User login
USPSTF: No recommendation on screening for celiac disease
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
AGA Resource
The AGA celiac disease patient education materials will help you discuss the disorder and management with your patients. Review and download the materials, in English and Spanish, at http://www.gastro.org/patient-care/conditions-diseases/celiac-disease.
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
AGA Resource
The AGA celiac disease patient education materials will help you discuss the disorder and management with your patients. Review and download the materials, in English and Spanish, at http://www.gastro.org/patient-care/conditions-diseases/celiac-disease.
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
AGA Resource
The AGA celiac disease patient education materials will help you discuss the disorder and management with your patients. Review and download the materials, in English and Spanish, at http://www.gastro.org/patient-care/conditions-diseases/celiac-disease.
FROM JAMA
Key clinical point: The current evidence is insufficient for the USPSTF to recommend either for or against routine screening of asymptomatic people for celiac disease.
Major finding: Only 4 studies out of the 3,036 that were examined addressed the question of screening adequately.
Data source: An assessment of the benefits and harms of screening based on a review of four studies.
Disclosures: The USPSTF’s work is supported by the U.S. Agency for Healthcare Research and Quality. The authors’ financial disclosures are available at www.uspreventiveservicestaskforce.org.
Nonoperative management of pediatric appendicitis appears feasible
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
FROM JAMA PEDIATRICS
Key clinical point: Nonoperative management of uncomplicated appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach.
Major finding: The rate of treatment success in the nonoperative group was 90.5% (152 of 168 patients).
Data source: A metaanalysis of five single-center clinical trials involving 404 patients aged 5-18 years.
Disclosures: No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
Gradual increase of nonoperative management of selected abdominal gunshot wounds
Selective nonoperative management of abdominal gunshot wounds has progressed from heresy a few years ago to established practice now, at least at Level I and Level II trauma centers across New England, according to a report published online in the Journal of the American College of Surgeons.
“Mandatory laparotomy used to be the only acceptable standard of care” for this patient population, but many surgeons now realize that a hole in the abdomen doesn’t necessitate immediate laparotomy. “Patients with a reliable clinical exam, who are hemodynamically stable and without signs of peritonitis, can be observed under structured protocols of close monitoring, frequent clinical exams, and appropriate imaging,” said Thomas Peponis, MD, of Massachusetts General Hospital, Boston, and his associates.
Two findings belie the main arguments that opponents of SNOM have made in favor of routine laparotomy: delaying surgery leads to devastating consequences and “unnecessary” laparotomies are virtually harmless.
The first finding was that the 18 patients in the SNOM group (1.9% of the entire study population) who eventually required a delayed laparotomy had few postoperative complications, and none of the complications appeared to be directly related to the delay in surgery. Those SNOM patients who eventually had surgery did not differ in terms of age, sex, location of the gunshot wound, vitals on ED presentation, Glasgow Coma Scale on ED presentation, presence of hemodynamic instability, or presence of diffuse abdominal tenderness on clinical exam.
The second finding concerned the 104 patients who underwent immediate exploratory laparotomy. Dr. Peponis and his associates deemed the immediacy to be nontherapeutic (unnecessary) because “the mere presence of a hole to the abdomen was the only indication” for the surgery. Nearly one in six patients operated on for an abdominal gunshot wound underwent a nontherapeutic laparotomy. Of those, 18 (17.3%) developed postoperative complications, including wound infections, ileus, pneumonia, pleural effusion requiring a chest tube, intra-abdominal abscess, acute kidney injury, sepsis, venous embolus, and a fistula related to a retained bullet.
The rate of abdominal gunshot wounds treated nonoperatively in the centers studied has grown from around 18% before 2010 to 27% in the following years. The increasing use of CT scans has bolstered the trend, but the clinical exam remains the critical element in deciding whether to operate immediately. The investigators recommended immediate surgery for all abdominal gunshot wound patients who are hemodynamically unstable or who exhibit diffuse abdominal tenderness. “There is no other place for a patient with an abdominal gunshot wound and definitively worsening clinical symptoms than the OR. The remaining patients are appropriate for SNOM under close observation, repeat clinical evaluations, and immediate OR availability in case the clinical picture changes.”
The limitations of the study are the following: First, it represents only Level I and II centers with experienced trauma teams. Second, there is no commonly established protocol across trauma centers for SNOM, giving rise to a variability in decision making and care. Third, the definition of immediate and delayed surgery was within a 2-hour window, a somewhat arbitrary time period.
The investigation was sponsored by the Research Consortium of New England Centers for Trauma (ReCoNECT). The authors had no disclosures.
Selective nonoperative management of abdominal gunshot wounds has progressed from heresy a few years ago to established practice now, at least at Level I and Level II trauma centers across New England, according to a report published online in the Journal of the American College of Surgeons.
“Mandatory laparotomy used to be the only acceptable standard of care” for this patient population, but many surgeons now realize that a hole in the abdomen doesn’t necessitate immediate laparotomy. “Patients with a reliable clinical exam, who are hemodynamically stable and without signs of peritonitis, can be observed under structured protocols of close monitoring, frequent clinical exams, and appropriate imaging,” said Thomas Peponis, MD, of Massachusetts General Hospital, Boston, and his associates.
Two findings belie the main arguments that opponents of SNOM have made in favor of routine laparotomy: delaying surgery leads to devastating consequences and “unnecessary” laparotomies are virtually harmless.
The first finding was that the 18 patients in the SNOM group (1.9% of the entire study population) who eventually required a delayed laparotomy had few postoperative complications, and none of the complications appeared to be directly related to the delay in surgery. Those SNOM patients who eventually had surgery did not differ in terms of age, sex, location of the gunshot wound, vitals on ED presentation, Glasgow Coma Scale on ED presentation, presence of hemodynamic instability, or presence of diffuse abdominal tenderness on clinical exam.
The second finding concerned the 104 patients who underwent immediate exploratory laparotomy. Dr. Peponis and his associates deemed the immediacy to be nontherapeutic (unnecessary) because “the mere presence of a hole to the abdomen was the only indication” for the surgery. Nearly one in six patients operated on for an abdominal gunshot wound underwent a nontherapeutic laparotomy. Of those, 18 (17.3%) developed postoperative complications, including wound infections, ileus, pneumonia, pleural effusion requiring a chest tube, intra-abdominal abscess, acute kidney injury, sepsis, venous embolus, and a fistula related to a retained bullet.
The rate of abdominal gunshot wounds treated nonoperatively in the centers studied has grown from around 18% before 2010 to 27% in the following years. The increasing use of CT scans has bolstered the trend, but the clinical exam remains the critical element in deciding whether to operate immediately. The investigators recommended immediate surgery for all abdominal gunshot wound patients who are hemodynamically unstable or who exhibit diffuse abdominal tenderness. “There is no other place for a patient with an abdominal gunshot wound and definitively worsening clinical symptoms than the OR. The remaining patients are appropriate for SNOM under close observation, repeat clinical evaluations, and immediate OR availability in case the clinical picture changes.”
The limitations of the study are the following: First, it represents only Level I and II centers with experienced trauma teams. Second, there is no commonly established protocol across trauma centers for SNOM, giving rise to a variability in decision making and care. Third, the definition of immediate and delayed surgery was within a 2-hour window, a somewhat arbitrary time period.
The investigation was sponsored by the Research Consortium of New England Centers for Trauma (ReCoNECT). The authors had no disclosures.
Selective nonoperative management of abdominal gunshot wounds has progressed from heresy a few years ago to established practice now, at least at Level I and Level II trauma centers across New England, according to a report published online in the Journal of the American College of Surgeons.
“Mandatory laparotomy used to be the only acceptable standard of care” for this patient population, but many surgeons now realize that a hole in the abdomen doesn’t necessitate immediate laparotomy. “Patients with a reliable clinical exam, who are hemodynamically stable and without signs of peritonitis, can be observed under structured protocols of close monitoring, frequent clinical exams, and appropriate imaging,” said Thomas Peponis, MD, of Massachusetts General Hospital, Boston, and his associates.
Two findings belie the main arguments that opponents of SNOM have made in favor of routine laparotomy: delaying surgery leads to devastating consequences and “unnecessary” laparotomies are virtually harmless.
The first finding was that the 18 patients in the SNOM group (1.9% of the entire study population) who eventually required a delayed laparotomy had few postoperative complications, and none of the complications appeared to be directly related to the delay in surgery. Those SNOM patients who eventually had surgery did not differ in terms of age, sex, location of the gunshot wound, vitals on ED presentation, Glasgow Coma Scale on ED presentation, presence of hemodynamic instability, or presence of diffuse abdominal tenderness on clinical exam.
The second finding concerned the 104 patients who underwent immediate exploratory laparotomy. Dr. Peponis and his associates deemed the immediacy to be nontherapeutic (unnecessary) because “the mere presence of a hole to the abdomen was the only indication” for the surgery. Nearly one in six patients operated on for an abdominal gunshot wound underwent a nontherapeutic laparotomy. Of those, 18 (17.3%) developed postoperative complications, including wound infections, ileus, pneumonia, pleural effusion requiring a chest tube, intra-abdominal abscess, acute kidney injury, sepsis, venous embolus, and a fistula related to a retained bullet.
The rate of abdominal gunshot wounds treated nonoperatively in the centers studied has grown from around 18% before 2010 to 27% in the following years. The increasing use of CT scans has bolstered the trend, but the clinical exam remains the critical element in deciding whether to operate immediately. The investigators recommended immediate surgery for all abdominal gunshot wound patients who are hemodynamically unstable or who exhibit diffuse abdominal tenderness. “There is no other place for a patient with an abdominal gunshot wound and definitively worsening clinical symptoms than the OR. The remaining patients are appropriate for SNOM under close observation, repeat clinical evaluations, and immediate OR availability in case the clinical picture changes.”
The limitations of the study are the following: First, it represents only Level I and II centers with experienced trauma teams. Second, there is no commonly established protocol across trauma centers for SNOM, giving rise to a variability in decision making and care. Third, the definition of immediate and delayed surgery was within a 2-hour window, a somewhat arbitrary time period.
The investigation was sponsored by the Research Consortium of New England Centers for Trauma (ReCoNECT). The authors had no disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Selective nonoperative management of abdominal gunshot wounds has progressed from heresy a few years ago to established practice now.
Major finding: 197 patients (91.6% of the nonoperative group and 21.4% of the entire study population) were successfully managed nonoperatively and were discharged without requiring any abdominal surgery.
Data source: A retrospective review of the medical records of 922 gunshot wound patients treated at 10 New England trauma centers during a 20-year period.
Disclosures: The investigation was a multicenter study of the Research Consortium of New England Centers for Trauma (ReCoNECT). Dr. Peponis and his associates reported having no relevant financial disclosures.
People often think functional neurological symptoms are feigned
Clinicians, other caregivers, and even patients often think that functional neurological symptoms and movement disorders are feigned or somewhat amenable to the patient’s control, according to two separate survey studies published online in the Journal of Neurology, Neurosurgery & Psychiatry.
In the first study, Sandra M. A. van der Salm, MD, and her associates reported their assessments of clinicians’ and patients’ views about “free will” pertaining to three movement disorders: functional (“psychogenic”) movement disorders, myoclonus, and tics. They administered survey questionnaires to 39 expert clinicians, 28 patients with functional movement disorders, 15 patients with myoclonus, 17 patients with tics, and 22 healthy control subjects.
Clinicians differed markedly from patients in their perceptions of patient control over movements and symptoms. The majority rated “raising one’s hand to vote” as completely voluntary, functional movement disorders as largely involuntary, tics as slightly more involuntary than voluntary, and myoclonus as completely involuntary. In contrast, most patients (other than some who said they were sometimes able to suppress tics) felt their movements and symptoms were well beyond their control, reported Dr. van der Salm of the department of neurology and clinical neurophysiology, Academic Medical Center, Amsterdam, and her associates (J Neurol Neurosurg Psychiatry. 2017 March 11. doi: 10.1136/jnnp-2016-315152).
“For clinicians, it may be helpful to realize that doctors and patients may have different views of voluntariness in movement disorders, in particular regarding tics and functional movement disorders. Keeping this gap in mind during consultation could prevent misunderstandings that might jeopardize the doctor-patient relationship,” the investigators wrote.
“Our results suggest that patients with functional movement disorders probably require different cognitive behavioral techniques than those used for tics, because patients with functional movement disorders experience less behavioral control” of their symptoms, they wrote.
In the second study, Richard A. A. Kanaan, MD, and Juen Mei Ding, MD, both in the department of psychiatry at the University of Melbourne, reported the results of their survey of 172 “people waiting in a general hospital outpatient department,” including patients, family members, and caregivers.
The questionnaire presented a hypothetical scenario to the respondent in which he or she had “leg weakness” for which all tests had come back negative. A doctor presented seven possible terms for the symptoms and then asked respondents whether such patients would be imagining their symptoms, faking their symptoms, mentally ill, or having a medical condition. The seven diagnoses were functional weakness, psychogenic weakness, medically unexplained weakness, somatic symptom disorder, dissociative disorder, conversion disorder, and stroke.
A total of 26% of the respondents said that at least one of the diagnoses connoted feigning, “confirming that suspicions of feigning in functional neurological symptoms remain a problem among patients and the public,” wrote Dr. Kanaan and Dr. Ding (J Neurol Neurosurg Psychiatry. 2017 March 9. doi: 10.1136/jnnp-2016-315224).
There was a strong correlation between the respondents’ opinions of “faking” and their level of education. Study participants with only a primary-school education were the most likely to think symptoms were feigned (32 of 94), while those with a tertiary education were very unlikely to think so (9 of 73), the investigators said.
It was encouraging that this correlation disappeared after a “simple remedy” was applied: briefly explaining the meaning of each diagnosis. Thus, “the problem appears to be largely one of public ignorance, rather than some inherent stigmatization of mental health,” Dr. Kanaan and Dr. Ding wrote.
Dr. van der Salm’s study was funded by the Academic Medical Center graduate school. No funding source was cited for the study by Dr. Kanaan and Dr. Ding. Both research groups reported having no relevant financial disclosures.
These two studies make interesting and worthwhile observations. They do make it clear that both the public and many physicians consider that many functional movement disorder patients have some voluntariness and hence are faking it. This is a stigma. A number of us have been trying to educate at least the physicians that almost all these patients do have involuntary movements. It is a problem for doctor-patient relationships and for patients to accept their diagnosis. We still have work to do!
Mark Hallett, MD, is chief of the National Institute of Neurological Disorders and Stroke’s Medical Neurology Branch and chief of its Human Motor Control Section. He is now president of the International Federation of Clinical Neurophysiology. He has been president of the Movement Disorder Society and vice president of the American Academy of Neurology.
These two studies make interesting and worthwhile observations. They do make it clear that both the public and many physicians consider that many functional movement disorder patients have some voluntariness and hence are faking it. This is a stigma. A number of us have been trying to educate at least the physicians that almost all these patients do have involuntary movements. It is a problem for doctor-patient relationships and for patients to accept their diagnosis. We still have work to do!
Mark Hallett, MD, is chief of the National Institute of Neurological Disorders and Stroke’s Medical Neurology Branch and chief of its Human Motor Control Section. He is now president of the International Federation of Clinical Neurophysiology. He has been president of the Movement Disorder Society and vice president of the American Academy of Neurology.
These two studies make interesting and worthwhile observations. They do make it clear that both the public and many physicians consider that many functional movement disorder patients have some voluntariness and hence are faking it. This is a stigma. A number of us have been trying to educate at least the physicians that almost all these patients do have involuntary movements. It is a problem for doctor-patient relationships and for patients to accept their diagnosis. We still have work to do!
Mark Hallett, MD, is chief of the National Institute of Neurological Disorders and Stroke’s Medical Neurology Branch and chief of its Human Motor Control Section. He is now president of the International Federation of Clinical Neurophysiology. He has been president of the Movement Disorder Society and vice president of the American Academy of Neurology.
Clinicians, other caregivers, and even patients often think that functional neurological symptoms and movement disorders are feigned or somewhat amenable to the patient’s control, according to two separate survey studies published online in the Journal of Neurology, Neurosurgery & Psychiatry.
In the first study, Sandra M. A. van der Salm, MD, and her associates reported their assessments of clinicians’ and patients’ views about “free will” pertaining to three movement disorders: functional (“psychogenic”) movement disorders, myoclonus, and tics. They administered survey questionnaires to 39 expert clinicians, 28 patients with functional movement disorders, 15 patients with myoclonus, 17 patients with tics, and 22 healthy control subjects.
Clinicians differed markedly from patients in their perceptions of patient control over movements and symptoms. The majority rated “raising one’s hand to vote” as completely voluntary, functional movement disorders as largely involuntary, tics as slightly more involuntary than voluntary, and myoclonus as completely involuntary. In contrast, most patients (other than some who said they were sometimes able to suppress tics) felt their movements and symptoms were well beyond their control, reported Dr. van der Salm of the department of neurology and clinical neurophysiology, Academic Medical Center, Amsterdam, and her associates (J Neurol Neurosurg Psychiatry. 2017 March 11. doi: 10.1136/jnnp-2016-315152).
“For clinicians, it may be helpful to realize that doctors and patients may have different views of voluntariness in movement disorders, in particular regarding tics and functional movement disorders. Keeping this gap in mind during consultation could prevent misunderstandings that might jeopardize the doctor-patient relationship,” the investigators wrote.
“Our results suggest that patients with functional movement disorders probably require different cognitive behavioral techniques than those used for tics, because patients with functional movement disorders experience less behavioral control” of their symptoms, they wrote.
In the second study, Richard A. A. Kanaan, MD, and Juen Mei Ding, MD, both in the department of psychiatry at the University of Melbourne, reported the results of their survey of 172 “people waiting in a general hospital outpatient department,” including patients, family members, and caregivers.
The questionnaire presented a hypothetical scenario to the respondent in which he or she had “leg weakness” for which all tests had come back negative. A doctor presented seven possible terms for the symptoms and then asked respondents whether such patients would be imagining their symptoms, faking their symptoms, mentally ill, or having a medical condition. The seven diagnoses were functional weakness, psychogenic weakness, medically unexplained weakness, somatic symptom disorder, dissociative disorder, conversion disorder, and stroke.
A total of 26% of the respondents said that at least one of the diagnoses connoted feigning, “confirming that suspicions of feigning in functional neurological symptoms remain a problem among patients and the public,” wrote Dr. Kanaan and Dr. Ding (J Neurol Neurosurg Psychiatry. 2017 March 9. doi: 10.1136/jnnp-2016-315224).
There was a strong correlation between the respondents’ opinions of “faking” and their level of education. Study participants with only a primary-school education were the most likely to think symptoms were feigned (32 of 94), while those with a tertiary education were very unlikely to think so (9 of 73), the investigators said.
It was encouraging that this correlation disappeared after a “simple remedy” was applied: briefly explaining the meaning of each diagnosis. Thus, “the problem appears to be largely one of public ignorance, rather than some inherent stigmatization of mental health,” Dr. Kanaan and Dr. Ding wrote.
Dr. van der Salm’s study was funded by the Academic Medical Center graduate school. No funding source was cited for the study by Dr. Kanaan and Dr. Ding. Both research groups reported having no relevant financial disclosures.
Clinicians, other caregivers, and even patients often think that functional neurological symptoms and movement disorders are feigned or somewhat amenable to the patient’s control, according to two separate survey studies published online in the Journal of Neurology, Neurosurgery & Psychiatry.
In the first study, Sandra M. A. van der Salm, MD, and her associates reported their assessments of clinicians’ and patients’ views about “free will” pertaining to three movement disorders: functional (“psychogenic”) movement disorders, myoclonus, and tics. They administered survey questionnaires to 39 expert clinicians, 28 patients with functional movement disorders, 15 patients with myoclonus, 17 patients with tics, and 22 healthy control subjects.
Clinicians differed markedly from patients in their perceptions of patient control over movements and symptoms. The majority rated “raising one’s hand to vote” as completely voluntary, functional movement disorders as largely involuntary, tics as slightly more involuntary than voluntary, and myoclonus as completely involuntary. In contrast, most patients (other than some who said they were sometimes able to suppress tics) felt their movements and symptoms were well beyond their control, reported Dr. van der Salm of the department of neurology and clinical neurophysiology, Academic Medical Center, Amsterdam, and her associates (J Neurol Neurosurg Psychiatry. 2017 March 11. doi: 10.1136/jnnp-2016-315152).
“For clinicians, it may be helpful to realize that doctors and patients may have different views of voluntariness in movement disorders, in particular regarding tics and functional movement disorders. Keeping this gap in mind during consultation could prevent misunderstandings that might jeopardize the doctor-patient relationship,” the investigators wrote.
“Our results suggest that patients with functional movement disorders probably require different cognitive behavioral techniques than those used for tics, because patients with functional movement disorders experience less behavioral control” of their symptoms, they wrote.
In the second study, Richard A. A. Kanaan, MD, and Juen Mei Ding, MD, both in the department of psychiatry at the University of Melbourne, reported the results of their survey of 172 “people waiting in a general hospital outpatient department,” including patients, family members, and caregivers.
The questionnaire presented a hypothetical scenario to the respondent in which he or she had “leg weakness” for which all tests had come back negative. A doctor presented seven possible terms for the symptoms and then asked respondents whether such patients would be imagining their symptoms, faking their symptoms, mentally ill, or having a medical condition. The seven diagnoses were functional weakness, psychogenic weakness, medically unexplained weakness, somatic symptom disorder, dissociative disorder, conversion disorder, and stroke.
A total of 26% of the respondents said that at least one of the diagnoses connoted feigning, “confirming that suspicions of feigning in functional neurological symptoms remain a problem among patients and the public,” wrote Dr. Kanaan and Dr. Ding (J Neurol Neurosurg Psychiatry. 2017 March 9. doi: 10.1136/jnnp-2016-315224).
There was a strong correlation between the respondents’ opinions of “faking” and their level of education. Study participants with only a primary-school education were the most likely to think symptoms were feigned (32 of 94), while those with a tertiary education were very unlikely to think so (9 of 73), the investigators said.
It was encouraging that this correlation disappeared after a “simple remedy” was applied: briefly explaining the meaning of each diagnosis. Thus, “the problem appears to be largely one of public ignorance, rather than some inherent stigmatization of mental health,” Dr. Kanaan and Dr. Ding wrote.
Dr. van der Salm’s study was funded by the Academic Medical Center graduate school. No funding source was cited for the study by Dr. Kanaan and Dr. Ding. Both research groups reported having no relevant financial disclosures.
FROM JOURNAL OF NEUROLOGY, NEUROSURGERY & PSYCHIATRY
Key clinical point:
Major finding: Most clinicians rated “raising one’s hand to vote” as completely voluntary, functional movement disorders as largely voluntary, tics as slightly more involuntary than voluntary, and myoclonus as completely involuntary.
Data source: Two separate survey questionnaires involving 172 respondents and 121 respondents, respectively.
Disclosures: Dr. van der Salm’s study was funded by the Academic Medical Center graduate school. No funding source was cited for the study by Dr. Kanaan and Dr. Ding. Both research groups reported having no relevant financial disclosures.
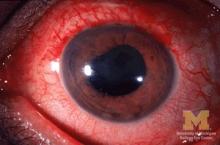
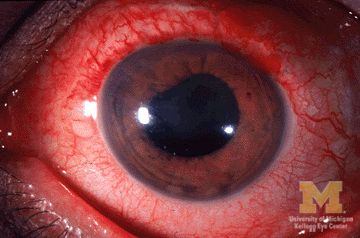
Etanercept found not optimal for reducing anterior uveitis in ankylosing spondylitis
Two anti–tumor necrosis factor monoclonal antibodies, adalimumab and infliximab, showed evidence of being markedly more effective than the anti-TNF–receptor inhibitor etanercept at reducing the rate of anterior uveitis in patients with ankylosing spondylitis in a retrospective Swedish cohort study.
To compare the efficacy of the three TNF inhibitors, researchers analyzed data in nationwide Swedish population-based registries for 1,365 ankylosing spondylitis (AS) patients who initiated treatment during a 7-year period. Treatment began with adalimumab in 406 patients, infliximab in 605, and etanercept in 354, said Elisabeth Lie, MD, of the department of rheumatology and inflammation research at the University of Gothenburg (Sweden), and her associates.
“Compared with the rates [of anterior uveitis] pretreatment, the rates increased when initiating treatment with etanercept, but decreased when starting adalimumab or infliximab,” the investigators wrote (Ann Rheum Dis. 2017 Mar 2. doi: 10.1136/annrheumdis-2016-210931).
The biological explanation for this discrepancy is unclear. It is possible that etanercept simply isn’t as protective as the other two agents, but it also appears possible that etanercept may act paradoxically to induce anterior uveitis in some patients. However, it should be noted that “previous studies have indicated that etanercept still reduces the number of uveitis flares more effectively than placebo,” Dr. Lie and her associates noted.
Regardless of the underlying reason, these findings, taken together with those of previous studies, “support the choice of another TNF inhibitor than etanercept in patients with AS with a history of anterior uveitis,” they said.
Dr. Lie also reported the results at the 2015 American College of Rheumatology annual meeting.
This study was supported by the Swedish Research Council, Gothenburg University, the Stockholm County Council, the Swedish National Rheumatism Association, the Swedish COMBINE public-private research program, the Swedish Cancer Society, the EU-IMI BT Cure project, and the Swedish Foundation for Strategic Research. Dr. Lie reported receiving personal fees from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, and UCB; her associates reported ties to numerous industry sources.
Two anti–tumor necrosis factor monoclonal antibodies, adalimumab and infliximab, showed evidence of being markedly more effective than the anti-TNF–receptor inhibitor etanercept at reducing the rate of anterior uveitis in patients with ankylosing spondylitis in a retrospective Swedish cohort study.
To compare the efficacy of the three TNF inhibitors, researchers analyzed data in nationwide Swedish population-based registries for 1,365 ankylosing spondylitis (AS) patients who initiated treatment during a 7-year period. Treatment began with adalimumab in 406 patients, infliximab in 605, and etanercept in 354, said Elisabeth Lie, MD, of the department of rheumatology and inflammation research at the University of Gothenburg (Sweden), and her associates.
“Compared with the rates [of anterior uveitis] pretreatment, the rates increased when initiating treatment with etanercept, but decreased when starting adalimumab or infliximab,” the investigators wrote (Ann Rheum Dis. 2017 Mar 2. doi: 10.1136/annrheumdis-2016-210931).
The biological explanation for this discrepancy is unclear. It is possible that etanercept simply isn’t as protective as the other two agents, but it also appears possible that etanercept may act paradoxically to induce anterior uveitis in some patients. However, it should be noted that “previous studies have indicated that etanercept still reduces the number of uveitis flares more effectively than placebo,” Dr. Lie and her associates noted.
Regardless of the underlying reason, these findings, taken together with those of previous studies, “support the choice of another TNF inhibitor than etanercept in patients with AS with a history of anterior uveitis,” they said.
Dr. Lie also reported the results at the 2015 American College of Rheumatology annual meeting.
This study was supported by the Swedish Research Council, Gothenburg University, the Stockholm County Council, the Swedish National Rheumatism Association, the Swedish COMBINE public-private research program, the Swedish Cancer Society, the EU-IMI BT Cure project, and the Swedish Foundation for Strategic Research. Dr. Lie reported receiving personal fees from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, and UCB; her associates reported ties to numerous industry sources.
Two anti–tumor necrosis factor monoclonal antibodies, adalimumab and infliximab, showed evidence of being markedly more effective than the anti-TNF–receptor inhibitor etanercept at reducing the rate of anterior uveitis in patients with ankylosing spondylitis in a retrospective Swedish cohort study.
To compare the efficacy of the three TNF inhibitors, researchers analyzed data in nationwide Swedish population-based registries for 1,365 ankylosing spondylitis (AS) patients who initiated treatment during a 7-year period. Treatment began with adalimumab in 406 patients, infliximab in 605, and etanercept in 354, said Elisabeth Lie, MD, of the department of rheumatology and inflammation research at the University of Gothenburg (Sweden), and her associates.
“Compared with the rates [of anterior uveitis] pretreatment, the rates increased when initiating treatment with etanercept, but decreased when starting adalimumab or infliximab,” the investigators wrote (Ann Rheum Dis. 2017 Mar 2. doi: 10.1136/annrheumdis-2016-210931).
The biological explanation for this discrepancy is unclear. It is possible that etanercept simply isn’t as protective as the other two agents, but it also appears possible that etanercept may act paradoxically to induce anterior uveitis in some patients. However, it should be noted that “previous studies have indicated that etanercept still reduces the number of uveitis flares more effectively than placebo,” Dr. Lie and her associates noted.
Regardless of the underlying reason, these findings, taken together with those of previous studies, “support the choice of another TNF inhibitor than etanercept in patients with AS with a history of anterior uveitis,” they said.
Dr. Lie also reported the results at the 2015 American College of Rheumatology annual meeting.
This study was supported by the Swedish Research Council, Gothenburg University, the Stockholm County Council, the Swedish National Rheumatism Association, the Swedish COMBINE public-private research program, the Swedish Cancer Society, the EU-IMI BT Cure project, and the Swedish Foundation for Strategic Research. Dr. Lie reported receiving personal fees from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, and UCB; her associates reported ties to numerous industry sources.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Etanercept was associated with nearly a fourfold higher risk of developing uveitis than was adalimumab (HR, 3.86) and a twofold higher risk than was infliximab (HR, 1.99), but there was no difference in risk between adalimumab and infliximab.
Data source: A retrospective cohort study involving 1,365 AS patients enrolled in nationwide Swedish registries during a 7-year period.
Disclosures: This study was supported by the Swedish Research Council, Gothenburg University, the Stockholm County Council, the Swedish National Rheumatism Association, the Swedish COMBINE public-private research program, the Swedish Cancer Society, the EU-IMI BT Cure project, and the Swedish Foundation for Strategic Research. Dr. Lie reported receiving personal fees from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, and UCB; her associates reported ties to numerous industry sources.
Reinforcing mesh at ostomy site prevents parastomal hernia
For patients undergoing elective permanent colostomy, prophylactic augmentation of the abdominal wall using mesh at the ostomy site prevents the development of parastomal hernia, according to a report published in the April issue of Annals of Surgery.
The incidence of parastomal hernia is expected to rise because of the increasing number of cancer patients surviving with a colostomy, and the rising number of obese patients who have increased tension on the abdominal wall because of their elevated intra-abdominal pressure and larger abdominal radius. Researchers in the Netherlands performed a prospective randomized study, the PREVENT trial, to assess whether augmenting the abdominal wall at the ostomy site, using a lightweight mesh, would be safe, feasible, and effective at preventing parastomal hernia. They reported their findings after 1 year of follow-up; the study will continue until longer-term results are available at 5 years.
In the intervention group, a retromuscular space was created to accommodate the mesh by dissecting the muscle from the posterior fascia or peritoneum to the lateral border via a median laparotomy. An incision was made in the center of the mesh to allow passage of the colon, and the mesh was placed on the posterior rectus sheath and anchored laterally with two absorbable sutures. “On the medial side, the mesh was incorporated in the running suture closing the fascia, thus preventing contact between the mesh and the viscera,” the investigators said (Ann Surg. 2017;265:663-9).
The primary end point – the incidence of parastomal hernia at 1 year – occurred in 3 patients (4.5%) in the intervention group and 16 (24.2%) in the control group, a significant difference. There were no mesh-related complications such as infection, strictures, or adhesions. “The majority of the parastomal hernias that required surgical repair were in the control group, which supports the concept that if a hernia develops in a patient with mesh, it is smaller and less likely to cause complaints,” Dr. Brandsma and his associates said.
Significantly fewer patients in the mesh group (9%) than in the control group (21%) reported stoma-related complaints such as pain, leakage, and skin problems. Scores on measures of quality of life and pain severity were no different between the two study groups.
“Prophylactic augmentation of the abdominal wall with a retromuscular polypropylene mesh at the ostomy site is a safe and feasible procedure with no adverse events. It significantly reduces the incidence of parastomal hernia,” the investigators concluded.
This study was supported by Canisius Wilhelmina Hospital’s surgery research fund, the Netherlands Organization for Health Research and Development, and Covidien/Medtronic. Dr. Brandsma and his associates reported having no relevant financial disclosures.
For patients undergoing elective permanent colostomy, prophylactic augmentation of the abdominal wall using mesh at the ostomy site prevents the development of parastomal hernia, according to a report published in the April issue of Annals of Surgery.
The incidence of parastomal hernia is expected to rise because of the increasing number of cancer patients surviving with a colostomy, and the rising number of obese patients who have increased tension on the abdominal wall because of their elevated intra-abdominal pressure and larger abdominal radius. Researchers in the Netherlands performed a prospective randomized study, the PREVENT trial, to assess whether augmenting the abdominal wall at the ostomy site, using a lightweight mesh, would be safe, feasible, and effective at preventing parastomal hernia. They reported their findings after 1 year of follow-up; the study will continue until longer-term results are available at 5 years.
In the intervention group, a retromuscular space was created to accommodate the mesh by dissecting the muscle from the posterior fascia or peritoneum to the lateral border via a median laparotomy. An incision was made in the center of the mesh to allow passage of the colon, and the mesh was placed on the posterior rectus sheath and anchored laterally with two absorbable sutures. “On the medial side, the mesh was incorporated in the running suture closing the fascia, thus preventing contact between the mesh and the viscera,” the investigators said (Ann Surg. 2017;265:663-9).
The primary end point – the incidence of parastomal hernia at 1 year – occurred in 3 patients (4.5%) in the intervention group and 16 (24.2%) in the control group, a significant difference. There were no mesh-related complications such as infection, strictures, or adhesions. “The majority of the parastomal hernias that required surgical repair were in the control group, which supports the concept that if a hernia develops in a patient with mesh, it is smaller and less likely to cause complaints,” Dr. Brandsma and his associates said.
Significantly fewer patients in the mesh group (9%) than in the control group (21%) reported stoma-related complaints such as pain, leakage, and skin problems. Scores on measures of quality of life and pain severity were no different between the two study groups.
“Prophylactic augmentation of the abdominal wall with a retromuscular polypropylene mesh at the ostomy site is a safe and feasible procedure with no adverse events. It significantly reduces the incidence of parastomal hernia,” the investigators concluded.
This study was supported by Canisius Wilhelmina Hospital’s surgery research fund, the Netherlands Organization for Health Research and Development, and Covidien/Medtronic. Dr. Brandsma and his associates reported having no relevant financial disclosures.
For patients undergoing elective permanent colostomy, prophylactic augmentation of the abdominal wall using mesh at the ostomy site prevents the development of parastomal hernia, according to a report published in the April issue of Annals of Surgery.
The incidence of parastomal hernia is expected to rise because of the increasing number of cancer patients surviving with a colostomy, and the rising number of obese patients who have increased tension on the abdominal wall because of their elevated intra-abdominal pressure and larger abdominal radius. Researchers in the Netherlands performed a prospective randomized study, the PREVENT trial, to assess whether augmenting the abdominal wall at the ostomy site, using a lightweight mesh, would be safe, feasible, and effective at preventing parastomal hernia. They reported their findings after 1 year of follow-up; the study will continue until longer-term results are available at 5 years.
In the intervention group, a retromuscular space was created to accommodate the mesh by dissecting the muscle from the posterior fascia or peritoneum to the lateral border via a median laparotomy. An incision was made in the center of the mesh to allow passage of the colon, and the mesh was placed on the posterior rectus sheath and anchored laterally with two absorbable sutures. “On the medial side, the mesh was incorporated in the running suture closing the fascia, thus preventing contact between the mesh and the viscera,” the investigators said (Ann Surg. 2017;265:663-9).
The primary end point – the incidence of parastomal hernia at 1 year – occurred in 3 patients (4.5%) in the intervention group and 16 (24.2%) in the control group, a significant difference. There were no mesh-related complications such as infection, strictures, or adhesions. “The majority of the parastomal hernias that required surgical repair were in the control group, which supports the concept that if a hernia develops in a patient with mesh, it is smaller and less likely to cause complaints,” Dr. Brandsma and his associates said.
Significantly fewer patients in the mesh group (9%) than in the control group (21%) reported stoma-related complaints such as pain, leakage, and skin problems. Scores on measures of quality of life and pain severity were no different between the two study groups.
“Prophylactic augmentation of the abdominal wall with a retromuscular polypropylene mesh at the ostomy site is a safe and feasible procedure with no adverse events. It significantly reduces the incidence of parastomal hernia,” the investigators concluded.
This study was supported by Canisius Wilhelmina Hospital’s surgery research fund, the Netherlands Organization for Health Research and Development, and Covidien/Medtronic. Dr. Brandsma and his associates reported having no relevant financial disclosures.
FROM THE ANNALS OF SURGERY
Key clinical point: For patients undergoing permanent colostomy, prophylactic augmentation of the abdominal wall using mesh at the ostomy site prevents the development of parastomal hernia.
Major finding: The primary end point – the incidence of parastomal hernia at 1 year – occurred in 3 patients (4.5%) in the intervention group and 16 (24.2%) in the control group.
Data source: A prospective, multicenter, randomized cohort study comparing prophylactic mesh against standard care in 133 adults undergoing elective end-colostomy during a 3-year period.
Disclosures: This study was supported by Canisius Wilhelmina Hospital’s surgery research fund, the Netherlands Organization for Health Research and Development, and Covidien/Medtronic. Dr. Brandsma and his associates reported having no relevant financial disclosures.
VIDEO: Subclinical leaflet thrombosis frequent after TAVR, SAVR
WASHINGTON – Subclinical leaflet thrombosis, as evidenced by reduced leaflet motion and corresponding hypoattenuating lesions on high-resolution CT, occurs frequently in bioprosthetic aortic valves implanted either by the transcatheter route or surgically, according to a report presented at the annual meeting of the American College of Cardiology and simultaneously published March 19 in the Lancet.
In an observational cohort study involving 890 patients enrolled in two single-center registries, reduced leaflet motion signaling the presence of thrombosis was detected in 106 patients (12%) by four-dimensional, volume-rendered CT. The thrombosis was hemodynamically silent and undetected by transthoracic echocardiograms done in a large subgroup of the study participants. Mean aortic valve gradients were numerically higher in affected patients, but still fell within the normal range in most of them, said Raj R. Makkar, MD, of Cedars-Sinai Heart Institute, Los Angeles.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Makkar and his associates studied this issue by analyzing data in the RESOLVE (Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and its Treatment with Anticoagulation) registry at Cedars-Sinai and the SAVORY (Subclinical Aortic Valve Biosprosthesis Thrombosis Assessed with Four-Dimensional CT) registry at the Rigshospitalet Heart Center in Copenhagen. Their study focused on enrolled patients who underwent either TAVR (626 patients) or SAVR (264 patients) and then had high-resolution CT scans done at various times after implantation. All the CT scans were analyzed in blinded fashion, and the study participants were followed for a mean of 540 days.
The prevalence of subclinical leaflet thrombosis was significantly higher in TAVR valves (13%) than in SAVR valves (4%). The severity of the thrombosis also was significantly higher in TAVR valves, when measured by the extent of leaflet motion restriction (71.0% vs. 56.9%).
One possible reason for this discrepancy is that traumatic injury to the pericardial leaflets, which theoretically could predispose to thrombus formation, may be more likely to occur during crimping and deployment of balloon-expandable and self-expanding stent valves in TAVR. Another possibility is that resection of the calcified native aortic valves during SAVR might improve flow dynamics after valve replacement, compared with leaving native aortic valve cusps in situ during TAVR. A third possibility is that inadvertent incomplete expansion or overexpansion of transcatheter valves might alter mechanical stress on the leaflets, predisposing them to thrombus formation, compared with the uniform expansion of valves placed surgically, the investigator said.
“Nevertheless, these findings should be interpreted in the context of findings from multiple randomized controlled trials showing similar mortality and stroke rates, better hemodynamics, and equivalent durability of transcatheter aortic valves at 5 years, compared with surgical valves,” he noted.
Patients taking novel oral anticoagulants (NOACs) or warfarin for anticoagulation before valve replacement were less likely to develop subclinical leaflet thrombosis than patients not taking those anticoagulants. And the thrombosis appeared to resolve in all patients treated with NOACs or warfarin for 30 days after it was found on CT, but it persisted or progressed in those not treated with anticoagulation. Thus, “anticoagulation with NOACs or warfarin was effective in prevention and treatment of reduced leaflet motion, but dual-antiplatelet therapy, which is the standard of care, was not,” the investigators said (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2.
“Our study challenges the American College of Cardiology and American Heart Association and European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines, which recommend dual-antiplatelet therapy after TAVR and do not recommend routine anticoagulation after TAVR,” he noted.
However, routine anticoagulation for all TAVR and SAVR patients, or even for all who develop leaflet thrombosis, cannot be recommended on the basis of results from a single observational study. The benefit of anticoagulation may not offset the risk of bleeding in this predominantly elderly population with multiple comorbidities, he said.
There were no significant differences between patients who developed subclinical leaflet thrombosis and those who did not regarding rates of death, MI, or stroke. However, “reduced leaflet wall motion was significantly associated with increased rates of all TIAs [transient ischemic attacks], nonprocedural TIAs, and post-CT TIAs.” Dr. Makkar said.
Based on his new findings, Dr. Makkar suggested that clinicians keep possible leaflet thrombosis on a replaced aortic valve in mind if they see clinical evidence for it, such as an increase in the valve’s pressure gradient or development of a TIA or stroke.* These events should trigger further investigation of the valve with CT angiography, and if thrombosis is then confirmed, the patient should receive treatment with an anticoagulant, although the appropriate duration of anticoagulant treatment remains unclear, he said in a video interview. Dr. Makkar also said that trials are needed to determine whether routine CT angiography assessment or routine prophylactic treatment with an anticoagulant is warranted because the antiplatelet therapy that patients currently receive after aortic valve replacement seems unable to prevent leaflet thrombosis.
Since this was an observational study based on data from nonrandomized registries, the findings cannot prove causality but only an association between leaflet thrombosis and TIA. They must be substantiated in further studies, including “the current Food and Drug Administration–mandated imaging substudies in randomized clinical trials.”
Dr. Makkar has received consultant fees and/or honoraria from Abbott Vascular, Medtronic, Cordis, and Entourage Medical, and research grants from Edwards Lifesciences and St. Jude Medical.
Mitchel L. Zoler contributed to this report.
Correction, 3/20/17: An earlier version of this article misstated the clinical evidence for possible leaflet thrombosis on a replaced aortic valve.
The study by Dr. Makkar and his coinvestigators provides important new information to guide future research, but changing clinical practice or guidelines on the basis of this information would be premature.
In particular, whether to recommend anticoagulation therapy to TAVR and SAVR patients is unknown, given the risks of chronic anticoagulation. We do not yet know whether such a recommendation should be guided by CT or echocardiographic imaging, and, if so, at what intervals? We don’t yet know the optimal duration of anticoagulation treatment, or whether NOAC’s are as good as or better than warfarin in this setting. And we don’t yet know whether the risk of bleeding is offset by a reduction in stroke or any other meaningful clinical sequelae.
Jeroen J. Bax, MD, is in the department of cardiology at Leiden (the Netherlands) University Medical Center. Gregg W. Stone, MD, is at Columbia University Medical Center and the Cardiovascular Research Foundation, both in New York. Dr. Bax’s institution has received unrestricted research grants from Biotronik, Medtronic, Boston Scientific, and Edwards LifeScience; Dr. Stone reported ties to numerous industry sources. Dr. Bax and Dr. Stone made these remarks in an editorial accompanying the Lancet report (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2).
The study by Dr. Makkar and his coinvestigators provides important new information to guide future research, but changing clinical practice or guidelines on the basis of this information would be premature.
In particular, whether to recommend anticoagulation therapy to TAVR and SAVR patients is unknown, given the risks of chronic anticoagulation. We do not yet know whether such a recommendation should be guided by CT or echocardiographic imaging, and, if so, at what intervals? We don’t yet know the optimal duration of anticoagulation treatment, or whether NOAC’s are as good as or better than warfarin in this setting. And we don’t yet know whether the risk of bleeding is offset by a reduction in stroke or any other meaningful clinical sequelae.
Jeroen J. Bax, MD, is in the department of cardiology at Leiden (the Netherlands) University Medical Center. Gregg W. Stone, MD, is at Columbia University Medical Center and the Cardiovascular Research Foundation, both in New York. Dr. Bax’s institution has received unrestricted research grants from Biotronik, Medtronic, Boston Scientific, and Edwards LifeScience; Dr. Stone reported ties to numerous industry sources. Dr. Bax and Dr. Stone made these remarks in an editorial accompanying the Lancet report (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2).
The study by Dr. Makkar and his coinvestigators provides important new information to guide future research, but changing clinical practice or guidelines on the basis of this information would be premature.
In particular, whether to recommend anticoagulation therapy to TAVR and SAVR patients is unknown, given the risks of chronic anticoagulation. We do not yet know whether such a recommendation should be guided by CT or echocardiographic imaging, and, if so, at what intervals? We don’t yet know the optimal duration of anticoagulation treatment, or whether NOAC’s are as good as or better than warfarin in this setting. And we don’t yet know whether the risk of bleeding is offset by a reduction in stroke or any other meaningful clinical sequelae.
Jeroen J. Bax, MD, is in the department of cardiology at Leiden (the Netherlands) University Medical Center. Gregg W. Stone, MD, is at Columbia University Medical Center and the Cardiovascular Research Foundation, both in New York. Dr. Bax’s institution has received unrestricted research grants from Biotronik, Medtronic, Boston Scientific, and Edwards LifeScience; Dr. Stone reported ties to numerous industry sources. Dr. Bax and Dr. Stone made these remarks in an editorial accompanying the Lancet report (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2).
WASHINGTON – Subclinical leaflet thrombosis, as evidenced by reduced leaflet motion and corresponding hypoattenuating lesions on high-resolution CT, occurs frequently in bioprosthetic aortic valves implanted either by the transcatheter route or surgically, according to a report presented at the annual meeting of the American College of Cardiology and simultaneously published March 19 in the Lancet.
In an observational cohort study involving 890 patients enrolled in two single-center registries, reduced leaflet motion signaling the presence of thrombosis was detected in 106 patients (12%) by four-dimensional, volume-rendered CT. The thrombosis was hemodynamically silent and undetected by transthoracic echocardiograms done in a large subgroup of the study participants. Mean aortic valve gradients were numerically higher in affected patients, but still fell within the normal range in most of them, said Raj R. Makkar, MD, of Cedars-Sinai Heart Institute, Los Angeles.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Makkar and his associates studied this issue by analyzing data in the RESOLVE (Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and its Treatment with Anticoagulation) registry at Cedars-Sinai and the SAVORY (Subclinical Aortic Valve Biosprosthesis Thrombosis Assessed with Four-Dimensional CT) registry at the Rigshospitalet Heart Center in Copenhagen. Their study focused on enrolled patients who underwent either TAVR (626 patients) or SAVR (264 patients) and then had high-resolution CT scans done at various times after implantation. All the CT scans were analyzed in blinded fashion, and the study participants were followed for a mean of 540 days.
The prevalence of subclinical leaflet thrombosis was significantly higher in TAVR valves (13%) than in SAVR valves (4%). The severity of the thrombosis also was significantly higher in TAVR valves, when measured by the extent of leaflet motion restriction (71.0% vs. 56.9%).
One possible reason for this discrepancy is that traumatic injury to the pericardial leaflets, which theoretically could predispose to thrombus formation, may be more likely to occur during crimping and deployment of balloon-expandable and self-expanding stent valves in TAVR. Another possibility is that resection of the calcified native aortic valves during SAVR might improve flow dynamics after valve replacement, compared with leaving native aortic valve cusps in situ during TAVR. A third possibility is that inadvertent incomplete expansion or overexpansion of transcatheter valves might alter mechanical stress on the leaflets, predisposing them to thrombus formation, compared with the uniform expansion of valves placed surgically, the investigator said.
“Nevertheless, these findings should be interpreted in the context of findings from multiple randomized controlled trials showing similar mortality and stroke rates, better hemodynamics, and equivalent durability of transcatheter aortic valves at 5 years, compared with surgical valves,” he noted.
Patients taking novel oral anticoagulants (NOACs) or warfarin for anticoagulation before valve replacement were less likely to develop subclinical leaflet thrombosis than patients not taking those anticoagulants. And the thrombosis appeared to resolve in all patients treated with NOACs or warfarin for 30 days after it was found on CT, but it persisted or progressed in those not treated with anticoagulation. Thus, “anticoagulation with NOACs or warfarin was effective in prevention and treatment of reduced leaflet motion, but dual-antiplatelet therapy, which is the standard of care, was not,” the investigators said (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2.
“Our study challenges the American College of Cardiology and American Heart Association and European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines, which recommend dual-antiplatelet therapy after TAVR and do not recommend routine anticoagulation after TAVR,” he noted.
However, routine anticoagulation for all TAVR and SAVR patients, or even for all who develop leaflet thrombosis, cannot be recommended on the basis of results from a single observational study. The benefit of anticoagulation may not offset the risk of bleeding in this predominantly elderly population with multiple comorbidities, he said.
There were no significant differences between patients who developed subclinical leaflet thrombosis and those who did not regarding rates of death, MI, or stroke. However, “reduced leaflet wall motion was significantly associated with increased rates of all TIAs [transient ischemic attacks], nonprocedural TIAs, and post-CT TIAs.” Dr. Makkar said.
Based on his new findings, Dr. Makkar suggested that clinicians keep possible leaflet thrombosis on a replaced aortic valve in mind if they see clinical evidence for it, such as an increase in the valve’s pressure gradient or development of a TIA or stroke.* These events should trigger further investigation of the valve with CT angiography, and if thrombosis is then confirmed, the patient should receive treatment with an anticoagulant, although the appropriate duration of anticoagulant treatment remains unclear, he said in a video interview. Dr. Makkar also said that trials are needed to determine whether routine CT angiography assessment or routine prophylactic treatment with an anticoagulant is warranted because the antiplatelet therapy that patients currently receive after aortic valve replacement seems unable to prevent leaflet thrombosis.
Since this was an observational study based on data from nonrandomized registries, the findings cannot prove causality but only an association between leaflet thrombosis and TIA. They must be substantiated in further studies, including “the current Food and Drug Administration–mandated imaging substudies in randomized clinical trials.”
Dr. Makkar has received consultant fees and/or honoraria from Abbott Vascular, Medtronic, Cordis, and Entourage Medical, and research grants from Edwards Lifesciences and St. Jude Medical.
Mitchel L. Zoler contributed to this report.
Correction, 3/20/17: An earlier version of this article misstated the clinical evidence for possible leaflet thrombosis on a replaced aortic valve.
WASHINGTON – Subclinical leaflet thrombosis, as evidenced by reduced leaflet motion and corresponding hypoattenuating lesions on high-resolution CT, occurs frequently in bioprosthetic aortic valves implanted either by the transcatheter route or surgically, according to a report presented at the annual meeting of the American College of Cardiology and simultaneously published March 19 in the Lancet.
In an observational cohort study involving 890 patients enrolled in two single-center registries, reduced leaflet motion signaling the presence of thrombosis was detected in 106 patients (12%) by four-dimensional, volume-rendered CT. The thrombosis was hemodynamically silent and undetected by transthoracic echocardiograms done in a large subgroup of the study participants. Mean aortic valve gradients were numerically higher in affected patients, but still fell within the normal range in most of them, said Raj R. Makkar, MD, of Cedars-Sinai Heart Institute, Los Angeles.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Makkar and his associates studied this issue by analyzing data in the RESOLVE (Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and its Treatment with Anticoagulation) registry at Cedars-Sinai and the SAVORY (Subclinical Aortic Valve Biosprosthesis Thrombosis Assessed with Four-Dimensional CT) registry at the Rigshospitalet Heart Center in Copenhagen. Their study focused on enrolled patients who underwent either TAVR (626 patients) or SAVR (264 patients) and then had high-resolution CT scans done at various times after implantation. All the CT scans were analyzed in blinded fashion, and the study participants were followed for a mean of 540 days.
The prevalence of subclinical leaflet thrombosis was significantly higher in TAVR valves (13%) than in SAVR valves (4%). The severity of the thrombosis also was significantly higher in TAVR valves, when measured by the extent of leaflet motion restriction (71.0% vs. 56.9%).
One possible reason for this discrepancy is that traumatic injury to the pericardial leaflets, which theoretically could predispose to thrombus formation, may be more likely to occur during crimping and deployment of balloon-expandable and self-expanding stent valves in TAVR. Another possibility is that resection of the calcified native aortic valves during SAVR might improve flow dynamics after valve replacement, compared with leaving native aortic valve cusps in situ during TAVR. A third possibility is that inadvertent incomplete expansion or overexpansion of transcatheter valves might alter mechanical stress on the leaflets, predisposing them to thrombus formation, compared with the uniform expansion of valves placed surgically, the investigator said.
“Nevertheless, these findings should be interpreted in the context of findings from multiple randomized controlled trials showing similar mortality and stroke rates, better hemodynamics, and equivalent durability of transcatheter aortic valves at 5 years, compared with surgical valves,” he noted.
Patients taking novel oral anticoagulants (NOACs) or warfarin for anticoagulation before valve replacement were less likely to develop subclinical leaflet thrombosis than patients not taking those anticoagulants. And the thrombosis appeared to resolve in all patients treated with NOACs or warfarin for 30 days after it was found on CT, but it persisted or progressed in those not treated with anticoagulation. Thus, “anticoagulation with NOACs or warfarin was effective in prevention and treatment of reduced leaflet motion, but dual-antiplatelet therapy, which is the standard of care, was not,” the investigators said (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2.
“Our study challenges the American College of Cardiology and American Heart Association and European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines, which recommend dual-antiplatelet therapy after TAVR and do not recommend routine anticoagulation after TAVR,” he noted.
However, routine anticoagulation for all TAVR and SAVR patients, or even for all who develop leaflet thrombosis, cannot be recommended on the basis of results from a single observational study. The benefit of anticoagulation may not offset the risk of bleeding in this predominantly elderly population with multiple comorbidities, he said.
There were no significant differences between patients who developed subclinical leaflet thrombosis and those who did not regarding rates of death, MI, or stroke. However, “reduced leaflet wall motion was significantly associated with increased rates of all TIAs [transient ischemic attacks], nonprocedural TIAs, and post-CT TIAs.” Dr. Makkar said.
Based on his new findings, Dr. Makkar suggested that clinicians keep possible leaflet thrombosis on a replaced aortic valve in mind if they see clinical evidence for it, such as an increase in the valve’s pressure gradient or development of a TIA or stroke.* These events should trigger further investigation of the valve with CT angiography, and if thrombosis is then confirmed, the patient should receive treatment with an anticoagulant, although the appropriate duration of anticoagulant treatment remains unclear, he said in a video interview. Dr. Makkar also said that trials are needed to determine whether routine CT angiography assessment or routine prophylactic treatment with an anticoagulant is warranted because the antiplatelet therapy that patients currently receive after aortic valve replacement seems unable to prevent leaflet thrombosis.
Since this was an observational study based on data from nonrandomized registries, the findings cannot prove causality but only an association between leaflet thrombosis and TIA. They must be substantiated in further studies, including “the current Food and Drug Administration–mandated imaging substudies in randomized clinical trials.”
Dr. Makkar has received consultant fees and/or honoraria from Abbott Vascular, Medtronic, Cordis, and Entourage Medical, and research grants from Edwards Lifesciences and St. Jude Medical.
Mitchel L. Zoler contributed to this report.
Correction, 3/20/17: An earlier version of this article misstated the clinical evidence for possible leaflet thrombosis on a replaced aortic valve.
AT ACC 17
Key clinical point: Subclinical leaflet thrombosis occurs frequently in bioprosthetic aortic valves implanted either by the transcatheter route or surgically.
Major finding: Reduced leaflet motion signaling the presence of thrombosis was detected in 106 patients (12%).
Data source: An observational cohort study involving 890 patients enrolled in two registries who underwent high-resolution CT to detect leaflet thrombosis after either TAVR or SAVR.
Disclosures: The RESOLVE study was funded by Cedars-Sinai Heart Institute and the SAVORY study was funded by Rigshospitalet. Dr. Makkar has received consultant fees and/or honoraria from Abbott Vascular, Medtronic, Cordis, and Entourage Medical, and research grants from Edwards Lifesciences and St. Jude Medical.
HCV ‘cure’ within the VA appears likely
The number of Veterans Affairs patients with hepatitis C who have achieved a sustained virologic response to antiviral therapy has escalated so rapidly and reached such a height that the disease may well be eradicated in that health care system within a few years, according to a report in Alimentary Pharmacology and Therapeutics.
The potential public health benefits are substantial, “considering that HCV infection is the most common cause of cirrhosis and liver cancer in the VA and the United States, that the benefits of SVR are long-lasting, and that HCV clearance reduces the risk of liver cancer by 76% and all-cause mortality by 50%,” said Andrew M. Moon, MD, of the division of general internal medicine, University of Washington, Seattle, and his associates.
An estimated 124,662 VA patients currently are infected, and curing them “would substantially reduce the burden of HCV within the entire country and prevent tens of thousands of deaths,” they noted.
The VA dramatically increased the number of patients who were offered treatment in recent years, because it was able to allocate nearly $700 million to offset the high costs of highly effective direct antiviral agents, which in turn made these better-tolerated drugs more widely available at clinics across the country. The VA also removed all treatment prioritization criteria, allowing all patients, not just those with severe disease, to receive highly effective direct antiviral agents. This is “in stark contrast to most health care systems, state Medicaid programs, and insurance carriers in the U.S., which still restrict access ... based on severity of liver disease,” the investigators said (Aliment Pharmacol Ther. 2017 March 8. doi: 10.1111/apt.14021).
To examine the impact of these changes, Dr. Moon and his associates performed a retrospective cohort study, analyzing the electronic medical records of all 105,369 HCV treatment regimens given to 78,947 VA patients (mean age, 56 years) during a 17-year period. They found that annual treatment rates more than doubled from 2,726 to 6,679 patients when pegylated interferon was introduced, declined for a while and then rose modestly to 4,900 patients when boceprevir and telaprevir were introduced, declined again to an all-time low of 2,609 and then rebounded to 9,180 patients when sofosbuvir and simeprevir were introduced, and finally skyrocketed to 31,028 patients when ledipasvir/sofosbuvir and paritaprevir/ritonavir/ombitasvir/dasabuvir were introduced.
Correspondingly, SVR rates rose from less than 25% at the beginning of the study period to a “remarkable” 90.5% at the end. The improvement in SVR rates was even more pronounced among traditionally “hard to treat” cases, such as patients with concomitant cirrhosis (from 11.0% to 87.0%), decompensated cirrhosis (from 14.6% to 85.2%), highly refractory infection (from 16.4% to 89.3%), and genotype-1 infection (from 1.3% to 91.7%). “The number of patients achieving SVR increased 21-fold from 1,313 in 2010 to an estimated 28,084 in 2015,” Dr. Moon and his associates said.
“We believe that our findings based on the VA health care system might be relevant and informative for other comprehensive health care systems,” providing proof-of-concept that similar results can be achieved if aggressive screening; affordable, tolerable treatment; and open access to all patients are implemented.
The number of Veterans Affairs patients with hepatitis C who have achieved a sustained virologic response to antiviral therapy has escalated so rapidly and reached such a height that the disease may well be eradicated in that health care system within a few years, according to a report in Alimentary Pharmacology and Therapeutics.
The potential public health benefits are substantial, “considering that HCV infection is the most common cause of cirrhosis and liver cancer in the VA and the United States, that the benefits of SVR are long-lasting, and that HCV clearance reduces the risk of liver cancer by 76% and all-cause mortality by 50%,” said Andrew M. Moon, MD, of the division of general internal medicine, University of Washington, Seattle, and his associates.
An estimated 124,662 VA patients currently are infected, and curing them “would substantially reduce the burden of HCV within the entire country and prevent tens of thousands of deaths,” they noted.
The VA dramatically increased the number of patients who were offered treatment in recent years, because it was able to allocate nearly $700 million to offset the high costs of highly effective direct antiviral agents, which in turn made these better-tolerated drugs more widely available at clinics across the country. The VA also removed all treatment prioritization criteria, allowing all patients, not just those with severe disease, to receive highly effective direct antiviral agents. This is “in stark contrast to most health care systems, state Medicaid programs, and insurance carriers in the U.S., which still restrict access ... based on severity of liver disease,” the investigators said (Aliment Pharmacol Ther. 2017 March 8. doi: 10.1111/apt.14021).
To examine the impact of these changes, Dr. Moon and his associates performed a retrospective cohort study, analyzing the electronic medical records of all 105,369 HCV treatment regimens given to 78,947 VA patients (mean age, 56 years) during a 17-year period. They found that annual treatment rates more than doubled from 2,726 to 6,679 patients when pegylated interferon was introduced, declined for a while and then rose modestly to 4,900 patients when boceprevir and telaprevir were introduced, declined again to an all-time low of 2,609 and then rebounded to 9,180 patients when sofosbuvir and simeprevir were introduced, and finally skyrocketed to 31,028 patients when ledipasvir/sofosbuvir and paritaprevir/ritonavir/ombitasvir/dasabuvir were introduced.
Correspondingly, SVR rates rose from less than 25% at the beginning of the study period to a “remarkable” 90.5% at the end. The improvement in SVR rates was even more pronounced among traditionally “hard to treat” cases, such as patients with concomitant cirrhosis (from 11.0% to 87.0%), decompensated cirrhosis (from 14.6% to 85.2%), highly refractory infection (from 16.4% to 89.3%), and genotype-1 infection (from 1.3% to 91.7%). “The number of patients achieving SVR increased 21-fold from 1,313 in 2010 to an estimated 28,084 in 2015,” Dr. Moon and his associates said.
“We believe that our findings based on the VA health care system might be relevant and informative for other comprehensive health care systems,” providing proof-of-concept that similar results can be achieved if aggressive screening; affordable, tolerable treatment; and open access to all patients are implemented.
The number of Veterans Affairs patients with hepatitis C who have achieved a sustained virologic response to antiviral therapy has escalated so rapidly and reached such a height that the disease may well be eradicated in that health care system within a few years, according to a report in Alimentary Pharmacology and Therapeutics.
The potential public health benefits are substantial, “considering that HCV infection is the most common cause of cirrhosis and liver cancer in the VA and the United States, that the benefits of SVR are long-lasting, and that HCV clearance reduces the risk of liver cancer by 76% and all-cause mortality by 50%,” said Andrew M. Moon, MD, of the division of general internal medicine, University of Washington, Seattle, and his associates.
An estimated 124,662 VA patients currently are infected, and curing them “would substantially reduce the burden of HCV within the entire country and prevent tens of thousands of deaths,” they noted.
The VA dramatically increased the number of patients who were offered treatment in recent years, because it was able to allocate nearly $700 million to offset the high costs of highly effective direct antiviral agents, which in turn made these better-tolerated drugs more widely available at clinics across the country. The VA also removed all treatment prioritization criteria, allowing all patients, not just those with severe disease, to receive highly effective direct antiviral agents. This is “in stark contrast to most health care systems, state Medicaid programs, and insurance carriers in the U.S., which still restrict access ... based on severity of liver disease,” the investigators said (Aliment Pharmacol Ther. 2017 March 8. doi: 10.1111/apt.14021).
To examine the impact of these changes, Dr. Moon and his associates performed a retrospective cohort study, analyzing the electronic medical records of all 105,369 HCV treatment regimens given to 78,947 VA patients (mean age, 56 years) during a 17-year period. They found that annual treatment rates more than doubled from 2,726 to 6,679 patients when pegylated interferon was introduced, declined for a while and then rose modestly to 4,900 patients when boceprevir and telaprevir were introduced, declined again to an all-time low of 2,609 and then rebounded to 9,180 patients when sofosbuvir and simeprevir were introduced, and finally skyrocketed to 31,028 patients when ledipasvir/sofosbuvir and paritaprevir/ritonavir/ombitasvir/dasabuvir were introduced.
Correspondingly, SVR rates rose from less than 25% at the beginning of the study period to a “remarkable” 90.5% at the end. The improvement in SVR rates was even more pronounced among traditionally “hard to treat” cases, such as patients with concomitant cirrhosis (from 11.0% to 87.0%), decompensated cirrhosis (from 14.6% to 85.2%), highly refractory infection (from 16.4% to 89.3%), and genotype-1 infection (from 1.3% to 91.7%). “The number of patients achieving SVR increased 21-fold from 1,313 in 2010 to an estimated 28,084 in 2015,” Dr. Moon and his associates said.
“We believe that our findings based on the VA health care system might be relevant and informative for other comprehensive health care systems,” providing proof-of-concept that similar results can be achieved if aggressive screening; affordable, tolerable treatment; and open access to all patients are implemented.
FROM ALIMENTARY PHARMACOLOGY AND THERAPEUTICS
Key clinical point: The number of VA patients with hepatitis C virus who have achieved a sustained virologic response has escalated so rapidly and so high that the disease may be eradicated in that health care system within a few years.
Major finding: SVR rates rose from less than 25% at the beginning of the study period to a “remarkable” 90.5% at the end; the number of patients achieving SVR increased 21-fold from 1,313 in 2010 to an estimated 28,084 in 2015.
Data source: A retrospective cohort study examining all 105,369 antiviral regimens administered within the VA in 1999-2016.
Disclosures: The VA Office of Research and Development funded the study. Dr. Moon and his associates reported having no relevant financial disclosures.
Universal preterm birth screening not ready for prime time
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
FROM JAMA
Key clinical point:
Major finding: With a threshold of transvaginal cervical length of 25 mm or less at 16-22 weeks’ gestation, 247 women would need to be screened to identify one case of spontaneous preterm birth before 37 weeks’ gestation.
Data source: A prospective multicenter observational cohort study involving 9,410 nulliparous women across the United States.
Disclosures: The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. An associate reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
Stroke in AF patients often preceded by inadequate anticoagulation
Of the 94,474 ischemic strokes that occurred in a nationwide registry among patients with known atrial fibrillation, 84% were preceded by inadequate anticoagulation, according to a report published online March 14 in JAMA.
Fully 30% of the patients in this retrospective cohort study weren’t taking any form of antithrombotic therapy before their stroke, even though all of them had atrial fibrillation (AF) and most of them had additional risk factors as well. And although nearly 20,000 of the study participants were taking warfarin before their stroke, 64% of them were taking subtherapeutic doses, said Ying Xian, MD, PhD, of the department of neurology at the Duke Clinical Research Institute, Durham, N.C., and his associates.
These findings highlight tens of thousands of missed opportunities for preventing stroke simply by following existing guidelines, the investigators said.
In previous studies, researchers have consistently found underuse of oral anticoagulants in community practice. To examine current trends since the rapid adoption of non–vitamin-K antagonist oral anticoagulants (NOACs), Dr. Xian and his associates analyzed data from the AHA/ASA Get With the Guidelines-Stroke Registry. They determined the prevalence of antithrombotic treatment among AF patients who developed ischemic stroke, focusing on patients (mean age, 79.9 years) enrolled in the registry after being admitted for ischemic stroke to 1,622 participating U.S. hospitals during a 2.5-year period.
A total of 79,008 (83.6%) were not receiving therapeutic anticoagulation at the time of their stroke. This included 28,583 who were not taking any antithrombotic treatment, 12,751 who were taking subtherapeutic doses of warfarin (international normalized ratio,or INR, of less than 2 at the time of their stroke), and 37,674 who were taking only antiplatelet drugs when additional treatment was indicated, the investigators said (JAMA. 2017 Mar 14;317[10]:1057-67).
Among the minority of AF patients who were receiving therapeutic anticoagulation at the time of their stroke, 7,176 were taking adequate warfarin and 8,290 were taking adequate NOACs such as dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Stroke severity, as measured by median scores on the National Institutes of Health Stroke Scale, was significantly greater among patients receiving no antithrombotic medication, antiplatelet agents alone, or subtherapeutic levels of warfarin, compared with patients receiving therapeutic levels of warfarin or NOACs. Similarly, patients who had adequate anticoagulation before their stroke were significantly more likely to have better functional outcomes than those who did not. “These findings reinforce the importance of INR monitoring and dose adjustment to ... keep the INR in the therapeutic range,” Dr. Xian and his associates wrote.
The reasons why patients weren’t receiving adequate anticoagulation before their stroke – including any direct contraindications to treatment – were not available from the registry data. But such reasons are likely to persist after a stroke, and study clinicians were required to document such reasons in the medical records at hospital discharge. So the investigators examined reasons listed for not prescribing oral anticoagulation at hospital discharge in 58,084 patients.
A striking 38,249 AF patients (65.8%) in this large subgroup of study participants had no documented reason for not receiving oral anticoagulation, even after they were hospitalized for ischemic stroke and even though such documentation was “required.” Among the minority of patients whose records did show such reasons, the most common reason cited was bleeding risk (16.3%), followed by risk of falling (10.3%), the presence of a terminal illness (6.2%), patient or family refusal of the medication (4.3%), the patient’s impaired mental status (1.1%), adverse effects of the medication (1.0%), and allergy to the medication (0.6%).
This study was supported by the Patient-Centered Outcomes Research Institute. Dr. Xian reported that his institution received research funding from the American Heart Association, Daiichi Sankyo, Janssen, and Genentech. His associates reported ties to numerous industry sources.
Of the 94,474 ischemic strokes that occurred in a nationwide registry among patients with known atrial fibrillation, 84% were preceded by inadequate anticoagulation, according to a report published online March 14 in JAMA.
Fully 30% of the patients in this retrospective cohort study weren’t taking any form of antithrombotic therapy before their stroke, even though all of them had atrial fibrillation (AF) and most of them had additional risk factors as well. And although nearly 20,000 of the study participants were taking warfarin before their stroke, 64% of them were taking subtherapeutic doses, said Ying Xian, MD, PhD, of the department of neurology at the Duke Clinical Research Institute, Durham, N.C., and his associates.
These findings highlight tens of thousands of missed opportunities for preventing stroke simply by following existing guidelines, the investigators said.
In previous studies, researchers have consistently found underuse of oral anticoagulants in community practice. To examine current trends since the rapid adoption of non–vitamin-K antagonist oral anticoagulants (NOACs), Dr. Xian and his associates analyzed data from the AHA/ASA Get With the Guidelines-Stroke Registry. They determined the prevalence of antithrombotic treatment among AF patients who developed ischemic stroke, focusing on patients (mean age, 79.9 years) enrolled in the registry after being admitted for ischemic stroke to 1,622 participating U.S. hospitals during a 2.5-year period.
A total of 79,008 (83.6%) were not receiving therapeutic anticoagulation at the time of their stroke. This included 28,583 who were not taking any antithrombotic treatment, 12,751 who were taking subtherapeutic doses of warfarin (international normalized ratio,or INR, of less than 2 at the time of their stroke), and 37,674 who were taking only antiplatelet drugs when additional treatment was indicated, the investigators said (JAMA. 2017 Mar 14;317[10]:1057-67).
Among the minority of AF patients who were receiving therapeutic anticoagulation at the time of their stroke, 7,176 were taking adequate warfarin and 8,290 were taking adequate NOACs such as dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Stroke severity, as measured by median scores on the National Institutes of Health Stroke Scale, was significantly greater among patients receiving no antithrombotic medication, antiplatelet agents alone, or subtherapeutic levels of warfarin, compared with patients receiving therapeutic levels of warfarin or NOACs. Similarly, patients who had adequate anticoagulation before their stroke were significantly more likely to have better functional outcomes than those who did not. “These findings reinforce the importance of INR monitoring and dose adjustment to ... keep the INR in the therapeutic range,” Dr. Xian and his associates wrote.
The reasons why patients weren’t receiving adequate anticoagulation before their stroke – including any direct contraindications to treatment – were not available from the registry data. But such reasons are likely to persist after a stroke, and study clinicians were required to document such reasons in the medical records at hospital discharge. So the investigators examined reasons listed for not prescribing oral anticoagulation at hospital discharge in 58,084 patients.
A striking 38,249 AF patients (65.8%) in this large subgroup of study participants had no documented reason for not receiving oral anticoagulation, even after they were hospitalized for ischemic stroke and even though such documentation was “required.” Among the minority of patients whose records did show such reasons, the most common reason cited was bleeding risk (16.3%), followed by risk of falling (10.3%), the presence of a terminal illness (6.2%), patient or family refusal of the medication (4.3%), the patient’s impaired mental status (1.1%), adverse effects of the medication (1.0%), and allergy to the medication (0.6%).
This study was supported by the Patient-Centered Outcomes Research Institute. Dr. Xian reported that his institution received research funding from the American Heart Association, Daiichi Sankyo, Janssen, and Genentech. His associates reported ties to numerous industry sources.
Of the 94,474 ischemic strokes that occurred in a nationwide registry among patients with known atrial fibrillation, 84% were preceded by inadequate anticoagulation, according to a report published online March 14 in JAMA.
Fully 30% of the patients in this retrospective cohort study weren’t taking any form of antithrombotic therapy before their stroke, even though all of them had atrial fibrillation (AF) and most of them had additional risk factors as well. And although nearly 20,000 of the study participants were taking warfarin before their stroke, 64% of them were taking subtherapeutic doses, said Ying Xian, MD, PhD, of the department of neurology at the Duke Clinical Research Institute, Durham, N.C., and his associates.
These findings highlight tens of thousands of missed opportunities for preventing stroke simply by following existing guidelines, the investigators said.
In previous studies, researchers have consistently found underuse of oral anticoagulants in community practice. To examine current trends since the rapid adoption of non–vitamin-K antagonist oral anticoagulants (NOACs), Dr. Xian and his associates analyzed data from the AHA/ASA Get With the Guidelines-Stroke Registry. They determined the prevalence of antithrombotic treatment among AF patients who developed ischemic stroke, focusing on patients (mean age, 79.9 years) enrolled in the registry after being admitted for ischemic stroke to 1,622 participating U.S. hospitals during a 2.5-year period.
A total of 79,008 (83.6%) were not receiving therapeutic anticoagulation at the time of their stroke. This included 28,583 who were not taking any antithrombotic treatment, 12,751 who were taking subtherapeutic doses of warfarin (international normalized ratio,or INR, of less than 2 at the time of their stroke), and 37,674 who were taking only antiplatelet drugs when additional treatment was indicated, the investigators said (JAMA. 2017 Mar 14;317[10]:1057-67).
Among the minority of AF patients who were receiving therapeutic anticoagulation at the time of their stroke, 7,176 were taking adequate warfarin and 8,290 were taking adequate NOACs such as dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Stroke severity, as measured by median scores on the National Institutes of Health Stroke Scale, was significantly greater among patients receiving no antithrombotic medication, antiplatelet agents alone, or subtherapeutic levels of warfarin, compared with patients receiving therapeutic levels of warfarin or NOACs. Similarly, patients who had adequate anticoagulation before their stroke were significantly more likely to have better functional outcomes than those who did not. “These findings reinforce the importance of INR monitoring and dose adjustment to ... keep the INR in the therapeutic range,” Dr. Xian and his associates wrote.
The reasons why patients weren’t receiving adequate anticoagulation before their stroke – including any direct contraindications to treatment – were not available from the registry data. But such reasons are likely to persist after a stroke, and study clinicians were required to document such reasons in the medical records at hospital discharge. So the investigators examined reasons listed for not prescribing oral anticoagulation at hospital discharge in 58,084 patients.
A striking 38,249 AF patients (65.8%) in this large subgroup of study participants had no documented reason for not receiving oral anticoagulation, even after they were hospitalized for ischemic stroke and even though such documentation was “required.” Among the minority of patients whose records did show such reasons, the most common reason cited was bleeding risk (16.3%), followed by risk of falling (10.3%), the presence of a terminal illness (6.2%), patient or family refusal of the medication (4.3%), the patient’s impaired mental status (1.1%), adverse effects of the medication (1.0%), and allergy to the medication (0.6%).
This study was supported by the Patient-Centered Outcomes Research Institute. Dr. Xian reported that his institution received research funding from the American Heart Association, Daiichi Sankyo, Janssen, and Genentech. His associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: 84% of ischemic strokes that occurred in patients with known atrial fibrillation were preceded by inadequate anticoagulation.
Major finding: A total of 30% of AF patients were not taking any antithrombotic treatment before their ischemic stroke, 14% were taking subtherapeutic doses of warfarin, and 40% were taking only antiplatelet drugs when additional treatment was indicated.
Data source: A retrospective observational cohort study involving 94,474 patients with AF enrolled in a nationwide stroke outcomes registry.
Disclosures: This study was supported by the Patient-Centered Outcomes Research Institute. Dr. Xian reported that his institution received research funding from the American Heart Association, Daiichi Sankyo, Janssen, and Genentech. His associates reported ties to numerous industry sources.