User login
DETOUR system shows early promise for long SFA lesions
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
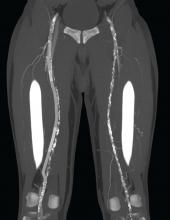
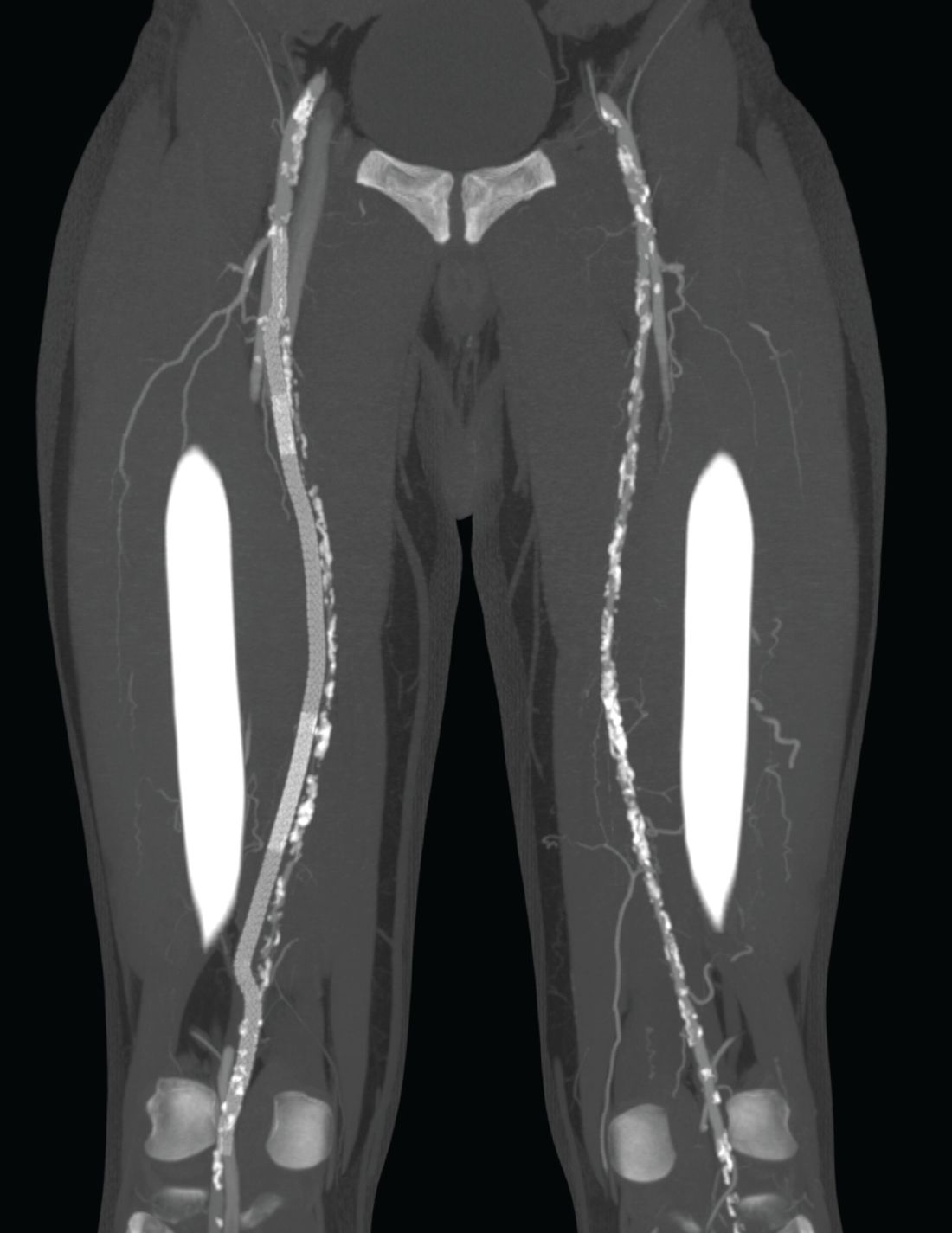
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
A new, percutaneous bypass system appeared safe and effective for treating long-segment blockages in the femoropopliteal artery, a subset of the DETOUR I trial showed. Data at 30 days showed low levels of major adverse events and the results showed that there was promising graft patency at 6 months.
Sean Lyden, MD, chairman of the department of vascular surgery at the Cleveland Clinic, presented the results in a late-breaking clinical trial session at the 2017 Vascular Interventional Advances meeting. The study evaluated the safety and effectiveness of the DETOUR System for treating long-segment (greater than 25 cm) blockages in the femoropopliteal artery.
To address this problem, the PQ Bypass DETOUR System was developed as a fully percutaneous bypass approach designed to achieve comparable end results as open bypass surgery, by using the femoral vein as a pathway for created a modular stent graft bypass.
“The DETOUR procedure creates a pathway around a lesion by placing stent grafts that cross from the superficial femoral artery (SFA) into the femoral vein and back into the artery. The new path through the stent grafts redirects oxygen-rich blood around the blockage and restores blood flow to the lower leg and foot of the patient,” according to a company press release.
The DETOUR 1 trial was a prospective, single-arm study of 77 patients (81 limbs) treated at eight global sites. Dr. Lyden reported on a subset analysis of 50 patients with long lesions (greater than 25 cm). The mean age of the patients was 65 years; 84% were men.
Comorbidities included diabetes (30%), history of renal insufficiency (26%), smoking (90%), and previous peripheral intervention (30%). There were 53 lesions treated in all, with a mean length of 33.5 cm. The percentage of total occlusions was 96% and the percentage of lesions with zero, one, two, or three runoff vessels was 0%, 4%, 26%, and 70%, respectively, according to Dr. Lyden.
The primary safety endpoint of 2% major adverse events defined as death, target vessel revascularization or amputation at 30 days was met, with no deaths or amputations and only one target vessel revascularization.
The primary patency was 89% at 6 months with optimal placement, with an overall primary patency of 77%.
Both the delivery and removal of the device was successful in all the lesions treated.
The Rutherford Class improved at least 2 grades in 92% of the patients, and there was a statistically significant improvement in ankle brachial index from 0.64 to 0.92 (P less than .0001).
“Percutaneous bypass using the femoral vein as a pathway may end up being an important step forward in the treatment of long-segment SFA disease,” Dr. Lyden concluded.
In March 2017, the DETOUR System received CE (Conformité Européenne) Mark approval, but the system is not yet approved by the Food and Drug Administration for sale in the United States.
The DETOUR trial was sponsored by PQ Bypass Inc. Dr. Lyden reported receiving fees Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
FROM VIVA 17
Key clinical point:
Major finding: Primary patency was 89% at 6 months, with low MAE at 30 days.
Data source: Subset analysis of 50 patients with long lesions in the multicenter, prospective, single-arm DETOUR 1 trial.
Disclosures: The DETOUR trial was sponsored by PQ Bypass. Dr. Lyden reported receiving fees from Spectranetics Corp and VIVA Physicians. He has no financial conflicts with regard to PQ Bypass.
IN.PACT Global: Promising 2-year data for drug-coated balloon performance for femoropopliteal PAD
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
The 2-year results of the largest real-world study of the use of drug-coated balloons (DCB) in patients with symptomatic femoropopliteal peripheral arterial disease showed a durable treatment effect with a 16.9% rate of reintervention, according to a presentation by Thomas Zeller, MD.
Dr. Zeller, who is director of the department of angiology at University Heart Center, Freiburg-Bad Krozingen, Germany, presented the 2-year results from the full clinical cohort of the IN.PACT Admiral Global Clinical Study as a late-breaking clinical trial presentation at the 2017 Vascular Interventional Advances meeting in Las Vegas.
The IN.PACT Global study is a real-world, prospective, multicenter, single-arm, independently adjudicated femoropopliteal study of the safety and effectiveness of a 150-mm drug-coated balloon treatment. The primary efficacy endpoint was freedom from clinically driven target lesion revascularization (CD-TLR) within 12 months. The primary safety endpoint was freedom from device- and procedure-related death through 30 days and freedom from target limb major amputation and CD-TLR within 12 months.
The study examined patients with symptomatic femoropopliteal disease at 64 non-U.S. sites. Mean lesion length in these patients was 12.1 cm: 18.0% were in-stent restenosis lesions, 35.5% were occluded lesions. In terms of comorbidities, 40% of the patients had diabetes, 40% had coronary heart disease, and 32% were current smokers. More than half (52%) of the patients had a previous peripheral revascularization. The mean patient age was 69 years and 68% were men.
Kaplan-Meier survival analysis showed a freedom from CD-TLR rate of 83% in the patient cohort through 2-year outcomes. The researchers’ safety and effectiveness outcomes included thrombosis rate (4.5%), occurrences of major target limb amputation (0.7%), and CD-TLR (16.9%) within 2 years, according to Dr. Zeller.
“These data continue to confirm the safety and strong performance of the IN.PACT DCB in this cohort of real-world patients,” he concluded.
The IN.PACT Admiral DCB was approved by the Food and Drug Administration in 2014 to treat superficial femoral and popliteal arteries and it received an expanded indication for treating in-stent restenosis in 2016.
The IN.PACT Global Clinical Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.
FROM VIVA 17
Key clinical point:
Major finding: The freedom from clinically driven target lesion revascularization (CD-TLR) rate at 2 years was 83%.
Data source: The prospective, multicenter, single-arm, IN.PACT Global Clinical Study.
Disclosures: The IN.PACT Global Study is sponsored by Medtronic Endovascular. Dr. Zeller reported receiving honoraria and research funding from and acting as a consultant to a number of device companies, including Medtronic.