User login
Uninterrupted ibrutinib with CAR T could improve CLL outcomes
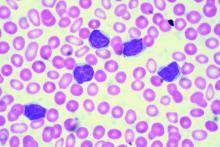
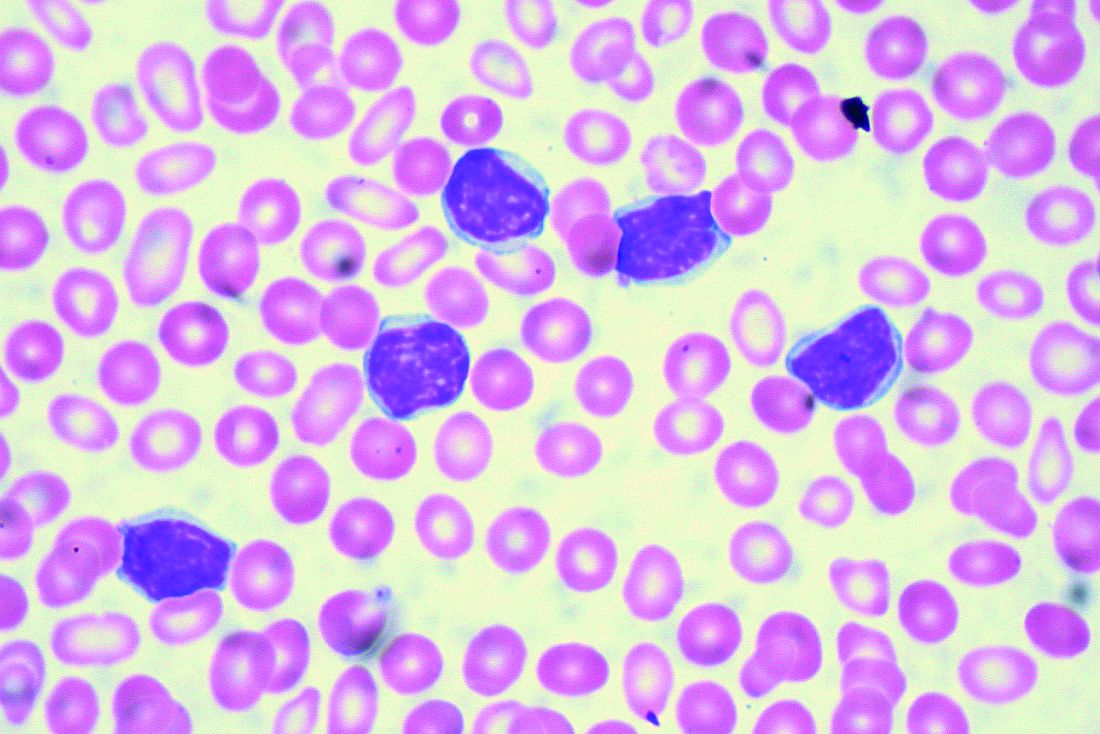
SAN DIEGO – Ibrutinib treatment continued before, during, and after infusion of the CD19-specific chimeric antigen receptor (CAR) T-cell therapy JCAR014 in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) appears to improve patient responses and decrease the risk of severe cytokine release syndrome.
The findings come from a comparison of sequential cohorts from a phase 1/2 study.
At 4 weeks after infusion, the approach was highly efficacious; overall response rates by 2008 International Workshop on CLL (IWCLL) criteria were 83% in 24 patients who received the uninterrupted ibrutinib regimen along with the JCAR014 therapy – a combination of CD4 and CD8 T cells – and 65% in 19 patients from a prior cohort who did not receive continuous ibrutinib, Jordan Gauthier, MD, reported at the annual meeting of the American Society of Hematology.
Concurrent ibrutinib was generally well tolerated, with 13 of 19 patients in the ibrutinib cohort receiving treatment as planned without discontinuation. The rates of grade 1 or higher cytokine release syndrome (CRS) were statistically similar in the ibrutinib and no-ibrutinib cohorts (74% and 92%, respectively). However, the rates of severe CRS (grade 3 or higher) were, strikingly, 0% and 25%, respectively, said Dr. Gauthier, a senior fellow in the Turtle Lab at Fred Hutchinson Cancer Center, Seattle.
Neurotoxicity occurred in 32% and 42% of patients in the groups; severe neurotoxicity occurred in 26% and 29%, respectively.
In the ibrutinib cohort, one patient with grade 2 CRS developed fatal presumed cardiac arrhythmia; in the no-ibrutinib cohort, one patient died from a CAR T cell–related toxicity.
Notably, a trend toward better expansion of CD8 CAR T cells and a significantly greater expansion of CD4 CAR T cells was observed in the ibrutinib cohort, he said.
The study was designed to assess JCAR014, and based on the initial cohort findings published in 2017, established a regimen of cyclophosphamide and fludarabine (Cy/Flu) lymphodepletion followed by JCAR014 infusion at 2 x 106 CAR T cells/kg. The study was not a randomized, head-to-head comparison but the groups were similar with respect to both patient and disease characteristics, Dr. Gauthier noted.
The outcomes in the first cohort were then compared retrospectively with those from the subsequent cohort of patients who received Cy/Flu with 2 x 106 CAR T cells/kg with concurrent ibrutinib administered at 420 mg per day from at least 2 weeks prior to leukapheresis until at least 3 months after JCAR014 infusion.
The rationale for uninterrupted ibrutinib in relapsed/refractory CLL patients receiving JCAR014 included potential prevention of tumor flare, mobilization of CLL cells into the blood from the lymph nodes, improvement of CAR T-cell function, and a decrease in CAR T-cell related toxicity, he said.
The concurrent administration of ibrutinib and JCAR014 was feasible for most patients. “[It] induced high response rates and deep responses early on at 4 weeks, and it was associated with higher in vivo expansion of CD4 CAR T cells and with lower rates of severe toxicity,” Dr. Gauthier said. “The next step is to hopefully validate these findings in a prospective phase 1/2 study.”
Dr. Gauthier reported having no financial disclosures.
SOURCE: Gauthier J et al. ASH 18, Abstract 299.
SAN DIEGO – Ibrutinib treatment continued before, during, and after infusion of the CD19-specific chimeric antigen receptor (CAR) T-cell therapy JCAR014 in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) appears to improve patient responses and decrease the risk of severe cytokine release syndrome.
The findings come from a comparison of sequential cohorts from a phase 1/2 study.
At 4 weeks after infusion, the approach was highly efficacious; overall response rates by 2008 International Workshop on CLL (IWCLL) criteria were 83% in 24 patients who received the uninterrupted ibrutinib regimen along with the JCAR014 therapy – a combination of CD4 and CD8 T cells – and 65% in 19 patients from a prior cohort who did not receive continuous ibrutinib, Jordan Gauthier, MD, reported at the annual meeting of the American Society of Hematology.
Concurrent ibrutinib was generally well tolerated, with 13 of 19 patients in the ibrutinib cohort receiving treatment as planned without discontinuation. The rates of grade 1 or higher cytokine release syndrome (CRS) were statistically similar in the ibrutinib and no-ibrutinib cohorts (74% and 92%, respectively). However, the rates of severe CRS (grade 3 or higher) were, strikingly, 0% and 25%, respectively, said Dr. Gauthier, a senior fellow in the Turtle Lab at Fred Hutchinson Cancer Center, Seattle.
Neurotoxicity occurred in 32% and 42% of patients in the groups; severe neurotoxicity occurred in 26% and 29%, respectively.
In the ibrutinib cohort, one patient with grade 2 CRS developed fatal presumed cardiac arrhythmia; in the no-ibrutinib cohort, one patient died from a CAR T cell–related toxicity.
Notably, a trend toward better expansion of CD8 CAR T cells and a significantly greater expansion of CD4 CAR T cells was observed in the ibrutinib cohort, he said.
The study was designed to assess JCAR014, and based on the initial cohort findings published in 2017, established a regimen of cyclophosphamide and fludarabine (Cy/Flu) lymphodepletion followed by JCAR014 infusion at 2 x 106 CAR T cells/kg. The study was not a randomized, head-to-head comparison but the groups were similar with respect to both patient and disease characteristics, Dr. Gauthier noted.
The outcomes in the first cohort were then compared retrospectively with those from the subsequent cohort of patients who received Cy/Flu with 2 x 106 CAR T cells/kg with concurrent ibrutinib administered at 420 mg per day from at least 2 weeks prior to leukapheresis until at least 3 months after JCAR014 infusion.
The rationale for uninterrupted ibrutinib in relapsed/refractory CLL patients receiving JCAR014 included potential prevention of tumor flare, mobilization of CLL cells into the blood from the lymph nodes, improvement of CAR T-cell function, and a decrease in CAR T-cell related toxicity, he said.
The concurrent administration of ibrutinib and JCAR014 was feasible for most patients. “[It] induced high response rates and deep responses early on at 4 weeks, and it was associated with higher in vivo expansion of CD4 CAR T cells and with lower rates of severe toxicity,” Dr. Gauthier said. “The next step is to hopefully validate these findings in a prospective phase 1/2 study.”
Dr. Gauthier reported having no financial disclosures.
SOURCE: Gauthier J et al. ASH 18, Abstract 299.
SAN DIEGO – Ibrutinib treatment continued before, during, and after infusion of the CD19-specific chimeric antigen receptor (CAR) T-cell therapy JCAR014 in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) appears to improve patient responses and decrease the risk of severe cytokine release syndrome.
The findings come from a comparison of sequential cohorts from a phase 1/2 study.
At 4 weeks after infusion, the approach was highly efficacious; overall response rates by 2008 International Workshop on CLL (IWCLL) criteria were 83% in 24 patients who received the uninterrupted ibrutinib regimen along with the JCAR014 therapy – a combination of CD4 and CD8 T cells – and 65% in 19 patients from a prior cohort who did not receive continuous ibrutinib, Jordan Gauthier, MD, reported at the annual meeting of the American Society of Hematology.
Concurrent ibrutinib was generally well tolerated, with 13 of 19 patients in the ibrutinib cohort receiving treatment as planned without discontinuation. The rates of grade 1 or higher cytokine release syndrome (CRS) were statistically similar in the ibrutinib and no-ibrutinib cohorts (74% and 92%, respectively). However, the rates of severe CRS (grade 3 or higher) were, strikingly, 0% and 25%, respectively, said Dr. Gauthier, a senior fellow in the Turtle Lab at Fred Hutchinson Cancer Center, Seattle.
Neurotoxicity occurred in 32% and 42% of patients in the groups; severe neurotoxicity occurred in 26% and 29%, respectively.
In the ibrutinib cohort, one patient with grade 2 CRS developed fatal presumed cardiac arrhythmia; in the no-ibrutinib cohort, one patient died from a CAR T cell–related toxicity.
Notably, a trend toward better expansion of CD8 CAR T cells and a significantly greater expansion of CD4 CAR T cells was observed in the ibrutinib cohort, he said.
The study was designed to assess JCAR014, and based on the initial cohort findings published in 2017, established a regimen of cyclophosphamide and fludarabine (Cy/Flu) lymphodepletion followed by JCAR014 infusion at 2 x 106 CAR T cells/kg. The study was not a randomized, head-to-head comparison but the groups were similar with respect to both patient and disease characteristics, Dr. Gauthier noted.
The outcomes in the first cohort were then compared retrospectively with those from the subsequent cohort of patients who received Cy/Flu with 2 x 106 CAR T cells/kg with concurrent ibrutinib administered at 420 mg per day from at least 2 weeks prior to leukapheresis until at least 3 months after JCAR014 infusion.
The rationale for uninterrupted ibrutinib in relapsed/refractory CLL patients receiving JCAR014 included potential prevention of tumor flare, mobilization of CLL cells into the blood from the lymph nodes, improvement of CAR T-cell function, and a decrease in CAR T-cell related toxicity, he said.
The concurrent administration of ibrutinib and JCAR014 was feasible for most patients. “[It] induced high response rates and deep responses early on at 4 weeks, and it was associated with higher in vivo expansion of CD4 CAR T cells and with lower rates of severe toxicity,” Dr. Gauthier said. “The next step is to hopefully validate these findings in a prospective phase 1/2 study.”
Dr. Gauthier reported having no financial disclosures.
SOURCE: Gauthier J et al. ASH 18, Abstract 299.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Severe cytokine release syndrome occurred in 0% versus 25% of patients in the ibrutinib and no-ibrutinib cohorts, respectively.
Study details: A retrospective comparison of 43 patients in two cohorts from a phase 1/2 study.
Disclosures: Dr. Gauthier reported having no financial disclosures.
Source: Gauthier J et al. ASH 2018, Abstract 299.
No evidence for disease-modifying effect of levodopa in Parkinson’s disease
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
This trial supports current clinical practice in two ways, according to Susan Bressman, MD, and Rachel Saunders‑Pullman, MD, MPH. On one hand, the study provides no evidence to suggest that levodopa slows Parkinson’s disease progression, Dr. Bressman and Dr. Saunders-Pullman wrote in an editorial accompanying the study. On the other hand, they added, it provides no evidence that clinicians should delay therapy when it is clinically indicated.
The LEAP trial (Levodopa in Early Parkinson’s Disease) was designed to resolve uncertainty over the potential effects of levodopa on disease progression, they noted. This was necessary because of the results of the placebo-controlled ELLDOPA trial, which was published about 14 years ago and suggested that patients randomized to 40 weeks of levodopa did not deteriorate clinically to the degree that was observed in patients randomized to placebo.
The primary end point of that trial was Unified Parkinson’s Disease Rating Scale (UPDRS) scores after a 2-week washout period.
While one interpretation of the UPDRS results from ELLDOPA was that levodopa slowed disease progression, another was that the 2-week washout period was too short, allowing for residual effects of levodopa on symptoms, suggested Dr. Bressman and Dr. Saunders-Pullman.
The randomized LEAP study now shows not only that there were no differences in UPDRS scores when using a delayed start trial design – which implies that there was no disease-modifying effect – but also that starting levodopa early did not have negative effects, the editorial authors wrote.
In particular, the researchers showed no differences in rates of dyskinesia or levodopa-related fluctuations in those started early versus those started later.
“The results of the current trial, taken together with those of other trials, support treatment that is guided by clinical need and that uses the lowest dose that provides a satisfactory clinical effect,” wrote the editorial’s authors.
Dr. Bressman and Dr. Saunders‑Pullman are with the Icahn School of Medicine at Mt. Sinai, New York. Their editorial appears in the New England Journal of Medicine (2019;380:389-90). Dr. Bressman reported disclosures related to Denali Therapeutics, the Michael J. Fox Foundation, and Prevail Therapeutics, while Dr. Saunders-Pullman reported disclosures with Denali Therapeutics, the National Institutes of Health, Genzyme Sanofi, and the Bigglesworth Family Foundation.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
investigators reported. The disease course was not significantly different for patients who had a full 80 weeks of levodopa/carbodopa therapy, compared with that seen with those who started treatment after a 40-week delay, according to the investigators.
“These findings imply that levodopa had no disease-modifying effect on Parkinson’s disease over the period of the trial,” wrote investigator Rob M. A. de Bie, MD, PhD, professor of movement disorders at the University of Amsterdam, and his colleagues in the New England Journal of Medicine.
By contrast, results of an earlier randomized, placebo-controlled trial suggested that levodopa had disease-modifying effects, though the findings of that study were inconclusive, according to authors of an editorial (see Views on the News).
In the current multicenter trial, known as LEAP (Levodopa in Early Parkinson’s Disease) a total of 445 patients with early Parkinson’s disease were randomized to either 80 weeks of levodopa and carbodopa or to 40 weeks of placebo followed by 40 weeks of levodopa/carbodopa.
Levodopa was dosed at 100 mg three times per day, and carbodopa at 25 mg three times per day, according to the report.
There was no significant difference between the early and delayed treatment groups for primary outcome of the trial, which was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) from baseline to week 80.
The mean change in UPDRS was –1.0 in the group of patients who had the full 80 weeks of levodopa/carbodopa and –2.0 for those who had delayed therapy, for a difference of 1 point (P = .44). Higher scores on the UPDRS signify worse disease.
At week 40, there was a change in UPDRS favoring the early-initiation strategy, which reflected the effects of levodopa on disease symptoms, investigators added.
Nausea was more common in the early-start group during the first 40 weeks of the trial. However, there were no differences between groups in other adverse events of particular interest, including dyskinesias and motor fluctuations related to levodopa, Dr. de Bie and his colleagues reported.
Taken together, these results suggest no beneficial or detrimental disease-modifying effect for an early treatment strategy, although further trials are warranted to evaluate other strategies, such as higher levodopa doses, longer administration, or starting the drug at later stages of disease, they wrote.
Dr. de Bie reported grants from ZonMw, Parkinson Vereniging, and Stichting Parkinsonfonds during the conduct of the study, as well as grants from GE Health and Medtronic outside the submitted work. Study authors provided disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
SOURCE: Verschuur CVM et al. N Engl J Med. 2019;380:315-24.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Levodopa did not have any significant disease-modifying effects in patients with early Parkinson’s disease.
Major finding: Change in the Unified Parkinson’s Disease Rating Scale (UPDRS) was –1.0 after 80 weeks of levodopa/carbodopa versus –2.0 for 40 weeks of placebo followed by 40 weeks of treatment (P = .44).
Study details: A delayed-start trial including 445 patients with early Parkinson’s disease randomized to 80 weeks of treatment or 40 weeks of placebo plus 40 weeks of treatment.
Disclosures: Study authors reported disclosures related to Netherlands Organization for Scientific Research, Michael J. Fox Foundation, UCB, AbbVie, Boston Scientific, Biogen, Merck, and others.
Source: Verschuur CVM et al. N Engl J Med. 2019;380:315-324.
Study hints at lacosamide’s efficacy for small fiber neuropathy
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
FROM BRAIN
Key clinical point:
Major finding: In the lacosamide group, 50.0% of patients reported mean average pain decreasing by at least 1 point, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213).
Study details: A randomized, placebo-controlled, double-blind, crossover-design study of 25 patients with Nav1.7-related small fiber neuropathy who received lacosamide followed by placebo, or vice versa.
Disclosures: The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
Source: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
Four-drug combo shows durable responses in relapsed/refractory lymphomas
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Seven patients responded, and three patients had responses lasting more than 24 months.
Study details: Phase 1 trial of 15 patients.
Disclosures: This research was supported by Celgene. The presenter reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
Autologous transplant linked to PFS benefit in MCL
Autologous hematopoietic cell transplantation (AHCT) was linked to improved progression-free survival, but not improved overall survival, in a retrospective cohort study including more than 1,000 mantle cell lymphoma (MCL) patients.
Use of consolidative AHCT was associated with improved progression-free survival even after the researchers controlled for disease severity in this cohort of younger, transplantation-eligible patients treated in the rituximab era, according to Stefan K. Barta, MD, of the University of Pennsylvania, Philadelphia, and his coinvestigators.
There was no such link between AHCT and improved overall survival in a propensity score–weighted analysis, though certain subgroups did seem to have an overall survival benefit, such as patients with high-risk features, Dr. Barta and his colleagues reported in the Journal of Clinical Oncology.
By contrast, other recent studies have found an association between consolidative AHCT and improved overall survival in MCL, including another recently reported retrospective analysis of 10,000 patients in the National Cancer Database.
The lack of overall survival improvement seen in the present study may be due to effective salvage therapy with novel agents or additional transplantation, abrogating any improvement that otherwise might be attributable to AHCT, Dr. Barta and his coauthors said in a discussion of their results.
The present analysis included 1,029 transplantation-eligible adults aged 65 years or younger with a new diagnosis of MCL, who were treated between 2000 and 2015 at one of 25 medical centers. About two-thirds of the patients underwent consolidative AHCT.
With a median follow-up of 76 months, median progression-free survival was 62 months, and median overall survival was 138 months, the investigators reported.
While AHCT was linked to improved progression-free survival and overall survival in an unadjusted analysis, subsequent analyses showed only a trend toward improvement or no improvement in overall survival.
Specifically, AHCT was associated with improved progression-free survival in multivariable regression analysis, with a hazard ratio (HR) of 0.53 (95% confidence interval, 0.43-0.66; P less than .01) and in propensity score–weighted analysis (HR, 0.70; 95% CI, 0.59-0.84; P less than .05).
By contrast, AHCT was associated with a trend toward improved overall survival in the multivariable analysis (HR, 0.77; 95% CI, 0.98 to 1.01; P = .06) and no improvement in overall survival in the propensity score–weighted analysis (HR, 0.87; 95% CI, 0.69 to 1.10; P = .24).
A subgroup analysis conducted after the multivariable analysis found that overall survival improvements were seen only in patients who received CHOP-like induction or induction without cytarabine, or those who had blastoid or pleomorphic variant or who scored high on the MCL International Prognostic Index.
Randomized trials are “urgently needed” to determine the true benefit of consolidative AHCT in MCL, Dr. Barta and his coauthors said, since some subgroups likely derive minimal benefit from it, including patients with TP53 mutations or those who are minimal residual disease (MRD) negative following induction.
One such study is EA4151, an ongoing randomized, phase 3 clinical trial, which is evaluating consolidation with AHCT followed by maintenance rituximab versus maintenance rituximab alone for MCL patients in MRD-negative first complete remission.
“With this and other well-designed prospective trials as well as with well-validated predictive biomarkers, clinicians will be better able to provide a more refined, risk-adapted approach to first-line management of MCL,” the investigators said.
Dr. Barta provided no disclosures related to the research. Coauthors had disclosures related to Sanofi, AstraZeneca, Celgene, Adaptive Biotechnologies, Janssen Oncology, Seattle Genetics, Genentech, Pharmacyclics, Merck, Bristol-Myers Squibb, and others.
SOURCE: Gerson JN et al. J Clin Oncol. 2019 Jan 7. doi: 10.1200/JCO.18.00690.
Autologous hematopoietic cell transplantation (AHCT) was linked to improved progression-free survival, but not improved overall survival, in a retrospective cohort study including more than 1,000 mantle cell lymphoma (MCL) patients.
Use of consolidative AHCT was associated with improved progression-free survival even after the researchers controlled for disease severity in this cohort of younger, transplantation-eligible patients treated in the rituximab era, according to Stefan K. Barta, MD, of the University of Pennsylvania, Philadelphia, and his coinvestigators.
There was no such link between AHCT and improved overall survival in a propensity score–weighted analysis, though certain subgroups did seem to have an overall survival benefit, such as patients with high-risk features, Dr. Barta and his colleagues reported in the Journal of Clinical Oncology.
By contrast, other recent studies have found an association between consolidative AHCT and improved overall survival in MCL, including another recently reported retrospective analysis of 10,000 patients in the National Cancer Database.
The lack of overall survival improvement seen in the present study may be due to effective salvage therapy with novel agents or additional transplantation, abrogating any improvement that otherwise might be attributable to AHCT, Dr. Barta and his coauthors said in a discussion of their results.
The present analysis included 1,029 transplantation-eligible adults aged 65 years or younger with a new diagnosis of MCL, who were treated between 2000 and 2015 at one of 25 medical centers. About two-thirds of the patients underwent consolidative AHCT.
With a median follow-up of 76 months, median progression-free survival was 62 months, and median overall survival was 138 months, the investigators reported.
While AHCT was linked to improved progression-free survival and overall survival in an unadjusted analysis, subsequent analyses showed only a trend toward improvement or no improvement in overall survival.
Specifically, AHCT was associated with improved progression-free survival in multivariable regression analysis, with a hazard ratio (HR) of 0.53 (95% confidence interval, 0.43-0.66; P less than .01) and in propensity score–weighted analysis (HR, 0.70; 95% CI, 0.59-0.84; P less than .05).
By contrast, AHCT was associated with a trend toward improved overall survival in the multivariable analysis (HR, 0.77; 95% CI, 0.98 to 1.01; P = .06) and no improvement in overall survival in the propensity score–weighted analysis (HR, 0.87; 95% CI, 0.69 to 1.10; P = .24).
A subgroup analysis conducted after the multivariable analysis found that overall survival improvements were seen only in patients who received CHOP-like induction or induction without cytarabine, or those who had blastoid or pleomorphic variant or who scored high on the MCL International Prognostic Index.
Randomized trials are “urgently needed” to determine the true benefit of consolidative AHCT in MCL, Dr. Barta and his coauthors said, since some subgroups likely derive minimal benefit from it, including patients with TP53 mutations or those who are minimal residual disease (MRD) negative following induction.
One such study is EA4151, an ongoing randomized, phase 3 clinical trial, which is evaluating consolidation with AHCT followed by maintenance rituximab versus maintenance rituximab alone for MCL patients in MRD-negative first complete remission.
“With this and other well-designed prospective trials as well as with well-validated predictive biomarkers, clinicians will be better able to provide a more refined, risk-adapted approach to first-line management of MCL,” the investigators said.
Dr. Barta provided no disclosures related to the research. Coauthors had disclosures related to Sanofi, AstraZeneca, Celgene, Adaptive Biotechnologies, Janssen Oncology, Seattle Genetics, Genentech, Pharmacyclics, Merck, Bristol-Myers Squibb, and others.
SOURCE: Gerson JN et al. J Clin Oncol. 2019 Jan 7. doi: 10.1200/JCO.18.00690.
Autologous hematopoietic cell transplantation (AHCT) was linked to improved progression-free survival, but not improved overall survival, in a retrospective cohort study including more than 1,000 mantle cell lymphoma (MCL) patients.
Use of consolidative AHCT was associated with improved progression-free survival even after the researchers controlled for disease severity in this cohort of younger, transplantation-eligible patients treated in the rituximab era, according to Stefan K. Barta, MD, of the University of Pennsylvania, Philadelphia, and his coinvestigators.
There was no such link between AHCT and improved overall survival in a propensity score–weighted analysis, though certain subgroups did seem to have an overall survival benefit, such as patients with high-risk features, Dr. Barta and his colleagues reported in the Journal of Clinical Oncology.
By contrast, other recent studies have found an association between consolidative AHCT and improved overall survival in MCL, including another recently reported retrospective analysis of 10,000 patients in the National Cancer Database.
The lack of overall survival improvement seen in the present study may be due to effective salvage therapy with novel agents or additional transplantation, abrogating any improvement that otherwise might be attributable to AHCT, Dr. Barta and his coauthors said in a discussion of their results.
The present analysis included 1,029 transplantation-eligible adults aged 65 years or younger with a new diagnosis of MCL, who were treated between 2000 and 2015 at one of 25 medical centers. About two-thirds of the patients underwent consolidative AHCT.
With a median follow-up of 76 months, median progression-free survival was 62 months, and median overall survival was 138 months, the investigators reported.
While AHCT was linked to improved progression-free survival and overall survival in an unadjusted analysis, subsequent analyses showed only a trend toward improvement or no improvement in overall survival.
Specifically, AHCT was associated with improved progression-free survival in multivariable regression analysis, with a hazard ratio (HR) of 0.53 (95% confidence interval, 0.43-0.66; P less than .01) and in propensity score–weighted analysis (HR, 0.70; 95% CI, 0.59-0.84; P less than .05).
By contrast, AHCT was associated with a trend toward improved overall survival in the multivariable analysis (HR, 0.77; 95% CI, 0.98 to 1.01; P = .06) and no improvement in overall survival in the propensity score–weighted analysis (HR, 0.87; 95% CI, 0.69 to 1.10; P = .24).
A subgroup analysis conducted after the multivariable analysis found that overall survival improvements were seen only in patients who received CHOP-like induction or induction without cytarabine, or those who had blastoid or pleomorphic variant or who scored high on the MCL International Prognostic Index.
Randomized trials are “urgently needed” to determine the true benefit of consolidative AHCT in MCL, Dr. Barta and his coauthors said, since some subgroups likely derive minimal benefit from it, including patients with TP53 mutations or those who are minimal residual disease (MRD) negative following induction.
One such study is EA4151, an ongoing randomized, phase 3 clinical trial, which is evaluating consolidation with AHCT followed by maintenance rituximab versus maintenance rituximab alone for MCL patients in MRD-negative first complete remission.
“With this and other well-designed prospective trials as well as with well-validated predictive biomarkers, clinicians will be better able to provide a more refined, risk-adapted approach to first-line management of MCL,” the investigators said.
Dr. Barta provided no disclosures related to the research. Coauthors had disclosures related to Sanofi, AstraZeneca, Celgene, Adaptive Biotechnologies, Janssen Oncology, Seattle Genetics, Genentech, Pharmacyclics, Merck, Bristol-Myers Squibb, and others.
SOURCE: Gerson JN et al. J Clin Oncol. 2019 Jan 7. doi: 10.1200/JCO.18.00690.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: AHCT was associated with improved progression-free survival in a propensity score–weighted analysis (hazard ratio, 0.70; P less than .05).
Study details: A retrospective cohort study including 1,029 transplantation-eligible adults aged 65 years or younger with newly diagnosed MCL.
Disclosures: The authors reported disclosures related to Sanofi, AstraZeneca, Celgene, Adaptive Biotechnologies, Janssen Oncology, Seattle Genetics, Genentech, Pharmacyclics, Merck, Bristol-Myers Squibb, and others.
Source: Gerson JN et al. J Clin Oncol. 2019 Jan 7. doi: 10.1200/JCO.18.00690.
Zanubrutinib receives breakthrough designation for MCL
The (MCL) who have received at least one prior therapy.
Zanubrutinib (BGB-3111) is a Bruton’s tyrosine kinase inhibitor being developed by BeiGene as a potential treatment for B-cell malignancies.
Researchers have evaluated zanubrutinib in a phase 2 trial (NCT03206970) of patients with relapsed/refractory MCL. Results from this trial were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 148).
As of March 27, 2018, 86 patients had been enrolled in the trial and received treatment. They had a median of two prior lines of therapy and they received zanubrutinib at 160 mg twice daily.
Eighty-five patients were evaluable for efficacy. The overall response rate was 83.5% (71/85), and the complete response rate was 58.8% (50/85). At a median follow-up of 24.1 weeks, the median duration of response and median progression-free survival had not been reached. The estimated 24-week progression-free survival rate was 82%. The most common adverse events (AEs) in this trial were decrease in neutrophil count (31.4%), rash (29.1%), upper respiratory tract infection (29.1%), and decrease in platelet count (22.1%). Common grade 3 or higher AEs included neutrophil count decrease (11.6%) and lung infection (5.8%).
Four patients had fatal treatment-emergent AEs. One death was caused by a traffic accident, one was due to cerebral hemorrhage, and one resulted from pneumonia. The fourth death occurred in a patient with infection, but the cause of death was unknown.
Breakthrough therapy designation is designed to expedite the development and review of a therapy for a serious or life-threatening disease, following preliminary clinical evidence indicating it demonstrates substantial improvement over existing therapies.
The (MCL) who have received at least one prior therapy.
Zanubrutinib (BGB-3111) is a Bruton’s tyrosine kinase inhibitor being developed by BeiGene as a potential treatment for B-cell malignancies.
Researchers have evaluated zanubrutinib in a phase 2 trial (NCT03206970) of patients with relapsed/refractory MCL. Results from this trial were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 148).
As of March 27, 2018, 86 patients had been enrolled in the trial and received treatment. They had a median of two prior lines of therapy and they received zanubrutinib at 160 mg twice daily.
Eighty-five patients were evaluable for efficacy. The overall response rate was 83.5% (71/85), and the complete response rate was 58.8% (50/85). At a median follow-up of 24.1 weeks, the median duration of response and median progression-free survival had not been reached. The estimated 24-week progression-free survival rate was 82%. The most common adverse events (AEs) in this trial were decrease in neutrophil count (31.4%), rash (29.1%), upper respiratory tract infection (29.1%), and decrease in platelet count (22.1%). Common grade 3 or higher AEs included neutrophil count decrease (11.6%) and lung infection (5.8%).
Four patients had fatal treatment-emergent AEs. One death was caused by a traffic accident, one was due to cerebral hemorrhage, and one resulted from pneumonia. The fourth death occurred in a patient with infection, but the cause of death was unknown.
Breakthrough therapy designation is designed to expedite the development and review of a therapy for a serious or life-threatening disease, following preliminary clinical evidence indicating it demonstrates substantial improvement over existing therapies.
The (MCL) who have received at least one prior therapy.
Zanubrutinib (BGB-3111) is a Bruton’s tyrosine kinase inhibitor being developed by BeiGene as a potential treatment for B-cell malignancies.
Researchers have evaluated zanubrutinib in a phase 2 trial (NCT03206970) of patients with relapsed/refractory MCL. Results from this trial were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 148).
As of March 27, 2018, 86 patients had been enrolled in the trial and received treatment. They had a median of two prior lines of therapy and they received zanubrutinib at 160 mg twice daily.
Eighty-five patients were evaluable for efficacy. The overall response rate was 83.5% (71/85), and the complete response rate was 58.8% (50/85). At a median follow-up of 24.1 weeks, the median duration of response and median progression-free survival had not been reached. The estimated 24-week progression-free survival rate was 82%. The most common adverse events (AEs) in this trial were decrease in neutrophil count (31.4%), rash (29.1%), upper respiratory tract infection (29.1%), and decrease in platelet count (22.1%). Common grade 3 or higher AEs included neutrophil count decrease (11.6%) and lung infection (5.8%).
Four patients had fatal treatment-emergent AEs. One death was caused by a traffic accident, one was due to cerebral hemorrhage, and one resulted from pneumonia. The fourth death occurred in a patient with infection, but the cause of death was unknown.
Breakthrough therapy designation is designed to expedite the development and review of a therapy for a serious or life-threatening disease, following preliminary clinical evidence indicating it demonstrates substantial improvement over existing therapies.
Frailty may affect the expression of dementia
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
The results of the study by Rockwood and colleagues confirm the strong links between frailty and Alzheimer’s disease and other dementias, said Francesco Panza, MD, PhD, of the University of Bari (Italy) Aldo Moro, and his colleagues in an accompanying editorial.
Frailty is primary or preclinical when it is not directly associated with a specific disease or when the patient has no substantial disability. Frailty is considered secondary or clinical when it is associated with known comorbidities (e.g., cardiovascular disease or depression). “This distinction is central in identifying frailty phenotypes with the potential to predict and prevent dementia, using novel models of risk that introduce modifiable factors,” wrote Dr. Panza and his colleagues.
“In light of current knowledge on the cognitive frailty phenotype, secondary preventive strategies for cognitive impairment and physical frailty can be suggested,” they added. “For instance, individualized multidomain interventions can target physical, nutritional, cognitive, and psychological domains that might delay the progression to overt dementia and secondary occurrence of adverse health-related outcomes, such as disability, hospitalization, and mortality.”
Dr. Panza, Madia Lozupone, MD, PhD , and Giancarlo Logroscino, MD, PhD , are affiliated with the neurodegenerative disease unit in the department of basic medicine, neuroscience, and sense organs at the University of Bari (Italy) Aldo Moro. The above remarks come from an editorial that these authors wrote to accompany the study by Rockwood et al. The authors declared no competing interests.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
according to research published online ahead of print Jan. 17 in Lancet Neurology. Data suggest that frailty reduces the threshold for Alzheimer’s disease pathology to cause cognitive decline. Frailty also may contribute to other mechanisms that cause dementia, such as inflammation and immunosenescence, said the investigators.
“While more research is needed, given that frailty is potentially reversible, it is possible that helping people to maintain function and independence in later life could reduce both dementia risk and the severity of debilitating symptoms common in this disease,” said Professor Kenneth Rockwood, MD, of the Nova Scotia Health Authority and Dalhousie University in Halifax, N.S., in a press release.
More susceptible to dementia?
The presence of amyloid plaques and neurofibrillary tangles is not a sufficient condition for the clinical expression of dementia. Some patients with a high degree of Alzheimer’s disease pathology have no apparent cognitive decline. Other factors therefore may modify the relationship between pathology and dementia.
Most people who develop Alzheimer’s disease dementia are older than 65 years, and many of these patients are frail. Frailty is understood as a decreased physiologic reserve and an increased risk for adverse health outcomes. Dr. Rockwood and his colleagues hypothesized that frailty moderates the clinical expression of dementia in relation to Alzheimer’s disease pathology.
To test their hypothesis, the investigators performed a cross-sectional analysis of data from the Rush Memory and Aging Project, which collects clinical and pathologic data from adults older than 59 years without dementia at baseline who live in Illinois. Since 1997, participants have undergone annual clinical and neuropsychological evaluations, and the cohort has been followed for 21 years. For their analysis, Dr. Rockwood and his colleagues included participants without dementia or with Alzheimer’s dementia at their last clinical assessment. Eligible participants had died, and complete autopsy data were available for them.
The researchers measured Alzheimer’s disease pathology using a summary measure of neurofibrillary tangles and neuritic and diffuse plaques. Clinical diagnoses of Alzheimer’s dementia were based on clinician consensus. Dr. Rockwood and his colleagues retrospectively created a 41-item frailty index from variables (e.g., symptoms, signs, comorbidities, and function) that were obtained at each clinical evaluation.
Logistic regression and moderation modeling allowed the investigators to evaluate relationships between Alzheimer’s disease pathology, frailty, and Alzheimer’s dementia. Dr. Rockwood and hus colleagues adjusted all analyses for age, sex, and education.
In all, 456 participants were included in the analysis. The sample’s mean age at death was 89.7 years, and 69% of participants were women. At participants’ last clinical assessment, 242 (53%) had possible or probable Alzheimer’s dementia.
The sample’s mean frailty index was 0.42. The median frailty index was 0.41, a value similar to the threshold commonly used to distinguish between moderate and severe frailty. People with high frailty index scores (i.e., 0.41 or greater) were older, had lower Mini-Mental State Examination scores, were more likely to have a diagnosis of dementia, and had a higher Braak stage than those with moderate or low frailty index scores.
Significant interaction between frailty and Alzheimer’s disease
After the investigators adjusted for age, sex, and education, frailty (odds ratio, 1.76) and Alzheimer’s disease pathology (OR, 4.81) were independently associated with Alzheimer’s dementia. When the investigators added frailty to the model for the relationship between Alzheimer’s disease pathology and Alzheimer’s dementia, the model fit improved. They found a significant interaction between frailty and Alzheimer’s disease pathology (OR, 0.73). People with a low amount of frailty were better able to tolerate Alzheimer’s disease pathology, and people with higher amounts of frailty were more likely to have more Alzheimer’s disease pathology and clinical dementia.
One of the study’s limitations is that it is a secondary analysis, according to Dr. Rockwood and his colleagues. In addition, frailty was measured close to participants’ time of death, and the measurements may thus reflect terminal decline. Participant deaths resulting from causes other than those related to dementia might have confounded the results. Finally, the sample came entirely from people living in retirement homes in Illinois, which might have introduced bias. Future research should use a population-based sample, said the authors.
Frailty could be a basis for risk stratification and could inform the management and treatment of older adults, said Dr. Rockwood and his colleagues. The study results have “the potential to improve our understanding of disease expression, explain failures in pharmacologic treatment, and aid in the development of more appropriate therapeutic targets, approaches, and measurements of success,” they concluded.
The study had no source of funding. The authors reported receiving fees and grants from DGI Clinical, GlaxoSmithKline, Pfizer, and Sanofi. Authors also received support from governmental bodies such as the National Institutes of Health and the Canadian Institutes of Health Research.
SOURCE: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
FROM LANCET NEUROLOGY
Key clinical point: Frailty modifies the association between Alzheimer’s disease pathology and Alzheimer dementia.
Major finding: Frailty index score (odds ratio, 1.76) is independently associated with dementia status.
Study details: A cross-sectional analysis of 456 deceased participants in the Rush Memory and Aging Project.
Disclosures: The study had no outside funding.
Source: Wallace LMK et al. Lancet Neurol. 2019;18:177-84.
Study supports need for less toxic therapies in FL
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: The cumulative risk of mortality from lymphoma was 10.3% at 10 years for patients with follicular lymphoma.
Study details: A pooled cohort study of 1,654 patients with follicular lymphoma in the United States and France.
Disclosures: The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
Source: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Obinutuzumab-based regimens yield durable remissions in CLL
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
FROM BLOOD
Key clinical point:
Major finding: With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Study details: Long-term follow-up of the phase 1b GALTON trial, including 41 patients with CLL.
Disclosures: The study was sponsored by Genentech. The study authors reported disclosures related to Genentech/Roche and other companies.
Source: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
DMTs, stem cell transplants both reduce disease progression in MS
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
The study by Brown et al. provides evidence that DMTs slow the appearance of persistent disabilities in patients with multiple sclerosis (MS), Harold Atkins, MD, wrote in an accompanying editorial (JAMA. 2019 Jan 15;321[2]:153-4). Although disease-modifying therapies (DMTs) may suppress clinical signs of disease activity for long periods in some patients, these therapies slow MS rather than halt it. DMTs require long-term administration and may cause intolerable side effects that impair patients’ quality of life. These therapies also may result in complications such as severe depression or progressive multifocal leukoencephalopathy.
“The study by Burt et al. ... provides a rigorous indication that HSCT [hematopoietic stem cell transplantation] can be an effective treatment for selected patients with MS,” Dr. Atkins said. Treating physicians, however, have concerns about this procedure, which is resource-intensive and “requires specialized medical and nursing expertise and dedicated hospital infrastructure to minimize its risks.” Many patients in the study had moderate to severe acute toxicity following treatment, and patient selection thus requires caution.
An important limitation of the study is that participants did not have access to alemtuzumab or ocrelizumab, which arguably are the most effective DMTs, Dr. Atkins said. The study began in 2005, when fewer DMTs were available. “The inclusion of patients who were less than optimally treated in the DMT group needs to be considered when interpreting the results of this study,” Dr. Atkins said.
Furthermore, Burt and colleagues studied patients with highly active MS, but “only a small proportion of the MS patient population exhibits this degree of activity,” he added. The results therefore may not be generalizable. Nevertheless, “even with the limitations of the trial, the results support a role for HSCT delivered at centers that are experienced in the clinical care of patients with highly active MS,” Dr. Atkins concluded.
Dr. Atkins is affiliated with the Ottawa Hospital Blood and Marrow Transplant Program at the University of Ottawa in Ontario. He reported no conflicts of interest.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
Disease-modifying therapies give patients with relapsing-remitting multiple sclerosis a lower risk of developing secondary progressive disease that may only be topped in specific patients with highly active disease by the use of nonmyeloablative hematopoietic stem cell transplantation, according to findings from two studies published online Jan. 15 in JAMA.
The first study found that interferon-beta, glatiramer acetate (Copaxone), fingolimod (Gilenya), natalizumab (Tysabri), and alemtuzumab (Lemtrada) are associated with a lower risk of conversion to secondary progressive MS, compared with no treatment. Initial treatment with the newer therapies provided a greater risk reduction, compared with initial treatment with interferon-beta or glatiramer acetate.
The second study, described as “the first randomized trial of HSCT [nonmyeloablative hematopoietic stem cell transplantation] in patients with relapsing-remitting MS,” suggests that HSCT prolongs the time to disease progression, compared with disease-modifying therapies (DMTs). It also suggests that HSCT can lead to clinical improvement.
DMTs reduced risk of conversion to secondary progressive MS
Few previous studies have examined the association between DMTs and the risk of conversion from relapsing-remitting MS to secondary progressive MS. Those that have analyzed this association have not used a validated definition of secondary progressive MS. J. William L. Brown, MD, of the University of Cambridge, England, and his colleagues used a validated definition of secondary progressive MS that was published in 2016 to investigate how DMTs affect the rate of conversion, compared with no treatment. The researchers also compared the risk reduction provided by fingolimod, alemtuzumab, or natalizumab with that provided by interferon-beta or glatiramer acetate.
Dr. Brown and his colleagues analyzed prospectively collected clinical data from an international observational cohort study called MSBase. Eligible participants had relapsing-remitting MS, the complete MSBase minimum data set, at least one Expanded Disability Status Scale (EDSS) score recorded within 6 months before baseline, and at least two EDSS scores recorded after baseline. Participants initiated a DMT or began clinical monitoring during 1988-2012. The population had a minimum follow-up duration of 4 years. Patients who stopped their initial therapy within 6 months and those participating in clinical trials were excluded.
The primary outcome was conversion to secondary progressive MS. Dr. Brown and his colleagues defined this outcome as an EDSS increase of 1 point for participants with a baseline EDSS score of 5.5 or less and as an increase of 0.5 points for participants with a baseline EDSS score higher than 5.5. This increase had to occur in the absence of relapses and be confirmed at a subsequent visit 3 or fewer months later. In addition, the increased EDSS score had to be 4 or more.
After excluding ineligible participants, the investigators matched 1,555 patients from 68 centers in 21 countries. Each therapy analyzed was associated with reduced risk of converting to secondary progressive MS, compared with no treatment. The hazard ratios for conversion were 0.71 for interferon-beta or glatiramer acetate, 0.37 for fingolimod, 0.61 for natalizumab, and 0.52 for alemtuzumab, compared with no treatment.
Treatment with interferon-beta or glatiramer acetate within 5 years of disease onset was associated with a reduced risk of conversion (HR, 0.77), compared with treatment later than 5 years after disease onset. Similarly, patients who escalated treatment from interferon-beta or glatiramer acetate to any of the other three DMTs within 5 years of disease onset had a significantly lower risk of conversion (HR, 0.76) than did those who escalated later. Furthermore, initial treatment with fingolimod, alemtuzumab, or natalizumab was associated with a significantly reduced risk of conversion (HR, 0.66), compared with initial treatment with interferon-beta or glatiramer acetate.
One of the study’s limitations is its observational design, which precludes the determination of causality, Dr. Brown and his colleagues said. In addition, functional score subcomponents of the EDSS were unavailable, which prevented the researchers from using the definition of secondary progressive MS with the best combination of sensitivity, specificity, and accuracy. Some analyses were limited by small numbers of patients, and the study did not evaluate the risks associated with DMTs. Nevertheless, “these findings, considered along with these therapies’ risks, may help inform decisions about DMT selection,” the authors concluded.
Financial support for this study was provided by the National Health and Medical Research Council of Australia and the University of Melbourne. Dr. Brown received a Next Generation Fellowship funded by the Grand Charity of the Freemasons and an MSBase 2017 Fellowship. Alemtuzumab studies conducted in Cambridge were supported by the National Institute for Health Research Cambridge Biomedical Research Centre and the MS Society UK.
HSCT delayed disease progression
In a previous case series, Richard K. Burt, MD, of Northwestern University in Chicago, and his colleagues found that patients with relapsing-remitting MS who underwent nonmyeloablative HSCT had neurologic improvement and a 70% likelihood of having a 4-year period of disease remission. Dr. Burt and his colleagues undertook the MS international stem cell transplant trial to compare the effects of nonmyeloablative HSCT with those of continued DMT treatment on disease progression in participants with highly active relapsing-remitting MS.
The researchers enrolled 110 participants at four international centers into their open-label trial. Eligible participants had two or more clinical relapses or one relapse and at least one gadolinium-enhancing lesion at a separate time within the previous 12 months, despite DMT treatment. The investigators also required participants to have an EDSS score between 2.0 and 6.0. Patients with primary or secondary progressive MS were excluded.
Dr. Burt and his colleagues randomized participants to receive HSCT or an approved DMT that was more effective or in a different class than the one they were receiving at baseline. Ocrelizumab (Ocrevus) was not administered during the study because it had not yet been approved. The investigators excluded alemtuzumab because of its association with persistent lymphopenia and autoimmune disorders. After 1 year of treatment, patients receiving a DMT who had disability progression could cross over to the HSCT arm. Patients randomized to HSCT stopped taking their usual DMT.
Time to disease progression was the study’s primary endpoint. The investigators defined disease progression as an increase in EDSS score of at least 1 point on two evaluations 6 months apart after at least 1 year of treatment. The increase was required to result from MS. The neurologist who recorded participants’ EDSS evaluations was blinded to treatment group assignment.
The researchers randomized 55 patients to each study arm. Approximately 66% of participants were women, and the sample’s mean age was 36 years. There were no significant baseline differences between groups on demographic, clinical, or imaging characteristics. Three patients in the HSCT group were withdrawn from the study, and four in the DMT group were lost to follow-up after seeking HSCT at outside facilities.
Three patients in the HSCT group and 34 patients in the DMT group had disease progression. Mean follow-up duration was 2.8 years. The investigators could not calculate the median time to progression in the HSCT group because too few events occurred. Median time to progression was 24 months in the DMT group (HR, 0.07). During the first year, mean EDSS scores decreased (indicating improvement) from 3.38 to 2.36 in the HSCT group. Mean EDSS scores increased from 3.31 to 3.98 in the DMT group. No participants died, and no patients who received HSCT-developed nonhematopoietic grade 4 toxicities.
“To our knowledge, this is the first randomized trial of HSCT in patients with relapsing-remitting MS,” Dr. Burt and his colleagues said. Although observational studies have found similar EDSS improvements following HSCT, “this degree of improvement has not been demonstrated in pharmaceutical trials even with more intensive DMT such as alemtuzumab,” they concluded.
The Danhakl Family Foundation, the Cumming Foundation, the McNamara Purcell Foundation, Morgan Stanley, and the National Institute for Health Research Sheffield Clinical Research Facility provided financial support for this study. No pharmaceutical companies supported the study.
SOURCEs: Brown JWL et al. JAMA. 2019;321(2):175-87. doi: 10.1001/jama.2018.20588.; and Burt RK et al. JAMA. 2019;321(2):165-74. doi: 10.1001/jama.2018.18743.
FROM JAMA