User login
Prioritize COVID-19 vaccination in both types of diabetes, say docs
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Noninvasive, low-cost CGM for type 2 diabetes coming in U.S. and EU
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
A novel lower-cost noninvasive continuous glucose monitor (CGM) combined with a digital education/guidance program is set to launch in the United States and Europe this month for use in type 2 diabetes.
With the goal of improving management, or even reversing the condition, Neumara’s SugarBEAT device is thought to be the world’s first noninvasive CGM.
Its cost is anticipated to be far lower than traditional CGM, and it’s aimed at a different patient population: those with type 2 diabetes or prediabetes who may or may not be performing fingerstick glucose monitoring, but if they are, they still aren’t using the information to guide management.
“This isn’t about handing out devices and letting patients get on about it on their own accord. This is really about supporting those individuals,” Faz Chowdhury, MD, Nemaura’s chief executive officer, said in an interview.
He pointed to studies showing improvements in glycemic control in patients with type 2 diabetes who were instructed to perform fingerstick blood glucose testing seven times a day for 3-4 days a month and given advice about how to respond to the data.
“This is well established. We’re saying we can make that process a lot more scalable and affordable and convenient for the patient. ... The behavior change side is digitized,” Dr. Chowdhury said. “We want to provide a program to help people reverse their diabetes or at least stabilize it as much as possible.”
Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, said in an interview: “It’s interesting. They’re taking a very different approach. I think there’s a lot of validity to what they’re looking at because we have great CGMs right now, but because of the price point it’s not accessible to a lot of people.
“I think they’re onto something that could prove to be useful to a larger group of patients,” he added.
Worn a few days per month and accurate despite being noninvasive
Instead of inserting a catheter under the skin with a needle, as do current CGMs, the device comprises a small rechargeable transmitter and adhesive patch with a sensor that sits on the top of the skin, typically the upper arm. Glucose molecules are drawn out of the interstitial fluid just below the skin and into a chamber where the transmitter measures the glucose level and transmits the data every 5 minutes via Bluetooth to a smartphone app.
Despite this noninvasive approach, the device appears to be about as accurate as traditional CGMs, with comparable mean absolute relative difference (MARD) from a gold standard glucose measure of about 11%-12% with once-daily calibration versus 10%-11% for the Abbott FreeStyle Libre.
Unlike traditional CGMs, SugarBEAT is meant to be worn for only 14 hours at a time during the day and for 2-4 days per month rather than every day.
It’s not aimed at patients with type 1 diabetes or those with type 2 diabetes who are at high risk for hypoglycemia. It requires once-daily fingerstick calibration and is not indicated to replace fingersticks for treatment decisions.
SugarBEAT received a CE Mark in Europe as a Class IIb medical device in May 2019. That version provides real-time glucose values visible to the wearer. In the United States the company submitted a premarketing approval application for the device to the Food and Drug Administration in July 2020, which awaits a decision.
However, FDA is allowing it to enter the U.S. market as a “wellness” device that won’t deliver real-time values for now but instead will generate retroactive reports available to the physician and the patient.
And last month, U.K.-based Neumara launched the BEATdiabetes site, which allows users to sign in and link to the device once it becomes available.
The site provides “scientifically validated, personalized coaching” based on a program developed at the Joslin Diabetes Clinic in Syracuse, N.Y., and will ultimately include monitoring of other cardiovascular risk factors with digital connectivity to a variety of wearables.
Fingerstick monitoring in type 2 diabetes is only so useful
“Fingerstick monitoring for type 2 diabetes is only so useful,” Dr. Argento said in an interview.
“It’s difficult to get people to monitor in a meaningful way.” If patients perform them only in the morning or at other sporadic times of the day, he said, “Then you get a one-dimensional picture ... and they don’t know what to do with the information anyway, so they stop doing it.”
In contrast, with SugarBEAT and BEATDiabetes, “I think it does address a need that fingerstick monitoring doesn’t.”
Dr. Argento did express a few caveats about the device, however. For one, it still requires one fingerstick a day for calibration. “If people don’t like needles, that might be a disincentive.”
Also, despite the apparently comparable mean absolute relative difference with that of conventional CGMs, that measure can still “hide” values that may be consistently either above or below target range.
“MARD is like A1c in that it’s useful but limited. ... It doesn’t tell you about variability or systemic bias,” he said.
Dr. Argento also said that he’d like to see data on the lag time between the interstitial fluid and blood glucose measures with this noninvasive method as compared with that of a subcutaneous catheter.
However, he acknowledged that these potentials for error would be less important for patients with type 2 diabetes who aren’t generally taking medications that increase their risk for hypoglycemia.
In all, he said, “stay tuned. I think this is part of a movement going away from point-in-time to looking at trends and wearables and data to enrich decision-making…There are still some unanswered questions I have but I think they’re onto a concept that’s useful for a broader population.”
Dr. Chowdhury is an employee of Neumara. Dr. Argento consults for Senseonics and Dexcom, and is also a speaker for Dexcom.
This article first appeared on Medscape.com.
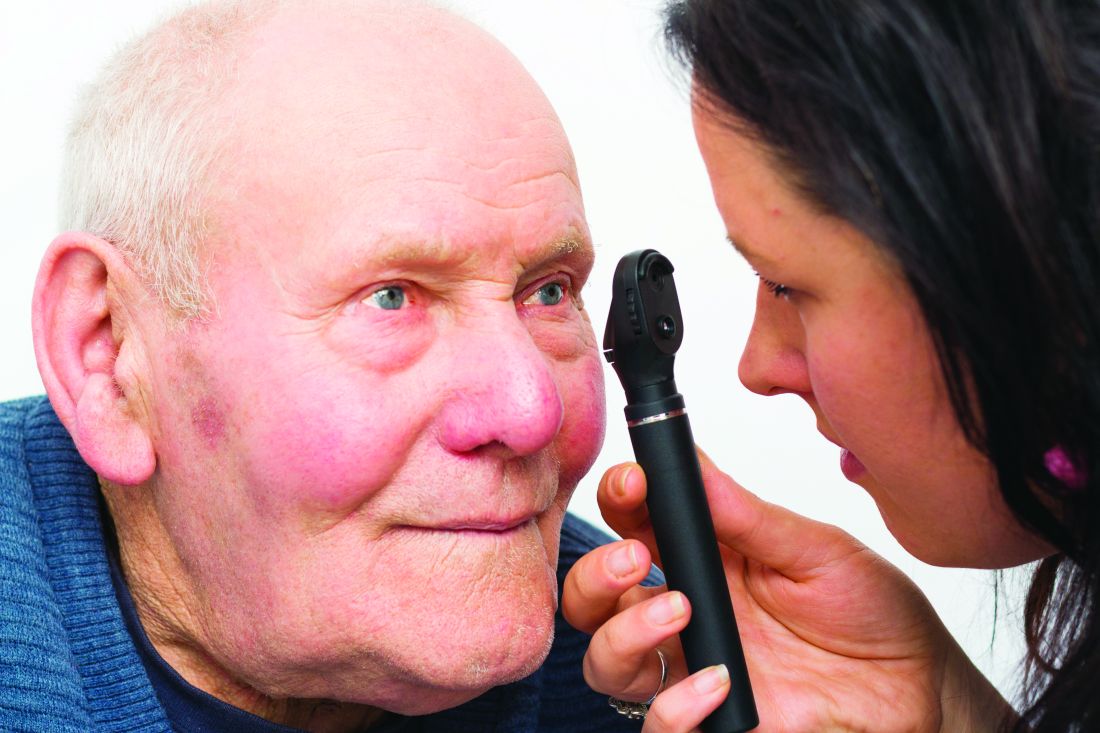
Blood glucose on admission predicts COVID-19 severity in all
Hyperglycemia at hospital admission – regardless of diabetes status – is a key predictor of COVID-19-related death and severity among noncritical patients, new research from Spain finds.
The observational study, the largest to date to investigate this association, was published online Nov. 23 in Annals of Medicine by Francisco Javier Carrasco-Sánchez, MD, PhD, and colleagues.
Among more than 11,000 patients with confirmed COVID-19 from March to May 2020 in a nationwide Spanish registry involving 109 hospitals, admission hyperglycemia independently predicted progression from noncritical to critical condition and death, regardless of prior diabetes history.
Those with abnormally high glucose levels were more than twice as likely to die from the virus than those with normal readings (41.4% vs 15.7%). They also had an increased need for a ventilator and intensive care unit (ICU) admission.
“These results provided a simple and practical way to stratify risk of death in hospitalized patients with COVID-19. Hence, admission hyperglycemia should not be overlooked, but rather detected and appropriately treated to improve the outcomes of COVID-19 patients with and without diabetes,” Dr. Carrasco-Sánchez and colleagues wrote.
The findings confirm those of previous retrospective observational studies, but the current study “has, by far, the biggest number of patients involved in this kind of study [to date]. All conclusions are consistent to other studies,” Dr. Carrasco-Sánchez, of University Hospital Juan Ramón Jiménez, Huelva, Spain, said in an interview.
However, a surprising finding, he said, “was how hyperglycemia works in the nondiabetic population and [that] glucose levels over 140 [mg/dL] ... increase the risk of death.”
Pay attention to even mild hyperglycemia from admission
The study also differs from some of the prior observational ones in that it examines outcome by admission glycemia rather than during the hospital stay, therefore eliminating the effect of any inpatient treatment, such as dexamethasone, he noted.
Although blood glucose measurement at admission is routine for all patients in Spain, as it is in the United States and elsewhere, a mildly elevated level in a person without a diagnosis of diabetes may not be recognized as important.
“In patients with diabetes we start the protocol to control and treat hyperglycemia during hospitalization. However, in nondiabetic patients blood glucose levels under 180 [mg/dL], and even greater, are usually overlooked. This means there is not a correct follow-up of the patients during hospitalization.
“After this study we learned that we need to pay attention to this population ... who develop hyperglycemia from the beginning,” he said.
The study was limited in that patients who had previously undiagnosed diabetes couldn’t always be distinguished from those with acute “stress hyperglycemia.”
However, both need to be managed during hospitalization, he said. “Unfortunately, there is high variability in inpatient glucose management. The working group of diabetes of the Spanish Society of Internal Medicine is working on specific protocols,” said Dr. Carrasco-Sánchez.
All-cause death, progress to critical care higher with hyperglycemia
The retrospective, multicenter study was based on data from 11,312 adult patients with confirmed COVID-19 in 109 hospitals participating in Spain’s SEMI-COVID-19 registry as of May 29, 2020. They had a mean age of 67 years, 57% were male, and 19% had a diagnosis of diabetes. A total of 20% (n = 2,289) died during hospitalization.
Overall all-cause mortality was 41.1% among those with admission blood glucose levels above 180 mg/dL, 33.0% for those with glucose levels 140-180 mg/dL, and 15.7% for levels below 140 mg/dL. All differences were significant (P < .0001), but there were no differences in mortality rates within each blood glucose category between patients with or without a previous diagnosis of diabetes.
After adjustment for confounding factors, elevated admission blood glucose level remained a significant predictor of death. Compared to < 140 mg/dL, the hazard ratios for 140-180 mg/dL and > 180 mg/dL were 1.48 and 1.50, respectively (both P < .001). (Adjustments included age, gender, hypertension, diabetes, chronic obstructive pulmonary disease, lymphopenia, anemia (hemoglobin < 10 g/dL), serum creatinine, C-reactive protein > 60 mg/L, lactate dehydrogenase > 400 U/L and D-dimer >1000 ng/mL.)
Length of stay was 12, 11.5, and 11.1 days for those with admission blood glucose levels > 180, 140-180, and < 140 mg/dL, respectively (P = .011).
Use of mechanical ventilation and admission to intensive care also rose with higher admission blood glucose levels. For the composite of death, mechanical ventilation, and/or ICU admission, odds ratios for 140-180 mg/dL and > 180 mg/dL compared with < 140 mg/dL were 1.70 and 2.02, respectively (both P < .001).
The study was supported by the Spanish Federation of Internal Medicine. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hyperglycemia at hospital admission – regardless of diabetes status – is a key predictor of COVID-19-related death and severity among noncritical patients, new research from Spain finds.
The observational study, the largest to date to investigate this association, was published online Nov. 23 in Annals of Medicine by Francisco Javier Carrasco-Sánchez, MD, PhD, and colleagues.
Among more than 11,000 patients with confirmed COVID-19 from March to May 2020 in a nationwide Spanish registry involving 109 hospitals, admission hyperglycemia independently predicted progression from noncritical to critical condition and death, regardless of prior diabetes history.
Those with abnormally high glucose levels were more than twice as likely to die from the virus than those with normal readings (41.4% vs 15.7%). They also had an increased need for a ventilator and intensive care unit (ICU) admission.
“These results provided a simple and practical way to stratify risk of death in hospitalized patients with COVID-19. Hence, admission hyperglycemia should not be overlooked, but rather detected and appropriately treated to improve the outcomes of COVID-19 patients with and without diabetes,” Dr. Carrasco-Sánchez and colleagues wrote.
The findings confirm those of previous retrospective observational studies, but the current study “has, by far, the biggest number of patients involved in this kind of study [to date]. All conclusions are consistent to other studies,” Dr. Carrasco-Sánchez, of University Hospital Juan Ramón Jiménez, Huelva, Spain, said in an interview.
However, a surprising finding, he said, “was how hyperglycemia works in the nondiabetic population and [that] glucose levels over 140 [mg/dL] ... increase the risk of death.”
Pay attention to even mild hyperglycemia from admission
The study also differs from some of the prior observational ones in that it examines outcome by admission glycemia rather than during the hospital stay, therefore eliminating the effect of any inpatient treatment, such as dexamethasone, he noted.
Although blood glucose measurement at admission is routine for all patients in Spain, as it is in the United States and elsewhere, a mildly elevated level in a person without a diagnosis of diabetes may not be recognized as important.
“In patients with diabetes we start the protocol to control and treat hyperglycemia during hospitalization. However, in nondiabetic patients blood glucose levels under 180 [mg/dL], and even greater, are usually overlooked. This means there is not a correct follow-up of the patients during hospitalization.
“After this study we learned that we need to pay attention to this population ... who develop hyperglycemia from the beginning,” he said.
The study was limited in that patients who had previously undiagnosed diabetes couldn’t always be distinguished from those with acute “stress hyperglycemia.”
However, both need to be managed during hospitalization, he said. “Unfortunately, there is high variability in inpatient glucose management. The working group of diabetes of the Spanish Society of Internal Medicine is working on specific protocols,” said Dr. Carrasco-Sánchez.
All-cause death, progress to critical care higher with hyperglycemia
The retrospective, multicenter study was based on data from 11,312 adult patients with confirmed COVID-19 in 109 hospitals participating in Spain’s SEMI-COVID-19 registry as of May 29, 2020. They had a mean age of 67 years, 57% were male, and 19% had a diagnosis of diabetes. A total of 20% (n = 2,289) died during hospitalization.
Overall all-cause mortality was 41.1% among those with admission blood glucose levels above 180 mg/dL, 33.0% for those with glucose levels 140-180 mg/dL, and 15.7% for levels below 140 mg/dL. All differences were significant (P < .0001), but there were no differences in mortality rates within each blood glucose category between patients with or without a previous diagnosis of diabetes.
After adjustment for confounding factors, elevated admission blood glucose level remained a significant predictor of death. Compared to < 140 mg/dL, the hazard ratios for 140-180 mg/dL and > 180 mg/dL were 1.48 and 1.50, respectively (both P < .001). (Adjustments included age, gender, hypertension, diabetes, chronic obstructive pulmonary disease, lymphopenia, anemia (hemoglobin < 10 g/dL), serum creatinine, C-reactive protein > 60 mg/L, lactate dehydrogenase > 400 U/L and D-dimer >1000 ng/mL.)
Length of stay was 12, 11.5, and 11.1 days for those with admission blood glucose levels > 180, 140-180, and < 140 mg/dL, respectively (P = .011).
Use of mechanical ventilation and admission to intensive care also rose with higher admission blood glucose levels. For the composite of death, mechanical ventilation, and/or ICU admission, odds ratios for 140-180 mg/dL and > 180 mg/dL compared with < 140 mg/dL were 1.70 and 2.02, respectively (both P < .001).
The study was supported by the Spanish Federation of Internal Medicine. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hyperglycemia at hospital admission – regardless of diabetes status – is a key predictor of COVID-19-related death and severity among noncritical patients, new research from Spain finds.
The observational study, the largest to date to investigate this association, was published online Nov. 23 in Annals of Medicine by Francisco Javier Carrasco-Sánchez, MD, PhD, and colleagues.
Among more than 11,000 patients with confirmed COVID-19 from March to May 2020 in a nationwide Spanish registry involving 109 hospitals, admission hyperglycemia independently predicted progression from noncritical to critical condition and death, regardless of prior diabetes history.
Those with abnormally high glucose levels were more than twice as likely to die from the virus than those with normal readings (41.4% vs 15.7%). They also had an increased need for a ventilator and intensive care unit (ICU) admission.
“These results provided a simple and practical way to stratify risk of death in hospitalized patients with COVID-19. Hence, admission hyperglycemia should not be overlooked, but rather detected and appropriately treated to improve the outcomes of COVID-19 patients with and without diabetes,” Dr. Carrasco-Sánchez and colleagues wrote.
The findings confirm those of previous retrospective observational studies, but the current study “has, by far, the biggest number of patients involved in this kind of study [to date]. All conclusions are consistent to other studies,” Dr. Carrasco-Sánchez, of University Hospital Juan Ramón Jiménez, Huelva, Spain, said in an interview.
However, a surprising finding, he said, “was how hyperglycemia works in the nondiabetic population and [that] glucose levels over 140 [mg/dL] ... increase the risk of death.”
Pay attention to even mild hyperglycemia from admission
The study also differs from some of the prior observational ones in that it examines outcome by admission glycemia rather than during the hospital stay, therefore eliminating the effect of any inpatient treatment, such as dexamethasone, he noted.
Although blood glucose measurement at admission is routine for all patients in Spain, as it is in the United States and elsewhere, a mildly elevated level in a person without a diagnosis of diabetes may not be recognized as important.
“In patients with diabetes we start the protocol to control and treat hyperglycemia during hospitalization. However, in nondiabetic patients blood glucose levels under 180 [mg/dL], and even greater, are usually overlooked. This means there is not a correct follow-up of the patients during hospitalization.
“After this study we learned that we need to pay attention to this population ... who develop hyperglycemia from the beginning,” he said.
The study was limited in that patients who had previously undiagnosed diabetes couldn’t always be distinguished from those with acute “stress hyperglycemia.”
However, both need to be managed during hospitalization, he said. “Unfortunately, there is high variability in inpatient glucose management. The working group of diabetes of the Spanish Society of Internal Medicine is working on specific protocols,” said Dr. Carrasco-Sánchez.
All-cause death, progress to critical care higher with hyperglycemia
The retrospective, multicenter study was based on data from 11,312 adult patients with confirmed COVID-19 in 109 hospitals participating in Spain’s SEMI-COVID-19 registry as of May 29, 2020. They had a mean age of 67 years, 57% were male, and 19% had a diagnosis of diabetes. A total of 20% (n = 2,289) died during hospitalization.
Overall all-cause mortality was 41.1% among those with admission blood glucose levels above 180 mg/dL, 33.0% for those with glucose levels 140-180 mg/dL, and 15.7% for levels below 140 mg/dL. All differences were significant (P < .0001), but there were no differences in mortality rates within each blood glucose category between patients with or without a previous diagnosis of diabetes.
After adjustment for confounding factors, elevated admission blood glucose level remained a significant predictor of death. Compared to < 140 mg/dL, the hazard ratios for 140-180 mg/dL and > 180 mg/dL were 1.48 and 1.50, respectively (both P < .001). (Adjustments included age, gender, hypertension, diabetes, chronic obstructive pulmonary disease, lymphopenia, anemia (hemoglobin < 10 g/dL), serum creatinine, C-reactive protein > 60 mg/L, lactate dehydrogenase > 400 U/L and D-dimer >1000 ng/mL.)
Length of stay was 12, 11.5, and 11.1 days for those with admission blood glucose levels > 180, 140-180, and < 140 mg/dL, respectively (P = .011).
Use of mechanical ventilation and admission to intensive care also rose with higher admission blood glucose levels. For the composite of death, mechanical ventilation, and/or ICU admission, odds ratios for 140-180 mg/dL and > 180 mg/dL compared with < 140 mg/dL were 1.70 and 2.02, respectively (both P < .001).
The study was supported by the Spanish Federation of Internal Medicine. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Golimumab preserves insulin production in type 1 diabetes
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
The human monoclonal antibody golimumab (Simponi) preserved endogenous insulin secretion in patients with new-onset type 1 diabetes and reduced their exogenous insulin requirements at 1 year, newly published phase 2 data indicate.
Results from the multicenter, double-blind, placebo-controlled trial were first reported as a poster at the virtual American Diabetes Association 80th Scientific Sessions in June. They were published online Nov. 18 in the New England Journal of Medicine.
In the 52-week study of 84 children and adults with new-onset type 1 diabetes, those given golimumab injections every 2 weeks had significantly higher levels of C-peptide, a marker of insulin secretion, and required less injected or infused insulin than did those who received placebo injections. There were no treatment-associated serious adverse events.
Golimumab is a human monoclonal antibody specific for tumor necrosis factor–alpha. It is approved for the treatment of several autoimmune diseases, including rheumatoid arthritis and ulcerative colitis, in the United States, Europe, and elsewhere.
An intermediate step toward a cure
Although none of the patients were able to stop taking insulin entirely, the results have important clinical implications, lead author Teresa Quattrin, MD, said in an interview.
“People want a cure, but the fact is, a cure is not available yet. So, this is an intermediate step towards a cure.... There are advantages to being on a small insulin dose,” including lower rates of hypoglycemia and maintenance of intraportal insulin, said Dr. Quattrin, of the State University of New York at Buffalo.
But in an accompanying editorial, Domenico Accili, MD, points to potential risks from immunotherapy and from attempting additional interventions at an “emotionally fraught” time when patients and families are coping with the new diabetes diagnosis.
He said of golimumab, “the effect is actually very small. ... There’s nothing wrong in and of itself with improving those outcomes. I just wouldn’t assign them as game changers.”
If this or a similar immunotherapeutic intervention were approved for this indication, “I would tell patients it exists and let them make the decision whether they want to try it. I wouldn’t say you must try it,” said Dr. Accili, of the Columbia University Diabetes and Endocrinology Research Center, New York.
With golimumab, higher C-peptide, lower insulin requirement
Of the 84 patients, who ranged in age from 6 to 21 years, 56 were randomly assigned within 100 days of being diagnosed with type 1 diabetes to receive golimumab, and 28 were assigned to receive placebo injections, given every 2 weeks.
The drug resulted in lower insulin use (0.51U/Kg per day vs. 0.69 U/kg per day), and the increase in insulin use over 52 weeks was less with golimumab than with placebo (0.07 vs. 0.24 U/kg per day; P = .001).
The mean percent decrease of C-peptide production from baseline was 12% with golimumab versus 56% with placebo.
Although the mean number of overall hypoglycemic events was similar, the mean number of level 2 hypoglycemic events (<54 mg/dL) was 36% lower with golimumab (11.5 vs. 17.6). There were no severe cases of hypoglycemia in either group.
No severe or serious infections occurred in either group, although mild to moderate infections were reported in 71% with golimumab versus 61% with placebo. More patients in the golimumab group experienced a decrease in neutrophils (29% vs. 19%).
Immunotherapy: Which one, and when should it start?
These findings come on the heels of the 2019 landmark results with another monoclonal antibody, the investigational anti-CD3 teplizumab (PRV-031). Among patients at risk, a diagnosis of type 1 diabetes was delayed by 2 years, and continued benefit was seen at 3 years.
However, Dr. Quattrin said teplizumab is limited by the fact that it must be administered via a 14-day infusion, whereas golimumab can be injected by patients themselves at home.
Moreover, the phase 2 teplizumab study was conducted in people who had antibodies that placed them at high risk for type 1 diabetes, but those patients did not yet have the condition. They were identified because they had close relatives with type 1 diabetes and were enrolled in the federally funded TrialNet screening program.
Dr. Quattrin is now participating in an ongoing phase 3 study of teplizumab that involves patients newly diagnosed with type 1 diabetes.
A Janssen spokesperson said in an interview that the company isn’t planning to further develop golimumab for use in type 1 diabetes.
“Our focus is to apply insights from the phase 2 ... proof-of-concept study to progress what we believe are novel, immunologically targeted pipeline candidates in stage 2 disease or presymptomatic stages of type 1 diabetes, which is consistent with our mission to intercept and prevent type 1 diabetes,” the spokesperson said.
To identify more individuals at risk for type 1 diabetes beyond the close relatives of those who already have it, so as to be able to intervene at a presymptomatic stage, Janssen is organizing a public-private effort to advocate for routine population screening for type 1 diabetes–related autoantibodies.
Dr. Quattrin said: “Preserving some insulin is key. Having somebody with beta cell functioning still is an intermediate step to a cure and will make their life easier, and that’s what people should care about.”
Dr. Accili, who cofounded and leads a company working on a novel approach to type 1 diabetes treatment, writes in his editorial: “We should also be mindful that this treatment debate is first world–centric.
“Current treatments for type 1 diabetes require resources not readily available in most parts of the world, where something as simple as refrigeration of insulin can become a logistic nightmare. While combinations of [approaches] tailored to individual risk and potential benefits are likely to make inroads in clinical practice, the need for a simpler, safer, and equally effective alternative to insulin remains,” he wrote.
Dr. Quattrin is a researcher and consultant for Janssen and conducts clinical trials for Provention Bio, Opko, and Ascendis. Dr. Accili is founder and director of Forkhead Therapeutics.
A version of this article originally appeared on Medscape.com.
‘Smart’ insulin pen with CGM first to launch in emerging field
Medtronic’s launch of a new version of its smart insulin pen with integrated continuous glucose monitoring (CGM) is the first such device for use by people with diabetes who use multiple daily injections (MDI) of insulin.
Initially launched by Companion Medical in 2017, the InPen system is a reusable insulin injector pen combined with a smartphone app that provides insulin dose calculation information and tracking.
Medtronic acquired Companion in September 2020 and now the new version, the InPen with Real-Time Guardian Connect CGM Data, allows users to view glucose readings and insulin dose information in the same app.
The InPen, a so-called “connected delivery device,” also provides reports that aggregate insulin, glucose, and carbohydrate information into graphical displays. As with other current CGM systems, the information can be sent wirelessly to a clinician. And as with insulin pumps, the pens are programmed with target blood glucose levels, insulin-to-carb ratios, and insulin sensitivity parameters. The device tracks “insulin on board” and delivers reminders for basal and bolus doses.
InPen delivers only short-acting insulin from cartridges, all the three major brands. Patients who need long-acting insulin still need to inject that separately.
Barry H. Ginsberg, MD, PhD, of Diabetes Technology Consultants, Arlington, Va., said in an interview, “People using pumps have had data integration for a while now. This is an excellent first step in data integration for people doing MDI and I am sure it will improve blood glucose control.”
Asked about comparative costs, Medtronic spokeswoman Pamela Reese said in an interview, “While insurance costs will vary, the smart pen is less expensive than the insulin pump.”
Smart pens: How large is the market?
Speaking on Nov. 14 at the Diabetes Technology Society conference, diabetes care and education specialist Hope Warshaw, RD, gave an overview of the current smart pen/connected delivery device landscape.
She noted that the patient population who might benefit from smart pens, those using MDI, which is defined as injecting both long-acting insulin and short-acting insulin before meals, may be larger than appreciated. There are about 1.6 million U.S. patients with type 1 diabetes, of whom just 30%-40% currently use insulin pumps. In addition, of the 5.8 million with type 2 diabetes who take insulin, about 29%, or 1.7 million, use MDI.
Among those with type 1 diabetes, she said that smart pens might be a good option for “people who don’t want to wear the physical pump. They can deal with the sensor, but for psychological reasons or they have dermatologic issues, they just can’t wear a pump.”
But, Ms. Warshaw stressed, the type 2 diabetes population shouldn’t be overlooked. “More and more people with type 2 diabetes are on MDI. ... In fact, there are more who use MDI than the entire population with type 1 diabetes. ... This is happening because people with type 2 are getting it earlier and living longer.”
Dr. Ginsberg views smart pens as a bridge between simple pen injectors to automated insulin delivery (AID) systems, those that link insulin pumps with CGMs.
Regarding patients with type 1 diabetes, he said, “I see pen users on MDI slowly moving to integrated systems and then, when comfortable with the technology, moving to AID, finances allowing.”
As for those with type 2 diabetes, he said that they “are less computer literate and less likely to move to integrated systems, but they will, over time.”
In all, Dr. Ginsberg said, “I see integrated pens as increasing, not decreasing, the AID market.”
Emerging field: “I think they’re here to stay”
The new Medtronic InPen system can still display information from other compatible CGM systems, but on a 3-hour delay. This is important since the Guardian is not currently approved for determining insulin doses. In order to do that, users must still either use readings from another CGM system on a separate app or perform fingerstick blood glucose measurements.
The InPen is the first CGM-integrated pen device but is not likely to be the last. Similar technologies are being pursued by all three of the major insulin manufacturers and some other companies.
Eli Lilly’s Humalog Tempo Pen, a modified version of KwikPen, is integrated with the Dexcom CGM. The pen itself has been cleared by the U.S. Food and Drug Administration, but some of the component parts await authorization.
Novo Nordisk is expected to file with the FDA in 2021 for its NovoPen Echo Plus.
For its part, in December 2019, Sanofi teamed up with Bioport to fit its SoloStar insulin pens with their technology called Mallya, which had received CE Mark in June 2019. That device, which clips onto the top and the button of most major pens, adds smart pen capacity via Bluetooth. BioCorp also has teamed up with other manufacturers including Roche and AgaMatrix.
Another major player, Bigfoot Biomedical, has filed with the FDA for its connected pen that works with the Abbott FreeStyle Libre 2 CGM.
Ms. Warshaw advised, “We need to start talking more about the ways that peoples’ wants, needs, and desires change and evolve over the person’s life as their diabetes evolves and as all this technology evolves.
“Time will tell how many people will be on the very expensive [AID] systems. ... Pens are cheaper. The main cost is insulin. I think they’re here to stay. The big insulin makers wouldn’t be doing it otherwise.”
Dr. Ginsberg has no disclosures. Ms. Warshaw is a consultant and writer for Companion Medical/Medtronic and a faculty member of LifeScan Diabetes Institute.
A version of this article originally appeared on Medscape.com.
Medtronic’s launch of a new version of its smart insulin pen with integrated continuous glucose monitoring (CGM) is the first such device for use by people with diabetes who use multiple daily injections (MDI) of insulin.
Initially launched by Companion Medical in 2017, the InPen system is a reusable insulin injector pen combined with a smartphone app that provides insulin dose calculation information and tracking.
Medtronic acquired Companion in September 2020 and now the new version, the InPen with Real-Time Guardian Connect CGM Data, allows users to view glucose readings and insulin dose information in the same app.
The InPen, a so-called “connected delivery device,” also provides reports that aggregate insulin, glucose, and carbohydrate information into graphical displays. As with other current CGM systems, the information can be sent wirelessly to a clinician. And as with insulin pumps, the pens are programmed with target blood glucose levels, insulin-to-carb ratios, and insulin sensitivity parameters. The device tracks “insulin on board” and delivers reminders for basal and bolus doses.
InPen delivers only short-acting insulin from cartridges, all the three major brands. Patients who need long-acting insulin still need to inject that separately.
Barry H. Ginsberg, MD, PhD, of Diabetes Technology Consultants, Arlington, Va., said in an interview, “People using pumps have had data integration for a while now. This is an excellent first step in data integration for people doing MDI and I am sure it will improve blood glucose control.”
Asked about comparative costs, Medtronic spokeswoman Pamela Reese said in an interview, “While insurance costs will vary, the smart pen is less expensive than the insulin pump.”
Smart pens: How large is the market?
Speaking on Nov. 14 at the Diabetes Technology Society conference, diabetes care and education specialist Hope Warshaw, RD, gave an overview of the current smart pen/connected delivery device landscape.
She noted that the patient population who might benefit from smart pens, those using MDI, which is defined as injecting both long-acting insulin and short-acting insulin before meals, may be larger than appreciated. There are about 1.6 million U.S. patients with type 1 diabetes, of whom just 30%-40% currently use insulin pumps. In addition, of the 5.8 million with type 2 diabetes who take insulin, about 29%, or 1.7 million, use MDI.
Among those with type 1 diabetes, she said that smart pens might be a good option for “people who don’t want to wear the physical pump. They can deal with the sensor, but for psychological reasons or they have dermatologic issues, they just can’t wear a pump.”
But, Ms. Warshaw stressed, the type 2 diabetes population shouldn’t be overlooked. “More and more people with type 2 diabetes are on MDI. ... In fact, there are more who use MDI than the entire population with type 1 diabetes. ... This is happening because people with type 2 are getting it earlier and living longer.”
Dr. Ginsberg views smart pens as a bridge between simple pen injectors to automated insulin delivery (AID) systems, those that link insulin pumps with CGMs.
Regarding patients with type 1 diabetes, he said, “I see pen users on MDI slowly moving to integrated systems and then, when comfortable with the technology, moving to AID, finances allowing.”
As for those with type 2 diabetes, he said that they “are less computer literate and less likely to move to integrated systems, but they will, over time.”
In all, Dr. Ginsberg said, “I see integrated pens as increasing, not decreasing, the AID market.”
Emerging field: “I think they’re here to stay”
The new Medtronic InPen system can still display information from other compatible CGM systems, but on a 3-hour delay. This is important since the Guardian is not currently approved for determining insulin doses. In order to do that, users must still either use readings from another CGM system on a separate app or perform fingerstick blood glucose measurements.
The InPen is the first CGM-integrated pen device but is not likely to be the last. Similar technologies are being pursued by all three of the major insulin manufacturers and some other companies.
Eli Lilly’s Humalog Tempo Pen, a modified version of KwikPen, is integrated with the Dexcom CGM. The pen itself has been cleared by the U.S. Food and Drug Administration, but some of the component parts await authorization.
Novo Nordisk is expected to file with the FDA in 2021 for its NovoPen Echo Plus.
For its part, in December 2019, Sanofi teamed up with Bioport to fit its SoloStar insulin pens with their technology called Mallya, which had received CE Mark in June 2019. That device, which clips onto the top and the button of most major pens, adds smart pen capacity via Bluetooth. BioCorp also has teamed up with other manufacturers including Roche and AgaMatrix.
Another major player, Bigfoot Biomedical, has filed with the FDA for its connected pen that works with the Abbott FreeStyle Libre 2 CGM.
Ms. Warshaw advised, “We need to start talking more about the ways that peoples’ wants, needs, and desires change and evolve over the person’s life as their diabetes evolves and as all this technology evolves.
“Time will tell how many people will be on the very expensive [AID] systems. ... Pens are cheaper. The main cost is insulin. I think they’re here to stay. The big insulin makers wouldn’t be doing it otherwise.”
Dr. Ginsberg has no disclosures. Ms. Warshaw is a consultant and writer for Companion Medical/Medtronic and a faculty member of LifeScan Diabetes Institute.
A version of this article originally appeared on Medscape.com.
Medtronic’s launch of a new version of its smart insulin pen with integrated continuous glucose monitoring (CGM) is the first such device for use by people with diabetes who use multiple daily injections (MDI) of insulin.
Initially launched by Companion Medical in 2017, the InPen system is a reusable insulin injector pen combined with a smartphone app that provides insulin dose calculation information and tracking.
Medtronic acquired Companion in September 2020 and now the new version, the InPen with Real-Time Guardian Connect CGM Data, allows users to view glucose readings and insulin dose information in the same app.
The InPen, a so-called “connected delivery device,” also provides reports that aggregate insulin, glucose, and carbohydrate information into graphical displays. As with other current CGM systems, the information can be sent wirelessly to a clinician. And as with insulin pumps, the pens are programmed with target blood glucose levels, insulin-to-carb ratios, and insulin sensitivity parameters. The device tracks “insulin on board” and delivers reminders for basal and bolus doses.
InPen delivers only short-acting insulin from cartridges, all the three major brands. Patients who need long-acting insulin still need to inject that separately.
Barry H. Ginsberg, MD, PhD, of Diabetes Technology Consultants, Arlington, Va., said in an interview, “People using pumps have had data integration for a while now. This is an excellent first step in data integration for people doing MDI and I am sure it will improve blood glucose control.”
Asked about comparative costs, Medtronic spokeswoman Pamela Reese said in an interview, “While insurance costs will vary, the smart pen is less expensive than the insulin pump.”
Smart pens: How large is the market?
Speaking on Nov. 14 at the Diabetes Technology Society conference, diabetes care and education specialist Hope Warshaw, RD, gave an overview of the current smart pen/connected delivery device landscape.
She noted that the patient population who might benefit from smart pens, those using MDI, which is defined as injecting both long-acting insulin and short-acting insulin before meals, may be larger than appreciated. There are about 1.6 million U.S. patients with type 1 diabetes, of whom just 30%-40% currently use insulin pumps. In addition, of the 5.8 million with type 2 diabetes who take insulin, about 29%, or 1.7 million, use MDI.
Among those with type 1 diabetes, she said that smart pens might be a good option for “people who don’t want to wear the physical pump. They can deal with the sensor, but for psychological reasons or they have dermatologic issues, they just can’t wear a pump.”
But, Ms. Warshaw stressed, the type 2 diabetes population shouldn’t be overlooked. “More and more people with type 2 diabetes are on MDI. ... In fact, there are more who use MDI than the entire population with type 1 diabetes. ... This is happening because people with type 2 are getting it earlier and living longer.”
Dr. Ginsberg views smart pens as a bridge between simple pen injectors to automated insulin delivery (AID) systems, those that link insulin pumps with CGMs.
Regarding patients with type 1 diabetes, he said, “I see pen users on MDI slowly moving to integrated systems and then, when comfortable with the technology, moving to AID, finances allowing.”
As for those with type 2 diabetes, he said that they “are less computer literate and less likely to move to integrated systems, but they will, over time.”
In all, Dr. Ginsberg said, “I see integrated pens as increasing, not decreasing, the AID market.”
Emerging field: “I think they’re here to stay”
The new Medtronic InPen system can still display information from other compatible CGM systems, but on a 3-hour delay. This is important since the Guardian is not currently approved for determining insulin doses. In order to do that, users must still either use readings from another CGM system on a separate app or perform fingerstick blood glucose measurements.
The InPen is the first CGM-integrated pen device but is not likely to be the last. Similar technologies are being pursued by all three of the major insulin manufacturers and some other companies.
Eli Lilly’s Humalog Tempo Pen, a modified version of KwikPen, is integrated with the Dexcom CGM. The pen itself has been cleared by the U.S. Food and Drug Administration, but some of the component parts await authorization.
Novo Nordisk is expected to file with the FDA in 2021 for its NovoPen Echo Plus.
For its part, in December 2019, Sanofi teamed up with Bioport to fit its SoloStar insulin pens with their technology called Mallya, which had received CE Mark in June 2019. That device, which clips onto the top and the button of most major pens, adds smart pen capacity via Bluetooth. BioCorp also has teamed up with other manufacturers including Roche and AgaMatrix.
Another major player, Bigfoot Biomedical, has filed with the FDA for its connected pen that works with the Abbott FreeStyle Libre 2 CGM.
Ms. Warshaw advised, “We need to start talking more about the ways that peoples’ wants, needs, and desires change and evolve over the person’s life as their diabetes evolves and as all this technology evolves.
“Time will tell how many people will be on the very expensive [AID] systems. ... Pens are cheaper. The main cost is insulin. I think they’re here to stay. The big insulin makers wouldn’t be doing it otherwise.”
Dr. Ginsberg has no disclosures. Ms. Warshaw is a consultant and writer for Companion Medical/Medtronic and a faculty member of LifeScan Diabetes Institute.
A version of this article originally appeared on Medscape.com.
Osteoporosis drugs don’t worsen COVID-19 risk, may help
New observational data are the first to support recommendations to continue osteoporosis medications during the COVID-19 pandemic, and even suggest that some agents may protect against the virus.
Findings from the cross-sectional study of 2,102 patients with osteoporosis, osteoarthritis, and/or fibromyalgia – so-called noninflammatory rheumatic conditions – during March 1 to May 3, 2020, were recently published in Aging by Josep Blanch-Rubió, MD, scientific clinical director of the Rheumatology Service, Hospital del Mar, Barcelona, and colleagues.
Patients taking denosumab, zoledronate, and calcium showed trends toward lower incidence of developing symptomatic presumed COVID-19 (polymerase chain reaction tests weren’t widely available at the time), as did those taking the antidepressant serotonin/norepinephrine inhibitor duloxetine.
Some analgesics, particularly pregabalin and most other antidepressants, were associated with higher incidences of COVID-19, while oral bisphosphonates, vitamin D, thiazide diuretics, antihypertensive drugs, and chronic nonsteroidal anti-inflammatory drugs had no effect on COVID-19 incidence.
These data are the first to support guidance issued in May 2020 by the American Society for Bone and Mineral Research and four other professional societies advising continuation of osteoporosis medications during the pandemic. That statement’s authors acknowledged that, lacking data, their recommendations were based primarily on expert opinion.
“There were guidelines without any scientific base. ... This is the first scientific evidence showing that indeed you should continue your osteoporosis treatment if you have COVID-19. This is the first study to provide scientific support for the guidelines,” study coauthor Rafael Maldonado, MD, PhD, of the Laboratory of Neuropharmacology, Universitat Pompeu Fabra, Barcelona, said in an interview.
And while the data don’t offer proof of benefit for any drug – all of the 95% confidence intervals crossed 1.0 – they do show trends that deserve further study, Dr. Maldonado said.
“What we observed is that there is no harm. Treatments should be continued.”
“But we obtained very interesting results with denosumab, zoledronate, calcium, and duloxetine. ... There is a clear tendency, and the message is we should promote studies to see if these four treatments provide benefit.”
Different mechanisms for each?
Asked to comment on the findings, Matthew T. Drake, MD, PhD, said in an interview, “I would agree that there’s no reason any of these medications should be stopped or discontinued since there’s no evidence that they make the risk for infection worse.”
“But how [some of them may] improve or reduce the infection risk in my mind is somewhat unclear. ... It’s hard to come up with a unifying explanation” because those mentioned as potentially beneficial “are fairly different,” he noted.
Dr. Drake, associate professor of medicine in the department of endocrinology at the Mayo Clinic, Rochester, Minn., said he agreed with the study authors that denosumab’s targeting of the RANK/RANKL system is a possible anti-COVID-19 mechanism for that drug because that system is involved in immune response.
Regarding zoledronate/zoledronic acid, both the Spanish authors and Dr. Drake pointed to a landmark study linking the intravenous drug to longer survival in patients with hip fracture. The study authors note that there could be several mechanisms for an overall survival benefit, but additionally, “zoledronate may make dendritic cells and their precursors less susceptible to SARS-CoV-2 infection, which could explain the beneficial effects here ... on COVID-19 incidence.”
And, the authors hypothesized, the reason for the lack of benefit with oral bisphosphonates might relate to the higher potency of the intravenous zoledronate. Dr. Drake added that its higher bioavailability may also play a role.
As for calcium, the authors suggest that the beneficial effect against COVID-19 could relate to its action in generating two immune cell types – T follicular helper cells and T follicular regulatory cells – which promote an appropriate immune response against infectious agents, including viruses.
Data supporting the guidelines
Of the 2,102 patients in the study by Blanch-Rubió and colleagues, 80.5% were women, and their mean age was 66.4 years. Overall, 63.7% had osteoarthritis, 43.5% had osteoporosis, and 27.2% had fibromyalgia. Treatments included vitamin D in 62%, calcium in 23.3%, denosumab in 12.6%, and intravenous zoledronate in 8.5%. Over half were taking analgesics and nearly a third antidepressants, with 9.9% taking duloxetine.
During the study period, 5.2%, or 109 individuals, were diagnosed with COVID-19 based on presenting for medical care with hallmark symptoms.
After adjustments for sex, age, diabetes, pulmonary disease, cardiovascular disease, chronic kidney disease, and active cancer or treatment, the relative risks for COVID-19 were 0.58 for denosumab, 0.62 for intravenous zoledronate, and 0.64 for calcium, all nonsignificant trends. No associations were found between COVID-19 and oral bisphosphonates, vitamin D, or thiazide diuretics. Increased but nonsignificant relative risks for COVID-19 were seen with analgesics, particularly pregabalin (1.55), gabapentin (1.39), and opioids (1.25).
Among antidepressants, there was a relative risk of 1.54 for selective serotonin reuptake inhibitors, 1.38 for amitriptyline, and 1.22 for all dual-action antidepressants together. In contrast, there was a negative association with the dual-action antidepressant duloxetine, with an adjusted relative risk of 0.68.
“The good news,” Dr. Drake said, “is that none of it appears bad.”
Dr. Blanch-Rubió has received grants or consulting fees from Amgen, Laboratorio Stada, Gedeon-Rhicter Ibérica, Lilly España, Pfizer, Gebro Pharma, and UCB Pharma. Dr. Maldonado has received research grants or consulting fees from Aelis, Almirall, Boehringer Ingelheim, BrainCo, Esteve, Ferrer, GlaxoSmithKline, Grünenthal, GW Pharmaceuticals, Janus, Lundbeck, Pharmaleads, Phytoplant, Rhodes, Sanofi, Spherium, Union de Pharmacologie Scientifique Appliquée, Upjohn, and Uriach. Dr. Drake has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New observational data are the first to support recommendations to continue osteoporosis medications during the COVID-19 pandemic, and even suggest that some agents may protect against the virus.
Findings from the cross-sectional study of 2,102 patients with osteoporosis, osteoarthritis, and/or fibromyalgia – so-called noninflammatory rheumatic conditions – during March 1 to May 3, 2020, were recently published in Aging by Josep Blanch-Rubió, MD, scientific clinical director of the Rheumatology Service, Hospital del Mar, Barcelona, and colleagues.
Patients taking denosumab, zoledronate, and calcium showed trends toward lower incidence of developing symptomatic presumed COVID-19 (polymerase chain reaction tests weren’t widely available at the time), as did those taking the antidepressant serotonin/norepinephrine inhibitor duloxetine.
Some analgesics, particularly pregabalin and most other antidepressants, were associated with higher incidences of COVID-19, while oral bisphosphonates, vitamin D, thiazide diuretics, antihypertensive drugs, and chronic nonsteroidal anti-inflammatory drugs had no effect on COVID-19 incidence.
These data are the first to support guidance issued in May 2020 by the American Society for Bone and Mineral Research and four other professional societies advising continuation of osteoporosis medications during the pandemic. That statement’s authors acknowledged that, lacking data, their recommendations were based primarily on expert opinion.
“There were guidelines without any scientific base. ... This is the first scientific evidence showing that indeed you should continue your osteoporosis treatment if you have COVID-19. This is the first study to provide scientific support for the guidelines,” study coauthor Rafael Maldonado, MD, PhD, of the Laboratory of Neuropharmacology, Universitat Pompeu Fabra, Barcelona, said in an interview.
And while the data don’t offer proof of benefit for any drug – all of the 95% confidence intervals crossed 1.0 – they do show trends that deserve further study, Dr. Maldonado said.
“What we observed is that there is no harm. Treatments should be continued.”
“But we obtained very interesting results with denosumab, zoledronate, calcium, and duloxetine. ... There is a clear tendency, and the message is we should promote studies to see if these four treatments provide benefit.”
Different mechanisms for each?
Asked to comment on the findings, Matthew T. Drake, MD, PhD, said in an interview, “I would agree that there’s no reason any of these medications should be stopped or discontinued since there’s no evidence that they make the risk for infection worse.”
“But how [some of them may] improve or reduce the infection risk in my mind is somewhat unclear. ... It’s hard to come up with a unifying explanation” because those mentioned as potentially beneficial “are fairly different,” he noted.
Dr. Drake, associate professor of medicine in the department of endocrinology at the Mayo Clinic, Rochester, Minn., said he agreed with the study authors that denosumab’s targeting of the RANK/RANKL system is a possible anti-COVID-19 mechanism for that drug because that system is involved in immune response.
Regarding zoledronate/zoledronic acid, both the Spanish authors and Dr. Drake pointed to a landmark study linking the intravenous drug to longer survival in patients with hip fracture. The study authors note that there could be several mechanisms for an overall survival benefit, but additionally, “zoledronate may make dendritic cells and their precursors less susceptible to SARS-CoV-2 infection, which could explain the beneficial effects here ... on COVID-19 incidence.”
And, the authors hypothesized, the reason for the lack of benefit with oral bisphosphonates might relate to the higher potency of the intravenous zoledronate. Dr. Drake added that its higher bioavailability may also play a role.
As for calcium, the authors suggest that the beneficial effect against COVID-19 could relate to its action in generating two immune cell types – T follicular helper cells and T follicular regulatory cells – which promote an appropriate immune response against infectious agents, including viruses.
Data supporting the guidelines
Of the 2,102 patients in the study by Blanch-Rubió and colleagues, 80.5% were women, and their mean age was 66.4 years. Overall, 63.7% had osteoarthritis, 43.5% had osteoporosis, and 27.2% had fibromyalgia. Treatments included vitamin D in 62%, calcium in 23.3%, denosumab in 12.6%, and intravenous zoledronate in 8.5%. Over half were taking analgesics and nearly a third antidepressants, with 9.9% taking duloxetine.
During the study period, 5.2%, or 109 individuals, were diagnosed with COVID-19 based on presenting for medical care with hallmark symptoms.
After adjustments for sex, age, diabetes, pulmonary disease, cardiovascular disease, chronic kidney disease, and active cancer or treatment, the relative risks for COVID-19 were 0.58 for denosumab, 0.62 for intravenous zoledronate, and 0.64 for calcium, all nonsignificant trends. No associations were found between COVID-19 and oral bisphosphonates, vitamin D, or thiazide diuretics. Increased but nonsignificant relative risks for COVID-19 were seen with analgesics, particularly pregabalin (1.55), gabapentin (1.39), and opioids (1.25).
Among antidepressants, there was a relative risk of 1.54 for selective serotonin reuptake inhibitors, 1.38 for amitriptyline, and 1.22 for all dual-action antidepressants together. In contrast, there was a negative association with the dual-action antidepressant duloxetine, with an adjusted relative risk of 0.68.
“The good news,” Dr. Drake said, “is that none of it appears bad.”
Dr. Blanch-Rubió has received grants or consulting fees from Amgen, Laboratorio Stada, Gedeon-Rhicter Ibérica, Lilly España, Pfizer, Gebro Pharma, and UCB Pharma. Dr. Maldonado has received research grants or consulting fees from Aelis, Almirall, Boehringer Ingelheim, BrainCo, Esteve, Ferrer, GlaxoSmithKline, Grünenthal, GW Pharmaceuticals, Janus, Lundbeck, Pharmaleads, Phytoplant, Rhodes, Sanofi, Spherium, Union de Pharmacologie Scientifique Appliquée, Upjohn, and Uriach. Dr. Drake has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New observational data are the first to support recommendations to continue osteoporosis medications during the COVID-19 pandemic, and even suggest that some agents may protect against the virus.
Findings from the cross-sectional study of 2,102 patients with osteoporosis, osteoarthritis, and/or fibromyalgia – so-called noninflammatory rheumatic conditions – during March 1 to May 3, 2020, were recently published in Aging by Josep Blanch-Rubió, MD, scientific clinical director of the Rheumatology Service, Hospital del Mar, Barcelona, and colleagues.
Patients taking denosumab, zoledronate, and calcium showed trends toward lower incidence of developing symptomatic presumed COVID-19 (polymerase chain reaction tests weren’t widely available at the time), as did those taking the antidepressant serotonin/norepinephrine inhibitor duloxetine.
Some analgesics, particularly pregabalin and most other antidepressants, were associated with higher incidences of COVID-19, while oral bisphosphonates, vitamin D, thiazide diuretics, antihypertensive drugs, and chronic nonsteroidal anti-inflammatory drugs had no effect on COVID-19 incidence.
These data are the first to support guidance issued in May 2020 by the American Society for Bone and Mineral Research and four other professional societies advising continuation of osteoporosis medications during the pandemic. That statement’s authors acknowledged that, lacking data, their recommendations were based primarily on expert opinion.
“There were guidelines without any scientific base. ... This is the first scientific evidence showing that indeed you should continue your osteoporosis treatment if you have COVID-19. This is the first study to provide scientific support for the guidelines,” study coauthor Rafael Maldonado, MD, PhD, of the Laboratory of Neuropharmacology, Universitat Pompeu Fabra, Barcelona, said in an interview.
And while the data don’t offer proof of benefit for any drug – all of the 95% confidence intervals crossed 1.0 – they do show trends that deserve further study, Dr. Maldonado said.
“What we observed is that there is no harm. Treatments should be continued.”
“But we obtained very interesting results with denosumab, zoledronate, calcium, and duloxetine. ... There is a clear tendency, and the message is we should promote studies to see if these four treatments provide benefit.”
Different mechanisms for each?
Asked to comment on the findings, Matthew T. Drake, MD, PhD, said in an interview, “I would agree that there’s no reason any of these medications should be stopped or discontinued since there’s no evidence that they make the risk for infection worse.”
“But how [some of them may] improve or reduce the infection risk in my mind is somewhat unclear. ... It’s hard to come up with a unifying explanation” because those mentioned as potentially beneficial “are fairly different,” he noted.
Dr. Drake, associate professor of medicine in the department of endocrinology at the Mayo Clinic, Rochester, Minn., said he agreed with the study authors that denosumab’s targeting of the RANK/RANKL system is a possible anti-COVID-19 mechanism for that drug because that system is involved in immune response.
Regarding zoledronate/zoledronic acid, both the Spanish authors and Dr. Drake pointed to a landmark study linking the intravenous drug to longer survival in patients with hip fracture. The study authors note that there could be several mechanisms for an overall survival benefit, but additionally, “zoledronate may make dendritic cells and their precursors less susceptible to SARS-CoV-2 infection, which could explain the beneficial effects here ... on COVID-19 incidence.”
And, the authors hypothesized, the reason for the lack of benefit with oral bisphosphonates might relate to the higher potency of the intravenous zoledronate. Dr. Drake added that its higher bioavailability may also play a role.
As for calcium, the authors suggest that the beneficial effect against COVID-19 could relate to its action in generating two immune cell types – T follicular helper cells and T follicular regulatory cells – which promote an appropriate immune response against infectious agents, including viruses.
Data supporting the guidelines
Of the 2,102 patients in the study by Blanch-Rubió and colleagues, 80.5% were women, and their mean age was 66.4 years. Overall, 63.7% had osteoarthritis, 43.5% had osteoporosis, and 27.2% had fibromyalgia. Treatments included vitamin D in 62%, calcium in 23.3%, denosumab in 12.6%, and intravenous zoledronate in 8.5%. Over half were taking analgesics and nearly a third antidepressants, with 9.9% taking duloxetine.
During the study period, 5.2%, or 109 individuals, were diagnosed with COVID-19 based on presenting for medical care with hallmark symptoms.
After adjustments for sex, age, diabetes, pulmonary disease, cardiovascular disease, chronic kidney disease, and active cancer or treatment, the relative risks for COVID-19 were 0.58 for denosumab, 0.62 for intravenous zoledronate, and 0.64 for calcium, all nonsignificant trends. No associations were found between COVID-19 and oral bisphosphonates, vitamin D, or thiazide diuretics. Increased but nonsignificant relative risks for COVID-19 were seen with analgesics, particularly pregabalin (1.55), gabapentin (1.39), and opioids (1.25).
Among antidepressants, there was a relative risk of 1.54 for selective serotonin reuptake inhibitors, 1.38 for amitriptyline, and 1.22 for all dual-action antidepressants together. In contrast, there was a negative association with the dual-action antidepressant duloxetine, with an adjusted relative risk of 0.68.
“The good news,” Dr. Drake said, “is that none of it appears bad.”
Dr. Blanch-Rubió has received grants or consulting fees from Amgen, Laboratorio Stada, Gedeon-Rhicter Ibérica, Lilly España, Pfizer, Gebro Pharma, and UCB Pharma. Dr. Maldonado has received research grants or consulting fees from Aelis, Almirall, Boehringer Ingelheim, BrainCo, Esteve, Ferrer, GlaxoSmithKline, Grünenthal, GW Pharmaceuticals, Janus, Lundbeck, Pharmaleads, Phytoplant, Rhodes, Sanofi, Spherium, Union de Pharmacologie Scientifique Appliquée, Upjohn, and Uriach. Dr. Drake has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Lancet panel calls for urgent global action to combat diabetes
The article was published online Nov. 12, just ahead of World Diabetes Day.
Of the 463 million people with diabetes worldwide in 2019, 80% live in low- and middle-income countries. The condition reduces life expectancy in middle-aged adults by 4-10 years, including increasing the risk of death from cardiovascular disease, kidney disease, and cancer by up to threefold. It is also a leading cause of nontraumatic amputation and blindness.
Use of evidence-based interventions, if implemented and managed properly, could prevent thousands of deaths globally every day, stressed the commission.
“There is an enormous amount of knowledge that we have amassed over the years. We need good preventive care and we need to ensure that diabetes patients, once diagnosed, have good continuous care. There is an urgent need for decision-makers, policymakers, and payers to make things happen,” the leader of the multidisciplinary commission, Juliana C.N. Chan, MBChB, MD, said in an interview.
And now diabetes has emerged as a major risk factor for death from COVID-19, particularly in the setting of inadequate glycemic control.
“COVID-19 has exposed the vulnerability of individuals with diabetes,” said Dr. Chan, of the Hong Kong Institute of Diabetes and Obesity. “We should use the pandemic as an opportunity to implement solutions.”
Physician education key, trickling down to field workers and patients
First on the agenda, she says, should be “physician education. There are many primary care providers and internal medicine physicians whose knowledge needs to be updated.”
“Then doctors need to transfer this information to other people, such as nurses and community field workers. We cannot just rely on doctors; we need to train nonmedics” so that knowledge about how to prevent, treat, and manage diabetes long term is communicated right down the health care chain, she explained.
“They need to know how to look at people’s eyes and feet, how to do blood and urine tests, and how to collect data. Then they need to educate patients on what they should be doing, on how to practice self-care,” she added.
“We need to change our way of thinking, redesign clinic flow and how you build a team. And those care teams need to know how to collect data, and then use that data to monitor patients and to stratify individual risk, to ensure that what has been said has been done, as well as to inform practice and policies” through, for example, the establishment of diabetes registers.
The focus needs to be on “lifelong integrated care, the right treatment at the right time,” she emphasized. History-taking, clinical and laboratory assessments, as well as monitoring of macrovascular and microvascular complications, comorbidities, and medications, are all key.
Just a few simple things, if properly implemented, could make a big difference, Dr. Chan stressed.
For example, implementing a structured lifestyle intervention and use of metformin can each prevent or delay type 2 diabetes in individuals with impaired glucose tolerance by 30%-50%, and sustained weight reduction in patients with obesity by 15 kg (33 lb) or more can induce remission of type 2 diabetes for up to 2 years.
And there are plenty of medications that are “very affordable even in low- and middle-income countries” to treat diabetes and associated risk factors, including metformin, “statins, and RAS inhibitors,” she noted.
For instance, the 10 low- and middle-income countries with the greatest burden of diabetes (China, India, Brazil, Mexico, Indonesia, Egypt, Pakistan, Bangladesh, Turkey, Thailand) account for 217 million cases of type 2 diabetes, representing nearly 50% of all diabetes cases.
The commission estimated that 3.2 million of these individuals would die in 3 years if not treated, with 1.3 million of these deaths due to cardiovascular disease.
By reducing hemoglobin A1c, blood pressure, and LDL-cholesterol through achieving a diagnosis rate of 50%, ensuring access to essential medicines in at least 70% of patients, and with a support system to sustain reductions in these risk factors over 3 years, up to 800,000 premature deaths could be avoided.
People with type 1 diabetes dying; WHO launches initiative
In an accompanying commentary (2020 Nov 12. doi: 10.1016/S0140-6736[20]32378-3), Katie Dain, chief executive officer of the Noncommunicable Diseases (NCD) Alliance, points out that only half of people living with diabetes around the world – and just one in seven in Africa – have reliable access to insulin.
“Lots of people with type 1 diabetes are still dying due to lack of insulin,” Dr. Chan said in an interview. “We need to elevate basic care to intermediate and ensure that basal-bolus insulin and glucose-monitoring tools are available and that patients are trained in self-care. In that way, 80% of type 1 diabetes deaths could be prevented.”
Ms. 3Dain agrees, stressing, “Political rhetoric and commitments have yet to translate into sufficient and sustainable action for people living with diabetes worldwide, and particularly for those in [low- and middle-income countries].”
The Lancet Commission document also emphasizes the importance of support for pregnant women with diabetes and attention to the psychosocial needs of people with diabetes.
And it stresses society-, population-, and community-based strategies for type 2 diabetes prevention including health awareness programs, food policies, and broad use of nonphysician personnel to deliver diabetes prevention efforts.
In tandem with World Diabetes Day, the World Health Organization will announce the development of the WHO Global Diabetes Compact, which will be launched in April 2021.
This will aim to implement the commission’s recommendations through partnerships with governments, care providers, patient advocates, and nongovernmental organizations.
Together, they will “support countries to mobilize resources and accelerate structural transformations, which will enable the scale-up of access to essential diabetes medicines and technologies, inclusion of diagnosis and treatment of diabetes in primary health care and universal health coverage packages, and reduction of major population-level diabetes risk factors such as obesity,” according to another Lancet editorial accompanying the report.
“The evidence-base for improving diabetes prevention and care is strong. The question now for diabetes advocates is how to achieve the comprehensive, systems-level change needed to translate this evidence into action.”
Dr. Chan has reported receiving grants from AstraZeneca, Lilly, Lee Powder, Hua Medicine, and Qualigenics, as well as grants and personal fees from Bayer, Boehringer Ingelheim, Sanofi, Novartis, Merck, and MSD outside the submitted work. She has reported being the chief executive officer (pro bono) of the Asia Diabetes Foundation and a cofounder of GemVCare. She also holds a patent for genetic markers for diabetes and its complications. Ms. Dain has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The article was published online Nov. 12, just ahead of World Diabetes Day.
Of the 463 million people with diabetes worldwide in 2019, 80% live in low- and middle-income countries. The condition reduces life expectancy in middle-aged adults by 4-10 years, including increasing the risk of death from cardiovascular disease, kidney disease, and cancer by up to threefold. It is also a leading cause of nontraumatic amputation and blindness.
Use of evidence-based interventions, if implemented and managed properly, could prevent thousands of deaths globally every day, stressed the commission.
“There is an enormous amount of knowledge that we have amassed over the years. We need good preventive care and we need to ensure that diabetes patients, once diagnosed, have good continuous care. There is an urgent need for decision-makers, policymakers, and payers to make things happen,” the leader of the multidisciplinary commission, Juliana C.N. Chan, MBChB, MD, said in an interview.
And now diabetes has emerged as a major risk factor for death from COVID-19, particularly in the setting of inadequate glycemic control.
“COVID-19 has exposed the vulnerability of individuals with diabetes,” said Dr. Chan, of the Hong Kong Institute of Diabetes and Obesity. “We should use the pandemic as an opportunity to implement solutions.”
Physician education key, trickling down to field workers and patients
First on the agenda, she says, should be “physician education. There are many primary care providers and internal medicine physicians whose knowledge needs to be updated.”
“Then doctors need to transfer this information to other people, such as nurses and community field workers. We cannot just rely on doctors; we need to train nonmedics” so that knowledge about how to prevent, treat, and manage diabetes long term is communicated right down the health care chain, she explained.
“They need to know how to look at people’s eyes and feet, how to do blood and urine tests, and how to collect data. Then they need to educate patients on what they should be doing, on how to practice self-care,” she added.
“We need to change our way of thinking, redesign clinic flow and how you build a team. And those care teams need to know how to collect data, and then use that data to monitor patients and to stratify individual risk, to ensure that what has been said has been done, as well as to inform practice and policies” through, for example, the establishment of diabetes registers.
The focus needs to be on “lifelong integrated care, the right treatment at the right time,” she emphasized. History-taking, clinical and laboratory assessments, as well as monitoring of macrovascular and microvascular complications, comorbidities, and medications, are all key.
Just a few simple things, if properly implemented, could make a big difference, Dr. Chan stressed.
For example, implementing a structured lifestyle intervention and use of metformin can each prevent or delay type 2 diabetes in individuals with impaired glucose tolerance by 30%-50%, and sustained weight reduction in patients with obesity by 15 kg (33 lb) or more can induce remission of type 2 diabetes for up to 2 years.
And there are plenty of medications that are “very affordable even in low- and middle-income countries” to treat diabetes and associated risk factors, including metformin, “statins, and RAS inhibitors,” she noted.
For instance, the 10 low- and middle-income countries with the greatest burden of diabetes (China, India, Brazil, Mexico, Indonesia, Egypt, Pakistan, Bangladesh, Turkey, Thailand) account for 217 million cases of type 2 diabetes, representing nearly 50% of all diabetes cases.
The commission estimated that 3.2 million of these individuals would die in 3 years if not treated, with 1.3 million of these deaths due to cardiovascular disease.
By reducing hemoglobin A1c, blood pressure, and LDL-cholesterol through achieving a diagnosis rate of 50%, ensuring access to essential medicines in at least 70% of patients, and with a support system to sustain reductions in these risk factors over 3 years, up to 800,000 premature deaths could be avoided.
People with type 1 diabetes dying; WHO launches initiative
In an accompanying commentary (2020 Nov 12. doi: 10.1016/S0140-6736[20]32378-3), Katie Dain, chief executive officer of the Noncommunicable Diseases (NCD) Alliance, points out that only half of people living with diabetes around the world – and just one in seven in Africa – have reliable access to insulin.
“Lots of people with type 1 diabetes are still dying due to lack of insulin,” Dr. Chan said in an interview. “We need to elevate basic care to intermediate and ensure that basal-bolus insulin and glucose-monitoring tools are available and that patients are trained in self-care. In that way, 80% of type 1 diabetes deaths could be prevented.”
Ms. 3Dain agrees, stressing, “Political rhetoric and commitments have yet to translate into sufficient and sustainable action for people living with diabetes worldwide, and particularly for those in [low- and middle-income countries].”
The Lancet Commission document also emphasizes the importance of support for pregnant women with diabetes and attention to the psychosocial needs of people with diabetes.
And it stresses society-, population-, and community-based strategies for type 2 diabetes prevention including health awareness programs, food policies, and broad use of nonphysician personnel to deliver diabetes prevention efforts.
In tandem with World Diabetes Day, the World Health Organization will announce the development of the WHO Global Diabetes Compact, which will be launched in April 2021.
This will aim to implement the commission’s recommendations through partnerships with governments, care providers, patient advocates, and nongovernmental organizations.
Together, they will “support countries to mobilize resources and accelerate structural transformations, which will enable the scale-up of access to essential diabetes medicines and technologies, inclusion of diagnosis and treatment of diabetes in primary health care and universal health coverage packages, and reduction of major population-level diabetes risk factors such as obesity,” according to another Lancet editorial accompanying the report.
“The evidence-base for improving diabetes prevention and care is strong. The question now for diabetes advocates is how to achieve the comprehensive, systems-level change needed to translate this evidence into action.”
Dr. Chan has reported receiving grants from AstraZeneca, Lilly, Lee Powder, Hua Medicine, and Qualigenics, as well as grants and personal fees from Bayer, Boehringer Ingelheim, Sanofi, Novartis, Merck, and MSD outside the submitted work. She has reported being the chief executive officer (pro bono) of the Asia Diabetes Foundation and a cofounder of GemVCare. She also holds a patent for genetic markers for diabetes and its complications. Ms. Dain has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The article was published online Nov. 12, just ahead of World Diabetes Day.
Of the 463 million people with diabetes worldwide in 2019, 80% live in low- and middle-income countries. The condition reduces life expectancy in middle-aged adults by 4-10 years, including increasing the risk of death from cardiovascular disease, kidney disease, and cancer by up to threefold. It is also a leading cause of nontraumatic amputation and blindness.
Use of evidence-based interventions, if implemented and managed properly, could prevent thousands of deaths globally every day, stressed the commission.
“There is an enormous amount of knowledge that we have amassed over the years. We need good preventive care and we need to ensure that diabetes patients, once diagnosed, have good continuous care. There is an urgent need for decision-makers, policymakers, and payers to make things happen,” the leader of the multidisciplinary commission, Juliana C.N. Chan, MBChB, MD, said in an interview.
And now diabetes has emerged as a major risk factor for death from COVID-19, particularly in the setting of inadequate glycemic control.
“COVID-19 has exposed the vulnerability of individuals with diabetes,” said Dr. Chan, of the Hong Kong Institute of Diabetes and Obesity. “We should use the pandemic as an opportunity to implement solutions.”
Physician education key, trickling down to field workers and patients
First on the agenda, she says, should be “physician education. There are many primary care providers and internal medicine physicians whose knowledge needs to be updated.”
“Then doctors need to transfer this information to other people, such as nurses and community field workers. We cannot just rely on doctors; we need to train nonmedics” so that knowledge about how to prevent, treat, and manage diabetes long term is communicated right down the health care chain, she explained.
“They need to know how to look at people’s eyes and feet, how to do blood and urine tests, and how to collect data. Then they need to educate patients on what they should be doing, on how to practice self-care,” she added.
“We need to change our way of thinking, redesign clinic flow and how you build a team. And those care teams need to know how to collect data, and then use that data to monitor patients and to stratify individual risk, to ensure that what has been said has been done, as well as to inform practice and policies” through, for example, the establishment of diabetes registers.
The focus needs to be on “lifelong integrated care, the right treatment at the right time,” she emphasized. History-taking, clinical and laboratory assessments, as well as monitoring of macrovascular and microvascular complications, comorbidities, and medications, are all key.
Just a few simple things, if properly implemented, could make a big difference, Dr. Chan stressed.
For example, implementing a structured lifestyle intervention and use of metformin can each prevent or delay type 2 diabetes in individuals with impaired glucose tolerance by 30%-50%, and sustained weight reduction in patients with obesity by 15 kg (33 lb) or more can induce remission of type 2 diabetes for up to 2 years.
And there are plenty of medications that are “very affordable even in low- and middle-income countries” to treat diabetes and associated risk factors, including metformin, “statins, and RAS inhibitors,” she noted.
For instance, the 10 low- and middle-income countries with the greatest burden of diabetes (China, India, Brazil, Mexico, Indonesia, Egypt, Pakistan, Bangladesh, Turkey, Thailand) account for 217 million cases of type 2 diabetes, representing nearly 50% of all diabetes cases.
The commission estimated that 3.2 million of these individuals would die in 3 years if not treated, with 1.3 million of these deaths due to cardiovascular disease.
By reducing hemoglobin A1c, blood pressure, and LDL-cholesterol through achieving a diagnosis rate of 50%, ensuring access to essential medicines in at least 70% of patients, and with a support system to sustain reductions in these risk factors over 3 years, up to 800,000 premature deaths could be avoided.
People with type 1 diabetes dying; WHO launches initiative
In an accompanying commentary (2020 Nov 12. doi: 10.1016/S0140-6736[20]32378-3), Katie Dain, chief executive officer of the Noncommunicable Diseases (NCD) Alliance, points out that only half of people living with diabetes around the world – and just one in seven in Africa – have reliable access to insulin.
“Lots of people with type 1 diabetes are still dying due to lack of insulin,” Dr. Chan said in an interview. “We need to elevate basic care to intermediate and ensure that basal-bolus insulin and glucose-monitoring tools are available and that patients are trained in self-care. In that way, 80% of type 1 diabetes deaths could be prevented.”
Ms. 3Dain agrees, stressing, “Political rhetoric and commitments have yet to translate into sufficient and sustainable action for people living with diabetes worldwide, and particularly for those in [low- and middle-income countries].”
The Lancet Commission document also emphasizes the importance of support for pregnant women with diabetes and attention to the psychosocial needs of people with diabetes.
And it stresses society-, population-, and community-based strategies for type 2 diabetes prevention including health awareness programs, food policies, and broad use of nonphysician personnel to deliver diabetes prevention efforts.
In tandem with World Diabetes Day, the World Health Organization will announce the development of the WHO Global Diabetes Compact, which will be launched in April 2021.
This will aim to implement the commission’s recommendations through partnerships with governments, care providers, patient advocates, and nongovernmental organizations.
Together, they will “support countries to mobilize resources and accelerate structural transformations, which will enable the scale-up of access to essential diabetes medicines and technologies, inclusion of diagnosis and treatment of diabetes in primary health care and universal health coverage packages, and reduction of major population-level diabetes risk factors such as obesity,” according to another Lancet editorial accompanying the report.
“The evidence-base for improving diabetes prevention and care is strong. The question now for diabetes advocates is how to achieve the comprehensive, systems-level change needed to translate this evidence into action.”
Dr. Chan has reported receiving grants from AstraZeneca, Lilly, Lee Powder, Hua Medicine, and Qualigenics, as well as grants and personal fees from Bayer, Boehringer Ingelheim, Sanofi, Novartis, Merck, and MSD outside the submitted work. She has reported being the chief executive officer (pro bono) of the Asia Diabetes Foundation and a cofounder of GemVCare. She also holds a patent for genetic markers for diabetes and its complications. Ms. Dain has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New guidelines address diabetes management in kidney disease
A new guideline from the Kidney Disease: Improving Global Outcomes group addressing issues around diabetes management in patients with chronic kidney disease (CKD) has just been published in synopsis form in Annals of Internal Medicine.
The full guideline, including 12 recommendations and 48 practice points for clinicians caring for patients with diabetes and CKD, was published last month in Kidney International and on the KDIGO website.
More than 40% of people with diabetes develop CKD, and a significant number develop kidney failure requiring dialysis or transplant. This is the first guidance from KDIGO to address the comorbidity.
The new synopsis is aimed at primary care and nonnephrology specialist clinicians who manage patients with diabetes and CKD, in addition to nephrologists, first author Sankar D. Navaneethan, MD, said in an interview.
“Most of these patients are in the hands of primary care, endocrinology, and cardiology. We want to emphasize when they see patients with different severities of kidney disease [is] what are some of the things they have to be cognizant of,” said Dr. Navaneethan, professor of medicine and director of clinical research in the section of nephrology at Baylor College of Medicine, Houston.
The synopsis summarizes key recommendations from the larger guidance regarding comprehensive care needs, glycemic monitoring and targets, lifestyle interventions, glucose-lowering therapies, and educational/integrated care approaches.
It does not depart from prior diabetes guidelines, but it does provide advice for specific situations relevant to CKD, such as the limitations of hemoglobin A1c when estimated glomerular filtration rate (eGFR) drops below 30 mL/min per 1.73m2, and dietary protein consumption. It is based on published evidence up until February 2020.
For the nephrologist audience in particular, Dr. Navaneethan said, “we wanted to highlight team-based care, interacting with other specialists and working with them.”
“We [nephrologists] are more used to team-based care in dialysis patients. ... So we wanted to highlight that self-management programs and team-based care are important for empowering patients.”
“As nephrologists, we might not be comfortable starting patients on an SGLT2 [sodium-glucose cotransporter 2] inhibitor. We may need to reach out to our endocrinology or primary care colleagues and learn from them,” he explained.
RAS inhibitor use, smoking cessation, glycemic targets
Under “comprehensive care,” the guideline panel recommends treatment with an ACE inhibitor or an angiotensin II receptor blocker – renin-angiotensin system (RAS) blockade – for patients with diabetes, hypertension, and albuminuria (albumin-creatinine ratio >30 mg/g).
These medications should be titrated to the highest approved tolerated dose, with close monitoring of serum potassium and serum creatinine levels within 2-4 weeks of initiation or change in dose.
The document guides clinicians on that monitoring, as well as on RAS blockade use in patient subgroups, use of alternative agents, and mitigation of adverse effects.
Patients with diabetes and CKD who use tobacco should be advised to quit.
The group recommended A1c to monitor glycemic control in patients with diabetes and CKD not receiving dialysis.
However, when eGFR is below 30 mL/min per 1.73m2, A1c levels tend to be lower because of shortened erythrocyte lifespan, which interpretation should take into account. Continuous glucose monitoring can be used as an alternative because it is not affected by CKD.
Glycemic targets should be individualized depending on hypoglycemia risk, ranging from 6.5% to 8.0% for A1c or time in range of 70-180 mg/dL for continuous glucose monitoring readings.
SGLT2 inhibitors, metformin, and GLP-1 agonists
The panel also recommends treatment with both metformin and an SGLT2 inhibitor for patients with type 2 diabetes, CKD, and an eGFR ≥30 mL/min per 1.73m2.
For those who do not achieve glycemic targets or who cannot take those medications, a long-acting glucagonlike peptide–1 receptor agonist can be used instead.
Clinical trial data are summarized for the SGLT2 inhibitor canagliflozin supporting its use in patients with CKD specifically, along with mitigation of adverse events. Last year, the Food and Drug Administration approved this agent to slow the progression of diabetic nephropathy based on the CREDENCE study.
Results from the DAPA-CKD trial showing CKD reduction with another SGLT2 inhibitor, dapagliflozin, were not available at the time the new document was written, nor was the recent study showing diabetic CKD benefit for the novel mineralocorticoid receptor antagonist finerenone, Dr. Navaneethan noted.
The panel determined that there is insufficient evidence for adding other glucose-lowering agents to insulin in patients with type 1 diabetes and CKD.
Lifestyle interventions: Dietary protein, sodium, and physical activity
Most of the dietary guidance for patients with diabetes and CKD is the same as for the general population, including a recommendation to eat a diet high in vegetables, fruits, whole grains, fiber, legumes, plant-based proteins, unsaturated fats, and nuts, and lower in processed meats, refined carbohydrates, and sweetened beverages.
However, the guideline details two key areas that differ, one with regard to protein intake and the other on sodium.
Although lower protein intake had been advised in the past for patients with CKD, clinical trial evidence has not shown protein restriction to reduce glomerular hyperfiltration or slow kidney disease progression.
Therefore, the same level recommended for the general population – 0.8 g/kg per day – is also advised for those with diabetes and CKD who are not on dialysis.
Those who are on dialysis can increase daily protein intake to 1.0-1.2 g/kg per day to offset catabolism and negative nitrogen imbalance.
Because kidney function decline is associated with sodium retention that can raise cardiovascular risk, sodium should be limited to less than 2 g/day (or less than 90 mmol or 5 g of sodium chloride per day).
The panel also recommended moderate-intensity physical activity for at least 150 minutes per week or to tolerance.
“We wanted to emphasize how important lifestyle is. It’s the foundation you want to build on. You can take medications without all these other things – exercise, diet, weight loss – but they won’t be nearly as effective,” Dr. Navaneethan commented.
Self-management education, team-based care
The final section of the synopsis advises that people with diabetes and CKD receive structured self-management educational programs, and that “policy makers and institutional decision-makers implement team-based, integrated care focused on risk evaluation and patient empowerment to provide comprehensive care in patients with diabetes and CKD.”
Despite limited data for those measures specifically in patients with diabetes and CKD, “the working group believed that well-informed patients would choose self-management as the cornerstone of any chronic care model; therefore, a high value was placed on the potential benefits of self-management education programs in persons with diabetes and CKD.”
And regarding team-based care, “despite a paucity of direct evidence, the working group judged that multidisciplinary integrated care for patients with diabetes and CKD would represent a good investment.”
The guidelines will likely be updated in the next 1-2 years, Dr. Navaneethan said in an interview.
Dr. Navaneethan has reported receiving consultancy fees from Bayer, Boehringer Ingelheim, Reata, and Tricida, and research support from Keryx.
A version of this article originally appeared on Medscape.com.
A new guideline from the Kidney Disease: Improving Global Outcomes group addressing issues around diabetes management in patients with chronic kidney disease (CKD) has just been published in synopsis form in Annals of Internal Medicine.
The full guideline, including 12 recommendations and 48 practice points for clinicians caring for patients with diabetes and CKD, was published last month in Kidney International and on the KDIGO website.
More than 40% of people with diabetes develop CKD, and a significant number develop kidney failure requiring dialysis or transplant. This is the first guidance from KDIGO to address the comorbidity.
The new synopsis is aimed at primary care and nonnephrology specialist clinicians who manage patients with diabetes and CKD, in addition to nephrologists, first author Sankar D. Navaneethan, MD, said in an interview.
“Most of these patients are in the hands of primary care, endocrinology, and cardiology. We want to emphasize when they see patients with different severities of kidney disease [is] what are some of the things they have to be cognizant of,” said Dr. Navaneethan, professor of medicine and director of clinical research in the section of nephrology at Baylor College of Medicine, Houston.
The synopsis summarizes key recommendations from the larger guidance regarding comprehensive care needs, glycemic monitoring and targets, lifestyle interventions, glucose-lowering therapies, and educational/integrated care approaches.
It does not depart from prior diabetes guidelines, but it does provide advice for specific situations relevant to CKD, such as the limitations of hemoglobin A1c when estimated glomerular filtration rate (eGFR) drops below 30 mL/min per 1.73m2, and dietary protein consumption. It is based on published evidence up until February 2020.
For the nephrologist audience in particular, Dr. Navaneethan said, “we wanted to highlight team-based care, interacting with other specialists and working with them.”
“We [nephrologists] are more used to team-based care in dialysis patients. ... So we wanted to highlight that self-management programs and team-based care are important for empowering patients.”
“As nephrologists, we might not be comfortable starting patients on an SGLT2 [sodium-glucose cotransporter 2] inhibitor. We may need to reach out to our endocrinology or primary care colleagues and learn from them,” he explained.
RAS inhibitor use, smoking cessation, glycemic targets
Under “comprehensive care,” the guideline panel recommends treatment with an ACE inhibitor or an angiotensin II receptor blocker – renin-angiotensin system (RAS) blockade – for patients with diabetes, hypertension, and albuminuria (albumin-creatinine ratio >30 mg/g).
These medications should be titrated to the highest approved tolerated dose, with close monitoring of serum potassium and serum creatinine levels within 2-4 weeks of initiation or change in dose.
The document guides clinicians on that monitoring, as well as on RAS blockade use in patient subgroups, use of alternative agents, and mitigation of adverse effects.
Patients with diabetes and CKD who use tobacco should be advised to quit.
The group recommended A1c to monitor glycemic control in patients with diabetes and CKD not receiving dialysis.
However, when eGFR is below 30 mL/min per 1.73m2, A1c levels tend to be lower because of shortened erythrocyte lifespan, which interpretation should take into account. Continuous glucose monitoring can be used as an alternative because it is not affected by CKD.
Glycemic targets should be individualized depending on hypoglycemia risk, ranging from 6.5% to 8.0% for A1c or time in range of 70-180 mg/dL for continuous glucose monitoring readings.
SGLT2 inhibitors, metformin, and GLP-1 agonists
The panel also recommends treatment with both metformin and an SGLT2 inhibitor for patients with type 2 diabetes, CKD, and an eGFR ≥30 mL/min per 1.73m2.
For those who do not achieve glycemic targets or who cannot take those medications, a long-acting glucagonlike peptide–1 receptor agonist can be used instead.
Clinical trial data are summarized for the SGLT2 inhibitor canagliflozin supporting its use in patients with CKD specifically, along with mitigation of adverse events. Last year, the Food and Drug Administration approved this agent to slow the progression of diabetic nephropathy based on the CREDENCE study.
Results from the DAPA-CKD trial showing CKD reduction with another SGLT2 inhibitor, dapagliflozin, were not available at the time the new document was written, nor was the recent study showing diabetic CKD benefit for the novel mineralocorticoid receptor antagonist finerenone, Dr. Navaneethan noted.
The panel determined that there is insufficient evidence for adding other glucose-lowering agents to insulin in patients with type 1 diabetes and CKD.
Lifestyle interventions: Dietary protein, sodium, and physical activity
Most of the dietary guidance for patients with diabetes and CKD is the same as for the general population, including a recommendation to eat a diet high in vegetables, fruits, whole grains, fiber, legumes, plant-based proteins, unsaturated fats, and nuts, and lower in processed meats, refined carbohydrates, and sweetened beverages.
However, the guideline details two key areas that differ, one with regard to protein intake and the other on sodium.
Although lower protein intake had been advised in the past for patients with CKD, clinical trial evidence has not shown protein restriction to reduce glomerular hyperfiltration or slow kidney disease progression.
Therefore, the same level recommended for the general population – 0.8 g/kg per day – is also advised for those with diabetes and CKD who are not on dialysis.
Those who are on dialysis can increase daily protein intake to 1.0-1.2 g/kg per day to offset catabolism and negative nitrogen imbalance.
Because kidney function decline is associated with sodium retention that can raise cardiovascular risk, sodium should be limited to less than 2 g/day (or less than 90 mmol or 5 g of sodium chloride per day).
The panel also recommended moderate-intensity physical activity for at least 150 minutes per week or to tolerance.
“We wanted to emphasize how important lifestyle is. It’s the foundation you want to build on. You can take medications without all these other things – exercise, diet, weight loss – but they won’t be nearly as effective,” Dr. Navaneethan commented.
Self-management education, team-based care
The final section of the synopsis advises that people with diabetes and CKD receive structured self-management educational programs, and that “policy makers and institutional decision-makers implement team-based, integrated care focused on risk evaluation and patient empowerment to provide comprehensive care in patients with diabetes and CKD.”
Despite limited data for those measures specifically in patients with diabetes and CKD, “the working group believed that well-informed patients would choose self-management as the cornerstone of any chronic care model; therefore, a high value was placed on the potential benefits of self-management education programs in persons with diabetes and CKD.”
And regarding team-based care, “despite a paucity of direct evidence, the working group judged that multidisciplinary integrated care for patients with diabetes and CKD would represent a good investment.”
The guidelines will likely be updated in the next 1-2 years, Dr. Navaneethan said in an interview.
Dr. Navaneethan has reported receiving consultancy fees from Bayer, Boehringer Ingelheim, Reata, and Tricida, and research support from Keryx.
A version of this article originally appeared on Medscape.com.
A new guideline from the Kidney Disease: Improving Global Outcomes group addressing issues around diabetes management in patients with chronic kidney disease (CKD) has just been published in synopsis form in Annals of Internal Medicine.
The full guideline, including 12 recommendations and 48 practice points for clinicians caring for patients with diabetes and CKD, was published last month in Kidney International and on the KDIGO website.
More than 40% of people with diabetes develop CKD, and a significant number develop kidney failure requiring dialysis or transplant. This is the first guidance from KDIGO to address the comorbidity.
The new synopsis is aimed at primary care and nonnephrology specialist clinicians who manage patients with diabetes and CKD, in addition to nephrologists, first author Sankar D. Navaneethan, MD, said in an interview.
“Most of these patients are in the hands of primary care, endocrinology, and cardiology. We want to emphasize when they see patients with different severities of kidney disease [is] what are some of the things they have to be cognizant of,” said Dr. Navaneethan, professor of medicine and director of clinical research in the section of nephrology at Baylor College of Medicine, Houston.
The synopsis summarizes key recommendations from the larger guidance regarding comprehensive care needs, glycemic monitoring and targets, lifestyle interventions, glucose-lowering therapies, and educational/integrated care approaches.
It does not depart from prior diabetes guidelines, but it does provide advice for specific situations relevant to CKD, such as the limitations of hemoglobin A1c when estimated glomerular filtration rate (eGFR) drops below 30 mL/min per 1.73m2, and dietary protein consumption. It is based on published evidence up until February 2020.
For the nephrologist audience in particular, Dr. Navaneethan said, “we wanted to highlight team-based care, interacting with other specialists and working with them.”
“We [nephrologists] are more used to team-based care in dialysis patients. ... So we wanted to highlight that self-management programs and team-based care are important for empowering patients.”
“As nephrologists, we might not be comfortable starting patients on an SGLT2 [sodium-glucose cotransporter 2] inhibitor. We may need to reach out to our endocrinology or primary care colleagues and learn from them,” he explained.
RAS inhibitor use, smoking cessation, glycemic targets
Under “comprehensive care,” the guideline panel recommends treatment with an ACE inhibitor or an angiotensin II receptor blocker – renin-angiotensin system (RAS) blockade – for patients with diabetes, hypertension, and albuminuria (albumin-creatinine ratio >30 mg/g).
These medications should be titrated to the highest approved tolerated dose, with close monitoring of serum potassium and serum creatinine levels within 2-4 weeks of initiation or change in dose.
The document guides clinicians on that monitoring, as well as on RAS blockade use in patient subgroups, use of alternative agents, and mitigation of adverse effects.
Patients with diabetes and CKD who use tobacco should be advised to quit.
The group recommended A1c to monitor glycemic control in patients with diabetes and CKD not receiving dialysis.
However, when eGFR is below 30 mL/min per 1.73m2, A1c levels tend to be lower because of shortened erythrocyte lifespan, which interpretation should take into account. Continuous glucose monitoring can be used as an alternative because it is not affected by CKD.
Glycemic targets should be individualized depending on hypoglycemia risk, ranging from 6.5% to 8.0% for A1c or time in range of 70-180 mg/dL for continuous glucose monitoring readings.
SGLT2 inhibitors, metformin, and GLP-1 agonists
The panel also recommends treatment with both metformin and an SGLT2 inhibitor for patients with type 2 diabetes, CKD, and an eGFR ≥30 mL/min per 1.73m2.
For those who do not achieve glycemic targets or who cannot take those medications, a long-acting glucagonlike peptide–1 receptor agonist can be used instead.
Clinical trial data are summarized for the SGLT2 inhibitor canagliflozin supporting its use in patients with CKD specifically, along with mitigation of adverse events. Last year, the Food and Drug Administration approved this agent to slow the progression of diabetic nephropathy based on the CREDENCE study.
Results from the DAPA-CKD trial showing CKD reduction with another SGLT2 inhibitor, dapagliflozin, were not available at the time the new document was written, nor was the recent study showing diabetic CKD benefit for the novel mineralocorticoid receptor antagonist finerenone, Dr. Navaneethan noted.
The panel determined that there is insufficient evidence for adding other glucose-lowering agents to insulin in patients with type 1 diabetes and CKD.
Lifestyle interventions: Dietary protein, sodium, and physical activity
Most of the dietary guidance for patients with diabetes and CKD is the same as for the general population, including a recommendation to eat a diet high in vegetables, fruits, whole grains, fiber, legumes, plant-based proteins, unsaturated fats, and nuts, and lower in processed meats, refined carbohydrates, and sweetened beverages.
However, the guideline details two key areas that differ, one with regard to protein intake and the other on sodium.
Although lower protein intake had been advised in the past for patients with CKD, clinical trial evidence has not shown protein restriction to reduce glomerular hyperfiltration or slow kidney disease progression.
Therefore, the same level recommended for the general population – 0.8 g/kg per day – is also advised for those with diabetes and CKD who are not on dialysis.
Those who are on dialysis can increase daily protein intake to 1.0-1.2 g/kg per day to offset catabolism and negative nitrogen imbalance.
Because kidney function decline is associated with sodium retention that can raise cardiovascular risk, sodium should be limited to less than 2 g/day (or less than 90 mmol or 5 g of sodium chloride per day).
The panel also recommended moderate-intensity physical activity for at least 150 minutes per week or to tolerance.
“We wanted to emphasize how important lifestyle is. It’s the foundation you want to build on. You can take medications without all these other things – exercise, diet, weight loss – but they won’t be nearly as effective,” Dr. Navaneethan commented.
Self-management education, team-based care
The final section of the synopsis advises that people with diabetes and CKD receive structured self-management educational programs, and that “policy makers and institutional decision-makers implement team-based, integrated care focused on risk evaluation and patient empowerment to provide comprehensive care in patients with diabetes and CKD.”
Despite limited data for those measures specifically in patients with diabetes and CKD, “the working group believed that well-informed patients would choose self-management as the cornerstone of any chronic care model; therefore, a high value was placed on the potential benefits of self-management education programs in persons with diabetes and CKD.”
And regarding team-based care, “despite a paucity of direct evidence, the working group judged that multidisciplinary integrated care for patients with diabetes and CKD would represent a good investment.”
The guidelines will likely be updated in the next 1-2 years, Dr. Navaneethan said in an interview.
Dr. Navaneethan has reported receiving consultancy fees from Bayer, Boehringer Ingelheim, Reata, and Tricida, and research support from Keryx.
A version of this article originally appeared on Medscape.com.
Proposed Medicare rule would expand CGM coverage
A new proposed rule from the Centers for Medicare & Medicaid Services (CMS) would expand coverage for continuous glucose monitors (CGMs) under Medicare to include devices that aren’t approved for making treatment decisions.
If accepted, the proposed rule would classify all approved CGMs as durable medical equipment under Medicare Part B and establish payment amounts for all related supplies. The move primarily affects Medtronic’s Guardian Connect System, which has not been approved by the U.S. Food and Drug Administration to replace the need for fingersticks in determining insulin or other glucose-lowering medication dosing.
Two other CGM systems, the Dexcom G6 and Abbott Libre, have “therapeutic” indications and are, therefore, already covered under Medicare, as is the combined insulin pump–CGM Tandem Diabetes Care Control-IQ Technology system.
According to a CMS statement, “CGMs that are not approved for use in making diabetes treatment decisions can be used to alert beneficiaries about potentially dangerous glucose levels while they sleep and that they should further test their glucose levels using a blood glucose monitor. ... This proposal would give Medicare beneficiaries and their physicians a wider range of technology and devices to choose from in managing diabetes.”
Sean Salmon, executive vice president and president of the Diabetes Group at Medtronic said in an interview that the company is “very encouraged” by the proposal. “Importantly, the proposed rule would enable continuity of therapy for people on Medtronic insulin pumps aging into Medicare – including Medtronic hybrid closed loop systems, which automatically adjust insulin delivery based on readings from the integrated CGM.”
The type 1 diabetes research and advocacy organization JDRF also applauded the proposed rule, noting in a statement, “CGM technology can be an integral component of artificial pancreas systems and important on its own to significantly improve diabetes management and enable users to avoid potential crises and risks for long-term complications. JDRF is heartened by this proposed change as it has long advocated for coverage, affordability and choice of all therapies to help ensure people with T1D have what they need to survive.”
The proposal is part of a broader set of proposed changes to Medicare Durable Medical Equipment, Prosthetics, Orthotic Devices and Supplies (DMEPOS) coverage and payment policies. Comments on the entire document can be submitted through Jan. 4, 2021 to the Federal Register.
A new proposed rule from the Centers for Medicare & Medicaid Services (CMS) would expand coverage for continuous glucose monitors (CGMs) under Medicare to include devices that aren’t approved for making treatment decisions.
If accepted, the proposed rule would classify all approved CGMs as durable medical equipment under Medicare Part B and establish payment amounts for all related supplies. The move primarily affects Medtronic’s Guardian Connect System, which has not been approved by the U.S. Food and Drug Administration to replace the need for fingersticks in determining insulin or other glucose-lowering medication dosing.
Two other CGM systems, the Dexcom G6 and Abbott Libre, have “therapeutic” indications and are, therefore, already covered under Medicare, as is the combined insulin pump–CGM Tandem Diabetes Care Control-IQ Technology system.
According to a CMS statement, “CGMs that are not approved for use in making diabetes treatment decisions can be used to alert beneficiaries about potentially dangerous glucose levels while they sleep and that they should further test their glucose levels using a blood glucose monitor. ... This proposal would give Medicare beneficiaries and their physicians a wider range of technology and devices to choose from in managing diabetes.”
Sean Salmon, executive vice president and president of the Diabetes Group at Medtronic said in an interview that the company is “very encouraged” by the proposal. “Importantly, the proposed rule would enable continuity of therapy for people on Medtronic insulin pumps aging into Medicare – including Medtronic hybrid closed loop systems, which automatically adjust insulin delivery based on readings from the integrated CGM.”
The type 1 diabetes research and advocacy organization JDRF also applauded the proposed rule, noting in a statement, “CGM technology can be an integral component of artificial pancreas systems and important on its own to significantly improve diabetes management and enable users to avoid potential crises and risks for long-term complications. JDRF is heartened by this proposed change as it has long advocated for coverage, affordability and choice of all therapies to help ensure people with T1D have what they need to survive.”
The proposal is part of a broader set of proposed changes to Medicare Durable Medical Equipment, Prosthetics, Orthotic Devices and Supplies (DMEPOS) coverage and payment policies. Comments on the entire document can be submitted through Jan. 4, 2021 to the Federal Register.
A new proposed rule from the Centers for Medicare & Medicaid Services (CMS) would expand coverage for continuous glucose monitors (CGMs) under Medicare to include devices that aren’t approved for making treatment decisions.
If accepted, the proposed rule would classify all approved CGMs as durable medical equipment under Medicare Part B and establish payment amounts for all related supplies. The move primarily affects Medtronic’s Guardian Connect System, which has not been approved by the U.S. Food and Drug Administration to replace the need for fingersticks in determining insulin or other glucose-lowering medication dosing.
Two other CGM systems, the Dexcom G6 and Abbott Libre, have “therapeutic” indications and are, therefore, already covered under Medicare, as is the combined insulin pump–CGM Tandem Diabetes Care Control-IQ Technology system.
According to a CMS statement, “CGMs that are not approved for use in making diabetes treatment decisions can be used to alert beneficiaries about potentially dangerous glucose levels while they sleep and that they should further test their glucose levels using a blood glucose monitor. ... This proposal would give Medicare beneficiaries and their physicians a wider range of technology and devices to choose from in managing diabetes.”
Sean Salmon, executive vice president and president of the Diabetes Group at Medtronic said in an interview that the company is “very encouraged” by the proposal. “Importantly, the proposed rule would enable continuity of therapy for people on Medtronic insulin pumps aging into Medicare – including Medtronic hybrid closed loop systems, which automatically adjust insulin delivery based on readings from the integrated CGM.”
The type 1 diabetes research and advocacy organization JDRF also applauded the proposed rule, noting in a statement, “CGM technology can be an integral component of artificial pancreas systems and important on its own to significantly improve diabetes management and enable users to avoid potential crises and risks for long-term complications. JDRF is heartened by this proposed change as it has long advocated for coverage, affordability and choice of all therapies to help ensure people with T1D have what they need to survive.”
The proposal is part of a broader set of proposed changes to Medicare Durable Medical Equipment, Prosthetics, Orthotic Devices and Supplies (DMEPOS) coverage and payment policies. Comments on the entire document can be submitted through Jan. 4, 2021 to the Federal Register.
Diabetes screening program in optometry offices to expand
The program is sponsored by VSP Vision Care, a vision benefits company with over 40,000 network optometrists and nearly 90 million consumer members worldwide. “Optometrists are often the first to detect signs of diabetes by looking at the blood vessels in the eye during a comprehensive eye exam,” the company said in a statement.
In the pilot program, conducted from May 2019 to February 2020* in 12 VSP practices in five states, 818 patients who had come in for their annual vision exam were given the American Diabetes Association Risk Test for type 2 diabetes, and 287 identified at risk were offered an in-office fingerstick hemoglobin A1c test.
Materials were provided free to the optometrists, who were paid a professional fee to perform the HbA1c screenings.
Of the 287 eligible for the HbA1c test, 85% took it. Of those 244, 31% and 5% had levels in the prediabetes and diabetes range, respectively. None had been aware of their status previously, and 92% rated the screening as an extremely or very positive experience.
Now, VSP is expanding the pilot program for another year with two large clients in Ohio covering about 90,000 members.
“Coupled with the fact that VSP members are more likely to get their annual eye exam over their annual physical exam with their primary care physician, HbA1c screenings provided by eye doctors offer another critical way to detect the chronic condition earlier and help prevent eye disease and even vision loss caused by diabetes,” according to the statement.
In an interview, a VSP spokesperson explained that if the patient provides their primary care provider information to the optometrist, the optometrist will send a referral with exam information to that provider and also instruct the patient to make an appointment with the provider for follow-up testing and care.
The optometrist also educates the patient about the connection between eye health and overall health and provides them with a flier that gives tips on lifestyle changes they can make to help slow or prevent the progression to type 2 diabetes, the spokesperson said.
Thirty states, including Ohio, allow optometrists to perform in-office blood testing, including HbA1c screening, provided they obtain a Clinical Laboratory Improvement Amendments Certificate of Waiver. VSP is providing online training for participating optometrists on administering the HbA1c screening.
The pilot program is part of an alliance between VSP and the American Diabetes Association formed in November 2019 to raise awareness of eye health in people with diabetes and those at risk for it.
*Correction: The original article included the wrong end date for the pilot program.
The program is sponsored by VSP Vision Care, a vision benefits company with over 40,000 network optometrists and nearly 90 million consumer members worldwide. “Optometrists are often the first to detect signs of diabetes by looking at the blood vessels in the eye during a comprehensive eye exam,” the company said in a statement.
In the pilot program, conducted from May 2019 to February 2020* in 12 VSP practices in five states, 818 patients who had come in for their annual vision exam were given the American Diabetes Association Risk Test for type 2 diabetes, and 287 identified at risk were offered an in-office fingerstick hemoglobin A1c test.
Materials were provided free to the optometrists, who were paid a professional fee to perform the HbA1c screenings.
Of the 287 eligible for the HbA1c test, 85% took it. Of those 244, 31% and 5% had levels in the prediabetes and diabetes range, respectively. None had been aware of their status previously, and 92% rated the screening as an extremely or very positive experience.
Now, VSP is expanding the pilot program for another year with two large clients in Ohio covering about 90,000 members.
“Coupled with the fact that VSP members are more likely to get their annual eye exam over their annual physical exam with their primary care physician, HbA1c screenings provided by eye doctors offer another critical way to detect the chronic condition earlier and help prevent eye disease and even vision loss caused by diabetes,” according to the statement.
In an interview, a VSP spokesperson explained that if the patient provides their primary care provider information to the optometrist, the optometrist will send a referral with exam information to that provider and also instruct the patient to make an appointment with the provider for follow-up testing and care.
The optometrist also educates the patient about the connection between eye health and overall health and provides them with a flier that gives tips on lifestyle changes they can make to help slow or prevent the progression to type 2 diabetes, the spokesperson said.
Thirty states, including Ohio, allow optometrists to perform in-office blood testing, including HbA1c screening, provided they obtain a Clinical Laboratory Improvement Amendments Certificate of Waiver. VSP is providing online training for participating optometrists on administering the HbA1c screening.
The pilot program is part of an alliance between VSP and the American Diabetes Association formed in November 2019 to raise awareness of eye health in people with diabetes and those at risk for it.
*Correction: The original article included the wrong end date for the pilot program.
The program is sponsored by VSP Vision Care, a vision benefits company with over 40,000 network optometrists and nearly 90 million consumer members worldwide. “Optometrists are often the first to detect signs of diabetes by looking at the blood vessels in the eye during a comprehensive eye exam,” the company said in a statement.
In the pilot program, conducted from May 2019 to February 2020* in 12 VSP practices in five states, 818 patients who had come in for their annual vision exam were given the American Diabetes Association Risk Test for type 2 diabetes, and 287 identified at risk were offered an in-office fingerstick hemoglobin A1c test.
Materials were provided free to the optometrists, who were paid a professional fee to perform the HbA1c screenings.
Of the 287 eligible for the HbA1c test, 85% took it. Of those 244, 31% and 5% had levels in the prediabetes and diabetes range, respectively. None had been aware of their status previously, and 92% rated the screening as an extremely or very positive experience.
Now, VSP is expanding the pilot program for another year with two large clients in Ohio covering about 90,000 members.
“Coupled with the fact that VSP members are more likely to get their annual eye exam over their annual physical exam with their primary care physician, HbA1c screenings provided by eye doctors offer another critical way to detect the chronic condition earlier and help prevent eye disease and even vision loss caused by diabetes,” according to the statement.
In an interview, a VSP spokesperson explained that if the patient provides their primary care provider information to the optometrist, the optometrist will send a referral with exam information to that provider and also instruct the patient to make an appointment with the provider for follow-up testing and care.
The optometrist also educates the patient about the connection between eye health and overall health and provides them with a flier that gives tips on lifestyle changes they can make to help slow or prevent the progression to type 2 diabetes, the spokesperson said.
Thirty states, including Ohio, allow optometrists to perform in-office blood testing, including HbA1c screening, provided they obtain a Clinical Laboratory Improvement Amendments Certificate of Waiver. VSP is providing online training for participating optometrists on administering the HbA1c screening.
The pilot program is part of an alliance between VSP and the American Diabetes Association formed in November 2019 to raise awareness of eye health in people with diabetes and those at risk for it.
*Correction: The original article included the wrong end date for the pilot program.