User login
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
THE DIAGNOSIS: Mpox Virus
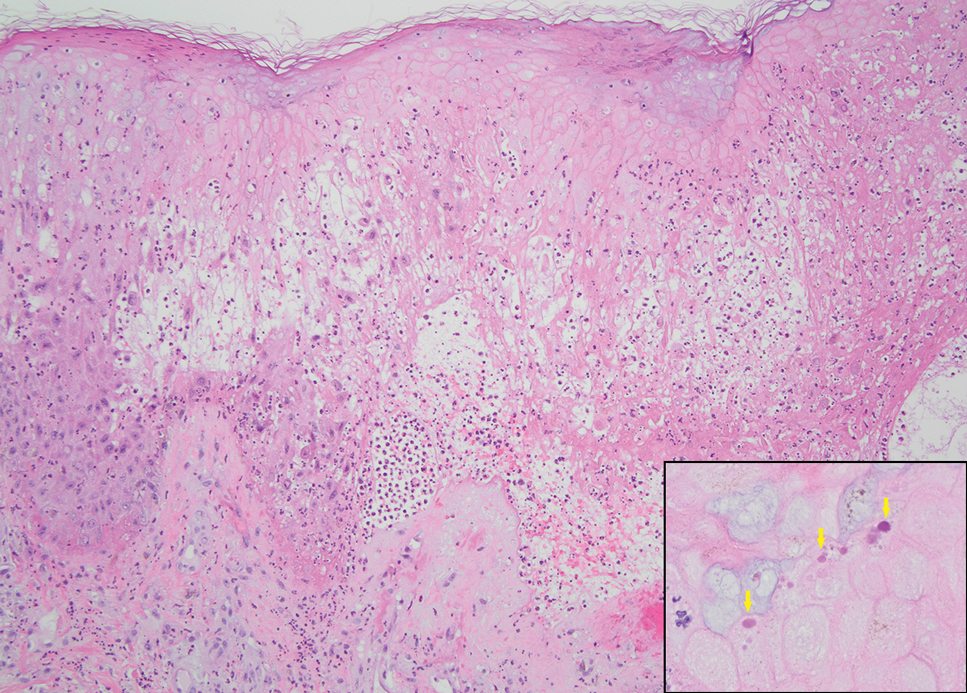
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.
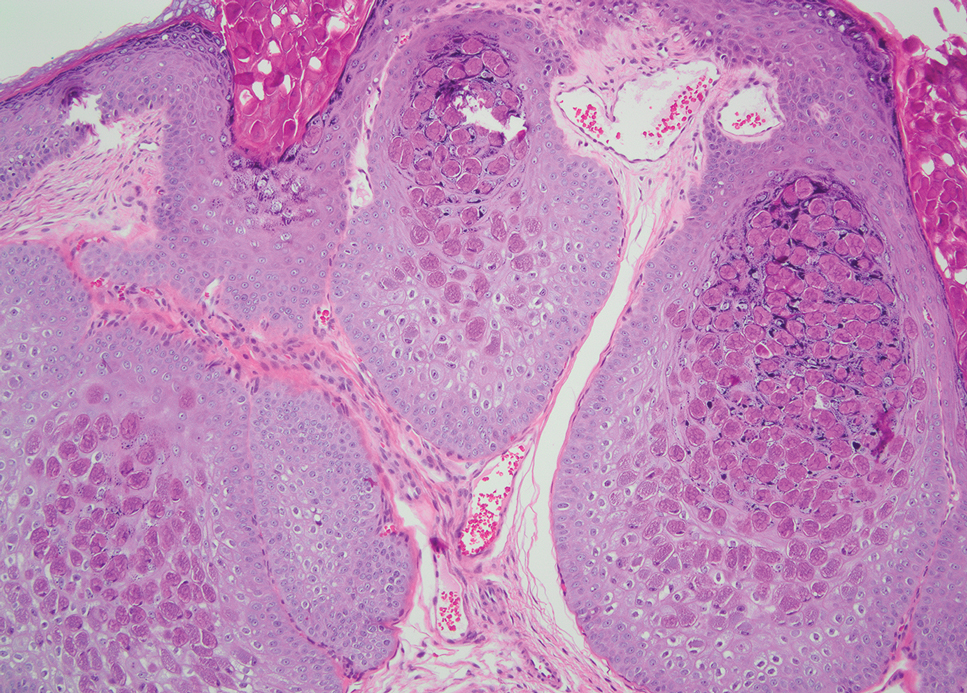
Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8
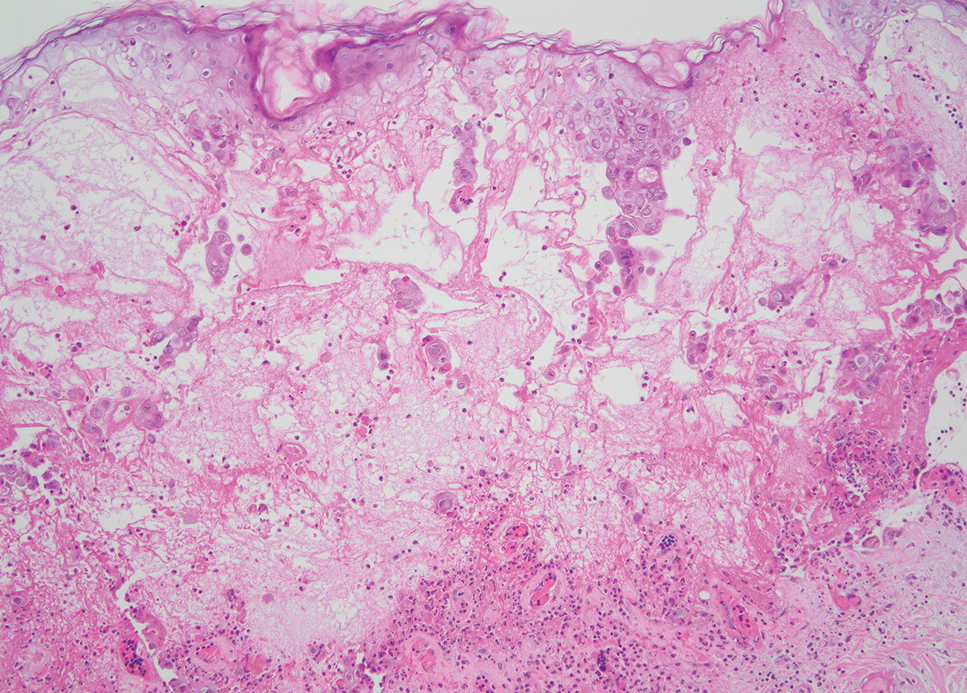
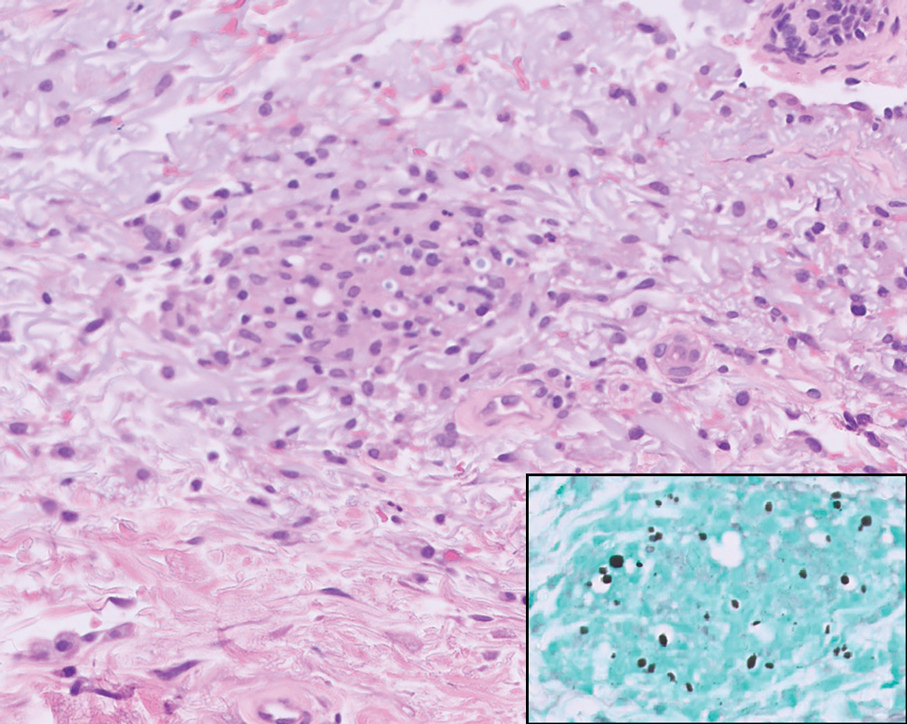
Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).
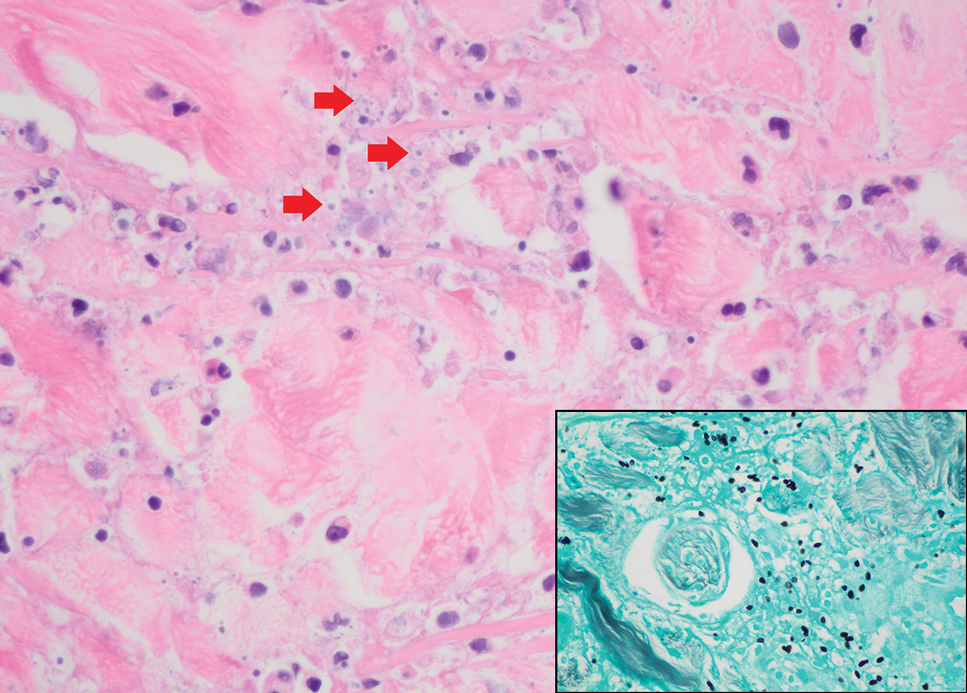
Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).
×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
THE DIAGNOSIS: Mpox Virus
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.

Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8

Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).

Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).

×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
THE DIAGNOSIS: Mpox Virus
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.

Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8

Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).

Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).

×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
A 42-year-old man with a history of multidrug-resistant HIV/AIDS presented to the emergency department for evaluation of pruritic, scattered, umbilicated papules on the left cheek, neck, and arms of 3 days’ duration. The patient’s most recent CD4+ T-cell count 6 weeks prior to the development of the rash was 1 cell/mm3. He was noncompliant with antiretroviral therapy. He reported that the lesions had progressed rapidly, starting on the face and extending down the neck and arms. Physical examination revealed scattered umbilicated and centrally crusted papules and plaques on the left cheek, neck, and arms. Erosions involving the oral mucosa also were noted, which the patient reported had been present for several weeks. An oral swab was positive for herpes simplex virus 2 on polymerase chain reaction. A shave biopsy of a lesion from the left cheek was performed.