User login
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
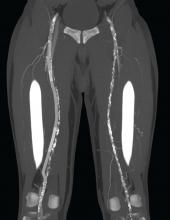
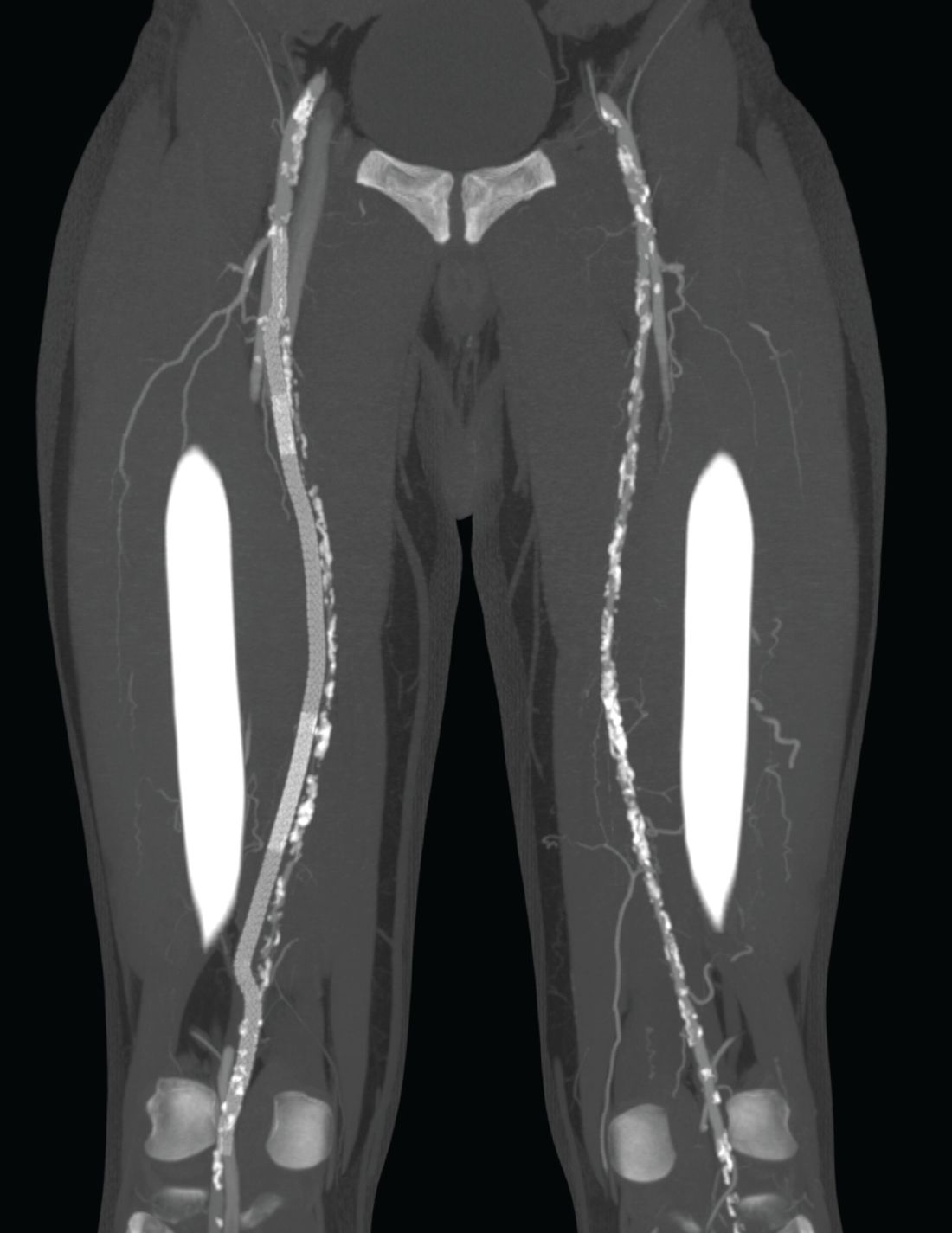
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
FROM VIVA 17
Key clinical point:
Major finding: Primary patency was 89% at 6 months, with low MAE at 30 days.
Data source: Subset analysis of 50 patients with long lesions in the multicenter, prospective, single-arm DETOUR 1 trial.
Disclosures: The DETOUR trial was sponsored by PQ Bypass. Dr. Lyden reported receiving fees from Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.