User login
Pfizer asks FDA to authorize COVID vaccine for children younger than 5
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
The FDA has accepted Pfizer’s application for a COVID-19 vaccine for children under age 5, which clears the way for approval and distribution in June.
Pfizer announced June 1 that it completed the application for a three-dose vaccine for kids between 6 months and 5 years old, and the FDA said it received the emergency use application.
Children in this age group – the last to be eligible for COVID-19 vaccines – could begin getting shots as early as June 21, according to White House COVID-19 response coordinator Ashish Jha, MD.
Meanwhile, COVID-19 cases are still high – an average of 100,000 cases a day – but death numbers are about 90% lower than they were when President Joe Biden first took office, Dr. Jha said.
The FDA’s advisory group, the Vaccines and Related Biological Products Advisory Committee, is scheduled to meet June 14 and June 15 to discuss data submitted by both Pfizer and Moderna.
If the FDA gives them the green light, the CDC will then weigh in.
“We know that many, many parents are eager to vaccinate their youngest kids, and it’s important to do this right,” Dr. Jha said at a White House press briefing on June 2. “We expect that vaccinations will begin in earnest as early as June 21 and really roll on throughout that week.”
States can place their orders as early as June 3, Dr. Jha said, and there will initially be 10 million doses available. If the FDA gives emergency use authorization for the vaccines, the government will begin shipping doses to thousands of sites across the country.
“The good news is we have plenty of supply of Pfizer and Moderna vaccines,” Dr. Jha said. “We’ve asked states to distribute to their highest priority sites, serving the highest risk and hardest to reach areas.”
Pfizer’s clinical trials found that three doses of the vaccine for children 6 months to under 5 years were safe and effective and proved to be 80% effective against Omicron.
The FDA announced its meeting information with a conversation about the Moderna vaccine for ages 6-17 scheduled for June 14 and a conversation about the Pfizer and Moderna vaccines for young children scheduled for June 15.
Moderna applied for FDA authorization of its two-dose vaccine for children under age 6 on April 28. The company said the vaccine was 51% effective against infections with symptoms for children ages 6 months to 2 years and 37% effective for ages 2-5.
Pfizer’s 3-microgram dose is one-tenth of its adult dose. Moderna’s 25-microgram dose is one-quarter of its adult dose.
A version of this article first appeared on Medscape.com.
Immunotherapy treatment combo charts new course for resectable NSCLC treatment
Among patients with resectable non–small cell lung cancer (NSCLC), and more frequent pathological complete response in patients than chemotherapy alone.
“Our data show that three cycles of neoadjuvant nivolumab plus chemotherapy improved long-term clinical outcomes in patients with resectable stage IB-IIIA NSCLC without impeding the feasibility of surgery or increasing the incidence of adverse events as compared with chemotherapy alone,” wrote the investigators, who were led by Patrick M. Forde, MB, BCh, Johns Hopkins Kimmel Cancer Center, Baltimore. The study was published online in the New England Journal of Medicine.
Nivolumab (Opdivo, Bristol-Myers Squibb), in combination with platinum-doublet chemotherapy, was approved in March by the Food and Drug Administration as a treatment for adults with early-stage, resectable NSCLC. It is the first approval of a neoadjuvant therapy for this patient population. The results of the study, called CheckMate 816, formed the basis of the approval.
About one in four NSCLC patients have resectable disease at diagnosis, but their mortality rate is 30%-55% even after surgery. Neoadjuvant chemotherapy improves survival in this group, but 5-year recurrence rates improve by just 5%-6%, and rates of pathological complete response are low.
In the neoadjuvant setting, the anti–programmed death 1 (PD-1) antibody nivolumab could reduce micrometastases and boost immune response against bulk tumor and tumor antigens. A phase 2 study published in the Journal of Thoracic Oncology showed that neoadjuvant nivolumab combined with chemotherapy conferred good 3-year overall survival (81.9%) and progression-free survival (69.6%) among patients with stage IIIA NSCLC.
Results from CheckMate 816
CheckMate 816 is an open-label, phase 3 trial in which 358 patients were randomized to a neoadjuvant course of 360 mg nivolumab and platinum-doublet chemotherapy or platinum-doublet chemotherapy alone. Treatments occurred every 3 weeks for three cycles.
Definitive surgery was performed in 83.2% of the combination group (R0, 83.2%) and 75.4% in the chemotherapy-only group (R0, 77.8%). 93.8% in the combined group and 84.7% in the chemotherapy-only group completed neoadjuvant treatment. 11.9% of the combination group and 22.2% in the chemotherapy-only group underwent adjuvant therapy. A total of 21.2% in the combination group had cancer therapy versus 43.6% of the chemotherapy-only group.
After a minimum follow-up of 21 months, the combination group had a median event-free survival of 31.6 months versus 20.8 months in the chemotherapy-only group (hazard ratio for disease progression, disease recurrence, or death, 0.63; P = .005). The interim analysis for overall survival showed a possible trend towards improved overall survival in the combination group (HR, 0.57; 99.67% confidence interval, 0.30-1.07; P = .0008).
A total of 24.0% of the combination therapy achieved a pathological complete response versus 2.2% in the chemotherapy-only group (odds ratio, 13.94; P < .001).
Grade 3 or 4 treatment-related adverse events occurred in 33.5% of the combination group and 36.9% of the chemotherapy-only group.
The researchers noted that 63.1% of patients in the study had stage IIIA tumors, which has a poor prognosis.
There were benefits to the combination treatment across PD-1–status subgroups, but event-free survival was higher where PD-L1 expression level was 1% or more.
The study is limited by its open-label nature. It was funded by Bristol-Myers Squibb.
Among patients with resectable non–small cell lung cancer (NSCLC), and more frequent pathological complete response in patients than chemotherapy alone.
“Our data show that three cycles of neoadjuvant nivolumab plus chemotherapy improved long-term clinical outcomes in patients with resectable stage IB-IIIA NSCLC without impeding the feasibility of surgery or increasing the incidence of adverse events as compared with chemotherapy alone,” wrote the investigators, who were led by Patrick M. Forde, MB, BCh, Johns Hopkins Kimmel Cancer Center, Baltimore. The study was published online in the New England Journal of Medicine.
Nivolumab (Opdivo, Bristol-Myers Squibb), in combination with platinum-doublet chemotherapy, was approved in March by the Food and Drug Administration as a treatment for adults with early-stage, resectable NSCLC. It is the first approval of a neoadjuvant therapy for this patient population. The results of the study, called CheckMate 816, formed the basis of the approval.
About one in four NSCLC patients have resectable disease at diagnosis, but their mortality rate is 30%-55% even after surgery. Neoadjuvant chemotherapy improves survival in this group, but 5-year recurrence rates improve by just 5%-6%, and rates of pathological complete response are low.
In the neoadjuvant setting, the anti–programmed death 1 (PD-1) antibody nivolumab could reduce micrometastases and boost immune response against bulk tumor and tumor antigens. A phase 2 study published in the Journal of Thoracic Oncology showed that neoadjuvant nivolumab combined with chemotherapy conferred good 3-year overall survival (81.9%) and progression-free survival (69.6%) among patients with stage IIIA NSCLC.
Results from CheckMate 816
CheckMate 816 is an open-label, phase 3 trial in which 358 patients were randomized to a neoadjuvant course of 360 mg nivolumab and platinum-doublet chemotherapy or platinum-doublet chemotherapy alone. Treatments occurred every 3 weeks for three cycles.
Definitive surgery was performed in 83.2% of the combination group (R0, 83.2%) and 75.4% in the chemotherapy-only group (R0, 77.8%). 93.8% in the combined group and 84.7% in the chemotherapy-only group completed neoadjuvant treatment. 11.9% of the combination group and 22.2% in the chemotherapy-only group underwent adjuvant therapy. A total of 21.2% in the combination group had cancer therapy versus 43.6% of the chemotherapy-only group.
After a minimum follow-up of 21 months, the combination group had a median event-free survival of 31.6 months versus 20.8 months in the chemotherapy-only group (hazard ratio for disease progression, disease recurrence, or death, 0.63; P = .005). The interim analysis for overall survival showed a possible trend towards improved overall survival in the combination group (HR, 0.57; 99.67% confidence interval, 0.30-1.07; P = .0008).
A total of 24.0% of the combination therapy achieved a pathological complete response versus 2.2% in the chemotherapy-only group (odds ratio, 13.94; P < .001).
Grade 3 or 4 treatment-related adverse events occurred in 33.5% of the combination group and 36.9% of the chemotherapy-only group.
The researchers noted that 63.1% of patients in the study had stage IIIA tumors, which has a poor prognosis.
There were benefits to the combination treatment across PD-1–status subgroups, but event-free survival was higher where PD-L1 expression level was 1% or more.
The study is limited by its open-label nature. It was funded by Bristol-Myers Squibb.
Among patients with resectable non–small cell lung cancer (NSCLC), and more frequent pathological complete response in patients than chemotherapy alone.
“Our data show that three cycles of neoadjuvant nivolumab plus chemotherapy improved long-term clinical outcomes in patients with resectable stage IB-IIIA NSCLC without impeding the feasibility of surgery or increasing the incidence of adverse events as compared with chemotherapy alone,” wrote the investigators, who were led by Patrick M. Forde, MB, BCh, Johns Hopkins Kimmel Cancer Center, Baltimore. The study was published online in the New England Journal of Medicine.
Nivolumab (Opdivo, Bristol-Myers Squibb), in combination with platinum-doublet chemotherapy, was approved in March by the Food and Drug Administration as a treatment for adults with early-stage, resectable NSCLC. It is the first approval of a neoadjuvant therapy for this patient population. The results of the study, called CheckMate 816, formed the basis of the approval.
About one in four NSCLC patients have resectable disease at diagnosis, but their mortality rate is 30%-55% even after surgery. Neoadjuvant chemotherapy improves survival in this group, but 5-year recurrence rates improve by just 5%-6%, and rates of pathological complete response are low.
In the neoadjuvant setting, the anti–programmed death 1 (PD-1) antibody nivolumab could reduce micrometastases and boost immune response against bulk tumor and tumor antigens. A phase 2 study published in the Journal of Thoracic Oncology showed that neoadjuvant nivolumab combined with chemotherapy conferred good 3-year overall survival (81.9%) and progression-free survival (69.6%) among patients with stage IIIA NSCLC.
Results from CheckMate 816
CheckMate 816 is an open-label, phase 3 trial in which 358 patients were randomized to a neoadjuvant course of 360 mg nivolumab and platinum-doublet chemotherapy or platinum-doublet chemotherapy alone. Treatments occurred every 3 weeks for three cycles.
Definitive surgery was performed in 83.2% of the combination group (R0, 83.2%) and 75.4% in the chemotherapy-only group (R0, 77.8%). 93.8% in the combined group and 84.7% in the chemotherapy-only group completed neoadjuvant treatment. 11.9% of the combination group and 22.2% in the chemotherapy-only group underwent adjuvant therapy. A total of 21.2% in the combination group had cancer therapy versus 43.6% of the chemotherapy-only group.
After a minimum follow-up of 21 months, the combination group had a median event-free survival of 31.6 months versus 20.8 months in the chemotherapy-only group (hazard ratio for disease progression, disease recurrence, or death, 0.63; P = .005). The interim analysis for overall survival showed a possible trend towards improved overall survival in the combination group (HR, 0.57; 99.67% confidence interval, 0.30-1.07; P = .0008).
A total of 24.0% of the combination therapy achieved a pathological complete response versus 2.2% in the chemotherapy-only group (odds ratio, 13.94; P < .001).
Grade 3 or 4 treatment-related adverse events occurred in 33.5% of the combination group and 36.9% of the chemotherapy-only group.
The researchers noted that 63.1% of patients in the study had stage IIIA tumors, which has a poor prognosis.
There were benefits to the combination treatment across PD-1–status subgroups, but event-free survival was higher where PD-L1 expression level was 1% or more.
The study is limited by its open-label nature. It was funded by Bristol-Myers Squibb.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
ADA 2022 preview: Tirzepatide and much more
The full results on Lilly’s tirzepatide for obesity will likely dominate the headlines from the annual scientific sessions of the American Diabetes Association, but the conference program is jam-packed with new findings – and new paradigms – in both type 1 and type 2 diabetes management and prevention.
Taking place June 3-7 both in person – for the first time in 3 years – in New Orleans, and virtually, the “hybrid” meeting is mandating COVID-19 vaccination and mask wearing for all on-site attendees.
A major topic will be new findings and thinking in the treatment of type 2 diabetes, including the new twincretin tirzepatide, as well as discussions about the role of weight loss and the concept of “remission.” In type 1 diabetes, sessions will examine intervention trials to prevent progression, progress in islet transplantation, and the latest findings in diabetes technology.
Other key conference themes include the often interrelated topics of disparities, mental health, and COVID-19.
“I think that the scientific planning committee has put together a really outstanding program this year, covering the entire spectrum of diabetes care and research and translation for both type 1 and type 2 diabetes,” Scientific Planning Committee Chair Dana Dabelea, MD, PhD, professor of epidemiology and pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, told this news organization.
Tirzepatide: The next big thing?
The presentation likely to generate the most buzz will take place Saturday morning, with the full detailed results from Lilly’s phase 3 SURMOUNT-1 trial of its dual-incretin tirzepatide for weight loss in people with obesity or overweight with at least one comorbidity but not diabetes.
Top-line results released by Lilly in April 2022 showed that the drug induced weight loss of up to 22%. Tirzepatide was approved May 13 by the Food and Drug Administration for type 2 diabetes under the brand name Mounjaro. It is not approved for weight loss.
“Certainly the general public will latch on to this idea that there is a drug they can lose 22% of their weight on,” Robert A. Gabbay, MD, PhD, ADA chief science and medical officer, told this news organization. “It’s hard to comment on a press release, so that’s why this presentation is going to be key.”
Another tirzepatide analysis, this one comparing its use to insulin glargine on kidney outcomes in participants with diabetes in the pivotal SURPASS-4 study, will be presented as an ADA Presidents’ Select Abstract on Friday afternoon.
“I think tirzepatide could be the great new thing, but I think we need to know a little bit more. Weight loss seems to be better than with glucagon-like peptide-1 (GLP-1) receptor agonists. Renal outcomes are important. Next will be to see if it has cardiovascular benefit. It makes one think about its use versus GLP-1 agonists,” Dr. Gabbay said.
Managing type 2 diabetes: Shifting paradigms
With the emergence of tirzepatide and other pharmacologic agents with benefits beyond glucose lowering, there has been much discussion in recent years about alternatives to the current metformin monotherapy first, stepwise approach to managing type 2 diabetes.
As has been done previously, on Monday afternoon, there will be a joint ADA/European Association for the Study of Diabetes (EASD) session during which a draft of the latest update will be presented on the management of hyperglycemia in type 2 diabetes. The final version will be presented at the EASD meeting in September.
While it won’t include tirzepatide, as the drug is not yet approved in Europe, there will be discussion about the role of weight loss goals in type 2 diabetes management, Dr. Gabbay said.
The concept of a 15% weight loss as a primary treatment goal of type 2 diabetes management is a new focus, initiated at the EASD 2021 annual meeting and published in The Lancet.
“With tirzepatide becoming available, there’s the opportunity for more significant weight loss. So, there’s been this debate, starting with the somewhat controversial opinion piece in Lancet ... Maybe it was stating things a bit too far but it certainly got everyone in the field thinking. You’ll see that come up in lots of places at this meeting,” Dr. Gabbay said.
Indeed, those sessions include a Sunday morning symposium titled: “Obesity Management as a Primary Treatment Goal for Type 2 Diabetes – It’s Time for a Paradigm Shift,” in which speakers will address both lifestyle and pharmacologic intervention. On Saturday afternoon, two speakers will debate the question: “Weighing the Evidence – Should Obesity Be the Primary Target of Treatment in Type 2 Diabetes?” Yet another session on Sunday afternoon, will cover “Incorporating Weight Management Strategies for Obesity Into Type 2 Diabetes Care – Medical Management and Surgery.”
From weight loss to type 2 diabetes ‘remission’?
Related to the issue of weight loss as first-line therapy is the concept of type 2 diabetes “remission.” “There is a school of thought that says early in the course of disease we probably want to be a lot more aggressive because there’s a greater chance of putting someone into remission,” Dr. Gabbay noted. “The opportunities for remission after someone has had diabetes for a number of years are relatively low.”
In September 2021, ADA, along with EASD, the Endocrine Society, and Diabetes UK, published a joint consensus statement aiming to standardize use of the term “remission” in type 2 diabetes.
At the ADA meeting, a symposium on Monday afternoon, titled, “Definition and Interpretation of Remission in Type 2 Diabetes,” will cover lifestyle, pharmacotherapy, and metabolic surgery approaches. One noteworthy talk in that session will address the question: “Can Type 2 Diabetes Remission Be Diagnosed While Glucose-Lowering Drugs Are Being Used?”
Asked how all of this – tirzepatide, weight loss, and “remission” – might play out clinically, Dr. Dabelea replied: “We are still debating the strategy. That’s why we’re having the scientific talks.
“I think they will be very interesting and very well-attended, but there isn’t a strategy yet ... The important thing is we have these ‘miracle drugs,’ if you want, and once we’ve learned all we need to know about how they act and who we should target, perhaps next year we can talk about a strategy.”
Type 1 diabetes: Progress in preventing, treating, and ... curing?
Type 1 diabetes also will be well represented at the conference, with topics covering prevention, treatment, and progress toward a cure. On Saturday afternoon, a symposium will cover data from a trial of low-dose IL-2 in people with recently diagnosed type 1 diabetes, while a Friday afternoon symposium will address “Emerging Approaches to Beta Cell Replacement.”
On Saturday afternoon, a symposium will provide an update on islet cell transplantation, including immune tolerance strategies, while an oral abstract session will cover “Clinical Outcomes in Islet and Pancreas Transplantation.” And on Monday afternoon, yet another symposium will examine “Emerging Data on Therapies to Treat the Underlying Autoimmunity in Type 1 Diabetes.”
As usual, there will also be numerous presentations on the latest in diabetes technology. Particularly noteworthy among these will be an oral abstract presentation on Monday afternoon, “The CREATE Trial: Randomized Clinical Trial Comparing Open-Source Automated Insulin Delivery With Sensor Augmented Pump Therapy in Type 1 Diabetes,” and results from the insulin-only “bionic pancreas” pivotal randomized clinical trial on Friday afternoon.
“I’m happy to see a plethora of studies in type 1 diabetes. Dr. Dabelea said. “As with tirzepatide in type 2 diabetes, we are witnessing discoveries and we need to have some time to really understand the results, understand who are they targeting, who is going to benefit, and then move into a strategy.”
However, she added, in both type 1 and type 2 diabetes, “we’re seeing these disparities [where] these novel technologies and therapeutics are not getting to the people who need them most,” which brings up another major meeting theme, health disparities.
Overlapping themes: Disparities, mental health, and COVID-19
The topics of health disparities in diabetes prevention, management, and care and promoting health equity, as well as the impact of COVID-19, are “certainly timely this year,” said Dr. Dabelea.
At least eight meeting sessions will address various aspects of disparity, including a Friday afternoon symposium, “Race, Racism, and Diabetes Research,” a Saturday morning oral presentation on “Mitigating Disparities in the Screening and Diagnosis of Diabetes,” and on Monday morning, the symposium “Disparities in the Use of Diabetes Medications and Technologies.”
A related topic, insulin access, will be addressed in a Friday morning “mini-symposium” that will cover the issue from U.S. and international perspectives, including humanitarian crisis situations. Related to that, on Sunday afternoon a panel will discuss the Ukraine situation specifically.
Regarding mental health, one noteworthy session is a symposium on Saturday afternoon: “Suicide and Self-Injury – Unveiling and Addressing the Hidden Nightmare in Diabetes.”
“It’s an underrecognized problem and we’ve devoted a symposium to really drill into it. I think that’s going to be an important story for all of us to think about,” Dr. Gabbay said.
Another mental health session on Saturday afternoon will examine “Stigma in Diabetes Care – Evidence and Solutions.” Dr. Dabelea noted, “Mental health is a rising concern in the United States, especially in people with chronic diseases in the wake of the pandemic ... Of course there’s overlap in mechanisms in type 1 and type 2, but I think there are also distinct pathways.”
COVID-19 will be somewhat less of a focus than in the past 2 years, but there will certainly still be plenty about it. A Friday morning mini-symposium will cover new findings in pathophysiology, another session on Monday afternoon will look at the impact of the pandemic on hypoglycemia risk, and COVID-19 will be the subject of several late-breaking posters on Sunday afternoon. One in particular will report a review of diabetes as a risk factor for long COVID.
Celebrating in person in the Big Easy
But unlike the past 2 years, COVID-19 has not kept ADA from meeting in person in 2022. “I think it’s going to be amazing ... We’re so excited to be in person and interacting,” Dr. Gabbay said.
He observed that virtual meetings – as ADA and most other medical societies have been forced into for the past 2 years during the pandemic – fail to capture “how science is advanced by the casual conversations in the hallway and collaborations and new ideas. It’s really this incredible incubator. For me, that’s the most exciting part.”
The location, New Orleans, also factors into his excitement: “What a great place to do this. It’s conducive to celebrating. It’s been a long couple of years.”
A version of this article first appeared on Medscape.com.
The full results on Lilly’s tirzepatide for obesity will likely dominate the headlines from the annual scientific sessions of the American Diabetes Association, but the conference program is jam-packed with new findings – and new paradigms – in both type 1 and type 2 diabetes management and prevention.
Taking place June 3-7 both in person – for the first time in 3 years – in New Orleans, and virtually, the “hybrid” meeting is mandating COVID-19 vaccination and mask wearing for all on-site attendees.
A major topic will be new findings and thinking in the treatment of type 2 diabetes, including the new twincretin tirzepatide, as well as discussions about the role of weight loss and the concept of “remission.” In type 1 diabetes, sessions will examine intervention trials to prevent progression, progress in islet transplantation, and the latest findings in diabetes technology.
Other key conference themes include the often interrelated topics of disparities, mental health, and COVID-19.
“I think that the scientific planning committee has put together a really outstanding program this year, covering the entire spectrum of diabetes care and research and translation for both type 1 and type 2 diabetes,” Scientific Planning Committee Chair Dana Dabelea, MD, PhD, professor of epidemiology and pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, told this news organization.
Tirzepatide: The next big thing?
The presentation likely to generate the most buzz will take place Saturday morning, with the full detailed results from Lilly’s phase 3 SURMOUNT-1 trial of its dual-incretin tirzepatide for weight loss in people with obesity or overweight with at least one comorbidity but not diabetes.
Top-line results released by Lilly in April 2022 showed that the drug induced weight loss of up to 22%. Tirzepatide was approved May 13 by the Food and Drug Administration for type 2 diabetes under the brand name Mounjaro. It is not approved for weight loss.
“Certainly the general public will latch on to this idea that there is a drug they can lose 22% of their weight on,” Robert A. Gabbay, MD, PhD, ADA chief science and medical officer, told this news organization. “It’s hard to comment on a press release, so that’s why this presentation is going to be key.”
Another tirzepatide analysis, this one comparing its use to insulin glargine on kidney outcomes in participants with diabetes in the pivotal SURPASS-4 study, will be presented as an ADA Presidents’ Select Abstract on Friday afternoon.
“I think tirzepatide could be the great new thing, but I think we need to know a little bit more. Weight loss seems to be better than with glucagon-like peptide-1 (GLP-1) receptor agonists. Renal outcomes are important. Next will be to see if it has cardiovascular benefit. It makes one think about its use versus GLP-1 agonists,” Dr. Gabbay said.
Managing type 2 diabetes: Shifting paradigms
With the emergence of tirzepatide and other pharmacologic agents with benefits beyond glucose lowering, there has been much discussion in recent years about alternatives to the current metformin monotherapy first, stepwise approach to managing type 2 diabetes.
As has been done previously, on Monday afternoon, there will be a joint ADA/European Association for the Study of Diabetes (EASD) session during which a draft of the latest update will be presented on the management of hyperglycemia in type 2 diabetes. The final version will be presented at the EASD meeting in September.
While it won’t include tirzepatide, as the drug is not yet approved in Europe, there will be discussion about the role of weight loss goals in type 2 diabetes management, Dr. Gabbay said.
The concept of a 15% weight loss as a primary treatment goal of type 2 diabetes management is a new focus, initiated at the EASD 2021 annual meeting and published in The Lancet.
“With tirzepatide becoming available, there’s the opportunity for more significant weight loss. So, there’s been this debate, starting with the somewhat controversial opinion piece in Lancet ... Maybe it was stating things a bit too far but it certainly got everyone in the field thinking. You’ll see that come up in lots of places at this meeting,” Dr. Gabbay said.
Indeed, those sessions include a Sunday morning symposium titled: “Obesity Management as a Primary Treatment Goal for Type 2 Diabetes – It’s Time for a Paradigm Shift,” in which speakers will address both lifestyle and pharmacologic intervention. On Saturday afternoon, two speakers will debate the question: “Weighing the Evidence – Should Obesity Be the Primary Target of Treatment in Type 2 Diabetes?” Yet another session on Sunday afternoon, will cover “Incorporating Weight Management Strategies for Obesity Into Type 2 Diabetes Care – Medical Management and Surgery.”
From weight loss to type 2 diabetes ‘remission’?
Related to the issue of weight loss as first-line therapy is the concept of type 2 diabetes “remission.” “There is a school of thought that says early in the course of disease we probably want to be a lot more aggressive because there’s a greater chance of putting someone into remission,” Dr. Gabbay noted. “The opportunities for remission after someone has had diabetes for a number of years are relatively low.”
In September 2021, ADA, along with EASD, the Endocrine Society, and Diabetes UK, published a joint consensus statement aiming to standardize use of the term “remission” in type 2 diabetes.
At the ADA meeting, a symposium on Monday afternoon, titled, “Definition and Interpretation of Remission in Type 2 Diabetes,” will cover lifestyle, pharmacotherapy, and metabolic surgery approaches. One noteworthy talk in that session will address the question: “Can Type 2 Diabetes Remission Be Diagnosed While Glucose-Lowering Drugs Are Being Used?”
Asked how all of this – tirzepatide, weight loss, and “remission” – might play out clinically, Dr. Dabelea replied: “We are still debating the strategy. That’s why we’re having the scientific talks.
“I think they will be very interesting and very well-attended, but there isn’t a strategy yet ... The important thing is we have these ‘miracle drugs,’ if you want, and once we’ve learned all we need to know about how they act and who we should target, perhaps next year we can talk about a strategy.”
Type 1 diabetes: Progress in preventing, treating, and ... curing?
Type 1 diabetes also will be well represented at the conference, with topics covering prevention, treatment, and progress toward a cure. On Saturday afternoon, a symposium will cover data from a trial of low-dose IL-2 in people with recently diagnosed type 1 diabetes, while a Friday afternoon symposium will address “Emerging Approaches to Beta Cell Replacement.”
On Saturday afternoon, a symposium will provide an update on islet cell transplantation, including immune tolerance strategies, while an oral abstract session will cover “Clinical Outcomes in Islet and Pancreas Transplantation.” And on Monday afternoon, yet another symposium will examine “Emerging Data on Therapies to Treat the Underlying Autoimmunity in Type 1 Diabetes.”
As usual, there will also be numerous presentations on the latest in diabetes technology. Particularly noteworthy among these will be an oral abstract presentation on Monday afternoon, “The CREATE Trial: Randomized Clinical Trial Comparing Open-Source Automated Insulin Delivery With Sensor Augmented Pump Therapy in Type 1 Diabetes,” and results from the insulin-only “bionic pancreas” pivotal randomized clinical trial on Friday afternoon.
“I’m happy to see a plethora of studies in type 1 diabetes. Dr. Dabelea said. “As with tirzepatide in type 2 diabetes, we are witnessing discoveries and we need to have some time to really understand the results, understand who are they targeting, who is going to benefit, and then move into a strategy.”
However, she added, in both type 1 and type 2 diabetes, “we’re seeing these disparities [where] these novel technologies and therapeutics are not getting to the people who need them most,” which brings up another major meeting theme, health disparities.
Overlapping themes: Disparities, mental health, and COVID-19
The topics of health disparities in diabetes prevention, management, and care and promoting health equity, as well as the impact of COVID-19, are “certainly timely this year,” said Dr. Dabelea.
At least eight meeting sessions will address various aspects of disparity, including a Friday afternoon symposium, “Race, Racism, and Diabetes Research,” a Saturday morning oral presentation on “Mitigating Disparities in the Screening and Diagnosis of Diabetes,” and on Monday morning, the symposium “Disparities in the Use of Diabetes Medications and Technologies.”
A related topic, insulin access, will be addressed in a Friday morning “mini-symposium” that will cover the issue from U.S. and international perspectives, including humanitarian crisis situations. Related to that, on Sunday afternoon a panel will discuss the Ukraine situation specifically.
Regarding mental health, one noteworthy session is a symposium on Saturday afternoon: “Suicide and Self-Injury – Unveiling and Addressing the Hidden Nightmare in Diabetes.”
“It’s an underrecognized problem and we’ve devoted a symposium to really drill into it. I think that’s going to be an important story for all of us to think about,” Dr. Gabbay said.
Another mental health session on Saturday afternoon will examine “Stigma in Diabetes Care – Evidence and Solutions.” Dr. Dabelea noted, “Mental health is a rising concern in the United States, especially in people with chronic diseases in the wake of the pandemic ... Of course there’s overlap in mechanisms in type 1 and type 2, but I think there are also distinct pathways.”
COVID-19 will be somewhat less of a focus than in the past 2 years, but there will certainly still be plenty about it. A Friday morning mini-symposium will cover new findings in pathophysiology, another session on Monday afternoon will look at the impact of the pandemic on hypoglycemia risk, and COVID-19 will be the subject of several late-breaking posters on Sunday afternoon. One in particular will report a review of diabetes as a risk factor for long COVID.
Celebrating in person in the Big Easy
But unlike the past 2 years, COVID-19 has not kept ADA from meeting in person in 2022. “I think it’s going to be amazing ... We’re so excited to be in person and interacting,” Dr. Gabbay said.
He observed that virtual meetings – as ADA and most other medical societies have been forced into for the past 2 years during the pandemic – fail to capture “how science is advanced by the casual conversations in the hallway and collaborations and new ideas. It’s really this incredible incubator. For me, that’s the most exciting part.”
The location, New Orleans, also factors into his excitement: “What a great place to do this. It’s conducive to celebrating. It’s been a long couple of years.”
A version of this article first appeared on Medscape.com.
The full results on Lilly’s tirzepatide for obesity will likely dominate the headlines from the annual scientific sessions of the American Diabetes Association, but the conference program is jam-packed with new findings – and new paradigms – in both type 1 and type 2 diabetes management and prevention.
Taking place June 3-7 both in person – for the first time in 3 years – in New Orleans, and virtually, the “hybrid” meeting is mandating COVID-19 vaccination and mask wearing for all on-site attendees.
A major topic will be new findings and thinking in the treatment of type 2 diabetes, including the new twincretin tirzepatide, as well as discussions about the role of weight loss and the concept of “remission.” In type 1 diabetes, sessions will examine intervention trials to prevent progression, progress in islet transplantation, and the latest findings in diabetes technology.
Other key conference themes include the often interrelated topics of disparities, mental health, and COVID-19.
“I think that the scientific planning committee has put together a really outstanding program this year, covering the entire spectrum of diabetes care and research and translation for both type 1 and type 2 diabetes,” Scientific Planning Committee Chair Dana Dabelea, MD, PhD, professor of epidemiology and pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, told this news organization.
Tirzepatide: The next big thing?
The presentation likely to generate the most buzz will take place Saturday morning, with the full detailed results from Lilly’s phase 3 SURMOUNT-1 trial of its dual-incretin tirzepatide for weight loss in people with obesity or overweight with at least one comorbidity but not diabetes.
Top-line results released by Lilly in April 2022 showed that the drug induced weight loss of up to 22%. Tirzepatide was approved May 13 by the Food and Drug Administration for type 2 diabetes under the brand name Mounjaro. It is not approved for weight loss.
“Certainly the general public will latch on to this idea that there is a drug they can lose 22% of their weight on,” Robert A. Gabbay, MD, PhD, ADA chief science and medical officer, told this news organization. “It’s hard to comment on a press release, so that’s why this presentation is going to be key.”
Another tirzepatide analysis, this one comparing its use to insulin glargine on kidney outcomes in participants with diabetes in the pivotal SURPASS-4 study, will be presented as an ADA Presidents’ Select Abstract on Friday afternoon.
“I think tirzepatide could be the great new thing, but I think we need to know a little bit more. Weight loss seems to be better than with glucagon-like peptide-1 (GLP-1) receptor agonists. Renal outcomes are important. Next will be to see if it has cardiovascular benefit. It makes one think about its use versus GLP-1 agonists,” Dr. Gabbay said.
Managing type 2 diabetes: Shifting paradigms
With the emergence of tirzepatide and other pharmacologic agents with benefits beyond glucose lowering, there has been much discussion in recent years about alternatives to the current metformin monotherapy first, stepwise approach to managing type 2 diabetes.
As has been done previously, on Monday afternoon, there will be a joint ADA/European Association for the Study of Diabetes (EASD) session during which a draft of the latest update will be presented on the management of hyperglycemia in type 2 diabetes. The final version will be presented at the EASD meeting in September.
While it won’t include tirzepatide, as the drug is not yet approved in Europe, there will be discussion about the role of weight loss goals in type 2 diabetes management, Dr. Gabbay said.
The concept of a 15% weight loss as a primary treatment goal of type 2 diabetes management is a new focus, initiated at the EASD 2021 annual meeting and published in The Lancet.
“With tirzepatide becoming available, there’s the opportunity for more significant weight loss. So, there’s been this debate, starting with the somewhat controversial opinion piece in Lancet ... Maybe it was stating things a bit too far but it certainly got everyone in the field thinking. You’ll see that come up in lots of places at this meeting,” Dr. Gabbay said.
Indeed, those sessions include a Sunday morning symposium titled: “Obesity Management as a Primary Treatment Goal for Type 2 Diabetes – It’s Time for a Paradigm Shift,” in which speakers will address both lifestyle and pharmacologic intervention. On Saturday afternoon, two speakers will debate the question: “Weighing the Evidence – Should Obesity Be the Primary Target of Treatment in Type 2 Diabetes?” Yet another session on Sunday afternoon, will cover “Incorporating Weight Management Strategies for Obesity Into Type 2 Diabetes Care – Medical Management and Surgery.”
From weight loss to type 2 diabetes ‘remission’?
Related to the issue of weight loss as first-line therapy is the concept of type 2 diabetes “remission.” “There is a school of thought that says early in the course of disease we probably want to be a lot more aggressive because there’s a greater chance of putting someone into remission,” Dr. Gabbay noted. “The opportunities for remission after someone has had diabetes for a number of years are relatively low.”
In September 2021, ADA, along with EASD, the Endocrine Society, and Diabetes UK, published a joint consensus statement aiming to standardize use of the term “remission” in type 2 diabetes.
At the ADA meeting, a symposium on Monday afternoon, titled, “Definition and Interpretation of Remission in Type 2 Diabetes,” will cover lifestyle, pharmacotherapy, and metabolic surgery approaches. One noteworthy talk in that session will address the question: “Can Type 2 Diabetes Remission Be Diagnosed While Glucose-Lowering Drugs Are Being Used?”
Asked how all of this – tirzepatide, weight loss, and “remission” – might play out clinically, Dr. Dabelea replied: “We are still debating the strategy. That’s why we’re having the scientific talks.
“I think they will be very interesting and very well-attended, but there isn’t a strategy yet ... The important thing is we have these ‘miracle drugs,’ if you want, and once we’ve learned all we need to know about how they act and who we should target, perhaps next year we can talk about a strategy.”
Type 1 diabetes: Progress in preventing, treating, and ... curing?
Type 1 diabetes also will be well represented at the conference, with topics covering prevention, treatment, and progress toward a cure. On Saturday afternoon, a symposium will cover data from a trial of low-dose IL-2 in people with recently diagnosed type 1 diabetes, while a Friday afternoon symposium will address “Emerging Approaches to Beta Cell Replacement.”
On Saturday afternoon, a symposium will provide an update on islet cell transplantation, including immune tolerance strategies, while an oral abstract session will cover “Clinical Outcomes in Islet and Pancreas Transplantation.” And on Monday afternoon, yet another symposium will examine “Emerging Data on Therapies to Treat the Underlying Autoimmunity in Type 1 Diabetes.”
As usual, there will also be numerous presentations on the latest in diabetes technology. Particularly noteworthy among these will be an oral abstract presentation on Monday afternoon, “The CREATE Trial: Randomized Clinical Trial Comparing Open-Source Automated Insulin Delivery With Sensor Augmented Pump Therapy in Type 1 Diabetes,” and results from the insulin-only “bionic pancreas” pivotal randomized clinical trial on Friday afternoon.
“I’m happy to see a plethora of studies in type 1 diabetes. Dr. Dabelea said. “As with tirzepatide in type 2 diabetes, we are witnessing discoveries and we need to have some time to really understand the results, understand who are they targeting, who is going to benefit, and then move into a strategy.”
However, she added, in both type 1 and type 2 diabetes, “we’re seeing these disparities [where] these novel technologies and therapeutics are not getting to the people who need them most,” which brings up another major meeting theme, health disparities.
Overlapping themes: Disparities, mental health, and COVID-19
The topics of health disparities in diabetes prevention, management, and care and promoting health equity, as well as the impact of COVID-19, are “certainly timely this year,” said Dr. Dabelea.
At least eight meeting sessions will address various aspects of disparity, including a Friday afternoon symposium, “Race, Racism, and Diabetes Research,” a Saturday morning oral presentation on “Mitigating Disparities in the Screening and Diagnosis of Diabetes,” and on Monday morning, the symposium “Disparities in the Use of Diabetes Medications and Technologies.”
A related topic, insulin access, will be addressed in a Friday morning “mini-symposium” that will cover the issue from U.S. and international perspectives, including humanitarian crisis situations. Related to that, on Sunday afternoon a panel will discuss the Ukraine situation specifically.
Regarding mental health, one noteworthy session is a symposium on Saturday afternoon: “Suicide and Self-Injury – Unveiling and Addressing the Hidden Nightmare in Diabetes.”
“It’s an underrecognized problem and we’ve devoted a symposium to really drill into it. I think that’s going to be an important story for all of us to think about,” Dr. Gabbay said.
Another mental health session on Saturday afternoon will examine “Stigma in Diabetes Care – Evidence and Solutions.” Dr. Dabelea noted, “Mental health is a rising concern in the United States, especially in people with chronic diseases in the wake of the pandemic ... Of course there’s overlap in mechanisms in type 1 and type 2, but I think there are also distinct pathways.”
COVID-19 will be somewhat less of a focus than in the past 2 years, but there will certainly still be plenty about it. A Friday morning mini-symposium will cover new findings in pathophysiology, another session on Monday afternoon will look at the impact of the pandemic on hypoglycemia risk, and COVID-19 will be the subject of several late-breaking posters on Sunday afternoon. One in particular will report a review of diabetes as a risk factor for long COVID.
Celebrating in person in the Big Easy
But unlike the past 2 years, COVID-19 has not kept ADA from meeting in person in 2022. “I think it’s going to be amazing ... We’re so excited to be in person and interacting,” Dr. Gabbay said.
He observed that virtual meetings – as ADA and most other medical societies have been forced into for the past 2 years during the pandemic – fail to capture “how science is advanced by the casual conversations in the hallway and collaborations and new ideas. It’s really this incredible incubator. For me, that’s the most exciting part.”
The location, New Orleans, also factors into his excitement: “What a great place to do this. It’s conducive to celebrating. It’s been a long couple of years.”
A version of this article first appeared on Medscape.com.
ADA prioritizes heart failure in patients with diabetes
All U.S. patients with diabetes should undergo annual biomarker testing to allow for early diagnosis of progressive but presymptomatic heart failure, and treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class should expand among such patients to include everyone with stage B heart failure (“pre–heart failure”) or more advanced stages.
That’s a recommendation from an American Diabetes Association consensus report published June 1 in Diabetes Care.
The report notes that until now, “implementation of available strategies to detect asymptomatic heart failure [in patients with diabetes] has been suboptimal.” The remedy for this is that, “among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and to implement strategies to prevent transition to symptomatic heart failure.”
Written by a 10-member panel, chaired by Rodica Pop-Busui, MD, PhD, and endorsed by the American College of Cardiology, the document also set threshold for levels of these biomarkers that are diagnostic for a more advanced stage (stage B) of heart failure in patients with diabetes but without heart failure symptoms:
- A B-type natriuretic peptide (BNP) level of ≥50 pg/mL;
- An N-terminal pro-BNP level of ≥125 pg/mL; or
- Any high sensitivity cardiac troponin value that’s above the usual upper reference limit set at >99th percentile.
‘Inexpensive’ biomarker testing
“Addition of relatively inexpensive biomarker testing as part of the standard of care may help to refine heart failure risk prediction in individuals with diabetes,” the report says.
“Substantial data indicate the ability of these biomarkers to identify those in stage A or B [heart failure] at highest risk of progressing to symptomatic heart failure or death,” and this identification is useful because “the risk in such individuals may be lowered through targeted intervention or multidisciplinary care.”
It is “impossible to understate the importance of early recognition of heart failure” in patients with heart failure, the authors declare. However, the report also cautions that, “using biomarkers to identify and in turn reduce risk for heart failure should always be done within the context of a thoughtful clinical evaluation, supported by all information available.”
The report, written during March 2021 – March 2022, cites the high prevalence and increasing incidence of heart failure in patients with diabetes as the rationale for the new recommendations.
For a person with diabetes who receives a heart failure diagnosis, the report details several management steps, starting with an evaluation for obstructive coronary artery disease, given the strong link between diabetes and atherosclerotic cardiovascular disease.
It highlights the importance of interventions that involve nutrition, smoking avoidance, minimized alcohol intake, exercise, weight loss, and relevant social determinants of health, but focuses in greater detail on a range of pharmacologic interventions. These include treatment of hypertension for people with early-stage heart failure with an ACE inhibitor or an angiotensin receptor blocker, a thiazide-type diuretic, and a mineralocorticoid receptor antagonist, such as spironolactone or the newer, nonsteroidal agent finerenone for patients with diabetic kidney disease.
Dr. Busui of the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor, and colleagues cite recent recommendations for using guidelines-directed medical therapy to treat patients with more advanced, symptomatic stages of heart failure, including heart failure with reduced or with preserved ejection fraction.
‘Prioritize’ the SGLT2-inhibitor class
The consensus report also summarizes the roles for agents in the various classes of antidiabetes drugs now available, with particular emphasis on the role for the SGLT2-inhibitor class.
SGLT2 inhibitors “are recommended for all individuals with [diabetes and] heart failure,” it says. “This consensus recommends prioritizing the use of SGLT2 inhibitors in individuals with stage B heart failure, and that SGLT2 inhibitors be an expected element of care in all individuals with diabetes and symptomatic heart failure.”
Other agents for glycemic control that receive endorsement from the report are those in the glucagonlike peptide 1 receptor agonist class. “Despite the lack of conclusive evidence of direct heart failure risk reduction” with this class, it gets a “should be considered” designation, based on its positive effects on weight loss, blood pressure, and atherothrombotic disease.
Similar acknowledgment of potential benefit in a “should be considered” role goes to metformin. But the report turned a thumb down for both the class of dipeptidyl peptidase 4 inhibitors and the thiazolidinedione class, and said that agents from the insulin and sulfonylurea classes should be used “judiciously.”
The report did not identify any commercial funding. Several of the writing committee members listed personal commercial disclosures.
All U.S. patients with diabetes should undergo annual biomarker testing to allow for early diagnosis of progressive but presymptomatic heart failure, and treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class should expand among such patients to include everyone with stage B heart failure (“pre–heart failure”) or more advanced stages.
That’s a recommendation from an American Diabetes Association consensus report published June 1 in Diabetes Care.
The report notes that until now, “implementation of available strategies to detect asymptomatic heart failure [in patients with diabetes] has been suboptimal.” The remedy for this is that, “among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and to implement strategies to prevent transition to symptomatic heart failure.”
Written by a 10-member panel, chaired by Rodica Pop-Busui, MD, PhD, and endorsed by the American College of Cardiology, the document also set threshold for levels of these biomarkers that are diagnostic for a more advanced stage (stage B) of heart failure in patients with diabetes but without heart failure symptoms:
- A B-type natriuretic peptide (BNP) level of ≥50 pg/mL;
- An N-terminal pro-BNP level of ≥125 pg/mL; or
- Any high sensitivity cardiac troponin value that’s above the usual upper reference limit set at >99th percentile.
‘Inexpensive’ biomarker testing
“Addition of relatively inexpensive biomarker testing as part of the standard of care may help to refine heart failure risk prediction in individuals with diabetes,” the report says.
“Substantial data indicate the ability of these biomarkers to identify those in stage A or B [heart failure] at highest risk of progressing to symptomatic heart failure or death,” and this identification is useful because “the risk in such individuals may be lowered through targeted intervention or multidisciplinary care.”
It is “impossible to understate the importance of early recognition of heart failure” in patients with heart failure, the authors declare. However, the report also cautions that, “using biomarkers to identify and in turn reduce risk for heart failure should always be done within the context of a thoughtful clinical evaluation, supported by all information available.”
The report, written during March 2021 – March 2022, cites the high prevalence and increasing incidence of heart failure in patients with diabetes as the rationale for the new recommendations.
For a person with diabetes who receives a heart failure diagnosis, the report details several management steps, starting with an evaluation for obstructive coronary artery disease, given the strong link between diabetes and atherosclerotic cardiovascular disease.
It highlights the importance of interventions that involve nutrition, smoking avoidance, minimized alcohol intake, exercise, weight loss, and relevant social determinants of health, but focuses in greater detail on a range of pharmacologic interventions. These include treatment of hypertension for people with early-stage heart failure with an ACE inhibitor or an angiotensin receptor blocker, a thiazide-type diuretic, and a mineralocorticoid receptor antagonist, such as spironolactone or the newer, nonsteroidal agent finerenone for patients with diabetic kidney disease.
Dr. Busui of the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor, and colleagues cite recent recommendations for using guidelines-directed medical therapy to treat patients with more advanced, symptomatic stages of heart failure, including heart failure with reduced or with preserved ejection fraction.
‘Prioritize’ the SGLT2-inhibitor class
The consensus report also summarizes the roles for agents in the various classes of antidiabetes drugs now available, with particular emphasis on the role for the SGLT2-inhibitor class.
SGLT2 inhibitors “are recommended for all individuals with [diabetes and] heart failure,” it says. “This consensus recommends prioritizing the use of SGLT2 inhibitors in individuals with stage B heart failure, and that SGLT2 inhibitors be an expected element of care in all individuals with diabetes and symptomatic heart failure.”
Other agents for glycemic control that receive endorsement from the report are those in the glucagonlike peptide 1 receptor agonist class. “Despite the lack of conclusive evidence of direct heart failure risk reduction” with this class, it gets a “should be considered” designation, based on its positive effects on weight loss, blood pressure, and atherothrombotic disease.
Similar acknowledgment of potential benefit in a “should be considered” role goes to metformin. But the report turned a thumb down for both the class of dipeptidyl peptidase 4 inhibitors and the thiazolidinedione class, and said that agents from the insulin and sulfonylurea classes should be used “judiciously.”
The report did not identify any commercial funding. Several of the writing committee members listed personal commercial disclosures.
All U.S. patients with diabetes should undergo annual biomarker testing to allow for early diagnosis of progressive but presymptomatic heart failure, and treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class should expand among such patients to include everyone with stage B heart failure (“pre–heart failure”) or more advanced stages.
That’s a recommendation from an American Diabetes Association consensus report published June 1 in Diabetes Care.
The report notes that until now, “implementation of available strategies to detect asymptomatic heart failure [in patients with diabetes] has been suboptimal.” The remedy for this is that, “among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and to implement strategies to prevent transition to symptomatic heart failure.”
Written by a 10-member panel, chaired by Rodica Pop-Busui, MD, PhD, and endorsed by the American College of Cardiology, the document also set threshold for levels of these biomarkers that are diagnostic for a more advanced stage (stage B) of heart failure in patients with diabetes but without heart failure symptoms:
- A B-type natriuretic peptide (BNP) level of ≥50 pg/mL;
- An N-terminal pro-BNP level of ≥125 pg/mL; or
- Any high sensitivity cardiac troponin value that’s above the usual upper reference limit set at >99th percentile.
‘Inexpensive’ biomarker testing
“Addition of relatively inexpensive biomarker testing as part of the standard of care may help to refine heart failure risk prediction in individuals with diabetes,” the report says.
“Substantial data indicate the ability of these biomarkers to identify those in stage A or B [heart failure] at highest risk of progressing to symptomatic heart failure or death,” and this identification is useful because “the risk in such individuals may be lowered through targeted intervention or multidisciplinary care.”
It is “impossible to understate the importance of early recognition of heart failure” in patients with heart failure, the authors declare. However, the report also cautions that, “using biomarkers to identify and in turn reduce risk for heart failure should always be done within the context of a thoughtful clinical evaluation, supported by all information available.”
The report, written during March 2021 – March 2022, cites the high prevalence and increasing incidence of heart failure in patients with diabetes as the rationale for the new recommendations.
For a person with diabetes who receives a heart failure diagnosis, the report details several management steps, starting with an evaluation for obstructive coronary artery disease, given the strong link between diabetes and atherosclerotic cardiovascular disease.
It highlights the importance of interventions that involve nutrition, smoking avoidance, minimized alcohol intake, exercise, weight loss, and relevant social determinants of health, but focuses in greater detail on a range of pharmacologic interventions. These include treatment of hypertension for people with early-stage heart failure with an ACE inhibitor or an angiotensin receptor blocker, a thiazide-type diuretic, and a mineralocorticoid receptor antagonist, such as spironolactone or the newer, nonsteroidal agent finerenone for patients with diabetic kidney disease.
Dr. Busui of the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor, and colleagues cite recent recommendations for using guidelines-directed medical therapy to treat patients with more advanced, symptomatic stages of heart failure, including heart failure with reduced or with preserved ejection fraction.
‘Prioritize’ the SGLT2-inhibitor class
The consensus report also summarizes the roles for agents in the various classes of antidiabetes drugs now available, with particular emphasis on the role for the SGLT2-inhibitor class.
SGLT2 inhibitors “are recommended for all individuals with [diabetes and] heart failure,” it says. “This consensus recommends prioritizing the use of SGLT2 inhibitors in individuals with stage B heart failure, and that SGLT2 inhibitors be an expected element of care in all individuals with diabetes and symptomatic heart failure.”
Other agents for glycemic control that receive endorsement from the report are those in the glucagonlike peptide 1 receptor agonist class. “Despite the lack of conclusive evidence of direct heart failure risk reduction” with this class, it gets a “should be considered” designation, based on its positive effects on weight loss, blood pressure, and atherothrombotic disease.
Similar acknowledgment of potential benefit in a “should be considered” role goes to metformin. But the report turned a thumb down for both the class of dipeptidyl peptidase 4 inhibitors and the thiazolidinedione class, and said that agents from the insulin and sulfonylurea classes should be used “judiciously.”
The report did not identify any commercial funding. Several of the writing committee members listed personal commercial disclosures.
FROM DIABETES CARE
Neoadjuvant denosumab ineffective in breast cancer
Results from a new phase IIb, 2x2 randomized, open-label trial (GeparSEPTO) showed no improvement in pathologic complete response (pCR) rates when the RANKL inhibitor denosumab was added to anthracycline/taxane-based neoadjuvant chemotherapy in the treatment of primary breast cancer. The study found that a weekly anthracycline/taxane-based neoadjuvant chemotherapy led to improved pCR compared to an every 3-week schedule both overall and in patients with triple-negative breast cancer (TNBC), though it was associated with greater toxicity.
“Currently, I do not see a place for antiosteolytic agents as part of the neoadjuvant therapy in breast cancer,” said lead author Sibylle Loibl, MD, PhD, who is a breast cancer researcher at Goethe (Germany) University. The researchers can’t exclude the possibility of a long-term benefit, and patients will be followed for disease-free and overall survival.
, prompting optimism that the agents might improve pCR rates and improve survival rates in the neoadjuvant setting.
The failure may be because of the shorter treatment duration, and it’s possible that pCR is not the best endpoint to study for a drug that has long-acting potential, according to Dr. Loibl.
Patients were randomized to 120 mg denosumab every 4 weeks for six cycles or to receive no supplementary treatment. Patients with or without denosumab either received nab-paclitaxel 125 mg/m2 weekly for 12 weeks or on days 1 and 8 every 3 weeks, over four cycles (eight total doses), then epirubicin/cyclophosphamide, 90/600 mg/m2 (every 2 and 3 weeks, respectively). Patients with triple-negative breast cancer also received carboplatin. Patients with ERBB2-positive breast cancer received the trastuzumab biosimilar ABP980 plus pertuzumab.
The study included 780 patients (1 male), with a median age of 49.0 years. There was no difference in pCR among denosumab recipients and nonrecipients (41.0% versus 42.8%; P = .58). Weekly nab-paclitaxel led to a higher pCR rate than a schedule of days 1 and 8 every 3 weeks (44.9% versus 39.0%; P = .06, significance level of alpha = .10). Among subgroups, there was only a difference in pCR rates among those with TNBC (60.4% versus 50.0%; P = .06). Grade 3-4 toxic effects were similar regardless of denosumab exposure, but nonhematologic grade 3-4 toxicity was higher with weekly nab-paclitaxel (33.7% versus 24.1%; P = .004).
“The overall pCR difference (with nab-paclitaxel as neoadjuvant chemotherapy) seems small, but looking at the data from the GeparSEPTO study we would expect a transformation into a better invasive disease-free survival. Looking specifically into patients with TNBC, there is clear pCR increase by using the weekly regimen. With just over 60%, this is the highest pCR rate reported so far for a chemotherapy-only regimen. I would prefer to use nab-paclitaxel also in early breast cancer and would use the weekly regimen for women with TNBC who are at high risk,” Dr. Loibl said.
The study was limited by an imbalance of tumor subtypes between the treatment groups. The authors wrote that the results should guide further research, but nab-paclitaxel should not currently be viewed as a standard neoadjuvant treatment of breast cancer.
The study was funded by the German Breast Group, Bristol Myers Squibb, and Amgen. Dr. Loibl has served on the advisory boards or given lectures for AbbVie, Amgen, Bayer, Celgene, EirGenix, GSK, Lilly, Merck, Puma, Seagen, AstraZeneca, Daiichi Sankyo, Novartis, Pierre Fabre, Prime/Medscape, Bristol Myers Squibb, Chugai, Ipsen, Roche, and Samsung. She also holds a related patent.
Results from a new phase IIb, 2x2 randomized, open-label trial (GeparSEPTO) showed no improvement in pathologic complete response (pCR) rates when the RANKL inhibitor denosumab was added to anthracycline/taxane-based neoadjuvant chemotherapy in the treatment of primary breast cancer. The study found that a weekly anthracycline/taxane-based neoadjuvant chemotherapy led to improved pCR compared to an every 3-week schedule both overall and in patients with triple-negative breast cancer (TNBC), though it was associated with greater toxicity.
“Currently, I do not see a place for antiosteolytic agents as part of the neoadjuvant therapy in breast cancer,” said lead author Sibylle Loibl, MD, PhD, who is a breast cancer researcher at Goethe (Germany) University. The researchers can’t exclude the possibility of a long-term benefit, and patients will be followed for disease-free and overall survival.
, prompting optimism that the agents might improve pCR rates and improve survival rates in the neoadjuvant setting.
The failure may be because of the shorter treatment duration, and it’s possible that pCR is not the best endpoint to study for a drug that has long-acting potential, according to Dr. Loibl.
Patients were randomized to 120 mg denosumab every 4 weeks for six cycles or to receive no supplementary treatment. Patients with or without denosumab either received nab-paclitaxel 125 mg/m2 weekly for 12 weeks or on days 1 and 8 every 3 weeks, over four cycles (eight total doses), then epirubicin/cyclophosphamide, 90/600 mg/m2 (every 2 and 3 weeks, respectively). Patients with triple-negative breast cancer also received carboplatin. Patients with ERBB2-positive breast cancer received the trastuzumab biosimilar ABP980 plus pertuzumab.
The study included 780 patients (1 male), with a median age of 49.0 years. There was no difference in pCR among denosumab recipients and nonrecipients (41.0% versus 42.8%; P = .58). Weekly nab-paclitaxel led to a higher pCR rate than a schedule of days 1 and 8 every 3 weeks (44.9% versus 39.0%; P = .06, significance level of alpha = .10). Among subgroups, there was only a difference in pCR rates among those with TNBC (60.4% versus 50.0%; P = .06). Grade 3-4 toxic effects were similar regardless of denosumab exposure, but nonhematologic grade 3-4 toxicity was higher with weekly nab-paclitaxel (33.7% versus 24.1%; P = .004).
“The overall pCR difference (with nab-paclitaxel as neoadjuvant chemotherapy) seems small, but looking at the data from the GeparSEPTO study we would expect a transformation into a better invasive disease-free survival. Looking specifically into patients with TNBC, there is clear pCR increase by using the weekly regimen. With just over 60%, this is the highest pCR rate reported so far for a chemotherapy-only regimen. I would prefer to use nab-paclitaxel also in early breast cancer and would use the weekly regimen for women with TNBC who are at high risk,” Dr. Loibl said.
The study was limited by an imbalance of tumor subtypes between the treatment groups. The authors wrote that the results should guide further research, but nab-paclitaxel should not currently be viewed as a standard neoadjuvant treatment of breast cancer.
The study was funded by the German Breast Group, Bristol Myers Squibb, and Amgen. Dr. Loibl has served on the advisory boards or given lectures for AbbVie, Amgen, Bayer, Celgene, EirGenix, GSK, Lilly, Merck, Puma, Seagen, AstraZeneca, Daiichi Sankyo, Novartis, Pierre Fabre, Prime/Medscape, Bristol Myers Squibb, Chugai, Ipsen, Roche, and Samsung. She also holds a related patent.
Results from a new phase IIb, 2x2 randomized, open-label trial (GeparSEPTO) showed no improvement in pathologic complete response (pCR) rates when the RANKL inhibitor denosumab was added to anthracycline/taxane-based neoadjuvant chemotherapy in the treatment of primary breast cancer. The study found that a weekly anthracycline/taxane-based neoadjuvant chemotherapy led to improved pCR compared to an every 3-week schedule both overall and in patients with triple-negative breast cancer (TNBC), though it was associated with greater toxicity.
“Currently, I do not see a place for antiosteolytic agents as part of the neoadjuvant therapy in breast cancer,” said lead author Sibylle Loibl, MD, PhD, who is a breast cancer researcher at Goethe (Germany) University. The researchers can’t exclude the possibility of a long-term benefit, and patients will be followed for disease-free and overall survival.
, prompting optimism that the agents might improve pCR rates and improve survival rates in the neoadjuvant setting.
The failure may be because of the shorter treatment duration, and it’s possible that pCR is not the best endpoint to study for a drug that has long-acting potential, according to Dr. Loibl.
Patients were randomized to 120 mg denosumab every 4 weeks for six cycles or to receive no supplementary treatment. Patients with or without denosumab either received nab-paclitaxel 125 mg/m2 weekly for 12 weeks or on days 1 and 8 every 3 weeks, over four cycles (eight total doses), then epirubicin/cyclophosphamide, 90/600 mg/m2 (every 2 and 3 weeks, respectively). Patients with triple-negative breast cancer also received carboplatin. Patients with ERBB2-positive breast cancer received the trastuzumab biosimilar ABP980 plus pertuzumab.
The study included 780 patients (1 male), with a median age of 49.0 years. There was no difference in pCR among denosumab recipients and nonrecipients (41.0% versus 42.8%; P = .58). Weekly nab-paclitaxel led to a higher pCR rate than a schedule of days 1 and 8 every 3 weeks (44.9% versus 39.0%; P = .06, significance level of alpha = .10). Among subgroups, there was only a difference in pCR rates among those with TNBC (60.4% versus 50.0%; P = .06). Grade 3-4 toxic effects were similar regardless of denosumab exposure, but nonhematologic grade 3-4 toxicity was higher with weekly nab-paclitaxel (33.7% versus 24.1%; P = .004).
“The overall pCR difference (with nab-paclitaxel as neoadjuvant chemotherapy) seems small, but looking at the data from the GeparSEPTO study we would expect a transformation into a better invasive disease-free survival. Looking specifically into patients with TNBC, there is clear pCR increase by using the weekly regimen. With just over 60%, this is the highest pCR rate reported so far for a chemotherapy-only regimen. I would prefer to use nab-paclitaxel also in early breast cancer and would use the weekly regimen for women with TNBC who are at high risk,” Dr. Loibl said.
The study was limited by an imbalance of tumor subtypes between the treatment groups. The authors wrote that the results should guide further research, but nab-paclitaxel should not currently be viewed as a standard neoadjuvant treatment of breast cancer.
The study was funded by the German Breast Group, Bristol Myers Squibb, and Amgen. Dr. Loibl has served on the advisory boards or given lectures for AbbVie, Amgen, Bayer, Celgene, EirGenix, GSK, Lilly, Merck, Puma, Seagen, AstraZeneca, Daiichi Sankyo, Novartis, Pierre Fabre, Prime/Medscape, Bristol Myers Squibb, Chugai, Ipsen, Roche, and Samsung. She also holds a related patent.
REPORTING FROM JAMA ONCOLOGY
FDA withdraws lymphoma drug approval after investigation
Umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
But safety concerns began to emerge in the phase 3 UNITY-CLL trial, which evaluated the drug in a related cancer type: chronic lymphocytic leukemia.
Last February, the FDA said it was investigating a possible increased risk of death associated with umbralisib.
Five months later, the results are in.
“Updated findings from the UNITY-CLL clinical trial continued to show a possible increased risk of death in patients receiving Ukoniq. As a result, we determined the risks of treatment with Ukoniq outweigh its benefits,” the FDA wrote in a drug safety communication published June 1.
In April, the drug manufacturer, TG Therapeutics, announced it was voluntarily withdrawing umbralisib from the market for its approved uses in marginal zone lymphoma and follicular lymphoma.
The FDA’s safety notice includes instructions for physicians and patients. The FDA urges health care professionals to “stop prescribing Ukoniq and switch patients to alternative treatments” and to “inform patients currently taking Ukoniq of the increased risk of death seen in the clinical trial and advise them to stop taking the medicine.”
In special instances in which a patient may be benefiting from the drug, the company plans to make umbralisib available under expanded access.
The FDA also recommends that patients who discontinue taking the drug dispose of unused umbralisib using a drug take-back location, such as a pharmacy, or throwing it away in the household trash after placing it in a sealed bag mixed with dirt or cat litter and removing personal identification information.
A version of this article first appeared on Medscape.com.
Umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
But safety concerns began to emerge in the phase 3 UNITY-CLL trial, which evaluated the drug in a related cancer type: chronic lymphocytic leukemia.
Last February, the FDA said it was investigating a possible increased risk of death associated with umbralisib.
Five months later, the results are in.
“Updated findings from the UNITY-CLL clinical trial continued to show a possible increased risk of death in patients receiving Ukoniq. As a result, we determined the risks of treatment with Ukoniq outweigh its benefits,” the FDA wrote in a drug safety communication published June 1.
In April, the drug manufacturer, TG Therapeutics, announced it was voluntarily withdrawing umbralisib from the market for its approved uses in marginal zone lymphoma and follicular lymphoma.
The FDA’s safety notice includes instructions for physicians and patients. The FDA urges health care professionals to “stop prescribing Ukoniq and switch patients to alternative treatments” and to “inform patients currently taking Ukoniq of the increased risk of death seen in the clinical trial and advise them to stop taking the medicine.”
In special instances in which a patient may be benefiting from the drug, the company plans to make umbralisib available under expanded access.
The FDA also recommends that patients who discontinue taking the drug dispose of unused umbralisib using a drug take-back location, such as a pharmacy, or throwing it away in the household trash after placing it in a sealed bag mixed with dirt or cat litter and removing personal identification information.
A version of this article first appeared on Medscape.com.
Umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
But safety concerns began to emerge in the phase 3 UNITY-CLL trial, which evaluated the drug in a related cancer type: chronic lymphocytic leukemia.
Last February, the FDA said it was investigating a possible increased risk of death associated with umbralisib.
Five months later, the results are in.
“Updated findings from the UNITY-CLL clinical trial continued to show a possible increased risk of death in patients receiving Ukoniq. As a result, we determined the risks of treatment with Ukoniq outweigh its benefits,” the FDA wrote in a drug safety communication published June 1.
In April, the drug manufacturer, TG Therapeutics, announced it was voluntarily withdrawing umbralisib from the market for its approved uses in marginal zone lymphoma and follicular lymphoma.
The FDA’s safety notice includes instructions for physicians and patients. The FDA urges health care professionals to “stop prescribing Ukoniq and switch patients to alternative treatments” and to “inform patients currently taking Ukoniq of the increased risk of death seen in the clinical trial and advise them to stop taking the medicine.”
In special instances in which a patient may be benefiting from the drug, the company plans to make umbralisib available under expanded access.
The FDA also recommends that patients who discontinue taking the drug dispose of unused umbralisib using a drug take-back location, such as a pharmacy, or throwing it away in the household trash after placing it in a sealed bag mixed with dirt or cat litter and removing personal identification information.
A version of this article first appeared on Medscape.com.
FDA clears Abbott Freestyle Libre 3 glucose sensor
The Food and Drug Administration has cleared Abbot’s Freestyle Libre 3 system for use by people aged 4 years and older with diabetes.
The new system was cleared for use for both iOS- and Android-compatible mobile apps, enabling real-time glucose readings in contrast to the “intermittently scanned” capability of prior Libre versions. The Libre 3 allows for optional alarms and notifications of urgent low or high glucose levels, as well as remote monitoring by health care professionals or the patient’s family members and/or friends.
The FreeStyle Libre 3 was granted a CE Mark in Europe in October 2020.
Smaller, thinner, and better integration
According to Abbott, the Libre 3 is the first continuous glucose monitoring (CGM) system to show a mean absolute relative difference (MARD) of less than 8% compared with a gold-standard glucose measure. The average Libre 3 MARD is 7.9%, compared with 9.3% for the Libre 2. The Libre 3 is also the “smallest and thinnest” CGM, roughly the size of two stacked U.S. pennies, worn on the upper arm.
And, the company said, the Libre 3 has a Bluetooth integration of up to 33 feet, a range 50% further than other CGMs.
This version follows the FreeStyle Libre 2, approved in June 2020, and its compatible iPhone app, approved in August 2021.
The Libre 3 will be priced the same as the Libre 2, at about one-third the cost of other CGM systems. However, it is not currently eligible for Medicare reimbursement. Medicaid eligibility may vary by state.
“I applaud Abbott for making their CGM system the most affordable and addressing disparities in care so patients living with diabetes can avoid complications and optimize their quality of life,” Eugene E. Wright Jr., MD, of Duke University, Durham, N.C., said in an Abbott statement.
“I have seen real-world evidence that diabetes technologies like CGMs have helped my patients safely achieve improved glycemic control,” he said.
The FreeStyle Libre 3 sensor will be available at participating pharmacies later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared Abbot’s Freestyle Libre 3 system for use by people aged 4 years and older with diabetes.
The new system was cleared for use for both iOS- and Android-compatible mobile apps, enabling real-time glucose readings in contrast to the “intermittently scanned” capability of prior Libre versions. The Libre 3 allows for optional alarms and notifications of urgent low or high glucose levels, as well as remote monitoring by health care professionals or the patient’s family members and/or friends.
The FreeStyle Libre 3 was granted a CE Mark in Europe in October 2020.
Smaller, thinner, and better integration
According to Abbott, the Libre 3 is the first continuous glucose monitoring (CGM) system to show a mean absolute relative difference (MARD) of less than 8% compared with a gold-standard glucose measure. The average Libre 3 MARD is 7.9%, compared with 9.3% for the Libre 2. The Libre 3 is also the “smallest and thinnest” CGM, roughly the size of two stacked U.S. pennies, worn on the upper arm.
And, the company said, the Libre 3 has a Bluetooth integration of up to 33 feet, a range 50% further than other CGMs.
This version follows the FreeStyle Libre 2, approved in June 2020, and its compatible iPhone app, approved in August 2021.
The Libre 3 will be priced the same as the Libre 2, at about one-third the cost of other CGM systems. However, it is not currently eligible for Medicare reimbursement. Medicaid eligibility may vary by state.
“I applaud Abbott for making their CGM system the most affordable and addressing disparities in care so patients living with diabetes can avoid complications and optimize their quality of life,” Eugene E. Wright Jr., MD, of Duke University, Durham, N.C., said in an Abbott statement.
“I have seen real-world evidence that diabetes technologies like CGMs have helped my patients safely achieve improved glycemic control,” he said.
The FreeStyle Libre 3 sensor will be available at participating pharmacies later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared Abbot’s Freestyle Libre 3 system for use by people aged 4 years and older with diabetes.
The new system was cleared for use for both iOS- and Android-compatible mobile apps, enabling real-time glucose readings in contrast to the “intermittently scanned” capability of prior Libre versions. The Libre 3 allows for optional alarms and notifications of urgent low or high glucose levels, as well as remote monitoring by health care professionals or the patient’s family members and/or friends.
The FreeStyle Libre 3 was granted a CE Mark in Europe in October 2020.
Smaller, thinner, and better integration
According to Abbott, the Libre 3 is the first continuous glucose monitoring (CGM) system to show a mean absolute relative difference (MARD) of less than 8% compared with a gold-standard glucose measure. The average Libre 3 MARD is 7.9%, compared with 9.3% for the Libre 2. The Libre 3 is also the “smallest and thinnest” CGM, roughly the size of two stacked U.S. pennies, worn on the upper arm.
And, the company said, the Libre 3 has a Bluetooth integration of up to 33 feet, a range 50% further than other CGMs.
This version follows the FreeStyle Libre 2, approved in June 2020, and its compatible iPhone app, approved in August 2021.
The Libre 3 will be priced the same as the Libre 2, at about one-third the cost of other CGM systems. However, it is not currently eligible for Medicare reimbursement. Medicaid eligibility may vary by state.
“I applaud Abbott for making their CGM system the most affordable and addressing disparities in care so patients living with diabetes can avoid complications and optimize their quality of life,” Eugene E. Wright Jr., MD, of Duke University, Durham, N.C., said in an Abbott statement.
“I have seen real-world evidence that diabetes technologies like CGMs have helped my patients safely achieve improved glycemic control,” he said.
The FreeStyle Libre 3 sensor will be available at participating pharmacies later this year.
A version of this article first appeared on Medscape.com.
Can lung cancer ID be as easy as breathing into an analyzer?
A study published in May in The Lancet journal eClinicalMedicine reports that
The tool was successfully used to identify, in 84 patients, 16 lung cancer–related carcinogenic volatile compounds (VOCs), such as aldehydes, hydrocarbons, ketones, carboxylic acids, and furan – some of which are compounds used in the production of common household goods, such as furniture, carpeting, and wood floors.
“The test is anticipated to be highlighted for primary screening of lung cancer but not the final diagnosis,” according to study authors who were led by Peiyu Wang, MD, PhD, chair of social medicine and health at Peking (China) University.
While early diagnosis and treatment are critical for improving lung cancer survival, early detection of lung cancer is challenging because of the lack of clinical manifestations and specific biomarkers. Annual CT scans are costly and include radiation exposure, Dr. Wang and his associates wrote.
Breathomics testing is considered a promising method for detection and screening for lung cancer. It has been under study for years and in 2014, researchers from Belgium published a review in Cancer Epidemiology Biomarkers and Prevention documenting the use of VOCs as early diagnostic or prognostic biomarkers for mesothelioma.
Lung cancer breath biomarkers identified in various studies have been highly heterogeneous because of differing sample collection methods, varying patient conditions, testing environments, and analysis methods. As a result, there currently is no breathomics test for lung cancer screening, Dr. Wang said in an interview.
In terms of its potential as a lung cancer screening tool, “Clinicians may introduce this test for people with high risk for lung cancer, such as elderly smokers, or people with suspected symptoms. It may also be introduced for young populations with subjective or objective needs to screen for lung cancer. As the proportion of lung adenocarcinoma in nonsmoking young women is increasing, the test may be a good method for lung cancer screening in this population,” Dr. Wang said.
After adjusting for age, sex, smoking, and comorbidities, researchers found elevated levels for 16 VOCs in patients with lung cancer. A diagnostic model including the 16 VOCs achieved an area under the curve of 0.952, sensitivity of 89.2%, specificity of 89.1%, and accuracy of 89.1% in lung cancer diagnosis. A model including the top eight VOCs achieved an area under the curve of 0.931, sensitivity of 86.0%, specificity of 87.2%, and accuracy of 86.9%.
After selecting 28 VOCs as candidates through a literature review, Dr. Wang and associates conducted a prospective discovery study from Sept. 1 to Dec. 31, 2020, using high-pressure photon ionization time-of-flight mass spectrometry to evaluate their performance for lung cancer diagnosis. The validation study included 157 lung cancer patients (mean age 57.0 years; 54.1 percent female) and 368 volunteers (mean age 44.5 years; 31.3% female).
“The external validation confirmed good performance of these biomarkers in lung cancer detection,” the researchers stated. It helped, they added, to solve the heterogeneity among published studies, establishing both 16 VOCs and 8 VOCS for lung cancer screening.
The authors stated that a large gap exists between breathomics research and clinical practices in lung cancer detection and screening. While the validated 16 VOCs, mainly aldehydes and hydrocarbon, showed potential for promoting this lung cancer screening strategy, more scientific studies are warranted to investigate the underlying mechanisms of identified lung cancer VOCs.
Dr. Wang declared no competing interests.
A study published in May in The Lancet journal eClinicalMedicine reports that
The tool was successfully used to identify, in 84 patients, 16 lung cancer–related carcinogenic volatile compounds (VOCs), such as aldehydes, hydrocarbons, ketones, carboxylic acids, and furan – some of which are compounds used in the production of common household goods, such as furniture, carpeting, and wood floors.
“The test is anticipated to be highlighted for primary screening of lung cancer but not the final diagnosis,” according to study authors who were led by Peiyu Wang, MD, PhD, chair of social medicine and health at Peking (China) University.
While early diagnosis and treatment are critical for improving lung cancer survival, early detection of lung cancer is challenging because of the lack of clinical manifestations and specific biomarkers. Annual CT scans are costly and include radiation exposure, Dr. Wang and his associates wrote.
Breathomics testing is considered a promising method for detection and screening for lung cancer. It has been under study for years and in 2014, researchers from Belgium published a review in Cancer Epidemiology Biomarkers and Prevention documenting the use of VOCs as early diagnostic or prognostic biomarkers for mesothelioma.
Lung cancer breath biomarkers identified in various studies have been highly heterogeneous because of differing sample collection methods, varying patient conditions, testing environments, and analysis methods. As a result, there currently is no breathomics test for lung cancer screening, Dr. Wang said in an interview.
In terms of its potential as a lung cancer screening tool, “Clinicians may introduce this test for people with high risk for lung cancer, such as elderly smokers, or people with suspected symptoms. It may also be introduced for young populations with subjective or objective needs to screen for lung cancer. As the proportion of lung adenocarcinoma in nonsmoking young women is increasing, the test may be a good method for lung cancer screening in this population,” Dr. Wang said.
After adjusting for age, sex, smoking, and comorbidities, researchers found elevated levels for 16 VOCs in patients with lung cancer. A diagnostic model including the 16 VOCs achieved an area under the curve of 0.952, sensitivity of 89.2%, specificity of 89.1%, and accuracy of 89.1% in lung cancer diagnosis. A model including the top eight VOCs achieved an area under the curve of 0.931, sensitivity of 86.0%, specificity of 87.2%, and accuracy of 86.9%.
After selecting 28 VOCs as candidates through a literature review, Dr. Wang and associates conducted a prospective discovery study from Sept. 1 to Dec. 31, 2020, using high-pressure photon ionization time-of-flight mass spectrometry to evaluate their performance for lung cancer diagnosis. The validation study included 157 lung cancer patients (mean age 57.0 years; 54.1 percent female) and 368 volunteers (mean age 44.5 years; 31.3% female).
“The external validation confirmed good performance of these biomarkers in lung cancer detection,” the researchers stated. It helped, they added, to solve the heterogeneity among published studies, establishing both 16 VOCs and 8 VOCS for lung cancer screening.
The authors stated that a large gap exists between breathomics research and clinical practices in lung cancer detection and screening. While the validated 16 VOCs, mainly aldehydes and hydrocarbon, showed potential for promoting this lung cancer screening strategy, more scientific studies are warranted to investigate the underlying mechanisms of identified lung cancer VOCs.
Dr. Wang declared no competing interests.
A study published in May in The Lancet journal eClinicalMedicine reports that
The tool was successfully used to identify, in 84 patients, 16 lung cancer–related carcinogenic volatile compounds (VOCs), such as aldehydes, hydrocarbons, ketones, carboxylic acids, and furan – some of which are compounds used in the production of common household goods, such as furniture, carpeting, and wood floors.
“The test is anticipated to be highlighted for primary screening of lung cancer but not the final diagnosis,” according to study authors who were led by Peiyu Wang, MD, PhD, chair of social medicine and health at Peking (China) University.
While early diagnosis and treatment are critical for improving lung cancer survival, early detection of lung cancer is challenging because of the lack of clinical manifestations and specific biomarkers. Annual CT scans are costly and include radiation exposure, Dr. Wang and his associates wrote.
Breathomics testing is considered a promising method for detection and screening for lung cancer. It has been under study for years and in 2014, researchers from Belgium published a review in Cancer Epidemiology Biomarkers and Prevention documenting the use of VOCs as early diagnostic or prognostic biomarkers for mesothelioma.
Lung cancer breath biomarkers identified in various studies have been highly heterogeneous because of differing sample collection methods, varying patient conditions, testing environments, and analysis methods. As a result, there currently is no breathomics test for lung cancer screening, Dr. Wang said in an interview.
In terms of its potential as a lung cancer screening tool, “Clinicians may introduce this test for people with high risk for lung cancer, such as elderly smokers, or people with suspected symptoms. It may also be introduced for young populations with subjective or objective needs to screen for lung cancer. As the proportion of lung adenocarcinoma in nonsmoking young women is increasing, the test may be a good method for lung cancer screening in this population,” Dr. Wang said.
After adjusting for age, sex, smoking, and comorbidities, researchers found elevated levels for 16 VOCs in patients with lung cancer. A diagnostic model including the 16 VOCs achieved an area under the curve of 0.952, sensitivity of 89.2%, specificity of 89.1%, and accuracy of 89.1% in lung cancer diagnosis. A model including the top eight VOCs achieved an area under the curve of 0.931, sensitivity of 86.0%, specificity of 87.2%, and accuracy of 86.9%.
After selecting 28 VOCs as candidates through a literature review, Dr. Wang and associates conducted a prospective discovery study from Sept. 1 to Dec. 31, 2020, using high-pressure photon ionization time-of-flight mass spectrometry to evaluate their performance for lung cancer diagnosis. The validation study included 157 lung cancer patients (mean age 57.0 years; 54.1 percent female) and 368 volunteers (mean age 44.5 years; 31.3% female).
“The external validation confirmed good performance of these biomarkers in lung cancer detection,” the researchers stated. It helped, they added, to solve the heterogeneity among published studies, establishing both 16 VOCs and 8 VOCS for lung cancer screening.
The authors stated that a large gap exists between breathomics research and clinical practices in lung cancer detection and screening. While the validated 16 VOCs, mainly aldehydes and hydrocarbon, showed potential for promoting this lung cancer screening strategy, more scientific studies are warranted to investigate the underlying mechanisms of identified lung cancer VOCs.
Dr. Wang declared no competing interests.
FROM ECLINICAL MEDICINE
Hearing, vision loss combo a colossal risk for cognitive decline
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).
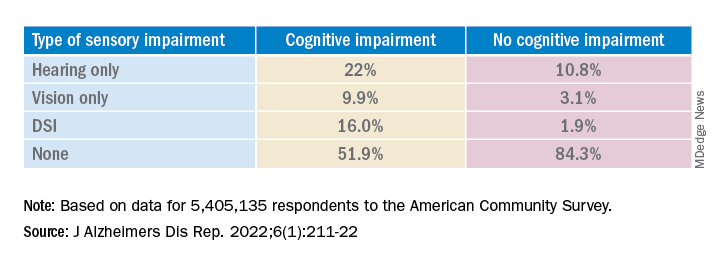
The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.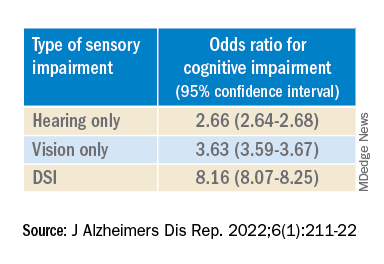
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.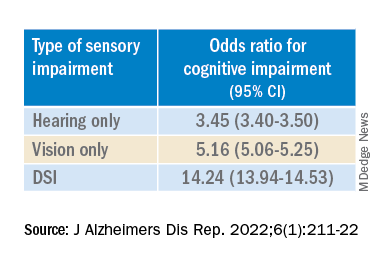
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.
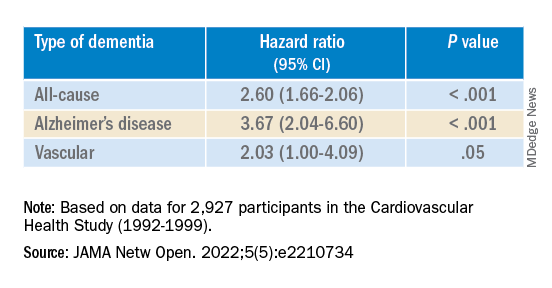
They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).

The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.

They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of hearing loss and vision loss is linked to an eightfold increased risk of cognitive impairment, new research shows.
Investigators analyzed data on more than 5 million U.S. seniors. Adjusted results show that participants with hearing impairment alone had more than twice the odds of also having cognitive impairment, while those with vision impairment alone had more than triple the odds of cognitive impairment.
However, those with dual sensory impairment (DSI) had an eightfold higher risk for cognitive impairment.
In addition, half of the participants with DSI also had cognitive impairment. Of those with cognitive impairment, 16% had DSI, compared with only about 2% of their peers without cognitive impairment.
“The findings of the present study may inform interventions that can support older people with concurrent sensory impairment and cognitive impairment,” said lead author Esme Fuller-Thomson, PhD, professor, Factor-Inwentash Faculty of Social Work, University of Toronto.
“Special attention, in particular, should be given to those aged 65-74 who have serious hearing and/or vision impairment [because], if the relationship with dementia is found to be causal, such interventions can potentially mitigate the development of cognitive impairment,” said Dr. Fuller-Thomson, who is also director of the Institute for Life Course and Aging and a professor in the department of family and community medicine and faculty of nursing, all at the University of Toronto.
The findings were published online in the Journal of Alzheimer’s Disease Reports.
Sensory isolation
Hearing and vision impairment increase with age; it is estimated that one-third of U.S. adults between the ages of 65 and 74 experience hearing loss, and 4% experience vision impairment, the investigators note.
“The link between dual hearing loss and seeing loss and mental health problems such as depression and social isolation have been well researched, but we were very interested in the link between dual sensory loss and cognitive problems,” Dr. Fuller-Thomson said.
Additionally, “there have been several studies in the past decade linking hearing loss to dementia and cognitive decline, but less attention has been paid to cognitive problems among those with DSI, despite this group being particularly isolated,” she said. Existing research into DSI suggests an association with cognitive decline; the current investigators sought to expand on this previous work.
To do so, they used merged data from 10 consecutive waves from 2008 to 2017 of the American Community Survey (ACS), which was conducted by the U.S. Census Bureau. The ACS is a nationally representative sample of 3.5 million randomly selected U.S. addresses and includes community-dwelling adults and those residing in institutional settings.
Participants aged 65 or older (n = 5,405,135; 56.4% women) were asked yes/no questions regarding serious cognitive impairment, hearing impairment, and vision impairment. A proxy, such as a family member or nursing home staff member, provided answers for individuals not capable of self-report.
Potential confounding variables included age, race/ethnicity, sex, education, and household income.
Potential mechanisms
Results showed that, among those with cognitive impairment, there was a higher prevalence of hearing impairment, vision impairment, and DSI than among their peers without cognitive impairment; in addition, a lower percentage of these persons had no sensory impairment (P < .001).

The prevalence of DSI climbed with age, from 1.5% for respondents aged 65-74 years to 2.6% for those aged 75-84 and to 10.8% in those 85 years and older.
Individuals with higher levels of poverty also had higher levels of DSI. Among those who had not completed high school, the prevalence of DSI was higher, compared with high school or university graduates (6.3% vs. 3.1% and 1.85, respectively).
After controlling for age, race, education, and income, the researchers found “substantially” higher odds of cognitive impairment in those with vs. those without sensory impairments.
“The magnitude of the odds of cognitive impairment by sensory impairment was greatest for the youngest cohort (age 65-74) and lowest for the oldest cohort (age 85+),” the investigators wrote. Among participants in the youngest cohort, there was a “dose-response relationship” for those with hearing impairment only, visual impairment only, and DSI.
Because the study was observational, it “does not provide sufficient information to determine the reasons behind the observed link between sensory loss and cognitive problems,” Dr. Fuller-Thomson said. However, there are “several potential causal mechanisms [that] warrant future research.”
The “sensory deprivation hypothesis” suggests that DSI could cause cognitive deterioration because of decreased auditory and visual input. The “resource allocation hypothesis” posits that hearing- or vision-impaired older adults “may use more cognitive resources to accommodate for sensory deficits, allocating fewer cognitive resources for higher-order memory processes,” the researchers wrote. Hearing impairment “may also lead to social disengagement among older adults, hastening cognitive decline due to isolation and lack of stimulation,” they added.
Reverse causality is also possible. In the “cognitive load on perception” hypothesis, cognitive decline may lead to declines in hearing and vision because of “decreased resources for sensory processing.”
In addition, the association may be noncausal. “The ‘common cause hypothesis’ theorizes that sensory impairment and cognitive impairment may be due to shared age-related degeneration of the central nervous system ... or frailty,” Dr. Fuller-Thomson said.
Parallel findings
The results are similar to those from a study conducted by Phillip Hwang, PhD, of the department of anatomy and neurobiology, Boston University, and colleagues that was published online in JAMA Network Open.

They analyzed data on 8 years of follow-up of 2,927 participants in the Cardiovascular Health Study (mean age, 74.6 years; 58.2% women).
Compared with no sensory impairment, DSI was associated with increased risk for all-cause dementia and Alzheimer’s disease, but not with vascular dementia.
“Future work in health care guidelines could consider incorporating screening of sensory impairment in older adults as part of risk assessment for dementia,” Nicholas Reed, AuD, and Esther Oh, MD, PhD, both of Johns Hopkins University, Baltimore, wrote in an accompanying editorial.
Accurate testing
Commenting on both studies, Heather Whitson, MD, professor of medicine (geriatrics) and ophthalmology and director at the Duke University Center for the Study of Aging and Human Development, Durham, N.C., said both “add further strength to the evidence base, which has really converged in the last few years to support that there is a link between sensory health and cognitive health.”
However, “we still don’t know whether hearing/vision loss causes cognitive decline, though there are plausible ways that sensory loss could affect cognitive abilities like memory, language, and executive function,” she said
Dr. Whitson, who was not involved with the research, is also codirector of the Duke/University of North Carolina Alzheimer’s Disease Research Center at Duke University, Durham, N.C., and the Durham VA Medical Center.
“The big question is whether we can improve patients’ cognitive performance by treating or accommodating their sensory impairments,” she said. “If safe and feasible things like hearing aids or cataract surgery improve cognitive health, even a little bit, it would be a huge benefit to society, because sensory loss is very common, and there are many treatment options,” Dr. Whitson added.
Dr. Fuller-Thomson emphasized that practitioners should “consider the full impact of sensory impairment on cognitive testing methods, as both auditory and visual testing methods may fail to take hearing and vision impairment into account.”
Thus, “when performing cognitive tests on older adults with sensory impairments, practitioners should ensure they are communicating audibly and/or using visual speech cues for hearing-impaired individuals, eliminating items from cognitive tests that rely on vision for those who are visually impaired, and using physical cues for individuals with hearing or dual sensory impairment, as this can help increase the accuracy of testing and prevent confounding,” she said.
The study by Fuller-Thomson et al. was funded by a donation from Janis Rotman. Its investigators have reported no relevant financial relationships. The study by Hwang et al. was funded by contracts from the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging. Dr. Hwang reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Reed received grants from the National Institute on Aging during the conduct of the study and has served on the advisory board of Neosensory outside the submitted work. Dr. Oh and Dr. Whitson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE REPORTS
Immunotherapy now first line for esophageal cancer
The new approval for the drug, a programmed cell death–ligand-1 inhibitor, is for use in this patient population regardless of PD-L1 status.
The indication also specifies that nivolumab is to be used together with chemotherapy (with a fluoropyrimidine- and platinum-containing regimen) or in combination with ipilimumab (Yervoy), an immunotherapy with a different mechanism of action.
“Today’s approvals bring two first-line immunotherapy-based treatment options at once ... to newly diagnosed patients with unresectable advanced or metastatic ESCC,” commented Adam Lenkowsky, a senior vice president at Bristol-Myers Squibb, which makes both nivolumab and ipilimumab.
The approval of the new indication by the Food and Drug Administration was based on improved survival shown in the phase 3 CheckMate-648 trial, which involved nearly 1,000 patients. The trial had three arms and compared nivolumab plus chemotherapy (n = 321) and nivolumab plus ipilimumab (n = 324) with chemotherapy alone (n = 324).
The results showed improved survival with both nivolumab combinations compared with chemotherapy (fluorouracil and cisplatin) alone. Overall survival was improved both in all randomized patients (a secondary endpoint) and in patients whose tumors expressed PD-L1 (≥ 1%), the primary endpoint.
For the combination of nivolumab plus chemotherapy, median overall survival was 13.2 versus 10.7 months, compared with chemotherapy alone in all randomized patients, and 15.4 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
For the combination of nivolumab plus ipilimumab, median overall survival was 12.8 versus 10.7 months with chemotherapy alone in all randomized patients and 13.7 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
However, progression-free survival did not reach statistical significance in any group.
“Unresectable advanced or metastatic ESCC is a challenging disease, and there’s a need for additional treatment options that may extend survival in the first-line setting,” commented Jaffer A. Ajani, MD, professor of gastrointestinal medical oncology at the University of Texas MD Anderson Cancer Center, Houston. He was also the lead U.S. investigator for CheckMate-648 and, in a company press release, said the “two nivolumab-based combinations showed a survival benefit compared to chemotherapy alone, offering new treatment options regardless of PD-L1 status.”
Results from the trial were presented at the 2021 annual meeting of the American Society of Clinical Oncology. At that time, trial investigator Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital in Sutton, England, told attendees that “nivolumab plus chemotherapy and nivolumab plus ipilimumab each represent a new potential first-line standard of care for patients with advanced ESCC.”
Commenting on that presentation, Samuel J. Klempner, MD, a gastrointestinal medical oncologist at the Massachusetts General Hospital Cancer Center, Boston, noted that the “prospect of a chemo-free regimen for advanced ESCC with the well-studied combination of ipilimumab and nivolumab would represent a welcome addition to our treatment armamentarium.”
No new safety signals
Dr. Chau noted there were no new safety signals with either of the immunotherapies.
Nivolumab and/or chemotherapy were discontinued in 39% of patients and delayed in 71% of patients for an adverse reaction.
Nivolumab and/or ipilimumab were discontinued in 23% of patients and delayed in 46% of patients for an adverse reaction.
The manufacturer cautioned that immunotherapy with nivolumab with or without ipilimumab has been associated with severe and fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, nephritis and renal dysfunction, dermatologic adverse reactions, and infusion-related reactions.
A version of this article first appeared on Medscape.com.
The new approval for the drug, a programmed cell death–ligand-1 inhibitor, is for use in this patient population regardless of PD-L1 status.
The indication also specifies that nivolumab is to be used together with chemotherapy (with a fluoropyrimidine- and platinum-containing regimen) or in combination with ipilimumab (Yervoy), an immunotherapy with a different mechanism of action.
“Today’s approvals bring two first-line immunotherapy-based treatment options at once ... to newly diagnosed patients with unresectable advanced or metastatic ESCC,” commented Adam Lenkowsky, a senior vice president at Bristol-Myers Squibb, which makes both nivolumab and ipilimumab.
The approval of the new indication by the Food and Drug Administration was based on improved survival shown in the phase 3 CheckMate-648 trial, which involved nearly 1,000 patients. The trial had three arms and compared nivolumab plus chemotherapy (n = 321) and nivolumab plus ipilimumab (n = 324) with chemotherapy alone (n = 324).
The results showed improved survival with both nivolumab combinations compared with chemotherapy (fluorouracil and cisplatin) alone. Overall survival was improved both in all randomized patients (a secondary endpoint) and in patients whose tumors expressed PD-L1 (≥ 1%), the primary endpoint.
For the combination of nivolumab plus chemotherapy, median overall survival was 13.2 versus 10.7 months, compared with chemotherapy alone in all randomized patients, and 15.4 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
For the combination of nivolumab plus ipilimumab, median overall survival was 12.8 versus 10.7 months with chemotherapy alone in all randomized patients and 13.7 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
However, progression-free survival did not reach statistical significance in any group.
“Unresectable advanced or metastatic ESCC is a challenging disease, and there’s a need for additional treatment options that may extend survival in the first-line setting,” commented Jaffer A. Ajani, MD, professor of gastrointestinal medical oncology at the University of Texas MD Anderson Cancer Center, Houston. He was also the lead U.S. investigator for CheckMate-648 and, in a company press release, said the “two nivolumab-based combinations showed a survival benefit compared to chemotherapy alone, offering new treatment options regardless of PD-L1 status.”
Results from the trial were presented at the 2021 annual meeting of the American Society of Clinical Oncology. At that time, trial investigator Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital in Sutton, England, told attendees that “nivolumab plus chemotherapy and nivolumab plus ipilimumab each represent a new potential first-line standard of care for patients with advanced ESCC.”
Commenting on that presentation, Samuel J. Klempner, MD, a gastrointestinal medical oncologist at the Massachusetts General Hospital Cancer Center, Boston, noted that the “prospect of a chemo-free regimen for advanced ESCC with the well-studied combination of ipilimumab and nivolumab would represent a welcome addition to our treatment armamentarium.”
No new safety signals
Dr. Chau noted there were no new safety signals with either of the immunotherapies.
Nivolumab and/or chemotherapy were discontinued in 39% of patients and delayed in 71% of patients for an adverse reaction.
Nivolumab and/or ipilimumab were discontinued in 23% of patients and delayed in 46% of patients for an adverse reaction.
The manufacturer cautioned that immunotherapy with nivolumab with or without ipilimumab has been associated with severe and fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, nephritis and renal dysfunction, dermatologic adverse reactions, and infusion-related reactions.
A version of this article first appeared on Medscape.com.
The new approval for the drug, a programmed cell death–ligand-1 inhibitor, is for use in this patient population regardless of PD-L1 status.
The indication also specifies that nivolumab is to be used together with chemotherapy (with a fluoropyrimidine- and platinum-containing regimen) or in combination with ipilimumab (Yervoy), an immunotherapy with a different mechanism of action.
“Today’s approvals bring two first-line immunotherapy-based treatment options at once ... to newly diagnosed patients with unresectable advanced or metastatic ESCC,” commented Adam Lenkowsky, a senior vice president at Bristol-Myers Squibb, which makes both nivolumab and ipilimumab.
The approval of the new indication by the Food and Drug Administration was based on improved survival shown in the phase 3 CheckMate-648 trial, which involved nearly 1,000 patients. The trial had three arms and compared nivolumab plus chemotherapy (n = 321) and nivolumab plus ipilimumab (n = 324) with chemotherapy alone (n = 324).
The results showed improved survival with both nivolumab combinations compared with chemotherapy (fluorouracil and cisplatin) alone. Overall survival was improved both in all randomized patients (a secondary endpoint) and in patients whose tumors expressed PD-L1 (≥ 1%), the primary endpoint.
For the combination of nivolumab plus chemotherapy, median overall survival was 13.2 versus 10.7 months, compared with chemotherapy alone in all randomized patients, and 15.4 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
For the combination of nivolumab plus ipilimumab, median overall survival was 12.8 versus 10.7 months with chemotherapy alone in all randomized patients and 13.7 versus 9.1 months in patients whose tumors express PD-L1 (≥ 1%).
However, progression-free survival did not reach statistical significance in any group.
“Unresectable advanced or metastatic ESCC is a challenging disease, and there’s a need for additional treatment options that may extend survival in the first-line setting,” commented Jaffer A. Ajani, MD, professor of gastrointestinal medical oncology at the University of Texas MD Anderson Cancer Center, Houston. He was also the lead U.S. investigator for CheckMate-648 and, in a company press release, said the “two nivolumab-based combinations showed a survival benefit compared to chemotherapy alone, offering new treatment options regardless of PD-L1 status.”
Results from the trial were presented at the 2021 annual meeting of the American Society of Clinical Oncology. At that time, trial investigator Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital in Sutton, England, told attendees that “nivolumab plus chemotherapy and nivolumab plus ipilimumab each represent a new potential first-line standard of care for patients with advanced ESCC.”
Commenting on that presentation, Samuel J. Klempner, MD, a gastrointestinal medical oncologist at the Massachusetts General Hospital Cancer Center, Boston, noted that the “prospect of a chemo-free regimen for advanced ESCC with the well-studied combination of ipilimumab and nivolumab would represent a welcome addition to our treatment armamentarium.”
No new safety signals
Dr. Chau noted there were no new safety signals with either of the immunotherapies.
Nivolumab and/or chemotherapy were discontinued in 39% of patients and delayed in 71% of patients for an adverse reaction.
Nivolumab and/or ipilimumab were discontinued in 23% of patients and delayed in 46% of patients for an adverse reaction.
The manufacturer cautioned that immunotherapy with nivolumab with or without ipilimumab has been associated with severe and fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, nephritis and renal dysfunction, dermatologic adverse reactions, and infusion-related reactions.
A version of this article first appeared on Medscape.com.

