User login
EVAR registry results show equivalence between the sexes
A global registry showed that men and women had equivalent outcomes after endovascular abdominal aortic aneurysm repair (EVAR) despite significant differences in baseline characteristics, anatomy, and off-instruction stent use.
The 5-year ENGAGE (Endurant Stent Graft Natural Selection Global Postmarket Registry) data were presented by Marc Schermerhorn, MD, chief, division of vascular and endovascular surgery, Beth Israel Deaconess Medical Center, Boston, in a late-breaking clinical trial at the Vascular Interventional Advances (VIVA) 2017 conference in Las Vegas.
The registry comprises data from 1,263 patients (133 women and 1,130 men) in 30 countries who received the Endurant II stent graft during for endovascular repair of the abdominal aortic aneurysm. ENGAGE participants are consecutively enrolled, and have 30-day and subsequent yearly evaluations, with independent data monitoring and event adjudication.
Despite many other equivalent demographic and clinical characteristics, including smoking status, hypertension, diabetes, and pulmonary disease, overall women were statistically significantly older, had less cardiac disease, had shorter and more angulated aneurysm necks, and had smaller iliac arteries. In addition they were more likely to be treated outside of the Endurant II instructions for use (IFU), according to Dr. Schemerhorn.
Women also had significantly longer hospital stays than men (7.9 days vs. 6.4 days), respectively, but had equivalent procedure duration, ICU times, and similarly successful stent delivery and deployment (99%).
But despite these differences in baseline characteristics and anatomy, the 5-year data showed equivalent outcomes between women and men at 30 days, and 1 year, and through 5 years. There were no significant differences across these periods in freedom from all-cause mortality (67.5% in men, 65.6% in women, P = 0.87), 5-year freedom from aneurysm-related mortality (97.5% men, 100% women, P = .09), 5-year freedom from rupture (98.4% men, 100% women, P = .23), and 5-year freedom from conversion (97.8% men, 99.2% women, P = .48). There were also no significant differences in type 1 endoleaks, 5-year freedom from conversion, and sac diameter changes, Dr. Schermerhorn concluded.
The ENGAGE registry is sponsored by Medtronic. Dr. Schermerhorn disclosed that he was a consultant for several medical device companies and a shareholder and owner of a health care company, but that he did not have any potential conflicts of interest for this particular study.
[email protected]
A global registry showed that men and women had equivalent outcomes after endovascular abdominal aortic aneurysm repair (EVAR) despite significant differences in baseline characteristics, anatomy, and off-instruction stent use.
The 5-year ENGAGE (Endurant Stent Graft Natural Selection Global Postmarket Registry) data were presented by Marc Schermerhorn, MD, chief, division of vascular and endovascular surgery, Beth Israel Deaconess Medical Center, Boston, in a late-breaking clinical trial at the Vascular Interventional Advances (VIVA) 2017 conference in Las Vegas.
The registry comprises data from 1,263 patients (133 women and 1,130 men) in 30 countries who received the Endurant II stent graft during for endovascular repair of the abdominal aortic aneurysm. ENGAGE participants are consecutively enrolled, and have 30-day and subsequent yearly evaluations, with independent data monitoring and event adjudication.
Despite many other equivalent demographic and clinical characteristics, including smoking status, hypertension, diabetes, and pulmonary disease, overall women were statistically significantly older, had less cardiac disease, had shorter and more angulated aneurysm necks, and had smaller iliac arteries. In addition they were more likely to be treated outside of the Endurant II instructions for use (IFU), according to Dr. Schemerhorn.
Women also had significantly longer hospital stays than men (7.9 days vs. 6.4 days), respectively, but had equivalent procedure duration, ICU times, and similarly successful stent delivery and deployment (99%).
But despite these differences in baseline characteristics and anatomy, the 5-year data showed equivalent outcomes between women and men at 30 days, and 1 year, and through 5 years. There were no significant differences across these periods in freedom from all-cause mortality (67.5% in men, 65.6% in women, P = 0.87), 5-year freedom from aneurysm-related mortality (97.5% men, 100% women, P = .09), 5-year freedom from rupture (98.4% men, 100% women, P = .23), and 5-year freedom from conversion (97.8% men, 99.2% women, P = .48). There were also no significant differences in type 1 endoleaks, 5-year freedom from conversion, and sac diameter changes, Dr. Schermerhorn concluded.
The ENGAGE registry is sponsored by Medtronic. Dr. Schermerhorn disclosed that he was a consultant for several medical device companies and a shareholder and owner of a health care company, but that he did not have any potential conflicts of interest for this particular study.
[email protected]
A global registry showed that men and women had equivalent outcomes after endovascular abdominal aortic aneurysm repair (EVAR) despite significant differences in baseline characteristics, anatomy, and off-instruction stent use.
The 5-year ENGAGE (Endurant Stent Graft Natural Selection Global Postmarket Registry) data were presented by Marc Schermerhorn, MD, chief, division of vascular and endovascular surgery, Beth Israel Deaconess Medical Center, Boston, in a late-breaking clinical trial at the Vascular Interventional Advances (VIVA) 2017 conference in Las Vegas.
The registry comprises data from 1,263 patients (133 women and 1,130 men) in 30 countries who received the Endurant II stent graft during for endovascular repair of the abdominal aortic aneurysm. ENGAGE participants are consecutively enrolled, and have 30-day and subsequent yearly evaluations, with independent data monitoring and event adjudication.
Despite many other equivalent demographic and clinical characteristics, including smoking status, hypertension, diabetes, and pulmonary disease, overall women were statistically significantly older, had less cardiac disease, had shorter and more angulated aneurysm necks, and had smaller iliac arteries. In addition they were more likely to be treated outside of the Endurant II instructions for use (IFU), according to Dr. Schemerhorn.
Women also had significantly longer hospital stays than men (7.9 days vs. 6.4 days), respectively, but had equivalent procedure duration, ICU times, and similarly successful stent delivery and deployment (99%).
But despite these differences in baseline characteristics and anatomy, the 5-year data showed equivalent outcomes between women and men at 30 days, and 1 year, and through 5 years. There were no significant differences across these periods in freedom from all-cause mortality (67.5% in men, 65.6% in women, P = 0.87), 5-year freedom from aneurysm-related mortality (97.5% men, 100% women, P = .09), 5-year freedom from rupture (98.4% men, 100% women, P = .23), and 5-year freedom from conversion (97.8% men, 99.2% women, P = .48). There were also no significant differences in type 1 endoleaks, 5-year freedom from conversion, and sac diameter changes, Dr. Schermerhorn concluded.
The ENGAGE registry is sponsored by Medtronic. Dr. Schermerhorn disclosed that he was a consultant for several medical device companies and a shareholder and owner of a health care company, but that he did not have any potential conflicts of interest for this particular study.
[email protected]
FROM VIVA 17
Key clinical point:
Major finding: The 5-year data showed equivalent outcomes between women and men at 30 days, and 1 year, and through 5 years.
Data source: ENGAGE global registry data from 1,263 patients (133 women and 1,130 men).
Disclosures: The ENGAGE registry is sponsored by Medtronic. Dr. Schermerhorn reported that he had no potential conflicts of interest for this study.
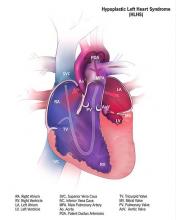
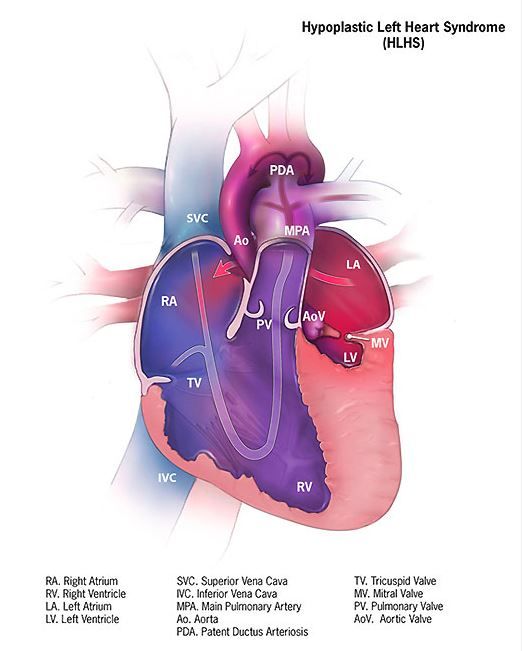
Cerebral NIRS may be flawed for assessing infant brains after stage 1 palliation of HLHS
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The use of postoperative cerebral venous oxygen saturation monitoring (ScvO2) through an internal jugular vein catheter allows better monitoring of circulation, which may lead to better outcomes, but it is invasive and challenging. NIRS, being noninvasive, has proved attractive, but clinical interpretation in terms of both absolute values and trends is difficult, Edward Buratto, MBBS, and his colleagues noted in their invited commentary (doi: 10.1016/j.jtcvs.2017.04.061).
Dr. Rescoe and her colleagues have analyzed the correlation of NIRS-derived data with ScvO2 measured by co-oximetry from the internal jugular vein in 73 neonates after stage 1 palliation for hypoplastic left heart syndrome. They demonstrated that cerebral rSO2 correlated poorly with low ScvO2, and they suggest that cerebral rSO2 not be used in isolation. This problem was somewhat ameliorated by correction of the signal for arterial contamination. NIRS appears to be too valuable a tool to be simply discarded, they said, suggesting that a perioperative risk assessment that would include multisite NIRS and hemodynamic monitoring might still allow early determination of low-cardiac output.
“Two numbers are better than one,” wrote Dr. Buratto and his colleagues. “Whether the NIRS technology will add any useful information to a simple bedside assessment by an astute clinician is yet to be seen.”
Edward Buratto, MBBS, Steve Horton, PhD, and Igor E. Konstantinov, MD, are from the Department of Cardiothoracic Surgery, The Royal Children’s Hospital; the Department of Pediatrics, University of Melbourne; and Murdoch Children’s Research Institute, Melbourne. They reported having no financial conflicts of interest.
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
The regional oxygenation index (rSO2) based on near infrared spectroscopy (NIRS) measurement is frequently used to assess the adequacy of oxygen delivery after stage 1 palliation of hypoplastic left heart syndrome (HLHS). However, a recent study showed that cerebral rSO2 has low sensitivity and should not be considered reassuring even at rSO2 of 50 or greater. In addition, values below 30 were not found to be sensitive for detecting compromised oxygen delivery, according to a report published online in the Journal of Thoracic and Cardiovascular Surgery.
Erin Rescoe, MD, of Boston Children’s Hospital, and her colleagues at Harvard Medical School, Boston, performed a retrospective study of 73 neonates assessed with cerebral venous oxyhemoglobin saturation (ScvO2) measured by co-oximetry from the internal jugular vein, which is considered the preferred method for assessing the adequacy of tissue oxygen delivery, compared with cerebral rSO2 after stage 1 palliation of HLHS (doi: 10.1016/j.jtcvs.2017.03.154).
To determine the suggested benefit of NIRS as an effective trend monitor, the researchers used their interpolated data to examine changes in rSO2 and changes in ScvO2 at hourly intervals and compared these values.Of particular concern is the result showing that, in all instances where ScvO2 was less than 30%, rSO2 was greater than 30%. In terms of the sensitivity (the true positive rate) and specificity (the true negative rate) of using NIRS, time-matched pairs of rSO2 and ScvO2 showed that the receiver operating characteristic curves for rSO2 as a diagnostic test to detect ScvO2 less than 30%, less than 40%, and less than 50% were 0.82, 0.84, and 0.87, respectively, showing good specificity, with a value of rSO2 less than 30% indicating that ScvO2 will be less than 30% 99% of the time.
“However, the sensitivity of rSO2 in the range of clinical interest in detecting ScvO2 less than 30% is extremely low,” according to the researchers. Thus, NIRS is likely to produce false negatives, missing patients with clinically low postoperative oxygen saturation.
In fact, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%. Similar results were seen in comparing values at the less than 40% mark (equivalent less than 1% of the time). Better results showed at the less than 50% mark, with equivalence seen 46% of the time.
NIRS measures a composite of arterial and venous blood, according to Dr. Rescoe and her colleagues. Therefore, to do a more direct comparison, they adjusted their NIRS results by calculating an rSO2-based ScvO2 designed to remove arterial contamination from the rSO2 signal: rSO2-based ScvO2 = (rSO2 arterial oxygen saturation x 0.3)/0.7.
This significantly improved the sensitivity of rSO2 to detect ScvO2 at less than 30% to 6.5%, to 29% for rSO2 at less than 40%, and 77.4% for rSO2 less than 50%.
The researchers “were surprised by the extremely low sensitivity of cerebral NIRS to detect even the most severe aberrations in DO2” (i.e., ScvO2 less than 30%, which has been found to be associated with poor outcomes).
“Cerebral rSO2 in isolation should not be used to detect low ScvO2, because its sensitivity is low, although correction of rSO2 for arterial contamination significantly improves sensitivity. Cerebral rSO2 of 50 or greater should not be considered reassuring with regard to ScvO2, although values less than 30 are specific for low ScvO2,” the researchers concluded.
The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:
Major finding: In terms of sensitivity, rSO2 was less than 30% less than 1% of the time that ScvO2 was less than 30%.
Data source: A retrospective single institution study of 73 neonates assessed after stage 1 palliation
Disclosures: The study was sponsored by the Gerber Foundation, the Hess Family Philanthropic Fund, and Boston Children’s Hospital Heart Center Strategic Investment Fund. The authors disclosed that they had no financial conflicts.
Sinus of Valsalva preserved in aortic valve replacement
The sinus of Valsalva segment can be preserved during aortic valve replacement irrespective of the type of valve pathology, according to a recent study by Rita Karianna Milewski, MD, and her colleagues at the Hospital of the University of Pennsylvania, Philadelphia.
Severe aortic root dilation coupled to aortic valve disease requires root replacement in patients with a tricuspid or bicuspid aortic valve. Commonly, an aortic valve replacement and supracoronary ascending aorta replacement (AVRSCAAR) procedure has been used for patients who have a mild to moderately dilated sinus segment. One advantage of the procedure is that it retains the sinus of Valsalva (SOV) and preserves the intact coronary ostia.
However, the long-term behavior and risk of aortic events for the retained SOV in both BAV and TAV patients remains unclear, according to Dr. Milewski and her colleagues.
Previous researchers have suggested that patients with BAV and TAV have different rates of complications of the remaining aorta and dilation of the proximal aorta and retained sinus segment. In addition, it has been suggested that the cause of aortic dilation is different in patients with aortic stenosis (AS) and aortic insufficiency (AI) and is based on TAV and BAV morphology, histology, and hemodynamic flow patterns.
However, in the August issue of the Journal of Thoracic and Cardiovascular Surgery, Dr. Milewski and her colleagues reported on their study showing that, in patients with nonaneurysmal SOV undergoing AVRSCAAR, the sinus of Valsalva segment can be preserved regardless of the type of valvular pathology (aortic stenosis vs. aortic insufficiency) or valvular morphology (BAV vs. TAV).
The researchers retrospectively reviewed a prospectively maintained institutional database to stratify all patients by BAV or TAV valvular pathology with concomitant ascending aortic aneurysm who underwent an elective AVRSCAAR from 2002 to 2015 (J Thorac Cardiovasc Surg. 2017;154:421-32).
The distribution of the 428 patients meeting inclusion criteria by subgroups was: BAV group (254 patients: BAV-AS = 178; BAV-AI = 76); TAV group (174 patients: TAV-AS = 61; TAV-AI =113). Preoperative sinus of Valsalva dimensions were divided into 3 subgroups (less than 40 mm, 40-45 mm, and greater than 45 mm).
The mean patient age for patients with BAV and TAV was 59 years and 72 years (P less than .001), respectively (with 78% with BAV being men and 57% with TAV being men). There was a significantly higher subpopulation of AS in the BAV cohort vs. TAV-AS (70% vs. 35%; P less than .001).
With regard to SOV sizing, there was no significant difference in mean preoperative aortic root diameters between BAV and TAV cohorts for the AS or AI subpopulations.
In-hospital/30-day mortality was significantly higher in patients with TAV (5.2%) than in patients with BAV (1.6%, P = .033). In addition, the incidence of transient ischemic attack/stroke was significantly higher in the TAV group (3.4%) vs. the BAV group (0.8%, P = .04).
Valvular morphology and pathology at baseline, preoperative SOV diameter, postoperative time course, and interaction effect of preoperative SOV diameters and postoperative time course were used as covariates to assess outcomes. Within-subject and within–stratified subgroup comparison failed to show main effects across the follow-up times on postoperative SOV size patterns (P = .935), implying that the SOV trends were stable and sustained (discharge to greater than or equal to 10 years) irrespective of valvular morphology and pathology (BAV-AI, BAV-AS, TAV-AI, and TAV-AS).
Preoperative SOV dimensions significantly affected the retained postoperative sinus dimensions (P less than .001), according to Dr. Milewski and her colleagues.
The data indicated that an initial and pronounced postoperative decrease in SOV dimensions occurs with AVRSCAAR independently of aortic valve morphology, aortic valve pathology, and age, they added.
The 10-year freedom from aortic reoperation rates were 97% and 95% in the BAV and TAV subgroups, respectively. The BAV group had significantly improved reoperation-free survival, compared with the TAV group (P less than .001), while the type of valvular pathology within each group did not show a significant survival difference.
“Irrespective of the aortic valve morphology or valve pathology, in patients with mild to moderate aortic root dilatation (less than 45 mm), preservation of the SOV segment in the context of an AVRSCAAR procedure is justified. Continued further follow-up will be important to understand the long-term outcomes of sinus preservation, especially in the younger population with BAVs,” the researchers concluded.
The authors reported having no financial conflicts to disclose.
With regard to the question, ‘‘Is it necessary to replace the sinuses of Valsalva in the setting of bicuspid aortic valve aortopathy?’’, the researchers “leverage their enormous institutional experience to find an answer. The results suggest that this answer is ‘no.’ At least not in all cases,” Thoralf M. Sundt, MD, wrote in his invited commentary on the paper (J Thorac Cardiovasc Surg. 2017;154:419-20).
“The findings of this study argue for us to take a step back and ask how much really needs be done,” he added. And although “it is hard to ask a surgeon to do less rather than more; however, the balance of judgment has to be between the operative risk of the more aggressive approach and the natural history of the disease. In other words, what does it ‘cost’ to be aggressive, and what do we gain?” he asked.
Bicuspid aortic valve aortopathy, it would appear, is not cancer after all. Regardless of theoretic arguments that are based on embryology and the migration of neural crest cells, it does not appear to require resection to ‘clean margins,’ even if we believe that the operative risk ‘in our hands’ is low,” concluded Dr. Sundt.
Thoralf M. Sundt, MD, is at Harvard Medical School, Boston. He reported having no disclosures.
With regard to the question, ‘‘Is it necessary to replace the sinuses of Valsalva in the setting of bicuspid aortic valve aortopathy?’’, the researchers “leverage their enormous institutional experience to find an answer. The results suggest that this answer is ‘no.’ At least not in all cases,” Thoralf M. Sundt, MD, wrote in his invited commentary on the paper (J Thorac Cardiovasc Surg. 2017;154:419-20).
“The findings of this study argue for us to take a step back and ask how much really needs be done,” he added. And although “it is hard to ask a surgeon to do less rather than more; however, the balance of judgment has to be between the operative risk of the more aggressive approach and the natural history of the disease. In other words, what does it ‘cost’ to be aggressive, and what do we gain?” he asked.
Bicuspid aortic valve aortopathy, it would appear, is not cancer after all. Regardless of theoretic arguments that are based on embryology and the migration of neural crest cells, it does not appear to require resection to ‘clean margins,’ even if we believe that the operative risk ‘in our hands’ is low,” concluded Dr. Sundt.
Thoralf M. Sundt, MD, is at Harvard Medical School, Boston. He reported having no disclosures.
With regard to the question, ‘‘Is it necessary to replace the sinuses of Valsalva in the setting of bicuspid aortic valve aortopathy?’’, the researchers “leverage their enormous institutional experience to find an answer. The results suggest that this answer is ‘no.’ At least not in all cases,” Thoralf M. Sundt, MD, wrote in his invited commentary on the paper (J Thorac Cardiovasc Surg. 2017;154:419-20).
“The findings of this study argue for us to take a step back and ask how much really needs be done,” he added. And although “it is hard to ask a surgeon to do less rather than more; however, the balance of judgment has to be between the operative risk of the more aggressive approach and the natural history of the disease. In other words, what does it ‘cost’ to be aggressive, and what do we gain?” he asked.
Bicuspid aortic valve aortopathy, it would appear, is not cancer after all. Regardless of theoretic arguments that are based on embryology and the migration of neural crest cells, it does not appear to require resection to ‘clean margins,’ even if we believe that the operative risk ‘in our hands’ is low,” concluded Dr. Sundt.
Thoralf M. Sundt, MD, is at Harvard Medical School, Boston. He reported having no disclosures.
The sinus of Valsalva segment can be preserved during aortic valve replacement irrespective of the type of valve pathology, according to a recent study by Rita Karianna Milewski, MD, and her colleagues at the Hospital of the University of Pennsylvania, Philadelphia.
Severe aortic root dilation coupled to aortic valve disease requires root replacement in patients with a tricuspid or bicuspid aortic valve. Commonly, an aortic valve replacement and supracoronary ascending aorta replacement (AVRSCAAR) procedure has been used for patients who have a mild to moderately dilated sinus segment. One advantage of the procedure is that it retains the sinus of Valsalva (SOV) and preserves the intact coronary ostia.
However, the long-term behavior and risk of aortic events for the retained SOV in both BAV and TAV patients remains unclear, according to Dr. Milewski and her colleagues.
Previous researchers have suggested that patients with BAV and TAV have different rates of complications of the remaining aorta and dilation of the proximal aorta and retained sinus segment. In addition, it has been suggested that the cause of aortic dilation is different in patients with aortic stenosis (AS) and aortic insufficiency (AI) and is based on TAV and BAV morphology, histology, and hemodynamic flow patterns.
However, in the August issue of the Journal of Thoracic and Cardiovascular Surgery, Dr. Milewski and her colleagues reported on their study showing that, in patients with nonaneurysmal SOV undergoing AVRSCAAR, the sinus of Valsalva segment can be preserved regardless of the type of valvular pathology (aortic stenosis vs. aortic insufficiency) or valvular morphology (BAV vs. TAV).
The researchers retrospectively reviewed a prospectively maintained institutional database to stratify all patients by BAV or TAV valvular pathology with concomitant ascending aortic aneurysm who underwent an elective AVRSCAAR from 2002 to 2015 (J Thorac Cardiovasc Surg. 2017;154:421-32).
The distribution of the 428 patients meeting inclusion criteria by subgroups was: BAV group (254 patients: BAV-AS = 178; BAV-AI = 76); TAV group (174 patients: TAV-AS = 61; TAV-AI =113). Preoperative sinus of Valsalva dimensions were divided into 3 subgroups (less than 40 mm, 40-45 mm, and greater than 45 mm).
The mean patient age for patients with BAV and TAV was 59 years and 72 years (P less than .001), respectively (with 78% with BAV being men and 57% with TAV being men). There was a significantly higher subpopulation of AS in the BAV cohort vs. TAV-AS (70% vs. 35%; P less than .001).
With regard to SOV sizing, there was no significant difference in mean preoperative aortic root diameters between BAV and TAV cohorts for the AS or AI subpopulations.
In-hospital/30-day mortality was significantly higher in patients with TAV (5.2%) than in patients with BAV (1.6%, P = .033). In addition, the incidence of transient ischemic attack/stroke was significantly higher in the TAV group (3.4%) vs. the BAV group (0.8%, P = .04).
Valvular morphology and pathology at baseline, preoperative SOV diameter, postoperative time course, and interaction effect of preoperative SOV diameters and postoperative time course were used as covariates to assess outcomes. Within-subject and within–stratified subgroup comparison failed to show main effects across the follow-up times on postoperative SOV size patterns (P = .935), implying that the SOV trends were stable and sustained (discharge to greater than or equal to 10 years) irrespective of valvular morphology and pathology (BAV-AI, BAV-AS, TAV-AI, and TAV-AS).
Preoperative SOV dimensions significantly affected the retained postoperative sinus dimensions (P less than .001), according to Dr. Milewski and her colleagues.
The data indicated that an initial and pronounced postoperative decrease in SOV dimensions occurs with AVRSCAAR independently of aortic valve morphology, aortic valve pathology, and age, they added.
The 10-year freedom from aortic reoperation rates were 97% and 95% in the BAV and TAV subgroups, respectively. The BAV group had significantly improved reoperation-free survival, compared with the TAV group (P less than .001), while the type of valvular pathology within each group did not show a significant survival difference.
“Irrespective of the aortic valve morphology or valve pathology, in patients with mild to moderate aortic root dilatation (less than 45 mm), preservation of the SOV segment in the context of an AVRSCAAR procedure is justified. Continued further follow-up will be important to understand the long-term outcomes of sinus preservation, especially in the younger population with BAVs,” the researchers concluded.
The authors reported having no financial conflicts to disclose.
The sinus of Valsalva segment can be preserved during aortic valve replacement irrespective of the type of valve pathology, according to a recent study by Rita Karianna Milewski, MD, and her colleagues at the Hospital of the University of Pennsylvania, Philadelphia.
Severe aortic root dilation coupled to aortic valve disease requires root replacement in patients with a tricuspid or bicuspid aortic valve. Commonly, an aortic valve replacement and supracoronary ascending aorta replacement (AVRSCAAR) procedure has been used for patients who have a mild to moderately dilated sinus segment. One advantage of the procedure is that it retains the sinus of Valsalva (SOV) and preserves the intact coronary ostia.
However, the long-term behavior and risk of aortic events for the retained SOV in both BAV and TAV patients remains unclear, according to Dr. Milewski and her colleagues.
Previous researchers have suggested that patients with BAV and TAV have different rates of complications of the remaining aorta and dilation of the proximal aorta and retained sinus segment. In addition, it has been suggested that the cause of aortic dilation is different in patients with aortic stenosis (AS) and aortic insufficiency (AI) and is based on TAV and BAV morphology, histology, and hemodynamic flow patterns.
However, in the August issue of the Journal of Thoracic and Cardiovascular Surgery, Dr. Milewski and her colleagues reported on their study showing that, in patients with nonaneurysmal SOV undergoing AVRSCAAR, the sinus of Valsalva segment can be preserved regardless of the type of valvular pathology (aortic stenosis vs. aortic insufficiency) or valvular morphology (BAV vs. TAV).
The researchers retrospectively reviewed a prospectively maintained institutional database to stratify all patients by BAV or TAV valvular pathology with concomitant ascending aortic aneurysm who underwent an elective AVRSCAAR from 2002 to 2015 (J Thorac Cardiovasc Surg. 2017;154:421-32).
The distribution of the 428 patients meeting inclusion criteria by subgroups was: BAV group (254 patients: BAV-AS = 178; BAV-AI = 76); TAV group (174 patients: TAV-AS = 61; TAV-AI =113). Preoperative sinus of Valsalva dimensions were divided into 3 subgroups (less than 40 mm, 40-45 mm, and greater than 45 mm).
The mean patient age for patients with BAV and TAV was 59 years and 72 years (P less than .001), respectively (with 78% with BAV being men and 57% with TAV being men). There was a significantly higher subpopulation of AS in the BAV cohort vs. TAV-AS (70% vs. 35%; P less than .001).
With regard to SOV sizing, there was no significant difference in mean preoperative aortic root diameters between BAV and TAV cohorts for the AS or AI subpopulations.
In-hospital/30-day mortality was significantly higher in patients with TAV (5.2%) than in patients with BAV (1.6%, P = .033). In addition, the incidence of transient ischemic attack/stroke was significantly higher in the TAV group (3.4%) vs. the BAV group (0.8%, P = .04).
Valvular morphology and pathology at baseline, preoperative SOV diameter, postoperative time course, and interaction effect of preoperative SOV diameters and postoperative time course were used as covariates to assess outcomes. Within-subject and within–stratified subgroup comparison failed to show main effects across the follow-up times on postoperative SOV size patterns (P = .935), implying that the SOV trends were stable and sustained (discharge to greater than or equal to 10 years) irrespective of valvular morphology and pathology (BAV-AI, BAV-AS, TAV-AI, and TAV-AS).
Preoperative SOV dimensions significantly affected the retained postoperative sinus dimensions (P less than .001), according to Dr. Milewski and her colleagues.
The data indicated that an initial and pronounced postoperative decrease in SOV dimensions occurs with AVRSCAAR independently of aortic valve morphology, aortic valve pathology, and age, they added.
The 10-year freedom from aortic reoperation rates were 97% and 95% in the BAV and TAV subgroups, respectively. The BAV group had significantly improved reoperation-free survival, compared with the TAV group (P less than .001), while the type of valvular pathology within each group did not show a significant survival difference.
“Irrespective of the aortic valve morphology or valve pathology, in patients with mild to moderate aortic root dilatation (less than 45 mm), preservation of the SOV segment in the context of an AVRSCAAR procedure is justified. Continued further follow-up will be important to understand the long-term outcomes of sinus preservation, especially in the younger population with BAVs,” the researchers concluded.
The authors reported having no financial conflicts to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:
Major finding: The 10-year freedom from aortic reoperation rates were 97% and 95% in the BAV and TAV subgroups, respectively.
Data source: A retrospective review of 428 patients in a prospectively maintained database who met study inclusion criteria and were operated on between 2002 and 2015.
Disclosures: The authors reported having no financial conflicts to disclose.
New-onset AF after aortic valve replacement did not affect long-term survival
New-onset atrial fibrillation after aortic valve replacement was not an independent risk factor for decreased long-term survival, according to the results of a single-center, retrospective study reported by Ben M. Swinkels, MD, of St Antonius Hospital, Nieuwegein, and his colleagues in the Netherlands.
Key to this success, however, is restoring normal sinus rhythm before hospital discharge, they said.
In this retrospective, longitudinal cohort study, 569 consecutive patients with no history of AF who underwent AVR with or without concomitant coronary artery bypass grafting during 1990-1993 were followed for a mean of 17.8 years (J Thorac Cardiovasc Surg. 2017;154:492-8).
Thirty-day and long-term survival rates were determined in the 241 patients (42%) with and the 328 patients (58%) without new-onset postoperative atrial fibrillation (POAF), which was defined as electrocardiographically documented AF lasting for at least several hours, and occurring after AVR while the patient was still admitted. Standard therapy to prevent new onset POAF was the use of sotalol in patients who were not on beta-blocker therapy, and continuation of beta-blocker therapy for those who were already on it.
There were no significant differences between the two groups in demographic characteristics. There were also no significant differences between the two groups in operative characteristics, postoperative in-hospital adverse events, and postoperative hospital lengths of stay until discharge home, except for mechanical ventilation time, which was significantly longer in the patients with new-onset POAF (P = .011).
Thirty-day mortality was 1.2% in the patients with POAF, and 2.7% in those without, a nonsignificant difference. There was no statistically significant difference between the two survival curves and the Kaplan-Meier overall cumulative survival rates at 15 years of follow-up in the patients with new-onset POAF vs. those without were not statistically different (41.5% vs. 41.3%, respectively).
In addition, the 18-year probability of long-term first adverse events, including recurrent AF, transient ischemic attack, ischemic or hemorrhagic stroke, peripheral venous thromboembolism, or major or minor bleeding was not significantly different between the two groups.
“New-onset POAF after AVR does not affect long-term survival when treatment is aimed to restore sinus rhythm before the patient is discharged home. Future studies with a prospective, randomized design should be done to confirm this finding in patients undergoing different kinds of cardiac surgery,” the researchers concluded.
The study was funded by the authors’ home institution; the authors reported they had nothing to disclose.
The incidence of atrial fibrillation after valve surgery has been described to be as high as 50%, Manuel J. Antunes, MD, said in an editorial commentary. “The adverse effect on long-term survival may not be related to the short-lived new-onset AF but rather to the underlying pathology associated to the arrhythmia, especially pathology that affects the myocardium, principally in atherosclerotic coronary artery disease,” he wrote. “It is not survival alone, however, that should be cause for concern; AF, even in episodes of limited duration, may result in transient ischemic attacks, ischemic, or hemorrhagic strokes, and peripheral thromboembolism, which is why affected patients should immediately be anticoagulated.”
This study, however, is at odds with previously published studies, with opposite conclusions, according to Dr. Antunes. Swinkels and his colleagues suggest that one of the reasons for the discrepancy was the homogeneous character of their series, which consisted almost entirely of patients who had isolated AVR. Dr. Antunes also adds that another important aspect to consider is that the antiarrhythmic drugs used prophylactically or therapeutically for this patient cohort (treated during 1990-1993) are no longer used or have been replaced by new and more efficacious pharmacologic agents.
Manuel J. Antunes, MD, of the University Hospital and Faculty of Medicine, Coimbra, Portugal, made these remarks in an invited editorial (J Thorac Cardiovasc Surg. 2017;154:490-1). He reported having nothing to disclose.
The incidence of atrial fibrillation after valve surgery has been described to be as high as 50%, Manuel J. Antunes, MD, said in an editorial commentary. “The adverse effect on long-term survival may not be related to the short-lived new-onset AF but rather to the underlying pathology associated to the arrhythmia, especially pathology that affects the myocardium, principally in atherosclerotic coronary artery disease,” he wrote. “It is not survival alone, however, that should be cause for concern; AF, even in episodes of limited duration, may result in transient ischemic attacks, ischemic, or hemorrhagic strokes, and peripheral thromboembolism, which is why affected patients should immediately be anticoagulated.”
This study, however, is at odds with previously published studies, with opposite conclusions, according to Dr. Antunes. Swinkels and his colleagues suggest that one of the reasons for the discrepancy was the homogeneous character of their series, which consisted almost entirely of patients who had isolated AVR. Dr. Antunes also adds that another important aspect to consider is that the antiarrhythmic drugs used prophylactically or therapeutically for this patient cohort (treated during 1990-1993) are no longer used or have been replaced by new and more efficacious pharmacologic agents.
Manuel J. Antunes, MD, of the University Hospital and Faculty of Medicine, Coimbra, Portugal, made these remarks in an invited editorial (J Thorac Cardiovasc Surg. 2017;154:490-1). He reported having nothing to disclose.
The incidence of atrial fibrillation after valve surgery has been described to be as high as 50%, Manuel J. Antunes, MD, said in an editorial commentary. “The adverse effect on long-term survival may not be related to the short-lived new-onset AF but rather to the underlying pathology associated to the arrhythmia, especially pathology that affects the myocardium, principally in atherosclerotic coronary artery disease,” he wrote. “It is not survival alone, however, that should be cause for concern; AF, even in episodes of limited duration, may result in transient ischemic attacks, ischemic, or hemorrhagic strokes, and peripheral thromboembolism, which is why affected patients should immediately be anticoagulated.”
This study, however, is at odds with previously published studies, with opposite conclusions, according to Dr. Antunes. Swinkels and his colleagues suggest that one of the reasons for the discrepancy was the homogeneous character of their series, which consisted almost entirely of patients who had isolated AVR. Dr. Antunes also adds that another important aspect to consider is that the antiarrhythmic drugs used prophylactically or therapeutically for this patient cohort (treated during 1990-1993) are no longer used or have been replaced by new and more efficacious pharmacologic agents.
Manuel J. Antunes, MD, of the University Hospital and Faculty of Medicine, Coimbra, Portugal, made these remarks in an invited editorial (J Thorac Cardiovasc Surg. 2017;154:490-1). He reported having nothing to disclose.
New-onset atrial fibrillation after aortic valve replacement was not an independent risk factor for decreased long-term survival, according to the results of a single-center, retrospective study reported by Ben M. Swinkels, MD, of St Antonius Hospital, Nieuwegein, and his colleagues in the Netherlands.
Key to this success, however, is restoring normal sinus rhythm before hospital discharge, they said.
In this retrospective, longitudinal cohort study, 569 consecutive patients with no history of AF who underwent AVR with or without concomitant coronary artery bypass grafting during 1990-1993 were followed for a mean of 17.8 years (J Thorac Cardiovasc Surg. 2017;154:492-8).
Thirty-day and long-term survival rates were determined in the 241 patients (42%) with and the 328 patients (58%) without new-onset postoperative atrial fibrillation (POAF), which was defined as electrocardiographically documented AF lasting for at least several hours, and occurring after AVR while the patient was still admitted. Standard therapy to prevent new onset POAF was the use of sotalol in patients who were not on beta-blocker therapy, and continuation of beta-blocker therapy for those who were already on it.
There were no significant differences between the two groups in demographic characteristics. There were also no significant differences between the two groups in operative characteristics, postoperative in-hospital adverse events, and postoperative hospital lengths of stay until discharge home, except for mechanical ventilation time, which was significantly longer in the patients with new-onset POAF (P = .011).
Thirty-day mortality was 1.2% in the patients with POAF, and 2.7% in those without, a nonsignificant difference. There was no statistically significant difference between the two survival curves and the Kaplan-Meier overall cumulative survival rates at 15 years of follow-up in the patients with new-onset POAF vs. those without were not statistically different (41.5% vs. 41.3%, respectively).
In addition, the 18-year probability of long-term first adverse events, including recurrent AF, transient ischemic attack, ischemic or hemorrhagic stroke, peripheral venous thromboembolism, or major or minor bleeding was not significantly different between the two groups.
“New-onset POAF after AVR does not affect long-term survival when treatment is aimed to restore sinus rhythm before the patient is discharged home. Future studies with a prospective, randomized design should be done to confirm this finding in patients undergoing different kinds of cardiac surgery,” the researchers concluded.
The study was funded by the authors’ home institution; the authors reported they had nothing to disclose.
New-onset atrial fibrillation after aortic valve replacement was not an independent risk factor for decreased long-term survival, according to the results of a single-center, retrospective study reported by Ben M. Swinkels, MD, of St Antonius Hospital, Nieuwegein, and his colleagues in the Netherlands.
Key to this success, however, is restoring normal sinus rhythm before hospital discharge, they said.
In this retrospective, longitudinal cohort study, 569 consecutive patients with no history of AF who underwent AVR with or without concomitant coronary artery bypass grafting during 1990-1993 were followed for a mean of 17.8 years (J Thorac Cardiovasc Surg. 2017;154:492-8).
Thirty-day and long-term survival rates were determined in the 241 patients (42%) with and the 328 patients (58%) without new-onset postoperative atrial fibrillation (POAF), which was defined as electrocardiographically documented AF lasting for at least several hours, and occurring after AVR while the patient was still admitted. Standard therapy to prevent new onset POAF was the use of sotalol in patients who were not on beta-blocker therapy, and continuation of beta-blocker therapy for those who were already on it.
There were no significant differences between the two groups in demographic characteristics. There were also no significant differences between the two groups in operative characteristics, postoperative in-hospital adverse events, and postoperative hospital lengths of stay until discharge home, except for mechanical ventilation time, which was significantly longer in the patients with new-onset POAF (P = .011).
Thirty-day mortality was 1.2% in the patients with POAF, and 2.7% in those without, a nonsignificant difference. There was no statistically significant difference between the two survival curves and the Kaplan-Meier overall cumulative survival rates at 15 years of follow-up in the patients with new-onset POAF vs. those without were not statistically different (41.5% vs. 41.3%, respectively).
In addition, the 18-year probability of long-term first adverse events, including recurrent AF, transient ischemic attack, ischemic or hemorrhagic stroke, peripheral venous thromboembolism, or major or minor bleeding was not significantly different between the two groups.
“New-onset POAF after AVR does not affect long-term survival when treatment is aimed to restore sinus rhythm before the patient is discharged home. Future studies with a prospective, randomized design should be done to confirm this finding in patients undergoing different kinds of cardiac surgery,” the researchers concluded.
The study was funded by the authors’ home institution; the authors reported they had nothing to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:
Major finding: Cumulative 15-year survival rates were similar in the patients with new-onset postop AF (41.5%) to those without (41.3%).
Data source: A retrospective longitudinal cohort study of 569 consecutive patients without a history of AF who were followed for a mean of 17.8 years after AVR with or without concomitant CABG.
Disclosures: The study was funded by the authors’ home institution and the authors reported they had nothing to disclose.
Elective open conversion after failed EVAR safer than emergent
Emergency open conversion after failed endovascular aortic aneurysm repair shows significantly higher mortality and morbidity, compared with elective conversion, according to the results of a retrospective, observational study of 31 patients at a single institution.
The primary endpoints of the study were 30-day and in-hospital mortality. Secondary endpoints included moderate to severe complications, secondary interventions, length of ICU stay, and length of hospital stay (LOS), according to I. Ben Abdallah, MD, of the Hôpital Européen Georges Pompidou and his colleagues.
During the study period, a total of 338 patients received EVAR at the institution. Of these, 31 patients underwent open conversion (19 elective, 12 emergent) after EVAR between August 2008 and September 2016. The median time from the index EVAR to the open conversion was 35 months, with the most common indications for intervention being endoleaks (24 patients, 77%), stent graft infection (3, 10%), thrombosis (3, 10%) and kinking (1, 3%). Stents removed were manufactured by various device makers, according to the report (Eur J Vasc Endovasc Surg. 2017;53:831-6).
The patient population had a mean age of 73 years and comprised 84% men. The two groups, elective and emergent, were highly similar in numerous comorbidities, with the only significant difference between them being a greater incidence of chronic renal disease among the emergent group, as compared with the elective (42% vs. 5%).
Overall in-hospital mortality was 10%, and significantly greater in emergent vs. elective conversion (25% vs. 0%). Renal and pulmonary complications were significantly higher in the emergency group (42% vs. 5% and 42% vs. 0%, respectively). There was no significant difference between elective and emergent hospital stay (14 days vs. 20 days), but ICU stay was significantly shorter for elective conversion (2 days vs. 7 days).
There were no late complications or death seen in either group after a mean follow-up of 18 months.
“In this series, open conversion seems to be significantly safer and more effective when performed electively with no mortality, a lower incidence of morbidity (renal and pulmonary), and shorter ICU stay. These results underline that close surveillance, allowing planned elective open conversion, is the key to better outcomes after failed EVAR,” the researchers concluded.
The authors reported that they had no conflicts of interest, and the study received no outside funding.
Emergency open conversion after failed endovascular aortic aneurysm repair shows significantly higher mortality and morbidity, compared with elective conversion, according to the results of a retrospective, observational study of 31 patients at a single institution.
The primary endpoints of the study were 30-day and in-hospital mortality. Secondary endpoints included moderate to severe complications, secondary interventions, length of ICU stay, and length of hospital stay (LOS), according to I. Ben Abdallah, MD, of the Hôpital Européen Georges Pompidou and his colleagues.
During the study period, a total of 338 patients received EVAR at the institution. Of these, 31 patients underwent open conversion (19 elective, 12 emergent) after EVAR between August 2008 and September 2016. The median time from the index EVAR to the open conversion was 35 months, with the most common indications for intervention being endoleaks (24 patients, 77%), stent graft infection (3, 10%), thrombosis (3, 10%) and kinking (1, 3%). Stents removed were manufactured by various device makers, according to the report (Eur J Vasc Endovasc Surg. 2017;53:831-6).
The patient population had a mean age of 73 years and comprised 84% men. The two groups, elective and emergent, were highly similar in numerous comorbidities, with the only significant difference between them being a greater incidence of chronic renal disease among the emergent group, as compared with the elective (42% vs. 5%).
Overall in-hospital mortality was 10%, and significantly greater in emergent vs. elective conversion (25% vs. 0%). Renal and pulmonary complications were significantly higher in the emergency group (42% vs. 5% and 42% vs. 0%, respectively). There was no significant difference between elective and emergent hospital stay (14 days vs. 20 days), but ICU stay was significantly shorter for elective conversion (2 days vs. 7 days).
There were no late complications or death seen in either group after a mean follow-up of 18 months.
“In this series, open conversion seems to be significantly safer and more effective when performed electively with no mortality, a lower incidence of morbidity (renal and pulmonary), and shorter ICU stay. These results underline that close surveillance, allowing planned elective open conversion, is the key to better outcomes after failed EVAR,” the researchers concluded.
The authors reported that they had no conflicts of interest, and the study received no outside funding.
Emergency open conversion after failed endovascular aortic aneurysm repair shows significantly higher mortality and morbidity, compared with elective conversion, according to the results of a retrospective, observational study of 31 patients at a single institution.
The primary endpoints of the study were 30-day and in-hospital mortality. Secondary endpoints included moderate to severe complications, secondary interventions, length of ICU stay, and length of hospital stay (LOS), according to I. Ben Abdallah, MD, of the Hôpital Européen Georges Pompidou and his colleagues.
During the study period, a total of 338 patients received EVAR at the institution. Of these, 31 patients underwent open conversion (19 elective, 12 emergent) after EVAR between August 2008 and September 2016. The median time from the index EVAR to the open conversion was 35 months, with the most common indications for intervention being endoleaks (24 patients, 77%), stent graft infection (3, 10%), thrombosis (3, 10%) and kinking (1, 3%). Stents removed were manufactured by various device makers, according to the report (Eur J Vasc Endovasc Surg. 2017;53:831-6).
The patient population had a mean age of 73 years and comprised 84% men. The two groups, elective and emergent, were highly similar in numerous comorbidities, with the only significant difference between them being a greater incidence of chronic renal disease among the emergent group, as compared with the elective (42% vs. 5%).
Overall in-hospital mortality was 10%, and significantly greater in emergent vs. elective conversion (25% vs. 0%). Renal and pulmonary complications were significantly higher in the emergency group (42% vs. 5% and 42% vs. 0%, respectively). There was no significant difference between elective and emergent hospital stay (14 days vs. 20 days), but ICU stay was significantly shorter for elective conversion (2 days vs. 7 days).
There were no late complications or death seen in either group after a mean follow-up of 18 months.
“In this series, open conversion seems to be significantly safer and more effective when performed electively with no mortality, a lower incidence of morbidity (renal and pulmonary), and shorter ICU stay. These results underline that close surveillance, allowing planned elective open conversion, is the key to better outcomes after failed EVAR,” the researchers concluded.
The authors reported that they had no conflicts of interest, and the study received no outside funding.
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point:
Major finding: Overall in-hospital mortality was 10% and significantly greater in emergent vs. elective conversion (25% vs. 0%).
Data source: A retrospective database analysis of 31 patients undergoing EVAR open conversion at a single institution.
Disclosures: The authors reported that they had no conflicts of interest, and the study received no outside funding.
Multiple factors predict surgical site infection risk after lower extremity revascularization
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point:
Major finding: SSIs occurred in 10.6% of lower extremity bypasses.
Data source: A retrospective analysis of 3,033 elective or urgent open LEB procedures performed over a 3.5-year period in a statewide cardiovascular consortium of 35 hospitals.
Disclosures: The authors reported having no conflicts of interest with regard to the study.
Factory contamination seen as likely source of postop endocarditis outbreak
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: Cardiac surgery–related patient isolates were all classified into the same group, in which all, except one, formed a distinct subgroup of Mycobacterium chimaera, which also comprised most isolates from LivaNova HCUs, and one from the equipment production site.
Data source: Phylogenetic analysis based on whole-genome sequencing of 250 M. chimaera isolates obtained from cardiac surgery patients, hospitals, and other sources.
Disclosures: Partly funded by the EU Horizon 2020 program and several German, Swiss, and U.K. infectious disease–related NGOs. The authors reported having no disclosures.
Endosonography can help eliminate false negatives
BOSTON – Patients at high risk for non–small cell lung cancer (NSCLC) metastases were found to have a significant rate of unsuspected lymph node metastases upon endosonographic assessment, even in the presence of radiologically normal mediastinal lymph nodes, according to a study reported by Pravachan Hegde, MD, of the University of Montreal. Dr. Hegde presented the results at the 2017 AATS Centennial meeting.
Positron-emission tomography (PET) with computed tomography (CT) is routinely utilized to investigate lymph node (LN) metastases in non-small cell lung cancer, according to Dr. Hegde. However, this method has been found to be less sensitive in normal-sized LNs.
Dr. Hegde and his colleagues retrospectively reviewed a single-institution prospectively maintained database. Patients were identified from a cohort between January 2009 and December 2014. Consecutive patients with NSCLC were identified in whom both the pre-endosonography CT and PET-CT were negative for mediastinal LN metastases.
Patients were staged if they had central tumor, tumor size greater than 3 cm, N1 lymph node involvement on PET-CT/CT, or if there was low SUV in the primary tumor. Combined endosonography (EBUS+EUS-FNA) was performed in all patients.
A total of 22 out of 161 patients with radiologically normal mediastinum were found to be positive on combined EBUS/EUS staging. Out of 21 patients upstaged, 71% had tumor size greater than 3 cm; 28% had N1 disease; 61% had N2 disease; and 9% had adrenal involvement. None of the patients that were upstaged had N1 LN involvement on PET-CT or CT scan, according to Dr. Hegde.
A total of 416 lymph nodes were biopsied in the 161 patients by combined endosonography, 147 by EBUS and 269 by EUS. Of the 22 patients upstaged with endosonography, 12 were upstaged with EBUS and 10 were upstaged with EUS.
“Given the significant rate of unsuspected lymph node metastases, combined endosonographic lymph node staging should be routinely performed in staging of NSCLC in high risk patients even in the presence of radiologically normal mediastinal lymph nodes,” Dr. Hegde concluded.
In an interview, Moishe Liberman, MD, a coauthor of the study stated: “Pre-operative lymph node staging of lung cancer has dramatically changed over the last decade due to the availability and improvements in technology in PET, CT, EBUS, and EUS. While imaging (CT and PET) definitely help in staging, these tests are imperfect with significant false-negative rates as seen in our study.
“Minimally invasive, endoscopic techniques can help to significantly decrease these false- negative rates by providing biopsy of the target lymph nodes. Surprise intra-operative findings not accurately picked up by PET and CT should be almost nonexistant in 2017 with aggressive endosonographic pre-operative staging. This ensures that patients who actually benefit from surgery undergo resection and those with higher stage disease get appropriate treatment,” Dr. Liberman added. ■
BOSTON – Patients at high risk for non–small cell lung cancer (NSCLC) metastases were found to have a significant rate of unsuspected lymph node metastases upon endosonographic assessment, even in the presence of radiologically normal mediastinal lymph nodes, according to a study reported by Pravachan Hegde, MD, of the University of Montreal. Dr. Hegde presented the results at the 2017 AATS Centennial meeting.
Positron-emission tomography (PET) with computed tomography (CT) is routinely utilized to investigate lymph node (LN) metastases in non-small cell lung cancer, according to Dr. Hegde. However, this method has been found to be less sensitive in normal-sized LNs.
Dr. Hegde and his colleagues retrospectively reviewed a single-institution prospectively maintained database. Patients were identified from a cohort between January 2009 and December 2014. Consecutive patients with NSCLC were identified in whom both the pre-endosonography CT and PET-CT were negative for mediastinal LN metastases.
Patients were staged if they had central tumor, tumor size greater than 3 cm, N1 lymph node involvement on PET-CT/CT, or if there was low SUV in the primary tumor. Combined endosonography (EBUS+EUS-FNA) was performed in all patients.
A total of 22 out of 161 patients with radiologically normal mediastinum were found to be positive on combined EBUS/EUS staging. Out of 21 patients upstaged, 71% had tumor size greater than 3 cm; 28% had N1 disease; 61% had N2 disease; and 9% had adrenal involvement. None of the patients that were upstaged had N1 LN involvement on PET-CT or CT scan, according to Dr. Hegde.
A total of 416 lymph nodes were biopsied in the 161 patients by combined endosonography, 147 by EBUS and 269 by EUS. Of the 22 patients upstaged with endosonography, 12 were upstaged with EBUS and 10 were upstaged with EUS.
“Given the significant rate of unsuspected lymph node metastases, combined endosonographic lymph node staging should be routinely performed in staging of NSCLC in high risk patients even in the presence of radiologically normal mediastinal lymph nodes,” Dr. Hegde concluded.
In an interview, Moishe Liberman, MD, a coauthor of the study stated: “Pre-operative lymph node staging of lung cancer has dramatically changed over the last decade due to the availability and improvements in technology in PET, CT, EBUS, and EUS. While imaging (CT and PET) definitely help in staging, these tests are imperfect with significant false-negative rates as seen in our study.
“Minimally invasive, endoscopic techniques can help to significantly decrease these false- negative rates by providing biopsy of the target lymph nodes. Surprise intra-operative findings not accurately picked up by PET and CT should be almost nonexistant in 2017 with aggressive endosonographic pre-operative staging. This ensures that patients who actually benefit from surgery undergo resection and those with higher stage disease get appropriate treatment,” Dr. Liberman added. ■
BOSTON – Patients at high risk for non–small cell lung cancer (NSCLC) metastases were found to have a significant rate of unsuspected lymph node metastases upon endosonographic assessment, even in the presence of radiologically normal mediastinal lymph nodes, according to a study reported by Pravachan Hegde, MD, of the University of Montreal. Dr. Hegde presented the results at the 2017 AATS Centennial meeting.
Positron-emission tomography (PET) with computed tomography (CT) is routinely utilized to investigate lymph node (LN) metastases in non-small cell lung cancer, according to Dr. Hegde. However, this method has been found to be less sensitive in normal-sized LNs.
Dr. Hegde and his colleagues retrospectively reviewed a single-institution prospectively maintained database. Patients were identified from a cohort between January 2009 and December 2014. Consecutive patients with NSCLC were identified in whom both the pre-endosonography CT and PET-CT were negative for mediastinal LN metastases.
Patients were staged if they had central tumor, tumor size greater than 3 cm, N1 lymph node involvement on PET-CT/CT, or if there was low SUV in the primary tumor. Combined endosonography (EBUS+EUS-FNA) was performed in all patients.
A total of 22 out of 161 patients with radiologically normal mediastinum were found to be positive on combined EBUS/EUS staging. Out of 21 patients upstaged, 71% had tumor size greater than 3 cm; 28% had N1 disease; 61% had N2 disease; and 9% had adrenal involvement. None of the patients that were upstaged had N1 LN involvement on PET-CT or CT scan, according to Dr. Hegde.
A total of 416 lymph nodes were biopsied in the 161 patients by combined endosonography, 147 by EBUS and 269 by EUS. Of the 22 patients upstaged with endosonography, 12 were upstaged with EBUS and 10 were upstaged with EUS.
“Given the significant rate of unsuspected lymph node metastases, combined endosonographic lymph node staging should be routinely performed in staging of NSCLC in high risk patients even in the presence of radiologically normal mediastinal lymph nodes,” Dr. Hegde concluded.
In an interview, Moishe Liberman, MD, a coauthor of the study stated: “Pre-operative lymph node staging of lung cancer has dramatically changed over the last decade due to the availability and improvements in technology in PET, CT, EBUS, and EUS. While imaging (CT and PET) definitely help in staging, these tests are imperfect with significant false-negative rates as seen in our study.
“Minimally invasive, endoscopic techniques can help to significantly decrease these false- negative rates by providing biopsy of the target lymph nodes. Surprise intra-operative findings not accurately picked up by PET and CT should be almost nonexistant in 2017 with aggressive endosonographic pre-operative staging. This ensures that patients who actually benefit from surgery undergo resection and those with higher stage disease get appropriate treatment,” Dr. Liberman added. ■
Surgeon volume tied to mitral valve surgery outcomes
CHICAGO – A total annual surgeon volume of fewer than 25 operations was associated with increased 1-year mortality and reoperation rates, according to a study presented at the 2017 American Association for Thoracic Surgery Centennial.
Improvements in repair rates, survival, and freedom from reoperation increased with increasing surgeon case volumes, Joanna Chikwe, MD, and her colleagues at the Icahn School of Medicine at Mount Sinai, New York, stated.
The researchers compared repair rates, long-term survival, and risk of postrepair reoperation in patients with degenerative disease according to total annual surgeon volume, which was defined as any mitral valve operation for any cause during the study period. The study was simultaneously published in the Journal of the American College of Cardiology (2017 May 16;69[19]:2397-406).
Mitral valve repair is the preferred treatment, compared with valve replacement, for the treatment of severe mitral valve regurgitation in patients who have degenerative valve disease with mitral valve prolapse, and both U.S. and European guidelines strongly recommend valve repair whenever possible, according to Dr. Chikwe and her colleagues.
But, “mitral valve replacement unfortunately remains relatively common in patients with degenerative valve disease,” they stated.
A total of 313 surgeons from 41 institutions met the study eligibility criteria, according to the researchers. They performed a median of 10 mitral valve operations per year (range, 1-230). The median annual institutional mitral valve volume was 59 mitral valve operations, ranging from a minimum of 6 to a maximum of 310 operations. Repair rates for primary mitral valve operations for any cause at all 41 institutions varied from 15% to 83%, and repair rates for degenerative mitral valve operations varied from 25% to 100%.
In the study cohort, surgeons with a total annual volume of less than 25 operations carried out 25% of procedures.
After multivariable adjustment, total annual surgeon volume was independently associated with the probability of mitral valve repair. The chance of repair increased significantly by 13% for every 10-case increment in total annual surgeon volume (P less than .001).
In addition, compared with patients operated on by surgeons with a total annual surgeon volume of 10 or fewer operations, patients operated on by surgeons with a total annual surgeon volume of greater than 50 operations were more than three times as likely to undergo mitral valve repair rather than replacement.
A total annual surgeon volume of less than 25 operations was associated with lower mitral valve repair rates and with increased 1-year mortality and mitral valve reoperation rates. Improvements in repair rates, survival, and freedom from reoperation increased with increasing surgeon case volumes.
After 1 year of repair or replacement, the actuarial survival of patients with degenerative mitral valve disease who were operated on by surgeons performing greater than 50 operations per year was 97.8%, compared with 94.1% for patients operated on by surgeons performing less than or equal to 10 operations a year.
Compared with replacement, mitral repair was significantly associated with better survival, but total annual surgeon volume still remained a significant independent predictor (P less than .001). In addition, for those patients who underwent mitral valve repair, total annual surgeon volume was a significant independent predictor of late death.
The results are important, the investigators noted, given that the median number of mitral valve operations performed annually by individual surgeons in the United States was five, according to an analysis of the Society of Thoracic Surgeons database – and that, in New York state, most surgeons actually performed less than one mitral operation per month.
There were significant differences seen in the patient characteristics across surgeons’ case volume groups. The prevalence of congestive heart failure was significantly higher in patients operated on by surgeons with lower volumes, and the proportion of patients undergoing urgent surgery was also significantly higher for lower-volume surgeons.
“This leads to a double jeopardy, where sicker patients are adversely affected by the lower repair rates and poorer outcomes seen with lower-volume surgeons, and it underscores the need to refer the highest-risk patients to high-volume surgeons,” said Dr. Chickwe and her colleagues.
However, “even among high-volume surgeons, there was an observed variability of degenerative disease repair rates, ranging from 19% to nearly 100%,” they added. “This finding reflects that surgeon volume is not the only factor for better outcomes, and it emphasizes the need for more transparency of surgeon-related factors and outcomes of degenerative mitral valve surgery for patients and referring cardiologists.
“Considering that there was an incremental improvement in survival and probability of repair with increasing volume over 25 operations, one could make the argument that a minimum volume target of 50, or even more, operations would be optimal,” the researchers noted. “Developing more very high-volume surgeons experienced in mitral valve repair would likely be particularly beneficial for patients with complex but repairable mitral valve disease, and for asymptomatic patients whose repair feasibility would optimally approach 100%.”
Dr. David Adams is the national coprincipal investigator of the Core Valve United States Pivotal Trial, supported by Medtronic. Dr. Chikwe received speaker honoraria from Edwards Lifesciences. The other coauthors had no disclosures to report.
CHICAGO – A total annual surgeon volume of fewer than 25 operations was associated with increased 1-year mortality and reoperation rates, according to a study presented at the 2017 American Association for Thoracic Surgery Centennial.
Improvements in repair rates, survival, and freedom from reoperation increased with increasing surgeon case volumes, Joanna Chikwe, MD, and her colleagues at the Icahn School of Medicine at Mount Sinai, New York, stated.
The researchers compared repair rates, long-term survival, and risk of postrepair reoperation in patients with degenerative disease according to total annual surgeon volume, which was defined as any mitral valve operation for any cause during the study period. The study was simultaneously published in the Journal of the American College of Cardiology (2017 May 16;69[19]:2397-406).
Mitral valve repair is the preferred treatment, compared with valve replacement, for the treatment of severe mitral valve regurgitation in patients who have degenerative valve disease with mitral valve prolapse, and both U.S. and European guidelines strongly recommend valve repair whenever possible, according to Dr. Chikwe and her colleagues.
But, “mitral valve replacement unfortunately remains relatively common in patients with degenerative valve disease,” they stated.
A total of 313 surgeons from 41 institutions met the study eligibility criteria, according to the researchers. They performed a median of 10 mitral valve operations per year (range, 1-230). The median annual institutional mitral valve volume was 59 mitral valve operations, ranging from a minimum of 6 to a maximum of 310 operations. Repair rates for primary mitral valve operations for any cause at all 41 institutions varied from 15% to 83%, and repair rates for degenerative mitral valve operations varied from 25% to 100%.
In the study cohort, surgeons with a total annual volume of less than 25 operations carried out 25% of procedures.
After multivariable adjustment, total annual surgeon volume was independently associated with the probability of mitral valve repair. The chance of repair increased significantly by 13% for every 10-case increment in total annual surgeon volume (P less than .001).
In addition, compared with patients operated on by surgeons with a total annual surgeon volume of 10 or fewer operations, patients operated on by surgeons with a total annual surgeon volume of greater than 50 operations were more than three times as likely to undergo mitral valve repair rather than replacement.
A total annual surgeon volume of less than 25 operations was associated with lower mitral valve repair rates and with increased 1-year mortality and mitral valve reoperation rates. Improvements in repair rates, survival, and freedom from reoperation increased with increasing surgeon case volumes.
After 1 year of repair or replacement, the actuarial survival of patients with degenerative mitral valve disease who were operated on by surgeons performing greater than 50 operations per year was 97.8%, compared with 94.1% for patients operated on by surgeons performing less than or equal to 10 operations a year.
Compared with replacement, mitral repair was significantly associated with better survival, but total annual surgeon volume still remained a significant independent predictor (P less than .001). In addition, for those patients who underwent mitral valve repair, total annual surgeon volume was a significant independent predictor of late death.
The results are important, the investigators noted, given that the median number of mitral valve operations performed annually by individual surgeons in the United States was five, according to an analysis of the Society of Thoracic Surgeons database – and that, in New York state, most surgeons actually performed less than one mitral operation per month.
There were significant differences seen in the patient characteristics across surgeons’ case volume groups. The prevalence of congestive heart failure was significantly higher in patients operated on by surgeons with lower volumes, and the proportion of patients undergoing urgent surgery was also significantly higher for lower-volume surgeons.
“This leads to a double jeopardy, where sicker patients are adversely affected by the lower repair rates and poorer outcomes seen with lower-volume surgeons, and it underscores the need to refer the highest-risk patients to high-volume surgeons,” said Dr. Chickwe and her colleagues.
However, “even among high-volume surgeons, there was an observed variability of degenerative disease repair rates, ranging from 19% to nearly 100%,” they added. “This finding reflects that surgeon volume is not the only factor for better outcomes, and it emphasizes the need for more transparency of surgeon-related factors and outcomes of degenerative mitral valve surgery for patients and referring cardiologists.
“Considering that there was an incremental improvement in survival and probability of repair with increasing volume over 25 operations, one could make the argument that a minimum volume target of 50, or even more, operations would be optimal,” the researchers noted. “Developing more very high-volume surgeons experienced in mitral valve repair would likely be particularly beneficial for patients with complex but repairable mitral valve disease, and for asymptomatic patients whose repair feasibility would optimally approach 100%.”
Dr. David Adams is the national coprincipal investigator of the Core Valve United States Pivotal Trial, supported by Medtronic. Dr. Chikwe received speaker honoraria from Edwards Lifesciences. The other coauthors had no disclosures to report.
CHICAGO – A total annual surgeon volume of fewer than 25 operations was associated with increased 1-year mortality and reoperation rates, according to a study presented at the 2017 American Association for Thoracic Surgery Centennial.
Improvements in repair rates, survival, and freedom from reoperation increased with increasing surgeon case volumes, Joanna Chikwe, MD, and her colleagues at the Icahn School of Medicine at Mount Sinai, New York, stated.
The researchers compared repair rates, long-term survival, and risk of postrepair reoperation in patients with degenerative disease according to total annual surgeon volume, which was defined as any mitral valve operation for any cause during the study period. The study was simultaneously published in the Journal of the American College of Cardiology (2017 May 16;69[19]:2397-406).
Mitral valve repair is the preferred treatment, compared with valve replacement, for the treatment of severe mitral valve regurgitation in patients who have degenerative valve disease with mitral valve prolapse, and both U.S. and European guidelines strongly recommend valve repair whenever possible, according to Dr. Chikwe and her colleagues.
But, “mitral valve replacement unfortunately remains relatively common in patients with degenerative valve disease,” they stated.
A total of 313 surgeons from 41 institutions met the study eligibility criteria, according to the researchers. They performed a median of 10 mitral valve operations per year (range, 1-230). The median annual institutional mitral valve volume was 59 mitral valve operations, ranging from a minimum of 6 to a maximum of 310 operations. Repair rates for primary mitral valve operations for any cause at all 41 institutions varied from 15% to 83%, and repair rates for degenerative mitral valve operations varied from 25% to 100%.
In the study cohort, surgeons with a total annual volume of less than 25 operations carried out 25% of procedures.
After multivariable adjustment, total annual surgeon volume was independently associated with the probability of mitral valve repair. The chance of repair increased significantly by 13% for every 10-case increment in total annual surgeon volume (P less than .001).
In addition, compared with patients operated on by surgeons with a total annual surgeon volume of 10 or fewer operations, patients operated on by surgeons with a total annual surgeon volume of greater than 50 operations were more than three times as likely to undergo mitral valve repair rather than replacement.
A total annual surgeon volume of less than 25 operations was associated with lower mitral valve repair rates and with increased 1-year mortality and mitral valve reoperation rates. Improvements in repair rates, survival, and freedom from reoperation increased with increasing surgeon case volumes.
After 1 year of repair or replacement, the actuarial survival of patients with degenerative mitral valve disease who were operated on by surgeons performing greater than 50 operations per year was 97.8%, compared with 94.1% for patients operated on by surgeons performing less than or equal to 10 operations a year.
Compared with replacement, mitral repair was significantly associated with better survival, but total annual surgeon volume still remained a significant independent predictor (P less than .001). In addition, for those patients who underwent mitral valve repair, total annual surgeon volume was a significant independent predictor of late death.
The results are important, the investigators noted, given that the median number of mitral valve operations performed annually by individual surgeons in the United States was five, according to an analysis of the Society of Thoracic Surgeons database – and that, in New York state, most surgeons actually performed less than one mitral operation per month.
There were significant differences seen in the patient characteristics across surgeons’ case volume groups. The prevalence of congestive heart failure was significantly higher in patients operated on by surgeons with lower volumes, and the proportion of patients undergoing urgent surgery was also significantly higher for lower-volume surgeons.
“This leads to a double jeopardy, where sicker patients are adversely affected by the lower repair rates and poorer outcomes seen with lower-volume surgeons, and it underscores the need to refer the highest-risk patients to high-volume surgeons,” said Dr. Chickwe and her colleagues.
However, “even among high-volume surgeons, there was an observed variability of degenerative disease repair rates, ranging from 19% to nearly 100%,” they added. “This finding reflects that surgeon volume is not the only factor for better outcomes, and it emphasizes the need for more transparency of surgeon-related factors and outcomes of degenerative mitral valve surgery for patients and referring cardiologists.
“Considering that there was an incremental improvement in survival and probability of repair with increasing volume over 25 operations, one could make the argument that a minimum volume target of 50, or even more, operations would be optimal,” the researchers noted. “Developing more very high-volume surgeons experienced in mitral valve repair would likely be particularly beneficial for patients with complex but repairable mitral valve disease, and for asymptomatic patients whose repair feasibility would optimally approach 100%.”
Dr. David Adams is the national coprincipal investigator of the Core Valve United States Pivotal Trial, supported by Medtronic. Dr. Chikwe received speaker honoraria from Edwards Lifesciences. The other coauthors had no disclosures to report.
FROM THE AATS ANNUAL MEETING AND THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point:
Major finding: Mitral valve reoperation rates steadily decreased with increasing surgeon volume until 25 operations per year, coupled to an improved 1-year survival for every 10 additional operations more than that.
Data source: A mandatory New York state database containing 5,475 patients who underwent mitral valve repair between 2002 and 2013.
Disclosures: Dr. David Adams is the national coprincipal investigator of the Core Valve United States Pivotal Trial, supported by Medtronic. Dr. Joanna Chikwe received speaker honoraria from Edwards Lifesciences. The other coauthors had no disclosures to report.
Consider invasive mediastinal staging in higher risk NSCLC patients, despite guidelines
Endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) appears to be cost effective for use in non–small cell lung cancer (NSCLC) staging if the prevalence of mediastinal lymph node metastasis (MLNM) is greater than or equal to 2.5%, according to the results of single institution modeling study. In addition, the study found that confirmatory mediastinoscopy should be performed in high-risk patients in cases of negative EBUS-TBNA.
Katarzyna Czarnecka-Kujawa, MD, of the University of Toronto and Toronto General Hospital, and her colleagues performed a decision analysis to compare health outcomes and costs of four mediastinal staging strategies. They assessed the following: no invasive staging, endobronchial ultrasound-guided transbronchial need aspiration (EBUS-TBNA), mediastinoscopy, and EBUS-TBNA followed by mediastinoscopy if EBUS-TBNA results were negative. They determined incremental cost-effectiveness ratios (ICER) for all strategies and performed comprehensive sensitivity analyses using a willingness to pay threshold of $80,000 [Canadian]/quality adjusted life-year (QALY).
They used data obtained for staging, outcomes, and costs from the patients in the lung cancer program at the Toronto General Hospital from Jan. 1, 2005 to Dec. 31, 2014, as detailed in a report published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2017. doi: 10.1016/j.jtcvs.2016.12.048).
After exclusions, they utilized a final case count of 499 cases for developing their surgical and procedure cost analysis, and a total of 750 cases in their endoscopy database for endoscopy analysis. For the base-case analysis, they assumed a prevalence of mediastinal metastasis of 9%, and obtained the prevalence of a pathologic lymph nodal stage disease following EBUS-TBNA from their institutional data.
Their results showed that EBUS-TBNA followed by mediastinoscopy was the strategy that resulted in the highest QALYs, but that it had a prohibitive ICER of greater than $1.4 million/QALY. Accordingly, it may not be justifiable to use mediastinoscopy after negative EBUS-TBNA in all patients, the researchers noted. However, the researchers’ data suggest that invasive screening may be justified in a very-low-risk population (MLNM above 2.5%).
In addition, the researchers stated that “[the] benefit conveyed by detecting mediastinal metastatic disease becomes more apparent as the prevalence of MLNM increases, with confirmatory mediastinoscopy becoming cost effective in cases of negative EBUS-TBNA in patients with moderate to high probability of MLNM” (greater than 57%).
Our model points out that there is a well-defined role for the use of different modalities, including mediastinoscopy. This stresses the need for ongoing focus on maintenance of competency and skill acquisition in mediastinoscopy and EBUS-TBNA by currently practicing and future thoracic surgeons respectively,” the researchers concluded.
Dr. Czarnecka-Kujawa disclosed that she is a research consultant with Olympus America. The study was funded in part by agencies of the Austrian government.
The authors make a compelling argument for invasive mediastinal staging in patients with clinical stage I non–small cell lung cancer and acknowledge that this conflicts with current guidelines, according to Biniam Kidane, MD, of the University of Manitoba, Winnipeg, in his invited comments on the study in the Journal of Thoracic and Cardiovascular Surgery (2017 Mar 10. doi: 10.1016/j.jtcvs.2017.02.051).
Their single-payer system is likely to have a different willingness-to-pay threshold, compared with those in other countries, especially the United States, where the EBUS-TBNA strategy without invasive staging is likely to remain the cost-effective choice.
“Cost-economic analyses such as these provide a window into the factors necessary to bridge guidelines from the realm of the abstract to the realm of local reality. When interpreting these findings, clinicians should consider: 1) What EBUS resources are available? (2) What is your local EBUS sensitivity? 3) What is the prevalence of MLNM?” Dr. Kidane concluded, with the caveat that such studies are not infallible and models are based on assumptions and must be treated with care.
Dr. Kidane reported no disclosures with regard to commercial support.
The authors make a compelling argument for invasive mediastinal staging in patients with clinical stage I non–small cell lung cancer and acknowledge that this conflicts with current guidelines, according to Biniam Kidane, MD, of the University of Manitoba, Winnipeg, in his invited comments on the study in the Journal of Thoracic and Cardiovascular Surgery (2017 Mar 10. doi: 10.1016/j.jtcvs.2017.02.051).
Their single-payer system is likely to have a different willingness-to-pay threshold, compared with those in other countries, especially the United States, where the EBUS-TBNA strategy without invasive staging is likely to remain the cost-effective choice.
“Cost-economic analyses such as these provide a window into the factors necessary to bridge guidelines from the realm of the abstract to the realm of local reality. When interpreting these findings, clinicians should consider: 1) What EBUS resources are available? (2) What is your local EBUS sensitivity? 3) What is the prevalence of MLNM?” Dr. Kidane concluded, with the caveat that such studies are not infallible and models are based on assumptions and must be treated with care.
Dr. Kidane reported no disclosures with regard to commercial support.
The authors make a compelling argument for invasive mediastinal staging in patients with clinical stage I non–small cell lung cancer and acknowledge that this conflicts with current guidelines, according to Biniam Kidane, MD, of the University of Manitoba, Winnipeg, in his invited comments on the study in the Journal of Thoracic and Cardiovascular Surgery (2017 Mar 10. doi: 10.1016/j.jtcvs.2017.02.051).
Their single-payer system is likely to have a different willingness-to-pay threshold, compared with those in other countries, especially the United States, where the EBUS-TBNA strategy without invasive staging is likely to remain the cost-effective choice.
“Cost-economic analyses such as these provide a window into the factors necessary to bridge guidelines from the realm of the abstract to the realm of local reality. When interpreting these findings, clinicians should consider: 1) What EBUS resources are available? (2) What is your local EBUS sensitivity? 3) What is the prevalence of MLNM?” Dr. Kidane concluded, with the caveat that such studies are not infallible and models are based on assumptions and must be treated with care.
Dr. Kidane reported no disclosures with regard to commercial support.
Endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) appears to be cost effective for use in non–small cell lung cancer (NSCLC) staging if the prevalence of mediastinal lymph node metastasis (MLNM) is greater than or equal to 2.5%, according to the results of single institution modeling study. In addition, the study found that confirmatory mediastinoscopy should be performed in high-risk patients in cases of negative EBUS-TBNA.
Katarzyna Czarnecka-Kujawa, MD, of the University of Toronto and Toronto General Hospital, and her colleagues performed a decision analysis to compare health outcomes and costs of four mediastinal staging strategies. They assessed the following: no invasive staging, endobronchial ultrasound-guided transbronchial need aspiration (EBUS-TBNA), mediastinoscopy, and EBUS-TBNA followed by mediastinoscopy if EBUS-TBNA results were negative. They determined incremental cost-effectiveness ratios (ICER) for all strategies and performed comprehensive sensitivity analyses using a willingness to pay threshold of $80,000 [Canadian]/quality adjusted life-year (QALY).
They used data obtained for staging, outcomes, and costs from the patients in the lung cancer program at the Toronto General Hospital from Jan. 1, 2005 to Dec. 31, 2014, as detailed in a report published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2017. doi: 10.1016/j.jtcvs.2016.12.048).
After exclusions, they utilized a final case count of 499 cases for developing their surgical and procedure cost analysis, and a total of 750 cases in their endoscopy database for endoscopy analysis. For the base-case analysis, they assumed a prevalence of mediastinal metastasis of 9%, and obtained the prevalence of a pathologic lymph nodal stage disease following EBUS-TBNA from their institutional data.
Their results showed that EBUS-TBNA followed by mediastinoscopy was the strategy that resulted in the highest QALYs, but that it had a prohibitive ICER of greater than $1.4 million/QALY. Accordingly, it may not be justifiable to use mediastinoscopy after negative EBUS-TBNA in all patients, the researchers noted. However, the researchers’ data suggest that invasive screening may be justified in a very-low-risk population (MLNM above 2.5%).
In addition, the researchers stated that “[the] benefit conveyed by detecting mediastinal metastatic disease becomes more apparent as the prevalence of MLNM increases, with confirmatory mediastinoscopy becoming cost effective in cases of negative EBUS-TBNA in patients with moderate to high probability of MLNM” (greater than 57%).
Our model points out that there is a well-defined role for the use of different modalities, including mediastinoscopy. This stresses the need for ongoing focus on maintenance of competency and skill acquisition in mediastinoscopy and EBUS-TBNA by currently practicing and future thoracic surgeons respectively,” the researchers concluded.
Dr. Czarnecka-Kujawa disclosed that she is a research consultant with Olympus America. The study was funded in part by agencies of the Austrian government.
Endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) appears to be cost effective for use in non–small cell lung cancer (NSCLC) staging if the prevalence of mediastinal lymph node metastasis (MLNM) is greater than or equal to 2.5%, according to the results of single institution modeling study. In addition, the study found that confirmatory mediastinoscopy should be performed in high-risk patients in cases of negative EBUS-TBNA.
Katarzyna Czarnecka-Kujawa, MD, of the University of Toronto and Toronto General Hospital, and her colleagues performed a decision analysis to compare health outcomes and costs of four mediastinal staging strategies. They assessed the following: no invasive staging, endobronchial ultrasound-guided transbronchial need aspiration (EBUS-TBNA), mediastinoscopy, and EBUS-TBNA followed by mediastinoscopy if EBUS-TBNA results were negative. They determined incremental cost-effectiveness ratios (ICER) for all strategies and performed comprehensive sensitivity analyses using a willingness to pay threshold of $80,000 [Canadian]/quality adjusted life-year (QALY).
They used data obtained for staging, outcomes, and costs from the patients in the lung cancer program at the Toronto General Hospital from Jan. 1, 2005 to Dec. 31, 2014, as detailed in a report published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2017. doi: 10.1016/j.jtcvs.2016.12.048).
After exclusions, they utilized a final case count of 499 cases for developing their surgical and procedure cost analysis, and a total of 750 cases in their endoscopy database for endoscopy analysis. For the base-case analysis, they assumed a prevalence of mediastinal metastasis of 9%, and obtained the prevalence of a pathologic lymph nodal stage disease following EBUS-TBNA from their institutional data.
Their results showed that EBUS-TBNA followed by mediastinoscopy was the strategy that resulted in the highest QALYs, but that it had a prohibitive ICER of greater than $1.4 million/QALY. Accordingly, it may not be justifiable to use mediastinoscopy after negative EBUS-TBNA in all patients, the researchers noted. However, the researchers’ data suggest that invasive screening may be justified in a very-low-risk population (MLNM above 2.5%).
In addition, the researchers stated that “[the] benefit conveyed by detecting mediastinal metastatic disease becomes more apparent as the prevalence of MLNM increases, with confirmatory mediastinoscopy becoming cost effective in cases of negative EBUS-TBNA in patients with moderate to high probability of MLNM” (greater than 57%).
Our model points out that there is a well-defined role for the use of different modalities, including mediastinoscopy. This stresses the need for ongoing focus on maintenance of competency and skill acquisition in mediastinoscopy and EBUS-TBNA by currently practicing and future thoracic surgeons respectively,” the researchers concluded.
Dr. Czarnecka-Kujawa disclosed that she is a research consultant with Olympus America. The study was funded in part by agencies of the Austrian government.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:
Major finding: Once the pathologic lymph nodal stage reaches 57%, EBUS-TBNA followed by mediastinoscopy is cost effective.
Data source: A model of health care outcomes and costs was developed from data obtained from patients treated over a 10-year period at a single institution.
Disclosures: Dr. Czarnecka-Kujawa disclosed that she is a research consultant with Olympus America. The study was funded in part by agencies of the Austrian government.