User login
Advances in CAR T-Cell Therapies (FULL)
Gene therapies, especially chimeric antigen receptor (CAR) T-cell therapies, experienced significant growth in 2017. The CAR T-cell therapies are among the most clinically important of the adoptive cell transfer therapies. In August, the FDA approved tisagenlecleucel for patients aged < 26 years with acute or relapsed lymphoblastic leukemia (ALL). In October, the FDA approved axicabtagene ciloleucel for treatment of adult patients nonresponsive to, or relapsed from treatment of, certain types of large B-cell lymphoma. And in November, the FDA granted breakthrough therapy designation to Celgene and Bluebird Bio for the bb2121 anti-B-cell maturation antigen (BCMA) CAR T-cell therapy for relapsed and refractory multiple myeloma (MM).
Chimeric antigen receptor T-cells circumvent the human major histocompatibility complex that T-cell receptors must navigate, shifting cell-based therapy away from identification of existing cells and toward creating T-cell products through genetic engineering. This broadens the potential for CAR T-cell applications and allows for rapid manufacture of tumor and patient-specific agents.1 Both Novartis’ Kymriah and Kite Pharma’s Yescarta are derived from investigations into anti-CD19 CAR therapy, which has been the most heavily researched of the CARs due to its links with B-cell malignancies, expression in most tumor cells, and absence from vital tissues.2 Studied in relation to a number of cancers, CD19 has not shown much success in either MM or solid tumor cancers.
Targeting the right antigen for myeloma is complicated: first because common MM antigens—CD38, CD56, CD138—also are expressed on essential normal cells, and second, because myeloma cells are synonymous with heterogeneity. The FDA based its designation of bb2121, or BCMA CAR T-cell therapy, on preliminary data from an ongoing phase 1 CRB-401 trial that, as of December 2017, concluded that 94% of 21 patients with MM treated with the highest doses showed complete or partial remissions and high rates of progression-free survival.3 The trial also showed that cytokine-release toxicity (CRS), although severe in some patients, was generally reversible and short lived.
Multiple myeloma BCMA is only one of several CAR targets under consideration for MM treatment; others include CD138, CD38, signaling lymphocyte-activating molecule 7, and κ light chain. However, B-cell maturation antigen is attractive to researchers because BCMA–specific CAR-expressing T lymphocytes recognize and kill B-cell maturation antigen–expressing tumor cells. Also, BCMA acts as a receptor for both a proliferation-inducing ligand and as a B-cell–activating factor and is a member of the tumor necrosis factor receptor superfamily, playing a key role in plasma cell survival. B-cell maturation antigen is expressed in most, if not all, myeloma cells but not in epithelial tissues. Finally, integration of CAR-Ts with other myeloma therapies is an important area of future research.4
Most of the 23 trials looking at CAR T-cell therapy for MM are in the U.S. or China, and several deal jointly with MM, leukemia, and lymphoma. The THINK (THerapeutic Immunotherapy with NKR-2) multinational open-label phase 1 study stands alone in assessing the safety and clinical activity of multiple administrations of autologous NKR-2 cells in 7 refractory cancers, including 5 solid tumors (colorectal, ovarian, bladder, triple-negative breast and pancreatic cancers) and 2 hematologic tumors (acute myeloid leukemia and MM). Unlike traditional CAR T-cell therapy, which targets only 1 tumor antigen, NK cell receptors enable a single receptor to recognize multiple tumor antigens.
Despite challenges of toxicity, costs, and restricted availability for immunotherapies, CAR T-cell therapies seem to offer great possibilities of groundbreaking treatments and possible cures for formerly hard to treat cancers, including MM.5
Click here to read the digital edition.
1. Almåsbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602.
2. Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392-3400.
3. C
4. Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130(24):2594-2602.
5. Vallet S, Pecherstorfer M, Podar K. Adoptive cell therapy in multiple myeloma. Expert Opin Biol Ther. 2017;17(12):1511-1522.
Gene therapies, especially chimeric antigen receptor (CAR) T-cell therapies, experienced significant growth in 2017. The CAR T-cell therapies are among the most clinically important of the adoptive cell transfer therapies. In August, the FDA approved tisagenlecleucel for patients aged < 26 years with acute or relapsed lymphoblastic leukemia (ALL). In October, the FDA approved axicabtagene ciloleucel for treatment of adult patients nonresponsive to, or relapsed from treatment of, certain types of large B-cell lymphoma. And in November, the FDA granted breakthrough therapy designation to Celgene and Bluebird Bio for the bb2121 anti-B-cell maturation antigen (BCMA) CAR T-cell therapy for relapsed and refractory multiple myeloma (MM).
Chimeric antigen receptor T-cells circumvent the human major histocompatibility complex that T-cell receptors must navigate, shifting cell-based therapy away from identification of existing cells and toward creating T-cell products through genetic engineering. This broadens the potential for CAR T-cell applications and allows for rapid manufacture of tumor and patient-specific agents.1 Both Novartis’ Kymriah and Kite Pharma’s Yescarta are derived from investigations into anti-CD19 CAR therapy, which has been the most heavily researched of the CARs due to its links with B-cell malignancies, expression in most tumor cells, and absence from vital tissues.2 Studied in relation to a number of cancers, CD19 has not shown much success in either MM or solid tumor cancers.
Targeting the right antigen for myeloma is complicated: first because common MM antigens—CD38, CD56, CD138—also are expressed on essential normal cells, and second, because myeloma cells are synonymous with heterogeneity. The FDA based its designation of bb2121, or BCMA CAR T-cell therapy, on preliminary data from an ongoing phase 1 CRB-401 trial that, as of December 2017, concluded that 94% of 21 patients with MM treated with the highest doses showed complete or partial remissions and high rates of progression-free survival.3 The trial also showed that cytokine-release toxicity (CRS), although severe in some patients, was generally reversible and short lived.
Multiple myeloma BCMA is only one of several CAR targets under consideration for MM treatment; others include CD138, CD38, signaling lymphocyte-activating molecule 7, and κ light chain. However, B-cell maturation antigen is attractive to researchers because BCMA–specific CAR-expressing T lymphocytes recognize and kill B-cell maturation antigen–expressing tumor cells. Also, BCMA acts as a receptor for both a proliferation-inducing ligand and as a B-cell–activating factor and is a member of the tumor necrosis factor receptor superfamily, playing a key role in plasma cell survival. B-cell maturation antigen is expressed in most, if not all, myeloma cells but not in epithelial tissues. Finally, integration of CAR-Ts with other myeloma therapies is an important area of future research.4
Most of the 23 trials looking at CAR T-cell therapy for MM are in the U.S. or China, and several deal jointly with MM, leukemia, and lymphoma. The THINK (THerapeutic Immunotherapy with NKR-2) multinational open-label phase 1 study stands alone in assessing the safety and clinical activity of multiple administrations of autologous NKR-2 cells in 7 refractory cancers, including 5 solid tumors (colorectal, ovarian, bladder, triple-negative breast and pancreatic cancers) and 2 hematologic tumors (acute myeloid leukemia and MM). Unlike traditional CAR T-cell therapy, which targets only 1 tumor antigen, NK cell receptors enable a single receptor to recognize multiple tumor antigens.
Despite challenges of toxicity, costs, and restricted availability for immunotherapies, CAR T-cell therapies seem to offer great possibilities of groundbreaking treatments and possible cures for formerly hard to treat cancers, including MM.5
Click here to read the digital edition.
Gene therapies, especially chimeric antigen receptor (CAR) T-cell therapies, experienced significant growth in 2017. The CAR T-cell therapies are among the most clinically important of the adoptive cell transfer therapies. In August, the FDA approved tisagenlecleucel for patients aged < 26 years with acute or relapsed lymphoblastic leukemia (ALL). In October, the FDA approved axicabtagene ciloleucel for treatment of adult patients nonresponsive to, or relapsed from treatment of, certain types of large B-cell lymphoma. And in November, the FDA granted breakthrough therapy designation to Celgene and Bluebird Bio for the bb2121 anti-B-cell maturation antigen (BCMA) CAR T-cell therapy for relapsed and refractory multiple myeloma (MM).
Chimeric antigen receptor T-cells circumvent the human major histocompatibility complex that T-cell receptors must navigate, shifting cell-based therapy away from identification of existing cells and toward creating T-cell products through genetic engineering. This broadens the potential for CAR T-cell applications and allows for rapid manufacture of tumor and patient-specific agents.1 Both Novartis’ Kymriah and Kite Pharma’s Yescarta are derived from investigations into anti-CD19 CAR therapy, which has been the most heavily researched of the CARs due to its links with B-cell malignancies, expression in most tumor cells, and absence from vital tissues.2 Studied in relation to a number of cancers, CD19 has not shown much success in either MM or solid tumor cancers.
Targeting the right antigen for myeloma is complicated: first because common MM antigens—CD38, CD56, CD138—also are expressed on essential normal cells, and second, because myeloma cells are synonymous with heterogeneity. The FDA based its designation of bb2121, or BCMA CAR T-cell therapy, on preliminary data from an ongoing phase 1 CRB-401 trial that, as of December 2017, concluded that 94% of 21 patients with MM treated with the highest doses showed complete or partial remissions and high rates of progression-free survival.3 The trial also showed that cytokine-release toxicity (CRS), although severe in some patients, was generally reversible and short lived.
Multiple myeloma BCMA is only one of several CAR targets under consideration for MM treatment; others include CD138, CD38, signaling lymphocyte-activating molecule 7, and κ light chain. However, B-cell maturation antigen is attractive to researchers because BCMA–specific CAR-expressing T lymphocytes recognize and kill B-cell maturation antigen–expressing tumor cells. Also, BCMA acts as a receptor for both a proliferation-inducing ligand and as a B-cell–activating factor and is a member of the tumor necrosis factor receptor superfamily, playing a key role in plasma cell survival. B-cell maturation antigen is expressed in most, if not all, myeloma cells but not in epithelial tissues. Finally, integration of CAR-Ts with other myeloma therapies is an important area of future research.4
Most of the 23 trials looking at CAR T-cell therapy for MM are in the U.S. or China, and several deal jointly with MM, leukemia, and lymphoma. The THINK (THerapeutic Immunotherapy with NKR-2) multinational open-label phase 1 study stands alone in assessing the safety and clinical activity of multiple administrations of autologous NKR-2 cells in 7 refractory cancers, including 5 solid tumors (colorectal, ovarian, bladder, triple-negative breast and pancreatic cancers) and 2 hematologic tumors (acute myeloid leukemia and MM). Unlike traditional CAR T-cell therapy, which targets only 1 tumor antigen, NK cell receptors enable a single receptor to recognize multiple tumor antigens.
Despite challenges of toxicity, costs, and restricted availability for immunotherapies, CAR T-cell therapies seem to offer great possibilities of groundbreaking treatments and possible cures for formerly hard to treat cancers, including MM.5
Click here to read the digital edition.
1. Almåsbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602.
2. Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392-3400.
3. C
4. Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130(24):2594-2602.
5. Vallet S, Pecherstorfer M, Podar K. Adoptive cell therapy in multiple myeloma. Expert Opin Biol Ther. 2017;17(12):1511-1522.
1. Almåsbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602.
2. Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392-3400.
3. C
4. Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130(24):2594-2602.
5. Vallet S, Pecherstorfer M, Podar K. Adoptive cell therapy in multiple myeloma. Expert Opin Biol Ther. 2017;17(12):1511-1522.
BCL expression intensity key in distinguishing FL lesions
Intensity of BCL2 expression, and to a lesser extent expression of t(14;18), may help distinguish common and indolent cutaneous lymphomas from poorer-prognosis cutaneous lesions secondary to systemic follicular lymphomas, results of a recent investigation show.
Strong expression of BCL2 was almost always associated with secondary cutaneous follicular lymphoma (SCFL), and infrequently associated with primary cutaneous follicular center-cell lymphoma (PCFCL), according to the study results.
The translocation t(14;18) was likewise linked to secondary lesions, occurring less frequently in PCFCL in the study, reported recently in the Journal of Cutaneous Pathology.
“BCL2 expression intensity is the single most valuable clue in differentiating PCFCL from SCFL cases on histopathological grounds,” said Ramon M. Pujol, MD, PhD, of Hospital del Mar, Barcelona, Spain, and colleagues.
One of the main cutaneous B-cell lymphoma subtypes, PCFCL is marked by frequent relapses, but little incidence of systemic spread, meaning that conservative, skin-based therapies are usually warranted. By contrast, patients with SCFLs have a poor prognosis and may require systemic therapy, the investigators noted in their report.
Previous investigations have yielded conflicting results on the role of BCL2 expression, CD10 expression, and presence of t(14;18) translocation in distinguishing PCFCL from SCFL.
While early studies suggested most PCFCLs were negative for these markers, some recent reports suggested BCL positivity in PCFCLs is as high as 86%, the investigators said.
Accordingly, Dr. Pujol and colleagues evaluated clinicopathologic and genetic features in a large series of patients, including 59 with PCFCL and 22 with SCFL.
Significant BCL2 expression was seen in 69% of PCFCLs and in 100% of SCFLs (P = .003) in this patient series; however, when looking at BCL2 intensity, investigators found strong expression almost exclusively in SCFL. Strong expression was seen in 46% of those patients with secondary lymphomas, versus just 4%, or two cases, in the PCFCL group (P = .001).
The t(14;18) translocation was seen in 64% of SCFLs and only 9.1% of PCFCLs (P = .001).
Similar to what was seen for BCL2, expression of CD10 was observed in 66% of PCFCLs and 91% of SCFLs, and again, intensity differences mattered. Strong CD10 expression was seen in 62% of secondary lymphomas and 16% of PCFCLs (P = .01). But the high number of positive PCFCLs made this marker less useful than BCL2, the investigators said.
“We believe that differences in BCL2 and CD10 expression between our results and older previous studies could reflect the improvement of antigen retrieval laboratory techniques,” they said.
The investigators did not report disclosures related to the research.
SOURCE: Servitje O et al. J Cutan Pathol. 2019;46:182-9.
Intensity of BCL2 expression, and to a lesser extent expression of t(14;18), may help distinguish common and indolent cutaneous lymphomas from poorer-prognosis cutaneous lesions secondary to systemic follicular lymphomas, results of a recent investigation show.
Strong expression of BCL2 was almost always associated with secondary cutaneous follicular lymphoma (SCFL), and infrequently associated with primary cutaneous follicular center-cell lymphoma (PCFCL), according to the study results.
The translocation t(14;18) was likewise linked to secondary lesions, occurring less frequently in PCFCL in the study, reported recently in the Journal of Cutaneous Pathology.
“BCL2 expression intensity is the single most valuable clue in differentiating PCFCL from SCFL cases on histopathological grounds,” said Ramon M. Pujol, MD, PhD, of Hospital del Mar, Barcelona, Spain, and colleagues.
One of the main cutaneous B-cell lymphoma subtypes, PCFCL is marked by frequent relapses, but little incidence of systemic spread, meaning that conservative, skin-based therapies are usually warranted. By contrast, patients with SCFLs have a poor prognosis and may require systemic therapy, the investigators noted in their report.
Previous investigations have yielded conflicting results on the role of BCL2 expression, CD10 expression, and presence of t(14;18) translocation in distinguishing PCFCL from SCFL.
While early studies suggested most PCFCLs were negative for these markers, some recent reports suggested BCL positivity in PCFCLs is as high as 86%, the investigators said.
Accordingly, Dr. Pujol and colleagues evaluated clinicopathologic and genetic features in a large series of patients, including 59 with PCFCL and 22 with SCFL.
Significant BCL2 expression was seen in 69% of PCFCLs and in 100% of SCFLs (P = .003) in this patient series; however, when looking at BCL2 intensity, investigators found strong expression almost exclusively in SCFL. Strong expression was seen in 46% of those patients with secondary lymphomas, versus just 4%, or two cases, in the PCFCL group (P = .001).
The t(14;18) translocation was seen in 64% of SCFLs and only 9.1% of PCFCLs (P = .001).
Similar to what was seen for BCL2, expression of CD10 was observed in 66% of PCFCLs and 91% of SCFLs, and again, intensity differences mattered. Strong CD10 expression was seen in 62% of secondary lymphomas and 16% of PCFCLs (P = .01). But the high number of positive PCFCLs made this marker less useful than BCL2, the investigators said.
“We believe that differences in BCL2 and CD10 expression between our results and older previous studies could reflect the improvement of antigen retrieval laboratory techniques,” they said.
The investigators did not report disclosures related to the research.
SOURCE: Servitje O et al. J Cutan Pathol. 2019;46:182-9.
Intensity of BCL2 expression, and to a lesser extent expression of t(14;18), may help distinguish common and indolent cutaneous lymphomas from poorer-prognosis cutaneous lesions secondary to systemic follicular lymphomas, results of a recent investigation show.
Strong expression of BCL2 was almost always associated with secondary cutaneous follicular lymphoma (SCFL), and infrequently associated with primary cutaneous follicular center-cell lymphoma (PCFCL), according to the study results.
The translocation t(14;18) was likewise linked to secondary lesions, occurring less frequently in PCFCL in the study, reported recently in the Journal of Cutaneous Pathology.
“BCL2 expression intensity is the single most valuable clue in differentiating PCFCL from SCFL cases on histopathological grounds,” said Ramon M. Pujol, MD, PhD, of Hospital del Mar, Barcelona, Spain, and colleagues.
One of the main cutaneous B-cell lymphoma subtypes, PCFCL is marked by frequent relapses, but little incidence of systemic spread, meaning that conservative, skin-based therapies are usually warranted. By contrast, patients with SCFLs have a poor prognosis and may require systemic therapy, the investigators noted in their report.
Previous investigations have yielded conflicting results on the role of BCL2 expression, CD10 expression, and presence of t(14;18) translocation in distinguishing PCFCL from SCFL.
While early studies suggested most PCFCLs were negative for these markers, some recent reports suggested BCL positivity in PCFCLs is as high as 86%, the investigators said.
Accordingly, Dr. Pujol and colleagues evaluated clinicopathologic and genetic features in a large series of patients, including 59 with PCFCL and 22 with SCFL.
Significant BCL2 expression was seen in 69% of PCFCLs and in 100% of SCFLs (P = .003) in this patient series; however, when looking at BCL2 intensity, investigators found strong expression almost exclusively in SCFL. Strong expression was seen in 46% of those patients with secondary lymphomas, versus just 4%, or two cases, in the PCFCL group (P = .001).
The t(14;18) translocation was seen in 64% of SCFLs and only 9.1% of PCFCLs (P = .001).
Similar to what was seen for BCL2, expression of CD10 was observed in 66% of PCFCLs and 91% of SCFLs, and again, intensity differences mattered. Strong CD10 expression was seen in 62% of secondary lymphomas and 16% of PCFCLs (P = .01). But the high number of positive PCFCLs made this marker less useful than BCL2, the investigators said.
“We believe that differences in BCL2 and CD10 expression between our results and older previous studies could reflect the improvement of antigen retrieval laboratory techniques,” they said.
The investigators did not report disclosures related to the research.
SOURCE: Servitje O et al. J Cutan Pathol. 2019;46:182-9.
FROM THE JOURNAL OF CUTANEOUS PATHOLOGY
Key clinical point:
Major finding: Strong BCL2 expression was seen in 46% of secondary lymphomas, versus just 4% of primary cutaneous follicular center-cell lymphomas (P = .001).
Study details: A comparative study evaluating clinicopathologic and genetic features in a series of patients, including 59 with PCFCL and 22 with SCFL.
Disclosures: Investigators did not report disclosures related to the research.
Source: Servitje O et al. J Cutan Pathol. 2019;46:182-9.
Combo appears to overcome aggressive L-NN-MCL
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Two patients with aggressive L-NN-MCL and P53 abnormalities who were treated with rituximab/ibrutinib and autologous stem cell transplantation remain free of disease 18 months later.
Study details: Two case reports.
Disclosures: The authors reported having no financial disclosures.
Source: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
Cloud of inconsistency hangs over cannabis data
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
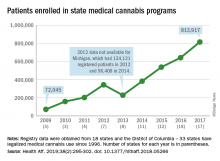
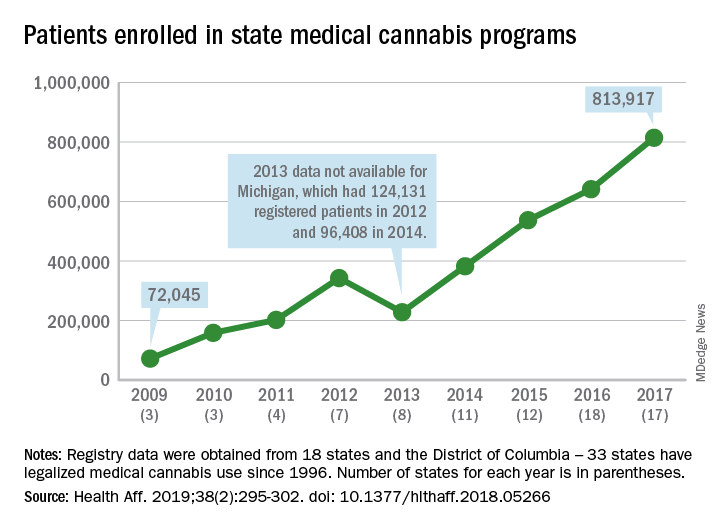
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
FROM HEALTH AFFAIRS
Clearance of Psoriasis After Ischemic Stroke
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
Practice Points
- Psoriasis is exacerbated in the presence of stress, and psoriatic lesions often have a symmetric distribution, which is evidence that the nervous system is involved in the pathophysiology of the condition.
- Various neuropeptides are involved in the pathophysiology of psoriasis, including substance P, nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide.
- Peripheral nerve damage results in decreased secretion of neuropeptides, which can lead to remission of psoriasis.
Barriers and Facilitators to the Use of Genomic-Based Targeted Therapy in the VA: Qualitative Findings(FULL)
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
Interview with Joseph R. Berger, MD, on the Financial Contribution of the MS Specialist
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Dr. Joseph Berger’s article The Financial Contribution of the Multiple Sclerosis Specialist
(Neurol Clin Pract. 2017;7:246-255) is an eye-opening examination of how multiple sclerosis (MS) specialists fit into the economic framework of large academic institutions. We sat down with Dr. Berger to discuss his findings.
How would you describe the downstream revenue generated from MS specialists at large academic institutions?
The downstream revenue generated from MS is highly dependent on whether the drugs prescribed to patients are provided by the academic institution, and whether the infusions and imaging studies are done at the academic institution.
Another component is whether that institution operates under a Medicare 340B, a law that enables the institutions that are providing care to the underserved to acquire drugs at a steeply discounted price. And, as cited in the paper, the Office of the Inspector General of the Department of Health and Human Services estimated that the profit margin is 58% for all the drugs being provided under 340B. It’s important to note that that statistic accounts for all drugs, not a specific drug or a specific class of drugs. But, for the sake of argument let’s assume that it’s 50% for MS drugs.
The typical MS drug costs $60,000 a year if not more, and that means that 50% of that total goes to the contribution margin of the MS provider or the MS clinic. If there are 1000 patients for whom the specialty pharmacy within the institution is providing drugs, that means an enormous amount of money is returning to the institution as a result of the contribution by the MS practitioners.
In addition to the cost of the drugs, there’s also the cost of infusions associated with the drug that contributes substantially to the bottom line of the institution.
MS specialists tend to do more imaging studies than any other discipline. At the time of diagnosis, individuals with MS get MRIs of brain, cervical and thoracic spine, and frequently the orbits. The frequency with which these images are repeated depends on the nature of the patient’s illness, the activity of their disease, etc.
How do you make a case to administrators for more funding in MS centers?
Unfortunately, the downstream revenue does not always find its way back to the MS centers. Moreover, MS practitioners are forced to prove their value to the institution before they can receive the resources that they need.
There are only two things that administrators in medical institutions respond to. The first is need. The second is the financial impact of the activity.
Here is an example from my own personal experience. I prescribed a specific drug for a patient who did not live close to the institution. It took three months for the patient to receive the drug. This was due, in large measure, to problems with the insurance company and the outside specialty pharmacies that we were dealing with. In that course of time the patient suffered two relapses from which she never fully recovered.
I thought we could do a much better job treating our patients if our own specialty pharmacy was providing the drug. Eventually, after some negotiating with the administration, we were able to provide all those drugs through our specialty pharmacy. That change resulted in a significant increase in terms of contribution margin for the MS team, and it was a great benefit for our patients.
How does MS compare with other neurology disciplines?
If you look at the contribution margin from MS and compare it to in any other division in neurology, it exceeds all of them combined by a significant percentage.
For example, the current contribution margin in the MS division at the University of Pennsylvania exceeds that of virtually any other line within the Neuroscience Center Service, which includes neurosurgery. It is on par with, and may exceed, that of spine surgery, which in the past had always been the biggest driver of the contribution margin from the Neuroscience service line.
Often, MS specialists aren’t getting the resources needed despite the fact that their growing practice would enhance the contribution margin.
Since this has been brought to the attention of the administration at the University of Pennsylvania, there have been increased resources available for the division; we now have more nurses and nurse practitioners, and we have pharmacists within the division. All of this has made a big difference in helping to provide the best care for our patients.
How would you characterize the compensation of the MS specialist?
One of the things that I did address in the article, but only obliquely, is the compensation of the MS neurologist.
Historically, the MS neurologist was among the least compensated of all the neurology disciplines, in academics as well as in private practice. The reason for this was simple. Until the early 1990s, there were very few drugs to treat MS. It was more a matter of diagnosing people and treating the symptoms as they arose. When drugs for MS emerged, they were not particularly complex to manage.
However, as new drugs have become available, and the efficacy of these drugs increased, so did their side effect profiles. A need arose for specialists to manage the treatment of patients with MS.
I hope to address this further in a future publication, but the underlying assertion is that the compensation of the MS neurologist needs to be revisited at both academic institutions and in the community.
Final thoughts?
The article was an attempt to educate not just the MS community, but the broader neurologic community as to the value of an MS specialist to an institution.
The purpose of this article was to encourage people to think about their worth and the worth of what they do as it applies to the financial well-being of the institution with which they’re associated.
Safety and Efficacy of Halobetasol Propionate Lotion 0.01% in the Treatment of Moderate to Severe Plaque Psoriasis: A Pooled Analysis of 2 Phase 3 Studies
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.
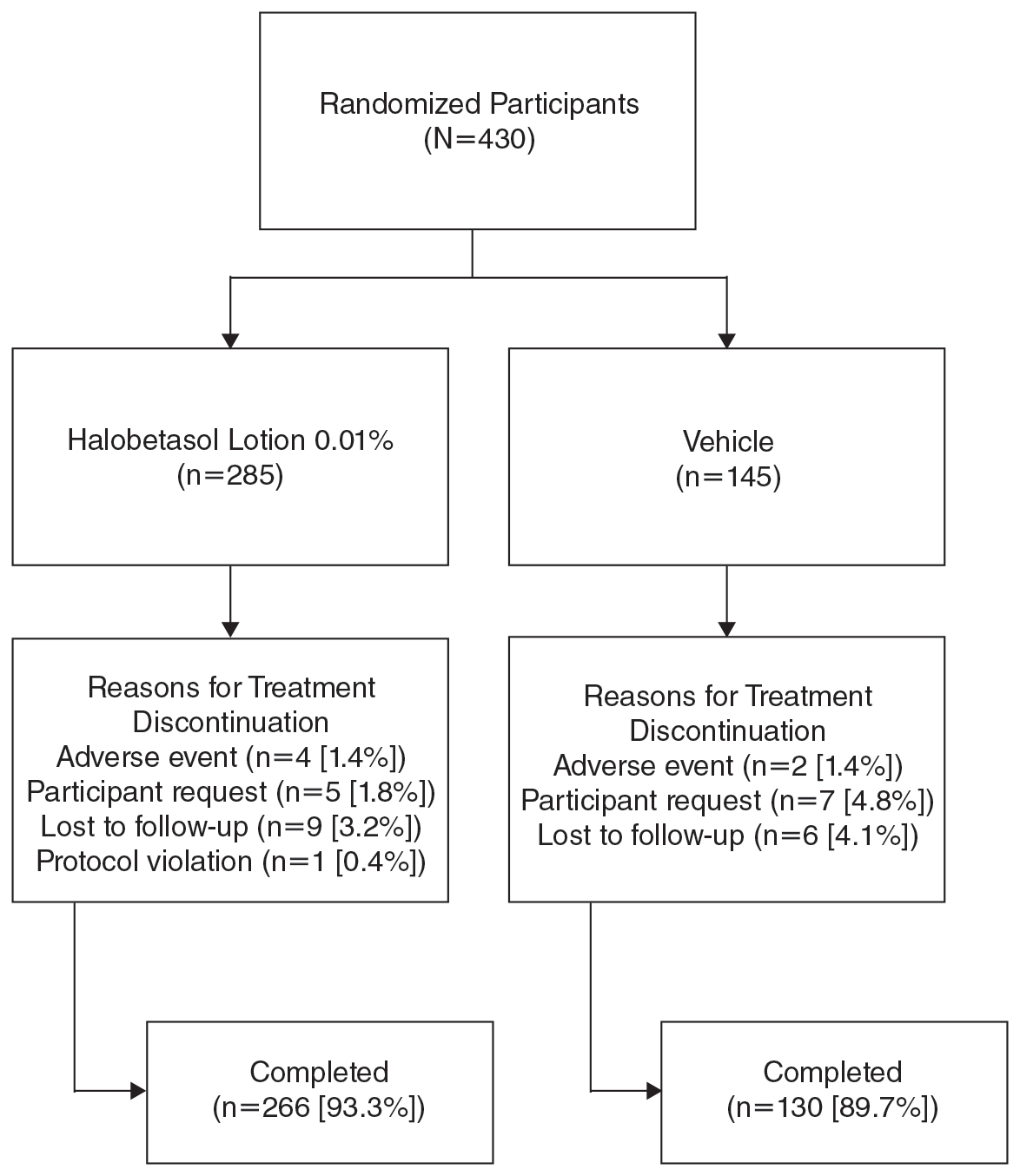
A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).
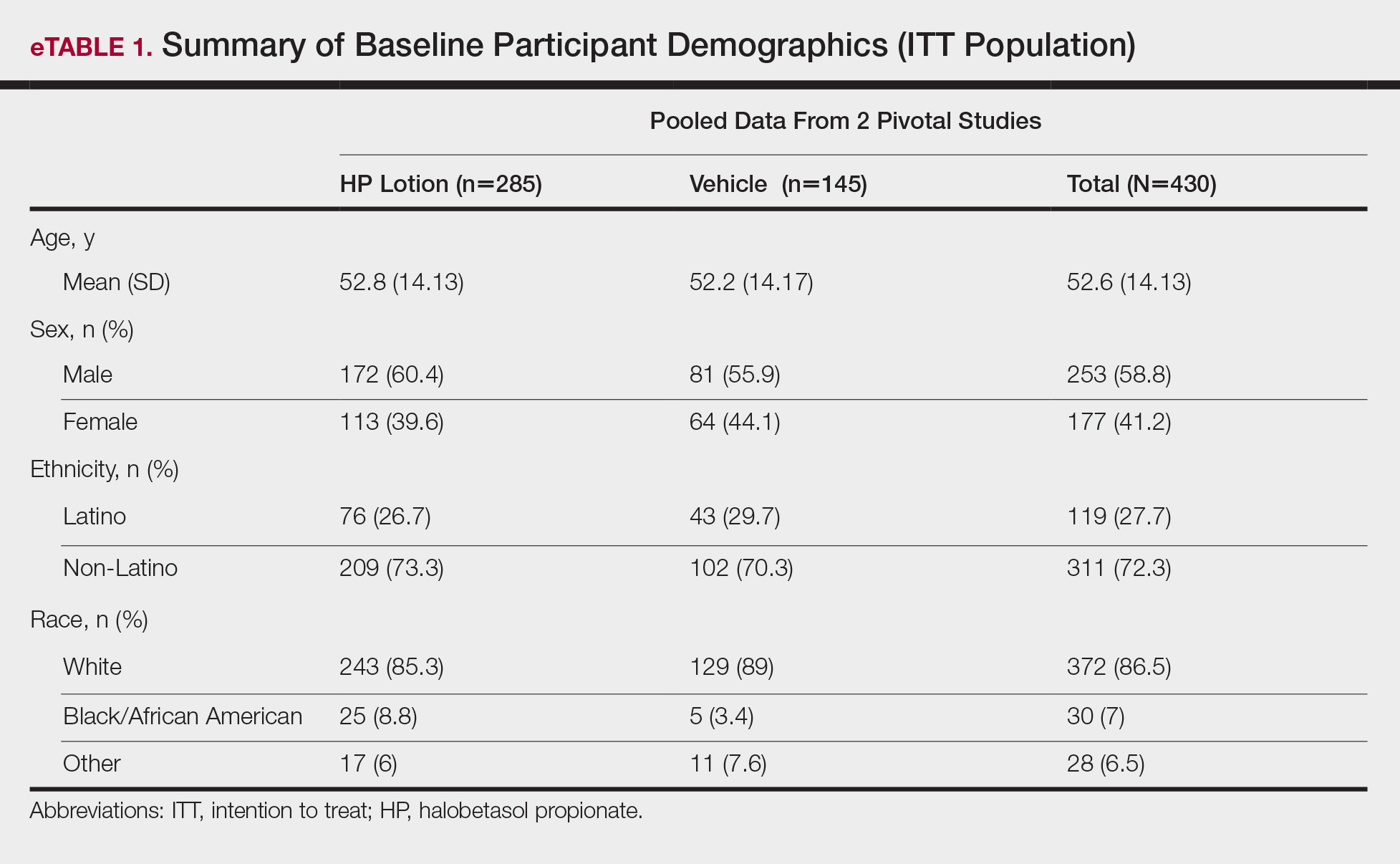
Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).
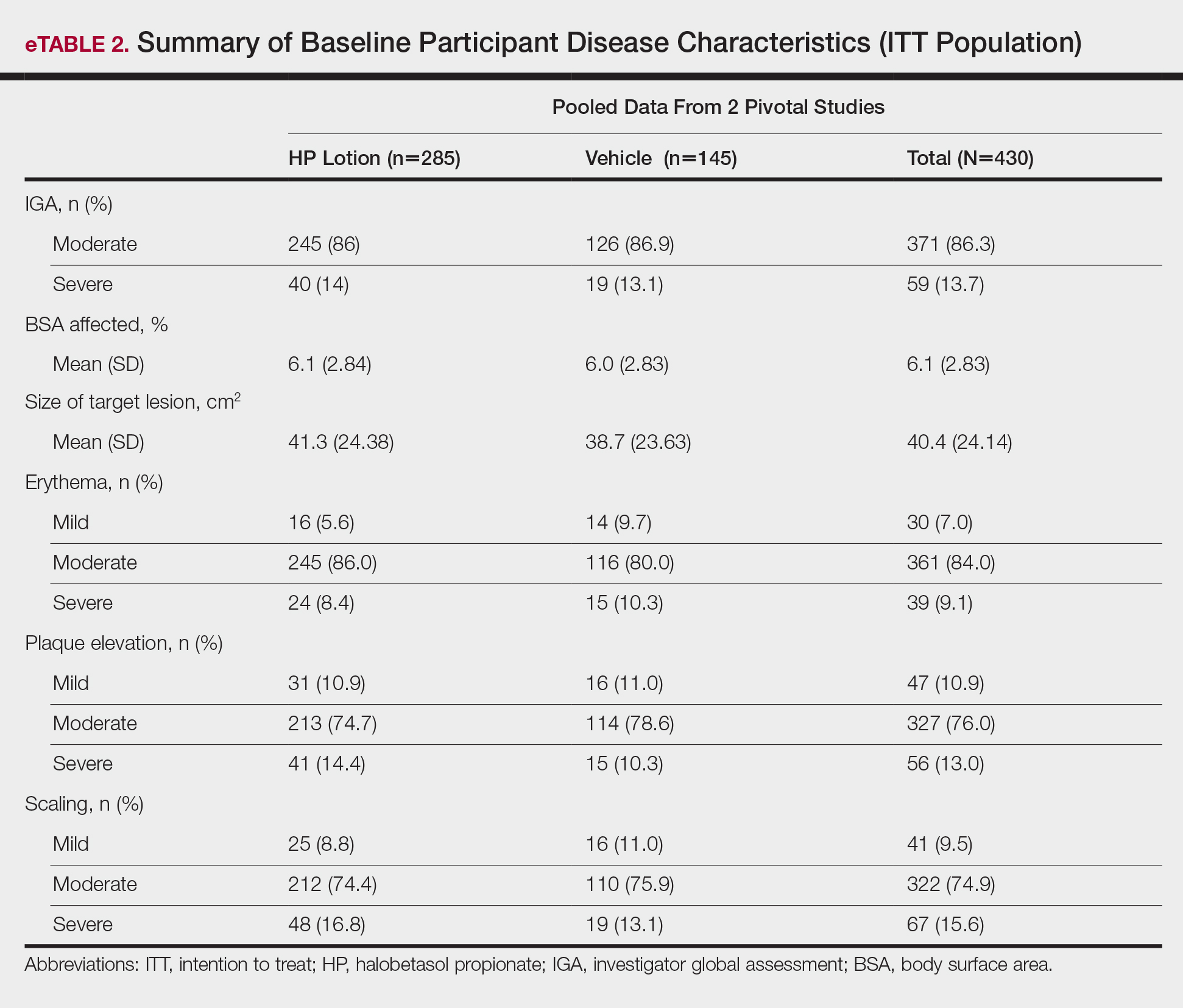
Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).
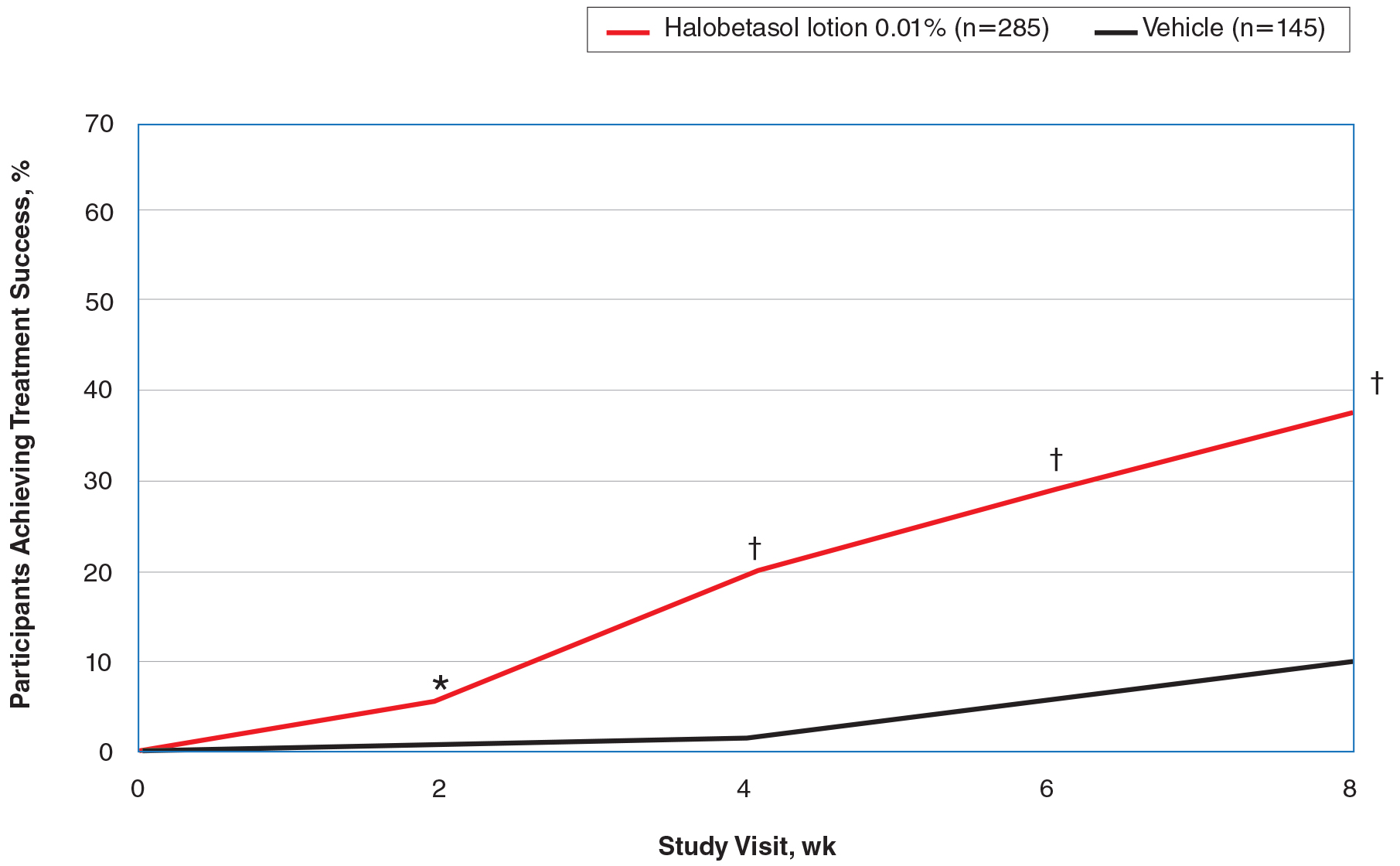
Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).
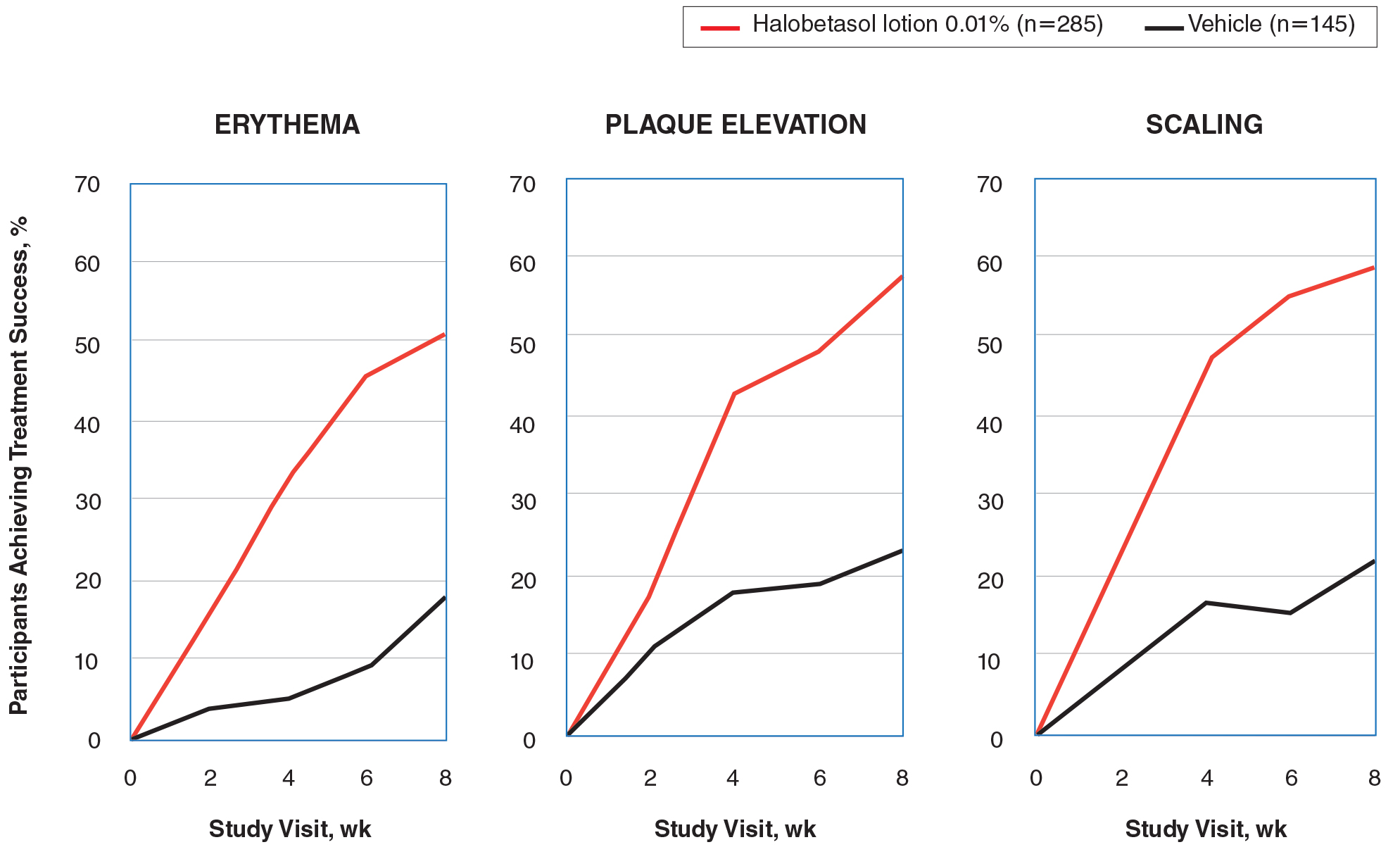
BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).
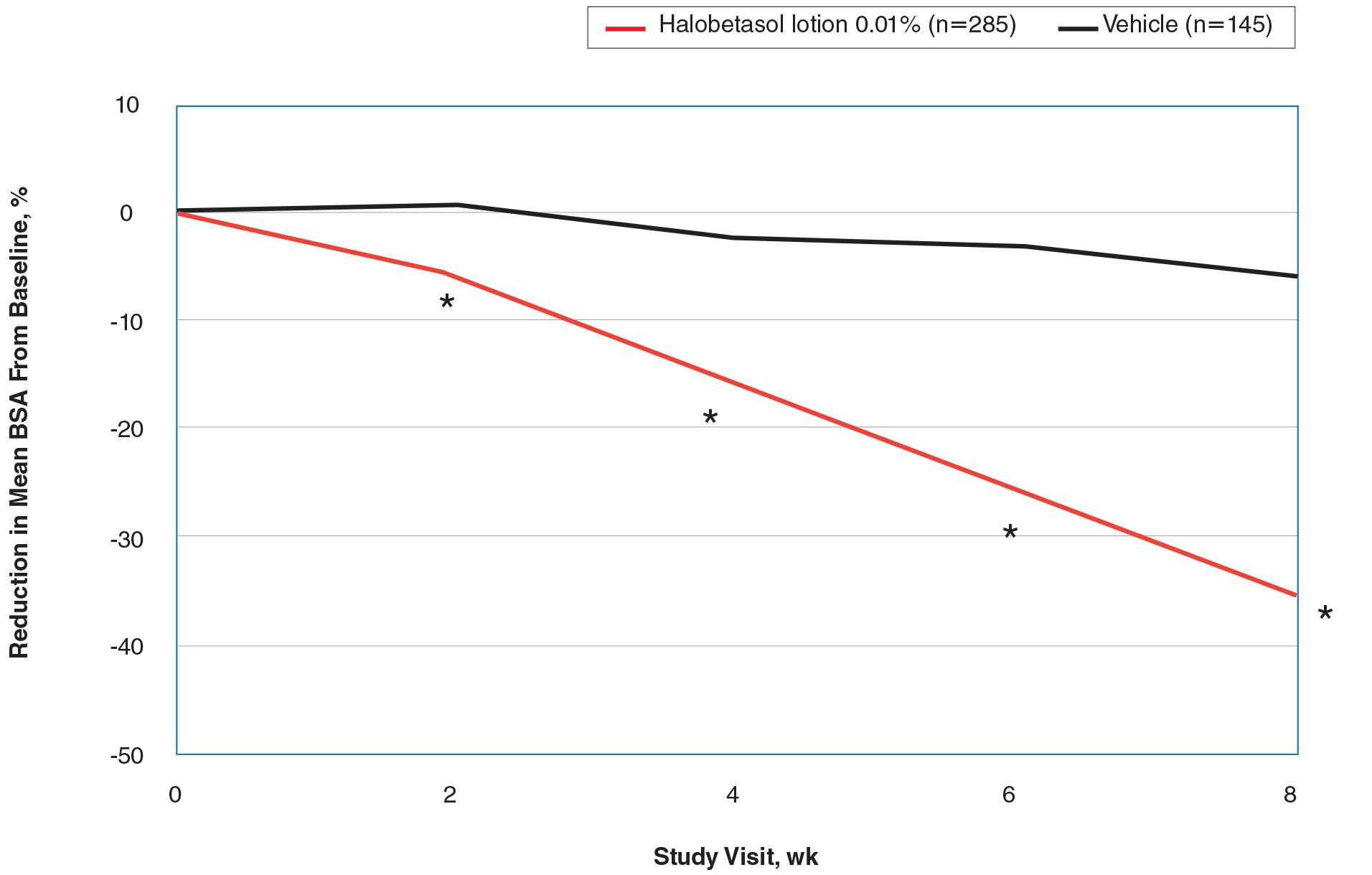
IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).
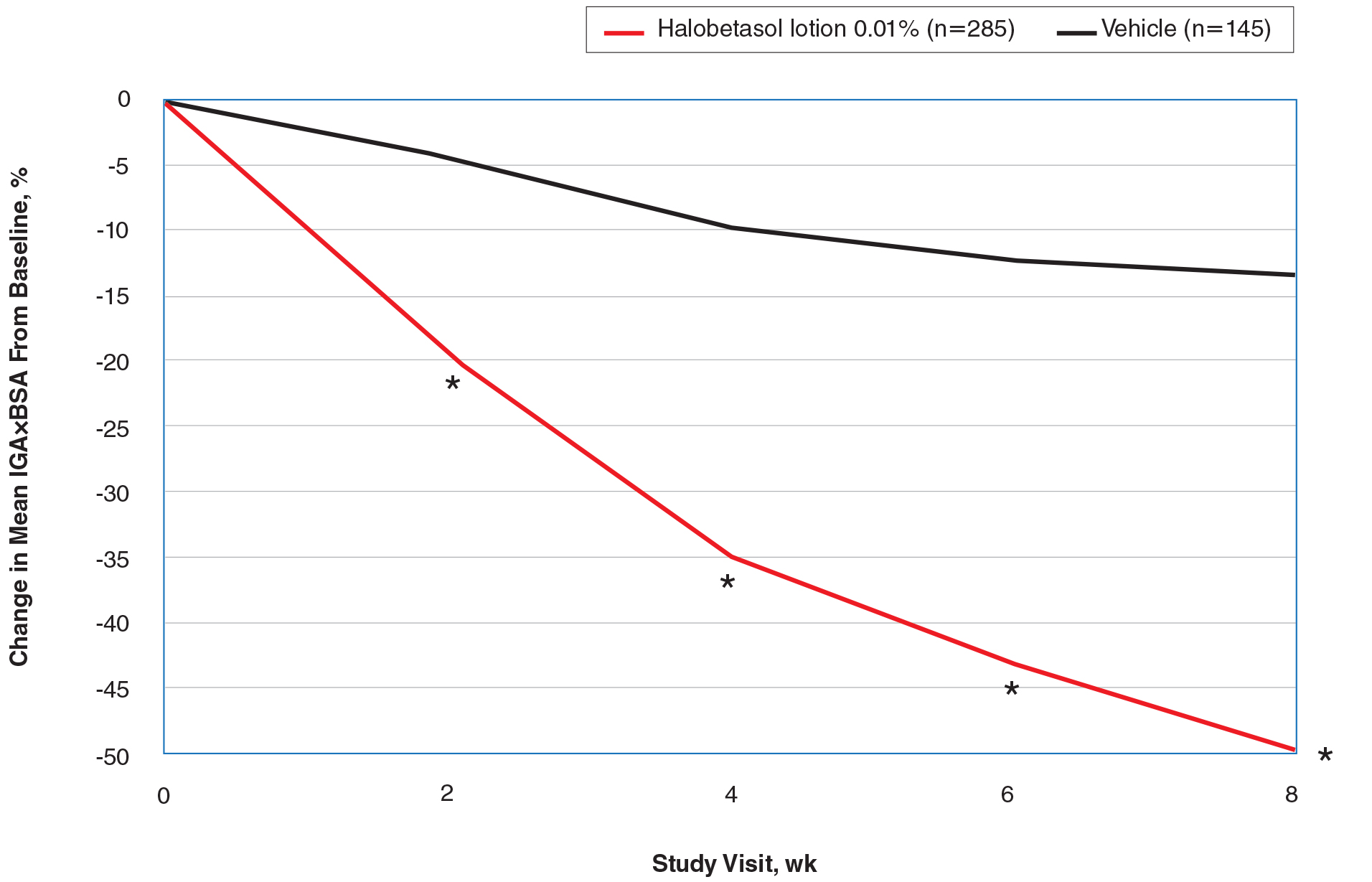
By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).
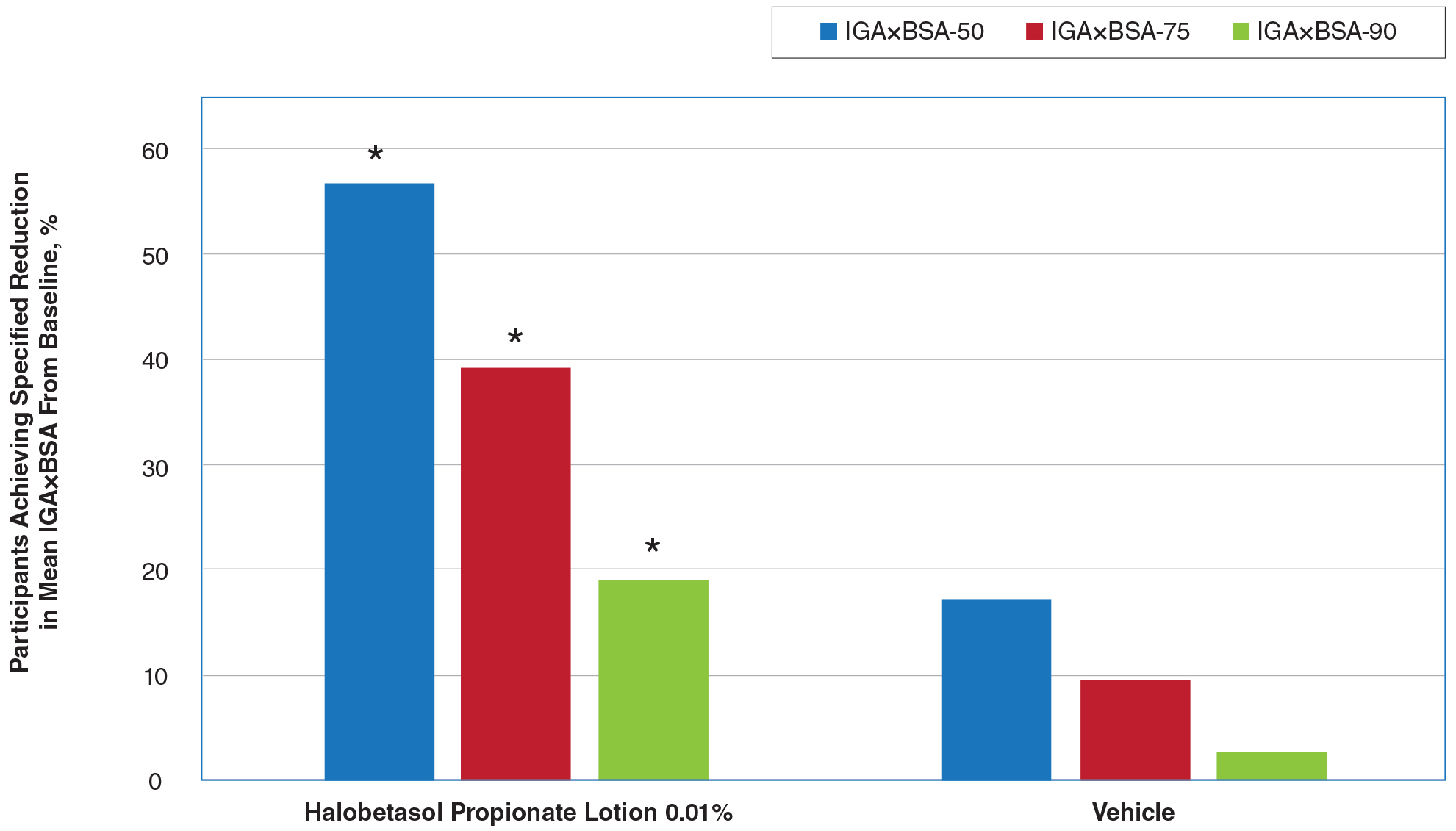
Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.
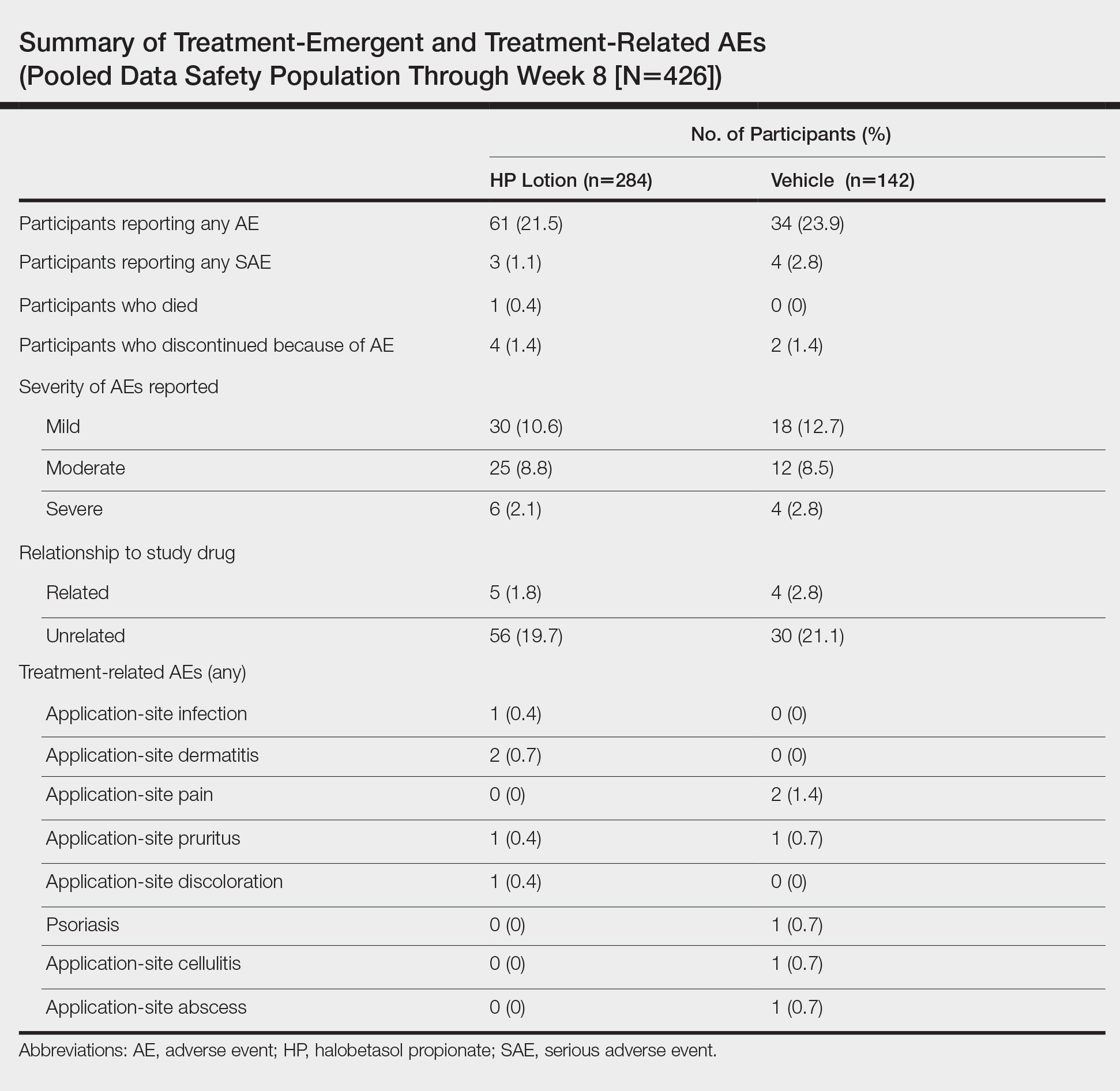
Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.

A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).

Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).

Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).

Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).

BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).

IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).

By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).

Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.

Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting almost 2% of the population.1-3 It is characterized by patches of raised reddish skin covered by silvery-white scales. Most patients have limited disease (<5% body surface area [BSA] involvement) that can be managed with topical agents.4 Topical corticosteroids (TCSs) are considered first-line therapy for mild to moderate disease because of the inflammatory nature of the condition and often are used in conjunction with systemic agents in more severe psoriasis.4
As many as 20% to 30% of patients with moderate to severe plaque psoriasis have inadequate disease control.5 Several factors may affect patient outcomes; however, drug selection and patient adherence are important given the chronic nature of the disease. A survey of 1200 patients with psoriasis reported nonadherence rates of 73% with topical therapy.6 In addition, patients tend to apply less than the recommended dose or abandon treatment altogether if rapid improvement does not occur7,8; it is not uncommon for patients with psoriasis to mistakenly believe treatment will improve their condition within 1 to 2 weeks.9 Patient satisfaction with topical treatments is low, partly because of these false expectations and formulation issues. Treatments can be greasy and sticky, with unpleasant odors and the potential to stain clothes and linens.7,10 Safety concerns with TCSs also limit their consecutive use beyond 2 to 4 weeks, which is not ideal for a disease that requires a long-term management strategy.
A potent/superpotent TCS that is administered once daily and has a safety profile that affords longer-term, once-daily treatment in an aesthetically pleasing formulation would seem ideal. Herein, we investigate the safety and tolerability of a novel low-concentration (0.01%) lotion formulation of halobetasol propionate (HP), reporting on the pooled data from 2 phase 3 clinical studies in participants with moderate to severe psoriasis.
METHODS
Study Design
We conducted 2 multicenter, double-blind, randomized, parallel-group phase 3 studies to assess the safety, tolerability, and efficacy of HP lotion 0.01% in participants with a clinical diagnosis of moderate to severe psoriasis with an investigator global assessment (IGA) score of 3 or 4 and an affected BSA of 3% to 12%. Participants were randomized (2:1) to receive HP lotion or vehicle applied topically to the affected area once daily for 8 weeks.
Inclusion and Exclusion Criteria
The studies included individuals of either sex aged 18 years or older. A target lesion was defined primarily to assess signs of psoriasis, measuring 16 to 100 cm2, with a score of 3 (moderate) or higher for 2 of 3 different psoriasis signs—erythema, plaque elevation, and scaling—and summed score of 8 or higher, with no sign scoring less than 2. Participants who had pustular psoriasis or used phototherapy, photochemotherapy, or systemic psoriasis therapy within the prior 4 weeks or biologics within the prior 3 months, or those who were diagnosed with skin conditions that would interfere with the interpretation of results were excluded from the studies.
Study Oversight
Participants provided written informed consent before study-related procedures were performed, and the protocol and consent were approved by institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Efficacy Assessment
A 5-point scale ranging from 0 (clear) to 4 (severe) was used by the investigator at each study visit to assess the overall psoriasis severity of the treatable areas. Treatment success (the percentage of participants with at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]) was evaluated at weeks 2, 4, 6, and 8, w
Signs of psoriasis at the target lesion were assessed at each visit using individual 5-point scales ranging from 0 (clear) to 4 (severe). Treatment success was defined as at least a 2-grade improvement from baseline score for each of the key signs—erythema, plaque elevation, and scaling—and reported at weeks 2, 4, 6, and 8, with a posttreatment follow-up at week 12.
Affected BSA also was evaluated at each visit. In addition, an IGA×BSA composite score was calculated by multiplying the IGA by the BSA (range, 9–48 [eg, maximum IGA=4 and maximum BSA=12]) at each time point. The mean percentage change in IGA×BSA from baseline was calculated for each study visit. Additional end points included the achievement of a 50%, 75%, and 90% or greater reduction from baseline IGA×BSA score—IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90—at week 8.
Safety Assessment
Safety evaluations including adverse events (AEs), local skin reactions (LSRs), vital signs, laboratory evaluations, and physical examinations were performed. Information on reported and observed AEs was obtained at each visit. Routine safety laboratory tests were performed at screening, week 4, and week 8. An abbreviated physical examination was performed at baseline, week 8 (end of treatment), and week 12 (end of study). Treatment areas also were examined by the investigator at baseline and each subsequent visit for the presence or absence of marked known drug-related AEs including skin atrophy, striae, telangiectasia, and folliculitis.
LSR Assessment
Local skin reactions such as itching, dryness, and burning/stinging were evaluated at each study visit using 4-point scales ranging from 0 (clear) to 3 (severe). Given the nature of the disease, the presence of LSRs and symptoms at baseline is commonplace, and as such, these evaluations identified both improvement and any emergent issues.
Statistical Analysis
The primary study goal was to assess differences in treatment efficacy between HP lotion and vehicle with respect to IGA. All statistical processing was performed using SAS unless otherwise stated; statistical tests were 2-sided and performed at the 0.05 level of significance. Markov Chain Monte Carlo multiple imputation was the primary method used to handle missing efficacy data. No imputations were made for missing safety data. All participants were randomized, and the dispensed study drug was included in the intention-to-treat analysis set. This analysis was considered primary for the evaluation of efficacy. Data were analyzed using Cochran-Mantel-Haenszel tests, stratified by analysis center.
Body surface area data were analyzed in a post hoc analysis of covariance with factors of treatment and analysis center and baseline BSA as a covariate. P values for comparisons of percentage change in IGA×BSA were derived from a Wilcoxon rank sum test. For IGA×BSA-50, IGA×BSA-75, and IGA×BSA-90, P values were derived from a Cochran-Mantel-Haenszel test. Last observation carried forward was used to impute data for IGA and BSA through week 8 prior to analysis.
The primary safety analysis was conducted at week 8 using the safety analysis set, which included all participants who were randomized, received at least 1 confirmed dose of the study drug, and had at least 1 postbaseline safety assessment. Adverse events were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA, Version 18.0). A post hoc Wilcoxon rank sum test was conducted to compare itching, dryness, and burning/stinging scores at week 8 for HP lotion versus vehicle.
RESULTS
Participant Disposition
Overall, 430 participants were randomized (2:1) to HP lotion (n=285) or vehicle (n=145)(eFigure 1) and included in the intention-to-treat population. Across the 2 studies, 93.3% (n=266) of participants treated with HP lotion and 89.7% (n=130) of participants treated with vehicle completed treatment. The main reasons for study discontinuation with HP lotion were lost to follow-up (3.2%; n=9), participant request (1.8%; n=5), and AEs (1.4%; n=4). Participant request (4.8%; n=7), lost to follow-up (4.1%; n=6), and AEs (1.4%; n=2) also were the main reasons for treatment discontinuation in the vehicle arm.

A total of 426 participants were included in the safety population, with no postbaseline safety evaluation in 4 participants.
Baseline Participant Demographics
Demographic data were comparable across the 2 studies. The mean age (SD) was 52.6 (14.13) years. Overall, the majority of participants were male (58.8%; n=253) and white (86.5%; n=372)(eTable 1).

Baseline disease characteristics also were comparable across the treatment groups. Participants had moderate (86.3%; n=371) or severe (13.7%; n=59) disease, with a mean BSA (SD) of 6.1% (2.83) and mean size of target lesion (SD) of 40.4 cm2 (24.14). The majority of participants had moderate (erythema, 84.0%; plaque elevation, 76.0%; and scaling, 74.9%) or severe (erythema, 9.1%; plaque elevation, 13.0%; and scaling, 15.6%) signs of psoriasis at the target lesion site (eTable 2).

Efficacy Evaluation
IGA of Disease Severity
Halobetasol propionate lotion was consistently more effective than its vehicle in achieving treatment success (at least a 2-grade improvement in baseline IGA score and a score of 0 [clear] or 1 [almost clear]). Halobetasol propionate lotion demonstrated statistically significant superiority over vehicle as early as week 2 (P=.003). By week 8, 37.43% of participants in the HP lotion group achieved treatment success compared with 10.03% in the vehicle group (P<.001)(Figure 1).

Overall, 39% of participants who had moderate disease (IGA score, 3) at baseline were treatment successes with HP lotion at week 8 compared with 11.53% of participants treated with vehicle; 27.97% of participants with severe disease (IGA score, 4) were treatment successes, with at least a 3-grade improvement in IGA. No participants with severe psoriasis who were treated with vehicle achieved treatment success at week 8. Efficacy was similar in female and male participants, allowing for vehicle effects.
Severity of Signs of Psoriasis (Erythema, Plaque Elevation, and Scaling) at Target Lesion Site
Halobetasol propionate lotion was statistically superior to vehicle in reducing the psoriasis signs of erythema, plaque elevation, and scaling at the target lesion from week 2. At week 8, treatment success (at least a 2-grade improvement from baseline) was achieved by 51.48% (erythema), 57.64% (plaque elevation), and 58.98% (scaling) of participants compared with 17.85%, 23.61%, and 22.82%, respectively, with vehicle (all P<.001)(Figure 2).

BSA Assessment
Halobetasol propionate lotion was statistically superior to vehicle in reducing BSA from week 2. At week 8 there was a 35.20% reduction in mean BSA for HP lotion compared to 5.85% for vehicle (P<.001)(eFigure 2).

IGA×BSA Composite Score
At baseline, the mean IGA×BSA scores for HP lotion and vehicle were similar: 19.3 and 18.8, respectively. By week 8, the percentage change in mean IGA×BSA score with HP lotion was 49.44% compared to 13.35% with vehicle (P<.001). Differences were significant from week 2 (P<.001)(Figure 3).

By week 8, 56.8% of participants (n=162) treated with HP lotion had achieved a 50% or greater reduction in baseline IGA×BSA compared to 17.2% of participants treated with vehicle (P<.001). Reductions of IGA×BSA-75 and IGA×BSA-90 were achieved in 39.3% and 19.3% of participants treated with HP lotion, respectively, compared with 9.7% and 2.8% of participants treated with vehicle (both P<.001)(eFigure 3).

Safety Evaluation
Adverse event reports were low and similar between the active and vehicle groups. Overall, 61 participants (21.5%) treated with HP lotion reported AEs compared with 34 participants (23.9%) treated with vehicle (Table). The majority of participants treated with HP lotion (90.2%) had AEs that were mild or moderate. There was 1 AE of telangiectasia, not considered treatment related. There were 5 treatment-related AEs for HP lotion, all at the application site: dermatitis (0.7%; n=2), infection (0.4%; n=1), pruritus (0.4%; n=1), and discoloration (0.4%; n=1). There were no AE reports of skin atrophy or folliculitis.

Local Skin Reactions
Most LSRs at baseline were mild to moderate in severity. Itching was the most common, present in 76.8% of participants. Participant-reported burning/stinging was less common, reported by 40.6% of participants. Investigator-reported dryness was noted in 65.7% of participants. There was a rapid improvement in participant-reported itching as early as week 2 that was sustained to the end of the studies, with more gradual improvements in skin dryness and burning/stinging.
COMMENT
Plaque psoriasis is a chronic condition. The rationale behind the development of HP lotion 0.01% was to provide optimal topical treatment of moderate to severe psoriasis, allowing for the potential of prolonged use beyond the 2-week consecutive use normally applied to HP cream 0.05% in a light, once-daily, aesthetically pleasing lotion formulation that patients would prefer.
Treatment success was rapid and achieved in more than 37% of participants by week 8, with significant improvements in psoriasis signs and symptoms (erythema, plaque elevation, and scaling) compared with vehicle. However, IGA does not consider BSA involvement, a key aspect of disease severity,11,12 and improvements in psoriasis signs of erythema, plaque elevation, and scaling were only assessed at the target lesion. Recently, the product of the IGA and BSA involvement (IGA×BSA) has been proposed as a simple alternative for assessing response to therapy that has been consistently shown to be highly correlated with the psoriasis area and severity index.13-19 Halobetasol propionate lotion 0.01% achieved a 50% reduction in IGA×BSA score by week 8. This efficacy compares well with results reported with apremilast in patients with moderate plaque psoriasis.20
Achieving clinically meaningful outcomes is an important aspect of disease management, especially in psoriasis with its disease burden and detriment to quality of life. It has been suggested that achieving a 75% or greater reduction from baseline IGA×BSA score (IGA×BSA-75) is an appropriate clinical goal.20 In our investigation, IGA×BSA-75 was achieved by 39% of participants treated with HP lotion by week 8, which again compares favorably with 35% of participants in the apremilast study who achieved IGA×BSA-75 at week 16.20
Physicians continue to have long-term safety concerns with TCSs,4,11,12 participants remain concerned about the risk for skin thinning,13 and product labelling restricts HP cream 0.05% consecutive use to 2 weeks. In clinical experience, HP cream 0.05% is well tolerated, with potential local AEs similar to those experienced with other superpotent TCSs. In short-term clinical trials, local AEs at the site of application were reported in up to 13% of patients21-26; itching, burning, or stinging were the most common local AEs (reported in 4.4% of patients).27
There were minimal safety concerns in our 2 studies using an 8-week, once-daily treatment regimen with HP lotion 0.01%. Local AEs at the application site were reported in less than 1% of participants. Baseline itching, dryness, and burning/stinging all improved with treatment.
CONCLUSION
Halobetasol propionate lotion 0.01% provides rapid improvement in disease severity. Halobetasol propionate lotion was consistently more effective than vehicle in achieving treatment success; reducing the BSA affected by the disease; reducing erythema, plaque elevation, and scaling at the target lesion; and improving IGA×BSA score over 8 weeks, which is a realistic time frame to see improvement in psoriasis with a topical steroid. There were minimal safety concerns with prolonged use. Halobetasol propionate lotion may provide an effective and reasonable treatment option in patients with moderate to severe plaque psoriasis.
Acknowledgment
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of this article. Ortho Dermatologics funded Mr. Bulley’s activities pertaining to this article.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25:535-546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8:1-12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759-764.
- Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157-178.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):61-67.
- Ersser SJ, Cowdell FC, Latter SM, et al. Self-management experiences in adults with mild-moderate psoriasis: an exploratory study and implications for improved support. Br J Dermatol. 2010;163:1044-1049.
- Choi CW, Kim BR, Ohn J, et al. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017;29:55-60.
- Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014;170:672-680.
- Spuls PI, Lecluse LL, Poulsen ML, et al. How good are clinical severity and outcome measures for psoriasis? quantitative evaluation in a systematic review. J Invest Dermatol. 2010;130:933-943.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bozek A, Reich A. The reliability of three psoriasis assessment tools: psoriasis area severity index, body surface area and physician global assessment. Adv Clin Exp Med. 2017;26:851-856.
- Walsh JA, McFadden M, Woodcock J, et al. Product of the Physician Global Assessment and body surface area: a simple static measure of psoriasis severity in a longitudinal cohort. J Am Acad Dermatol. 2013;69:931-937.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Duffin KC, Papp KA, Bagel J, et al. Evaluation of the Physician Global Assessment and body surface area composite tool for assessing psoriasis response to apremilast therapy: results from ESTEEM 1 and ESTEEM 2. J Drugs Dermatol. 2017;16:147-153.
- Chiesa Fuxench ZC, Callis DK, Siegel M, et al. Validity of the Simple Measure for Assessing Psoriasis Activity (S-MAPA) for objectively evaluating disease severity in patients with plaque psoriasis. J Am Acad Dermatol. 2015;73:868-870.
- Walsh J. Comparative assessment of PASI and variations of PGA×BSA as measures of psoriasis severity in a clinical trial of moderate to severe psoriasis [poster 1830]. Presented at: Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, CA.
- Gottlieb AB, Merola JF, Chen R, et al. Assessing clinical response and defining minimal disease activity in plaque psoriasis with the Physician Global Assessment and body surface area (PGA×BSA) composite tool: An analysis of apremilast phase 3 ESTEEM data. J Am Acad Dermatol. 2017;77:1178-1180.
- Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16:801-808.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Ultravate [package insert]. Jacksonville, FL: Ranbaxy; 2012.
Psoriasis Treatment in Patients With Sickle Cell Disease
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
Plaque psoriasis is a chronic inflammatory disease with a complex pathogenesis. Cutaneous dendritic cells drive the activation and proliferation of T cells with production of several immunomodulators, such as tumor necrosis factor (TNF) α, IL-17, IL-12, and IL-23. Because multiple systemic therapies are efficacious, treatment selection depends on side-effect profiles, availability, and patient preference. Activation of the TNF-α pathway is not unique to psoriasis. Tumor necrosis factor α plays a key role in multiple inflammatory conditions, including psoriatic arthritis, rheumatoid arthritis, and hidradenitis suppurativa. One study in mice demonstrated that TNF-α drives endothelial and vascular wall dysfunction in sickle cell anemia. In this study, use of the TNF-α blocker etanercept in mice with homozygous sickle cell anemia (HbSS) disease resulted in amelioration of TNF-mediated clinical features shared by sickle mice and humans.1
Sickle cell anemia is caused by a structural defect in hemoglobin that results in hemolysis and chronic anemia. The most common type of hemoglobin in adults without sickle cell anemia is HbAA. Homozygous sickle cell anemia patients carry 2 abnormal S alleles, whereas in sickle cell trait, patients carry both the S and normal A alleles (HbSA). Hemoglobin C is a structural variant of HbA that results in lower solubility in red blood cells. Patients with hemoglobin SC disease (HbSC) have S and C alleles.2 We present a case of a patient with moderate to severe plaque psoriasis and heterozygous sickle cell anemia treated with adalimumab.
Case Report
A 31-year-old woman presented with moderate to severe plaque psoriasis (70% body surface area) and HbSC. She reported chronic dull arthralgia in the ankles that was worse at night. Radiographs of the feet and ankles showed erosive changes of the distal tarsal row and metatarsal bases. The diffuse bone pain had gradually worsened over the years and was treated by hematology with ibuprofen and ketorolac. At presentation, her HbSC pain was 8/10 on a visual analog scale. She described her sickle cell pain crises as sharp 10/10 pain in the back, elbows, and ankles, associated with mild edema lasting 1 to 2 days. Radiographs of the spine, hands, and ankles were unremarkable.
Adalimumab was chosen as a systemic therapy for psoriasis based on the potential for improvement in HbSC. Within 17 weeks of starting adalimumab, the psoriasis body surface area decreased from 70% to 40%, and the HbSC pain decreased from 8/10 to 4/10 at 8-week follow-up and to 0/10 at 17-week follow-up. After initiation of adalimumab, she reported decreased use of pain medication with no sickle cell pain crises.
Comment
Tumor necrosis factor α blockers are commonly used for moderate to severe plaque psoriasis. To our knowledge, there have been no reported human studies showing TNF-α blockade as a potential treatment of sickle cell disease. Increased levels of TNF-α have been shown to contribute to the onset of sickle cell crises and severity of sickle cell disease by playing an integral role in the development of vascular wall dysfunction and ischemia.3 Inflammatory mediators in HbSS disease, such as heparan sulfate from the endothelial glycocalyx and heme from hemolysis, act on monocytes to release TNF-α.1 Through this effect on the endothelium, TNF-α impedes blood flow during sickle cell crisis, leading to worsening ischemia and resultant painful infarction.3 Analysis of cytokine levels in HbSS patients showed significantly (P<.05) elevated levels of TNF
Although these findings were observational and limited to a single patient, the 50% decrease in pain level and use of pain medications reported to her hematologist independent of her dermatology visits coincided with the initiation of adalimumab. Although radiographs showed possible psoriatic changes of the distal metatarsal row, her described sickle cell pain and pain crises were atypical for psoriatic arthralgia. Tumor necrosis factor α inhibitors could be the drug of choice to treat patients with psoriasis with concomitant HbSS or HbSC disease due to the blockade of a common inflammatory mediator. Further studies are indicated to analyze the in vivo role of TNF-α inhibition in sickle cell disease.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
- Solovey A, Somani A, Belcher JD, et al. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol. 2017;92:1119-1130.
- Mais DD. Diseases of red blood cells. In: Laposata M, ed. Laposata’s Laboratory Medicine: Diagnosis of Disease in the Clinical Laboratory. 3rd ed. New York, NY: McGraw-Hill; 2018:247-280.
- Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
Practice Points
• Tumor necrosis factor α contributes both to the vascular inflammatory state seen in sickle cell disease as well as the cycle of inflammation seen in the development of psoriasis.
• Tumor necrosis factor α inhibitors may be the drug of choice for patients with both psoriasis and sickle cell disease.
What’s New in Topical Treatments for Psoriasis
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.