User login
Richard Pizzi is editor of The Hospitalist. He has been an editor at Frontline Medical Communications since 2015, and previously served as editor of MDedge publications Hospitalist News and ID Practitioner. He has also worked as an editor and in editorial management roles for HIMSS Media, MedTech Media, and the American Association for Clinical Chemistry. Follow him on Twitter @richpizzi
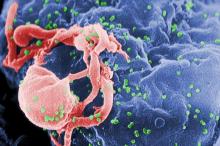
HIV research update: Early January 2016
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
A review article in Current Opinion in HIV & AIDS suggests that a better understanding of the role of opportunistic infections at the initiation of antiretroviral therapy, and the pathogenesis and long-term consequences of immune reconstitution inflammatory syndrome (IRIS), will assist in developing improved strategies for HIV prevention and treatment. The essay also notes that chronic viral coinfections with herpes viruses and hepatitis C virus are important factors in persistent immune activation in chronic-treated HIV.
Researchers in King County, Washington, examined trends in time from HIV diagnosis to viral load suppression to gauge improvement in the region’s HIV care continuum from 2007-2013. IN an article published in Sexually Transmitted Diseases, the authors discovered that the time from HIV diagnosis to viral suppression dramatically declined between 2007 and 2013, and more than three-quarters of recently HIV-diagnosed individuals in King County now achieve viral suppression within a year of diagnosis.
Another review essay in Current Opinion in HIV & AIDS examined the impactof HIV-associated immune activation on AIDS and non-AIDS morbidity and mortality. The authors summarize recent evidence helping to elucidate the immunologic pathways that appear most strongly predictive of infectious and noninfectious morbidity, highlighting the likelihood that not all root drivers of immune activation are likely to produce the same disease manifestations and/or be equally attenuated by early antiretroviral therapy initiation.
A study in AIDS examined the potential for combination antiretroviral therapy (cART)-free remission following analytic treatment interruption (ATI) in chronically HIV-infected patients with ultralow cell-associated DNA. In a highly selected population of 10 patients with excellent immune status, durable virologic suppression and ultralow reservoir, the success rate of ATI was 10% and 9 of 10 patients had prompt rebound of plasma viremia. Resumption of antiretroviral therapy led to a return to baseline cell-associated total DNA.
A population-based cohort-study found that estimated median survival time for HIV-infected individuals from aged 50 years has increased by more than 10 years from 1996-1999 to 2006-2014, but is still substantially lower than in the background population. Even among well-treated HIV-infected patients 50 years or older without comorbidity or AIDS-defining events, the estimated median survival time remains lower than in the general population.
Finally, a retrospective cohort study of 16,070 HIV-infected U.S. veterans discovered that the role of bicarbonate concentrations as a tool to monitor kidney health in HIV-infected persons may be limited in settings of tenofovir disoproxil fumarate use.
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
A review article in Current Opinion in HIV & AIDS suggests that a better understanding of the role of opportunistic infections at the initiation of antiretroviral therapy, and the pathogenesis and long-term consequences of immune reconstitution inflammatory syndrome (IRIS), will assist in developing improved strategies for HIV prevention and treatment. The essay also notes that chronic viral coinfections with herpes viruses and hepatitis C virus are important factors in persistent immune activation in chronic-treated HIV.
Researchers in King County, Washington, examined trends in time from HIV diagnosis to viral load suppression to gauge improvement in the region’s HIV care continuum from 2007-2013. IN an article published in Sexually Transmitted Diseases, the authors discovered that the time from HIV diagnosis to viral suppression dramatically declined between 2007 and 2013, and more than three-quarters of recently HIV-diagnosed individuals in King County now achieve viral suppression within a year of diagnosis.
Another review essay in Current Opinion in HIV & AIDS examined the impactof HIV-associated immune activation on AIDS and non-AIDS morbidity and mortality. The authors summarize recent evidence helping to elucidate the immunologic pathways that appear most strongly predictive of infectious and noninfectious morbidity, highlighting the likelihood that not all root drivers of immune activation are likely to produce the same disease manifestations and/or be equally attenuated by early antiretroviral therapy initiation.
A study in AIDS examined the potential for combination antiretroviral therapy (cART)-free remission following analytic treatment interruption (ATI) in chronically HIV-infected patients with ultralow cell-associated DNA. In a highly selected population of 10 patients with excellent immune status, durable virologic suppression and ultralow reservoir, the success rate of ATI was 10% and 9 of 10 patients had prompt rebound of plasma viremia. Resumption of antiretroviral therapy led to a return to baseline cell-associated total DNA.
A population-based cohort-study found that estimated median survival time for HIV-infected individuals from aged 50 years has increased by more than 10 years from 1996-1999 to 2006-2014, but is still substantially lower than in the background population. Even among well-treated HIV-infected patients 50 years or older without comorbidity or AIDS-defining events, the estimated median survival time remains lower than in the general population.
Finally, a retrospective cohort study of 16,070 HIV-infected U.S. veterans discovered that the role of bicarbonate concentrations as a tool to monitor kidney health in HIV-infected persons may be limited in settings of tenofovir disoproxil fumarate use.
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
A review article in Current Opinion in HIV & AIDS suggests that a better understanding of the role of opportunistic infections at the initiation of antiretroviral therapy, and the pathogenesis and long-term consequences of immune reconstitution inflammatory syndrome (IRIS), will assist in developing improved strategies for HIV prevention and treatment. The essay also notes that chronic viral coinfections with herpes viruses and hepatitis C virus are important factors in persistent immune activation in chronic-treated HIV.
Researchers in King County, Washington, examined trends in time from HIV diagnosis to viral load suppression to gauge improvement in the region’s HIV care continuum from 2007-2013. IN an article published in Sexually Transmitted Diseases, the authors discovered that the time from HIV diagnosis to viral suppression dramatically declined between 2007 and 2013, and more than three-quarters of recently HIV-diagnosed individuals in King County now achieve viral suppression within a year of diagnosis.
Another review essay in Current Opinion in HIV & AIDS examined the impactof HIV-associated immune activation on AIDS and non-AIDS morbidity and mortality. The authors summarize recent evidence helping to elucidate the immunologic pathways that appear most strongly predictive of infectious and noninfectious morbidity, highlighting the likelihood that not all root drivers of immune activation are likely to produce the same disease manifestations and/or be equally attenuated by early antiretroviral therapy initiation.
A study in AIDS examined the potential for combination antiretroviral therapy (cART)-free remission following analytic treatment interruption (ATI) in chronically HIV-infected patients with ultralow cell-associated DNA. In a highly selected population of 10 patients with excellent immune status, durable virologic suppression and ultralow reservoir, the success rate of ATI was 10% and 9 of 10 patients had prompt rebound of plasma viremia. Resumption of antiretroviral therapy led to a return to baseline cell-associated total DNA.
A population-based cohort-study found that estimated median survival time for HIV-infected individuals from aged 50 years has increased by more than 10 years from 1996-1999 to 2006-2014, but is still substantially lower than in the background population. Even among well-treated HIV-infected patients 50 years or older without comorbidity or AIDS-defining events, the estimated median survival time remains lower than in the general population.
Finally, a retrospective cohort study of 16,070 HIV-infected U.S. veterans discovered that the role of bicarbonate concentrations as a tool to monitor kidney health in HIV-infected persons may be limited in settings of tenofovir disoproxil fumarate use.
On Twitter @richpizzi
‘D-drugs’ linked to liver disease, cancer in HIV patients
The cumulative use of dideoxynucleoside analogues, or ‘d-drugs,’ in the treatment of HIV-positive patients was independently associated with increased rates of end-stage liver disease and hepatocellular carcinoma, according to a new study.
D-drugs, in particular didanosine (ddI) and stavudine (d4T), have been associated with alterations in liver function and severe steatosis/fibrosis development, and ddI use has been linked with the development of noncirrhotic portal hypertension. Use of d-drugs for HIV-positive patients is rare in most high-income countries because of serious adverse effects, but their use was common in the past and research indicates previous exposure may have adverse impacts on liver function.
A research team led by Dr. Lene Ryom of the department of infectious diseases at the University of Copenhagen, Denmark, assessed end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) events in patients participating in the Data Collection on Adverse Events of Anti-HIV Drugs Study (D:A:D), a prospective cohort collaboration established in 1999, which follows more than 49,000 HIV-1-positive individuals in Europe, the United States, and Australia. They followed patients from the date of enrollment in the study or Jan. 2, 2004, if this was later, until the first of an ESLD/HCC event, death, 6 months after their last visit or Jan. 2, 2014.
Ryom and coauthors investigated associations between ESLD/HCC and cumulative use of individual antiretrovirals, including d-drugs, by using Poisson regression adjusting for potential confounders. They found that cumulative exposure to d4T, ddI, tenofovir, and (fos)amprenavir was associated with increased ESLD/HCC rates. Longer exposure to emtricitabine and nevirapine was associated with lower ESLD/HCC rates.
Because of the independent association with increased ESLD/HCC risk, the authors said that the use of d-drugs to treat HIV-positive patients should be avoided where alternatives are available, and intensified monitoring of liver function should be considered among all individuals exposed to these drugs for longer time periods.
Read the study in the journal AIDS (AIDS. 2016 Jan 8. doi:10.1097/QAD.0000000000001018).
On Twitter @richpizzi
The cumulative use of dideoxynucleoside analogues, or ‘d-drugs,’ in the treatment of HIV-positive patients was independently associated with increased rates of end-stage liver disease and hepatocellular carcinoma, according to a new study.
D-drugs, in particular didanosine (ddI) and stavudine (d4T), have been associated with alterations in liver function and severe steatosis/fibrosis development, and ddI use has been linked with the development of noncirrhotic portal hypertension. Use of d-drugs for HIV-positive patients is rare in most high-income countries because of serious adverse effects, but their use was common in the past and research indicates previous exposure may have adverse impacts on liver function.
A research team led by Dr. Lene Ryom of the department of infectious diseases at the University of Copenhagen, Denmark, assessed end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) events in patients participating in the Data Collection on Adverse Events of Anti-HIV Drugs Study (D:A:D), a prospective cohort collaboration established in 1999, which follows more than 49,000 HIV-1-positive individuals in Europe, the United States, and Australia. They followed patients from the date of enrollment in the study or Jan. 2, 2004, if this was later, until the first of an ESLD/HCC event, death, 6 months after their last visit or Jan. 2, 2014.
Ryom and coauthors investigated associations between ESLD/HCC and cumulative use of individual antiretrovirals, including d-drugs, by using Poisson regression adjusting for potential confounders. They found that cumulative exposure to d4T, ddI, tenofovir, and (fos)amprenavir was associated with increased ESLD/HCC rates. Longer exposure to emtricitabine and nevirapine was associated with lower ESLD/HCC rates.
Because of the independent association with increased ESLD/HCC risk, the authors said that the use of d-drugs to treat HIV-positive patients should be avoided where alternatives are available, and intensified monitoring of liver function should be considered among all individuals exposed to these drugs for longer time periods.
Read the study in the journal AIDS (AIDS. 2016 Jan 8. doi:10.1097/QAD.0000000000001018).
On Twitter @richpizzi
The cumulative use of dideoxynucleoside analogues, or ‘d-drugs,’ in the treatment of HIV-positive patients was independently associated with increased rates of end-stage liver disease and hepatocellular carcinoma, according to a new study.
D-drugs, in particular didanosine (ddI) and stavudine (d4T), have been associated with alterations in liver function and severe steatosis/fibrosis development, and ddI use has been linked with the development of noncirrhotic portal hypertension. Use of d-drugs for HIV-positive patients is rare in most high-income countries because of serious adverse effects, but their use was common in the past and research indicates previous exposure may have adverse impacts on liver function.
A research team led by Dr. Lene Ryom of the department of infectious diseases at the University of Copenhagen, Denmark, assessed end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) events in patients participating in the Data Collection on Adverse Events of Anti-HIV Drugs Study (D:A:D), a prospective cohort collaboration established in 1999, which follows more than 49,000 HIV-1-positive individuals in Europe, the United States, and Australia. They followed patients from the date of enrollment in the study or Jan. 2, 2004, if this was later, until the first of an ESLD/HCC event, death, 6 months after their last visit or Jan. 2, 2014.
Ryom and coauthors investigated associations between ESLD/HCC and cumulative use of individual antiretrovirals, including d-drugs, by using Poisson regression adjusting for potential confounders. They found that cumulative exposure to d4T, ddI, tenofovir, and (fos)amprenavir was associated with increased ESLD/HCC rates. Longer exposure to emtricitabine and nevirapine was associated with lower ESLD/HCC rates.
Because of the independent association with increased ESLD/HCC risk, the authors said that the use of d-drugs to treat HIV-positive patients should be avoided where alternatives are available, and intensified monitoring of liver function should be considered among all individuals exposed to these drugs for longer time periods.
Read the study in the journal AIDS (AIDS. 2016 Jan 8. doi:10.1097/QAD.0000000000001018).
On Twitter @richpizzi
FROM AIDS
HIV-TB coinfection strongly associated with poor TB treatment outcomes
A large European study published in the journal AIDS has confirmed that HIV-positive status is a strong risk factor for an adverse tuberculosis treatment outcome.
Researchers at the Robert Koch Institute in Berlin analyzed the treatment outcomes of 61,138 TB cases reported from nine European countries during 2010-2012, and investigated the effect of HIV on TB outcomes using multilevel and multinomial logistic models. They also considered the interaction between HIV and multidrug-resistant TB (MDR-TB).
According to lead author Basel Karo and his colleagues, 5.5% of the TB cases analyzed were HIV positive. HIV coinfected cases had a significantly lower TB treatment success rate, compared with HIV-negative cases (57% vs. 79%). In the multilevel model adjusted for age and an interaction with MDR-TB, HIV was significantly associated with lower treatment success in all MDR strata, while in the multinomial regression model, HIV-positive cases had significantly higher relative risk ratio for death. Mr. Karo and coauthors said an increased risk of still being on treatment (more than 12 months for non-MDR-TB; more than 24 months for MDR-TB) is another indicator of less successful TB regimens in HIV-positive patients. The research team did not find any significant association between HIV and TB treatment failure.
Negative treatment outcomes may be explained by difficulties in diagnosis of TB, sometimes due to “alternation of the clinical manifestation of TB and lack of a rapid and sensitive TB diagnostic test” in HIV coinfected patients, which may lead to a delayed diagnosis and treatment. TB treatment of TB in HIV coinfected patients also “presents with major challenges regarding the drug interactions between the rifamycins and some antiretroviral agents, overlapping toxic effects, and the occurrence of immune reconstitution inflammatory syndrome (IRIS),” the investigators said.
The results of the trial should encourage future studies “including randomized clinical trials to investigate the optimal duration of TB treatment in HIV coinfected individuals,” Mr. Karo said.
Read the study in the journal AIDS (AIDS. 2016 Jan 8. doi:10.1097/QAD.0000000000001016).
On Twitter @richpizzi
A large European study published in the journal AIDS has confirmed that HIV-positive status is a strong risk factor for an adverse tuberculosis treatment outcome.
Researchers at the Robert Koch Institute in Berlin analyzed the treatment outcomes of 61,138 TB cases reported from nine European countries during 2010-2012, and investigated the effect of HIV on TB outcomes using multilevel and multinomial logistic models. They also considered the interaction between HIV and multidrug-resistant TB (MDR-TB).
According to lead author Basel Karo and his colleagues, 5.5% of the TB cases analyzed were HIV positive. HIV coinfected cases had a significantly lower TB treatment success rate, compared with HIV-negative cases (57% vs. 79%). In the multilevel model adjusted for age and an interaction with MDR-TB, HIV was significantly associated with lower treatment success in all MDR strata, while in the multinomial regression model, HIV-positive cases had significantly higher relative risk ratio for death. Mr. Karo and coauthors said an increased risk of still being on treatment (more than 12 months for non-MDR-TB; more than 24 months for MDR-TB) is another indicator of less successful TB regimens in HIV-positive patients. The research team did not find any significant association between HIV and TB treatment failure.
Negative treatment outcomes may be explained by difficulties in diagnosis of TB, sometimes due to “alternation of the clinical manifestation of TB and lack of a rapid and sensitive TB diagnostic test” in HIV coinfected patients, which may lead to a delayed diagnosis and treatment. TB treatment of TB in HIV coinfected patients also “presents with major challenges regarding the drug interactions between the rifamycins and some antiretroviral agents, overlapping toxic effects, and the occurrence of immune reconstitution inflammatory syndrome (IRIS),” the investigators said.
The results of the trial should encourage future studies “including randomized clinical trials to investigate the optimal duration of TB treatment in HIV coinfected individuals,” Mr. Karo said.
Read the study in the journal AIDS (AIDS. 2016 Jan 8. doi:10.1097/QAD.0000000000001016).
On Twitter @richpizzi
A large European study published in the journal AIDS has confirmed that HIV-positive status is a strong risk factor for an adverse tuberculosis treatment outcome.
Researchers at the Robert Koch Institute in Berlin analyzed the treatment outcomes of 61,138 TB cases reported from nine European countries during 2010-2012, and investigated the effect of HIV on TB outcomes using multilevel and multinomial logistic models. They also considered the interaction between HIV and multidrug-resistant TB (MDR-TB).
According to lead author Basel Karo and his colleagues, 5.5% of the TB cases analyzed were HIV positive. HIV coinfected cases had a significantly lower TB treatment success rate, compared with HIV-negative cases (57% vs. 79%). In the multilevel model adjusted for age and an interaction with MDR-TB, HIV was significantly associated with lower treatment success in all MDR strata, while in the multinomial regression model, HIV-positive cases had significantly higher relative risk ratio for death. Mr. Karo and coauthors said an increased risk of still being on treatment (more than 12 months for non-MDR-TB; more than 24 months for MDR-TB) is another indicator of less successful TB regimens in HIV-positive patients. The research team did not find any significant association between HIV and TB treatment failure.
Negative treatment outcomes may be explained by difficulties in diagnosis of TB, sometimes due to “alternation of the clinical manifestation of TB and lack of a rapid and sensitive TB diagnostic test” in HIV coinfected patients, which may lead to a delayed diagnosis and treatment. TB treatment of TB in HIV coinfected patients also “presents with major challenges regarding the drug interactions between the rifamycins and some antiretroviral agents, overlapping toxic effects, and the occurrence of immune reconstitution inflammatory syndrome (IRIS),” the investigators said.
The results of the trial should encourage future studies “including randomized clinical trials to investigate the optimal duration of TB treatment in HIV coinfected individuals,” Mr. Karo said.
Read the study in the journal AIDS (AIDS. 2016 Jan 8. doi:10.1097/QAD.0000000000001016).
On Twitter @richpizzi
FROM AIDS
Patients may safely self-administer long-term IV antibiotics
Uninsured patients can be trained to safely and efficiently self-administer long-term intravenous antibiotics, according to a 4-year outcomes study published in PLOS Medicine.
Between 2010 and 2013, 994 uninsured patients at Parkland Hospital in Dallas were enrolled in a self-administered outpatient parenteral antimicrobial therapy (S-OPAT) program, and 224 insured patients were discharged to a health care–administered OPAT program. Patients in the S-OPAT group were trained to self-administer intravenous antimicrobials, tested for their ability to treat themselves before discharge, and then monitored by weekly visits to the S-OPAT outpatient clinic. The 224 insured patients in the H-OPAT program had antibiotics administered by a health care worker.
A research team led by Dr. Kavita Bhavan of University of Texas Southwestern Medical Center, Dallas, estimated the effect of S-OPAT versus H-OPAT on 30-day all-cause readmission and 1-year all-cause mortality after controlling for selection bias with a propensity score developed using baseline clinical and sociodemographic information collected from the patients.
The 30-day readmission rate was 47% lower in the S-OPAT group than in the H-OPAT group, and the 1-year mortality rate did not differ significantly between the two groups. Because the S-OPAT program resulted in patients spending fewer days having inpatient infusions, 27,666 inpatient days were avoided over the study period.
Thus, S-OPAT was associated with similar or better outcomes than H-OPAT, meaning S-OPAT may be an acceptable model of treatment for uninsured, medically stable patients to complete extended courses of intravenous antimicrobials at home.
Read the full study online at PLOS Medicine (PLoS Med. 2015 Dec 15;12[12]. doi: 10.1371/journal.pmed.1001922).
On Twitter @richpizzi
Uninsured patients can be trained to safely and efficiently self-administer long-term intravenous antibiotics, according to a 4-year outcomes study published in PLOS Medicine.
Between 2010 and 2013, 994 uninsured patients at Parkland Hospital in Dallas were enrolled in a self-administered outpatient parenteral antimicrobial therapy (S-OPAT) program, and 224 insured patients were discharged to a health care–administered OPAT program. Patients in the S-OPAT group were trained to self-administer intravenous antimicrobials, tested for their ability to treat themselves before discharge, and then monitored by weekly visits to the S-OPAT outpatient clinic. The 224 insured patients in the H-OPAT program had antibiotics administered by a health care worker.
A research team led by Dr. Kavita Bhavan of University of Texas Southwestern Medical Center, Dallas, estimated the effect of S-OPAT versus H-OPAT on 30-day all-cause readmission and 1-year all-cause mortality after controlling for selection bias with a propensity score developed using baseline clinical and sociodemographic information collected from the patients.
The 30-day readmission rate was 47% lower in the S-OPAT group than in the H-OPAT group, and the 1-year mortality rate did not differ significantly between the two groups. Because the S-OPAT program resulted in patients spending fewer days having inpatient infusions, 27,666 inpatient days were avoided over the study period.
Thus, S-OPAT was associated with similar or better outcomes than H-OPAT, meaning S-OPAT may be an acceptable model of treatment for uninsured, medically stable patients to complete extended courses of intravenous antimicrobials at home.
Read the full study online at PLOS Medicine (PLoS Med. 2015 Dec 15;12[12]. doi: 10.1371/journal.pmed.1001922).
On Twitter @richpizzi
Uninsured patients can be trained to safely and efficiently self-administer long-term intravenous antibiotics, according to a 4-year outcomes study published in PLOS Medicine.
Between 2010 and 2013, 994 uninsured patients at Parkland Hospital in Dallas were enrolled in a self-administered outpatient parenteral antimicrobial therapy (S-OPAT) program, and 224 insured patients were discharged to a health care–administered OPAT program. Patients in the S-OPAT group were trained to self-administer intravenous antimicrobials, tested for their ability to treat themselves before discharge, and then monitored by weekly visits to the S-OPAT outpatient clinic. The 224 insured patients in the H-OPAT program had antibiotics administered by a health care worker.
A research team led by Dr. Kavita Bhavan of University of Texas Southwestern Medical Center, Dallas, estimated the effect of S-OPAT versus H-OPAT on 30-day all-cause readmission and 1-year all-cause mortality after controlling for selection bias with a propensity score developed using baseline clinical and sociodemographic information collected from the patients.
The 30-day readmission rate was 47% lower in the S-OPAT group than in the H-OPAT group, and the 1-year mortality rate did not differ significantly between the two groups. Because the S-OPAT program resulted in patients spending fewer days having inpatient infusions, 27,666 inpatient days were avoided over the study period.
Thus, S-OPAT was associated with similar or better outcomes than H-OPAT, meaning S-OPAT may be an acceptable model of treatment for uninsured, medically stable patients to complete extended courses of intravenous antimicrobials at home.
Read the full study online at PLOS Medicine (PLoS Med. 2015 Dec 15;12[12]. doi: 10.1371/journal.pmed.1001922).
On Twitter @richpizzi
FROM PLOS MEDICINE
Ebola update: Dec. 2015
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed over the past month, ID Practitioner is offering some notable news items and journal articles.
Three studies published online in Emerging Infectious Diseases addressed multiple clinical concerns related to treating Ebola virus disease (EVD). Samuel Crowe, Ph.D., of the Centers for Disease Control and Prevention and his colleagues found that two readily available indicatorspredicted survival among patients with EVD in Sierra Leone and may be useful for clinicians making treatment decisions or managing patient or family expectations. The indicators were time from symptom onset to health care facility admission and quantitative real-time reverse transcription polymerase chain reaction cycle threshold, a surrogate for viral load, in first Ebola virus–positive blood sample tested.
In another research dispatch, an international team led by Thomas Hoenen, Ph.D., of the Friedrich-Loeffler-Institut in Greifswald–Insel Riems, Germany, successfully used a novel, pocket-sized nanopore sequencer at a field diagnostic laboratory in Liberia during the current Ebola virus outbreak. The tool was used for rapid sequencing of RNA/DNA from pathogen samples and may offer “tremendous potential” for use in remote and resource-limited areas by revolutionizing the capacity of public health professionals to perform sequencing during future disease outbreaks.
Finally, Dr. J.J. Mark Haverkort of the University Medical Centre of Utrecht, the Netherlands, and his coauthors detailed his hospital’s preparations for an outbreak of viral hemorrhagic fever and its experience during admission of a patient with EVD. The patient’s admission proceeded without incident but put considerable demands on hospital staff. The researchers found that the use of a buddy system, frequent training, and information sessions for staff and their relatives greatly increased the sense of safety and motivation among staff.
The eighth annual G-FINDER report, released in early December by the Australia-based research group Policy Cures, found that $3.4 billion was invested in neglected disease research and development in 2014, but that new funding for Ebola R&D (generated in response to the West African Ebola outbreak) was entirely responsible for the $150 million increase in overall neglected disease R&D funding. Funding for all other neglected diseases dropped by $14 million, or 0.4%. A total of $165 million was invested in Ebola R&D in 2014, more than for any other neglected disease except HIV/AIDS, malaria, tuberculosis and diarrheal diseases. Most of the funding ($101 million) came from the U.S. government, representing 86% of all public funding for Ebola R&D.
According to a studypublished in the journal Infection Control & Hospital Epidemiology, the United States has sufficient capacity for treating another outbreak of the Ebola virus, but financial, staffing, and resource challenges remain a hurdle for hospitals and health systems. Researchers found that the designation of 55 Ebola treatment centers in 2014 heightened nationwide preparedness levels, but challenges remained for those sites providing treatment, which strained institutional capacity in key areas such as waste disposal, staffing, and pediatric care.
The ongoing West African outbreak of EVD is the largest ever recorded, with more than 28,000 reported cases. In a studyrecently published in Cell Host & Microbe, researchers used genome sequencing to trace the introduction and spread of the virus in Liberia, the second-worst–affected country. It seems the majority of Liberian EVD cases are consistent with a single introduction, followed by spread and diversification within the country. Reintroductions of the virus from Liberia served as an important source for the continuation of the already-ongoing EVD outbreak in Guinea. The study suggested that the Ebola virus did not appear to further adapt to humans during the outbreak, but the authors said additional research is needed to understand how the virus transitioned to humans at the beginning of the outbreak.
A meta-analysis published in BMC Infectious Diseases compared the relative frequencies of three typical hemorrhagic symptoms (conjunctival, nasal, and gingival bleedings) in the East-Central African and West African Ebola virus outbreaks. The study found that, along with the increased Ebola virus mutation rate and the decreased case-fatality rate that occurred during the West African outbreak, that outbreak was also characterized by a significant decrease in the proportion of patients with bleeding features. Both nasal and gingival bleeding almost disappeared. The authors concluded that the data support their hypothesis that in West Africa, the Ebola virus’ long human-to-human transmission cycle occurred and gave rise to human adaptation and consequent immune escape.
A study in Clinical Infectious Diseases concludes that current field experience supports the use of convalescent plasma (CP) against EVD as acceptable, feasible, and safe, although efficacy data are pending. Researchers performing an overview of nonrandomized comparative clinical trials in Guinea, Sierra Leone, and Liberia said that CP sourced from within the outbreak is the most readily available source of anti-EVD antibodies, and until the advent of effective antivirals or monoclonal antibodies, CP merits further evaluation by clinicians treating the disease. But longer-term follow-up as well as data from non–trial settings and evidence on the scalability of the intervention are required.
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed over the past month, ID Practitioner is offering some notable news items and journal articles.
Three studies published online in Emerging Infectious Diseases addressed multiple clinical concerns related to treating Ebola virus disease (EVD). Samuel Crowe, Ph.D., of the Centers for Disease Control and Prevention and his colleagues found that two readily available indicatorspredicted survival among patients with EVD in Sierra Leone and may be useful for clinicians making treatment decisions or managing patient or family expectations. The indicators were time from symptom onset to health care facility admission and quantitative real-time reverse transcription polymerase chain reaction cycle threshold, a surrogate for viral load, in first Ebola virus–positive blood sample tested.
In another research dispatch, an international team led by Thomas Hoenen, Ph.D., of the Friedrich-Loeffler-Institut in Greifswald–Insel Riems, Germany, successfully used a novel, pocket-sized nanopore sequencer at a field diagnostic laboratory in Liberia during the current Ebola virus outbreak. The tool was used for rapid sequencing of RNA/DNA from pathogen samples and may offer “tremendous potential” for use in remote and resource-limited areas by revolutionizing the capacity of public health professionals to perform sequencing during future disease outbreaks.
Finally, Dr. J.J. Mark Haverkort of the University Medical Centre of Utrecht, the Netherlands, and his coauthors detailed his hospital’s preparations for an outbreak of viral hemorrhagic fever and its experience during admission of a patient with EVD. The patient’s admission proceeded without incident but put considerable demands on hospital staff. The researchers found that the use of a buddy system, frequent training, and information sessions for staff and their relatives greatly increased the sense of safety and motivation among staff.
The eighth annual G-FINDER report, released in early December by the Australia-based research group Policy Cures, found that $3.4 billion was invested in neglected disease research and development in 2014, but that new funding for Ebola R&D (generated in response to the West African Ebola outbreak) was entirely responsible for the $150 million increase in overall neglected disease R&D funding. Funding for all other neglected diseases dropped by $14 million, or 0.4%. A total of $165 million was invested in Ebola R&D in 2014, more than for any other neglected disease except HIV/AIDS, malaria, tuberculosis and diarrheal diseases. Most of the funding ($101 million) came from the U.S. government, representing 86% of all public funding for Ebola R&D.
According to a studypublished in the journal Infection Control & Hospital Epidemiology, the United States has sufficient capacity for treating another outbreak of the Ebola virus, but financial, staffing, and resource challenges remain a hurdle for hospitals and health systems. Researchers found that the designation of 55 Ebola treatment centers in 2014 heightened nationwide preparedness levels, but challenges remained for those sites providing treatment, which strained institutional capacity in key areas such as waste disposal, staffing, and pediatric care.
The ongoing West African outbreak of EVD is the largest ever recorded, with more than 28,000 reported cases. In a studyrecently published in Cell Host & Microbe, researchers used genome sequencing to trace the introduction and spread of the virus in Liberia, the second-worst–affected country. It seems the majority of Liberian EVD cases are consistent with a single introduction, followed by spread and diversification within the country. Reintroductions of the virus from Liberia served as an important source for the continuation of the already-ongoing EVD outbreak in Guinea. The study suggested that the Ebola virus did not appear to further adapt to humans during the outbreak, but the authors said additional research is needed to understand how the virus transitioned to humans at the beginning of the outbreak.
A meta-analysis published in BMC Infectious Diseases compared the relative frequencies of three typical hemorrhagic symptoms (conjunctival, nasal, and gingival bleedings) in the East-Central African and West African Ebola virus outbreaks. The study found that, along with the increased Ebola virus mutation rate and the decreased case-fatality rate that occurred during the West African outbreak, that outbreak was also characterized by a significant decrease in the proportion of patients with bleeding features. Both nasal and gingival bleeding almost disappeared. The authors concluded that the data support their hypothesis that in West Africa, the Ebola virus’ long human-to-human transmission cycle occurred and gave rise to human adaptation and consequent immune escape.
A study in Clinical Infectious Diseases concludes that current field experience supports the use of convalescent plasma (CP) against EVD as acceptable, feasible, and safe, although efficacy data are pending. Researchers performing an overview of nonrandomized comparative clinical trials in Guinea, Sierra Leone, and Liberia said that CP sourced from within the outbreak is the most readily available source of anti-EVD antibodies, and until the advent of effective antivirals or monoclonal antibodies, CP merits further evaluation by clinicians treating the disease. But longer-term follow-up as well as data from non–trial settings and evidence on the scalability of the intervention are required.
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed over the past month, ID Practitioner is offering some notable news items and journal articles.
Three studies published online in Emerging Infectious Diseases addressed multiple clinical concerns related to treating Ebola virus disease (EVD). Samuel Crowe, Ph.D., of the Centers for Disease Control and Prevention and his colleagues found that two readily available indicatorspredicted survival among patients with EVD in Sierra Leone and may be useful for clinicians making treatment decisions or managing patient or family expectations. The indicators were time from symptom onset to health care facility admission and quantitative real-time reverse transcription polymerase chain reaction cycle threshold, a surrogate for viral load, in first Ebola virus–positive blood sample tested.
In another research dispatch, an international team led by Thomas Hoenen, Ph.D., of the Friedrich-Loeffler-Institut in Greifswald–Insel Riems, Germany, successfully used a novel, pocket-sized nanopore sequencer at a field diagnostic laboratory in Liberia during the current Ebola virus outbreak. The tool was used for rapid sequencing of RNA/DNA from pathogen samples and may offer “tremendous potential” for use in remote and resource-limited areas by revolutionizing the capacity of public health professionals to perform sequencing during future disease outbreaks.
Finally, Dr. J.J. Mark Haverkort of the University Medical Centre of Utrecht, the Netherlands, and his coauthors detailed his hospital’s preparations for an outbreak of viral hemorrhagic fever and its experience during admission of a patient with EVD. The patient’s admission proceeded without incident but put considerable demands on hospital staff. The researchers found that the use of a buddy system, frequent training, and information sessions for staff and their relatives greatly increased the sense of safety and motivation among staff.
The eighth annual G-FINDER report, released in early December by the Australia-based research group Policy Cures, found that $3.4 billion was invested in neglected disease research and development in 2014, but that new funding for Ebola R&D (generated in response to the West African Ebola outbreak) was entirely responsible for the $150 million increase in overall neglected disease R&D funding. Funding for all other neglected diseases dropped by $14 million, or 0.4%. A total of $165 million was invested in Ebola R&D in 2014, more than for any other neglected disease except HIV/AIDS, malaria, tuberculosis and diarrheal diseases. Most of the funding ($101 million) came from the U.S. government, representing 86% of all public funding for Ebola R&D.
According to a studypublished in the journal Infection Control & Hospital Epidemiology, the United States has sufficient capacity for treating another outbreak of the Ebola virus, but financial, staffing, and resource challenges remain a hurdle for hospitals and health systems. Researchers found that the designation of 55 Ebola treatment centers in 2014 heightened nationwide preparedness levels, but challenges remained for those sites providing treatment, which strained institutional capacity in key areas such as waste disposal, staffing, and pediatric care.
The ongoing West African outbreak of EVD is the largest ever recorded, with more than 28,000 reported cases. In a studyrecently published in Cell Host & Microbe, researchers used genome sequencing to trace the introduction and spread of the virus in Liberia, the second-worst–affected country. It seems the majority of Liberian EVD cases are consistent with a single introduction, followed by spread and diversification within the country. Reintroductions of the virus from Liberia served as an important source for the continuation of the already-ongoing EVD outbreak in Guinea. The study suggested that the Ebola virus did not appear to further adapt to humans during the outbreak, but the authors said additional research is needed to understand how the virus transitioned to humans at the beginning of the outbreak.
A meta-analysis published in BMC Infectious Diseases compared the relative frequencies of three typical hemorrhagic symptoms (conjunctival, nasal, and gingival bleedings) in the East-Central African and West African Ebola virus outbreaks. The study found that, along with the increased Ebola virus mutation rate and the decreased case-fatality rate that occurred during the West African outbreak, that outbreak was also characterized by a significant decrease in the proportion of patients with bleeding features. Both nasal and gingival bleeding almost disappeared. The authors concluded that the data support their hypothesis that in West Africa, the Ebola virus’ long human-to-human transmission cycle occurred and gave rise to human adaptation and consequent immune escape.
A study in Clinical Infectious Diseases concludes that current field experience supports the use of convalescent plasma (CP) against EVD as acceptable, feasible, and safe, although efficacy data are pending. Researchers performing an overview of nonrandomized comparative clinical trials in Guinea, Sierra Leone, and Liberia said that CP sourced from within the outbreak is the most readily available source of anti-EVD antibodies, and until the advent of effective antivirals or monoclonal antibodies, CP merits further evaluation by clinicians treating the disease. But longer-term follow-up as well as data from non–trial settings and evidence on the scalability of the intervention are required.
On Twitter @richpizzi
WHO identifies top emerging diseases
The World Health Organization has generated a list of top emerging pathogens likely to cause severe outbreaks in the near future and for which few or no medical countermeasures exist.
The diseases designated by WHO were revealed by the organization last week in a public statement and provide the basis for its ongoing work on a “Blueprint for R&D preparedness” to help control potential future outbreaks. The Blueprint will advocate for the enhancement of the research and development process to develop diagnostics, vaccines, and therapeutics for the diseases. In addition to jump-starting R&D, WHO said the Blueprint will also consider behavioral interventions to allow the design of better disease control measures.
WHO’s initial list of eight diseases needing urgent attention includes:
• Crimean Congo hemorrhagic fever
• Middle East respiratory syndrome coronavirus (MERS-CoV)
• Severe acute respiratory syndrome (SARS)
Three other diseases were designated by WHO as “serious,” requiring attention “as soon as possible”:
• Severe fever with thrombocytopenia syndrome
According to WHO, the list will be reviewed and the prioritization methodology fine-tuned annually or when new diseases emerge.
WHO said other diseases with epidemic potential – such as HIV/AIDS, tuberculosis, malaria, avian influenza, and dengue – were not included in the list because there are major disease control and research networks for these infections and an existing pipeline for improved interventions.
On Twitter @richpizzi
This article was updated 2/12/16.
The World Health Organization has generated a list of top emerging pathogens likely to cause severe outbreaks in the near future and for which few or no medical countermeasures exist.
The diseases designated by WHO were revealed by the organization last week in a public statement and provide the basis for its ongoing work on a “Blueprint for R&D preparedness” to help control potential future outbreaks. The Blueprint will advocate for the enhancement of the research and development process to develop diagnostics, vaccines, and therapeutics for the diseases. In addition to jump-starting R&D, WHO said the Blueprint will also consider behavioral interventions to allow the design of better disease control measures.
WHO’s initial list of eight diseases needing urgent attention includes:
• Crimean Congo hemorrhagic fever
• Middle East respiratory syndrome coronavirus (MERS-CoV)
• Severe acute respiratory syndrome (SARS)
Three other diseases were designated by WHO as “serious,” requiring attention “as soon as possible”:
• Severe fever with thrombocytopenia syndrome
According to WHO, the list will be reviewed and the prioritization methodology fine-tuned annually or when new diseases emerge.
WHO said other diseases with epidemic potential – such as HIV/AIDS, tuberculosis, malaria, avian influenza, and dengue – were not included in the list because there are major disease control and research networks for these infections and an existing pipeline for improved interventions.
On Twitter @richpizzi
This article was updated 2/12/16.
The World Health Organization has generated a list of top emerging pathogens likely to cause severe outbreaks in the near future and for which few or no medical countermeasures exist.
The diseases designated by WHO were revealed by the organization last week in a public statement and provide the basis for its ongoing work on a “Blueprint for R&D preparedness” to help control potential future outbreaks. The Blueprint will advocate for the enhancement of the research and development process to develop diagnostics, vaccines, and therapeutics for the diseases. In addition to jump-starting R&D, WHO said the Blueprint will also consider behavioral interventions to allow the design of better disease control measures.
WHO’s initial list of eight diseases needing urgent attention includes:
• Crimean Congo hemorrhagic fever
• Middle East respiratory syndrome coronavirus (MERS-CoV)
• Severe acute respiratory syndrome (SARS)
Three other diseases were designated by WHO as “serious,” requiring attention “as soon as possible”:
• Severe fever with thrombocytopenia syndrome
According to WHO, the list will be reviewed and the prioritization methodology fine-tuned annually or when new diseases emerge.
WHO said other diseases with epidemic potential – such as HIV/AIDS, tuberculosis, malaria, avian influenza, and dengue – were not included in the list because there are major disease control and research networks for these infections and an existing pipeline for improved interventions.
On Twitter @richpizzi
This article was updated 2/12/16.
Viremia in Ebola patients a strong predictor of death
The levels of virus present in the blood for patients with Ebola virus disease (EVD) are strong predictors of fatality, according to a new study in PLOS Medicine.
A research team at the Pasteur Institute in Paris and Dakar, Senegal, analyzed the laboratory and epidemiologic records of 699 patients with EVD to determine the association between viremia and case fatality ratio (CFR). The EVD diagnoses of patients hospitalized in the Conakry area, Guinea, between March 1, 2014, and Feb. 28, 2015, were confirmed by reverse transcription polymerase chain reaction. Researchers discovered that, in the week following EVD symptom onset, patient viremia remained stable, and that the CFR increased with level of viremia.
The CFR for patients with low-, intermediate-, and high viremia was 21%, 53%, and 81%, respectively. Compared with adults aged 15-44 years, children younger than 5 years and adults aged 45 years and older had higher CFR, but children aged 5-14 years had a lower CFR. When the average viremia increased 10-fold in July 2014, CFR increased as well, by 14%.
Study authors cautioned that their findings may not translate to cases outside the hospital setting, but that knowledge of the relationship between viremia levels and CFR could help clinicians more accurately assess the efficacy of treatments for EVD in nonrandomized trials.
“This finding suggests that heterogeneity in historical CFR estimates among patients, [Ebola treatment centers], and over time may at least partly be explained by variations in viremia and underscores that more valid estimates of the influence of other factors, including treatment effects, might be obtained by adjusting for differing levels of viremia among patients,” the authors wrote.
The study was funded by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases,” the U.S. National Institute of General Medical Sciences MIDAS (Models of Infectious Disease Agent Study) initiative, the AXA Research Fund, and the Seventh Framework Programme of the European Union. The authors declared no conflicts of interest.
Read the entire study on the PLOS Medicine website.
On Twitter @richpizzi
The levels of virus present in the blood for patients with Ebola virus disease (EVD) are strong predictors of fatality, according to a new study in PLOS Medicine.
A research team at the Pasteur Institute in Paris and Dakar, Senegal, analyzed the laboratory and epidemiologic records of 699 patients with EVD to determine the association between viremia and case fatality ratio (CFR). The EVD diagnoses of patients hospitalized in the Conakry area, Guinea, between March 1, 2014, and Feb. 28, 2015, were confirmed by reverse transcription polymerase chain reaction. Researchers discovered that, in the week following EVD symptom onset, patient viremia remained stable, and that the CFR increased with level of viremia.
The CFR for patients with low-, intermediate-, and high viremia was 21%, 53%, and 81%, respectively. Compared with adults aged 15-44 years, children younger than 5 years and adults aged 45 years and older had higher CFR, but children aged 5-14 years had a lower CFR. When the average viremia increased 10-fold in July 2014, CFR increased as well, by 14%.
Study authors cautioned that their findings may not translate to cases outside the hospital setting, but that knowledge of the relationship between viremia levels and CFR could help clinicians more accurately assess the efficacy of treatments for EVD in nonrandomized trials.
“This finding suggests that heterogeneity in historical CFR estimates among patients, [Ebola treatment centers], and over time may at least partly be explained by variations in viremia and underscores that more valid estimates of the influence of other factors, including treatment effects, might be obtained by adjusting for differing levels of viremia among patients,” the authors wrote.
The study was funded by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases,” the U.S. National Institute of General Medical Sciences MIDAS (Models of Infectious Disease Agent Study) initiative, the AXA Research Fund, and the Seventh Framework Programme of the European Union. The authors declared no conflicts of interest.
Read the entire study on the PLOS Medicine website.
On Twitter @richpizzi
The levels of virus present in the blood for patients with Ebola virus disease (EVD) are strong predictors of fatality, according to a new study in PLOS Medicine.
A research team at the Pasteur Institute in Paris and Dakar, Senegal, analyzed the laboratory and epidemiologic records of 699 patients with EVD to determine the association between viremia and case fatality ratio (CFR). The EVD diagnoses of patients hospitalized in the Conakry area, Guinea, between March 1, 2014, and Feb. 28, 2015, were confirmed by reverse transcription polymerase chain reaction. Researchers discovered that, in the week following EVD symptom onset, patient viremia remained stable, and that the CFR increased with level of viremia.
The CFR for patients with low-, intermediate-, and high viremia was 21%, 53%, and 81%, respectively. Compared with adults aged 15-44 years, children younger than 5 years and adults aged 45 years and older had higher CFR, but children aged 5-14 years had a lower CFR. When the average viremia increased 10-fold in July 2014, CFR increased as well, by 14%.
Study authors cautioned that their findings may not translate to cases outside the hospital setting, but that knowledge of the relationship between viremia levels and CFR could help clinicians more accurately assess the efficacy of treatments for EVD in nonrandomized trials.
“This finding suggests that heterogeneity in historical CFR estimates among patients, [Ebola treatment centers], and over time may at least partly be explained by variations in viremia and underscores that more valid estimates of the influence of other factors, including treatment effects, might be obtained by adjusting for differing levels of viremia among patients,” the authors wrote.
The study was funded by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases,” the U.S. National Institute of General Medical Sciences MIDAS (Models of Infectious Disease Agent Study) initiative, the AXA Research Fund, and the Seventh Framework Programme of the European Union. The authors declared no conflicts of interest.
Read the entire study on the PLOS Medicine website.
On Twitter @richpizzi
FROM PLOS MEDICINE
Study questions efficacy of NRTIs in HIV salvage therapy
HIV-infected patients in whom antiretroviral therapy has failed are not benefited by treatment regimens that include nucleotide reverse transcriptase inhibitors (NRTIs), as the drugs may not contribute to virologic suppression, a new study reports.
The study, published online in the Annals of Internal Medicine, suggests that treatment-experienced HIV-infected patients (those who have used antiretroviral drugs) may safely omit NRTIs from their drug regimens, which appears to contradict standard of care. However, this new research indicates that, because treatment-experienced patients have HIV isolates with mutations that significantly compromise NRTI activity, a treatment regimen omitting NRTIs is a real option if a new optimized regimen contains several fully or partially active antiretroviral medications with a continuous phenotypic susceptibility score greater than 2.0; the continuous phenotypic susceptibility score is a research measure of retroviral activity (Ann Intern Med. 2015 Nov;163. doi: 10.7326/M15-0949).
The study, called the OPTIONS (Optimized Treatment That Includes or Omits NRTIs) trial, was a multicenter, randomized, controlled trial in patients for whom current proteinase inhibitor–based therapy that included NRTIs had failed. Researchers, led by Dr. Karen Tashima of the Miriam Hospital and the Alpert Medical School of Brown University, both in Providence, R.I., compared treatment success between 360 HIV-infected patients who omitted versus added NRTIs with an optimized antiretroviral regimen of three or more agents.
A total of 93% of the randomly assigned participants completed a 48-week visit. The cumulative probability of regimen failure was 29.8% in the omit-NRTIs group vs. 25.9% in the add-NRTIs group (difference, 3.2 percentage points; 95% confidence interval, 6.1-12.5 percentage points). No significant between-group differences were found in the primary safety end points or the proportion of participants with HIV RNA level less than 50 copies/mL. No deaths occurred in the omit-NRTIs group, compared with seven deaths in the add-NRTIs group.
“The potential benefits of omitting NRTIs include reduced pill burden, reduced cost, and, probably, a decrease in NRTI-associated toxicity over the long term,” said Dr. Tashima and her coauthors. “These results have been incorporated in recent antiretroviral guideline recommendations for treatment-experienced patients.”
The study was funded by the National Institute of Allergy and Infectious Diseases, Boehringer Ingelheim, Janssen, Merck, ViiV Healthcare, Roche, and Monogram Biosciences.
Read the entire study on the Annals of Internal Medicine website.
On Twitter @richpizzi
HIV-infected patients in whom antiretroviral therapy has failed are not benefited by treatment regimens that include nucleotide reverse transcriptase inhibitors (NRTIs), as the drugs may not contribute to virologic suppression, a new study reports.
The study, published online in the Annals of Internal Medicine, suggests that treatment-experienced HIV-infected patients (those who have used antiretroviral drugs) may safely omit NRTIs from their drug regimens, which appears to contradict standard of care. However, this new research indicates that, because treatment-experienced patients have HIV isolates with mutations that significantly compromise NRTI activity, a treatment regimen omitting NRTIs is a real option if a new optimized regimen contains several fully or partially active antiretroviral medications with a continuous phenotypic susceptibility score greater than 2.0; the continuous phenotypic susceptibility score is a research measure of retroviral activity (Ann Intern Med. 2015 Nov;163. doi: 10.7326/M15-0949).
The study, called the OPTIONS (Optimized Treatment That Includes or Omits NRTIs) trial, was a multicenter, randomized, controlled trial in patients for whom current proteinase inhibitor–based therapy that included NRTIs had failed. Researchers, led by Dr. Karen Tashima of the Miriam Hospital and the Alpert Medical School of Brown University, both in Providence, R.I., compared treatment success between 360 HIV-infected patients who omitted versus added NRTIs with an optimized antiretroviral regimen of three or more agents.
A total of 93% of the randomly assigned participants completed a 48-week visit. The cumulative probability of regimen failure was 29.8% in the omit-NRTIs group vs. 25.9% in the add-NRTIs group (difference, 3.2 percentage points; 95% confidence interval, 6.1-12.5 percentage points). No significant between-group differences were found in the primary safety end points or the proportion of participants with HIV RNA level less than 50 copies/mL. No deaths occurred in the omit-NRTIs group, compared with seven deaths in the add-NRTIs group.
“The potential benefits of omitting NRTIs include reduced pill burden, reduced cost, and, probably, a decrease in NRTI-associated toxicity over the long term,” said Dr. Tashima and her coauthors. “These results have been incorporated in recent antiretroviral guideline recommendations for treatment-experienced patients.”
The study was funded by the National Institute of Allergy and Infectious Diseases, Boehringer Ingelheim, Janssen, Merck, ViiV Healthcare, Roche, and Monogram Biosciences.
Read the entire study on the Annals of Internal Medicine website.
On Twitter @richpizzi
HIV-infected patients in whom antiretroviral therapy has failed are not benefited by treatment regimens that include nucleotide reverse transcriptase inhibitors (NRTIs), as the drugs may not contribute to virologic suppression, a new study reports.
The study, published online in the Annals of Internal Medicine, suggests that treatment-experienced HIV-infected patients (those who have used antiretroviral drugs) may safely omit NRTIs from their drug regimens, which appears to contradict standard of care. However, this new research indicates that, because treatment-experienced patients have HIV isolates with mutations that significantly compromise NRTI activity, a treatment regimen omitting NRTIs is a real option if a new optimized regimen contains several fully or partially active antiretroviral medications with a continuous phenotypic susceptibility score greater than 2.0; the continuous phenotypic susceptibility score is a research measure of retroviral activity (Ann Intern Med. 2015 Nov;163. doi: 10.7326/M15-0949).
The study, called the OPTIONS (Optimized Treatment That Includes or Omits NRTIs) trial, was a multicenter, randomized, controlled trial in patients for whom current proteinase inhibitor–based therapy that included NRTIs had failed. Researchers, led by Dr. Karen Tashima of the Miriam Hospital and the Alpert Medical School of Brown University, both in Providence, R.I., compared treatment success between 360 HIV-infected patients who omitted versus added NRTIs with an optimized antiretroviral regimen of three or more agents.
A total of 93% of the randomly assigned participants completed a 48-week visit. The cumulative probability of regimen failure was 29.8% in the omit-NRTIs group vs. 25.9% in the add-NRTIs group (difference, 3.2 percentage points; 95% confidence interval, 6.1-12.5 percentage points). No significant between-group differences were found in the primary safety end points or the proportion of participants with HIV RNA level less than 50 copies/mL. No deaths occurred in the omit-NRTIs group, compared with seven deaths in the add-NRTIs group.
“The potential benefits of omitting NRTIs include reduced pill burden, reduced cost, and, probably, a decrease in NRTI-associated toxicity over the long term,” said Dr. Tashima and her coauthors. “These results have been incorporated in recent antiretroviral guideline recommendations for treatment-experienced patients.”
The study was funded by the National Institute of Allergy and Infectious Diseases, Boehringer Ingelheim, Janssen, Merck, ViiV Healthcare, Roche, and Monogram Biosciences.
Read the entire study on the Annals of Internal Medicine website.
On Twitter @richpizzi
FROM ANNALS OF INTERNAL MEDICINE
U.S. hospitalization rates overestimated for some causes
Patient hospitalization rates based on discharge data that include repeat hospitalizations may overestimate disease incidence in individuals, particularly for conditions with a high proportion of repeat hospitalizations, a new study found.
Stephanie Benjamin, Ph.D., of the department of health sciences at California State University, Northridge, and her associates said that this is the first study to quantify the overestimation of hospitalization rates of individuals for common diabetes-related causes from the inclusion of repeat hospitalizations, and to determine whether this in turn affects comparisons of diabetes to nondiabetes rates. The results of the study, published online in Preventing Chronic Diseases, suggest that the use of repeat hospitalizations substantially overestimated hospitalization rates for both the diabetic and nondiabetic populations and that this overestimation varied by cause (2015 Nov 19;12:150274 ).
The research team analyzed 2011 hospitalization data for adults aged 18 years or older from the State Inpatient Databases of the Agency for Healthcare Research and Quality. The researchers examined data (a total of 10,384,306 hospital discharges) from 12 states (Arkansas, California, Florida, Hawaii, Iowa, Massachusetts, Mississippi, Nebraska, New Mexico, New York, Vermont, and Washington) whose discharge data distinguished repeat hospitalizations among individuals. They then calculated percentage increases from repeat hospitalizations in rates and compared the ratio of diabetes with nondiabetes rates while excluding and including repeat hospitalizations.
Regardless of diabetes status, hospitalization rates were considerably higher when repeat hospitalizations within a calendar year were included. The magnitude of the differences varied by condition, Dr. Benjamin and her associates found. Among adults with diabetes, rates ranged from 13.0% higher for stroke to 41.6% higher for heart failure; for adults without diabetes, these rates ranged from 9.5% higher for stroke to 25.2% higher for heart failure. Ratios of diabetes versus nondiabetes rates were similar with and without repeat hospitalizations.
While the study’s findings may not be generalizable to the national population or any one region of the country, Dr. Benjamin said, the impact of repeat hospitalizations on rates and rate ratios were consistent across all 12 states, suggesting that the relationships described “may be robust, especially because the 12 states evaluated were distributed across the country.”
Dr. Benjamin and her coauthors suggest that, when such data are used to examine trends, consideration should be given to the possible impact of any change in frequency of repeat hospitalizations. For example, successful efforts to reduce 30-day readmission rates could reduce repeat hospitalizations without reducing disease incidence rates among individuals.
Read the entire study at the PCD website.
On Twitter @richpizzi
Patient hospitalization rates based on discharge data that include repeat hospitalizations may overestimate disease incidence in individuals, particularly for conditions with a high proportion of repeat hospitalizations, a new study found.
Stephanie Benjamin, Ph.D., of the department of health sciences at California State University, Northridge, and her associates said that this is the first study to quantify the overestimation of hospitalization rates of individuals for common diabetes-related causes from the inclusion of repeat hospitalizations, and to determine whether this in turn affects comparisons of diabetes to nondiabetes rates. The results of the study, published online in Preventing Chronic Diseases, suggest that the use of repeat hospitalizations substantially overestimated hospitalization rates for both the diabetic and nondiabetic populations and that this overestimation varied by cause (2015 Nov 19;12:150274 ).
The research team analyzed 2011 hospitalization data for adults aged 18 years or older from the State Inpatient Databases of the Agency for Healthcare Research and Quality. The researchers examined data (a total of 10,384,306 hospital discharges) from 12 states (Arkansas, California, Florida, Hawaii, Iowa, Massachusetts, Mississippi, Nebraska, New Mexico, New York, Vermont, and Washington) whose discharge data distinguished repeat hospitalizations among individuals. They then calculated percentage increases from repeat hospitalizations in rates and compared the ratio of diabetes with nondiabetes rates while excluding and including repeat hospitalizations.
Regardless of diabetes status, hospitalization rates were considerably higher when repeat hospitalizations within a calendar year were included. The magnitude of the differences varied by condition, Dr. Benjamin and her associates found. Among adults with diabetes, rates ranged from 13.0% higher for stroke to 41.6% higher for heart failure; for adults without diabetes, these rates ranged from 9.5% higher for stroke to 25.2% higher for heart failure. Ratios of diabetes versus nondiabetes rates were similar with and without repeat hospitalizations.
While the study’s findings may not be generalizable to the national population or any one region of the country, Dr. Benjamin said, the impact of repeat hospitalizations on rates and rate ratios were consistent across all 12 states, suggesting that the relationships described “may be robust, especially because the 12 states evaluated were distributed across the country.”
Dr. Benjamin and her coauthors suggest that, when such data are used to examine trends, consideration should be given to the possible impact of any change in frequency of repeat hospitalizations. For example, successful efforts to reduce 30-day readmission rates could reduce repeat hospitalizations without reducing disease incidence rates among individuals.
Read the entire study at the PCD website.
On Twitter @richpizzi
Patient hospitalization rates based on discharge data that include repeat hospitalizations may overestimate disease incidence in individuals, particularly for conditions with a high proportion of repeat hospitalizations, a new study found.
Stephanie Benjamin, Ph.D., of the department of health sciences at California State University, Northridge, and her associates said that this is the first study to quantify the overestimation of hospitalization rates of individuals for common diabetes-related causes from the inclusion of repeat hospitalizations, and to determine whether this in turn affects comparisons of diabetes to nondiabetes rates. The results of the study, published online in Preventing Chronic Diseases, suggest that the use of repeat hospitalizations substantially overestimated hospitalization rates for both the diabetic and nondiabetic populations and that this overestimation varied by cause (2015 Nov 19;12:150274 ).
The research team analyzed 2011 hospitalization data for adults aged 18 years or older from the State Inpatient Databases of the Agency for Healthcare Research and Quality. The researchers examined data (a total of 10,384,306 hospital discharges) from 12 states (Arkansas, California, Florida, Hawaii, Iowa, Massachusetts, Mississippi, Nebraska, New Mexico, New York, Vermont, and Washington) whose discharge data distinguished repeat hospitalizations among individuals. They then calculated percentage increases from repeat hospitalizations in rates and compared the ratio of diabetes with nondiabetes rates while excluding and including repeat hospitalizations.
Regardless of diabetes status, hospitalization rates were considerably higher when repeat hospitalizations within a calendar year were included. The magnitude of the differences varied by condition, Dr. Benjamin and her associates found. Among adults with diabetes, rates ranged from 13.0% higher for stroke to 41.6% higher for heart failure; for adults without diabetes, these rates ranged from 9.5% higher for stroke to 25.2% higher for heart failure. Ratios of diabetes versus nondiabetes rates were similar with and without repeat hospitalizations.
While the study’s findings may not be generalizable to the national population or any one region of the country, Dr. Benjamin said, the impact of repeat hospitalizations on rates and rate ratios were consistent across all 12 states, suggesting that the relationships described “may be robust, especially because the 12 states evaluated were distributed across the country.”
Dr. Benjamin and her coauthors suggest that, when such data are used to examine trends, consideration should be given to the possible impact of any change in frequency of repeat hospitalizations. For example, successful efforts to reduce 30-day readmission rates could reduce repeat hospitalizations without reducing disease incidence rates among individuals.
Read the entire study at the PCD website.
On Twitter @richpizzi
FROM PREVENTING CHRONIC DISEASES
CDC encourages improved antibiotic stewardship
The Centers for Disease Control and Prevention is highlighting a series of new and ongoing initiatives to combat antibiotic resistance this week, officially designated “Get Smart About Antibiotics Week” by President Barack Obama.
The CDC estimates that 2 million Americans are infected with an antibiotic-resistant bacteria annually and that 23,000 of those patients die. The agency has made combating antibiotic resistance a top priority.
“Antibiotic resistance is one of the deadliest health threats facing the world,” CDC Director Tom Frieden said in a statement. “These pledges will help protect the antibiotics we have so we can use these miracle drugs to save lives for years to come.”
The CDC is tracking antibiotic use and the spread of antibiotic-resistant infections in the United States and exploring new ways to stop the rise of antibiotic resistance. The agency is using data to identify hotspots in need of attention, and the CDC-run isolate bank assists industry in developing new antibiotics and rapid diagnostic tests, contributing to the global effort to combat antibiotic resistance.
The CDC is highlighting a number of projects this week to promote antibiotic stewardship:
Ascension Health launches stewardship initiative: Ascension, the largest nonprofit health system in the United States, is creating stewardship programs throughout its care sites. A new center of excellence will focus on antimicrobial stewardship efforts system-wide.
Hospital Corporation of America: HCA joined with the CDC to track antibiotic prescriptions in HCA facilities by automatically collecting and reporting monthly antibiotic-use data using CDC’s National Healthcare Safety Network. Data can be analyzed and fed back to caregivers to guide patient-care decisions.
Premier Safety Institute: First launch of a collaborative of more than 50 hospitals working to implement CDC Core Elements of an Antibiotic Stewardship Programs as well as reducing the overuse of specific antimicrobials that were identified in research conducted by Premier and in collaboration with the CDC.
Walmart public service announcement (PSA) on appropriate antibiotic use: Walmart created educational videos for checkout lines across the country so that customers get clear information on antibiotic resistance and what they can do to improve antibiotic use.
Major airlines run in-flight PSA: State health departments are partnering to improve educational awareness about antibiotic stewardship. An in-flight PSA about antibiotic stewardship, produced by the Michigan Antibiotic Resistance Reduction (MARR) Coalition, is now featured on Jet Blue and other airlines.
Pew Charitable Trust briefing on Capitol Hill: A Pew coalition of “Supermoms against superbugs” will join the Pew Charitable Trust and CDC director Dr. Frieden at a Capitol Hill briefing on Nov. 18, 2015. Pew also is partnering with the CDC to establish national targets to improve the use of antibiotics in support of the goals outlined in the National Action Plan on Combating Antibiotic-Resistant Bacteria.
Consumer Reports: The CDC is partnering with Consumer Reports and the American Board of Internal Medicine Foundation in support of the Choosing Wisely campaign.
U.S. State Department toolkit: The State Department is piloting a toolkit for use by 10 U.S. embassies with a focus on improving antibiotic use.
Society for Hospital Medicine’s educational campaign for hospitalists: The society’s antibiotic stewardship campaign targets hospitalists – an important group for improving antibiotic use.
Global Twitter chat: Hosted by the European Union’s Antibiotic Awareness Day (Nov. 18, 2015), the 24-hour chat will use the hashtag #antibioticresistance and unite CDC experts and partners in a global conversation about antibiotic resistance. CDC experts will lead the conversation from 2 p.m. to 4 p.m. ET.
The latest U.S. antibiotic prescribing rate map: Although overuse of antibiotics is happening in every state across the country, community antibiotic prescribing rates in some states are two times greater than in other states, suggesting opportunities for improvement.
U.S. hospital stewardship programs map: The percentage of hospitals reported by states as having antibiotic stewardship programs that follow all seven of CDC Core Elements of Antibiotic Stewardship Programs varies from state to state from a low of 7% to a high of 58%. The national goal is for 100% of all U.S. hospitals to have antibiotic stewardship programs by 2020.
On Twitter @richpizzi
The Centers for Disease Control and Prevention is highlighting a series of new and ongoing initiatives to combat antibiotic resistance this week, officially designated “Get Smart About Antibiotics Week” by President Barack Obama.
The CDC estimates that 2 million Americans are infected with an antibiotic-resistant bacteria annually and that 23,000 of those patients die. The agency has made combating antibiotic resistance a top priority.
“Antibiotic resistance is one of the deadliest health threats facing the world,” CDC Director Tom Frieden said in a statement. “These pledges will help protect the antibiotics we have so we can use these miracle drugs to save lives for years to come.”
The CDC is tracking antibiotic use and the spread of antibiotic-resistant infections in the United States and exploring new ways to stop the rise of antibiotic resistance. The agency is using data to identify hotspots in need of attention, and the CDC-run isolate bank assists industry in developing new antibiotics and rapid diagnostic tests, contributing to the global effort to combat antibiotic resistance.
The CDC is highlighting a number of projects this week to promote antibiotic stewardship:
Ascension Health launches stewardship initiative: Ascension, the largest nonprofit health system in the United States, is creating stewardship programs throughout its care sites. A new center of excellence will focus on antimicrobial stewardship efforts system-wide.
Hospital Corporation of America: HCA joined with the CDC to track antibiotic prescriptions in HCA facilities by automatically collecting and reporting monthly antibiotic-use data using CDC’s National Healthcare Safety Network. Data can be analyzed and fed back to caregivers to guide patient-care decisions.
Premier Safety Institute: First launch of a collaborative of more than 50 hospitals working to implement CDC Core Elements of an Antibiotic Stewardship Programs as well as reducing the overuse of specific antimicrobials that were identified in research conducted by Premier and in collaboration with the CDC.
Walmart public service announcement (PSA) on appropriate antibiotic use: Walmart created educational videos for checkout lines across the country so that customers get clear information on antibiotic resistance and what they can do to improve antibiotic use.
Major airlines run in-flight PSA: State health departments are partnering to improve educational awareness about antibiotic stewardship. An in-flight PSA about antibiotic stewardship, produced by the Michigan Antibiotic Resistance Reduction (MARR) Coalition, is now featured on Jet Blue and other airlines.
Pew Charitable Trust briefing on Capitol Hill: A Pew coalition of “Supermoms against superbugs” will join the Pew Charitable Trust and CDC director Dr. Frieden at a Capitol Hill briefing on Nov. 18, 2015. Pew also is partnering with the CDC to establish national targets to improve the use of antibiotics in support of the goals outlined in the National Action Plan on Combating Antibiotic-Resistant Bacteria.
Consumer Reports: The CDC is partnering with Consumer Reports and the American Board of Internal Medicine Foundation in support of the Choosing Wisely campaign.
U.S. State Department toolkit: The State Department is piloting a toolkit for use by 10 U.S. embassies with a focus on improving antibiotic use.
Society for Hospital Medicine’s educational campaign for hospitalists: The society’s antibiotic stewardship campaign targets hospitalists – an important group for improving antibiotic use.
Global Twitter chat: Hosted by the European Union’s Antibiotic Awareness Day (Nov. 18, 2015), the 24-hour chat will use the hashtag #antibioticresistance and unite CDC experts and partners in a global conversation about antibiotic resistance. CDC experts will lead the conversation from 2 p.m. to 4 p.m. ET.
The latest U.S. antibiotic prescribing rate map: Although overuse of antibiotics is happening in every state across the country, community antibiotic prescribing rates in some states are two times greater than in other states, suggesting opportunities for improvement.
U.S. hospital stewardship programs map: The percentage of hospitals reported by states as having antibiotic stewardship programs that follow all seven of CDC Core Elements of Antibiotic Stewardship Programs varies from state to state from a low of 7% to a high of 58%. The national goal is for 100% of all U.S. hospitals to have antibiotic stewardship programs by 2020.
On Twitter @richpizzi
The Centers for Disease Control and Prevention is highlighting a series of new and ongoing initiatives to combat antibiotic resistance this week, officially designated “Get Smart About Antibiotics Week” by President Barack Obama.
The CDC estimates that 2 million Americans are infected with an antibiotic-resistant bacteria annually and that 23,000 of those patients die. The agency has made combating antibiotic resistance a top priority.
“Antibiotic resistance is one of the deadliest health threats facing the world,” CDC Director Tom Frieden said in a statement. “These pledges will help protect the antibiotics we have so we can use these miracle drugs to save lives for years to come.”
The CDC is tracking antibiotic use and the spread of antibiotic-resistant infections in the United States and exploring new ways to stop the rise of antibiotic resistance. The agency is using data to identify hotspots in need of attention, and the CDC-run isolate bank assists industry in developing new antibiotics and rapid diagnostic tests, contributing to the global effort to combat antibiotic resistance.
The CDC is highlighting a number of projects this week to promote antibiotic stewardship:
Ascension Health launches stewardship initiative: Ascension, the largest nonprofit health system in the United States, is creating stewardship programs throughout its care sites. A new center of excellence will focus on antimicrobial stewardship efforts system-wide.
Hospital Corporation of America: HCA joined with the CDC to track antibiotic prescriptions in HCA facilities by automatically collecting and reporting monthly antibiotic-use data using CDC’s National Healthcare Safety Network. Data can be analyzed and fed back to caregivers to guide patient-care decisions.
Premier Safety Institute: First launch of a collaborative of more than 50 hospitals working to implement CDC Core Elements of an Antibiotic Stewardship Programs as well as reducing the overuse of specific antimicrobials that were identified in research conducted by Premier and in collaboration with the CDC.
Walmart public service announcement (PSA) on appropriate antibiotic use: Walmart created educational videos for checkout lines across the country so that customers get clear information on antibiotic resistance and what they can do to improve antibiotic use.
Major airlines run in-flight PSA: State health departments are partnering to improve educational awareness about antibiotic stewardship. An in-flight PSA about antibiotic stewardship, produced by the Michigan Antibiotic Resistance Reduction (MARR) Coalition, is now featured on Jet Blue and other airlines.
Pew Charitable Trust briefing on Capitol Hill: A Pew coalition of “Supermoms against superbugs” will join the Pew Charitable Trust and CDC director Dr. Frieden at a Capitol Hill briefing on Nov. 18, 2015. Pew also is partnering with the CDC to establish national targets to improve the use of antibiotics in support of the goals outlined in the National Action Plan on Combating Antibiotic-Resistant Bacteria.
Consumer Reports: The CDC is partnering with Consumer Reports and the American Board of Internal Medicine Foundation in support of the Choosing Wisely campaign.
U.S. State Department toolkit: The State Department is piloting a toolkit for use by 10 U.S. embassies with a focus on improving antibiotic use.
Society for Hospital Medicine’s educational campaign for hospitalists: The society’s antibiotic stewardship campaign targets hospitalists – an important group for improving antibiotic use.
Global Twitter chat: Hosted by the European Union’s Antibiotic Awareness Day (Nov. 18, 2015), the 24-hour chat will use the hashtag #antibioticresistance and unite CDC experts and partners in a global conversation about antibiotic resistance. CDC experts will lead the conversation from 2 p.m. to 4 p.m. ET.
The latest U.S. antibiotic prescribing rate map: Although overuse of antibiotics is happening in every state across the country, community antibiotic prescribing rates in some states are two times greater than in other states, suggesting opportunities for improvement.
U.S. hospital stewardship programs map: The percentage of hospitals reported by states as having antibiotic stewardship programs that follow all seven of CDC Core Elements of Antibiotic Stewardship Programs varies from state to state from a low of 7% to a high of 58%. The national goal is for 100% of all U.S. hospitals to have antibiotic stewardship programs by 2020.
On Twitter @richpizzi