User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Add PCSK9 inhibitor to high-intensity statin at primary PCI, proposes sham-controlled EPIC-STEMI
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Yes, we should talk politics and religion with patients
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
Legacy of neutral renal denervation trial recast by long-term outcomes: SYMPLICITY HTN-3
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.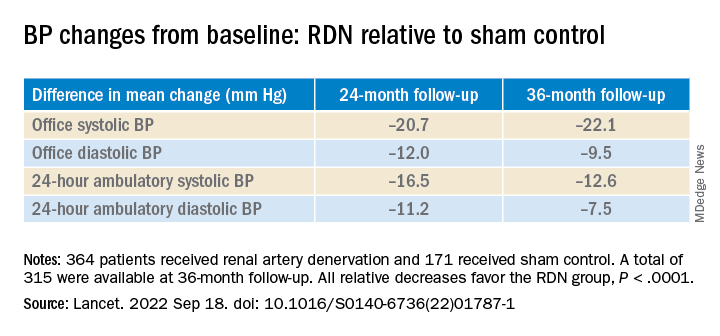
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
BOSTON – There’s an intriguing plot twist in the story of SYMPLICITY HTN-3, the sham-controlled clinical trial that nearly put the kibosh on renal denervation (RDN) therapy as a promising approach to treatment-resistant hypertension (HTN).
The trial famously showed no benefit for systolic blood pressure (BP) from the invasive procedure at 6 months and 12 months, dampening enthusiasm for RDN in HTN for both physicians and industry. But it turns out that disappointment in the study may have been premature.
The procedure led to significant improvements in systolic BP, whether in-office or ambulatory, compared with a sham control procedure, in a new analysis that followed the trial’s patients out to 3 years. Those who underwent RDN also required less intense antihypertensive drug therapy.
“These findings support that durable blood pressure reductions with radiofrequency renal artery denervation, in the presence of lifestyle modification and maximal medical therapy, are safely achievable,” Deepak L. Bhatt, MD, said in a Sept. 18 presentation at the Transcatheter Cardiovascular Therapeutics annual meeting, which was sponsored by the Cardiovascular Research Foundation.
Dr. Bhatt, of Boston’s Brigham and Women’s Hospital and Harvard Medical School, is lead author on the report published in The Lancet simultaneously with his presentation.
Strides in RDN technology and trial design since the neutral primary SYMPLICITY HTN-3 results were reported in 2014 have long since restored faith in the procedure, which is currently in advanced stages of clinical trials and expected to eventually make a mark on practice.
But Roxana Mehran, MD, not connected to SYMPLICITY HTN-3, expressed caution in interpreting the current analysis based on secondary endpoints and extended follow-up time.
And elsewhere at the TCT sessions, observers of the trial as well as Dr. Bhatt urged similar cautions interpreting “positive” secondary results from trials that were “negative” in their primary analyses.
Still, “I believe there is no question that we have now enough evidence to say that renal denervation on top of medications is probably something that we’re going to be seeing in the future,” Dr. Mehran, of the Icahn School of Medicine at Mount Sinai, New York, told this news organization.
Importantly, and a bit controversially, the RDN group in the 36-month SYMPLICITY HTN-3 analysis includes patients originally assigned to the sham control group who crossed over to receive RDN after the trial was unblinded. Their “control” BP responses were thereafter imputed by accepted statistical methodology that Dr. Bhatt characterized as “last observation carried forward.”
That’s another reason to be circumspect about the current results, observed Naomi Fisher, MD, also of Brigham and Women’s and Harvard Medical School, as a panelist following Dr. Bhatt’s formal presentation.
“With all the missing data and imputational calculations,” she said, “I think we have to apply caution in the interpretation.”
She also pointed out that blinding in the trial was lifted at 6 months, allowing patients to learn their treatment assignment, and potentially influencing subsequent changes to medications.
They were prescribed, on average, about five antihypertensive meds, Dr. Fisher noted, and “that’s already a red flag. Patients taking that many medications generally aren’t universally taking them. There’s very high likelihood that there could have been variable adherence.”
Patients who learned they were in the sham control group, for example, could have “fallen off” taking their medications, potentially worsening outcomes and amplifying the apparent benefit of RDN. Such an effect, Dr. Fisher said, “could have contributed” to the study’s long-term results.
As previously reported, the single-blind SYMPLICITY HTN-3 had randomly assigned 535 patients to either RDN or a sham control procedure, 364 and 171 patients respectively, at 88 U.S. centers. The trial used the Symplicity Flex RDN radiofrequency ablation catheter (Medtronic).
For study entry, patients were required to have office systolic BP of at least 160 mm Hg and 24-hour ambulatory systolic BP of at least 135 mm Hg despite stable, maximally tolerated dosages of a diuretic plus at least two other antihypertensive agents.
Blinding was lifted at 6 months, per protocol, after which patients in the sham control group who still met the trial’s BP entry criteria were allowed to cross over and undergo RDN. The 101 controls who crossed over were combined with the original active-therapy cohort for the current analysis.
From baseline to 36 months, mean number of medication classes per patient maintained between 4.5 and 5, with no significant difference between groups at any point.
However, medication burden expressed as number of doses daily held steady between 9.7 to 10.2 for controls while the RDN group showed a steady decline from 10.2 to 8.4. Differences between RDN patients and controls were significant at both 24 months (P = .01) and 36 months (P = .005), Dr. Bhatt reported.
All relative decreases favor the RDN group, P < .0001
The RDN group spent a longer percentage of time with systolic BP at goal compared to those in the sham control group in an analysis that did not involve imputation of data, Dr. Bhatt reported. The proportions of time in therapeutic range were 18% for RDN patients and 9% for controls (P < .0001).
As in the 6- and 12-month analyses, there was no adverse safety signal associated with RDN in follow-up out to both 36 and 48 months. As Dr. Bhatt reported, the rates of the composite safety endpoint in RDN patients, crossovers, and noncrossover controls were 15%, 14%, and 14%, respectively.
The safety endpoint included death, new end-stage renal disease, significant embolic events causing end-organ damage, vascular complications, renal-artery reintervention, and “hypertensive emergency unrelated to nonadherence to medications,” Dr. Bhatt reported.
There are many patients with “out of control” HTN “who cannot remain compliant on their medications,” Dr. Mehran observed for this news organization. “I believe having an adjunct to medical management of these patients,” that is RDN, “is going to be tremendously important.”
SYMPLICITY HTN-3 was funded by Medtronic. Dr. Bhatt has disclosed ties with many companies, as well as WebMD, Medscape Cardiology, and other publications or organizations. Dr. Mehran disclosed ties to Abbott Vascular, AstraZeneca, Bayer, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo/Eli Lilly, Medtronic, Novartis, OrbusNeich, Abiomed; Boston Scientific, Alleviant, Amgen, AM-Pharma, Applied Therapeutics, Arena, BAIM, Biosensors, Biotronik, CardiaWave, CellAegis, Concept Medical, CeloNova, CERC, Chiesi, Cytosorbents, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe, Philips, RenalPro, Vivasure, and Zoll; as well as Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical. Dr. Fisher disclosed ties to Medtronic, Recor Medical, and Aktiia; and receiving grants or hold research contracts with Recor Medical and Aktiia.
A version of this article first appeared on Medscape.com.
AT TCT 2022
Post-PCI FFR in multivessel disease predicts target vessel failure: FAME 3 analysis
Risk by FFR is continuous variable
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
Risk by FFR is continuous variable
Risk by FFR is continuous variable
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
In a new analysis of the previously published FAME 3 trial, which compared fractional flow reserve–guided percutaneous coronary interventions to coronary artery bypass surgery (CABG) in patients with three-vessel disease, post-PCI FFR was shown to predict both target vessel failure (TVF) and risk of cardiac events.
“We found that the post-PCI FFR had prognostic value both for the vessel and for the patient,” reported Zsolt Piroth, MD, PhD, deputy head, adult cardiology, György Gottsegen Institute of Cardiology, Budapest.
In this post hoc analysis, which was not a prespecified FAME 3 substudy, the goal was to look at the prognostic value of both post-PCI FFR and intravascular ultrasound, which were recommended in the study protocol. Several studies have addressed the value of these measures previously, according to Dr. Piroth, but he said the clinical value “has remained poorly defined” despite the currently available data.
The FAME 3 trial, published in the New England Journal of Medicine, was negative. It failed to confirm the study hypothesis that FFR-guided PCI is noninferior to CABG for the outcome of major adverse cardiac events (MACE) at 12 months.
However, this multinational trial has generated a large body of data with which to explore other issues relevant to revascularization. In this analysis, the goal was to evaluate whether post-PCI FFR predicted outcomes in complex multivessel revascularizations as it has been shown previously to do in single-vessel disease.
Presented at the Transcatheter Cardiovascular Therapeutics annual meeting, the focus of this analysis was on the 461 (61%) of patients in the 757-patient PCI arm of FAME 3 who underwent post-PCI FFR. The authors also looked at the predictive value of intravascular ultrasound, even though this was performed in just 11% of this group of trial participants.
As a continuous value, each 0.1-unit change in the post-PCI FFR was found to be prognostically significant for the outcome of TVF, defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization (only postprocedural events were counted in this analysis). Specifically, for each 0.1-unit increase on a univariate analysis, the risk of TVF was reduced by about one-third (hazard ratio, 0.67; P = .0165).
On a patient level, a 0.1-unit increase in lowest post-PCI FFR of any assessed vessel was also associated with the same relative risk reduction (HR, 0.65; P = .0074) in the outcomes of cardiac death, target vessel MI, or target vessel revascularization, according to Dr. Piroth. On a receiver operating characteristic curve analysis, a value of 0.88 or below was predictive of TVF.
Although several other patient characteristics were also risk predictors of TVF on univariate analysis, only renal disease and the single lowest post-PCI FFR (as a continuous variable) emerged as predictors of TVF on multivariable analysis after adjustment for key clinical parameters, Dr. Piroth reported.
The reason why post-PCI FFR was not performed in almost 40% of patients randomized to PCI is unclear, but Dr. Piroth reported that the baseline characteristics of those who were or were not assessed with FFR after their procedure did not differ to any major degree.
Despite “a trend for improved outcomes in those who underwent post-PCI FFR,” Dr. Piroth, whose substudy was published in Circulation: Cardiovascular Interventions simultaneously with his TCT presentation, acknowledged that the reasons for a potential benefit cannot be derived from this post hoc analysis.
As for the prognostic value of IVUS, any conclusions are limited by the small proportion of patients who underwent this form of imaging. Overall, IVUS imaging was associated with longer procedures and more stents and “if anything, a signal for harm” in this analysis, but Dr. Piroth cautioned against any conclusions because of the small data pool.
The prognostic value of post-PCI FFR in complex multivessel disease is supported by these data, but the analysis was not designed to determine whether post-PCI FFR has relevance to intervention.
According to J. Dawn Abbott, MD, an FFR analysis conducted to identify lesions that are candidates for treatment should not be confused with FFR for physiologically guided PCI to optimize outcomes.
Noting that post-PCI FFR was encouraged in this study but not mandated and that these FFR values did not typically or necessarily lead to a change in management, take home messages about the value of post-PCI FFR in multivessel disease remain limited, said Dr. Abbott, director of interventional cardiology fellowship training, Brown University, Providence, R.I.
“There was a trend toward improved outcomes in patients who had this measurement done, but, unfortunately, we do not have data regarding whether these patients had further interventions performed,” Dr. Piroth acknowledged.
The post-PCI FFR values were made available to the treating physicians, but Dr. Piroth reiterated that it is unknown whether the physicians considered this information actionable. Moreover, “the vast majority had a nonsignificant post-PCI FFR” result, and “all of the patients had an angiographically successful PCI,” Dr. Piroth added.
Dr. Piroth has financial relationships with Abbott Vascular and Boston Scientific. Dr. Abbott reports financial relationships with Abbott Vascular, Boston Scientific, Medtronic, Microport, Philips, Penumbra, Recor, and Shockwave.
FROM TCT 2022
Unsure on the best T2D drug choice? Let patients decide
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
STOCKHOLM – When a clinician is unsure which of several equally viable drug options is best for a specific patient with type 2 diabetes, a rational approach is to run a serial trial with each one and then let each patient decide which agent works best for them.
That concept underwent successful testing in a recent trial with 457 patients with type 2 diabetes and already on treatment with metformin or metformin plus a sulfonylurea but needed further glycemic control. After cycling through 4-month trials (when tolerated) of canagliflozin (Invokana), pioglitazone (Actos), and sitagliptin (Januvia), 24% identified pioglitazone as the one that made them feel best, 33% favored sitagliptin, 37% said canagliflozin was tops, and 6% had no preference, Beverley Shields, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
After making these selections based on just their qualitative self-appraisals, researchers told patients about their hemoglobin A1c status on each of the three agents. It barely budged their choices, which became 25% calling pioglitazone best, 35% naming sitagliptin their preference, 38% opting for canagliflozin, with 2% having no preference.
Further analysis showed that the drug patients preferred was also the one that produced their lowest A1c level when compared with their 8 months on each of the two other agents tested, showing a link between lower A1c levels and improved well-being. The same relationship existed for the drug that caused the fewest adverse events for each patient.
Patients prefer feeling better
“Patients tended to prefer the drug that they ‘felt better’ on, with the lowest A1c level and the lowest number of side effects,” explained Dr. Shields, a medical statistician at the University of Exeter (England). Changes in weight appeared less important to patients for establishing a preference.
“This is for when there is equipoise” among drug options, Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in an interview. “When you are unsure what to prescribe and there is no clear indication for one drug over another, try 4 months of one and 4 months of the other, then let the patient decide.
“Patients had overwhelming positivity about being able to choose their drug,” added Dr. Hattersley, who is also professor of molecular medicine at the University of Exeter.
“This has implications across medicine,” he added. “Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects.”
“I’m a bit worried by this approach, but it is something new” and worth considering, commented Drazenka P. Barlovic, MD, an endocrinologist at the University Medical Center in Ljubljana, Slovenia, who chaired the session where Dr. Shields gave her report. “We should also have the courage to challenge metformin, as there is no longer an obligation to make it the first drug,” she said in an interview.
The study ran as a secondary analysis of the TriMaster study, which had the primary objective of identifying patient characteristics that could predict which of the three drug options tested worked best for certain patient subgroups. That analysis, presented at the 2021 EASD annual meeting, found that factors such as body mass index and kidney function significantly linked with the clinical responses patients had to each of the three tested agents.
The new analysis focused on 457 of the TriMaster participants who had provided preference information after they had tried all three agents. By design, none of the participants enrolled in the study had a contraindication for any of the tested drugs.
Patients quickly identify adverse effects
“We picked 4 months because it not too long, but long enough to see adverse effects, and to measure on-treatment A1c. Patients quickly identify their adverse events,” Dr. Shields said in an interview.
“This could come into practice now; there is no cost involved. Do it when you’re not certain which drug to prescribe,” Dr. Hattersley suggested. “We can’t know which drug a patient might prefer.” He also stressed telling patients to return quicker than 4 months if they can’t tolerate a new drug.
The findings have already changed Dr. Hattersley’s practice, and he believes it will catch on as he introduces it to local primary care physicians.
The study received no commercial funding. Dr. Shields, Dr. Hattersley, and Dr. Barlovic had no disclosures.
AT EASD 2022
Desperate long COVID patients turn to unproven alternative therapies
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Early age at hysterectomy ups type 2 diabetes risk
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
Data from a large French cohort study suggest that women who have a hysterectomy before 40-45 years of age may be at particular risk of subsequently developing type 2 diabetes.
A 20% increase in the risk for incident diabetes was found comparing women of all ages who had and had not had a hysterectomy (P = .0003).
This risk jumped to a 52% increase when only women below the age of 45 were considered (P < .0001) and was still 38% higher if only women under 40 years were analyzed (P = .005).
“Our findings clearly show that hysterectomy is a risk marker for diabetes,” Fabrice Bonnet, MD, PhD, of Centre Hospitalier Universitaire (CHU) de Rennes (France), said at the annual meeting of the European Association for the Study of Diabetes.
Importantly, this risk appears to occur “independently of any hormonal therapy, any reproductive factors, physical activity, and diet,” Dr. Bonnet added.
Findings challenged
“I would like to challenge your findings,” said Peter Nilsson, MD, PhD, a professor at Lund (Sweden) University, during the postpresentation discussion period.
“Could there be a detection bias?” queried Dr. Nilsson. “If you undergo surgery like this, there will be several postoperative visits to a physician and there’s a higher likelihood of somebody taking blood samples and detecting diabetes.
“So, if this is true, it could mean that postoperative controls of goiter or thyroid surgery would bring the same findings,” Dr. Nilsson suggested.
“It is an epidemiological cohort of woman followed for a long time,” Dr. Bonnet responded. “So of course, there probably was more blood testing than in the usual population, but we did not observe the association for another type of surgery and type 2 diabetes.”
Clarifying further, Dr. Bonnet said that they had looked at thyroid surgery but not any other types of abdominal surgery.
Assessing the risk of incident diabetes
Hysterectomy is a common surgery among women – more than 400,000 are estimated to be performed every year in the United States, and 80,000 in France, with a rising rate in developing countries, Dr. Bonnet said in an interview.
“We don’t know exactly why that is, but it could have long-term consequences in terms of metabolic effects and the incidence of diabetes,” he said.
Prior research has linked having a hysterectomy with an increased rate of hypertension and cardiovascular risk, and there have also been a few studies linking it to diabetes.
“Our aim was to analyze the relationship between the past history of hysterectomies and the risk of incident diabetes; and specifically, we assessed the influence of age,” Dr. Bonnet said.
To do so, data on more than 83,000 women who had participated in The French E3N Prospective Cohort Study (E3N) were obtained. This large epidemiologic study is the French component of the long-running EPIC study.
For inclusion in the analysis, women had to have no diabetes at baseline, to have had their uterus, ovaries, or both removed for benign gynecologic reasons, and to have had their surgeries performed before any diagnosis of diabetes had been made. A diagnosis of diabetes was identified through the women’s responses to self-report questionnaires and prescriptions for antidiabetic medications.
In all, 2,672 women were found to have developed diabetes during the 16-year follow-up period.
The hazard ratio for the risk of diabetes in women who had and had not had a hysterectomy was 1.30 (95% confidence interval, 1.17-1.43; P < .0001), taking age into account and stratifying for birth generation.
The association held, when there was adjustment for other factors such as smoking status, physical activity, history of diabetes, weight, and adherence to a Mediterranean diet (HR 1.27; 95% CI 1.02-1.05; P = .02).
And, after adjustment for age at menarche, menopausal status, age at which menopause was reached, oral contraceptive and hormone therapy use, and the number of pregnancies, the risk for type 2 diabetes was still apparent in those who had undergoing a hysterectomy (HR, 1.20; 95% CI, 1.09-1.33; P = .0003).
Risk increased with oophorectomy
“Women who had both hysterectomy with bilateral oophorectomy had the highest rates of incident diabetes, as compared to women without hysterectomy and no oophorectomy,” said Dr. Bonnet (HR, 1.26; 95% CI, 1.11-1.42; P = .0003).
“This suggests preserving ovarian function is of importance,” he added. “Try to keep the ovaries in place, so just have hysterectomy alone,” he suggested might be the advice to fellow clinicians.
“So, identifying women at higher risk could be followed by a prevention program,” he suggested. “We do this for women who have gestational diabetes,” but for women who have had a hysterectomy, “we didn’t pay attention to this until now.”
No increased risk for endometriosis
While hysterectomy appears to up the risk for diabetes, having endometriosis does not. In a separate analysis of data from the E3N cohort, no effect was seen despite the association between endometriosis and other cardiometabolic risk factors.
The HR for incident type 2 diabetes comparing women with and without endometriosis was 10.06 in a fully adjusted statistical model (95% CI, 0.87-1.29). While there was an increase in the risk for diabetes if a woman had endometriosis and had also had a hysterectomy, this was not significant (HR, 1.22; 95% CI, 0.96-1.54).
The E3N study was sponsored by the French Institute for Health and Research. Dr. Bonnet and Dr. Nilsson had no relevant conflicts of interest to disclose.
FROM EASD 2022
Amulet, Watchman 2.5 LAAO outcomes neck and neck at 3 years
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
The Amplatzer Amulet (Abbott) and first-generation Watchman 2.5 (Boston Scientific) devices provide relatively comparable results out to 3 years after left atrial appendage occlusion (LAAO), longer follow-up from the Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE) trial shows.
“The dual-seal Amplatzer Amulet left atrial appendage occluder continued to demonstrate safety and effectiveness through 3 years,” principal investigator Dhanunjaya Lakkireddy, MD, said in a late-breaking session at the recent Transcatheter Cardiovascular Therapeutics annual meeting.
Preliminary results, reported last year, showed that procedural complications were higher with the Amplatzer but that it provided superior closure of the left atrial appendage (LAA) at 45 days and was noninferior with respect to safety at 12 months and efficacy at 18 months.
Amulet IDE is the largest head-to-head comparison of the two devices, enrolling 1,878 high-risk patients with nonvalvular atrial fibrillation undergoing LAA closure to reduce the risk of stroke.
Three-year follow-up was higher with the Amulet device than with the Watchman, at 721 vs. 659 patients, driven by increased deaths (85 vs. 63) and withdrawals (50 vs. 23) in the Watchman group within 18 months, noted Dr. Lakkireddy, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan.
Use of oral anticoagulation was higher in the Watchman group at 6 months (2.8% vs. 4.7%; P = .04), 18 months (3.1% vs. 5.6%; P = .01), and 3 years (3.7% vs. 7.3%; P < .01).
This was primarily driven by more late device-related thrombus (DRT) after 6 months with the Watchman device than with the Amulet occluder (23 vs. 10). “Perhaps the dual-closure mechanism of the Amulet explains this fundamental difference, where you have a nice smooth disc that covers the ostium,” he posited.
At 3 years, rates of cardiovascular death trended lower with Amulet than with Watchman (6.6% vs. 8.5%; P = .14), as did all-cause deaths (14.6% vs. 17.9%; P = .07).
Most cardiovascular deaths in the Amulet group were not preceded by a device factor, whereas DRT (1 vs. 4) and peridevice leak 3 mm or more (5 vs. 15) frequently preceded these deaths in the Watchman group, Dr. Lakkireddy observed. No pericardial effusion-related deaths occurred in either group.
Major bleeding, however, trended higher for the Amulet, at 16.1%, compared with 14.7% for the Watchman (P = .46). Ischemic stroke and systemic embolic rates also trended higher for Amulet, at 5%, and 4.6% for Watchman.
The protocol recommended aspirin only for both groups after 6 months. None of the 29 Amulet and 3 of the 29 Watchman patients with an ischemic stroke were on oral anticoagulation at the time of the stroke.
Device factors, however, frequently preceded ischemic strokes in the Watchman group, Dr. Lakkireddy said. DRT occurred in 1 patient with Amulet and 2 patients with Watchman and peridevice leak in 3 with Amulet and 15 with Watchman. “Again, the peridevice leak issue really stands out as an important factor,” he said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Based on “data from the large trials, it’s clearly evident that the presence of peridevice leak significantly raises the risk of stroke in follow-up,” he said. “So, attention has to be paid to the choice of the device and how we can mitigate the risk of peridevice leaks in these patients.”
The composite of stroke, systemic embolism, and cardiovascular death occurred in 11.1% of patients with Amulet and 12.7% with Watchman (P = .31).
Asked following the formal presentation whether the results justify use of one device over the other for LAA occlusion, Dr. Lakkireddy said he likes the dual closure mechanism of the Amulet and is more likely to use it in patients with proximal lobes, very large appendages, or a relatively shallow appendage. “In the rest of the cases, I think it’s a toss-up.”
As for how generalizable the results are, he noted that the study tested the Amulet against the legacy Watchman 2.5 but that the second-generation Watchman FLX is available in a larger size and has shown improved performance.
The Amplatzer Amulet does not require oral anticoagulants at discharge. However, the indication for the Watchman FLX was recently expanded to include 45-day dual antiplatelet therapy as a postprocedure alternative to oral anticoagulation plus aspirin.
Going forward, the “next evolution” is to test the Watchman FLX and Amulet on either single antiplatelet or a dual antiplatelet regimen without oral anticoagulation, he suggested.
Results from SWISS APERO, the first randomized trial to compare the Amulet and Watchman FLX (and a handful of 2.5 devices) in 221 patients, showed that the devices are not interchangeable for rates of complications or leaks.
During a press conference prior to the presentation, discussant Federico Asch, MD, MedStar Health Research Institute, Washington, said, “the most exciting thing here is that we have good options. We now can start to tease out which patients will benefit best from one or the other because we actually have two options.”
The Amulet IDE trial was funded by Abbott. Dr. Lakkireddy reports that he or his spouse/partner have received grant/research support from Abbott, AtriCure, Alta Thera, Medtronic, Biosense Webster, Biotronik, and Boston Scientific; and speaker honoraria from Abbott, Medtronic, Biotronik, and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Limiting antibiotic overprescription in pandemics: New guidelines
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Type 1 diabetes complication risk rises with A1c, duration
Long-term A1c from the time of type 1 diabetes diagnosis strongly predicts the development of severe retinopathy and nephropathy, new data suggest.
“[Weighted] HbA1c followed from diagnosis is a very strong biomarker for pan-retinal laser-treated diabetic retinopathy (PDR) and nephropathy, [and] the prevalence of both is still increasing 32 years after diagnosis,” say Hans J. Arnqvist, MD, and colleagues in their study published online Sept. 12 in Diabetes Care.
The results are from a 32-year follow-up of 447 patients from time of diagnosis of type 1 diabetes at age 0-34 in the Vascular Diabetic Complications in Southeast Sweden study.
“To avoid PDR and macroalbuminuria in patients with type 1 diabetes, A1c less than 7.0% (53 mmol/mol) and as normal as possible should be recommended when achievable without severe hypoglycemia and with good quality of life,” stress Dr. Arnqvist, department of endocrinology, Linköping University (Sweden), and coauthors.
At the time of the 20- to 24-year VISS follow-up, severe eye complications, defined as PDR, or nephropathy, defined as macroalbuminuria, were not present in participants with a long-term weighted mean A1c less than 7.6% (60 mmol/mol), they write.
Is explanation an increase in glycemic burden with diabetes duration?
By years 32-36, the prevalence of PDR had risen from 14% to 27%, and macroalbuminuria from 4% to 8%, with prevalence strongly correlated with A1c levels. At the same time, the threshold for the appearance of those severe complications dropped, with the lowest A1c values for appearance of PDR decreasing from 7.6% to 7.3% and for macroalbuminuria from 8.4% to 8.1%.
“A possible explanation for the lowered threshold for development of severe microangiopathy is the increase in ‘glycemic burden’ with diabetes duration,” the authors speculate.
In all A1c categories above 6.7% (> 50 mmol/mol), the cumulative proportion with PDR and/or macroproteinuria continued to increase up to at least 32 years of diabetes duration.
At the highest A1c quintile, greater than 9.5% (> 80mmol/mol), 75% had developed PDR and 44.2% had macroalbuminuria.
These findings align with guidelines from both the International Society for Pediatric and Adolescent Diabetes, which recommend A1c less than 7% (53 mmol/mol) as a treatment goal, and the UK National Institute for Health and Care Excellence, which advises a target A1c of 6.5% (48 mmol/mol) or lower in children and adults with type 1 diabetes.
The American Diabetes Association recommends individualized A1c targets ranging from 6.5% to 8.0%.
The study was supported by Barndiabetesfonden (Swedish Children’s Diabetes Foundation) and Region Ostergotlands Stiftelsefonder. The authors reported no further disclosures.
A version of this article first appeared on Medscape.com.
Long-term A1c from the time of type 1 diabetes diagnosis strongly predicts the development of severe retinopathy and nephropathy, new data suggest.
“[Weighted] HbA1c followed from diagnosis is a very strong biomarker for pan-retinal laser-treated diabetic retinopathy (PDR) and nephropathy, [and] the prevalence of both is still increasing 32 years after diagnosis,” say Hans J. Arnqvist, MD, and colleagues in their study published online Sept. 12 in Diabetes Care.
The results are from a 32-year follow-up of 447 patients from time of diagnosis of type 1 diabetes at age 0-34 in the Vascular Diabetic Complications in Southeast Sweden study.
“To avoid PDR and macroalbuminuria in patients with type 1 diabetes, A1c less than 7.0% (53 mmol/mol) and as normal as possible should be recommended when achievable without severe hypoglycemia and with good quality of life,” stress Dr. Arnqvist, department of endocrinology, Linköping University (Sweden), and coauthors.
At the time of the 20- to 24-year VISS follow-up, severe eye complications, defined as PDR, or nephropathy, defined as macroalbuminuria, were not present in participants with a long-term weighted mean A1c less than 7.6% (60 mmol/mol), they write.
Is explanation an increase in glycemic burden with diabetes duration?
By years 32-36, the prevalence of PDR had risen from 14% to 27%, and macroalbuminuria from 4% to 8%, with prevalence strongly correlated with A1c levels. At the same time, the threshold for the appearance of those severe complications dropped, with the lowest A1c values for appearance of PDR decreasing from 7.6% to 7.3% and for macroalbuminuria from 8.4% to 8.1%.
“A possible explanation for the lowered threshold for development of severe microangiopathy is the increase in ‘glycemic burden’ with diabetes duration,” the authors speculate.
In all A1c categories above 6.7% (> 50 mmol/mol), the cumulative proportion with PDR and/or macroproteinuria continued to increase up to at least 32 years of diabetes duration.
At the highest A1c quintile, greater than 9.5% (> 80mmol/mol), 75% had developed PDR and 44.2% had macroalbuminuria.
These findings align with guidelines from both the International Society for Pediatric and Adolescent Diabetes, which recommend A1c less than 7% (53 mmol/mol) as a treatment goal, and the UK National Institute for Health and Care Excellence, which advises a target A1c of 6.5% (48 mmol/mol) or lower in children and adults with type 1 diabetes.
The American Diabetes Association recommends individualized A1c targets ranging from 6.5% to 8.0%.
The study was supported by Barndiabetesfonden (Swedish Children’s Diabetes Foundation) and Region Ostergotlands Stiftelsefonder. The authors reported no further disclosures.
A version of this article first appeared on Medscape.com.
Long-term A1c from the time of type 1 diabetes diagnosis strongly predicts the development of severe retinopathy and nephropathy, new data suggest.
“[Weighted] HbA1c followed from diagnosis is a very strong biomarker for pan-retinal laser-treated diabetic retinopathy (PDR) and nephropathy, [and] the prevalence of both is still increasing 32 years after diagnosis,” say Hans J. Arnqvist, MD, and colleagues in their study published online Sept. 12 in Diabetes Care.
The results are from a 32-year follow-up of 447 patients from time of diagnosis of type 1 diabetes at age 0-34 in the Vascular Diabetic Complications in Southeast Sweden study.
“To avoid PDR and macroalbuminuria in patients with type 1 diabetes, A1c less than 7.0% (53 mmol/mol) and as normal as possible should be recommended when achievable without severe hypoglycemia and with good quality of life,” stress Dr. Arnqvist, department of endocrinology, Linköping University (Sweden), and coauthors.
At the time of the 20- to 24-year VISS follow-up, severe eye complications, defined as PDR, or nephropathy, defined as macroalbuminuria, were not present in participants with a long-term weighted mean A1c less than 7.6% (60 mmol/mol), they write.
Is explanation an increase in glycemic burden with diabetes duration?
By years 32-36, the prevalence of PDR had risen from 14% to 27%, and macroalbuminuria from 4% to 8%, with prevalence strongly correlated with A1c levels. At the same time, the threshold for the appearance of those severe complications dropped, with the lowest A1c values for appearance of PDR decreasing from 7.6% to 7.3% and for macroalbuminuria from 8.4% to 8.1%.
“A possible explanation for the lowered threshold for development of severe microangiopathy is the increase in ‘glycemic burden’ with diabetes duration,” the authors speculate.
In all A1c categories above 6.7% (> 50 mmol/mol), the cumulative proportion with PDR and/or macroproteinuria continued to increase up to at least 32 years of diabetes duration.
At the highest A1c quintile, greater than 9.5% (> 80mmol/mol), 75% had developed PDR and 44.2% had macroalbuminuria.
These findings align with guidelines from both the International Society for Pediatric and Adolescent Diabetes, which recommend A1c less than 7% (53 mmol/mol) as a treatment goal, and the UK National Institute for Health and Care Excellence, which advises a target A1c of 6.5% (48 mmol/mol) or lower in children and adults with type 1 diabetes.
The American Diabetes Association recommends individualized A1c targets ranging from 6.5% to 8.0%.
The study was supported by Barndiabetesfonden (Swedish Children’s Diabetes Foundation) and Region Ostergotlands Stiftelsefonder. The authors reported no further disclosures.
A version of this article first appeared on Medscape.com.












