User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Meet our newest genetically engineered frenemy, herpes
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Out-of-state telehealth visits could help more patients if restrictions eased: Study
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM
Heart failure drug a new treatment option for alcoholism?
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.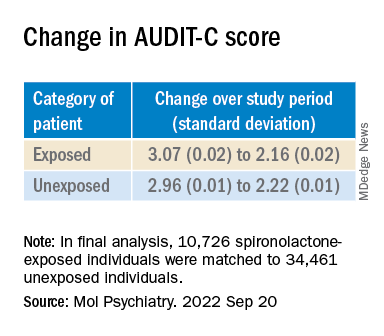
“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
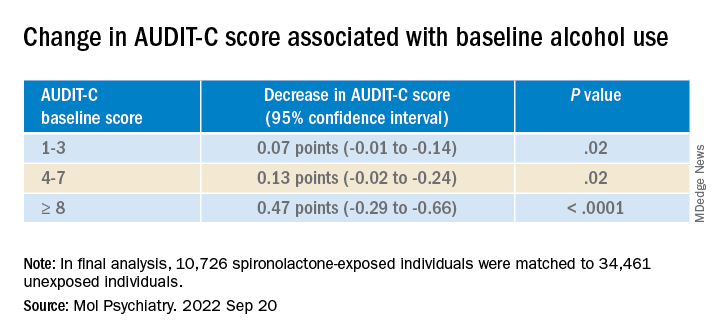
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.

“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(AUD), new research suggests.
Researchers at the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and Yale University, New Haven, Conn., investigated the impact of spironolactone on AUD.
Initially, they studied rodents and found that spironolactone reduced binge drinking in mice and reduced self-administration of alcohol in rats without adversely affecting food or water intake or causing motor or coordination problems.
They also analyzed electronic health records of patients drawn from the United States Veterans Affairs health care system to explore potential changes in alcohol use after spironolactone treatment was initiated for other conditions and found a significant link between spironolactone treatment and reduction in self-reported alcohol consumption, with the largest effects observed among those who reported hazardous/heavy episodic alcohol use prior to starting spironolactone treatment.
“Combining findings across three species and different types of research studies, and then seeing similarities in these data, gives us confidence that we are onto something potentially important scientifically and clinically,” senior coauthor Lorenzo Leggio, MD, PhD, senior investigator in the Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, a joint NIDA and NIAAA laboratory, said in a news release.
The study was published online in Molecular Psychiatry.
There is a “critical need to increase the armamentarium of pharmacotherapies to treat individuals with AUD,” the authors note, adding that neuroendocrine systems involved in alcohol craving and drinking “offer promising pharmacologic targets in this regard.”
“Both our team and others have observed that patients with AUD often present with changes in peripheral hormones, including aldosterone, which plays a key role in regulating blood pressure and electrolytes,” Dr. Leggio said in an interview.
Spironolactone is a nonselective mineralocorticoid receptor (MT) antagonist. In studies in animal models, investigators said they found “an inverse correlation between alcohol drinking and the expression of the MR in the amygdala, a key brain region in the development and maintenance of AUD and addiction in general.”
Taken together, this led them to hypothesize that blocking the MR, which is the mechanism of action of spironolactone, “could be a novel pharmacotherapeutic approach for AUD,” he said.
Previous research by the same group of researchers suggested spironolactone “may be a potential new medication to treat patients with AUD.” The present study expanded on those findings and consisted of a three-part investigation.
In the current study, the investigators tested different dosages of spironolactone on binge-like alcohol consumption in male and female mice and assessed food and water intake, blood alcohol levels, motor coordination, and spontaneous locomotion.
They then tested the effects of different dosages of spironolactone injections on operant alcohol self-administration in alcohol-dependent and nondependent male and female rats, also testing blood alcohol levels and motor coordination.
Finally, they analyzed health records of veterans to examine the association between at least 60 continuous days of spironolactone treatment and self-reported alcohol consumption (measured by the Alcohol Use Disorders Identification Test-Consumption [AUDIT-C]).
Each of the spironolactone-exposed patients was matched using propensity scores with up to five unexposed patients who had reported alcohol consumption in the 2 years prior to the index date.
The final analysis included a matched cohort of 10,726 spironolactone-exposed individuals who were matched to 34,461 unexposed individuals.
New targets
Spironolactone reduced alcohol intake in mice drinking a sweetened alcohol solution; a 2-way ANOVA revealed a main effect of dose (F 4,52 = 9.09; P < .0001) and sex, with female mice drinking more alcohol, compared to male mice (F 1,13 = 6.05; P = .02).
Post hoc comparisons showed that spironolactone at doses of 50, 100, and 200 mg/kg significantly reduced alcohol intake (P values = .007, .002, and .0001, respectively).
In mice drinking an unsweetened alcohol solution, the 2-way repeated measures ANOVA similarly found a main effect of dose (F 4,52 = 5.77; P = .0006), but not of sex (F 1,13 = 1.41; P = .25).
Spironolactone had no effect on the mice’s intake of a sweet solution without alcohol and had no impact on the consumption of food and water or on locomotion and coordination.
In rats, a 2-way ANOVA revealed a significant spironolactone effect of dose (F 3,66 = 43.95; P < .001), with a post hoc test indicating that spironolactone at 25, 50, and 75 mg/kg reduced alcohol self-administration in alcohol-dependent and nondependent rats (all P values = .0001).
In humans, among the exposed individuals in the matched cohort, 25%, 57%, and 18% received daily doses of spironolactone of less than 25 mg/day, 25-49 mg/day, and 50 mg/day or higher, respectively, with a median follow-up time of 542 (interquartile range, 337-730) days.
The AUDIT-C scores decreased during the study period in both treatment groups, with a larger decrease in average AUDIT-C scores among the exposed vs. unexposed individuals.

“These are very exciting times because, thanks to the progress in the addiction biomedical research field, we are increasing our understanding of the mechanisms how some people develop AUD; hence we can use this knowledge to identify new targets.” The current study “is an example of these ongoing efforts,” said Dr. Leggio.
“It is important to note that [these results] are important but preliminary.” At this juncture, “it would be too premature to think about prescribing spironolactone to treat AUD,” he added.
Exciting findings
Commenting on the study, Joyce Besheer, PhD, professor, department of psychiatry and Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, called the study an “elegant demonstration of translational science.”
“While clinical trials will be needed to determine whether this medication is effective at reducing drinking in patients with AUD, these findings are exciting as they suggest that spironolactone may be a promising compound and new treatment options for AUD are much needed,” said Dr. Besheer, who was not involved with the current study.
Dr. Leggio agreed. “We now need prospective, placebo-controlled studies to assess the potential safety and efficacy of spironolactone in people with AUD,” he said.
This work was supported by the National Institutes of Health and the NIAAA. Dr. Leggio, study coauthors, and Dr. Besheer declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
Coffee linked to reduced cardiovascular disease and mortality risk
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Drinking two to three daily cups of – ground, instant, or decaffeinated – is associated with significant reductions in new cardiovascular disease (CVD) and mortality risk, compared with avoiding coffee, a new analysis of the prospective UK Biobank suggests.
Ground and instant coffee, but not decaffeinated coffee, also was associated with reduced risk of new-onset arrhythmia, including atrial fibrillation.
“Our study is the first to look at differences in coffee subtypes to tease out important differences which may explain some of the mechanisms through which coffee works,” Peter M. Kistler, MD, of the Alfred Hospital and Baker Heart and Diabetes Institute, Melbourne, Australia, told this news organization.
“Daily coffee intake should not be discouraged by physicians but rather considered part of a healthy diet,” Dr. Kistler said.
“This study supports that coffee is safe and even potentially beneficial, which is consistent with most of the prior evidence,” Carl “Chip” Lavie, MD, who wasn’t involved in the study, told this news organization.
“We do not prescribe coffee to patients, but for the majority who like coffee, they can be encouraged it is fine to take a few cups daily,” said Dr. Lavie, with the Ochsner Heart and Vascular Institute in New Orleans.
The study was published online in the European Journal of Preventive Cardiology.
Clear cardiovascular benefits
A total of 449,563 UK Biobank participants (median age 58 years; 55% women), who were free of arrhythmias or other CVD at baseline, reported in questionnaires their level of daily coffee intake and preferred type of coffee.
During more than 12.5 years of follow-up, 27,809 participants (6.2%) died.
Drinking one to five cups per day of ground or instant coffee (but not decaffeinated coffee) was associated with a significant reduction in incident arrhythmia. The lowest risk was with four to five cups per day for ground coffee (hazard ratio [HR] 0.83; 95% confidence interval [CI], 0.76-0.91; P < .0001) and two to three cups per day for instant coffee (HR, 0.88; 95% CI, 0.85-0.92; P < .0001).
Habitual coffee drinking of up to five cups perday was also associated with significant reductions in the risk of incident CVD, when compared with nondrinkers.
Significant reductions in the risk of incident coronary heart disease (CHD) were associated with habitual coffee intake of up to five cups per day, with the lowest risk for CHD observed in those who consumed two to three cups per day (HR 0.89; 95% CI, 0.86-0.91; P < .0001).
Coffee consumption at all levels was linked to significant reduction in the risk of congestive cardiac failure (CCF) and ischemic stroke. The lowest risks were observed in those who consumed two to three cups per day, with HR, 0.83 (95% CI, 0.79-0.87; P < .0001) for CCF and HR, 0.84 (95% CI, 0.78-0.90; P < .0001) for ischemic stroke.
Death from any cause was significantly reduced for all coffee subtypes, with the greatest risk reduction seen with two to three cups per day for decaffeinated (HR, 0.86; 95% CI, 0.81-0.91; P < .0001); ground (HR, 0.73; 95% CI, 0.69-0.78; P < .0001); and instant coffee (HR, 0.89; 95% CI, 0.86-0.93; P < .0001).
“Coffee consumption is associated with cardiovascular benefits and should not empirically be discontinued in those with underlying heart rhythm disorders or cardiovascular disease,” Dr. Kistler told this news organization.
Plausible mechanisms
There are a number of proposed mechanisms to explain the benefits of coffee on CVD.
“Caffeine has antiarrhythmic properties through adenosine A1 and A2A receptor inhibition, hence the difference in effects of decaf vs. full-strength coffee on heart rhythm disorders,” Dr. Kistler explained.
Coffee has vasodilatory effects and coffee also contains antioxidant polyphenols, which reduce oxidative stress and modulate metabolism.
“The explanation for improved survival with habitual coffee consumption remains unclear,” Dr. Kistler said.
“Putative mechanisms include improved endothelial function, circulating antioxidants, improved insulin sensitivity, and reduced inflammation. Another potential mechanism includes the beneficial effects of coffee on metabolic syndrome,” he said.
“Caffeine has a role in weight loss through inhibition of gut fatty acid absorption and increase in basal metabolic rate. Furthermore, coffee has been associated with a significantly lower incidence of type 2 diabetes mellitus,” Dr. Kistler added.
Direction of relationship unclear
Charlotte Mills, PhD, University of Reading, England, said this study “adds to the body of evidence from observational trials associating moderate coffee consumption with cardioprotection, which looks promising.”
However, with the observational design, it’s unclear “which direction the relationship goes – for example, does coffee make you healthy or do inherently healthier people consume coffee? Randomized controlled trials are needed to fully understand the relationship between coffee and health before recommendations can be made,” Dr. Mills told the UK nonprofit Science Media Centre.
Annette Creedon, PhD, nutrition scientist with the British Nutrition Foundation, said it’s possible that respondents over- or underestimated the amount of coffee that they were consuming at the start of the study when they self-reported their intake.
“It is therefore difficult to determine whether the outcomes can be directly associated with the behaviors in coffee consumption reported at the start of the study,” she told the Science Media Centre.
The study had no funding. Dr. Kistler has received funding from Abbott Medical for consultancy and speaking engagements and fellowship support from Biosense Webster. Dr. Lavie has no relevant disclosures. Dr. Mills has worked in collaboration with Nestle on research relating to coffee and health funded by UKRI. Dr. Creedon has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
What we know about long COVID so far
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Apremilast may have some cardiometabolic benefits in patients with psoriasis
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Type 1 diabetes cases poised to double worldwide by 2040
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – The number of people living with type 1 diabetes worldwide is expected to double by 2040, with most new cases among adults living in low- and middle-income countries, new modeling data suggest.
The forecast, developed from available data collected in the newly established open-source Type 1 Diabetes Index, provides estimates for type 1 diabetes prevalence, incidence, associated mortality, and life expectancy for 201 countries for 2021.
The model also projects estimates for prevalent cases in 2040. It is the first type 1 diabetes dataset to account for the lack of prevalence because of premature mortality, particularly in low- and middle-income countries.
“The worldwide prevalence of type 1 diabetes is substantial and growing. Improved surveillance – particularly in adults who make up most of the population living with type 1 diabetes – is essential to enable improvements to care and outcomes. There is an opportunity to save millions of lives in the coming decades by raising the standard of care (including ensuring universal access to insulin and other essential supplies) and increasing awareness of the signs and symptoms of type 1 diabetes to enable a 100% rate of diagnosis in all countries,” the authors write.
“This work spells out the need for early diagnosis of type 1 diabetes and timely access to quality care,” said Chantal Mathieu, MD, at the European Association for the Study of Diabetes annual meeting.
One in five deaths from type 1 diabetes in under 25s
The new findings were published in Lancet Diabetes & Endocrinology by Gabriel A. Gregory, MD, of Life for a Child Program, New South Wales, Australia, and colleagues. The T1D Index Project database was published Sept. 21, 2022.
According to the model, about 8.4 million people were living with type 1 diabetes in 2021, with one-fifth from low- and middle-income countries. An additional 3.7 million died prematurely and would have been added to that count had they lived. One in five of all deaths caused by type 1 diabetes in 2021 is estimated to have occurred in people younger than age 25 years because of nondiagnosis.
“It is unacceptable that, in 2022, some 35,000 people worldwide are dying undiagnosed within a year of onset of symptoms. There also continues to be a huge disparity in life expectancy for people with type 1 diabetes, hitting those in the poorest countries hardest,” noted Dr. Mathieu, who is senior vice-president of EASD and an endocrinologist based at KU Leuven, Belgium.
By 2040, the model predicts that between 13.5 million and 17.4 million people will be living with the condition, with the largest relative increase from 2021 in low-income and lower-middle-income countries. The majority of incident and prevalent cases of type 1 diabetes are in adults, with an estimated 62% of 510,000 new diagnoses worldwide in 2021 occurring in people aged 20 years and older.
Type 1 diabetes is not predominantly a disease of childhood
Dr. Mathieu also noted that the data dispute the long-held view of type 1 diabetes as a predominantly pediatric condition. Indeed, worldwide, the median age for a person living with type 1 diabetes is 37 years.
“While type 1 diabetes is often referred to as ‘child-onset’ diabetes, this important study shows that only around one in five living with the condition are aged 20 years or younger, two-thirds are aged 20-64 years, and a further one in five are aged 65 years or older.”
“This condition does not stop at age 18 years – the children become adults, and the adults become elderly. All countries must examine and strengthen their diagnosis and care pathways for people of all ages living with type 1 diabetes,” Dr. Mathieu emphasized.
And in an accompanying editorial, Serena Jingchuan Guo, MD, PhD, and Hui Shao, MD, PhD, point out that most studies that estimate diabetes burden have focused on type 2 diabetes, noting, “type 1 diabetes faces the challenges of misdiagnosis, underdiagnosis, high risk of complications, and premature mortality.”
The insulin affordability issue is central, point out Dr. Guo and Dr. Shao of the Center for Drug Evaluation and Safety, department of pharmaceutical evaluation and policy, University of Florida College of Pharmacy, Gainesville.
“Countries need to strengthen the price regulation and reimbursement policy for insulin while building subsidy programs to ensure insulin access and to cope with the growing demand for insulin. Meanwhile, optimizing the insulin supply chain between manufacturers and patients while seeking alternative treatment options (for example, biosimilar products) will also improve the current situation,” they conclude.
The study was funded by JDRF, of which four coauthors are employees. The editorialists have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Cre8 EVO stent loses sweet spot in diabetes at 2 years: SUGAR
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
BOSTON – Despite a promising start, extended follow-up from the SUGAR trial found that the Cre8 EVO drug-eluting stent could not maintain superiority over the Resolute Onyx DES at 2 years in patients with diabetes undergoing revascularization for coronary artery disease.
The Cre8 EVO stent (Alvimedica) is not available in the United States but, as previously reported, caused a stir last year after demonstrating a 35% relative risk reduction in the primary endpoint of target lesion failure (TLF) at 1 year in a prespecified superiority analysis.
At 2 years, however, the TLF rate was 10.4% with the polymer-free Cre8 EVO amphilimus-eluting stent and 12.1% with the durable polymer Resolute Onyx (Medtronic) zotarolimus-eluting stent, which did not achieve superiority (hazard ratio, 0.84; 95% confidence interval, 0.60-1.19).
Rates were numerically lower with the Cre8 EVO stent for the endpoint’s individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%).
Results were also similar between the Cre8 EVO and Resolute Onyx stents for all-cause mortality (7.1% vs. 6.8%), any MI (9.0% vs. 9.2%), target vessel revascularization (5.5% vs. 5.1%), all new revascularizations (7.6% vs. 9.4%), definite stent thrombosis (1.0% vs. 1.2%), and major adverse cardiac events (18.3% vs. 20.8%), Pablo Salinas, MD, PhD, of Hospital Clinico San Carlos, Madrid, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
He noted that all-cause mortality was 7% in just 2 years in the diabetic cohort, or twice the number of cardiac deaths. “In other words, these patients had the same chance of dying from cardiac causes and noncardiac causes, so we need a more comprehensive approach to the disease. Also, if you look at all new revascularizations, roughly 50% were off target, so there is disease progression at 2 years in this population.”
Among the 586 Cre8 EVO and 589 Resolute Onyx patients who underwent percutaneous coronary intervention (PCI), roughly half had multivessel coronary artery disease, 83% had hypertension, 81% had dyslipidemia, and 21% were current smokers. Nearly all patients had diabetes type 2 for an average of 10.6 years for Cre8 EVO and 11.4 years for Resolute Onyx, with hemoglobin A1c levels of 7.4% and 7.5%, respectively.
Although there is “insufficient evidence” the Cre8 EVO stent is superior to the Resolute Onyx stent with regard to TLF, Dr. Salinas concluded extended follow-up until 5 years is warranted.
During a discussion of the results, Dr. Salinas said he expects the 5-year results will “probably go parallel” but that it’s worth following this very valuable cohort. “There are not so many trials with 1,000 diabetic patients. We always speak about how complex they are, the results are bad, but we don’t use the diabetic population in trials,” he said at the meeting sponsored by the Cardiovascular Research Foundation.
Asked during a TCT press conference what could have caused the catch-up in TLF at 2 years, Dr. Salinas said there were only 25 primary events from years 1 to 2, driven primarily by periprocedural MI, but that the timing of restenosis was different. Events accrued “drop by drop” with the Cre8 EVO, whereas with the Resolute Onyx there was a “bump in restenosis” after 6 months “but then it is very nice to see it is flat, which means that durable polymers are also safe because we have not seen late events.”
Press conference discussant Carlo Di Mario, MD, from Careggi University Hospital, Florence, Italy, who was not involved in the study, said the reversal of superiority for the Cre8 EVO might be a “bitter note” for the investigators but “maybe it is not bitter for us because overall, the percentage of figures are so low that it’s very difficult to find a difference” between the two stents.
Roxana Mehran, MD, of Icahn School of Medicine at Mount Sinai, New York, who previously described the 1-year results as “almost too good to be true,” commented to this news organization, “We just saw in this trial, no benefit whatsoever at 2 years in terms of target lesion failure. So it’s very important for us to evaluate this going forward.”
She continued, “We’ve always been talking about these biodegradable polymers and then going back to the bare metal stent – oh that’s great because polymers aren’t so good – but now we’re seeing durable polymers may be okay, especially with the current technology.”
Asked whether Cre8 EVO, which is CE mark certified in Europe, remains an option in light of the new results, Dr. Mehran said, “I don’t think it kills it. It’s not worse; it’s another stent that’s available.”
Nevertheless, “what we’re looking for is some efficacious benefit for diabetic patients. We don’t have one yet,” observed Dr. Mehran, who is leading the ABILITY Diabetes Global trial, which just finished enrolling 3,000 patients with diabetes and is testing PCI with the Abluminus DES+ sirolimus-eluting stent system vs. the Xience everolimus-eluting stent. The study is estimated to be complete in August 2024.
The study was funded by the Spanish Society of Cardiology. Dr. Salinas reported consulting fees/honoraria from Boston Scientific, Abbott Vascular, Biomenco, and Medtronic.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Emphasis on weight loss in new type 2 diabetes guidance
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Weight loss should be a co–primary management goal for type 2 diabetes in adults, according to a new comprehensive joint consensus report from the European Association for the Study of Diabetes and the American Diabetes Association.
And while metformin is still recommended as first-line therapy for patients with type 2 diabetes with no other comorbidities, the statement expands the indications for use of other agents or combinations of agents as initial therapy for subgroups of patients, as part of individualized and patient-centered decision-making.
Last updated in 2019, the new “Management of Hyperglycemia in Type 2 Diabetes” statement also places increased emphasis on social determinants of health, incorporates recent clinical trial data for cardiovascular and kidney outcomes for sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) agonists to broaden recommendations for cardiorenal protection, and discusses health behaviors such as sleep and sitting. It also targets a wider audience than in the past by addressing health system organization to optimize delivery of diabetes care.
The new statement was presented during a 90-minute session at the annual meeting of the EASD, with 12 of its 14 European and American authors as presenters. The document was simultaneously published in Diabetologia and Diabetes Care.
During the discussion, panel member Jennifer Brigitte Green, MD, commented: “Many of these recommendations are not new. They’re modest revisions of recommendations that have been in place for years, but we know that actual implementation rates of use of these drugs in patients with established comorbidities are very low.”
“I think it’s time for communities, health care systems, etc, to actually introduce these as expectations of care... to assess quality because unless it’s considered formally to be a requirement of care I just don’t think we’re going to move that needle very much,” added Dr. Green, who is professor of medicine at Duke University, Durham, N.C.
Vanita R. Aroda, MD, of the division of endocrinology, diabetes, and hypertension at Brigham and Women’s Hospital, Boston, commented: “In the past, sometimes these recommendations created fodder for debate, but I don’t think this one will. It’s just really solidly evidence based, with the rationales presented throughout, including the figures. I think just having very clear evidence-based directions should support their dissemination and use.”
Weight management plays a prominent role in treatment
In an interview, writing panel cochair John B. Buse, MD, PhD, said: “We are saying that the four major components of type 2 diabetes care are glycemic management, cardiovascular risk management, weight management, and prevention of end-organ damage, particularly with regard to cardiorenal risk.”
“The weight management piece is much more explicit now,” said Dr. Buse, director of the Diabetes Center at the University of North Carolina at Chapel Hill.
He noted that recent evidence from the intensive lifestyle trial DiRECT, conducted in the United Kingdom, the bariatric surgery literature, and the emergence of potent weight-loss drugs have meant that “achieving 10%-15% body weight loss is now possible.
“So, aiming for remission is something that might be attractive to patients and providers. This could be based on weight management, with the [chosen] method based on shared decision-making.”
According to the new report: “Weight loss of 5%-10% confers metabolic improvement; weight loss of 10%-15% or more can have a disease-modifying effect and lead to remission of diabetes, defined as normal blood glucose levels for 3 months or more in the absence of pharmacological therapy in a 2021 consensus report.”
“Weight loss may exert benefits that extend beyond glycemic management to improve risk factors for cardiometabolic disease and quality of life,” it adds.
Individualization featured throughout
The report’s sections cover principles of care, including the importance of diabetes self-management education and support and avoidance of therapeutic inertia. Detailed guidance addresses therapeutic options including lifestyle, weight management, and pharmacotherapy for treating type 2 diabetes.
Another entire section is devoted to personalizing treatment approaches based on individual characteristics, including new evidence from cardiorenal outcomes studies for SGLT2 inhibitors and GLP-1 agonists that have come out since the last consensus report.
The document advises: “Consider initial combination therapy with glucose-lowering agents, especially in those with high [hemoglobin] A1c at diagnosis (that is, > 70 mmol/mol [> 8.5%]), in younger people with type 2 diabetes (regardless of A1c), and in those in whom a stepwise approach would delay access to agents that provide cardiorenal protection beyond their glucose-lowering effects.”
Designed to be used and user-friendly
Under the “Putting it all together: strategies for implementation” section, several lists of “practical tips for clinicians” are provided for many of the topics covered.
A series of colorful infographics are included as well, addressing the “decision cycle for person-centered glycemic management in type 2 diabetes,” including a chart summarizing characteristics of available glucose-lowering medications, including cardiorenal protection.
Also mentioned is the importance of 24-hour physical behaviors (including sleep, sitting, and sweating) and the impact on cardiometabolic health, use of a “holistic person-centered approach” to type 2 diabetes management, and an algorithm on insulin use.
Dr. Buse has financial ties to numerous drug and device companies. Dr. Green is a consultant for AstraZeneca, Pfizer, Boehringer Ingelheim/Lilly, Bayer, Sanofi, Anji, Vertex/ICON, and Valo. Dr. Aroda has served as a consultant for Applied Therapeutics, Duke, Fractyl, Novo Nordisk, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Uncontrolled BP linked to one-third of ED visits for CVD
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A look at the top cardiovascular disease (CVD) diagnoses in U.S. emergency departments (EDs) suggests that many heart-related emergencies are due to poorly controlled high blood pressure.
In a study of more than 20 million ED visits, about one-third of CVD-related ED visits in the United States were for hypertension-related conditions.
Overall, 13% of ED visits, representing more than 2.7 million individuals, were for essential hypertension.
The fact that these visits rarely led to an inpatient admission (< 3%) or death (< 0.1%) suggests they were “mostly related to the management of hypertension,” lead author Mamas A. Mamas, MD, Keele University, Staffordshire, England, said in a news release.
The study was published online in the Journal of the American Heart Association.
Nationwide sample
The researchers studied more than 20.6 million ED encounters in adults with a primary CVD diagnosis using data from the Nationwide Emergency Department Sample between 2016 and 2018.
In the sample, 49% were women, and the median age was 67 years. Men had poorer overall baseline cardiometabolic profiles, but women had higher rates of obesity, hypertension, and cerebrovascular disease. The majority had Medicare or Medicaid insurance.
In women, essential hypertension was the most common reason for an ED visit (16%), followed by hypertensive heart or kidney disease (14%) and atrial fibrillation (AF)/flutter (10%).
In men, the top three reasons were hypertensive heart or kidney disease (15%), essential hypertension (11%), and acute myocardial infarction (AMI, 11%).
On presentation, women were significantly more likely to have essential hypertension, hypertensive crisis, AF/flutter, supraventricular tachycardia, pulmonary embolism, or ischemic stroke, while men were more likely to have AMI, or cardiac arrest.
“Previous studies have shown sex differences in patterns of CVD among hospitalized patients,” Dr. Mamas noted. “However, examining CVD encounters in the ED provides a more complete picture of the cardiovascular healthcare needs of men and women, as it captures encounters prior to hospitalization.”
He noted that previous studies of CVD emergency visits are limited to suspected MI visits. “Therefore, this analysis of 15 CVD conditions helps to better understand the full spectrum of acute CVD needs, including sex disparities in hospitalization and risk of death,” Dr. Mamas said.
Sex differences in outcomes
The study found that outcomes from the emergency CVD visits were slightly different for men and women.
Overall, women were less likely than were men to die (3.3% vs. 4.3%) or be hospitalized (49.1% vs. 52.3%) after an ED visit for CVD. The difference may be due to women’s generally lower-risk diagnoses, Dr. Mamas said, but there could be an underestimation of deaths in women.
In logistic regression models adjusted for baseline covariates, women with intracranial hemorrhage (ICH) had a higher risk of being admitted to hospital or dying compared with men with ICH.
Men were more likely to die if they presented with hypertensive heart or kidney disease, AF/flutter, AMI or cardiac arrest, the researchers found.
“We did not track deaths outside of the hospital setting,” Dr. Mamas pointed out. Given past evidence that women are more likely to be inappropriately discharged from the ED, and strong evidence for the systemic undertreatment of women, further study is warranted to track outcomes beyond the ED visit,” he added.
The researchers called for further research into understanding the underlying factors driving the differences in CVD patterns and outcomes between men and women.
Reached for comment, Maryann McLaughlin, MD, a cardiologist at Mount Sinai Hospital, New York, said: “Hypertension is a silent killer” and this study “reiterates that people need to get their blood pressure checked more regularly.
“In the very least, if they do present to the hospital as not feeling well or whatever it is, and they are identified as having high blood pressure, that’s an important opportunity to really teach them about hypertension and have them follow-up with it,” Dr. McLaughlin told this news organization.
The study was supported by Health Data Research UK. Dr. Keele and Dr. McLaughlin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.