User login
Checkpoint inhibitors linked to rare, but serious immune-related side effects
Checkpoint inhibitors can cause rare, but serious, hematological immune-related adverse events (hem-irAEs), which require early detection and intervention, according to a recent French study.
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs in the population, reported lead author, Nicolas Delanoy, MD, of Gustave Roussy, Université Paris-Saclay, Villejuif, France, and his colleagues.
“About 71% of patients treated have any-grade irAEs and 10% have grade 3-4 irAEs after anti-PD-1 immunotherapy,” the investigators wrote. The report is in The Lancet Haematology. “In most cases, they involve the skin, gastrointestinal tract, thyroid or endocrine glands, liver, lungs, or joints. However, all organs can potentially be affected, including the hemopoietic system.”
Despite this possibility, few reports detail the frequency or character of hematological toxicities from immunotherapy.
The present study involved 948 patients who entered into three French registries between 2014 and 2018. The first registry, consisting of 745 patients, was observed prospectively during checkpoint inhibitor therapy. The other two registries provided retrospective data on confirmed irAEs or hem-irAEs.
Among 745 patients followed during checkpoint inhibitor therapy, four developed hem-irAEs, providing an incidence rate of 0.5%. The other two databases added 31 patients with confirmed hem-irAEs, allowing for characterization of 35 total cases.
The group of 35 patients had a median age of 65 years, with more men (n = 21) than women (n = 14). Melanoma was the most common type of malignancy (43%), followed by non–small-cell lung cancer (34%), lymphoma (11%), and others. The majority of patients received nivolumab (57%), slightly fewer received pembrolizumab (40%), and a small minority received atezolizumab (3%).
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs, each occurring in nine patients (26%). Five patients (14%) had aplastic anemia or pancytopenia, two patients had bicytopenia (6%; neutropenia and anemia or thrombocytopenia and anemia), and one patient had pure red cell aplasia (3%).
Hem-irAEs resolved in 60% of patients, but two patients (6%) died due to febrile neutropenia. Overall, 71% of hem-irAEs were grade 4.
These findings suggest that hem-irAEs are rare, but they are often serious, and potentially life-threatening, the researchers noted.
In 7 of 35 patients (20%) who were rechallenged with checkpoint inhibitor therapy, 3 (43%) had recurrence of hem-irAEs. This finding should elicit caution and close monitoring if rechallenge is elected.
“This observational study encourages further, in-depth investigations of hematological immune toxicities, to search for biomarkers that can be helpful for earlier detection,” the investigators concluded.
This study was funded by Gustave Roussy and the Gustave Roussy Immunotherapy Program. Dr. Delanoy reported nonfinancial support from Sanofi and other authors reported financial relationships with pharmaceutical companies.
SOURCE: Delanoy N et al. Lancet Haematol. 2018 Dec 4. doi: 10.1016/S2352-3026(18)30175-3.
Checkpoint inhibitors can cause rare, but serious, hematological immune-related adverse events (hem-irAEs), which require early detection and intervention, according to a recent French study.
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs in the population, reported lead author, Nicolas Delanoy, MD, of Gustave Roussy, Université Paris-Saclay, Villejuif, France, and his colleagues.
“About 71% of patients treated have any-grade irAEs and 10% have grade 3-4 irAEs after anti-PD-1 immunotherapy,” the investigators wrote. The report is in The Lancet Haematology. “In most cases, they involve the skin, gastrointestinal tract, thyroid or endocrine glands, liver, lungs, or joints. However, all organs can potentially be affected, including the hemopoietic system.”
Despite this possibility, few reports detail the frequency or character of hematological toxicities from immunotherapy.
The present study involved 948 patients who entered into three French registries between 2014 and 2018. The first registry, consisting of 745 patients, was observed prospectively during checkpoint inhibitor therapy. The other two registries provided retrospective data on confirmed irAEs or hem-irAEs.
Among 745 patients followed during checkpoint inhibitor therapy, four developed hem-irAEs, providing an incidence rate of 0.5%. The other two databases added 31 patients with confirmed hem-irAEs, allowing for characterization of 35 total cases.
The group of 35 patients had a median age of 65 years, with more men (n = 21) than women (n = 14). Melanoma was the most common type of malignancy (43%), followed by non–small-cell lung cancer (34%), lymphoma (11%), and others. The majority of patients received nivolumab (57%), slightly fewer received pembrolizumab (40%), and a small minority received atezolizumab (3%).
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs, each occurring in nine patients (26%). Five patients (14%) had aplastic anemia or pancytopenia, two patients had bicytopenia (6%; neutropenia and anemia or thrombocytopenia and anemia), and one patient had pure red cell aplasia (3%).
Hem-irAEs resolved in 60% of patients, but two patients (6%) died due to febrile neutropenia. Overall, 71% of hem-irAEs were grade 4.
These findings suggest that hem-irAEs are rare, but they are often serious, and potentially life-threatening, the researchers noted.
In 7 of 35 patients (20%) who were rechallenged with checkpoint inhibitor therapy, 3 (43%) had recurrence of hem-irAEs. This finding should elicit caution and close monitoring if rechallenge is elected.
“This observational study encourages further, in-depth investigations of hematological immune toxicities, to search for biomarkers that can be helpful for earlier detection,” the investigators concluded.
This study was funded by Gustave Roussy and the Gustave Roussy Immunotherapy Program. Dr. Delanoy reported nonfinancial support from Sanofi and other authors reported financial relationships with pharmaceutical companies.
SOURCE: Delanoy N et al. Lancet Haematol. 2018 Dec 4. doi: 10.1016/S2352-3026(18)30175-3.
Checkpoint inhibitors can cause rare, but serious, hematological immune-related adverse events (hem-irAEs), which require early detection and intervention, according to a recent French study.
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs in the population, reported lead author, Nicolas Delanoy, MD, of Gustave Roussy, Université Paris-Saclay, Villejuif, France, and his colleagues.
“About 71% of patients treated have any-grade irAEs and 10% have grade 3-4 irAEs after anti-PD-1 immunotherapy,” the investigators wrote. The report is in The Lancet Haematology. “In most cases, they involve the skin, gastrointestinal tract, thyroid or endocrine glands, liver, lungs, or joints. However, all organs can potentially be affected, including the hemopoietic system.”
Despite this possibility, few reports detail the frequency or character of hematological toxicities from immunotherapy.
The present study involved 948 patients who entered into three French registries between 2014 and 2018. The first registry, consisting of 745 patients, was observed prospectively during checkpoint inhibitor therapy. The other two registries provided retrospective data on confirmed irAEs or hem-irAEs.
Among 745 patients followed during checkpoint inhibitor therapy, four developed hem-irAEs, providing an incidence rate of 0.5%. The other two databases added 31 patients with confirmed hem-irAEs, allowing for characterization of 35 total cases.
The group of 35 patients had a median age of 65 years, with more men (n = 21) than women (n = 14). Melanoma was the most common type of malignancy (43%), followed by non–small-cell lung cancer (34%), lymphoma (11%), and others. The majority of patients received nivolumab (57%), slightly fewer received pembrolizumab (40%), and a small minority received atezolizumab (3%).
Immune thrombocytopenia, hemolytic anemia, and neutropenia were the most common hem-irAEs, each occurring in nine patients (26%). Five patients (14%) had aplastic anemia or pancytopenia, two patients had bicytopenia (6%; neutropenia and anemia or thrombocytopenia and anemia), and one patient had pure red cell aplasia (3%).
Hem-irAEs resolved in 60% of patients, but two patients (6%) died due to febrile neutropenia. Overall, 71% of hem-irAEs were grade 4.
These findings suggest that hem-irAEs are rare, but they are often serious, and potentially life-threatening, the researchers noted.
In 7 of 35 patients (20%) who were rechallenged with checkpoint inhibitor therapy, 3 (43%) had recurrence of hem-irAEs. This finding should elicit caution and close monitoring if rechallenge is elected.
“This observational study encourages further, in-depth investigations of hematological immune toxicities, to search for biomarkers that can be helpful for earlier detection,” the investigators concluded.
This study was funded by Gustave Roussy and the Gustave Roussy Immunotherapy Program. Dr. Delanoy reported nonfinancial support from Sanofi and other authors reported financial relationships with pharmaceutical companies.
SOURCE: Delanoy N et al. Lancet Haematol. 2018 Dec 4. doi: 10.1016/S2352-3026(18)30175-3.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: Checkpoint inhibitor therapy led to hematological toxicity in 0.5% of patients.
Study details: A study of 948 patients in French registries who were observed prospectively or retrospectively, including a case series of 35 patients treated with checkpoint inhibitor therapy who developed hematologic, immune-related adverse events.
Disclosures: This study was funded by Gustave Roussy and the Gustave Roussy Immunotherapy Program. Dr. Delanoy reported nonfinancial support from Sanofi and other authors reported financial relationships with pharmaceutical companies.
Source: Delanoy N et al. Lancet Haematol. 2018 Dec 4. doi: 10.1016/S2352-3026(18)30175-3.
Tic disorders are associated with obesity and diabetes
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
FROM JAMA NEUROLOGY
Key clinical point: Monitor cardiometabolic health in patients with Tourette syndrome or chronic tic disorder.
Major finding: Patients with Tourette syndrome or chronic tic disorder have a higher risk of metabolic or cardiovascular disorders, compared with the general population (adjusted hazard ratio, 1.99) and sibling controls (adjusted hazard ratio, 1.37).
Study details: A Swedish longitudinal, population-based cohort study of 7,804 individuals with Tourette syndrome or chronic tic disorder.
Disclosures: The study was supported by a research grant from Tourettes Action. Authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
Source: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
Adding umbralisib to ibrutinib produced responses in MCL, CLL
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
FROM LANCET HAEMATOLOGY
Key clinical point:
Major finding: Of CLL patients, 90% achieved an overall response.
Study details: Phase 1-1b trial of umbralisib and ibrutinib in patients with relapsed or refractory MCL or CLL.
Disclosures: The study was supported by TG Therapeutics and the Leukemia and Lymphoma Therapy Accelerator Program. Dr. Davids and his coauthors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
Source: Davids MS et al. Lancet Haematol. 2019;6:e38-47.
As deep sleep decreases, Alzheimer’s pathology – particularly tau – increases
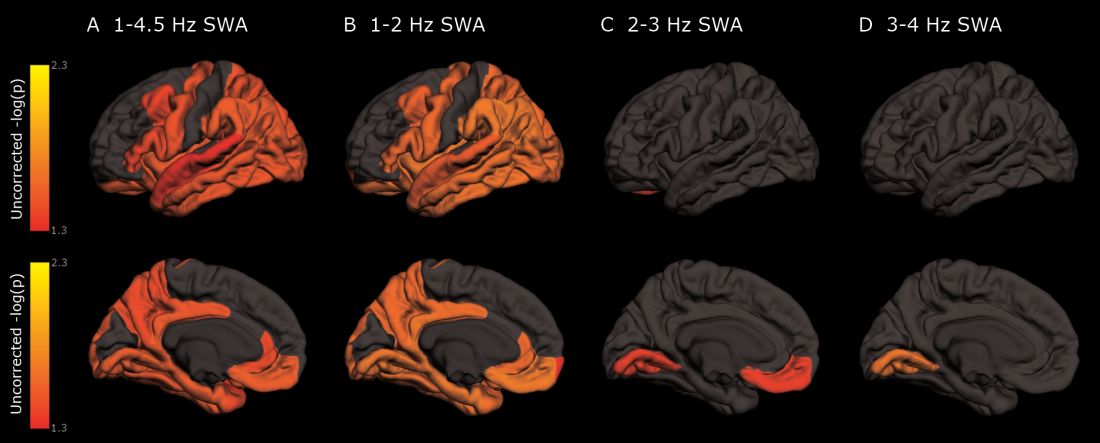
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Cognitively normal subjects with tau deposition experience altered sleep patterns.
Major finding: Decreased time in non-REM deep sleep was associated with increased tau pathology in Alzheimer’s-affected brain regions and in cerebrospinal fluid.
Study details: The prospective longitudinal study comprised 119 subjects.
Disclosures: The authors reported no relevant financial disclosures.
Source: Lucey BP et al. Sci Transl Med. 2019 Jan 9;11:eaau6550.
Alcohol use, psychological distress associated with possible RBD
(RBD), according to a population-based cohort study published in Neurology. In addition, the results also replicate previous findings of an association between possible RBD and smoking, low education, and male sex.
The risk factors for RBD have been studied comparatively little. “While much is still unknown about RBD, it can be caused by medications or it may be an early sign of another neurologic condition like Parkinson’s disease, dementia with Lewy bodies, or multiple system atrophy,” according to Ronald B. Postuma, MD, an associate professor at McGill University, Montreal. “Identifying lifestyle and personal risk factors linked to this sleep disorder may lead to finding ways to reduce the chances of developing it.”
To assess sociodemographic, socioeconomic, and clinical correlates of possible RBD, Dr. Postuma and his colleagues examined baseline data collected between 2012 and 2015 in the Canadian Longitudinal Study on Aging (CLSA), which included 30,097 participants. To screen for possible RBD, the CLSA researchers asked patients, “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep [e.g., punching, flailing your arms in the air, making running movements, etc.]?” Participants answered additional questions to rule out RBD mimics. Patients with symptom onset before age 20 years, positive apnea screen, or a diagnosis of dementia, Alzheimer’s disease, parkinsonism, or Parkinson’s disease were excluded from analysis.
In all, 3,271 participants screened positive for possible RBD. After the investigators excluded participants with potential mimics, 958 patients (about 3.2% of the total population) remained in the analysis. Approximately 59% of patients with possible RBD were male, compared with 42% of controls. Patients with possible RBD were more likely to be married, in a common-law relationship, or widowed.
Participants with possible RBD had slightly less education (estimated mean, 13.2 years vs. 13.6 years) and lower income, compared with controls. Participants with possible RBD retired at a slightly younger age (57.5 years vs. 58.6 years) and were more likely to have retired because of health concerns (28.9% vs. 22.0%), compared with controls.
In addition, patients with possible RBD were more likely to drink more and to be moderate to heavy drinkers than controls; they were also more likely to be current or past smokers. Antidepressant use was more frequent and psychological distress was greater among participants with possible RBD.
When the investigators performed a multivariable logistic regression analysis, the associations between possible RBD and male sex and relationship status remained. Lower educational level, but not income level, also remained associated with possible RBD. Furthermore, retirement age and having reported retirement because of health concerns remained significantly associated with possible RBD, as did the amount of alcohol consumed weekly and moderate to heavy drinking. Sensitivity analyses did not change the results significantly.
One of the study’s limitations is its reliance on self-report to identify participants with possible RBD, the authors wrote. The prevalence of possible RBD in the study was 3.2%, but research using polysomnography has found a prevalence of about 1%. Thus, the majority of cases in this study may have other disorders such as restless legs syndrome or periodic limb movements. Furthermore, many participants who enact their dreams (such as unmarried people) are likely unaware of it. Finally, the researchers did not measure several variables of interest, such as consumption of caffeinated products.
“The main advantages of our current study are the large sample size; the systematic population-based sampling; the capacity to adjust for diverse potential confounding variables, including mental illness; and the ability to screen out RBD mimics,” the authors concluded.
SOURCE: Postuma RB et al. Neurology. 2018 Dec 26. doi: 10.1212/WNL.0000000000006849.
(RBD), according to a population-based cohort study published in Neurology. In addition, the results also replicate previous findings of an association between possible RBD and smoking, low education, and male sex.
The risk factors for RBD have been studied comparatively little. “While much is still unknown about RBD, it can be caused by medications or it may be an early sign of another neurologic condition like Parkinson’s disease, dementia with Lewy bodies, or multiple system atrophy,” according to Ronald B. Postuma, MD, an associate professor at McGill University, Montreal. “Identifying lifestyle and personal risk factors linked to this sleep disorder may lead to finding ways to reduce the chances of developing it.”
To assess sociodemographic, socioeconomic, and clinical correlates of possible RBD, Dr. Postuma and his colleagues examined baseline data collected between 2012 and 2015 in the Canadian Longitudinal Study on Aging (CLSA), which included 30,097 participants. To screen for possible RBD, the CLSA researchers asked patients, “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep [e.g., punching, flailing your arms in the air, making running movements, etc.]?” Participants answered additional questions to rule out RBD mimics. Patients with symptom onset before age 20 years, positive apnea screen, or a diagnosis of dementia, Alzheimer’s disease, parkinsonism, or Parkinson’s disease were excluded from analysis.
In all, 3,271 participants screened positive for possible RBD. After the investigators excluded participants with potential mimics, 958 patients (about 3.2% of the total population) remained in the analysis. Approximately 59% of patients with possible RBD were male, compared with 42% of controls. Patients with possible RBD were more likely to be married, in a common-law relationship, or widowed.
Participants with possible RBD had slightly less education (estimated mean, 13.2 years vs. 13.6 years) and lower income, compared with controls. Participants with possible RBD retired at a slightly younger age (57.5 years vs. 58.6 years) and were more likely to have retired because of health concerns (28.9% vs. 22.0%), compared with controls.
In addition, patients with possible RBD were more likely to drink more and to be moderate to heavy drinkers than controls; they were also more likely to be current or past smokers. Antidepressant use was more frequent and psychological distress was greater among participants with possible RBD.
When the investigators performed a multivariable logistic regression analysis, the associations between possible RBD and male sex and relationship status remained. Lower educational level, but not income level, also remained associated with possible RBD. Furthermore, retirement age and having reported retirement because of health concerns remained significantly associated with possible RBD, as did the amount of alcohol consumed weekly and moderate to heavy drinking. Sensitivity analyses did not change the results significantly.
One of the study’s limitations is its reliance on self-report to identify participants with possible RBD, the authors wrote. The prevalence of possible RBD in the study was 3.2%, but research using polysomnography has found a prevalence of about 1%. Thus, the majority of cases in this study may have other disorders such as restless legs syndrome or periodic limb movements. Furthermore, many participants who enact their dreams (such as unmarried people) are likely unaware of it. Finally, the researchers did not measure several variables of interest, such as consumption of caffeinated products.
“The main advantages of our current study are the large sample size; the systematic population-based sampling; the capacity to adjust for diverse potential confounding variables, including mental illness; and the ability to screen out RBD mimics,” the authors concluded.
SOURCE: Postuma RB et al. Neurology. 2018 Dec 26. doi: 10.1212/WNL.0000000000006849.
(RBD), according to a population-based cohort study published in Neurology. In addition, the results also replicate previous findings of an association between possible RBD and smoking, low education, and male sex.
The risk factors for RBD have been studied comparatively little. “While much is still unknown about RBD, it can be caused by medications or it may be an early sign of another neurologic condition like Parkinson’s disease, dementia with Lewy bodies, or multiple system atrophy,” according to Ronald B. Postuma, MD, an associate professor at McGill University, Montreal. “Identifying lifestyle and personal risk factors linked to this sleep disorder may lead to finding ways to reduce the chances of developing it.”
To assess sociodemographic, socioeconomic, and clinical correlates of possible RBD, Dr. Postuma and his colleagues examined baseline data collected between 2012 and 2015 in the Canadian Longitudinal Study on Aging (CLSA), which included 30,097 participants. To screen for possible RBD, the CLSA researchers asked patients, “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep [e.g., punching, flailing your arms in the air, making running movements, etc.]?” Participants answered additional questions to rule out RBD mimics. Patients with symptom onset before age 20 years, positive apnea screen, or a diagnosis of dementia, Alzheimer’s disease, parkinsonism, or Parkinson’s disease were excluded from analysis.
In all, 3,271 participants screened positive for possible RBD. After the investigators excluded participants with potential mimics, 958 patients (about 3.2% of the total population) remained in the analysis. Approximately 59% of patients with possible RBD were male, compared with 42% of controls. Patients with possible RBD were more likely to be married, in a common-law relationship, or widowed.
Participants with possible RBD had slightly less education (estimated mean, 13.2 years vs. 13.6 years) and lower income, compared with controls. Participants with possible RBD retired at a slightly younger age (57.5 years vs. 58.6 years) and were more likely to have retired because of health concerns (28.9% vs. 22.0%), compared with controls.
In addition, patients with possible RBD were more likely to drink more and to be moderate to heavy drinkers than controls; they were also more likely to be current or past smokers. Antidepressant use was more frequent and psychological distress was greater among participants with possible RBD.
When the investigators performed a multivariable logistic regression analysis, the associations between possible RBD and male sex and relationship status remained. Lower educational level, but not income level, also remained associated with possible RBD. Furthermore, retirement age and having reported retirement because of health concerns remained significantly associated with possible RBD, as did the amount of alcohol consumed weekly and moderate to heavy drinking. Sensitivity analyses did not change the results significantly.
One of the study’s limitations is its reliance on self-report to identify participants with possible RBD, the authors wrote. The prevalence of possible RBD in the study was 3.2%, but research using polysomnography has found a prevalence of about 1%. Thus, the majority of cases in this study may have other disorders such as restless legs syndrome or periodic limb movements. Furthermore, many participants who enact their dreams (such as unmarried people) are likely unaware of it. Finally, the researchers did not measure several variables of interest, such as consumption of caffeinated products.
“The main advantages of our current study are the large sample size; the systematic population-based sampling; the capacity to adjust for diverse potential confounding variables, including mental illness; and the ability to screen out RBD mimics,” the authors concluded.
SOURCE: Postuma RB et al. Neurology. 2018 Dec 26. doi: 10.1212/WNL.0000000000006849.
FROM NEUROLOGY
Key clinical point: Alcohol use and psychological distress are associated with possible REM sleep behavior disorder.
Major finding: A self-report questionnaire yielded a 3.2% prevalence of possible REM sleep behavior disorder.
Study details: A prospective, population-based cohort study of 30,097 participants.
Disclosures: The Canadian government provided funding for the research.
Source: Postuma RB et al. Neurology. 2018 Dec 26. doi: 10.1212/WNL.0000000000006849.
MD Anderson–led alliance seeks to advance leukemia drug development
The primarily for leukemia.
The collaboration, led by Hagop Kantarjian, MD, chair of leukemia at MD Anderson, will use Ascentage’s proprietary Protein-Protein Interaction drug discovery technology platform to develop the company’s apoptosis-targeted and tyrosine kinase inhibitor drug candidates.
The drug candidates will be studied as single-agent therapies and in combinations with other approved or investigational therapeutics. The candidates, chosen for their potential to treat acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), myeloproliferative neoplasms, and myelofibrosis, include:
- HQP1351, a third-generation BCR-ABL inhibitor that has been shown to be safe and “highly active” in treating patients with chronic- or accelerated-phase CML, with or without the T3151 mutation. Preliminary results of the phase 1 study were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 791).
- APG-1252, a highly potent Bcl-2 family inhibitor, has high binding affinities to Bcl-2, Bcl-xL and Bcl-w. It has achieved tumor regression in small cell lung cancer, colon, breast, and ALL xenografts. A phase 1, dose-escalating study is currently being conducted (NCT03387332).
- APG-2575, a selective Bcl-2 inhibitor, is being studied in a phase 1, multicenter, single-agent trial in patients with B-cell hematologic malignancies, including multiple myeloma, chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, non-Hodgkin lymphomas, and AML (NCT03537482).
- APG-1387, an inhibitor of apoptosis protein, is being studied in solid tumors and hematologic malignancies (NCT03386526). Investigators asserted that combining it with an anti–programmed death 1 antibody would be “a very attractive approach” for cancer therapy. In advanced solid tumors it has been well tolerated with manageable adverse events, according to a study presented at the 2018 annual meeting of the American Society of Clinical Oncology (Abstract 2593).
- APG-115 is an MDM2-p53 inhibitor that, when combined with radiotherapy, has been shown to enhance the antitumor effect in gastric adenocarcinoma, according to a paper published in the Journal of Experimental & Clinical Cancer Research.
“We will be investigating this pipeline of candidate therapies, and we are interested in the novel mechanism of their actions,” Dr. Kantarjian said in a statement.
The primarily for leukemia.
The collaboration, led by Hagop Kantarjian, MD, chair of leukemia at MD Anderson, will use Ascentage’s proprietary Protein-Protein Interaction drug discovery technology platform to develop the company’s apoptosis-targeted and tyrosine kinase inhibitor drug candidates.
The drug candidates will be studied as single-agent therapies and in combinations with other approved or investigational therapeutics. The candidates, chosen for their potential to treat acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), myeloproliferative neoplasms, and myelofibrosis, include:
- HQP1351, a third-generation BCR-ABL inhibitor that has been shown to be safe and “highly active” in treating patients with chronic- or accelerated-phase CML, with or without the T3151 mutation. Preliminary results of the phase 1 study were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 791).
- APG-1252, a highly potent Bcl-2 family inhibitor, has high binding affinities to Bcl-2, Bcl-xL and Bcl-w. It has achieved tumor regression in small cell lung cancer, colon, breast, and ALL xenografts. A phase 1, dose-escalating study is currently being conducted (NCT03387332).
- APG-2575, a selective Bcl-2 inhibitor, is being studied in a phase 1, multicenter, single-agent trial in patients with B-cell hematologic malignancies, including multiple myeloma, chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, non-Hodgkin lymphomas, and AML (NCT03537482).
- APG-1387, an inhibitor of apoptosis protein, is being studied in solid tumors and hematologic malignancies (NCT03386526). Investigators asserted that combining it with an anti–programmed death 1 antibody would be “a very attractive approach” for cancer therapy. In advanced solid tumors it has been well tolerated with manageable adverse events, according to a study presented at the 2018 annual meeting of the American Society of Clinical Oncology (Abstract 2593).
- APG-115 is an MDM2-p53 inhibitor that, when combined with radiotherapy, has been shown to enhance the antitumor effect in gastric adenocarcinoma, according to a paper published in the Journal of Experimental & Clinical Cancer Research.
“We will be investigating this pipeline of candidate therapies, and we are interested in the novel mechanism of their actions,” Dr. Kantarjian said in a statement.
The primarily for leukemia.
The collaboration, led by Hagop Kantarjian, MD, chair of leukemia at MD Anderson, will use Ascentage’s proprietary Protein-Protein Interaction drug discovery technology platform to develop the company’s apoptosis-targeted and tyrosine kinase inhibitor drug candidates.
The drug candidates will be studied as single-agent therapies and in combinations with other approved or investigational therapeutics. The candidates, chosen for their potential to treat acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), myeloproliferative neoplasms, and myelofibrosis, include:
- HQP1351, a third-generation BCR-ABL inhibitor that has been shown to be safe and “highly active” in treating patients with chronic- or accelerated-phase CML, with or without the T3151 mutation. Preliminary results of the phase 1 study were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 791).
- APG-1252, a highly potent Bcl-2 family inhibitor, has high binding affinities to Bcl-2, Bcl-xL and Bcl-w. It has achieved tumor regression in small cell lung cancer, colon, breast, and ALL xenografts. A phase 1, dose-escalating study is currently being conducted (NCT03387332).
- APG-2575, a selective Bcl-2 inhibitor, is being studied in a phase 1, multicenter, single-agent trial in patients with B-cell hematologic malignancies, including multiple myeloma, chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, non-Hodgkin lymphomas, and AML (NCT03537482).
- APG-1387, an inhibitor of apoptosis protein, is being studied in solid tumors and hematologic malignancies (NCT03386526). Investigators asserted that combining it with an anti–programmed death 1 antibody would be “a very attractive approach” for cancer therapy. In advanced solid tumors it has been well tolerated with manageable adverse events, according to a study presented at the 2018 annual meeting of the American Society of Clinical Oncology (Abstract 2593).
- APG-115 is an MDM2-p53 inhibitor that, when combined with radiotherapy, has been shown to enhance the antitumor effect in gastric adenocarcinoma, according to a paper published in the Journal of Experimental & Clinical Cancer Research.
“We will be investigating this pipeline of candidate therapies, and we are interested in the novel mechanism of their actions,” Dr. Kantarjian said in a statement.
Daclizumab beta may be superior to interferon beta on MS disability progression
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
FROM MULTIPLE SCLEROSIS JOURNAL
Key clinical point: Daclizumab beta reduces the risk of 24-week sustained worsening on the MSFC by 20%, compared with interferon beta-1a.
Major finding: Daclizumab appears superior to interferon beta-1a regardless of patients’ demographic or disease characteristics.
Study details: A post hoc analysis of the DECIDE study, which included 1,841 patients with relapsing-remitting MS.
Disclosures: Biogen and AbbVie Biotherapeutics supported the DECIDE study.
Source: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
AAN publishes position statement on brain death
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
FROM NEUROLOGY
Key clinical point: The AAN calls for uniform brain death laws, policies, and practices.
Major finding: The association published a position statement online on January 2.
Study details: The AAN’s Brain Death Working Group drafted the statement.
Disclosures: The authors reported no relevant disclosures, and the American Academy of Neurology funded their work.
Source: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
Does rituximab delay disability progression in patients with secondary progressive MS?
, according to a retrospective analysis published online Jan. 7 in JAMA Neurology.
The results suggest that “B-cell depletion by rituximab may be therapeutically beneficial in these patients,” said study author Yvonne Naegelin, MD, of the department of neurology at University Hospital Basel, Switzerland, and her colleagues. “A prospective randomized clinical trial with a better level of evidence is needed to confirm the efficacy of rituximab in such patients.”
Research indicates that B cells play a role in the pathogenesis of relapsing-remitting and secondary progressive MS, and rituximab, a monoclonal CD20 antibody, may deplete B cells in the peripheral immune system and CNS. “Owing to the limited treatment options for secondary progressive MS and the extrapolation of results in relapsing-remitting MS and primary progressive MS, rituximab was used off-label for the treatment of secondary progressive MS,” the authors said. They compared disability progression in patients who were treated with rituximab at MS centers in Switzerland with disability of control patients with secondary progressive MS who did not receive rituximab. The control patients were part of an observational cohort study at MS centers in Switzerland and the Netherlands. Data for the present analysis were collected between 2004 and 2017.
The investigators matched rituximab-treated and control patients 1:1 using propensity scores. Matching variables were sex, age, EDSS score, and disease duration at baseline. Rituximab-treated patients had a mean age of 49.7 years, mean disease duration of 18.2 years, and mean EDSS score of 5.9; 59% were women. Controls had a mean age of 51.3 years, mean disease duration of 19.4 years, and mean EDSS score of 5.7; 61% were women.
A covariate-adjusted analysis of the matched set found that rituximab-treated patients had a significantly lower EDSS score during a mean follow-up of 3.5 years (mean difference, –0.52). In addition, time to confirmed disability progression was delayed in the rituximab-treated group (hazard ratio, 0.49). “Approximately 75% of untreated and 50% of treated individuals in our cohorts developed clinically significant confirmed progression for the 10-year period,” Dr. Naegelin and her colleagues reported. Complications, mainly related to infections, occurred in five cases during treatment. The researchers did not identify major safety concerns, however.
Dr. Naegelin had no conflict of interest disclosures. Several coauthors disclosed research support and compensation from pharmaceutical companies.
SOURCE: Naegelin Y et al. JAMA Neurol. 2019 Jan 7. doi: 10.1001/jamaneurol.2018.4239.
, according to a retrospective analysis published online Jan. 7 in JAMA Neurology.
The results suggest that “B-cell depletion by rituximab may be therapeutically beneficial in these patients,” said study author Yvonne Naegelin, MD, of the department of neurology at University Hospital Basel, Switzerland, and her colleagues. “A prospective randomized clinical trial with a better level of evidence is needed to confirm the efficacy of rituximab in such patients.”
Research indicates that B cells play a role in the pathogenesis of relapsing-remitting and secondary progressive MS, and rituximab, a monoclonal CD20 antibody, may deplete B cells in the peripheral immune system and CNS. “Owing to the limited treatment options for secondary progressive MS and the extrapolation of results in relapsing-remitting MS and primary progressive MS, rituximab was used off-label for the treatment of secondary progressive MS,” the authors said. They compared disability progression in patients who were treated with rituximab at MS centers in Switzerland with disability of control patients with secondary progressive MS who did not receive rituximab. The control patients were part of an observational cohort study at MS centers in Switzerland and the Netherlands. Data for the present analysis were collected between 2004 and 2017.
The investigators matched rituximab-treated and control patients 1:1 using propensity scores. Matching variables were sex, age, EDSS score, and disease duration at baseline. Rituximab-treated patients had a mean age of 49.7 years, mean disease duration of 18.2 years, and mean EDSS score of 5.9; 59% were women. Controls had a mean age of 51.3 years, mean disease duration of 19.4 years, and mean EDSS score of 5.7; 61% were women.
A covariate-adjusted analysis of the matched set found that rituximab-treated patients had a significantly lower EDSS score during a mean follow-up of 3.5 years (mean difference, –0.52). In addition, time to confirmed disability progression was delayed in the rituximab-treated group (hazard ratio, 0.49). “Approximately 75% of untreated and 50% of treated individuals in our cohorts developed clinically significant confirmed progression for the 10-year period,” Dr. Naegelin and her colleagues reported. Complications, mainly related to infections, occurred in five cases during treatment. The researchers did not identify major safety concerns, however.
Dr. Naegelin had no conflict of interest disclosures. Several coauthors disclosed research support and compensation from pharmaceutical companies.
SOURCE: Naegelin Y et al. JAMA Neurol. 2019 Jan 7. doi: 10.1001/jamaneurol.2018.4239.
, according to a retrospective analysis published online Jan. 7 in JAMA Neurology.
The results suggest that “B-cell depletion by rituximab may be therapeutically beneficial in these patients,” said study author Yvonne Naegelin, MD, of the department of neurology at University Hospital Basel, Switzerland, and her colleagues. “A prospective randomized clinical trial with a better level of evidence is needed to confirm the efficacy of rituximab in such patients.”
Research indicates that B cells play a role in the pathogenesis of relapsing-remitting and secondary progressive MS, and rituximab, a monoclonal CD20 antibody, may deplete B cells in the peripheral immune system and CNS. “Owing to the limited treatment options for secondary progressive MS and the extrapolation of results in relapsing-remitting MS and primary progressive MS, rituximab was used off-label for the treatment of secondary progressive MS,” the authors said. They compared disability progression in patients who were treated with rituximab at MS centers in Switzerland with disability of control patients with secondary progressive MS who did not receive rituximab. The control patients were part of an observational cohort study at MS centers in Switzerland and the Netherlands. Data for the present analysis were collected between 2004 and 2017.
The investigators matched rituximab-treated and control patients 1:1 using propensity scores. Matching variables were sex, age, EDSS score, and disease duration at baseline. Rituximab-treated patients had a mean age of 49.7 years, mean disease duration of 18.2 years, and mean EDSS score of 5.9; 59% were women. Controls had a mean age of 51.3 years, mean disease duration of 19.4 years, and mean EDSS score of 5.7; 61% were women.
A covariate-adjusted analysis of the matched set found that rituximab-treated patients had a significantly lower EDSS score during a mean follow-up of 3.5 years (mean difference, –0.52). In addition, time to confirmed disability progression was delayed in the rituximab-treated group (hazard ratio, 0.49). “Approximately 75% of untreated and 50% of treated individuals in our cohorts developed clinically significant confirmed progression for the 10-year period,” Dr. Naegelin and her colleagues reported. Complications, mainly related to infections, occurred in five cases during treatment. The researchers did not identify major safety concerns, however.
Dr. Naegelin had no conflict of interest disclosures. Several coauthors disclosed research support and compensation from pharmaceutical companies.
SOURCE: Naegelin Y et al. JAMA Neurol. 2019 Jan 7. doi: 10.1001/jamaneurol.2018.4239.
FROM JAMA NEUROLOGY
Key clinical point: Among patients with secondary progressive MS, those treated with rituximab may accrue less disability.
Major finding: Rituximab-treated patients, compared with controls, had a significantly lower EDSS score during a mean follow-up of 3.5 years (mean difference, –0.52).
Study details: A retrospective study of 88 propensity score–matched patients with secondary progressive MS.
Disclosures: Dr. Naegelin had no disclosures. Several coauthors disclosed research support and compensation from pharmaceutical companies.
Source: Naegelin Y et al. JAMA Neurol. 2019 Jan 7. doi: 10.1001/jamaneurol.2018.4239.
Armored CAR protects T cells, induces remissions
SAN DIEGO – A second-generation CD19-specific “armored” chimeric antigen receptor (CAR) T-cell construct was associated with high complete remission rates in diffuse large B-cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1 trial.
The CAR T construct – labeled 1928z-41BBL – also induced “encouraging” complete remission rates in patients with chronic lymphocytic leukemia (CLL) with Richter’s transformation, reported Jae H. Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC), New York, and his colleagues.
“Interestingly and encouragingly, severe [cytokine release syndrome] was not seen and grade 3 neurotoxicity was observed in less than 10%, with no grade 4 neurotoxicity, so there appears to be a favorable side effect profile,” Dr. Park said at the annual meeting of the American Society of Hematology.
Just as armored cars are designed to protect their valuable contents from people with bad intent, armored CAR T cells are engineered to protect the modified T-cells from a hostile tumor microenvironment and simultaneously recruit non-modified T cells to the target to produce a more robust immune response against malignant cells.
MSKCC investigators had previously shown that in contrast to other CAR T-cell constructs, the 1928z-41BBL configuration, which consists of two signaling domains (CD28 and CD3zeta) and the 4-1BB ligand, hit the sweet spot between tumor-killing function and T-cell persistence (Cancer Cell. 2015 Oct 12;28[4]:415-28).
In the current study, they enrolled 35 adults with relapsed or refractory CD19-positive hematologic malignancies, 29 of whom eventually underwent CAR T-cell infusions. The treated population comprised 14 patients with CLL (4 of whom had Richter’s transformation), 9 with DLBCL, 5 with indolent NHL, and 1 with acute lymphoblastic leukemia.
The patients with CLL had received a median of 5.5 prior lines of therapy, including ibrutinib (Imbruvica) and venetoclax (Venclexta).
There were 15 complete remissions (CR), with CR rates of 78% in DLBCL, 20% in CLL, 67% in CLL with Richter’s transformation, 60% in patients with indolent NHL, as well as CR in the single patient with ALL.
There were eight partial remissions. One patient with CLL had stable disease, and four patients had disease progression (one patient each with DLBCL, CLL, CLL with Richter’s, and indolent NHL).
Dr. Park noted that T cells are being detected in peripheral blood more than 6 months after T-cell infusion.
There were no cases of severe cytokine release syndrome, defined as requiring vasopressors and/or mechanical ventilation for hypoxia, and just three cases of grade 3 neurotoxicity. There were no cases of grade 4 neurotoxicity, no deaths related to neurotoxicity, and no cases of cerebral edema – a serious complication that has been seen in earlier CAR T-cell studies.
Split or multiple infusions of CAR T cells or incorporation of the technique into earlier lines of therapy might generate higher response rates, Dr. Park said.
The study was supported by Juno Therapeutics. Dr. Park reported consulting for and research funding from Juno, and financial relationships with other companies.
SOURCE: Park JH et al. ASH 2018, Abstract 224.
SAN DIEGO – A second-generation CD19-specific “armored” chimeric antigen receptor (CAR) T-cell construct was associated with high complete remission rates in diffuse large B-cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1 trial.
The CAR T construct – labeled 1928z-41BBL – also induced “encouraging” complete remission rates in patients with chronic lymphocytic leukemia (CLL) with Richter’s transformation, reported Jae H. Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC), New York, and his colleagues.
“Interestingly and encouragingly, severe [cytokine release syndrome] was not seen and grade 3 neurotoxicity was observed in less than 10%, with no grade 4 neurotoxicity, so there appears to be a favorable side effect profile,” Dr. Park said at the annual meeting of the American Society of Hematology.
Just as armored cars are designed to protect their valuable contents from people with bad intent, armored CAR T cells are engineered to protect the modified T-cells from a hostile tumor microenvironment and simultaneously recruit non-modified T cells to the target to produce a more robust immune response against malignant cells.
MSKCC investigators had previously shown that in contrast to other CAR T-cell constructs, the 1928z-41BBL configuration, which consists of two signaling domains (CD28 and CD3zeta) and the 4-1BB ligand, hit the sweet spot between tumor-killing function and T-cell persistence (Cancer Cell. 2015 Oct 12;28[4]:415-28).
In the current study, they enrolled 35 adults with relapsed or refractory CD19-positive hematologic malignancies, 29 of whom eventually underwent CAR T-cell infusions. The treated population comprised 14 patients with CLL (4 of whom had Richter’s transformation), 9 with DLBCL, 5 with indolent NHL, and 1 with acute lymphoblastic leukemia.
The patients with CLL had received a median of 5.5 prior lines of therapy, including ibrutinib (Imbruvica) and venetoclax (Venclexta).
There were 15 complete remissions (CR), with CR rates of 78% in DLBCL, 20% in CLL, 67% in CLL with Richter’s transformation, 60% in patients with indolent NHL, as well as CR in the single patient with ALL.
There were eight partial remissions. One patient with CLL had stable disease, and four patients had disease progression (one patient each with DLBCL, CLL, CLL with Richter’s, and indolent NHL).
Dr. Park noted that T cells are being detected in peripheral blood more than 6 months after T-cell infusion.
There were no cases of severe cytokine release syndrome, defined as requiring vasopressors and/or mechanical ventilation for hypoxia, and just three cases of grade 3 neurotoxicity. There were no cases of grade 4 neurotoxicity, no deaths related to neurotoxicity, and no cases of cerebral edema – a serious complication that has been seen in earlier CAR T-cell studies.
Split or multiple infusions of CAR T cells or incorporation of the technique into earlier lines of therapy might generate higher response rates, Dr. Park said.
The study was supported by Juno Therapeutics. Dr. Park reported consulting for and research funding from Juno, and financial relationships with other companies.
SOURCE: Park JH et al. ASH 2018, Abstract 224.
SAN DIEGO – A second-generation CD19-specific “armored” chimeric antigen receptor (CAR) T-cell construct was associated with high complete remission rates in diffuse large B-cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1 trial.
The CAR T construct – labeled 1928z-41BBL – also induced “encouraging” complete remission rates in patients with chronic lymphocytic leukemia (CLL) with Richter’s transformation, reported Jae H. Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC), New York, and his colleagues.
“Interestingly and encouragingly, severe [cytokine release syndrome] was not seen and grade 3 neurotoxicity was observed in less than 10%, with no grade 4 neurotoxicity, so there appears to be a favorable side effect profile,” Dr. Park said at the annual meeting of the American Society of Hematology.
Just as armored cars are designed to protect their valuable contents from people with bad intent, armored CAR T cells are engineered to protect the modified T-cells from a hostile tumor microenvironment and simultaneously recruit non-modified T cells to the target to produce a more robust immune response against malignant cells.
MSKCC investigators had previously shown that in contrast to other CAR T-cell constructs, the 1928z-41BBL configuration, which consists of two signaling domains (CD28 and CD3zeta) and the 4-1BB ligand, hit the sweet spot between tumor-killing function and T-cell persistence (Cancer Cell. 2015 Oct 12;28[4]:415-28).
In the current study, they enrolled 35 adults with relapsed or refractory CD19-positive hematologic malignancies, 29 of whom eventually underwent CAR T-cell infusions. The treated population comprised 14 patients with CLL (4 of whom had Richter’s transformation), 9 with DLBCL, 5 with indolent NHL, and 1 with acute lymphoblastic leukemia.
The patients with CLL had received a median of 5.5 prior lines of therapy, including ibrutinib (Imbruvica) and venetoclax (Venclexta).
There were 15 complete remissions (CR), with CR rates of 78% in DLBCL, 20% in CLL, 67% in CLL with Richter’s transformation, 60% in patients with indolent NHL, as well as CR in the single patient with ALL.
There were eight partial remissions. One patient with CLL had stable disease, and four patients had disease progression (one patient each with DLBCL, CLL, CLL with Richter’s, and indolent NHL).
Dr. Park noted that T cells are being detected in peripheral blood more than 6 months after T-cell infusion.
There were no cases of severe cytokine release syndrome, defined as requiring vasopressors and/or mechanical ventilation for hypoxia, and just three cases of grade 3 neurotoxicity. There were no cases of grade 4 neurotoxicity, no deaths related to neurotoxicity, and no cases of cerebral edema – a serious complication that has been seen in earlier CAR T-cell studies.
Split or multiple infusions of CAR T cells or incorporation of the technique into earlier lines of therapy might generate higher response rates, Dr. Park said.
The study was supported by Juno Therapeutics. Dr. Park reported consulting for and research funding from Juno, and financial relationships with other companies.
SOURCE: Park JH et al. ASH 2018, Abstract 224.
REPORTING FROM ASH 2018
Key clinical point: The 1928z-41BBL CAR T-cell construct induced high rates of complete remissions.
Major finding: The CAR T product was associated with a 78% complete remission rate in patients with heavily pretreated diffuse large B-cell lymphoma.
Study details: A phase 1 trial in 29 patients with CD19-positive hematologic malignancies.
Disclosures: Juno Therapeutics supported the study. Dr. Park reported consulting for and research funding from Juno, and financial relationships with other companies.
Source: Park JH et al. ASH 2018, Abstract 224.