User login
Multidrug-resistant TB response boosted by six-drug therapy
Using more than five agents to treat multidrug-resistant tuberculosis markedly increases the cure rate by as much as 65%, according to a report published online Dec. 29 in PLOS Medicine.
At present, the World Health Organization recommends a regimen of pyrazinamide plus at least four second-line drugs that are likely to be effective, based on the patient’s previous exposure, background resistance levels in the community, and any drug susceptibility testing results from known cases in contact with the patient. But recent evidence suggested that including even more drugs in the regimen might improve clinical outcomes, said Courtney M. Yuen, Ph.D., of the Centers for Disease Control and Prevention, and her associates.
The researchers performed a secondary analysis of data for 1,137 participants in the Preserving Effective Tuberculosis Treatment Study (PETTS), an international prospective cohort study of patients with multidrug-resistant pulmonary TB. These patients were followed for a median of 20 months, undergoing sputum cultures for TB every month. The researchers used time to sputum culture conversion as the indicator of treatment effectiveness.
Receiving at least six potentially effective drugs per day raised the likelihood of sputum culture conversion by 36%, compared with using the recommended five drugs. In addition, for patients receiving at least one untested drug – any antituberculosis agent given empirically, without susceptibility testing – in their five-drug regimen, adding an extra potentially effective drug raised the likelihood of sputum culture conversion by 65%. Even adding an extra untested drug to a five-drug regimen improved the likelihood of sputum culture conversion by 33%, Dr. Yuen and her associates said (PLOS Med. 2015 Dec 29. doi:10.1371/pmed.1001932).
“We observed a benefit to receiving a greater number of potentially effective drugs ... as well as an interaction in which the presence of more effective drugs enhanced the benefit of untested drugs. Both of these results add to existing evidence that increasing the number of drugs in multidrug-resistant TB regimens is advantageous,” they noted.
The WHO initially recommended a regimen of four drugs for these patients in 2006, then raised that number to five in 2011. “Our results suggest that treatment might be further fortified by adding additional potentially effective drugs,” the investigators said.
Using more than five agents to treat multidrug-resistant tuberculosis markedly increases the cure rate by as much as 65%, according to a report published online Dec. 29 in PLOS Medicine.
At present, the World Health Organization recommends a regimen of pyrazinamide plus at least four second-line drugs that are likely to be effective, based on the patient’s previous exposure, background resistance levels in the community, and any drug susceptibility testing results from known cases in contact with the patient. But recent evidence suggested that including even more drugs in the regimen might improve clinical outcomes, said Courtney M. Yuen, Ph.D., of the Centers for Disease Control and Prevention, and her associates.
The researchers performed a secondary analysis of data for 1,137 participants in the Preserving Effective Tuberculosis Treatment Study (PETTS), an international prospective cohort study of patients with multidrug-resistant pulmonary TB. These patients were followed for a median of 20 months, undergoing sputum cultures for TB every month. The researchers used time to sputum culture conversion as the indicator of treatment effectiveness.
Receiving at least six potentially effective drugs per day raised the likelihood of sputum culture conversion by 36%, compared with using the recommended five drugs. In addition, for patients receiving at least one untested drug – any antituberculosis agent given empirically, without susceptibility testing – in their five-drug regimen, adding an extra potentially effective drug raised the likelihood of sputum culture conversion by 65%. Even adding an extra untested drug to a five-drug regimen improved the likelihood of sputum culture conversion by 33%, Dr. Yuen and her associates said (PLOS Med. 2015 Dec 29. doi:10.1371/pmed.1001932).
“We observed a benefit to receiving a greater number of potentially effective drugs ... as well as an interaction in which the presence of more effective drugs enhanced the benefit of untested drugs. Both of these results add to existing evidence that increasing the number of drugs in multidrug-resistant TB regimens is advantageous,” they noted.
The WHO initially recommended a regimen of four drugs for these patients in 2006, then raised that number to five in 2011. “Our results suggest that treatment might be further fortified by adding additional potentially effective drugs,” the investigators said.
Using more than five agents to treat multidrug-resistant tuberculosis markedly increases the cure rate by as much as 65%, according to a report published online Dec. 29 in PLOS Medicine.
At present, the World Health Organization recommends a regimen of pyrazinamide plus at least four second-line drugs that are likely to be effective, based on the patient’s previous exposure, background resistance levels in the community, and any drug susceptibility testing results from known cases in contact with the patient. But recent evidence suggested that including even more drugs in the regimen might improve clinical outcomes, said Courtney M. Yuen, Ph.D., of the Centers for Disease Control and Prevention, and her associates.
The researchers performed a secondary analysis of data for 1,137 participants in the Preserving Effective Tuberculosis Treatment Study (PETTS), an international prospective cohort study of patients with multidrug-resistant pulmonary TB. These patients were followed for a median of 20 months, undergoing sputum cultures for TB every month. The researchers used time to sputum culture conversion as the indicator of treatment effectiveness.
Receiving at least six potentially effective drugs per day raised the likelihood of sputum culture conversion by 36%, compared with using the recommended five drugs. In addition, for patients receiving at least one untested drug – any antituberculosis agent given empirically, without susceptibility testing – in their five-drug regimen, adding an extra potentially effective drug raised the likelihood of sputum culture conversion by 65%. Even adding an extra untested drug to a five-drug regimen improved the likelihood of sputum culture conversion by 33%, Dr. Yuen and her associates said (PLOS Med. 2015 Dec 29. doi:10.1371/pmed.1001932).
“We observed a benefit to receiving a greater number of potentially effective drugs ... as well as an interaction in which the presence of more effective drugs enhanced the benefit of untested drugs. Both of these results add to existing evidence that increasing the number of drugs in multidrug-resistant TB regimens is advantageous,” they noted.
The WHO initially recommended a regimen of four drugs for these patients in 2006, then raised that number to five in 2011. “Our results suggest that treatment might be further fortified by adding additional potentially effective drugs,” the investigators said.
FROM PLOS MEDICINE
Key clinical point: Using more than the recommended five drugs to treat multidrug-resistant TB increased the cure rate by as much as 65%.
Major finding: Receiving at least six potentially effective drugs per day raised the likelihood of sputum culture conversion by 36%, compared with using the recommended five drugs.
Data source: A secondary analysis of data regarding 1,137 participants in an international prospective cohort study.
Disclosures: This study was supported by the U.S. Agency for International Development, the CDC, the National Institute for Allergy and Infectious Diseases, and the Korean Ministry of Health and Welfare. Dr. Yuen and her associates reported having no relevant financial disclosures.
Aortic valve replacement: Transcatheter soars past surgical
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.
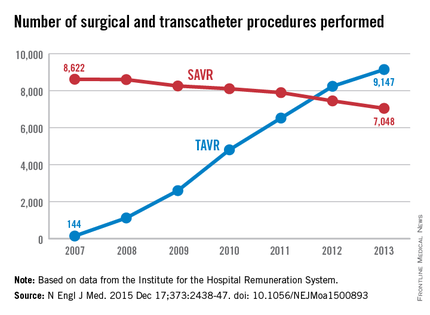
To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.

To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.

To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement occurred among patients unsuited for a surgical approach.
Major finding: The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of surgical procedures declined only slightly, from 8,622 to 7,048 per year.
Data source: A retrospective analysis of all 88,573 surgical and TAVR performed in Germany in 2007-2013.
Disclosures: This study was supported by the Heart Center at Freiburg University. Dr. Reinöhl and one of his associates reported receiving personal fees from Edwards Lifesciences and Direct Flow Medical.
ACC, AHA Update Performance Measures for Lipid Management
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
ACC, AHA update performance measures for lipid management
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
Updated performance measures regarding lipid management for secondary prevention, introducing and placing great emphasis on shared decision making between clinicians and patients, have been released jointly by the American College of Cardiology and the American Heart Association.
“These measures respect the wishes of patients regarding the use of statins and do not penalize physicians who may have a patient decline to take the medications for personal reasons,” Dr. Joseph P. Drozda Jr., chair of the update’s writing committee, said in a statement accompanying the release.
“Integrating patient values, preferences, and personal context with evidence-based medicine and guidelines is novel and changes the focus from recommending and prescribing statins ... to promoting choice by an informed patient,” said Dr. Drozda, director of outcomes research at Mercy Health, St. Louis.
Performance measures are intended to accelerate the translation of scientific evidence into clinical practice, and they are rapidly updated whenever there are changes to a relevant ACC/AHA guideline. In this case, lipid management performance measures needed updating because of new recommendations in the most recent ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). The new recommendations emphasized treatment with high-intensity statin therapy instead of achieving LDL-cholesterol targets.
In accordance with that, the lipid performance measures have been revised in four areas of lipid management: in secondary prevention for patients who have peripheral artery disease, STEMI or non-STEMI myocardial infarction, percutaneous coronary intervention, and coronary artery disease/hypertension. In addition, a new performance measure has been added addressing clinical atherosclerotic cardiovascular disease.
Abundant research has shown that only a fraction of patients with peripheral artery disease, MI, percutaneous coronary intervention, and coronary artery disease/hypertension who would benefit greatly from statin therapy are actually taking it, and that those who do take statins generally are receiving suboptimal doses. Studies also have clearly demonstrated that more intensive statin regimens reduce adverse cardiovascular events even further in these patient populations, Dr. Drozda and his associates said (J Am Coll Cardiol. 2015 Dec 13 [doi:10.1016/j.jacc.2015.02.003]).
“Better patient outcomes are realized only if patients agree with, act on, and adhere to [statin recommendations] for 5-10 years.” At present, up to half of patients prescribed statins for secondary prevention discontinue the drugs within 1-2 years, they noted.
“Clinicians need to embrace the concept that evidence-based medicine and guidelines alone are not sufficient to make a [treatment] recommendation or a decision; rather, the evidence has to be considered from the viewpoint of what matters to individual patients. Hence, the clinical encounter transforms from one where the clinician strives to convince the patient of the ‘right answer’ to one where the clinician and patient collaborate, deliberate, and arrive at the ‘best answer’ that fits patient preferences, values, and context,” Dr. Drozda and his associates said.
Clinicians may not be aware of all the factors contributing to statin discontinuation and missed doses. The cost of the drug or the copay amount may be unaffordable. The label instructions may be unclear. The patient may be too forgetful to take medications reliably, or too embarrassed to discuss the agent’s adverse effects. The patient may dislike having to take any medication, or may not understand its importance when he or she has no symptoms. One strategy to address all possible reasons for nonadherence and to accomplish shared decision making is for the clinician at every office visit to review the medication list; ask about adverse effects, cost, and adherence; and discuss barriers to adherence, the investigators said.
The updated lipid performance measures are available at www.acc.org and www.my.americanheart.org.
This work was supported exclusively by the ACC and the AHA. The financial disclosures of Dr. Drozda and the other members of the writing committee are available from the ACC and the AHA.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Poor response to third anti-TNF agent seen in most psoriatic arthritis patients
Patients with psoriatic arthritis who don’t respond to or cannot tolerate two different anti–tumor necrosis factor (TNF) agents are likely to respond poorly, if at all, to a third one, according to findings from a prospective, open-label, longitudinal study.
Dr. Lars Erik Kristensen of the department of rheumatology, Parker Institute, Copenhagen, and the department of rheumatology, Lund (Sweden) University Hospital, and his coinvestigators assessed treatment responses in patients treated at 11 European rheumatology centers during a 9-year period to build on the “rather sparse” data concerning second or third courses of anti-TNF treatment in psoriatic arthritis patients. “Our results suggest that other therapeutic options be considered after two courses of anti-TNF treatment have failed,” such as biological disease-modifying antirheumatic drugs that have different modes of action, they wrote.
The study participants were 217 patients with psoriatic arthritis who were switching from one anti-TNF agent to another and 57 who had tried two anti-TNF agents and were switching to a third. The drugs included etanercept, adalimumab, certolizumab pegol, golimumab, and infliximab.
In general, the treatment response rates among patients trying their second agent were markedly greater than those of patients trying their third. Nearly half (47%) of first-time switchers met the primary outcome measure – an ACR 20 response at 3 months – compared with only 22% of second-time switchers, the investigators said (J Rheumatol. 2015 Dec 1. doi: 10.3899/jrheum.150744).
The median drug survival (time on treatment) was 64 months for the first group, compared with only 14 months for the second group. The estimated 5-year drug survival was 51% for patients trying their second anti-TNF agent, compared with only 23% for patients trying their third.
This study was supported by the Osterlund Foundation, the Kock Foundation, the King Gustav V 80-Year Fund, Lund University Hospital, the Reumatikerforbundet, and the Oak Foundation. No information was available regarding Dr. Kristensen’s and his associates’ financial disclosures.
Patients with psoriatic arthritis who don’t respond to or cannot tolerate two different anti–tumor necrosis factor (TNF) agents are likely to respond poorly, if at all, to a third one, according to findings from a prospective, open-label, longitudinal study.
Dr. Lars Erik Kristensen of the department of rheumatology, Parker Institute, Copenhagen, and the department of rheumatology, Lund (Sweden) University Hospital, and his coinvestigators assessed treatment responses in patients treated at 11 European rheumatology centers during a 9-year period to build on the “rather sparse” data concerning second or third courses of anti-TNF treatment in psoriatic arthritis patients. “Our results suggest that other therapeutic options be considered after two courses of anti-TNF treatment have failed,” such as biological disease-modifying antirheumatic drugs that have different modes of action, they wrote.
The study participants were 217 patients with psoriatic arthritis who were switching from one anti-TNF agent to another and 57 who had tried two anti-TNF agents and were switching to a third. The drugs included etanercept, adalimumab, certolizumab pegol, golimumab, and infliximab.
In general, the treatment response rates among patients trying their second agent were markedly greater than those of patients trying their third. Nearly half (47%) of first-time switchers met the primary outcome measure – an ACR 20 response at 3 months – compared with only 22% of second-time switchers, the investigators said (J Rheumatol. 2015 Dec 1. doi: 10.3899/jrheum.150744).
The median drug survival (time on treatment) was 64 months for the first group, compared with only 14 months for the second group. The estimated 5-year drug survival was 51% for patients trying their second anti-TNF agent, compared with only 23% for patients trying their third.
This study was supported by the Osterlund Foundation, the Kock Foundation, the King Gustav V 80-Year Fund, Lund University Hospital, the Reumatikerforbundet, and the Oak Foundation. No information was available regarding Dr. Kristensen’s and his associates’ financial disclosures.
Patients with psoriatic arthritis who don’t respond to or cannot tolerate two different anti–tumor necrosis factor (TNF) agents are likely to respond poorly, if at all, to a third one, according to findings from a prospective, open-label, longitudinal study.
Dr. Lars Erik Kristensen of the department of rheumatology, Parker Institute, Copenhagen, and the department of rheumatology, Lund (Sweden) University Hospital, and his coinvestigators assessed treatment responses in patients treated at 11 European rheumatology centers during a 9-year period to build on the “rather sparse” data concerning second or third courses of anti-TNF treatment in psoriatic arthritis patients. “Our results suggest that other therapeutic options be considered after two courses of anti-TNF treatment have failed,” such as biological disease-modifying antirheumatic drugs that have different modes of action, they wrote.
The study participants were 217 patients with psoriatic arthritis who were switching from one anti-TNF agent to another and 57 who had tried two anti-TNF agents and were switching to a third. The drugs included etanercept, adalimumab, certolizumab pegol, golimumab, and infliximab.
In general, the treatment response rates among patients trying their second agent were markedly greater than those of patients trying their third. Nearly half (47%) of first-time switchers met the primary outcome measure – an ACR 20 response at 3 months – compared with only 22% of second-time switchers, the investigators said (J Rheumatol. 2015 Dec 1. doi: 10.3899/jrheum.150744).
The median drug survival (time on treatment) was 64 months for the first group, compared with only 14 months for the second group. The estimated 5-year drug survival was 51% for patients trying their second anti-TNF agent, compared with only 23% for patients trying their third.
This study was supported by the Osterlund Foundation, the Kock Foundation, the King Gustav V 80-Year Fund, Lund University Hospital, the Reumatikerforbundet, and the Oak Foundation. No information was available regarding Dr. Kristensen’s and his associates’ financial disclosures.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: Patients with psoriatic arthritis who don’t respond to or cannot tolerate two different anti-TNF agents are likely to respond poorly, if at all, to a third one.
Major finding: 47% of first-time switchers met the primary outcome measure – an ACR 20 response at 3 months – compared with only 22% of second-time switchers.
Data source: A prospective, open-label, longitudinal study involving 217 patients who switched anti-TNF therapy once and 57 who switched twice during a 9-year period.
Disclosures: This study was supported by the Osterlund Foundation, the Kock Foundation, the King Gustav V 80-Year Fund, Lund University Hospital, the Reumatikerforbundet, and the Oak Foundation. No information was available regarding Dr. Kristensen’s and his associates’ financial disclosures.
Stem-cell therapy doesn’t induce sustained Crohn’s remission
Autologous hematopoietic stem-cell transplantation didn’t induce sustained remission of refractory Crohn’s disease in an international randomized controlled trial, which was reported online December 15 in JAMA.
Exploratory analyses of the trial data suggested that slightly more patients receiving hematopoietic stem-cell transplantation (HSCT) than usual care were able to discontinue immunosuppressive therapy, achieve nonsustained clinical remission, and show improvement in clinical and endoscopic measures of disease activity. Nevertheless, the procedure is associated with numerous and serious adverse effects. Given its failure to induce sustained remission, “HSCT is unlikely to alter the natural history of Crohn’s disease, and our findings argue against extension of HSCT to a wider group of patients outside of future additional trials,” said Dr. Christopher J. Hawkey, professor of gastroenterology at the Digestive Diseases Centre, Nottingham University, England, and his associates in the Autologous Stem Cell Transplantation International Crohn’s Disease (ASTIC) trial.
The study was conducted at 11 transplantation centers in the U.K., France, Spain, Switzerland, Italy, and Belgium and involved 45 adults with established Crohn’s disease refractory to treatment with corticosteroids and at least three immunosuppressive or biologic agents. Their disease was not amenable to surgery and continued to severely impair their everyday function and quality of life.
The study participants were randomly assigned to undergo HSCT immediately (23 patients) or to delay the procedure for 1 year (22 patients). Every 6 weeks they underwent physical examination; laboratory testing; and assessment of disease activity, adverse events, medication use, use of medical services, and effects of Crohn’s on employment. Both study groups also were assessed for quality of life at 6 and 12 months.
The primary endpoint, sustained disease remission after 12 months, was defined as a Crohn’s Disease Activity Index score of less than 150 for at least the preceding 3 months, no active treatment for at least the preceding 3 months, and no mucosal erosion or ulceration anywhere in the GI tract on endoscopy and GI imaging. Admittedly, this endpoint was stringent, but it was chosen “to reflect the benefit we judged HSCT would need to yield to justify treatment toxicity,” the investigators noted.
Only two patients in the HSCT group (8.7%) and one in the control group (4.5%) achieved the primary endpoint, which was a nonsignificant difference. There also were no differences between the two groups in quality of life as measured by the European Quality of Life Visual Analogue Scale, the EuroQoL 5 Dimensions Questionnaire, the Inflammatory Bowel Disease Questionnaire, or the Karnofsky index.
However, the data slightly favored HSCT on the secondary endpoints of decreased disease activity as measured by the Crohn’s Disease Activity Index score, the Simple Endoscopic Score for Crohn’s Disease, and the need for medical therapy or consultation. These benefits “may still be clinically meaningful in this group of patients, who have no other therapeutic options and a markedly impaired quality of life,” Dr. Hawkey and his associates said (JAMA. 2015 Dec 15. doi: 10.1001/jama.2015.16700).
The HSCT group had more frequent adverse events, particularly infections (13 vs. 0) such as neutropenic sepsis, pneumonia, and perianal abscesses. The infections included reactivations of Epstein-Barr virus, cytomegalovirus, BK virus, intestinal adenovirus, and varicella zoster virus. One patient died from infection.
“These findings do not support the widespread use of HSCT for patients with refractory Crohn’s disease,” the investigators said.
Autologous hematopoietic stem-cell transplantation didn’t induce sustained remission of refractory Crohn’s disease in an international randomized controlled trial, which was reported online December 15 in JAMA.
Exploratory analyses of the trial data suggested that slightly more patients receiving hematopoietic stem-cell transplantation (HSCT) than usual care were able to discontinue immunosuppressive therapy, achieve nonsustained clinical remission, and show improvement in clinical and endoscopic measures of disease activity. Nevertheless, the procedure is associated with numerous and serious adverse effects. Given its failure to induce sustained remission, “HSCT is unlikely to alter the natural history of Crohn’s disease, and our findings argue against extension of HSCT to a wider group of patients outside of future additional trials,” said Dr. Christopher J. Hawkey, professor of gastroenterology at the Digestive Diseases Centre, Nottingham University, England, and his associates in the Autologous Stem Cell Transplantation International Crohn’s Disease (ASTIC) trial.
The study was conducted at 11 transplantation centers in the U.K., France, Spain, Switzerland, Italy, and Belgium and involved 45 adults with established Crohn’s disease refractory to treatment with corticosteroids and at least three immunosuppressive or biologic agents. Their disease was not amenable to surgery and continued to severely impair their everyday function and quality of life.
The study participants were randomly assigned to undergo HSCT immediately (23 patients) or to delay the procedure for 1 year (22 patients). Every 6 weeks they underwent physical examination; laboratory testing; and assessment of disease activity, adverse events, medication use, use of medical services, and effects of Crohn’s on employment. Both study groups also were assessed for quality of life at 6 and 12 months.
The primary endpoint, sustained disease remission after 12 months, was defined as a Crohn’s Disease Activity Index score of less than 150 for at least the preceding 3 months, no active treatment for at least the preceding 3 months, and no mucosal erosion or ulceration anywhere in the GI tract on endoscopy and GI imaging. Admittedly, this endpoint was stringent, but it was chosen “to reflect the benefit we judged HSCT would need to yield to justify treatment toxicity,” the investigators noted.
Only two patients in the HSCT group (8.7%) and one in the control group (4.5%) achieved the primary endpoint, which was a nonsignificant difference. There also were no differences between the two groups in quality of life as measured by the European Quality of Life Visual Analogue Scale, the EuroQoL 5 Dimensions Questionnaire, the Inflammatory Bowel Disease Questionnaire, or the Karnofsky index.
However, the data slightly favored HSCT on the secondary endpoints of decreased disease activity as measured by the Crohn’s Disease Activity Index score, the Simple Endoscopic Score for Crohn’s Disease, and the need for medical therapy or consultation. These benefits “may still be clinically meaningful in this group of patients, who have no other therapeutic options and a markedly impaired quality of life,” Dr. Hawkey and his associates said (JAMA. 2015 Dec 15. doi: 10.1001/jama.2015.16700).
The HSCT group had more frequent adverse events, particularly infections (13 vs. 0) such as neutropenic sepsis, pneumonia, and perianal abscesses. The infections included reactivations of Epstein-Barr virus, cytomegalovirus, BK virus, intestinal adenovirus, and varicella zoster virus. One patient died from infection.
“These findings do not support the widespread use of HSCT for patients with refractory Crohn’s disease,” the investigators said.
Autologous hematopoietic stem-cell transplantation didn’t induce sustained remission of refractory Crohn’s disease in an international randomized controlled trial, which was reported online December 15 in JAMA.
Exploratory analyses of the trial data suggested that slightly more patients receiving hematopoietic stem-cell transplantation (HSCT) than usual care were able to discontinue immunosuppressive therapy, achieve nonsustained clinical remission, and show improvement in clinical and endoscopic measures of disease activity. Nevertheless, the procedure is associated with numerous and serious adverse effects. Given its failure to induce sustained remission, “HSCT is unlikely to alter the natural history of Crohn’s disease, and our findings argue against extension of HSCT to a wider group of patients outside of future additional trials,” said Dr. Christopher J. Hawkey, professor of gastroenterology at the Digestive Diseases Centre, Nottingham University, England, and his associates in the Autologous Stem Cell Transplantation International Crohn’s Disease (ASTIC) trial.
The study was conducted at 11 transplantation centers in the U.K., France, Spain, Switzerland, Italy, and Belgium and involved 45 adults with established Crohn’s disease refractory to treatment with corticosteroids and at least three immunosuppressive or biologic agents. Their disease was not amenable to surgery and continued to severely impair their everyday function and quality of life.
The study participants were randomly assigned to undergo HSCT immediately (23 patients) or to delay the procedure for 1 year (22 patients). Every 6 weeks they underwent physical examination; laboratory testing; and assessment of disease activity, adverse events, medication use, use of medical services, and effects of Crohn’s on employment. Both study groups also were assessed for quality of life at 6 and 12 months.
The primary endpoint, sustained disease remission after 12 months, was defined as a Crohn’s Disease Activity Index score of less than 150 for at least the preceding 3 months, no active treatment for at least the preceding 3 months, and no mucosal erosion or ulceration anywhere in the GI tract on endoscopy and GI imaging. Admittedly, this endpoint was stringent, but it was chosen “to reflect the benefit we judged HSCT would need to yield to justify treatment toxicity,” the investigators noted.
Only two patients in the HSCT group (8.7%) and one in the control group (4.5%) achieved the primary endpoint, which was a nonsignificant difference. There also were no differences between the two groups in quality of life as measured by the European Quality of Life Visual Analogue Scale, the EuroQoL 5 Dimensions Questionnaire, the Inflammatory Bowel Disease Questionnaire, or the Karnofsky index.
However, the data slightly favored HSCT on the secondary endpoints of decreased disease activity as measured by the Crohn’s Disease Activity Index score, the Simple Endoscopic Score for Crohn’s Disease, and the need for medical therapy or consultation. These benefits “may still be clinically meaningful in this group of patients, who have no other therapeutic options and a markedly impaired quality of life,” Dr. Hawkey and his associates said (JAMA. 2015 Dec 15. doi: 10.1001/jama.2015.16700).
The HSCT group had more frequent adverse events, particularly infections (13 vs. 0) such as neutropenic sepsis, pneumonia, and perianal abscesses. The infections included reactivations of Epstein-Barr virus, cytomegalovirus, BK virus, intestinal adenovirus, and varicella zoster virus. One patient died from infection.
“These findings do not support the widespread use of HSCT for patients with refractory Crohn’s disease,” the investigators said.
FROM JAMA
Key clinical point: Autologous hematopoietic stem-cell transplantation doesn’t induce sustained remission of refractory Crohn’s disease, compared with usual care.
Major finding: Only two patients in the HSCT group (8.7%) and one in the control group (4.5%) achieved sustained remission, which was a nonsignificant difference.
Data source: An international randomized clinical trial involving 45 adults followed for 1 year.
Disclosures: This study was sponsored by the European Group for Blood and Marrow Transplantation and the European Crohn’s and Colitis Organisation. Dr. Hawkey and his associates reported having no relevant financial disclosures.
Study characterizes intracerebral hemorrhage with new oral anticoagulants
Intracerebral hemorrhage related to non–vitamin-K antagonist oral anticoagulants carries a high mortality and frequently involves hematoma expansion, according to a report published online Dec. 14 in JAMA Neurology.
The characteristics and natural history of acute-phase non–vitamin-K antagonist oral anticoagulant (NOAC)-associated intracerebral hemorrhage (ICH) “are largely unknown,” and there are no prospective data concerning hematoma expansion or the effectiveness of prothrombin complex concentrate in limiting that expansion by reversing anticoagulation. Nevertheless, current recommendations suggest that clinicians consider administering prothrombin complex concentrate in this patient population, said Dr. Jan C. Purrucker of the department of neurology at Heidelberg (Germany) University and his associates (JAMA Neurol. 2015 Dec 14. doi: 10.1001/jamaneurol.2015.3682).
To characterize the clinical and radiologic course, management, and outcome of NOAC-associated intracerebral hemorrhage in routine clinical practice, Dr. Purrucker and his associates performed the ICH substudy of the Registry of Acute Stroke Under New Oral Anticoagulants (RASUNOA). This is a prospective registry involving 38 neurology departments with certified stroke units across Germany. For their substudy, the investigators focused on 61 adults with a mean age of 76 years (range, 46-97 years) who were taking NOACs (apixaban [Eliquis], dabigatran etexilate [Pradaxa], or rivaroxaban [Xarelto]) and had moderate to severe neurologic deficit and a median hematoma volume of 10.8 mL at presentation. Thirty-five of these patients (57%) were treated with prothrombin complex concentrate.
Mortality was high, at 16% (10 patients) during the acute inpatient stay and 28% (17 patients) at 3 months; 65% of the survivors had an unfavorable outcome. Substantial hematoma expansion – defined as a 33% or greater relative increase or 6 mL or greater absolute increase in ICH volume – was common, affecting 38% of patients. “This proportion was within the range reported for vitamin-K antagonist–associated intracerebral hemorrhage (36%-56%) and is higher, compared with that related to intracerebral hemorrhage in patients not receiving anticoagulation (12%-26%),” the investigators wrote.
Both larger hematoma volume at baseline (odds ratio, 2.37) and intraventricular extension at baseline (OR, 8.13) strongly correlated with adverse outcomes. In contrast, prothrombin complex concentrate failed to limit lesion expansion or avert adverse outcomes. This might be because patients given the treatment tended to have more severe initial neurologic deficits and more unfavorable hematoma location than did those who weren’t given prothrombin complex concentrate. In any case, “our study design, the limited sample size, and the potential for confounding by indication do not allow any [firm] conclusions regarding a potential association between prothrombin complex concentrate treatment and outcome,” they noted.
The RASUNOA registry was supported by the University Hospital Heidelberg. Dr. Purrucker reported receiving support from Pfizer unrelated to this study, and his associates reported ties to numerous industry sources.
It’s important to note that in the study by Dr. Purrucker and his colleagues, the median time from symptom onset to the first brain imaging was 14 hours and that fully 25% of patients presented for treatment more than 22 hours after noticing their initial symptoms.

|
Dr. Stephan A. Mayer |
In contrast, patients with spontaneous hypertensive intracerebral hemorrhage present much earlier, usually within 6 hours. This indicates that the bleeding in NOAC-associated hemorrhagic stroke often is gradual and prolonged, an “oozing” process rather than the explosive type of process seen in spontaneous hemorrhagic stroke.
It is almost certain that if this cohort had undergone imaging at 3 hours rather than at 14 hours after symptom onset, the frequency of hematoma expansion would have approached 100% rather than 38%.
Dr. Stephan A. Mayer is at Mount Sinai University, New York. He reported having no relevant financial disclosures. Dr. Mayer made these remarks in an editorial accompanying Dr. Purrucker’s report (JAMA Neurol. 2015 Dec 14. doi:10.1001/jamaneurol.2015.3884).
It’s important to note that in the study by Dr. Purrucker and his colleagues, the median time from symptom onset to the first brain imaging was 14 hours and that fully 25% of patients presented for treatment more than 22 hours after noticing their initial symptoms.

|
Dr. Stephan A. Mayer |
In contrast, patients with spontaneous hypertensive intracerebral hemorrhage present much earlier, usually within 6 hours. This indicates that the bleeding in NOAC-associated hemorrhagic stroke often is gradual and prolonged, an “oozing” process rather than the explosive type of process seen in spontaneous hemorrhagic stroke.
It is almost certain that if this cohort had undergone imaging at 3 hours rather than at 14 hours after symptom onset, the frequency of hematoma expansion would have approached 100% rather than 38%.
Dr. Stephan A. Mayer is at Mount Sinai University, New York. He reported having no relevant financial disclosures. Dr. Mayer made these remarks in an editorial accompanying Dr. Purrucker’s report (JAMA Neurol. 2015 Dec 14. doi:10.1001/jamaneurol.2015.3884).
It’s important to note that in the study by Dr. Purrucker and his colleagues, the median time from symptom onset to the first brain imaging was 14 hours and that fully 25% of patients presented for treatment more than 22 hours after noticing their initial symptoms.

|
Dr. Stephan A. Mayer |
In contrast, patients with spontaneous hypertensive intracerebral hemorrhage present much earlier, usually within 6 hours. This indicates that the bleeding in NOAC-associated hemorrhagic stroke often is gradual and prolonged, an “oozing” process rather than the explosive type of process seen in spontaneous hemorrhagic stroke.
It is almost certain that if this cohort had undergone imaging at 3 hours rather than at 14 hours after symptom onset, the frequency of hematoma expansion would have approached 100% rather than 38%.
Dr. Stephan A. Mayer is at Mount Sinai University, New York. He reported having no relevant financial disclosures. Dr. Mayer made these remarks in an editorial accompanying Dr. Purrucker’s report (JAMA Neurol. 2015 Dec 14. doi:10.1001/jamaneurol.2015.3884).
Intracerebral hemorrhage related to non–vitamin-K antagonist oral anticoagulants carries a high mortality and frequently involves hematoma expansion, according to a report published online Dec. 14 in JAMA Neurology.
The characteristics and natural history of acute-phase non–vitamin-K antagonist oral anticoagulant (NOAC)-associated intracerebral hemorrhage (ICH) “are largely unknown,” and there are no prospective data concerning hematoma expansion or the effectiveness of prothrombin complex concentrate in limiting that expansion by reversing anticoagulation. Nevertheless, current recommendations suggest that clinicians consider administering prothrombin complex concentrate in this patient population, said Dr. Jan C. Purrucker of the department of neurology at Heidelberg (Germany) University and his associates (JAMA Neurol. 2015 Dec 14. doi: 10.1001/jamaneurol.2015.3682).
To characterize the clinical and radiologic course, management, and outcome of NOAC-associated intracerebral hemorrhage in routine clinical practice, Dr. Purrucker and his associates performed the ICH substudy of the Registry of Acute Stroke Under New Oral Anticoagulants (RASUNOA). This is a prospective registry involving 38 neurology departments with certified stroke units across Germany. For their substudy, the investigators focused on 61 adults with a mean age of 76 years (range, 46-97 years) who were taking NOACs (apixaban [Eliquis], dabigatran etexilate [Pradaxa], or rivaroxaban [Xarelto]) and had moderate to severe neurologic deficit and a median hematoma volume of 10.8 mL at presentation. Thirty-five of these patients (57%) were treated with prothrombin complex concentrate.
Mortality was high, at 16% (10 patients) during the acute inpatient stay and 28% (17 patients) at 3 months; 65% of the survivors had an unfavorable outcome. Substantial hematoma expansion – defined as a 33% or greater relative increase or 6 mL or greater absolute increase in ICH volume – was common, affecting 38% of patients. “This proportion was within the range reported for vitamin-K antagonist–associated intracerebral hemorrhage (36%-56%) and is higher, compared with that related to intracerebral hemorrhage in patients not receiving anticoagulation (12%-26%),” the investigators wrote.
Both larger hematoma volume at baseline (odds ratio, 2.37) and intraventricular extension at baseline (OR, 8.13) strongly correlated with adverse outcomes. In contrast, prothrombin complex concentrate failed to limit lesion expansion or avert adverse outcomes. This might be because patients given the treatment tended to have more severe initial neurologic deficits and more unfavorable hematoma location than did those who weren’t given prothrombin complex concentrate. In any case, “our study design, the limited sample size, and the potential for confounding by indication do not allow any [firm] conclusions regarding a potential association between prothrombin complex concentrate treatment and outcome,” they noted.
The RASUNOA registry was supported by the University Hospital Heidelberg. Dr. Purrucker reported receiving support from Pfizer unrelated to this study, and his associates reported ties to numerous industry sources.
Intracerebral hemorrhage related to non–vitamin-K antagonist oral anticoagulants carries a high mortality and frequently involves hematoma expansion, according to a report published online Dec. 14 in JAMA Neurology.
The characteristics and natural history of acute-phase non–vitamin-K antagonist oral anticoagulant (NOAC)-associated intracerebral hemorrhage (ICH) “are largely unknown,” and there are no prospective data concerning hematoma expansion or the effectiveness of prothrombin complex concentrate in limiting that expansion by reversing anticoagulation. Nevertheless, current recommendations suggest that clinicians consider administering prothrombin complex concentrate in this patient population, said Dr. Jan C. Purrucker of the department of neurology at Heidelberg (Germany) University and his associates (JAMA Neurol. 2015 Dec 14. doi: 10.1001/jamaneurol.2015.3682).
To characterize the clinical and radiologic course, management, and outcome of NOAC-associated intracerebral hemorrhage in routine clinical practice, Dr. Purrucker and his associates performed the ICH substudy of the Registry of Acute Stroke Under New Oral Anticoagulants (RASUNOA). This is a prospective registry involving 38 neurology departments with certified stroke units across Germany. For their substudy, the investigators focused on 61 adults with a mean age of 76 years (range, 46-97 years) who were taking NOACs (apixaban [Eliquis], dabigatran etexilate [Pradaxa], or rivaroxaban [Xarelto]) and had moderate to severe neurologic deficit and a median hematoma volume of 10.8 mL at presentation. Thirty-five of these patients (57%) were treated with prothrombin complex concentrate.
Mortality was high, at 16% (10 patients) during the acute inpatient stay and 28% (17 patients) at 3 months; 65% of the survivors had an unfavorable outcome. Substantial hematoma expansion – defined as a 33% or greater relative increase or 6 mL or greater absolute increase in ICH volume – was common, affecting 38% of patients. “This proportion was within the range reported for vitamin-K antagonist–associated intracerebral hemorrhage (36%-56%) and is higher, compared with that related to intracerebral hemorrhage in patients not receiving anticoagulation (12%-26%),” the investigators wrote.
Both larger hematoma volume at baseline (odds ratio, 2.37) and intraventricular extension at baseline (OR, 8.13) strongly correlated with adverse outcomes. In contrast, prothrombin complex concentrate failed to limit lesion expansion or avert adverse outcomes. This might be because patients given the treatment tended to have more severe initial neurologic deficits and more unfavorable hematoma location than did those who weren’t given prothrombin complex concentrate. In any case, “our study design, the limited sample size, and the potential for confounding by indication do not allow any [firm] conclusions regarding a potential association between prothrombin complex concentrate treatment and outcome,” they noted.
The RASUNOA registry was supported by the University Hospital Heidelberg. Dr. Purrucker reported receiving support from Pfizer unrelated to this study, and his associates reported ties to numerous industry sources.
FROM JAMA NEUROLOGY
Key clinical point: Intracerebral hemorrhage related to new oral anticoagulants frequently involves hematoma expansion and doesn’t appear to respond to prothrombin complex concentrate.
Major finding: Mortality was 28%, 65% of survivors had unfavorable outcomes, and substantial hematoma expansion occurred in 38% of patients.
Data source: A prospective, multicenter, observational study involving 61 patients treated during a 3-year period in Germany.
Disclosures: The RASUNOA registry was supported by the University Hospital Heidelberg. Dr. Purrucker reported receiving support from Pfizer unrelated to this study, and his associates reported ties to numerous industry sources.
Mass drug administration controls scabies in endemic areas
Mass drug administration, particularly with ivermectin, controls scabies and its burdensome complications in endemic regions of the world, according to a report published online Dec. 10 in the New England Journal of Medicine.
Scabies, a skin condition caused by a microscopic mite and transmitted by person-to-person contact, causes debilitating itching, sleep disturbance, reduced ability to concentrate and social stigmatization. In many developing countries, it is an important cause of impetigo, which in turn can lead to septicemia, glomerulonephritis, and rheumatic heart disease. Scabies is estimated to affect 100 million people worldwide every year, said Lucia Romani of the Kirby Institute, University of New South Wales (Australia), and her associates.
Even though effective treatments are available, scabies reinfestation is common. The investigators performed a comparative trial to determine whether mass drug administration, similar to that used for several other tropical diseases, would be more effective than usual care would be at controlling scabies. They randomly assigned three relatively isolated island communities in Fiji to receive three different treatment approaches during a 1-year period: topical permethrin given to all affected persons and their contacts (standard care), mass administration of topical permethrin to the entire community (permethrin group), or mass administration of oral ivermectin (ivermectin group). Treatment was administered at a single nurse-staffed health clinic in each community.
A total of 2,051 residents of all ages agreed to participate in the study, which represents more than 85% of the population. The prevalence of scabies was high at baseline: 37% of the 803 participants were given standard care, 42% of the 532 participants were given permethrin, and 32% of the 716 participants were given ivermectin. The severity of disease was similar across the three study groups.
Receipt of treatment was directly observed by clinic staff much more frequently with ivermectin (96%) than with permethrin (58%), mostly because of the different routes of administration.
The primary outcome measure – decline in the prevalence of scabies from baseline to 12 months – occurred in all three study groups, but a significantly greater reduction occurred in the ivermectin group. The relative reduction in scabies prevalence was 94% with ivermectin, 62% with permethrin, and 49% with standard care. The combined relative reduction in the two groups that had mass drug administration was 78%, Ms. Romani and her associates wrote (N Engl J Med. 2015 Dec 10;373:2305-13. doi:10.1056/NEJMoa1500987).
The prevalence of impetigo showed a similar pattern, with a relative reduction of 67% for ivermectin, 54% for permethrin, and 32% for standard care. The number of clinic visits also declined, with 10.6 fewer visits per 100 persons for ivermectin, 7.0 fewer for permethrin, and 13.7 fewer for standard care.
Scabies declined in all age groups and in both sexes. In addition to reductions in prevalence, there also were significant declines in the severity of scabies cases that did develop.
Adverse events were common, but all were mild and resolved quickly. Itching was the most frequent event and likely was caused by an inflammatory response to dead mite antigens. A total of 5.3% of the ivermectin group and 3.6% of the permethrin group reported itching, and 3.8% of the ivermectin group and 0.9% of the permethrin group reported headache.
The study findings show that mass drug administration may become an important control strategy in countries where scabies is endemic. Its effectiveness must now be studied in larger populations, with an eye toward establishing the optimal number of treatment cycles and mode of delivery, mechanisms to track efficacy and safety, and cost effectiveness. The effect that controlling scabies and impetigo exerts on downstream complications also should be assessed, they added.
The study was funded by the Australian National Health and Medical Research Council. Ms. Romani reported receiving nonfinancial support from Merck, Sharp & Dohme; her associates reported having no relevant financial disclosures. Merck, Sharp & Dohme provided the ivermectin but had no other role in the study.
This elegant study demonstrates that, although the administration of topical permethrin and oral ivermectin may show similar efficacy in individual patients with scabies, the practical aspects of oral therapy translate to superior effectiveness on a large scale.
Scabies is a major underlying cause of the ubiquitous bacterial skin infections and their septic sequelae in tropical countries. It was particularly encouraging that after mass administration of ivermectin in this study, the prevalence of impetigo decreased from approximately 25% to just 8% after only 1 year.
Dr. Bart J. Currie is at the Menzies School of Health Research at Charles Darwin University, Casuarina, and also in the department of infectious diseases at Royal Darwin Hospital, Tiwi, both in the Northern Territory, Australia. He reported having no relevant financial disclosures. Dr. Currie made these remarks in an editorial accompanying Ms. Romani’s report (N Engl J Med. 2015 Dec 10;373:2371-2. doi: 10.1056/NEJMe1511805).
This elegant study demonstrates that, although the administration of topical permethrin and oral ivermectin may show similar efficacy in individual patients with scabies, the practical aspects of oral therapy translate to superior effectiveness on a large scale.
Scabies is a major underlying cause of the ubiquitous bacterial skin infections and their septic sequelae in tropical countries. It was particularly encouraging that after mass administration of ivermectin in this study, the prevalence of impetigo decreased from approximately 25% to just 8% after only 1 year.
Dr. Bart J. Currie is at the Menzies School of Health Research at Charles Darwin University, Casuarina, and also in the department of infectious diseases at Royal Darwin Hospital, Tiwi, both in the Northern Territory, Australia. He reported having no relevant financial disclosures. Dr. Currie made these remarks in an editorial accompanying Ms. Romani’s report (N Engl J Med. 2015 Dec 10;373:2371-2. doi: 10.1056/NEJMe1511805).
This elegant study demonstrates that, although the administration of topical permethrin and oral ivermectin may show similar efficacy in individual patients with scabies, the practical aspects of oral therapy translate to superior effectiveness on a large scale.
Scabies is a major underlying cause of the ubiquitous bacterial skin infections and their septic sequelae in tropical countries. It was particularly encouraging that after mass administration of ivermectin in this study, the prevalence of impetigo decreased from approximately 25% to just 8% after only 1 year.
Dr. Bart J. Currie is at the Menzies School of Health Research at Charles Darwin University, Casuarina, and also in the department of infectious diseases at Royal Darwin Hospital, Tiwi, both in the Northern Territory, Australia. He reported having no relevant financial disclosures. Dr. Currie made these remarks in an editorial accompanying Ms. Romani’s report (N Engl J Med. 2015 Dec 10;373:2371-2. doi: 10.1056/NEJMe1511805).
Mass drug administration, particularly with ivermectin, controls scabies and its burdensome complications in endemic regions of the world, according to a report published online Dec. 10 in the New England Journal of Medicine.
Scabies, a skin condition caused by a microscopic mite and transmitted by person-to-person contact, causes debilitating itching, sleep disturbance, reduced ability to concentrate and social stigmatization. In many developing countries, it is an important cause of impetigo, which in turn can lead to septicemia, glomerulonephritis, and rheumatic heart disease. Scabies is estimated to affect 100 million people worldwide every year, said Lucia Romani of the Kirby Institute, University of New South Wales (Australia), and her associates.
Even though effective treatments are available, scabies reinfestation is common. The investigators performed a comparative trial to determine whether mass drug administration, similar to that used for several other tropical diseases, would be more effective than usual care would be at controlling scabies. They randomly assigned three relatively isolated island communities in Fiji to receive three different treatment approaches during a 1-year period: topical permethrin given to all affected persons and their contacts (standard care), mass administration of topical permethrin to the entire community (permethrin group), or mass administration of oral ivermectin (ivermectin group). Treatment was administered at a single nurse-staffed health clinic in each community.
A total of 2,051 residents of all ages agreed to participate in the study, which represents more than 85% of the population. The prevalence of scabies was high at baseline: 37% of the 803 participants were given standard care, 42% of the 532 participants were given permethrin, and 32% of the 716 participants were given ivermectin. The severity of disease was similar across the three study groups.
Receipt of treatment was directly observed by clinic staff much more frequently with ivermectin (96%) than with permethrin (58%), mostly because of the different routes of administration.
The primary outcome measure – decline in the prevalence of scabies from baseline to 12 months – occurred in all three study groups, but a significantly greater reduction occurred in the ivermectin group. The relative reduction in scabies prevalence was 94% with ivermectin, 62% with permethrin, and 49% with standard care. The combined relative reduction in the two groups that had mass drug administration was 78%, Ms. Romani and her associates wrote (N Engl J Med. 2015 Dec 10;373:2305-13. doi:10.1056/NEJMoa1500987).
The prevalence of impetigo showed a similar pattern, with a relative reduction of 67% for ivermectin, 54% for permethrin, and 32% for standard care. The number of clinic visits also declined, with 10.6 fewer visits per 100 persons for ivermectin, 7.0 fewer for permethrin, and 13.7 fewer for standard care.
Scabies declined in all age groups and in both sexes. In addition to reductions in prevalence, there also were significant declines in the severity of scabies cases that did develop.
Adverse events were common, but all were mild and resolved quickly. Itching was the most frequent event and likely was caused by an inflammatory response to dead mite antigens. A total of 5.3% of the ivermectin group and 3.6% of the permethrin group reported itching, and 3.8% of the ivermectin group and 0.9% of the permethrin group reported headache.
The study findings show that mass drug administration may become an important control strategy in countries where scabies is endemic. Its effectiveness must now be studied in larger populations, with an eye toward establishing the optimal number of treatment cycles and mode of delivery, mechanisms to track efficacy and safety, and cost effectiveness. The effect that controlling scabies and impetigo exerts on downstream complications also should be assessed, they added.
The study was funded by the Australian National Health and Medical Research Council. Ms. Romani reported receiving nonfinancial support from Merck, Sharp & Dohme; her associates reported having no relevant financial disclosures. Merck, Sharp & Dohme provided the ivermectin but had no other role in the study.
Mass drug administration, particularly with ivermectin, controls scabies and its burdensome complications in endemic regions of the world, according to a report published online Dec. 10 in the New England Journal of Medicine.
Scabies, a skin condition caused by a microscopic mite and transmitted by person-to-person contact, causes debilitating itching, sleep disturbance, reduced ability to concentrate and social stigmatization. In many developing countries, it is an important cause of impetigo, which in turn can lead to septicemia, glomerulonephritis, and rheumatic heart disease. Scabies is estimated to affect 100 million people worldwide every year, said Lucia Romani of the Kirby Institute, University of New South Wales (Australia), and her associates.
Even though effective treatments are available, scabies reinfestation is common. The investigators performed a comparative trial to determine whether mass drug administration, similar to that used for several other tropical diseases, would be more effective than usual care would be at controlling scabies. They randomly assigned three relatively isolated island communities in Fiji to receive three different treatment approaches during a 1-year period: topical permethrin given to all affected persons and their contacts (standard care), mass administration of topical permethrin to the entire community (permethrin group), or mass administration of oral ivermectin (ivermectin group). Treatment was administered at a single nurse-staffed health clinic in each community.
A total of 2,051 residents of all ages agreed to participate in the study, which represents more than 85% of the population. The prevalence of scabies was high at baseline: 37% of the 803 participants were given standard care, 42% of the 532 participants were given permethrin, and 32% of the 716 participants were given ivermectin. The severity of disease was similar across the three study groups.
Receipt of treatment was directly observed by clinic staff much more frequently with ivermectin (96%) than with permethrin (58%), mostly because of the different routes of administration.
The primary outcome measure – decline in the prevalence of scabies from baseline to 12 months – occurred in all three study groups, but a significantly greater reduction occurred in the ivermectin group. The relative reduction in scabies prevalence was 94% with ivermectin, 62% with permethrin, and 49% with standard care. The combined relative reduction in the two groups that had mass drug administration was 78%, Ms. Romani and her associates wrote (N Engl J Med. 2015 Dec 10;373:2305-13. doi:10.1056/NEJMoa1500987).
The prevalence of impetigo showed a similar pattern, with a relative reduction of 67% for ivermectin, 54% for permethrin, and 32% for standard care. The number of clinic visits also declined, with 10.6 fewer visits per 100 persons for ivermectin, 7.0 fewer for permethrin, and 13.7 fewer for standard care.
Scabies declined in all age groups and in both sexes. In addition to reductions in prevalence, there also were significant declines in the severity of scabies cases that did develop.
Adverse events were common, but all were mild and resolved quickly. Itching was the most frequent event and likely was caused by an inflammatory response to dead mite antigens. A total of 5.3% of the ivermectin group and 3.6% of the permethrin group reported itching, and 3.8% of the ivermectin group and 0.9% of the permethrin group reported headache.
The study findings show that mass drug administration may become an important control strategy in countries where scabies is endemic. Its effectiveness must now be studied in larger populations, with an eye toward establishing the optimal number of treatment cycles and mode of delivery, mechanisms to track efficacy and safety, and cost effectiveness. The effect that controlling scabies and impetigo exerts on downstream complications also should be assessed, they added.
The study was funded by the Australian National Health and Medical Research Council. Ms. Romani reported receiving nonfinancial support from Merck, Sharp & Dohme; her associates reported having no relevant financial disclosures. Merck, Sharp & Dohme provided the ivermectin but had no other role in the study.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Mass drug administration, particularly with ivermectin, controls scabies in endemic regions.
Major finding: The primary outcome measure – the relative decline in the prevalence of scabies from baseline to 12 months – was 94% with ivermectin, 62% with permethrin, and 49% with standard care.
Data source: A 1-year prospective randomized trial comparing three treatment strategies in 2,051 free-living children and adults in the Fiji Islands.
Disclosures: This study was funded by the Australian National Health and Medical Research Council. Ms. Romani reported receiving nonfinancial support from Merck, Sharp & Dohme; her associates reported having no relevant financial disclosures. Merck, Sharp & Dohme provided the ivermectin but had no other role in the study.
Endobronchial valves improve pulmonary function in emphysema
Endobronchial valves improved pulmonary function, exercise capacity, and quality of life in a prospective randomized controlled trial involving 68 adults with severe emphysema, according to a report published online Dec. 10 in the New England Journal of Medicine.
“The improvements we found were of greater magnitude than those noted with pharmacologic treatment in comparable patients and were similar to improvements with surgical lung-volume reduction, but with significantly less morbidity,” said Karin Klooster of the department of pulmonary diseases, University Medical Center Groningen (the Netherlands) and her associates.
Previous research suggested that bronchoscopic lung-volume reduction using one-way endobronchial valves to block inspiratory but not expiratory air flow would be most effective in patients who had a complete rather than an incomplete fissure between the targeted lobe and the adjacent lobe on high-resolution CT. “A complete fissure on HRCT [high-resolution computed tomography] is a surrogate finding for the absence of interlobar collateral ventilation; if there is collateral ventilation, an occluded lobe can be reinflated through its collaterals,” defeating the purpose of the procedure, the researchers wrote.
During a 3-year period, Ms. Klooster and her associates studied emphysema patients who were older than 35 years (mean age, 58-59) and had a postbronchodilator forced expiratory volume in 1 second (FEV1) less than 60% of predicted volume, a total lung capacity more than 100% of the predicted value, and residual volume more than 150% of predicted volume. On HRCT, all the study participants showed a complete or nearly complete fissure between the targeted lobe and the adjacent lobe. They were randomly assigned to receive endobronchial valves (34 patients) or usual care (34 control subjects) and followed for 6 months. At that time, control subjects were allowed to crossover and receive endobronchial valves as well.
The median procedure time was 18 minutes (range, 6-51 minutes), and the median number of valves placed in each patient was 4 (range, 2-7 valves). The median hospital stay was 1 day (range, 1-13 days).
Compared with the control subjects, patients who received endobronchial valves showed a reduction in target lobar volume of 1,366 mL. This was accompanied by improvements in FEV1 by 191 mL, in forced vital capacity by 442 mL, in residual lung volume, in longer 6-minute walk distance by 106 meters, in scores on the Clinical COPD Questionnaire measuring daily functioning, and in scores on the St. George’s Respiratory Questionnaire measuring quality of life. The results for the control subjects who crossed over to the active-treatment group were very similar, the investigators said (N Engl J Med. 2015 Dec 10;373:2325-35. doi:10.1056/NEJMoa1507807).
However, several adverse effects occurred, and close monitoring of this patient population is crucial. The most common complication was pneumothorax, which developed in 6 of the 34 patients (18%), usually within 1 day of undergoing the procedure. Pneumothorax resolved spontaneously in one patient but required chest-tube drainage in the other five; it resolved in one patient after temporary removal of the valves to promote healing, and in another after permanent removal of all valves.
Other adverse effects, some of which required repeat bronchoscopy, included torsion of the lower-lobe bronchus after upper-lobe treatment (two patients), pneumonia distal to the valves (one patient), increased dyspnea and sputum production (two patients), valve migration (two patients), valve dislocation because of granulation-tissue formation (one patient), and persistent cough (one patient). Despite these setbacks, “the overall outcome of treatment was positive,” Ms. Klooster and her associates said.
All patients who underwent valve removal recovered without any further adverse effects, indicating that this treatment “is fully reversible and doesn’t preclude further therapeutic options,” they added.
The study was supported by the Netherlands Organization for Health Research and Development and the University Medical Center Groningen. Ms. Klooster reported receiving fees, devices, travel support, and grant support from Pulmonx and PneumRx/BTG; her associates reported ties to numerous industry sponsors. Pulmonx commercially supplied the endobronchial valves for the study.
Endobronchial valves improved pulmonary function, exercise capacity, and quality of life in a prospective randomized controlled trial involving 68 adults with severe emphysema, according to a report published online Dec. 10 in the New England Journal of Medicine.
“The improvements we found were of greater magnitude than those noted with pharmacologic treatment in comparable patients and were similar to improvements with surgical lung-volume reduction, but with significantly less morbidity,” said Karin Klooster of the department of pulmonary diseases, University Medical Center Groningen (the Netherlands) and her associates.
Previous research suggested that bronchoscopic lung-volume reduction using one-way endobronchial valves to block inspiratory but not expiratory air flow would be most effective in patients who had a complete rather than an incomplete fissure between the targeted lobe and the adjacent lobe on high-resolution CT. “A complete fissure on HRCT [high-resolution computed tomography] is a surrogate finding for the absence of interlobar collateral ventilation; if there is collateral ventilation, an occluded lobe can be reinflated through its collaterals,” defeating the purpose of the procedure, the researchers wrote.
During a 3-year period, Ms. Klooster and her associates studied emphysema patients who were older than 35 years (mean age, 58-59) and had a postbronchodilator forced expiratory volume in 1 second (FEV1) less than 60% of predicted volume, a total lung capacity more than 100% of the predicted value, and residual volume more than 150% of predicted volume. On HRCT, all the study participants showed a complete or nearly complete fissure between the targeted lobe and the adjacent lobe. They were randomly assigned to receive endobronchial valves (34 patients) or usual care (34 control subjects) and followed for 6 months. At that time, control subjects were allowed to crossover and receive endobronchial valves as well.
The median procedure time was 18 minutes (range, 6-51 minutes), and the median number of valves placed in each patient was 4 (range, 2-7 valves). The median hospital stay was 1 day (range, 1-13 days).
Compared with the control subjects, patients who received endobronchial valves showed a reduction in target lobar volume of 1,366 mL. This was accompanied by improvements in FEV1 by 191 mL, in forced vital capacity by 442 mL, in residual lung volume, in longer 6-minute walk distance by 106 meters, in scores on the Clinical COPD Questionnaire measuring daily functioning, and in scores on the St. George’s Respiratory Questionnaire measuring quality of life. The results for the control subjects who crossed over to the active-treatment group were very similar, the investigators said (N Engl J Med. 2015 Dec 10;373:2325-35. doi:10.1056/NEJMoa1507807).
However, several adverse effects occurred, and close monitoring of this patient population is crucial. The most common complication was pneumothorax, which developed in 6 of the 34 patients (18%), usually within 1 day of undergoing the procedure. Pneumothorax resolved spontaneously in one patient but required chest-tube drainage in the other five; it resolved in one patient after temporary removal of the valves to promote healing, and in another after permanent removal of all valves.
Other adverse effects, some of which required repeat bronchoscopy, included torsion of the lower-lobe bronchus after upper-lobe treatment (two patients), pneumonia distal to the valves (one patient), increased dyspnea and sputum production (two patients), valve migration (two patients), valve dislocation because of granulation-tissue formation (one patient), and persistent cough (one patient). Despite these setbacks, “the overall outcome of treatment was positive,” Ms. Klooster and her associates said.
All patients who underwent valve removal recovered without any further adverse effects, indicating that this treatment “is fully reversible and doesn’t preclude further therapeutic options,” they added.
The study was supported by the Netherlands Organization for Health Research and Development and the University Medical Center Groningen. Ms. Klooster reported receiving fees, devices, travel support, and grant support from Pulmonx and PneumRx/BTG; her associates reported ties to numerous industry sponsors. Pulmonx commercially supplied the endobronchial valves for the study.
Endobronchial valves improved pulmonary function, exercise capacity, and quality of life in a prospective randomized controlled trial involving 68 adults with severe emphysema, according to a report published online Dec. 10 in the New England Journal of Medicine.
“The improvements we found were of greater magnitude than those noted with pharmacologic treatment in comparable patients and were similar to improvements with surgical lung-volume reduction, but with significantly less morbidity,” said Karin Klooster of the department of pulmonary diseases, University Medical Center Groningen (the Netherlands) and her associates.
Previous research suggested that bronchoscopic lung-volume reduction using one-way endobronchial valves to block inspiratory but not expiratory air flow would be most effective in patients who had a complete rather than an incomplete fissure between the targeted lobe and the adjacent lobe on high-resolution CT. “A complete fissure on HRCT [high-resolution computed tomography] is a surrogate finding for the absence of interlobar collateral ventilation; if there is collateral ventilation, an occluded lobe can be reinflated through its collaterals,” defeating the purpose of the procedure, the researchers wrote.
During a 3-year period, Ms. Klooster and her associates studied emphysema patients who were older than 35 years (mean age, 58-59) and had a postbronchodilator forced expiratory volume in 1 second (FEV1) less than 60% of predicted volume, a total lung capacity more than 100% of the predicted value, and residual volume more than 150% of predicted volume. On HRCT, all the study participants showed a complete or nearly complete fissure between the targeted lobe and the adjacent lobe. They were randomly assigned to receive endobronchial valves (34 patients) or usual care (34 control subjects) and followed for 6 months. At that time, control subjects were allowed to crossover and receive endobronchial valves as well.
The median procedure time was 18 minutes (range, 6-51 minutes), and the median number of valves placed in each patient was 4 (range, 2-7 valves). The median hospital stay was 1 day (range, 1-13 days).
Compared with the control subjects, patients who received endobronchial valves showed a reduction in target lobar volume of 1,366 mL. This was accompanied by improvements in FEV1 by 191 mL, in forced vital capacity by 442 mL, in residual lung volume, in longer 6-minute walk distance by 106 meters, in scores on the Clinical COPD Questionnaire measuring daily functioning, and in scores on the St. George’s Respiratory Questionnaire measuring quality of life. The results for the control subjects who crossed over to the active-treatment group were very similar, the investigators said (N Engl J Med. 2015 Dec 10;373:2325-35. doi:10.1056/NEJMoa1507807).
However, several adverse effects occurred, and close monitoring of this patient population is crucial. The most common complication was pneumothorax, which developed in 6 of the 34 patients (18%), usually within 1 day of undergoing the procedure. Pneumothorax resolved spontaneously in one patient but required chest-tube drainage in the other five; it resolved in one patient after temporary removal of the valves to promote healing, and in another after permanent removal of all valves.
Other adverse effects, some of which required repeat bronchoscopy, included torsion of the lower-lobe bronchus after upper-lobe treatment (two patients), pneumonia distal to the valves (one patient), increased dyspnea and sputum production (two patients), valve migration (two patients), valve dislocation because of granulation-tissue formation (one patient), and persistent cough (one patient). Despite these setbacks, “the overall outcome of treatment was positive,” Ms. Klooster and her associates said.
All patients who underwent valve removal recovered without any further adverse effects, indicating that this treatment “is fully reversible and doesn’t preclude further therapeutic options,” they added.
The study was supported by the Netherlands Organization for Health Research and Development and the University Medical Center Groningen. Ms. Klooster reported receiving fees, devices, travel support, and grant support from Pulmonx and PneumRx/BTG; her associates reported ties to numerous industry sponsors. Pulmonx commercially supplied the endobronchial valves for the study.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Endobronchial valves improved pulmonary function, exercise capacity, and QOL in severe emphysema.
Major finding: Patients who received endobronchial valves showed improved FEV1 by 191 mL, forced vital capacity by 442 mL, residual lung volume, 6-minute walk distance by 106 meters, and QOL scores.
Data source: A prospective randomized controlled trial involving 68 patients treated during a 3-year period at a single medical center.
Disclosures: The Netherlands Organization for Health Research and Development and the University Medical Center Groningen funded the study. Ms. Klooster reported receiving fees, devices, travel support, and grant support from Pulmonx and PneumRx/BTG; her associates reported ties to numerous industry sponsors. Pulmonx commercially supplied the endobronchial valves for this study.
One-step gestational diabetes screen not beneficial
Switching to the new one-step screen for gestational diabetes, recommended by the International Association of the Diabetes and Pregnancy Study Group and endorsed by the World Health Organization, failed to reduce the rates of large-for-gestational-age neonates, macrosomia, neonatal intensive care unit admissions, preterm births, preeclampsia, and shoulder dystocia, according to a new study.
Clinicians in the United States have traditionally screened for gestational diabetes using a two-step test early in gestation in those patients considered high risk, with a second test at 24-28 weeks of gestation for average-risk patients. Values were considered abnormal if they exceeded those recommended by either the Carpenter and Coustan and Fourth International Workshop–Conference Criteria or the values designated by the National Diabetes Group. The American College of Obstetricians and Gynecologists continues to recommend this two-step approach, according to Dr. R. Klara Feldman of the department of ob.gyn., Kaiser Permanente at Baldwin Park, Montebello, Calif., and her associates.
But based on the results of observational studies, some in the ob.gyn. community thought that expanding the number of women diagnosed and treated early would decrease the number of LGA neonates and related complications. In 2010, the IADPSG and the WHO recommended that instead of the two-step screen, clinicians should perform a hemoglobin A1c test, a random plasma glucose test, or a fasting plasma glucose test at the first prenatal visit to identify undiagnosed pregestational diabetes. For all women not found to have pregestational diabetes, a one-step, 2-hour glucose tolerance test is recommended at 24-28 weeks.
To determine whether switching to the new screening method decreased the number of LGA neonates as hoped, Dr. Feldman and her associates performed a before-after retrospective cohort study comparing the outcomes of 2,972 singleton pregnancies before their hospital and its affiliated clinics switched from the two-step Carpenter-Coustan screen to the one-step IADPSG screen, with 3,094 singleton pregnancies after the change.
The care of patients with gestational diabetes did not change during this period, and the two study groups showed no significant differences in mother’s prenatal weight, mother’s body mass index at initial prenatal visit, the number of office visits during pregnancy, or the percentage of deliveries requiring labor induction.
The rate of diagnosed gestational diabetes increased as expected after the new screen was implemented, from 17% to 27%. However, the primary outcome measure – the percentage of LGA neonates – did not decrease significantly. That rate was 10% in the “before” group and 9% in the “after” group. Similarly, rates of macrosomia, NICU admission, preterm birth, preeclampsia, and shoulder dystocia remained unchanged after implementation of the new screening approach (Obstet Gynecol. 2016;127:10-7. doi: 10.1097/AOG.0000000000001132).
“The cutoffs chosen by the IADPSG may be too low and thus result in too many patients being treated as having gestational diabetes,” they added. “Different cutoff values need to be evaluated, and more attention needs to be focused on controlling prepregnancy BMI.”
The Kaiser Permanente Regional Research Committee supported the study. Dr. Feldman and her associates reported having no relevant financial disclosures.
Switching to the new one-step screen for gestational diabetes, recommended by the International Association of the Diabetes and Pregnancy Study Group and endorsed by the World Health Organization, failed to reduce the rates of large-for-gestational-age neonates, macrosomia, neonatal intensive care unit admissions, preterm births, preeclampsia, and shoulder dystocia, according to a new study.
Clinicians in the United States have traditionally screened for gestational diabetes using a two-step test early in gestation in those patients considered high risk, with a second test at 24-28 weeks of gestation for average-risk patients. Values were considered abnormal if they exceeded those recommended by either the Carpenter and Coustan and Fourth International Workshop–Conference Criteria or the values designated by the National Diabetes Group. The American College of Obstetricians and Gynecologists continues to recommend this two-step approach, according to Dr. R. Klara Feldman of the department of ob.gyn., Kaiser Permanente at Baldwin Park, Montebello, Calif., and her associates.
But based on the results of observational studies, some in the ob.gyn. community thought that expanding the number of women diagnosed and treated early would decrease the number of LGA neonates and related complications. In 2010, the IADPSG and the WHO recommended that instead of the two-step screen, clinicians should perform a hemoglobin A1c test, a random plasma glucose test, or a fasting plasma glucose test at the first prenatal visit to identify undiagnosed pregestational diabetes. For all women not found to have pregestational diabetes, a one-step, 2-hour glucose tolerance test is recommended at 24-28 weeks.
To determine whether switching to the new screening method decreased the number of LGA neonates as hoped, Dr. Feldman and her associates performed a before-after retrospective cohort study comparing the outcomes of 2,972 singleton pregnancies before their hospital and its affiliated clinics switched from the two-step Carpenter-Coustan screen to the one-step IADPSG screen, with 3,094 singleton pregnancies after the change.
The care of patients with gestational diabetes did not change during this period, and the two study groups showed no significant differences in mother’s prenatal weight, mother’s body mass index at initial prenatal visit, the number of office visits during pregnancy, or the percentage of deliveries requiring labor induction.
The rate of diagnosed gestational diabetes increased as expected after the new screen was implemented, from 17% to 27%. However, the primary outcome measure – the percentage of LGA neonates – did not decrease significantly. That rate was 10% in the “before” group and 9% in the “after” group. Similarly, rates of macrosomia, NICU admission, preterm birth, preeclampsia, and shoulder dystocia remained unchanged after implementation of the new screening approach (Obstet Gynecol. 2016;127:10-7. doi: 10.1097/AOG.0000000000001132).
“The cutoffs chosen by the IADPSG may be too low and thus result in too many patients being treated as having gestational diabetes,” they added. “Different cutoff values need to be evaluated, and more attention needs to be focused on controlling prepregnancy BMI.”
The Kaiser Permanente Regional Research Committee supported the study. Dr. Feldman and her associates reported having no relevant financial disclosures.
Switching to the new one-step screen for gestational diabetes, recommended by the International Association of the Diabetes and Pregnancy Study Group and endorsed by the World Health Organization, failed to reduce the rates of large-for-gestational-age neonates, macrosomia, neonatal intensive care unit admissions, preterm births, preeclampsia, and shoulder dystocia, according to a new study.
Clinicians in the United States have traditionally screened for gestational diabetes using a two-step test early in gestation in those patients considered high risk, with a second test at 24-28 weeks of gestation for average-risk patients. Values were considered abnormal if they exceeded those recommended by either the Carpenter and Coustan and Fourth International Workshop–Conference Criteria or the values designated by the National Diabetes Group. The American College of Obstetricians and Gynecologists continues to recommend this two-step approach, according to Dr. R. Klara Feldman of the department of ob.gyn., Kaiser Permanente at Baldwin Park, Montebello, Calif., and her associates.
But based on the results of observational studies, some in the ob.gyn. community thought that expanding the number of women diagnosed and treated early would decrease the number of LGA neonates and related complications. In 2010, the IADPSG and the WHO recommended that instead of the two-step screen, clinicians should perform a hemoglobin A1c test, a random plasma glucose test, or a fasting plasma glucose test at the first prenatal visit to identify undiagnosed pregestational diabetes. For all women not found to have pregestational diabetes, a one-step, 2-hour glucose tolerance test is recommended at 24-28 weeks.
To determine whether switching to the new screening method decreased the number of LGA neonates as hoped, Dr. Feldman and her associates performed a before-after retrospective cohort study comparing the outcomes of 2,972 singleton pregnancies before their hospital and its affiliated clinics switched from the two-step Carpenter-Coustan screen to the one-step IADPSG screen, with 3,094 singleton pregnancies after the change.
The care of patients with gestational diabetes did not change during this period, and the two study groups showed no significant differences in mother’s prenatal weight, mother’s body mass index at initial prenatal visit, the number of office visits during pregnancy, or the percentage of deliveries requiring labor induction.
The rate of diagnosed gestational diabetes increased as expected after the new screen was implemented, from 17% to 27%. However, the primary outcome measure – the percentage of LGA neonates – did not decrease significantly. That rate was 10% in the “before” group and 9% in the “after” group. Similarly, rates of macrosomia, NICU admission, preterm birth, preeclampsia, and shoulder dystocia remained unchanged after implementation of the new screening approach (Obstet Gynecol. 2016;127:10-7. doi: 10.1097/AOG.0000000000001132).
“The cutoffs chosen by the IADPSG may be too low and thus result in too many patients being treated as having gestational diabetes,” they added. “Different cutoff values need to be evaluated, and more attention needs to be focused on controlling prepregnancy BMI.”
The Kaiser Permanente Regional Research Committee supported the study. Dr. Feldman and her associates reported having no relevant financial disclosures.
FROM OBSTETRICS AND GYNECOLOGY
Key clinical point: Switching to the new 1-step screen for gestational diabetes recommended by the International Association of the Diabetes and Pregnancy Study Group failed to reduce the rates of large-for-gestational age neonates, macrosomia, neonatal intensive care unit admissions, preterm births, preeclampsia, or shoulder dystocia.
Major finding: The primary outcome measure – the percentage of LGA neonates – was 10% before switching to the new screen and 9% afterward, a nonsignificant difference.
Data source: A before-after retrospective cohort study comparing outcomes of 2,972 pregnancies before the switch to the new screen against 3,094 afterward.
Disclosures: The Kaiser Permanente Regional Research Committee supported the study. Dr. Feldman and her associates reported having no relevant financial disclosures.