User login
Plant-based diet tied to healthier blood lipid levels
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN HEART JOURNAL
Does Ozempic cause hair loss?
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Should people be concerned about possible hair loss when taking Wegovy, Ozempic, or Mounjaro for weight loss (where the latter two drugs are being used off label) – as was recently claimed by some people on social media and reported in news stories?
The consensus among dermatologists and endocrinologists is no.
It’s up to the individual to weigh the benefits of treating obesity against the risks of the therapy, including the low risk of developing temporary hair loss, says one expert.
Wegovy, Ozempic, and Mounjaro
Of these three newer medications, only the glucagonlike peptide–1 (GLP-1) receptor agonist semaglutide (Wegovy) is approved by the Food and Drug Administration (since June 2021) for weight management – specifically for people with either obesity (body mass index ≥ 30 kg/m2) or overweight (BMI ≥ 27) plus at least one weight-related comorbidity such as hypertension, type 2 diabetes, and high cholesterol – with a dosage up to a 2.4-mg weekly injection.
When there was a short supply of Wegovy soon after it became available, some people turned to the same drug – semaglutide, but marketed as Ozempic for type 2 diabetes, which is titrated up to a 2-mg weekly injection. Still others opted for tirzepatide (Mounjaro), a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) agonist. Tirzepatide is approved for type 2 diabetes in the United States but is not yet approved for weight loss.
Wegovy shortages continue to be reported.
; of interest, it was more common after bariatric surgery.
In clinical trials, 3% of patients receiving Wegovy (a 2.4-mg/wk injection) versus 1% of patients receiving placebo reported alopecia. Hair loss was not reported as a side effect in clinical trials of Ozempic (a 2-mg/wk injection) for type 2 diabetes. In a clinical trial of tirzepatide for weight loss in obesity, 5.7% of patients taking the highest dose (a 15-mg once-weekly injection) reported alopecia vs 1% of those who got a placebo.
In contrast, a review of 18 mostly observational studies reported that 57% of patients had hair loss after bariatric surgery.
Is it the drug or the rapid weight loss?
None of the experts consulted for this article had seen patients who came to them about hair loss while taking these drugs for weight loss.
“I have not seen patients complaining of hair loss from these medications, but perhaps it is just a matter of time,” said Lynne J. Goldberg, MD, a professor of dermatology and pathology and laboratory medicine, at Boston University, and director of the hair clinic at Boston Medical Center.
“Some of my patients lose hair when they lose weight, generally as a result of the weight loss itself and not as a side effect of these medications,” said Katharine H. Saunders, MD, an obesity medicine physician, cofounder of Intellihealth, and an assistant professor of medicine at Weill Cornell Medicine, New York.
“Hair loss from rapid weight loss is very common [and] not necessarily a side effect of the medication itself but more as a result of how quickly the weight loss occurs,” echoed Susan Massick, MD, associate professor of dermatology, Ohio State University, and a dermatologist at Ohio State’s Wexner Medical Center, both in Columbus.
“Hair loss is tricky,” observed Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles. “Losing weight and/or changing your diet causes hair loss. Stress can cause hair loss. So, it is hard to separate weight loss from medication effect.”
Telogen effluvium (stress shedding) with rapid weight loss
The hair loss seems to be associated with rapid weight loss, the experts agreed.
“It is rare, but we can see patients who have a period of diffuse hair loss, called telogen effluvium, or ‘stress shedding’ with rapid weight loss,” said Michael A. Weintraub, MD, an endocrinologist at NYU Langone Health, New York.
This hair loss occurs in relation to either physical (surgery, pregnancy, illness) or emotional stress, added Dr. Weintraub, who is an assistant professor at NYU Grossman School of Medicine.
Hair loss caused by rapid weight loss could be caused by an antiobesity medication, but it could also occur with other obesity treatments, such as bariatric surgery or drastic dietary changes, he said. The hair shedding is typically short lived and reversible.
About 80%-85% of hair is in the anagen (growth) phase, about 5% is in a transitional (catagen) phase, and the rest is in telogen (resting, or shedding) phase, Dr. Massick explained. In telogen effluvium, hairs that are normally in the growth phase get suddenly shifted to telogen phase and are shed rapidly.
“Telogen effluvium can be caused by rapid weight loss, major surgery, severe COVID infection, high fever, or death in the family,” she noted. “You will not go bald with telogen effluvium, but you might find that you may lose a good volume of hair,” much more than the normal loss of up to 100 hairs a day.
“I counsel my patients about the possibility of losing hair before they undergo bariatric surgery,” Dr. Saunders said. “Generally, the health benefits of weight loss and weight maintenance outweigh the risk of temporary hair loss.”
Nutritional deficiencies and malnutrition can contribute to hair loss as well, and iron deficiency is sometimes a culprit, she added.
“If someone is worried” about hair loss associated with weight loss, “they should see their doctor,” Dr. Peters said. “If they are on thyroid hormone, in particular, the levels should be retested after weight loss.”
Hair loss appears more common after bariatric surgery than with antiobesity medications,” Dr. Weintraub observed, and it is unclear whether this is because the weight loss is more dramatic after surgery and thus a greater stressor, or whether it is caused by nutrient deficiency or a different mechanism entirely.
“Unlike certain forms of bariatric surgery, which can lead to malabsorption (e.g., Roux-en-Y gastric bypass), medications such as GLP-1 agonists and GLP-1/GIP dual agonists do not cause malabsorption,” Dr. Weintraub noted. “So nutritional deficiencies are less likely to be the cause of new hair loss in those taking antiobesity medications than [in] someone who underwent bariatric surgery.”
Iron and vitamin D deficiencies are the most common nutritional deficiencies that can cause hair loss, he noted.
Slow and steady weight loss rather than rapid
“I would suggest that patients try to keep the weight loss slow and steady, rather than rapid,” Dr. Goldberg said, “and follow any vitamin/mineral supplementation plan that they are given. Patients with bariatric surgery have nutritional guidance and a supplementation plan.”
“Follow a well-balanced dietary strategy with ample protein, vegetables, and some fruit,” Dr. Saunders said. Health care providers should monitor lab tests to check for and treat vitamin deficiencies, and registered dietitians can be crucial to ensure proper nutrition. She advises patients: “Find coping strategies to reduce stress and get enough sleep. If iron levels are low, start an iron supplement under your provider’s supervision.”
“Some of my patients swear by biotin supplements, prenatal vitamins or ‘hair, skin, and nails’ vitamins,” she added. If hair loss doesn’t stop, a dermatologist can look for other contributors and discuss strategies for hair restoration.
Individuals who undergo bariatric surgery require lifelong vitamin supplementation and yearly (or more frequent) lab testing, she noted.
“With, for example, bariatric surgery or any type of diet change you want to make sure you still maintain a balanced diet, whether its calories, protein, iron, zinc, vitamins (vitamin D for example),” Dr. Massick echoed.
Similarly, Dr. Peters advised: “I would say to maintain a normal healthy diet even if eating less. Exercise. Do all those healthy things. Taking a daily multivitamin isn’t a bad idea. Talk with a nutritionist. Use the appetite suppression of the medication to combine with healthy eating.”
“If someone is having new hair loss, they should see their clinician to evaluate for all possible causes,” Dr. Weintraub said. “Their provider can evaluate for underlying causes like thyroid dysfunction, iron deficiency, and vitamin D deficiency.”
However, if a patient’s pattern of hair loss is not diffuse but occurs in patches, this has an entirely different set of etiologies probably unrelated to antiobesity medication and should be evaluated.
Working with a nutritionist to ensure that patients have sufficient protein and micronutrient intake can lower the risk of developing hair loss and other complications, Dr. Weintraub said. “This is particularly important for certain forms of bariatric surgery such as Roux-en-Y gastric bypass, since that can lead to malabsorption of specific vitamins and minerals that need to be periodically measured and supplemented.”
In individuals starting an antiobesity medication, beginning a daily multivitamin has little harm, he added, and can ensure they are getting essential minerals and vitamins. However, no studies have specifically investigated this yet.
“Ultimately, it’s important to weigh the benefits of antiobesity medications against the potential risks, as we do with any medical intervention,” according to Dr. Weintraub.
“The purpose of treating obesity,” he stressed, “is to reduce the risk of heart disease, stroke, and multiple types of cancers. It’s up to the individual to weigh these benefits against the risks of the treatment, including the low risk of developing temporary hair loss.”
Dr. Peters writes a column for Medscape and disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care; received a research grant from Abbott Diabetes Care; and received stock options from Teladoc and Omada Health. Dr. Goldberg, Dr. Saunders, Dr. Massick, and Dr. Weintraub declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Could vitamin D supplementation help in long COVID?
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, in a retrospective, case-matched study.
The lower levels of vitamin D in patients with long COVID were most notable in those with brain fog.
These findings, by Luigi di Filippo, MD, and colleagues, were recently presented at the European Congress of Endocrinology and published in the Journal of Clinical Endocrinology & Metabolism.
“Our data suggest that vitamin D levels should be evaluated in COVID-19 patients after hospital discharge,” wrote the researchers, from San Raffaele Hospital, Milan.
“The role of vitamin D supplementation as a preventive strategy of COVID-19 sequelae should be tested in randomized controlled trials,” they urged.
The researchers also stressed that this was a controlled study in a homogeneous population, it included multiple signs and symptoms of long COVID, and it had a longer follow-up than most previous studies (6 vs. 3 months).
“The highly controlled nature of our study helps us better understand the role of vitamin D deficiency in long COVID and establish that there is likely a link between vitamin D deficiency and long COVID,” senior author Andrea Giustina, MD, said in a press release from the ECE.
“Our study shows that COVID-19 patients with low vitamin D levels are more likely to develop long COVID, but it is not yet known whether vitamin D supplements could improve the symptoms or reduce this risk altogether,” he cautioned.
“If confirmed in large, interventional, randomized controlled trials, [our data suggest] that vitamin D supplementation could represent a possible preventive strategy in reducing the burden of COVID-19 sequelae,” Dr. Giustina and colleagues wrote.
Reasonable to test vitamin D levels, consider supplementation
Invited to comment, Amiel Dror, MD, PhD, who led a related study that showed that people with a vitamin D deficiency were more likely to have severe COVID-19, agreed.
“The novelty and significance of this [new] study lie in the fact that it expands on our current understanding of the interplay between vitamin D and COVID-19, taking it beyond the acute phase of the disease,” said Dr. Dror, from Bar-Ilan University, Safed, Israel.
“It’s striking to see how vitamin D levels continue to influence patients’ health even after recovery from the initial infection,” he noted.
“The findings certainly add weight to the argument for conducting a randomized control trial [RCT],” he continued, which “would enable us to conclusively determine whether vitamin D supplementation can effectively reduce the risk or severity of long COVID.”
“In the interim,” Dr. Dror said, “given the safety profile of vitamin D and its broad health benefits, it could be reasonable to test for vitamin D levels in patients admitted with COVID-19. If levels are found to be low, supplementation could be considered.”
“However, it’s important to note that this should be done under medical supervision,” he cautioned, “and further studies are needed to establish the optimal timing and dosage of supplementation.”
“I anticipate that we’ll see more RCTs [of this] in the future,” he speculated.
Low vitamin D and risk of long COVID
Long COVID is an emerging syndrome that affects 50%-70% of COVID-19 survivors.
Low levels of vitamin D have been associated with increased likelihood of needing mechanical ventilation and worse survival in patients hospitalized with COVID-19, but the risk of long COVID associated with vitamin D has not been known.
Researchers analyzed data from adults aged 18 and older hospitalized at San Raffaele Hospital with a confirmed diagnosis of COVID-19 and discharged during the first pandemic wave, from March to May 2020, and then seen 6-months later for follow-up.
Patients were excluded if they had been admitted to the intensive care unit during hospitalization or had missing medical data or blood samples available to determine (OH) vitamin D levels, at admission and the 6-month follow-up.
Long COVID-19 was defined based on the U.K. National Institute for Health and Care Excellence guidelines as the concomitant presence of at least two or more of 17 signs and symptoms that were absent prior to the COVID-19 infection and could only be attributed to that acute disease.
Researchers identified 50 patients with long COVID at the 6-month follow-up and matched them with 50 patients without long COVID at that time point, based on age, sex, concomitant comorbidities, need for noninvasive mechanical ventilation, and week of evaluation.
Patients were a mean age of 61 years (range, 51-73) and 56% were men; 28% had been on a ventilator during hospitalization for COVID-19.
The most frequent signs and symptoms at 6 months in the patients with long COVID were asthenia (weakness, 38% of patients), dysgeusia (bad taste in the mouth, 34%), dyspnea (shortness of breath, 34%), and anosmia (loss of sense of smell, 24%).
Most symptoms were related to the cardiorespiratory system (42%), the feeling of well-being (42%), or the senses (36%), and fewer patients had symptoms related to neurocognitive impairment (headache or brain fog, 14%), or ear, nose, and throat (12%), or gastrointestinal system (4%).
Patients with long COVID had lower mean 25(OH) vitamin D levels than patients without long COVID (20.1 vs 23.2 ng/mL; P = .03). However, actual vitamin D deficiency levels were similar in both groups.
Two-thirds of patients with low vitamin D levels at hospital admission still presented with low levels at the 6-month follow-up.
Vitamin D levels were significantly lower in patients with neurocognitive symptoms at follow-up (n = 7) than in those without such symptoms (n = 93) (14.6 vs. 20.6 ng/mL; P = .042).
In patients with vitamin D deficiency (< 20 ng/mL) at admission and at follow-up (n = 42), those with long COVID (n = 22) had lower vitamin D levels at follow-up than those without long COVID (n = 20) (12.7 vs. 15.2 ng/mL; P = .041).
And in multiple regression analyses, a lower 25(OH) vitamin D level at follow-up was the only variable that was significantly associated with long COVID (odds ratio, 1.09; 95% confidence interval, 1.01-1.16; P = .008).
The findings “strongly reinforce the clinical usefulness of 25(OH) vitamin D evaluation as a possible modifiable pathophysiological factor underlying this emerging worldwide critical health issue,” the researchers concluded.
The study was supported by Abiogen Pharma. One study author is an employee at Abiogen. Dr. Giustina has reported being a consultant for Abiogen and Takeda and receiving a research grant to his institution from Takeda. Dr. Di Filippo and the other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECE 2023
CGM completes picture of A1c in type 2 diabetes
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETES THERAPY
Lifestyle med experts tell how to deprescribe diabetes meds
Nine lifestyle medicine practitioners describe how they safely and effectively deprescribe glucose-lowering medications after patients demonstrate a reduced need for such medications following lifestyle changes.
The report by Michael D. Bradley, PharmD, and colleagues was recently published as a feature article in Clinical Diabetes.
“Lifestyle medicine uses an evidence-based lifestyle therapeutic approach to treat lifestyle-related chronic disease,” they wrote, and it includes “a whole-food, predominantly plant-based eating plan, regular physical activity, restorative sleep, stress management, avoidance of risky substances, and positive social connection.”
“Medication deprescribing,” senior author Micaela C. Karlsen, PhD, said in an email, “is a planned and supervised process of dose reduction or discontinuation of a medication that may be causing harm, or no longer providing benefit to a patient.”
According to the authors, the article “is the first account published of the medication de-escalation methods used by lifestyle medicine providers when patients demonstrate a decreased need for pharmacotherapy.” It “supports the feasibility of de-escalating glucose-lowering medications in this context and provides pilot data on protocols from individual practitioners experienced in deprescribing glucose-lowering medications.”
The study was not designed to cover deprescribing glucose-lowering medications following weight loss and diabetes remission after bariatric surgery.
“A key takeaway [from the current study] for general practitioners and endocrinologists is that, while deprescribing is already known to be beneficial to reduce polypharmacy, it may be appropriate following lifestyle interventions,” said Dr. Karlsen, who is senior director of the American College of Lifestyle Medicine in Chesterfield, Md.
“The protocols presented can serve as a model for how to do so,” she continued.
The American Diabetes Association and the American Association of Clinical Endocrinology recommend lifestyle optimization as part of medical care for type 2 diabetes.
According to the ACLM, “remission of type 2 diabetes should be a clinical goal and may be achieved with a whole-food, plant-based dietary pattern coupled with moderate exercise,” the researchers noted.
“Remission,” they wrote, “can be defined as attainment of a [hemoglobin] A1c less than 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for the specific purpose of lowering blood glucose.”
In ACLM’s recent expert consensus statement on dietary interventions for type 2 diabetes remission, which was also endorsed by AACE, supported by the Academy of Nutrition and Dietetics, and cosponsored by the Endocrine Society, panel members agreed that remission is a realistic and achievable goal for some adults with type 2 diabetes, and a high-intensity dietary intervention can result in remission, Dr. Karlsen said.
To avoid hypoglycemia when deprescribing antiglycemic drugs, medications known to cause hypoglycemia – notably sulfonylurea and insulin – are often deprescribed first, she noted.
“Our biggest hope,” she said, “is that [type 2 diabetes] remission may come to the forefront as a clinical goal in treatment and that other organizations will more strongly emphasize lifestyle in standards of care.”
“We hope that clinicians reading this paper will be made aware that de-escalation of glucose-lowering medications is feasible, is a desirable outcome, and can be necessary in a lifestyle medicine context,” she added.
Further research is needed to prospectively track the likelihood of type 2 diabetes remission, factors that predict successful remission, and decision-making protocols followed by practitioners, Dr. Karlsen said.
Deprescribing antiglycemic meds in lifestyle medicine
Researchers at the Bruyère Research Institute, Ottawa, and Université de Montréal provide algorithms for deprescribing antihyperglycemic medications specifically for older individuals.
In the current study, the authors conducted individual, 30-minute to 1-hour interviews with nine lifestyle medicine practitioners to document their protocols for deprescribing glucose-lowering medications after lifestyle interventions with a goal of potential type 2 diabetes remission.
Three practitioners reported medication deprescribing in an intensive therapeutic lifestyle program (longer, more frequent treatment with greater monitoring). The others provide deprescribing in a nonintensive program (similar to primary care practice) or both.
Deprescribing is necessary when using intensive therapeutic lifestyle change, as substantial and rapid drops in glucose levels aren’t adjusted for, the authors noted.
Most practitioners work with a team of allied health care providers.
During the deprescribing process, most protocols require that patients get a basic or comprehensive metabolic panel of blood tests, with variations in laboratory tests for A1c, C-peptide, and renal function.
Most practitioners recommend a target blood glucose less than 120 mg/dL for further deprescribing.
Currently, there is no clinical guidance for use of continuous glucose monitoring (CGM) during medication de-escalation, the authors note.
Most practitioners reported they consider patient expenses associated with CGM and third-party payor coverage in their decision-making.
Most practitioners prefer to deprescribe sulfonylureas, insulin, and other medications known to cause hypoglycemia first.
Conversely, most prefer to defer deprescribing medications that have demonstrated cardiovascular and/or renal benefits (that is, glucagonlike peptide–1 receptor agonists and sodium-glucose cotransporter 2 inhibitors), as well as those with a less severe adverse effect profile (that is, metformin and GLP-1 receptor agonists) until after other medications are deprescribed.
The study was funded by the Ardmore Institute of Health. The authors reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Nine lifestyle medicine practitioners describe how they safely and effectively deprescribe glucose-lowering medications after patients demonstrate a reduced need for such medications following lifestyle changes.
The report by Michael D. Bradley, PharmD, and colleagues was recently published as a feature article in Clinical Diabetes.
“Lifestyle medicine uses an evidence-based lifestyle therapeutic approach to treat lifestyle-related chronic disease,” they wrote, and it includes “a whole-food, predominantly plant-based eating plan, regular physical activity, restorative sleep, stress management, avoidance of risky substances, and positive social connection.”
“Medication deprescribing,” senior author Micaela C. Karlsen, PhD, said in an email, “is a planned and supervised process of dose reduction or discontinuation of a medication that may be causing harm, or no longer providing benefit to a patient.”
According to the authors, the article “is the first account published of the medication de-escalation methods used by lifestyle medicine providers when patients demonstrate a decreased need for pharmacotherapy.” It “supports the feasibility of de-escalating glucose-lowering medications in this context and provides pilot data on protocols from individual practitioners experienced in deprescribing glucose-lowering medications.”
The study was not designed to cover deprescribing glucose-lowering medications following weight loss and diabetes remission after bariatric surgery.
“A key takeaway [from the current study] for general practitioners and endocrinologists is that, while deprescribing is already known to be beneficial to reduce polypharmacy, it may be appropriate following lifestyle interventions,” said Dr. Karlsen, who is senior director of the American College of Lifestyle Medicine in Chesterfield, Md.
“The protocols presented can serve as a model for how to do so,” she continued.
The American Diabetes Association and the American Association of Clinical Endocrinology recommend lifestyle optimization as part of medical care for type 2 diabetes.
According to the ACLM, “remission of type 2 diabetes should be a clinical goal and may be achieved with a whole-food, plant-based dietary pattern coupled with moderate exercise,” the researchers noted.
“Remission,” they wrote, “can be defined as attainment of a [hemoglobin] A1c less than 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for the specific purpose of lowering blood glucose.”
In ACLM’s recent expert consensus statement on dietary interventions for type 2 diabetes remission, which was also endorsed by AACE, supported by the Academy of Nutrition and Dietetics, and cosponsored by the Endocrine Society, panel members agreed that remission is a realistic and achievable goal for some adults with type 2 diabetes, and a high-intensity dietary intervention can result in remission, Dr. Karlsen said.
To avoid hypoglycemia when deprescribing antiglycemic drugs, medications known to cause hypoglycemia – notably sulfonylurea and insulin – are often deprescribed first, she noted.
“Our biggest hope,” she said, “is that [type 2 diabetes] remission may come to the forefront as a clinical goal in treatment and that other organizations will more strongly emphasize lifestyle in standards of care.”
“We hope that clinicians reading this paper will be made aware that de-escalation of glucose-lowering medications is feasible, is a desirable outcome, and can be necessary in a lifestyle medicine context,” she added.
Further research is needed to prospectively track the likelihood of type 2 diabetes remission, factors that predict successful remission, and decision-making protocols followed by practitioners, Dr. Karlsen said.
Deprescribing antiglycemic meds in lifestyle medicine
Researchers at the Bruyère Research Institute, Ottawa, and Université de Montréal provide algorithms for deprescribing antihyperglycemic medications specifically for older individuals.
In the current study, the authors conducted individual, 30-minute to 1-hour interviews with nine lifestyle medicine practitioners to document their protocols for deprescribing glucose-lowering medications after lifestyle interventions with a goal of potential type 2 diabetes remission.
Three practitioners reported medication deprescribing in an intensive therapeutic lifestyle program (longer, more frequent treatment with greater monitoring). The others provide deprescribing in a nonintensive program (similar to primary care practice) or both.
Deprescribing is necessary when using intensive therapeutic lifestyle change, as substantial and rapid drops in glucose levels aren’t adjusted for, the authors noted.
Most practitioners work with a team of allied health care providers.
During the deprescribing process, most protocols require that patients get a basic or comprehensive metabolic panel of blood tests, with variations in laboratory tests for A1c, C-peptide, and renal function.
Most practitioners recommend a target blood glucose less than 120 mg/dL for further deprescribing.
Currently, there is no clinical guidance for use of continuous glucose monitoring (CGM) during medication de-escalation, the authors note.
Most practitioners reported they consider patient expenses associated with CGM and third-party payor coverage in their decision-making.
Most practitioners prefer to deprescribe sulfonylureas, insulin, and other medications known to cause hypoglycemia first.
Conversely, most prefer to defer deprescribing medications that have demonstrated cardiovascular and/or renal benefits (that is, glucagonlike peptide–1 receptor agonists and sodium-glucose cotransporter 2 inhibitors), as well as those with a less severe adverse effect profile (that is, metformin and GLP-1 receptor agonists) until after other medications are deprescribed.
The study was funded by the Ardmore Institute of Health. The authors reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Nine lifestyle medicine practitioners describe how they safely and effectively deprescribe glucose-lowering medications after patients demonstrate a reduced need for such medications following lifestyle changes.
The report by Michael D. Bradley, PharmD, and colleagues was recently published as a feature article in Clinical Diabetes.
“Lifestyle medicine uses an evidence-based lifestyle therapeutic approach to treat lifestyle-related chronic disease,” they wrote, and it includes “a whole-food, predominantly plant-based eating plan, regular physical activity, restorative sleep, stress management, avoidance of risky substances, and positive social connection.”
“Medication deprescribing,” senior author Micaela C. Karlsen, PhD, said in an email, “is a planned and supervised process of dose reduction or discontinuation of a medication that may be causing harm, or no longer providing benefit to a patient.”
According to the authors, the article “is the first account published of the medication de-escalation methods used by lifestyle medicine providers when patients demonstrate a decreased need for pharmacotherapy.” It “supports the feasibility of de-escalating glucose-lowering medications in this context and provides pilot data on protocols from individual practitioners experienced in deprescribing glucose-lowering medications.”
The study was not designed to cover deprescribing glucose-lowering medications following weight loss and diabetes remission after bariatric surgery.
“A key takeaway [from the current study] for general practitioners and endocrinologists is that, while deprescribing is already known to be beneficial to reduce polypharmacy, it may be appropriate following lifestyle interventions,” said Dr. Karlsen, who is senior director of the American College of Lifestyle Medicine in Chesterfield, Md.
“The protocols presented can serve as a model for how to do so,” she continued.
The American Diabetes Association and the American Association of Clinical Endocrinology recommend lifestyle optimization as part of medical care for type 2 diabetes.
According to the ACLM, “remission of type 2 diabetes should be a clinical goal and may be achieved with a whole-food, plant-based dietary pattern coupled with moderate exercise,” the researchers noted.
“Remission,” they wrote, “can be defined as attainment of a [hemoglobin] A1c less than 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for the specific purpose of lowering blood glucose.”
In ACLM’s recent expert consensus statement on dietary interventions for type 2 diabetes remission, which was also endorsed by AACE, supported by the Academy of Nutrition and Dietetics, and cosponsored by the Endocrine Society, panel members agreed that remission is a realistic and achievable goal for some adults with type 2 diabetes, and a high-intensity dietary intervention can result in remission, Dr. Karlsen said.
To avoid hypoglycemia when deprescribing antiglycemic drugs, medications known to cause hypoglycemia – notably sulfonylurea and insulin – are often deprescribed first, she noted.
“Our biggest hope,” she said, “is that [type 2 diabetes] remission may come to the forefront as a clinical goal in treatment and that other organizations will more strongly emphasize lifestyle in standards of care.”
“We hope that clinicians reading this paper will be made aware that de-escalation of glucose-lowering medications is feasible, is a desirable outcome, and can be necessary in a lifestyle medicine context,” she added.
Further research is needed to prospectively track the likelihood of type 2 diabetes remission, factors that predict successful remission, and decision-making protocols followed by practitioners, Dr. Karlsen said.
Deprescribing antiglycemic meds in lifestyle medicine
Researchers at the Bruyère Research Institute, Ottawa, and Université de Montréal provide algorithms for deprescribing antihyperglycemic medications specifically for older individuals.
In the current study, the authors conducted individual, 30-minute to 1-hour interviews with nine lifestyle medicine practitioners to document their protocols for deprescribing glucose-lowering medications after lifestyle interventions with a goal of potential type 2 diabetes remission.
Three practitioners reported medication deprescribing in an intensive therapeutic lifestyle program (longer, more frequent treatment with greater monitoring). The others provide deprescribing in a nonintensive program (similar to primary care practice) or both.
Deprescribing is necessary when using intensive therapeutic lifestyle change, as substantial and rapid drops in glucose levels aren’t adjusted for, the authors noted.
Most practitioners work with a team of allied health care providers.
During the deprescribing process, most protocols require that patients get a basic or comprehensive metabolic panel of blood tests, with variations in laboratory tests for A1c, C-peptide, and renal function.
Most practitioners recommend a target blood glucose less than 120 mg/dL for further deprescribing.
Currently, there is no clinical guidance for use of continuous glucose monitoring (CGM) during medication de-escalation, the authors note.
Most practitioners reported they consider patient expenses associated with CGM and third-party payor coverage in their decision-making.
Most practitioners prefer to deprescribe sulfonylureas, insulin, and other medications known to cause hypoglycemia first.
Conversely, most prefer to defer deprescribing medications that have demonstrated cardiovascular and/or renal benefits (that is, glucagonlike peptide–1 receptor agonists and sodium-glucose cotransporter 2 inhibitors), as well as those with a less severe adverse effect profile (that is, metformin and GLP-1 receptor agonists) until after other medications are deprescribed.
The study was funded by the Ardmore Institute of Health. The authors reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CLINICAL DIABETES
Obesity drugs overpriced, change needed to tackle issue
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).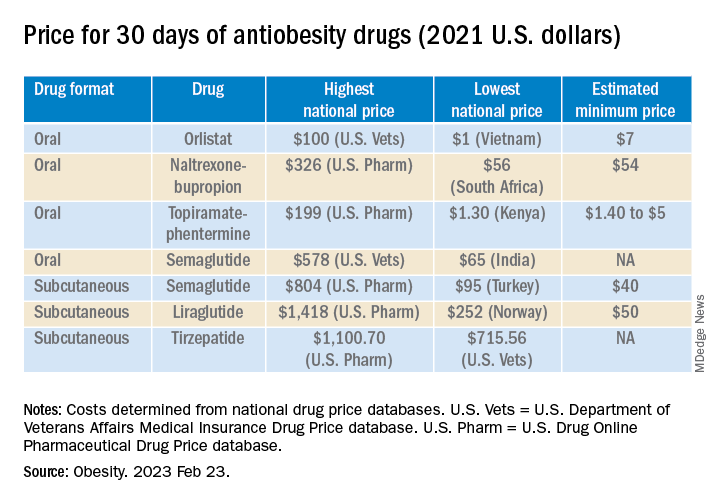
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Use age, not weight, to screen for diabetes; assess over 35s
Universal screening of all U.S. adults aged 35-70 years for prediabetes and type 2 diabetes, regardless of body mass index, would provide the fairest means of detection, according to a new analysis.
This would better detect prediabetes and diabetes in ethnic groups that have a higher risk of diabetes at lower cutoffs. Compared with White individuals, Black or Hispanic adults have a higher risk of developing type 2 diabetes at a younger age, and Asian, Hispanic, and Black Americans all have a higher risk of developing it at a lower BMI.
In the new study, researchers examined six different screening scenarios in a nationally representative sample without diabetes.
They compared screening for prediabetes and type 2 diabetes using criteria from the 2021 U.S. Preventive Services Task Force (USPSTF) recommendations with the 2015 USPSTF recommendations, as well as four other screening thresholds with lower age or weight.
Universal screening for prediabetes and diabetes at age 35-70, regardless of BMI – which appears to be the sweet spot for most equitable detection in different races – may be easier to put into practice because it will mean clinicians don’t have to remember alternate cutoffs for different patient groups, the researchers suggested.
“All major racial and ethnic minority groups develop diabetes at lower weights than White adults, and it’s most pronounced for Asian Americans,” lead author Matthew J. O’Brien, MD, explained in a press release.
“If we make decisions about diabetes testing based on weight we will miss some people from racial and ethnic minority groups who are developing prediabetes and diabetes at lower weights,” said Dr. O’Brien, of Northwestern University, Chicago.
Going forward, to achieve equity in diagnosing prediabetes and diabetes “also requires addressing structural barriers [facing racial and ethnic minorities], which include not having a usual source of primary care, lacking health insurance, or having copays for screening tests based on insurance coverage,” the authors noted in their paper, published online in the American Journal of Preventive Medicine.
There is also a need for further study to examine the cost-effectiveness of any approach, and to study the impact of screening criteria on diagnosis, treatment, and outcomes in diverse populations.
Nationally representative sample, six screening scenarios
In the overall U.S. population, 81% of adults with prediabetes are unaware they have it, said Dr. O’Brien and colleagues, and 23% of diabetes cases are undiagnosed.
And Black, Hispanic, or Asian individuals have a nearly twofold higher prevalence of diabetes compared with White individuals.
The 2021 USPSTF recommendations state that clinicians should screen asymptomatic adults aged 35-70 years with overweight/obesity (BMI ≥ 25 kg/m2) and “should consider screening at an earlier age in persons from groups with disproportionately high incidence and prevalence (American Indian/Alaska Native, Asian American, Black, Hispanic/Latino, or Native Hawaiian/Pacific Islander persons) or in persons who have a family history of diabetes, a history of gestational diabetes, or a history of polycystic ovarian syndrome, and at a lower BMI in Asian American persons. Data suggest that a BMI of 23 or greater may be an appropriate cut point in Asian American persons.”
Dr. O’Brien and colleagues identified 3,243 nonpregnant adults without diagnosed diabetes who participated in the National Health and Nutrition Examination Survey (NHANES) in 2017-2020 and had an A1c blood test. (Half also had a fasting plasma glucose test.)
First, they compared screening using the more recent and earlier USPSTF criteria: BMI of at least 25 kg/m2 and age 35-70 (2021 criteria) and BMI of at least 25 kg/m2 and age 40-70 (2015 criteria).
They estimated that 13.9 million more adults would be eligible for screening using the 2021 versus the 2015 screening criteria.
The increases in screening eligibility were highest in Hispanic individuals (30.6%), followed by Asian individuals (17.9%), White individuals (14.0%), and Black individuals (13.9%).
Using the USPSTF 2021 versus 2015 screening criteria resulted in marginally higher sensitivity (58.6% vs. 52.9%) but lower specificity (69.3% vs. 76.4%) overall, as well as within each racial group.
Next, the researchers examined screening at two lower age cutoffs and two lower BMI cutoffs: BMI of at least 25 kg/m2 and age 30-70, BMI of at least 25 kg/m2 and age 18-70, age 35-70 and BMI of at least 23 kg/m2, and age 35-70 and any BMI.
Screening at these lower age and weight thresholds resulted in even greater sensitivity and lower specificity than using the 2021 USPSTF criteria, especially among Hispanic, non-Hispanic Black, and Asian adults.
However, screening all adults aged 35-70 years regardless of BMI yielded the most equitable detection of prediabetes and diabetes – with a sensitivity of 67.8% and a specificity of 52.1% in the overall population, and a sensitivity of 70.1%, 70.4%, 68.4%, and 67.6%, and a specificity of 53.8%, 59.9%, 56.2%, and 48.9%, in the Asian, Black, Hispanic, and White subgroups, respectively.
The American Diabetes Association currently recommends screening all adults aged ≥ 35 years, or at any age if they have overweight/obesity and an additional diabetes risk factor, the researchers noted.
The study was partly funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Universal screening of all U.S. adults aged 35-70 years for prediabetes and type 2 diabetes, regardless of body mass index, would provide the fairest means of detection, according to a new analysis.
This would better detect prediabetes and diabetes in ethnic groups that have a higher risk of diabetes at lower cutoffs. Compared with White individuals, Black or Hispanic adults have a higher risk of developing type 2 diabetes at a younger age, and Asian, Hispanic, and Black Americans all have a higher risk of developing it at a lower BMI.
In the new study, researchers examined six different screening scenarios in a nationally representative sample without diabetes.
They compared screening for prediabetes and type 2 diabetes using criteria from the 2021 U.S. Preventive Services Task Force (USPSTF) recommendations with the 2015 USPSTF recommendations, as well as four other screening thresholds with lower age or weight.
Universal screening for prediabetes and diabetes at age 35-70, regardless of BMI – which appears to be the sweet spot for most equitable detection in different races – may be easier to put into practice because it will mean clinicians don’t have to remember alternate cutoffs for different patient groups, the researchers suggested.
“All major racial and ethnic minority groups develop diabetes at lower weights than White adults, and it’s most pronounced for Asian Americans,” lead author Matthew J. O’Brien, MD, explained in a press release.
“If we make decisions about diabetes testing based on weight we will miss some people from racial and ethnic minority groups who are developing prediabetes and diabetes at lower weights,” said Dr. O’Brien, of Northwestern University, Chicago.
Going forward, to achieve equity in diagnosing prediabetes and diabetes “also requires addressing structural barriers [facing racial and ethnic minorities], which include not having a usual source of primary care, lacking health insurance, or having copays for screening tests based on insurance coverage,” the authors noted in their paper, published online in the American Journal of Preventive Medicine.
There is also a need for further study to examine the cost-effectiveness of any approach, and to study the impact of screening criteria on diagnosis, treatment, and outcomes in diverse populations.
Nationally representative sample, six screening scenarios
In the overall U.S. population, 81% of adults with prediabetes are unaware they have it, said Dr. O’Brien and colleagues, and 23% of diabetes cases are undiagnosed.
And Black, Hispanic, or Asian individuals have a nearly twofold higher prevalence of diabetes compared with White individuals.
The 2021 USPSTF recommendations state that clinicians should screen asymptomatic adults aged 35-70 years with overweight/obesity (BMI ≥ 25 kg/m2) and “should consider screening at an earlier age in persons from groups with disproportionately high incidence and prevalence (American Indian/Alaska Native, Asian American, Black, Hispanic/Latino, or Native Hawaiian/Pacific Islander persons) or in persons who have a family history of diabetes, a history of gestational diabetes, or a history of polycystic ovarian syndrome, and at a lower BMI in Asian American persons. Data suggest that a BMI of 23 or greater may be an appropriate cut point in Asian American persons.”
Dr. O’Brien and colleagues identified 3,243 nonpregnant adults without diagnosed diabetes who participated in the National Health and Nutrition Examination Survey (NHANES) in 2017-2020 and had an A1c blood test. (Half also had a fasting plasma glucose test.)
First, they compared screening using the more recent and earlier USPSTF criteria: BMI of at least 25 kg/m2 and age 35-70 (2021 criteria) and BMI of at least 25 kg/m2 and age 40-70 (2015 criteria).
They estimated that 13.9 million more adults would be eligible for screening using the 2021 versus the 2015 screening criteria.
The increases in screening eligibility were highest in Hispanic individuals (30.6%), followed by Asian individuals (17.9%), White individuals (14.0%), and Black individuals (13.9%).
Using the USPSTF 2021 versus 2015 screening criteria resulted in marginally higher sensitivity (58.6% vs. 52.9%) but lower specificity (69.3% vs. 76.4%) overall, as well as within each racial group.
Next, the researchers examined screening at two lower age cutoffs and two lower BMI cutoffs: BMI of at least 25 kg/m2 and age 30-70, BMI of at least 25 kg/m2 and age 18-70, age 35-70 and BMI of at least 23 kg/m2, and age 35-70 and any BMI.
Screening at these lower age and weight thresholds resulted in even greater sensitivity and lower specificity than using the 2021 USPSTF criteria, especially among Hispanic, non-Hispanic Black, and Asian adults.
However, screening all adults aged 35-70 years regardless of BMI yielded the most equitable detection of prediabetes and diabetes – with a sensitivity of 67.8% and a specificity of 52.1% in the overall population, and a sensitivity of 70.1%, 70.4%, 68.4%, and 67.6%, and a specificity of 53.8%, 59.9%, 56.2%, and 48.9%, in the Asian, Black, Hispanic, and White subgroups, respectively.
The American Diabetes Association currently recommends screening all adults aged ≥ 35 years, or at any age if they have overweight/obesity and an additional diabetes risk factor, the researchers noted.
The study was partly funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Universal screening of all U.S. adults aged 35-70 years for prediabetes and type 2 diabetes, regardless of body mass index, would provide the fairest means of detection, according to a new analysis.
This would better detect prediabetes and diabetes in ethnic groups that have a higher risk of diabetes at lower cutoffs. Compared with White individuals, Black or Hispanic adults have a higher risk of developing type 2 diabetes at a younger age, and Asian, Hispanic, and Black Americans all have a higher risk of developing it at a lower BMI.
In the new study, researchers examined six different screening scenarios in a nationally representative sample without diabetes.
They compared screening for prediabetes and type 2 diabetes using criteria from the 2021 U.S. Preventive Services Task Force (USPSTF) recommendations with the 2015 USPSTF recommendations, as well as four other screening thresholds with lower age or weight.
Universal screening for prediabetes and diabetes at age 35-70, regardless of BMI – which appears to be the sweet spot for most equitable detection in different races – may be easier to put into practice because it will mean clinicians don’t have to remember alternate cutoffs for different patient groups, the researchers suggested.
“All major racial and ethnic minority groups develop diabetes at lower weights than White adults, and it’s most pronounced for Asian Americans,” lead author Matthew J. O’Brien, MD, explained in a press release.
“If we make decisions about diabetes testing based on weight we will miss some people from racial and ethnic minority groups who are developing prediabetes and diabetes at lower weights,” said Dr. O’Brien, of Northwestern University, Chicago.
Going forward, to achieve equity in diagnosing prediabetes and diabetes “also requires addressing structural barriers [facing racial and ethnic minorities], which include not having a usual source of primary care, lacking health insurance, or having copays for screening tests based on insurance coverage,” the authors noted in their paper, published online in the American Journal of Preventive Medicine.
There is also a need for further study to examine the cost-effectiveness of any approach, and to study the impact of screening criteria on diagnosis, treatment, and outcomes in diverse populations.
Nationally representative sample, six screening scenarios
In the overall U.S. population, 81% of adults with prediabetes are unaware they have it, said Dr. O’Brien and colleagues, and 23% of diabetes cases are undiagnosed.
And Black, Hispanic, or Asian individuals have a nearly twofold higher prevalence of diabetes compared with White individuals.
The 2021 USPSTF recommendations state that clinicians should screen asymptomatic adults aged 35-70 years with overweight/obesity (BMI ≥ 25 kg/m2) and “should consider screening at an earlier age in persons from groups with disproportionately high incidence and prevalence (American Indian/Alaska Native, Asian American, Black, Hispanic/Latino, or Native Hawaiian/Pacific Islander persons) or in persons who have a family history of diabetes, a history of gestational diabetes, or a history of polycystic ovarian syndrome, and at a lower BMI in Asian American persons. Data suggest that a BMI of 23 or greater may be an appropriate cut point in Asian American persons.”
Dr. O’Brien and colleagues identified 3,243 nonpregnant adults without diagnosed diabetes who participated in the National Health and Nutrition Examination Survey (NHANES) in 2017-2020 and had an A1c blood test. (Half also had a fasting plasma glucose test.)
First, they compared screening using the more recent and earlier USPSTF criteria: BMI of at least 25 kg/m2 and age 35-70 (2021 criteria) and BMI of at least 25 kg/m2 and age 40-70 (2015 criteria).
They estimated that 13.9 million more adults would be eligible for screening using the 2021 versus the 2015 screening criteria.
The increases in screening eligibility were highest in Hispanic individuals (30.6%), followed by Asian individuals (17.9%), White individuals (14.0%), and Black individuals (13.9%).
Using the USPSTF 2021 versus 2015 screening criteria resulted in marginally higher sensitivity (58.6% vs. 52.9%) but lower specificity (69.3% vs. 76.4%) overall, as well as within each racial group.
Next, the researchers examined screening at two lower age cutoffs and two lower BMI cutoffs: BMI of at least 25 kg/m2 and age 30-70, BMI of at least 25 kg/m2 and age 18-70, age 35-70 and BMI of at least 23 kg/m2, and age 35-70 and any BMI.
Screening at these lower age and weight thresholds resulted in even greater sensitivity and lower specificity than using the 2021 USPSTF criteria, especially among Hispanic, non-Hispanic Black, and Asian adults.
However, screening all adults aged 35-70 years regardless of BMI yielded the most equitable detection of prediabetes and diabetes – with a sensitivity of 67.8% and a specificity of 52.1% in the overall population, and a sensitivity of 70.1%, 70.4%, 68.4%, and 67.6%, and a specificity of 53.8%, 59.9%, 56.2%, and 48.9%, in the Asian, Black, Hispanic, and White subgroups, respectively.
The American Diabetes Association currently recommends screening all adults aged ≥ 35 years, or at any age if they have overweight/obesity and an additional diabetes risk factor, the researchers noted.
The study was partly funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Suicidal thoughts decline in endocrinologists: Survey
Rates of suicidal thoughts and attempted suicide among endocrinologists declined from 2022 and now rank similar to the average rate among physicians overall, but these rates are still higher than the general public, according to survey findings.
The current report about suicide among endocrinologists, titled, “Doctors’ Burden: Endocrinologist Suicide Report 2023,” was recently published.
A report about suicide among physicians overall, based on the same survey, titled, “Doctors’ Burden: Medscape Physician Suicide Report 2023,” was published previously.
Improved rates among 28 medical specialties
In the 2022 survey of a representative national sample of 13,069 U.S. physicians, 10% of endocrinologists reported having suicidal thoughts, ranking the specialty sixth among 29 medical specialties that year.
The 2023 survey found that in a representative national sample of 9,175 U.S. physicians, 8% of endocrinologists reported having suicidal thoughts, roughly the average rate among clinicians overall, ranking it 20th among 29 medical specialties.
The highest rates of thoughts of suicide in the latest survey were reported by physicians in otolaryngology (13%), followed by physicians in psychiatry, family medicine, anesthesiology, obstetrics/gynecology, and emergency medicine (roughly 12% in each specialty).
The rate of attempted suicide was 1% among endocrinologists, which was also the rate among physicians overall.
More female than male endocrinologists reported contemplating suicide (8% versus 5%). In addition, 1% of male endocrinologists reported that they had attempted suicide and 2% of female endocrinologists replied they preferred not to answer the question about attempted suicide.
In contrast, in 2020, an estimated 4.9% of U.S. adults aged 18 and older had serious thoughts about suicide and 0.5% attempted suicide, according to the National Institutes of Health website, the latest report states.
Rates of suicidal thoughts and suicide attempts among physicians overall “are worryingly high numbers,” Peter Yellowlees, MBBS, MD, emeritus professor of psychiatry at University of California, Davis, Health, and chief executive officer, Asynchealth, said in the report.
Confiding in others, good mental health habits, resources
In the 2023 survey, half of the endocrinologists who had thought about suicide had confided in a therapist and 41% had spoken to a family member, but none had told a colleague or a friend, or phoned a suicide hotline.
On the other hand, 7% of male and 10% of female endocrinologists, and 9% of male and 11% of female physicians overall, reported that a colleague had shared suicidal thoughts with them.
“It’s pleasing that physicians overall have shown themselves slightly more likely to bring ideas about suicide to a therapist and less likely to keep their distress entirely to themselves,” Dr. Yellowlees said.
“It’s possible that the need for health care is becoming less stigmatized nationally, with large and increasing emphasis on physician well-being during and after the COVID-19 pandemic,” he suggested.
Endocrinologists reported that to keep happy and have good mental health, they engaged in activities and hobbies (70%), exercised (66%), spent time with family and friends (63%), got enough sleep (56%), ate healthy (48%), went to therapy (11%), or did other things (8%), which was similar to that reported by physicians overall.
The report lists several resources that are specific for physicians having suicidal thoughts (Physician Support Line, 988 Suicide and Crisis Lifeline, Peer RxMed, International Association for Suicide Prevention, and the American Foundation for Suicide Prevention) along with contact information.
The 2023 survey was conducted from June 28, 2022, to Oct. 3, 2022, and the 2022 survey was conducted from June 29, 2021, to Sept. 26, 2021.
A version of this article first appeared on Medscape.com.
Rates of suicidal thoughts and attempted suicide among endocrinologists declined from 2022 and now rank similar to the average rate among physicians overall, but these rates are still higher than the general public, according to survey findings.
The current report about suicide among endocrinologists, titled, “Doctors’ Burden: Endocrinologist Suicide Report 2023,” was recently published.
A report about suicide among physicians overall, based on the same survey, titled, “Doctors’ Burden: Medscape Physician Suicide Report 2023,” was published previously.
Improved rates among 28 medical specialties
In the 2022 survey of a representative national sample of 13,069 U.S. physicians, 10% of endocrinologists reported having suicidal thoughts, ranking the specialty sixth among 29 medical specialties that year.
The 2023 survey found that in a representative national sample of 9,175 U.S. physicians, 8% of endocrinologists reported having suicidal thoughts, roughly the average rate among clinicians overall, ranking it 20th among 29 medical specialties.
The highest rates of thoughts of suicide in the latest survey were reported by physicians in otolaryngology (13%), followed by physicians in psychiatry, family medicine, anesthesiology, obstetrics/gynecology, and emergency medicine (roughly 12% in each specialty).
The rate of attempted suicide was 1% among endocrinologists, which was also the rate among physicians overall.
More female than male endocrinologists reported contemplating suicide (8% versus 5%). In addition, 1% of male endocrinologists reported that they had attempted suicide and 2% of female endocrinologists replied they preferred not to answer the question about attempted suicide.
In contrast, in 2020, an estimated 4.9% of U.S. adults aged 18 and older had serious thoughts about suicide and 0.5% attempted suicide, according to the National Institutes of Health website, the latest report states.
Rates of suicidal thoughts and suicide attempts among physicians overall “are worryingly high numbers,” Peter Yellowlees, MBBS, MD, emeritus professor of psychiatry at University of California, Davis, Health, and chief executive officer, Asynchealth, said in the report.
Confiding in others, good mental health habits, resources
In the 2023 survey, half of the endocrinologists who had thought about suicide had confided in a therapist and 41% had spoken to a family member, but none had told a colleague or a friend, or phoned a suicide hotline.
On the other hand, 7% of male and 10% of female endocrinologists, and 9% of male and 11% of female physicians overall, reported that a colleague had shared suicidal thoughts with them.
“It’s pleasing that physicians overall have shown themselves slightly more likely to bring ideas about suicide to a therapist and less likely to keep their distress entirely to themselves,” Dr. Yellowlees said.
“It’s possible that the need for health care is becoming less stigmatized nationally, with large and increasing emphasis on physician well-being during and after the COVID-19 pandemic,” he suggested.
Endocrinologists reported that to keep happy and have good mental health, they engaged in activities and hobbies (70%), exercised (66%), spent time with family and friends (63%), got enough sleep (56%), ate healthy (48%), went to therapy (11%), or did other things (8%), which was similar to that reported by physicians overall.
The report lists several resources that are specific for physicians having suicidal thoughts (Physician Support Line, 988 Suicide and Crisis Lifeline, Peer RxMed, International Association for Suicide Prevention, and the American Foundation for Suicide Prevention) along with contact information.
The 2023 survey was conducted from June 28, 2022, to Oct. 3, 2022, and the 2022 survey was conducted from June 29, 2021, to Sept. 26, 2021.
A version of this article first appeared on Medscape.com.
Rates of suicidal thoughts and attempted suicide among endocrinologists declined from 2022 and now rank similar to the average rate among physicians overall, but these rates are still higher than the general public, according to survey findings.
The current report about suicide among endocrinologists, titled, “Doctors’ Burden: Endocrinologist Suicide Report 2023,” was recently published.
A report about suicide among physicians overall, based on the same survey, titled, “Doctors’ Burden: Medscape Physician Suicide Report 2023,” was published previously.
Improved rates among 28 medical specialties
In the 2022 survey of a representative national sample of 13,069 U.S. physicians, 10% of endocrinologists reported having suicidal thoughts, ranking the specialty sixth among 29 medical specialties that year.
The 2023 survey found that in a representative national sample of 9,175 U.S. physicians, 8% of endocrinologists reported having suicidal thoughts, roughly the average rate among clinicians overall, ranking it 20th among 29 medical specialties.
The highest rates of thoughts of suicide in the latest survey were reported by physicians in otolaryngology (13%), followed by physicians in psychiatry, family medicine, anesthesiology, obstetrics/gynecology, and emergency medicine (roughly 12% in each specialty).
The rate of attempted suicide was 1% among endocrinologists, which was also the rate among physicians overall.
More female than male endocrinologists reported contemplating suicide (8% versus 5%). In addition, 1% of male endocrinologists reported that they had attempted suicide and 2% of female endocrinologists replied they preferred not to answer the question about attempted suicide.
In contrast, in 2020, an estimated 4.9% of U.S. adults aged 18 and older had serious thoughts about suicide and 0.5% attempted suicide, according to the National Institutes of Health website, the latest report states.
Rates of suicidal thoughts and suicide attempts among physicians overall “are worryingly high numbers,” Peter Yellowlees, MBBS, MD, emeritus professor of psychiatry at University of California, Davis, Health, and chief executive officer, Asynchealth, said in the report.
Confiding in others, good mental health habits, resources
In the 2023 survey, half of the endocrinologists who had thought about suicide had confided in a therapist and 41% had spoken to a family member, but none had told a colleague or a friend, or phoned a suicide hotline.
On the other hand, 7% of male and 10% of female endocrinologists, and 9% of male and 11% of female physicians overall, reported that a colleague had shared suicidal thoughts with them.
“It’s pleasing that physicians overall have shown themselves slightly more likely to bring ideas about suicide to a therapist and less likely to keep their distress entirely to themselves,” Dr. Yellowlees said.
“It’s possible that the need for health care is becoming less stigmatized nationally, with large and increasing emphasis on physician well-being during and after the COVID-19 pandemic,” he suggested.
Endocrinologists reported that to keep happy and have good mental health, they engaged in activities and hobbies (70%), exercised (66%), spent time with family and friends (63%), got enough sleep (56%), ate healthy (48%), went to therapy (11%), or did other things (8%), which was similar to that reported by physicians overall.
The report lists several resources that are specific for physicians having suicidal thoughts (Physician Support Line, 988 Suicide and Crisis Lifeline, Peer RxMed, International Association for Suicide Prevention, and the American Foundation for Suicide Prevention) along with contact information.
The 2023 survey was conducted from June 28, 2022, to Oct. 3, 2022, and the 2022 survey was conducted from June 29, 2021, to Sept. 26, 2021.
A version of this article first appeared on Medscape.com.
Parathyroidectomy does not preserve kidney function in seniors
Early parathyroidectomy within 1 year of diagnosis of primary hyperparathyroidism (PHPT) did not reduce the risk of a sustained decline in kidney function, measured by a decline in estimated glomerular filtration rate (eGFR) of at least 50%, compared with observation (no surgery) in adults aged 60 and older.
Early parathyroidectomy was, however, associated with a reduced adjusted risk of this decline in kidney function in patients with newly diagnosed PHPT who were younger than age 60.
The findings, based on data from close to 43,000 veterans, were published online in Annals of Internal Medicine.
“The important takeaway from our study is that for older adults [age 60 or older] with primary hyperparathyroidism, preservation of kidney function should not be a primary consideration when making decisions about whether to undergo parathyroidectomy,” lead author Carolyn D. Seib, MD, told this news organization.
“It is important that physicians also discuss with their patients the potential long-term benefits of parathyroidectomy related to a reduced risk of fractures, kidney stones, and cardiovascular disease, and improved quality of life, in addition to the need for lifelong surveillance if surgery is declined, weighing these against an individual patient’s risk of surgery,” said Dr. Seib, a surgeon at Palo Alto (Calif.) VA Medical Center.
“However, in patients younger than 60, early parathyroidectomy may prevent progression to chronic kidney disease (CKD) and should be more strongly considered,” she noted.
Parathyroidectomy, she observed, is a low-risk outpatient surgery for most adults.
“Potential complications of surgery include temporary or permanent hoarseness, hypoparathyroidism (low postoperative parathyroid function), bleeding requiring return to the operating room, and complications related to general anesthesia, all of which are rare,” said Dr. Seib.
“Surgery by a high-volume surgeon is associated with a reduced risk of complications, so patients should seek out an experienced parathyroid surgeon,” she emphasized.
Moreover, parathyroidectomy is the only treatment for primary hyperparathyroidism.
Does parathyroidectomy slow loss of kidney function?
Multidisciplinary guidelines recommend parathyroidectomy, at least in part to mitigate the risk for, and effects related to, the progression of CKD in patients with PHPT and an eGFR below 60 mL/min per 1.73 m2, the researchers wrote.
However, whether parathyroidectomy slows the loss of kidney function in adults with PHPT is not clear.
Guidelines also state that “observation for PHPT disease progression can be considered when patients have no obvious end organ damage (i.e., eGFR > 60 mL/min per 1.73 m2, normal bone mineral density, and no history of kidney stones or fractures),” Dr. Seib noted.
To address the evidence gap, the researchers emulated a randomized target trial using observational data.
In this type of study, Dr. Seib explained, “although patients aren’t randomly assigned to a treatment, complex statistical methods are used to adjust for baseline confounders in an attempt to emulate random treatment assignment and account for bias that may affect the timing of when patients receive treatment.”
Using national Veterans Health Administration data, researchers identified 43,697 veterans with a new biochemical diagnosis of PHPT, defined as elevated parathyroid hormone (> 65 ng/mL) within 6 months of an elevated serum calcium level (> 32.55 mmol/L or >10.2 mg/dL), from 2000 to 2019.
Of these patients, 3,804 underwent parathyroidectomy within 1 year of diagnosis of PHPT, and 39,893 did not, and instead, a watchful waiting approach was adopted.
To be included in the analysis, patients had to have an eGFR above 30 mL/min per 1.73 m2 for 12 months before PHPT diagnosis to exclude secondary or tertiary hyperparathyroidism.
The primary outcome was a sustained decline in eGFR of at least 50% from baseline.
In the overall cohort, patients had a mean pretreatment eGFR of 71.8 mL/min per 1.73 m2. The mean age of patients was 67, 88% were men, and 68% were White.
After a median follow-up of 4.9 years, 6.7% of the patients had a decline in eGFR of at least 50%.
The cumulative incidence of this decline in eGFR was 5.1% at 5 years and 10.8% at 10 years in patients who had had early parathyroidectomy compared with 5.1% and 12.0%, respectively, in patients who did not undergo surgery.
In the overall population, the risk of at least a 50% decline in eGFR was similar in the early parathyroidectomy group versus the observation group (adjusted hazard ratio [HR], 0.98, 95% confidence interval [CI], 0.82-1.16).
However, diving deeper showed that parathyroidectomy was associated with a reduced risk of the primary outcome among patients younger than 60 years (adjusted HR, 0.75, 95% CI, 0.59-0.93) but not among those aged 60 or older (adjusted HR, 1.08, 95% CI, 0.87-1.34).
“When participating in shared decision-making for older adults [age 60 and older] with PHPT, clinicians should not consider parathyroidectomy for potential benefits of preservation of kidney function,” the researchers reiterated.
“For younger patients, clinicians should discuss the potential benefit of parathyroidectomy to reduce the risk for CKD and associated complications in adults with PHPT,” they concluded.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early parathyroidectomy within 1 year of diagnosis of primary hyperparathyroidism (PHPT) did not reduce the risk of a sustained decline in kidney function, measured by a decline in estimated glomerular filtration rate (eGFR) of at least 50%, compared with observation (no surgery) in adults aged 60 and older.
Early parathyroidectomy was, however, associated with a reduced adjusted risk of this decline in kidney function in patients with newly diagnosed PHPT who were younger than age 60.
The findings, based on data from close to 43,000 veterans, were published online in Annals of Internal Medicine.
“The important takeaway from our study is that for older adults [age 60 or older] with primary hyperparathyroidism, preservation of kidney function should not be a primary consideration when making decisions about whether to undergo parathyroidectomy,” lead author Carolyn D. Seib, MD, told this news organization.
“It is important that physicians also discuss with their patients the potential long-term benefits of parathyroidectomy related to a reduced risk of fractures, kidney stones, and cardiovascular disease, and improved quality of life, in addition to the need for lifelong surveillance if surgery is declined, weighing these against an individual patient’s risk of surgery,” said Dr. Seib, a surgeon at Palo Alto (Calif.) VA Medical Center.
“However, in patients younger than 60, early parathyroidectomy may prevent progression to chronic kidney disease (CKD) and should be more strongly considered,” she noted.
Parathyroidectomy, she observed, is a low-risk outpatient surgery for most adults.
“Potential complications of surgery include temporary or permanent hoarseness, hypoparathyroidism (low postoperative parathyroid function), bleeding requiring return to the operating room, and complications related to general anesthesia, all of which are rare,” said Dr. Seib.
“Surgery by a high-volume surgeon is associated with a reduced risk of complications, so patients should seek out an experienced parathyroid surgeon,” she emphasized.
Moreover, parathyroidectomy is the only treatment for primary hyperparathyroidism.
Does parathyroidectomy slow loss of kidney function?
Multidisciplinary guidelines recommend parathyroidectomy, at least in part to mitigate the risk for, and effects related to, the progression of CKD in patients with PHPT and an eGFR below 60 mL/min per 1.73 m2, the researchers wrote.
However, whether parathyroidectomy slows the loss of kidney function in adults with PHPT is not clear.
Guidelines also state that “observation for PHPT disease progression can be considered when patients have no obvious end organ damage (i.e., eGFR > 60 mL/min per 1.73 m2, normal bone mineral density, and no history of kidney stones or fractures),” Dr. Seib noted.
To address the evidence gap, the researchers emulated a randomized target trial using observational data.
In this type of study, Dr. Seib explained, “although patients aren’t randomly assigned to a treatment, complex statistical methods are used to adjust for baseline confounders in an attempt to emulate random treatment assignment and account for bias that may affect the timing of when patients receive treatment.”
Using national Veterans Health Administration data, researchers identified 43,697 veterans with a new biochemical diagnosis of PHPT, defined as elevated parathyroid hormone (> 65 ng/mL) within 6 months of an elevated serum calcium level (> 32.55 mmol/L or >10.2 mg/dL), from 2000 to 2019.
Of these patients, 3,804 underwent parathyroidectomy within 1 year of diagnosis of PHPT, and 39,893 did not, and instead, a watchful waiting approach was adopted.
To be included in the analysis, patients had to have an eGFR above 30 mL/min per 1.73 m2 for 12 months before PHPT diagnosis to exclude secondary or tertiary hyperparathyroidism.
The primary outcome was a sustained decline in eGFR of at least 50% from baseline.
In the overall cohort, patients had a mean pretreatment eGFR of 71.8 mL/min per 1.73 m2. The mean age of patients was 67, 88% were men, and 68% were White.
After a median follow-up of 4.9 years, 6.7% of the patients had a decline in eGFR of at least 50%.
The cumulative incidence of this decline in eGFR was 5.1% at 5 years and 10.8% at 10 years in patients who had had early parathyroidectomy compared with 5.1% and 12.0%, respectively, in patients who did not undergo surgery.
In the overall population, the risk of at least a 50% decline in eGFR was similar in the early parathyroidectomy group versus the observation group (adjusted hazard ratio [HR], 0.98, 95% confidence interval [CI], 0.82-1.16).
However, diving deeper showed that parathyroidectomy was associated with a reduced risk of the primary outcome among patients younger than 60 years (adjusted HR, 0.75, 95% CI, 0.59-0.93) but not among those aged 60 or older (adjusted HR, 1.08, 95% CI, 0.87-1.34).
“When participating in shared decision-making for older adults [age 60 and older] with PHPT, clinicians should not consider parathyroidectomy for potential benefits of preservation of kidney function,” the researchers reiterated.
“For younger patients, clinicians should discuss the potential benefit of parathyroidectomy to reduce the risk for CKD and associated complications in adults with PHPT,” they concluded.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early parathyroidectomy within 1 year of diagnosis of primary hyperparathyroidism (PHPT) did not reduce the risk of a sustained decline in kidney function, measured by a decline in estimated glomerular filtration rate (eGFR) of at least 50%, compared with observation (no surgery) in adults aged 60 and older.
Early parathyroidectomy was, however, associated with a reduced adjusted risk of this decline in kidney function in patients with newly diagnosed PHPT who were younger than age 60.
The findings, based on data from close to 43,000 veterans, were published online in Annals of Internal Medicine.
“The important takeaway from our study is that for older adults [age 60 or older] with primary hyperparathyroidism, preservation of kidney function should not be a primary consideration when making decisions about whether to undergo parathyroidectomy,” lead author Carolyn D. Seib, MD, told this news organization.
“It is important that physicians also discuss with their patients the potential long-term benefits of parathyroidectomy related to a reduced risk of fractures, kidney stones, and cardiovascular disease, and improved quality of life, in addition to the need for lifelong surveillance if surgery is declined, weighing these against an individual patient’s risk of surgery,” said Dr. Seib, a surgeon at Palo Alto (Calif.) VA Medical Center.
“However, in patients younger than 60, early parathyroidectomy may prevent progression to chronic kidney disease (CKD) and should be more strongly considered,” she noted.
Parathyroidectomy, she observed, is a low-risk outpatient surgery for most adults.
“Potential complications of surgery include temporary or permanent hoarseness, hypoparathyroidism (low postoperative parathyroid function), bleeding requiring return to the operating room, and complications related to general anesthesia, all of which are rare,” said Dr. Seib.
“Surgery by a high-volume surgeon is associated with a reduced risk of complications, so patients should seek out an experienced parathyroid surgeon,” she emphasized.
Moreover, parathyroidectomy is the only treatment for primary hyperparathyroidism.
Does parathyroidectomy slow loss of kidney function?
Multidisciplinary guidelines recommend parathyroidectomy, at least in part to mitigate the risk for, and effects related to, the progression of CKD in patients with PHPT and an eGFR below 60 mL/min per 1.73 m2, the researchers wrote.
However, whether parathyroidectomy slows the loss of kidney function in adults with PHPT is not clear.
Guidelines also state that “observation for PHPT disease progression can be considered when patients have no obvious end organ damage (i.e., eGFR > 60 mL/min per 1.73 m2, normal bone mineral density, and no history of kidney stones or fractures),” Dr. Seib noted.
To address the evidence gap, the researchers emulated a randomized target trial using observational data.
In this type of study, Dr. Seib explained, “although patients aren’t randomly assigned to a treatment, complex statistical methods are used to adjust for baseline confounders in an attempt to emulate random treatment assignment and account for bias that may affect the timing of when patients receive treatment.”
Using national Veterans Health Administration data, researchers identified 43,697 veterans with a new biochemical diagnosis of PHPT, defined as elevated parathyroid hormone (> 65 ng/mL) within 6 months of an elevated serum calcium level (> 32.55 mmol/L or >10.2 mg/dL), from 2000 to 2019.
Of these patients, 3,804 underwent parathyroidectomy within 1 year of diagnosis of PHPT, and 39,893 did not, and instead, a watchful waiting approach was adopted.
To be included in the analysis, patients had to have an eGFR above 30 mL/min per 1.73 m2 for 12 months before PHPT diagnosis to exclude secondary or tertiary hyperparathyroidism.
The primary outcome was a sustained decline in eGFR of at least 50% from baseline.
In the overall cohort, patients had a mean pretreatment eGFR of 71.8 mL/min per 1.73 m2. The mean age of patients was 67, 88% were men, and 68% were White.
After a median follow-up of 4.9 years, 6.7% of the patients had a decline in eGFR of at least 50%.
The cumulative incidence of this decline in eGFR was 5.1% at 5 years and 10.8% at 10 years in patients who had had early parathyroidectomy compared with 5.1% and 12.0%, respectively, in patients who did not undergo surgery.
In the overall population, the risk of at least a 50% decline in eGFR was similar in the early parathyroidectomy group versus the observation group (adjusted hazard ratio [HR], 0.98, 95% confidence interval [CI], 0.82-1.16).
However, diving deeper showed that parathyroidectomy was associated with a reduced risk of the primary outcome among patients younger than 60 years (adjusted HR, 0.75, 95% CI, 0.59-0.93) but not among those aged 60 or older (adjusted HR, 1.08, 95% CI, 0.87-1.34).
“When participating in shared decision-making for older adults [age 60 and older] with PHPT, clinicians should not consider parathyroidectomy for potential benefits of preservation of kidney function,” the researchers reiterated.
“For younger patients, clinicians should discuss the potential benefit of parathyroidectomy to reduce the risk for CKD and associated complications in adults with PHPT,” they concluded.
The study was funded by the National Institute on Aging. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
COVID-19 in pregnancy affects growth in child’s first year of life
in a new analysis.
This “exaggerated growth pattern observed among infants with COVID-19 exposure may in some cases be a catch-up response to a prenatal growth deficit,” Mollie W. Ockene and colleagues wrote in a report published recently in the Journal of Clinical Endocrinology & Metabolism.
But given that lower birth weight and accelerated postnatal weight gain are risk factors for cardiometabolic disease, the findings “raise concern” about whether children born to mothers with prenatal COVID-19 go on to develop obesity, diabetes, or cardiovascular disease, senior coauthors Andrea G. Edlow, MD, and Lindsay T. Fourman, MD, of Massachusetts General Hospital, Boston, told this news organization.
Further studies in larger numbers of patients with longer follow-up and detailed assessments are needed, the researchers said, but this points to “a potentially increased cardiometabolic disease risk for the large global population of children with in utero COVID-19 exposure.”
It will be “important for clinicians caring for children with in utero exposure to maternal COVID-19 to be aware of this history,” Dr. Edlow and Dr. Fourman added, “and to view the child’s growth trajectory and metabolic risk factors in a holistic context that includes this prenatal infection exposure.”
COVID-19 vaccination important during and prior to pregnancy
The study also underscores the importance of primary prevention of COVID-19 among women who are contemplating pregnancy or who are already pregnant, the researchers noted, “including the need for widespread implementation of protective measures such as indoor masking and COVID-19 vaccination and boosting during or prior to pregnancy.”
Dr. Edlow and Dr. Fourman added, “Given the disproportionate impact that COVID-19 has had on historically marginalized populations, adverse health outcomes following in utero exposure to maternal COVID-19 may threaten to widen existing disparities in child health.”
On the other hand, although “COVID-19 vaccination rates lagged behind in minority populations following the initial vaccine rollout,” they noted, “these differences have fortunately narrowed over time, particularly for Hispanic individuals, though they do still persist in the Black population,” according to a recent report.
BMI trajectories during first year of life
In utero exposure to COVID-19 has been linked to fetal/neonatal morbidity and mortality, including stillbirth, preterm birth, preeclampsia, and gestational hypertension, but less is known about infant outcomes during the first year of life.
The researchers aimed to compare weight, length, and BMI trajectories over the first year of life in infants with, versus without, in utero exposure to COVID-19.
They identified 149 infants with in utero exposure to COVID-19 and 127 unexposed infants; all were born between March 30, 2020, and May 30, 2021, to mothers who participated in the Mass General Brigham COVID-19 Perinatal Biorepository.
The study excluded infants whose mothers received the vaccine (n = 5) or who had unclear vaccination status during pregnancy (n = 4) to reduce sample heterogeneity.
At the time of the study, few women had received the COVID-19 vaccine because vaccines were approved by the Food and Drug Administration for emergency use in December 2020 and the CDC recommended them for all pregnant women much later, in August 2021.
The researchers examined the weight, length, and BMI of the infants at birth, and at 2, 6, and 12 months, standardized using World Health Organization (WHO) growth charts.
Compared with mothers who did not have COVID-19 during pregnancy, those who had COVID-19 were younger (mean age, 32 vs. 34 years) and had a higher earliest BMI during pregnancy (29 vs. 26 kg/m2) and greater parity (previous births, excluding the index pregnancy, 1.2 vs. 0.9), and they were more likely to be Hispanic or Black and less likely to have private insurance.
Compared with infants exposed to COVID-19 in utero, infants who were not exposed were more likely to be male (47% vs. 55%).
Both infant groups were equally likely to be breastfed (90%).
Compared with the unexposed infants, infants born to mothers with prenatal COVID-19 had lower BMI z-scores at birth (effect size, −0.35; P = .03) and greater gain in BMI z-scores from birth to 12 months (effect size, 0.53; P = .03), but they had similar length at birth and over 12 months, after adjustment for maternal age at delivery, ethnicity, parity, insurance status, and earliest BMI during pregnancy, as well as infant sex, date of birth, and if applicable, history of breastfeeding.
The study received funding from the National Institutes of Health, Harvard Nutrition Obesity Research Center, Boston Area Diabetes Endocrinology Research Centers, American Heart Association, and Simons Foundation. Ms. Ockene has reported no relevant financial relationships. Dr. Edlow has reported being a consultant for Mirvie and receiving research funding from Merck outside the study. Dr. Fourman has reported serving as a consultant and receiving grant funding to her institution from Amryt outside the study. Disclosures for the other authors are listed with the article.
in a new analysis.
This “exaggerated growth pattern observed among infants with COVID-19 exposure may in some cases be a catch-up response to a prenatal growth deficit,” Mollie W. Ockene and colleagues wrote in a report published recently in the Journal of Clinical Endocrinology & Metabolism.
But given that lower birth weight and accelerated postnatal weight gain are risk factors for cardiometabolic disease, the findings “raise concern” about whether children born to mothers with prenatal COVID-19 go on to develop obesity, diabetes, or cardiovascular disease, senior coauthors Andrea G. Edlow, MD, and Lindsay T. Fourman, MD, of Massachusetts General Hospital, Boston, told this news organization.
Further studies in larger numbers of patients with longer follow-up and detailed assessments are needed, the researchers said, but this points to “a potentially increased cardiometabolic disease risk for the large global population of children with in utero COVID-19 exposure.”
It will be “important for clinicians caring for children with in utero exposure to maternal COVID-19 to be aware of this history,” Dr. Edlow and Dr. Fourman added, “and to view the child’s growth trajectory and metabolic risk factors in a holistic context that includes this prenatal infection exposure.”
COVID-19 vaccination important during and prior to pregnancy
The study also underscores the importance of primary prevention of COVID-19 among women who are contemplating pregnancy or who are already pregnant, the researchers noted, “including the need for widespread implementation of protective measures such as indoor masking and COVID-19 vaccination and boosting during or prior to pregnancy.”
Dr. Edlow and Dr. Fourman added, “Given the disproportionate impact that COVID-19 has had on historically marginalized populations, adverse health outcomes following in utero exposure to maternal COVID-19 may threaten to widen existing disparities in child health.”
On the other hand, although “COVID-19 vaccination rates lagged behind in minority populations following the initial vaccine rollout,” they noted, “these differences have fortunately narrowed over time, particularly for Hispanic individuals, though they do still persist in the Black population,” according to a recent report.
BMI trajectories during first year of life
In utero exposure to COVID-19 has been linked to fetal/neonatal morbidity and mortality, including stillbirth, preterm birth, preeclampsia, and gestational hypertension, but less is known about infant outcomes during the first year of life.
The researchers aimed to compare weight, length, and BMI trajectories over the first year of life in infants with, versus without, in utero exposure to COVID-19.
They identified 149 infants with in utero exposure to COVID-19 and 127 unexposed infants; all were born between March 30, 2020, and May 30, 2021, to mothers who participated in the Mass General Brigham COVID-19 Perinatal Biorepository.
The study excluded infants whose mothers received the vaccine (n = 5) or who had unclear vaccination status during pregnancy (n = 4) to reduce sample heterogeneity.
At the time of the study, few women had received the COVID-19 vaccine because vaccines were approved by the Food and Drug Administration for emergency use in December 2020 and the CDC recommended them for all pregnant women much later, in August 2021.
The researchers examined the weight, length, and BMI of the infants at birth, and at 2, 6, and 12 months, standardized using World Health Organization (WHO) growth charts.
Compared with mothers who did not have COVID-19 during pregnancy, those who had COVID-19 were younger (mean age, 32 vs. 34 years) and had a higher earliest BMI during pregnancy (29 vs. 26 kg/m2) and greater parity (previous births, excluding the index pregnancy, 1.2 vs. 0.9), and they were more likely to be Hispanic or Black and less likely to have private insurance.
Compared with infants exposed to COVID-19 in utero, infants who were not exposed were more likely to be male (47% vs. 55%).
Both infant groups were equally likely to be breastfed (90%).
Compared with the unexposed infants, infants born to mothers with prenatal COVID-19 had lower BMI z-scores at birth (effect size, −0.35; P = .03) and greater gain in BMI z-scores from birth to 12 months (effect size, 0.53; P = .03), but they had similar length at birth and over 12 months, after adjustment for maternal age at delivery, ethnicity, parity, insurance status, and earliest BMI during pregnancy, as well as infant sex, date of birth, and if applicable, history of breastfeeding.
The study received funding from the National Institutes of Health, Harvard Nutrition Obesity Research Center, Boston Area Diabetes Endocrinology Research Centers, American Heart Association, and Simons Foundation. Ms. Ockene has reported no relevant financial relationships. Dr. Edlow has reported being a consultant for Mirvie and receiving research funding from Merck outside the study. Dr. Fourman has reported serving as a consultant and receiving grant funding to her institution from Amryt outside the study. Disclosures for the other authors are listed with the article.
in a new analysis.
This “exaggerated growth pattern observed among infants with COVID-19 exposure may in some cases be a catch-up response to a prenatal growth deficit,” Mollie W. Ockene and colleagues wrote in a report published recently in the Journal of Clinical Endocrinology & Metabolism.
But given that lower birth weight and accelerated postnatal weight gain are risk factors for cardiometabolic disease, the findings “raise concern” about whether children born to mothers with prenatal COVID-19 go on to develop obesity, diabetes, or cardiovascular disease, senior coauthors Andrea G. Edlow, MD, and Lindsay T. Fourman, MD, of Massachusetts General Hospital, Boston, told this news organization.
Further studies in larger numbers of patients with longer follow-up and detailed assessments are needed, the researchers said, but this points to “a potentially increased cardiometabolic disease risk for the large global population of children with in utero COVID-19 exposure.”
It will be “important for clinicians caring for children with in utero exposure to maternal COVID-19 to be aware of this history,” Dr. Edlow and Dr. Fourman added, “and to view the child’s growth trajectory and metabolic risk factors in a holistic context that includes this prenatal infection exposure.”
COVID-19 vaccination important during and prior to pregnancy
The study also underscores the importance of primary prevention of COVID-19 among women who are contemplating pregnancy or who are already pregnant, the researchers noted, “including the need for widespread implementation of protective measures such as indoor masking and COVID-19 vaccination and boosting during or prior to pregnancy.”
Dr. Edlow and Dr. Fourman added, “Given the disproportionate impact that COVID-19 has had on historically marginalized populations, adverse health outcomes following in utero exposure to maternal COVID-19 may threaten to widen existing disparities in child health.”
On the other hand, although “COVID-19 vaccination rates lagged behind in minority populations following the initial vaccine rollout,” they noted, “these differences have fortunately narrowed over time, particularly for Hispanic individuals, though they do still persist in the Black population,” according to a recent report.
BMI trajectories during first year of life
In utero exposure to COVID-19 has been linked to fetal/neonatal morbidity and mortality, including stillbirth, preterm birth, preeclampsia, and gestational hypertension, but less is known about infant outcomes during the first year of life.
The researchers aimed to compare weight, length, and BMI trajectories over the first year of life in infants with, versus without, in utero exposure to COVID-19.
They identified 149 infants with in utero exposure to COVID-19 and 127 unexposed infants; all were born between March 30, 2020, and May 30, 2021, to mothers who participated in the Mass General Brigham COVID-19 Perinatal Biorepository.
The study excluded infants whose mothers received the vaccine (n = 5) or who had unclear vaccination status during pregnancy (n = 4) to reduce sample heterogeneity.
At the time of the study, few women had received the COVID-19 vaccine because vaccines were approved by the Food and Drug Administration for emergency use in December 2020 and the CDC recommended them for all pregnant women much later, in August 2021.
The researchers examined the weight, length, and BMI of the infants at birth, and at 2, 6, and 12 months, standardized using World Health Organization (WHO) growth charts.
Compared with mothers who did not have COVID-19 during pregnancy, those who had COVID-19 were younger (mean age, 32 vs. 34 years) and had a higher earliest BMI during pregnancy (29 vs. 26 kg/m2) and greater parity (previous births, excluding the index pregnancy, 1.2 vs. 0.9), and they were more likely to be Hispanic or Black and less likely to have private insurance.
Compared with infants exposed to COVID-19 in utero, infants who were not exposed were more likely to be male (47% vs. 55%).
Both infant groups were equally likely to be breastfed (90%).
Compared with the unexposed infants, infants born to mothers with prenatal COVID-19 had lower BMI z-scores at birth (effect size, −0.35; P = .03) and greater gain in BMI z-scores from birth to 12 months (effect size, 0.53; P = .03), but they had similar length at birth and over 12 months, after adjustment for maternal age at delivery, ethnicity, parity, insurance status, and earliest BMI during pregnancy, as well as infant sex, date of birth, and if applicable, history of breastfeeding.
The study received funding from the National Institutes of Health, Harvard Nutrition Obesity Research Center, Boston Area Diabetes Endocrinology Research Centers, American Heart Association, and Simons Foundation. Ms. Ockene has reported no relevant financial relationships. Dr. Edlow has reported being a consultant for Mirvie and receiving research funding from Merck outside the study. Dr. Fourman has reported serving as a consultant and receiving grant funding to her institution from Amryt outside the study. Disclosures for the other authors are listed with the article.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM








