’t have to look far to be reminded of it.
Unfortunately, on occasion we all have to interact with other human beings. If you suffer from social anxiety, this is not a fun thing to do. But new research indicates that there may be a way to alleviate the stress for those with social anxiety: armpits.
Specifically, sweat from the armpits of other people. Yes, this means a group of scientists gathered up some volunteers and collected their armpit sweat while the volunteers watched a variety of movies (horror, comedy, romance, etc.). Our condolences to the poor unpaid interns tasked with gathering the sweat.
Once they had their precious new medicine, the researchers took a group of women and administered a round of mindfulness therapy. Some of the participants then received the various sweats, while the rest were forced to smell only clean air. (The horror!) Lo and behold, the sweat groups had their anxiety scores reduced by about 40% after their therapy, compared with just 17% in the control group.
The researchers also found that the source of the sweat didn’t matter. Their study subjects responded the same to sweat excreted during a scary movie as they did to sweat from a comedy, a result that surprised the researchers. They suggested chemosignals in the sweat may affect the treatment response and advised further research. Which means more sweat collection! They plan on testing emotionally neutral movies next time, and if we can make a humble suggestion, they also should try the sweatiest movies.
Before the Food and Drug Administration can approve armpit sweat as a treatment for social anxiety, we have some advice for those shut-in introverts out there. Next time you have to interact with rabid extroverts, instead of shaking their hands, walk up to them and take a deep whiff of their armpits. Establish dominance. Someone will feel awkward, and science has proved it won’t be you.
The puff that vaccinates
Ever been shot with a Nerf gun or hit with a foam pool tube? More annoying than painful, right? If we asked if you’d rather get pelted with one of those than receive a traditional vaccine injection, you would choose the former. Maybe someday you actually will.
During the boredom of the early pandemic lockdown, Jeremiah Gassensmith, PhD, of the department of chemistry and biochemistry at the University of Texas, Dallas, ordered a compressed gas–powered jet injection system to fool around with at home. Hey, who didn’t? Anyway, when it was time to go back to the lab he handed it over to one of his grad students, Yalini Wijesundara, and asked her to see what could be done with it.
In her tinkering she found that the jet injector could deliver metal-organic frameworks (MOFs) that can hold a bunch of different materials, like proteins and nucleic acids, through the skin.
Thus the “MOF-Jet” was born!
Jet injectors are nothing new, but they hurt. The MOF-Jet, however, is practically painless and cheaper than the gene guns that veterinarians use to inject biological cargo attached to the surface of a metal microparticle.
Changing the carrier gas also changes the time needed to break down the MOF and thus alters delivery of the drug inside. “If you shoot it with carbon dioxide, it will release its cargo faster within cells; if you use regular air, it will take 4 or 5 days,” Ms. Wijesundara explained in a written statement. That means the same drug could be released over different timescales without changing its formulation.
While testing on onion cells and mice, Ms. Wijesundara noted that it was as easy as “pointing and shooting” to distribute the puff of gas into the cells. A saving grace to those with needle anxiety. Not that we would know anything about needle anxiety.
More testing needs to be done before bringing this technology to human use, obviously, but we’re looking forward to saying goodbye to that dreaded prick and hello to a puff.
Your hippocampus is showing
Brain anatomy is one of the many, many things that’s not really our thing, but we do know a cool picture when we see one. Case in point: The image just below, which happens to be a full-scale, single-cell resolution model of the CA1 region of the hippocampus that “replicates the structure and architecture of the area, along with the position and relative connectivity of the neurons,” according to a statement from the Human Brain Project.
“We have performed a data mining operation on high resolution images of the human hippocampus, obtained from the BigBrain database. The position of individual neurons has been derived from a detailed analysis of these images,” said senior author Michele Migliore, PhD, of the Italian National Research Council’s Institute of Biophysics in Palermo.
Yes, he did say BigBrain database. BigBrain is – we checked and it’s definitely not this – a 3D model of a brain that was sectioned into 7,404 slices just 20 micrometers thick and then scanned by MRI. Digital reconstruction of those slices was done by supercomputer and the results are now available for analysis.
Dr. Migliore and his associates developed an image-processing algorithm to obtain neuronal positioning distribution and an algorithm to generate neuronal connectivity by approximating the shapes of dendrites and axons. (Our brains are starting to hurt just trying to write this.) “Some fit into narrow cones, others have a broad complex extension that can be approximated by dedicated geometrical volumes, and the connectivity to nearby neurons changes accordingly,” explained lead author Daniela Gandolfi of the University of Modena (Italy) and Reggio Emilia.
The investigators have made their dataset and the extraction methodology available on the EBRAINS platform and through the Human Brain Project and are moving on to other brain regions. And then, once everyone can find their way in and around the old gray matter, it should bring an end to conversations like this, which no doubt occur between male and female neuroscientists every day:
“Arnold, I think we’re lost.”
“Don’t worry, Bev, I know where I’m going.”
“Stop and ask this lady for directions.”
“I said I can find it.”
“Just ask her.”
“Fine. Excuse me, ma’am, can you tell us how to get to the corpora quadrigemina from here?
Mucus plugging phenotype associated with adverse features
In a real-life clinic setting study aimed at determining phenotypic associations of mucus plugging
Rory Chan, MBChB, of the University of Dundee (Scotland) and colleagues found conversely that the presence of these features was associated with an increased likelihood of mucus plugging.
Important pathophysiological characteristics of persistent asthma include mucus plugging, goblet cell hyperplasia, smooth muscle hypertrophy, and eosinophilic infiltration. Mucus plugging contributes significantly to airway obstruction and death in acute asthma, the investigators stated, noting further that the understanding of mucus plugging’s role in chronic asthma is increasing.
Their retrospective cohort study included 126 patients with respiratory physician-diagnosed moderate to severe asthma who had attended their clinic (January 2016–March 2022) and were receiving daily doses of inhaled corticosteroid (ICS) (≥ 800 mcg) and a second-line controller. All had prior high-resolution CT (HRCT) scans with mucus plugs identified by an experienced thoracic radiologist. Prior to the start of biologic therapy, a mucus plug score (MPS) signifying the number of affected lung segments (0-20) was calculated subsequently and considered along with pulmonary function testing, T2 inflammatory markers, asthma control data, and measures of peripheral blood eosinophils (PBE), as well as total IgG and IgE antibodies to Apergillus fumigatus.
The analysis showed that reduced forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio (OR, 3.01; 95% confidence interval, 1.14-7.97), two or more exacerbations per year (OR, 5.00; 95% CI, 1.55-16.11), raised PBE (OR, 3.23; 95% CI, 1.16-8.96), raised total IgE (OR, 3.20; 95% CI, 1.09-9.37), and Aspergillus fumigatus IgE titers (OR, 9.37; 95% CI, 1.82-48.20) all conferred significantly higher likelihood of the presence of mucus plugging. Highest prevalence of mucus plugs was in the right and left lower lung lobes (about 26% vs. about 10% and 14% in the middle and upper lobes).
Adjusted ORs in patients with impaired FEV1/FVC, showed the likelihood of mucus plugging to be 67% higher. In those with frequent exacerbations, they were 80% higher, and in those with raised PBE and IgE, 69% higher. Patients without mucus plugging had preserved FEV1 and FEV1/FVC.
Asthma patients with mucus plugging in the study exhibited higher levels of routinely measured T2 biomarkers, including blood eosinophils, FeNO, and total IgE, with median values all exceeding traditionally accepted cut points. Although patients with mucus plugging were receiving significantly higher ICS doses, and despite the suppressive effect of ICS on FeNO, they still had higher FeNO levels. “We therefore postulate that asthma patients with the MP phenotype might potentially experience greater treatment response to biologics targeting the underlying inflammatory endotype,” the investigators stated, adding that “the presence of mucus plugging should be recognized as a treatable trait for patients with severe asthma in terms of targeting therapy with biologics.”
They wrote that, “in a real-life clinic setting, the presence of mucus plugging detected on HRCT was associated with more severe exacerbations, more severe airflow obstruction, and greater T2 inflammation. This, in turn, suggests that imaging should be part of the routine workup of patients with poorly controlled severe asthma.”
In an accompanying editorial, Jorge Cedano, MD, Jiwoong Choi, PhD, and Mario Castro, MD, MPH, of the University of Kansas, Kansas City, cited that the prevalence and contribution of mucus plugging in the pathophysiology and morbidity of uncontrolled asthma is much greater than has been appreciated. They focused particularly on the suggestion that, even after adjusting for confounders, molds such as Apergillus may play a causal role, along with blood eosinophils, fractional exhaled nitric oxide, and total IgE, in T2 inflammation.
While current biologic therapies targeting the T2 phenotype have not yet been shown to reverse the progressive loss of lung function or lung remodeling process, the editorialists referenced a recent post hoc analysis of the CASCADE study showing mucus plugging reduction with the biologic tezepelumab versus placebo correlated with lung function improvement. “At least 20% of patients with moderate to severe asthma will experience progressive decline in lung function, more exacerbations, and worse asthma control despite the use of controller therapies. If physicians could identify the MP phenotype using computed tomography, then potentially earlier treatment with biologic therapy may improve asthma control and prevent future decline in lung function,” they said.
The study limitations listed included its retrospective observational nature and the fact that the study had only one senior thoracic radiologist interpreting the lung scans.
The study authors cited numerous conflicts of interest with pharmaceutical companies and medical societies. Dr. Castro reported affiliation or involvement in multiple organizations or entities with a financial or nonfinancial interest in the subject matter or materials discussed in the article.
In a real-life clinic setting study aimed at determining phenotypic associations of mucus plugging
Rory Chan, MBChB, of the University of Dundee (Scotland) and colleagues found conversely that the presence of these features was associated with an increased likelihood of mucus plugging.
Important pathophysiological characteristics of persistent asthma include mucus plugging, goblet cell hyperplasia, smooth muscle hypertrophy, and eosinophilic infiltration. Mucus plugging contributes significantly to airway obstruction and death in acute asthma, the investigators stated, noting further that the understanding of mucus plugging’s role in chronic asthma is increasing.
Their retrospective cohort study included 126 patients with respiratory physician-diagnosed moderate to severe asthma who had attended their clinic (January 2016–March 2022) and were receiving daily doses of inhaled corticosteroid (ICS) (≥ 800 mcg) and a second-line controller. All had prior high-resolution CT (HRCT) scans with mucus plugs identified by an experienced thoracic radiologist. Prior to the start of biologic therapy, a mucus plug score (MPS) signifying the number of affected lung segments (0-20) was calculated subsequently and considered along with pulmonary function testing, T2 inflammatory markers, asthma control data, and measures of peripheral blood eosinophils (PBE), as well as total IgG and IgE antibodies to Apergillus fumigatus.
The analysis showed that reduced forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio (OR, 3.01; 95% confidence interval, 1.14-7.97), two or more exacerbations per year (OR, 5.00; 95% CI, 1.55-16.11), raised PBE (OR, 3.23; 95% CI, 1.16-8.96), raised total IgE (OR, 3.20; 95% CI, 1.09-9.37), and Aspergillus fumigatus IgE titers (OR, 9.37; 95% CI, 1.82-48.20) all conferred significantly higher likelihood of the presence of mucus plugging. Highest prevalence of mucus plugs was in the right and left lower lung lobes (about 26% vs. about 10% and 14% in the middle and upper lobes).
Adjusted ORs in patients with impaired FEV1/FVC, showed the likelihood of mucus plugging to be 67% higher. In those with frequent exacerbations, they were 80% higher, and in those with raised PBE and IgE, 69% higher. Patients without mucus plugging had preserved FEV1 and FEV1/FVC.
Asthma patients with mucus plugging in the study exhibited higher levels of routinely measured T2 biomarkers, including blood eosinophils, FeNO, and total IgE, with median values all exceeding traditionally accepted cut points. Although patients with mucus plugging were receiving significantly higher ICS doses, and despite the suppressive effect of ICS on FeNO, they still had higher FeNO levels. “We therefore postulate that asthma patients with the MP phenotype might potentially experience greater treatment response to biologics targeting the underlying inflammatory endotype,” the investigators stated, adding that “the presence of mucus plugging should be recognized as a treatable trait for patients with severe asthma in terms of targeting therapy with biologics.”
They wrote that, “in a real-life clinic setting, the presence of mucus plugging detected on HRCT was associated with more severe exacerbations, more severe airflow obstruction, and greater T2 inflammation. This, in turn, suggests that imaging should be part of the routine workup of patients with poorly controlled severe asthma.”
In an accompanying editorial, Jorge Cedano, MD, Jiwoong Choi, PhD, and Mario Castro, MD, MPH, of the University of Kansas, Kansas City, cited that the prevalence and contribution of mucus plugging in the pathophysiology and morbidity of uncontrolled asthma is much greater than has been appreciated. They focused particularly on the suggestion that, even after adjusting for confounders, molds such as Apergillus may play a causal role, along with blood eosinophils, fractional exhaled nitric oxide, and total IgE, in T2 inflammation.
While current biologic therapies targeting the T2 phenotype have not yet been shown to reverse the progressive loss of lung function or lung remodeling process, the editorialists referenced a recent post hoc analysis of the CASCADE study showing mucus plugging reduction with the biologic tezepelumab versus placebo correlated with lung function improvement. “At least 20% of patients with moderate to severe asthma will experience progressive decline in lung function, more exacerbations, and worse asthma control despite the use of controller therapies. If physicians could identify the MP phenotype using computed tomography, then potentially earlier treatment with biologic therapy may improve asthma control and prevent future decline in lung function,” they said.
The study limitations listed included its retrospective observational nature and the fact that the study had only one senior thoracic radiologist interpreting the lung scans.
The study authors cited numerous conflicts of interest with pharmaceutical companies and medical societies. Dr. Castro reported affiliation or involvement in multiple organizations or entities with a financial or nonfinancial interest in the subject matter or materials discussed in the article.
In a real-life clinic setting study aimed at determining phenotypic associations of mucus plugging
Rory Chan, MBChB, of the University of Dundee (Scotland) and colleagues found conversely that the presence of these features was associated with an increased likelihood of mucus plugging.
Important pathophysiological characteristics of persistent asthma include mucus plugging, goblet cell hyperplasia, smooth muscle hypertrophy, and eosinophilic infiltration. Mucus plugging contributes significantly to airway obstruction and death in acute asthma, the investigators stated, noting further that the understanding of mucus plugging’s role in chronic asthma is increasing.
Their retrospective cohort study included 126 patients with respiratory physician-diagnosed moderate to severe asthma who had attended their clinic (January 2016–March 2022) and were receiving daily doses of inhaled corticosteroid (ICS) (≥ 800 mcg) and a second-line controller. All had prior high-resolution CT (HRCT) scans with mucus plugs identified by an experienced thoracic radiologist. Prior to the start of biologic therapy, a mucus plug score (MPS) signifying the number of affected lung segments (0-20) was calculated subsequently and considered along with pulmonary function testing, T2 inflammatory markers, asthma control data, and measures of peripheral blood eosinophils (PBE), as well as total IgG and IgE antibodies to Apergillus fumigatus.
The analysis showed that reduced forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio (OR, 3.01; 95% confidence interval, 1.14-7.97), two or more exacerbations per year (OR, 5.00; 95% CI, 1.55-16.11), raised PBE (OR, 3.23; 95% CI, 1.16-8.96), raised total IgE (OR, 3.20; 95% CI, 1.09-9.37), and Aspergillus fumigatus IgE titers (OR, 9.37; 95% CI, 1.82-48.20) all conferred significantly higher likelihood of the presence of mucus plugging. Highest prevalence of mucus plugs was in the right and left lower lung lobes (about 26% vs. about 10% and 14% in the middle and upper lobes).
Adjusted ORs in patients with impaired FEV1/FVC, showed the likelihood of mucus plugging to be 67% higher. In those with frequent exacerbations, they were 80% higher, and in those with raised PBE and IgE, 69% higher. Patients without mucus plugging had preserved FEV1 and FEV1/FVC.
Asthma patients with mucus plugging in the study exhibited higher levels of routinely measured T2 biomarkers, including blood eosinophils, FeNO, and total IgE, with median values all exceeding traditionally accepted cut points. Although patients with mucus plugging were receiving significantly higher ICS doses, and despite the suppressive effect of ICS on FeNO, they still had higher FeNO levels. “We therefore postulate that asthma patients with the MP phenotype might potentially experience greater treatment response to biologics targeting the underlying inflammatory endotype,” the investigators stated, adding that “the presence of mucus plugging should be recognized as a treatable trait for patients with severe asthma in terms of targeting therapy with biologics.”
They wrote that, “in a real-life clinic setting, the presence of mucus plugging detected on HRCT was associated with more severe exacerbations, more severe airflow obstruction, and greater T2 inflammation. This, in turn, suggests that imaging should be part of the routine workup of patients with poorly controlled severe asthma.”
In an accompanying editorial, Jorge Cedano, MD, Jiwoong Choi, PhD, and Mario Castro, MD, MPH, of the University of Kansas, Kansas City, cited that the prevalence and contribution of mucus plugging in the pathophysiology and morbidity of uncontrolled asthma is much greater than has been appreciated. They focused particularly on the suggestion that, even after adjusting for confounders, molds such as Apergillus may play a causal role, along with blood eosinophils, fractional exhaled nitric oxide, and total IgE, in T2 inflammation.
While current biologic therapies targeting the T2 phenotype have not yet been shown to reverse the progressive loss of lung function or lung remodeling process, the editorialists referenced a recent post hoc analysis of the CASCADE study showing mucus plugging reduction with the biologic tezepelumab versus placebo correlated with lung function improvement. “At least 20% of patients with moderate to severe asthma will experience progressive decline in lung function, more exacerbations, and worse asthma control despite the use of controller therapies. If physicians could identify the MP phenotype using computed tomography, then potentially earlier treatment with biologic therapy may improve asthma control and prevent future decline in lung function,” they said.
The study limitations listed included its retrospective observational nature and the fact that the study had only one senior thoracic radiologist interpreting the lung scans.
The study authors cited numerous conflicts of interest with pharmaceutical companies and medical societies. Dr. Castro reported affiliation or involvement in multiple organizations or entities with a financial or nonfinancial interest in the subject matter or materials discussed in the article.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
COVID-19 potentially induced adult-onset IgA vasculitis
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM CUREUS
De Marco gift to CHEST makes more than one dream possible
As a member of CHEST leadership for years, Bob De Marco, MD, FCCP, ruminated over new, exciting ways to increase support of the philanthropic efforts of the American College of Chest Physicians.
Dr. De Marco knows all too well that the percentage of CHEST members who donate to support CHEST’s philanthropic initiatives is – in a word - underwhelming. For those who are involved, they do so greatly and with their whole selves, but Dr. De Marco believed more could be done.
In the months leading up to the CHEST Annual Meeting 2022 in Nashville, Dr. De Marco discussed fundraising with CHEST staff and was already thinking ahead to CHEST 2023 in Hawai’i.
“That’s when it hit me – we could leverage Hawai’i to get donations and to expose people to CHEST philanthropy,” said Dr. De Marco. “Hawai’i is a dream destination, and that might be the exact motivation it would take to get that first donation from someone.”
Having a good idea is one thing, but making sure it happens requires individual commitment. Dr. DeMarco personally pledged to cover the cost of first-class airfare for two to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu. For a minimum donation of $250 to CHEST between September and the end of 2022, each donor would be entered into a drawing for a chance to win this dream trip.
“I thought to myself, who wouldn’t want this prize?” said Dr. De Marco.
“You get to go to paradise for free – with a guest – and attend a top tier educational conference. Knowing your entry supported an organization as deserving as CHEST is the cherry on top,” he added.
In launching the Hawai’i trip fundraiser before and during CHEST 2022, attendees from around the world were introduced to CHEST’s philanthropic efforts and its mission to champion lung health. Over $180,000 was donated during this time period, in no small part because of the Hawai’i travel reward.
“I’m happy to say that the fundraiser did a lot better than I expected, and I was elated to see all of the new donors,” said Dr. De Marco.
“It’s my hope that those first-time donors continue their support for all that we do to provide grants – community, research, and diversity – and support CHEST initiatives that impact patient care and change lives.”
During CHEST 2022, Dr. De Marco and other donors reflected on the organization’s philanthropic accomplishments and impact over the past decades.
Former grant recipients were invited to celebrate with donors and speak to what they were able to accomplish because of the support they received.
The celebration also introduced new CHEST initiatives, the First 5 Minutes® program and Bridging Specialties™: Timely Diagnosis for ILD. The former improves patient care through strengthened patient/clinician relationships, and the latter aims to eliminate gaps in diagnosing complex lung diseases like pulmonary fibrosis.
To all who donated to CHEST in 2022, Dr. De Marco said, “A sincere thank you to each and every one of you for helping us fulfill our mission. To the first-time donors, hopefully this will inspire you and your friends to be an active part of the CHEST family.”
And, to the winner of the trip, Dr. De Marco said, “A sincere congratulations and I hope you enjoy beautiful Hawai’i and your time at the meeting.”
Those who are interested in getting involved and supporting the philanthropic work of CHEST can contact [email protected].
Out of the 150+ donors who gave $250 or more to CHEST between September 2022 and the end of 2022, longtime friend of CHEST, Noah Dorsky, was the recipient of two first-class tickets to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu.
Noah donated specifically to the Mark J. Rosen, MD, Master FCCP Endowment in honor of his late friend, Dr. Mark J. Rosen, who served as CHEST President from 2006 to 2007 and died in 2019.
"Mark was a remarkable doctor and valued life-long friend,” Noah said. “My continued support for CHEST is my way of honoring his memory and how much he meant to me and others."
Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. Before serving as President, Dr. Rosen served on the CHEST Board of Regents for many years. He held positions as Chair or member on numerous CHEST committees, including Education, Nominations, Membership, Marketing, and Finance.
Following his passing, Dr. Rosen’s wife, Ilene, stayed engaged with CHEST by creating the endowment in his name and attending the CHEST Annual Meeting every year to award the Rosen Cup to the winners of the annual CHEST Challenge.
Congratulations, Noah, and thank you for your faithful giving to support the work of CHEST.
As a member of CHEST leadership for years, Bob De Marco, MD, FCCP, ruminated over new, exciting ways to increase support of the philanthropic efforts of the American College of Chest Physicians.
Dr. De Marco knows all too well that the percentage of CHEST members who donate to support CHEST’s philanthropic initiatives is – in a word - underwhelming. For those who are involved, they do so greatly and with their whole selves, but Dr. De Marco believed more could be done.
In the months leading up to the CHEST Annual Meeting 2022 in Nashville, Dr. De Marco discussed fundraising with CHEST staff and was already thinking ahead to CHEST 2023 in Hawai’i.
“That’s when it hit me – we could leverage Hawai’i to get donations and to expose people to CHEST philanthropy,” said Dr. De Marco. “Hawai’i is a dream destination, and that might be the exact motivation it would take to get that first donation from someone.”
Having a good idea is one thing, but making sure it happens requires individual commitment. Dr. DeMarco personally pledged to cover the cost of first-class airfare for two to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu. For a minimum donation of $250 to CHEST between September and the end of 2022, each donor would be entered into a drawing for a chance to win this dream trip.
“I thought to myself, who wouldn’t want this prize?” said Dr. De Marco.
“You get to go to paradise for free – with a guest – and attend a top tier educational conference. Knowing your entry supported an organization as deserving as CHEST is the cherry on top,” he added.
In launching the Hawai’i trip fundraiser before and during CHEST 2022, attendees from around the world were introduced to CHEST’s philanthropic efforts and its mission to champion lung health. Over $180,000 was donated during this time period, in no small part because of the Hawai’i travel reward.
“I’m happy to say that the fundraiser did a lot better than I expected, and I was elated to see all of the new donors,” said Dr. De Marco.
“It’s my hope that those first-time donors continue their support for all that we do to provide grants – community, research, and diversity – and support CHEST initiatives that impact patient care and change lives.”
During CHEST 2022, Dr. De Marco and other donors reflected on the organization’s philanthropic accomplishments and impact over the past decades.
Former grant recipients were invited to celebrate with donors and speak to what they were able to accomplish because of the support they received.
The celebration also introduced new CHEST initiatives, the First 5 Minutes® program and Bridging Specialties™: Timely Diagnosis for ILD. The former improves patient care through strengthened patient/clinician relationships, and the latter aims to eliminate gaps in diagnosing complex lung diseases like pulmonary fibrosis.
To all who donated to CHEST in 2022, Dr. De Marco said, “A sincere thank you to each and every one of you for helping us fulfill our mission. To the first-time donors, hopefully this will inspire you and your friends to be an active part of the CHEST family.”
And, to the winner of the trip, Dr. De Marco said, “A sincere congratulations and I hope you enjoy beautiful Hawai’i and your time at the meeting.”
Those who are interested in getting involved and supporting the philanthropic work of CHEST can contact [email protected].
Out of the 150+ donors who gave $250 or more to CHEST between September 2022 and the end of 2022, longtime friend of CHEST, Noah Dorsky, was the recipient of two first-class tickets to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu.
Noah donated specifically to the Mark J. Rosen, MD, Master FCCP Endowment in honor of his late friend, Dr. Mark J. Rosen, who served as CHEST President from 2006 to 2007 and died in 2019.
"Mark was a remarkable doctor and valued life-long friend,” Noah said. “My continued support for CHEST is my way of honoring his memory and how much he meant to me and others."
Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. Before serving as President, Dr. Rosen served on the CHEST Board of Regents for many years. He held positions as Chair or member on numerous CHEST committees, including Education, Nominations, Membership, Marketing, and Finance.
Following his passing, Dr. Rosen’s wife, Ilene, stayed engaged with CHEST by creating the endowment in his name and attending the CHEST Annual Meeting every year to award the Rosen Cup to the winners of the annual CHEST Challenge.
Congratulations, Noah, and thank you for your faithful giving to support the work of CHEST.
As a member of CHEST leadership for years, Bob De Marco, MD, FCCP, ruminated over new, exciting ways to increase support of the philanthropic efforts of the American College of Chest Physicians.
Dr. De Marco knows all too well that the percentage of CHEST members who donate to support CHEST’s philanthropic initiatives is – in a word - underwhelming. For those who are involved, they do so greatly and with their whole selves, but Dr. De Marco believed more could be done.
In the months leading up to the CHEST Annual Meeting 2022 in Nashville, Dr. De Marco discussed fundraising with CHEST staff and was already thinking ahead to CHEST 2023 in Hawai’i.
“That’s when it hit me – we could leverage Hawai’i to get donations and to expose people to CHEST philanthropy,” said Dr. De Marco. “Hawai’i is a dream destination, and that might be the exact motivation it would take to get that first donation from someone.”
Having a good idea is one thing, but making sure it happens requires individual commitment. Dr. DeMarco personally pledged to cover the cost of first-class airfare for two to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu. For a minimum donation of $250 to CHEST between September and the end of 2022, each donor would be entered into a drawing for a chance to win this dream trip.
“I thought to myself, who wouldn’t want this prize?” said Dr. De Marco.
“You get to go to paradise for free – with a guest – and attend a top tier educational conference. Knowing your entry supported an organization as deserving as CHEST is the cherry on top,” he added.
In launching the Hawai’i trip fundraiser before and during CHEST 2022, attendees from around the world were introduced to CHEST’s philanthropic efforts and its mission to champion lung health. Over $180,000 was donated during this time period, in no small part because of the Hawai’i travel reward.
“I’m happy to say that the fundraiser did a lot better than I expected, and I was elated to see all of the new donors,” said Dr. De Marco.
“It’s my hope that those first-time donors continue their support for all that we do to provide grants – community, research, and diversity – and support CHEST initiatives that impact patient care and change lives.”
During CHEST 2022, Dr. De Marco and other donors reflected on the organization’s philanthropic accomplishments and impact over the past decades.
Former grant recipients were invited to celebrate with donors and speak to what they were able to accomplish because of the support they received.
The celebration also introduced new CHEST initiatives, the First 5 Minutes® program and Bridging Specialties™: Timely Diagnosis for ILD. The former improves patient care through strengthened patient/clinician relationships, and the latter aims to eliminate gaps in diagnosing complex lung diseases like pulmonary fibrosis.
To all who donated to CHEST in 2022, Dr. De Marco said, “A sincere thank you to each and every one of you for helping us fulfill our mission. To the first-time donors, hopefully this will inspire you and your friends to be an active part of the CHEST family.”
And, to the winner of the trip, Dr. De Marco said, “A sincere congratulations and I hope you enjoy beautiful Hawai’i and your time at the meeting.”
Those who are interested in getting involved and supporting the philanthropic work of CHEST can contact [email protected].
Out of the 150+ donors who gave $250 or more to CHEST between September 2022 and the end of 2022, longtime friend of CHEST, Noah Dorsky, was the recipient of two first-class tickets to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu.
Noah donated specifically to the Mark J. Rosen, MD, Master FCCP Endowment in honor of his late friend, Dr. Mark J. Rosen, who served as CHEST President from 2006 to 2007 and died in 2019.
"Mark was a remarkable doctor and valued life-long friend,” Noah said. “My continued support for CHEST is my way of honoring his memory and how much he meant to me and others."
Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. Before serving as President, Dr. Rosen served on the CHEST Board of Regents for many years. He held positions as Chair or member on numerous CHEST committees, including Education, Nominations, Membership, Marketing, and Finance.
Following his passing, Dr. Rosen’s wife, Ilene, stayed engaged with CHEST by creating the endowment in his name and attending the CHEST Annual Meeting every year to award the Rosen Cup to the winners of the annual CHEST Challenge.
Congratulations, Noah, and thank you for your faithful giving to support the work of CHEST.
Dupilumab moves forward as possible COPD treatment
of more than 900 adults with uncontrolled chronic obstructive pulmonary disease.
In the study, known as the BOREAS trial, dupilumab met its primary and secondary endpoints, with a significant reduction compared with placebo in exacerbations for adults with chronic obstructive pulmonary disease (COPD) that was uncontrolled despite use of the maximal standard-of-care inhaled therapy (triple therapy), according to a press release from manufacturers Regeneron and Sanofi.
Dupilumab, which inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways, is currently approved in multiple countries for certain patients with conditions including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, or prurigo nodularis in different age groups. The drug is not an immunosuppressant, and would be the first biologic approved for COPD, according to the manufacturers.
In the BOREAS trial, 468 adults with COPD who were current or former smokers aged 40-80 years were randomized to dupilumab and 471 to placebo; both groups continued to receive maximal standard of care.
Over 52 weeks, patients in the dupilumab group experienced a 30% reduction in moderate to severe COPD exacerbations compared with placebo (P = .0005).
In addition, patients treated with dupilumab met the key secondary endpoints of significant improvement in lung function from baseline to 12 weeks compared with placebo (160 mL vs. 77 mL, P < .0001); this difference persisted at 52 weeks (P = .0003).
Dupilumab also met endpoints for improvement in patient-reported health-related quality of life based on the St. George’s Respiratory Questionnaire (SGRQ) and reduction in the severity of respiratory symptoms of COPD based on the Evaluation Respiratory Symptoms: COPD (E-RS: COPD) Scale, according to the companies’ statement.
The results represent a previously unreported magnitude of improvement for COPD patients treated with a biologic, principal investigator George D. Yancopoulos, MD, said in the statement. “These results also validate the role type 2 inflammation plays in driving COPD in these patients, advancing the scientific community’s understanding of the underlying biology of this disease,” he added.
The safety results in the BOREAS trial were generally consistent with the known safety profile of Dupixent in its approved indications. Overall adverse event rates were similar for dupilumab and placebo patients (77% and 76%, respectively) and the overall safety profiles were consistent with the currently approved dupilumab indications, according to the manufacturers.
The adverse events that were more common in dupilumab patients compared with placebo patients were headache (8.1% vs. 6.8%), diarrhea (5.3% vs. 3.6%), and back pain (5.1% vs. 3.4%).
Adverse events leading to deaths were similar between the groups (1.7% in placebo patients and 1.5% in dupilumab patients).
Complete safety and efficacy results from the BOREAS trial are scheduled to be presented in a future scientific forum, and a second phase 3 trial of dupilumab for COPD, known as NOTUS, is ongoing, with data expected in 2024, according to the manufacturers.
The Boreas trial was sponsored by Sanofi and Regeneron Pharmaceuticals.
of more than 900 adults with uncontrolled chronic obstructive pulmonary disease.
In the study, known as the BOREAS trial, dupilumab met its primary and secondary endpoints, with a significant reduction compared with placebo in exacerbations for adults with chronic obstructive pulmonary disease (COPD) that was uncontrolled despite use of the maximal standard-of-care inhaled therapy (triple therapy), according to a press release from manufacturers Regeneron and Sanofi.
Dupilumab, which inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways, is currently approved in multiple countries for certain patients with conditions including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, or prurigo nodularis in different age groups. The drug is not an immunosuppressant, and would be the first biologic approved for COPD, according to the manufacturers.
In the BOREAS trial, 468 adults with COPD who were current or former smokers aged 40-80 years were randomized to dupilumab and 471 to placebo; both groups continued to receive maximal standard of care.
Over 52 weeks, patients in the dupilumab group experienced a 30% reduction in moderate to severe COPD exacerbations compared with placebo (P = .0005).
In addition, patients treated with dupilumab met the key secondary endpoints of significant improvement in lung function from baseline to 12 weeks compared with placebo (160 mL vs. 77 mL, P < .0001); this difference persisted at 52 weeks (P = .0003).
Dupilumab also met endpoints for improvement in patient-reported health-related quality of life based on the St. George’s Respiratory Questionnaire (SGRQ) and reduction in the severity of respiratory symptoms of COPD based on the Evaluation Respiratory Symptoms: COPD (E-RS: COPD) Scale, according to the companies’ statement.
The results represent a previously unreported magnitude of improvement for COPD patients treated with a biologic, principal investigator George D. Yancopoulos, MD, said in the statement. “These results also validate the role type 2 inflammation plays in driving COPD in these patients, advancing the scientific community’s understanding of the underlying biology of this disease,” he added.
The safety results in the BOREAS trial were generally consistent with the known safety profile of Dupixent in its approved indications. Overall adverse event rates were similar for dupilumab and placebo patients (77% and 76%, respectively) and the overall safety profiles were consistent with the currently approved dupilumab indications, according to the manufacturers.
The adverse events that were more common in dupilumab patients compared with placebo patients were headache (8.1% vs. 6.8%), diarrhea (5.3% vs. 3.6%), and back pain (5.1% vs. 3.4%).
Adverse events leading to deaths were similar between the groups (1.7% in placebo patients and 1.5% in dupilumab patients).
Complete safety and efficacy results from the BOREAS trial are scheduled to be presented in a future scientific forum, and a second phase 3 trial of dupilumab for COPD, known as NOTUS, is ongoing, with data expected in 2024, according to the manufacturers.
The Boreas trial was sponsored by Sanofi and Regeneron Pharmaceuticals.
of more than 900 adults with uncontrolled chronic obstructive pulmonary disease.
In the study, known as the BOREAS trial, dupilumab met its primary and secondary endpoints, with a significant reduction compared with placebo in exacerbations for adults with chronic obstructive pulmonary disease (COPD) that was uncontrolled despite use of the maximal standard-of-care inhaled therapy (triple therapy), according to a press release from manufacturers Regeneron and Sanofi.
Dupilumab, which inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways, is currently approved in multiple countries for certain patients with conditions including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, or prurigo nodularis in different age groups. The drug is not an immunosuppressant, and would be the first biologic approved for COPD, according to the manufacturers.
In the BOREAS trial, 468 adults with COPD who were current or former smokers aged 40-80 years were randomized to dupilumab and 471 to placebo; both groups continued to receive maximal standard of care.
Over 52 weeks, patients in the dupilumab group experienced a 30% reduction in moderate to severe COPD exacerbations compared with placebo (P = .0005).
In addition, patients treated with dupilumab met the key secondary endpoints of significant improvement in lung function from baseline to 12 weeks compared with placebo (160 mL vs. 77 mL, P < .0001); this difference persisted at 52 weeks (P = .0003).
Dupilumab also met endpoints for improvement in patient-reported health-related quality of life based on the St. George’s Respiratory Questionnaire (SGRQ) and reduction in the severity of respiratory symptoms of COPD based on the Evaluation Respiratory Symptoms: COPD (E-RS: COPD) Scale, according to the companies’ statement.
The results represent a previously unreported magnitude of improvement for COPD patients treated with a biologic, principal investigator George D. Yancopoulos, MD, said in the statement. “These results also validate the role type 2 inflammation plays in driving COPD in these patients, advancing the scientific community’s understanding of the underlying biology of this disease,” he added.
The safety results in the BOREAS trial were generally consistent with the known safety profile of Dupixent in its approved indications. Overall adverse event rates were similar for dupilumab and placebo patients (77% and 76%, respectively) and the overall safety profiles were consistent with the currently approved dupilumab indications, according to the manufacturers.
The adverse events that were more common in dupilumab patients compared with placebo patients were headache (8.1% vs. 6.8%), diarrhea (5.3% vs. 3.6%), and back pain (5.1% vs. 3.4%).
Adverse events leading to deaths were similar between the groups (1.7% in placebo patients and 1.5% in dupilumab patients).
Complete safety and efficacy results from the BOREAS trial are scheduled to be presented in a future scientific forum, and a second phase 3 trial of dupilumab for COPD, known as NOTUS, is ongoing, with data expected in 2024, according to the manufacturers.
The Boreas trial was sponsored by Sanofi and Regeneron Pharmaceuticals.
Luxe vacations, private jets: Medical device maker, surgeon to pay $46 million penalty in kickback scheme
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
Tofacitinib may have possible protective effect against ILD in RA
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) were 69% less likely to develop interstitial lung disease (ILD), compared with those treated with adalimumab (Humira), according to a new retrospective study.
About 10% of RA patients develop ILD, but data on how different biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) may affect the risk of developing ILD is lacking, the authors wrote. Identifying treatments that may have protective effects could be useful when prescribing treatments for patients with RA who are at higher risk for ILD, first author Matthew C. Baker, MD, clinical chief in the division of immunology and rheumatology at Stanford (Calif.) University, said in an interview.
In the analysis, published in JAMA Network Open researchers used the Optum Clinformatics Data Mart to identify claims data for patients with RA who were taking b/tsDMARDs from December 2003 to December 2019. Patients were excluded if they had a preexisting diagnosis of ILD or if they had less than 1 year of continuous enrollment in the data set.
The researchers identified 28,559 patients with RA who were treated with adalimumab (13,326), abatacept (Orencia; 5,676), rituximab (Rituxan; 5,444), tocilizumab (Actemra; 2,548), and tofacitinib (1,565). More than three-fourths of patients were female (78%), and their average age was 55.6 years old. During the study period, 276 developed ILD. An adjusted model showed a 69% lower incidence of ILD in patients treated with tofacitinib, compared with those treated with adalimumab (adjusted hazard ratio, 0.31; 95% confidence interval, 0.12-0.78; P = .009). An additional sensitivity analysis also showed a similar reduction in ILD risk in those taking tofacitinib, compared with adalimumab (aHR, 0.32; 95% CI, 0.13-0.82; P < .001). There was no significant difference in risk of developing ILD in the abatacept, rituximab, or tocilizumab groups, compared with the adalimumab group.
“Patients who generally looked similar with RA, but were given different treatments, had different risks of developing ILD,” Dr. Baker said. “Based on what we found, most of the biologic therapies had similar rates of developing ILD, but the JAK inhibitor tofacitinib had a reduced risk.” Additional research is necessary to see if tofacitinib shows the same benefit in prospective studies, he said.
“Even though this wasn’t a clinical trial, it suggested that one of the medications that we use to treat RA could potentially prevent the development of ILD,” Elizabeth Volkmann, MD, codirector of the Connective Tissue Disease-Related Interstitial Lung Disease Program at the University of California, Los Angeles, told this news organization. She was not involved with the study.
With retrospective studies, it is difficult to account for all confounding factors, even with adjusted models, she said. For example, the authors did not have data on patients’ history of smoking, a known risk factor for ILD that could have affected which treatment was selected, they acknowledged. The tofacitinib group was also smaller than other treatment groups, which “may have contributed to a small number of events,” the authors wrote. “However, the follow-up time was similar across all groups, and we used Cox proportional hazard models to investigate the association between time-to-event and use of treatment while controlling for the other baseline characteristics.”
Both Dr. Baker and Dr. Volkmann agreed that future research could also investigate whether tofacitinib prevents the progression of ILD in patients with RA who already have the lung condition. “That’s never been looked at before,” Dr. Volkmann said.
Dr. Baker and a coauthor received support for this work from grants from the National Institutes of Health. Dr. Baker and Dr. Volkmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) were 69% less likely to develop interstitial lung disease (ILD), compared with those treated with adalimumab (Humira), according to a new retrospective study.
About 10% of RA patients develop ILD, but data on how different biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) may affect the risk of developing ILD is lacking, the authors wrote. Identifying treatments that may have protective effects could be useful when prescribing treatments for patients with RA who are at higher risk for ILD, first author Matthew C. Baker, MD, clinical chief in the division of immunology and rheumatology at Stanford (Calif.) University, said in an interview.
In the analysis, published in JAMA Network Open researchers used the Optum Clinformatics Data Mart to identify claims data for patients with RA who were taking b/tsDMARDs from December 2003 to December 2019. Patients were excluded if they had a preexisting diagnosis of ILD or if they had less than 1 year of continuous enrollment in the data set.
The researchers identified 28,559 patients with RA who were treated with adalimumab (13,326), abatacept (Orencia; 5,676), rituximab (Rituxan; 5,444), tocilizumab (Actemra; 2,548), and tofacitinib (1,565). More than three-fourths of patients were female (78%), and their average age was 55.6 years old. During the study period, 276 developed ILD. An adjusted model showed a 69% lower incidence of ILD in patients treated with tofacitinib, compared with those treated with adalimumab (adjusted hazard ratio, 0.31; 95% confidence interval, 0.12-0.78; P = .009). An additional sensitivity analysis also showed a similar reduction in ILD risk in those taking tofacitinib, compared with adalimumab (aHR, 0.32; 95% CI, 0.13-0.82; P < .001). There was no significant difference in risk of developing ILD in the abatacept, rituximab, or tocilizumab groups, compared with the adalimumab group.
“Patients who generally looked similar with RA, but were given different treatments, had different risks of developing ILD,” Dr. Baker said. “Based on what we found, most of the biologic therapies had similar rates of developing ILD, but the JAK inhibitor tofacitinib had a reduced risk.” Additional research is necessary to see if tofacitinib shows the same benefit in prospective studies, he said.
“Even though this wasn’t a clinical trial, it suggested that one of the medications that we use to treat RA could potentially prevent the development of ILD,” Elizabeth Volkmann, MD, codirector of the Connective Tissue Disease-Related Interstitial Lung Disease Program at the University of California, Los Angeles, told this news organization. She was not involved with the study.
With retrospective studies, it is difficult to account for all confounding factors, even with adjusted models, she said. For example, the authors did not have data on patients’ history of smoking, a known risk factor for ILD that could have affected which treatment was selected, they acknowledged. The tofacitinib group was also smaller than other treatment groups, which “may have contributed to a small number of events,” the authors wrote. “However, the follow-up time was similar across all groups, and we used Cox proportional hazard models to investigate the association between time-to-event and use of treatment while controlling for the other baseline characteristics.”
Both Dr. Baker and Dr. Volkmann agreed that future research could also investigate whether tofacitinib prevents the progression of ILD in patients with RA who already have the lung condition. “That’s never been looked at before,” Dr. Volkmann said.
Dr. Baker and a coauthor received support for this work from grants from the National Institutes of Health. Dr. Baker and Dr. Volkmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) were 69% less likely to develop interstitial lung disease (ILD), compared with those treated with adalimumab (Humira), according to a new retrospective study.
About 10% of RA patients develop ILD, but data on how different biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) may affect the risk of developing ILD is lacking, the authors wrote. Identifying treatments that may have protective effects could be useful when prescribing treatments for patients with RA who are at higher risk for ILD, first author Matthew C. Baker, MD, clinical chief in the division of immunology and rheumatology at Stanford (Calif.) University, said in an interview.
In the analysis, published in JAMA Network Open researchers used the Optum Clinformatics Data Mart to identify claims data for patients with RA who were taking b/tsDMARDs from December 2003 to December 2019. Patients were excluded if they had a preexisting diagnosis of ILD or if they had less than 1 year of continuous enrollment in the data set.
The researchers identified 28,559 patients with RA who were treated with adalimumab (13,326), abatacept (Orencia; 5,676), rituximab (Rituxan; 5,444), tocilizumab (Actemra; 2,548), and tofacitinib (1,565). More than three-fourths of patients were female (78%), and their average age was 55.6 years old. During the study period, 276 developed ILD. An adjusted model showed a 69% lower incidence of ILD in patients treated with tofacitinib, compared with those treated with adalimumab (adjusted hazard ratio, 0.31; 95% confidence interval, 0.12-0.78; P = .009). An additional sensitivity analysis also showed a similar reduction in ILD risk in those taking tofacitinib, compared with adalimumab (aHR, 0.32; 95% CI, 0.13-0.82; P < .001). There was no significant difference in risk of developing ILD in the abatacept, rituximab, or tocilizumab groups, compared with the adalimumab group.
“Patients who generally looked similar with RA, but were given different treatments, had different risks of developing ILD,” Dr. Baker said. “Based on what we found, most of the biologic therapies had similar rates of developing ILD, but the JAK inhibitor tofacitinib had a reduced risk.” Additional research is necessary to see if tofacitinib shows the same benefit in prospective studies, he said.
“Even though this wasn’t a clinical trial, it suggested that one of the medications that we use to treat RA could potentially prevent the development of ILD,” Elizabeth Volkmann, MD, codirector of the Connective Tissue Disease-Related Interstitial Lung Disease Program at the University of California, Los Angeles, told this news organization. She was not involved with the study.
With retrospective studies, it is difficult to account for all confounding factors, even with adjusted models, she said. For example, the authors did not have data on patients’ history of smoking, a known risk factor for ILD that could have affected which treatment was selected, they acknowledged. The tofacitinib group was also smaller than other treatment groups, which “may have contributed to a small number of events,” the authors wrote. “However, the follow-up time was similar across all groups, and we used Cox proportional hazard models to investigate the association between time-to-event and use of treatment while controlling for the other baseline characteristics.”
Both Dr. Baker and Dr. Volkmann agreed that future research could also investigate whether tofacitinib prevents the progression of ILD in patients with RA who already have the lung condition. “That’s never been looked at before,” Dr. Volkmann said.
Dr. Baker and a coauthor received support for this work from grants from the National Institutes of Health. Dr. Baker and Dr. Volkmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Dabigatran recalled over potential carcinogen
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
eNose knows S. aureus in children with cystic fibrosis
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
FROM THE JOURNAL OF CYSTIC FIBROSIS