User login
Immunotherapy may hold the key to defeating virally associated cancers
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
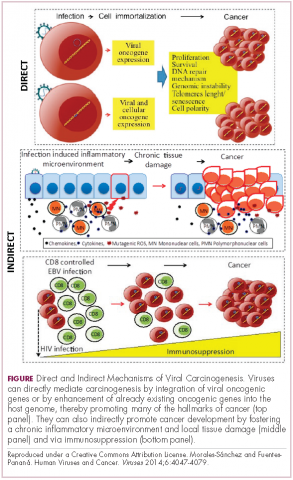
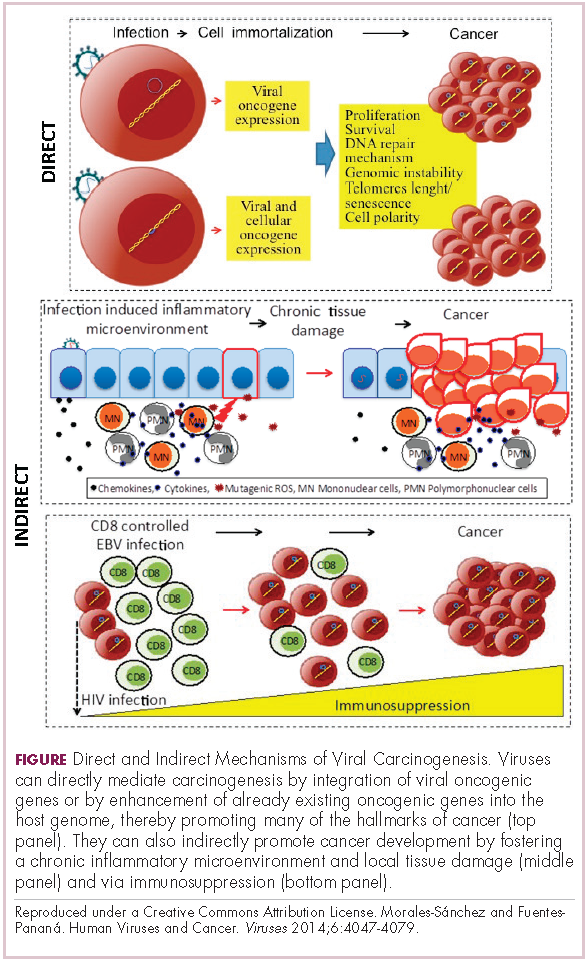
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
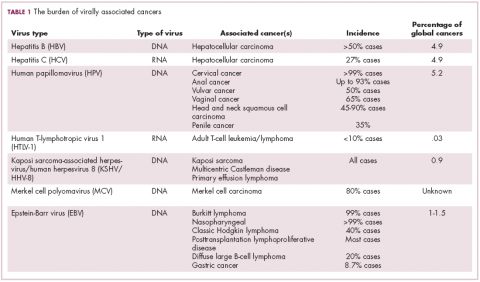
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
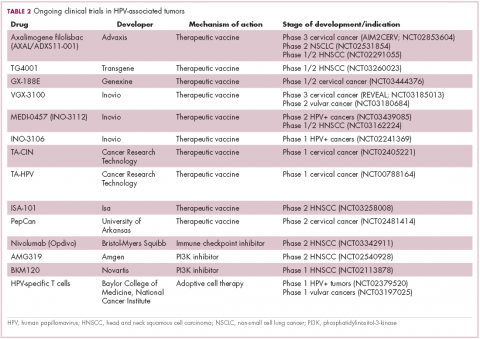
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
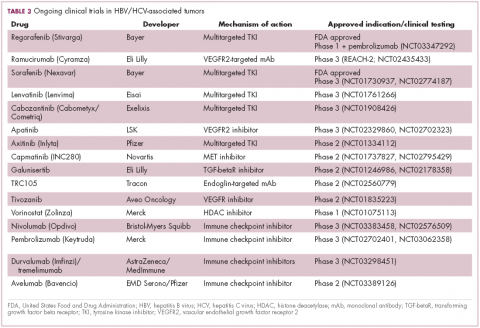
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
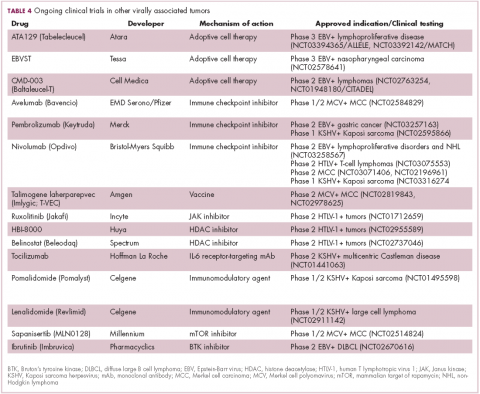
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
Infection with certain viruses has been causally linked to the development of cancer. In recent years, an improved understanding of the unique pathology and molecular underpinnings of these virally associated cancers has prompted the development of more personalized treatment strategies, with a particular focus on immunotherapy. Here, we describe some of the latest developments.
The link between viruses and cancer
Suspicions about a possible role of viral infections in the development of cancer were first aroused in the early 1900s. The seminal discovery is traced back to Peyton Rous, who showed that a malignant tumor growing in a chicken could be transferred to a healthy bird by injecting it with tumor extracts that contained no actual tumor cells.1
The infectious etiology of human cancer, however, remained controversial until many years later when the first cancer-causing virus, Epstein-Barr virus (EBV), was identified in cell cultures from patients with Burkitt lymphoma. Shortly afterward, the Rous sarcoma virus was unveiled as the oncogenic agent behind Rous’ observations.2Seven viruses have now been linked to the development of cancers and are thought to be responsible for around 12% of all cancer cases worldwide. The burden is likely to increase as technological advancements make it easier to establish a causal link between viruses and cancer development.3
In addition to making these links, researchers have also made significant headway in understanding how viruses cause cancer. Cancerous transformation of host cells occurs in only a minority of those who are infected with oncogenic viruses and often occurs in the setting of chronic infection.
Viruses can mediate carcinogenesis by direct and/or indirect mechanisms (Figure 1). Many of the hallmarks of cancer, the key attributes that drive the transformation from a normal cell to a malignant one, are compatible with the virus’s needs, such as needing to avoid cell death, increasing cell proliferation, and avoiding detection by the immune system.
Viruses hijack the cellular machinery to meet those needs and they can do this either by producing viral proteins that have an oncogenic effect or by integrating their genetic material into the host cell genome. When the latter occurs, the process of integration can also cause damage to the DNA, which further increases the risk of cancer-promoting changes occurring in the host genome.
Viruses can indirectly contribute to carcinogenesis by fostering a microenvironment of chronic inflammation, causing oxidative stress and local tissue damage, and by suppressing the antitumor immune response.4,5
Screening and prevention efforts have helped to reduce the burden of several different virally associated cancers. However, for the substantial proportion of patients who are still affected by these cancers, there is a pressing need for new therapeutic options, particularly since genome sequencing studies have revealed that these cancers can often have distinct underlying molecular mechanisms.
Vaccines lead the charge in HPV-driven cancers
German virologist Harald zur Hausen received the Nobel Prize in 2008 for his discovery of the oncogenic role of human papillomaviruses (HPVs), a large family of more than 100 DNA viruses that infect the epithelial cells of the skin and mucous membranes. They are responsible for the largest number of virally associated cancer cases globally – around 5% (Table 1).
A number of different cancer types are linked to HPV infection, but it is best known as the cause of cervical cancer. The development of diagnostic blood tests and prophylactic vaccines for prevention and early intervention in HPV infection has helped to reduce the incidence of cervical cancer. Conversely, another type of HPV-associated cancer, head and neck squamous cell carcinoma (HNSCC), has seen increased incidence in recent years.
HPVs are categorized according to their oncogenic potential as high, intermediate, or low risk. The high-risk HPV16 and HPV18 strains are most commonly associated with cancer. They are thought to cause cancer predominantly through integration into the host genome. The HPV genome is composed of 8 genes encoding proteins that regulate viral replication and assembly. The E6 and E7 genes are the most highly oncogenic; as the HPV DNA is inserted into the host genome, the transcriptional regulator of E6/E7 is lost, leading to their increased expression. These genes have significant oncogenic potential because of their interaction with 2 tumor suppressor proteins, p53 and pRb.6,7
The largest investment in therapeutic development for HPV-positive cancers has been in the realm of immunotherapy in an effort to boost the anti-tumor immune response. In particular, there has been a focus on the development of therapeutic vaccines, designed to prime the anti-tumor immune response to recognize viral antigens. A variety of different types of vaccines are being developed, including live, attenuated and inactivated vaccines that are protein, DNA, or peptide based. Most developed to date target the E6/E7 proteins from the HPV16/18 strains (Table 2).8,9
Other immunotherapies are also being evaluated, including immune checkpoint inhibitors, antibodies designed to target one of the principal mechanisms of immune evasion exploited by cancer cells. The combination of immune checkpoint inhibitors with vaccines is a particularly promising strategy in HPV-associated cancers. At the European Society for Medical Oncology Congress in 2017, the results of a phase 2 trial of nivolumab in combination with ISA-101 were presented.
Among 24 patients with HPV-positive tumors, the majority oropharyngeal cancers, the combination elicited an overall response rate (ORR) of 33%, including 2 complete responses (CRs). Most adverse events (AEs) were mild to moderate in severity and included fever, injection site reactions, fatigue and nausea.14
Hepatocellular carcinoma: a tale of two viruses
The hepatitis viruses are a group of 5 unrelated viruses that causes inflammation of the liver. Hepatitis B (HBV), a DNA virus, and hepatitis C (HCV), an RNA virus, are also oncoviruses; HBV in particular is one of the main causes of hepatocellular carcinoma (HCC), the most common type of liver cancer.
The highly inflammatory environment fostered by HBV and HCV infection causes liver damage that often leads to cirrhosis. Continued infection can drive permanent damage to the hepatocytes, leading to genetic and epigenetic damage and driving oncogenesis. As an RNA virus, HCV doesn’t integrate into the genome and no confirmed viral oncoproteins have been identified to date, therefore it mostly drives cancer through these indirect mechanisms, which is also reflected in the fact that HCV-associated HCC predominantly occurs against a backdrop of liver cirrhosis.
HBV does integrate into the host genome. Genome sequencing studies revealed hundreds of integration sites, but most commonly they disrupted host genes involved in telomere stability and cell cycle regulation, providing some insight into the mechanisms by which HBV-associated HCC develops. In addition, HBV produces several oncoproteins, including HBx, which disrupts gene transcription, cell signaling pathways, cell cycle progress, apoptosis and other cellular processes.15,16
Multitargeted tyrosine kinase inhibitors (TKIs) have been the focal point of therapeutic development in HCC. However, following the approval of sorafenib in 2008, there was a dearth of effective new treatment options despite substantial efforts and numerous phase 3 trials. More recently, immunotherapy has also come to the forefront, especially immune checkpoint inhibitors.
Last year marked the first new drug approvals in nearly a decade – the TKI regorafenib (Stivarga) and immune checkpoint inhibitor nivolumab (Opdivo), both in the second-line setting after failure of sorafenib. Treatment options in this setting may continue to expand, with the TKIs cabozantinib and lenvatinib and the immune checkpoint inhibitor pembrolizumab and the combination of durvalumab and tremelimumab hot on their heels.17-20 Many of these drugs are also being evaluated in the front-line setting in comparison with sorafenib (Table 3).
At the current time, the treatment strategy for patients with HCC is independent of etiology, however, there are significant ongoing efforts to try to tease out the implications of infection for treatment efficacy. A recent meta-analysis of patients treated with sorafenib in 3 randomized phase 3 trials (n = 3,526) suggested that it improved overall survival (OS) among patients who were HCV-positive, but HBV-negative.21
Studies of the vascular endothelial growth factor receptor 2-targeting monoclonal antibody ramucirumab, on the other hand, suggested that it may have a greater OS benefit in patients with HBV, while regorafenib seemed to have a comparable OS benefit in both subgroups.22-25 The immune checkpoint inhibitors studied thus far seem to elicit responses irrespective of infection status.
A phase 2 trial of the immune checkpoint inhibitor tremelimumab was conducted specifically in patients with advanced HCC and chronic HCV infection. The disease control rate (DCR) was 76.4%, with 17.6% partial response (PR) rate. There was also a significant drop in viral load, suggesting that tremelimumab may have antiviral effects.26,27,28
Adoptive cell therapy promising in EBV-positive cancers
More than 90% of the global population is infected with EBV, making it one of the most common human viruses. It is a member of the herpesvirus family that is probably best known as the cause of infectious mononucleosis. On rare occasions, however, EBV can cause tumor development, though our understanding of its exact pathogenic role in cancer is still incomplete.
EBV is a DNA virus that doesn’t tend to integrate into the host genome, but instead remains in the nucleus in the form of episomes and produces several oncoproteins, including latent membrane protein-1. It is associated with a range of different cancer types, including Burkitt lymphoma and other B-cell malignancies. It also infects epithelial cells and can cause nasopharyngeal carcinoma and gastric cancer, however, much less is known about the molecular underpinnings of these EBV-positive cancer types.26,27Gastric cancers actually comprise the largest group of EBV-associated tumors because of the global incidence of this cancer type. The Cancer Genome Atlas Research Network recently characterized gastric cancer on a molecular level and identified an EBV-positive subgroup as a distinct clinical entity with unique molecular characteristics.29
The focus of therapeutic development has again been on immunotherapy, however in this case the idea of collecting the patients T cells, engineering them to recognize EBV, and then reinfusing them into the patient – adoptive cell therapy – has gained the most traction (Table 4).
Two presentations at the American Society of Hematology annual meeting in 2017 detailed ongoing clinical trials of Atara Biotherapeutics’ ATA129 and Cell Medica’s CMD-003. ATA129 was associated with a high response rate and a low rate of serious AEs in patients with posttransplant lymphoproliferative disorder; ORR was 80% in 6 patients treated after hematopoietic stem cell transplantation, and 83% in 6 patients after solid organ transplant.30
CMD-003, meanwhile, demonstrated preliminary signs of activity and safety in patients with relapsed extranodal NK/T-cell lymphoma, according to early results from the phase 2 CITADEL trial. Among 6 evaluable patients, the ORR was 50% and the DCR was 67%.31
Newest oncovirus on the block
The most recently discovered cancer-associated virus is Merkel cell polyomavirus (MCV), a DNA virus that was identified in 2008. Like EBV, virtually the whole global adult population is infected with MCV. It is linked to the development of a highly aggressive and lethal, though rare, form of skin cancer – Merkel cell carcinoma.
MCV is found in around 80% of MCC cases and in fewer than 10% of melanomas and other skin cancers. Thus far, several direct mechanisms of oncogenesis have been described, including integration of MCV into the host genome and the production of viral oncogenes, though their precise function is as yet unclear.32-34
The American Cancer Society estimates that only 1500 cases of MCC are diagnosed each year in the United States.35 Its rarity makes it difficult to conduct clinical trials with sufficient power, yet some headway has still been made.
Around half of MCCs express the programmed cell death ligand 1 (PD-L1) on their surface, making them a logical candidate for immune checkpoint inhibition. In 2017, avelumab became the first FDA-approved drug for the treatment of MCC. Approval was based on the JAVELIN Merkel 200 study in which 88 patients received avelumab. After 1 year of follow-up the ORR was 31.8%, with a CR rate of 9%.36
Genome sequencing studies suggest that the mutational profile of MCV-positive tumors is quite different to those that are MCV-negative, which could have therapeutic implications. To date, these implications have not been delineated, given the challenge of small patient numbers, however an ongoing phase 1/2 trial is evaluating the combination of avelumab and radiation therapy or recombinant interferon beta, with or without MCV-specific cytotoxic T cells in patients with MCC and MCV infection.
The 2 other known cancer-causing viruses are human T-lymphotropic virus 1 (HTLV-1), a retrovirus associated with adult T-cell leukemia/lymphoma (ATL) and Kaposi sarcoma herpesvirus (KSHV). The latter is the causative agent of Kaposi sarcoma, often in combination with human immunodeficiency virus (HIV), a rare skin tumor that became renowned in the 1980s as an AIDS-defining illness.
The incidence of HTLV-1- and KSHV-positive tumors is substantially lower than the other virally associated cancers and, like MCC, this makes studying them and conducting clinical trials of novel therapeutic options a challenge. Nonetheless, several trials of targeted therapies and immunotherapies are underway.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
1. Rous PA. Transmissible avain neoplasm. (Sarcoma of the common fowl). J Exp Med. 1910;12(5):696-705.
2. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1(7335):702-703.
3. Mesri Enrique A, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host & Microbe. 2014;15(3):266-282.
4. Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3(1):106-112.
5. Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27(1):59-73.
6. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80-85.
7. Tulay P, Serakinci N. The route to HPV-associated neoplastic transformation: a review of the literature. Crit Rev Eukaryot Gene Expr. 2016;26(1):27-39.
8. Smola S. Immunopathogenesis of HPV-associated cancers and prospects for immunotherapy. Viruses. 2017;9(9).
9. Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World Journal of Clinical Oncology. 2014;5(5):1002-1019.
10. Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract. 2017;4:10.
11. Miles BA, Monk BJ, Safran HP. Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract. 2017;4:9.
12. Huh W, Dizon D, Powell M, Landrum L, Leath C. A prospective phase II trial of the listeria-based human papillomavirus immunotherapy axalimogene filolisbac in second and third-line metastatic cervical cancer: A NRG oncology group trial. Paper presented at: Annual Meeting on Women's Cancer; March 12-15, 2017, 2017; National Harbor, MD.
13. Petit RG, Mehta A, Jain M, et al. ADXS11-001 immunotherapy targeting HPV-E7: final results from a Phase II study in Indian women with recurrent cervical cancer. Journal for Immunotherapy of Cancer. 2014;2(Suppl 3):P92-P92.
14. Glisson B, Massarelli E, William W, et al. Nivolumab and ISA 101 HPV vaccine in incurable HPV-16+ cancer. Ann Oncol. 2017;28(suppl_5):v403-v427.
15. Ding X-X, Zhu Q-G, Zhang S-M, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715-55730.
16. Ringehan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160274.
17. Abou-Alfa G, Meyer T, Cheng AL, et al. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from the randomized phase III CELESTIAL trial. J Clin Oncol. 2017;36(Suppl 4S):abstr 207.
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018.
19. Zhu AX, Finn RS, Cattan S, et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36(Suppl 4S):Abstr 209.
20. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. Journal of Clinical Oncology. 2017;35(15_suppl):4073-4073.
21. Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. Journal of Clinical Oncology. 2017;35(6):622-628.
22. Kudo M. Molecular Targeted Agents for Hepatocellular Carcinoma: Current Status and Future Perspectives. Liver Cancer. 2017;6(2):101-112.
23. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer. 1974;13(5):650-656.
24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
25. Bruix J, Tak WY, Gasbarrini A, et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer. 2013;49(16):3412-3419.
26. Neparidze N, Lacy J. Malignancies associated with epstein-barr virus: pathobiology, clinical features, and evolving treatments. Clin Adv Hematol Oncol. 2014;12(6):358-371.
27. Ozoya OO, Sokol L, Dalia S. EBV-Related Malignancies, Outcomes and Novel Prevention Strategies. Infect Disord Drug Targets. 2016;16(1):4-21.
28. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81-88.
29. The Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202.
30. Prockop S, Li A, Baiocchi R, et al. Efficacy and safety of ATA129, partially matched allogeneic third-party Epstein-Barr virus-targeted cytotoxic T lymphocytes in a multicenter study for post-transplant lymphoproliferative disorder. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
31. Kim W, Ardeshna K, Lin Y, et al. Autologous EBV-specific T cells (CMD-003): Early results from a multicenter, multinational Phase 2 trial for treatment of EBV-associated NK/T-cell lymphoma. Paper presented at: 59th Annual Meeting of the American Society of Hematology; December 9-12, 2017, 2017; Atlanta, GA.
32. Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. European Journal of Cancer. 2017;71:53-69.
33. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology. 2013;435(1):118-130.
34. Tello TL, Coggshall K, Yom SS, Yu SS. Merkel cell carcinoma: An update and review: Current and future therapy. J Am Acad Dermatol. 2018;78(3):445-454.
35. American Cancer Society. Key Statistics for Merkel Cell Carcinoma. 2015; https://www.cancer.org/cancer/merkel-cell-skin-cancer/about/key-statistics.html#written_by. Accessed March 7th, 2017.
36. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. The Lancet Oncology.17(10):1374-1385.
ASH 2018 coming attractions look at the big picture
In the closest thing the medical world has to movie trailers, the American Society of Hematology held a press conference offering
Shorter R-CHOP regimen for DLBCL
Under the heading “Big Trials, Big Results” will be data from the FLYER trial, a phase 3, randomized, deescalation trial in 592 patients aged 18-60 years with favorable-prognosis diffuse large B-cell lymphoma. The investigators report that both progression-free survival and overall survival with four cycles of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) were noninferior to those for patients treated with six cycles of R-CHOP (abstract 781).
Ibrutinib mastery in CLL
Also on the program are results of a study showing that ibrutinib (Imbruvica), either alone or in combination with rituximab, is associated with superior progression-free survival than bendamustine and rituximab in older patients with chronic lymphocytic leukemia (CLL).
The trial, the Alliance North American Intergroup Study A041202 (abstract 6) is the first major trial to pit ibrutinib against the modern standard of immunochemotherapy rather than the older standard of chlorambucil, Dr. Brodsky noted.
Anemia support in beta-thalassemia, MDS
In nonmalignant disease, investigators in the randomized, phase 3 BELIEVE trial are reporting results of their study showing that the first-in-class erythroid maturation agent luspatercept was associated with significant reductions in the need for RBC transfusion in adults with transfusion-dependent beta-thalassemia.
The investigators report that the experimental agent was “generally well tolerated” (abstract 163).
“Beyond a proof of principle, [this is] certainly a very exciting advancement in this group of patients who otherwise had very few treatment options,” said Alexis A. Thompson, MD, associate director of equity and minority health at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, and the current ASH president.
Dr. Thompson also highlighted the MEDALIST trial (abstract 1), a phase 3, randomized study showing that luspatercept significantly reduced transfusion burden, compared with placebo, in patients with anemia caused by very low–, low-, or intermediate-risk myelodysplastic syndrome with ring sideroblasts who require RBC transfusions.
“This group of patients were individuals who were refractory or were not responders or did not tolerate erythropoietic stimulating agents and therefore were requiring regular transfusion,” Dr. Thompson said.
Worth the wait
The late-breaking abstract program was stretched from the usual six abstracts to seven this year because of the unusually high quality of the science, Dr. Brodsky said.
Among these star attractions are results of a phase 3, randomized study of daratumumab (Darzalex) plus lenalidomide and dexamethasone versus lenalidomide-dexamethasone alone for patients with newly diagnosed multiple myeloma who are ineligible for transplant.
The investigators found that adding daratumumab reduced the risk of disease progression or death by close to 50%, supporting the combination as a new standard of care in these patients, according to Thierry Facon, MD, from the Hospital Claude Huriez in Lille, France, and colleagues (abstract LBA-2).
Two other late-breakers deal with CLL. The first, a randomized, phase 3 study of ibrutinib-based therapy versus standard fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy in younger patients with untreated CLL, found that ibrutinib and rituximab provided significantly better progression-free survival and overall survival (abstract LBA-4).
“These findings have immediate practice-changing implications and establish ibrutinib-based therapy as the most efficacious first-line therapy for patients with CLL,” wrote Tait D. Shanafelt, MD, from Stanford (Calif.) University, and colleagues.
On a less positive note, Australian researchers report their discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax (Venclexta) in patients with progressive CLL (abstract LBA-7).
“This mutation provides new insights into the pathobiology of venetoclax resistance and provides a potential biomarker of impending clinical relapse,” wrote Piers Blombery, MBBS, from the University of Melbourne, and colleagues.
Finally, investigators from children’s hospitals in the United States and Europe report promising findings on the safety and efficacy of emapalumab for the treatment of patients with the rare genetic disorder primary hemophagocytic lymphohistiocytosis (HLH).
The drug, newly approved by the Food and Drug Administration, was able to control HLH’s hyperinflammatory activity, and allowed a substantial proportion of patients to survive to hematopoietic stem cell transplantation, the investigators said (abstract LBA-6).
In the closest thing the medical world has to movie trailers, the American Society of Hematology held a press conference offering
Shorter R-CHOP regimen for DLBCL
Under the heading “Big Trials, Big Results” will be data from the FLYER trial, a phase 3, randomized, deescalation trial in 592 patients aged 18-60 years with favorable-prognosis diffuse large B-cell lymphoma. The investigators report that both progression-free survival and overall survival with four cycles of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) were noninferior to those for patients treated with six cycles of R-CHOP (abstract 781).
Ibrutinib mastery in CLL
Also on the program are results of a study showing that ibrutinib (Imbruvica), either alone or in combination with rituximab, is associated with superior progression-free survival than bendamustine and rituximab in older patients with chronic lymphocytic leukemia (CLL).
The trial, the Alliance North American Intergroup Study A041202 (abstract 6) is the first major trial to pit ibrutinib against the modern standard of immunochemotherapy rather than the older standard of chlorambucil, Dr. Brodsky noted.
Anemia support in beta-thalassemia, MDS
In nonmalignant disease, investigators in the randomized, phase 3 BELIEVE trial are reporting results of their study showing that the first-in-class erythroid maturation agent luspatercept was associated with significant reductions in the need for RBC transfusion in adults with transfusion-dependent beta-thalassemia.
The investigators report that the experimental agent was “generally well tolerated” (abstract 163).
“Beyond a proof of principle, [this is] certainly a very exciting advancement in this group of patients who otherwise had very few treatment options,” said Alexis A. Thompson, MD, associate director of equity and minority health at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, and the current ASH president.
Dr. Thompson also highlighted the MEDALIST trial (abstract 1), a phase 3, randomized study showing that luspatercept significantly reduced transfusion burden, compared with placebo, in patients with anemia caused by very low–, low-, or intermediate-risk myelodysplastic syndrome with ring sideroblasts who require RBC transfusions.
“This group of patients were individuals who were refractory or were not responders or did not tolerate erythropoietic stimulating agents and therefore were requiring regular transfusion,” Dr. Thompson said.
Worth the wait
The late-breaking abstract program was stretched from the usual six abstracts to seven this year because of the unusually high quality of the science, Dr. Brodsky said.
Among these star attractions are results of a phase 3, randomized study of daratumumab (Darzalex) plus lenalidomide and dexamethasone versus lenalidomide-dexamethasone alone for patients with newly diagnosed multiple myeloma who are ineligible for transplant.
The investigators found that adding daratumumab reduced the risk of disease progression or death by close to 50%, supporting the combination as a new standard of care in these patients, according to Thierry Facon, MD, from the Hospital Claude Huriez in Lille, France, and colleagues (abstract LBA-2).
Two other late-breakers deal with CLL. The first, a randomized, phase 3 study of ibrutinib-based therapy versus standard fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy in younger patients with untreated CLL, found that ibrutinib and rituximab provided significantly better progression-free survival and overall survival (abstract LBA-4).
“These findings have immediate practice-changing implications and establish ibrutinib-based therapy as the most efficacious first-line therapy for patients with CLL,” wrote Tait D. Shanafelt, MD, from Stanford (Calif.) University, and colleagues.
On a less positive note, Australian researchers report their discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax (Venclexta) in patients with progressive CLL (abstract LBA-7).
“This mutation provides new insights into the pathobiology of venetoclax resistance and provides a potential biomarker of impending clinical relapse,” wrote Piers Blombery, MBBS, from the University of Melbourne, and colleagues.
Finally, investigators from children’s hospitals in the United States and Europe report promising findings on the safety and efficacy of emapalumab for the treatment of patients with the rare genetic disorder primary hemophagocytic lymphohistiocytosis (HLH).
The drug, newly approved by the Food and Drug Administration, was able to control HLH’s hyperinflammatory activity, and allowed a substantial proportion of patients to survive to hematopoietic stem cell transplantation, the investigators said (abstract LBA-6).
In the closest thing the medical world has to movie trailers, the American Society of Hematology held a press conference offering
Shorter R-CHOP regimen for DLBCL
Under the heading “Big Trials, Big Results” will be data from the FLYER trial, a phase 3, randomized, deescalation trial in 592 patients aged 18-60 years with favorable-prognosis diffuse large B-cell lymphoma. The investigators report that both progression-free survival and overall survival with four cycles of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) were noninferior to those for patients treated with six cycles of R-CHOP (abstract 781).
Ibrutinib mastery in CLL
Also on the program are results of a study showing that ibrutinib (Imbruvica), either alone or in combination with rituximab, is associated with superior progression-free survival than bendamustine and rituximab in older patients with chronic lymphocytic leukemia (CLL).
The trial, the Alliance North American Intergroup Study A041202 (abstract 6) is the first major trial to pit ibrutinib against the modern standard of immunochemotherapy rather than the older standard of chlorambucil, Dr. Brodsky noted.
Anemia support in beta-thalassemia, MDS
In nonmalignant disease, investigators in the randomized, phase 3 BELIEVE trial are reporting results of their study showing that the first-in-class erythroid maturation agent luspatercept was associated with significant reductions in the need for RBC transfusion in adults with transfusion-dependent beta-thalassemia.
The investigators report that the experimental agent was “generally well tolerated” (abstract 163).
“Beyond a proof of principle, [this is] certainly a very exciting advancement in this group of patients who otherwise had very few treatment options,” said Alexis A. Thompson, MD, associate director of equity and minority health at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, and the current ASH president.
Dr. Thompson also highlighted the MEDALIST trial (abstract 1), a phase 3, randomized study showing that luspatercept significantly reduced transfusion burden, compared with placebo, in patients with anemia caused by very low–, low-, or intermediate-risk myelodysplastic syndrome with ring sideroblasts who require RBC transfusions.
“This group of patients were individuals who were refractory or were not responders or did not tolerate erythropoietic stimulating agents and therefore were requiring regular transfusion,” Dr. Thompson said.
Worth the wait
The late-breaking abstract program was stretched from the usual six abstracts to seven this year because of the unusually high quality of the science, Dr. Brodsky said.
Among these star attractions are results of a phase 3, randomized study of daratumumab (Darzalex) plus lenalidomide and dexamethasone versus lenalidomide-dexamethasone alone for patients with newly diagnosed multiple myeloma who are ineligible for transplant.
The investigators found that adding daratumumab reduced the risk of disease progression or death by close to 50%, supporting the combination as a new standard of care in these patients, according to Thierry Facon, MD, from the Hospital Claude Huriez in Lille, France, and colleagues (abstract LBA-2).
Two other late-breakers deal with CLL. The first, a randomized, phase 3 study of ibrutinib-based therapy versus standard fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy in younger patients with untreated CLL, found that ibrutinib and rituximab provided significantly better progression-free survival and overall survival (abstract LBA-4).
“These findings have immediate practice-changing implications and establish ibrutinib-based therapy as the most efficacious first-line therapy for patients with CLL,” wrote Tait D. Shanafelt, MD, from Stanford (Calif.) University, and colleagues.
On a less positive note, Australian researchers report their discovery of a recurrent mutation in BCL2 that confers resistance to venetoclax (Venclexta) in patients with progressive CLL (abstract LBA-7).
“This mutation provides new insights into the pathobiology of venetoclax resistance and provides a potential biomarker of impending clinical relapse,” wrote Piers Blombery, MBBS, from the University of Melbourne, and colleagues.
Finally, investigators from children’s hospitals in the United States and Europe report promising findings on the safety and efficacy of emapalumab for the treatment of patients with the rare genetic disorder primary hemophagocytic lymphohistiocytosis (HLH).
The drug, newly approved by the Food and Drug Administration, was able to control HLH’s hyperinflammatory activity, and allowed a substantial proportion of patients to survive to hematopoietic stem cell transplantation, the investigators said (abstract LBA-6).
ASH preview: Studies target CAR T-cell improvements
, according to investigators in two separate studies.
Meanwhile, responses to tisagenlecleucel appear to be even more durable with longer follow-up, according to preliminary results from two more CAR T-cell therapy studies slated for presentation at the annual meeting of the American Society of Hematology.
A fifth study will show that bone marrow transplant may effectively consolidate remission after CAR T-cell therapy, according to Robert A. Brodsky, MD, ASH secretary, who highlighted the studies during a media briefing.
The ibrutinib study (abstract 299) shows that administering this BTK inhibitor starting 2 weeks prior to leukapheresis and continuing until 3 months after JCAR014 could improve responses and may decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Of 16 patients in an ibrutinib cohort and 18 patients in a no-ibrutinib cohort, the proportion of responders was 88% and 56%, respectively, according to preliminary data reported in the abstract. Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients in the no-ibrutinib cohort, and 0 of 17 patients in the ibrutinib cohort.
Those findings are “early and preliminary, but very exciting” for ibrutinib in combination with this CD-19 specific CAR T-cell therapy said Dr. Brodsky, director of the division of hematology at Johns Hopkins University in Baltimore.
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy in patients with relapsed B-cell acute lymphoblastic leukemia (ALL).
In data to date, 14 patients with early CAR T-cell loss, partial response, or no response to CAR T-cell therapy received a PD-1 inhibitor.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said. “Sure enough, they were able to see that in roughly half of the patients. So again, very small, preliminary data, but very exciting that it is safe to give checkpoint inhibitors with CAR T-cells and it may be efficacious at getting the immune response back.”
One of the two tisagenlecleucel updates (abstract 895) showed that in the ELIANA trial, which included pediatric and young adults patients with relapsed/refractory ALL, the probability of relapse-free survival at 18 months was 66%.
“These are some very fast-growing tumors and these are refractory resistant patients, so as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
In the other tisagenlecleucel update (abstract 1684), investigators showed sustained disease control in Juliet, the global trial including adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), with 40% of patients still in remission at 18 months, according to Dr. Brodsky.
One more report Dr. Brodsky highlighted (abstract 967) will look at long-term follow-up after administration of SCRI-CAR19v1, a CD19-specific CAR T-cell product. Preliminary data suggest a survival advantage when hematopoietic stem cell transplantation is done after CAR T-cell induced remission.
“This study is very small and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
, according to investigators in two separate studies.
Meanwhile, responses to tisagenlecleucel appear to be even more durable with longer follow-up, according to preliminary results from two more CAR T-cell therapy studies slated for presentation at the annual meeting of the American Society of Hematology.
A fifth study will show that bone marrow transplant may effectively consolidate remission after CAR T-cell therapy, according to Robert A. Brodsky, MD, ASH secretary, who highlighted the studies during a media briefing.
The ibrutinib study (abstract 299) shows that administering this BTK inhibitor starting 2 weeks prior to leukapheresis and continuing until 3 months after JCAR014 could improve responses and may decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Of 16 patients in an ibrutinib cohort and 18 patients in a no-ibrutinib cohort, the proportion of responders was 88% and 56%, respectively, according to preliminary data reported in the abstract. Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients in the no-ibrutinib cohort, and 0 of 17 patients in the ibrutinib cohort.
Those findings are “early and preliminary, but very exciting” for ibrutinib in combination with this CD-19 specific CAR T-cell therapy said Dr. Brodsky, director of the division of hematology at Johns Hopkins University in Baltimore.
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy in patients with relapsed B-cell acute lymphoblastic leukemia (ALL).
In data to date, 14 patients with early CAR T-cell loss, partial response, or no response to CAR T-cell therapy received a PD-1 inhibitor.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said. “Sure enough, they were able to see that in roughly half of the patients. So again, very small, preliminary data, but very exciting that it is safe to give checkpoint inhibitors with CAR T-cells and it may be efficacious at getting the immune response back.”
One of the two tisagenlecleucel updates (abstract 895) showed that in the ELIANA trial, which included pediatric and young adults patients with relapsed/refractory ALL, the probability of relapse-free survival at 18 months was 66%.
“These are some very fast-growing tumors and these are refractory resistant patients, so as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
In the other tisagenlecleucel update (abstract 1684), investigators showed sustained disease control in Juliet, the global trial including adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), with 40% of patients still in remission at 18 months, according to Dr. Brodsky.
One more report Dr. Brodsky highlighted (abstract 967) will look at long-term follow-up after administration of SCRI-CAR19v1, a CD19-specific CAR T-cell product. Preliminary data suggest a survival advantage when hematopoietic stem cell transplantation is done after CAR T-cell induced remission.
“This study is very small and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
, according to investigators in two separate studies.
Meanwhile, responses to tisagenlecleucel appear to be even more durable with longer follow-up, according to preliminary results from two more CAR T-cell therapy studies slated for presentation at the annual meeting of the American Society of Hematology.
A fifth study will show that bone marrow transplant may effectively consolidate remission after CAR T-cell therapy, according to Robert A. Brodsky, MD, ASH secretary, who highlighted the studies during a media briefing.
The ibrutinib study (abstract 299) shows that administering this BTK inhibitor starting 2 weeks prior to leukapheresis and continuing until 3 months after JCAR014 could improve responses and may decrease the incidence of severe cytokine release syndrome in patients with relapsed or refractory chronic lymphocytic leukemia (CLL).
Of 16 patients in an ibrutinib cohort and 18 patients in a no-ibrutinib cohort, the proportion of responders was 88% and 56%, respectively, according to preliminary data reported in the abstract. Grade 3-5 cytokine release syndrome occurred in 5 of 19 patients in the no-ibrutinib cohort, and 0 of 17 patients in the ibrutinib cohort.
Those findings are “early and preliminary, but very exciting” for ibrutinib in combination with this CD-19 specific CAR T-cell therapy said Dr. Brodsky, director of the division of hematology at Johns Hopkins University in Baltimore.
Early results of the checkpoint inhibitor study (abstract 556) suggest that pembrolizumab or nivolumab may augment CD19-directed CAR T-cell therapy in patients with relapsed B-cell acute lymphoblastic leukemia (ALL).
In data to date, 14 patients with early CAR T-cell loss, partial response, or no response to CAR T-cell therapy received a PD-1 inhibitor.
“The idea was if you can give pembrolizumab, you can take the brakes off, and maybe you can reinitiate the immune attack,” Dr. Brodsky said. “Sure enough, they were able to see that in roughly half of the patients. So again, very small, preliminary data, but very exciting that it is safe to give checkpoint inhibitors with CAR T-cells and it may be efficacious at getting the immune response back.”
One of the two tisagenlecleucel updates (abstract 895) showed that in the ELIANA trial, which included pediatric and young adults patients with relapsed/refractory ALL, the probability of relapse-free survival at 18 months was 66%.
“These are some very fast-growing tumors and these are refractory resistant patients, so as we get further and further out, it’s more encouraging to see that there are durable responses,” Dr. Brodsky said.
In the other tisagenlecleucel update (abstract 1684), investigators showed sustained disease control in Juliet, the global trial including adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), with 40% of patients still in remission at 18 months, according to Dr. Brodsky.
One more report Dr. Brodsky highlighted (abstract 967) will look at long-term follow-up after administration of SCRI-CAR19v1, a CD19-specific CAR T-cell product. Preliminary data suggest a survival advantage when hematopoietic stem cell transplantation is done after CAR T-cell induced remission.
“This study is very small and it’s retrospective, but it suggests that bone marrow transplant is a good way to consolidate the remission after CAR T-cell therapy,” Dr. Brodsky said.
Biosimilar deemed equivalent to rituximab in FL
Phase 3 results suggest the biosimilar product CT-P10 is equivalent to rituximab in patients with low-tumor-burden follicular lymphoma (FL).
Overall response rates were similar—both exceeding 80%—in patients who received CT-P10 and those who received rituximab.
In addition, adverse event (AE) profiles were comparable between the treatment arms.
Larry W. Kwak, MD, PhD, of City of Hope in Duarte, California, and his colleagues reported these results in The Lancet Haematology.
CT-P10 was approved by the European Commission in 2017 and was recommended for approval by the U.S. Food and Drug Administration’s Oncologic Drugs Advisory Committee last month.
The phase 3 trial of CT-P10 included 258 patients with stage II-IV low-tumor-burden FL. They were randomized to receive CT-P10 (n=130) or rituximab (n=128).
Patients received intravenous CT-P10 or rituximab weekly for 4 weeks as induction therapy. Patients experiencing disease control went on to a maintenance phase with their assigned treatment, given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
Efficacy
The overall response rate at 7 months was 83% in patients randomized to CT-P10 and 81% in those randomized to rituximab.
The complete response rates were 28% and 34%, respectively. The unconfirmed complete response rates were 5% and 2%, respectively. And the partial response rates were 51% and 46%, respectively.
The two treatments were deemed therapeutically equivalent, as the two-sided 90% confidence intervals for the difference in proportion of responders between CT-P10 and rituximab were within the prespecified equivalence margin of 17%.
Safety
Treatment-emergent AEs occurred in 71% of patients in the CT-P10 arm and 67% of those in the rituximab arm.
The most common treatment-emergent AEs (in the CT-P10 and rituximab arms, respectively) were:
- Infusion-related reactions (31% and 29%)
- Infections (27% and 21%)
- Worsening neutropenia (22% for both)
- Upper respiratory tract infection (12% and 11%)
- Worsening anemia (10% and 14%)
- Worsening thrombocytopenia (8% and 7%)
- Fatigue (7% and 9%)
- Diarrhea (5% for both)
- Nausea (5% for both)
- Urinary tract infection (4% and 5%)
- Headache (3% and 5%).
Serious AEs were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
Two serious AEs—myocardial infarction and constipation—in the CT-P10 arm were considered related to treatment. None of the serious AEs in the rituximab arm were considered treatment-related.
Two patients in the CT-P10 arm discontinued treatment due to AEs—one due to myocardial infarction and one due to dermatitis. There were no AE-related discontinuations in the rituximab arm.
There were two deaths in the CT-P10 arm as of the cutoff date (January 4, 2018). One was due to myocardial infarction, and one was due to respiratory failure. The myocardial infarction was considered possibly related to treatment.
This trial was sponsored by Celltrion, the company developing CT-P10. Three study authors are employees of the company.
Dr. Kwak and several other authors not employed by Celltrion reported disclosures related to the company. Authors also reported relationships with Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
Phase 3 results suggest the biosimilar product CT-P10 is equivalent to rituximab in patients with low-tumor-burden follicular lymphoma (FL).
Overall response rates were similar—both exceeding 80%—in patients who received CT-P10 and those who received rituximab.
In addition, adverse event (AE) profiles were comparable between the treatment arms.
Larry W. Kwak, MD, PhD, of City of Hope in Duarte, California, and his colleagues reported these results in The Lancet Haematology.
CT-P10 was approved by the European Commission in 2017 and was recommended for approval by the U.S. Food and Drug Administration’s Oncologic Drugs Advisory Committee last month.
The phase 3 trial of CT-P10 included 258 patients with stage II-IV low-tumor-burden FL. They were randomized to receive CT-P10 (n=130) or rituximab (n=128).
Patients received intravenous CT-P10 or rituximab weekly for 4 weeks as induction therapy. Patients experiencing disease control went on to a maintenance phase with their assigned treatment, given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
Efficacy
The overall response rate at 7 months was 83% in patients randomized to CT-P10 and 81% in those randomized to rituximab.
The complete response rates were 28% and 34%, respectively. The unconfirmed complete response rates were 5% and 2%, respectively. And the partial response rates were 51% and 46%, respectively.
The two treatments were deemed therapeutically equivalent, as the two-sided 90% confidence intervals for the difference in proportion of responders between CT-P10 and rituximab were within the prespecified equivalence margin of 17%.
Safety
Treatment-emergent AEs occurred in 71% of patients in the CT-P10 arm and 67% of those in the rituximab arm.
The most common treatment-emergent AEs (in the CT-P10 and rituximab arms, respectively) were:
- Infusion-related reactions (31% and 29%)
- Infections (27% and 21%)
- Worsening neutropenia (22% for both)
- Upper respiratory tract infection (12% and 11%)
- Worsening anemia (10% and 14%)
- Worsening thrombocytopenia (8% and 7%)
- Fatigue (7% and 9%)
- Diarrhea (5% for both)
- Nausea (5% for both)
- Urinary tract infection (4% and 5%)
- Headache (3% and 5%).
Serious AEs were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
Two serious AEs—myocardial infarction and constipation—in the CT-P10 arm were considered related to treatment. None of the serious AEs in the rituximab arm were considered treatment-related.
Two patients in the CT-P10 arm discontinued treatment due to AEs—one due to myocardial infarction and one due to dermatitis. There were no AE-related discontinuations in the rituximab arm.
There were two deaths in the CT-P10 arm as of the cutoff date (January 4, 2018). One was due to myocardial infarction, and one was due to respiratory failure. The myocardial infarction was considered possibly related to treatment.
This trial was sponsored by Celltrion, the company developing CT-P10. Three study authors are employees of the company.
Dr. Kwak and several other authors not employed by Celltrion reported disclosures related to the company. Authors also reported relationships with Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
Phase 3 results suggest the biosimilar product CT-P10 is equivalent to rituximab in patients with low-tumor-burden follicular lymphoma (FL).
Overall response rates were similar—both exceeding 80%—in patients who received CT-P10 and those who received rituximab.
In addition, adverse event (AE) profiles were comparable between the treatment arms.
Larry W. Kwak, MD, PhD, of City of Hope in Duarte, California, and his colleagues reported these results in The Lancet Haematology.
CT-P10 was approved by the European Commission in 2017 and was recommended for approval by the U.S. Food and Drug Administration’s Oncologic Drugs Advisory Committee last month.
The phase 3 trial of CT-P10 included 258 patients with stage II-IV low-tumor-burden FL. They were randomized to receive CT-P10 (n=130) or rituximab (n=128).
Patients received intravenous CT-P10 or rituximab weekly for 4 weeks as induction therapy. Patients experiencing disease control went on to a maintenance phase with their assigned treatment, given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
Efficacy
The overall response rate at 7 months was 83% in patients randomized to CT-P10 and 81% in those randomized to rituximab.
The complete response rates were 28% and 34%, respectively. The unconfirmed complete response rates were 5% and 2%, respectively. And the partial response rates were 51% and 46%, respectively.
The two treatments were deemed therapeutically equivalent, as the two-sided 90% confidence intervals for the difference in proportion of responders between CT-P10 and rituximab were within the prespecified equivalence margin of 17%.
Safety
Treatment-emergent AEs occurred in 71% of patients in the CT-P10 arm and 67% of those in the rituximab arm.
The most common treatment-emergent AEs (in the CT-P10 and rituximab arms, respectively) were:
- Infusion-related reactions (31% and 29%)
- Infections (27% and 21%)
- Worsening neutropenia (22% for both)
- Upper respiratory tract infection (12% and 11%)
- Worsening anemia (10% and 14%)
- Worsening thrombocytopenia (8% and 7%)
- Fatigue (7% and 9%)
- Diarrhea (5% for both)
- Nausea (5% for both)
- Urinary tract infection (4% and 5%)
- Headache (3% and 5%).
Serious AEs were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
Two serious AEs—myocardial infarction and constipation—in the CT-P10 arm were considered related to treatment. None of the serious AEs in the rituximab arm were considered treatment-related.
Two patients in the CT-P10 arm discontinued treatment due to AEs—one due to myocardial infarction and one due to dermatitis. There were no AE-related discontinuations in the rituximab arm.
There were two deaths in the CT-P10 arm as of the cutoff date (January 4, 2018). One was due to myocardial infarction, and one was due to respiratory failure. The myocardial infarction was considered possibly related to treatment.
This trial was sponsored by Celltrion, the company developing CT-P10. Three study authors are employees of the company.
Dr. Kwak and several other authors not employed by Celltrion reported disclosures related to the company. Authors also reported relationships with Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
Elderly NHL patients have higher NRM after HSCT
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.
A retrospective study suggests elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within a year of allogeneic hematopoietic stem cell transplant (allo-HSCT).
The rate of non-relapse mortality (NRM) at 1 year was significantly higher for elderly patients than for middle-aged or young patients.
However, the 3-year rate of relapse was similar across the age groups.
Charalampia Kyriakou, MD, PhD, of University College London in the U.K., and her colleagues reported these findings in Biology of Blood and Marrow Transplantation.
The investigators analyzed 3,919 patients with NHL who underwent allo-HSCT between 2003 and 2013.
The patients had follicular lymphoma (n=1,461), diffuse large B-cell lymphoma (n=1,192), mantle cell lymphoma (n=823), and peripheral T-cell lymphoma (n=443).
At the time of transplant, about 85% of patients were chemo-sensitive, with the remainder being chemo-refractory.
Results
The investigators compared outcomes in patients assigned to three age groups—young (18-50), middle-aged (51-65), and elderly (66-77).
NRM at 1 year was 13% for young patients, 20% for middle-aged patients, and 33% for elderly patients (P<0.001).
Overall survival at 3 years was 60% in young patients, 54% in middle-aged patients, and 38% in the elderly (P<0.001).
In contrast to these significant associations between age and survival, the rate of relapse at 3 years remained relatively consistent—30% in young patients, 31% in middle-aged patients, and 28% in elderly patients (P=0.355).
The increased risk of NRM in elderly patients could not be fully explained by comorbidities, although these were more common in the elderly.
After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.”
Therefore, age remains an independent risk factor.
The investigators did not report conflicts of interest.
Rituximab biosimilar looks equivalent in follicular lymphoma
The rituximab biosimilar CT-P10 has equivalent efficacy, compared with rituximab, and is well tolerated in the treatment of low–tumor-burden follicular lymphoma, according to results from a multinational, randomized, phase 3 study.
Overall response after 7 months of treatment exceeded 80% for patients assigned to CT-P10 and for those assigned to rituximab, investigators reported in the Lancet Haematology.
Adverse event profiles were comparable for rituximab and the biosimilar over that time period, while pharmacokinetics, pharmacodynamics, and immunogenicity were likewise comparable between arms, according to investigators.
“Thus, CT-P10 monotherapy is suggested as a new therapeutic option for patients with low–tumor-burden follicular lymphoma,” wrote senior author Larry W Kwak, MD, PhD, of the Comprehensive Cancer Center, City of Hope, Duarte, Calif., and his colleagues.
CT-P10, the first rituximab biosimilar to be authorized by the European Medicines Agency, has been recommended for approval in the United States by the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
If approved by the FDA, CT-P10 would be the first rituximab biosimilar available in the United States, according to the company, which noted three proposed indications in non-Hodgkin lymphoma.
In the current randomized, double-blind, parallel-group, phase 3 trial, 258 patients with stage II-IV low–tumor-burden follicular lymphoma were randomly assigned to CT-P10 (130 patients) or rituximab sourced in the United States (128 patients).
Treatment consisted of an induction period of intravenous CT-P10 or rituximab weekly for 4 weeks, while patients experiencing disease control went on to a maintenance phase with their assigned treatment given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
The primary endpoint of the study was overall response at 7 months, defined as a complete response, unconfirmed complete response, or partial response.
Overall response was seen in 83% of patients randomized to CT-P10 and 81% of patients randomized to rituximab at 7 months, Dr. Kwak and his colleagues reported.
The two treatments were deemed therapeutically equivalent, as illustrated by 90% confidence intervals within a prespecified equivalence margin of 17%, investigators said.
The most common treatment-emergent adverse events in either group were infusion-related reactions, which were of grade 1-2, except for one grade 3 reaction reported in the CT-P10 group, according to the report. Other common adverse events were upper respiratory tract infections and fatigue.
Serious adverse events were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
The availability of a rituximab biosimilar is anticipated to reduce the cost of treatment and improve patient access, according to investigators.
Introduction of CT-P10 in the European Union was projected to save between 90 and 150 million euros over a year, enabling more than 12,500 new patients to be treated with the biosimilar, according to results of a budget impact analysis investigators cited in their report.
“Widespread adoption of a rituximab biosimilar could have a substantial effect on health care budgets and might also have effects at a societal level,” Dr. Kwak and his coauthors said in the report.
The trial was sponsored by Celltrion and three coauthors of the study were employees of the company. Dr. Kwak and several other coinvestigators not employed by Celltrion reported disclosures related to the company. Other disclosures provided related to Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
SOURCE: Ogura M et al. Lancet Haematol. 2018 Nov;5(11):e543-53.
The rituximab biosimilar CT-P10 has equivalent efficacy, compared with rituximab, and is well tolerated in the treatment of low–tumor-burden follicular lymphoma, according to results from a multinational, randomized, phase 3 study.
Overall response after 7 months of treatment exceeded 80% for patients assigned to CT-P10 and for those assigned to rituximab, investigators reported in the Lancet Haematology.
Adverse event profiles were comparable for rituximab and the biosimilar over that time period, while pharmacokinetics, pharmacodynamics, and immunogenicity were likewise comparable between arms, according to investigators.
“Thus, CT-P10 monotherapy is suggested as a new therapeutic option for patients with low–tumor-burden follicular lymphoma,” wrote senior author Larry W Kwak, MD, PhD, of the Comprehensive Cancer Center, City of Hope, Duarte, Calif., and his colleagues.
CT-P10, the first rituximab biosimilar to be authorized by the European Medicines Agency, has been recommended for approval in the United States by the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
If approved by the FDA, CT-P10 would be the first rituximab biosimilar available in the United States, according to the company, which noted three proposed indications in non-Hodgkin lymphoma.
In the current randomized, double-blind, parallel-group, phase 3 trial, 258 patients with stage II-IV low–tumor-burden follicular lymphoma were randomly assigned to CT-P10 (130 patients) or rituximab sourced in the United States (128 patients).
Treatment consisted of an induction period of intravenous CT-P10 or rituximab weekly for 4 weeks, while patients experiencing disease control went on to a maintenance phase with their assigned treatment given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
The primary endpoint of the study was overall response at 7 months, defined as a complete response, unconfirmed complete response, or partial response.
Overall response was seen in 83% of patients randomized to CT-P10 and 81% of patients randomized to rituximab at 7 months, Dr. Kwak and his colleagues reported.
The two treatments were deemed therapeutically equivalent, as illustrated by 90% confidence intervals within a prespecified equivalence margin of 17%, investigators said.
The most common treatment-emergent adverse events in either group were infusion-related reactions, which were of grade 1-2, except for one grade 3 reaction reported in the CT-P10 group, according to the report. Other common adverse events were upper respiratory tract infections and fatigue.
Serious adverse events were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
The availability of a rituximab biosimilar is anticipated to reduce the cost of treatment and improve patient access, according to investigators.
Introduction of CT-P10 in the European Union was projected to save between 90 and 150 million euros over a year, enabling more than 12,500 new patients to be treated with the biosimilar, according to results of a budget impact analysis investigators cited in their report.
“Widespread adoption of a rituximab biosimilar could have a substantial effect on health care budgets and might also have effects at a societal level,” Dr. Kwak and his coauthors said in the report.
The trial was sponsored by Celltrion and three coauthors of the study were employees of the company. Dr. Kwak and several other coinvestigators not employed by Celltrion reported disclosures related to the company. Other disclosures provided related to Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
SOURCE: Ogura M et al. Lancet Haematol. 2018 Nov;5(11):e543-53.
The rituximab biosimilar CT-P10 has equivalent efficacy, compared with rituximab, and is well tolerated in the treatment of low–tumor-burden follicular lymphoma, according to results from a multinational, randomized, phase 3 study.
Overall response after 7 months of treatment exceeded 80% for patients assigned to CT-P10 and for those assigned to rituximab, investigators reported in the Lancet Haematology.
Adverse event profiles were comparable for rituximab and the biosimilar over that time period, while pharmacokinetics, pharmacodynamics, and immunogenicity were likewise comparable between arms, according to investigators.
“Thus, CT-P10 monotherapy is suggested as a new therapeutic option for patients with low–tumor-burden follicular lymphoma,” wrote senior author Larry W Kwak, MD, PhD, of the Comprehensive Cancer Center, City of Hope, Duarte, Calif., and his colleagues.
CT-P10, the first rituximab biosimilar to be authorized by the European Medicines Agency, has been recommended for approval in the United States by the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
If approved by the FDA, CT-P10 would be the first rituximab biosimilar available in the United States, according to the company, which noted three proposed indications in non-Hodgkin lymphoma.
In the current randomized, double-blind, parallel-group, phase 3 trial, 258 patients with stage II-IV low–tumor-burden follicular lymphoma were randomly assigned to CT-P10 (130 patients) or rituximab sourced in the United States (128 patients).
Treatment consisted of an induction period of intravenous CT-P10 or rituximab weekly for 4 weeks, while patients experiencing disease control went on to a maintenance phase with their assigned treatment given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
The primary endpoint of the study was overall response at 7 months, defined as a complete response, unconfirmed complete response, or partial response.
Overall response was seen in 83% of patients randomized to CT-P10 and 81% of patients randomized to rituximab at 7 months, Dr. Kwak and his colleagues reported.
The two treatments were deemed therapeutically equivalent, as illustrated by 90% confidence intervals within a prespecified equivalence margin of 17%, investigators said.
The most common treatment-emergent adverse events in either group were infusion-related reactions, which were of grade 1-2, except for one grade 3 reaction reported in the CT-P10 group, according to the report. Other common adverse events were upper respiratory tract infections and fatigue.
Serious adverse events were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
The availability of a rituximab biosimilar is anticipated to reduce the cost of treatment and improve patient access, according to investigators.
Introduction of CT-P10 in the European Union was projected to save between 90 and 150 million euros over a year, enabling more than 12,500 new patients to be treated with the biosimilar, according to results of a budget impact analysis investigators cited in their report.
“Widespread adoption of a rituximab biosimilar could have a substantial effect on health care budgets and might also have effects at a societal level,” Dr. Kwak and his coauthors said in the report.
The trial was sponsored by Celltrion and three coauthors of the study were employees of the company. Dr. Kwak and several other coinvestigators not employed by Celltrion reported disclosures related to the company. Other disclosures provided related to Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
SOURCE: Ogura M et al. Lancet Haematol. 2018 Nov;5(11):e543-53.
FROM LANCET HAEMATOLOGY
Key clinical point:
Major finding: Overall response after 7 months of treatment was seen in 83% of patients randomized to CT-P10 and 81% of patients randomized to rituximab.
Study details: Analysis of 258 patients randomized to CT-P10 or rituximab in a phase 3, double-blind, parallel-group trial.
Disclosures: The trial was sponsored by Celltrion and three coauthors of the study were employees of the company. Other study coauthors reported disclosures related to Celltrion, Novartis, Roche, AbbVie, Celgene, and Takeda, among other companies.
Source: Ogura M et al. Lancet Haematol. 2018 Nov;5(11):e543-53.
Panel Provides Recommendations for Managing Cognitive Changes in MS
Baseline screening and periodic reassessments aid in the monitoring of treatment response and disease progression.
The National Multiple Sclerosis (MS) Society has developed recommendations for the identification and management of cognitive impairment in MS. The recommendations, which were endorsed by the Consortium of MS Centers and the International MS Cognition Society, were published online ahead of print October 10 in Multiple Sclerosis Journal.
Cognitive change may affect between 34% and 65% of adults with MS. Decreases in information processing and memory are the most common changes, and cognitive impairment may arise before MRI abnormalities that indicate MS. These changes can affect patients’ ability to work, drive, manage money, and participate in activities.
Patients and Clinicians Need Information
Patients and caregivers should receive information about common cognitive changes in MS and how they affect everyday life, said Rosalind Kalb, PhD, Vice President of the Professional Resource Center at the National MS Society in New York, and coauthors. Patients and caregivers also should be told about the high prevalence of cognitive symptoms in MS and the need for ongoing assessments. Similarly, clinicians need information about how cognitive impairments affect medical decision-making and adherence, said the authors. Clinicians also need referral resources for cognitive assessment and treatment.
All adults and children age 8 or older diagnosed with MS should, as a minimum, undergo early baseline screening with the Symbol Digit Modalities Test (SDMT) or another validated screening tool, according to the recommendations. These patients should be reassessed with the same instrument annually or more often, as needed, to detect disease activity, assess for treatment effects or relapse recovery, monitor progression of cognitive impairment, and screen for new cognitive problems.
In addition to the SDMT, screening tools that have been validated in patients with MS include the Processing Speed Test, Computerized Speed Cognitive Test, MS Neuropsychological Screening Questionnaire, Brief International Cognitive Assessment for MS, Brief Repeatable Neuropsychological Battery, and Minimal Assessment of Cognitive Function in MS.
Interventions May Improve or Maintain Function
An adult who tests positive for cognitive impairment on initial screening should undergo a more comprehensive assessment, especially if the person has comorbidities that raise concerns or is applying for disability due to cognitive impairment. A child with an unexplained change in school performance should receive a neuropsychologic evaluation, said Dr. Kalb and colleagues.
Furthermore, adults and children should be offered remedial interventions or accommodations to improve function at home, work, or school. Appropriately trained professionals should deliver these interventions to address “objectively measured deficits in attention, processing speed, memory and learning, and performance of everyday functional tasks,” said the authors. Clinicians can consider contextualized treatment (eg, self-generated learning tasks) and noncontextualized treatment (eg, memory-retrieval practice and computer-based attention interventions) for remediation of everyday activities, according to the recommendations.
Emerging research supports the potential for exercise to benefit cognitive processing speed in patients with MS. Trials of symptomatic pharmacologic treatments for cognitive impairment related to MS have yielded inconclusive results, however. In addition, few pivotal trials of disease-modifying therapies have incorporated cognitive outcome measures.
—Erik Greb
Suggested Reading
Kalb R, Beier M, Benedict RH, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler. 2018 Oct 10 [Epub ahead of print].
Baseline screening and periodic reassessments aid in the monitoring of treatment response and disease progression.
Baseline screening and periodic reassessments aid in the monitoring of treatment response and disease progression.
The National Multiple Sclerosis (MS) Society has developed recommendations for the identification and management of cognitive impairment in MS. The recommendations, which were endorsed by the Consortium of MS Centers and the International MS Cognition Society, were published online ahead of print October 10 in Multiple Sclerosis Journal.
Cognitive change may affect between 34% and 65% of adults with MS. Decreases in information processing and memory are the most common changes, and cognitive impairment may arise before MRI abnormalities that indicate MS. These changes can affect patients’ ability to work, drive, manage money, and participate in activities.
Patients and Clinicians Need Information
Patients and caregivers should receive information about common cognitive changes in MS and how they affect everyday life, said Rosalind Kalb, PhD, Vice President of the Professional Resource Center at the National MS Society in New York, and coauthors. Patients and caregivers also should be told about the high prevalence of cognitive symptoms in MS and the need for ongoing assessments. Similarly, clinicians need information about how cognitive impairments affect medical decision-making and adherence, said the authors. Clinicians also need referral resources for cognitive assessment and treatment.
All adults and children age 8 or older diagnosed with MS should, as a minimum, undergo early baseline screening with the Symbol Digit Modalities Test (SDMT) or another validated screening tool, according to the recommendations. These patients should be reassessed with the same instrument annually or more often, as needed, to detect disease activity, assess for treatment effects or relapse recovery, monitor progression of cognitive impairment, and screen for new cognitive problems.
In addition to the SDMT, screening tools that have been validated in patients with MS include the Processing Speed Test, Computerized Speed Cognitive Test, MS Neuropsychological Screening Questionnaire, Brief International Cognitive Assessment for MS, Brief Repeatable Neuropsychological Battery, and Minimal Assessment of Cognitive Function in MS.
Interventions May Improve or Maintain Function
An adult who tests positive for cognitive impairment on initial screening should undergo a more comprehensive assessment, especially if the person has comorbidities that raise concerns or is applying for disability due to cognitive impairment. A child with an unexplained change in school performance should receive a neuropsychologic evaluation, said Dr. Kalb and colleagues.
Furthermore, adults and children should be offered remedial interventions or accommodations to improve function at home, work, or school. Appropriately trained professionals should deliver these interventions to address “objectively measured deficits in attention, processing speed, memory and learning, and performance of everyday functional tasks,” said the authors. Clinicians can consider contextualized treatment (eg, self-generated learning tasks) and noncontextualized treatment (eg, memory-retrieval practice and computer-based attention interventions) for remediation of everyday activities, according to the recommendations.
Emerging research supports the potential for exercise to benefit cognitive processing speed in patients with MS. Trials of symptomatic pharmacologic treatments for cognitive impairment related to MS have yielded inconclusive results, however. In addition, few pivotal trials of disease-modifying therapies have incorporated cognitive outcome measures.
—Erik Greb
Suggested Reading
Kalb R, Beier M, Benedict RH, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler. 2018 Oct 10 [Epub ahead of print].
The National Multiple Sclerosis (MS) Society has developed recommendations for the identification and management of cognitive impairment in MS. The recommendations, which were endorsed by the Consortium of MS Centers and the International MS Cognition Society, were published online ahead of print October 10 in Multiple Sclerosis Journal.
Cognitive change may affect between 34% and 65% of adults with MS. Decreases in information processing and memory are the most common changes, and cognitive impairment may arise before MRI abnormalities that indicate MS. These changes can affect patients’ ability to work, drive, manage money, and participate in activities.
Patients and Clinicians Need Information
Patients and caregivers should receive information about common cognitive changes in MS and how they affect everyday life, said Rosalind Kalb, PhD, Vice President of the Professional Resource Center at the National MS Society in New York, and coauthors. Patients and caregivers also should be told about the high prevalence of cognitive symptoms in MS and the need for ongoing assessments. Similarly, clinicians need information about how cognitive impairments affect medical decision-making and adherence, said the authors. Clinicians also need referral resources for cognitive assessment and treatment.
All adults and children age 8 or older diagnosed with MS should, as a minimum, undergo early baseline screening with the Symbol Digit Modalities Test (SDMT) or another validated screening tool, according to the recommendations. These patients should be reassessed with the same instrument annually or more often, as needed, to detect disease activity, assess for treatment effects or relapse recovery, monitor progression of cognitive impairment, and screen for new cognitive problems.
In addition to the SDMT, screening tools that have been validated in patients with MS include the Processing Speed Test, Computerized Speed Cognitive Test, MS Neuropsychological Screening Questionnaire, Brief International Cognitive Assessment for MS, Brief Repeatable Neuropsychological Battery, and Minimal Assessment of Cognitive Function in MS.
Interventions May Improve or Maintain Function
An adult who tests positive for cognitive impairment on initial screening should undergo a more comprehensive assessment, especially if the person has comorbidities that raise concerns or is applying for disability due to cognitive impairment. A child with an unexplained change in school performance should receive a neuropsychologic evaluation, said Dr. Kalb and colleagues.
Furthermore, adults and children should be offered remedial interventions or accommodations to improve function at home, work, or school. Appropriately trained professionals should deliver these interventions to address “objectively measured deficits in attention, processing speed, memory and learning, and performance of everyday functional tasks,” said the authors. Clinicians can consider contextualized treatment (eg, self-generated learning tasks) and noncontextualized treatment (eg, memory-retrieval practice and computer-based attention interventions) for remediation of everyday activities, according to the recommendations.
Emerging research supports the potential for exercise to benefit cognitive processing speed in patients with MS. Trials of symptomatic pharmacologic treatments for cognitive impairment related to MS have yielded inconclusive results, however. In addition, few pivotal trials of disease-modifying therapies have incorporated cognitive outcome measures.
—Erik Greb
Suggested Reading
Kalb R, Beier M, Benedict RH, et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler. 2018 Oct 10 [Epub ahead of print].
R-CHOP effective as first-line treatment in FL
Long-term data suggest R-CHOP can be effective as first-line treatment for patients with follicular lymphoma (FL).
In a phase 2-3 trial, investigators compared R-CHOP-21 and R-CHOP-14 in a cohort of patients with indolent lymphomas, most of whom had FL.
Ten-year survival rates were similar between the R-CHOP-21 and R-CHOP-14 groups, with progression-free survival (PFS) rates of 33% and 39%, respectively, and overall survival (OS) rates of 81% and 85%, respectively.
The investigators did note that 9% of patients in each treatment group developed secondary malignancies, and grade 3 infections were a concern as well.
Takashi Watanabe, MD, PhD, of Mie University in Japan, and his colleagues reported these results in The Lancet Haematology.
The trial (JCOG0203) included 300 patients with stage III or IV indolent B-cell lymphomas from 44 Japanese hospitals.
Most patients (n=248) had grade 1-3a FL, 17 had grade 3b FL, 6 had marginal zone lymphoma, 6 had diffuse large B-cell lymphoma, 4 had mantle cell lymphoma, 2 had small lymphocytic lymphoma, 1 had plasmacytoma, 13 had other indolent B-cell lymphomas, and 3 had other lymphomas.
The patients were randomly assigned to receive six cycles of R-CHOP 21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) or R-CHOP 14 (R-CHOP every 2 weeks with granulocyte-colony stimulating factor support). Neither group received rituximab maintenance.
Overall results
The median follow-up was 11.2 years (interquartile range, 10.1 to 12.7 years).
The 10-year PFS was 33% in the R-CHOP-21 group and 39% in the R-CHOP-14 group (hazard ratio=0.89). The 10-year OS was 81% and 85%, respectively (hazard ratio=0.87).
At 10 years, the incidence of secondary malignancies was 9% in both the R-CHOP-21 group (14/148) and the R-CHOP-14 group (14/151).
The most frequent solid tumor malignancies were stomach (n=5), lung (n=4), colon (n=3), bladder (n=2), and prostate (n=2) cancers. Hematologic malignancies included myelodysplastic syndromes (n=6), acute myeloid leukemia (n=2), acute lymphoblastic leukemia (n=1), and chronic myeloid leukemia (n=1).
There were nine deaths from secondary malignancies, four in the R-CHOP-21 group and five in the R-CHOP-14 group.
The rate of grade 3 adverse events was 18% (n=53) for the entire cohort. Grade 3 infections occurred in 23% of the R-CHOP-21 group and 12% of the R-CHOP-14 group.
Focus on grade 1-3a FL
Among the 248 patients with grade 1-3a FL, the PFS (for both treatment groups) was 45% at 5 years, 39% at 8 years, and 36% at 10 years. The OS was 94% at 5 years, 87% at 8 years, and 85% at 10 years.
Histological transformation was observed in 11% of the patients who had grade 1-3a FL at enrollment. The cumulative incidence of histological transformation was 2.4% at 3 years, 3.2% at 5 years, 8.5% at 8 years, and 9.3% at 10 years.
Secondary malignancies occurred in 10% (12/125) of the R-CHOP-21 group and 11% (13/123) of the R-CHOP-14 group.
The cumulative incidence of hematologic secondary malignancies at 10 years was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known. They emphasized that patients should be followed beyond 10 years to ensure the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up—both of which could lead to death,” the investigators wrote.
This study was supported by the Ministry of Health, Labour and Welfare of Japan and the National Cancer Center Research and Development Fund of Japan.
Dr. Wantanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple co-authors reported similar relationships.
Long-term data suggest R-CHOP can be effective as first-line treatment for patients with follicular lymphoma (FL).
In a phase 2-3 trial, investigators compared R-CHOP-21 and R-CHOP-14 in a cohort of patients with indolent lymphomas, most of whom had FL.
Ten-year survival rates were similar between the R-CHOP-21 and R-CHOP-14 groups, with progression-free survival (PFS) rates of 33% and 39%, respectively, and overall survival (OS) rates of 81% and 85%, respectively.
The investigators did note that 9% of patients in each treatment group developed secondary malignancies, and grade 3 infections were a concern as well.
Takashi Watanabe, MD, PhD, of Mie University in Japan, and his colleagues reported these results in The Lancet Haematology.
The trial (JCOG0203) included 300 patients with stage III or IV indolent B-cell lymphomas from 44 Japanese hospitals.
Most patients (n=248) had grade 1-3a FL, 17 had grade 3b FL, 6 had marginal zone lymphoma, 6 had diffuse large B-cell lymphoma, 4 had mantle cell lymphoma, 2 had small lymphocytic lymphoma, 1 had plasmacytoma, 13 had other indolent B-cell lymphomas, and 3 had other lymphomas.
The patients were randomly assigned to receive six cycles of R-CHOP 21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) or R-CHOP 14 (R-CHOP every 2 weeks with granulocyte-colony stimulating factor support). Neither group received rituximab maintenance.
Overall results
The median follow-up was 11.2 years (interquartile range, 10.1 to 12.7 years).
The 10-year PFS was 33% in the R-CHOP-21 group and 39% in the R-CHOP-14 group (hazard ratio=0.89). The 10-year OS was 81% and 85%, respectively (hazard ratio=0.87).
At 10 years, the incidence of secondary malignancies was 9% in both the R-CHOP-21 group (14/148) and the R-CHOP-14 group (14/151).
The most frequent solid tumor malignancies were stomach (n=5), lung (n=4), colon (n=3), bladder (n=2), and prostate (n=2) cancers. Hematologic malignancies included myelodysplastic syndromes (n=6), acute myeloid leukemia (n=2), acute lymphoblastic leukemia (n=1), and chronic myeloid leukemia (n=1).
There were nine deaths from secondary malignancies, four in the R-CHOP-21 group and five in the R-CHOP-14 group.
The rate of grade 3 adverse events was 18% (n=53) for the entire cohort. Grade 3 infections occurred in 23% of the R-CHOP-21 group and 12% of the R-CHOP-14 group.
Focus on grade 1-3a FL
Among the 248 patients with grade 1-3a FL, the PFS (for both treatment groups) was 45% at 5 years, 39% at 8 years, and 36% at 10 years. The OS was 94% at 5 years, 87% at 8 years, and 85% at 10 years.
Histological transformation was observed in 11% of the patients who had grade 1-3a FL at enrollment. The cumulative incidence of histological transformation was 2.4% at 3 years, 3.2% at 5 years, 8.5% at 8 years, and 9.3% at 10 years.
Secondary malignancies occurred in 10% (12/125) of the R-CHOP-21 group and 11% (13/123) of the R-CHOP-14 group.
The cumulative incidence of hematologic secondary malignancies at 10 years was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known. They emphasized that patients should be followed beyond 10 years to ensure the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up—both of which could lead to death,” the investigators wrote.
This study was supported by the Ministry of Health, Labour and Welfare of Japan and the National Cancer Center Research and Development Fund of Japan.
Dr. Wantanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple co-authors reported similar relationships.
Long-term data suggest R-CHOP can be effective as first-line treatment for patients with follicular lymphoma (FL).
In a phase 2-3 trial, investigators compared R-CHOP-21 and R-CHOP-14 in a cohort of patients with indolent lymphomas, most of whom had FL.
Ten-year survival rates were similar between the R-CHOP-21 and R-CHOP-14 groups, with progression-free survival (PFS) rates of 33% and 39%, respectively, and overall survival (OS) rates of 81% and 85%, respectively.
The investigators did note that 9% of patients in each treatment group developed secondary malignancies, and grade 3 infections were a concern as well.
Takashi Watanabe, MD, PhD, of Mie University in Japan, and his colleagues reported these results in The Lancet Haematology.
The trial (JCOG0203) included 300 patients with stage III or IV indolent B-cell lymphomas from 44 Japanese hospitals.
Most patients (n=248) had grade 1-3a FL, 17 had grade 3b FL, 6 had marginal zone lymphoma, 6 had diffuse large B-cell lymphoma, 4 had mantle cell lymphoma, 2 had small lymphocytic lymphoma, 1 had plasmacytoma, 13 had other indolent B-cell lymphomas, and 3 had other lymphomas.
The patients were randomly assigned to receive six cycles of R-CHOP 21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) or R-CHOP 14 (R-CHOP every 2 weeks with granulocyte-colony stimulating factor support). Neither group received rituximab maintenance.
Overall results
The median follow-up was 11.2 years (interquartile range, 10.1 to 12.7 years).
The 10-year PFS was 33% in the R-CHOP-21 group and 39% in the R-CHOP-14 group (hazard ratio=0.89). The 10-year OS was 81% and 85%, respectively (hazard ratio=0.87).
At 10 years, the incidence of secondary malignancies was 9% in both the R-CHOP-21 group (14/148) and the R-CHOP-14 group (14/151).
The most frequent solid tumor malignancies were stomach (n=5), lung (n=4), colon (n=3), bladder (n=2), and prostate (n=2) cancers. Hematologic malignancies included myelodysplastic syndromes (n=6), acute myeloid leukemia (n=2), acute lymphoblastic leukemia (n=1), and chronic myeloid leukemia (n=1).
There were nine deaths from secondary malignancies, four in the R-CHOP-21 group and five in the R-CHOP-14 group.
The rate of grade 3 adverse events was 18% (n=53) for the entire cohort. Grade 3 infections occurred in 23% of the R-CHOP-21 group and 12% of the R-CHOP-14 group.
Focus on grade 1-3a FL
Among the 248 patients with grade 1-3a FL, the PFS (for both treatment groups) was 45% at 5 years, 39% at 8 years, and 36% at 10 years. The OS was 94% at 5 years, 87% at 8 years, and 85% at 10 years.
Histological transformation was observed in 11% of the patients who had grade 1-3a FL at enrollment. The cumulative incidence of histological transformation was 2.4% at 3 years, 3.2% at 5 years, 8.5% at 8 years, and 9.3% at 10 years.
Secondary malignancies occurred in 10% (12/125) of the R-CHOP-21 group and 11% (13/123) of the R-CHOP-14 group.
The cumulative incidence of hematologic secondary malignancies at 10 years was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known. They emphasized that patients should be followed beyond 10 years to ensure the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up—both of which could lead to death,” the investigators wrote.
This study was supported by the Ministry of Health, Labour and Welfare of Japan and the National Cancer Center Research and Development Fund of Japan.
Dr. Wantanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple co-authors reported similar relationships.
R-CHOP looks viable as first line in follicular lymphoma
A decade of follow-up data suggest that patients with newly diagnosed follicular lymphoma may derive long-term benefit from first-line therapy with the R-CHOP regimen, according to investigators in Japan.
Among patients with untreated follicular and other indolent B-cell lymphomas enrolled in a randomized phase 2/3 trial, the 10-year progression-free survival (PFS) rate for patients assigned to R-CHOP-21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) was 33%, and the 10-year PFS rate for patients assigned to R-CHOP-14 (R-CHOP every 2 weeks with granulocyte colony-stimulating factor [G-CSF] support] was 39%, and these PFS rates did not differ between the treatment arms, Takashi Watanabe, MD, PhD, from Mie University in Tsu, Japan, and his colleagues reported in the Lancet Haematology.
They also found that, compared with therapeutic regimens prior to the introduction of rituximab, R-CHOP was associated with a reduced likelihood of histological transformation of follicular lymphoma into a poor-prognosis diffuse large B-cell lymphoma (DLBCL), with no apparent increase in risk of secondary malignancies.
“The gold-standard, first-line treatment in patients with advanced-stage follicular lymphoma remains undetermined. However, because of the reduction in incidence of transformation without an increase in either secondary malignancies or fatal infectious events, the R-CHOP regimen should be a candidate for standard treatment, particularly from the viewpoint of long-term follow-up,” the investigators wrote
The JCOG0203 trial, which began enrollment in September 2002, included patients with stage III or IV indolent B-cell lymphomas, including grades 1-3 follicular lymphoma, from 44 Japanese hospitals. The patients were randomly assigned to receive six cycles of either R-CHOP-14 plus G-CSF or R-CHOP-21 administered once daily for 6 days beginning on day 8 of every cycle). Patients did not receive rituximab maintenance in either group.
In the primary analysis of the trial, published in 2011, the investigators reported that in 299 patients there were no significant differences in either PFS or 6-year overall survival (OS).
In the current analysis, the investigators reported that, for 248 patients with grade 1-3a follicular lymphoma, the 8-year PFS rate was 39% and the 10-year PFS rate was 36%.
The cumulative incidence of histological transformation was 3.2% at 5 years, 8.5% at 8 years, and 9.3% 10 years after the last patient was enrolled.
“In our study, survival after histological transformation was still poor; therefore, reducing histological transformation remains a crucial issue for patients with follicular lymphoma,” the investigators wrote.
The cumulative incidence of secondary malignancies at 10 years was 8.1%, and the cumulative incidence of hematological secondary malignancies was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known and emphasized that patients should be followed beyond 10 years to ensure that the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up – both of which could lead to death,” they advised.
The study was supported by the Ministry of Health, Labour and Welfare of Japan and by the National Cancer Center Research and Development Fund of Japan. Dr. Watanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple coauthors reported similar relationships.
SOURCE: Watanabe T et al. Lancet Haematol. 2018 Nov;5(11):e520-31.
A decade of follow-up data suggest that patients with newly diagnosed follicular lymphoma may derive long-term benefit from first-line therapy with the R-CHOP regimen, according to investigators in Japan.
Among patients with untreated follicular and other indolent B-cell lymphomas enrolled in a randomized phase 2/3 trial, the 10-year progression-free survival (PFS) rate for patients assigned to R-CHOP-21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) was 33%, and the 10-year PFS rate for patients assigned to R-CHOP-14 (R-CHOP every 2 weeks with granulocyte colony-stimulating factor [G-CSF] support] was 39%, and these PFS rates did not differ between the treatment arms, Takashi Watanabe, MD, PhD, from Mie University in Tsu, Japan, and his colleagues reported in the Lancet Haematology.
They also found that, compared with therapeutic regimens prior to the introduction of rituximab, R-CHOP was associated with a reduced likelihood of histological transformation of follicular lymphoma into a poor-prognosis diffuse large B-cell lymphoma (DLBCL), with no apparent increase in risk of secondary malignancies.
“The gold-standard, first-line treatment in patients with advanced-stage follicular lymphoma remains undetermined. However, because of the reduction in incidence of transformation without an increase in either secondary malignancies or fatal infectious events, the R-CHOP regimen should be a candidate for standard treatment, particularly from the viewpoint of long-term follow-up,” the investigators wrote
The JCOG0203 trial, which began enrollment in September 2002, included patients with stage III or IV indolent B-cell lymphomas, including grades 1-3 follicular lymphoma, from 44 Japanese hospitals. The patients were randomly assigned to receive six cycles of either R-CHOP-14 plus G-CSF or R-CHOP-21 administered once daily for 6 days beginning on day 8 of every cycle). Patients did not receive rituximab maintenance in either group.
In the primary analysis of the trial, published in 2011, the investigators reported that in 299 patients there were no significant differences in either PFS or 6-year overall survival (OS).
In the current analysis, the investigators reported that, for 248 patients with grade 1-3a follicular lymphoma, the 8-year PFS rate was 39% and the 10-year PFS rate was 36%.
The cumulative incidence of histological transformation was 3.2% at 5 years, 8.5% at 8 years, and 9.3% 10 years after the last patient was enrolled.
“In our study, survival after histological transformation was still poor; therefore, reducing histological transformation remains a crucial issue for patients with follicular lymphoma,” the investigators wrote.
The cumulative incidence of secondary malignancies at 10 years was 8.1%, and the cumulative incidence of hematological secondary malignancies was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known and emphasized that patients should be followed beyond 10 years to ensure that the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up – both of which could lead to death,” they advised.
The study was supported by the Ministry of Health, Labour and Welfare of Japan and by the National Cancer Center Research and Development Fund of Japan. Dr. Watanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple coauthors reported similar relationships.
SOURCE: Watanabe T et al. Lancet Haematol. 2018 Nov;5(11):e520-31.
A decade of follow-up data suggest that patients with newly diagnosed follicular lymphoma may derive long-term benefit from first-line therapy with the R-CHOP regimen, according to investigators in Japan.
Among patients with untreated follicular and other indolent B-cell lymphomas enrolled in a randomized phase 2/3 trial, the 10-year progression-free survival (PFS) rate for patients assigned to R-CHOP-21 (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone every 3 weeks) was 33%, and the 10-year PFS rate for patients assigned to R-CHOP-14 (R-CHOP every 2 weeks with granulocyte colony-stimulating factor [G-CSF] support] was 39%, and these PFS rates did not differ between the treatment arms, Takashi Watanabe, MD, PhD, from Mie University in Tsu, Japan, and his colleagues reported in the Lancet Haematology.
They also found that, compared with therapeutic regimens prior to the introduction of rituximab, R-CHOP was associated with a reduced likelihood of histological transformation of follicular lymphoma into a poor-prognosis diffuse large B-cell lymphoma (DLBCL), with no apparent increase in risk of secondary malignancies.
“The gold-standard, first-line treatment in patients with advanced-stage follicular lymphoma remains undetermined. However, because of the reduction in incidence of transformation without an increase in either secondary malignancies or fatal infectious events, the R-CHOP regimen should be a candidate for standard treatment, particularly from the viewpoint of long-term follow-up,” the investigators wrote
The JCOG0203 trial, which began enrollment in September 2002, included patients with stage III or IV indolent B-cell lymphomas, including grades 1-3 follicular lymphoma, from 44 Japanese hospitals. The patients were randomly assigned to receive six cycles of either R-CHOP-14 plus G-CSF or R-CHOP-21 administered once daily for 6 days beginning on day 8 of every cycle). Patients did not receive rituximab maintenance in either group.
In the primary analysis of the trial, published in 2011, the investigators reported that in 299 patients there were no significant differences in either PFS or 6-year overall survival (OS).
In the current analysis, the investigators reported that, for 248 patients with grade 1-3a follicular lymphoma, the 8-year PFS rate was 39% and the 10-year PFS rate was 36%.
The cumulative incidence of histological transformation was 3.2% at 5 years, 8.5% at 8 years, and 9.3% 10 years after the last patient was enrolled.
“In our study, survival after histological transformation was still poor; therefore, reducing histological transformation remains a crucial issue for patients with follicular lymphoma,” the investigators wrote.
The cumulative incidence of secondary malignancies at 10 years was 8.1%, and the cumulative incidence of hematological secondary malignancies was 2.9%.
The investigators noted that the actual incidence of secondary solid tumors or hematologic malignancies apart from the setting of autologous stem cell transplants is not known and emphasized that patients should be followed beyond 10 years to ensure that the risk of secondary malignancies is not underestimated.
“Clinicians choosing a first-line treatment for patients with follicular lymphoma should be cautious of secondary malignancies caused by immunochemotherapy and severe complications of infectious diseases in the long-term follow-up – both of which could lead to death,” they advised.
The study was supported by the Ministry of Health, Labour and Welfare of Japan and by the National Cancer Center Research and Development Fund of Japan. Dr. Watanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy at Mie University. Multiple coauthors reported similar relationships.
SOURCE: Watanabe T et al. Lancet Haematol. 2018 Nov;5(11):e520-31.
FROM LANCET HAEMATOLOGY
Key clinical point:
Major finding: Approximately one-third of patients treated with R-CHOP-14 plus G-CSF or R-CHOP-21 had no disease progression after 10 years of follow-up.
Study details: Long-term analysis of a randomized phase 2/3 trial in 299 patients with indolent B-cell lymphomas, including follicular lymphoma.
Disclosures: The study was supported by the Ministry of Health, Labor and Welfare of Japan and by the National Cancer Center Research and Development Fund of Japan. Dr. Wantanabe has received honoraria from Bristol-Myers Squibb, Takeda, Taisho Toyama, Celgene, Nippon Shinyaku, and Novartis and funding resources from TakaraBio and United Immunity to support the Department of Immuno-Gene Therapy in Mie University. Multiple coauthors reported similar relationships.
Source: Wantanabe T et al. Lancet Haematol. 2018 Nov;5(11):e520-31.
Bortezomib may unlock resistance in WM with mutations
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
FROM BLOOD
Key clinical point:
Major finding: Progression-free and overall survival were not significantly different between WM patients with CXCR4 mutations and those with CXCR4 wild type (log-rank, P = .994 and P = .407, respectively).
Study details: An analysis of 43 WM patients treated with bortezomib/rituximab and genotyped to determine CXCR4 mutation status.
Disclosures: The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
Source: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.