User login
Topical timolol improved chronic leg ulcer healing
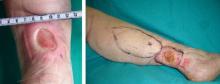
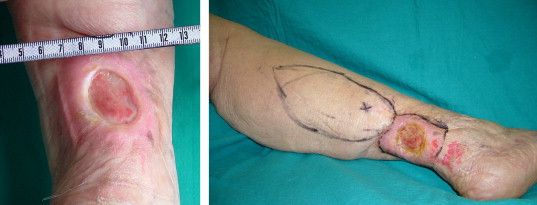
The use of topical timolol maleate as a treatment for chronic diabetic and chronic venous ulcers showed increased wound healing compared with controls, according to the results of a prospective observational study of 60 patients.
In the treatment group, 30 patients with chronic leg ulcer (15 diabetic ulcers; 15 venous) received topical application of 0.5% timolol maleate (a beta-blocker) plus conventional antibiotic and wound dressing therapy. In the control group, 30 patients (identical split between diabetic and venous ulcers) were treated with conventional therapy alone, according to a report published in the November issue of the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
The researchers found no significant difference in healing rates due to sex, between smokers and nonsmokers, or alcohol consumers vs. nonconsumers and they saw no major adverse effects due to timolol application (J Vasc Surg: Venous and Lym Dis 2017;5:844-50).
They reported that the limitations of their study included the lack of randomization and a formal power assessment.
“Topical application of timolol maleate is an effective therapeutic option for the treatment of chronic diabetic ulcer and chronic venous ulcer patients to improve ulcer healing by promoting keratinocyte migration,” the researchers concluded.
They reported having no relevant conflicts.
The use of topical timolol maleate as a treatment for chronic diabetic and chronic venous ulcers showed increased wound healing compared with controls, according to the results of a prospective observational study of 60 patients.
In the treatment group, 30 patients with chronic leg ulcer (15 diabetic ulcers; 15 venous) received topical application of 0.5% timolol maleate (a beta-blocker) plus conventional antibiotic and wound dressing therapy. In the control group, 30 patients (identical split between diabetic and venous ulcers) were treated with conventional therapy alone, according to a report published in the November issue of the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
The researchers found no significant difference in healing rates due to sex, between smokers and nonsmokers, or alcohol consumers vs. nonconsumers and they saw no major adverse effects due to timolol application (J Vasc Surg: Venous and Lym Dis 2017;5:844-50).
They reported that the limitations of their study included the lack of randomization and a formal power assessment.
“Topical application of timolol maleate is an effective therapeutic option for the treatment of chronic diabetic ulcer and chronic venous ulcer patients to improve ulcer healing by promoting keratinocyte migration,” the researchers concluded.
They reported having no relevant conflicts.
The use of topical timolol maleate as a treatment for chronic diabetic and chronic venous ulcers showed increased wound healing compared with controls, according to the results of a prospective observational study of 60 patients.
In the treatment group, 30 patients with chronic leg ulcer (15 diabetic ulcers; 15 venous) received topical application of 0.5% timolol maleate (a beta-blocker) plus conventional antibiotic and wound dressing therapy. In the control group, 30 patients (identical split between diabetic and venous ulcers) were treated with conventional therapy alone, according to a report published in the November issue of the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
The researchers found no significant difference in healing rates due to sex, between smokers and nonsmokers, or alcohol consumers vs. nonconsumers and they saw no major adverse effects due to timolol application (J Vasc Surg: Venous and Lym Dis 2017;5:844-50).
They reported that the limitations of their study included the lack of randomization and a formal power assessment.
“Topical application of timolol maleate is an effective therapeutic option for the treatment of chronic diabetic ulcer and chronic venous ulcer patients to improve ulcer healing by promoting keratinocyte migration,” the researchers concluded.
They reported having no relevant conflicts.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Caprini score is not a good predictor of PE in patients with DVT
The Caprini score, commonly used to risk stratify patients for the development of venous thromboembolism and to determine the optimal dose of prophylaxis, failed to predict the development of pulmonary embolism and hemodynamically significant PE in patients presenting with deep vein thrombosis (DVT), according to the results of a large, retrospective single-center study.
Recent surgery was not associated with the development of hemodynamically significant PE, but the presence of proximal DVT was, according to a report published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (2017. doi: 10.1016/j.jvsv.2017.08.015).
Their results showed that patients who had undergone recent surgery were less likely to develop hemodynamically significant PE (13.3% vs. 27.2%; P = .01). In contrast, patients with proximal DVT were at higher risk for development of hemodynamically significant PE (80.7% vs. 64.2%; P = .007). They found no association between Caprini score and PE severity (P = .17) or the Caprini score and proximal DVT (P = .89).
“This study shows that the Caprini score does not correlate with the occurrence of PE or the severity of PE. On the other hand, a proximal location of DVT seems to have a high association with hemodynamically significant PE. Such patients may benefit from more aggressive anticoagulant therapy and work-up for PE,” the researchers concluded.
The authors reported that they had no conflicts of interest.
The Caprini score, commonly used to risk stratify patients for the development of venous thromboembolism and to determine the optimal dose of prophylaxis, failed to predict the development of pulmonary embolism and hemodynamically significant PE in patients presenting with deep vein thrombosis (DVT), according to the results of a large, retrospective single-center study.
Recent surgery was not associated with the development of hemodynamically significant PE, but the presence of proximal DVT was, according to a report published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (2017. doi: 10.1016/j.jvsv.2017.08.015).
Their results showed that patients who had undergone recent surgery were less likely to develop hemodynamically significant PE (13.3% vs. 27.2%; P = .01). In contrast, patients with proximal DVT were at higher risk for development of hemodynamically significant PE (80.7% vs. 64.2%; P = .007). They found no association between Caprini score and PE severity (P = .17) or the Caprini score and proximal DVT (P = .89).
“This study shows that the Caprini score does not correlate with the occurrence of PE or the severity of PE. On the other hand, a proximal location of DVT seems to have a high association with hemodynamically significant PE. Such patients may benefit from more aggressive anticoagulant therapy and work-up for PE,” the researchers concluded.
The authors reported that they had no conflicts of interest.
The Caprini score, commonly used to risk stratify patients for the development of venous thromboembolism and to determine the optimal dose of prophylaxis, failed to predict the development of pulmonary embolism and hemodynamically significant PE in patients presenting with deep vein thrombosis (DVT), according to the results of a large, retrospective single-center study.
Recent surgery was not associated with the development of hemodynamically significant PE, but the presence of proximal DVT was, according to a report published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (2017. doi: 10.1016/j.jvsv.2017.08.015).
Their results showed that patients who had undergone recent surgery were less likely to develop hemodynamically significant PE (13.3% vs. 27.2%; P = .01). In contrast, patients with proximal DVT were at higher risk for development of hemodynamically significant PE (80.7% vs. 64.2%; P = .007). They found no association between Caprini score and PE severity (P = .17) or the Caprini score and proximal DVT (P = .89).
“This study shows that the Caprini score does not correlate with the occurrence of PE or the severity of PE. On the other hand, a proximal location of DVT seems to have a high association with hemodynamically significant PE. Such patients may benefit from more aggressive anticoagulant therapy and work-up for PE,” the researchers concluded.
The authors reported that they had no conflicts of interest.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point:
Major finding: Among 838 patients presenting with DVT, nearly 26% had concomitant PE, more than half of which was hemodynamically significant.
Data source: A single-center, retrospective review of 838 patients diagnosed with DVT.
Disclosures: The authors reported that they had no conflicts of interest.
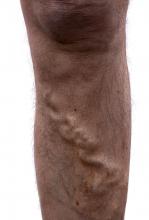
Beware the risks associated with greater saphenous vein thrombosis
Isolated greater saphenous vein thrombosis is not as benign as generally thought, according to the results of a single institution retrospective study of 61 patients (67 limbs) with isolated GSVT.
Instead, patients had a significant risk of persistent symptoms, recurrence, deep vein thrombosis (DVT), and pulmonary embolism (PE), according to a report published in the Annals of Vascular Surgery by Elizabeth Kudlaty, MD, Ohio State University, Columbus, and her colleagues.
Location of the GSVT within 5 cm of the saphenous vein junction (SVJ; 32 patients) as compared with GSVT greater than 5 cm from the SVJ (29 patients) was significantly associated with malignancy (37.5% vs. 6.9%, respectively; P = .01), in-patient status (71.9% vs. 41.4%; P = .02), and diabetes (37.5% vs. 10.3%; P = .02). PE also was significantly greater in patients with GSVT within 5 cm of the SVJ (18.8% vs. 0.0%; P = .02). Patients with GSVT greater than 5 cm from the SVJ showed significantly more GSVT propagation/new saphenous vein thrombosis (0% vs. 31.3%, P = .048). There was a nonsignificant trend toward greater mortality for patients with GSVT within 5 cm of the SVJ (P = .052).
Dr. Kudlaty and her colleagues found that the different management options used, including anticoagulation, observation, and aspirin use, did not significantly affect outcomes (Ann Vasc Surg. 2017 Nov;45:154-9).
“Isolated GSVT can be viewed as a marker of more serious systemic diseases, notably diabetes and cancer, and leaves patients at high risk for thromboembolic events, recurrence, and persistent symptoms, despite a variety of managements,” the researchers concluded.
The authors reported that they had no conflicts of interest and that there were no outside funding sources.
Isolated greater saphenous vein thrombosis is not as benign as generally thought, according to the results of a single institution retrospective study of 61 patients (67 limbs) with isolated GSVT.
Instead, patients had a significant risk of persistent symptoms, recurrence, deep vein thrombosis (DVT), and pulmonary embolism (PE), according to a report published in the Annals of Vascular Surgery by Elizabeth Kudlaty, MD, Ohio State University, Columbus, and her colleagues.
Location of the GSVT within 5 cm of the saphenous vein junction (SVJ; 32 patients) as compared with GSVT greater than 5 cm from the SVJ (29 patients) was significantly associated with malignancy (37.5% vs. 6.9%, respectively; P = .01), in-patient status (71.9% vs. 41.4%; P = .02), and diabetes (37.5% vs. 10.3%; P = .02). PE also was significantly greater in patients with GSVT within 5 cm of the SVJ (18.8% vs. 0.0%; P = .02). Patients with GSVT greater than 5 cm from the SVJ showed significantly more GSVT propagation/new saphenous vein thrombosis (0% vs. 31.3%, P = .048). There was a nonsignificant trend toward greater mortality for patients with GSVT within 5 cm of the SVJ (P = .052).
Dr. Kudlaty and her colleagues found that the different management options used, including anticoagulation, observation, and aspirin use, did not significantly affect outcomes (Ann Vasc Surg. 2017 Nov;45:154-9).
“Isolated GSVT can be viewed as a marker of more serious systemic diseases, notably diabetes and cancer, and leaves patients at high risk for thromboembolic events, recurrence, and persistent symptoms, despite a variety of managements,” the researchers concluded.
The authors reported that they had no conflicts of interest and that there were no outside funding sources.
Isolated greater saphenous vein thrombosis is not as benign as generally thought, according to the results of a single institution retrospective study of 61 patients (67 limbs) with isolated GSVT.
Instead, patients had a significant risk of persistent symptoms, recurrence, deep vein thrombosis (DVT), and pulmonary embolism (PE), according to a report published in the Annals of Vascular Surgery by Elizabeth Kudlaty, MD, Ohio State University, Columbus, and her colleagues.
Location of the GSVT within 5 cm of the saphenous vein junction (SVJ; 32 patients) as compared with GSVT greater than 5 cm from the SVJ (29 patients) was significantly associated with malignancy (37.5% vs. 6.9%, respectively; P = .01), in-patient status (71.9% vs. 41.4%; P = .02), and diabetes (37.5% vs. 10.3%; P = .02). PE also was significantly greater in patients with GSVT within 5 cm of the SVJ (18.8% vs. 0.0%; P = .02). Patients with GSVT greater than 5 cm from the SVJ showed significantly more GSVT propagation/new saphenous vein thrombosis (0% vs. 31.3%, P = .048). There was a nonsignificant trend toward greater mortality for patients with GSVT within 5 cm of the SVJ (P = .052).
Dr. Kudlaty and her colleagues found that the different management options used, including anticoagulation, observation, and aspirin use, did not significantly affect outcomes (Ann Vasc Surg. 2017 Nov;45:154-9).
“Isolated GSVT can be viewed as a marker of more serious systemic diseases, notably diabetes and cancer, and leaves patients at high risk for thromboembolic events, recurrence, and persistent symptoms, despite a variety of managements,” the researchers concluded.
The authors reported that they had no conflicts of interest and that there were no outside funding sources.
FROM THE ANNALS OF VASCULAR SURGERY
Key clinical point:
Major finding: Patients with GSVT show a significant risk of persistent symptoms, recurrence, deep vein thrombosis, and pulmonary embolism.
Data source: A retrospective review of all 61 patients with isolated GSVT at a single institution between 2008 and 2014.
Disclosures: The authors reported that they had no conflicts of interest and that there were no outside funding sources.
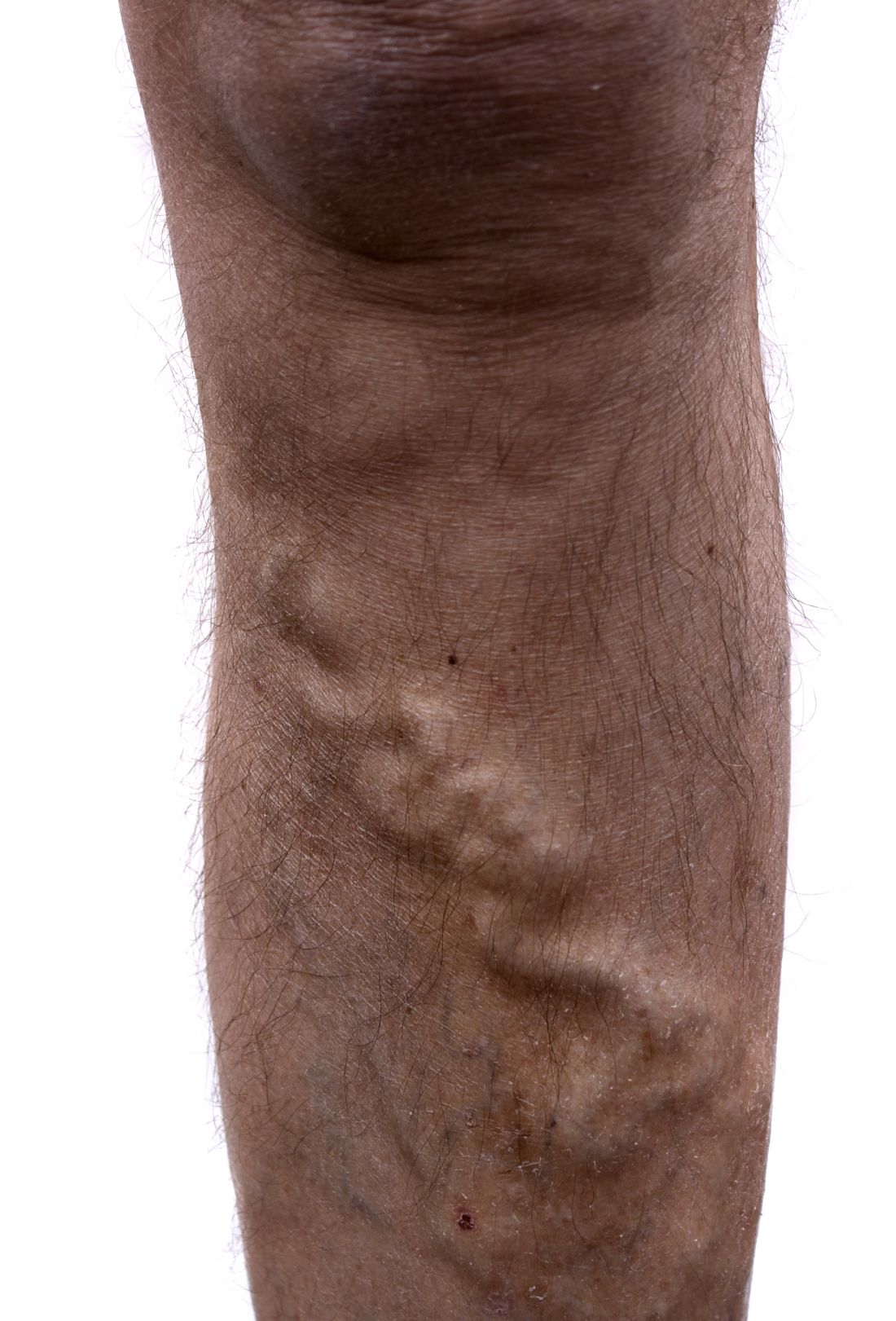
Study: Varicose vein procedures should be offered to patients 65 years and older
Older and younger patients benefited from varicose vein procedures, a finding that calls into question the use of compression therapy as the primary treatment for patients aged 65 years and older, according to the results of a prospectively maintained database study of all patients in the Vascular Quality Initiative Varicose Vein Registry–participating centers.
Procedures for varicose veins in 1,068 patients aged 65 years or older showed similar improvement in Clinical, Etiology, Anatomy, and Pathophysiology class and Venous Clinical Severity Score, compared with a group of 2,691 younger patients, according to a report published in Journal of Vascular Surgery: Venous and Lymphatic Disorders. Among patients younger than 65 years, 57.4% had an improvement, compared with 52% of patients aged 65 years or older.
However, the younger patients had more improvement in patient-reported outcomes, according to Danielle C. Sutzko, MD, of the University of Michigan, Ann Arbor, and her colleagues (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:647-57).
One of the main limitations of the study was the fact that only 62% of procedures within the Vascular Quality Initiative Varicose Vein Registry had follow-up.
“Patients older than 65 years appear to benefit from appropriate varicose vein procedures and should not be denied interventions on their varicose veins and venous insufficiency on the basis of their age only,” the researchers concluded.
Dr. Sutzko and her colleagues reported having no conflicts of interest.
Older and younger patients benefited from varicose vein procedures, a finding that calls into question the use of compression therapy as the primary treatment for patients aged 65 years and older, according to the results of a prospectively maintained database study of all patients in the Vascular Quality Initiative Varicose Vein Registry–participating centers.
Procedures for varicose veins in 1,068 patients aged 65 years or older showed similar improvement in Clinical, Etiology, Anatomy, and Pathophysiology class and Venous Clinical Severity Score, compared with a group of 2,691 younger patients, according to a report published in Journal of Vascular Surgery: Venous and Lymphatic Disorders. Among patients younger than 65 years, 57.4% had an improvement, compared with 52% of patients aged 65 years or older.
However, the younger patients had more improvement in patient-reported outcomes, according to Danielle C. Sutzko, MD, of the University of Michigan, Ann Arbor, and her colleagues (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:647-57).
One of the main limitations of the study was the fact that only 62% of procedures within the Vascular Quality Initiative Varicose Vein Registry had follow-up.
“Patients older than 65 years appear to benefit from appropriate varicose vein procedures and should not be denied interventions on their varicose veins and venous insufficiency on the basis of their age only,” the researchers concluded.
Dr. Sutzko and her colleagues reported having no conflicts of interest.
Older and younger patients benefited from varicose vein procedures, a finding that calls into question the use of compression therapy as the primary treatment for patients aged 65 years and older, according to the results of a prospectively maintained database study of all patients in the Vascular Quality Initiative Varicose Vein Registry–participating centers.
Procedures for varicose veins in 1,068 patients aged 65 years or older showed similar improvement in Clinical, Etiology, Anatomy, and Pathophysiology class and Venous Clinical Severity Score, compared with a group of 2,691 younger patients, according to a report published in Journal of Vascular Surgery: Venous and Lymphatic Disorders. Among patients younger than 65 years, 57.4% had an improvement, compared with 52% of patients aged 65 years or older.
However, the younger patients had more improvement in patient-reported outcomes, according to Danielle C. Sutzko, MD, of the University of Michigan, Ann Arbor, and her colleagues (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:647-57).
One of the main limitations of the study was the fact that only 62% of procedures within the Vascular Quality Initiative Varicose Vein Registry had follow-up.
“Patients older than 65 years appear to benefit from appropriate varicose vein procedures and should not be denied interventions on their varicose veins and venous insufficiency on the basis of their age only,” the researchers concluded.
Dr. Sutzko and her colleagues reported having no conflicts of interest.
FROM JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point:
Major finding: Among patients younger than 65, 57.4% had an improvement, compared to 52% of patients aged 65 years or older.
Data source: Prospectively captured data for all patients in the Vascular Quality Initiative Varicose Vein Registry–participating centers.
Disclosures: Dr. Sutzko and her colleagues reported having no conflicts of interest.
Venography for stenting led to good results for patients with May-Thurner syndrome
Stenting of the left common iliac vein of patients with May-Thurner syndrome provided good short-term results as compared with nonstenting, according to the results of a retrospective, single-center registry study.
When to treat patients with May-Thurner syndrome (MTS) who have mild symptoms and what degree of compression should trigger intervention are in considerable question. Approximately 50% of the general population has some degree of left common iliac vein (LCIV) compression as detected using intravascular ultrasound (IVUS) and axial imaging, according to Johnathon C. Rollo, MD, of the University of Washington, Seattle, and his colleagues at the University of California, Los Angeles. They performed their study in order to address the debate over what were the optimal IVUS and venography criteria for stent implantation in these patients.
Of 102 patients in a registry, 63 had clear evidence of LCIV compression by the overlying right common iliac artery by IVUS assessment or venography. Nonthrombotic MTS patients who presented with chronic leg swelling or venous claudication underwent duplex ultrasound to rule out deep-vein thrombosis (DVT) were placed in compression therapy, and venography was performed to assess for iliac vein involvement
Iliac vein stenting was offered to those patients who met the following criteria:
• Sufficiently severe symptoms of swelling, venous claudication, or pain to affect their quality of life despite compression therapy.
• Diagnostic venogram imaging showing evidence of physiologically significant MTS compression, including contrast stagnation within the proximal left common and external iliac vein, contralateral cross-filling to the right iliac venous • circulation via hypogastric collateral networks, and/or significant retroperitoneal collateralization.
• IVUS assessment demonstrating greater than 50% luminal narrowing of the LCIV or extensive intravascular webs.
Patients who did not meet one of these criteria (generally the venogram findings) were treated with continued conservative management, which consisted of compression therapy, weight loss and exercise programs, and other conservative measures (J Vasc Surg: Venous Lymphatic Disorders. 2017;5:667-76).
Of the 63 patients in the final study group, a total of 44 were treated with iliofemoral stents, with or without thrombolysis, and 19 conservatively managed patients who were not treated with stents served as controls. The mean age of the patients was 46 years, and 76% of them were women. With regard to comorbidities, 63% had a patient-reported history of DVT, and 22% had a patient-reported history of pulmonary embolism. Of the 63 patients, 32 had nonthrombotic MTS.
Stent diameter was based on IVUS measurement, with the goal of achieving normal vein diameter, and undersizing was avoided. Stenting was performed under local anesthesia.
A total of 44 patients (70%) underwent primary stenting (70%) or thrombolysis and stenting (30%), whereas 19 patients were not stented. Of these latter, 14 were nonthrombotic and were treated conservatively with compression therapy alone; the remaining 5 patients with thrombotic MTS were treated with lysis or angioplasty alone. Technical success was achieved in 100% of patients who had an intervention.
Primary and secondary patency rates in the stented thrombotic population were 87% and 93% at 24 months, respectively, by Kaplan-Meier analysis and were not significantly different from the results of the nonthrombotic stented patients.
Clinical improvement was significantly more likely in stented patients, compared with those managed without stenting (95% vs. 58%, respectively; P less than .001), Complete clinical resolution, defined as an absence of swelling or any other venous symptoms, was three times more likely in stented patients than in nonstented patients (64% vs. 21%, respectively; P less than .001), according to the researchers.
“MTS patients are typically young and relatively healthy. Whereas several series have demonstrated good intermediate-term results out to 7-10 years, the durability of these stents 20-30 years or more after implantation is unknown. For this reason, our group has been conservative in offering stent implantation to nonthrombotic patients,” Dr. Rollo and his colleagues stated.
“Regardless of the differential in clinical outcomes between stented and nonstented patients, this selective approach to stenting is reasonable in that those believed to be best managed with conservative therapy can be re-evaluated at regular intervals for clinical deterioration,” they concluded.
The authors reported that they had no disclosures.
Most vascular specialists, including our group in New York, have relied heavily on intravascular ultrasound demonstrating greater than 50% stenosis to decide whether or not stenting is indicated. Dr. Rollo and his associates emphasized the importance of using venography-guided findings (contrast stagnation within the external iliac vein, contralateral cross-filling to the right iliac system, and/or significant retroperitoneal collateralization) to decide on stent indication. They did, however, use IVUS to guide in stent sizing and placement.
Todd Berland, MD , is the director, outpatient vascular interventions, NYU Langone Health, New York, N.Y. He had no relevant financial disclosures.
Most vascular specialists, including our group in New York, have relied heavily on intravascular ultrasound demonstrating greater than 50% stenosis to decide whether or not stenting is indicated. Dr. Rollo and his associates emphasized the importance of using venography-guided findings (contrast stagnation within the external iliac vein, contralateral cross-filling to the right iliac system, and/or significant retroperitoneal collateralization) to decide on stent indication. They did, however, use IVUS to guide in stent sizing and placement.
Todd Berland, MD , is the director, outpatient vascular interventions, NYU Langone Health, New York, N.Y. He had no relevant financial disclosures.
Most vascular specialists, including our group in New York, have relied heavily on intravascular ultrasound demonstrating greater than 50% stenosis to decide whether or not stenting is indicated. Dr. Rollo and his associates emphasized the importance of using venography-guided findings (contrast stagnation within the external iliac vein, contralateral cross-filling to the right iliac system, and/or significant retroperitoneal collateralization) to decide on stent indication. They did, however, use IVUS to guide in stent sizing and placement.
Todd Berland, MD , is the director, outpatient vascular interventions, NYU Langone Health, New York, N.Y. He had no relevant financial disclosures.
Stenting of the left common iliac vein of patients with May-Thurner syndrome provided good short-term results as compared with nonstenting, according to the results of a retrospective, single-center registry study.
When to treat patients with May-Thurner syndrome (MTS) who have mild symptoms and what degree of compression should trigger intervention are in considerable question. Approximately 50% of the general population has some degree of left common iliac vein (LCIV) compression as detected using intravascular ultrasound (IVUS) and axial imaging, according to Johnathon C. Rollo, MD, of the University of Washington, Seattle, and his colleagues at the University of California, Los Angeles. They performed their study in order to address the debate over what were the optimal IVUS and venography criteria for stent implantation in these patients.
Of 102 patients in a registry, 63 had clear evidence of LCIV compression by the overlying right common iliac artery by IVUS assessment or venography. Nonthrombotic MTS patients who presented with chronic leg swelling or venous claudication underwent duplex ultrasound to rule out deep-vein thrombosis (DVT) were placed in compression therapy, and venography was performed to assess for iliac vein involvement
Iliac vein stenting was offered to those patients who met the following criteria:
• Sufficiently severe symptoms of swelling, venous claudication, or pain to affect their quality of life despite compression therapy.
• Diagnostic venogram imaging showing evidence of physiologically significant MTS compression, including contrast stagnation within the proximal left common and external iliac vein, contralateral cross-filling to the right iliac venous • circulation via hypogastric collateral networks, and/or significant retroperitoneal collateralization.
• IVUS assessment demonstrating greater than 50% luminal narrowing of the LCIV or extensive intravascular webs.
Patients who did not meet one of these criteria (generally the venogram findings) were treated with continued conservative management, which consisted of compression therapy, weight loss and exercise programs, and other conservative measures (J Vasc Surg: Venous Lymphatic Disorders. 2017;5:667-76).
Of the 63 patients in the final study group, a total of 44 were treated with iliofemoral stents, with or without thrombolysis, and 19 conservatively managed patients who were not treated with stents served as controls. The mean age of the patients was 46 years, and 76% of them were women. With regard to comorbidities, 63% had a patient-reported history of DVT, and 22% had a patient-reported history of pulmonary embolism. Of the 63 patients, 32 had nonthrombotic MTS.
Stent diameter was based on IVUS measurement, with the goal of achieving normal vein diameter, and undersizing was avoided. Stenting was performed under local anesthesia.
A total of 44 patients (70%) underwent primary stenting (70%) or thrombolysis and stenting (30%), whereas 19 patients were not stented. Of these latter, 14 were nonthrombotic and were treated conservatively with compression therapy alone; the remaining 5 patients with thrombotic MTS were treated with lysis or angioplasty alone. Technical success was achieved in 100% of patients who had an intervention.
Primary and secondary patency rates in the stented thrombotic population were 87% and 93% at 24 months, respectively, by Kaplan-Meier analysis and were not significantly different from the results of the nonthrombotic stented patients.
Clinical improvement was significantly more likely in stented patients, compared with those managed without stenting (95% vs. 58%, respectively; P less than .001), Complete clinical resolution, defined as an absence of swelling or any other venous symptoms, was three times more likely in stented patients than in nonstented patients (64% vs. 21%, respectively; P less than .001), according to the researchers.
“MTS patients are typically young and relatively healthy. Whereas several series have demonstrated good intermediate-term results out to 7-10 years, the durability of these stents 20-30 years or more after implantation is unknown. For this reason, our group has been conservative in offering stent implantation to nonthrombotic patients,” Dr. Rollo and his colleagues stated.
“Regardless of the differential in clinical outcomes between stented and nonstented patients, this selective approach to stenting is reasonable in that those believed to be best managed with conservative therapy can be re-evaluated at regular intervals for clinical deterioration,” they concluded.
The authors reported that they had no disclosures.
Stenting of the left common iliac vein of patients with May-Thurner syndrome provided good short-term results as compared with nonstenting, according to the results of a retrospective, single-center registry study.
When to treat patients with May-Thurner syndrome (MTS) who have mild symptoms and what degree of compression should trigger intervention are in considerable question. Approximately 50% of the general population has some degree of left common iliac vein (LCIV) compression as detected using intravascular ultrasound (IVUS) and axial imaging, according to Johnathon C. Rollo, MD, of the University of Washington, Seattle, and his colleagues at the University of California, Los Angeles. They performed their study in order to address the debate over what were the optimal IVUS and venography criteria for stent implantation in these patients.
Of 102 patients in a registry, 63 had clear evidence of LCIV compression by the overlying right common iliac artery by IVUS assessment or venography. Nonthrombotic MTS patients who presented with chronic leg swelling or venous claudication underwent duplex ultrasound to rule out deep-vein thrombosis (DVT) were placed in compression therapy, and venography was performed to assess for iliac vein involvement
Iliac vein stenting was offered to those patients who met the following criteria:
• Sufficiently severe symptoms of swelling, venous claudication, or pain to affect their quality of life despite compression therapy.
• Diagnostic venogram imaging showing evidence of physiologically significant MTS compression, including contrast stagnation within the proximal left common and external iliac vein, contralateral cross-filling to the right iliac venous • circulation via hypogastric collateral networks, and/or significant retroperitoneal collateralization.
• IVUS assessment demonstrating greater than 50% luminal narrowing of the LCIV or extensive intravascular webs.
Patients who did not meet one of these criteria (generally the venogram findings) were treated with continued conservative management, which consisted of compression therapy, weight loss and exercise programs, and other conservative measures (J Vasc Surg: Venous Lymphatic Disorders. 2017;5:667-76).
Of the 63 patients in the final study group, a total of 44 were treated with iliofemoral stents, with or without thrombolysis, and 19 conservatively managed patients who were not treated with stents served as controls. The mean age of the patients was 46 years, and 76% of them were women. With regard to comorbidities, 63% had a patient-reported history of DVT, and 22% had a patient-reported history of pulmonary embolism. Of the 63 patients, 32 had nonthrombotic MTS.
Stent diameter was based on IVUS measurement, with the goal of achieving normal vein diameter, and undersizing was avoided. Stenting was performed under local anesthesia.
A total of 44 patients (70%) underwent primary stenting (70%) or thrombolysis and stenting (30%), whereas 19 patients were not stented. Of these latter, 14 were nonthrombotic and were treated conservatively with compression therapy alone; the remaining 5 patients with thrombotic MTS were treated with lysis or angioplasty alone. Technical success was achieved in 100% of patients who had an intervention.
Primary and secondary patency rates in the stented thrombotic population were 87% and 93% at 24 months, respectively, by Kaplan-Meier analysis and were not significantly different from the results of the nonthrombotic stented patients.
Clinical improvement was significantly more likely in stented patients, compared with those managed without stenting (95% vs. 58%, respectively; P less than .001), Complete clinical resolution, defined as an absence of swelling or any other venous symptoms, was three times more likely in stented patients than in nonstented patients (64% vs. 21%, respectively; P less than .001), according to the researchers.
“MTS patients are typically young and relatively healthy. Whereas several series have demonstrated good intermediate-term results out to 7-10 years, the durability of these stents 20-30 years or more after implantation is unknown. For this reason, our group has been conservative in offering stent implantation to nonthrombotic patients,” Dr. Rollo and his colleagues stated.
“Regardless of the differential in clinical outcomes between stented and nonstented patients, this selective approach to stenting is reasonable in that those believed to be best managed with conservative therapy can be re-evaluated at regular intervals for clinical deterioration,” they concluded.
The authors reported that they had no disclosures.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point:
Major finding: May-Thurner patients treated with iliofemoral stents had significantly better (95%) clinical improvement than 21 patients not treated with stents (58%).
Data source: Retrospective analysis of a single-center registry of 65 patients with May-Thurner syndrome.
Disclosures: The authors reported that they had no disclosures.
Adaptive pneumatic compression device found comparable to compression stockings
The use of an adaptive pneumatic compression device showed comparable results to compression stockings in a two-arm, randomized multicenter pilot study of previously noncompliant patients with chronic venous disease.
In addition, patient satisfaction regarding ease of use was higher in for the device, compared with the stockings, according to Fedor Lurie, MD, PhD, of the Jobst Vascular Institute, Toledo, Ohio, and his colleague.
A total of 89 subjects with unilateral or bilateral chronic venous insufficiency were randomized and included in the final analysis of the study. The patients comprised 44% women, with a median age of nearly 63 years, the majority (53%) of whom had bilateral chronic venous insufficiency (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:699-706).
Significantly more patients found the ACTitouch device easy to apply (71%) versus the compression stockings (37.5%; P = .0001) and easy to remove (89% vs. 59%; P = .0001). However, compliance and average time of use were not significantly different between the two treatment groups.
In terms of limb volume reduction, the device group demonstrated a significant volume reduction (44%), compared with the standard compression garment use (17%) in obese patients (P = .019) but not in nonobese patients.
The device was easy to put on and remove and was considered comfortable, according to the researchers. “These are characteristics usually associated with good potential for long-term acceptance by patients. The observed trend that suggested an associated benefit in achieving limb volume reduction (magnified in obese patients) was greater with the AT device than with [compression stockings],” the researchers concluded.
Tactile Medical, which manufactures the device, was the study sponsor and provided funding for the study costs. The authors received no specific funding for their work.
The use of an adaptive pneumatic compression device showed comparable results to compression stockings in a two-arm, randomized multicenter pilot study of previously noncompliant patients with chronic venous disease.
In addition, patient satisfaction regarding ease of use was higher in for the device, compared with the stockings, according to Fedor Lurie, MD, PhD, of the Jobst Vascular Institute, Toledo, Ohio, and his colleague.
A total of 89 subjects with unilateral or bilateral chronic venous insufficiency were randomized and included in the final analysis of the study. The patients comprised 44% women, with a median age of nearly 63 years, the majority (53%) of whom had bilateral chronic venous insufficiency (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:699-706).
Significantly more patients found the ACTitouch device easy to apply (71%) versus the compression stockings (37.5%; P = .0001) and easy to remove (89% vs. 59%; P = .0001). However, compliance and average time of use were not significantly different between the two treatment groups.
In terms of limb volume reduction, the device group demonstrated a significant volume reduction (44%), compared with the standard compression garment use (17%) in obese patients (P = .019) but not in nonobese patients.
The device was easy to put on and remove and was considered comfortable, according to the researchers. “These are characteristics usually associated with good potential for long-term acceptance by patients. The observed trend that suggested an associated benefit in achieving limb volume reduction (magnified in obese patients) was greater with the AT device than with [compression stockings],” the researchers concluded.
Tactile Medical, which manufactures the device, was the study sponsor and provided funding for the study costs. The authors received no specific funding for their work.
The use of an adaptive pneumatic compression device showed comparable results to compression stockings in a two-arm, randomized multicenter pilot study of previously noncompliant patients with chronic venous disease.
In addition, patient satisfaction regarding ease of use was higher in for the device, compared with the stockings, according to Fedor Lurie, MD, PhD, of the Jobst Vascular Institute, Toledo, Ohio, and his colleague.
A total of 89 subjects with unilateral or bilateral chronic venous insufficiency were randomized and included in the final analysis of the study. The patients comprised 44% women, with a median age of nearly 63 years, the majority (53%) of whom had bilateral chronic venous insufficiency (J Vasc Surg Venous Lymphat Disord. 2017 Sep;5[5]:699-706).
Significantly more patients found the ACTitouch device easy to apply (71%) versus the compression stockings (37.5%; P = .0001) and easy to remove (89% vs. 59%; P = .0001). However, compliance and average time of use were not significantly different between the two treatment groups.
In terms of limb volume reduction, the device group demonstrated a significant volume reduction (44%), compared with the standard compression garment use (17%) in obese patients (P = .019) but not in nonobese patients.
The device was easy to put on and remove and was considered comfortable, according to the researchers. “These are characteristics usually associated with good potential for long-term acceptance by patients. The observed trend that suggested an associated benefit in achieving limb volume reduction (magnified in obese patients) was greater with the AT device than with [compression stockings],” the researchers concluded.
Tactile Medical, which manufactures the device, was the study sponsor and provided funding for the study costs. The authors received no specific funding for their work.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Vascular surgery trainees perceive weakness in venous education, case volumes
Venous training during vascular residency programs is perceived to be lacking in both case volume and didactic education, based on the results of a national survey of vascular trainees.
The majority of respondents (82%) believed that treating venous disease is part of a standard vascular practice, and 75% indicated a desire for increased venous training, according to article in press published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
In terms of case loads, the responders reported the following:
- 63% had performed fewer than 10 inferior vena cava stents.
- 64% had performed fewer than 10 vein stripping/ligation procedures.
- 50% had performed fewer than 10 iliac stents.
- 92% had performed fewer than 10 venous bypasses.
In contrast, 74% of responders reported having performed as many as 20 cases of endothermal ablation.
Currently, the Accreditation Council for Graduate Medical Education does not demand a minimum number of venous cases before graduation from a vascular training program, Dr. Hicks and her colleagues wrote.
Although integrated and traditional vascular surgery trainees showed no overall differences in reported venous procedure volumes (P less than or equal to .28), integrated students reported receiving significantly more didactic education than their traditionally trained peers (P less than or equal to .01).
Both integrated and traditional vascular surgery trainees recognized a need for a more comprehensive educational curriculum in venous disease in terms of both didactic education and case exposure, the authors reported.
“Our data suggest that expansion of the venous training curriculum with clear training standards is warranted and that trainees would welcome such a change,” wrote Dr. Hicks and her colleagues.
“Further study will be required to determine if the perceived deficits affect recent graduates’ experiences with venous disease in their developing practice and if increasing training in venous disease during vascular residency will increase the venous work performed by practicing vascular surgeons,” they concluded.
The authors reported that they had no conflicts of interest.
Venous training during vascular residency programs is perceived to be lacking in both case volume and didactic education, based on the results of a national survey of vascular trainees.
The majority of respondents (82%) believed that treating venous disease is part of a standard vascular practice, and 75% indicated a desire for increased venous training, according to article in press published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
In terms of case loads, the responders reported the following:
- 63% had performed fewer than 10 inferior vena cava stents.
- 64% had performed fewer than 10 vein stripping/ligation procedures.
- 50% had performed fewer than 10 iliac stents.
- 92% had performed fewer than 10 venous bypasses.
In contrast, 74% of responders reported having performed as many as 20 cases of endothermal ablation.
Currently, the Accreditation Council for Graduate Medical Education does not demand a minimum number of venous cases before graduation from a vascular training program, Dr. Hicks and her colleagues wrote.
Although integrated and traditional vascular surgery trainees showed no overall differences in reported venous procedure volumes (P less than or equal to .28), integrated students reported receiving significantly more didactic education than their traditionally trained peers (P less than or equal to .01).
Both integrated and traditional vascular surgery trainees recognized a need for a more comprehensive educational curriculum in venous disease in terms of both didactic education and case exposure, the authors reported.
“Our data suggest that expansion of the venous training curriculum with clear training standards is warranted and that trainees would welcome such a change,” wrote Dr. Hicks and her colleagues.
“Further study will be required to determine if the perceived deficits affect recent graduates’ experiences with venous disease in their developing practice and if increasing training in venous disease during vascular residency will increase the venous work performed by practicing vascular surgeons,” they concluded.
The authors reported that they had no conflicts of interest.
Venous training during vascular residency programs is perceived to be lacking in both case volume and didactic education, based on the results of a national survey of vascular trainees.
The majority of respondents (82%) believed that treating venous disease is part of a standard vascular practice, and 75% indicated a desire for increased venous training, according to article in press published online in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
In terms of case loads, the responders reported the following:
- 63% had performed fewer than 10 inferior vena cava stents.
- 64% had performed fewer than 10 vein stripping/ligation procedures.
- 50% had performed fewer than 10 iliac stents.
- 92% had performed fewer than 10 venous bypasses.
In contrast, 74% of responders reported having performed as many as 20 cases of endothermal ablation.
Currently, the Accreditation Council for Graduate Medical Education does not demand a minimum number of venous cases before graduation from a vascular training program, Dr. Hicks and her colleagues wrote.
Although integrated and traditional vascular surgery trainees showed no overall differences in reported venous procedure volumes (P less than or equal to .28), integrated students reported receiving significantly more didactic education than their traditionally trained peers (P less than or equal to .01).
Both integrated and traditional vascular surgery trainees recognized a need for a more comprehensive educational curriculum in venous disease in terms of both didactic education and case exposure, the authors reported.
“Our data suggest that expansion of the venous training curriculum with clear training standards is warranted and that trainees would welcome such a change,” wrote Dr. Hicks and her colleagues.
“Further study will be required to determine if the perceived deficits affect recent graduates’ experiences with venous disease in their developing practice and if increasing training in venous disease during vascular residency will increase the venous work performed by practicing vascular surgeons,” they concluded.
The authors reported that they had no conflicts of interest.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point:
Major finding: Of the of vascular trainees who responded to the survey, 75% reported a desire for increased venous training.
Data source: Nationwide U.S. survey of vascular trainees resulting in a 104/464 (22%) response rate.
Disclosures: The authors reported having no conflicts of interest.
FDA warns of endoleaks associated with endovascular grafts for AAA
The Food and Drug Administration has reported an apparent increase in device-related adverse events from the use of endovascular graft repair (EVAR) to treat abdominal aortic aneurysms (AAA).
In a Letter to Health Care Providers issued on Sept. 28, the FDA indicated that “recent information from several sources, including FDA’s Medical Device Reporting system and Annual Clinical Updates to Physicians by the manufacturers, suggests an increase in the occurrence of Type III endoleaks.”
A Type III endoleak is defined by the failure to completely exclude the AAA from blood flow, thereby allowing a systematic arterial pressurization of the aneurysm sac, increasing the risk of rupture, which is a life-threatening event.
The FDA stated that predictors of Type III endoleaks included treatment with early-generation graft materials, the presence of calcified plaque, and inadequate overlap between graft components.
Secondary interventions to treat Type III endoleaks carry their own risk of adverse events.
It is recommended that health care providers should do the following:
- Consider lifelong surveillance of patients who have been treated with EVAR.
- Consider type III endoleaks in the differential diagnosis of patients who present with symptoms of potential aneurysm expansion or rupture.
- Discuss all treatment options in depth with patients before deciding on the best treatment for Type III endoleaks.
- Report any early or late device-related adverse events, including Type IIIa and Type IIIb endoleaks, associated with EVAR, as well as any device-related adverse events that occur as the result of a secondary intervention to treat Type III endoleaks.
These events should be reported to MedWatch, using the FDA’s Safety Information and Adverse Event Reporting Program Online Voluntary Reporting Form. A form also can be requested by calling 800-332-1088.
The FDA stated that it “continues to work with all manufacturers of endovascular graft systems to better understand this issue,” and that the agency would keep the public informed when significant new information becomes available.
The Food and Drug Administration has reported an apparent increase in device-related adverse events from the use of endovascular graft repair (EVAR) to treat abdominal aortic aneurysms (AAA).
In a Letter to Health Care Providers issued on Sept. 28, the FDA indicated that “recent information from several sources, including FDA’s Medical Device Reporting system and Annual Clinical Updates to Physicians by the manufacturers, suggests an increase in the occurrence of Type III endoleaks.”
A Type III endoleak is defined by the failure to completely exclude the AAA from blood flow, thereby allowing a systematic arterial pressurization of the aneurysm sac, increasing the risk of rupture, which is a life-threatening event.
The FDA stated that predictors of Type III endoleaks included treatment with early-generation graft materials, the presence of calcified plaque, and inadequate overlap between graft components.
Secondary interventions to treat Type III endoleaks carry their own risk of adverse events.
It is recommended that health care providers should do the following:
- Consider lifelong surveillance of patients who have been treated with EVAR.
- Consider type III endoleaks in the differential diagnosis of patients who present with symptoms of potential aneurysm expansion or rupture.
- Discuss all treatment options in depth with patients before deciding on the best treatment for Type III endoleaks.
- Report any early or late device-related adverse events, including Type IIIa and Type IIIb endoleaks, associated with EVAR, as well as any device-related adverse events that occur as the result of a secondary intervention to treat Type III endoleaks.
These events should be reported to MedWatch, using the FDA’s Safety Information and Adverse Event Reporting Program Online Voluntary Reporting Form. A form also can be requested by calling 800-332-1088.
The FDA stated that it “continues to work with all manufacturers of endovascular graft systems to better understand this issue,” and that the agency would keep the public informed when significant new information becomes available.
The Food and Drug Administration has reported an apparent increase in device-related adverse events from the use of endovascular graft repair (EVAR) to treat abdominal aortic aneurysms (AAA).
In a Letter to Health Care Providers issued on Sept. 28, the FDA indicated that “recent information from several sources, including FDA’s Medical Device Reporting system and Annual Clinical Updates to Physicians by the manufacturers, suggests an increase in the occurrence of Type III endoleaks.”
A Type III endoleak is defined by the failure to completely exclude the AAA from blood flow, thereby allowing a systematic arterial pressurization of the aneurysm sac, increasing the risk of rupture, which is a life-threatening event.
The FDA stated that predictors of Type III endoleaks included treatment with early-generation graft materials, the presence of calcified plaque, and inadequate overlap between graft components.
Secondary interventions to treat Type III endoleaks carry their own risk of adverse events.
It is recommended that health care providers should do the following:
- Consider lifelong surveillance of patients who have been treated with EVAR.
- Consider type III endoleaks in the differential diagnosis of patients who present with symptoms of potential aneurysm expansion or rupture.
- Discuss all treatment options in depth with patients before deciding on the best treatment for Type III endoleaks.
- Report any early or late device-related adverse events, including Type IIIa and Type IIIb endoleaks, associated with EVAR, as well as any device-related adverse events that occur as the result of a secondary intervention to treat Type III endoleaks.
These events should be reported to MedWatch, using the FDA’s Safety Information and Adverse Event Reporting Program Online Voluntary Reporting Form. A form also can be requested by calling 800-332-1088.
The FDA stated that it “continues to work with all manufacturers of endovascular graft systems to better understand this issue,” and that the agency would keep the public informed when significant new information becomes available.
Key clinical point:
Major finding: FDA monitoring sources have detected an apparent increase in the occurrence of Type III endoleaks following EVAR.
Data source: FDA’s Medical Device Reporting System and the Annual Clinical Updates to Physicians by the manufacturers.
Disclosures: None.
DETOUR system shows early promise for long SFA lesions
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
FROM VIVA 17
Key clinical point:
Major finding: Primary patency was 89% at 6 months, with low MAE at 30 days.
Data source: Subset analysis of 50 patients with long lesions in the multicenter, prospective, single-arm DETOUR 1 trial.
Disclosures: The DETOUR trial was sponsored by PQ Bypass. Dr. Lyden reported receiving fees from Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
IN.PACT Global: Promising 2-year data for drug-coated balloon performance for femoropopliteal PAD
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
FROM VIVA 17
Key clinical point:
Major finding: The freedom from clinically driven target lesion revascularization (CD-TLR) rate at 2 years was 83%.
Data source: The prospective, multicenter, single-arm, IN.PACT Global Clinical Study.
Disclosures: The IN.PACT Global Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.