User login
Interview with Joseph R. Berger, MD, on the Financial Contribution of the MS Specialist
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Safety and Efficacy of Halobetasol Propionate Lotion 0.01% in the Treatment of Moderate to Severe Plaque Psoriasis: A Pooled Analysis of 2 Phase 3 Studies
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
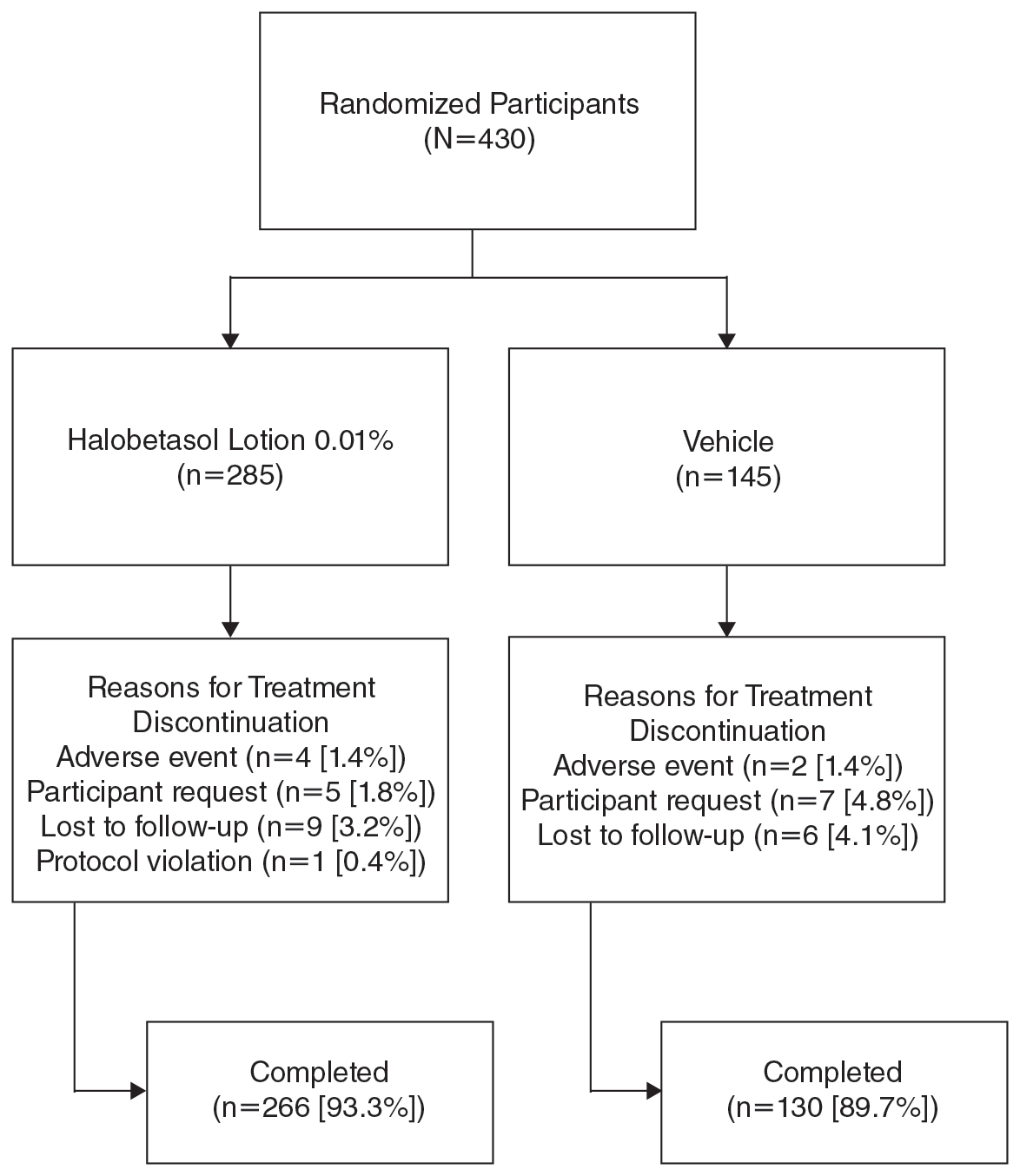
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
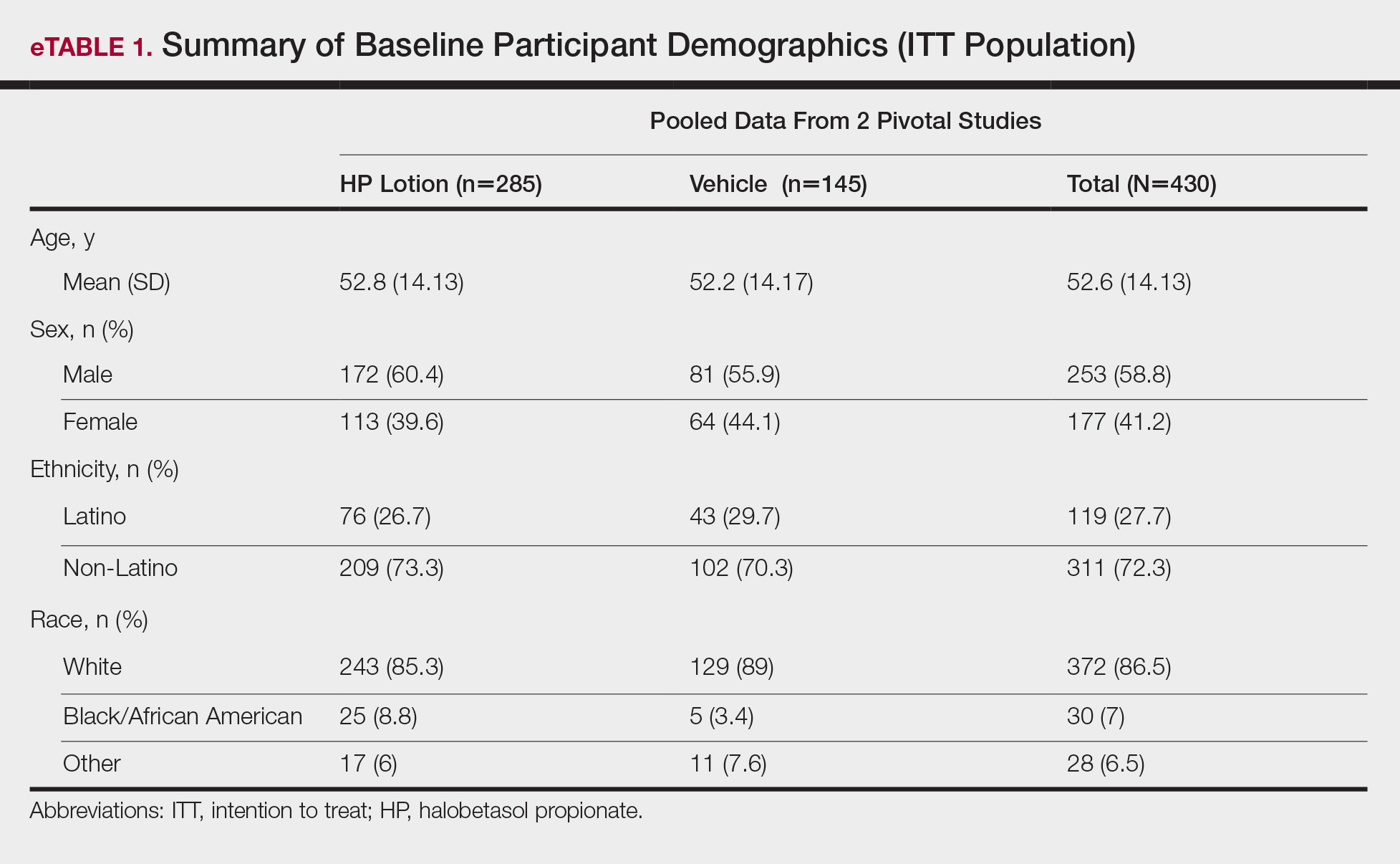
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
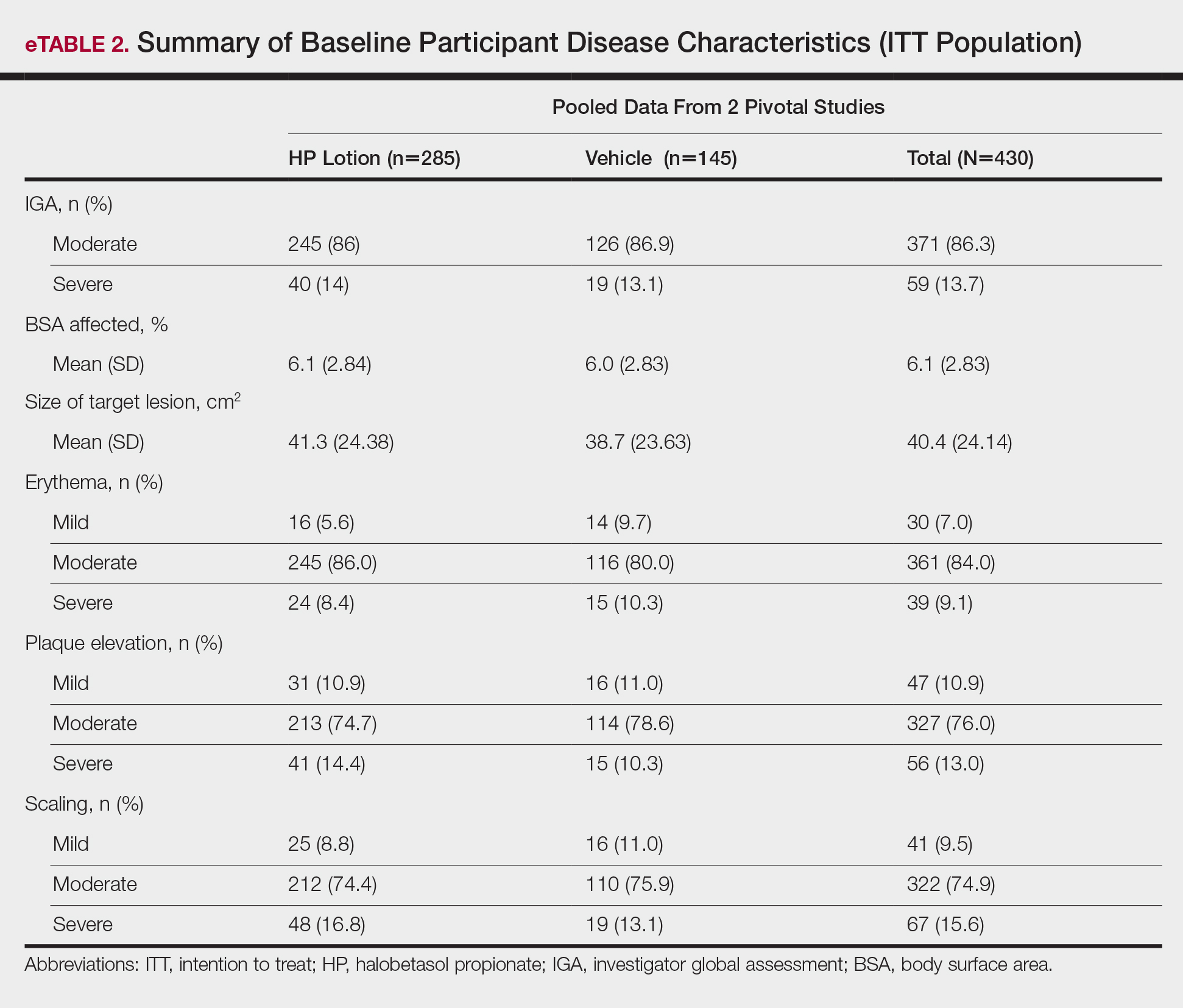
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
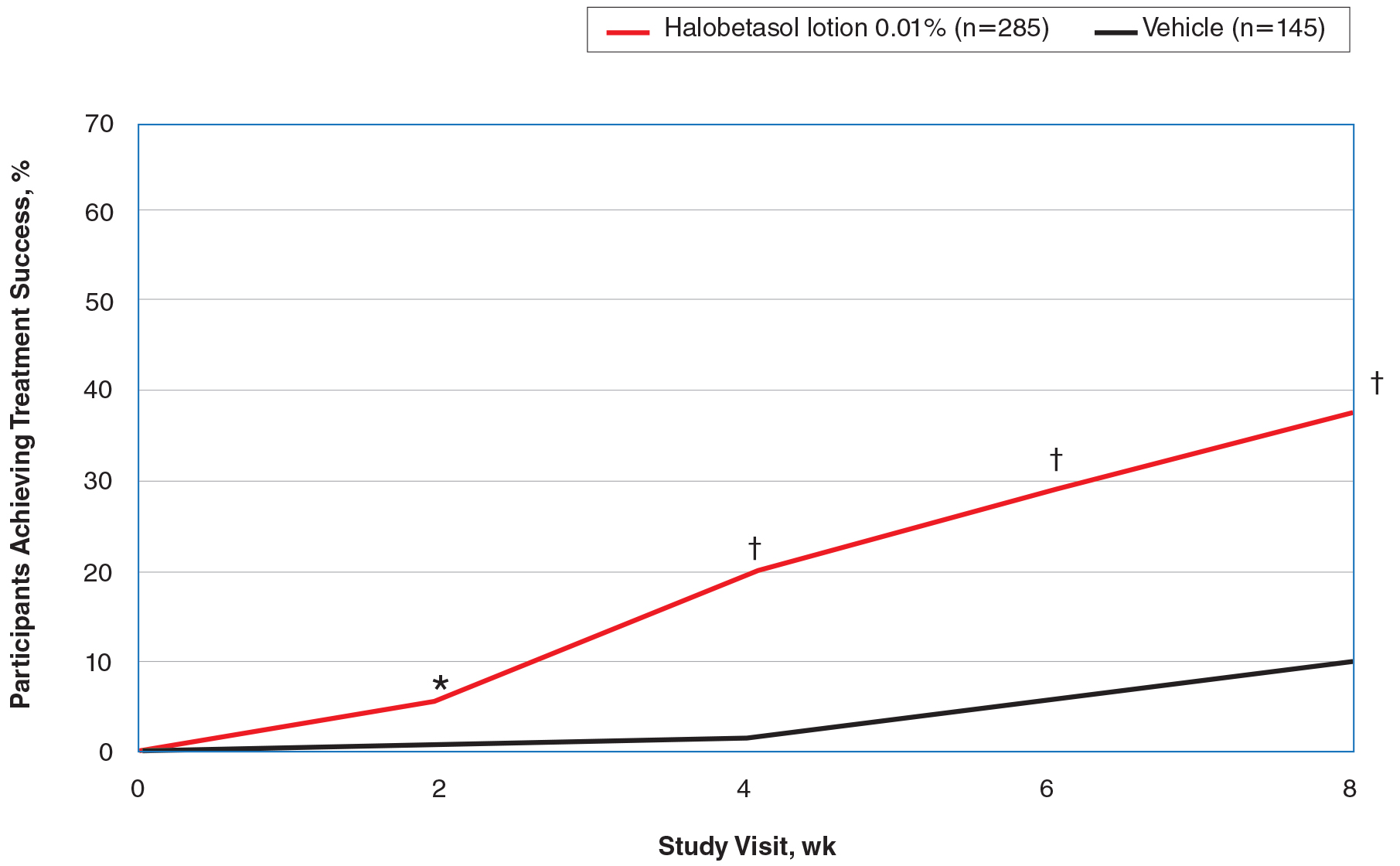
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
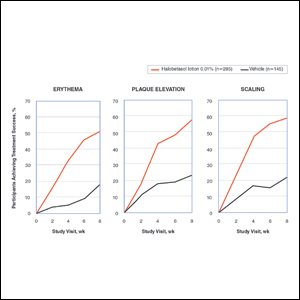
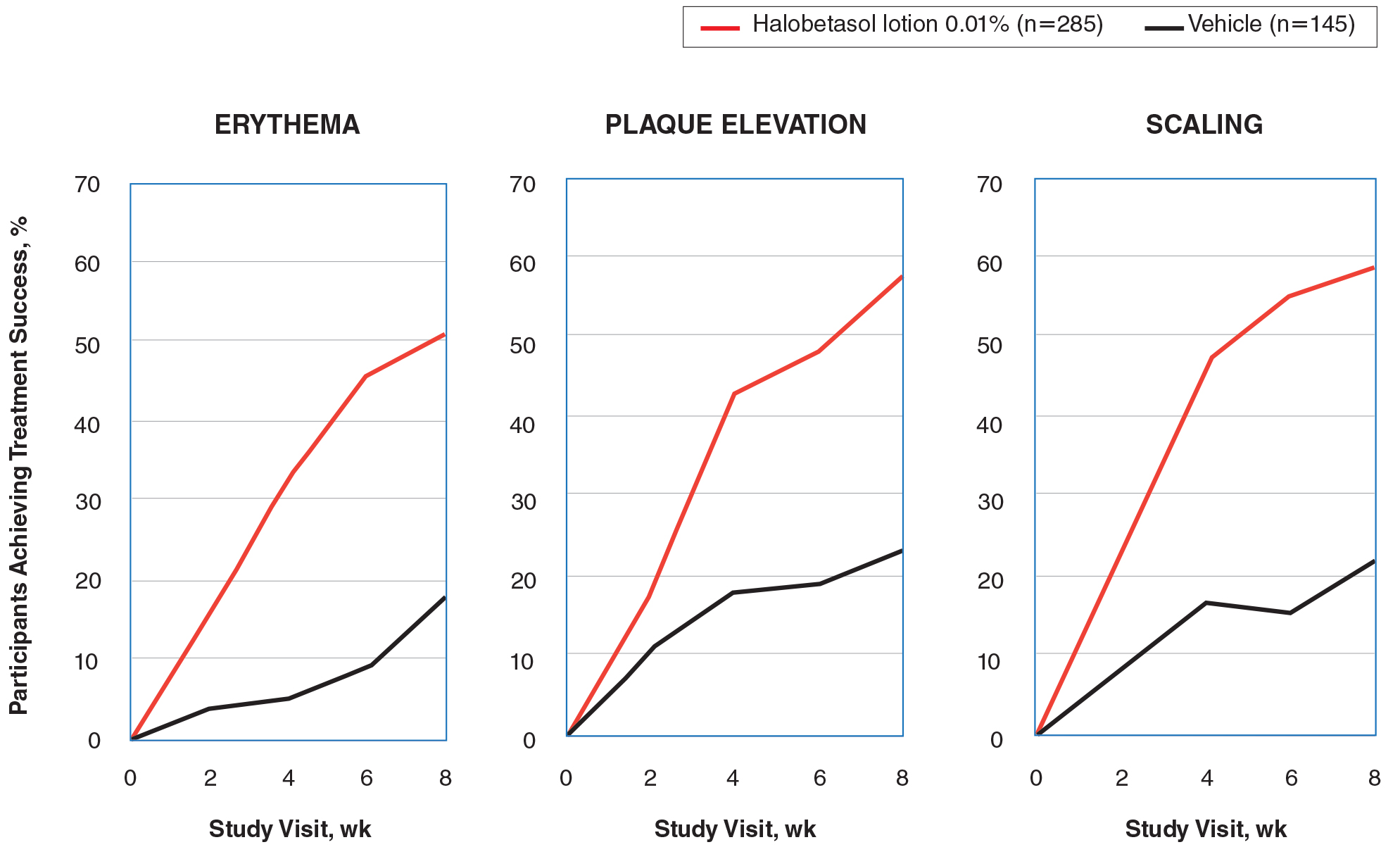
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
BSA Assessment
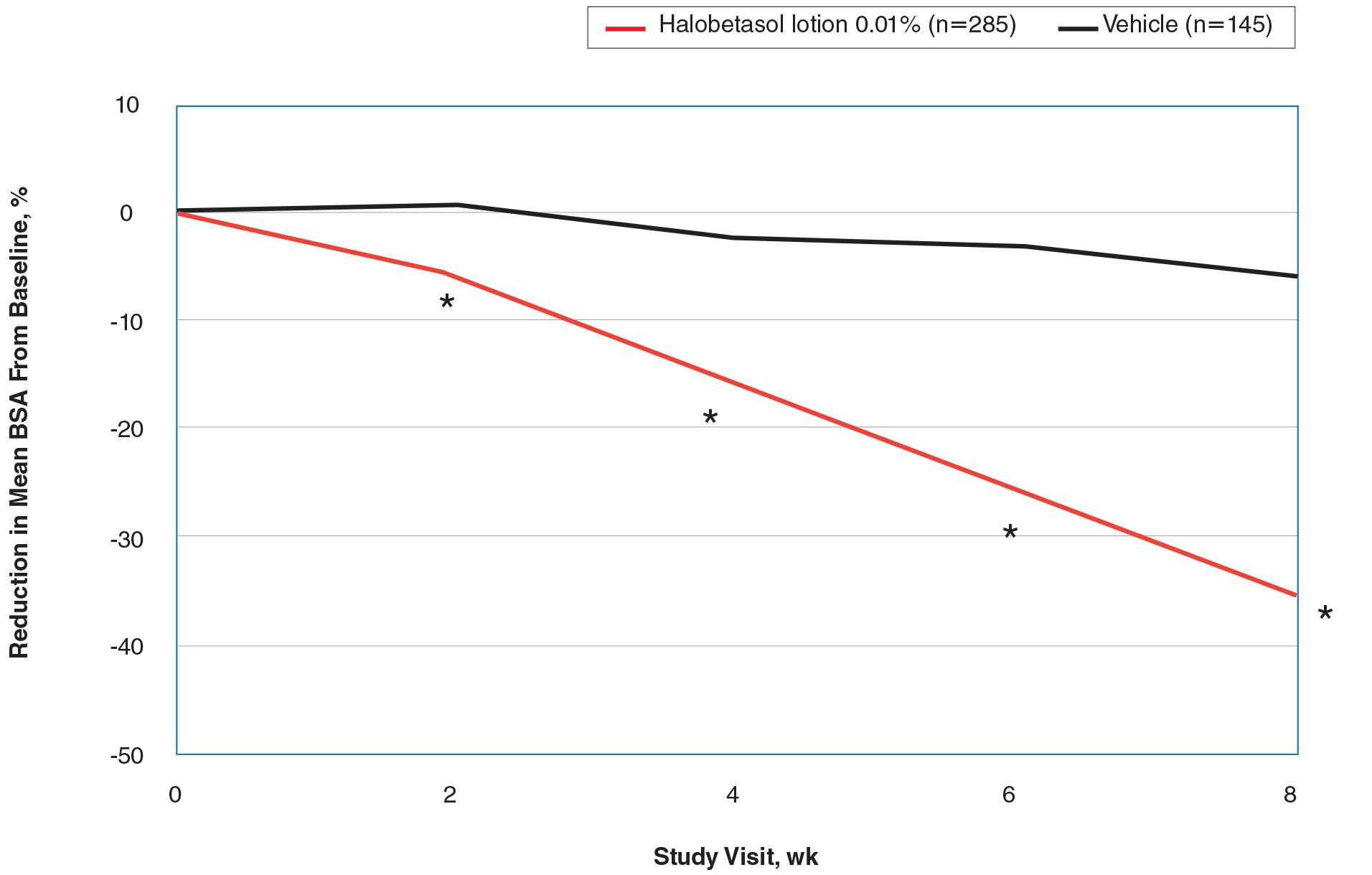
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
IGA×BSA Composite Score
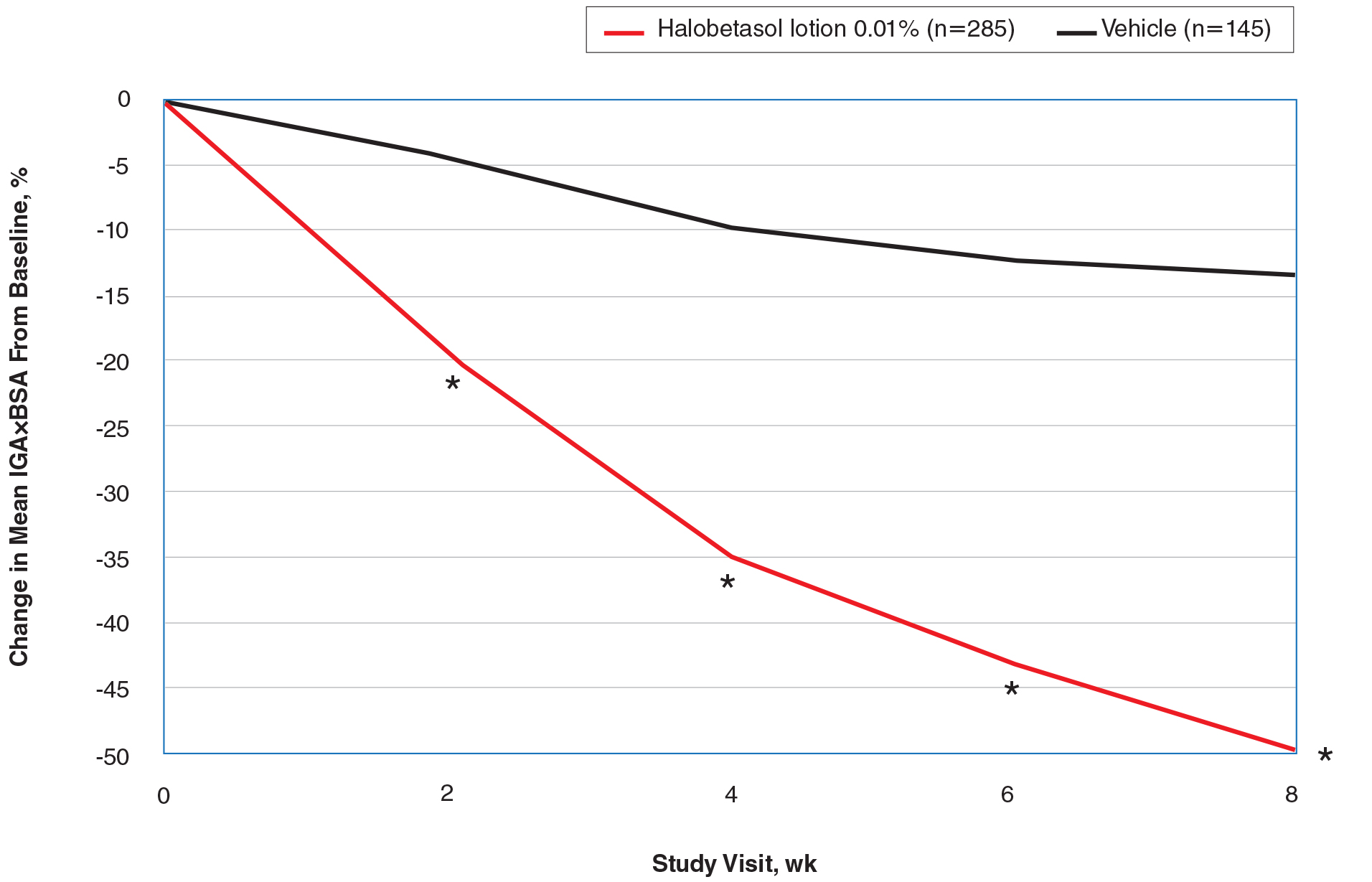
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
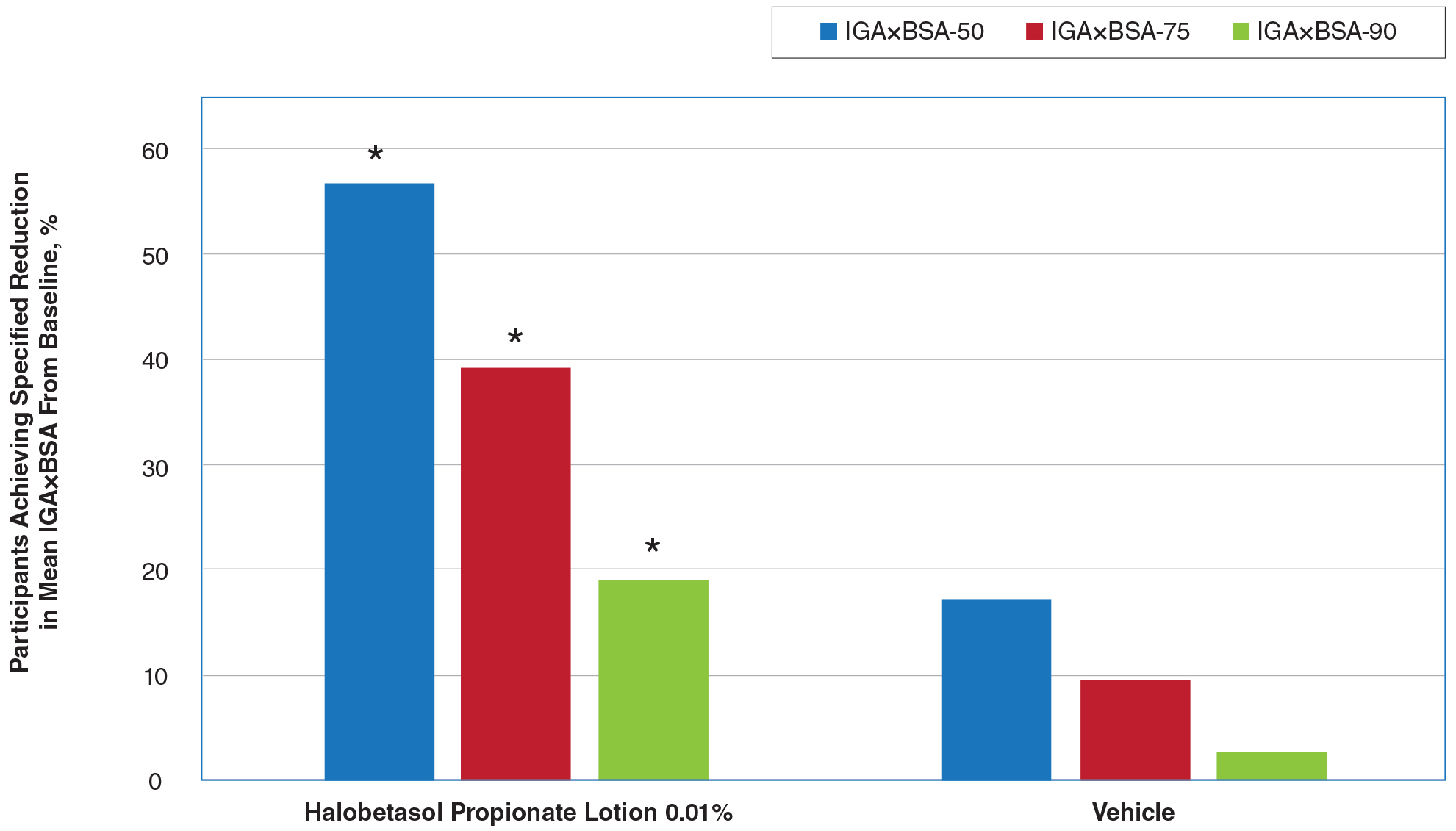
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
Safety Evaluation
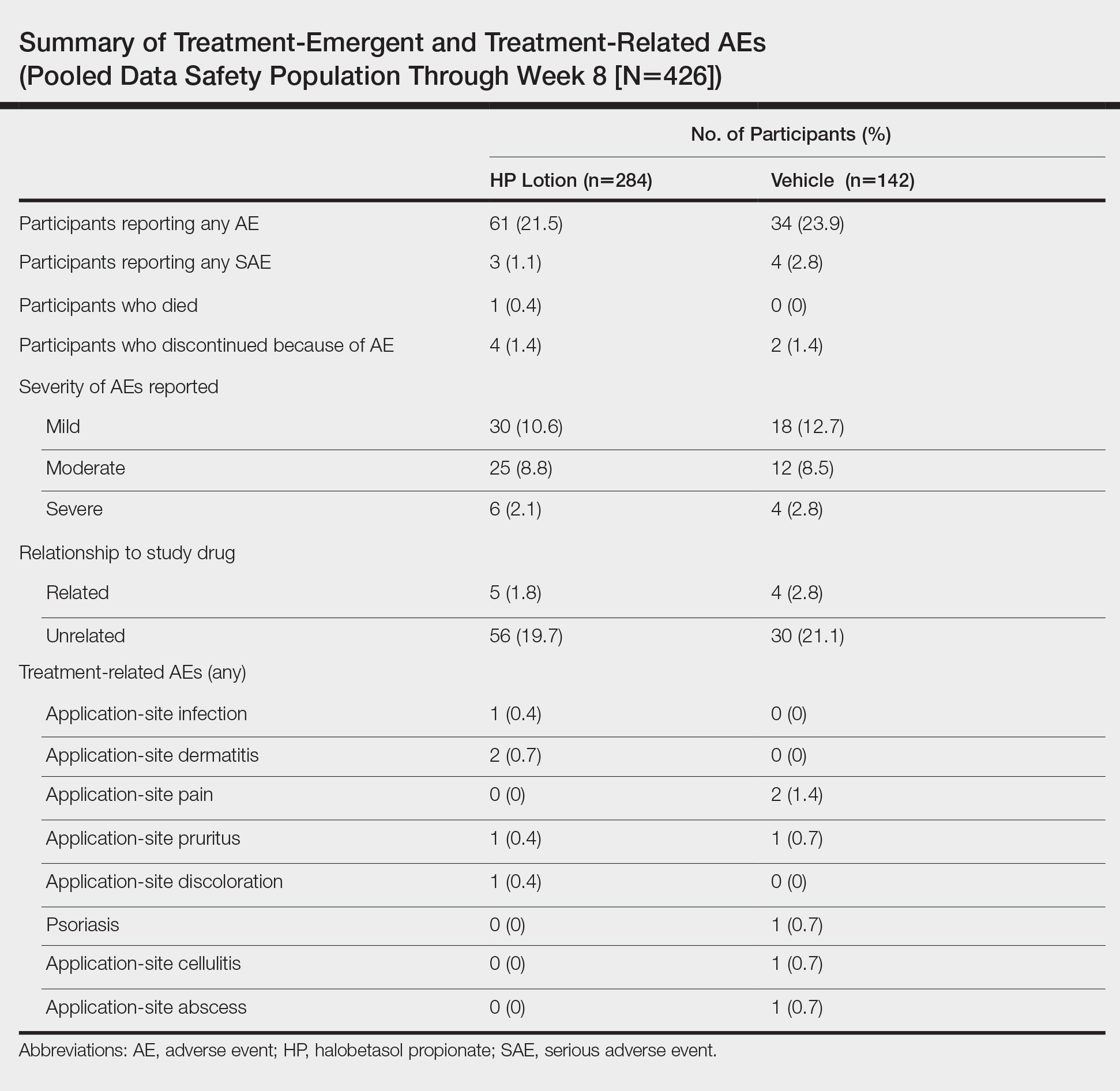
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.

A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).

Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).

Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).

Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).

BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).

IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).

By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).

Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.

Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.

A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).

Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).

Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).

Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).

BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).

IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).

By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).

Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.

Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis Treatment in Patients With Sickle Cell Disease
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Practice Points
• Tumor necrosis factor α contributes both to the vascular inflammatory state seen in sickle cell disease as well as the cycle of inflammation seen in the development of psoriasis.
• Tumor necrosis factor α inhibitors may be the drug of choice for patients with both psoriasis and sickle cell disease.
What’s New in Topical Treatments for Psoriasis
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
Clinical relevance of tamoxifen pharmacogenetics in question
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
The clinical relevance of tamoxifen pharmacogenetics and therapeutic drug monitoring has been challenged, according to findings from an observational study.
“The objective of the prospective CYPTAM study was to associate endoxifen concentrations and CYP2D6 genotypes with clinical outcome[s] in patients with early-stage breast cancer receiving tamoxifen,” wrote Anabel Sanchez-Spitman, PharmD, of the Leiden University Medical Center, the Netherlands, and her colleagues in the Journal of Clinical Oncology.
“Although, in a retrospective study, an endoxifen threshold of 5.9 ng/mL for efficacy [has been] described, confirmation based on prospective studies is lacking,” they wrote.
The researchers followed 667 patients with early-stage breast malignancy who were treated with 20 mg of oral tamoxifen daily.
Dr. Sanchez-Spitman and her colleagues performed CYP2D6 genotype testing and measured blood levels of endoxifen, an active metabolite of tamoxifen, in study participants. Both of these measures were linked with relapse-free survival via statistical analysis.
After analysis, no significant association was found between endoxifen concentrations (P = .691) or CYP2D6 genotyping (P = .799) and relapse-free survival. These data were the same in both univariable and multivariable regression analysis.
“Neither categorizing endoxifen concentrations into quartiles nor using 5.9 ng/mL as threshold altered these results,” they wrote.
The authors acknowledged that a key limitation of the study was the absence of tamoxifen adherence testing, but the availability of persistence data was well collected, lessening this drawback.
“Our data do not justify therapeutic drug monitoring based on endoxifen concentrations in patients with breast cancer receiving tamoxifen,” they concluded.
The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
SOURCE: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: An endoxifen concentration of greater than 5.9 ng/mL was not linked with clinical efficacy in patients with breast malignancy.
Study details: A prospective study of 667 patients with early-stage breast malignancy treated with adjuvant tamoxifen.
Disclosures: The study was supported by grant funding provided by ZOLEON. The authors reported no conflicts of interest.
Source: Sanchez-Spitman A et al. J Clin Oncol. 2019 Jan 24. doi: 10.1200/JCO.18.00307.
The Use of Immuno-Oncology Treatments in the VA (FULL)
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.
Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants (FULL)
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
A National WestlawNext Database Analysis of Malpractice Litigation in Radiation Oncology (FULL)
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.


Click here to read the digital edition.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.


Click here to read the digital edition.
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.


Click here to read the digital edition.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules (FULL)
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
Single-cell genomics drive progress toward human breast cell atlas development
SAN ANTONIO – Researchers at MD Anderson Cancer Center in Houston and the University of New South Wales (UNSW) in Sydney are among teams from around the world working toward human breast cell atlas development using single-cell genomics, and their efforts to date have yielded new understanding of both the normal breast cell ecosystem and the breast cancer tumor microenvironment.
The work at MD Anderson, for example, has led to the identification of a number of new gene markers and multiple cell states within breast cell types, according to Tapsi Kumar Seth, who reported early findings from an analysis of more than 32,000 cells from normal breast tissue during a presentation at the San Antonio Breast Cancer Symposium.
At the UNSW’s Garvan Institute of Medical Research, Alexander Swarbrick, PhD, and his colleagues are working to better define the tumor microenvironment at the single-cell level. At the symposium, Dr. Swarbrick presented interim findings from cellular analyses in the first 23 breast cancer cases of about 200 that will be studied in the course of the project.
Improved understanding of the cellular landscape of both normal breast tissue and breast cancer tissue should lead to new stromal- and immune-based therapies for the treatment of breast cancer, the investigators said.
The normal breast cell ecosystem
The MD Anderson researchers studied 32,148 stromal cells from pathologically normal breast tissues collected from 11 women who underwent mastectomy at the center.
Unbiased expression analysis identified three major cell types, including epithelial cells, fibroblasts, and endothelial cells, as well as several minor cell types such as macrophages, T-cells, apocrine cells, pericytes, and others, said Ms. Seth, a graduate student in the department of genetics at the center and a member of the Navin Laboratory there.
The work is designed to help identify the presence and function of cells and explain how they behave in a normal breast ecosystem, she said.
“We know that a female breast undergoes a lot of changes due to age, pregnancy, or when there is a disease such as cancer, so it’s essential to chart out what a normal cell reference would look like,” she said.
Toward that goal, a protocol was developed to dissociate the tissue samples within 2 hours due to the decline in viability seen in cells and RNA over time. Analysis of the cell states revealed different transcriptional programs in luminal epithelial cells (hormone receptor positive and secretory), basal epithelial cells (myoepithelial or basement-like), endothelial cells (lymphatic or vascular), macrophages (M1 or M2) and fibroblasts (three subgroups) and provided insight into progenitors of each cell types, she said.
A map was created to show gene expression and to identify transcriptomally similar cells.
“We were able to identify most of the major cell types that are present in human breasts,” she said. “What was interesting was that the composition of these cells also varied across women.”
For example, the proportion of fibroblasts was lower in 3 of the 11 patients, and even though the cells were pathologically normal, immune cell populations, including T-cells and macrophages, were also seen.
Adipocytes cannot be evaluated using this technology because they are large and the layer of fat cells must be removed during dissociation to prevent clogging of the machines, she noted, adding that “this is really a limitation of our technology.”
A closer look was taken at each of the major cell types identified.
Epithelial cells
Both canonical and new gene markers were used to identify luminal and basal epithelial cells, Ms. Seth noted.
Among the known markers were KRT18 for luminal epithelial cells and KRT5, KRT6B, KRT14, and KRT15 for basal epithelial cells. Among the new markers were SLC39A6, EFHD1 and HES1 for the luminal epithelial cells, and CITED4, CCK28, MMP7, and MDRG2 for the basal epithelial cells.
“We went on and validated these markers on the tissue section using methods like spatial transcriptomics,” she said, explaining that this “really helps capture the RNA expression spatially,” and can resolve the localization of cell types markers in anatomical structures.
For these cells, the expression of the newly identified gene markers was mostly confined to ducts and lobules.
In addition, an analysis of cell states within the luminal epithelial cells showed four different cell states, each of which have “different kinds of genes that they express, and also different pathways that they express, suggesting that these might be transcriptomally different,” Ms. Seth said.
Of note, these cells and cells states are not biased to a specific condition or patient, suggesting that they are coming from all of the patients, she added.
Two of the four cell states – the secretory and hormone responsive states – have previously been reported, but Ms. Seth and her colleagues identified two additional cell states that may have different biological functions and are present in the different anatomical regions of the breast.
Fibroblasts
Fibroblasts, the cells of the connective tissue, were the most abundant cell type. Like the epithelial cells, both canonical collagen markers (COL6A3, MMP2, FBN1, FBLN2, FBN, and COL1A1) and newly identified gene markers (TNXB, AEBP1, CFH, CTSK, TPPP3, MEG3, HTRA1, LHFP, and OGN) were used to identify them.
Endothelial cells
Breast tissue is highly vascular, so endothelial cells, which line the walls of veins, arteries, and lymphatic vessels, are plentiful.
“Again, for both these cell types, we identified them using the canonical marker CD31, and we identified some new gene markers,” she said, noting that the new markers include CCL21, CLDN5, MMRN1, LYVE1, and PROX1 for lymphatic endothelial cells, and RNASE1 and IFI27 for vascular endothelial cells.
Two different groups – or states – of vascular endothelial cells were identified, with each expressing “very different genes as well as very different pathways, again suggesting that they might have different biological functions, which we are still investigating,” she said.
Additional findings and future directions
In addition to stromal cells, some immune cells were also seen. These included T cells that came mostly from two patients, as well as macrophages and monocytes, which comprised the most abundant immune cell population.
Of note, all of these cells are also found in the tumor microenvironment, but they are in a transformed state. For example cancer-associated fibroblasts, tumor endothelial cells, tumor-associated macrophages, and tumor-associated adipocytes have been seen in that environment, she said.
“So what we are trying to do with this project is ... learn how these cells are, and how these cells behave in the normal ecosystem,” she explained, noting that the hope is to provide a valuable reference for the research community with new insights about how normal cell types are transformed in the tumor microenvironment.
In an effort to overcome the adipocyte-associated limitation of the technology, adipocytes are “now being isolated by single nucleus RNA sequencing.”
“This [sequencing] technology has helped us identify multiple cell states within a cell type; and most of these cell states may have different biological functions, which probably can be investigated by spatial transcriptomic methods,” she said.
Spatial transcriptomics also continue to be used for validation of the new gene markers identified in the course of this research, she noted.
The breast tumor microenvironment
At the Garvan Institute, current work is focusing more on defining the landscape of the breast tumor microenvironment at single-cell resolution, according to Dr. Swarbrick, a senior research fellow and head of the Tumour Progression Laboratory there.
“Breast cancers ... are complex cellular ecosystems, and it’s really the sum of the interactions between the cell types that play major roles in determining the etiology of disease and its response to therapy,” he said. “So I think that going forward toward a new age of diagnostics and therapeutics, there’s wonderful potential in capitalizing on the tumor microenvironment for new developments, but this has to be built on a really deep understanding of the tumor microenvironment, and – I might say – a new taxonomy of the breast cellular environment.”
Therefore, in an effort to address “this limitation in our knowledge base,” his lab is also working toward development of a breast cell atlas.
A fresh tissue collection program was established to collect early breast cancer tissues at the time of surgery, metastatic biopsies, and metastatic lesions from autopsies. The tissues are quickly dissociated into their cellular components and they undergo massively parallel capture and sequencing using the 10x genomics platform, he said.
Thousands of cells per case are analyzed using single-cell RNA sequencing (RNA-seq), as well as “RAGE-seq” and “CITE-seq,” which are performed in parallel to the RNA sequencing to address some of the limitations of the RNA sequencing alone and to “try to gain a multi-omic insight into the cell biology,” he explained.
RAGE-seq, which Dr. Swarbrick and his team developed, “is essentially a method to do targeted long-read sequencing in parallel to the short-read sequencing that we use for RNA-seq,” and CITE-seq is “a really fantastic method developed at the New York Genome Center that essentially allows us to gather proteomic data in parallel to the RNA data,” he said.
Based on findings from the analyses of about 125,000 cells from 25 patients, a map was created that showed the cell clusters identified by both canonical markers and gene expression signatures.
“We find the cell types we would expect to be present in a breast cancer,” he said.
The map shows clusters of myeloid, epithelial-1 and -2, cancer-associated fibroblast (CAF)-1 and -2, endothelial, T Reg, B, and CD8 and CD4 T cells.
Next, each cell type is quantified in each patient, and a graphic representation of the findings shows large variability in the proportions of each cell type in each patient.
“Ultimately, our goal is to be able to relate the frequencies of cell types and molecular features to each other, but also to clinical-pathological features from these patients,” he noted.
A closer look at the findings on an individual case level demonstrates the potential for development of better therapies.
For example, a case involving a high-grade triple-negative invasive ductal carcinoma exhibited each of the cell types found overall.
“One of the things that strikes us early on is we see a number of malignant epithelial populations,” he said, noting that proliferation is one of the drivers of the heterogeneity, but that heterogeneity was also seen for “other clinically relevant features such as basal cytokeratins,” which were heterogeneously expressed in different cell-type clusters.
“This was kind of paralleled in the immunohistochemistry results that we obtained from this patient,” he said. “We could also apply other clinically used tests that we’ve developed on bulk (such as PAM50 intrinsic subtyping) and ask whether they can be applied at the single-cell resolution.
“We think that these are going to be great tools to try to now get in and understand the significance of this heterogeneity and try to identify the lethal cells within this patient, and potentially therapeutic strategies to eradicate those cells,” he added.
Fibroblasts
A notable finding of this project was the presence of “not one, but two populations of fibroblasts,” Dr. Swarbrick said, noting that fibroblasts are typically discussed as a single entity.
“This is arguing that there are at least two major types present within the breast, and almost every case has these populations present at roughly equal amounts,” he said.
This is of particular interest, because it has been shown in prior studies that targeting fibroblasts can have therapeutic outcomes.
“So we think this is a very important population within the tumor microenvironment,” he added.
With respect to gene expression features, CAF-1 is dominated by signatures of extracellular matrix deposition and remodeling, which “look like the classic myofibroblasts that we typically think of when we study cancer-associated fibroblasts.”
“In contrast, the CAF-2 population ... have what appears to be quite a predominant secretory function, so we see a lot of cytokines being produced by these cells, but we also see a very high level of expression of a number immune checkpoint ligands,” he said, adding that his team is actively pursuing whether these cells may be undergoing signaling events with infiltrating lymphocytes in the tumor microenvironment.
The signatures for both CAF types are prognostic within large breast cancer data sets, suggesting that they do actually have an important role in disease, he noted.
Markers for these cells include ACTA2, which was previously known to be a marker, and which is almost exclusively restricted to CAF-1, and the cell surface protein CD34 – a progenitor marker in many different cellular systems, “which is actually beautifully expressed on the CAF-2 population” as demonstrated using CITE-seq.
“So we’re now using this as a way to prospectively identify these cells, pull them out of tumors, and conduct biologic assays to learn more about them,” he said.
The immune milieu
“We’re in the age of immunotherapy, and this is an area of huge interest, but we have a long way to go in making it as effective as possible for breast cancer patients,” Dr. Swarbrick said. “I believe part of that is through a very deep understanding of the taxonomy.”
RNA data alone are useful but insufficient to fully identify subsets of immune cells due to a “relatively low-resolution ability to resolve T cells.”
“But because we’re now using the panel of 125 antibodies in parallel, we can now start to use protein levels to split up these populations and we can start to now identify, with higher resolution, more unique populations within the environment,” he said, noting that the availability of protein data not only helps identify subtypes, but is also therapeutically important as it allows for certainty regarding whether the protein target of therapeutic antibodies is expressed on the surface of cells.
Ultimately the hope is that this effort to build a multi-omic breast cancer atlas will continue to drive new discoveries in personalized medicine for breast cancer, Dr. Swarbrick concluded, adding that the field is moving fast, and it will be very important for labs like his and the Navin Lab to communicate to avoid needlessly duplicating efforts.
“I think it’s going to be really exciting to start to put some of these [findings] together,” he said.
The MD Anderson project is funded by the Chan Zuckerberg Initiative as part of its work in supporting the Human Cell Atlas project. Ms. Seth reported having no disclosures. Dr. Swarbrick’s research is funded by the Australian Government/National Health and Medical Research Council and the National Breast Cancer Foundation. He reported having no relevant disclosures.
SOURCE: Seth T et al. SABCS 2018, Abstract GS1-02; Swarbrick A et al. SABCS 2018, Abstract GS1-01
SAN ANTONIO – Researchers at MD Anderson Cancer Center in Houston and the University of New South Wales (UNSW) in Sydney are among teams from around the world working toward human breast cell atlas development using single-cell genomics, and their efforts to date have yielded new understanding of both the normal breast cell ecosystem and the breast cancer tumor microenvironment.
The work at MD Anderson, for example, has led to the identification of a number of new gene markers and multiple cell states within breast cell types, according to Tapsi Kumar Seth, who reported early findings from an analysis of more than 32,000 cells from normal breast tissue during a presentation at the San Antonio Breast Cancer Symposium.
At the UNSW’s Garvan Institute of Medical Research, Alexander Swarbrick, PhD, and his colleagues are working to better define the tumor microenvironment at the single-cell level. At the symposium, Dr. Swarbrick presented interim findings from cellular analyses in the first 23 breast cancer cases of about 200 that will be studied in the course of the project.
Improved understanding of the cellular landscape of both normal breast tissue and breast cancer tissue should lead to new stromal- and immune-based therapies for the treatment of breast cancer, the investigators said.
The normal breast cell ecosystem
The MD Anderson researchers studied 32,148 stromal cells from pathologically normal breast tissues collected from 11 women who underwent mastectomy at the center.
Unbiased expression analysis identified three major cell types, including epithelial cells, fibroblasts, and endothelial cells, as well as several minor cell types such as macrophages, T-cells, apocrine cells, pericytes, and others, said Ms. Seth, a graduate student in the department of genetics at the center and a member of the Navin Laboratory there.
The work is designed to help identify the presence and function of cells and explain how they behave in a normal breast ecosystem, she said.
“We know that a female breast undergoes a lot of changes due to age, pregnancy, or when there is a disease such as cancer, so it’s essential to chart out what a normal cell reference would look like,” she said.
Toward that goal, a protocol was developed to dissociate the tissue samples within 2 hours due to the decline in viability seen in cells and RNA over time. Analysis of the cell states revealed different transcriptional programs in luminal epithelial cells (hormone receptor positive and secretory), basal epithelial cells (myoepithelial or basement-like), endothelial cells (lymphatic or vascular), macrophages (M1 or M2) and fibroblasts (three subgroups) and provided insight into progenitors of each cell types, she said.
A map was created to show gene expression and to identify transcriptomally similar cells.
“We were able to identify most of the major cell types that are present in human breasts,” she said. “What was interesting was that the composition of these cells also varied across women.”
For example, the proportion of fibroblasts was lower in 3 of the 11 patients, and even though the cells were pathologically normal, immune cell populations, including T-cells and macrophages, were also seen.
Adipocytes cannot be evaluated using this technology because they are large and the layer of fat cells must be removed during dissociation to prevent clogging of the machines, she noted, adding that “this is really a limitation of our technology.”
A closer look was taken at each of the major cell types identified.
Epithelial cells
Both canonical and new gene markers were used to identify luminal and basal epithelial cells, Ms. Seth noted.
Among the known markers were KRT18 for luminal epithelial cells and KRT5, KRT6B, KRT14, and KRT15 for basal epithelial cells. Among the new markers were SLC39A6, EFHD1 and HES1 for the luminal epithelial cells, and CITED4, CCK28, MMP7, and MDRG2 for the basal epithelial cells.
“We went on and validated these markers on the tissue section using methods like spatial transcriptomics,” she said, explaining that this “really helps capture the RNA expression spatially,” and can resolve the localization of cell types markers in anatomical structures.
For these cells, the expression of the newly identified gene markers was mostly confined to ducts and lobules.
In addition, an analysis of cell states within the luminal epithelial cells showed four different cell states, each of which have “different kinds of genes that they express, and also different pathways that they express, suggesting that these might be transcriptomally different,” Ms. Seth said.
Of note, these cells and cells states are not biased to a specific condition or patient, suggesting that they are coming from all of the patients, she added.
Two of the four cell states – the secretory and hormone responsive states – have previously been reported, but Ms. Seth and her colleagues identified two additional cell states that may have different biological functions and are present in the different anatomical regions of the breast.
Fibroblasts
Fibroblasts, the cells of the connective tissue, were the most abundant cell type. Like the epithelial cells, both canonical collagen markers (COL6A3, MMP2, FBN1, FBLN2, FBN, and COL1A1) and newly identified gene markers (TNXB, AEBP1, CFH, CTSK, TPPP3, MEG3, HTRA1, LHFP, and OGN) were used to identify them.
Endothelial cells
Breast tissue is highly vascular, so endothelial cells, which line the walls of veins, arteries, and lymphatic vessels, are plentiful.
“Again, for both these cell types, we identified them using the canonical marker CD31, and we identified some new gene markers,” she said, noting that the new markers include CCL21, CLDN5, MMRN1, LYVE1, and PROX1 for lymphatic endothelial cells, and RNASE1 and IFI27 for vascular endothelial cells.
Two different groups – or states – of vascular endothelial cells were identified, with each expressing “very different genes as well as very different pathways, again suggesting that they might have different biological functions, which we are still investigating,” she said.
Additional findings and future directions
In addition to stromal cells, some immune cells were also seen. These included T cells that came mostly from two patients, as well as macrophages and monocytes, which comprised the most abundant immune cell population.
Of note, all of these cells are also found in the tumor microenvironment, but they are in a transformed state. For example cancer-associated fibroblasts, tumor endothelial cells, tumor-associated macrophages, and tumor-associated adipocytes have been seen in that environment, she said.
“So what we are trying to do with this project is ... learn how these cells are, and how these cells behave in the normal ecosystem,” she explained, noting that the hope is to provide a valuable reference for the research community with new insights about how normal cell types are transformed in the tumor microenvironment.
In an effort to overcome the adipocyte-associated limitation of the technology, adipocytes are “now being isolated by single nucleus RNA sequencing.”
“This [sequencing] technology has helped us identify multiple cell states within a cell type; and most of these cell states may have different biological functions, which probably can be investigated by spatial transcriptomic methods,” she said.
Spatial transcriptomics also continue to be used for validation of the new gene markers identified in the course of this research, she noted.
The breast tumor microenvironment
At the Garvan Institute, current work is focusing more on defining the landscape of the breast tumor microenvironment at single-cell resolution, according to Dr. Swarbrick, a senior research fellow and head of the Tumour Progression Laboratory there.
“Breast cancers ... are complex cellular ecosystems, and it’s really the sum of the interactions between the cell types that play major roles in determining the etiology of disease and its response to therapy,” he said. “So I think that going forward toward a new age of diagnostics and therapeutics, there’s wonderful potential in capitalizing on the tumor microenvironment for new developments, but this has to be built on a really deep understanding of the tumor microenvironment, and – I might say – a new taxonomy of the breast cellular environment.”
Therefore, in an effort to address “this limitation in our knowledge base,” his lab is also working toward development of a breast cell atlas.
A fresh tissue collection program was established to collect early breast cancer tissues at the time of surgery, metastatic biopsies, and metastatic lesions from autopsies. The tissues are quickly dissociated into their cellular components and they undergo massively parallel capture and sequencing using the 10x genomics platform, he said.
Thousands of cells per case are analyzed using single-cell RNA sequencing (RNA-seq), as well as “RAGE-seq” and “CITE-seq,” which are performed in parallel to the RNA sequencing to address some of the limitations of the RNA sequencing alone and to “try to gain a multi-omic insight into the cell biology,” he explained.
RAGE-seq, which Dr. Swarbrick and his team developed, “is essentially a method to do targeted long-read sequencing in parallel to the short-read sequencing that we use for RNA-seq,” and CITE-seq is “a really fantastic method developed at the New York Genome Center that essentially allows us to gather proteomic data in parallel to the RNA data,” he said.
Based on findings from the analyses of about 125,000 cells from 25 patients, a map was created that showed the cell clusters identified by both canonical markers and gene expression signatures.
“We find the cell types we would expect to be present in a breast cancer,” he said.
The map shows clusters of myeloid, epithelial-1 and -2, cancer-associated fibroblast (CAF)-1 and -2, endothelial, T Reg, B, and CD8 and CD4 T cells.
Next, each cell type is quantified in each patient, and a graphic representation of the findings shows large variability in the proportions of each cell type in each patient.
“Ultimately, our goal is to be able to relate the frequencies of cell types and molecular features to each other, but also to clinical-pathological features from these patients,” he noted.
A closer look at the findings on an individual case level demonstrates the potential for development of better therapies.
For example, a case involving a high-grade triple-negative invasive ductal carcinoma exhibited each of the cell types found overall.
“One of the things that strikes us early on is we see a number of malignant epithelial populations,” he said, noting that proliferation is one of the drivers of the heterogeneity, but that heterogeneity was also seen for “other clinically relevant features such as basal cytokeratins,” which were heterogeneously expressed in different cell-type clusters.
“This was kind of paralleled in the immunohistochemistry results that we obtained from this patient,” he said. “We could also apply other clinically used tests that we’ve developed on bulk (such as PAM50 intrinsic subtyping) and ask whether they can be applied at the single-cell resolution.
“We think that these are going to be great tools to try to now get in and understand the significance of this heterogeneity and try to identify the lethal cells within this patient, and potentially therapeutic strategies to eradicate those cells,” he added.
Fibroblasts
A notable finding of this project was the presence of “not one, but two populations of fibroblasts,” Dr. Swarbrick said, noting that fibroblasts are typically discussed as a single entity.
“This is arguing that there are at least two major types present within the breast, and almost every case has these populations present at roughly equal amounts,” he said.
This is of particular interest, because it has been shown in prior studies that targeting fibroblasts can have therapeutic outcomes.
“So we think this is a very important population within the tumor microenvironment,” he added.
With respect to gene expression features, CAF-1 is dominated by signatures of extracellular matrix deposition and remodeling, which “look like the classic myofibroblasts that we typically think of when we study cancer-associated fibroblasts.”
“In contrast, the CAF-2 population ... have what appears to be quite a predominant secretory function, so we see a lot of cytokines being produced by these cells, but we also see a very high level of expression of a number immune checkpoint ligands,” he said, adding that his team is actively pursuing whether these cells may be undergoing signaling events with infiltrating lymphocytes in the tumor microenvironment.
The signatures for both CAF types are prognostic within large breast cancer data sets, suggesting that they do actually have an important role in disease, he noted.
Markers for these cells include ACTA2, which was previously known to be a marker, and which is almost exclusively restricted to CAF-1, and the cell surface protein CD34 – a progenitor marker in many different cellular systems, “which is actually beautifully expressed on the CAF-2 population” as demonstrated using CITE-seq.
“So we’re now using this as a way to prospectively identify these cells, pull them out of tumors, and conduct biologic assays to learn more about them,” he said.
The immune milieu
“We’re in the age of immunotherapy, and this is an area of huge interest, but we have a long way to go in making it as effective as possible for breast cancer patients,” Dr. Swarbrick said. “I believe part of that is through a very deep understanding of the taxonomy.”
RNA data alone are useful but insufficient to fully identify subsets of immune cells due to a “relatively low-resolution ability to resolve T cells.”
“But because we’re now using the panel of 125 antibodies in parallel, we can now start to use protein levels to split up these populations and we can start to now identify, with higher resolution, more unique populations within the environment,” he said, noting that the availability of protein data not only helps identify subtypes, but is also therapeutically important as it allows for certainty regarding whether the protein target of therapeutic antibodies is expressed on the surface of cells.
Ultimately the hope is that this effort to build a multi-omic breast cancer atlas will continue to drive new discoveries in personalized medicine for breast cancer, Dr. Swarbrick concluded, adding that the field is moving fast, and it will be very important for labs like his and the Navin Lab to communicate to avoid needlessly duplicating efforts.
“I think it’s going to be really exciting to start to put some of these [findings] together,” he said.
The MD Anderson project is funded by the Chan Zuckerberg Initiative as part of its work in supporting the Human Cell Atlas project. Ms. Seth reported having no disclosures. Dr. Swarbrick’s research is funded by the Australian Government/National Health and Medical Research Council and the National Breast Cancer Foundation. He reported having no relevant disclosures.
SOURCE: Seth T et al. SABCS 2018, Abstract GS1-02; Swarbrick A et al. SABCS 2018, Abstract GS1-01
SAN ANTONIO – Researchers at MD Anderson Cancer Center in Houston and the University of New South Wales (UNSW) in Sydney are among teams from around the world working toward human breast cell atlas development using single-cell genomics, and their efforts to date have yielded new understanding of both the normal breast cell ecosystem and the breast cancer tumor microenvironment.
The work at MD Anderson, for example, has led to the identification of a number of new gene markers and multiple cell states within breast cell types, according to Tapsi Kumar Seth, who reported early findings from an analysis of more than 32,000 cells from normal breast tissue during a presentation at the San Antonio Breast Cancer Symposium.
At the UNSW’s Garvan Institute of Medical Research, Alexander Swarbrick, PhD, and his colleagues are working to better define the tumor microenvironment at the single-cell level. At the symposium, Dr. Swarbrick presented interim findings from cellular analyses in the first 23 breast cancer cases of about 200 that will be studied in the course of the project.
Improved understanding of the cellular landscape of both normal breast tissue and breast cancer tissue should lead to new stromal- and immune-based therapies for the treatment of breast cancer, the investigators said.
The normal breast cell ecosystem
The MD Anderson researchers studied 32,148 stromal cells from pathologically normal breast tissues collected from 11 women who underwent mastectomy at the center.
Unbiased expression analysis identified three major cell types, including epithelial cells, fibroblasts, and endothelial cells, as well as several minor cell types such as macrophages, T-cells, apocrine cells, pericytes, and others, said Ms. Seth, a graduate student in the department of genetics at the center and a member of the Navin Laboratory there.
The work is designed to help identify the presence and function of cells and explain how they behave in a normal breast ecosystem, she said.
“We know that a female breast undergoes a lot of changes due to age, pregnancy, or when there is a disease such as cancer, so it’s essential to chart out what a normal cell reference would look like,” she said.
Toward that goal, a protocol was developed to dissociate the tissue samples within 2 hours due to the decline in viability seen in cells and RNA over time. Analysis of the cell states revealed different transcriptional programs in luminal epithelial cells (hormone receptor positive and secretory), basal epithelial cells (myoepithelial or basement-like), endothelial cells (lymphatic or vascular), macrophages (M1 or M2) and fibroblasts (three subgroups) and provided insight into progenitors of each cell types, she said.
A map was created to show gene expression and to identify transcriptomally similar cells.
“We were able to identify most of the major cell types that are present in human breasts,” she said. “What was interesting was that the composition of these cells also varied across women.”
For example, the proportion of fibroblasts was lower in 3 of the 11 patients, and even though the cells were pathologically normal, immune cell populations, including T-cells and macrophages, were also seen.
Adipocytes cannot be evaluated using this technology because they are large and the layer of fat cells must be removed during dissociation to prevent clogging of the machines, she noted, adding that “this is really a limitation of our technology.”
A closer look was taken at each of the major cell types identified.
Epithelial cells
Both canonical and new gene markers were used to identify luminal and basal epithelial cells, Ms. Seth noted.
Among the known markers were KRT18 for luminal epithelial cells and KRT5, KRT6B, KRT14, and KRT15 for basal epithelial cells. Among the new markers were SLC39A6, EFHD1 and HES1 for the luminal epithelial cells, and CITED4, CCK28, MMP7, and MDRG2 for the basal epithelial cells.
“We went on and validated these markers on the tissue section using methods like spatial transcriptomics,” she said, explaining that this “really helps capture the RNA expression spatially,” and can resolve the localization of cell types markers in anatomical structures.
For these cells, the expression of the newly identified gene markers was mostly confined to ducts and lobules.
In addition, an analysis of cell states within the luminal epithelial cells showed four different cell states, each of which have “different kinds of genes that they express, and also different pathways that they express, suggesting that these might be transcriptomally different,” Ms. Seth said.
Of note, these cells and cells states are not biased to a specific condition or patient, suggesting that they are coming from all of the patients, she added.
Two of the four cell states – the secretory and hormone responsive states – have previously been reported, but Ms. Seth and her colleagues identified two additional cell states that may have different biological functions and are present in the different anatomical regions of the breast.
Fibroblasts
Fibroblasts, the cells of the connective tissue, were the most abundant cell type. Like the epithelial cells, both canonical collagen markers (COL6A3, MMP2, FBN1, FBLN2, FBN, and COL1A1) and newly identified gene markers (TNXB, AEBP1, CFH, CTSK, TPPP3, MEG3, HTRA1, LHFP, and OGN) were used to identify them.
Endothelial cells
Breast tissue is highly vascular, so endothelial cells, which line the walls of veins, arteries, and lymphatic vessels, are plentiful.
“Again, for both these cell types, we identified them using the canonical marker CD31, and we identified some new gene markers,” she said, noting that the new markers include CCL21, CLDN5, MMRN1, LYVE1, and PROX1 for lymphatic endothelial cells, and RNASE1 and IFI27 for vascular endothelial cells.
Two different groups – or states – of vascular endothelial cells were identified, with each expressing “very different genes as well as very different pathways, again suggesting that they might have different biological functions, which we are still investigating,” she said.
Additional findings and future directions
In addition to stromal cells, some immune cells were also seen. These included T cells that came mostly from two patients, as well as macrophages and monocytes, which comprised the most abundant immune cell population.
Of note, all of these cells are also found in the tumor microenvironment, but they are in a transformed state. For example cancer-associated fibroblasts, tumor endothelial cells, tumor-associated macrophages, and tumor-associated adipocytes have been seen in that environment, she said.
“So what we are trying to do with this project is ... learn how these cells are, and how these cells behave in the normal ecosystem,” she explained, noting that the hope is to provide a valuable reference for the research community with new insights about how normal cell types are transformed in the tumor microenvironment.
In an effort to overcome the adipocyte-associated limitation of the technology, adipocytes are “now being isolated by single nucleus RNA sequencing.”
“This [sequencing] technology has helped us identify multiple cell states within a cell type; and most of these cell states may have different biological functions, which probably can be investigated by spatial transcriptomic methods,” she said.
Spatial transcriptomics also continue to be used for validation of the new gene markers identified in the course of this research, she noted.
The breast tumor microenvironment
At the Garvan Institute, current work is focusing more on defining the landscape of the breast tumor microenvironment at single-cell resolution, according to Dr. Swarbrick, a senior research fellow and head of the Tumour Progression Laboratory there.
“Breast cancers ... are complex cellular ecosystems, and it’s really the sum of the interactions between the cell types that play major roles in determining the etiology of disease and its response to therapy,” he said. “So I think that going forward toward a new age of diagnostics and therapeutics, there’s wonderful potential in capitalizing on the tumor microenvironment for new developments, but this has to be built on a really deep understanding of the tumor microenvironment, and – I might say – a new taxonomy of the breast cellular environment.”
Therefore, in an effort to address “this limitation in our knowledge base,” his lab is also working toward development of a breast cell atlas.
A fresh tissue collection program was established to collect early breast cancer tissues at the time of surgery, metastatic biopsies, and metastatic lesions from autopsies. The tissues are quickly dissociated into their cellular components and they undergo massively parallel capture and sequencing using the 10x genomics platform, he said.
Thousands of cells per case are analyzed using single-cell RNA sequencing (RNA-seq), as well as “RAGE-seq” and “CITE-seq,” which are performed in parallel to the RNA sequencing to address some of the limitations of the RNA sequencing alone and to “try to gain a multi-omic insight into the cell biology,” he explained.
RAGE-seq, which Dr. Swarbrick and his team developed, “is essentially a method to do targeted long-read sequencing in parallel to the short-read sequencing that we use for RNA-seq,” and CITE-seq is “a really fantastic method developed at the New York Genome Center that essentially allows us to gather proteomic data in parallel to the RNA data,” he said.
Based on findings from the analyses of about 125,000 cells from 25 patients, a map was created that showed the cell clusters identified by both canonical markers and gene expression signatures.
“We find the cell types we would expect to be present in a breast cancer,” he said.
The map shows clusters of myeloid, epithelial-1 and -2, cancer-associated fibroblast (CAF)-1 and -2, endothelial, T Reg, B, and CD8 and CD4 T cells.
Next, each cell type is quantified in each patient, and a graphic representation of the findings shows large variability in the proportions of each cell type in each patient.
“Ultimately, our goal is to be able to relate the frequencies of cell types and molecular features to each other, but also to clinical-pathological features from these patients,” he noted.
A closer look at the findings on an individual case level demonstrates the potential for development of better therapies.
For example, a case involving a high-grade triple-negative invasive ductal carcinoma exhibited each of the cell types found overall.
“One of the things that strikes us early on is we see a number of malignant epithelial populations,” he said, noting that proliferation is one of the drivers of the heterogeneity, but that heterogeneity was also seen for “other clinically relevant features such as basal cytokeratins,” which were heterogeneously expressed in different cell-type clusters.
“This was kind of paralleled in the immunohistochemistry results that we obtained from this patient,” he said. “We could also apply other clinically used tests that we’ve developed on bulk (such as PAM50 intrinsic subtyping) and ask whether they can be applied at the single-cell resolution.
“We think that these are going to be great tools to try to now get in and understand the significance of this heterogeneity and try to identify the lethal cells within this patient, and potentially therapeutic strategies to eradicate those cells,” he added.
Fibroblasts
A notable finding of this project was the presence of “not one, but two populations of fibroblasts,” Dr. Swarbrick said, noting that fibroblasts are typically discussed as a single entity.
“This is arguing that there are at least two major types present within the breast, and almost every case has these populations present at roughly equal amounts,” he said.
This is of particular interest, because it has been shown in prior studies that targeting fibroblasts can have therapeutic outcomes.
“So we think this is a very important population within the tumor microenvironment,” he added.
With respect to gene expression features, CAF-1 is dominated by signatures of extracellular matrix deposition and remodeling, which “look like the classic myofibroblasts that we typically think of when we study cancer-associated fibroblasts.”
“In contrast, the CAF-2 population ... have what appears to be quite a predominant secretory function, so we see a lot of cytokines being produced by these cells, but we also see a very high level of expression of a number immune checkpoint ligands,” he said, adding that his team is actively pursuing whether these cells may be undergoing signaling events with infiltrating lymphocytes in the tumor microenvironment.
The signatures for both CAF types are prognostic within large breast cancer data sets, suggesting that they do actually have an important role in disease, he noted.
Markers for these cells include ACTA2, which was previously known to be a marker, and which is almost exclusively restricted to CAF-1, and the cell surface protein CD34 – a progenitor marker in many different cellular systems, “which is actually beautifully expressed on the CAF-2 population” as demonstrated using CITE-seq.
“So we’re now using this as a way to prospectively identify these cells, pull them out of tumors, and conduct biologic assays to learn more about them,” he said.
The immune milieu
“We’re in the age of immunotherapy, and this is an area of huge interest, but we have a long way to go in making it as effective as possible for breast cancer patients,” Dr. Swarbrick said. “I believe part of that is through a very deep understanding of the taxonomy.”
RNA data alone are useful but insufficient to fully identify subsets of immune cells due to a “relatively low-resolution ability to resolve T cells.”
“But because we’re now using the panel of 125 antibodies in parallel, we can now start to use protein levels to split up these populations and we can start to now identify, with higher resolution, more unique populations within the environment,” he said, noting that the availability of protein data not only helps identify subtypes, but is also therapeutically important as it allows for certainty regarding whether the protein target of therapeutic antibodies is expressed on the surface of cells.
Ultimately the hope is that this effort to build a multi-omic breast cancer atlas will continue to drive new discoveries in personalized medicine for breast cancer, Dr. Swarbrick concluded, adding that the field is moving fast, and it will be very important for labs like his and the Navin Lab to communicate to avoid needlessly duplicating efforts.
“I think it’s going to be really exciting to start to put some of these [findings] together,” he said.
The MD Anderson project is funded by the Chan Zuckerberg Initiative as part of its work in supporting the Human Cell Atlas project. Ms. Seth reported having no disclosures. Dr. Swarbrick’s research is funded by the Australian Government/National Health and Medical Research Council and the National Breast Cancer Foundation. He reported having no relevant disclosures.
SOURCE: Seth T et al. SABCS 2018, Abstract GS1-02; Swarbrick A et al. SABCS 2018, Abstract GS1-01
REPORTING FROM SABCS 2018
Key clinical point: Improved understanding of the cellular landscape of both normal breast tissue and breast cancer could lead to new stromal- and immune-based therapies.
Major finding: From pathologically normal breast tissues expression, investigators identified three major cell types, as well as several minor cell types. In analyses of cells from breast cancer patients, a map was created that showed the cell clusters identified by both canonical markers and gene expression signatures.
Study details: An analysis of 32,138 breast cells from 11 women, and another of about 125,000 cells from 25 patients.
Disclosures: The MD Anderson research is part of the Human Cell Atlas project and is funded by the Chan Zuckerberg Initiative. Ms. Seth reported having no disclosures. Dr. Swarbrick’s research is funded by the Australian Government/National Health and Medical Research Council and the National Breast Cancer Foundation. He reported having no relevant disclosures.
Source: Seth T et al. SABCS 2018: Abstract GS1-02; Swarbrick A et al. SABCS 2018: Abstract GS1-01.