User login
PARADISE-MI results obscured as post hoc analysis finds flaws
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
A post hoc analysis of the PARADISE-MI trial, although not intended to alter the conclusions generated by the published data, suggests that clinically relevant benefits were obscured, providing the basis for recommending different analyses for future studies that are more suited to capture the most clinically significant endpoints.
“What these data show us is that we need clinical trial designs moving towards more pragmatic information that better reflect clinical practice,” reported Otavio Berwanger, MD, PhD, director of the Academic Research Organization at Hospital Israelita Albert Einstein, São Paulo, Brazil.
The reevaluation of the PARADISE-MI data, presented at the annual congress of the European Society of Cardiology in Barcelona, was based on a win ratio analysis and on the inclusion of investigator-reported endpoints, not just adjudicated events. Both appear to reveal clinically meaningful benefits not reflected in the published study, according to Dr. Berwanger.
In PARADISE-MI, which was published in the New England Journal of Medicine last year, more than 5,500 patients were randomized to the angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan or the ACE inhibitor ramipril after a myocardial infarction. A reduced left ventricular ejection fraction (LVEF), pulmonary congestion, or both were required for enrollment.
For the primary composite outcomes of death from cardiovascular (CV) causes or incident heart failure, the ARNI had a 10% numerical advantage, but it did not reach statistical significance (hazard ratio [HR], 0.90; P = .17).
“PARADISE-MI was a neutral trial. This post hoc analysis will not change that result,” Dr. Berwanger emphasized. However, the post hoc analysis does provide a basis for exploring why conventional trial designs might not be providing answers that are relevant and helpful for clinical practice.
New analysis provides positive trial result
When the data from PARADISE-MI are reevaluated in a hierarchical win ratio analysis with CV death serving as the most severe and important outcome, the principal conclusion changes. Whether events are reevaluated in this format by the clinical events committee (CEC) or by investigators, there is a greater number of total wins than total losses for the ARNI. Combined, sacubitril/valsartan was associated with a win ratio of 1.17 (95% confidence interval, 1.03-1.33; P = 0.015) over ramipril.
Using a sports analogy, Dr. Berwanger explained that the win ratio analysis divides the total number of wins to the total number of losses to provide a much more clinically relevant approach to keeping score. It also used a hierarchical analysis so that the most serious and important events are considered first.
In addition to CV death, this analysis included first hospitalization for heart failure and first outpatient heart failure events. CEC-defined events and events reported by investigators were evaluated separately.
The ARNI had more wins than losses in every category for all outcomes, whether CEC adjudicated or investigator reported, but most of this benefit was generated by the endpoint of CEC-adjudicated CV deaths. This accounted for 36.9% of all events (investigator-documented CV death accounted for 0.7%). This is important because PARADISE-MI, like many standard trials, was conducted on a time-to-primary event basis.
“In this type of analysis, the first event is what counts. Usually time-to-first-event analyses are dominated by nonfatal events,” Dr. Berwanger explained. He believes that placing more weight on the most serious events results in an emphasis on what outcomes are of greatest clinical interest.
In addition, Dr. Berwanger argued that it is important to consider investigator-reported events, not just CEC-adjudicated events. While adjudicated events improve the rigor of the data, Dr. Berwanger suggested it omits outcomes with which clinicians are most concerned.
Investigator, adjudicated outcomes differ
Again, using PARADISE-MI as an example, he reevaluated the primary outcome based on investigator reports. When investigator-reported events are included, the number of events increased in both the ARNI (443 vs. 338) and ramipril (516 vs. 373) arms, but the advantage of the ARNI over the ACE inhibitors now reached statistical significance (HR, 0.85; P = .01).
“The data suggest that maybe we should find definitions for adjudication that are closer to clinical judgment in the real world and clinical practice,” Dr. Berwanger said.
One possible explanation for the neutral result in PARADISE-MI is that benefit of an ARNI over an ACE inhibitor would only be expected in those at risk for progressive left ventricular dysfunction, and it is likely that a substantial proportion of patients enrolled in this trial recovered, according to Johann Bauersachs, MD, PhD, professor and head of cardiology at Hannover (Germany) Medical School.
“You cannot predict which patients with reduced LV function following an MI will go on to chronic remodeling and which will recover,” said Dr. Bauersachs, who was an ESC-invited discussant of Dr. Berwanger’s post hoc analysis.
He agreed that Dr. Berwanger has raised several important issues in standard trial design that might have prevented PARADISE-MI from showing a benefit from an ARNI, but he pointed out that there are other potential issues, such as the low use of mineralocorticoid antagonists in PARADISE-MI, that may have skewed results.
However, he agreed generally with the premise that there is a need for trial design likely to generate more clinically useful information.
“We have now seen the win-ratio approach used in several studies,” said Dr. Bauersachs, citing in particular the EMPULSE trial presented at the 2022 meeting of the American College of Cardiology. “It is a very useful tool, and I think we will be seeing it used more in the future.”
However, he indicated that the issues raised by Dr. Berwanger are not necessarily easily resolved. Dr. Bauersachs endorsed the effort to consider trial designs that generate data that are more immediately clinically applicable but suggested that different types of designs may be required for different types of clinical questions.
Dr. Berwanger reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Pfizer, Servier, and Novartis, which provided funding for the PARADISE-MI trial. Dr. Bauersachs reports financial relationships with Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Cardior, Corvia, CVRx, Novartis, Pfizer, Vifor, and Zoll.
FROM ESC CONGRESS 2022
Polypodium leucotomos found to reverse AK skin damage
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
AT THE EADV CONGRESS
AI-assisted reading of echocardiograms readily detects severe aortic stenosis
AI might facilitate early intervention
Patients with aortic stenosis (AS) of sufficient severity to portend a high likelihood of early mortality can be detected by an artificial intelligence (AI) algorithm employed in the reading of routine echocardiograms, according to a study that tested this tool in a large national database.
The artificial intelligence decision support algorithm (AI-DSA) “automatically identified patients with moderate to severe forms of AS associated with poor survival if left untreated,” reported Geoffrey A. Strange, PhD, professor, faculty of medicine, University of Sydney.
The AS-DSA was trained not only to recognize adverse changes in aortic valve morphology but to evaluate indices of impaired valve function, including those related to the left ventricle, the left atrium, and pulmonary circulation, according to Dr. Strange.
AI algorithm based on more than 800K echos
The training was performed on more than 1 million echocardiograms obtained from 630,000 patients in the National Echo Database (NEDA) of Australia. The testing phase of the study, called AI ENHANCED AS, was carried out on 179,054 individuals from the same database.
In the testing phase, mortality was compared for those determined by AI to have a low probability of clinically significant AS, a moderate to severe AS, or severe AS.
In the nearly 200,000 patients evaluated from the database, the AI-DSA classified 2.5% as having moderate to severe AS and 1.4% as having severe AS. Relative to a 22.9% mortality at 5 years in the low-risk reference group, the rates were 56.2% and 67.9% in the moderate to severe and severe groups, respectively.
When expressed as odds ratios, the mortality risk for the moderate to severe group (OR, 1.8; P < .001) and severe group (HR, 2.8; P < .001) “were about two to three times higher than the low probability group,” Dr. Strange reported.
All severe AS by guidelines AI identified
The algorithm picked up all patients identified with severe AS in current guidelines, but it also identified patients “missed by conventional definitions,” Dr. Strange reported.
The findings support the idea “that the AI algorithm could be used in clinical practice to alert physicians to patients who should undergo further investigations to determine if they qualify for aortic valve replacement,” he added.
Missing clinically significant AS is an important clinical problem, according to Catherine Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle.
“We focus on the patients who already have a diagnosis of AS,” she said. “The bigger issue is identification of patients with unknown AS.”
She praised the effort to develop AI that improves detection of AS, but also said that there are immediate steps to improve detection of AS even in the absence of AI support. In addition to the variability in the quality of how echocardiograms are read, she said a substantial proportion of echo reports omit key variables.
“We do not need AI to measure the aortic valve. It is simple to do in clinical practice,” she said. However, studies have repeatedly shown that values, such as maximal aortic jet velocity (Vmax) and the pressure difference across the ventricular septal defect (delta P), are not included. When AS is present, some reports do not include a characterization of the severity.
The AI-DSA described by Dr. Strange takes into account all of these variables along with additional information, but he acknowledged that it does have limitations. For example, the presence of cardiac impairments other than AS will not be included, and these can be relevant to prognostication and treatment.
AI does not eliminate clinical decision-making
“This algorithm is definitely not meant to take away from clinical decision-making,” Dr. Strange said, but he argued that there is an unmet need to do better in the detection of AS. He presented data to show that “even moderate AS is not benign” in regard to 5-year outcomes, and he believes AI-DSA can allow clinicians to detect significant disease earlier and intervene in a timelier manner.
“It is time to revisit the practice of watchful waiting and consider more proactive attempts to identify those at risk,” he said.
The next step is to determine if AI-DSA makes a clinical difference,
“Research is now needed to determine if aortic valve replacement in patients identified as being at risk by AI-DSA improves survival and quality of life, particularly in those who do not meet current guideline definitions of clinically significant disease,” he said.
Dr. Strange reports financial relationships with Edwards, Medtronic, Novartis, Pfizer, and Echo IQ, which is developing the artificial algorithm studied in this trial. Dr. Otto reports no relevant conflicts of interest.
AI might facilitate early intervention
AI might facilitate early intervention
Patients with aortic stenosis (AS) of sufficient severity to portend a high likelihood of early mortality can be detected by an artificial intelligence (AI) algorithm employed in the reading of routine echocardiograms, according to a study that tested this tool in a large national database.
The artificial intelligence decision support algorithm (AI-DSA) “automatically identified patients with moderate to severe forms of AS associated with poor survival if left untreated,” reported Geoffrey A. Strange, PhD, professor, faculty of medicine, University of Sydney.
The AS-DSA was trained not only to recognize adverse changes in aortic valve morphology but to evaluate indices of impaired valve function, including those related to the left ventricle, the left atrium, and pulmonary circulation, according to Dr. Strange.
AI algorithm based on more than 800K echos
The training was performed on more than 1 million echocardiograms obtained from 630,000 patients in the National Echo Database (NEDA) of Australia. The testing phase of the study, called AI ENHANCED AS, was carried out on 179,054 individuals from the same database.
In the testing phase, mortality was compared for those determined by AI to have a low probability of clinically significant AS, a moderate to severe AS, or severe AS.
In the nearly 200,000 patients evaluated from the database, the AI-DSA classified 2.5% as having moderate to severe AS and 1.4% as having severe AS. Relative to a 22.9% mortality at 5 years in the low-risk reference group, the rates were 56.2% and 67.9% in the moderate to severe and severe groups, respectively.
When expressed as odds ratios, the mortality risk for the moderate to severe group (OR, 1.8; P < .001) and severe group (HR, 2.8; P < .001) “were about two to three times higher than the low probability group,” Dr. Strange reported.
All severe AS by guidelines AI identified
The algorithm picked up all patients identified with severe AS in current guidelines, but it also identified patients “missed by conventional definitions,” Dr. Strange reported.
The findings support the idea “that the AI algorithm could be used in clinical practice to alert physicians to patients who should undergo further investigations to determine if they qualify for aortic valve replacement,” he added.
Missing clinically significant AS is an important clinical problem, according to Catherine Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle.
“We focus on the patients who already have a diagnosis of AS,” she said. “The bigger issue is identification of patients with unknown AS.”
She praised the effort to develop AI that improves detection of AS, but also said that there are immediate steps to improve detection of AS even in the absence of AI support. In addition to the variability in the quality of how echocardiograms are read, she said a substantial proportion of echo reports omit key variables.
“We do not need AI to measure the aortic valve. It is simple to do in clinical practice,” she said. However, studies have repeatedly shown that values, such as maximal aortic jet velocity (Vmax) and the pressure difference across the ventricular septal defect (delta P), are not included. When AS is present, some reports do not include a characterization of the severity.
The AI-DSA described by Dr. Strange takes into account all of these variables along with additional information, but he acknowledged that it does have limitations. For example, the presence of cardiac impairments other than AS will not be included, and these can be relevant to prognostication and treatment.
AI does not eliminate clinical decision-making
“This algorithm is definitely not meant to take away from clinical decision-making,” Dr. Strange said, but he argued that there is an unmet need to do better in the detection of AS. He presented data to show that “even moderate AS is not benign” in regard to 5-year outcomes, and he believes AI-DSA can allow clinicians to detect significant disease earlier and intervene in a timelier manner.
“It is time to revisit the practice of watchful waiting and consider more proactive attempts to identify those at risk,” he said.
The next step is to determine if AI-DSA makes a clinical difference,
“Research is now needed to determine if aortic valve replacement in patients identified as being at risk by AI-DSA improves survival and quality of life, particularly in those who do not meet current guideline definitions of clinically significant disease,” he said.
Dr. Strange reports financial relationships with Edwards, Medtronic, Novartis, Pfizer, and Echo IQ, which is developing the artificial algorithm studied in this trial. Dr. Otto reports no relevant conflicts of interest.
Patients with aortic stenosis (AS) of sufficient severity to portend a high likelihood of early mortality can be detected by an artificial intelligence (AI) algorithm employed in the reading of routine echocardiograms, according to a study that tested this tool in a large national database.
The artificial intelligence decision support algorithm (AI-DSA) “automatically identified patients with moderate to severe forms of AS associated with poor survival if left untreated,” reported Geoffrey A. Strange, PhD, professor, faculty of medicine, University of Sydney.
The AS-DSA was trained not only to recognize adverse changes in aortic valve morphology but to evaluate indices of impaired valve function, including those related to the left ventricle, the left atrium, and pulmonary circulation, according to Dr. Strange.
AI algorithm based on more than 800K echos
The training was performed on more than 1 million echocardiograms obtained from 630,000 patients in the National Echo Database (NEDA) of Australia. The testing phase of the study, called AI ENHANCED AS, was carried out on 179,054 individuals from the same database.
In the testing phase, mortality was compared for those determined by AI to have a low probability of clinically significant AS, a moderate to severe AS, or severe AS.
In the nearly 200,000 patients evaluated from the database, the AI-DSA classified 2.5% as having moderate to severe AS and 1.4% as having severe AS. Relative to a 22.9% mortality at 5 years in the low-risk reference group, the rates were 56.2% and 67.9% in the moderate to severe and severe groups, respectively.
When expressed as odds ratios, the mortality risk for the moderate to severe group (OR, 1.8; P < .001) and severe group (HR, 2.8; P < .001) “were about two to three times higher than the low probability group,” Dr. Strange reported.
All severe AS by guidelines AI identified
The algorithm picked up all patients identified with severe AS in current guidelines, but it also identified patients “missed by conventional definitions,” Dr. Strange reported.
The findings support the idea “that the AI algorithm could be used in clinical practice to alert physicians to patients who should undergo further investigations to determine if they qualify for aortic valve replacement,” he added.
Missing clinically significant AS is an important clinical problem, according to Catherine Otto, MD, director of the heart valve clinic and a professor of cardiology at the University of Washington Medical Center, Seattle.
“We focus on the patients who already have a diagnosis of AS,” she said. “The bigger issue is identification of patients with unknown AS.”
She praised the effort to develop AI that improves detection of AS, but also said that there are immediate steps to improve detection of AS even in the absence of AI support. In addition to the variability in the quality of how echocardiograms are read, she said a substantial proportion of echo reports omit key variables.
“We do not need AI to measure the aortic valve. It is simple to do in clinical practice,” she said. However, studies have repeatedly shown that values, such as maximal aortic jet velocity (Vmax) and the pressure difference across the ventricular septal defect (delta P), are not included. When AS is present, some reports do not include a characterization of the severity.
The AI-DSA described by Dr. Strange takes into account all of these variables along with additional information, but he acknowledged that it does have limitations. For example, the presence of cardiac impairments other than AS will not be included, and these can be relevant to prognostication and treatment.
AI does not eliminate clinical decision-making
“This algorithm is definitely not meant to take away from clinical decision-making,” Dr. Strange said, but he argued that there is an unmet need to do better in the detection of AS. He presented data to show that “even moderate AS is not benign” in regard to 5-year outcomes, and he believes AI-DSA can allow clinicians to detect significant disease earlier and intervene in a timelier manner.
“It is time to revisit the practice of watchful waiting and consider more proactive attempts to identify those at risk,” he said.
The next step is to determine if AI-DSA makes a clinical difference,
“Research is now needed to determine if aortic valve replacement in patients identified as being at risk by AI-DSA improves survival and quality of life, particularly in those who do not meet current guideline definitions of clinically significant disease,” he said.
Dr. Strange reports financial relationships with Edwards, Medtronic, Novartis, Pfizer, and Echo IQ, which is developing the artificial algorithm studied in this trial. Dr. Otto reports no relevant conflicts of interest.
FROM ESC CONGRESS 2022
ARBs, beta-blockers independently inhibit Marfan syndrome progression
Early start might delay surgery
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
Early start might delay surgery
Early start might delay surgery
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
Beta-blockers have long been recommended to prevent aortic dissection associated with Marfan syndrome despite limited evidence, but a new analysis also supports a benefit from angiotensin receptors blockers (ARBs) and further suggests that beta-blockers and ARBs exert independent effects.
For the endpoint of inhibition of growth of the aortic root, “there is no evidence of any interaction between the effects of ARBs with beta-blockers, and so we think that the treatment effects are likely to be additive,” reported Alex Pitcher, BMBCh, DPhil, Oxford (England) University Hospitals, NHS Trust.
Based on these data, Dr. Pitcher recommended considering ARBs and beta-blockers together soon after the diagnosis of Marfan syndrome. This includes young children.
“We think that medical treatments can delay surgery and dissection substantially if given for a number of years,” he added.
In this study, undertaken by the Marfan Treatment Trialists (MTT) collaboration, data were available from 1,442 Marfan syndrome patients participating in seven treatment trials. The primary outcome was aortic root enlargement, a predictor of life-threatening aortic dissection and rupture. Rather than a meta-analysis of the pooled data, the meta-analysis was conducted with individual patient data that involved collaboration with the original trialists.
Four of the studies with 746 patients compared ARBs to placebo or a control medication. A second group of three trials with 766 patients compared ARBs to beta-blockers.
From the two sets of data, a calculation of the effect of beta-blockers was indirectly estimated.
ARBs slow annualized aortic growth rate significantly
In the first set of trials, the analysis showed a significantly slower annualized aortic root growth rate for those treated with ARBs relative to controls (0.07 vs. 0.13), producing a statistically significant absolute difference (0.7%; P = .01) in favor of the ARB.
“In other words, the rate of growth was nearly double in the control arm,” Dr. Pitcher said.
In the three trials comparing ARBs to beta-blockers, the annualized growth rate among those taking an ARB was similar (0.8%) to that seen in the previous set of controlled trials. This rate of annualized growth was not significantly different from the 0.11% annualized rate of growth in patients receiving beta-blockers. When an analysis of the impact of beta-blockers was conducted by indirectly evaluating the change in growth relative to controls, the estimated impact was an annualized growth rate of 0.9% (P = .042).
A second set of data provided the basis for suggesting that the effects of beta ARBs and beta-blockers are independent and potentially additive.
“We were able to look at subgroups of patients in the ARB trials that were broken down by whether they were or were not on beta-blockers at baseline, and so by doing able to estimate independent effects,” Dr. Pitcher said. The lack of any interactions led Dr. Pitcher to conclude that benefits are likely additive.
Of patients genotyped in the ARB studies, more than 80% had the FBN1 pathogenic variant of Marfan syndrome. When the data were analyzed by subgroups, including age or blood pressure, there were no differences in treatment effect except for those with the FBN1 mutation in whom the benefit of ARB therapy was greater relative to those without.
As FBN1 is one of the most common genetic signatures of Marfan syndrome, the “greater effect of ARBs in this group makes it more plausible that the effect is real,” Dr. Pitcher said.
Results could change treatment guidelines
Current guidelines recommend beta-blockers in Marfan syndrome prior to a dilatation size of 4.5 to 5 cm when surgery is indicated, according to Dr. Pitcher, but he said these data might change guidelines. While reinforcing the benefit of beta-blockers, this analysis suggests ARBs should also be considered, possibly in combination with beta-blockers.
“What I hope this meta-analysis does is add substantially to the certainty with which physicians can discuss treatments with patients.”
As for the mechanism, it is reasonable to speculate the antihypertensive effect of both medications is relevant, but each has plausible independent activities that might contribute to modifying aortic growth, according to Roland R.J. van Kimmenade, MD, PhD, a specialist in aortic diseases and heart failure at Raboud University Medical Center, Nijmegan, the Netherlands.
Citing several studies, he suggested that the benefit of beta-blockers could also stem from their ability to reduce heart rate and aortic stiffness while ARBs are likely to inhibit the interaction between the renin-angiotensin system (RAS) and TGF-beta pathway. Each of these might participate in risk of aortic root growth, according to Dr. van Kimmenade, who was invited by ESC to discuss this study.
On the basis of these data as well as past studies, he agreed that the combination of beta-blockers and ARBs might not just be additive but “even a little bit synergistic.”
While Dr. Pitcher suggested that the evidence supports starting both beta-blockers and ARBs soon after the diagnosis, Dr. van Kimmenade said, “I don’t like using beta-blockers in young patients, but ARBs are now shown to be an excellent alternative.”
Ultimately, “the prescription pencil will not replace the surgical knife” in a disease that is likely to eventually require surgery to prevent life-threatening events, according to Dr. van Kimmenade, but he agreed that these data provide more certainty about the value of beta-blockers and ARBs for slowing progression.
Dr. Pitcher reports no potential conflicts of interest. Dr. van Kimmenade has financial relationships with Bayer and Novartis.
FROM ESC CONGRESS 2022
New ovulatory disorder classifications from FIGO replace 50-year-old system
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
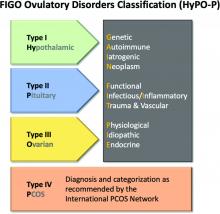
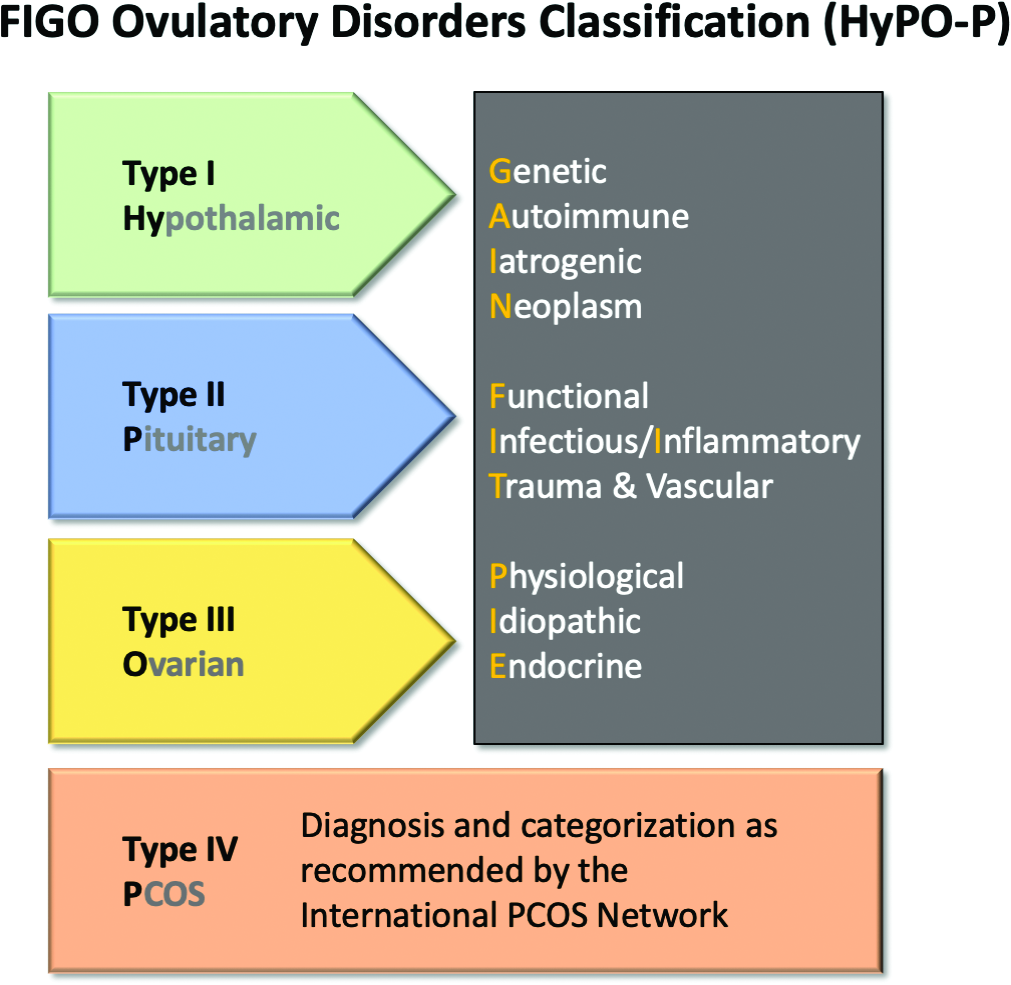
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
FROM INTERNATIONAL JOURNAL OF GYNECOLOGY AND OBSTETRICS
Artificial intelligence poised to change paradigm of CV risk prevention
Causal-based algorithm personalizes strategies
Typically, artificial intelligence (AI) is applied to analyze a complex set of variables to make correlations not readily made by unassisted observation. But an AI offshoot, sometimes referred to as causal AI, incorporates causation not just association, and it appears capable of changing the paradigm for preventing cardiovascular (CV) events.
“Causal AI is a new generation of AI algorithms that empowers AI to move beyond prediction to help guide clinical decision-making for each individual,” reported Brian A. Ference, MD, director of research in translational therapeutics, University of Cambridge (England).
In a novel study testing this premise, called CAUSAL AI, this approach was explored with two major risk factors, elevated LDL cholesterol (LDL-C) and elevated systolic BP (SBP). Based on a deep learning algorithm that studied the impact of these risk factors on the biology of atherosclerosis, causal effects of these risk factors were assessed and then embedded in risk estimation.
Causal AI can predict treatment effect
The study showed that the accuracy of risk prediction can be improved markedly with causal AI, but, more importantly, it suggests that causal AI can predict the impact of specific actions to reduce this risk in the context of the patient’s trajectory toward CV events.
“Risk-estimating algorithms are used to select patients at high risk who may benefit from interventions to reduce risk, but they do not include the causal effects of changes in LDL-C and SBP,” Dr. Ference explained.
As a result, they “may not accurately estimate the baseline risk of cardiovascular events caused by a person’s LDL-C or SBP level or the benefit of treating these risk factors,” he added.
In the CAUSAL AI study, presented at the annual congress of the European Society of Cardiology, risk prediction embedded with causal AI demonstrated the ability to match predicted events with actual events in several large sets of patient data.
“Embedding causal effects into risk-estimating algorithms accurately estimates baseline cardiovascular risk caused by LDL and SBP and the benefit of lowering LDL, SBP, or both beginning at any age and extending for any duration,” Dr. Ference said.
Deep-learning AI evaluated more than 300 gene variants
The deep-learning AI was based on Mendelian randomization studies evaluating 140 gene variants associated with LDL-C and 202 variants associated with SBP.
In one test of the predictive impact of causal AI, risk prediction was first conducted in 445,771 participants in the UK Biobank with the Joint British Societies (JBS3) risk calculator. Relative to actual events in this population, the JBS3 alone “consistently underestimated the increased risk caused by elevated LDL, blood pressure, or both” over the lifetime of the patient, according to Dr. Ference.
It also systematically overestimated the risk of cardiovascular events among participants with lower LDL-C, blood pressure, or both.
However, after embedding the causal effect of LDL and blood pressure, “the same algorithm was able to precisely predict the risk of cardiovascular events,” Dr. Ference said. The improved accuracy resulted in “nearly superimposable observed and predicted event curves over time.”
Embedded causal effects precisely predicts outcomes
Causal AI, embedded into risk analyses, was also able to correct for inaccurate risk benefit derived from short-term clinical trials. These also “systematically underestimate the benefit of lowering LDL, blood pressure, or both,” according to Dr. Ference.
“By contrast, after embedding causal effects of LDL and blood pressure into the algorithm, the same algorithm precisely predicted the benefit of lowering LDL, blood pressure, or both at every age, once again producing superimposable observed and predicted event curves.
In another evaluation conducted by Dr. Ference and coinvestigators, the JBS3 algorithm was applied to several major trials, such as the Heart Protection Trial and HOPE-3. By itself, the JBS3 algorithm predicted less benefit than actually observed.
“After embedding causal effects of LDL and blood pressure, the same algorithm was able to precisely predict the benefit of lowering LDL, blood pressure, or both observed in the trials after 3-5 years,” Dr. Ference reported.
In a sensitivity analysis, the accuracy of the prediction remained largely similar across stratifications by risk factors, such as male sex, presence of diabetes, family history of cardiovascular disease, and other variables. It was also similar across participant age prior to a cardiovascular event and all durations of follow-up.
The data presented by Dr. Ference provides compelling evidence that JBS3, which is widely used in the United Kingdom for risk estimates, does not accurately estimate the risk of cardiovascular disease caused by elevated LDL or SBP. It also fails to estimate the benefit of treating these risk factors.
“Therefore, they cannot be used to determine the optimal timing, intensity, and duration of therapies to prevent cardiovascular events,” Dr. Ference said.
By embedding the causal effects of LDL-C and blood pressure through an AI-based algorithm, the benefit of treatment can be estimated accurately “beginning at any age and lasting for any duration, thus providing the essential information to inform individual treatment decisions about ultimate timing, intensity, and duration,” according to Dr. Ference.
Routine application awaits further steps
Despite the promise of this concept, there are many steps to be taken before it is introduced into the clinic, asserted designated discussant Folkert Asselbergs, MD, PhD. In addition to testing the accuracy in multiple populations, “we have to do the trials as well,” meaning prospective evaluations to validate the concept is meaningful for improving outcomes.
However, he does not doubt that the concept of causal AI is promising and likely to have a meaningful impact on cardiology after further validation.
“Causal AI is a crucial step that we need to take for more efficient health care,” he said. One reason he expressed caution is that several risk scores enhanced by AI, although not necessarily causal AI, have shown only “modest predictive value” in several studies that he cited.
“Hopefully the data presented from the CAUSAL AI study will really help us take a step up in the discussion to see how we can really benefit by including genetic information in an AI framework to include causality in predicting risk and predicting benefit of treatment,” said Dr. Asselbergs, professor of precision medicine, University of Utrecht (the Netherlands) Medical Center.
Dr. Ference reported financial relationships with more than 15 pharmaceutical companies. Dr. Asselbergs reported no potential conflicts of interest.
Causal-based algorithm personalizes strategies
Causal-based algorithm personalizes strategies
Typically, artificial intelligence (AI) is applied to analyze a complex set of variables to make correlations not readily made by unassisted observation. But an AI offshoot, sometimes referred to as causal AI, incorporates causation not just association, and it appears capable of changing the paradigm for preventing cardiovascular (CV) events.
“Causal AI is a new generation of AI algorithms that empowers AI to move beyond prediction to help guide clinical decision-making for each individual,” reported Brian A. Ference, MD, director of research in translational therapeutics, University of Cambridge (England).
In a novel study testing this premise, called CAUSAL AI, this approach was explored with two major risk factors, elevated LDL cholesterol (LDL-C) and elevated systolic BP (SBP). Based on a deep learning algorithm that studied the impact of these risk factors on the biology of atherosclerosis, causal effects of these risk factors were assessed and then embedded in risk estimation.
Causal AI can predict treatment effect
The study showed that the accuracy of risk prediction can be improved markedly with causal AI, but, more importantly, it suggests that causal AI can predict the impact of specific actions to reduce this risk in the context of the patient’s trajectory toward CV events.
“Risk-estimating algorithms are used to select patients at high risk who may benefit from interventions to reduce risk, but they do not include the causal effects of changes in LDL-C and SBP,” Dr. Ference explained.
As a result, they “may not accurately estimate the baseline risk of cardiovascular events caused by a person’s LDL-C or SBP level or the benefit of treating these risk factors,” he added.
In the CAUSAL AI study, presented at the annual congress of the European Society of Cardiology, risk prediction embedded with causal AI demonstrated the ability to match predicted events with actual events in several large sets of patient data.
“Embedding causal effects into risk-estimating algorithms accurately estimates baseline cardiovascular risk caused by LDL and SBP and the benefit of lowering LDL, SBP, or both beginning at any age and extending for any duration,” Dr. Ference said.
Deep-learning AI evaluated more than 300 gene variants
The deep-learning AI was based on Mendelian randomization studies evaluating 140 gene variants associated with LDL-C and 202 variants associated with SBP.
In one test of the predictive impact of causal AI, risk prediction was first conducted in 445,771 participants in the UK Biobank with the Joint British Societies (JBS3) risk calculator. Relative to actual events in this population, the JBS3 alone “consistently underestimated the increased risk caused by elevated LDL, blood pressure, or both” over the lifetime of the patient, according to Dr. Ference.
It also systematically overestimated the risk of cardiovascular events among participants with lower LDL-C, blood pressure, or both.
However, after embedding the causal effect of LDL and blood pressure, “the same algorithm was able to precisely predict the risk of cardiovascular events,” Dr. Ference said. The improved accuracy resulted in “nearly superimposable observed and predicted event curves over time.”
Embedded causal effects precisely predicts outcomes
Causal AI, embedded into risk analyses, was also able to correct for inaccurate risk benefit derived from short-term clinical trials. These also “systematically underestimate the benefit of lowering LDL, blood pressure, or both,” according to Dr. Ference.
“By contrast, after embedding causal effects of LDL and blood pressure into the algorithm, the same algorithm precisely predicted the benefit of lowering LDL, blood pressure, or both at every age, once again producing superimposable observed and predicted event curves.
In another evaluation conducted by Dr. Ference and coinvestigators, the JBS3 algorithm was applied to several major trials, such as the Heart Protection Trial and HOPE-3. By itself, the JBS3 algorithm predicted less benefit than actually observed.
“After embedding causal effects of LDL and blood pressure, the same algorithm was able to precisely predict the benefit of lowering LDL, blood pressure, or both observed in the trials after 3-5 years,” Dr. Ference reported.
In a sensitivity analysis, the accuracy of the prediction remained largely similar across stratifications by risk factors, such as male sex, presence of diabetes, family history of cardiovascular disease, and other variables. It was also similar across participant age prior to a cardiovascular event and all durations of follow-up.
The data presented by Dr. Ference provides compelling evidence that JBS3, which is widely used in the United Kingdom for risk estimates, does not accurately estimate the risk of cardiovascular disease caused by elevated LDL or SBP. It also fails to estimate the benefit of treating these risk factors.
“Therefore, they cannot be used to determine the optimal timing, intensity, and duration of therapies to prevent cardiovascular events,” Dr. Ference said.
By embedding the causal effects of LDL-C and blood pressure through an AI-based algorithm, the benefit of treatment can be estimated accurately “beginning at any age and lasting for any duration, thus providing the essential information to inform individual treatment decisions about ultimate timing, intensity, and duration,” according to Dr. Ference.
Routine application awaits further steps
Despite the promise of this concept, there are many steps to be taken before it is introduced into the clinic, asserted designated discussant Folkert Asselbergs, MD, PhD. In addition to testing the accuracy in multiple populations, “we have to do the trials as well,” meaning prospective evaluations to validate the concept is meaningful for improving outcomes.
However, he does not doubt that the concept of causal AI is promising and likely to have a meaningful impact on cardiology after further validation.
“Causal AI is a crucial step that we need to take for more efficient health care,” he said. One reason he expressed caution is that several risk scores enhanced by AI, although not necessarily causal AI, have shown only “modest predictive value” in several studies that he cited.
“Hopefully the data presented from the CAUSAL AI study will really help us take a step up in the discussion to see how we can really benefit by including genetic information in an AI framework to include causality in predicting risk and predicting benefit of treatment,” said Dr. Asselbergs, professor of precision medicine, University of Utrecht (the Netherlands) Medical Center.
Dr. Ference reported financial relationships with more than 15 pharmaceutical companies. Dr. Asselbergs reported no potential conflicts of interest.
Typically, artificial intelligence (AI) is applied to analyze a complex set of variables to make correlations not readily made by unassisted observation. But an AI offshoot, sometimes referred to as causal AI, incorporates causation not just association, and it appears capable of changing the paradigm for preventing cardiovascular (CV) events.
“Causal AI is a new generation of AI algorithms that empowers AI to move beyond prediction to help guide clinical decision-making for each individual,” reported Brian A. Ference, MD, director of research in translational therapeutics, University of Cambridge (England).
In a novel study testing this premise, called CAUSAL AI, this approach was explored with two major risk factors, elevated LDL cholesterol (LDL-C) and elevated systolic BP (SBP). Based on a deep learning algorithm that studied the impact of these risk factors on the biology of atherosclerosis, causal effects of these risk factors were assessed and then embedded in risk estimation.
Causal AI can predict treatment effect
The study showed that the accuracy of risk prediction can be improved markedly with causal AI, but, more importantly, it suggests that causal AI can predict the impact of specific actions to reduce this risk in the context of the patient’s trajectory toward CV events.
“Risk-estimating algorithms are used to select patients at high risk who may benefit from interventions to reduce risk, but they do not include the causal effects of changes in LDL-C and SBP,” Dr. Ference explained.
As a result, they “may not accurately estimate the baseline risk of cardiovascular events caused by a person’s LDL-C or SBP level or the benefit of treating these risk factors,” he added.
In the CAUSAL AI study, presented at the annual congress of the European Society of Cardiology, risk prediction embedded with causal AI demonstrated the ability to match predicted events with actual events in several large sets of patient data.
“Embedding causal effects into risk-estimating algorithms accurately estimates baseline cardiovascular risk caused by LDL and SBP and the benefit of lowering LDL, SBP, or both beginning at any age and extending for any duration,” Dr. Ference said.
Deep-learning AI evaluated more than 300 gene variants
The deep-learning AI was based on Mendelian randomization studies evaluating 140 gene variants associated with LDL-C and 202 variants associated with SBP.
In one test of the predictive impact of causal AI, risk prediction was first conducted in 445,771 participants in the UK Biobank with the Joint British Societies (JBS3) risk calculator. Relative to actual events in this population, the JBS3 alone “consistently underestimated the increased risk caused by elevated LDL, blood pressure, or both” over the lifetime of the patient, according to Dr. Ference.
It also systematically overestimated the risk of cardiovascular events among participants with lower LDL-C, blood pressure, or both.
However, after embedding the causal effect of LDL and blood pressure, “the same algorithm was able to precisely predict the risk of cardiovascular events,” Dr. Ference said. The improved accuracy resulted in “nearly superimposable observed and predicted event curves over time.”
Embedded causal effects precisely predicts outcomes
Causal AI, embedded into risk analyses, was also able to correct for inaccurate risk benefit derived from short-term clinical trials. These also “systematically underestimate the benefit of lowering LDL, blood pressure, or both,” according to Dr. Ference.
“By contrast, after embedding causal effects of LDL and blood pressure into the algorithm, the same algorithm precisely predicted the benefit of lowering LDL, blood pressure, or both at every age, once again producing superimposable observed and predicted event curves.
In another evaluation conducted by Dr. Ference and coinvestigators, the JBS3 algorithm was applied to several major trials, such as the Heart Protection Trial and HOPE-3. By itself, the JBS3 algorithm predicted less benefit than actually observed.
“After embedding causal effects of LDL and blood pressure, the same algorithm was able to precisely predict the benefit of lowering LDL, blood pressure, or both observed in the trials after 3-5 years,” Dr. Ference reported.
In a sensitivity analysis, the accuracy of the prediction remained largely similar across stratifications by risk factors, such as male sex, presence of diabetes, family history of cardiovascular disease, and other variables. It was also similar across participant age prior to a cardiovascular event and all durations of follow-up.
The data presented by Dr. Ference provides compelling evidence that JBS3, which is widely used in the United Kingdom for risk estimates, does not accurately estimate the risk of cardiovascular disease caused by elevated LDL or SBP. It also fails to estimate the benefit of treating these risk factors.
“Therefore, they cannot be used to determine the optimal timing, intensity, and duration of therapies to prevent cardiovascular events,” Dr. Ference said.
By embedding the causal effects of LDL-C and blood pressure through an AI-based algorithm, the benefit of treatment can be estimated accurately “beginning at any age and lasting for any duration, thus providing the essential information to inform individual treatment decisions about ultimate timing, intensity, and duration,” according to Dr. Ference.
Routine application awaits further steps
Despite the promise of this concept, there are many steps to be taken before it is introduced into the clinic, asserted designated discussant Folkert Asselbergs, MD, PhD. In addition to testing the accuracy in multiple populations, “we have to do the trials as well,” meaning prospective evaluations to validate the concept is meaningful for improving outcomes.
However, he does not doubt that the concept of causal AI is promising and likely to have a meaningful impact on cardiology after further validation.
“Causal AI is a crucial step that we need to take for more efficient health care,” he said. One reason he expressed caution is that several risk scores enhanced by AI, although not necessarily causal AI, have shown only “modest predictive value” in several studies that he cited.
“Hopefully the data presented from the CAUSAL AI study will really help us take a step up in the discussion to see how we can really benefit by including genetic information in an AI framework to include causality in predicting risk and predicting benefit of treatment,” said Dr. Asselbergs, professor of precision medicine, University of Utrecht (the Netherlands) Medical Center.
Dr. Ference reported financial relationships with more than 15 pharmaceutical companies. Dr. Asselbergs reported no potential conflicts of interest.
FROM ESC CONGRESS 2022
In blinded trial, artificial intelligence beats sonographers for echo accuracy
Video-based artificial intelligence provided a more accurate and consistent reading of echocardiograms than did experienced sonographers in a blinded trial, a result suggesting that this technology is no longer experimental.
“We are planning to deploy this at Cedars, so this is essentially ready for use,” said David Ouyang, MD, who is affiliated with the Cedars-Sinai Medical School and is an instructor of cardiology at the University of California, both in Los Angeles.
The primary outcome of this trial, called EchoNet-RCT, was the proportion of cases in which cardiologists changed the left ventricular ejection fraction (LVEF) reading by more than 5%. They were blinded to the origin of the reports.
This endpoint was reached in 27.2% of reports generated by sonographers but just 16.8% of reports generated by AI, a mean difference of 10.5% (P < .001).
The AI tested in the trial is called EchoNet-Dynamic. It employs a video-based deep learning algorithm that permits beat-by-beat evaluation of ejection fraction. The specifics of this system were described in a study published 2 years ago in Nature. In that evaluation of the model training set, the absolute error rate was 6% in the more than 10,000 annotated echocardiogram videos.
Echo-Net is first blinded AI echo trial
Although AI is already being employed for image evaluation in many areas of medicine, the EchoNet-RCT study “is the first blinded trial of AI in cardiology,” Dr. Ouyang said. Indeed, he noted that no prior study has even been randomized.
After a run-in period, 3,495 echocardiograms were randomizly assigned to be read by AI or by a sonographer. The reports generated by these two approaches were then evaluated by the blinded cardiologists. The sonographers and the cardiologists participating in this study had a mean of 14.1 years and 12.7 years of experience, respectively.
Each reading by both sonographers and AI was based on a single beat, but this presumably was a relative handicap for the potential advantage of AI technology, which is capable of evaluating ejection fraction across multiple cardiac cycles. The evaluation of multiple cycles has been shown previously to improve accuracy, but it is tedious and not commonly performed in routine practice, according to Dr. Ouyang.
AI favored for all major endpoints
The superiority of AI was calculated after noninferiority was demonstrated. AI also showed superiority for the secondary safety outcome which involved a test-retest evaluation. Historical AI and sonographer echocardiogram reports were again blindly assessed. Although the retest variability was lower for both (6.29% vs. 7.23%), the difference was still highly significant in favor of AI (P < .001)
The relative efficiency of AI to sonographer assessment was also tested and showed meaningful reductions in work time. While AI eliminates the labor of the sonographer completely (0 vs. a median of 119 seconds, P < .001), it was also associated with a highly significant reduction in median cardiologist time spent on echo evaluation (54 vs. 64 seconds, P < .001).
Assuming that AI is integrated into the routine workflow of a busy center, AI “could be very effective at not only improving the quality of echo reading output but also increasing efficiencies in time and effort spent by sonographers and cardiologists by simplifying otherwise tedious but important tasks,” Dr. Ouyang said.
The trial enrolled a relatively typical population. The median age was 66 years, 57% were male, and comorbidities such as diabetes and chronic kidney disease were common. When AI was compared with sonographer evaluation in groups stratified by these variables as well as by race, image quality, and location of the evaluation (inpatient vs. outpatient), the advantage of AI was consistent.
Cardiologists cannot detect AI-read echos
Identifying potential limitations of this study, James D. Thomas, MD, professor of medicine, Northwestern University, Chicago, pointed out that it was a single-center trial, and he questioned a potential bias from cardiologists able to guess accurately which of the reports they were evaluating were generated by AI.
Dr. Ouyang acknowledged that this study was limited to patients at UCLA, but he pointed out that the training model was developed at Stanford (Calif.) University, so there were two sets of patients involved in testing the machine learning algorithm. He also noted that it was exceptionally large, providing a robust dataset.
As for the bias, this was evaluated as predefined endpoint.
“We asked the cardiologists to tell us [whether] they knew which reports were generated by AI,” Dr. Ouyang said. In 43% of cases, they reported they were not sure. However, when they did express confidence that the report was generated by AI, they were correct in only 32% of the cases and incorrect in 24%. Dr. Ouyang suggested these numbers argue against a substantial role for a bias affecting the trial results.
Dr. Thomas, who has an interest in the role of AI for cardiology, cautioned that there are “technical, privacy, commercial, maintenance, and regulatory barriers” that must be circumvented before AI is widely incorporated into clinical practice, but he praised this blinded trial for advancing the field. Even accounting for any limitations, he clearly shared Dr. Ouyang’s enthusiasm about the future of AI for EF assessment.
Dr. Ouyang reports financial relationships with EchoIQ, Ultromics, and InVision. Dr. Thomas reports financial relationships with Abbott, GE, egnite, EchoIQ, and Caption Health.
Video-based artificial intelligence provided a more accurate and consistent reading of echocardiograms than did experienced sonographers in a blinded trial, a result suggesting that this technology is no longer experimental.
“We are planning to deploy this at Cedars, so this is essentially ready for use,” said David Ouyang, MD, who is affiliated with the Cedars-Sinai Medical School and is an instructor of cardiology at the University of California, both in Los Angeles.
The primary outcome of this trial, called EchoNet-RCT, was the proportion of cases in which cardiologists changed the left ventricular ejection fraction (LVEF) reading by more than 5%. They were blinded to the origin of the reports.
This endpoint was reached in 27.2% of reports generated by sonographers but just 16.8% of reports generated by AI, a mean difference of 10.5% (P < .001).
The AI tested in the trial is called EchoNet-Dynamic. It employs a video-based deep learning algorithm that permits beat-by-beat evaluation of ejection fraction. The specifics of this system were described in a study published 2 years ago in Nature. In that evaluation of the model training set, the absolute error rate was 6% in the more than 10,000 annotated echocardiogram videos.
Echo-Net is first blinded AI echo trial
Although AI is already being employed for image evaluation in many areas of medicine, the EchoNet-RCT study “is the first blinded trial of AI in cardiology,” Dr. Ouyang said. Indeed, he noted that no prior study has even been randomized.
After a run-in period, 3,495 echocardiograms were randomizly assigned to be read by AI or by a sonographer. The reports generated by these two approaches were then evaluated by the blinded cardiologists. The sonographers and the cardiologists participating in this study had a mean of 14.1 years and 12.7 years of experience, respectively.
Each reading by both sonographers and AI was based on a single beat, but this presumably was a relative handicap for the potential advantage of AI technology, which is capable of evaluating ejection fraction across multiple cardiac cycles. The evaluation of multiple cycles has been shown previously to improve accuracy, but it is tedious and not commonly performed in routine practice, according to Dr. Ouyang.
AI favored for all major endpoints
The superiority of AI was calculated after noninferiority was demonstrated. AI also showed superiority for the secondary safety outcome which involved a test-retest evaluation. Historical AI and sonographer echocardiogram reports were again blindly assessed. Although the retest variability was lower for both (6.29% vs. 7.23%), the difference was still highly significant in favor of AI (P < .001)
The relative efficiency of AI to sonographer assessment was also tested and showed meaningful reductions in work time. While AI eliminates the labor of the sonographer completely (0 vs. a median of 119 seconds, P < .001), it was also associated with a highly significant reduction in median cardiologist time spent on echo evaluation (54 vs. 64 seconds, P < .001).
Assuming that AI is integrated into the routine workflow of a busy center, AI “could be very effective at not only improving the quality of echo reading output but also increasing efficiencies in time and effort spent by sonographers and cardiologists by simplifying otherwise tedious but important tasks,” Dr. Ouyang said.
The trial enrolled a relatively typical population. The median age was 66 years, 57% were male, and comorbidities such as diabetes and chronic kidney disease were common. When AI was compared with sonographer evaluation in groups stratified by these variables as well as by race, image quality, and location of the evaluation (inpatient vs. outpatient), the advantage of AI was consistent.
Cardiologists cannot detect AI-read echos
Identifying potential limitations of this study, James D. Thomas, MD, professor of medicine, Northwestern University, Chicago, pointed out that it was a single-center trial, and he questioned a potential bias from cardiologists able to guess accurately which of the reports they were evaluating were generated by AI.
Dr. Ouyang acknowledged that this study was limited to patients at UCLA, but he pointed out that the training model was developed at Stanford (Calif.) University, so there were two sets of patients involved in testing the machine learning algorithm. He also noted that it was exceptionally large, providing a robust dataset.
As for the bias, this was evaluated as predefined endpoint.
“We asked the cardiologists to tell us [whether] they knew which reports were generated by AI,” Dr. Ouyang said. In 43% of cases, they reported they were not sure. However, when they did express confidence that the report was generated by AI, they were correct in only 32% of the cases and incorrect in 24%. Dr. Ouyang suggested these numbers argue against a substantial role for a bias affecting the trial results.
Dr. Thomas, who has an interest in the role of AI for cardiology, cautioned that there are “technical, privacy, commercial, maintenance, and regulatory barriers” that must be circumvented before AI is widely incorporated into clinical practice, but he praised this blinded trial for advancing the field. Even accounting for any limitations, he clearly shared Dr. Ouyang’s enthusiasm about the future of AI for EF assessment.
Dr. Ouyang reports financial relationships with EchoIQ, Ultromics, and InVision. Dr. Thomas reports financial relationships with Abbott, GE, egnite, EchoIQ, and Caption Health.
Video-based artificial intelligence provided a more accurate and consistent reading of echocardiograms than did experienced sonographers in a blinded trial, a result suggesting that this technology is no longer experimental.
“We are planning to deploy this at Cedars, so this is essentially ready for use,” said David Ouyang, MD, who is affiliated with the Cedars-Sinai Medical School and is an instructor of cardiology at the University of California, both in Los Angeles.
The primary outcome of this trial, called EchoNet-RCT, was the proportion of cases in which cardiologists changed the left ventricular ejection fraction (LVEF) reading by more than 5%. They were blinded to the origin of the reports.
This endpoint was reached in 27.2% of reports generated by sonographers but just 16.8% of reports generated by AI, a mean difference of 10.5% (P < .001).
The AI tested in the trial is called EchoNet-Dynamic. It employs a video-based deep learning algorithm that permits beat-by-beat evaluation of ejection fraction. The specifics of this system were described in a study published 2 years ago in Nature. In that evaluation of the model training set, the absolute error rate was 6% in the more than 10,000 annotated echocardiogram videos.
Echo-Net is first blinded AI echo trial
Although AI is already being employed for image evaluation in many areas of medicine, the EchoNet-RCT study “is the first blinded trial of AI in cardiology,” Dr. Ouyang said. Indeed, he noted that no prior study has even been randomized.
After a run-in period, 3,495 echocardiograms were randomizly assigned to be read by AI or by a sonographer. The reports generated by these two approaches were then evaluated by the blinded cardiologists. The sonographers and the cardiologists participating in this study had a mean of 14.1 years and 12.7 years of experience, respectively.
Each reading by both sonographers and AI was based on a single beat, but this presumably was a relative handicap for the potential advantage of AI technology, which is capable of evaluating ejection fraction across multiple cardiac cycles. The evaluation of multiple cycles has been shown previously to improve accuracy, but it is tedious and not commonly performed in routine practice, according to Dr. Ouyang.
AI favored for all major endpoints
The superiority of AI was calculated after noninferiority was demonstrated. AI also showed superiority for the secondary safety outcome which involved a test-retest evaluation. Historical AI and sonographer echocardiogram reports were again blindly assessed. Although the retest variability was lower for both (6.29% vs. 7.23%), the difference was still highly significant in favor of AI (P < .001)
The relative efficiency of AI to sonographer assessment was also tested and showed meaningful reductions in work time. While AI eliminates the labor of the sonographer completely (0 vs. a median of 119 seconds, P < .001), it was also associated with a highly significant reduction in median cardiologist time spent on echo evaluation (54 vs. 64 seconds, P < .001).
Assuming that AI is integrated into the routine workflow of a busy center, AI “could be very effective at not only improving the quality of echo reading output but also increasing efficiencies in time and effort spent by sonographers and cardiologists by simplifying otherwise tedious but important tasks,” Dr. Ouyang said.
The trial enrolled a relatively typical population. The median age was 66 years, 57% were male, and comorbidities such as diabetes and chronic kidney disease were common. When AI was compared with sonographer evaluation in groups stratified by these variables as well as by race, image quality, and location of the evaluation (inpatient vs. outpatient), the advantage of AI was consistent.
Cardiologists cannot detect AI-read echos
Identifying potential limitations of this study, James D. Thomas, MD, professor of medicine, Northwestern University, Chicago, pointed out that it was a single-center trial, and he questioned a potential bias from cardiologists able to guess accurately which of the reports they were evaluating were generated by AI.
Dr. Ouyang acknowledged that this study was limited to patients at UCLA, but he pointed out that the training model was developed at Stanford (Calif.) University, so there were two sets of patients involved in testing the machine learning algorithm. He also noted that it was exceptionally large, providing a robust dataset.
As for the bias, this was evaluated as predefined endpoint.
“We asked the cardiologists to tell us [whether] they knew which reports were generated by AI,” Dr. Ouyang said. In 43% of cases, they reported they were not sure. However, when they did express confidence that the report was generated by AI, they were correct in only 32% of the cases and incorrect in 24%. Dr. Ouyang suggested these numbers argue against a substantial role for a bias affecting the trial results.
Dr. Thomas, who has an interest in the role of AI for cardiology, cautioned that there are “technical, privacy, commercial, maintenance, and regulatory barriers” that must be circumvented before AI is widely incorporated into clinical practice, but he praised this blinded trial for advancing the field. Even accounting for any limitations, he clearly shared Dr. Ouyang’s enthusiasm about the future of AI for EF assessment.
Dr. Ouyang reports financial relationships with EchoIQ, Ultromics, and InVision. Dr. Thomas reports financial relationships with Abbott, GE, egnite, EchoIQ, and Caption Health.
FROM ESC CONGRESS 2022
Secondary CV prevention benefit from polypill promises global health benefit
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
FROM ESC CONGRESS 2022
Second opinions on melanocytic lesions swayed when first opinion is known
, diminishing the value and accuracy of an independent analysis.
In a novel effort to determine whether previous interpretations sway second opinions, 149 dermatopathologists were asked to read melanocytic skin biopsy specimens without access to the initial pathology report. A year or more later they read them again but now with access to the initial reading.
The study showed that the participants, independent of many variables, such as years of experience or frequency with which they offered second options, were more likely to upgrade or downgrade the severity of the specimens in accordance with the initial report even if their original reading was correct.
If the goal of a second dermatopathologist opinion is to obtain an independent diagnostic opinion, the message from this study is that they “should be blinded to first opinions,” according to the authors of this study, led by Joann G. Elmore, MD, professor of medicine, University of California, Los Angeles. The study was published online in JAMA Dermatology.
Two-phase study has 1-year washout
The study was conducted in two phases. In phase 1, a nationally representative sample of volunteer dermatopathologists performed 878 interpretations. In phase 2, conducted after a washout period of 12 months or more, the dermatopathologists read a random subset of the same cases evaluated in phase 1, but this time, unlike the first, they were first exposed to prior pathology reports.
Ultimately, “the dermatologists provided more than 5,000 interpretations of study cases, which was a big contribution of time,” Dr. Elmore said in an interview. Grateful for their critical contribution, she speculated that they were driven by the importance of the question being asked.
When categorized by the Melanocytic Pathology Assessment Tool (MPAT), which rates specimens from benign (class 1) to pT1b invasive melanoma (class 4), the influence of the prior report went in both directions, so that the likelihood of upgrading or downgrading went in accordance with the grading in the original dermatopathology report.
As a result, the risk of a less severe interpretation on the second relative to the first reading was 38% greater if the initial dermatopathology report had a lower grade (relative risk, 1.38; 95% confidence interval [CI], 1.19-1.59). The risk of upgrading the second report if the initial pathology report had a higher grade was increased by more than 50% (RR, 1.52; 95% CI, 1.34-1.73).
The greater likelihood of upgrading than downgrading is “understandable,” Dr. Elmore said. “I think this is consistent with the concern about missing something,” she explained.
According to Dr. Elmore, one of the greatest concerns regarding the bias imposed by the original pathology report is that the switch of opinions often went from one that was accurate to one that was inaccurate.
If the phase 1 diagnosis was accurate but upgraded in the phase 2 diagnosis, the risk of inaccuracy was almost doubled (RR, 1.96; 95% CI, 1.31-2.93). If the phase 1 report was inaccurate, the relative risk of changing the phase 2 diagnosis was still high but lower than if it was accurate (RR, 1.46; 95% CI, 1.27-1.68).
“That is, even when the phase 1 diagnoses agreed with the consensus reference diagnosis, they were swayed away from the correct diagnosis in phase 2 [when the initial pathology report characterized the specimen as higher grade],” Dr. Elmore reported.
Conversely, the risk of downgrading was about the same whether the phase 1 evaluation was accurate (RR, 1.37; 95% CI, 1.14-1.64) or inaccurate (RR 1.32; 95% CI, 1.07-1.64).
Downward and upward shifts in severity from an accurate diagnosis are concerning because of the likelihood they will lead to overtreatment or undertreatment. The problem, according to data from this study, is that dermatologists making a second opinion cannot judge their own susceptibility to being swayed by the original report.
Pathologists might be unaware of bias
At baseline, the participants were asked whether they thought they were influenced by the first interpretation when providing a second opinion. Although 69% acknowledged that they might be “somewhat influenced,” 31% maintained that they do not take initial reports into consideration. When the two groups were compared, the risk of downgrading was nearly identical. The risk of upgrading was lower in those claiming to disregard initial reports (RR, 1.29) relative to those who said they were “somewhat influenced” by a previous diagnosis (RR, 1.64), but the difference was not significant.
The actual risk of bias incurred by prior pathology reports might be greater than that captured in this study for several reasons, according to the investigators. They pointed out that all participants were experienced and board-certified and might therefore be expected to be more confident in their interpretations than an unselected group of dermatopathologists. In addition, participants might have been more careful in their interpretations knowing they were participating in a study.
“There are a lot of data to support the value of second opinions [in dermatopathology and other areas], but we need to consider the process of how they are being obtained,” Dr. Elmore said. “There needs to be a greater emphasis on providing an independent analysis.”
More than 60% of the dermatologists participating in this study reported that they agreed or strongly agreed with the premise that they prefer to have the original dermatopathology report when they offer a second opinion. Dr. Elmore said that the desire of those offering a second opinion to have as much information in front of them as possible is understandable, but the bias imposed by the original report weakens the value of the second opinion.
Blind reading of pathology reports needed
“These data suggest that seeing the original report sways opinions and that includes swaying opinions away from an accurate reading,” Dr. Elmore said. She thinks that for dermatopathologists to render a valuable and independent second opinion, the specimens should be examined “at least initially” without access to the first report.
The results of this study were not surprising to Vishal Anil Patel, MD, director of the Cutaneous Oncology Program, George Washington University Cancer Center, Washington. He made the point that physicians “are human first and foremost and not perfect machines.” As a result, he suggested bias and error are inevitable.
Although strategies to avoid bias are likely to offer some protection against inaccuracy, he said that diagnostic support tools such as artificial intelligence might be the right direction for improving inter- and intra-rater reliability.
Ruifeng Guo, MD, PhD, a consultant in the division of anatomic pathology at the Mayo Clinic, Rochester, Minn., agreed with the basic premise of the study, but he cautioned that restricting access to the initial pathology report might not always be the right approach.
It is true that “dermatopathologists providing a second opinion in diagnosing cutaneous melanoma are mostly unaware of the risk of bias if they read the initial pathology report,” said Dr. Guo, but restricting access comes with risks.
“There are also times critical information may be contained in the initial pathology report that needs to be considered when providing a second opinion consultation,” he noted. Ultimately, the decision to read or not read the initial report should be decided “on an individual basis.”
The study was funded by grants from the National Cancer Institute. Dr. Elmore, Dr. Patel, and Dr. Guo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, diminishing the value and accuracy of an independent analysis.
In a novel effort to determine whether previous interpretations sway second opinions, 149 dermatopathologists were asked to read melanocytic skin biopsy specimens without access to the initial pathology report. A year or more later they read them again but now with access to the initial reading.
The study showed that the participants, independent of many variables, such as years of experience or frequency with which they offered second options, were more likely to upgrade or downgrade the severity of the specimens in accordance with the initial report even if their original reading was correct.
If the goal of a second dermatopathologist opinion is to obtain an independent diagnostic opinion, the message from this study is that they “should be blinded to first opinions,” according to the authors of this study, led by Joann G. Elmore, MD, professor of medicine, University of California, Los Angeles. The study was published online in JAMA Dermatology.
Two-phase study has 1-year washout
The study was conducted in two phases. In phase 1, a nationally representative sample of volunteer dermatopathologists performed 878 interpretations. In phase 2, conducted after a washout period of 12 months or more, the dermatopathologists read a random subset of the same cases evaluated in phase 1, but this time, unlike the first, they were first exposed to prior pathology reports.
Ultimately, “the dermatologists provided more than 5,000 interpretations of study cases, which was a big contribution of time,” Dr. Elmore said in an interview. Grateful for their critical contribution, she speculated that they were driven by the importance of the question being asked.
When categorized by the Melanocytic Pathology Assessment Tool (MPAT), which rates specimens from benign (class 1) to pT1b invasive melanoma (class 4), the influence of the prior report went in both directions, so that the likelihood of upgrading or downgrading went in accordance with the grading in the original dermatopathology report.
As a result, the risk of a less severe interpretation on the second relative to the first reading was 38% greater if the initial dermatopathology report had a lower grade (relative risk, 1.38; 95% confidence interval [CI], 1.19-1.59). The risk of upgrading the second report if the initial pathology report had a higher grade was increased by more than 50% (RR, 1.52; 95% CI, 1.34-1.73).
The greater likelihood of upgrading than downgrading is “understandable,” Dr. Elmore said. “I think this is consistent with the concern about missing something,” she explained.
According to Dr. Elmore, one of the greatest concerns regarding the bias imposed by the original pathology report is that the switch of opinions often went from one that was accurate to one that was inaccurate.
If the phase 1 diagnosis was accurate but upgraded in the phase 2 diagnosis, the risk of inaccuracy was almost doubled (RR, 1.96; 95% CI, 1.31-2.93). If the phase 1 report was inaccurate, the relative risk of changing the phase 2 diagnosis was still high but lower than if it was accurate (RR, 1.46; 95% CI, 1.27-1.68).
“That is, even when the phase 1 diagnoses agreed with the consensus reference diagnosis, they were swayed away from the correct diagnosis in phase 2 [when the initial pathology report characterized the specimen as higher grade],” Dr. Elmore reported.
Conversely, the risk of downgrading was about the same whether the phase 1 evaluation was accurate (RR, 1.37; 95% CI, 1.14-1.64) or inaccurate (RR 1.32; 95% CI, 1.07-1.64).
Downward and upward shifts in severity from an accurate diagnosis are concerning because of the likelihood they will lead to overtreatment or undertreatment. The problem, according to data from this study, is that dermatologists making a second opinion cannot judge their own susceptibility to being swayed by the original report.
Pathologists might be unaware of bias
At baseline, the participants were asked whether they thought they were influenced by the first interpretation when providing a second opinion. Although 69% acknowledged that they might be “somewhat influenced,” 31% maintained that they do not take initial reports into consideration. When the two groups were compared, the risk of downgrading was nearly identical. The risk of upgrading was lower in those claiming to disregard initial reports (RR, 1.29) relative to those who said they were “somewhat influenced” by a previous diagnosis (RR, 1.64), but the difference was not significant.
The actual risk of bias incurred by prior pathology reports might be greater than that captured in this study for several reasons, according to the investigators. They pointed out that all participants were experienced and board-certified and might therefore be expected to be more confident in their interpretations than an unselected group of dermatopathologists. In addition, participants might have been more careful in their interpretations knowing they were participating in a study.
“There are a lot of data to support the value of second opinions [in dermatopathology and other areas], but we need to consider the process of how they are being obtained,” Dr. Elmore said. “There needs to be a greater emphasis on providing an independent analysis.”
More than 60% of the dermatologists participating in this study reported that they agreed or strongly agreed with the premise that they prefer to have the original dermatopathology report when they offer a second opinion. Dr. Elmore said that the desire of those offering a second opinion to have as much information in front of them as possible is understandable, but the bias imposed by the original report weakens the value of the second opinion.
Blind reading of pathology reports needed
“These data suggest that seeing the original report sways opinions and that includes swaying opinions away from an accurate reading,” Dr. Elmore said. She thinks that for dermatopathologists to render a valuable and independent second opinion, the specimens should be examined “at least initially” without access to the first report.
The results of this study were not surprising to Vishal Anil Patel, MD, director of the Cutaneous Oncology Program, George Washington University Cancer Center, Washington. He made the point that physicians “are human first and foremost and not perfect machines.” As a result, he suggested bias and error are inevitable.
Although strategies to avoid bias are likely to offer some protection against inaccuracy, he said that diagnostic support tools such as artificial intelligence might be the right direction for improving inter- and intra-rater reliability.
Ruifeng Guo, MD, PhD, a consultant in the division of anatomic pathology at the Mayo Clinic, Rochester, Minn., agreed with the basic premise of the study, but he cautioned that restricting access to the initial pathology report might not always be the right approach.
It is true that “dermatopathologists providing a second opinion in diagnosing cutaneous melanoma are mostly unaware of the risk of bias if they read the initial pathology report,” said Dr. Guo, but restricting access comes with risks.
“There are also times critical information may be contained in the initial pathology report that needs to be considered when providing a second opinion consultation,” he noted. Ultimately, the decision to read or not read the initial report should be decided “on an individual basis.”
The study was funded by grants from the National Cancer Institute. Dr. Elmore, Dr. Patel, and Dr. Guo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, diminishing the value and accuracy of an independent analysis.
In a novel effort to determine whether previous interpretations sway second opinions, 149 dermatopathologists were asked to read melanocytic skin biopsy specimens without access to the initial pathology report. A year or more later they read them again but now with access to the initial reading.
The study showed that the participants, independent of many variables, such as years of experience or frequency with which they offered second options, were more likely to upgrade or downgrade the severity of the specimens in accordance with the initial report even if their original reading was correct.
If the goal of a second dermatopathologist opinion is to obtain an independent diagnostic opinion, the message from this study is that they “should be blinded to first opinions,” according to the authors of this study, led by Joann G. Elmore, MD, professor of medicine, University of California, Los Angeles. The study was published online in JAMA Dermatology.
Two-phase study has 1-year washout
The study was conducted in two phases. In phase 1, a nationally representative sample of volunteer dermatopathologists performed 878 interpretations. In phase 2, conducted after a washout period of 12 months or more, the dermatopathologists read a random subset of the same cases evaluated in phase 1, but this time, unlike the first, they were first exposed to prior pathology reports.
Ultimately, “the dermatologists provided more than 5,000 interpretations of study cases, which was a big contribution of time,” Dr. Elmore said in an interview. Grateful for their critical contribution, she speculated that they were driven by the importance of the question being asked.
When categorized by the Melanocytic Pathology Assessment Tool (MPAT), which rates specimens from benign (class 1) to pT1b invasive melanoma (class 4), the influence of the prior report went in both directions, so that the likelihood of upgrading or downgrading went in accordance with the grading in the original dermatopathology report.
As a result, the risk of a less severe interpretation on the second relative to the first reading was 38% greater if the initial dermatopathology report had a lower grade (relative risk, 1.38; 95% confidence interval [CI], 1.19-1.59). The risk of upgrading the second report if the initial pathology report had a higher grade was increased by more than 50% (RR, 1.52; 95% CI, 1.34-1.73).
The greater likelihood of upgrading than downgrading is “understandable,” Dr. Elmore said. “I think this is consistent with the concern about missing something,” she explained.
According to Dr. Elmore, one of the greatest concerns regarding the bias imposed by the original pathology report is that the switch of opinions often went from one that was accurate to one that was inaccurate.
If the phase 1 diagnosis was accurate but upgraded in the phase 2 diagnosis, the risk of inaccuracy was almost doubled (RR, 1.96; 95% CI, 1.31-2.93). If the phase 1 report was inaccurate, the relative risk of changing the phase 2 diagnosis was still high but lower than if it was accurate (RR, 1.46; 95% CI, 1.27-1.68).
“That is, even when the phase 1 diagnoses agreed with the consensus reference diagnosis, they were swayed away from the correct diagnosis in phase 2 [when the initial pathology report characterized the specimen as higher grade],” Dr. Elmore reported.
Conversely, the risk of downgrading was about the same whether the phase 1 evaluation was accurate (RR, 1.37; 95% CI, 1.14-1.64) or inaccurate (RR 1.32; 95% CI, 1.07-1.64).
Downward and upward shifts in severity from an accurate diagnosis are concerning because of the likelihood they will lead to overtreatment or undertreatment. The problem, according to data from this study, is that dermatologists making a second opinion cannot judge their own susceptibility to being swayed by the original report.
Pathologists might be unaware of bias
At baseline, the participants were asked whether they thought they were influenced by the first interpretation when providing a second opinion. Although 69% acknowledged that they might be “somewhat influenced,” 31% maintained that they do not take initial reports into consideration. When the two groups were compared, the risk of downgrading was nearly identical. The risk of upgrading was lower in those claiming to disregard initial reports (RR, 1.29) relative to those who said they were “somewhat influenced” by a previous diagnosis (RR, 1.64), but the difference was not significant.
The actual risk of bias incurred by prior pathology reports might be greater than that captured in this study for several reasons, according to the investigators. They pointed out that all participants were experienced and board-certified and might therefore be expected to be more confident in their interpretations than an unselected group of dermatopathologists. In addition, participants might have been more careful in their interpretations knowing they were participating in a study.
“There are a lot of data to support the value of second opinions [in dermatopathology and other areas], but we need to consider the process of how they are being obtained,” Dr. Elmore said. “There needs to be a greater emphasis on providing an independent analysis.”
More than 60% of the dermatologists participating in this study reported that they agreed or strongly agreed with the premise that they prefer to have the original dermatopathology report when they offer a second opinion. Dr. Elmore said that the desire of those offering a second opinion to have as much information in front of them as possible is understandable, but the bias imposed by the original report weakens the value of the second opinion.
Blind reading of pathology reports needed
“These data suggest that seeing the original report sways opinions and that includes swaying opinions away from an accurate reading,” Dr. Elmore said. She thinks that for dermatopathologists to render a valuable and independent second opinion, the specimens should be examined “at least initially” without access to the first report.
The results of this study were not surprising to Vishal Anil Patel, MD, director of the Cutaneous Oncology Program, George Washington University Cancer Center, Washington. He made the point that physicians “are human first and foremost and not perfect machines.” As a result, he suggested bias and error are inevitable.
Although strategies to avoid bias are likely to offer some protection against inaccuracy, he said that diagnostic support tools such as artificial intelligence might be the right direction for improving inter- and intra-rater reliability.
Ruifeng Guo, MD, PhD, a consultant in the division of anatomic pathology at the Mayo Clinic, Rochester, Minn., agreed with the basic premise of the study, but he cautioned that restricting access to the initial pathology report might not always be the right approach.
It is true that “dermatopathologists providing a second opinion in diagnosing cutaneous melanoma are mostly unaware of the risk of bias if they read the initial pathology report,” said Dr. Guo, but restricting access comes with risks.
“There are also times critical information may be contained in the initial pathology report that needs to be considered when providing a second opinion consultation,” he noted. Ultimately, the decision to read or not read the initial report should be decided “on an individual basis.”
The study was funded by grants from the National Cancer Institute. Dr. Elmore, Dr. Patel, and Dr. Guo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How nonadherence complicates cardiology, in two trials
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Each study adds new twist
Each study adds new twist
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
Two very different sets of clinical evidence have offered new twists on how nonadherence to cardiovascular medicines not only leads to suboptimal outcomes, but also complicates the data from clinical studies.
One study, a subanalysis of a major trial, outlined how taking more than the assigned therapy – that is, nonadherence by taking too much rather than too little – skewed results. The other was a trial demonstrating that early use of an invasive procedure is not a strategy to compensate for nonadherence to guideline-directed medical therapy (GDMT).
“Both studies provide a fresh reminder that nonadherence is a significant problem in cardiology overall, but also in the trial setting when we are trying to interpret study results,” explained Usam Baber, MD, director of interventional cardiology, University of Oklahoma Health, Oklahoma City, coauthor of an editorial accompanying the two published studies.
Dr. Baber was the first author of a unifying editorial that addressed the issues raised by each. In an interview, Dr. Baber said the studies had unique take-home messages but together highlight important issues of nonadherence.
MASTER DAPT: Too much medicine
The subanalysis was performed on data generated by MASTER DAPT, a study evaluating whether a relatively short course of dual-antiplatelet therapy (DAPT) in patients at high risk of bleeding could preserve protection against major adverse cardiovascular events (MACE) while reducing risk of adverse events. The problem was that nonadherence muddied the primary message.
In MASTER DAPT, 1 month of DAPT was compared with a standard therapy of at least 2 additional months of DAPT following revascularization and placement of a biodegradable polymer stent. Enrollment in the study was restricted to those with a high risk of bleeding, the report of the primary results showed.
The major message of MASTER DAPT was that the abbreviated course of DAPT was noninferior for preventing MACE but resulted in lower rates of clinically relevant bleeding in those patients without an indication for oral anticoagulation (OAC). In the subgroup with an indication for OAC, there was no bleeding benefit.
However, when the results were reexamined in the context of adherence, the benefit of the shorter course was found to be underestimated. Relative to 9.4% in the standard-therapy arm, the nonadherence rate in the experimental arm was 20.2%, most of whom did not stop therapy at 1 month. They instead remained on the antiplatelet therapy, failing to adhere to the study protocol.
This form of nonadherence, taking more DAPT than assigned, was particularly common in the group with an indication for oral anticoagulation (OAC). In this group, nearly 25% assigned to an abbreviated course remained on DAPT for more than 6 months.
In the intention-to-treat analysis, there was no difference between abbreviated and standard DAPT for MACE whether or not patients had an indication for OAC. In other words, the new analysis showed a reduced risk of bleeding among all patients, whether taking OAC or not after controlling for nonadherence.
In addition, this MASTER DAPT analysis found that a high proportion of patients taking OAC did not discontinue their single-antiplatelet therapy (SAPT) after 6 months as specified.
When correcting for this failure to adhere to the MASTER DAPT protocol in a patient population at high bleeding risk, the new analysis “suggests for the first time that discontinuation of SAPT at 6 months after percutaneous intervention is associated with less bleeding without an increase in ischemic events,” Marco Valgimigli, MD, PhD, director of clinical research, Inselspital University Hospital, Bern, Switzerland, reported in the Journal of the American College of Cardiology.
The findings “reinforce the importance of accounting and correcting for nonadherence” in order to reduce bias in the assessment of treatment effects, according to Dr. Valgimigli, principal investigator of MASTER DAPT and this substudy.
“The first interesting message from this study is that clinicians are reluctant to stop SAPT in these patients even in the setting of a randomized controlled trial,” Dr. Valgimigli said in an interview.
In addition, this substudy, which was prespecified in the MASTER DAPT protocol and employed “a very sophisticated methodology” to control for the effect of adherence, extends the value of a conservative approach to those who are candidates for OAC.
“The main clinical message is that SAPT needs to be discontinued after 6 months in OAC patients, and clinicians need to stop being reluctant to do so,” Dr. Valgimigli said. The data show “prolongation of SAPT increases bleeding risk without decreasing ischemic risk.”
In evaluating trial relevance, regulators prefer ITT analyses, but Dr. Baber pointed out that these can obscure the evidence of risk or benefit of a per-protocol analysis when patients take their medicine as prescribed.
“The technical message is that, when we are trying to apply results of a clinical trial to daily practice, we must understand nonadherence,” Dr. Baber said.
Dr. Baber pointed out that the lack of adherence in the case of MASTER DAPT appears to relate more to clinicians managing the patients than to the patients themselves, but it still speaks to the importance of understanding the effects of treatment in the context of the medicine rather than adherence to the medicine.
ISCHEMIA: Reconsidering adherence
In the ISCHEMIA trial, the goal was to evaluate whether an early invasive intervention might compensate to at least some degree for the persistent problem of nonadherence.
“If you are managing a patient that you know is at high risk of noncompliance, many clinicians are tempted to perform early revascularization. This was my bias. The thinking is that by offering an invasive therapy we are at least doing something to control their disease,” John A. Spertus, MD, clinical director of outcomes research, St. Luke’s Mid America Heart Institute, Kansas City, Mo., explained in an interview.
The study did not support the hypothesis. Patients with chronic coronary disease were randomized to a strategy of angiography and, if indicated, revascularization, or to receive GDMT alone. The health status was followed with the Seattle Angina Questionnaire (SAQ-7).
At 12 months, patients who were adherent to GDMT had better SAQ-7 scores than those who were nonadherent, regardless of the arm to which they were randomized. Conversely, there was no difference in SAQ-7 scores between the two groups when the nonadherent subgroups in each arm were compared.
“I think these data suggest that an interventional therapy does not absolve clinicians from the responsibility of educating patients about the importance of adhering to GDMT,” Dr. Spertus said.
In ISCHEMIA, 4,480 patients were randomized. At baseline assessment 27.8% were nonadherent to GDMT. The baselines SAQ-7 scores were worse in these patients relative to those who were adherent. At 12 months, nonadherence still correlated with worse SAQ-7 scores.
“These data dispel the belief that we might be benefiting nonadherent patients by moving more quickly to invasive procedures,” Dr. Spertus said.
In cardiovascular disease, particularly heart failure, adherence to GDMT has been associated numerous times with improved quality of life, according to Dr. Baber. However, he said, the ability of invasive procedures to modify the adverse impact of poor adherence to GDMT has not been well studied. This ISCHEMIA subanalysis only reinforces the message that GDMT adherence is a meaningful predictor of improved quality of life.
However, urging clinicians to work with patients to improve adherence is not a novel idea, according to Dr. Baber. The unmet need is effective and reliable strategies.
“There are so many different reasons that patients are nonadherent, so there are limited gains by focusing on just one of the issues,” Dr. Baber said. “I think the answer is a patient-centric approach in which clinicians deal with the specific issues facing the patient in front of them. I think there are data go suggest this yields better results.”
These two very different studies also show that poor adherence is an insidious issue. While the MASTER DAPT data reveal how nonadherence confuse trial data, the ISCHEMIA trial shows that some assumptions about circumventing the effects of nonadherence might not be accurate.
According to Dr. Baber, effective strategies to reduce nonadherence are available, but the problem deserves to be addressed more proactively in clinical trials and in patient care.
Dr. Baber reported financial relationships with AstraZeneca and Amgen. Dr. Spertus has financial relationships with Abbott, Bayer, Bristol-Myers Squibb, Corvia, Janssen, Merck, Novartis, Pfizer and Terumo. Dr. Valgimigli has financial relationships with more than 15 pharmaceutical companies, including Terumo, which provided funding for the MASTER DAPT trial.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY