User login
The Use of Immuno-Oncology Treatments in the VA (FULL)
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
The following is a lightly edited transcript of a teleconference discussion recorded in April 2018.
Suman Kambhampati, MD. Immuno-oncology is a paradigm-shifting treatment approach. It is an easy-to-understand term for both providers and for patients. The underlying principle is that the body’s own immune system is used or stimulated to fight cancer, and there are drugs that clearly have shown huge promise for this, not only in oncology, but also for other diseases. Time will tell whether that really pans out or not, but to begin with, the emphasis has been inoncology, and therefore, the term immunooncology is fitting.
Dr. Kaster. It was encouraging at first, especially when ipilimumab came out, to see the effects on patients with melanoma. Then the KEYNOTE-024 trial came out, and we were able to jump in anduse monoclonal antibodies directed against programmed death 1 (PD-1) in the first line, which is when things got exciting.1 We have a smaller populationin Boise, so PD-1s in lung cancer have had the biggest impact on our patients so far.
Ellen Nason, RN, MSN. Patients are open to immunotherapies.They’re excited about it. And as the other panelists have said, you can start broadly, as the body fights the cancer on its own, to providing more specific details as a patient wants more information. Immuno-oncology is definitely accepted by patients, and they’re very excited about it, especially with all the news about new therapies.
Dr. Kambhampati. For the Department of Veteran Affairs (VA) population, lung cancer has seen significant impact, and now it’s translating into other diseases through more research, trials, and better understanding about how these drugs are used and work.
The paradigm is shifting toward offering these drugs not only in metastatic cancers, but also in the surgically resectable tumors. The 2018 American Association for Cancer Research (AACR) meeting, just concluded. At the meeting several abstracts reported instances where immunooncology drugs are being introduced in the early phases of lung cancer and showing outstanding results. It’s very much possible that we’re going to see less use of traditional chemotherapy in the near future.
Ms. Nason. I primarily work with solid tumors,and the majority of the population I work with have lung cancer. So we’re excited about some of the results that we’ve seen and the lower toxicity involved. Recently, we’ve begun using durvalumab with patients with stage III disease. We have about 5 people now that are using it as a maintenance or consolidative treatment vs just using it for patients with stage IV disease. Hopefully, we’ll see some of the same results describedin the paper published on it.2
Dr. Kaster. Yes, we are incorporating these new changes into care as they're coming out. As Ms. Nason mentioned, we're already using immunotherapies in earlier settings, and we are seeing as much research that could be translated into care soon, like combining immunotherapies
in first-line settings, as we see in the Checkmate-227 study with nivolumab and ipilimumab.3,4 The landscape is going to change dramatically in the next couple of years.
Accessing Testing For First-Line Treatments
Dr. Lynch. There has been an ongoing discussionin the literature on accessing appropriate testing—delays in testing can result in patients who are not able to access the best targeted drugs on a first-line basis. The drug companiesand the VA have become highly sensitized to ensuring that veterans are accessing the appropriate testing. We are expanding the capability of VA labs to do that testing.
Ms. Nason. I want to put in a plug for the VA Precision Oncology Program (POP). It’s about 2 years into its existence, and Neil Spector, MD, is the director. The POP pays for sequencing the tumor samples.
A new sequencing contract will go into effect October 2018 and will include sequencing for hematologic malignancies in addition to the current testing of solid tumors. Patients from New York who have been unable to receive testing through the current vendors used by POP, will be included in the new contract. It is important to note that POP is working closely with the National Pharmacy Benefit Management Service (PBM) to develop a policy for approving off-label use of US Food and Drug Administration-approved targeted therapies based on sequenced data collected on patients tested through POP.
In addition, the leadership of POP is working to leverage the molecular testing results conducted through POP to improve veterans' access to clinical trials, both inside and outside the VA. Within the VA people can access information at tinyurl.com/precisiononcology. There is no reason why any eligible patient with cancer in the VA health care system should not have their tumor tissue sequenced through POP, particularly once the new contract goes into effect.
Dr. Lynch. Fortunately, the cost of next-generation sequencing has come down so much that most VA contracted reference laboratories offer next-generation sequencing, including LabCorp (Burlington,NC), Quest Diagnostics (Secaucus, NJ), Fulgent (Temple City, CA), and academic partners such as Oregon Health Sciences University and University of Washington.
Ms. Nason. At the Durham VAMC, sometimes a lack of tissue has been a barrier, but we now have the ability to send blood (liquid biopsy) for next-generation sequencing. Hopefully that will open up options for veterans with inadequate tissue. Importantly, all VA facilities can request liquid biopsiesthrough POP.
Dr. Lynch. That’s an important point. There have been huge advances in liquid biopsy testing.The VA Salt Lake City Health Care System (VASLCHCS) was in talks with Genomic Health (Redwood City, CA) to do a study as part of clinical operations to look at the concordance between the liquid biopsy testing and the precision oncology data. But Genomic Health eventually abandoned its liquid biopsy testing. Currently, the VA is only reimbursing or encouraging liquid biopsy if the tissue is not available or if the veteran has too high a level of comorbidities to undergo tissue biopsy. The main point for the discussion today is that access to testing is a key component of access to all of these advanced drugs.
Dr. Kambhampati. The precision medicine piece will be a game changer—no question about that. Liquid biopsy is very timely. Many patients have difficulty getting rebiopsied, so liquid biopsy is definitely a big, big step forward.
Still, there has not been consistency across the VA as there should be. Perhaps there are a few select centers, including our site in Kansas City, where access to precision medicine is readily available and liquid biopsies are available. We use the PlasmaSELECT test from Personal Genome Diagnostics (Baltimore, MD). We have just added Foundation Medicine (Cambridge, MA) also in hematology. Access to mutational profilingis absolutely a must for precision medicine.
All that being said, the unique issue with immuno-oncology is that it pretty much transcends the mutational profile and perhaps has leveled the playing field, irrespective of the tumor mutation profile or burden. In some solid tumors these immuno-oncology drugs have been shown to work across tumor types and across different mutation types. And there is a hint now in the recent data presented at AACR and in the New England Journalof Medicine showing that the tumor mutational burden is a predictor of pathologic response to at least PD-1 blockade in the resectable stages of lung cancer.1,3 To me, that’s a very important piece of data because that’s something that can be tested and can have a prognostic impact in immuno-oncology, particularly in the early stages of lung cancer and is further proof of the broad value of immunotherapics in targeting tumors irrespective of the precise tumor targets.
Dr. Kaster. Yes, it’s nice to see other options like tumor mutational burden and Lung Immune Prognostic Index being studied.5 It would be nice if we could rely a little more on these, and not PD-L1, which as we all know is a variable and an unreliable target.
Dr. Kambhampati. I agree.
Rural Challenges In A Veterans Population
Dr. Lynch. Providing high-quality cancer care to rural veterans care can be a challenge but it is a VA priority. The VA National Genomic Medicine Services offers better access for rural veterans to germline genetic testing than any other healthcare system in the country. In terms of access to somatic testing and next-generation sequencing, we are working toward providing the same level of cancer care as patients would receive at National Cancer Institute (NCI) cancer centers. The VA oncology leadership has done teleconsults and virtual tumor boards, but for some rural VAMCs, fellowsare leading the clinical care. As we expand use of oral agents for oncology treatment, it will be easier to ensure that rural veterans receive the same standard of care for POP that veterans being cared for at VASLCHCS, Kansas City VAMC, or Durham VAMC get.
Dr. Kambhampati. The Kansas City VAMC in its catchment area includes underserved areas, such as Topeka and Leavenworth, Kansas. What we’ve been able to do here is something that’s unique—Kansas City VAMC is the only standalone VA in the country to be recognized as a primary SWOG (Southwestern Oncology Group) institution, which provides access to many trials, such as the Lung-MAP trial and others. And that has allowed us to use the full expanse of precision medicine without financial barriers. The research has helped us improve the standard of
care for patients across VISN 15.
Dr. Lynch. In precision oncology, the chief of pathology is an important figure in access to advanced care. I’ve worked with Sharad Mathur,MD, of the Kansas City VAMC on many clinical trials. He’s on the Kansas City VAMC Institutional Review Board and the cancer committee and is tuned in to veterans’ access to precision oncology. Kansas City was ordering Foundation One for select patients that met the criteria probably sooner than any other VA and participated in NCI Cooperative Group clinical trials. It is a great example of how veterans are getting access to
the same level of care as are patients who gettreated at NCI partners.
Comorbidities
Dr. Kambhampati. I don’t treat a lot of patients with lung cancer, but I find it easier to use these immuno-oncology drugs than platinums and etoposide. I consider them absolutely nasty chemotherapy drugs now in this era of immuno-oncology and targeted therapy.
Dr. Lynch. The VA is very important in translational lung cancer research and clinical care. It used to be thought that African American patients don’t get epidermal growth factor receptor mutations. And that’s because not enough African American patients with lung cancer were included in the NCI-based clinical trial.There are7,000 veterans who get lung cancer each year, and 20% to 25% of those are African Americans. Prevalence of various mutations and the pharmacogenetics of some of these drugs differ by patient ancestry. Including veterans with lung
cancer in precision oncology clinical trials and clinical care is not just a priority for the VA but a priority for NCI and internationally. I can’t emphasize this enough—veterans with lung cancer should be included in these studies and should be getting the same level of care that our partners are getting at NCI cancer centers. In the VA we’re positioned to do this because of our nationalelectronic health record (EHR) and becauseof our ability to identify patients with specific variants and enroll them in clinical trials.
Ms. Nason. One of the barriers that I find withsome of the patients that I have treated is getting them to a trial. If the trial isn’t available locally, specifically there are socioeconomic and distance issues that are hard to overcome.
Dr. Kaster. For smaller medical centers, getting patients to clinical trials can be difficult. The Boise VAMC is putting together a proposal now to justify hiring a research pharmacist in order to get trials atour site. The goal is to offer trial participation to our patients who otherwise might not be able to participate while offsetting some of the costs of immunotherapy. We are trying to make what could be a negative into a positive.
Measuring Success
Dr. Kambhampati. Unfortunately, we do not have any calculators to incorporate the quality of lives saved to the society. I know there are clearmetrics in transplant and in hematology, but unfortunately, there are no established metrics in solid tumor treatment that allow us to predict the cost savings to the health care system or to society or the benefit to the society. I don’t use any such predictive models or metrics in my decision making. These decisions are made based on existing evidence, and the existing evidence overwhelmingly supports use of immuno-oncology in certain types of solid tumors and in a select group of hematologic malignancies.
Dr. Kaster. This is where you can get more bang for your buck with an oncology pharmacist these days. A pharmacist can make a minor dosing change that will allow the same benefit for the patient, but could equal tens of thousands of dollars in cost-benefit for the VA. They can also be the second set of eyes when adjudicating a nonformulary request to ensure that a patient will benefit.
Dr. Lynch. Inappropriate prescribing is far more expensive than appropriate treatment. And the care for veterans whose long-term health outcomes could be improved by the new immunotherapies. It’s cheaper for veterans to be healthy and live longer than it is to take care of them in
their last 6 weeks of life. Unfortunately, there are not a lot of studies that have demonstrated that empirically, but I think it’s important to do those studies.
Role of Pharmacists
Dr. Lynch. I was at a meeting recently talking about how to improve veteran access to clinical trials. Francesca Cunningham, PharmD, director of the VA Center for Medication Safety of the VA Pharmacy Benefit Management Service (PBM) described the commitment that pharmacy has in taking a leadership role in the integration of precision medicine. Linking veterans’ tumor mutation status and pharmacogenetic variants to pharmacy databases is the best way to ensure treatment is informed by genetics. We have to be realistic about what we’re asking community oncologists to do. With the onset of precision oncology, 10 cancers have become really 100 cancers. In the prior model of care, it was the oncologist, maybe in collaboration with a pathologist, but it was mostly oncologists who determined care.
And in the evolution of precision oncology, Ithink that it’s become an interdisciplinary adventure. Pharmacy is going to play an increasinglyimportant role in precision medicine around all of the molecular alterations, even immuno-oncology regardless of molecular status in which the VA has an advantage. We’re not talking about some community pharmacist. We’re talking about a national health care system where there’s a national EHR, where there’s national PBM systems. So my thoughts on this aspect is that it’s an intricate multidisciplinary team who can ensure that veteran sget the best care possible: the best most cost-effective care possible.
Dr. Kaster. As an oncology pharmacist, I have to second that.
Ms. Nason. As Dr. Kaster said earlier, having a dedicated oncology pharmacist is tremendouslybeneficial. The oncology/hematology pharmacists are following the patients closely and notice when dose adjustments need to be made, optimizing the drug benefit and providing additional safety. Not to mention the cost benefit that can be realized with appropriate adjustment and the expertise they bring to managing possible interactionsand pharmacodynamics.
Dr. Kambhampati. To brag about the Kansas City VAMC program, we have published in Federal Practitioner our best practices showing the collaboration between a pharmacist and providers.6 And we have used several examples of cost savings, which have basically helped us build the research program, and several examples of dual monitoring oral chemotherapy monitoring. And we have created these templates within the EHR that allow everyone to get a quick snapshot of where things are, what needs to be done, and what needs to be monitored.
Now, we are taking it a step further to determine when to stop chemotherapy or when to stop treatments. For example, for chronic myeloid leukemia (CML), there are good data onstopping tyrosine kinase inhibitors.7 And that alone, if implemented across the VA, could bring
in huge cost savings, which perhaps could be put into investments in immuno-oncology or other efforts. We have several examples here that we have published, and we continue to increaseand strengthen our collaboration withour oncology pharmacist. We are very lucky and privileged to have a dedicated oncology pharmacistfor clinics and for research.
Dr. Lynch. The example of CML is perfect, because precision oncology has increased the complexity of care substantially. The VA is wellpositioned to be a leader in this area when care becomes this complex because of its ability to measure access to testing, to translate the results
of testing to pharmacy, to have pharmacists take the lead on prescribing, to have pathologists take the lead on molecular alterations, and to have oncologists take the lead on delivering the cancer care to the patients.
With hematologic malignancies, adherence in the early stages can result in patients getting offcare sooner, which is cost savings. But that requires access to testing, monitoring that testing, and working in partnership with pharmacy. This is a great story about how the VA is positioned to lead in this area of care.
Dr. Kaster. I would like to put a plug in for advanced practice providers and the use of nurse practitioners (NPs) and physician assistants (PAs).The VA is well positioned because it often has established interdisciplinary teams with these providers, pharmacy, nursing, and often social work, to coordinate the care and manage symptoms outside of oncologist visits.
Dr. Lynch. In the NCI cancer center model, once the patient has become stable, the ongoing careis designated to the NP or PA. Then as soon as there’s a change and it requires reevaluation, the oncologist becomes involved again. That pointabout the oncology treatment team is totally in line
with some of the previous comments.
Areas For Further Investigation
Dr. Kaster. There are so many nuances that we’re finding out all of the time about immunotherapies. A recent study brought up the role of antibiotics in the 30 or possibly 60 days prior to immunotherapy.3 How does that change treatment? Which patients are more likely to benefit from immunotherapies, and which are susceptible to “hyperprogression”? How do we integrate palliative care discussions into the carenow that patients are feeling better on treatment and may be less likely to want to discuss palliative care?
Ms. Nason. I absolutely agree with that, especially keeping palliative care integrated within our services. Our focus is now a little different, in thatwe have more optimistic outcomes in mind, butthere still are symptoms and issues where our colleaguesin palliative care are invaluable.
Dr. Lynch. I third that motion. What I would really like to see come out of this discussion is how veterans are getting access to leading oncology care. We just published an analysis of Medicare data and access to EGFR testing. The result of that analysis showed that testing in the VA was consistent with testing in Medicare.
For palliative care, I think the VA does a better job. And it’s just so discouraging as VA employees and as clinicians treating veterans to see publicationsthat suggest that veterans are getting a lower quality of care and that they would be better if care was privatized or outsourced. It’s just fundamentally not the case.
In CML, we see it. We’ve analyzed the data, in that there’s a far lower number of patients with CML who are included in the registry because patients who are diagnosed outside the VA are incorporated in other cancer registries.8 But as soon as their copays increase for access to targeted drugs, they immediately activate their VA benefits so that theycan get their drugs at the VA. For hematologic malignancies that are diagnosed outside the VA and are captured in other cancer registries, as soon as the drugs become expensive, they start getting their care in the VA. I don’t think there’s beena lot of empirical research that’s shown this, but we have the data to illustrate this trend. I hope thatthere are more publications that show that veterans with cancer are getting really good care inside the VA in the existing VA health care system.
Ms. Nason. It is disheartening to see negativepublicity, knowing that I work with colleagues who are strongly committed to providing up-to-date and relevant oncology care.
Dr. Lynch. As we record this conversation, I am in Rotterdam, Netherlands, in a meeting about genomewide testing. In hematologic malignancies, prostate cancer, and breast cancer, it’s a huge issue. And that is the other area that MVP (Million Veteran Program) is leading the way with the MVP biorepository data. Frankly, there’s no other biorepository that has this many patients, that has so many African Americans, and that has such rich EHR data. So inthat other area, the VA is doing really well.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.
1. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab vs chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
2. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non–smallcell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
3. Hellmann MD, Ciuleanu T-E, Pluzansk A, et al. Nivolumab plus ipilimumab in Lung Cancer with a high tumor mutational burden. N Engl J Med. 2018 April 16. [Epub ahead of print.]
4. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate214 Investigators. Nivolumab plus ipilimumab versus sunitinibin advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
5. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small cell
lung cancer. Ann Oncol. 2018 March 30. [Epub ahead of print.]
6. Heinrichs A, Dessars B, El Housni H, et al. Identification of chronic myeloid leukemia patients treated with imatinib who are potentially eligible for treatment discontinuation by assessingreal-life molecular responses on the international scale in a EUTOS-certified lab. Leuk Res. 2018;67:27-31.
7. Keefe S, Kambhampati S, Powers B. An electronic chemotherapy ordering process and template. Fed Pract. 2015;32(suppl 1):21S-25S.
8. Lynch JA, Berse B, Rabb M, et al. Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010 - 2013. BMC Cancer. 2018;18(1):306.
Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants (FULL)
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
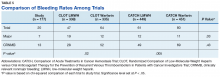
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
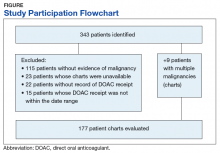
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
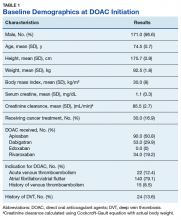
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
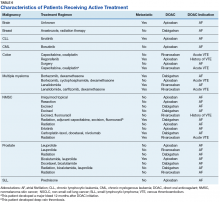
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
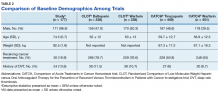
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
A National WestlawNext Database Analysis of Malpractice Litigation in Radiation Oncology (FULL)
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.


Click here to read the digital edition.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.


Click here to read the digital edition.
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.


Click here to read the digital edition.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules (FULL)
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
Although historically associated with a low risk of malignancy, hyperthyroidism is no longer thought to be protective against the occurrence of thyroid cancer. The incidence of malignancy has been reported in Graves disease at 2% and as high as 9% in toxic multinodular goiters.1,2
In evaluating patients with thyroid nodules and low thyroid stimulating hormone (TSH), which may indicate hyperthyroidism, the American Thyroid Association (ATA) recommends a radioiodine thyroid scan to determine whether a thyroid nodule is autonomous (hot) or nonfunctional (cold).3 Hot thyroid nodules are nodular areas of hyperfunctioning activity on radioiodine scan where tracer uptake is greater than the surrounding normal thyroid.
Historically, hot nodules have been associated with a low risk of malignancy and typically did not receive further ultrasound evaluation. However, recent studies have documented that the incidence of thyroid cancer in hot nodules may be underestimated. Mirfakhraee and colleagues performed a literature review in 2013 that revealed the prevalence of thyroid carcinoma in hot nodules managed by thyroidectomy ranged from 0% to 12.5% and averaged 3.1%.4 These findings may underestimate the prevalence of malignancy, because most hot nodules are not managed by thyroidectomy.
Given findings of hot nodules harboring malignancy, the authors investigated the role of thyroid ultrasound in patients with hyperthyroidism to identify suspicious features concerning for possible malignancies. The study objective was to estimate the prevalence of hot nodules with sonographic features concerning for malignancy in patients with hyperthyroidism in a Department of Veterans Affairs (VA) health care system.
Methods
This retrospective chart review consisted of 149,549 patients seen between January 2010 and December 2015 at the VA Northern California Health Care System (VANCHCS). The institutional review board approved the study and informed consent was waived.
Seven hundred sixty veterans were identified in the Computerized Patient Record System (CPRS) using the following ICD-9 codes: 242.9 (hyperthyroidism), 242.2 (toxic multinodular goiter), 242.3 (toxic nodular goiter), 242.1 (toxic uninodular goiter), and 241.9 (adenomatous goiter) (Figure 1).
Manual review of thyroid ultrasound scans for suspicious characteristics concerning for thyroid carcinoma were based on the 2015 ATA Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer.3 Per the ATA guidelines, sonographic patterns that are highly suspicious for malignancy were solid hypoechoic nodule or solid hypoechoic component of a partially cystic nodule with one or more of the following features: irregular margins (infiltrative, microlobulated), microcalcifications, taller than wide on transverse view, and rim calcifications with small extrusive soft-tissue component. Sonographic patterns with intermediate suspicion were hypoechoic solid nodule with smooth margins without microcalcifications, extrathyroidal extension, or taller than wide shape.3
Results
Of the 760 identified veterans, 230 had thyroid ultrasounds, and 113 had radioiodine thyroid scans. Of these, 70 patients had both ultrasound and radioiodine thyroid scans. This cohort consisted of 84.3% (59) males and 15.7% women (11). Ages ranged from 32 to 93 (mean age 62.9) years.
A total of 121 nodules were identified among the remaining 43 patients (11 individuals with cold thyroid scans and 16 individuals with no nodules were excluded). Of the 121 nodules, 44 were hot nodules, 29 were coexisting nodules found in patients with hot nodules, and 48 were other nodules found in patients without coexisting hot nodules (Figure 2).
Of the 44 hot nodules, the analysis identified 16 hot nodules with suspicious features on ultrasound and 28 nodules without suspicious findings. Breakdown of specific suspicious features included 11 that were solid hypoechoic, 3 nodules that had microcalcifications, and 2 nodules that had both characteristics (Table).
Twelve patients had hot nodules with suspicious ultrasound findings. Of this group, 6 patients had no further workup, 1 patient was lost to follow-up, and 1 patient was planned for fine needle aspiration (FNA) biopsy. Four patients underwent FNA, and all results were benign.
Discussion
Although most veterans identified with hyperthyroidism did not undergo imaging studies, of those who did, a remarkable number had unexpected ultrasonographically suspicious nodules. Of the 44 hot nodules identified on radioiodine studies, 16 had suspicious ultrasound findings that raised concern for malignancy based on the most recent ATA guidelines. In contrast to recent studies that have suggested an increased incidence of thyroid carcinoma in hot nodules, no cancers were detected in this cohort.4 However, only 4 patients in this study underwent FNA.
Worth noting is that the most common suspicious feature found in this study’s cohort was hypoechoic solid nodules, which is a feature that has a sensitivity of 81% however a low specificity of 53% in detecting thyroid malignancy.5 This appearance also is found in 55% of benign thyroid nodules.6 The overlap of hypoechoic nodules as a feature in both benign and malignant thyroid nodules can present as a diagnostic challenge in differentiating between the two.
The 2015 ATA guideline recommends that low TSH warrants a radioiodine scan, and FNA should be considered for isofunctioning or nonfunctioning nodules with suspicious sonographic features. Hot nodules found on scintigraphy need no further cytologic evaluation because they are mostly benign.3 There is no clear stance on the use of ultrasound in hot nodules.
The answer to whether patients with hot nodules should undergo ultrasound still remains unclear. This study showed a surprising number of hot nodules with worrisome architecture found on ultrasound. However, whether that correlates to actual malignant findings remains unknown as most individuals in the cohort did not undergo biopsy. Also, given the high prevalence of suspicious findings, it may be difficult to use ultrasound as a diagnostic tool in patients with hot nodules as false positives may lead to unnecessary interventions such as biopsy.
Limitations
The patient population consisted mostly of men (84.3%) and cannot be applied to the general population. Thyroid nodules are 4 times more common in women than they are in men.7 Another limitation was the lack of data on patients’ radiation exposure while in military service or as civilians. Finally, as a retrospective study, there was unavoidable selection bias.
Conclusion
The prevalence of suspicious findings concerning for malignancy in hot nodules was 36.3% (16/44) based on the 2015 ATA guidelines. This study’s preliminary observation suggests that although ultrasound is a noninvasive and relatively inexpensive diagnostic modality, it has a limited role in the evaluation of hot nodules given the high prevalence of suspicious findings. Clinicians may still consider its use in patients who also have high-risk historic features. This was a thought-generating, retrospective study, and further prospective studies in larger populations are needed to validate the study’s results.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
1. Stocker DJ, Burch HB. Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol. 2003;28(3):205-212.
2. Cerci C, Cerci SS, Eroglu E, et al. Thyroid cancer in toxic and non-toxic multinodular goiter. J Postgrad Med. 2007;53(3):157-160.
3. Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
4. Mirfakhraee S, Mathews D, Peng L, Woodruff S, Zigman JM. A solitary hyperfunctioning thyroid nodule harboring thyroid carcinoma: review of the literature. Thyroid Res. 2013;6(1):7.
5. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-1946.
6. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553-559.
7. Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37(2):401-417.
FDA grants BI-1206 orphan designation for MCL
The Food and Drug Administration has granted orphan designation to BI-1206 for the treatment of mantle cell lymphoma (MCL).
BI-1206 is a monoclonal antibody being developed by BioInvent International.
The company says BI-1206 works by inhibiting FcgRIIB (CD32B), which is associated with poor prognosis in MCL and other non-Hodgkin lymphomas. By inhibiting FcgRIIB, BI-1206 is expected to enhance the activity of rituximab or other anti-CD20 monoclonal antibodies.
BioInvent is conducting a phase 1/2a study (NCT03571568) of BI-1206 in combination with rituximab in patients with indolent, relapsed/refractory B-cell non-Hodgkin lymphomas, including MCL. The first patient began receiving treatment with BI-1206 in September 2018.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases or disorders that affect fewer than 200,000 people in the United States. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The Food and Drug Administration has granted orphan designation to BI-1206 for the treatment of mantle cell lymphoma (MCL).
BI-1206 is a monoclonal antibody being developed by BioInvent International.
The company says BI-1206 works by inhibiting FcgRIIB (CD32B), which is associated with poor prognosis in MCL and other non-Hodgkin lymphomas. By inhibiting FcgRIIB, BI-1206 is expected to enhance the activity of rituximab or other anti-CD20 monoclonal antibodies.
BioInvent is conducting a phase 1/2a study (NCT03571568) of BI-1206 in combination with rituximab in patients with indolent, relapsed/refractory B-cell non-Hodgkin lymphomas, including MCL. The first patient began receiving treatment with BI-1206 in September 2018.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases or disorders that affect fewer than 200,000 people in the United States. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The Food and Drug Administration has granted orphan designation to BI-1206 for the treatment of mantle cell lymphoma (MCL).
BI-1206 is a monoclonal antibody being developed by BioInvent International.
The company says BI-1206 works by inhibiting FcgRIIB (CD32B), which is associated with poor prognosis in MCL and other non-Hodgkin lymphomas. By inhibiting FcgRIIB, BI-1206 is expected to enhance the activity of rituximab or other anti-CD20 monoclonal antibodies.
BioInvent is conducting a phase 1/2a study (NCT03571568) of BI-1206 in combination with rituximab in patients with indolent, relapsed/refractory B-cell non-Hodgkin lymphomas, including MCL. The first patient began receiving treatment with BI-1206 in September 2018.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases or disorders that affect fewer than 200,000 people in the United States. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
Mohs Micrographic Surgery in the VHA (FULL)
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Clinical benefits persist 5 years after thymectomy for myasthenia gravis
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
FROM LANCET NEUROLOGY
Key clinical point: The benefits of thymectomy for myasthenia gravis persist 5 years after the procedure.
Major finding: Patients who undergo thymectomy and receive prednisone have lower time-weighted average Quantitative Myasthenia Gravis scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who receive prednisone alone.
Study details: A rater-blinded 2-year extension study that enrolled 68 patients who had completed a 3-year randomized controlled trial.
Disclosures: The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Other authors reported working with and receiving funds from various agencies, foundations, and pharmaceutical companies.
Source: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
FDA approves ibrutinib plus obinutuzumab for CLL/SLL
The Food and Drug Administration has approved ibrutinib (Imbruvica) for use in combination with obinutuzumab to treat adults with previously untreated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
This is the tenth FDA approval for ibrutinib, a Bruton tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics, an AbbVie company, and Janssen Biotech.
The approval is supported by the phase 3 iLLUMINATE trial (NCT02264574).
Results from this study were recently presented at the annual meeting of the American Society of Hematology (Blood. 2018;132:691) and published in the Lancet Oncology (2019 Jan;20[1]:43-56).
The iLLUMINATE trial enrolled newly diagnosed CLL patients who were randomized to receive ibrutinib plus obinutuzumab (n = 113) or chlorambucil plus obinutuzumab (n = 116).
The median follow-up was 31.3 months. The overall response rate was 88% in the ibrutinib arm and 73% in the chlorambucil arm. The complete response rate, including complete response with incomplete marrow recovery, was 19% and 8%, respectively.
The median progression-free survival was not reached in the ibrutinib arm and was 19.0 months in the chlorambucil arm (hazard ratio, 0.23; 95% confidence interval, 0.15-0.37; P less than .0001). The estimated 30-month progression-free survival was 79% and 31%, respectively.
The most common grade 3/4 adverse events in both arms were neutropenia (36% in the ibrutinib arm and 46% in the chlorambucil arm) and thrombocytopenia (19% and 10%, respectively).
There were 10 deaths caused by adverse events in the ibrutinib arm and 3 in the chlorambucil arm. One death was considered possibly related to ibrutinib (sudden death), and another was considered possibly related to chlorambucil (neuroendocrine carcinoma of the skin).
The Food and Drug Administration has approved ibrutinib (Imbruvica) for use in combination with obinutuzumab to treat adults with previously untreated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
This is the tenth FDA approval for ibrutinib, a Bruton tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics, an AbbVie company, and Janssen Biotech.
The approval is supported by the phase 3 iLLUMINATE trial (NCT02264574).
Results from this study were recently presented at the annual meeting of the American Society of Hematology (Blood. 2018;132:691) and published in the Lancet Oncology (2019 Jan;20[1]:43-56).
The iLLUMINATE trial enrolled newly diagnosed CLL patients who were randomized to receive ibrutinib plus obinutuzumab (n = 113) or chlorambucil plus obinutuzumab (n = 116).
The median follow-up was 31.3 months. The overall response rate was 88% in the ibrutinib arm and 73% in the chlorambucil arm. The complete response rate, including complete response with incomplete marrow recovery, was 19% and 8%, respectively.
The median progression-free survival was not reached in the ibrutinib arm and was 19.0 months in the chlorambucil arm (hazard ratio, 0.23; 95% confidence interval, 0.15-0.37; P less than .0001). The estimated 30-month progression-free survival was 79% and 31%, respectively.
The most common grade 3/4 adverse events in both arms were neutropenia (36% in the ibrutinib arm and 46% in the chlorambucil arm) and thrombocytopenia (19% and 10%, respectively).
There were 10 deaths caused by adverse events in the ibrutinib arm and 3 in the chlorambucil arm. One death was considered possibly related to ibrutinib (sudden death), and another was considered possibly related to chlorambucil (neuroendocrine carcinoma of the skin).
The Food and Drug Administration has approved ibrutinib (Imbruvica) for use in combination with obinutuzumab to treat adults with previously untreated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
This is the tenth FDA approval for ibrutinib, a Bruton tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics, an AbbVie company, and Janssen Biotech.
The approval is supported by the phase 3 iLLUMINATE trial (NCT02264574).
Results from this study were recently presented at the annual meeting of the American Society of Hematology (Blood. 2018;132:691) and published in the Lancet Oncology (2019 Jan;20[1]:43-56).
The iLLUMINATE trial enrolled newly diagnosed CLL patients who were randomized to receive ibrutinib plus obinutuzumab (n = 113) or chlorambucil plus obinutuzumab (n = 116).
The median follow-up was 31.3 months. The overall response rate was 88% in the ibrutinib arm and 73% in the chlorambucil arm. The complete response rate, including complete response with incomplete marrow recovery, was 19% and 8%, respectively.
The median progression-free survival was not reached in the ibrutinib arm and was 19.0 months in the chlorambucil arm (hazard ratio, 0.23; 95% confidence interval, 0.15-0.37; P less than .0001). The estimated 30-month progression-free survival was 79% and 31%, respectively.
The most common grade 3/4 adverse events in both arms were neutropenia (36% in the ibrutinib arm and 46% in the chlorambucil arm) and thrombocytopenia (19% and 10%, respectively).
There were 10 deaths caused by adverse events in the ibrutinib arm and 3 in the chlorambucil arm. One death was considered possibly related to ibrutinib (sudden death), and another was considered possibly related to chlorambucil (neuroendocrine carcinoma of the skin).
Novel bispecific CAR shows promise in B-cell malignancies
SAN DIEGO – A chimeric antigen receptor (CAR) targeting both CD19 and CD22 shows promising safety and efficacy for the treatment of relapsed or refractory B-cell malignancies in adults, according to early findings from a phase 1 trial of the novel bispecific CAR.
Of six patients with diffuse large B-cell lymphoma (DLBCL) and two patients with B-cell acute lymphoblastic leukemia (B-ALL) enrolled in the single-institution dose escalation study and available for safety analysis after the bispecific CAR T-cell infusion, five developed reversible grade 1 cytokine release syndrome (CRS) and one developed grade 2 CRS requiring treatment with tocilizumab, Nasheed Hossain, MD, reported at the annual meeting of the American Society of Hematology.
Additionally, two patients developed grade 1 neurotoxicity, and one developed grade 2 neurotoxicity requiring treatment with dexamethasone.
“But no dose-limiting toxicities have been encountered thus far,” said Dr. Hossain of Loyola University Medical Center, Chicago. “With regard to efficacy, the DLBCL overall response rate is 60%, with 1 [complete response] and 2 [partial responses] at day 28 and day 90, and the ALL overall response rate is 100%, with 1 CR and 1 PR at day 28.
“With longer follow-up, five patients have relapsed and biopsies at the time of progression all showed ongoing CD19 expression,” he said, adding that all enrolled patients are alive, except for one patient who died from disease progression.
Study participants were adults aged 35-75 years with DLBCL or B-ALL that was refractory to standard therapies.
“Our primary objectives are twofold: One is to determine the feasibility of making our CAR ... and [the other] is to assess the safety using an escalating CAR dose following standard cyclophosphamide/fludarabine conditioning,” Dr. Hossain said.
The dose assessed in the current analysis was 1 x 106 CAR T cells/kg; other planned doses include 3 x 106 CAR T cells/kg and 1 x 107 CAR T cells/kg, he said.
All patients underwent lymphodepletion with cyclophosphamide (500 mg/m2 daily x 3 doses) and fludarabine (30 mg/m2 daily x 3 doses) followed by CAR T-cell infusion 2 days later.
The findings of this ongoing study – the first in-human study of a bispecific loop CAR in the United States – suggest that the novel CAR has low toxicity and promising efficacy, Dr. Hossain said.
Currently approved therapies target CD19 alone, he said, noting that they all use the same anti-CD19 domain, but different costimulatory domains, and have good clinical efficacy of greater than 70% CRs in ALL and up to 52% CRs in DLBCL.
“But questions remain about determining the durability of response and the causes of therapy failure,” he said.
One common cause of treatment failure is CD19 antigen loss, and efforts to reduce such antigen loss using bispecific loop CARs targeting both CD19 and CD22 have shown promise. The CAR construct evaluated in this study was developed to target CD19 and CD22 with intracellular signaling domains incorporating 4-1BB and CD3-zeta to overcome CD19 immune escape.
“We have now escalated the dose to 3 x 106 CAR T cells/kg ... and an expansion study of 60 patients will follow,” Dr. Hossain said.
A companion phase 1 pediatric trial using the same CAR construct is also underway, with preliminary data presented at the ASH meeting demonstrating safety and tolerability in children with relapsed or refractory B-cell ALL.
Dr. Hossain reported having no financial disclosures.
SOURCE: Hossain N et al. ASH 2018, Abstract 490.
SAN DIEGO – A chimeric antigen receptor (CAR) targeting both CD19 and CD22 shows promising safety and efficacy for the treatment of relapsed or refractory B-cell malignancies in adults, according to early findings from a phase 1 trial of the novel bispecific CAR.
Of six patients with diffuse large B-cell lymphoma (DLBCL) and two patients with B-cell acute lymphoblastic leukemia (B-ALL) enrolled in the single-institution dose escalation study and available for safety analysis after the bispecific CAR T-cell infusion, five developed reversible grade 1 cytokine release syndrome (CRS) and one developed grade 2 CRS requiring treatment with tocilizumab, Nasheed Hossain, MD, reported at the annual meeting of the American Society of Hematology.
Additionally, two patients developed grade 1 neurotoxicity, and one developed grade 2 neurotoxicity requiring treatment with dexamethasone.
“But no dose-limiting toxicities have been encountered thus far,” said Dr. Hossain of Loyola University Medical Center, Chicago. “With regard to efficacy, the DLBCL overall response rate is 60%, with 1 [complete response] and 2 [partial responses] at day 28 and day 90, and the ALL overall response rate is 100%, with 1 CR and 1 PR at day 28.
“With longer follow-up, five patients have relapsed and biopsies at the time of progression all showed ongoing CD19 expression,” he said, adding that all enrolled patients are alive, except for one patient who died from disease progression.
Study participants were adults aged 35-75 years with DLBCL or B-ALL that was refractory to standard therapies.
“Our primary objectives are twofold: One is to determine the feasibility of making our CAR ... and [the other] is to assess the safety using an escalating CAR dose following standard cyclophosphamide/fludarabine conditioning,” Dr. Hossain said.
The dose assessed in the current analysis was 1 x 106 CAR T cells/kg; other planned doses include 3 x 106 CAR T cells/kg and 1 x 107 CAR T cells/kg, he said.
All patients underwent lymphodepletion with cyclophosphamide (500 mg/m2 daily x 3 doses) and fludarabine (30 mg/m2 daily x 3 doses) followed by CAR T-cell infusion 2 days later.
The findings of this ongoing study – the first in-human study of a bispecific loop CAR in the United States – suggest that the novel CAR has low toxicity and promising efficacy, Dr. Hossain said.
Currently approved therapies target CD19 alone, he said, noting that they all use the same anti-CD19 domain, but different costimulatory domains, and have good clinical efficacy of greater than 70% CRs in ALL and up to 52% CRs in DLBCL.
“But questions remain about determining the durability of response and the causes of therapy failure,” he said.
One common cause of treatment failure is CD19 antigen loss, and efforts to reduce such antigen loss using bispecific loop CARs targeting both CD19 and CD22 have shown promise. The CAR construct evaluated in this study was developed to target CD19 and CD22 with intracellular signaling domains incorporating 4-1BB and CD3-zeta to overcome CD19 immune escape.
“We have now escalated the dose to 3 x 106 CAR T cells/kg ... and an expansion study of 60 patients will follow,” Dr. Hossain said.
A companion phase 1 pediatric trial using the same CAR construct is also underway, with preliminary data presented at the ASH meeting demonstrating safety and tolerability in children with relapsed or refractory B-cell ALL.
Dr. Hossain reported having no financial disclosures.
SOURCE: Hossain N et al. ASH 2018, Abstract 490.
SAN DIEGO – A chimeric antigen receptor (CAR) targeting both CD19 and CD22 shows promising safety and efficacy for the treatment of relapsed or refractory B-cell malignancies in adults, according to early findings from a phase 1 trial of the novel bispecific CAR.
Of six patients with diffuse large B-cell lymphoma (DLBCL) and two patients with B-cell acute lymphoblastic leukemia (B-ALL) enrolled in the single-institution dose escalation study and available for safety analysis after the bispecific CAR T-cell infusion, five developed reversible grade 1 cytokine release syndrome (CRS) and one developed grade 2 CRS requiring treatment with tocilizumab, Nasheed Hossain, MD, reported at the annual meeting of the American Society of Hematology.
Additionally, two patients developed grade 1 neurotoxicity, and one developed grade 2 neurotoxicity requiring treatment with dexamethasone.
“But no dose-limiting toxicities have been encountered thus far,” said Dr. Hossain of Loyola University Medical Center, Chicago. “With regard to efficacy, the DLBCL overall response rate is 60%, with 1 [complete response] and 2 [partial responses] at day 28 and day 90, and the ALL overall response rate is 100%, with 1 CR and 1 PR at day 28.
“With longer follow-up, five patients have relapsed and biopsies at the time of progression all showed ongoing CD19 expression,” he said, adding that all enrolled patients are alive, except for one patient who died from disease progression.
Study participants were adults aged 35-75 years with DLBCL or B-ALL that was refractory to standard therapies.
“Our primary objectives are twofold: One is to determine the feasibility of making our CAR ... and [the other] is to assess the safety using an escalating CAR dose following standard cyclophosphamide/fludarabine conditioning,” Dr. Hossain said.
The dose assessed in the current analysis was 1 x 106 CAR T cells/kg; other planned doses include 3 x 106 CAR T cells/kg and 1 x 107 CAR T cells/kg, he said.
All patients underwent lymphodepletion with cyclophosphamide (500 mg/m2 daily x 3 doses) and fludarabine (30 mg/m2 daily x 3 doses) followed by CAR T-cell infusion 2 days later.
The findings of this ongoing study – the first in-human study of a bispecific loop CAR in the United States – suggest that the novel CAR has low toxicity and promising efficacy, Dr. Hossain said.
Currently approved therapies target CD19 alone, he said, noting that they all use the same anti-CD19 domain, but different costimulatory domains, and have good clinical efficacy of greater than 70% CRs in ALL and up to 52% CRs in DLBCL.
“But questions remain about determining the durability of response and the causes of therapy failure,” he said.
One common cause of treatment failure is CD19 antigen loss, and efforts to reduce such antigen loss using bispecific loop CARs targeting both CD19 and CD22 have shown promise. The CAR construct evaluated in this study was developed to target CD19 and CD22 with intracellular signaling domains incorporating 4-1BB and CD3-zeta to overcome CD19 immune escape.
“We have now escalated the dose to 3 x 106 CAR T cells/kg ... and an expansion study of 60 patients will follow,” Dr. Hossain said.
A companion phase 1 pediatric trial using the same CAR construct is also underway, with preliminary data presented at the ASH meeting demonstrating safety and tolerability in children with relapsed or refractory B-cell ALL.
Dr. Hossain reported having no financial disclosures.
SOURCE: Hossain N et al. ASH 2018, Abstract 490.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Grade 1 cytokine release syndrome occurred in five patients, and grade 2 CRS occurred in one patient; there were no dose-limiting toxicities.
Study details: A phase 1 dose escalation study of nine patients.
Disclosures: Dr. Hossain reported having no financial disclosures.
Source: Hossain N et al. ASH 2018, Abstract 490.
Imaging, radiotherapy clarified in new PMBCL guidelines
Fertility preservation, imaging and radiotherapy guidelines, and best practices in relapse or salvage therapy for primary mediastinal B-cell lymphoma (PMBCL) are all highlighted in a new good practice paper from the British Society for Haematology.
Though PMBCL was previously thought of as a subtype of diffuse large B-cell lymphoma, “gene expression profiling data has shown it to be a separate clinicopathological entity with evidence of an overlap with classic Hodgkin lymphoma,” said Kate Cwynarski, MD, PhD, of University College London Hospitals NHS Foundation Trust in England, and her coauthors. The recommendations were published in the British Journal of Haematology.
PMBCL makes up 2%-4% of non-Hodgkin lymphomas, they said; a bulky anterior mediastinal mass is the usual initial presentation. PMBCL does not usually spread beyond the thoracic cavity.
Biopsy, which should be reviewed by a hematopathologist, is required for a histological diagnosis of PMBCL. A multidisciplinary team should review the clinical presentation, pathology, and management plan, according to the good practice paper authors. This was a strong recommendation backed by a high level of evidence.
In addition, patients should receive positron emission tomography–computed tomography (PET/CT) at diagnosis, before steroids are administered, if possible, as standard of care. Results from the PET/CT should be reported in accordance with international guidelines. These strong recommendations are backed by high-quality evidence.
If PET/CT is performed, then “a bone marrow biopsy is not considered essential,” said Dr. Cwynarski and her coauthors. However, if the findings would influence management, such as when there is extranodal disease that presents central nervous system opportunities, then bone marrow biopsy should be performed. It should also be performed when cytotoxic therapy was initiated before PET/CT could be done. This is a weak recommendation supported by moderate evidence.
Since patients with PMBCL are usually young adults at presentation, it’s important to consider fertility preservation in the face of chemotherapy. For males, semen preservation should be offered. Female patients may not be able to postpone treatment long enough to accomplish egg harvesting. The risk of infertility and premature ovarian failure will depend on the treatment regimen, so “the risks of each individual therapeutic regimen should be discussed with the patient,” Dr. Cwynarski and her colleagues said.
If a patient is diagnosed with PMBCL while pregnant, treatment should be managed in conjunction with high-risk obstetrics and anesthesia specialists. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has been used in pregnancy, and immunotherapy without antimetabolites can be considered in the second and third trimesters, according to the good practice paper. These are strong fertility and pregnancy recommendations, backed by moderate to low-quality evidence.
If superior vena cava obstruction causes thrombosis, local standard of care for anticoagulation should be used, but therapy-induced thrombocytopenia should be taken into consideration.
There is a lack of prospective, randomized studies to guide treatment decisions in PMBCL, according to the paper. Still, adding rituximab improves both response rates and duration of remission, they noted.
The standard of care for treatment is six cycles of R-CHOP and involved site radiotherapy (ISRT). If the patient is being cared for at a site that can manage the complexities of dose adjustment and monitoring, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) without ISRT is an alternative, according to the good practice paper.
All patients should be offered clinical trial participation when feasible, a strong recommendation based on high-quality evidence.
To assess the response to therapy, R-CHOP and ISRT recipients not participating in a clinical trial should receive a PET-CT scan 2-3 months after treatment is completed, and DA-EPOCH-R patients should receive their scan 6 weeks after the end of therapy. For all patients, Deauville criteria should be used in reporting response scan results. These strong recommendations about posttherapy imaging are based on moderate-quality evidence.
The rate of relapse and refractory disease is relatively low at about 10%-30%, Dr. Cwynarski and her colleagues said. Relapse usually happens within the first year and is rare after 2 years; extranodal disease is common, but usually spares the central nervous system and bone marrow. The good practice paper authors strongly recommend, based on high-quality evidence, that biopsy and fluorodeoxyglucose-PET/CT should be performed with relapse.
Radiotherapy can be considered if the relapse is localized and the patient didn’t receive initial radiotherapy, a strong recommendation with moderate evidence to support it.
Salvage regimens for patients who have not previously achieved complete metabolic response lack a disease-specific evidence base, noted Dr. Cwynarski and her colleagues. Taking this into consideration, a PMBCL salvage regimen should be the same as that offered to patients with relapsed diffused large B-cell lymphoma. High-dose therapy and autologous stem cell transplantation is appropriate for responsive disease.
If radiotherapy had not been given previously, it should be considered either pre- or post transplant. This, along with the other salvage therapy guidance, is a weak recommendation, backed by moderate evidence.
For longer-term follow-up, asymptomatic patients should not have routine imaging, a strong recommendation with moderate evidence. “[P]atients who remain in remission may be considered for discharge back to primary care,” Dr. Cwynarski and her coauthors said, making a weak recommendation based on low-quality evidence. Patients and their primary care providers should know about the potential for such long-term complications as cardiac toxicities and second malignancies.
SOURCE: Cwynarski K et al. Br J Haematol. 2019 Jan 4. doi:10.1111/bjh.15731
Fertility preservation, imaging and radiotherapy guidelines, and best practices in relapse or salvage therapy for primary mediastinal B-cell lymphoma (PMBCL) are all highlighted in a new good practice paper from the British Society for Haematology.
Though PMBCL was previously thought of as a subtype of diffuse large B-cell lymphoma, “gene expression profiling data has shown it to be a separate clinicopathological entity with evidence of an overlap with classic Hodgkin lymphoma,” said Kate Cwynarski, MD, PhD, of University College London Hospitals NHS Foundation Trust in England, and her coauthors. The recommendations were published in the British Journal of Haematology.
PMBCL makes up 2%-4% of non-Hodgkin lymphomas, they said; a bulky anterior mediastinal mass is the usual initial presentation. PMBCL does not usually spread beyond the thoracic cavity.
Biopsy, which should be reviewed by a hematopathologist, is required for a histological diagnosis of PMBCL. A multidisciplinary team should review the clinical presentation, pathology, and management plan, according to the good practice paper authors. This was a strong recommendation backed by a high level of evidence.
In addition, patients should receive positron emission tomography–computed tomography (PET/CT) at diagnosis, before steroids are administered, if possible, as standard of care. Results from the PET/CT should be reported in accordance with international guidelines. These strong recommendations are backed by high-quality evidence.
If PET/CT is performed, then “a bone marrow biopsy is not considered essential,” said Dr. Cwynarski and her coauthors. However, if the findings would influence management, such as when there is extranodal disease that presents central nervous system opportunities, then bone marrow biopsy should be performed. It should also be performed when cytotoxic therapy was initiated before PET/CT could be done. This is a weak recommendation supported by moderate evidence.
Since patients with PMBCL are usually young adults at presentation, it’s important to consider fertility preservation in the face of chemotherapy. For males, semen preservation should be offered. Female patients may not be able to postpone treatment long enough to accomplish egg harvesting. The risk of infertility and premature ovarian failure will depend on the treatment regimen, so “the risks of each individual therapeutic regimen should be discussed with the patient,” Dr. Cwynarski and her colleagues said.
If a patient is diagnosed with PMBCL while pregnant, treatment should be managed in conjunction with high-risk obstetrics and anesthesia specialists. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has been used in pregnancy, and immunotherapy without antimetabolites can be considered in the second and third trimesters, according to the good practice paper. These are strong fertility and pregnancy recommendations, backed by moderate to low-quality evidence.
If superior vena cava obstruction causes thrombosis, local standard of care for anticoagulation should be used, but therapy-induced thrombocytopenia should be taken into consideration.
There is a lack of prospective, randomized studies to guide treatment decisions in PMBCL, according to the paper. Still, adding rituximab improves both response rates and duration of remission, they noted.
The standard of care for treatment is six cycles of R-CHOP and involved site radiotherapy (ISRT). If the patient is being cared for at a site that can manage the complexities of dose adjustment and monitoring, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) without ISRT is an alternative, according to the good practice paper.
All patients should be offered clinical trial participation when feasible, a strong recommendation based on high-quality evidence.
To assess the response to therapy, R-CHOP and ISRT recipients not participating in a clinical trial should receive a PET-CT scan 2-3 months after treatment is completed, and DA-EPOCH-R patients should receive their scan 6 weeks after the end of therapy. For all patients, Deauville criteria should be used in reporting response scan results. These strong recommendations about posttherapy imaging are based on moderate-quality evidence.
The rate of relapse and refractory disease is relatively low at about 10%-30%, Dr. Cwynarski and her colleagues said. Relapse usually happens within the first year and is rare after 2 years; extranodal disease is common, but usually spares the central nervous system and bone marrow. The good practice paper authors strongly recommend, based on high-quality evidence, that biopsy and fluorodeoxyglucose-PET/CT should be performed with relapse.
Radiotherapy can be considered if the relapse is localized and the patient didn’t receive initial radiotherapy, a strong recommendation with moderate evidence to support it.
Salvage regimens for patients who have not previously achieved complete metabolic response lack a disease-specific evidence base, noted Dr. Cwynarski and her colleagues. Taking this into consideration, a PMBCL salvage regimen should be the same as that offered to patients with relapsed diffused large B-cell lymphoma. High-dose therapy and autologous stem cell transplantation is appropriate for responsive disease.
If radiotherapy had not been given previously, it should be considered either pre- or post transplant. This, along with the other salvage therapy guidance, is a weak recommendation, backed by moderate evidence.
For longer-term follow-up, asymptomatic patients should not have routine imaging, a strong recommendation with moderate evidence. “[P]atients who remain in remission may be considered for discharge back to primary care,” Dr. Cwynarski and her coauthors said, making a weak recommendation based on low-quality evidence. Patients and their primary care providers should know about the potential for such long-term complications as cardiac toxicities and second malignancies.
SOURCE: Cwynarski K et al. Br J Haematol. 2019 Jan 4. doi:10.1111/bjh.15731
Fertility preservation, imaging and radiotherapy guidelines, and best practices in relapse or salvage therapy for primary mediastinal B-cell lymphoma (PMBCL) are all highlighted in a new good practice paper from the British Society for Haematology.
Though PMBCL was previously thought of as a subtype of diffuse large B-cell lymphoma, “gene expression profiling data has shown it to be a separate clinicopathological entity with evidence of an overlap with classic Hodgkin lymphoma,” said Kate Cwynarski, MD, PhD, of University College London Hospitals NHS Foundation Trust in England, and her coauthors. The recommendations were published in the British Journal of Haematology.
PMBCL makes up 2%-4% of non-Hodgkin lymphomas, they said; a bulky anterior mediastinal mass is the usual initial presentation. PMBCL does not usually spread beyond the thoracic cavity.
Biopsy, which should be reviewed by a hematopathologist, is required for a histological diagnosis of PMBCL. A multidisciplinary team should review the clinical presentation, pathology, and management plan, according to the good practice paper authors. This was a strong recommendation backed by a high level of evidence.
In addition, patients should receive positron emission tomography–computed tomography (PET/CT) at diagnosis, before steroids are administered, if possible, as standard of care. Results from the PET/CT should be reported in accordance with international guidelines. These strong recommendations are backed by high-quality evidence.
If PET/CT is performed, then “a bone marrow biopsy is not considered essential,” said Dr. Cwynarski and her coauthors. However, if the findings would influence management, such as when there is extranodal disease that presents central nervous system opportunities, then bone marrow biopsy should be performed. It should also be performed when cytotoxic therapy was initiated before PET/CT could be done. This is a weak recommendation supported by moderate evidence.
Since patients with PMBCL are usually young adults at presentation, it’s important to consider fertility preservation in the face of chemotherapy. For males, semen preservation should be offered. Female patients may not be able to postpone treatment long enough to accomplish egg harvesting. The risk of infertility and premature ovarian failure will depend on the treatment regimen, so “the risks of each individual therapeutic regimen should be discussed with the patient,” Dr. Cwynarski and her colleagues said.
If a patient is diagnosed with PMBCL while pregnant, treatment should be managed in conjunction with high-risk obstetrics and anesthesia specialists. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has been used in pregnancy, and immunotherapy without antimetabolites can be considered in the second and third trimesters, according to the good practice paper. These are strong fertility and pregnancy recommendations, backed by moderate to low-quality evidence.
If superior vena cava obstruction causes thrombosis, local standard of care for anticoagulation should be used, but therapy-induced thrombocytopenia should be taken into consideration.
There is a lack of prospective, randomized studies to guide treatment decisions in PMBCL, according to the paper. Still, adding rituximab improves both response rates and duration of remission, they noted.
The standard of care for treatment is six cycles of R-CHOP and involved site radiotherapy (ISRT). If the patient is being cared for at a site that can manage the complexities of dose adjustment and monitoring, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) without ISRT is an alternative, according to the good practice paper.
All patients should be offered clinical trial participation when feasible, a strong recommendation based on high-quality evidence.
To assess the response to therapy, R-CHOP and ISRT recipients not participating in a clinical trial should receive a PET-CT scan 2-3 months after treatment is completed, and DA-EPOCH-R patients should receive their scan 6 weeks after the end of therapy. For all patients, Deauville criteria should be used in reporting response scan results. These strong recommendations about posttherapy imaging are based on moderate-quality evidence.
The rate of relapse and refractory disease is relatively low at about 10%-30%, Dr. Cwynarski and her colleagues said. Relapse usually happens within the first year and is rare after 2 years; extranodal disease is common, but usually spares the central nervous system and bone marrow. The good practice paper authors strongly recommend, based on high-quality evidence, that biopsy and fluorodeoxyglucose-PET/CT should be performed with relapse.
Radiotherapy can be considered if the relapse is localized and the patient didn’t receive initial radiotherapy, a strong recommendation with moderate evidence to support it.
Salvage regimens for patients who have not previously achieved complete metabolic response lack a disease-specific evidence base, noted Dr. Cwynarski and her colleagues. Taking this into consideration, a PMBCL salvage regimen should be the same as that offered to patients with relapsed diffused large B-cell lymphoma. High-dose therapy and autologous stem cell transplantation is appropriate for responsive disease.
If radiotherapy had not been given previously, it should be considered either pre- or post transplant. This, along with the other salvage therapy guidance, is a weak recommendation, backed by moderate evidence.
For longer-term follow-up, asymptomatic patients should not have routine imaging, a strong recommendation with moderate evidence. “[P]atients who remain in remission may be considered for discharge back to primary care,” Dr. Cwynarski and her coauthors said, making a weak recommendation based on low-quality evidence. Patients and their primary care providers should know about the potential for such long-term complications as cardiac toxicities and second malignancies.
SOURCE: Cwynarski K et al. Br J Haematol. 2019 Jan 4. doi:10.1111/bjh.15731
FROM BRITISH JOURNAL OF HAEMATOLOGY