User login
Treatment failure reduced in Barrett’s esophagus with endoscopic mucosal resection
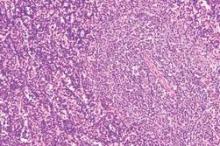
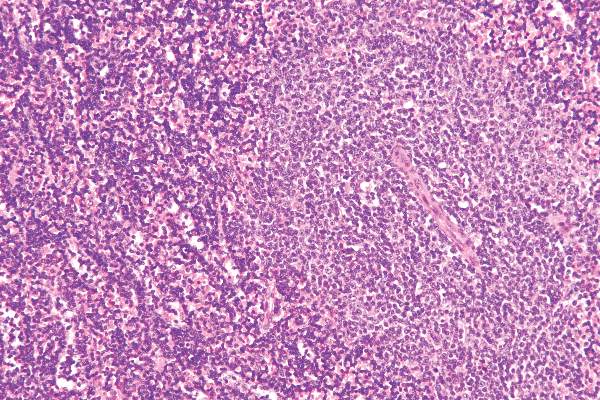
The use of endoscopic mucosal resection (EMR) before radiofrequency ablation significantly reduced the risk for treatment failure among patients with Barrett’s esophagus–associated intramucosal adenocarcinoma (IMC) and dysplasia, according to new data published in the American Journal of Surgical Pathology.
Complete eradication of IMC/dysplasia on the first follow-up endoscopy after treatment was achieved in 86% of patients, while durable eradication, defined as a complete recurrence that persisted until the last follow-up, was achieved in 78% of patients. However, there was significant variation between the different study sites (P = .03) and outcomes were significantly impacted by the baseline extent of IMC and the use of EMR prior to radiofrequency ablation (RFA) therapy.
In addition, almost a quarter of all patients developed treatment-related strictures, usually in the setting of multiple EMRs, and recurrence occurred as a malignant stricture in one patient.
Radiofrequency ablation used with or without EMR, is a safe, effective, and durable treatment option for the treatment of dysplasia associated with Barrett’s esophagus. However, studies that have assessed the predictors of treatment failure in Barrett’s esophagus-associated intramucosal adenocarcinoma (IMC) are limited.
In this study, Dr. Agoston T. Agoston of the department of pathology at Brigham and Women’s Hospital, Boston, and his colleagues investigated the rate of Barrett’s esophagus–associated IMC eradication when using RFA, with or without EMR, in a multicenter setting. In addition, they attempted to identify clinical and pathologic predictors of treatment failure.
“We anticipate that these data will have significant implications for a personalized treatment approach to patients with BE [Barrett’s esophagus]–associated IMC,” wrote the authors.
They conducted a retrospective review of medical records from four tertiary care academic medical centers, and identified 78 patients who underwent RFA with or without EMR as the primary treatment for biopsy-proven IMC.
Some notable baseline differences were observed in patient characteristics at the different study sites, including baseline Barrett’s esophagus segment length (P = .06), baseline nodularity (P = .08), and percentage of tissue involved by IMC at pretreatment endoscopy and biopsy (P = .01).
Over a mean follow-up time of 26.4 months (range, 2-116 months), 86% of patients achieved complete eradication and 78% durable eradication of IMC/dysplasia.
Within the cohort, 11 patients failed to achieve complete eradication, and of the 67 patients who initially did, 6 patients (9.0%) had a subsequent recurrence of neoplasia (3.91 recurrences per 100 patient-years). This extrapolated to an overall rate of 22% for treatment failure (17/78 patients). Of the 17 patients who failed the treatment, 3 subsequently underwent esophagectomy, 1 received palliative measures in the setting of advanced neurological disease, and 1 patient is currently undergoing a repeat ablation procedure with curative intent.
Dr. Agoston and his team also identified 2 clinicopathologic factors that were significantly associated with treatment failure, and both remained significant on univariate and multivariate analysis. The first was that the use of EMR prior to RFA was associated with a significantly reduced risk for treatment failure (hazard ratio, 0.15; 95% confidence interval, 0.05-0.48; P = .001), and the second was that the extent of IMC involving at least 50% of the columnar metaplastic area was associated with a significantly increased risk for treatment failure (HR, 4.24; 95% CI, 1.53-11.7; P = .005).
They also observed similar results when the analysis of extent of IMC as a predictor was restricted to a subset of 43 cases in which the diagnosis of IMC was made on EMR specimens only (HR, 10.8; 95% CI, 2.30-50.8; P = .003).
“In conclusion, we have identified endoscopic and pathologic factors associated with treatment success in patients with BE-associated IMC treated with RFA with or without EMR,” they wrote. “Utilization of these predictors can help in identifying patients with a high probability of success and also those patients with a higher risk for treatment failure for whom a more aggressive initial approach may be justified” (Am J Surg Pathol. 2015 Dec 5. doi: 10.1097/PAS.0000000000000566).
Dr. Rothstein and Dr. Abrams have received research support previously from Barrx/ Covidien and C2 Therapeutics/Covidien, respectively. None of the other authors reported significant conflicts of interest.
The use of endoscopic mucosal resection (EMR) before radiofrequency ablation significantly reduced the risk for treatment failure among patients with Barrett’s esophagus–associated intramucosal adenocarcinoma (IMC) and dysplasia, according to new data published in the American Journal of Surgical Pathology.
Complete eradication of IMC/dysplasia on the first follow-up endoscopy after treatment was achieved in 86% of patients, while durable eradication, defined as a complete recurrence that persisted until the last follow-up, was achieved in 78% of patients. However, there was significant variation between the different study sites (P = .03) and outcomes were significantly impacted by the baseline extent of IMC and the use of EMR prior to radiofrequency ablation (RFA) therapy.
In addition, almost a quarter of all patients developed treatment-related strictures, usually in the setting of multiple EMRs, and recurrence occurred as a malignant stricture in one patient.
Radiofrequency ablation used with or without EMR, is a safe, effective, and durable treatment option for the treatment of dysplasia associated with Barrett’s esophagus. However, studies that have assessed the predictors of treatment failure in Barrett’s esophagus-associated intramucosal adenocarcinoma (IMC) are limited.
In this study, Dr. Agoston T. Agoston of the department of pathology at Brigham and Women’s Hospital, Boston, and his colleagues investigated the rate of Barrett’s esophagus–associated IMC eradication when using RFA, with or without EMR, in a multicenter setting. In addition, they attempted to identify clinical and pathologic predictors of treatment failure.
“We anticipate that these data will have significant implications for a personalized treatment approach to patients with BE [Barrett’s esophagus]–associated IMC,” wrote the authors.
They conducted a retrospective review of medical records from four tertiary care academic medical centers, and identified 78 patients who underwent RFA with or without EMR as the primary treatment for biopsy-proven IMC.
Some notable baseline differences were observed in patient characteristics at the different study sites, including baseline Barrett’s esophagus segment length (P = .06), baseline nodularity (P = .08), and percentage of tissue involved by IMC at pretreatment endoscopy and biopsy (P = .01).
Over a mean follow-up time of 26.4 months (range, 2-116 months), 86% of patients achieved complete eradication and 78% durable eradication of IMC/dysplasia.
Within the cohort, 11 patients failed to achieve complete eradication, and of the 67 patients who initially did, 6 patients (9.0%) had a subsequent recurrence of neoplasia (3.91 recurrences per 100 patient-years). This extrapolated to an overall rate of 22% for treatment failure (17/78 patients). Of the 17 patients who failed the treatment, 3 subsequently underwent esophagectomy, 1 received palliative measures in the setting of advanced neurological disease, and 1 patient is currently undergoing a repeat ablation procedure with curative intent.
Dr. Agoston and his team also identified 2 clinicopathologic factors that were significantly associated with treatment failure, and both remained significant on univariate and multivariate analysis. The first was that the use of EMR prior to RFA was associated with a significantly reduced risk for treatment failure (hazard ratio, 0.15; 95% confidence interval, 0.05-0.48; P = .001), and the second was that the extent of IMC involving at least 50% of the columnar metaplastic area was associated with a significantly increased risk for treatment failure (HR, 4.24; 95% CI, 1.53-11.7; P = .005).
They also observed similar results when the analysis of extent of IMC as a predictor was restricted to a subset of 43 cases in which the diagnosis of IMC was made on EMR specimens only (HR, 10.8; 95% CI, 2.30-50.8; P = .003).
“In conclusion, we have identified endoscopic and pathologic factors associated with treatment success in patients with BE-associated IMC treated with RFA with or without EMR,” they wrote. “Utilization of these predictors can help in identifying patients with a high probability of success and also those patients with a higher risk for treatment failure for whom a more aggressive initial approach may be justified” (Am J Surg Pathol. 2015 Dec 5. doi: 10.1097/PAS.0000000000000566).
Dr. Rothstein and Dr. Abrams have received research support previously from Barrx/ Covidien and C2 Therapeutics/Covidien, respectively. None of the other authors reported significant conflicts of interest.
The use of endoscopic mucosal resection (EMR) before radiofrequency ablation significantly reduced the risk for treatment failure among patients with Barrett’s esophagus–associated intramucosal adenocarcinoma (IMC) and dysplasia, according to new data published in the American Journal of Surgical Pathology.
Complete eradication of IMC/dysplasia on the first follow-up endoscopy after treatment was achieved in 86% of patients, while durable eradication, defined as a complete recurrence that persisted until the last follow-up, was achieved in 78% of patients. However, there was significant variation between the different study sites (P = .03) and outcomes were significantly impacted by the baseline extent of IMC and the use of EMR prior to radiofrequency ablation (RFA) therapy.
In addition, almost a quarter of all patients developed treatment-related strictures, usually in the setting of multiple EMRs, and recurrence occurred as a malignant stricture in one patient.
Radiofrequency ablation used with or without EMR, is a safe, effective, and durable treatment option for the treatment of dysplasia associated with Barrett’s esophagus. However, studies that have assessed the predictors of treatment failure in Barrett’s esophagus-associated intramucosal adenocarcinoma (IMC) are limited.
In this study, Dr. Agoston T. Agoston of the department of pathology at Brigham and Women’s Hospital, Boston, and his colleagues investigated the rate of Barrett’s esophagus–associated IMC eradication when using RFA, with or without EMR, in a multicenter setting. In addition, they attempted to identify clinical and pathologic predictors of treatment failure.
“We anticipate that these data will have significant implications for a personalized treatment approach to patients with BE [Barrett’s esophagus]–associated IMC,” wrote the authors.
They conducted a retrospective review of medical records from four tertiary care academic medical centers, and identified 78 patients who underwent RFA with or without EMR as the primary treatment for biopsy-proven IMC.
Some notable baseline differences were observed in patient characteristics at the different study sites, including baseline Barrett’s esophagus segment length (P = .06), baseline nodularity (P = .08), and percentage of tissue involved by IMC at pretreatment endoscopy and biopsy (P = .01).
Over a mean follow-up time of 26.4 months (range, 2-116 months), 86% of patients achieved complete eradication and 78% durable eradication of IMC/dysplasia.
Within the cohort, 11 patients failed to achieve complete eradication, and of the 67 patients who initially did, 6 patients (9.0%) had a subsequent recurrence of neoplasia (3.91 recurrences per 100 patient-years). This extrapolated to an overall rate of 22% for treatment failure (17/78 patients). Of the 17 patients who failed the treatment, 3 subsequently underwent esophagectomy, 1 received palliative measures in the setting of advanced neurological disease, and 1 patient is currently undergoing a repeat ablation procedure with curative intent.
Dr. Agoston and his team also identified 2 clinicopathologic factors that were significantly associated with treatment failure, and both remained significant on univariate and multivariate analysis. The first was that the use of EMR prior to RFA was associated with a significantly reduced risk for treatment failure (hazard ratio, 0.15; 95% confidence interval, 0.05-0.48; P = .001), and the second was that the extent of IMC involving at least 50% of the columnar metaplastic area was associated with a significantly increased risk for treatment failure (HR, 4.24; 95% CI, 1.53-11.7; P = .005).
They also observed similar results when the analysis of extent of IMC as a predictor was restricted to a subset of 43 cases in which the diagnosis of IMC was made on EMR specimens only (HR, 10.8; 95% CI, 2.30-50.8; P = .003).
“In conclusion, we have identified endoscopic and pathologic factors associated with treatment success in patients with BE-associated IMC treated with RFA with or without EMR,” they wrote. “Utilization of these predictors can help in identifying patients with a high probability of success and also those patients with a higher risk for treatment failure for whom a more aggressive initial approach may be justified” (Am J Surg Pathol. 2015 Dec 5. doi: 10.1097/PAS.0000000000000566).
Dr. Rothstein and Dr. Abrams have received research support previously from Barrx/ Covidien and C2 Therapeutics/Covidien, respectively. None of the other authors reported significant conflicts of interest.
FROM THE AMERICAN JOURNAL OF SURGICAL PATHOLOGY
Key clinical point: The use of EMR before radiofrequency ablation significantly reduced the risk for treatment failure for IMC associated with Barrett’s esophagus.
Major finding: The overall rate of complete and durable IMC eradication in Barrett’s esophagus was 86% and 78%, respectively, during a mean follow-up of about 2 years, but was significantly impacted by the baseline extent of IMC and the use of EMR.
Data source: A retrospective review of data from four tertiary care academic medical centers that included 78 patients and was conducted to determine the rate of IMC eradication when using RFA and EMR.
Disclosures: Dr. Rothstein and Dr. Abrams have received research support previously from Barrx/ Covidien and C2 Therapeutics/Covidien, respectively. None of the other authors reported significant conflicts of interest.
November 2015: Click for Credit
Here are 8 articles in the November issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Low-risk Prostate Cancer: Immediate Contemplation, Not Immediate Intervention
To take the posttest, go to http://bit.ly/1Vz6Cok
VITALS
Key clinical point: Men with favorable-risk prostate cancer have a low risk for progression to a lethal phenotype and should consider active surveillance.
Major finding: Of 1,298 men with favorable-risk prostate cancer who were enrolled in an active surveillance program, overall, cancer-specific, and metastasis-free survival rates were 69%, 99.9%, and 99.4%, respectively, at 15 years.
Data source: A follow-up of a cohort of men with favorable-risk prostate cancer receiving active surveillance at a single institution that used a clearly defined protocol for enrollment, monitoring, and intervention.
Disclosures: There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
2. Diabetes in Seniors Increases Dementia Risk
To take the posttest, go to http://bit.ly/1Q1bITm
VITALS
Key clinical point: Even short-term hyperglycemia in late life can trigger or accelerate cognitive decline, and incident diabetes is a risk factor for dementia after adjustment for differences in cardiovascular disease and other common risk factors.
Major finding: Individuals diagnosed with diabetes later in life have a 16% higher risk for dementia than do those without diabetes.
Data source: A population-based matched cohort study in 225,045 seniors newly diagnosed with diabetes and 668,070 nondiabetic controls.
Disclosures: The Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Institutes of Health Research, the University of Toronto, and the Ontario Ministry of Health and Long-Term Care supported the study. One author reported an unrestricted grant from Amgen, but there were no other conflicts of interest declared.
3. Extremes of Sleep Linked With Early Signs of CVD
To take the posttest, go to http://bit.ly/1FSvLmw
VITALS
Key clinical point: Individuals with very long or short sleep, or poor sleep quality, showed signs of early cardiovascular disease.
Major finding: Extremely short and extremely long sleep duration were associated with significantly increased levels of coronary artery calcification (CAC) and increased brachial-ankle pulse wave velocity (baPWV).
Data source: Cross-sectional study of more than 47,000 healthy adult men and women who reported sleep duration and quality and underwent either measurement of CAC.
Disclosures: The funding source was not reported. The authors reported no disclosures.
4. Sunscreens With DNA Repair Enzymes Might Lessen AK Progression
To take the posttest, go to http://bit.ly/1LdZWFf
VITALS
Key clinical point: Sunscreen containing DNA repair enzymes might prevent malignant progression of actinic keratosis better than sunscreen alone.
Major finding: Field cancerization and cyclobutane pyrimidine dimer levels improved significantly more with sunscreen plus enzymes than with sunscreen only (P < .0001 for each).
Data source: Six-month randomized trial of 28 patients with actinic keratosis.
Disclosures: Biodue S.p.A. provided the methyl aminolevulinate used in the study. Dr. Enzo Emanuele, the study’s senior author, is a major shareholder of Living Research S.A.S., a privately held biomedical research organization that provided funding for the work. The other researchers reported no conflicts of interest.
5. Breastfeeding Protects Against Postpartum MS Relapse
To take the posttest, go to http://bit.ly/1OSYU49
VITALS
Key clinical point: Don’t discourage new mothers with multiple sclerosis from breastfeeding.
Major finding: Among 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first six postpartum months, compared with 29 women (24.2%) among the 120 who intended to breastfeed their children exclusively for at least two months (adjusted HR, 1.70).
Data source: A prospective study of 201 pregnant women with relapsing-remitting MS who were followed for one year post partum.
Disclosures: The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer HealthCare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
6. S aureus Seen in 1% of Pediatric CAP Cases
To take the posttest, go to http://bit.ly/1FPJnQ3
VITALS
Key clinical point: About 1% of children presenting to a hospital with community-acquired pneumonia had Staphylococcus aureus infections, which do not respond to recommended firstline narrow-spectrum antibiotics for CAP.
Major finding: In a cohort of 554 children admitted with CAP, seven had S aureus infections, six classified as complicated. All received vancomycin within 24 hours of admission; anemia incidence was significantly higher in S aureus patients than for the rest of the cohort.
Data source: Retrospective cohort study of more than 3,400 children.
Disclosures: The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
7. Higher Arrhythmia Risk for Psoriasis Patients
To take the posttest, go to http://bit.ly/1VBdbS6
VITALS
Key clinical point: Patients with psoriasis are at increased risk for arrhythmia compared to those without psoriasis.
Major finding: After researchers adjusted for history and medication use, patients with psoriasis were at increased risk for overall arrhythmia (adjusted hazard ratio, 1.34; 95% confidence interval, 1.29-1.39).
Data source: A retrospective cohort study using data from almost 41,000 psoriasis patients identified from the Taiwan National Health Insurance Research Database, and almost 163,000 age- and sex-matched cohorts from the same database.
Disclosures: The study was institutionally funded. Dr. Chiu, Ms. Chang, and three other authors had no disclosures; one author disclosed having conducted clinical trials or received honoraria from several companies, including Pfizer and Novartis, and having received speaking fees from AbbVie.
8. Hepatitis C Drove Steep Rises in Cirrhosis, HCC, and Related Deaths
To take the posttest, go to http://bit.ly/1jyNrdp
VITALS
Key clinical point: Cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality rose substantially among Veterans Affairs (VA) patients over the past 12 years, mainly driven by hepatitis C virus infection.
Major finding: The prevalence of cirrhosis nearly doubled between 2001 and 2013, while cirrhosis-related deaths rose by about 50% and the incidence of HCC almost tripled.
Data source: A retrospective cohort study of 129,998 VA patients with cirrhosis and 21,326 VA patients with HCC between 2001 and 2013.
Disclosures: The Department of VA and the Veterans Health Administration funded the study. The investigators declared no competing interests.
Here are 8 articles in the November issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Low-risk Prostate Cancer: Immediate Contemplation, Not Immediate Intervention
To take the posttest, go to http://bit.ly/1Vz6Cok
VITALS
Key clinical point: Men with favorable-risk prostate cancer have a low risk for progression to a lethal phenotype and should consider active surveillance.
Major finding: Of 1,298 men with favorable-risk prostate cancer who were enrolled in an active surveillance program, overall, cancer-specific, and metastasis-free survival rates were 69%, 99.9%, and 99.4%, respectively, at 15 years.
Data source: A follow-up of a cohort of men with favorable-risk prostate cancer receiving active surveillance at a single institution that used a clearly defined protocol for enrollment, monitoring, and intervention.
Disclosures: There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
2. Diabetes in Seniors Increases Dementia Risk
To take the posttest, go to http://bit.ly/1Q1bITm
VITALS
Key clinical point: Even short-term hyperglycemia in late life can trigger or accelerate cognitive decline, and incident diabetes is a risk factor for dementia after adjustment for differences in cardiovascular disease and other common risk factors.
Major finding: Individuals diagnosed with diabetes later in life have a 16% higher risk for dementia than do those without diabetes.
Data source: A population-based matched cohort study in 225,045 seniors newly diagnosed with diabetes and 668,070 nondiabetic controls.
Disclosures: The Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Institutes of Health Research, the University of Toronto, and the Ontario Ministry of Health and Long-Term Care supported the study. One author reported an unrestricted grant from Amgen, but there were no other conflicts of interest declared.
3. Extremes of Sleep Linked With Early Signs of CVD
To take the posttest, go to http://bit.ly/1FSvLmw
VITALS
Key clinical point: Individuals with very long or short sleep, or poor sleep quality, showed signs of early cardiovascular disease.
Major finding: Extremely short and extremely long sleep duration were associated with significantly increased levels of coronary artery calcification (CAC) and increased brachial-ankle pulse wave velocity (baPWV).
Data source: Cross-sectional study of more than 47,000 healthy adult men and women who reported sleep duration and quality and underwent either measurement of CAC.
Disclosures: The funding source was not reported. The authors reported no disclosures.
4. Sunscreens With DNA Repair Enzymes Might Lessen AK Progression
To take the posttest, go to http://bit.ly/1LdZWFf
VITALS
Key clinical point: Sunscreen containing DNA repair enzymes might prevent malignant progression of actinic keratosis better than sunscreen alone.
Major finding: Field cancerization and cyclobutane pyrimidine dimer levels improved significantly more with sunscreen plus enzymes than with sunscreen only (P < .0001 for each).
Data source: Six-month randomized trial of 28 patients with actinic keratosis.
Disclosures: Biodue S.p.A. provided the methyl aminolevulinate used in the study. Dr. Enzo Emanuele, the study’s senior author, is a major shareholder of Living Research S.A.S., a privately held biomedical research organization that provided funding for the work. The other researchers reported no conflicts of interest.
5. Breastfeeding Protects Against Postpartum MS Relapse
To take the posttest, go to http://bit.ly/1OSYU49
VITALS
Key clinical point: Don’t discourage new mothers with multiple sclerosis from breastfeeding.
Major finding: Among 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first six postpartum months, compared with 29 women (24.2%) among the 120 who intended to breastfeed their children exclusively for at least two months (adjusted HR, 1.70).
Data source: A prospective study of 201 pregnant women with relapsing-remitting MS who were followed for one year post partum.
Disclosures: The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer HealthCare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
6. S aureus Seen in 1% of Pediatric CAP Cases
To take the posttest, go to http://bit.ly/1FPJnQ3
VITALS
Key clinical point: About 1% of children presenting to a hospital with community-acquired pneumonia had Staphylococcus aureus infections, which do not respond to recommended firstline narrow-spectrum antibiotics for CAP.
Major finding: In a cohort of 554 children admitted with CAP, seven had S aureus infections, six classified as complicated. All received vancomycin within 24 hours of admission; anemia incidence was significantly higher in S aureus patients than for the rest of the cohort.
Data source: Retrospective cohort study of more than 3,400 children.
Disclosures: The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
7. Higher Arrhythmia Risk for Psoriasis Patients
To take the posttest, go to http://bit.ly/1VBdbS6
VITALS
Key clinical point: Patients with psoriasis are at increased risk for arrhythmia compared to those without psoriasis.
Major finding: After researchers adjusted for history and medication use, patients with psoriasis were at increased risk for overall arrhythmia (adjusted hazard ratio, 1.34; 95% confidence interval, 1.29-1.39).
Data source: A retrospective cohort study using data from almost 41,000 psoriasis patients identified from the Taiwan National Health Insurance Research Database, and almost 163,000 age- and sex-matched cohorts from the same database.
Disclosures: The study was institutionally funded. Dr. Chiu, Ms. Chang, and three other authors had no disclosures; one author disclosed having conducted clinical trials or received honoraria from several companies, including Pfizer and Novartis, and having received speaking fees from AbbVie.
8. Hepatitis C Drove Steep Rises in Cirrhosis, HCC, and Related Deaths
To take the posttest, go to http://bit.ly/1jyNrdp
VITALS
Key clinical point: Cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality rose substantially among Veterans Affairs (VA) patients over the past 12 years, mainly driven by hepatitis C virus infection.
Major finding: The prevalence of cirrhosis nearly doubled between 2001 and 2013, while cirrhosis-related deaths rose by about 50% and the incidence of HCC almost tripled.
Data source: A retrospective cohort study of 129,998 VA patients with cirrhosis and 21,326 VA patients with HCC between 2001 and 2013.
Disclosures: The Department of VA and the Veterans Health Administration funded the study. The investigators declared no competing interests.
Here are 8 articles in the November issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Low-risk Prostate Cancer: Immediate Contemplation, Not Immediate Intervention
To take the posttest, go to http://bit.ly/1Vz6Cok
VITALS
Key clinical point: Men with favorable-risk prostate cancer have a low risk for progression to a lethal phenotype and should consider active surveillance.
Major finding: Of 1,298 men with favorable-risk prostate cancer who were enrolled in an active surveillance program, overall, cancer-specific, and metastasis-free survival rates were 69%, 99.9%, and 99.4%, respectively, at 15 years.
Data source: A follow-up of a cohort of men with favorable-risk prostate cancer receiving active surveillance at a single institution that used a clearly defined protocol for enrollment, monitoring, and intervention.
Disclosures: There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
2. Diabetes in Seniors Increases Dementia Risk
To take the posttest, go to http://bit.ly/1Q1bITm
VITALS
Key clinical point: Even short-term hyperglycemia in late life can trigger or accelerate cognitive decline, and incident diabetes is a risk factor for dementia after adjustment for differences in cardiovascular disease and other common risk factors.
Major finding: Individuals diagnosed with diabetes later in life have a 16% higher risk for dementia than do those without diabetes.
Data source: A population-based matched cohort study in 225,045 seniors newly diagnosed with diabetes and 668,070 nondiabetic controls.
Disclosures: The Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Institutes of Health Research, the University of Toronto, and the Ontario Ministry of Health and Long-Term Care supported the study. One author reported an unrestricted grant from Amgen, but there were no other conflicts of interest declared.
3. Extremes of Sleep Linked With Early Signs of CVD
To take the posttest, go to http://bit.ly/1FSvLmw
VITALS
Key clinical point: Individuals with very long or short sleep, or poor sleep quality, showed signs of early cardiovascular disease.
Major finding: Extremely short and extremely long sleep duration were associated with significantly increased levels of coronary artery calcification (CAC) and increased brachial-ankle pulse wave velocity (baPWV).
Data source: Cross-sectional study of more than 47,000 healthy adult men and women who reported sleep duration and quality and underwent either measurement of CAC.
Disclosures: The funding source was not reported. The authors reported no disclosures.
4. Sunscreens With DNA Repair Enzymes Might Lessen AK Progression
To take the posttest, go to http://bit.ly/1LdZWFf
VITALS
Key clinical point: Sunscreen containing DNA repair enzymes might prevent malignant progression of actinic keratosis better than sunscreen alone.
Major finding: Field cancerization and cyclobutane pyrimidine dimer levels improved significantly more with sunscreen plus enzymes than with sunscreen only (P < .0001 for each).
Data source: Six-month randomized trial of 28 patients with actinic keratosis.
Disclosures: Biodue S.p.A. provided the methyl aminolevulinate used in the study. Dr. Enzo Emanuele, the study’s senior author, is a major shareholder of Living Research S.A.S., a privately held biomedical research organization that provided funding for the work. The other researchers reported no conflicts of interest.
5. Breastfeeding Protects Against Postpartum MS Relapse
To take the posttest, go to http://bit.ly/1OSYU49
VITALS
Key clinical point: Don’t discourage new mothers with multiple sclerosis from breastfeeding.
Major finding: Among 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first six postpartum months, compared with 29 women (24.2%) among the 120 who intended to breastfeed their children exclusively for at least two months (adjusted HR, 1.70).
Data source: A prospective study of 201 pregnant women with relapsing-remitting MS who were followed for one year post partum.
Disclosures: The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer HealthCare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
6. S aureus Seen in 1% of Pediatric CAP Cases
To take the posttest, go to http://bit.ly/1FPJnQ3
VITALS
Key clinical point: About 1% of children presenting to a hospital with community-acquired pneumonia had Staphylococcus aureus infections, which do not respond to recommended firstline narrow-spectrum antibiotics for CAP.
Major finding: In a cohort of 554 children admitted with CAP, seven had S aureus infections, six classified as complicated. All received vancomycin within 24 hours of admission; anemia incidence was significantly higher in S aureus patients than for the rest of the cohort.
Data source: Retrospective cohort study of more than 3,400 children.
Disclosures: The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
7. Higher Arrhythmia Risk for Psoriasis Patients
To take the posttest, go to http://bit.ly/1VBdbS6
VITALS
Key clinical point: Patients with psoriasis are at increased risk for arrhythmia compared to those without psoriasis.
Major finding: After researchers adjusted for history and medication use, patients with psoriasis were at increased risk for overall arrhythmia (adjusted hazard ratio, 1.34; 95% confidence interval, 1.29-1.39).
Data source: A retrospective cohort study using data from almost 41,000 psoriasis patients identified from the Taiwan National Health Insurance Research Database, and almost 163,000 age- and sex-matched cohorts from the same database.
Disclosures: The study was institutionally funded. Dr. Chiu, Ms. Chang, and three other authors had no disclosures; one author disclosed having conducted clinical trials or received honoraria from several companies, including Pfizer and Novartis, and having received speaking fees from AbbVie.
8. Hepatitis C Drove Steep Rises in Cirrhosis, HCC, and Related Deaths
To take the posttest, go to http://bit.ly/1jyNrdp
VITALS
Key clinical point: Cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality rose substantially among Veterans Affairs (VA) patients over the past 12 years, mainly driven by hepatitis C virus infection.
Major finding: The prevalence of cirrhosis nearly doubled between 2001 and 2013, while cirrhosis-related deaths rose by about 50% and the incidence of HCC almost tripled.
Data source: A retrospective cohort study of 129,998 VA patients with cirrhosis and 21,326 VA patients with HCC between 2001 and 2013.
Disclosures: The Department of VA and the Veterans Health Administration funded the study. The investigators declared no competing interests.
Urinary biomarkers miss the mark for bladder cancer
When used alone, urinary biomarkers miss a substantial proportion of patients with bladder cancer and are subject to false-positive results in others, and the accuracy is poor for low-stage and low-grade tumors, according to a study published online in Annals of Internal Medicine.
The diagnostic accuracy of biomarkers may be slightly higher for the initial diagnosis of bladder cancer in patients who present with signs and symptoms, rather than just for surveillance.
“Urinary biomarkers in combination with cytologic evaluation are more accurate than biomarkers alone; research is needed to understand how the use of urinary biomarkers with other diagnostic tests affects the use of cystoscopy and clinical outcomes,” wrote Dr. Roger Chou of Oregon Health and Science University, Portland, and his colleagues. (Ann Intern Med. 2015 Oct 26. doi:10.7326/M15-0997).
At the current time, six urinary biomarkers have been approved by the Food and Drug Administration for the diagnosis or surveillance of bladder cancer. These include quantitative nuclear matrix protein 22 (NMP22) (Alere NMP22 [Alere]), qualitative NMP22 (BladderChek [Alere]), qualitative bladder tumor antigen (BTA) (BTA stat [Polymedco]), quantitative BTA (BTA TRAK [Polymedco]), fluorescent in situ hybridization (FISH) (UroVysion [Abbott Molecular]), and fluorescent immunohistochemistry (ImmunoCyt [Scimedx]).
Dr. Chou and his team systematically reviewed the evidence on the accuracy of urinary biomarkers for diagnosing bladder cancer in adults who are experiencing signs or symptoms suggestive of the disease or who are undergoing surveillance for recurrent disease.
Their review was conducted as part of a larger analysis for the evaluation and treatment of non–muscle-invasive bladder cancer, which was nominated by the American Urological Association to the Agency for Healthcare Research and Quality, to be used for updating its guidelines.
The authors identified 57 studies that met their inclusion criteria and evaluated the diagnostic accuracy of the urinary biomarkers. They found that across all biomarkers, sensitivities ranged from 0.57 to 0.82 and specificities ranged from 0.74 to 0.88. Positive likelihood ratios ranged from 2.52 to 5.53, while negative ones ranged from 0.21 to 0.48.
Overall, evidence was strongest for quantitative NMP22, qualitative BTA, FISH, and ImmunoCyt (moderate strength of evidence) but relatively sparse for other biomarkers (low strength of evidence). For all of the biomarkers, sensitivity was greater for higher-stage and higher-grade tumors (high strength of evidence).
In addition, only a few of the studies looked at the effects of patient characteristics on the diagnostic accuracy of urinary biomarkers. But the diagnostic accuracy clearly did not differ according to factors such as age, gender, smoking status, or receipt of prior intravesical therapy. Also, eight of the studies also did not find any consistent differences in specificity according to factors that included other types of urological cancers, renal calculi, prostatitis, benign prostatic hypertrophy, urinary tract infection, or hematuria. However, specificity was higher in some of the studies in the absence of other urological conditions.
Up to 30% of patients with newly diagnosed bladder cancer will have muscle-invasive disease at the time of their initial diagnosis, suggesting an opportunity for screening tests to identify disease in earlier stages before the onset of hematuria. But screening requires the availability of a highly sensitive and specific test to properly identify patients who need further work-up while avoiding unnecessary testing and costs of evaluating true-negative results. Therefore, an unmet need exists for better noninvasive screening techniques, such as urine-based biomarkers.
In addition, patients with superficial bladder cancer tend to have recurrent but not progressive disease, necessitating life-long surveillance, and surveillance would be another setting in which urinary biomarkers would be valuable.
Are urinary biomarkers, with or without cytologic evaluation, adequate to avoid cystoscopy in patients with low-grade or low-stage disease? Also, what is the cost of missing a low-grade or low-stage tumor if a cytologic result is negative in the absence of cystoscopy? Few data exist to answer these questions decisively. To evaluate cost-effectiveness, studies need to evaluate presymptomatic patients or those with early-onset hematuria with one or all of the tests, or a combination, in addition to cystoscopy, cytologic evaluation, and imaging.
Biomarkers would provide value only if they circumvented the need for invasive evaluation or the need for more costly management if diagnoses were made earlier. Cost-effectiveness analyses would also need to incorporate estimated costs of missed diagnoses and overdiagnoses.
Thus, until urinary biomarkers become available that are sufficiently accurate to supplant the current recommended detection algorithms in biomarker-negative patients, they will not be a cost-effective addition to strategies to detect bladder cancer.
Dr. Phillip H. Abbosh and Dr. Elizabeth R. Plimack are with the Fox Chase Cancer Center in Philadelphia. These remarks were taken from their editorial accompanying Dr. Chou’s report (Ann Intern Med. 2015 Oct 26. doi:10.7326/M15-2445).
Up to 30% of patients with newly diagnosed bladder cancer will have muscle-invasive disease at the time of their initial diagnosis, suggesting an opportunity for screening tests to identify disease in earlier stages before the onset of hematuria. But screening requires the availability of a highly sensitive and specific test to properly identify patients who need further work-up while avoiding unnecessary testing and costs of evaluating true-negative results. Therefore, an unmet need exists for better noninvasive screening techniques, such as urine-based biomarkers.
In addition, patients with superficial bladder cancer tend to have recurrent but not progressive disease, necessitating life-long surveillance, and surveillance would be another setting in which urinary biomarkers would be valuable.
Are urinary biomarkers, with or without cytologic evaluation, adequate to avoid cystoscopy in patients with low-grade or low-stage disease? Also, what is the cost of missing a low-grade or low-stage tumor if a cytologic result is negative in the absence of cystoscopy? Few data exist to answer these questions decisively. To evaluate cost-effectiveness, studies need to evaluate presymptomatic patients or those with early-onset hematuria with one or all of the tests, or a combination, in addition to cystoscopy, cytologic evaluation, and imaging.
Biomarkers would provide value only if they circumvented the need for invasive evaluation or the need for more costly management if diagnoses were made earlier. Cost-effectiveness analyses would also need to incorporate estimated costs of missed diagnoses and overdiagnoses.
Thus, until urinary biomarkers become available that are sufficiently accurate to supplant the current recommended detection algorithms in biomarker-negative patients, they will not be a cost-effective addition to strategies to detect bladder cancer.
Dr. Phillip H. Abbosh and Dr. Elizabeth R. Plimack are with the Fox Chase Cancer Center in Philadelphia. These remarks were taken from their editorial accompanying Dr. Chou’s report (Ann Intern Med. 2015 Oct 26. doi:10.7326/M15-2445).
Up to 30% of patients with newly diagnosed bladder cancer will have muscle-invasive disease at the time of their initial diagnosis, suggesting an opportunity for screening tests to identify disease in earlier stages before the onset of hematuria. But screening requires the availability of a highly sensitive and specific test to properly identify patients who need further work-up while avoiding unnecessary testing and costs of evaluating true-negative results. Therefore, an unmet need exists for better noninvasive screening techniques, such as urine-based biomarkers.
In addition, patients with superficial bladder cancer tend to have recurrent but not progressive disease, necessitating life-long surveillance, and surveillance would be another setting in which urinary biomarkers would be valuable.
Are urinary biomarkers, with or without cytologic evaluation, adequate to avoid cystoscopy in patients with low-grade or low-stage disease? Also, what is the cost of missing a low-grade or low-stage tumor if a cytologic result is negative in the absence of cystoscopy? Few data exist to answer these questions decisively. To evaluate cost-effectiveness, studies need to evaluate presymptomatic patients or those with early-onset hematuria with one or all of the tests, or a combination, in addition to cystoscopy, cytologic evaluation, and imaging.
Biomarkers would provide value only if they circumvented the need for invasive evaluation or the need for more costly management if diagnoses were made earlier. Cost-effectiveness analyses would also need to incorporate estimated costs of missed diagnoses and overdiagnoses.
Thus, until urinary biomarkers become available that are sufficiently accurate to supplant the current recommended detection algorithms in biomarker-negative patients, they will not be a cost-effective addition to strategies to detect bladder cancer.
Dr. Phillip H. Abbosh and Dr. Elizabeth R. Plimack are with the Fox Chase Cancer Center in Philadelphia. These remarks were taken from their editorial accompanying Dr. Chou’s report (Ann Intern Med. 2015 Oct 26. doi:10.7326/M15-2445).
When used alone, urinary biomarkers miss a substantial proportion of patients with bladder cancer and are subject to false-positive results in others, and the accuracy is poor for low-stage and low-grade tumors, according to a study published online in Annals of Internal Medicine.
The diagnostic accuracy of biomarkers may be slightly higher for the initial diagnosis of bladder cancer in patients who present with signs and symptoms, rather than just for surveillance.
“Urinary biomarkers in combination with cytologic evaluation are more accurate than biomarkers alone; research is needed to understand how the use of urinary biomarkers with other diagnostic tests affects the use of cystoscopy and clinical outcomes,” wrote Dr. Roger Chou of Oregon Health and Science University, Portland, and his colleagues. (Ann Intern Med. 2015 Oct 26. doi:10.7326/M15-0997).
At the current time, six urinary biomarkers have been approved by the Food and Drug Administration for the diagnosis or surveillance of bladder cancer. These include quantitative nuclear matrix protein 22 (NMP22) (Alere NMP22 [Alere]), qualitative NMP22 (BladderChek [Alere]), qualitative bladder tumor antigen (BTA) (BTA stat [Polymedco]), quantitative BTA (BTA TRAK [Polymedco]), fluorescent in situ hybridization (FISH) (UroVysion [Abbott Molecular]), and fluorescent immunohistochemistry (ImmunoCyt [Scimedx]).
Dr. Chou and his team systematically reviewed the evidence on the accuracy of urinary biomarkers for diagnosing bladder cancer in adults who are experiencing signs or symptoms suggestive of the disease or who are undergoing surveillance for recurrent disease.
Their review was conducted as part of a larger analysis for the evaluation and treatment of non–muscle-invasive bladder cancer, which was nominated by the American Urological Association to the Agency for Healthcare Research and Quality, to be used for updating its guidelines.
The authors identified 57 studies that met their inclusion criteria and evaluated the diagnostic accuracy of the urinary biomarkers. They found that across all biomarkers, sensitivities ranged from 0.57 to 0.82 and specificities ranged from 0.74 to 0.88. Positive likelihood ratios ranged from 2.52 to 5.53, while negative ones ranged from 0.21 to 0.48.
Overall, evidence was strongest for quantitative NMP22, qualitative BTA, FISH, and ImmunoCyt (moderate strength of evidence) but relatively sparse for other biomarkers (low strength of evidence). For all of the biomarkers, sensitivity was greater for higher-stage and higher-grade tumors (high strength of evidence).
In addition, only a few of the studies looked at the effects of patient characteristics on the diagnostic accuracy of urinary biomarkers. But the diagnostic accuracy clearly did not differ according to factors such as age, gender, smoking status, or receipt of prior intravesical therapy. Also, eight of the studies also did not find any consistent differences in specificity according to factors that included other types of urological cancers, renal calculi, prostatitis, benign prostatic hypertrophy, urinary tract infection, or hematuria. However, specificity was higher in some of the studies in the absence of other urological conditions.
When used alone, urinary biomarkers miss a substantial proportion of patients with bladder cancer and are subject to false-positive results in others, and the accuracy is poor for low-stage and low-grade tumors, according to a study published online in Annals of Internal Medicine.
The diagnostic accuracy of biomarkers may be slightly higher for the initial diagnosis of bladder cancer in patients who present with signs and symptoms, rather than just for surveillance.
“Urinary biomarkers in combination with cytologic evaluation are more accurate than biomarkers alone; research is needed to understand how the use of urinary biomarkers with other diagnostic tests affects the use of cystoscopy and clinical outcomes,” wrote Dr. Roger Chou of Oregon Health and Science University, Portland, and his colleagues. (Ann Intern Med. 2015 Oct 26. doi:10.7326/M15-0997).
At the current time, six urinary biomarkers have been approved by the Food and Drug Administration for the diagnosis or surveillance of bladder cancer. These include quantitative nuclear matrix protein 22 (NMP22) (Alere NMP22 [Alere]), qualitative NMP22 (BladderChek [Alere]), qualitative bladder tumor antigen (BTA) (BTA stat [Polymedco]), quantitative BTA (BTA TRAK [Polymedco]), fluorescent in situ hybridization (FISH) (UroVysion [Abbott Molecular]), and fluorescent immunohistochemistry (ImmunoCyt [Scimedx]).
Dr. Chou and his team systematically reviewed the evidence on the accuracy of urinary biomarkers for diagnosing bladder cancer in adults who are experiencing signs or symptoms suggestive of the disease or who are undergoing surveillance for recurrent disease.
Their review was conducted as part of a larger analysis for the evaluation and treatment of non–muscle-invasive bladder cancer, which was nominated by the American Urological Association to the Agency for Healthcare Research and Quality, to be used for updating its guidelines.
The authors identified 57 studies that met their inclusion criteria and evaluated the diagnostic accuracy of the urinary biomarkers. They found that across all biomarkers, sensitivities ranged from 0.57 to 0.82 and specificities ranged from 0.74 to 0.88. Positive likelihood ratios ranged from 2.52 to 5.53, while negative ones ranged from 0.21 to 0.48.
Overall, evidence was strongest for quantitative NMP22, qualitative BTA, FISH, and ImmunoCyt (moderate strength of evidence) but relatively sparse for other biomarkers (low strength of evidence). For all of the biomarkers, sensitivity was greater for higher-stage and higher-grade tumors (high strength of evidence).
In addition, only a few of the studies looked at the effects of patient characteristics on the diagnostic accuracy of urinary biomarkers. But the diagnostic accuracy clearly did not differ according to factors such as age, gender, smoking status, or receipt of prior intravesical therapy. Also, eight of the studies also did not find any consistent differences in specificity according to factors that included other types of urological cancers, renal calculi, prostatitis, benign prostatic hypertrophy, urinary tract infection, or hematuria. However, specificity was higher in some of the studies in the absence of other urological conditions.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point:Urinary biomarkers used alone miss a substantial proportion of patients with bladder cancer and are subject to false-positive results in others.
Major finding: Urinary biomarkers were associated with sensitivities for bladder cancer that ranged from 0.57 to 0.82 and specificities that ranged from 0.74 to 0.88.
Data source: Systematic review of 57 studies of the comparative accuracy of urinary biomarkers for diagnosis of bladder cancer.
Disclosures: The Agency for Healthcare Research and Quality supported the study. Dr. Chou, Dr. Gore, and Dr. Fu reported receiving grants from the AHRQ during the study.
Five epigenetic biomarkers define three CLL subgroups
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
Researchers have devised a simple and reproducible method of tracking the cellular origin of chronic lymphocytic leukemia (CLL) by applying five epigenetic biomarkers. By using this strategy, CLL patients can be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes – naive B-cell-like, intermediate, and memory B-cell-like CLL, according to a paper published in Leukemia.
Being able to identify CLL patients early on who are destined to progress would greatly help their clinical management, said Dr. Ana C. Queirós of the University of Barcelona and colleagues (Leukemia. 2015;29:598-605.
“We believe that the most relevant information obtained by the five epigenetic biomarkers is to classify CLL patients based on the putative cell of origin of the disease rather than being a mere additional prognostic biomarker,” the investigators wrote. “The recent advance in the genetics and cellular biology of CLL, including the present epigenetic classification, could result in the use of targeted therapies for specific subgroups of patients.”
In previous research, the authors identified the presence of three subgroups of CLL with different clinicobiologic features, and in this study they hypothesized that DNA methylation patterns associated with normal B cells could be used to classify CLL into three novel subgroups.
To test their hypothesis and to develop a clinically useful strategy, they identified five epigenetic biomarkers and established new quantitative DNA methylation assays, applying them to two independent series of CLL patients of different geographical origin.
The first epigenetic classification was determined in an initial cohort of 211 CLL patients and then validated in a series of 97 additional CLL patients.
To test the stability of these markers over time and after treatment, two or three sequential samples were analyzed from 27 CLL patients with a median difference between samples of 59 months (range, 5-114). In addition, specimens from 13 patients, from before and after treatment, also were analyzed.
In the initial 211 patients, the three subgroups had different levels of immunoglobulin heavy-chain locus (IGHV) mutation (P <.001) and VH gene usage (P <.03). There also were different clinical features and outcomes in regard to their time to first treatment and overall survival (P <.001), Dr. Queirós and associates reported.
After a Cox multivariate analysis, the final model showed that the epigenetic signature related to the cellular origin of CLL was the most important variable in predicting time to first treatment. Other important variables in the model were Binet stage, CD38 expression, LDH levels, and SF3B1 mutations.
The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
FROM LEUKEMIA
Key clinical point: A new strategy allows CLL patients to be categorized into three epigenetic subgroups with differential clinicobiologic features and outcomes.
Major finding: Epigenetic classification was the strongest predictor of time to treatment (P <.001), along with Binet stage (P <.001); these findings were corroborated in a validation series (n = 97).
Data source: A prediction model formulated using five epigenetic biomarkers that was able to classify CLL patients accurately into the three subgroups.
Disclosures: The study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III (ISCIII) and the Red Temática de Investigación del Cáncer (RTICC) of the ISCIII and project SAF2009-08663, the UK Medical Research Council, and the European Union’s Seventh Framework Programme through the Blueprint Consortium. The authors declared no conflicts of interest.
Sofosbuvir reduces liver fibrosis in chronic hepatitis C
Antiviral treatment with sofosbuvir reduced liver fibrosis, as measured by three independent noninvasive predictors for liver fibrosis, and liver stiffness, in patients with chronic hepatitis C (CHC), according to new research published in the Digestive and Liver Disease (doi:10.1016/j.dld.2015.09.015).
“Despite the relatively small sample size, the effects on fibrosis parameters are impressive, implying clinically significant fibrosis regression and potential reduction of cirrhosis-associated mortality in successfully treated patients with CHC,” wrote Dr. Sebastian Bernuth of Cirrhosis Center Mainz (CCM), Johannes Gutenberg University Mainz, Germany, and colleagues.
CHC is a major cause of liver-associated mortality, and is responsible for approximately 25% of primary hepatocellular carcinomas and 25% of liver cirrhosis. Successful antiviral therapy with a sustained virological response (SVR) can reduce liver-related morbidity and mortality, including a decreased need for transplantation.
In 2014, the first NS5B RNA-polymerase inhibitor, sofosbuvir, was approved for use in conjunction with pegylated interferon-alpha (PEG-IFN) and/or ribavirin to treat hepatitis C infection, and yielded high SVR rates of around 90% after short-term therapy in genotype 1-3-infected patients.In this study, Dr. Benuth and his colleagues evaluated early changes in dynamic fibrosis-related parameters using the enhanced liver fibrosis (ELF) panel, combined with liver stiffness measurement (LSM), along with metabolic alterations in insulin resistance, and lipid and iron metabolism during sofosbuvir-based antiviral therapy.
A total of 32 patients were included in the analysis, and all received treatment with sofosbuvir, 19 in combination with PEG-IFN, 29 in combination with ribavirin, and four in combination with simeprevir.Patients experienced a biochemical and virological response within 4 weeks of starting treatment. At 12 weeks, the SVR was 93.8% and two patients experienced a relapse.There was a significant decrease from baseline to 12-week posttreatment follow-up in ELF (10.00 vs. 9.37; P = .007), and the median of the LSM (measured by Fibroscan) significantly decreased over the whole observation period, from 8 kPa (METAVIR F2) at baseline to 6.8 kPa (METAVIR F0-1) at 12 weeks (P = .016). This suggests a significant regression of liver fibrosis at 12 weeks, compared to baseline.The liver enzymes (ALT, AST, and gamma-GT) rapidly normalized under treatment, and total bilirubin also decreased from the baseline upper normal values (0.91 mg/dL) to 0.74 mg/dL (P = .034).“In conclusion, this is the first study implying significant and clinically relevant reduction in liver fibrosis measures by three independent noninvasive predictors for liver fibrosis assessment and liver stiffness measurement under highly effective antiviral regimens with sofosbuvir,” said the authors.
The study was supported by intramural funding of the University of Mainz (Inneruniversitäre Forschungsförderung Stufe I grant) to Dr. Tim Zimmermann, who has also received consultant/lecture fees and/or travel support from Abbvie, BMS, Gilead, Janssen-Cilag, Merck, and Roche. Dr. Martin F. Sprinzl received research funding from Gilead and lecture fees from Roche. All other authors have no conflicts of interest to declare in terms of this manuscript.
AGA ResourceThrough the AGA Roadmap to the Future of Practice, AGA offers a hepatitis C clinical service line to help support high-quality patient care at http://www.gastro.org/patient-care/conditions-
diseases/hepatitis-c.
Antiviral treatment with sofosbuvir reduced liver fibrosis, as measured by three independent noninvasive predictors for liver fibrosis, and liver stiffness, in patients with chronic hepatitis C (CHC), according to new research published in the Digestive and Liver Disease (doi:10.1016/j.dld.2015.09.015).
“Despite the relatively small sample size, the effects on fibrosis parameters are impressive, implying clinically significant fibrosis regression and potential reduction of cirrhosis-associated mortality in successfully treated patients with CHC,” wrote Dr. Sebastian Bernuth of Cirrhosis Center Mainz (CCM), Johannes Gutenberg University Mainz, Germany, and colleagues.
CHC is a major cause of liver-associated mortality, and is responsible for approximately 25% of primary hepatocellular carcinomas and 25% of liver cirrhosis. Successful antiviral therapy with a sustained virological response (SVR) can reduce liver-related morbidity and mortality, including a decreased need for transplantation.
In 2014, the first NS5B RNA-polymerase inhibitor, sofosbuvir, was approved for use in conjunction with pegylated interferon-alpha (PEG-IFN) and/or ribavirin to treat hepatitis C infection, and yielded high SVR rates of around 90% after short-term therapy in genotype 1-3-infected patients.In this study, Dr. Benuth and his colleagues evaluated early changes in dynamic fibrosis-related parameters using the enhanced liver fibrosis (ELF) panel, combined with liver stiffness measurement (LSM), along with metabolic alterations in insulin resistance, and lipid and iron metabolism during sofosbuvir-based antiviral therapy.
A total of 32 patients were included in the analysis, and all received treatment with sofosbuvir, 19 in combination with PEG-IFN, 29 in combination with ribavirin, and four in combination with simeprevir.Patients experienced a biochemical and virological response within 4 weeks of starting treatment. At 12 weeks, the SVR was 93.8% and two patients experienced a relapse.There was a significant decrease from baseline to 12-week posttreatment follow-up in ELF (10.00 vs. 9.37; P = .007), and the median of the LSM (measured by Fibroscan) significantly decreased over the whole observation period, from 8 kPa (METAVIR F2) at baseline to 6.8 kPa (METAVIR F0-1) at 12 weeks (P = .016). This suggests a significant regression of liver fibrosis at 12 weeks, compared to baseline.The liver enzymes (ALT, AST, and gamma-GT) rapidly normalized under treatment, and total bilirubin also decreased from the baseline upper normal values (0.91 mg/dL) to 0.74 mg/dL (P = .034).“In conclusion, this is the first study implying significant and clinically relevant reduction in liver fibrosis measures by three independent noninvasive predictors for liver fibrosis assessment and liver stiffness measurement under highly effective antiviral regimens with sofosbuvir,” said the authors.
The study was supported by intramural funding of the University of Mainz (Inneruniversitäre Forschungsförderung Stufe I grant) to Dr. Tim Zimmermann, who has also received consultant/lecture fees and/or travel support from Abbvie, BMS, Gilead, Janssen-Cilag, Merck, and Roche. Dr. Martin F. Sprinzl received research funding from Gilead and lecture fees from Roche. All other authors have no conflicts of interest to declare in terms of this manuscript.
AGA ResourceThrough the AGA Roadmap to the Future of Practice, AGA offers a hepatitis C clinical service line to help support high-quality patient care at http://www.gastro.org/patient-care/conditions-
diseases/hepatitis-c.
Antiviral treatment with sofosbuvir reduced liver fibrosis, as measured by three independent noninvasive predictors for liver fibrosis, and liver stiffness, in patients with chronic hepatitis C (CHC), according to new research published in the Digestive and Liver Disease (doi:10.1016/j.dld.2015.09.015).
“Despite the relatively small sample size, the effects on fibrosis parameters are impressive, implying clinically significant fibrosis regression and potential reduction of cirrhosis-associated mortality in successfully treated patients with CHC,” wrote Dr. Sebastian Bernuth of Cirrhosis Center Mainz (CCM), Johannes Gutenberg University Mainz, Germany, and colleagues.
CHC is a major cause of liver-associated mortality, and is responsible for approximately 25% of primary hepatocellular carcinomas and 25% of liver cirrhosis. Successful antiviral therapy with a sustained virological response (SVR) can reduce liver-related morbidity and mortality, including a decreased need for transplantation.
In 2014, the first NS5B RNA-polymerase inhibitor, sofosbuvir, was approved for use in conjunction with pegylated interferon-alpha (PEG-IFN) and/or ribavirin to treat hepatitis C infection, and yielded high SVR rates of around 90% after short-term therapy in genotype 1-3-infected patients.In this study, Dr. Benuth and his colleagues evaluated early changes in dynamic fibrosis-related parameters using the enhanced liver fibrosis (ELF) panel, combined with liver stiffness measurement (LSM), along with metabolic alterations in insulin resistance, and lipid and iron metabolism during sofosbuvir-based antiviral therapy.
A total of 32 patients were included in the analysis, and all received treatment with sofosbuvir, 19 in combination with PEG-IFN, 29 in combination with ribavirin, and four in combination with simeprevir.Patients experienced a biochemical and virological response within 4 weeks of starting treatment. At 12 weeks, the SVR was 93.8% and two patients experienced a relapse.There was a significant decrease from baseline to 12-week posttreatment follow-up in ELF (10.00 vs. 9.37; P = .007), and the median of the LSM (measured by Fibroscan) significantly decreased over the whole observation period, from 8 kPa (METAVIR F2) at baseline to 6.8 kPa (METAVIR F0-1) at 12 weeks (P = .016). This suggests a significant regression of liver fibrosis at 12 weeks, compared to baseline.The liver enzymes (ALT, AST, and gamma-GT) rapidly normalized under treatment, and total bilirubin also decreased from the baseline upper normal values (0.91 mg/dL) to 0.74 mg/dL (P = .034).“In conclusion, this is the first study implying significant and clinically relevant reduction in liver fibrosis measures by three independent noninvasive predictors for liver fibrosis assessment and liver stiffness measurement under highly effective antiviral regimens with sofosbuvir,” said the authors.
The study was supported by intramural funding of the University of Mainz (Inneruniversitäre Forschungsförderung Stufe I grant) to Dr. Tim Zimmermann, who has also received consultant/lecture fees and/or travel support from Abbvie, BMS, Gilead, Janssen-Cilag, Merck, and Roche. Dr. Martin F. Sprinzl received research funding from Gilead and lecture fees from Roche. All other authors have no conflicts of interest to declare in terms of this manuscript.
AGA ResourceThrough the AGA Roadmap to the Future of Practice, AGA offers a hepatitis C clinical service line to help support high-quality patient care at http://www.gastro.org/patient-care/conditions-
diseases/hepatitis-c.
FROM DIGESTIVE AND LIVER DISEASE
Key clinical point: Treatment with sofosbuvir achieved clinically significant fibrosis regression and potential reduction of cirrhosis-associated mortality in patients with hepatitis C.
Major finding: There was a sustained virological response rate at 12-week follow-up (93.8%), and a significant decrease from baseline to 12-week posttreatment follow-up in enhanced liver fibrosis (ELF) scores (10.00 vs. 9.37; P = .007) and FibroScan (8.0 vs. 6.8 kPa; P = .016)
Data source: A total of 32 hepatitis C patients were treated prospectively with sofosbuvir and ELF scores and FibroScan measurements were taken at baseline, week 4, end of treatment, and 12 weeks thereafter.
Disclosures: The study was supported by intramural funding of the University of Mainz (Inneruniversitäre Forschungsförderung Stufe I grant) to Dr. Tim Zimmermann, who has also received consultant/lecture fees and/or travel support from Abbvie, BMS, Gilead, Janssen-Cilag, Merck, and Roche. Dr. Martin F. Sprinzl received research funding from Gilead and lecture fees from Roche. All other authors have no conflicts of interest to declare in terms of this manuscript.
Sofosbuvir reduces liver fibrosis in chronic hepatitis C
Antiviral treatment with sofosbuvir reduced liver fibrosis, as measured by three independent noninvasive predictors for liver fibrosis, and liver stiffness, in patients with chronic hepatitis C (CHC), according to new research published in the Digestive and Liver Disease (doi:10.1016/j.dld.2015.09.015).
“Despite the relatively small sample size, the effects on fibrosis parameters are impressive, implying clinically significant fibrosis regression and potential reduction of cirrhosis-associated mortality in successfully treated patients with CHC,” wrote Dr. Sebastian Bernuth of Cirrhosis Center Mainz (CCM), Johannes Gutenberg University Mainz, Germany, and colleagues.
CHC is a major cause of liver-associated mortality, and is responsible for approximately 25% of primary hepatocellular carcinomas and 25% of liver cirrhosis. Successful antiviral therapy with a sustained virological response (SVR) can reduce liver-related morbidity and mortality, including a decreased need for transplantation.
In 2014, the first NS5B RNA-polymerase inhibitor, sofosbuvir, was approved for use in conjunction with pegylated interferon-alpha (PEG-IFN) and/or ribavirin to treat hepatitis C infection, and yielded high SVR rates of around 90% after short-term therapy in genotype 1-3-infected patients.In this study, Dr. Benuth and his colleagues evaluated early changes in dynamic fibrosis-related parameters using the enhanced liver fibrosis (ELF) panel, combined with liver stiffness measurement (LSM), along with metabolic alterations in insulin resistance, and lipid and iron metabolism during sofosbuvir-based antiviral therapy.
A total of 32 patients were included in the analysis, and all received treatment with sofosbuvir, 19 in combination with PEG-IFN, 29 in combination with ribavirin, and four in combination with simeprevir.Patients experienced a biochemical and virological response within 4 weeks of starting treatment. At 12 weeks, the SVR was 93.8% and two patients experienced a relapse.There was a significant decrease from baseline to 12-week posttreatment follow-up in ELF (10.00 vs. 9.37; P = .007), and the median of the LSM (measured by Fibroscan) significantly decreased over the whole observation period, from 8 kPa (METAVIR F2) at baseline to 6.8 kPa (METAVIR F0-1) at 12 weeks (P = .016). This suggests a significant regression of liver fibrosis at 12 weeks, compared to baseline.The liver enzymes (ALT, AST, and gamma-GT) rapidly normalized under treatment, and total bilirubin also decreased from the baseline upper normal values (0.91 mg/dL) to 0.74 mg/dL (P = .034).“In conclusion, this is the first study implying significant and clinically relevant reduction in liver fibrosis measures by three independent noninvasive predictors for liver fibrosis assessment and liver stiffness measurement under highly effective antiviral regimens with sofosbuvir,” said the authors.
The study was supported by intramural funding of the University of Mainz (Inneruniversitäre Forschungsförderung Stufe I grant) to Dr. Tim Zimmermann, who has also received consultant/lecture fees and/or travel support from Abbvie, BMS, Gilead, Janssen-Cilag, Merck, and Roche. Dr. Martin F. Sprinzl received research funding from Gilead and lecture fees from Roche. All other authors have no conflicts of interest to declare in terms of this manuscript.
Antiviral treatment with sofosbuvir reduced liver fibrosis, as measured by three independent noninvasive predictors for liver fibrosis, and liver stiffness, in patients with chronic hepatitis C (CHC), according to new research published in the Digestive and Liver Disease (doi:10.1016/j.dld.2015.09.015).
“Despite the relatively small sample size, the effects on fibrosis parameters are impressive, implying clinically significant fibrosis regression and potential reduction of cirrhosis-associated mortality in successfully treated patients with CHC,” wrote Dr. Sebastian Bernuth of Cirrhosis Center Mainz (CCM), Johannes Gutenberg University Mainz, Germany, and colleagues.
CHC is a major cause of liver-associated mortality, and is responsible for approximately 25% of primary hepatocellular carcinomas and 25% of liver cirrhosis. Successful antiviral therapy with a sustained virological response (SVR) can reduce liver-related morbidity and mortality, including a decreased need for transplantation.
In 2014, the first NS5B RNA-polymerase inhibitor, sofosbuvir, was approved for use in conjunction with pegylated interferon-alpha (PEG-IFN) and/or ribavirin to treat hepatitis C infection, and yielded high SVR rates of around 90% after short-term therapy in genotype 1-3-infected patients.In this study, Dr. Benuth and his colleagues evaluated early changes in dynamic fibrosis-related parameters using the enhanced liver fibrosis (ELF) panel, combined with liver stiffness measurement (LSM), along with metabolic alterations in insulin resistance, and lipid and iron metabolism during sofosbuvir-based antiviral therapy.
A total of 32 patients were included in the analysis, and all received treatment with sofosbuvir, 19 in combination with PEG-IFN, 29 in combination with ribavirin, and four in combination with simeprevir.Patients experienced a biochemical and virological response within 4 weeks of starting treatment. At 12 weeks, the SVR was 93.8% and two patients experienced a relapse.There was a significant decrease from baseline to 12-week posttreatment follow-up in ELF (10.00 vs. 9.37; P = .007), and the median of the LSM (measured by Fibroscan) significantly decreased over the whole observation period, from 8 kPa (METAVIR F2) at baseline to 6.8 kPa (METAVIR F0-1) at 12 weeks (P = .016). This suggests a significant regression of liver fibrosis at 12 weeks, compared to baseline.The liver enzymes (ALT, AST, and gamma-GT) rapidly normalized under treatment, and total bilirubin also decreased from the baseline upper normal values (0.91 mg/dL) to 0.74 mg/dL (P = .034).“In conclusion, this is the first study implying significant and clinically relevant reduction in liver fibrosis measures by three independent noninvasive predictors for liver fibrosis assessment and liver stiffness measurement under highly effective antiviral regimens with sofosbuvir,” said the authors.
The study was supported by intramural funding of the University of Mainz (Inneruniversitäre Forschungsförderung Stufe I grant) to Dr. Tim Zimmermann, who has also received consultant/lecture fees and/or travel support from Abbvie, BMS, Gilead, Janssen-Cilag, Merck, and Roche. Dr. Martin F. Sprinzl received research funding from Gilead and lecture fees from Roche. All other authors have no conflicts of interest to declare in terms of this manuscript.
Antiviral treatment with sofosbuvir reduced liver fibrosis, as measured by three independent noninvasive predictors for liver fibrosis, and liver stiffness, in patients with chronic hepatitis C (CHC), according to new research published in the Digestive and Liver Disease (doi:10.1016/j.dld.2015.09.015).
“Despite the relatively small sample size, the effects on fibrosis parameters are impressive, implying clinically significant fibrosis regression and potential reduction of cirrhosis-associated mortality in successfully treated patients with CHC,” wrote Dr. Sebastian Bernuth of Cirrhosis Center Mainz (CCM), Johannes Gutenberg University Mainz, Germany, and colleagues.
CHC is a major cause of liver-associated mortality, and is responsible for approximately 25% of primary hepatocellular carcinomas and 25% of liver cirrhosis. Successful antiviral therapy with a sustained virological response (SVR) can reduce liver-related morbidity and mortality, including a decreased need for transplantation.
In 2014, the first NS5B RNA-polymerase inhibitor, sofosbuvir, was approved for use in conjunction with pegylated interferon-alpha (PEG-IFN) and/or ribavirin to treat hepatitis C infection, and yielded high SVR rates of around 90% after short-term therapy in genotype 1-3-infected patients.In this study, Dr. Benuth and his colleagues evaluated early changes in dynamic fibrosis-related parameters using the enhanced liver fibrosis (ELF) panel, combined with liver stiffness measurement (LSM), along with metabolic alterations in insulin resistance, and lipid and iron metabolism during sofosbuvir-based antiviral therapy.
A total of 32 patients were included in the analysis, and all received treatment with sofosbuvir, 19 in combination with PEG-IFN, 29 in combination with ribavirin, and four in combination with simeprevir.Patients experienced a biochemical and virological response within 4 weeks of starting treatment. At 12 weeks, the SVR was 93.8% and two patients experienced a relapse.There was a significant decrease from baseline to 12-week posttreatment follow-up in ELF (10.00 vs. 9.37; P = .007), and the median of the LSM (measured by Fibroscan) significantly decreased over the whole observation period, from 8 kPa (METAVIR F2) at baseline to 6.8 kPa (METAVIR F0-1) at 12 weeks (P = .016). This suggests a significant regression of liver fibrosis at 12 weeks, compared to baseline.The liver enzymes (ALT, AST, and gamma-GT) rapidly normalized under treatment, and total bilirubin also decreased from the baseline upper normal values (0.91 mg/dL) to 0.74 mg/dL (P = .034).“In conclusion, this is the first study implying significant and clinically relevant reduction in liver fibrosis measures by three independent noninvasive predictors for liver fibrosis assessment and liver stiffness measurement under highly effective antiviral regimens with sofosbuvir,” said the authors.
The study was supported by intramural funding of the University of Mainz (Inneruniversitäre Forschungsförderung Stufe I grant) to Dr. Tim Zimmermann, who has also received consultant/lecture fees and/or travel support from Abbvie, BMS, Gilead, Janssen-Cilag, Merck, and Roche. Dr. Martin F. Sprinzl received research funding from Gilead and lecture fees from Roche. All other authors have no conflicts of interest to declare in terms of this manuscript.
FROM DIGESTIVE AND LIVER DISEASE
Key clinical point: Treatment with sofosbuvir achieved clinically significant fibrosis regression and potential reduction of cirrhosis-associated mortality in patients with hepatitis C.
Major finding: There was a sustained virological response rate at 12-week follow-up (93.8%), and a significant decrease from baseline to 12-week posttreatment follow-up in enhanced liver fibrosis (ELF) scores (10.00 vs. 9.37; P = .007) and FibroScan (8.0 vs. 6.8 kPa; P = .016)
Data source: A total of 32 hepatitis C patients were treated prospectively with sofosbuvir and ELF scores and FibroScan measurements were taken at baseline, week 4, end of treatment, and 12 weeks thereafter.
Disclosures: The study was supported by intramural funding of the University of Mainz (Inneruniversitäre Forschungsförderung Stufe I grant) to Dr. Tim Zimmermann, who has also received consultant/lecture fees and/or travel support from Abbvie, BMS, Gilead, Janssen-Cilag, Merck, and Roche. Dr. Martin F. Sprinzl received research funding from Gilead and lecture fees from Roche. All other authors have no conflicts of interest to declare in terms of this manuscript.
Race, age, BMI had no impact on pCR rates
SAN FRANCISCO – Age, race, and body mass index (BMI) do not appear to be related to achieving pathologic complete response (pCR) in breast cancer patients, according to data presented at the ASCO Breast Cancer Symposium.
“We did see some suggestions that maybe age and race were associated, but in our modeling, once we accounted for tumor characteristics such as tumor size and lymph node involvement, that association was attenuated to nonsignificance,” said Dr. Erica T. Warner of the Harvard T.H. Chan School of Public Health, Boston.
She noted that some previous research has found a higher pCR among young women and a lower pCR among overweight and obese women.
“Overall we had pretty high pCR rates, much higher than observed in these other studies,” said Dr. Warner. “It is likely due to our definition of pCR, the tumor subtypes that were included in our study, and the studies we included had combination therapy in addition to neoadjuvant therapy.”
Pathologic complete response is an important prognostic indicator and surrogate endpoint, and this is particularly true for patients with hormone receptor–negative breast cancer. Prior research has suggested there may be a differential response to preoperative therapy by age and BMI, although no association has been observed by race.
“Our motivation for this trial was that we know that young age, black race, and obesity are associated with poorer survival, and we wanted to try to better understand the mechanisms behind that,” explained Dr. Warner.
In this study, Dr. Warner and her colleagues conducted an analysis of 1,146 women with breast cancer, who were enrolled in four clinical trials of neoadjuvant chemotherapy (CALGB 40601 and 40603; ACOSOG Z1031 and Z1041). They used logistic regression models to determine the association of race/ethnicity and age at diagnosis with pCR.
Within the cohort, 156 patients (13.6%) were black; 590 tumors (51.5%) were HER2+, 169 (14.7%) were ER+/HER2-, and 387 (33.8%) were triple negative.
In multivariate analyses that controlled for tumor characteristics and other factors, black race (OR, 1.07; 95% CI, 0.67-1.70), age (OR, 0.99; 95% CI, 0.97-1.00), and BMI (OR, 0.99; 95% CI, 0.96-1.01) were not significant predictors of pCR. The researchers found similar associations when they stratified the data by subtype, and found no interaction between age and black race (P = .06), black race and BMI (P = .54), or age and BMI (P = .73).
For the ER+ HER2- subtype, there was a suggestion of an inverse association; per 5-year increase the probability of pCR decreased 17%, but again it was not statistically significant.
There were higher rates of pCR among HER2+ and triple-negative tumors, but there was no difference in pCR according to race (white women, 43.5%; black women, 44.2%; and other/unknown, 46.1%), Dr. Warner pointed out.
There was a lower median age for achieving pCR, as the median age for women who did not achieve pCR was 53, compared with 49 for those who did (P <less than .0001). And for BMI, women who didn’t achieve pCR had an average BMI of 28.6, compared with 28 for those who did (P = .03).
“But there was no association of black race with pCR for any subtype, no association between BMI and pCR for any subtype, and no association for age,” she said.
SAN FRANCISCO – Age, race, and body mass index (BMI) do not appear to be related to achieving pathologic complete response (pCR) in breast cancer patients, according to data presented at the ASCO Breast Cancer Symposium.
“We did see some suggestions that maybe age and race were associated, but in our modeling, once we accounted for tumor characteristics such as tumor size and lymph node involvement, that association was attenuated to nonsignificance,” said Dr. Erica T. Warner of the Harvard T.H. Chan School of Public Health, Boston.
She noted that some previous research has found a higher pCR among young women and a lower pCR among overweight and obese women.
“Overall we had pretty high pCR rates, much higher than observed in these other studies,” said Dr. Warner. “It is likely due to our definition of pCR, the tumor subtypes that were included in our study, and the studies we included had combination therapy in addition to neoadjuvant therapy.”
Pathologic complete response is an important prognostic indicator and surrogate endpoint, and this is particularly true for patients with hormone receptor–negative breast cancer. Prior research has suggested there may be a differential response to preoperative therapy by age and BMI, although no association has been observed by race.
“Our motivation for this trial was that we know that young age, black race, and obesity are associated with poorer survival, and we wanted to try to better understand the mechanisms behind that,” explained Dr. Warner.
In this study, Dr. Warner and her colleagues conducted an analysis of 1,146 women with breast cancer, who were enrolled in four clinical trials of neoadjuvant chemotherapy (CALGB 40601 and 40603; ACOSOG Z1031 and Z1041). They used logistic regression models to determine the association of race/ethnicity and age at diagnosis with pCR.
Within the cohort, 156 patients (13.6%) were black; 590 tumors (51.5%) were HER2+, 169 (14.7%) were ER+/HER2-, and 387 (33.8%) were triple negative.
In multivariate analyses that controlled for tumor characteristics and other factors, black race (OR, 1.07; 95% CI, 0.67-1.70), age (OR, 0.99; 95% CI, 0.97-1.00), and BMI (OR, 0.99; 95% CI, 0.96-1.01) were not significant predictors of pCR. The researchers found similar associations when they stratified the data by subtype, and found no interaction between age and black race (P = .06), black race and BMI (P = .54), or age and BMI (P = .73).
For the ER+ HER2- subtype, there was a suggestion of an inverse association; per 5-year increase the probability of pCR decreased 17%, but again it was not statistically significant.
There were higher rates of pCR among HER2+ and triple-negative tumors, but there was no difference in pCR according to race (white women, 43.5%; black women, 44.2%; and other/unknown, 46.1%), Dr. Warner pointed out.
There was a lower median age for achieving pCR, as the median age for women who did not achieve pCR was 53, compared with 49 for those who did (P <less than .0001). And for BMI, women who didn’t achieve pCR had an average BMI of 28.6, compared with 28 for those who did (P = .03).
“But there was no association of black race with pCR for any subtype, no association between BMI and pCR for any subtype, and no association for age,” she said.
SAN FRANCISCO – Age, race, and body mass index (BMI) do not appear to be related to achieving pathologic complete response (pCR) in breast cancer patients, according to data presented at the ASCO Breast Cancer Symposium.
“We did see some suggestions that maybe age and race were associated, but in our modeling, once we accounted for tumor characteristics such as tumor size and lymph node involvement, that association was attenuated to nonsignificance,” said Dr. Erica T. Warner of the Harvard T.H. Chan School of Public Health, Boston.
She noted that some previous research has found a higher pCR among young women and a lower pCR among overweight and obese women.
“Overall we had pretty high pCR rates, much higher than observed in these other studies,” said Dr. Warner. “It is likely due to our definition of pCR, the tumor subtypes that were included in our study, and the studies we included had combination therapy in addition to neoadjuvant therapy.”
Pathologic complete response is an important prognostic indicator and surrogate endpoint, and this is particularly true for patients with hormone receptor–negative breast cancer. Prior research has suggested there may be a differential response to preoperative therapy by age and BMI, although no association has been observed by race.
“Our motivation for this trial was that we know that young age, black race, and obesity are associated with poorer survival, and we wanted to try to better understand the mechanisms behind that,” explained Dr. Warner.
In this study, Dr. Warner and her colleagues conducted an analysis of 1,146 women with breast cancer, who were enrolled in four clinical trials of neoadjuvant chemotherapy (CALGB 40601 and 40603; ACOSOG Z1031 and Z1041). They used logistic regression models to determine the association of race/ethnicity and age at diagnosis with pCR.
Within the cohort, 156 patients (13.6%) were black; 590 tumors (51.5%) were HER2+, 169 (14.7%) were ER+/HER2-, and 387 (33.8%) were triple negative.
In multivariate analyses that controlled for tumor characteristics and other factors, black race (OR, 1.07; 95% CI, 0.67-1.70), age (OR, 0.99; 95% CI, 0.97-1.00), and BMI (OR, 0.99; 95% CI, 0.96-1.01) were not significant predictors of pCR. The researchers found similar associations when they stratified the data by subtype, and found no interaction between age and black race (P = .06), black race and BMI (P = .54), or age and BMI (P = .73).
For the ER+ HER2- subtype, there was a suggestion of an inverse association; per 5-year increase the probability of pCR decreased 17%, but again it was not statistically significant.
There were higher rates of pCR among HER2+ and triple-negative tumors, but there was no difference in pCR according to race (white women, 43.5%; black women, 44.2%; and other/unknown, 46.1%), Dr. Warner pointed out.
There was a lower median age for achieving pCR, as the median age for women who did not achieve pCR was 53, compared with 49 for those who did (P <less than .0001). And for BMI, women who didn’t achieve pCR had an average BMI of 28.6, compared with 28 for those who did (P = .03).
“But there was no association of black race with pCR for any subtype, no association between BMI and pCR for any subtype, and no association for age,” she said.
FROM THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Race, age, and body mass index do not appear to affect pathologic complete response in breast cancer.
Major finding: In multivariate analyses controlling for tumor characteristics and other factors, black race (OR, 1.07; 95% CI, 0.67-1.70), age (OR, 0.99; 95% CI, 0.97-1.00) and BMI (OR, 0.99; 95% CI, 0.96-1.01) were not significant predictors of pCR.
Data source: An analysis of four clinical trials of neoadjuvant chemotherapy that included 1,146 women with breast cancer.
Disclosures: Dr. Warner had no relevant financial disclosures, and there was no information on outside sponsorship of the study.
Microemboli play role in certain migraine auras
The prevalence and frequency of interictal microembolic signals (MES) were higher in migraine patients with higher cortical dysfunction (HCD) during aura, as compared with migraine patients without HCD during aura and with healthy controls, according to a study published in the journal Cephalalgia (2015 Sep 29. doi: 10.1177/0333102415607191).
Dr. Jasna Zidverc-Trajkovic of the Neurology Clinic, Clinical Center of Serbia, Belgrade, and her colleagues used transcranial Doppler ultrasound to test their hypothesis that the complexity of migraine aura is dependent on hypoperfusion caused by microemboli that occur in different regions of the brain. The goal was to evaluate the prevalence and clinical impact of interictal MES in migraine patients with HCD during aura.
The investigators enrolled 34 individuals with migraines who experienced language and memory impairment during aura (HCD group), 31 patients who had migraines with only visual or visual and somatosensory symptoms during aura (control group I), and 34 healthy individuals who comprised a control group (control group II). A Doppler instrument was used to detect microemboli, and the researchers also analyzed demographic data, disease features, and the detection of MES between these groups, as well as the predictors of HCD during the aura.
The duration of aura (34.71 plus or minus 18.05 minutes vs. 23.87 plus or minus 13.64 minutes; P = .002) was significantly longer and the frequency of aura per year (16.29 plus or minus 14.21 vs. 10.10 plus or minus 11.00; P = .029) was significantly higher in the HCD group, as compared with control group I. The presence of somatosensory symptoms during the aura was also significantly higher in the HCD group as well (P less than .001).
A binary logistic regression identified three independent predictors of HCD occurrence in patients with migraine aura. These were longer duration of the aura, presence of somatosensory symptoms during the aura, and positive MES detection.
MES were interictally detected in 29% of patients with migraines who experienced migraines with HCD during the aura, but in only 3% of those with visual and/or somatosensory aura. In addition, the number of detected MES in a single patient was as high as 85 among those in the HCD group, as compared with only 8 that were detected in other examined patients and healthy controls.
The detection of MES and investigating of the origin of microembolism could be a valuable tool for screening individuals with migraines with aura for ischemic stroke risk, as well as investigating links between migraine with aura and ischemic stroke, Dr. Zidverc-Trajkovic and her colleagues wrote.
The investigators speculated that in patients who have memory and language impairment during migraine aura, the cerebral cortex may be affected by cortical spreading depression (CSD) in regions beyond the occipital lobe, and microemboli may trigger CSD. This in turn might contribute to the pathophysiology of migraine aura.
“Further research should include analysis of the influence of microemboli on the neuron-glial interaction or the network modulation and pathophysiology of cortical spreading depression,” the investigators wrote.
Cortical spreading depression has been shown to occur in humans in ischemic stroke, severe traumatic brain lesions, and following hypoxia, and the scientifically relevant question is how spreading depression is triggered in patients who suffer from migraines with aura. The current study proposes that microemboli are a potential trigger.

|
Dr. Hans-Christoph Diener |
Microembolic signals, or MES, were detected in 29.4% of patients with higher cortical aura, which was much higher than in the other two groups. But what does this finding implicate? The nature of MES is unknown in patients without atherosclerotic plaques, and one assumed mechanism is right-left shunt either through a patent foramen ovale or through pulmonary shunts. Patent foramen ovale is associated with a higher prevalence of migraine with aura.
The major shortcoming of the study is the recording of MES outside migraine attacks. It is difficult to understand how MES occurring outside migraine attacks should play a role in the rare events of complex auras, and many of the assumptions and implications of the authors in the discussion are not supported by scientific evidence.
In summary, this is an interesting observation with very limited relationship to the pathophysiology of migraine aura, and further research is needed.
Dr. Hans-Christoph Diener is from the department of neurology, University of Essen (Germany), and has disclosed a number of financial relationships with industry. These remarks were taken from his editorial accompanying Dr. Zidverc-Trajkovic’s report (Cephalalgia. 2015 Sep 28. doi: 10.1177/0333102415607177).
Cortical spreading depression has been shown to occur in humans in ischemic stroke, severe traumatic brain lesions, and following hypoxia, and the scientifically relevant question is how spreading depression is triggered in patients who suffer from migraines with aura. The current study proposes that microemboli are a potential trigger.

|
Dr. Hans-Christoph Diener |
Microembolic signals, or MES, were detected in 29.4% of patients with higher cortical aura, which was much higher than in the other two groups. But what does this finding implicate? The nature of MES is unknown in patients without atherosclerotic plaques, and one assumed mechanism is right-left shunt either through a patent foramen ovale or through pulmonary shunts. Patent foramen ovale is associated with a higher prevalence of migraine with aura.
The major shortcoming of the study is the recording of MES outside migraine attacks. It is difficult to understand how MES occurring outside migraine attacks should play a role in the rare events of complex auras, and many of the assumptions and implications of the authors in the discussion are not supported by scientific evidence.
In summary, this is an interesting observation with very limited relationship to the pathophysiology of migraine aura, and further research is needed.
Dr. Hans-Christoph Diener is from the department of neurology, University of Essen (Germany), and has disclosed a number of financial relationships with industry. These remarks were taken from his editorial accompanying Dr. Zidverc-Trajkovic’s report (Cephalalgia. 2015 Sep 28. doi: 10.1177/0333102415607177).
Cortical spreading depression has been shown to occur in humans in ischemic stroke, severe traumatic brain lesions, and following hypoxia, and the scientifically relevant question is how spreading depression is triggered in patients who suffer from migraines with aura. The current study proposes that microemboli are a potential trigger.

|
Dr. Hans-Christoph Diener |
Microembolic signals, or MES, were detected in 29.4% of patients with higher cortical aura, which was much higher than in the other two groups. But what does this finding implicate? The nature of MES is unknown in patients without atherosclerotic plaques, and one assumed mechanism is right-left shunt either through a patent foramen ovale or through pulmonary shunts. Patent foramen ovale is associated with a higher prevalence of migraine with aura.
The major shortcoming of the study is the recording of MES outside migraine attacks. It is difficult to understand how MES occurring outside migraine attacks should play a role in the rare events of complex auras, and many of the assumptions and implications of the authors in the discussion are not supported by scientific evidence.
In summary, this is an interesting observation with very limited relationship to the pathophysiology of migraine aura, and further research is needed.
Dr. Hans-Christoph Diener is from the department of neurology, University of Essen (Germany), and has disclosed a number of financial relationships with industry. These remarks were taken from his editorial accompanying Dr. Zidverc-Trajkovic’s report (Cephalalgia. 2015 Sep 28. doi: 10.1177/0333102415607177).
The prevalence and frequency of interictal microembolic signals (MES) were higher in migraine patients with higher cortical dysfunction (HCD) during aura, as compared with migraine patients without HCD during aura and with healthy controls, according to a study published in the journal Cephalalgia (2015 Sep 29. doi: 10.1177/0333102415607191).
Dr. Jasna Zidverc-Trajkovic of the Neurology Clinic, Clinical Center of Serbia, Belgrade, and her colleagues used transcranial Doppler ultrasound to test their hypothesis that the complexity of migraine aura is dependent on hypoperfusion caused by microemboli that occur in different regions of the brain. The goal was to evaluate the prevalence and clinical impact of interictal MES in migraine patients with HCD during aura.
The investigators enrolled 34 individuals with migraines who experienced language and memory impairment during aura (HCD group), 31 patients who had migraines with only visual or visual and somatosensory symptoms during aura (control group I), and 34 healthy individuals who comprised a control group (control group II). A Doppler instrument was used to detect microemboli, and the researchers also analyzed demographic data, disease features, and the detection of MES between these groups, as well as the predictors of HCD during the aura.
The duration of aura (34.71 plus or minus 18.05 minutes vs. 23.87 plus or minus 13.64 minutes; P = .002) was significantly longer and the frequency of aura per year (16.29 plus or minus 14.21 vs. 10.10 plus or minus 11.00; P = .029) was significantly higher in the HCD group, as compared with control group I. The presence of somatosensory symptoms during the aura was also significantly higher in the HCD group as well (P less than .001).
A binary logistic regression identified three independent predictors of HCD occurrence in patients with migraine aura. These were longer duration of the aura, presence of somatosensory symptoms during the aura, and positive MES detection.
MES were interictally detected in 29% of patients with migraines who experienced migraines with HCD during the aura, but in only 3% of those with visual and/or somatosensory aura. In addition, the number of detected MES in a single patient was as high as 85 among those in the HCD group, as compared with only 8 that were detected in other examined patients and healthy controls.
The detection of MES and investigating of the origin of microembolism could be a valuable tool for screening individuals with migraines with aura for ischemic stroke risk, as well as investigating links between migraine with aura and ischemic stroke, Dr. Zidverc-Trajkovic and her colleagues wrote.
The investigators speculated that in patients who have memory and language impairment during migraine aura, the cerebral cortex may be affected by cortical spreading depression (CSD) in regions beyond the occipital lobe, and microemboli may trigger CSD. This in turn might contribute to the pathophysiology of migraine aura.
“Further research should include analysis of the influence of microemboli on the neuron-glial interaction or the network modulation and pathophysiology of cortical spreading depression,” the investigators wrote.
The prevalence and frequency of interictal microembolic signals (MES) were higher in migraine patients with higher cortical dysfunction (HCD) during aura, as compared with migraine patients without HCD during aura and with healthy controls, according to a study published in the journal Cephalalgia (2015 Sep 29. doi: 10.1177/0333102415607191).
Dr. Jasna Zidverc-Trajkovic of the Neurology Clinic, Clinical Center of Serbia, Belgrade, and her colleagues used transcranial Doppler ultrasound to test their hypothesis that the complexity of migraine aura is dependent on hypoperfusion caused by microemboli that occur in different regions of the brain. The goal was to evaluate the prevalence and clinical impact of interictal MES in migraine patients with HCD during aura.
The investigators enrolled 34 individuals with migraines who experienced language and memory impairment during aura (HCD group), 31 patients who had migraines with only visual or visual and somatosensory symptoms during aura (control group I), and 34 healthy individuals who comprised a control group (control group II). A Doppler instrument was used to detect microemboli, and the researchers also analyzed demographic data, disease features, and the detection of MES between these groups, as well as the predictors of HCD during the aura.
The duration of aura (34.71 plus or minus 18.05 minutes vs. 23.87 plus or minus 13.64 minutes; P = .002) was significantly longer and the frequency of aura per year (16.29 plus or minus 14.21 vs. 10.10 plus or minus 11.00; P = .029) was significantly higher in the HCD group, as compared with control group I. The presence of somatosensory symptoms during the aura was also significantly higher in the HCD group as well (P less than .001).
A binary logistic regression identified three independent predictors of HCD occurrence in patients with migraine aura. These were longer duration of the aura, presence of somatosensory symptoms during the aura, and positive MES detection.
MES were interictally detected in 29% of patients with migraines who experienced migraines with HCD during the aura, but in only 3% of those with visual and/or somatosensory aura. In addition, the number of detected MES in a single patient was as high as 85 among those in the HCD group, as compared with only 8 that were detected in other examined patients and healthy controls.
The detection of MES and investigating of the origin of microembolism could be a valuable tool for screening individuals with migraines with aura for ischemic stroke risk, as well as investigating links between migraine with aura and ischemic stroke, Dr. Zidverc-Trajkovic and her colleagues wrote.
The investigators speculated that in patients who have memory and language impairment during migraine aura, the cerebral cortex may be affected by cortical spreading depression (CSD) in regions beyond the occipital lobe, and microemboli may trigger CSD. This in turn might contribute to the pathophysiology of migraine aura.
“Further research should include analysis of the influence of microemboli on the neuron-glial interaction or the network modulation and pathophysiology of cortical spreading depression,” the investigators wrote.
FROM CEPHALALGIA
Key clinical point: A higher prevalence and frequency of microembolic signals were detected in migraine patients with higher cortical dysfunction during migraine aura.
Major finding: MES were detected in 29.4% of patients in the HCD group, which was significantly higher than was 3.2% and 5.9% in the control groups.
Data source: The study included 34 persons with migraines with language and memory impairment during aura, 31 with only visual or visual and somatosensory symptoms during aura, and 34 healthy controls.
Disclosures: This work was supported by a grant from the Ministry of Education, Science, and Technological development of the Republic of Serbia. The authors have no disclosures.
ASCO: APF530 superior to ondansetron in prevention of CINV
SAN FRANCISCO – APF530 is a long-acting 5-HT3 antagonist formulation in development for the prevention of chemotherapy-induced nausea and vomiting, and new data presented at the 2015 Breast Cancer Symposium suggest that it may have an edge over the standard regimen.
When administered with fosaprepitant and dexamethasone, it was superior to ondansetron, in combination with the same two agents, for preventing chemotherapy-induced nausea and vomiting (CINV) associated with delayed ( > 24-120 h) highly emetogenic chemotherapy (HEC).
Among the 450 patients who received the three-drug APF530 combination, 263 (58.4%) experienced a complete response, defined as having no emesis or need for rescue medication, as compared with 239/452 (53%) who received the ondansetron regimen (P= .092).
“APF530 is the first and only 5-HT3 receptor antagonist to demonstrate superiority in a phase III, three-drug versus three-drug regimen efficacy trial for the prevention of CINV in patients receiving HEC,” said Dr. Ian D. Schnadig, from Compass Oncology, The US Oncology Network, Tualatin, OR. “Managing delayed-phase CINV associated with HEC is an unmet need.”
APF530 is a novel, injectable, extended-release version of granisetron that provides sustained release over ≥ 5 days to prevent both acute (0-24 h) and delayed CINV. A large randomized double blind phase III trial has already demonstrated that this new agent is non-inferior to palonosetron in this setting.
In this double-blind, multicenter study known as the MAGIC trial, Dr. Schnadig randomized 902 patients to receive APF530 500 mg by subcutaneous injection (10 mg granisetron) or ondansetron 0.15 mg/kg IV, and the cohort was stratified by planned cisplatin therapy ( ≥ 50 mg/m2).
The primary end point was delayed-phase complete response, and secondary end points included complete response in acute and overall phases and complete control (complete response and no more than mild nausea) in acute, delayed, and overall phases.
A significantly higher percentage of patients in the APF530 cohort (65%) had delayed-phase complete response, as compared to those with ondansetron (57%) (P= .014). In addition, a significantly higher percentage (61% vs. 53%) of patients had delayed-phase complete control (P= .022).
The rates of complete response and complete control in acute and overall phases were numerically higher with APF530 compared with ondansetron, but this did not reach statistical significance.
In looking at exploratory efficacy end points, the authors observed that the proportion of patients who experienced an episode of nausea was generally higher with ondansetron than with APF530. Rates of no nausea were also numerically higher with APF530 compared with the ondansetron regimen in the delayed 49.7% vs. 44.25%; P= .099) and overall phases (45.3% vs. 44.2%; P= .138), but there were no statistically significant differences.
The regimen containing APR530 was generally well tolerated, without any new safety signals identified. The most common treatment related events were injection-site reactions, which were mostly mild or moderate. Injection site reactions were similar between the two groups, Dr. Schnadig noted.
In a discussion of the paper, Dr. Clifford Hudis reiterated that there was a higher complete response rate in late phase with APF530—“that’s an advantage and it was 8% higher in absolute terms, and it was 11% higher in the cisplatin group.”
If the drug is approved and it’s comparably priced to other agents and available, “why wouldn’t you use it?” he asked. “Its more convenient, it’s a little more effective, and its one less thing for all of us practicing to think very much about.”
SAN FRANCISCO – APF530 is a long-acting 5-HT3 antagonist formulation in development for the prevention of chemotherapy-induced nausea and vomiting, and new data presented at the 2015 Breast Cancer Symposium suggest that it may have an edge over the standard regimen.
When administered with fosaprepitant and dexamethasone, it was superior to ondansetron, in combination with the same two agents, for preventing chemotherapy-induced nausea and vomiting (CINV) associated with delayed ( > 24-120 h) highly emetogenic chemotherapy (HEC).
Among the 450 patients who received the three-drug APF530 combination, 263 (58.4%) experienced a complete response, defined as having no emesis or need for rescue medication, as compared with 239/452 (53%) who received the ondansetron regimen (P= .092).
“APF530 is the first and only 5-HT3 receptor antagonist to demonstrate superiority in a phase III, three-drug versus three-drug regimen efficacy trial for the prevention of CINV in patients receiving HEC,” said Dr. Ian D. Schnadig, from Compass Oncology, The US Oncology Network, Tualatin, OR. “Managing delayed-phase CINV associated with HEC is an unmet need.”
APF530 is a novel, injectable, extended-release version of granisetron that provides sustained release over ≥ 5 days to prevent both acute (0-24 h) and delayed CINV. A large randomized double blind phase III trial has already demonstrated that this new agent is non-inferior to palonosetron in this setting.
In this double-blind, multicenter study known as the MAGIC trial, Dr. Schnadig randomized 902 patients to receive APF530 500 mg by subcutaneous injection (10 mg granisetron) or ondansetron 0.15 mg/kg IV, and the cohort was stratified by planned cisplatin therapy ( ≥ 50 mg/m2).
The primary end point was delayed-phase complete response, and secondary end points included complete response in acute and overall phases and complete control (complete response and no more than mild nausea) in acute, delayed, and overall phases.
A significantly higher percentage of patients in the APF530 cohort (65%) had delayed-phase complete response, as compared to those with ondansetron (57%) (P= .014). In addition, a significantly higher percentage (61% vs. 53%) of patients had delayed-phase complete control (P= .022).
The rates of complete response and complete control in acute and overall phases were numerically higher with APF530 compared with ondansetron, but this did not reach statistical significance.
In looking at exploratory efficacy end points, the authors observed that the proportion of patients who experienced an episode of nausea was generally higher with ondansetron than with APF530. Rates of no nausea were also numerically higher with APF530 compared with the ondansetron regimen in the delayed 49.7% vs. 44.25%; P= .099) and overall phases (45.3% vs. 44.2%; P= .138), but there were no statistically significant differences.
The regimen containing APR530 was generally well tolerated, without any new safety signals identified. The most common treatment related events were injection-site reactions, which were mostly mild or moderate. Injection site reactions were similar between the two groups, Dr. Schnadig noted.
In a discussion of the paper, Dr. Clifford Hudis reiterated that there was a higher complete response rate in late phase with APF530—“that’s an advantage and it was 8% higher in absolute terms, and it was 11% higher in the cisplatin group.”
If the drug is approved and it’s comparably priced to other agents and available, “why wouldn’t you use it?” he asked. “Its more convenient, it’s a little more effective, and its one less thing for all of us practicing to think very much about.”
SAN FRANCISCO – APF530 is a long-acting 5-HT3 antagonist formulation in development for the prevention of chemotherapy-induced nausea and vomiting, and new data presented at the 2015 Breast Cancer Symposium suggest that it may have an edge over the standard regimen.
When administered with fosaprepitant and dexamethasone, it was superior to ondansetron, in combination with the same two agents, for preventing chemotherapy-induced nausea and vomiting (CINV) associated with delayed ( > 24-120 h) highly emetogenic chemotherapy (HEC).
Among the 450 patients who received the three-drug APF530 combination, 263 (58.4%) experienced a complete response, defined as having no emesis or need for rescue medication, as compared with 239/452 (53%) who received the ondansetron regimen (P= .092).
“APF530 is the first and only 5-HT3 receptor antagonist to demonstrate superiority in a phase III, three-drug versus three-drug regimen efficacy trial for the prevention of CINV in patients receiving HEC,” said Dr. Ian D. Schnadig, from Compass Oncology, The US Oncology Network, Tualatin, OR. “Managing delayed-phase CINV associated with HEC is an unmet need.”
APF530 is a novel, injectable, extended-release version of granisetron that provides sustained release over ≥ 5 days to prevent both acute (0-24 h) and delayed CINV. A large randomized double blind phase III trial has already demonstrated that this new agent is non-inferior to palonosetron in this setting.
In this double-blind, multicenter study known as the MAGIC trial, Dr. Schnadig randomized 902 patients to receive APF530 500 mg by subcutaneous injection (10 mg granisetron) or ondansetron 0.15 mg/kg IV, and the cohort was stratified by planned cisplatin therapy ( ≥ 50 mg/m2).
The primary end point was delayed-phase complete response, and secondary end points included complete response in acute and overall phases and complete control (complete response and no more than mild nausea) in acute, delayed, and overall phases.
A significantly higher percentage of patients in the APF530 cohort (65%) had delayed-phase complete response, as compared to those with ondansetron (57%) (P= .014). In addition, a significantly higher percentage (61% vs. 53%) of patients had delayed-phase complete control (P= .022).
The rates of complete response and complete control in acute and overall phases were numerically higher with APF530 compared with ondansetron, but this did not reach statistical significance.
In looking at exploratory efficacy end points, the authors observed that the proportion of patients who experienced an episode of nausea was generally higher with ondansetron than with APF530. Rates of no nausea were also numerically higher with APF530 compared with the ondansetron regimen in the delayed 49.7% vs. 44.25%; P= .099) and overall phases (45.3% vs. 44.2%; P= .138), but there were no statistically significant differences.
The regimen containing APR530 was generally well tolerated, without any new safety signals identified. The most common treatment related events were injection-site reactions, which were mostly mild or moderate. Injection site reactions were similar between the two groups, Dr. Schnadig noted.
In a discussion of the paper, Dr. Clifford Hudis reiterated that there was a higher complete response rate in late phase with APF530—“that’s an advantage and it was 8% higher in absolute terms, and it was 11% higher in the cisplatin group.”
If the drug is approved and it’s comparably priced to other agents and available, “why wouldn’t you use it?” he asked. “Its more convenient, it’s a little more effective, and its one less thing for all of us practicing to think very much about.”
FROM THE ASCO BREAST CANCER SYMPOSIUM 2015
Key clinical point:APF530 combined with fosaprepitant and dexamethasone was superior to ondansetron combined with the same agents in the prevention of chemotherapy induced vomiting.
Major finding: A significantly higher percentage of patients in the APF530 cohort (65%) had delayed-phase complete response, as compared to those with on-dansetron (57%) (P= .014), the primary endpoint of the study.
Data source: A phase III randomized trial that included 902 patients.
Disclosures: Research support for the study was provided by Heron Therapeutics. Dr. Schnadig is employed by Compass Oncology and reports relationships with HERON, Tesaro, and McKesson. Several of the co-authors also have disclosures with industry.
ASCO: Potentially targetable biomarkers identified in geriatric breast cancer tumors
SAN FRANCISCO – Using multiplatform profiling, potentially targetable biomarker aberrations were identified in a large sample of tumors taken from older breast cancer patients, reported researchers at the 2015 Breast Cancer Symposium.
In this cross sectional study, 1,189 tumors were collected from breast cancer patients between the ages of 70-97 years, and the samples were assayed for potential targetable biomarkers, and then the findings were compared with samples obtained from younger patients.
“Most of the differences we found were in triple negative patients, and they are related to PIK3CA mutations,” said study author Dr. Paula Pohlmann, from the Lombardi Comprehensive Cancer Center, MedStar Georgetown University Hospital, Washington.
“Also, I am very puzzled that the BRCA1 mutations were not found in older triple negative patients, as compared with the younger patients,” she noted. “Even taking into account the limitations of the study, it was still interesting.”
ERBB2 mutations were identified in non-amplifed breast cancers and less frequently in amplified ones. “And another interesting point was that both PD-1 and PD-L1 positivity by immunohistochemistry were found in all groups, and not only triple negative,” said Dr. Pohlmann. “The checkpoints are similar for both older and younger populations.”
Dr. Pohlmann explained that while she expected to see the PD-1 and PD-L1 positivity, it is worth mentioning since all the treatments focusing on PD-1 and PD-L1 in breast cancer are with triple negative disease. “But we found this expression in Her2 positive and hormone receptor positive disease.”
However, she emphasized that this doesn’t mean that they are necessarily a target. “If they will respond to that therapy, we don’t know, that’s a different story, but the markers are there,” Dr. Pohlmann said.
In this study a total of 1,189 tumors were collected from breast cancer patients (male= 21/female = 1,168) who were aged 70 years and older. The tumors were analyzed (breast biopsy n = 512; metastatic site n = 677), and of 1,088 tumors with available immunohistochemistry (IHC) of ER, PR and IHC and/or in situ hybridization (ISH) of HER2, 613 (56%) were HR+/HER2-, 72 (7%) were HR+/HER2+, 346 (32%) were triple negative, and 57 (5%) were HR-/HER2+.
Overall, 39 of 47 genes that were sequenced carried mutations with frequencies ranging from 0.2% to 37%. The highest mutation rates were seen in PIK3CA (37%), TP53 (37%), BRCA2 (12%), PTEN (5.8%), AKT1 (4.2%), cMET (3.9%), ERBB2 (3.5%), BRCA1 (3.3%), and ATM (3.2%).
Among 13 patients with ERBB2 mutation, 3 had it amplified. PD-L1 expression on tumor cells was detected in 13% of tumors and PD-1 expression on tumor-infiltrating lymphocytes was seen in 46%, but the triple negative subtype had the highest expression: 20% and 60%, respectively.
The researchers also noted that TOPO1, TLE3, AR, TOP2A, and SPARC were overexpressed in 61%, 58%, 55%, 51%, and 37% of tumors, respectively. These observations suggest that there is potential sensitivity to irinotecan, taxanes, anthracyclines, and nab-paclitaxel.
In addition, TS, RRM1, and ERCC1 were under-expressed in 69%, 69%, and 53% of tumors, respectively, which suggests that there may be potential sensitivity to fluoropyrimidines, gemcitabine, and platinums
“This study provides key elements for the design of clinical trials focusing on geriatric patient population, particularly in the subgroup of triple negative breast cancer,” said Dr. Pohlmann. “In addition, ERBB2 abnormalities in older patients may also warrant further investigation.”
Dr. Charles E. Geyer, Jr., of Virginia Commonwealth University Massey Cancer Center, Richmond, in his discussion of the paper, pointed out that while there were some differences in the groups, it wasn’t clear that they are clinically significant differences. “The most interesting were PD-1 and PD-L1, which were in higher numbers than are being reported.”
But the data from this study suggest that focus needs to be on inclusion of geriatric patients in targeted therapy trials, he said.
He also noted that in general, patients frequently have some unrealistic expectations when it comes to genetic testing. “The advocacy often gets a little ahead of the science,” Dr. Geyer said.
SAN FRANCISCO – Using multiplatform profiling, potentially targetable biomarker aberrations were identified in a large sample of tumors taken from older breast cancer patients, reported researchers at the 2015 Breast Cancer Symposium.
In this cross sectional study, 1,189 tumors were collected from breast cancer patients between the ages of 70-97 years, and the samples were assayed for potential targetable biomarkers, and then the findings were compared with samples obtained from younger patients.
“Most of the differences we found were in triple negative patients, and they are related to PIK3CA mutations,” said study author Dr. Paula Pohlmann, from the Lombardi Comprehensive Cancer Center, MedStar Georgetown University Hospital, Washington.
“Also, I am very puzzled that the BRCA1 mutations were not found in older triple negative patients, as compared with the younger patients,” she noted. “Even taking into account the limitations of the study, it was still interesting.”
ERBB2 mutations were identified in non-amplifed breast cancers and less frequently in amplified ones. “And another interesting point was that both PD-1 and PD-L1 positivity by immunohistochemistry were found in all groups, and not only triple negative,” said Dr. Pohlmann. “The checkpoints are similar for both older and younger populations.”
Dr. Pohlmann explained that while she expected to see the PD-1 and PD-L1 positivity, it is worth mentioning since all the treatments focusing on PD-1 and PD-L1 in breast cancer are with triple negative disease. “But we found this expression in Her2 positive and hormone receptor positive disease.”
However, she emphasized that this doesn’t mean that they are necessarily a target. “If they will respond to that therapy, we don’t know, that’s a different story, but the markers are there,” Dr. Pohlmann said.
In this study a total of 1,189 tumors were collected from breast cancer patients (male= 21/female = 1,168) who were aged 70 years and older. The tumors were analyzed (breast biopsy n = 512; metastatic site n = 677), and of 1,088 tumors with available immunohistochemistry (IHC) of ER, PR and IHC and/or in situ hybridization (ISH) of HER2, 613 (56%) were HR+/HER2-, 72 (7%) were HR+/HER2+, 346 (32%) were triple negative, and 57 (5%) were HR-/HER2+.
Overall, 39 of 47 genes that were sequenced carried mutations with frequencies ranging from 0.2% to 37%. The highest mutation rates were seen in PIK3CA (37%), TP53 (37%), BRCA2 (12%), PTEN (5.8%), AKT1 (4.2%), cMET (3.9%), ERBB2 (3.5%), BRCA1 (3.3%), and ATM (3.2%).
Among 13 patients with ERBB2 mutation, 3 had it amplified. PD-L1 expression on tumor cells was detected in 13% of tumors and PD-1 expression on tumor-infiltrating lymphocytes was seen in 46%, but the triple negative subtype had the highest expression: 20% and 60%, respectively.
The researchers also noted that TOPO1, TLE3, AR, TOP2A, and SPARC were overexpressed in 61%, 58%, 55%, 51%, and 37% of tumors, respectively. These observations suggest that there is potential sensitivity to irinotecan, taxanes, anthracyclines, and nab-paclitaxel.
In addition, TS, RRM1, and ERCC1 were under-expressed in 69%, 69%, and 53% of tumors, respectively, which suggests that there may be potential sensitivity to fluoropyrimidines, gemcitabine, and platinums
“This study provides key elements for the design of clinical trials focusing on geriatric patient population, particularly in the subgroup of triple negative breast cancer,” said Dr. Pohlmann. “In addition, ERBB2 abnormalities in older patients may also warrant further investigation.”
Dr. Charles E. Geyer, Jr., of Virginia Commonwealth University Massey Cancer Center, Richmond, in his discussion of the paper, pointed out that while there were some differences in the groups, it wasn’t clear that they are clinically significant differences. “The most interesting were PD-1 and PD-L1, which were in higher numbers than are being reported.”
But the data from this study suggest that focus needs to be on inclusion of geriatric patients in targeted therapy trials, he said.
He also noted that in general, patients frequently have some unrealistic expectations when it comes to genetic testing. “The advocacy often gets a little ahead of the science,” Dr. Geyer said.
SAN FRANCISCO – Using multiplatform profiling, potentially targetable biomarker aberrations were identified in a large sample of tumors taken from older breast cancer patients, reported researchers at the 2015 Breast Cancer Symposium.
In this cross sectional study, 1,189 tumors were collected from breast cancer patients between the ages of 70-97 years, and the samples were assayed for potential targetable biomarkers, and then the findings were compared with samples obtained from younger patients.
“Most of the differences we found were in triple negative patients, and they are related to PIK3CA mutations,” said study author Dr. Paula Pohlmann, from the Lombardi Comprehensive Cancer Center, MedStar Georgetown University Hospital, Washington.
“Also, I am very puzzled that the BRCA1 mutations were not found in older triple negative patients, as compared with the younger patients,” she noted. “Even taking into account the limitations of the study, it was still interesting.”
ERBB2 mutations were identified in non-amplifed breast cancers and less frequently in amplified ones. “And another interesting point was that both PD-1 and PD-L1 positivity by immunohistochemistry were found in all groups, and not only triple negative,” said Dr. Pohlmann. “The checkpoints are similar for both older and younger populations.”
Dr. Pohlmann explained that while she expected to see the PD-1 and PD-L1 positivity, it is worth mentioning since all the treatments focusing on PD-1 and PD-L1 in breast cancer are with triple negative disease. “But we found this expression in Her2 positive and hormone receptor positive disease.”
However, she emphasized that this doesn’t mean that they are necessarily a target. “If they will respond to that therapy, we don’t know, that’s a different story, but the markers are there,” Dr. Pohlmann said.
In this study a total of 1,189 tumors were collected from breast cancer patients (male= 21/female = 1,168) who were aged 70 years and older. The tumors were analyzed (breast biopsy n = 512; metastatic site n = 677), and of 1,088 tumors with available immunohistochemistry (IHC) of ER, PR and IHC and/or in situ hybridization (ISH) of HER2, 613 (56%) were HR+/HER2-, 72 (7%) were HR+/HER2+, 346 (32%) were triple negative, and 57 (5%) were HR-/HER2+.
Overall, 39 of 47 genes that were sequenced carried mutations with frequencies ranging from 0.2% to 37%. The highest mutation rates were seen in PIK3CA (37%), TP53 (37%), BRCA2 (12%), PTEN (5.8%), AKT1 (4.2%), cMET (3.9%), ERBB2 (3.5%), BRCA1 (3.3%), and ATM (3.2%).
Among 13 patients with ERBB2 mutation, 3 had it amplified. PD-L1 expression on tumor cells was detected in 13% of tumors and PD-1 expression on tumor-infiltrating lymphocytes was seen in 46%, but the triple negative subtype had the highest expression: 20% and 60%, respectively.
The researchers also noted that TOPO1, TLE3, AR, TOP2A, and SPARC were overexpressed in 61%, 58%, 55%, 51%, and 37% of tumors, respectively. These observations suggest that there is potential sensitivity to irinotecan, taxanes, anthracyclines, and nab-paclitaxel.
In addition, TS, RRM1, and ERCC1 were under-expressed in 69%, 69%, and 53% of tumors, respectively, which suggests that there may be potential sensitivity to fluoropyrimidines, gemcitabine, and platinums
“This study provides key elements for the design of clinical trials focusing on geriatric patient population, particularly in the subgroup of triple negative breast cancer,” said Dr. Pohlmann. “In addition, ERBB2 abnormalities in older patients may also warrant further investigation.”
Dr. Charles E. Geyer, Jr., of Virginia Commonwealth University Massey Cancer Center, Richmond, in his discussion of the paper, pointed out that while there were some differences in the groups, it wasn’t clear that they are clinically significant differences. “The most interesting were PD-1 and PD-L1, which were in higher numbers than are being reported.”
But the data from this study suggest that focus needs to be on inclusion of geriatric patients in targeted therapy trials, he said.
He also noted that in general, patients frequently have some unrealistic expectations when it comes to genetic testing. “The advocacy often gets a little ahead of the science,” Dr. Geyer said.
FROM THE ASCO BREAST CANCER SYMPOSIUM 2015
Key clinical point: Multiplatform profiling identified potentially targetable biomarker aberrations in a large sample of tumor taken from older breast cancer patients.
Major finding: Most of the differences found between younger and older patients were in triple negative patients, and these were primarily related to PIK3CA mutations.
Data source: A cross sectional study comprised of 1189 tumors collected from breast cancer patients aged 70-97 years, and assayed for potential targetable biomarkers.
Disclosures: Dr. Pohlmann has relationships with Immunonet BioSciences, OncoPlex Diagnostics; Personalized Cancer Therapy and patents/royalties and other intellectual property for Immunological Compositions as Cancer Therapeutics. Dr. Joanne Xiu and Dr. Sandeep K. Reddy are employees of Caris Life Sciences, who conducted the genetic analysis.