User login
Mohs Micrographic Surgery in the VHA (FULL)
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
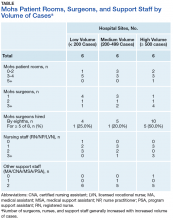
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
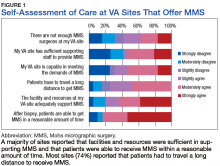
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
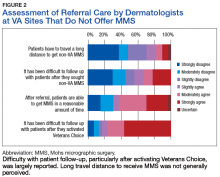
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Clinical benefits persist 5 years after thymectomy for myasthenia gravis
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
FROM LANCET NEUROLOGY
Key clinical point: The benefits of thymectomy for myasthenia gravis persist 5 years after the procedure.
Major finding: Patients who undergo thymectomy and receive prednisone have lower time-weighted average Quantitative Myasthenia Gravis scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who receive prednisone alone.
Study details: A rater-blinded 2-year extension study that enrolled 68 patients who had completed a 3-year randomized controlled trial.
Disclosures: The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Other authors reported working with and receiving funds from various agencies, foundations, and pharmaceutical companies.
Source: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
No evidence for disease-modifying effect of levodopa in Parkinson’s disease
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Levodopa did not have any significant disease-modifying effects in patients with early Parkinson’s disease.
Major finding: Change in the Unified Parkinson’s Disease Rating Scale (UPDRS) was –1.0 after 80 weeks of levodopa/carbodopa versus –2.0 for 40 weeks of placebo followed by 40 weeks of treatment (P = .44).
Study details: A delayed-start trial including 445 patients with early Parkinson’s disease randomized to 80 weeks of treatment or 40 weeks of placebo plus 40 weeks of treatment.
Disclosures: Study authors reported disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
Source: Verschuur CVM et al. N Engl J Med. 2019;380:315-324.
Study hints at lacosamide’s efficacy for small fiber neuropathy
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
FROM BRAIN
Key clinical point:
Major finding: In the lacosamide group, 50.0% of patients reported mean average pain decreasing by at least 1 point, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213).
Study details: A randomized, placebo-controlled, double-blind, crossover-design study of 25 patients with Nav1.7-related small fiber neuropathy who received lacosamide followed by placebo, or vice versa.
Disclosures: The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
Source: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
AML, myeloma risk higher for breast cancer survivors
Breast cancer survivors should continue to be monitored for hematologic malignancies, especially acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), results of a population-based study from France suggest.
Among nearly 440,000 women with an incident breast cancer diagnosis, the incidence of AML was nearly three times higher and the incidence of MDS was five times higher than that of women in the general population. Women with breast cancer also were at higher risk for multiple myeloma (MM) and acute lymphoblastic leukemia/lymphocytic lymphoma (ALL/LL) compared with the background population, reported Marie Joelle Jabagi, PharmD, MPH, of the University of Paris Sud, France, and her colleagues.
“These findings serve to better inform practicing oncologists, and breast cancer survivors should be advised of the increased risk of developing certain hematologic malignant neoplasms after their first cancer diagnosis,” they wrote in JAMA Network Open.
Breast cancers are the malignant solid tumors most frequently associated with risk for myeloid neoplasms, but there is little information on the risk for secondary lymphoid malignancies among breast cancer patients, the investigators stated.
“In addition, real-life data on secondary hematologic malignant neoplasm incidence are scarce, especially in the recent period marked by major advances in breast cancer treatments,” they wrote.
To get better estimates of the incidence of myeloid and lymphoid neoplasms in this population, they conducted a retrospective review of information from the French National Health Data System on all French women from the ages of 20 to 85 years who had an incident breast cancer diagnosis from July 1, 2006, through Dec. 31, 2015.
In all, 439,704 women with a median age of 59 years were identified. They were followed until a diagnosis of a hematologic malignancy, death, or loss to follow-up, or until Dec. 31, 2016.
Data on the breast cancer patients were compared with those for all French women in the general population who were registered in the general national health insurance program from January 2007 through the end of 2016.
During a median follow-up of 5 years, there were 3,046 cases of hematologic neoplasms among the breast cancer patients, including 509 cases of AML, for a crude incidence rate (CIR) of 24.5 per 100,000 person-years (py); 832 cases of MDS for a CIR of 40.1/100,000 py; and 267 cases of myeloproliferative neoplasms (MPN), for a CIR of 12.8/100,000 py.
In addition, there were 420 cases of MM for a CIR of 20.3/100,000 py; 912 cases of Hodgkin or non-Hodgkin lymphoma (HL/NHL) for a CIR of 44.4/100,000 py, and 106 cases of ALL/LL for a CIR of 5.1/100,000 py.
Breast cancer survivors had significantly higher incidences, compared with the general population, of AML (standardized incidence ratio [SIR] 2.8, 95% confidence interval [CI], 2.5-3.2), MDS (SIR 5.0, CI, 4.4-5.7), MM (SIR 1.5, CI, 1.3-17), and ALL/LL (SIR 2.0, CI, 1.3-3.0). There was a trend toward significance for both MPN and HL/NHL, but the lower limit of the confidence intervals for these conditions either crossed or touched 1.
In a review of the literature, the authors found that “[s]everal studies linked AML and MDS to chemotherapeutic agents, radiation treatment, and supportive treatment with granulocyte colony-stimulating factor. These results are consistent with other available data showing a 2½-fold to 3½-fold increased risk of AML.”
They noted that their estimate of a five-fold increase in risk for MDS was higher than the 3.7-fold risk reported in a previous registry cohort analysis, suggesting that risk for MDS among breast cancer patients may be underestimated.
“The recent discovery of the gene signatures that guide treatment decisions in early-stage breast cancer might reduce the number of patients exposed to cytotoxic chemotherapy and its complications, including hematologic malignant neoplasm. Therefore, continuing to monitor hematologic malignant neoplasm trends is necessary, especially given that approaches to cancer treatment are rapidly evolving. Further research is also required to assess the modality of treatment for and the genetic predisposition to these secondary malignant neoplasms,” the authors concluded.
SOURCE: Jabagi MJ et al. JAMA Network Open. 2019 Jan 18. doi: 10.1001/jamanetworkopen.2018.7147.
Breast cancer survivors should continue to be monitored for hematologic malignancies, especially acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), results of a population-based study from France suggest.
Among nearly 440,000 women with an incident breast cancer diagnosis, the incidence of AML was nearly three times higher and the incidence of MDS was five times higher than that of women in the general population. Women with breast cancer also were at higher risk for multiple myeloma (MM) and acute lymphoblastic leukemia/lymphocytic lymphoma (ALL/LL) compared with the background population, reported Marie Joelle Jabagi, PharmD, MPH, of the University of Paris Sud, France, and her colleagues.
“These findings serve to better inform practicing oncologists, and breast cancer survivors should be advised of the increased risk of developing certain hematologic malignant neoplasms after their first cancer diagnosis,” they wrote in JAMA Network Open.
Breast cancers are the malignant solid tumors most frequently associated with risk for myeloid neoplasms, but there is little information on the risk for secondary lymphoid malignancies among breast cancer patients, the investigators stated.
“In addition, real-life data on secondary hematologic malignant neoplasm incidence are scarce, especially in the recent period marked by major advances in breast cancer treatments,” they wrote.
To get better estimates of the incidence of myeloid and lymphoid neoplasms in this population, they conducted a retrospective review of information from the French National Health Data System on all French women from the ages of 20 to 85 years who had an incident breast cancer diagnosis from July 1, 2006, through Dec. 31, 2015.
In all, 439,704 women with a median age of 59 years were identified. They were followed until a diagnosis of a hematologic malignancy, death, or loss to follow-up, or until Dec. 31, 2016.
Data on the breast cancer patients were compared with those for all French women in the general population who were registered in the general national health insurance program from January 2007 through the end of 2016.
During a median follow-up of 5 years, there were 3,046 cases of hematologic neoplasms among the breast cancer patients, including 509 cases of AML, for a crude incidence rate (CIR) of 24.5 per 100,000 person-years (py); 832 cases of MDS for a CIR of 40.1/100,000 py; and 267 cases of myeloproliferative neoplasms (MPN), for a CIR of 12.8/100,000 py.
In addition, there were 420 cases of MM for a CIR of 20.3/100,000 py; 912 cases of Hodgkin or non-Hodgkin lymphoma (HL/NHL) for a CIR of 44.4/100,000 py, and 106 cases of ALL/LL for a CIR of 5.1/100,000 py.
Breast cancer survivors had significantly higher incidences, compared with the general population, of AML (standardized incidence ratio [SIR] 2.8, 95% confidence interval [CI], 2.5-3.2), MDS (SIR 5.0, CI, 4.4-5.7), MM (SIR 1.5, CI, 1.3-17), and ALL/LL (SIR 2.0, CI, 1.3-3.0). There was a trend toward significance for both MPN and HL/NHL, but the lower limit of the confidence intervals for these conditions either crossed or touched 1.
In a review of the literature, the authors found that “[s]everal studies linked AML and MDS to chemotherapeutic agents, radiation treatment, and supportive treatment with granulocyte colony-stimulating factor. These results are consistent with other available data showing a 2½-fold to 3½-fold increased risk of AML.”
They noted that their estimate of a five-fold increase in risk for MDS was higher than the 3.7-fold risk reported in a previous registry cohort analysis, suggesting that risk for MDS among breast cancer patients may be underestimated.
“The recent discovery of the gene signatures that guide treatment decisions in early-stage breast cancer might reduce the number of patients exposed to cytotoxic chemotherapy and its complications, including hematologic malignant neoplasm. Therefore, continuing to monitor hematologic malignant neoplasm trends is necessary, especially given that approaches to cancer treatment are rapidly evolving. Further research is also required to assess the modality of treatment for and the genetic predisposition to these secondary malignant neoplasms,” the authors concluded.
SOURCE: Jabagi MJ et al. JAMA Network Open. 2019 Jan 18. doi: 10.1001/jamanetworkopen.2018.7147.
Breast cancer survivors should continue to be monitored for hematologic malignancies, especially acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), results of a population-based study from France suggest.
Among nearly 440,000 women with an incident breast cancer diagnosis, the incidence of AML was nearly three times higher and the incidence of MDS was five times higher than that of women in the general population. Women with breast cancer also were at higher risk for multiple myeloma (MM) and acute lymphoblastic leukemia/lymphocytic lymphoma (ALL/LL) compared with the background population, reported Marie Joelle Jabagi, PharmD, MPH, of the University of Paris Sud, France, and her colleagues.
“These findings serve to better inform practicing oncologists, and breast cancer survivors should be advised of the increased risk of developing certain hematologic malignant neoplasms after their first cancer diagnosis,” they wrote in JAMA Network Open.
Breast cancers are the malignant solid tumors most frequently associated with risk for myeloid neoplasms, but there is little information on the risk for secondary lymphoid malignancies among breast cancer patients, the investigators stated.
“In addition, real-life data on secondary hematologic malignant neoplasm incidence are scarce, especially in the recent period marked by major advances in breast cancer treatments,” they wrote.
To get better estimates of the incidence of myeloid and lymphoid neoplasms in this population, they conducted a retrospective review of information from the French National Health Data System on all French women from the ages of 20 to 85 years who had an incident breast cancer diagnosis from July 1, 2006, through Dec. 31, 2015.
In all, 439,704 women with a median age of 59 years were identified. They were followed until a diagnosis of a hematologic malignancy, death, or loss to follow-up, or until Dec. 31, 2016.
Data on the breast cancer patients were compared with those for all French women in the general population who were registered in the general national health insurance program from January 2007 through the end of 2016.
During a median follow-up of 5 years, there were 3,046 cases of hematologic neoplasms among the breast cancer patients, including 509 cases of AML, for a crude incidence rate (CIR) of 24.5 per 100,000 person-years (py); 832 cases of MDS for a CIR of 40.1/100,000 py; and 267 cases of myeloproliferative neoplasms (MPN), for a CIR of 12.8/100,000 py.
In addition, there were 420 cases of MM for a CIR of 20.3/100,000 py; 912 cases of Hodgkin or non-Hodgkin lymphoma (HL/NHL) for a CIR of 44.4/100,000 py, and 106 cases of ALL/LL for a CIR of 5.1/100,000 py.
Breast cancer survivors had significantly higher incidences, compared with the general population, of AML (standardized incidence ratio [SIR] 2.8, 95% confidence interval [CI], 2.5-3.2), MDS (SIR 5.0, CI, 4.4-5.7), MM (SIR 1.5, CI, 1.3-17), and ALL/LL (SIR 2.0, CI, 1.3-3.0). There was a trend toward significance for both MPN and HL/NHL, but the lower limit of the confidence intervals for these conditions either crossed or touched 1.
In a review of the literature, the authors found that “[s]everal studies linked AML and MDS to chemotherapeutic agents, radiation treatment, and supportive treatment with granulocyte colony-stimulating factor. These results are consistent with other available data showing a 2½-fold to 3½-fold increased risk of AML.”
They noted that their estimate of a five-fold increase in risk for MDS was higher than the 3.7-fold risk reported in a previous registry cohort analysis, suggesting that risk for MDS among breast cancer patients may be underestimated.
“The recent discovery of the gene signatures that guide treatment decisions in early-stage breast cancer might reduce the number of patients exposed to cytotoxic chemotherapy and its complications, including hematologic malignant neoplasm. Therefore, continuing to monitor hematologic malignant neoplasm trends is necessary, especially given that approaches to cancer treatment are rapidly evolving. Further research is also required to assess the modality of treatment for and the genetic predisposition to these secondary malignant neoplasms,” the authors concluded.
SOURCE: Jabagi MJ et al. JAMA Network Open. 2019 Jan 18. doi: 10.1001/jamanetworkopen.2018.7147.
FROM JAMA NETWORK OPEN
Key clinical point: Breast cancer survivors should be monitored for hematologic malignancies.
Major finding: The standardized incidence ratio for AML was 2.8 and the SIR for multiple myeloma was 5.0 among French breast cancer survivors compared with women in the general French population.
Study details: Retrospective analysis of data on 439,704 women aged 20-85 years with a breast cancer diagnosis.
Disclosures: The authors did not report a study funding source. Coauthor Anthony Goncalves, MD, reported nonfinancial support from Roche, Novartis, Pfizer, Celgene, MSD, Lilly, and Astra Zeneca outside of the submitted work. No other disclosures were reported.
Source: Jabagi MJ et al. JAMA Network Open. 2019 Jan 18. doi: 10.1001/jamanetworkopen.2018.7147.
FDA approves third trastuzumab biosimilar
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
The Food and Drug Administration .
Ontruzant (trastuzumab-dttb), marketed by Samsung Bioepis, is the third approved biosimilar to Genentech’s Herceptin in the United States.
Patients should be selected for treatment with Ontruzant using an FDA-approved companion diagnostic for a trastuzumab product.
The prescribing information for the newly approved biosimilar includes a boxed warning highlighting the risk of cardiomyopathy, infusion reactions, embryo-fetal toxicity, and pulmonary toxicity associated with the drug.
Ontruzant was shown to be equivalent to Herceptin in a phase 3 study (Eur J Cancer. 2018 Apr;93:19-27).
The trial included 875 patients with HER2-positive early breast cancer. Patients were randomized to receive Ontruzant or Herceptin for eight cycles concurrently with chemotherapy. The chemotherapy consisted of four cycles of docetaxel followed by four cycles of 5-fluorouracil/epirubicin/cyclophosphamide.
The patients then underwent surgery, which was followed by 10 cycles of Ontruzant (n=380) or Herceptin (n=384).
The median follow-up was 437 days in the Ontruzant arm and 438 days in the Herceptin arm. Safety and efficacy results were similar between the treatment arms.
Treatment-emergent adverse events occurred in 97.5% of patients in the Ontruzant arm and 96.1% of those in the Herceptin arm.
The 12-month event-free survival rate was 93.7% in the Ontruzant arm and 93.4% in the Herceptin arm.
Frailty may affect the expression of dementia
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
FROM LANCET NEUROLOGY
Key clinical point: Frailty modifies the association between Alzheimer’s disease pathology and Alzheimer dementia.
Major finding: Frailty index score (odds ratio, 1.76) is independently associated with dementia status.
Study details: A cross-sectional analysis of 456 deceased participants in the Rush Memory and Aging Project.
Disclosures: The study had no outside funding.
Source: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
High postpartum breast cancer metastasis risk may persist for a decade
Increased risk of metastasis associated with postpartum breast cancer (PPBC) in women 45 years or younger may persist for 10 years after childbirth, a finding that may give reason to extend the 5-year window currently defining PPBC.
Analysis of more than 700 patients showed that risk of metastasis was approximately twofold higher for a decade after childbirth, with risks about 3.5- to fivefold higher in women diagnosed with stage I or II disease, reported lead author Erica Goddard, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues. Regardless of parity status, patients diagnosed with stage III disease had poor outcomes.
“The high risk for metastasis is independent of poor prognostic indicators, including biological subtype, stage, age, or year of diagnosis,” the investigators wrote in JAMA Network Open. “Yet, PPBC is an underrecognized subset of breast cancer, and few studies address the associated high risk for metastasis.”
The cohort study involved 701 women 45 years or younger who were diagnosed with breast cancer between 1981 and 2014. Early cases were retrospective, until the study switched to a prospective method in 2004. The investigators analyzed rates of distant metastasis and looked for associations with tumor cell proliferation, lymphovascular invasion, lymph node involvement, and other clinical attributes. Distant metastasis was defined by spread beyond the ipsilateral breast or local draining lymph node, as detected by physical exam, imaging, and/or pathological testing. The investigators also stained available tumor samples for Ki67 positivity, which is used for prognostic purposes, and to distinguish between ER-positive luminal A versus ER-positive luminal B disease.
Compared with nulliparous patients, women under 45 who were diagnosed with PPBC within 5 years of childbirth were 2.13 times as likely to develop metastasis (P = .009). This risk persisted for 5 more years. Women diagnosed within 5-10 years of childbirth showed a similar hazard ratio, of 2.23 (P = .006). After 10 years, the hazard ratio dropped to 1.6, but this value was statistically insignificant (P = .13). Patients identified with stage I or II disease had more dramatic risk profiles, with hazard ratios of 3.5 and 5.2, for diagnoses up to 5 years postpartum, and diagnoses 5-10 years postpartum, respectively. These findings suggest that, for some patients, the 5- to 10-year window may be the riskiest time for metastasis, and, incidentally, one that has historically been excluded from the definition of PPBC.
In addition, patients diagnosed with estrogen receptor–positive breast cancer within 10 years of childbirth had outcomes similar to those of nulliparous women with estrogen receptor–negative breast cancer, and postpartum women with estrogen receptor–negative breast cancer had worse outcomes than did nulliparous women with the same subtype. Furthermore, PPBC was associated with higher rates of lymph node involvement and lymphovascular invasion. Collectively, these findings suggest that PPBC is generally more aggressive than nulliparous breast cancer. In contrast, Ki67 positivity, identifying the luminal B subtype, was associated with worse outcome regardless of parity status, but this finding was statistically insignificant.
“[T]hese data suggest that stages I and II breast cancer in patients with PPBC diagnosed within 10 years of parturition may be underestimated in their risk for metastasis, as parity status is not currently factored into clinical decision-making algorithms, such as the National Comprehensive Cancer Network guidelines,” the investigators concluded. “In sum, we suggest that poor-prognostic PPBC is an increasing problem that merits more dedicated research.”
The study was funded by the National Cancer Institute, the National Institutes of Health, the U.S. Department of Defense, and other organizations. Dr. Goddard reported funding from the NCI and NIH. Dr. Mori reported financial support from the Department of Defense.
SOURCE: Goddard et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.
Increased risk of metastasis associated with postpartum breast cancer (PPBC) in women 45 years or younger may persist for 10 years after childbirth, a finding that may give reason to extend the 5-year window currently defining PPBC.
Analysis of more than 700 patients showed that risk of metastasis was approximately twofold higher for a decade after childbirth, with risks about 3.5- to fivefold higher in women diagnosed with stage I or II disease, reported lead author Erica Goddard, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues. Regardless of parity status, patients diagnosed with stage III disease had poor outcomes.
“The high risk for metastasis is independent of poor prognostic indicators, including biological subtype, stage, age, or year of diagnosis,” the investigators wrote in JAMA Network Open. “Yet, PPBC is an underrecognized subset of breast cancer, and few studies address the associated high risk for metastasis.”
The cohort study involved 701 women 45 years or younger who were diagnosed with breast cancer between 1981 and 2014. Early cases were retrospective, until the study switched to a prospective method in 2004. The investigators analyzed rates of distant metastasis and looked for associations with tumor cell proliferation, lymphovascular invasion, lymph node involvement, and other clinical attributes. Distant metastasis was defined by spread beyond the ipsilateral breast or local draining lymph node, as detected by physical exam, imaging, and/or pathological testing. The investigators also stained available tumor samples for Ki67 positivity, which is used for prognostic purposes, and to distinguish between ER-positive luminal A versus ER-positive luminal B disease.
Compared with nulliparous patients, women under 45 who were diagnosed with PPBC within 5 years of childbirth were 2.13 times as likely to develop metastasis (P = .009). This risk persisted for 5 more years. Women diagnosed within 5-10 years of childbirth showed a similar hazard ratio, of 2.23 (P = .006). After 10 years, the hazard ratio dropped to 1.6, but this value was statistically insignificant (P = .13). Patients identified with stage I or II disease had more dramatic risk profiles, with hazard ratios of 3.5 and 5.2, for diagnoses up to 5 years postpartum, and diagnoses 5-10 years postpartum, respectively. These findings suggest that, for some patients, the 5- to 10-year window may be the riskiest time for metastasis, and, incidentally, one that has historically been excluded from the definition of PPBC.
In addition, patients diagnosed with estrogen receptor–positive breast cancer within 10 years of childbirth had outcomes similar to those of nulliparous women with estrogen receptor–negative breast cancer, and postpartum women with estrogen receptor–negative breast cancer had worse outcomes than did nulliparous women with the same subtype. Furthermore, PPBC was associated with higher rates of lymph node involvement and lymphovascular invasion. Collectively, these findings suggest that PPBC is generally more aggressive than nulliparous breast cancer. In contrast, Ki67 positivity, identifying the luminal B subtype, was associated with worse outcome regardless of parity status, but this finding was statistically insignificant.
“[T]hese data suggest that stages I and II breast cancer in patients with PPBC diagnosed within 10 years of parturition may be underestimated in their risk for metastasis, as parity status is not currently factored into clinical decision-making algorithms, such as the National Comprehensive Cancer Network guidelines,” the investigators concluded. “In sum, we suggest that poor-prognostic PPBC is an increasing problem that merits more dedicated research.”
The study was funded by the National Cancer Institute, the National Institutes of Health, the U.S. Department of Defense, and other organizations. Dr. Goddard reported funding from the NCI and NIH. Dr. Mori reported financial support from the Department of Defense.
SOURCE: Goddard et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.
Increased risk of metastasis associated with postpartum breast cancer (PPBC) in women 45 years or younger may persist for 10 years after childbirth, a finding that may give reason to extend the 5-year window currently defining PPBC.
Analysis of more than 700 patients showed that risk of metastasis was approximately twofold higher for a decade after childbirth, with risks about 3.5- to fivefold higher in women diagnosed with stage I or II disease, reported lead author Erica Goddard, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues. Regardless of parity status, patients diagnosed with stage III disease had poor outcomes.
“The high risk for metastasis is independent of poor prognostic indicators, including biological subtype, stage, age, or year of diagnosis,” the investigators wrote in JAMA Network Open. “Yet, PPBC is an underrecognized subset of breast cancer, and few studies address the associated high risk for metastasis.”
The cohort study involved 701 women 45 years or younger who were diagnosed with breast cancer between 1981 and 2014. Early cases were retrospective, until the study switched to a prospective method in 2004. The investigators analyzed rates of distant metastasis and looked for associations with tumor cell proliferation, lymphovascular invasion, lymph node involvement, and other clinical attributes. Distant metastasis was defined by spread beyond the ipsilateral breast or local draining lymph node, as detected by physical exam, imaging, and/or pathological testing. The investigators also stained available tumor samples for Ki67 positivity, which is used for prognostic purposes, and to distinguish between ER-positive luminal A versus ER-positive luminal B disease.
Compared with nulliparous patients, women under 45 who were diagnosed with PPBC within 5 years of childbirth were 2.13 times as likely to develop metastasis (P = .009). This risk persisted for 5 more years. Women diagnosed within 5-10 years of childbirth showed a similar hazard ratio, of 2.23 (P = .006). After 10 years, the hazard ratio dropped to 1.6, but this value was statistically insignificant (P = .13). Patients identified with stage I or II disease had more dramatic risk profiles, with hazard ratios of 3.5 and 5.2, for diagnoses up to 5 years postpartum, and diagnoses 5-10 years postpartum, respectively. These findings suggest that, for some patients, the 5- to 10-year window may be the riskiest time for metastasis, and, incidentally, one that has historically been excluded from the definition of PPBC.
In addition, patients diagnosed with estrogen receptor–positive breast cancer within 10 years of childbirth had outcomes similar to those of nulliparous women with estrogen receptor–negative breast cancer, and postpartum women with estrogen receptor–negative breast cancer had worse outcomes than did nulliparous women with the same subtype. Furthermore, PPBC was associated with higher rates of lymph node involvement and lymphovascular invasion. Collectively, these findings suggest that PPBC is generally more aggressive than nulliparous breast cancer. In contrast, Ki67 positivity, identifying the luminal B subtype, was associated with worse outcome regardless of parity status, but this finding was statistically insignificant.
“[T]hese data suggest that stages I and II breast cancer in patients with PPBC diagnosed within 10 years of parturition may be underestimated in their risk for metastasis, as parity status is not currently factored into clinical decision-making algorithms, such as the National Comprehensive Cancer Network guidelines,” the investigators concluded. “In sum, we suggest that poor-prognostic PPBC is an increasing problem that merits more dedicated research.”
The study was funded by the National Cancer Institute, the National Institutes of Health, the U.S. Department of Defense, and other organizations. Dr. Goddard reported funding from the NCI and NIH. Dr. Mori reported financial support from the Department of Defense.
SOURCE: Goddard et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.
FROM JAMA NETWORK OPEN
Key clinical point: Increased risk of metastasis associated with postpartum breast cancer in women 45 years or younger may persist for 10 years after childbirth, instead of 5 years, as previously reported.
Major finding: Compared with nulliparous breast cancer patients, women 45 years or younger diagnosed with breast cancer within 5-10 years of childbirth were 2.23 times as likely to develop metastasis.
Study details: A retrospective and prospective cohort study involving 701 women with stage I, II, or III breast cancer who were 45 years or younger at time of diagnosis.
Disclosures: The study was funded by the National Cancer Institute, the National Institutes of Health, the U.S. Department of Defense, and other organizations. Dr. Goddard reported funding from the NCI and NIH. Dr. Mori reported financial support from the Department of Defense.
Source: Goddard et al. JAMA Netw Open. 2019 Jan 11. doi: 10.1001/jamanetworkopen.2018.6997.
DMTs, stem cell transplants both reduce disease progression in MS
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
FROM JAMA
Progress in cancer research slowed by broken system
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”
NEW YORK – Conflicts of interest, whether reported or unreported, are just one component of a broken system that has misaligned incentives with meaningful advances in breast cancer research, agreed a panel of experts who participated in an evening program sponsored by SHARE, a breast cancer patient advocacy group.
“The issue of disclosures, while it is important, is not really the key issue. The key issue for me is about the amazing amount of money that is changing hands,” said Fran Visco, president of the National Breast Cancer Coalition, Washington. She contended not enough money in breast cancer research is being applied to save women’s lives rather than driving profits for institutions, physicians, and the pharmaceutical industry.
The other two participants in this panel, called Conflicts of Interest in Research and Treatment: What Breast Cancer Patients Should Know, largely agreed. In turn, they each explained that major sums of money in play for physicians and institutions collaborating with industry are earmarked for generating income much more than improving outcomes.
“Most of where this money is going is for these little tiny improvements and there is no association between the outcome increment that you get and what you pay for that outcome increment,” said Robert Cook-Deegan, MD, a bioethicist and a professor at the School for Innovation in Society, Arizona State University, Tempe.
Leaving aside whether physician-scientists working at nonprofit research institutions can avoid conflicts of interest when money is funneled to them from start-up investment opportunities, pharmaceutical board memberships, and direct compensation deals for consultancies and other services, Dr. Cook-Deegan was referring to the bigger problem of misaligned incentives. Large financial rewards are available for any drug with market potential instead of being limited to those that save lives, he said.
Tracing the current system to the Bayh-Dole Act in 1980, which permitted researchers to claim patents for intellectual property developed with federal funding, Dr. Cook-Deegan described a system of minnows, fish, and sharks. Researchers, who are the minnows, seek potential new therapeutics to be developed by for-profit startups or other commercial entities, which are the fish. If the drug continues to show promise, it brings investors, who are the sharks.
Once the new therapy reaches the sharks, the excitement about a new and more effective treatment has been overshadowed by the excitement about profits, which are essential to pay for the large investment required for the clinical development that brings new drugs to market. If the drug is marketable, it is considered a success even when the clinical benefits barely register.
“There have been about 20 new [cancer] drugs approved by the [Food and Drug Administration] in the last 2 years. The increased median disease-free survival is usually about 3 months,” said Otis W. Brawley, MD, who recently joined the Kimmel Cancer Center at Johns Hopkins University after resigning his position as chief medical officer at the American Cancer Society (ACS).
Dr. Brawley joined the others in declaring: “The system is broken.” The flow of money is not the only incentive for researchers to participate in clinical development programs focused on minor advances rather than blockbuster discoveries. Unrestricted research grants are now much harder to obtain, according to Dr. Brawley, making collaboration with industry essential not just for research funding but career advancement. Research at major cancer centers is still being conducted at the highest level, but there are unavoidable interdependent relationships for nonprofit institutions and for-profit enterprises.
“The doc who prescribes the drug to the average patient probably does not have this conflict of interest,” he said. “However, they are influenced by people who have these conflicts.”
The SHARE panel on conflicts of interest was convened after a series of articles co-reported by ProPublica and the New York Times described the failure of the chief medical officer at Memorial Sloan Kettering Cancer Center, José Baselga, MD, to disclose his financial conflicts of interest in publications, such as the New England Journal of Medicine, where they are required. Dr. Baselga resigned his position.
However, the failure to disclose financial relationships with industry was a minor topic during the SHARE panel. Robert Bazell, PhD, an adjunct professor in the division of molecular, cellular, and developmental biology at Yale University, New Haven, Conn., moderated the panel. He said in his opening remarks that the issues “go much deeper.”
While the articles did not reveal “anything illegal, it opened a lot of peoples’ eyes as to how much money there was in the system,” said Dr. Bazell, who was for many years the chief science and health correspondent for NBC News.
Specifically, it has heightened concern about whether profit motives are subverting the goals of science, according to Dr. Bazell. “This money bomb has fallen on a lot of science but it has particularly fallen on oncology,” he said.
Not everyone, including the bioethicist, agreed that profits by themselves are the problem. Big rewards may be a reasonable price for discoveries that save lives, but all agreed that the system does not necessarily reserve big rewards for life-saving advances.
“The focus isn’t really on drugs that will save lives, its just on more drugs,” said Ms. Visco, echoing the incentive system described by Dr. Cook-Deegan. She believes the current system handsomely rewards scientists and physicians for developing drugs with little or no significant clinical benefit. When a drug shows a progression-free survival benefit relative to a previous standard, even of modest statistical or clinical significance, it will be prescribed and profits will be generated.
Early in her tenure at the National Breast Cancer Coalition, Ms. Visco, who was a practicing lawyer prior to taking the helm of the coalition, considered fund raising for research the primary goal. She hoped that the coalition could help the breast cancer research community identify and fund priorities, but her perspective has changed.
“I used to think that we should be collaborators but now I am coming to the conclusion that we should be in charge. Educated, trained patient advocates with a constituency should be in charge, because our only agenda is to save lives and end breast cancer,” Ms. Visco said. She no longer believes in simply increasing funding even at research centers such as the National Cancer Institute without a fundamental reorganization of priorities.
Many leaders in medicine are aware of the problem, according to Dr. Brawley, who cited a recent statement by the American Society of Clinical Oncology that expressed concern about the plethora of cancer trials showing very small gains. He did agree with the others, however, that incentives are now misaligned.
“We are designing clinical trials not to look for big gains,” Dr. Brawley agreed. But he also cautioned that the system is complex. One reason that drugs offering modest or little gain over a previous standard are highly marketable is that patients themselves demand them. Direct-to-consumer marketing supports drugs with little or even nothing to offer.
“I see patients who want drugs that they should not get,” said Dr. Brawley, who indicated that clinicians are under pressure to offer something to desperate patients even when options are expensive and not shown to provide any change in outcome.
Overall, in the shake-up at Memorial Sloan Kettering, it was the failure to disclose significant financial relationships rather than the financial relationships themselves that represented an important breach in ethics, according to Dr. Brawley, who indicated that researcher relationships with industry are not inherently wrong. In an article published in November 2018, the New York Times reported that Dr. Brawley left his post at the ACS because of “his dismay” over some partnerships the ACS had formed with industry, but Dr. Brawley would not confirm or deny this report.
Relative to conflicts of interest at major research institutions, Dr. Cook-Deegan was more circumspect about whether disclosure is enough. Although he agreed with the premise that close collaboration with industry might be clinically valuable, which one investigator at Memorial Sloan Kettering claimed when speaking with the New York Times, he questioned whether there is a line over which the relationship is too intertwined.
“Do you really need to serve on the board? Do you really need the types of financial ties that have the potential to influence clinical decisions?” he asked.
Although Dr. Cook-Deegan agreed with the others that he does not know exactly how best to fix the system, he believes a fix may be coming.
“I think we are at an inflection point,” he said. “The symptoms of a system that has been running off the rails for a while are getting severe enough that we are starting to pay attention,” he said. One sign of movement is that both political parties have “introduced bills to address pricing, which is directly related to the problems we are talking about.”