User login
What do patients want in a migraine preventive?
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
, according to the results of a study published in Headache. When offered hypothetical preventive migraine medicines with a wide array of attributes, patients leaned toward those with a reduction in migraine days and an avoidance of weight gain, according to an analysis of responses to a discrete-choice experiment survey.
“We found that respondents had a significant willingness to pay for medicines with higher efficacy and less-severe adverse events,” wrote Carol Mansfield, PhD, of RTI Health Solutions in North Carolina, and coauthors.
To evaluate patient preferences for theoretical migraine medicine, the researchers conducted a discrete-choice experiment via a web-based survey. Respondents met eligibility criteria if they were adults aged 18 years or older who self-reported 6 or more migraine days per month and completed the survey in full. They were asked to choose between options defined by six attributes: reduction in headache days per month, frequency of limitations with physical activities, cognition problems, weight gain, how the medicine is taken, and monthly out-of-pocket cost.
Of the 300 respondents included in the analysis, 72% indicated that migraines make physical activities difficult all or most of the time, and 81% had taken a prescription migraine preventive in the last 6 months. Respondents reported, on average, approximately 16 headache days per month. Among noncost attributes, respondents valued a change from a 10% reduction in migraine days to a 50% reduction more highly than avoiding the worst levels of adverse events – defined as memory problems and 10% weight gain – but were willing to trade off efficacy for less-severe adverse events. Avoiding memory problems was more important than avoiding thinking problems. Avoiding a 10% weight gain was more important than avoiding thinking and memory problems. Respondents preferred a once-monthly injection or daily pill to twice-monthly injections. Respondents, on average, were willing to pay $116 per month for an improvement from 10% to 50% in reduced headache days (95% confidence interval [CI], $91-$141) and $43 for an improvement from 10% to 25% (95% CI, $34-$53). They were also willing to pay $84 per month to avoid a 10% weight gain (95% CI, $64-$103), $59 per month to avoid memory problems (95% CI, $42-$76), and $32 per month to avoid thinking problems (95% CI, $18-$46).
The coauthors acknowledged their study’s limitations, including all migraine diagnoses being self-reported and the study sample not necessarily being representative of patients with migraine overall. In addition, though the potential medicinal attributes used were prominent in clinical literature and focus groups, they could choose only a limited amount and so their analysis “did not address other attributes that may be important to patients.”
Given their findings, the researchers recommended that “clinicians should work with patients to select treatments that meet each patient’s needs.”
Amgen and Novartis funded the study. The authors reported numerous conflicts of interest, including receiving grants, consulting fees, and royalties from pharmaceutical companies and organizations. During the study, three of the authors were employed at RTI Health Solutions, a non-for-profit organization that conducts research with pharmaceutical companies such as the study’s sponsor.
SOURCE: Mansfield C et al. Headache. 2019 May;59(5):715-26. doi: 10.1111/head.13498.
FROM HEADACHE
AD biomarker not tied to increased interest in physician-assisted death
Being diagnosed with an elevated amyloid-beta biomarker that indicates greater risk of Alzheimer’s disease did not lead to increased consideration of physician-assisted death (PAD), according to an analysis of patients interviewed during clinical trials on cognitive decline.
“Our findings suggest that learning one’s amyloid imaging result does not change baseline attitudes regarding the acceptability of PAD,” wrote Emily A. Largent, PhD, of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and coauthors. The study was published as a research letter in JAMA Neurology.
Participants were recruited from two ongoing clinical trials, one of which included patients with elevated amyloid-beta (n = 50), whereas the other did not (n = 30). All participants completed an interview 4-12 weeks after receiving their biomarker results; 47 and 30 participants, respectively, also completed a follow-up interview at 12 months.
When asked whether they had considered PAD, nearly two-thirds of interviewees with the Alzheimer’s disease biomarker stated that they neither had nor would. Roughly one in five from that group said they would pursue PAD if they began to suffer from cognitive impairment or became a burden on others. Interviewees who did not have elevated amyloid beta, when asked whether a reversed result would have led to PAD or suicide, showed interest in roughly similar proportion to their at-risk counterparts.
The coauthors acknowledged the limitations of their study, including not asking about other end-of-life preferences or perceived quality of life for people with dementia. They also noted that, although their sample mirrors the populations of the two studies they drew from, “its homogeneity limits generalizability.” As such, they stressed that
The study was supported by grants from the Alzheimer’s Association and the National Institute on Aging. One author reported receiving grants from those two organizations during the study; another reported receiving grants from Lilly and Novartis. No other conflicts of interest were reported.
SOURCE: Largent EA et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0797.
The fascinating thing about this study is that the idea for it arose when some of the individuals spontaneously mentioned assisted suicide during their initial interview, Annette L. Hanson, MD, said in an interview.
“Would these subjects have thought of suicide in the absence of the Brittany Maynard publicity campaign? I doubt it.”
Dr. Hanson, a forensic psychiatrist, is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
The fascinating thing about this study is that the idea for it arose when some of the individuals spontaneously mentioned assisted suicide during their initial interview, Annette L. Hanson, MD, said in an interview.
“Would these subjects have thought of suicide in the absence of the Brittany Maynard publicity campaign? I doubt it.”
Dr. Hanson, a forensic psychiatrist, is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
The fascinating thing about this study is that the idea for it arose when some of the individuals spontaneously mentioned assisted suicide during their initial interview, Annette L. Hanson, MD, said in an interview.
“Would these subjects have thought of suicide in the absence of the Brittany Maynard publicity campaign? I doubt it.”
Dr. Hanson, a forensic psychiatrist, is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
Being diagnosed with an elevated amyloid-beta biomarker that indicates greater risk of Alzheimer’s disease did not lead to increased consideration of physician-assisted death (PAD), according to an analysis of patients interviewed during clinical trials on cognitive decline.
“Our findings suggest that learning one’s amyloid imaging result does not change baseline attitudes regarding the acceptability of PAD,” wrote Emily A. Largent, PhD, of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and coauthors. The study was published as a research letter in JAMA Neurology.
Participants were recruited from two ongoing clinical trials, one of which included patients with elevated amyloid-beta (n = 50), whereas the other did not (n = 30). All participants completed an interview 4-12 weeks after receiving their biomarker results; 47 and 30 participants, respectively, also completed a follow-up interview at 12 months.
When asked whether they had considered PAD, nearly two-thirds of interviewees with the Alzheimer’s disease biomarker stated that they neither had nor would. Roughly one in five from that group said they would pursue PAD if they began to suffer from cognitive impairment or became a burden on others. Interviewees who did not have elevated amyloid beta, when asked whether a reversed result would have led to PAD or suicide, showed interest in roughly similar proportion to their at-risk counterparts.
The coauthors acknowledged the limitations of their study, including not asking about other end-of-life preferences or perceived quality of life for people with dementia. They also noted that, although their sample mirrors the populations of the two studies they drew from, “its homogeneity limits generalizability.” As such, they stressed that
The study was supported by grants from the Alzheimer’s Association and the National Institute on Aging. One author reported receiving grants from those two organizations during the study; another reported receiving grants from Lilly and Novartis. No other conflicts of interest were reported.
SOURCE: Largent EA et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0797.
Being diagnosed with an elevated amyloid-beta biomarker that indicates greater risk of Alzheimer’s disease did not lead to increased consideration of physician-assisted death (PAD), according to an analysis of patients interviewed during clinical trials on cognitive decline.
“Our findings suggest that learning one’s amyloid imaging result does not change baseline attitudes regarding the acceptability of PAD,” wrote Emily A. Largent, PhD, of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and coauthors. The study was published as a research letter in JAMA Neurology.
Participants were recruited from two ongoing clinical trials, one of which included patients with elevated amyloid-beta (n = 50), whereas the other did not (n = 30). All participants completed an interview 4-12 weeks after receiving their biomarker results; 47 and 30 participants, respectively, also completed a follow-up interview at 12 months.
When asked whether they had considered PAD, nearly two-thirds of interviewees with the Alzheimer’s disease biomarker stated that they neither had nor would. Roughly one in five from that group said they would pursue PAD if they began to suffer from cognitive impairment or became a burden on others. Interviewees who did not have elevated amyloid beta, when asked whether a reversed result would have led to PAD or suicide, showed interest in roughly similar proportion to their at-risk counterparts.
The coauthors acknowledged the limitations of their study, including not asking about other end-of-life preferences or perceived quality of life for people with dementia. They also noted that, although their sample mirrors the populations of the two studies they drew from, “its homogeneity limits generalizability.” As such, they stressed that
The study was supported by grants from the Alzheimer’s Association and the National Institute on Aging. One author reported receiving grants from those two organizations during the study; another reported receiving grants from Lilly and Novartis. No other conflicts of interest were reported.
SOURCE: Largent EA et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0797.
FROM JAMA NEUROLOGY
Depression treatment rates rose with expanded insurance coverage
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
FROM JAMA PSYCHIATRY
Key clinical point: Treatment for – and spending on – depression both saw modest increases from 1998 to 2015.
Major finding: Rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 in 1998 to 3.47 (95% CI, 3.16-3.79) per 100 in 2015.
Study details: An analysis of 86,216 individuals from the 1998, 2007, and 2015 Medical Expenditure Panel Surveys.
Disclosures: The study was supported in part by the Commonwealth Fund, and the lead author also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
Source: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
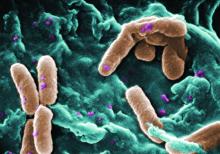
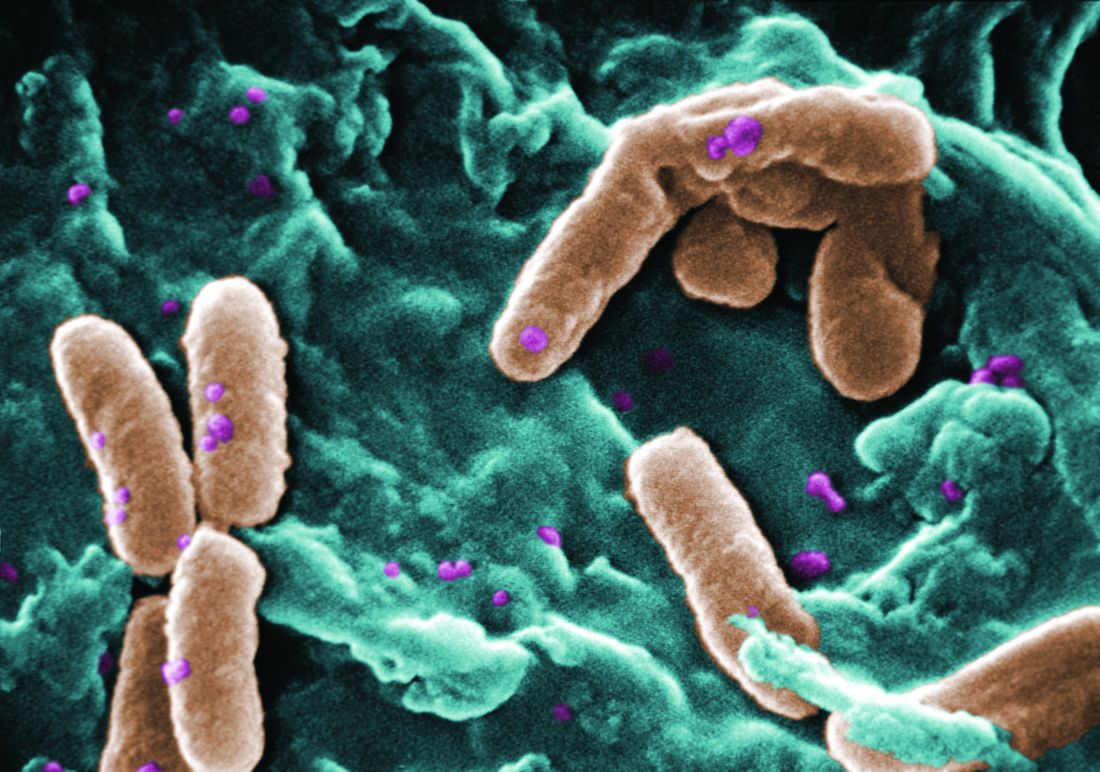
Filamentous bacteriophage linked to lung infections in patients with cystic fibrosis
according to a study of the prevalence and clinical relevance of Pf phage in two patient cohorts.
“Our data from both the Stanford and Danish CF cohorts together suggest that either patients with CF acquire Pf phage–producing strains of P. aeruginosa or the Pf phage–negative P. aeruginosa become infected with Pf phage as patients age and their disease progresses,” wrote Elizabeth B. Burgener, MD, of Stanford (Calif.) University, and her coauthors. The study was published in Science Translational Medicine.The study analyzed a previous Danish longitudinal cohort of 34 patients and a prospective cross-sectional cohort of 76 patients at Stanford, 58 of which had P. aeruginosa. The researchers also reviewed a collection of genetic sequences called the Pseudomonas Genome Database to determine the prevalence of Pf phage, finding evidence in 1,159 of 2,226 P. aeruginosa sequences (52.1%).
In the Danish cohort, 21 of the 34 CF patients (61.8%; 95% confidence interval, 43.6%-77.8%) had at least one P. aeruginosa isolate containing Pf phage; 9 (26.5%) of the patients were found to be consistently positive for Pf phage. Those who were consistently positive were also older than those who never had Pf phage detected (19.1 years vs. 13.9 years; P = .046), suggesting that “there may be a tendency for P. aeruginosa strains that produce Pf phage to dominate in the sputum of individual patients with CF over time.”
In the Stanford cohort, the prevalence of Pf phage was 36.2% (21 of 58; 95% CI, 24.0%-49.9%) in patients with P. aeruginosa infection and 27.6% (21 of 76; 95% CI, 18.0%-39.1%) in all patients. No Pf phage was detected in any P. aeruginosa–negative samples. Patients positive for Pf phage in this cohort were also older than patients who were negative.
The authors acknowledged their study’s limitations, describing the methods used to collect and sequence the analyzed samples as “highly heterogeneous.” In addition, the two cohorts were CF specific while the Genome Database is not. Finally, the CF cohorts only had a single dominant strain sampled, though multiple P. aeruginosa lineages are often present in CF patients.
One author reported receiving grants from the Cystic Fibrosis Foundation, clinical trial support, and consulting fees from industry. Two other authors are inventors on a patent application that covers the development of a vaccine that targets Pf phage. The others reported no conflicts of interest.
SOURCE: Burgener EB et al. Sci Transl Med. 2019 Apr 17. doi: 10.1126/scitranslmed.aau9748.
according to a study of the prevalence and clinical relevance of Pf phage in two patient cohorts.
“Our data from both the Stanford and Danish CF cohorts together suggest that either patients with CF acquire Pf phage–producing strains of P. aeruginosa or the Pf phage–negative P. aeruginosa become infected with Pf phage as patients age and their disease progresses,” wrote Elizabeth B. Burgener, MD, of Stanford (Calif.) University, and her coauthors. The study was published in Science Translational Medicine.The study analyzed a previous Danish longitudinal cohort of 34 patients and a prospective cross-sectional cohort of 76 patients at Stanford, 58 of which had P. aeruginosa. The researchers also reviewed a collection of genetic sequences called the Pseudomonas Genome Database to determine the prevalence of Pf phage, finding evidence in 1,159 of 2,226 P. aeruginosa sequences (52.1%).
In the Danish cohort, 21 of the 34 CF patients (61.8%; 95% confidence interval, 43.6%-77.8%) had at least one P. aeruginosa isolate containing Pf phage; 9 (26.5%) of the patients were found to be consistently positive for Pf phage. Those who were consistently positive were also older than those who never had Pf phage detected (19.1 years vs. 13.9 years; P = .046), suggesting that “there may be a tendency for P. aeruginosa strains that produce Pf phage to dominate in the sputum of individual patients with CF over time.”
In the Stanford cohort, the prevalence of Pf phage was 36.2% (21 of 58; 95% CI, 24.0%-49.9%) in patients with P. aeruginosa infection and 27.6% (21 of 76; 95% CI, 18.0%-39.1%) in all patients. No Pf phage was detected in any P. aeruginosa–negative samples. Patients positive for Pf phage in this cohort were also older than patients who were negative.
The authors acknowledged their study’s limitations, describing the methods used to collect and sequence the analyzed samples as “highly heterogeneous.” In addition, the two cohorts were CF specific while the Genome Database is not. Finally, the CF cohorts only had a single dominant strain sampled, though multiple P. aeruginosa lineages are often present in CF patients.
One author reported receiving grants from the Cystic Fibrosis Foundation, clinical trial support, and consulting fees from industry. Two other authors are inventors on a patent application that covers the development of a vaccine that targets Pf phage. The others reported no conflicts of interest.
SOURCE: Burgener EB et al. Sci Transl Med. 2019 Apr 17. doi: 10.1126/scitranslmed.aau9748.
according to a study of the prevalence and clinical relevance of Pf phage in two patient cohorts.
“Our data from both the Stanford and Danish CF cohorts together suggest that either patients with CF acquire Pf phage–producing strains of P. aeruginosa or the Pf phage–negative P. aeruginosa become infected with Pf phage as patients age and their disease progresses,” wrote Elizabeth B. Burgener, MD, of Stanford (Calif.) University, and her coauthors. The study was published in Science Translational Medicine.The study analyzed a previous Danish longitudinal cohort of 34 patients and a prospective cross-sectional cohort of 76 patients at Stanford, 58 of which had P. aeruginosa. The researchers also reviewed a collection of genetic sequences called the Pseudomonas Genome Database to determine the prevalence of Pf phage, finding evidence in 1,159 of 2,226 P. aeruginosa sequences (52.1%).
In the Danish cohort, 21 of the 34 CF patients (61.8%; 95% confidence interval, 43.6%-77.8%) had at least one P. aeruginosa isolate containing Pf phage; 9 (26.5%) of the patients were found to be consistently positive for Pf phage. Those who were consistently positive were also older than those who never had Pf phage detected (19.1 years vs. 13.9 years; P = .046), suggesting that “there may be a tendency for P. aeruginosa strains that produce Pf phage to dominate in the sputum of individual patients with CF over time.”
In the Stanford cohort, the prevalence of Pf phage was 36.2% (21 of 58; 95% CI, 24.0%-49.9%) in patients with P. aeruginosa infection and 27.6% (21 of 76; 95% CI, 18.0%-39.1%) in all patients. No Pf phage was detected in any P. aeruginosa–negative samples. Patients positive for Pf phage in this cohort were also older than patients who were negative.
The authors acknowledged their study’s limitations, describing the methods used to collect and sequence the analyzed samples as “highly heterogeneous.” In addition, the two cohorts were CF specific while the Genome Database is not. Finally, the CF cohorts only had a single dominant strain sampled, though multiple P. aeruginosa lineages are often present in CF patients.
One author reported receiving grants from the Cystic Fibrosis Foundation, clinical trial support, and consulting fees from industry. Two other authors are inventors on a patent application that covers the development of a vaccine that targets Pf phage. The others reported no conflicts of interest.
SOURCE: Burgener EB et al. Sci Transl Med. 2019 Apr 17. doi: 10.1126/scitranslmed.aau9748.
FROM SCIENCE TRANSLATIONAL MEDICINE
Atorvastatin appears to lower cardiovascular risk in RA patients
Atorvastatin proved safe and potentially effective in preventing cardiovascular events in RA patients, according to the prematurely terminated TRACE RA trial.
“TRACE RA suggests that atorvastatin 40 mg daily is safe for the primary prevention of [CV events] in patients with RA and appears to confer a similar degree of risk reduction in these patients as in other populations,” wrote George D. Kitas, MD, of the Dudley (England) Group NHS Foundation Trust. The study was published in Arthritis & Rheumatology.
TRACE RA was a multicenter, double-blind, randomized trial that compared atorvastatin with placebo in preventing CV events by reducing LDL cholesterol. Its 3,002 patients with RA were randomized to receive either atorvastatin (1,504) or placebo (1,498). The goal was to follow the participants for 5 years. However, because of an unexpectedly low event rate, the trial was terminated early, resulting in a mean follow-up of 2.5 years.
At the end of the trial, those in the atorvastatin group had 0.77 mmol/L lower LDL cholesterol levels, compared with the placebo group (P less than .0001). Of the patients who received atorvastatin, 24 (1.6%) had a cardiac event versus 36 (2.4%) for placebo (hazard ratio, 0.66; 95% confidence interval, 0.39-1.11; P = .115). The estimated CV event risk reduction per 1 mmol/L reduction in LDL cholesterol was 42% (95% CI, –14% to 70%).
The coauthors acknowledged the study’s limitations, including the fact that it was terminated early because of a lower-than-expected CV event rate. This led to their results not being deemed statistically significant. They noted several reasons why this might have occurred – among them TRACE RA purposely excluding patients with the highest CV event risk – but also recognized that “the low event rate shows that there is a sizeable population of RA patients who have a relatively low CVD risk.”
“This does not support prescribing statins to all RA patients,” they added. “Instead, the decision to prescribe should be based on assessment of the individual RA patient’s risk using, at present, the relevant national or international recommendations and risk assessment tools.”
The study was funded by Arthritis Research UK and the British Heart Foundation. The coauthors report numerous potential conflicts of interest, including receiving honoraria for lectures and advisory boards participation, grant support, and consulting fees from various pharmaceutical companies.
SOURCE: Kitas GD et al. Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40892.
Although it did not accomplish exactly what it set out to do, the TRACE RA study is a firm step in the right direction, according to Katherine P. Liao, MD, and Daniel H. Solomon, MD, of Brigham and Women’s Hospital in Boston.
To illustrate their point, Dr. Liao and Dr. Solomon presented a hypothetical RA patient called TR. She is firmly “average,” especially among the population represented in this study. Though she doesn’t seem like a glaring candidate for a statin, we can rightfully assume that – because of RA and a C-reactive protein above 2 mg/dL – her cardiovascular risk is higher than a member of the general population. The next step is determining if a statin will benefit such a patient, something relatively unexplored thus far.
Despite its abrupt termination, the coauthors “laud the investigators of TRACE RA, as this is the first trial among RA patients that was designed to study hard CVD endpoints.” At the very least, the study reinforced that statins are not associated with side effects when paired with typical RA treatments. In the future, Dr. Liao and Dr. Solomon suggested a focus on “better methods for identifying the appropriate patient population in RA to target for CV risk reduction strategies.”
These comments are adapted from an accompanying editorial (Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40891). Dr. Solomon reported receiving salary support through research contracts from AbbVie, Amgen, Corrona, Genentech, Janssen, and Pfizer.
Although it did not accomplish exactly what it set out to do, the TRACE RA study is a firm step in the right direction, according to Katherine P. Liao, MD, and Daniel H. Solomon, MD, of Brigham and Women’s Hospital in Boston.
To illustrate their point, Dr. Liao and Dr. Solomon presented a hypothetical RA patient called TR. She is firmly “average,” especially among the population represented in this study. Though she doesn’t seem like a glaring candidate for a statin, we can rightfully assume that – because of RA and a C-reactive protein above 2 mg/dL – her cardiovascular risk is higher than a member of the general population. The next step is determining if a statin will benefit such a patient, something relatively unexplored thus far.
Despite its abrupt termination, the coauthors “laud the investigators of TRACE RA, as this is the first trial among RA patients that was designed to study hard CVD endpoints.” At the very least, the study reinforced that statins are not associated with side effects when paired with typical RA treatments. In the future, Dr. Liao and Dr. Solomon suggested a focus on “better methods for identifying the appropriate patient population in RA to target for CV risk reduction strategies.”
These comments are adapted from an accompanying editorial (Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40891). Dr. Solomon reported receiving salary support through research contracts from AbbVie, Amgen, Corrona, Genentech, Janssen, and Pfizer.
Although it did not accomplish exactly what it set out to do, the TRACE RA study is a firm step in the right direction, according to Katherine P. Liao, MD, and Daniel H. Solomon, MD, of Brigham and Women’s Hospital in Boston.
To illustrate their point, Dr. Liao and Dr. Solomon presented a hypothetical RA patient called TR. She is firmly “average,” especially among the population represented in this study. Though she doesn’t seem like a glaring candidate for a statin, we can rightfully assume that – because of RA and a C-reactive protein above 2 mg/dL – her cardiovascular risk is higher than a member of the general population. The next step is determining if a statin will benefit such a patient, something relatively unexplored thus far.
Despite its abrupt termination, the coauthors “laud the investigators of TRACE RA, as this is the first trial among RA patients that was designed to study hard CVD endpoints.” At the very least, the study reinforced that statins are not associated with side effects when paired with typical RA treatments. In the future, Dr. Liao and Dr. Solomon suggested a focus on “better methods for identifying the appropriate patient population in RA to target for CV risk reduction strategies.”
These comments are adapted from an accompanying editorial (Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40891). Dr. Solomon reported receiving salary support through research contracts from AbbVie, Amgen, Corrona, Genentech, Janssen, and Pfizer.
Atorvastatin proved safe and potentially effective in preventing cardiovascular events in RA patients, according to the prematurely terminated TRACE RA trial.
“TRACE RA suggests that atorvastatin 40 mg daily is safe for the primary prevention of [CV events] in patients with RA and appears to confer a similar degree of risk reduction in these patients as in other populations,” wrote George D. Kitas, MD, of the Dudley (England) Group NHS Foundation Trust. The study was published in Arthritis & Rheumatology.
TRACE RA was a multicenter, double-blind, randomized trial that compared atorvastatin with placebo in preventing CV events by reducing LDL cholesterol. Its 3,002 patients with RA were randomized to receive either atorvastatin (1,504) or placebo (1,498). The goal was to follow the participants for 5 years. However, because of an unexpectedly low event rate, the trial was terminated early, resulting in a mean follow-up of 2.5 years.
At the end of the trial, those in the atorvastatin group had 0.77 mmol/L lower LDL cholesterol levels, compared with the placebo group (P less than .0001). Of the patients who received atorvastatin, 24 (1.6%) had a cardiac event versus 36 (2.4%) for placebo (hazard ratio, 0.66; 95% confidence interval, 0.39-1.11; P = .115). The estimated CV event risk reduction per 1 mmol/L reduction in LDL cholesterol was 42% (95% CI, –14% to 70%).
The coauthors acknowledged the study’s limitations, including the fact that it was terminated early because of a lower-than-expected CV event rate. This led to their results not being deemed statistically significant. They noted several reasons why this might have occurred – among them TRACE RA purposely excluding patients with the highest CV event risk – but also recognized that “the low event rate shows that there is a sizeable population of RA patients who have a relatively low CVD risk.”
“This does not support prescribing statins to all RA patients,” they added. “Instead, the decision to prescribe should be based on assessment of the individual RA patient’s risk using, at present, the relevant national or international recommendations and risk assessment tools.”
The study was funded by Arthritis Research UK and the British Heart Foundation. The coauthors report numerous potential conflicts of interest, including receiving honoraria for lectures and advisory boards participation, grant support, and consulting fees from various pharmaceutical companies.
SOURCE: Kitas GD et al. Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40892.
Atorvastatin proved safe and potentially effective in preventing cardiovascular events in RA patients, according to the prematurely terminated TRACE RA trial.
“TRACE RA suggests that atorvastatin 40 mg daily is safe for the primary prevention of [CV events] in patients with RA and appears to confer a similar degree of risk reduction in these patients as in other populations,” wrote George D. Kitas, MD, of the Dudley (England) Group NHS Foundation Trust. The study was published in Arthritis & Rheumatology.
TRACE RA was a multicenter, double-blind, randomized trial that compared atorvastatin with placebo in preventing CV events by reducing LDL cholesterol. Its 3,002 patients with RA were randomized to receive either atorvastatin (1,504) or placebo (1,498). The goal was to follow the participants for 5 years. However, because of an unexpectedly low event rate, the trial was terminated early, resulting in a mean follow-up of 2.5 years.
At the end of the trial, those in the atorvastatin group had 0.77 mmol/L lower LDL cholesterol levels, compared with the placebo group (P less than .0001). Of the patients who received atorvastatin, 24 (1.6%) had a cardiac event versus 36 (2.4%) for placebo (hazard ratio, 0.66; 95% confidence interval, 0.39-1.11; P = .115). The estimated CV event risk reduction per 1 mmol/L reduction in LDL cholesterol was 42% (95% CI, –14% to 70%).
The coauthors acknowledged the study’s limitations, including the fact that it was terminated early because of a lower-than-expected CV event rate. This led to their results not being deemed statistically significant. They noted several reasons why this might have occurred – among them TRACE RA purposely excluding patients with the highest CV event risk – but also recognized that “the low event rate shows that there is a sizeable population of RA patients who have a relatively low CVD risk.”
“This does not support prescribing statins to all RA patients,” they added. “Instead, the decision to prescribe should be based on assessment of the individual RA patient’s risk using, at present, the relevant national or international recommendations and risk assessment tools.”
The study was funded by Arthritis Research UK and the British Heart Foundation. The coauthors report numerous potential conflicts of interest, including receiving honoraria for lectures and advisory boards participation, grant support, and consulting fees from various pharmaceutical companies.
SOURCE: Kitas GD et al. Arthritis Rheumatol. 2019 Apr 15. doi: 10.1002/art.40892.
FROM ARTHRITIS & RHEUMATOLOGY
Direct-to-consumer telemedicine visits may lead to pediatric antibiotic overprescribing
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
These findings from this study illustrate the issues with direct-to-consumer (DTC) telemedicine, especially when treating children, according to Jeffrey S. Gerber, MD, medical director of the antimicrobial stewardship program at Children’s Hospital of Philadelphia.
The best way to get a 5-star rating after a DTC telemedicine visit is to prescribe an antibiotic, Dr. Gerber wrote, so it shouldn’t be surprising that doctors are handing them out at a higher rate than after an urgent care or a primary care visit. It should also be noted that this study covers a very specific privately insured population and that DTC telemedicine remains a “small piece of the pie,” for now, in terms of patient care.
But, he added, the most problematic element of this study may be that none of the 3 most common pediatric acute respiratory tract infection (ARTI) diagnoses should be followed with an immediate prescription, especially after a virtual visit.
“It could be argued that essentially no ARTI encounters should lead to antibiotic prescriptions solely on the basis of a DTC telemedicine visit,” he wrote, recognizing that – though there may be value for telemedicine in a screening capacity – the DTC version seems to be a “low quality encounter” at best and “a vehicle for antibiotic overuse” at worst.
These comments are adapted from an accompanying editorial (Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2019-0631 ). Dr. Gerber reported receiving personal fees from Medtronic outside the submitted work.
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
(ARIs), according to a study of antibiotic prescriptions for ARIs across 3 clinical settings.
“These differences in antibiotic prescribing for children contrast with previous studies of DTC telemedicine quality among adult patients in which quality differences have been smaller or nonexistent,” wrote Kristin N. Ray, MD, of Children’s Hospital of Pittsburgh, and her coauthors. The study was published in Pediatrics.
To determine quality of care during pediatric DTC telemedicine visits, the researchers embarked on a retrospective cohort study using 2015–2016 claims data from a large national commercial health plan. They identified visits for ARIs and matched them across 3 settings: DTC telemedicine, urgent care, and PCP offices. The matched sample included 4,604 DTC telemedicine visits, 38,408 urgent care visits, and 485,201 PCP visits.
Their analysis showed that children were more likely to be prescribed antibiotics at DTC telemedicine visits than in other settings (52% versus 42% for urgent care and 31% for PCP, P less than .001). In addition, they were less likely to receive guideline-concordant antibiotic management (59% versus 67% and 78%, P less than .001). This was primarily attributed to “antibiotic prescribing for visits with viral ARI diagnoses that do not warrant antibiotics,” antibiotics were appropriately not prescribed in only 54% of those DTC telemedicine visits, compared with 66% for urgent care and 80% for PCP (P less than .001).
The authors shared the limitations of their study, including a lack of sociodemographic or clinical data stemming from a reliance on insurance claims. They also noted that their analysis was limited to a specific health plan and its contracted DTC telemedicine vendor, recognizing that “antibiotic prescribing among other DTC telemedicine companies, models, and populations may differ.”
The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
SOURCE: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
FROM PEDIATRICS
Key clinical point: For children diagnosed with acute respiratory infections, antibiotic prescribing was higher and guideline-concordant antibiotic management was lower at direct-to-consumer (DTC) telemedicine visits.
Major finding: Children at DTC telemedicine visits were prescribed antibiotics for respiratory infections 52% of the time, compared with 42% at urgent care visits and 31% at primary care provider visits.
Study details: A retrospective cohort study of DTC telemedicine, urgent care, and primary care provider visits for acute respiratory infections and subsequent antibiotic prescriptions.
Disclosures: The study was funded by the National Institutes of Health and supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gifts from Melvin Hall. The authors reported no conflicts of interest.
Source: Ray KN et al. Pediatrics. 2019 Apr 8. doi: 10.1542/peds.2018-2491.
More chest compression–only CPR leads to increased survival rates
according to a Swedish study of out-of-hospital cardiac arrests and subsequent CPR.
“These findings support continuous endorsement of chest compression–only CPR as an option in future CPR guidelines because it is associated with higher CPR rates and survival in out-of-hospital cardiac arrests,” wrote Gabriel Riva, MD, of the Karolinska Institutet, Stockholm, and his coauthors. The study was published in Circulation.
To determine changes in the rate and type of CPR performed before emergency medical services (EMS) arrival, the researchers compared all bystander-witnessed out-of-hospital cardiac arrests (OHCAs) reported in Sweden between 2000 and 2017. In all, 30,445 patients were included; the time periods compared were 2000-2005, 2006-2010, and 2011-2017. Patients were categorized as receiving either no CPR (NO-CPR), standard CPR (S-CPR), or chest compression–only CPR (CO-CPR). In 2005, CO-CPR was introduced in national CPR guidelines as an option for bystanders; in 2010, it was recommended for anyone untrained in CPR.
The proportion of patients who received CPR in general increased from 41% in 2000-2005 to 59% in 2006-2010 to 68% in 2011-2017. S-CPR changed from 35% to 45% to 38% over the three periods, while CO-CPR increased from 5% to 14% to 30%. In regard to 30-day survival rates, the S-CPR group saw an increase from 9% to 13% to 16% and the CO-CPR group increased from 8% to 12% to 14%, compared with 4% to 6% to 7% for the NO-CPR group.
The authors noted the limitations of their study, including the results being based on register data and therefore subject to misclassification and missing data. In addition, missing data negated any reporting on the neurological function of survivors; analyzing witnessed OHCAs only also meant the findings could not be validated for nonwitnessed OHCA.
The Swedish Heart and Lung Foundation funded the study. The authors made no disclosures.
SOURCE: Riva G et al. Circulation. 2019 Apr 1. doi: 10.1161/CIRCULATIONAHA.118.038179.
according to a Swedish study of out-of-hospital cardiac arrests and subsequent CPR.
“These findings support continuous endorsement of chest compression–only CPR as an option in future CPR guidelines because it is associated with higher CPR rates and survival in out-of-hospital cardiac arrests,” wrote Gabriel Riva, MD, of the Karolinska Institutet, Stockholm, and his coauthors. The study was published in Circulation.
To determine changes in the rate and type of CPR performed before emergency medical services (EMS) arrival, the researchers compared all bystander-witnessed out-of-hospital cardiac arrests (OHCAs) reported in Sweden between 2000 and 2017. In all, 30,445 patients were included; the time periods compared were 2000-2005, 2006-2010, and 2011-2017. Patients were categorized as receiving either no CPR (NO-CPR), standard CPR (S-CPR), or chest compression–only CPR (CO-CPR). In 2005, CO-CPR was introduced in national CPR guidelines as an option for bystanders; in 2010, it was recommended for anyone untrained in CPR.
The proportion of patients who received CPR in general increased from 41% in 2000-2005 to 59% in 2006-2010 to 68% in 2011-2017. S-CPR changed from 35% to 45% to 38% over the three periods, while CO-CPR increased from 5% to 14% to 30%. In regard to 30-day survival rates, the S-CPR group saw an increase from 9% to 13% to 16% and the CO-CPR group increased from 8% to 12% to 14%, compared with 4% to 6% to 7% for the NO-CPR group.
The authors noted the limitations of their study, including the results being based on register data and therefore subject to misclassification and missing data. In addition, missing data negated any reporting on the neurological function of survivors; analyzing witnessed OHCAs only also meant the findings could not be validated for nonwitnessed OHCA.
The Swedish Heart and Lung Foundation funded the study. The authors made no disclosures.
SOURCE: Riva G et al. Circulation. 2019 Apr 1. doi: 10.1161/CIRCULATIONAHA.118.038179.
according to a Swedish study of out-of-hospital cardiac arrests and subsequent CPR.
“These findings support continuous endorsement of chest compression–only CPR as an option in future CPR guidelines because it is associated with higher CPR rates and survival in out-of-hospital cardiac arrests,” wrote Gabriel Riva, MD, of the Karolinska Institutet, Stockholm, and his coauthors. The study was published in Circulation.
To determine changes in the rate and type of CPR performed before emergency medical services (EMS) arrival, the researchers compared all bystander-witnessed out-of-hospital cardiac arrests (OHCAs) reported in Sweden between 2000 and 2017. In all, 30,445 patients were included; the time periods compared were 2000-2005, 2006-2010, and 2011-2017. Patients were categorized as receiving either no CPR (NO-CPR), standard CPR (S-CPR), or chest compression–only CPR (CO-CPR). In 2005, CO-CPR was introduced in national CPR guidelines as an option for bystanders; in 2010, it was recommended for anyone untrained in CPR.
The proportion of patients who received CPR in general increased from 41% in 2000-2005 to 59% in 2006-2010 to 68% in 2011-2017. S-CPR changed from 35% to 45% to 38% over the three periods, while CO-CPR increased from 5% to 14% to 30%. In regard to 30-day survival rates, the S-CPR group saw an increase from 9% to 13% to 16% and the CO-CPR group increased from 8% to 12% to 14%, compared with 4% to 6% to 7% for the NO-CPR group.
The authors noted the limitations of their study, including the results being based on register data and therefore subject to misclassification and missing data. In addition, missing data negated any reporting on the neurological function of survivors; analyzing witnessed OHCAs only also meant the findings could not be validated for nonwitnessed OHCA.
The Swedish Heart and Lung Foundation funded the study. The authors made no disclosures.
SOURCE: Riva G et al. Circulation. 2019 Apr 1. doi: 10.1161/CIRCULATIONAHA.118.038179.
FROM CIRCULATION
Key clinical point: Since chest compression-only CPR was introduced and recommended as an alternative for bystanders witnessing a cardiac arrest, CPR rates and survival rates have increased.
Major finding: From 2001-2005 to 2011-2017, 30-day survival rates increased from 9% to 16% for the standard CPR group and from 8% to 14% for the chest compression–only group, compared with 4%-7% for the no CPR group.
Study details: An observational nationwide cohort study of 30,445 Swedish patients who suffered out-of-hospital cardiac arrest.
Disclosures: The Swedish Heart and Lung Foundation funded the study. The authors made no disclosures.
Source: Riva G et al. Circulation. 2019 Apr 1. doi: 10.1161/CIRCULATIONAHA.118.038179.
Food allergy can be revealed in the epidermis of children with atopic dermatitis
according to a study of children with and without AD and FA.
The researchers included 62 children aged 4-17 years, who were divided into three groups: atopic dermatitis and food allergy (AD FA+, n = 21), atopic dermatitis and no food allergy (AD FA−, n = 19), and nonatopic controls (NA, n = 22).
“In this prospective clinical study with laboratory personnel blinded to minimize bias, we demonstrate that children with AD FA+ represent a unique endotype that can be distinguished from AD FA− or NA,” wrote Donald Y. M. Leung, MD, of National Jewish Health, Denver, and his coauthors. Their work was published online in Science Translational Medicine.
According to three different scoring systems, the two AD groups were measured to have similar skin disease severity. Dr. Leung and colleagues then used skin tape stripping to measure the first layer of skin tissue for transepidermal water loss (TEWL) and stratum corneum (SC) composition, along with other variables that would indicate a difference between AD FA+ and the other groups.
Upon analysis, children in the AD FA+ group were found to have “a constellation of SC attributes,” including increased TEWL and lower levels of filaggrin gene breakdown products (urocanic acid and pyroglutamic acid) at nonlesional layers. In addition, there was an increase of Staphylococcus aureus on the nonlesional skin of AD FA+, compared with NA.
The coauthors shared the study’s limitations, which included transcriptome analysis being successful for only a fraction of the patients and the lack of skin biopsies, which would be useful to confirm “the potential role of changes in the deeper layers of skin.” However, they also noted that using minimally invasive STS led to more patients providing samples, and thus less bias in collection. “Although future studies are needed to validate our findings,” Dr. Leung and his associates wrote, “our current data support the concept that primary and secondary prevention of AD and FA in this subset of AD should focus on improving skin barrier function.”
The study was funded by the National Institute of Health/The National Institute of Allergy and Infectious Diseases’ Atopic Dermatitis Research Network, with partial support from the Edelstein Family Chair for Pediatric Allergy at NIH and a NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards grant. Three of the authors declared being inventors of a patent that covers methods of identifying AD with FA as a unique endotype. No other conflicts of interest were reported.
SOURCE: Leung DYM et al. Sci Transl Med. 2019 Feb 20. doi: 10.1126/scitranslmed.aav2685.
according to a study of children with and without AD and FA.
The researchers included 62 children aged 4-17 years, who were divided into three groups: atopic dermatitis and food allergy (AD FA+, n = 21), atopic dermatitis and no food allergy (AD FA−, n = 19), and nonatopic controls (NA, n = 22).
“In this prospective clinical study with laboratory personnel blinded to minimize bias, we demonstrate that children with AD FA+ represent a unique endotype that can be distinguished from AD FA− or NA,” wrote Donald Y. M. Leung, MD, of National Jewish Health, Denver, and his coauthors. Their work was published online in Science Translational Medicine.
According to three different scoring systems, the two AD groups were measured to have similar skin disease severity. Dr. Leung and colleagues then used skin tape stripping to measure the first layer of skin tissue for transepidermal water loss (TEWL) and stratum corneum (SC) composition, along with other variables that would indicate a difference between AD FA+ and the other groups.
Upon analysis, children in the AD FA+ group were found to have “a constellation of SC attributes,” including increased TEWL and lower levels of filaggrin gene breakdown products (urocanic acid and pyroglutamic acid) at nonlesional layers. In addition, there was an increase of Staphylococcus aureus on the nonlesional skin of AD FA+, compared with NA.
The coauthors shared the study’s limitations, which included transcriptome analysis being successful for only a fraction of the patients and the lack of skin biopsies, which would be useful to confirm “the potential role of changes in the deeper layers of skin.” However, they also noted that using minimally invasive STS led to more patients providing samples, and thus less bias in collection. “Although future studies are needed to validate our findings,” Dr. Leung and his associates wrote, “our current data support the concept that primary and secondary prevention of AD and FA in this subset of AD should focus on improving skin barrier function.”
The study was funded by the National Institute of Health/The National Institute of Allergy and Infectious Diseases’ Atopic Dermatitis Research Network, with partial support from the Edelstein Family Chair for Pediatric Allergy at NIH and a NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards grant. Three of the authors declared being inventors of a patent that covers methods of identifying AD with FA as a unique endotype. No other conflicts of interest were reported.
SOURCE: Leung DYM et al. Sci Transl Med. 2019 Feb 20. doi: 10.1126/scitranslmed.aav2685.
according to a study of children with and without AD and FA.
The researchers included 62 children aged 4-17 years, who were divided into three groups: atopic dermatitis and food allergy (AD FA+, n = 21), atopic dermatitis and no food allergy (AD FA−, n = 19), and nonatopic controls (NA, n = 22).
“In this prospective clinical study with laboratory personnel blinded to minimize bias, we demonstrate that children with AD FA+ represent a unique endotype that can be distinguished from AD FA− or NA,” wrote Donald Y. M. Leung, MD, of National Jewish Health, Denver, and his coauthors. Their work was published online in Science Translational Medicine.
According to three different scoring systems, the two AD groups were measured to have similar skin disease severity. Dr. Leung and colleagues then used skin tape stripping to measure the first layer of skin tissue for transepidermal water loss (TEWL) and stratum corneum (SC) composition, along with other variables that would indicate a difference between AD FA+ and the other groups.
Upon analysis, children in the AD FA+ group were found to have “a constellation of SC attributes,” including increased TEWL and lower levels of filaggrin gene breakdown products (urocanic acid and pyroglutamic acid) at nonlesional layers. In addition, there was an increase of Staphylococcus aureus on the nonlesional skin of AD FA+, compared with NA.
The coauthors shared the study’s limitations, which included transcriptome analysis being successful for only a fraction of the patients and the lack of skin biopsies, which would be useful to confirm “the potential role of changes in the deeper layers of skin.” However, they also noted that using minimally invasive STS led to more patients providing samples, and thus less bias in collection. “Although future studies are needed to validate our findings,” Dr. Leung and his associates wrote, “our current data support the concept that primary and secondary prevention of AD and FA in this subset of AD should focus on improving skin barrier function.”
The study was funded by the National Institute of Health/The National Institute of Allergy and Infectious Diseases’ Atopic Dermatitis Research Network, with partial support from the Edelstein Family Chair for Pediatric Allergy at NIH and a NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards grant. Three of the authors declared being inventors of a patent that covers methods of identifying AD with FA as a unique endotype. No other conflicts of interest were reported.
SOURCE: Leung DYM et al. Sci Transl Med. 2019 Feb 20. doi: 10.1126/scitranslmed.aav2685.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Children with both atopic dermatitis and food allergy can be distinguished from those with just atopic dermatitis via their nonlesional skin surface.
Major finding: Those in the AD FA+ group were found to have “a constellation of stratum corneum attributes,” including increased TEWL and lower levels of filaggrin gene breakdown products.
Study details: A prospective clinical study of 62 children aged 4-17 years who were divided into three groups: atopic dermatitis and food allergy, atopic dermatitis and no food allergy, and nonatopic controls.
Disclosures: The study was funded by the National Institute of Health/The National Institute of Allergy and Infectious Diseases’ Atopic Dermatitis Research Network, with partial support from the Edelstein Family Chair for Pediatric Allergy at NIH and a NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards grant. Three of the authors declared being inventors of a patent that covers methods of identifying AD with FA as a unique endotype. No other conflicts of interest were reported.
Source: Leung DYM et al. Sci Transl Med. 2019 Feb 20. doi: 10.1126/scitranslmed.aav2685.
Patients at risk of RA may already have abnormal aortic stiffness
according to a study of potential RA patients who underwent cardiac MRI.
“To our knowledge, this is the first study showing subclinical increase in aortic stiffness in at-risk individuals for RA, with values numerically close to those seen in early, treatment-naive RA,” wrote Graham Fent, MBChB, of the University of Leeds (England) and his associates. The study was published in Annals of the Rheumatic Diseases.
Hypothesizing that patients with no systemic inflammation but circulating anti–cyclic citrullinated peptide (CCP) antibodies may already have cardiovascular concerns, Dr. Fent and his colleagues recruited 18 individuals at risk of developing RA and 30 healthy controls. The groups were matched for age and gender and then underwent multiparametric 3.0 Tesla cardiac MRI with late gadolinium enhancement. The at-risk individuals were classified as being at either low (n = 10) or high (n = 8) risk of RA. Over 12 months, five of the at-risk patients progressed to RA.
According to the cardiac MRI findings, aortic distensibility was lower – and thus arterial stiffness was greater – in the at-risk group (3.6 x 10–3 per mm Hg) versus the healthy controls (4.9 x 10–3 per mm Hg). The difference was even more distinct in the high-risk group (3.1 x 10–3 per mm Hg), compared with the low-risk group (4.2 x 10–3 per mm Hg). The group who eventually progressed to RA also showed lower levels of distensibility (3.2 x 10–3 per mm Hg).
The coauthors acknowledged that the major limitation of their study was a lack of control groups. However, they noted that such a pronounced level of aortic stiffness in the high-risk and RA groups should be seen as “implying a particular role of CCP antibodies.”
The study was supported by the U.K. National Institute for Health Research. One author reported being funded by a National Institute for Health Research grant; another reported being funded by a British Heart Foundation Personal Chair.
SOURCE: Fent G et al. Ann Rheum Dis. 2019 Mar 9. doi: 10.1136/annrheumdis-2018-214975.
according to a study of potential RA patients who underwent cardiac MRI.
“To our knowledge, this is the first study showing subclinical increase in aortic stiffness in at-risk individuals for RA, with values numerically close to those seen in early, treatment-naive RA,” wrote Graham Fent, MBChB, of the University of Leeds (England) and his associates. The study was published in Annals of the Rheumatic Diseases.
Hypothesizing that patients with no systemic inflammation but circulating anti–cyclic citrullinated peptide (CCP) antibodies may already have cardiovascular concerns, Dr. Fent and his colleagues recruited 18 individuals at risk of developing RA and 30 healthy controls. The groups were matched for age and gender and then underwent multiparametric 3.0 Tesla cardiac MRI with late gadolinium enhancement. The at-risk individuals were classified as being at either low (n = 10) or high (n = 8) risk of RA. Over 12 months, five of the at-risk patients progressed to RA.
According to the cardiac MRI findings, aortic distensibility was lower – and thus arterial stiffness was greater – in the at-risk group (3.6 x 10–3 per mm Hg) versus the healthy controls (4.9 x 10–3 per mm Hg). The difference was even more distinct in the high-risk group (3.1 x 10–3 per mm Hg), compared with the low-risk group (4.2 x 10–3 per mm Hg). The group who eventually progressed to RA also showed lower levels of distensibility (3.2 x 10–3 per mm Hg).
The coauthors acknowledged that the major limitation of their study was a lack of control groups. However, they noted that such a pronounced level of aortic stiffness in the high-risk and RA groups should be seen as “implying a particular role of CCP antibodies.”
The study was supported by the U.K. National Institute for Health Research. One author reported being funded by a National Institute for Health Research grant; another reported being funded by a British Heart Foundation Personal Chair.
SOURCE: Fent G et al. Ann Rheum Dis. 2019 Mar 9. doi: 10.1136/annrheumdis-2018-214975.
according to a study of potential RA patients who underwent cardiac MRI.
“To our knowledge, this is the first study showing subclinical increase in aortic stiffness in at-risk individuals for RA, with values numerically close to those seen in early, treatment-naive RA,” wrote Graham Fent, MBChB, of the University of Leeds (England) and his associates. The study was published in Annals of the Rheumatic Diseases.
Hypothesizing that patients with no systemic inflammation but circulating anti–cyclic citrullinated peptide (CCP) antibodies may already have cardiovascular concerns, Dr. Fent and his colleagues recruited 18 individuals at risk of developing RA and 30 healthy controls. The groups were matched for age and gender and then underwent multiparametric 3.0 Tesla cardiac MRI with late gadolinium enhancement. The at-risk individuals were classified as being at either low (n = 10) or high (n = 8) risk of RA. Over 12 months, five of the at-risk patients progressed to RA.
According to the cardiac MRI findings, aortic distensibility was lower – and thus arterial stiffness was greater – in the at-risk group (3.6 x 10–3 per mm Hg) versus the healthy controls (4.9 x 10–3 per mm Hg). The difference was even more distinct in the high-risk group (3.1 x 10–3 per mm Hg), compared with the low-risk group (4.2 x 10–3 per mm Hg). The group who eventually progressed to RA also showed lower levels of distensibility (3.2 x 10–3 per mm Hg).
The coauthors acknowledged that the major limitation of their study was a lack of control groups. However, they noted that such a pronounced level of aortic stiffness in the high-risk and RA groups should be seen as “implying a particular role of CCP antibodies.”
The study was supported by the U.K. National Institute for Health Research. One author reported being funded by a National Institute for Health Research grant; another reported being funded by a British Heart Foundation Personal Chair.
SOURCE: Fent G et al. Ann Rheum Dis. 2019 Mar 9. doi: 10.1136/annrheumdis-2018-214975.
FROM ANNALS OF THE RHEUMATIC DISEASES
CREOLE: Amlodipine may be preferable for lowering blood pressure in black patients
Amlodipine plus either hydrochlorothiazide or perindopril effectively reduced blood pressure better than perindopril and hydrochlorothiazide in black African patients with hypertension, based on data presented at the annual meeting of the American College of Cardiology.
“(A) long-acting dihydropyridine calcium-channel blocker (in this case, amlodipine) may be critical to more efficacious blood-pressure lowering among black patients as part of the two-drug combinations used (in Africa),” wrote lead author Dike B. Ojji, PhD, of the University of Abuja in Gwagwalada, Abuja, Nigeria, and his coauthors. “These results contrast with recommendations for black patients in the most recent U.S. guidelines” which recommend either a calcium channel blocker or a diuretic in combination with a different drug class.
The study was published simultaneously in the New England Journal of Medicine.
In the CREOLE study, Dr. Ojji and his colleagues enrolled 728 black patients, mean age 51 years and 63% of them women, in a randomized, single-blind, three-group trial across six countries in sub-Saharan Africa. All patients had uncontrolled hypertension and were assigned to one of three treatment groups: amlodipine plus hydrochlorothiazide (n = 244), amlodipine plus perindopril (n = 243), and perindopril plus hydrochlorothiazide (n = 241). Patients underwent 24-hour ambulatory blood-pressure measuring at baseline and at 6 months.
Of the 621 patients who completed the trial, those in the two groups receiving amlodipine had a larger mean reduction in systolic blood pressure after 6 months than the group receiving perindopril plus hydrochlorothiazide. Compared with the perindopril plus hydrochlorothiazide group, the amlodipine plus hydrochlorothiazide group had an additional -3.14 mm Hg reduction in systolic blood pressure from baseline (95% confidence interval, -5.90 to -0.38, P = 0.03) while the amlodipine plus perindopril group had an additional -3.00 mm Hg reduction (95% CI, -5.81 to -0.20, P = 0.04). The difference between the amlodipine plus hydrochlorothiazide group and the amlodipine plus perindopril group was -0.14 mm Hg (95% CI, -2.90 to 2.61, P = 0.92).
The limitations of the study included using nonmatching trial drugs and not adjusting the P values for the three comparisons of the primary end point, the authors wrote. It’s uncertain whether the findings can be extrapolated to black patients with diabetes or to those living outside of sub-Saharan Africa.
The study was sponsored by a grant from the GlaxoSmithKline Africa Noncommunicable Disease Open Lab. Trial drugs were donated by Aspen Pharmacare. Two authors reported receiving grants and personal fees from numerous pharmaceutical companies.
SOURCE: Ojji DB et al. NEJM. 2019 Mar 18. doi: 10.1056/NEJMoa1901113.
Amlodipine plus either hydrochlorothiazide or perindopril effectively reduced blood pressure better than perindopril and hydrochlorothiazide in black African patients with hypertension, based on data presented at the annual meeting of the American College of Cardiology.
“(A) long-acting dihydropyridine calcium-channel blocker (in this case, amlodipine) may be critical to more efficacious blood-pressure lowering among black patients as part of the two-drug combinations used (in Africa),” wrote lead author Dike B. Ojji, PhD, of the University of Abuja in Gwagwalada, Abuja, Nigeria, and his coauthors. “These results contrast with recommendations for black patients in the most recent U.S. guidelines” which recommend either a calcium channel blocker or a diuretic in combination with a different drug class.
The study was published simultaneously in the New England Journal of Medicine.
In the CREOLE study, Dr. Ojji and his colleagues enrolled 728 black patients, mean age 51 years and 63% of them women, in a randomized, single-blind, three-group trial across six countries in sub-Saharan Africa. All patients had uncontrolled hypertension and were assigned to one of three treatment groups: amlodipine plus hydrochlorothiazide (n = 244), amlodipine plus perindopril (n = 243), and perindopril plus hydrochlorothiazide (n = 241). Patients underwent 24-hour ambulatory blood-pressure measuring at baseline and at 6 months.
Of the 621 patients who completed the trial, those in the two groups receiving amlodipine had a larger mean reduction in systolic blood pressure after 6 months than the group receiving perindopril plus hydrochlorothiazide. Compared with the perindopril plus hydrochlorothiazide group, the amlodipine plus hydrochlorothiazide group had an additional -3.14 mm Hg reduction in systolic blood pressure from baseline (95% confidence interval, -5.90 to -0.38, P = 0.03) while the amlodipine plus perindopril group had an additional -3.00 mm Hg reduction (95% CI, -5.81 to -0.20, P = 0.04). The difference between the amlodipine plus hydrochlorothiazide group and the amlodipine plus perindopril group was -0.14 mm Hg (95% CI, -2.90 to 2.61, P = 0.92).
The limitations of the study included using nonmatching trial drugs and not adjusting the P values for the three comparisons of the primary end point, the authors wrote. It’s uncertain whether the findings can be extrapolated to black patients with diabetes or to those living outside of sub-Saharan Africa.
The study was sponsored by a grant from the GlaxoSmithKline Africa Noncommunicable Disease Open Lab. Trial drugs were donated by Aspen Pharmacare. Two authors reported receiving grants and personal fees from numerous pharmaceutical companies.
SOURCE: Ojji DB et al. NEJM. 2019 Mar 18. doi: 10.1056/NEJMoa1901113.
Amlodipine plus either hydrochlorothiazide or perindopril effectively reduced blood pressure better than perindopril and hydrochlorothiazide in black African patients with hypertension, based on data presented at the annual meeting of the American College of Cardiology.
“(A) long-acting dihydropyridine calcium-channel blocker (in this case, amlodipine) may be critical to more efficacious blood-pressure lowering among black patients as part of the two-drug combinations used (in Africa),” wrote lead author Dike B. Ojji, PhD, of the University of Abuja in Gwagwalada, Abuja, Nigeria, and his coauthors. “These results contrast with recommendations for black patients in the most recent U.S. guidelines” which recommend either a calcium channel blocker or a diuretic in combination with a different drug class.
The study was published simultaneously in the New England Journal of Medicine.
In the CREOLE study, Dr. Ojji and his colleagues enrolled 728 black patients, mean age 51 years and 63% of them women, in a randomized, single-blind, three-group trial across six countries in sub-Saharan Africa. All patients had uncontrolled hypertension and were assigned to one of three treatment groups: amlodipine plus hydrochlorothiazide (n = 244), amlodipine plus perindopril (n = 243), and perindopril plus hydrochlorothiazide (n = 241). Patients underwent 24-hour ambulatory blood-pressure measuring at baseline and at 6 months.
Of the 621 patients who completed the trial, those in the two groups receiving amlodipine had a larger mean reduction in systolic blood pressure after 6 months than the group receiving perindopril plus hydrochlorothiazide. Compared with the perindopril plus hydrochlorothiazide group, the amlodipine plus hydrochlorothiazide group had an additional -3.14 mm Hg reduction in systolic blood pressure from baseline (95% confidence interval, -5.90 to -0.38, P = 0.03) while the amlodipine plus perindopril group had an additional -3.00 mm Hg reduction (95% CI, -5.81 to -0.20, P = 0.04). The difference between the amlodipine plus hydrochlorothiazide group and the amlodipine plus perindopril group was -0.14 mm Hg (95% CI, -2.90 to 2.61, P = 0.92).
The limitations of the study included using nonmatching trial drugs and not adjusting the P values for the three comparisons of the primary end point, the authors wrote. It’s uncertain whether the findings can be extrapolated to black patients with diabetes or to those living outside of sub-Saharan Africa.
The study was sponsored by a grant from the GlaxoSmithKline Africa Noncommunicable Disease Open Lab. Trial drugs were donated by Aspen Pharmacare. Two authors reported receiving grants and personal fees from numerous pharmaceutical companies.
SOURCE: Ojji DB et al. NEJM. 2019 Mar 18. doi: 10.1056/NEJMoa1901113.
FROM ACC 2019