User login
RECAM vs. RUCAM: Finding a better way to diagnose DILI
Researchers looking for a better way to diagnose drug-induced liver injury (DILI) have found evidence to support the use of the Revised Electronic Causality Assessment Method (RECAM).
The broadly used Roussel Uclaf Causality Assessment Method (RUCAM), introduced in 1993, “has been a valuable clinical framework for DILI diagnosis,” but it has been clouded by subjectivity and poor reliability, wrote the authors, led by Paul H. Hayashi, MD, MPH, with the Food and Drug Administration, in Hepatology. Citing a review from the Journal of Hepatology, Dr. Hayashi and colleagues noted three major problems: “(1) unclear operating instructions and subjectivity leading to poor reliability and usability, (2) unclear validity due to lack of an accepted gold standard and (3) domain criteria that are not evidence-based.”
Currently, a diagnosis of DILI is primarily based on clinicians’ judgment and ruling out alternative diagnoses, the authors of this study wrote. The lack of an evidence-based and reliable diagnostic tool is a significant obstacle in clinical care and research .
Reaching a new method
The researchers used classification tree analysis to set diagnostic cut-offs for RECAM and then compared RECAM with RUCAM for correlation with expert opinion diagnostic categories in 194 DILI cases (98 from the Drug-Induced Liver Injury Network, 96 from the Spanish DILI Registry).
The area under receiver operator curves for identifying at least probable DILI were the same at 0.89 for both RECAM and RUCAM.
The authors wrote, “However, RECAM diagnostic categories have better observed overall agreement with expert opinion (0.62 vs. 0.56 weighted kappa, P = .14), and had better sensitivity to detect extreme diagnostic categories (73 vs. 54 for highly likely or high probable, P = .02; 65 vs. 48 for unlikely/excluded, P = .08) than RUCAM diagnostic categories.”
They concluded that RECAM “is at least as capable as RUCAM in diagnosing DILI compared to expert opinion but is better than RUCAM at the diagnostic extremes.”
RECAM appears to add objectivity and clarity that can improve precision and reliability when diagnosing DILI and improve diagnostic standardization, according to authors. It has automated scoring, which reduces subjective input and should lead to better reliability among raters, something that has limited RUCAM’s adaptation in clinical practice and research.
RECAM has automatic warnings for data inconsistencies, which DILI and RUCAM do not. In RUCAM, a different diagnosis or other data could rule out DILI, but the case would still gain points in other criteria.
The authors explained, “Even when data clearly diagnose acute viral hepatitis or autoimmune hepatitis by simplified autoimmune hepatitis score, points are still given for latency, dechallenge, or underlying hepatotoxicity risk of the drug. In these situations of highly implausible DILI, RECAM gives warnings to stop with an imputed total score of –6. One can over-ride these warnings, if one believes DILI may be concurrent with the non-DILI diagnosis. However, –6 points are still assessed.”
Diagnosis of exclusion
Paul Martin, MD, chief, division of digestive health and liver diseases, Mandel Chair in Gastroenterology and professor of medicine at the University of Miami, said in an interview that he hopes RECAM will become widely used and better address a condition that sometimes doesn’t get enough attention. DILI remains underappreciated, he said, despite it being a major cause of morbidity and mortality in some patients.
“Any algorithm or criteria that can improve diagnostic accuracy is useful because typically it is a diagnosis of exclusion,” Dr. Martin said. “This new system seems to be as good as any other prior algorithms to diagnose drug-induced liver injury.”
He added, “This should help clinicians with individual patients with unexplained liver disease.”
The authors noted some limitations. RECAM was developed in U.S. and Spanish cohorts, so its performance in other regions is unclear. Both registries have minimum enrollment requirements for liver enzyme and bilirubin elevation, so it is not known how effective RECAM is in less severe cases. It also needs to be tested by other clinicians, including nonhepatologists.
The authors also added, “It is currently limited to single-agent medication cases leaving the user to score each medication individually in multidrug cases. However, any competing medication causing loss of points in the RUCAM, probably deserves its own RECAM score.”
The DILIN is structured as a cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Hayashi is employed by the FDA, but the conclusions of this paper do not reflect any opinion of the FDA. One coauthor has advised Pfizer, GSK, and NuCANA through Nottingham University Consultants, and another has received support from Gilead and AbbVie and consulted for Sanofi. The remaining authors have no conflicts. Dr. Martin reports no relevant financial relationships.
Researchers looking for a better way to diagnose drug-induced liver injury (DILI) have found evidence to support the use of the Revised Electronic Causality Assessment Method (RECAM).
The broadly used Roussel Uclaf Causality Assessment Method (RUCAM), introduced in 1993, “has been a valuable clinical framework for DILI diagnosis,” but it has been clouded by subjectivity and poor reliability, wrote the authors, led by Paul H. Hayashi, MD, MPH, with the Food and Drug Administration, in Hepatology. Citing a review from the Journal of Hepatology, Dr. Hayashi and colleagues noted three major problems: “(1) unclear operating instructions and subjectivity leading to poor reliability and usability, (2) unclear validity due to lack of an accepted gold standard and (3) domain criteria that are not evidence-based.”
Currently, a diagnosis of DILI is primarily based on clinicians’ judgment and ruling out alternative diagnoses, the authors of this study wrote. The lack of an evidence-based and reliable diagnostic tool is a significant obstacle in clinical care and research .
Reaching a new method
The researchers used classification tree analysis to set diagnostic cut-offs for RECAM and then compared RECAM with RUCAM for correlation with expert opinion diagnostic categories in 194 DILI cases (98 from the Drug-Induced Liver Injury Network, 96 from the Spanish DILI Registry).
The area under receiver operator curves for identifying at least probable DILI were the same at 0.89 for both RECAM and RUCAM.
The authors wrote, “However, RECAM diagnostic categories have better observed overall agreement with expert opinion (0.62 vs. 0.56 weighted kappa, P = .14), and had better sensitivity to detect extreme diagnostic categories (73 vs. 54 for highly likely or high probable, P = .02; 65 vs. 48 for unlikely/excluded, P = .08) than RUCAM diagnostic categories.”
They concluded that RECAM “is at least as capable as RUCAM in diagnosing DILI compared to expert opinion but is better than RUCAM at the diagnostic extremes.”
RECAM appears to add objectivity and clarity that can improve precision and reliability when diagnosing DILI and improve diagnostic standardization, according to authors. It has automated scoring, which reduces subjective input and should lead to better reliability among raters, something that has limited RUCAM’s adaptation in clinical practice and research.
RECAM has automatic warnings for data inconsistencies, which DILI and RUCAM do not. In RUCAM, a different diagnosis or other data could rule out DILI, but the case would still gain points in other criteria.
The authors explained, “Even when data clearly diagnose acute viral hepatitis or autoimmune hepatitis by simplified autoimmune hepatitis score, points are still given for latency, dechallenge, or underlying hepatotoxicity risk of the drug. In these situations of highly implausible DILI, RECAM gives warnings to stop with an imputed total score of –6. One can over-ride these warnings, if one believes DILI may be concurrent with the non-DILI diagnosis. However, –6 points are still assessed.”
Diagnosis of exclusion
Paul Martin, MD, chief, division of digestive health and liver diseases, Mandel Chair in Gastroenterology and professor of medicine at the University of Miami, said in an interview that he hopes RECAM will become widely used and better address a condition that sometimes doesn’t get enough attention. DILI remains underappreciated, he said, despite it being a major cause of morbidity and mortality in some patients.
“Any algorithm or criteria that can improve diagnostic accuracy is useful because typically it is a diagnosis of exclusion,” Dr. Martin said. “This new system seems to be as good as any other prior algorithms to diagnose drug-induced liver injury.”
He added, “This should help clinicians with individual patients with unexplained liver disease.”
The authors noted some limitations. RECAM was developed in U.S. and Spanish cohorts, so its performance in other regions is unclear. Both registries have minimum enrollment requirements for liver enzyme and bilirubin elevation, so it is not known how effective RECAM is in less severe cases. It also needs to be tested by other clinicians, including nonhepatologists.
The authors also added, “It is currently limited to single-agent medication cases leaving the user to score each medication individually in multidrug cases. However, any competing medication causing loss of points in the RUCAM, probably deserves its own RECAM score.”
The DILIN is structured as a cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Hayashi is employed by the FDA, but the conclusions of this paper do not reflect any opinion of the FDA. One coauthor has advised Pfizer, GSK, and NuCANA through Nottingham University Consultants, and another has received support from Gilead and AbbVie and consulted for Sanofi. The remaining authors have no conflicts. Dr. Martin reports no relevant financial relationships.
Researchers looking for a better way to diagnose drug-induced liver injury (DILI) have found evidence to support the use of the Revised Electronic Causality Assessment Method (RECAM).
The broadly used Roussel Uclaf Causality Assessment Method (RUCAM), introduced in 1993, “has been a valuable clinical framework for DILI diagnosis,” but it has been clouded by subjectivity and poor reliability, wrote the authors, led by Paul H. Hayashi, MD, MPH, with the Food and Drug Administration, in Hepatology. Citing a review from the Journal of Hepatology, Dr. Hayashi and colleagues noted three major problems: “(1) unclear operating instructions and subjectivity leading to poor reliability and usability, (2) unclear validity due to lack of an accepted gold standard and (3) domain criteria that are not evidence-based.”
Currently, a diagnosis of DILI is primarily based on clinicians’ judgment and ruling out alternative diagnoses, the authors of this study wrote. The lack of an evidence-based and reliable diagnostic tool is a significant obstacle in clinical care and research .
Reaching a new method
The researchers used classification tree analysis to set diagnostic cut-offs for RECAM and then compared RECAM with RUCAM for correlation with expert opinion diagnostic categories in 194 DILI cases (98 from the Drug-Induced Liver Injury Network, 96 from the Spanish DILI Registry).
The area under receiver operator curves for identifying at least probable DILI were the same at 0.89 for both RECAM and RUCAM.
The authors wrote, “However, RECAM diagnostic categories have better observed overall agreement with expert opinion (0.62 vs. 0.56 weighted kappa, P = .14), and had better sensitivity to detect extreme diagnostic categories (73 vs. 54 for highly likely or high probable, P = .02; 65 vs. 48 for unlikely/excluded, P = .08) than RUCAM diagnostic categories.”
They concluded that RECAM “is at least as capable as RUCAM in diagnosing DILI compared to expert opinion but is better than RUCAM at the diagnostic extremes.”
RECAM appears to add objectivity and clarity that can improve precision and reliability when diagnosing DILI and improve diagnostic standardization, according to authors. It has automated scoring, which reduces subjective input and should lead to better reliability among raters, something that has limited RUCAM’s adaptation in clinical practice and research.
RECAM has automatic warnings for data inconsistencies, which DILI and RUCAM do not. In RUCAM, a different diagnosis or other data could rule out DILI, but the case would still gain points in other criteria.
The authors explained, “Even when data clearly diagnose acute viral hepatitis or autoimmune hepatitis by simplified autoimmune hepatitis score, points are still given for latency, dechallenge, or underlying hepatotoxicity risk of the drug. In these situations of highly implausible DILI, RECAM gives warnings to stop with an imputed total score of –6. One can over-ride these warnings, if one believes DILI may be concurrent with the non-DILI diagnosis. However, –6 points are still assessed.”
Diagnosis of exclusion
Paul Martin, MD, chief, division of digestive health and liver diseases, Mandel Chair in Gastroenterology and professor of medicine at the University of Miami, said in an interview that he hopes RECAM will become widely used and better address a condition that sometimes doesn’t get enough attention. DILI remains underappreciated, he said, despite it being a major cause of morbidity and mortality in some patients.
“Any algorithm or criteria that can improve diagnostic accuracy is useful because typically it is a diagnosis of exclusion,” Dr. Martin said. “This new system seems to be as good as any other prior algorithms to diagnose drug-induced liver injury.”
He added, “This should help clinicians with individual patients with unexplained liver disease.”
The authors noted some limitations. RECAM was developed in U.S. and Spanish cohorts, so its performance in other regions is unclear. Both registries have minimum enrollment requirements for liver enzyme and bilirubin elevation, so it is not known how effective RECAM is in less severe cases. It also needs to be tested by other clinicians, including nonhepatologists.
The authors also added, “It is currently limited to single-agent medication cases leaving the user to score each medication individually in multidrug cases. However, any competing medication causing loss of points in the RUCAM, probably deserves its own RECAM score.”
The DILIN is structured as a cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Hayashi is employed by the FDA, but the conclusions of this paper do not reflect any opinion of the FDA. One coauthor has advised Pfizer, GSK, and NuCANA through Nottingham University Consultants, and another has received support from Gilead and AbbVie and consulted for Sanofi. The remaining authors have no conflicts. Dr. Martin reports no relevant financial relationships.
FROM HEPATOLOGY
Antibody mix may prevent COVID symptoms in some asymptomatic people
over 28 days, new research shows.
Results of the study by Meagan P. O’Brien, MD, from Regeneron Pharmaceuticals and one of the study’s funders, and coauthors were published online Jan. 14, 2022, in an original investigation in JAMA.
The results suggest new potential for monoclonal antibodies currently used for postexposure prophylaxis and treatment of symptomatic SARS-CoV-2. It has not been clear whether monoclonal antibodies can benefit people with asymptomatic SARS-CoV-2 infection.
The trial included 314 participants (mean age, 41 years; 51.6% women). Of the participants, 310 (99.7%) completed the efficacy assessment period, and 204 were asymptomatic and tested negative at baseline and were included in the primary efficacy analysis.
The subcutaneous combination of casirivimab and imdevimab, 1,200 mg (600 mg each), significantly prevented progression to symptomatic disease (29/100 [29.0%] vs. 44/104 [42.3%] with placebo; odds ratio, 0.54 [95% confidence interval, 0.30-0.97]; P = .04; absolute risk difference, −13.3% [95% CI, −26.3% to −0.3%]).
These results were part of a randomized, double-blind, placebo-controlled, phase 3 trial of close household contacts of a SARS-CoV-2–infected person at 112 sites in the United States, Romania, and Moldova. They were enrolled between July 13, 2020, and Jan. 28, 2021; follow-up ended March 11, 2021.
Asymptomatic people at least 12 years old were eligible if identified within 96 hours of index case positive test collection and were randomly assigned 1:1 to receive one dose of subcutaneous casirivimab and imdevimab (n = 158), or placebo (n = 156).
COVID-19 vaccination was prohibited before enrollment but was allowed after completing the 28-day efficacy assessment period.
Caution warranted
In an accompanying editorial, however, Jonathan Z. Li, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and Rajesh T. Gandhi, MD, Massachusetts General Hospital, Boston, and Harvard Medical School, urged caution in interpreting the results.
They wrote that, although monoclonal antibodies are generally used in individuals at high risk for severe COVID-19, this study population was less vulnerable, with an average age of 41, and 30% had no risk for the disease.
“Of the remainder, the most common risk factor was being overweight (which confers less risk than other factors),” the editorialists wrote.
They pointed out, as did the study authors, that enrollment came before the emergence of the Delta and Omicron variants, and that both casirivimab and imdevimab maintain their activity against Delta but not against Omicron.
“While prevention of symptomatic infection has benefits,” they wrote, “the primary goal of monoclonal antibody therapy is to prevent progression to severe disease; however, this trial was unable to assess this outcome because there were only three hospitalizations (all in the placebo group). Also, this study was conducted prior to widespread COVID-19 vaccination; whether monoclonal antibodies have the same benefit in people who have breakthrough infection after vaccination is not known.”
The editorialists highlighted the subcutaneous delivery in this study.
They wrote that Dr. O’Brien and coauthors provide evidence that subcutaneous administration is effective in infected individuals. “However, high serum monoclonal antibody levels are achieved more quickly after intravenous administration than following subcutaneous injection; it is unknown whether intravenous administration might have led to even greater efficacy for individuals with asymptomatic SARS-CoV-2 infection.”
The authors of the study also add that, despite efforts to recruit non-White participants, relatively few non-White people were enrolled. Additionally, few adolescents were enrolled.
The sample size was also relatively small, they acknowledge, because of a study design in which the infection status of asymptomatic participants was not confirmed at inclusion.
Several of the authors are employees/stockholders of Regeneron, and have a patent pending, which has been licensed and is receiving royalties. The study was supported by Regeneron and F. Hoffmann–La Roche. This trial was conducted jointly with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. The CoVPN (COVID-19 Prevention Network) is supported by cooperative agreement awards from the NIAID and NIH.
A version of this article first appeared on Medscape.com.
over 28 days, new research shows.
Results of the study by Meagan P. O’Brien, MD, from Regeneron Pharmaceuticals and one of the study’s funders, and coauthors were published online Jan. 14, 2022, in an original investigation in JAMA.
The results suggest new potential for monoclonal antibodies currently used for postexposure prophylaxis and treatment of symptomatic SARS-CoV-2. It has not been clear whether monoclonal antibodies can benefit people with asymptomatic SARS-CoV-2 infection.
The trial included 314 participants (mean age, 41 years; 51.6% women). Of the participants, 310 (99.7%) completed the efficacy assessment period, and 204 were asymptomatic and tested negative at baseline and were included in the primary efficacy analysis.
The subcutaneous combination of casirivimab and imdevimab, 1,200 mg (600 mg each), significantly prevented progression to symptomatic disease (29/100 [29.0%] vs. 44/104 [42.3%] with placebo; odds ratio, 0.54 [95% confidence interval, 0.30-0.97]; P = .04; absolute risk difference, −13.3% [95% CI, −26.3% to −0.3%]).
These results were part of a randomized, double-blind, placebo-controlled, phase 3 trial of close household contacts of a SARS-CoV-2–infected person at 112 sites in the United States, Romania, and Moldova. They were enrolled between July 13, 2020, and Jan. 28, 2021; follow-up ended March 11, 2021.
Asymptomatic people at least 12 years old were eligible if identified within 96 hours of index case positive test collection and were randomly assigned 1:1 to receive one dose of subcutaneous casirivimab and imdevimab (n = 158), or placebo (n = 156).
COVID-19 vaccination was prohibited before enrollment but was allowed after completing the 28-day efficacy assessment period.
Caution warranted
In an accompanying editorial, however, Jonathan Z. Li, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and Rajesh T. Gandhi, MD, Massachusetts General Hospital, Boston, and Harvard Medical School, urged caution in interpreting the results.
They wrote that, although monoclonal antibodies are generally used in individuals at high risk for severe COVID-19, this study population was less vulnerable, with an average age of 41, and 30% had no risk for the disease.
“Of the remainder, the most common risk factor was being overweight (which confers less risk than other factors),” the editorialists wrote.
They pointed out, as did the study authors, that enrollment came before the emergence of the Delta and Omicron variants, and that both casirivimab and imdevimab maintain their activity against Delta but not against Omicron.
“While prevention of symptomatic infection has benefits,” they wrote, “the primary goal of monoclonal antibody therapy is to prevent progression to severe disease; however, this trial was unable to assess this outcome because there were only three hospitalizations (all in the placebo group). Also, this study was conducted prior to widespread COVID-19 vaccination; whether monoclonal antibodies have the same benefit in people who have breakthrough infection after vaccination is not known.”
The editorialists highlighted the subcutaneous delivery in this study.
They wrote that Dr. O’Brien and coauthors provide evidence that subcutaneous administration is effective in infected individuals. “However, high serum monoclonal antibody levels are achieved more quickly after intravenous administration than following subcutaneous injection; it is unknown whether intravenous administration might have led to even greater efficacy for individuals with asymptomatic SARS-CoV-2 infection.”
The authors of the study also add that, despite efforts to recruit non-White participants, relatively few non-White people were enrolled. Additionally, few adolescents were enrolled.
The sample size was also relatively small, they acknowledge, because of a study design in which the infection status of asymptomatic participants was not confirmed at inclusion.
Several of the authors are employees/stockholders of Regeneron, and have a patent pending, which has been licensed and is receiving royalties. The study was supported by Regeneron and F. Hoffmann–La Roche. This trial was conducted jointly with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. The CoVPN (COVID-19 Prevention Network) is supported by cooperative agreement awards from the NIAID and NIH.
A version of this article first appeared on Medscape.com.
over 28 days, new research shows.
Results of the study by Meagan P. O’Brien, MD, from Regeneron Pharmaceuticals and one of the study’s funders, and coauthors were published online Jan. 14, 2022, in an original investigation in JAMA.
The results suggest new potential for monoclonal antibodies currently used for postexposure prophylaxis and treatment of symptomatic SARS-CoV-2. It has not been clear whether monoclonal antibodies can benefit people with asymptomatic SARS-CoV-2 infection.
The trial included 314 participants (mean age, 41 years; 51.6% women). Of the participants, 310 (99.7%) completed the efficacy assessment period, and 204 were asymptomatic and tested negative at baseline and were included in the primary efficacy analysis.
The subcutaneous combination of casirivimab and imdevimab, 1,200 mg (600 mg each), significantly prevented progression to symptomatic disease (29/100 [29.0%] vs. 44/104 [42.3%] with placebo; odds ratio, 0.54 [95% confidence interval, 0.30-0.97]; P = .04; absolute risk difference, −13.3% [95% CI, −26.3% to −0.3%]).
These results were part of a randomized, double-blind, placebo-controlled, phase 3 trial of close household contacts of a SARS-CoV-2–infected person at 112 sites in the United States, Romania, and Moldova. They were enrolled between July 13, 2020, and Jan. 28, 2021; follow-up ended March 11, 2021.
Asymptomatic people at least 12 years old were eligible if identified within 96 hours of index case positive test collection and were randomly assigned 1:1 to receive one dose of subcutaneous casirivimab and imdevimab (n = 158), or placebo (n = 156).
COVID-19 vaccination was prohibited before enrollment but was allowed after completing the 28-day efficacy assessment period.
Caution warranted
In an accompanying editorial, however, Jonathan Z. Li, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and Rajesh T. Gandhi, MD, Massachusetts General Hospital, Boston, and Harvard Medical School, urged caution in interpreting the results.
They wrote that, although monoclonal antibodies are generally used in individuals at high risk for severe COVID-19, this study population was less vulnerable, with an average age of 41, and 30% had no risk for the disease.
“Of the remainder, the most common risk factor was being overweight (which confers less risk than other factors),” the editorialists wrote.
They pointed out, as did the study authors, that enrollment came before the emergence of the Delta and Omicron variants, and that both casirivimab and imdevimab maintain their activity against Delta but not against Omicron.
“While prevention of symptomatic infection has benefits,” they wrote, “the primary goal of monoclonal antibody therapy is to prevent progression to severe disease; however, this trial was unable to assess this outcome because there were only three hospitalizations (all in the placebo group). Also, this study was conducted prior to widespread COVID-19 vaccination; whether monoclonal antibodies have the same benefit in people who have breakthrough infection after vaccination is not known.”
The editorialists highlighted the subcutaneous delivery in this study.
They wrote that Dr. O’Brien and coauthors provide evidence that subcutaneous administration is effective in infected individuals. “However, high serum monoclonal antibody levels are achieved more quickly after intravenous administration than following subcutaneous injection; it is unknown whether intravenous administration might have led to even greater efficacy for individuals with asymptomatic SARS-CoV-2 infection.”
The authors of the study also add that, despite efforts to recruit non-White participants, relatively few non-White people were enrolled. Additionally, few adolescents were enrolled.
The sample size was also relatively small, they acknowledge, because of a study design in which the infection status of asymptomatic participants was not confirmed at inclusion.
Several of the authors are employees/stockholders of Regeneron, and have a patent pending, which has been licensed and is receiving royalties. The study was supported by Regeneron and F. Hoffmann–La Roche. This trial was conducted jointly with the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. The CoVPN (COVID-19 Prevention Network) is supported by cooperative agreement awards from the NIAID and NIH.
A version of this article first appeared on Medscape.com.
FROM JAMA
Childhood trauma may influence vaccine hesitancy
, data published Feb. 1 suggest.
The findings by Mark A. Bellis, DSc, College of Human Sciences, Bangor (Wales) University, and colleagues were published online in BMJ Open.
The results are especially significant, the authors say, because of the prevalence of adverse childhood experiences (ACEs) globally, with proportions of people having multiple traumas in some countries at 10% or more of the population.
The authors wrote that hesitancy or refusal to get the vaccine increased with the number of traumas reported.
For example, hesitancy was three times higher among people who had experienced four or more types of childhood trauma than among those who did not report any traumatic events.
Dr. Bellis told this news organization that though their work suggests that higher levels of ACEs are linked with higher vaccine hesitancy, it is by no means the only reason people choose not to get vaccinated.
However, he said, the association they found may have key messages for clinicians.
“For clinicians, simply being trauma informed can help,” Dr. Bellis said. “Understanding how such childhood adversity can affect people may help them when discussing vaccines, and in understanding resistance to what is a complex medical issue and one that requires considerable trust. What can appear routine to a clinician may be a difficult leap of faith especially for those who have poorer experiences of trusting even within family settings.”
More trauma, less trust
The authors used responses to a nationally representative telephone survey of adults in Wales taken between December 2020 and March 2021, when COVID-19 restrictions were in force. Out of 6,763 people contacted, 2,285 met all criteria and answered all the questions and were included in the final analysis.
The survey asked about nine types of ACEs before the age of 18, including: parental separation; physical, verbal, and sexual abuse; exposure to domestic violence; and living with a household member who has mental illness, misuses alcohol and/or drugs, or who was incarcerated.
It also included personal details and long-term health information.
About half of the respondents said they hadn’t experienced any childhood trauma. Of those who did, one in five said they had experienced one type, 17% reported two to three types, and 10% reported four or more.
According to the authors, prevalence of ACEs reported was consistent with other comparable population surveys, including those conducted face to face.
They also investigated measures of trust and preference for different health regulations.
People with more ACEs were more likely to have low trust in National Health Service COVID-19 information.
“Other sociodemographics and a history of either chronic disease or COVID-19 infection were not significantly associated with low trust,” the authors pointed out.
People reporting higher ACEs also were more likely to report that they felt they were unfairly restricted by the government. People with four or more ACEs were twice as likely than were those with no ACEs to say they felt unfairly restricted and wanted rules such as mandatory masking to stop.
People with four or more types of trauma were almost twice as likely to ignore the restrictions as were those who hadn’t experienced any – 38% versus 21% – to ignore the restrictions, even after the researchers accounted for associations with sociodemographic factors and previous COVID-19 infection or a history of long-term conditions.
“Clinicians can be a powerful voice to counter more alarmist or even conspiratorial messages that might otherwise resonate with those who find trust difficult,” Dr. Bellis said.
He said that the effect of childhood adversity needs to be considered at all levels in health systems. Overarching public health strategists should include ways to earn trust to counter resistance in some of the most vulnerable communities where ACEs can be higher.
It will also be important in the short-term to “provide reassurance, build community champions, and understand the low base from which trust needs to be built,” he said.
Loss of control
“Past traumatic experiences can predispose someone to avoid things that remind them of that trauma. This avoidance protects them from re-experiencing the negative symptoms and behaviors that come with it. Whether this results into hesitancy of something that would benefit their health is not well known,” Consuelo Cagande, MD, senior associate program director and fellowship adviser in the department of child and adolescent psychiatry and behavioral sciences, Children’s Hospital of Philadelphia, told this news organization.
She pointed out a limitation the authors mention that is common when using ACEs as a measure linking to future negative behaviors – that people self-report them and may misremember or misreport them.
Another limitation is the potential for self-selection bias, as participation level was 36.4%, though the authors noted that is not unusual for unsolicited telephone surveys.
Dr. Cagande said that fearing loss of control may be another factor at play in having to follow restrictions, such as quarantining and masking, social distancing, or mandated vaccinations.
She said it’s important to understand a person’s reason for hesitancy to vaccines and work with the person with the help of the community, to help them trust and feel safe.
Young adults of particular concern
The 18- to 29-year-old age group is of particular concern, Dr. Bellis said.
The researchers estimated the likely rates of vaccine hesitancy according to childhood trauma and age, and the numbers ranged from around 3.5% among those aged 70 and older with no experience of childhood adversity to 38% among 18- to 29-year-olds who had experienced four or more types of childhood trauma.
“Childhood adversity can be an especially raw issue in this group,” he explained. “Some have already been obliged to sacrifice substantial proportions of their teenage lives and some will have suffered greater exposure to adverse childhood experiences as a result of being isolated during the pandemic, sometimes in difficult home environments. Our results suggest that this age group and especially those with high levels of ACEs are some of the most likely to be vaccine hesitant.”
This work was supported by Public Health Wales. The study authors and Dr. Cagande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, data published Feb. 1 suggest.
The findings by Mark A. Bellis, DSc, College of Human Sciences, Bangor (Wales) University, and colleagues were published online in BMJ Open.
The results are especially significant, the authors say, because of the prevalence of adverse childhood experiences (ACEs) globally, with proportions of people having multiple traumas in some countries at 10% or more of the population.
The authors wrote that hesitancy or refusal to get the vaccine increased with the number of traumas reported.
For example, hesitancy was three times higher among people who had experienced four or more types of childhood trauma than among those who did not report any traumatic events.
Dr. Bellis told this news organization that though their work suggests that higher levels of ACEs are linked with higher vaccine hesitancy, it is by no means the only reason people choose not to get vaccinated.
However, he said, the association they found may have key messages for clinicians.
“For clinicians, simply being trauma informed can help,” Dr. Bellis said. “Understanding how such childhood adversity can affect people may help them when discussing vaccines, and in understanding resistance to what is a complex medical issue and one that requires considerable trust. What can appear routine to a clinician may be a difficult leap of faith especially for those who have poorer experiences of trusting even within family settings.”
More trauma, less trust
The authors used responses to a nationally representative telephone survey of adults in Wales taken between December 2020 and March 2021, when COVID-19 restrictions were in force. Out of 6,763 people contacted, 2,285 met all criteria and answered all the questions and were included in the final analysis.
The survey asked about nine types of ACEs before the age of 18, including: parental separation; physical, verbal, and sexual abuse; exposure to domestic violence; and living with a household member who has mental illness, misuses alcohol and/or drugs, or who was incarcerated.
It also included personal details and long-term health information.
About half of the respondents said they hadn’t experienced any childhood trauma. Of those who did, one in five said they had experienced one type, 17% reported two to three types, and 10% reported four or more.
According to the authors, prevalence of ACEs reported was consistent with other comparable population surveys, including those conducted face to face.
They also investigated measures of trust and preference for different health regulations.
People with more ACEs were more likely to have low trust in National Health Service COVID-19 information.
“Other sociodemographics and a history of either chronic disease or COVID-19 infection were not significantly associated with low trust,” the authors pointed out.
People reporting higher ACEs also were more likely to report that they felt they were unfairly restricted by the government. People with four or more ACEs were twice as likely than were those with no ACEs to say they felt unfairly restricted and wanted rules such as mandatory masking to stop.
People with four or more types of trauma were almost twice as likely to ignore the restrictions as were those who hadn’t experienced any – 38% versus 21% – to ignore the restrictions, even after the researchers accounted for associations with sociodemographic factors and previous COVID-19 infection or a history of long-term conditions.
“Clinicians can be a powerful voice to counter more alarmist or even conspiratorial messages that might otherwise resonate with those who find trust difficult,” Dr. Bellis said.
He said that the effect of childhood adversity needs to be considered at all levels in health systems. Overarching public health strategists should include ways to earn trust to counter resistance in some of the most vulnerable communities where ACEs can be higher.
It will also be important in the short-term to “provide reassurance, build community champions, and understand the low base from which trust needs to be built,” he said.
Loss of control
“Past traumatic experiences can predispose someone to avoid things that remind them of that trauma. This avoidance protects them from re-experiencing the negative symptoms and behaviors that come with it. Whether this results into hesitancy of something that would benefit their health is not well known,” Consuelo Cagande, MD, senior associate program director and fellowship adviser in the department of child and adolescent psychiatry and behavioral sciences, Children’s Hospital of Philadelphia, told this news organization.
She pointed out a limitation the authors mention that is common when using ACEs as a measure linking to future negative behaviors – that people self-report them and may misremember or misreport them.
Another limitation is the potential for self-selection bias, as participation level was 36.4%, though the authors noted that is not unusual for unsolicited telephone surveys.
Dr. Cagande said that fearing loss of control may be another factor at play in having to follow restrictions, such as quarantining and masking, social distancing, or mandated vaccinations.
She said it’s important to understand a person’s reason for hesitancy to vaccines and work with the person with the help of the community, to help them trust and feel safe.
Young adults of particular concern
The 18- to 29-year-old age group is of particular concern, Dr. Bellis said.
The researchers estimated the likely rates of vaccine hesitancy according to childhood trauma and age, and the numbers ranged from around 3.5% among those aged 70 and older with no experience of childhood adversity to 38% among 18- to 29-year-olds who had experienced four or more types of childhood trauma.
“Childhood adversity can be an especially raw issue in this group,” he explained. “Some have already been obliged to sacrifice substantial proportions of their teenage lives and some will have suffered greater exposure to adverse childhood experiences as a result of being isolated during the pandemic, sometimes in difficult home environments. Our results suggest that this age group and especially those with high levels of ACEs are some of the most likely to be vaccine hesitant.”
This work was supported by Public Health Wales. The study authors and Dr. Cagande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, data published Feb. 1 suggest.
The findings by Mark A. Bellis, DSc, College of Human Sciences, Bangor (Wales) University, and colleagues were published online in BMJ Open.
The results are especially significant, the authors say, because of the prevalence of adverse childhood experiences (ACEs) globally, with proportions of people having multiple traumas in some countries at 10% or more of the population.
The authors wrote that hesitancy or refusal to get the vaccine increased with the number of traumas reported.
For example, hesitancy was three times higher among people who had experienced four or more types of childhood trauma than among those who did not report any traumatic events.
Dr. Bellis told this news organization that though their work suggests that higher levels of ACEs are linked with higher vaccine hesitancy, it is by no means the only reason people choose not to get vaccinated.
However, he said, the association they found may have key messages for clinicians.
“For clinicians, simply being trauma informed can help,” Dr. Bellis said. “Understanding how such childhood adversity can affect people may help them when discussing vaccines, and in understanding resistance to what is a complex medical issue and one that requires considerable trust. What can appear routine to a clinician may be a difficult leap of faith especially for those who have poorer experiences of trusting even within family settings.”
More trauma, less trust
The authors used responses to a nationally representative telephone survey of adults in Wales taken between December 2020 and March 2021, when COVID-19 restrictions were in force. Out of 6,763 people contacted, 2,285 met all criteria and answered all the questions and were included in the final analysis.
The survey asked about nine types of ACEs before the age of 18, including: parental separation; physical, verbal, and sexual abuse; exposure to domestic violence; and living with a household member who has mental illness, misuses alcohol and/or drugs, or who was incarcerated.
It also included personal details and long-term health information.
About half of the respondents said they hadn’t experienced any childhood trauma. Of those who did, one in five said they had experienced one type, 17% reported two to three types, and 10% reported four or more.
According to the authors, prevalence of ACEs reported was consistent with other comparable population surveys, including those conducted face to face.
They also investigated measures of trust and preference for different health regulations.
People with more ACEs were more likely to have low trust in National Health Service COVID-19 information.
“Other sociodemographics and a history of either chronic disease or COVID-19 infection were not significantly associated with low trust,” the authors pointed out.
People reporting higher ACEs also were more likely to report that they felt they were unfairly restricted by the government. People with four or more ACEs were twice as likely than were those with no ACEs to say they felt unfairly restricted and wanted rules such as mandatory masking to stop.
People with four or more types of trauma were almost twice as likely to ignore the restrictions as were those who hadn’t experienced any – 38% versus 21% – to ignore the restrictions, even after the researchers accounted for associations with sociodemographic factors and previous COVID-19 infection or a history of long-term conditions.
“Clinicians can be a powerful voice to counter more alarmist or even conspiratorial messages that might otherwise resonate with those who find trust difficult,” Dr. Bellis said.
He said that the effect of childhood adversity needs to be considered at all levels in health systems. Overarching public health strategists should include ways to earn trust to counter resistance in some of the most vulnerable communities where ACEs can be higher.
It will also be important in the short-term to “provide reassurance, build community champions, and understand the low base from which trust needs to be built,” he said.
Loss of control
“Past traumatic experiences can predispose someone to avoid things that remind them of that trauma. This avoidance protects them from re-experiencing the negative symptoms and behaviors that come with it. Whether this results into hesitancy of something that would benefit their health is not well known,” Consuelo Cagande, MD, senior associate program director and fellowship adviser in the department of child and adolescent psychiatry and behavioral sciences, Children’s Hospital of Philadelphia, told this news organization.
She pointed out a limitation the authors mention that is common when using ACEs as a measure linking to future negative behaviors – that people self-report them and may misremember or misreport them.
Another limitation is the potential for self-selection bias, as participation level was 36.4%, though the authors noted that is not unusual for unsolicited telephone surveys.
Dr. Cagande said that fearing loss of control may be another factor at play in having to follow restrictions, such as quarantining and masking, social distancing, or mandated vaccinations.
She said it’s important to understand a person’s reason for hesitancy to vaccines and work with the person with the help of the community, to help them trust and feel safe.
Young adults of particular concern
The 18- to 29-year-old age group is of particular concern, Dr. Bellis said.
The researchers estimated the likely rates of vaccine hesitancy according to childhood trauma and age, and the numbers ranged from around 3.5% among those aged 70 and older with no experience of childhood adversity to 38% among 18- to 29-year-olds who had experienced four or more types of childhood trauma.
“Childhood adversity can be an especially raw issue in this group,” he explained. “Some have already been obliged to sacrifice substantial proportions of their teenage lives and some will have suffered greater exposure to adverse childhood experiences as a result of being isolated during the pandemic, sometimes in difficult home environments. Our results suggest that this age group and especially those with high levels of ACEs are some of the most likely to be vaccine hesitant.”
This work was supported by Public Health Wales. The study authors and Dr. Cagande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Kids’ mask use linked with fewer childcare closings
Mask-wearing in childcare programs is linked with fewer COVID-19–related program closures, new data released suggest.
Researchers included 6,654 childcare professionals in a prospective, 1-year, longitudinal electronic survey study of home- and center-based childcare programs in all 50 states.
Findings by Thomas S. Murray, MD, PhD, with the department of pediatrics, Yale University, New Haven, Conn., and coauthors, were published in JAMA Network Open on Jan. 28, 2022.
They found that mask-wearing from the May 22, 2020, baseline to June 8, 2020, was associated with a 13% reduction in program closures within the following year (adjusted relative risk, 0.87; 95% confidence interval, 0.77-0.99). Continued mask-wearing throughout the 1-year follow-up was associated with a 14% reduction in program closures (aRR, 0.86; 95% CI, 0.74-1.00).
The authors said the evidence supports current masking recommendation in younger children provided by the Centers for Disease Control and Prevention.
They wrote: “This finding has important public health policy implications for families that rely on childcare to sustain employment.”
The benefits of masking in preventing COVID-19 transmission within kindergarten through 12th-grade classes are well documented. Masks are particularly important in areas where vaccinations are not widespread.
Masks can be worn safely by young children without harming respiratory function, studies have shown.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., pointed out that the American Academy of Pediatrics has said there are no noteworthy effects on breathing function for most children.
“There’s been so much discussion about the contribution of masks to reducing the risk of COVID that it’s nice to have the data,” he said, adding that this is a relationship that has been difficult to study, but this analysis was able to make the connection with hard numbers.
“It’s an important outcome,” he said in an interview.
The authors pointed out there is evidence that school-age children can identify most emotions in masked faces.
They added that “2-year-old children recognize spoken words better through an opaque mask, compared with a clear face shield, suggesting verbal communication to infants is not harmed by face masks.”
Studies have shown that childhood infection with other respiratory viruses also decreased and asthma symptoms were not reported when preschool children wore masks and used other preventative steps.
The authors wrote that a potential reason for that may be that those who wear masks have less face touching, known to increase the spread of COVID-19.
Paloma Beamer, PhD, an engineer and exposure scientist at University of Arizona, Tucson, who also has a 3-year-old son who wears masks at his daycare center, said in an interview that she works closely with his school on training kids how to wear their masks because getting young children to keep them on and finding ones that fit is challenging.
“We need layered controls and protections in place at schools as much as possible,” she said, adding that the authors didn’t mention ventilation, but that’s another important component as well.
“We’re fortunate in Arizona that we are in an old school and the windows are open as much as possible,” she said.
She said this study shows that “masks are a great form of additional control.” Her son is on his third quarantine this month after three kids tested positive, she added.
She said: “I think these newer variants perhaps make the findings of this study more compelling and it will be interesting to see if the researchers do a follow-up study.”
Strengths of the study include that it utilized prospective data from a large national cohort of childcare professionals. Additionally, the retention rate was high at 1 year. And the self-reported information likely gives better information than looking at policies that may or may not be well followed.
Limitations include potential reporting bias because the self-reports were not independently confirmed. Also, family behavior outside childcare, such as social gatherings where masking is not enforced, also influence COVID-19 cases when children gather and may affect the numbers of closures.
Having the option of childcare centers benefits kids with in-person early education and social interactions with staff, the authors noted. The centers also help parents return to work without interruptions at home.
“Our findings support current national recommendations endorsed by many local and state governments for masking children 2 years and older in childcare programs when community COVID-19 transmission levels are elevated,” the authors wrote.
Dr. Schaffner said the results have implications outside of childcare centers and should be included in discussions of masking in schools and in the general public.
All phases of this study were supported by and coauthors report grants from the Andrew & Julie Klingenstein Family Fund, Esther A. & Joseph Klingenstein Fund, Heising-Simons Foundation, W.K. Kellogg Foundation, Foundation for Child Development, Early Educator Investment Collaborative, and Scholastic. The study was partially funded by the Yale Institute for Global Health. Dr. Schaffner and Dr. Beamer reported no relevant financial relationships.
Mask-wearing in childcare programs is linked with fewer COVID-19–related program closures, new data released suggest.
Researchers included 6,654 childcare professionals in a prospective, 1-year, longitudinal electronic survey study of home- and center-based childcare programs in all 50 states.
Findings by Thomas S. Murray, MD, PhD, with the department of pediatrics, Yale University, New Haven, Conn., and coauthors, were published in JAMA Network Open on Jan. 28, 2022.
They found that mask-wearing from the May 22, 2020, baseline to June 8, 2020, was associated with a 13% reduction in program closures within the following year (adjusted relative risk, 0.87; 95% confidence interval, 0.77-0.99). Continued mask-wearing throughout the 1-year follow-up was associated with a 14% reduction in program closures (aRR, 0.86; 95% CI, 0.74-1.00).
The authors said the evidence supports current masking recommendation in younger children provided by the Centers for Disease Control and Prevention.
They wrote: “This finding has important public health policy implications for families that rely on childcare to sustain employment.”
The benefits of masking in preventing COVID-19 transmission within kindergarten through 12th-grade classes are well documented. Masks are particularly important in areas where vaccinations are not widespread.
Masks can be worn safely by young children without harming respiratory function, studies have shown.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., pointed out that the American Academy of Pediatrics has said there are no noteworthy effects on breathing function for most children.
“There’s been so much discussion about the contribution of masks to reducing the risk of COVID that it’s nice to have the data,” he said, adding that this is a relationship that has been difficult to study, but this analysis was able to make the connection with hard numbers.
“It’s an important outcome,” he said in an interview.
The authors pointed out there is evidence that school-age children can identify most emotions in masked faces.
They added that “2-year-old children recognize spoken words better through an opaque mask, compared with a clear face shield, suggesting verbal communication to infants is not harmed by face masks.”
Studies have shown that childhood infection with other respiratory viruses also decreased and asthma symptoms were not reported when preschool children wore masks and used other preventative steps.
The authors wrote that a potential reason for that may be that those who wear masks have less face touching, known to increase the spread of COVID-19.
Paloma Beamer, PhD, an engineer and exposure scientist at University of Arizona, Tucson, who also has a 3-year-old son who wears masks at his daycare center, said in an interview that she works closely with his school on training kids how to wear their masks because getting young children to keep them on and finding ones that fit is challenging.
“We need layered controls and protections in place at schools as much as possible,” she said, adding that the authors didn’t mention ventilation, but that’s another important component as well.
“We’re fortunate in Arizona that we are in an old school and the windows are open as much as possible,” she said.
She said this study shows that “masks are a great form of additional control.” Her son is on his third quarantine this month after three kids tested positive, she added.
She said: “I think these newer variants perhaps make the findings of this study more compelling and it will be interesting to see if the researchers do a follow-up study.”
Strengths of the study include that it utilized prospective data from a large national cohort of childcare professionals. Additionally, the retention rate was high at 1 year. And the self-reported information likely gives better information than looking at policies that may or may not be well followed.
Limitations include potential reporting bias because the self-reports were not independently confirmed. Also, family behavior outside childcare, such as social gatherings where masking is not enforced, also influence COVID-19 cases when children gather and may affect the numbers of closures.
Having the option of childcare centers benefits kids with in-person early education and social interactions with staff, the authors noted. The centers also help parents return to work without interruptions at home.
“Our findings support current national recommendations endorsed by many local and state governments for masking children 2 years and older in childcare programs when community COVID-19 transmission levels are elevated,” the authors wrote.
Dr. Schaffner said the results have implications outside of childcare centers and should be included in discussions of masking in schools and in the general public.
All phases of this study were supported by and coauthors report grants from the Andrew & Julie Klingenstein Family Fund, Esther A. & Joseph Klingenstein Fund, Heising-Simons Foundation, W.K. Kellogg Foundation, Foundation for Child Development, Early Educator Investment Collaborative, and Scholastic. The study was partially funded by the Yale Institute for Global Health. Dr. Schaffner and Dr. Beamer reported no relevant financial relationships.
Mask-wearing in childcare programs is linked with fewer COVID-19–related program closures, new data released suggest.
Researchers included 6,654 childcare professionals in a prospective, 1-year, longitudinal electronic survey study of home- and center-based childcare programs in all 50 states.
Findings by Thomas S. Murray, MD, PhD, with the department of pediatrics, Yale University, New Haven, Conn., and coauthors, were published in JAMA Network Open on Jan. 28, 2022.
They found that mask-wearing from the May 22, 2020, baseline to June 8, 2020, was associated with a 13% reduction in program closures within the following year (adjusted relative risk, 0.87; 95% confidence interval, 0.77-0.99). Continued mask-wearing throughout the 1-year follow-up was associated with a 14% reduction in program closures (aRR, 0.86; 95% CI, 0.74-1.00).
The authors said the evidence supports current masking recommendation in younger children provided by the Centers for Disease Control and Prevention.
They wrote: “This finding has important public health policy implications for families that rely on childcare to sustain employment.”
The benefits of masking in preventing COVID-19 transmission within kindergarten through 12th-grade classes are well documented. Masks are particularly important in areas where vaccinations are not widespread.
Masks can be worn safely by young children without harming respiratory function, studies have shown.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., pointed out that the American Academy of Pediatrics has said there are no noteworthy effects on breathing function for most children.
“There’s been so much discussion about the contribution of masks to reducing the risk of COVID that it’s nice to have the data,” he said, adding that this is a relationship that has been difficult to study, but this analysis was able to make the connection with hard numbers.
“It’s an important outcome,” he said in an interview.
The authors pointed out there is evidence that school-age children can identify most emotions in masked faces.
They added that “2-year-old children recognize spoken words better through an opaque mask, compared with a clear face shield, suggesting verbal communication to infants is not harmed by face masks.”
Studies have shown that childhood infection with other respiratory viruses also decreased and asthma symptoms were not reported when preschool children wore masks and used other preventative steps.
The authors wrote that a potential reason for that may be that those who wear masks have less face touching, known to increase the spread of COVID-19.
Paloma Beamer, PhD, an engineer and exposure scientist at University of Arizona, Tucson, who also has a 3-year-old son who wears masks at his daycare center, said in an interview that she works closely with his school on training kids how to wear their masks because getting young children to keep them on and finding ones that fit is challenging.
“We need layered controls and protections in place at schools as much as possible,” she said, adding that the authors didn’t mention ventilation, but that’s another important component as well.
“We’re fortunate in Arizona that we are in an old school and the windows are open as much as possible,” she said.
She said this study shows that “masks are a great form of additional control.” Her son is on his third quarantine this month after three kids tested positive, she added.
She said: “I think these newer variants perhaps make the findings of this study more compelling and it will be interesting to see if the researchers do a follow-up study.”
Strengths of the study include that it utilized prospective data from a large national cohort of childcare professionals. Additionally, the retention rate was high at 1 year. And the self-reported information likely gives better information than looking at policies that may or may not be well followed.
Limitations include potential reporting bias because the self-reports were not independently confirmed. Also, family behavior outside childcare, such as social gatherings where masking is not enforced, also influence COVID-19 cases when children gather and may affect the numbers of closures.
Having the option of childcare centers benefits kids with in-person early education and social interactions with staff, the authors noted. The centers also help parents return to work without interruptions at home.
“Our findings support current national recommendations endorsed by many local and state governments for masking children 2 years and older in childcare programs when community COVID-19 transmission levels are elevated,” the authors wrote.
Dr. Schaffner said the results have implications outside of childcare centers and should be included in discussions of masking in schools and in the general public.
All phases of this study were supported by and coauthors report grants from the Andrew & Julie Klingenstein Family Fund, Esther A. & Joseph Klingenstein Fund, Heising-Simons Foundation, W.K. Kellogg Foundation, Foundation for Child Development, Early Educator Investment Collaborative, and Scholastic. The study was partially funded by the Yale Institute for Global Health. Dr. Schaffner and Dr. Beamer reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
COVID at 2 years: Preparing for a different ‘normal’
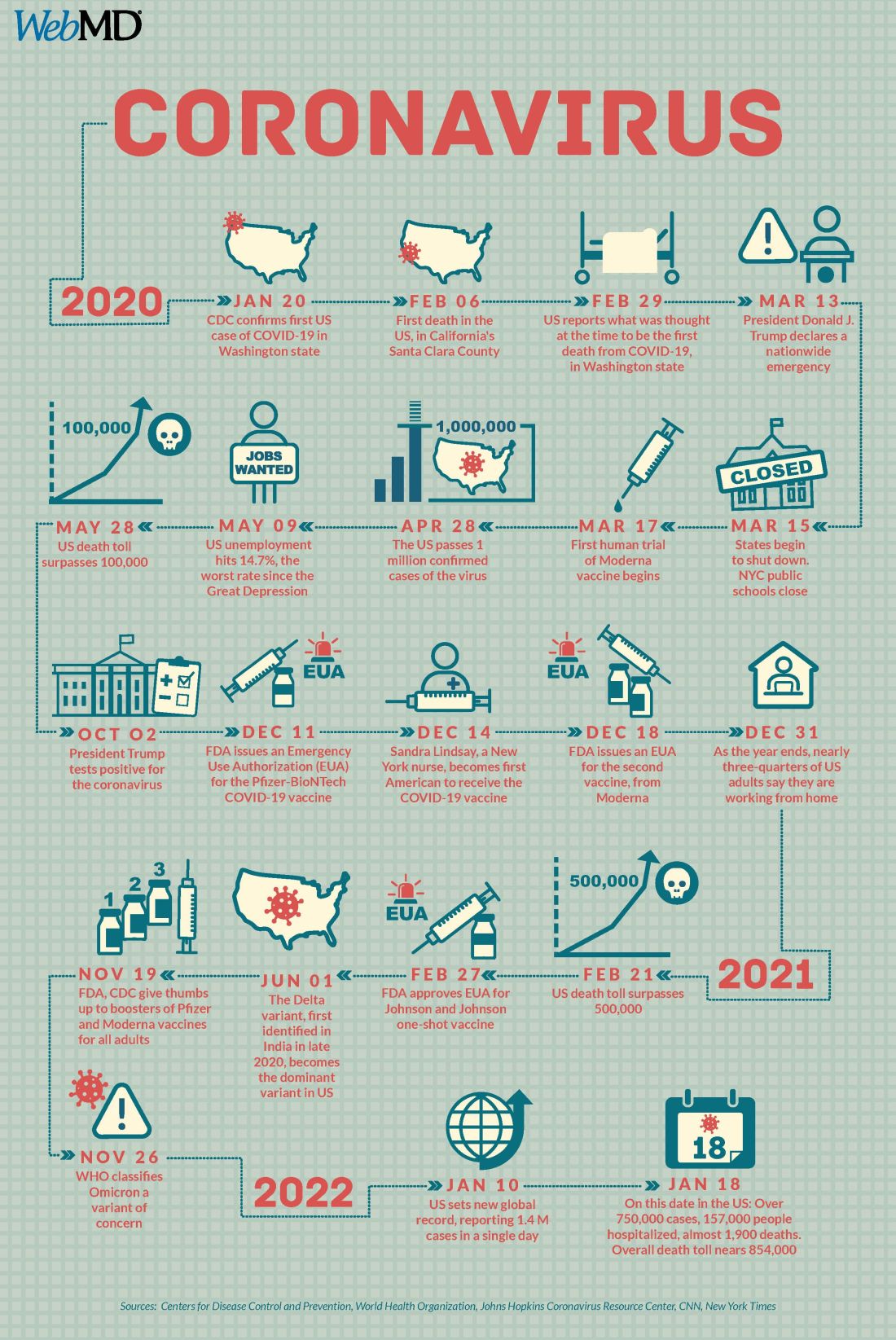
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.
A version of this article first appeared on WebMD.com.
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.

A version of this article first appeared on WebMD.com.
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.

A version of this article first appeared on WebMD.com.
Parent group warns of social media/eating disorders link
A parents’ advocacy group with more than 2.5 million members nationwide sent an advisory to its members on Jan. 11, warning that social media’s January onslaught of messages for dieting and weight loss may be particularly harmful to kids struggling with weight and body image.
The guidance from ParentsTogether noted that such messages can trigger eating disorders and body dysmorphia. But some are particularly dangerous.
A Wall Street Journal investigation recently found that TikTok is distributing videos of rapid-weight-loss competitions and ways to purge food.
According to the ParentsTogether advisory, the Wall Street Journal also found TikTok has sent thousands of videos to teen accounts with messages such as “how to eat only 300 calories a day” or ”how to hide not eating from parents.” The group says similar messages appear on other social media platforms children use daily.
The seasonal January barrage of ads comes on top of a pandemic trend of worsening eating disorder patterns in young people worldwide.
Amanda Kloer, an organizer of the campaign behind the advisory and mother of two teenagers, said in an interview: “We know that January is a particularly sensitive month for this because of the amount of ad spending the wellness industry does.
“We wanted parents to be aware that while these risks exist year round, if they have a kid who is at risk, who is struggling a bit, they should pay particular attention to what they’re seeing on social media in January.”
Ms. Kloer sets up accounts on different platforms to test the messages a teen might receive and says the algorithms ramp up the frequency and the severity of the content as interest by the user grows.
“It sends kids down an extremely dangerous rabbit hole,” she said.
Debra Katzman, MD, with the division of adolescent medicine, department of pediatrics, University of Toronto, wrote in the Journal of Adolescent Health: “The COVID-19 pandemic has had a severe impact on individuals with eating disorders. Since the onset of the COVID-19 pandemic, eating disorder experts from across the globe have observed a substantial increase in the number and severity of new and preexisting young people suffering with eating disorders compared to prior years.”
Contributors beyond social media include lockdowns that bring steady access to food, distancing from peers, anxiety over school closures, and lack of a steady routine.
Eleanor Benner, PsyD, with Children’s Hospital of Philadelphia, said in an interview that awareness is growing regarding the increase in eating disorders correlated with social media use.
Researchers and experts have acknowledged that social media use has increased and changed during the pandemic. Awareness is heightened as parents have been home with kids and noticing what kids are seeing online.
Dr. Benner, a psychologist for the eating disorder assessment and treatment program at CHOP, said platforms have made attempts to limit eating disorder content, but “the reality is that content producers can find ways around this, and unfortunately, we don’t know for whom exactly that content poses greatest risk of contributing to the onset of an eating disorder.”
The most important change for physicians and families to watch for is weight loss, Dr. Benner said.
“Weight loss or lack of weight in children and teenagers is not okay,” she said. “Kids and adolescents should be continually growing and gaining weight through their early 20s.”
Signs of trouble may include diet changes, rejections of favorite foods, and abnormal changes in physical activity, mood, and personality.
Dr. Benner said parents should feel empowered to share these changes with their pediatrician and request that the doctor not discuss weight in front of their children.
Parents should initiate conversations around what kids are seeing to help encourage critical questioning of social media content, Dr. Benner said.
“Parents can also promote body neutrality, the idea that bodies are neither good nor bad, that we don’t have to love our bodies, but acknowledge what they do for us and go about our lives without getting stuck on what they look or feel like,” she said.
Neutrality also extends to categorizing food, and Dr. Benner advised calling foods what they are – ice cream or broccoli, not “junk” or “healthy,” she said. “Food should not be a moral issue. Moralizing and labeling foods perpetuates diet culture and can contribute to shame and guilt around eating.”
ParentsTogether also called on social media platforms to:
- Remove extreme content and stop sending weight-loss material to kids’ accounts: Social media platforms should remove the most extreme and dangerous content such as promoting skin lightening, the group said.
- Create parental account settings. That way, parents can see what their kids see and initiate conversations about bodies and health.
- Feature diverse content creators. The group urges platforms to promote creators with diverse personal appearances and backgrounds and those who support body acceptance and self-love.
ParentsTogether had collected more than 2,700 signatures by Jan. 13 on an online petition asking Instagram and TikTok to “Stop pushing extreme weight loss and dieting on kids.”
Pinterest became the first major platform to prohibit all weight loss ads, according to its announcement in July 2021.
The platform announced, “It’s an expansion of our ad policies that have long prohibited body shaming and dangerous weight loss products or claims. We encourage others in the industry to do the same and acknowledge, once and for all, that there’s no such thing as one size fits all.”
Ms. Kloer and Dr. Benner report no relevant financial relationships.
A parents’ advocacy group with more than 2.5 million members nationwide sent an advisory to its members on Jan. 11, warning that social media’s January onslaught of messages for dieting and weight loss may be particularly harmful to kids struggling with weight and body image.
The guidance from ParentsTogether noted that such messages can trigger eating disorders and body dysmorphia. But some are particularly dangerous.
A Wall Street Journal investigation recently found that TikTok is distributing videos of rapid-weight-loss competitions and ways to purge food.
According to the ParentsTogether advisory, the Wall Street Journal also found TikTok has sent thousands of videos to teen accounts with messages such as “how to eat only 300 calories a day” or ”how to hide not eating from parents.” The group says similar messages appear on other social media platforms children use daily.
The seasonal January barrage of ads comes on top of a pandemic trend of worsening eating disorder patterns in young people worldwide.
Amanda Kloer, an organizer of the campaign behind the advisory and mother of two teenagers, said in an interview: “We know that January is a particularly sensitive month for this because of the amount of ad spending the wellness industry does.
“We wanted parents to be aware that while these risks exist year round, if they have a kid who is at risk, who is struggling a bit, they should pay particular attention to what they’re seeing on social media in January.”
Ms. Kloer sets up accounts on different platforms to test the messages a teen might receive and says the algorithms ramp up the frequency and the severity of the content as interest by the user grows.
“It sends kids down an extremely dangerous rabbit hole,” she said.
Debra Katzman, MD, with the division of adolescent medicine, department of pediatrics, University of Toronto, wrote in the Journal of Adolescent Health: “The COVID-19 pandemic has had a severe impact on individuals with eating disorders. Since the onset of the COVID-19 pandemic, eating disorder experts from across the globe have observed a substantial increase in the number and severity of new and preexisting young people suffering with eating disorders compared to prior years.”
Contributors beyond social media include lockdowns that bring steady access to food, distancing from peers, anxiety over school closures, and lack of a steady routine.
Eleanor Benner, PsyD, with Children’s Hospital of Philadelphia, said in an interview that awareness is growing regarding the increase in eating disorders correlated with social media use.
Researchers and experts have acknowledged that social media use has increased and changed during the pandemic. Awareness is heightened as parents have been home with kids and noticing what kids are seeing online.
Dr. Benner, a psychologist for the eating disorder assessment and treatment program at CHOP, said platforms have made attempts to limit eating disorder content, but “the reality is that content producers can find ways around this, and unfortunately, we don’t know for whom exactly that content poses greatest risk of contributing to the onset of an eating disorder.”
The most important change for physicians and families to watch for is weight loss, Dr. Benner said.
“Weight loss or lack of weight in children and teenagers is not okay,” she said. “Kids and adolescents should be continually growing and gaining weight through their early 20s.”
Signs of trouble may include diet changes, rejections of favorite foods, and abnormal changes in physical activity, mood, and personality.
Dr. Benner said parents should feel empowered to share these changes with their pediatrician and request that the doctor not discuss weight in front of their children.
Parents should initiate conversations around what kids are seeing to help encourage critical questioning of social media content, Dr. Benner said.
“Parents can also promote body neutrality, the idea that bodies are neither good nor bad, that we don’t have to love our bodies, but acknowledge what they do for us and go about our lives without getting stuck on what they look or feel like,” she said.
Neutrality also extends to categorizing food, and Dr. Benner advised calling foods what they are – ice cream or broccoli, not “junk” or “healthy,” she said. “Food should not be a moral issue. Moralizing and labeling foods perpetuates diet culture and can contribute to shame and guilt around eating.”
ParentsTogether also called on social media platforms to:
- Remove extreme content and stop sending weight-loss material to kids’ accounts: Social media platforms should remove the most extreme and dangerous content such as promoting skin lightening, the group said.
- Create parental account settings. That way, parents can see what their kids see and initiate conversations about bodies and health.
- Feature diverse content creators. The group urges platforms to promote creators with diverse personal appearances and backgrounds and those who support body acceptance and self-love.
ParentsTogether had collected more than 2,700 signatures by Jan. 13 on an online petition asking Instagram and TikTok to “Stop pushing extreme weight loss and dieting on kids.”
Pinterest became the first major platform to prohibit all weight loss ads, according to its announcement in July 2021.
The platform announced, “It’s an expansion of our ad policies that have long prohibited body shaming and dangerous weight loss products or claims. We encourage others in the industry to do the same and acknowledge, once and for all, that there’s no such thing as one size fits all.”
Ms. Kloer and Dr. Benner report no relevant financial relationships.
A parents’ advocacy group with more than 2.5 million members nationwide sent an advisory to its members on Jan. 11, warning that social media’s January onslaught of messages for dieting and weight loss may be particularly harmful to kids struggling with weight and body image.
The guidance from ParentsTogether noted that such messages can trigger eating disorders and body dysmorphia. But some are particularly dangerous.
A Wall Street Journal investigation recently found that TikTok is distributing videos of rapid-weight-loss competitions and ways to purge food.
According to the ParentsTogether advisory, the Wall Street Journal also found TikTok has sent thousands of videos to teen accounts with messages such as “how to eat only 300 calories a day” or ”how to hide not eating from parents.” The group says similar messages appear on other social media platforms children use daily.
The seasonal January barrage of ads comes on top of a pandemic trend of worsening eating disorder patterns in young people worldwide.
Amanda Kloer, an organizer of the campaign behind the advisory and mother of two teenagers, said in an interview: “We know that January is a particularly sensitive month for this because of the amount of ad spending the wellness industry does.
“We wanted parents to be aware that while these risks exist year round, if they have a kid who is at risk, who is struggling a bit, they should pay particular attention to what they’re seeing on social media in January.”
Ms. Kloer sets up accounts on different platforms to test the messages a teen might receive and says the algorithms ramp up the frequency and the severity of the content as interest by the user grows.
“It sends kids down an extremely dangerous rabbit hole,” she said.
Debra Katzman, MD, with the division of adolescent medicine, department of pediatrics, University of Toronto, wrote in the Journal of Adolescent Health: “The COVID-19 pandemic has had a severe impact on individuals with eating disorders. Since the onset of the COVID-19 pandemic, eating disorder experts from across the globe have observed a substantial increase in the number and severity of new and preexisting young people suffering with eating disorders compared to prior years.”
Contributors beyond social media include lockdowns that bring steady access to food, distancing from peers, anxiety over school closures, and lack of a steady routine.
Eleanor Benner, PsyD, with Children’s Hospital of Philadelphia, said in an interview that awareness is growing regarding the increase in eating disorders correlated with social media use.
Researchers and experts have acknowledged that social media use has increased and changed during the pandemic. Awareness is heightened as parents have been home with kids and noticing what kids are seeing online.
Dr. Benner, a psychologist for the eating disorder assessment and treatment program at CHOP, said platforms have made attempts to limit eating disorder content, but “the reality is that content producers can find ways around this, and unfortunately, we don’t know for whom exactly that content poses greatest risk of contributing to the onset of an eating disorder.”
The most important change for physicians and families to watch for is weight loss, Dr. Benner said.
“Weight loss or lack of weight in children and teenagers is not okay,” she said. “Kids and adolescents should be continually growing and gaining weight through their early 20s.”
Signs of trouble may include diet changes, rejections of favorite foods, and abnormal changes in physical activity, mood, and personality.
Dr. Benner said parents should feel empowered to share these changes with their pediatrician and request that the doctor not discuss weight in front of their children.
Parents should initiate conversations around what kids are seeing to help encourage critical questioning of social media content, Dr. Benner said.
“Parents can also promote body neutrality, the idea that bodies are neither good nor bad, that we don’t have to love our bodies, but acknowledge what they do for us and go about our lives without getting stuck on what they look or feel like,” she said.
Neutrality also extends to categorizing food, and Dr. Benner advised calling foods what they are – ice cream or broccoli, not “junk” or “healthy,” she said. “Food should not be a moral issue. Moralizing and labeling foods perpetuates diet culture and can contribute to shame and guilt around eating.”
ParentsTogether also called on social media platforms to:
- Remove extreme content and stop sending weight-loss material to kids’ accounts: Social media platforms should remove the most extreme and dangerous content such as promoting skin lightening, the group said.
- Create parental account settings. That way, parents can see what their kids see and initiate conversations about bodies and health.
- Feature diverse content creators. The group urges platforms to promote creators with diverse personal appearances and backgrounds and those who support body acceptance and self-love.
ParentsTogether had collected more than 2,700 signatures by Jan. 13 on an online petition asking Instagram and TikTok to “Stop pushing extreme weight loss and dieting on kids.”
Pinterest became the first major platform to prohibit all weight loss ads, according to its announcement in July 2021.
The platform announced, “It’s an expansion of our ad policies that have long prohibited body shaming and dangerous weight loss products or claims. We encourage others in the industry to do the same and acknowledge, once and for all, that there’s no such thing as one size fits all.”
Ms. Kloer and Dr. Benner report no relevant financial relationships.
Medicaid expansion curbs disparities, increases immigrant access, in postpartum care
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
FROM JAMA HEALTH FORUM AND JAMA NETWORK OPEN
Super-low uric acid may not be best for erosive gout
Lowering the serum urate target to less than 0.20 mmol/L (<3.6 mg/dL) for patients with erosive gout does not achieve better gout outcomes and leads to more medication use and subsequent side effects, according to findings from a 2-year, double-blind, randomized, controlled trial.
Nicola Dalbeth, MD, of the bone and joint research group, department of medicine, faculty of medical and health sciences at University of Auckland (New Zealand), and coauthors noted that intensive serum urate lowering is difficult to achieve with oral urate-lowering therapy (ULT) and their findings suggest lower is not always better.
Their data, published in Arthritis & Rheumatology, suggest the less-intensive standard target of less than 0.30 mmol/L (<5.4 mg/dL), currently recommended by rheumatology guidelines, is sufficient.
The more intensive target leads to a high medication burden and does not improve bone erosion score in erosive gout, the authors found.
Rheumatologist Angelo Gaffo, MD, associate professor of medicine at the University of Alabama at Birmingham, who was not part of the study, said erosion scores are the best way to test outcomes and this study provides support for current gout treatment approaches.
“It is reassuring that the approach of treating to target is a good approach,” Dr. Gaffo said. “The very, very low targets were not better than the [standard target].”
The trial included 104 participants with erosive gout on oral ULT who were randomized either to a serum urate target of less than 0.20 mmol/L or less than 0.30 mmol/L.
Ninety participants completed the study: 44 (85%) in the intensive target group and 46 (88%) in the standard target group. All were included in the primary intention-to-treat analysis. Participants were mostly men with an average age of 61. Average period of disease was 19 years and about half had a gout flare in the 3 months before enrollment in the study.
Fewer in intensive group hit target
The researchers found that serum urate at year 2 was significantly lower in the intensive target group, compared with the level in the standard target group (P = .002), but fewer participants in the intensive group hit their target, compared with those in the standard group (62% vs. 83%; P < .05).
The intensive group also required more medication. Participants in that group needed higher doses of the first-line treatment allopurinol (mean, 746 mg/day vs. 496 mg/day; P < .001). They also used more combination therapy (P = .0004).
Bone erosion scores were slightly better in both groups over 2 years, but there was no between-group difference (P = .20).
Rates of adverse and serious adverse events were similar between the groups.
The authors noted that a previous study has shown that escalating doses of allopurinol to achieve a target lower than 0.36 mmol/L (6.48 mg/dL) can reduce progression of bone erosion in gout.
“However, improved erosion scores were not observed in this study,” the authors noted.
The authors said that emerging data on intensive serum urate lowering “may lead to erosion healing in gout,” particularly with pegloticase (Krystexxa), a treatment that leads to profound reductions in serum urate.
They highlighted a small longitudinal study of patients treated with pegloticase in whom researchers observed the filling in of bone erosions over a year.
Pegloticase not available outside United States
However, the authors explained, use of pegloticase is unlikely to be widespread for erosive gout because of its lack of availability outside the United States and the need for infusions every 2 weeks. Therefore, more feasible strategies are needed.
Guidelines suggest the serum urate target of less than 0.30 mmol/L (5.4 mg/dL) for people with severe gout, including those with chronic arthropathy.
Managing gout is a long-term process
Herbert S.B. Baraf, MD, a rheumatologist in a large group practice in the Washington, D.C., area and clinical professor of medicine at George Washington University, Washington, who was not part of this study, said he would not come to the conclusion that some cynics might draw that there’s no point in trying to continually lower uric acid.
“Managing gout is a long-term proposition, and the long-term benefit of continuous uric acid lowering continue to accumulate over a period of time,” Dr. Baraf said.
He agreed with Dr. Dalbeth and colleagues that trying to get serum uric acid to less than 0.20 mmol/L is very difficult to achieve with oral drugs.
He said: “The study was not able to show a change in erosions because the amount of uric acid lowering wasn’t profound enough over a short enough period of time to show that, but over a longer period of time it might well show that.”
He said oral therapies work more slowly than enzyme-based therapies, such as pegloticase, but agreed there are barriers to using pegloticase.
“A drug like pegloticase costs about $26,000 per infusion every 2 weeks for a 6-month period. It’s not practical, and we tend to use it for people who are severely functionally impaired,” said Dr. Baraf.
It would still be a goal to keep the arthritis from progressing by using oral therapies, he said.
“I wouldn’t denigrate the fact that oral therapies are effective in decreasing flares over time, decreasing tophaceous deposits and probably – over a longer period of time allowing bone to heal. But 2 years is not enough time to show that.” He said showing benefit on erosions may take 5-10 years instead.
The study authors noted that the trial’s results “are not relevant to those without erosive disease, and to health care systems without access to a broad range of urate-lowering agents.”
Dr. Dalbeth reports personal fees (all less than $10,000) from AstraZeneca, Dyve BioSciences, Selecta, Arthrosi, Horizon, AbbVie, JW Pharmaceuticals, and PK Med outside the submitted work. The other authors have no disclosures. Dr. Gaffo reported no relevant financial relationships. Dr. Baraf has been an investigator/consultant and speaker for Horizon Therapeutics, maker of pegloticase; is an investigator and a consultant to Selecta Biosciences; and has been an investigator, speaker, and consultant for Takeda.
Lowering the serum urate target to less than 0.20 mmol/L (<3.6 mg/dL) for patients with erosive gout does not achieve better gout outcomes and leads to more medication use and subsequent side effects, according to findings from a 2-year, double-blind, randomized, controlled trial.
Nicola Dalbeth, MD, of the bone and joint research group, department of medicine, faculty of medical and health sciences at University of Auckland (New Zealand), and coauthors noted that intensive serum urate lowering is difficult to achieve with oral urate-lowering therapy (ULT) and their findings suggest lower is not always better.
Their data, published in Arthritis & Rheumatology, suggest the less-intensive standard target of less than 0.30 mmol/L (<5.4 mg/dL), currently recommended by rheumatology guidelines, is sufficient.
The more intensive target leads to a high medication burden and does not improve bone erosion score in erosive gout, the authors found.
Rheumatologist Angelo Gaffo, MD, associate professor of medicine at the University of Alabama at Birmingham, who was not part of the study, said erosion scores are the best way to test outcomes and this study provides support for current gout treatment approaches.
“It is reassuring that the approach of treating to target is a good approach,” Dr. Gaffo said. “The very, very low targets were not better than the [standard target].”
The trial included 104 participants with erosive gout on oral ULT who were randomized either to a serum urate target of less than 0.20 mmol/L or less than 0.30 mmol/L.
Ninety participants completed the study: 44 (85%) in the intensive target group and 46 (88%) in the standard target group. All were included in the primary intention-to-treat analysis. Participants were mostly men with an average age of 61. Average period of disease was 19 years and about half had a gout flare in the 3 months before enrollment in the study.
Fewer in intensive group hit target
The researchers found that serum urate at year 2 was significantly lower in the intensive target group, compared with the level in the standard target group (P = .002), but fewer participants in the intensive group hit their target, compared with those in the standard group (62% vs. 83%; P < .05).
The intensive group also required more medication. Participants in that group needed higher doses of the first-line treatment allopurinol (mean, 746 mg/day vs. 496 mg/day; P < .001). They also used more combination therapy (P = .0004).
Bone erosion scores were slightly better in both groups over 2 years, but there was no between-group difference (P = .20).
Rates of adverse and serious adverse events were similar between the groups.
The authors noted that a previous study has shown that escalating doses of allopurinol to achieve a target lower than 0.36 mmol/L (6.48 mg/dL) can reduce progression of bone erosion in gout.
“However, improved erosion scores were not observed in this study,” the authors noted.
The authors said that emerging data on intensive serum urate lowering “may lead to erosion healing in gout,” particularly with pegloticase (Krystexxa), a treatment that leads to profound reductions in serum urate.
They highlighted a small longitudinal study of patients treated with pegloticase in whom researchers observed the filling in of bone erosions over a year.
Pegloticase not available outside United States
However, the authors explained, use of pegloticase is unlikely to be widespread for erosive gout because of its lack of availability outside the United States and the need for infusions every 2 weeks. Therefore, more feasible strategies are needed.
Guidelines suggest the serum urate target of less than 0.30 mmol/L (5.4 mg/dL) for people with severe gout, including those with chronic arthropathy.
Managing gout is a long-term process
Herbert S.B. Baraf, MD, a rheumatologist in a large group practice in the Washington, D.C., area and clinical professor of medicine at George Washington University, Washington, who was not part of this study, said he would not come to the conclusion that some cynics might draw that there’s no point in trying to continually lower uric acid.
“Managing gout is a long-term proposition, and the long-term benefit of continuous uric acid lowering continue to accumulate over a period of time,” Dr. Baraf said.
He agreed with Dr. Dalbeth and colleagues that trying to get serum uric acid to less than 0.20 mmol/L is very difficult to achieve with oral drugs.
He said: “The study was not able to show a change in erosions because the amount of uric acid lowering wasn’t profound enough over a short enough period of time to show that, but over a longer period of time it might well show that.”
He said oral therapies work more slowly than enzyme-based therapies, such as pegloticase, but agreed there are barriers to using pegloticase.
“A drug like pegloticase costs about $26,000 per infusion every 2 weeks for a 6-month period. It’s not practical, and we tend to use it for people who are severely functionally impaired,” said Dr. Baraf.
It would still be a goal to keep the arthritis from progressing by using oral therapies, he said.
“I wouldn’t denigrate the fact that oral therapies are effective in decreasing flares over time, decreasing tophaceous deposits and probably – over a longer period of time allowing bone to heal. But 2 years is not enough time to show that.” He said showing benefit on erosions may take 5-10 years instead.
The study authors noted that the trial’s results “are not relevant to those without erosive disease, and to health care systems without access to a broad range of urate-lowering agents.”
Dr. Dalbeth reports personal fees (all less than $10,000) from AstraZeneca, Dyve BioSciences, Selecta, Arthrosi, Horizon, AbbVie, JW Pharmaceuticals, and PK Med outside the submitted work. The other authors have no disclosures. Dr. Gaffo reported no relevant financial relationships. Dr. Baraf has been an investigator/consultant and speaker for Horizon Therapeutics, maker of pegloticase; is an investigator and a consultant to Selecta Biosciences; and has been an investigator, speaker, and consultant for Takeda.
Lowering the serum urate target to less than 0.20 mmol/L (<3.6 mg/dL) for patients with erosive gout does not achieve better gout outcomes and leads to more medication use and subsequent side effects, according to findings from a 2-year, double-blind, randomized, controlled trial.
Nicola Dalbeth, MD, of the bone and joint research group, department of medicine, faculty of medical and health sciences at University of Auckland (New Zealand), and coauthors noted that intensive serum urate lowering is difficult to achieve with oral urate-lowering therapy (ULT) and their findings suggest lower is not always better.
Their data, published in Arthritis & Rheumatology, suggest the less-intensive standard target of less than 0.30 mmol/L (<5.4 mg/dL), currently recommended by rheumatology guidelines, is sufficient.
The more intensive target leads to a high medication burden and does not improve bone erosion score in erosive gout, the authors found.
Rheumatologist Angelo Gaffo, MD, associate professor of medicine at the University of Alabama at Birmingham, who was not part of the study, said erosion scores are the best way to test outcomes and this study provides support for current gout treatment approaches.
“It is reassuring that the approach of treating to target is a good approach,” Dr. Gaffo said. “The very, very low targets were not better than the [standard target].”
The trial included 104 participants with erosive gout on oral ULT who were randomized either to a serum urate target of less than 0.20 mmol/L or less than 0.30 mmol/L.
Ninety participants completed the study: 44 (85%) in the intensive target group and 46 (88%) in the standard target group. All were included in the primary intention-to-treat analysis. Participants were mostly men with an average age of 61. Average period of disease was 19 years and about half had a gout flare in the 3 months before enrollment in the study.
Fewer in intensive group hit target
The researchers found that serum urate at year 2 was significantly lower in the intensive target group, compared with the level in the standard target group (P = .002), but fewer participants in the intensive group hit their target, compared with those in the standard group (62% vs. 83%; P < .05).
The intensive group also required more medication. Participants in that group needed higher doses of the first-line treatment allopurinol (mean, 746 mg/day vs. 496 mg/day; P < .001). They also used more combination therapy (P = .0004).
Bone erosion scores were slightly better in both groups over 2 years, but there was no between-group difference (P = .20).
Rates of adverse and serious adverse events were similar between the groups.
The authors noted that a previous study has shown that escalating doses of allopurinol to achieve a target lower than 0.36 mmol/L (6.48 mg/dL) can reduce progression of bone erosion in gout.
“However, improved erosion scores were not observed in this study,” the authors noted.
The authors said that emerging data on intensive serum urate lowering “may lead to erosion healing in gout,” particularly with pegloticase (Krystexxa), a treatment that leads to profound reductions in serum urate.
They highlighted a small longitudinal study of patients treated with pegloticase in whom researchers observed the filling in of bone erosions over a year.
Pegloticase not available outside United States
However, the authors explained, use of pegloticase is unlikely to be widespread for erosive gout because of its lack of availability outside the United States and the need for infusions every 2 weeks. Therefore, more feasible strategies are needed.
Guidelines suggest the serum urate target of less than 0.30 mmol/L (5.4 mg/dL) for people with severe gout, including those with chronic arthropathy.
Managing gout is a long-term process
Herbert S.B. Baraf, MD, a rheumatologist in a large group practice in the Washington, D.C., area and clinical professor of medicine at George Washington University, Washington, who was not part of this study, said he would not come to the conclusion that some cynics might draw that there’s no point in trying to continually lower uric acid.
“Managing gout is a long-term proposition, and the long-term benefit of continuous uric acid lowering continue to accumulate over a period of time,” Dr. Baraf said.
He agreed with Dr. Dalbeth and colleagues that trying to get serum uric acid to less than 0.20 mmol/L is very difficult to achieve with oral drugs.
He said: “The study was not able to show a change in erosions because the amount of uric acid lowering wasn’t profound enough over a short enough period of time to show that, but over a longer period of time it might well show that.”
He said oral therapies work more slowly than enzyme-based therapies, such as pegloticase, but agreed there are barriers to using pegloticase.
“A drug like pegloticase costs about $26,000 per infusion every 2 weeks for a 6-month period. It’s not practical, and we tend to use it for people who are severely functionally impaired,” said Dr. Baraf.
It would still be a goal to keep the arthritis from progressing by using oral therapies, he said.
“I wouldn’t denigrate the fact that oral therapies are effective in decreasing flares over time, decreasing tophaceous deposits and probably – over a longer period of time allowing bone to heal. But 2 years is not enough time to show that.” He said showing benefit on erosions may take 5-10 years instead.
The study authors noted that the trial’s results “are not relevant to those without erosive disease, and to health care systems without access to a broad range of urate-lowering agents.”
Dr. Dalbeth reports personal fees (all less than $10,000) from AstraZeneca, Dyve BioSciences, Selecta, Arthrosi, Horizon, AbbVie, JW Pharmaceuticals, and PK Med outside the submitted work. The other authors have no disclosures. Dr. Gaffo reported no relevant financial relationships. Dr. Baraf has been an investigator/consultant and speaker for Horizon Therapeutics, maker of pegloticase; is an investigator and a consultant to Selecta Biosciences; and has been an investigator, speaker, and consultant for Takeda.
FROM ARTHRITIS & RHEUMATOLOGY
Pediatric antibiotic prescriptions plummeted in pandemic
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
FROM PEDIATRICS
CDC panel recommends Pfizer COVID-19 boosters for ages 12-15
The CDC had already said 16- and 17-year-olds “may” receive a Pfizer booster but the new recommendation adds the 12- to 15-year-old group and strengthens the “may” to “should” for 16- and 17-year-olds.
The committee voted 13-1 to recommend the booster for ages 12-17. CDC Director Rochelle Walensky, MD, must still approve the recommendation for it to take effect.
The vote comes after the FDA on Jan. 3 authorized the Pfizer vaccine booster dose for 12- to 15-year-olds.
The FDA action updated the authorization for the Pfizer vaccine, and the agency also shortened the recommended time between a second dose and the booster to 5 months or more (from 6 months). A third primary series dose is also now authorized for certain immunocompromised children between 5 and 11 years old. Full details are available in an FDA news release.
The CDC on Jan. 4 also backed the shortened time frame and a third primary series dose for some immunocompromised children 5-11 years old. But the CDC delayed a decision on a booster for 12- to 15-year-olds until it heard from its Advisory Committee on Immunization Practices on Jan. 5.
The decision came as school districts nationwide are wrestling with decisions of whether to keep schools open or revert to a virtual format as cases surge, and as pediatric COVID-19 cases and hospitalizations reach new highs.
The only dissenting vote came from Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University in Nashville, Tenn.
She said after the vote, “I am just fine with kids getting a booster. This is not me against all boosters. I just really want the U.S. to move forward with all kids.”
Dr. Talbot said earlier in the comment period, “If we divert our public health from the unvaccinated to the vaccinated, we are not going to make a big impact. Boosters are incredibly important but they won’t solve this problem of the crowded hospitals.”
She said vaccinating the unvaccinated must be the priority.
“If you are a parent out there who has not yet vaccinated your child because you have questions, please, please talk to a health care provider,” she said.
Among the 13 supporters of the recommendation was Oliver Brooks, MD, chief medical officer of Watts HealthCare Corporation in Los Angeles.
Dr. Brooks said extending the population for boosters is another tool in the toolbox.
“If it’s a hammer, we should hit that nail hard,” he said.
Sara Oliver, MD, ACIP’s lead for the COVID-19 work group, presented the case behind the recommendation.
She noted the soaring Omicron cases.
“As of Jan. 3, the 7-day average had reached an all-time high of nearly 500,000 cases,” Dr. Oliver noted.
Since this summer, she said, adolescents have had a higher rate of incidence than that of adults.
“The majority of COVID cases continue to occur among the unvaccinated,” she said, “with unvaccinated 12- to 17-year-olds having a 7-times-higher risk of testing positive for SARS-CoV-2 compared to vaccinated 12- to 17-year-olds. Unvaccinated 12- to 17-year-olds have around 11 times higher risk of hospitalization than vaccinated 12- to 17-year-olds.
“Vaccine effectiveness in adolescents 12-15 years old remains high,” Dr. Oliver said, but evidence shows there may be “some waning over time.”
Discussion of risk centered on myocarditis.
Dr. Oliver said myocarditis rates reported after the Pfizer vaccine in Israel across all populations as of Dec. 15 show that “the rates of myocarditis after a third dose are lower than what is seen after the second dose.”
A version of this article first appeared on WebMD.com.
The CDC had already said 16- and 17-year-olds “may” receive a Pfizer booster but the new recommendation adds the 12- to 15-year-old group and strengthens the “may” to “should” for 16- and 17-year-olds.
The committee voted 13-1 to recommend the booster for ages 12-17. CDC Director Rochelle Walensky, MD, must still approve the recommendation for it to take effect.
The vote comes after the FDA on Jan. 3 authorized the Pfizer vaccine booster dose for 12- to 15-year-olds.
The FDA action updated the authorization for the Pfizer vaccine, and the agency also shortened the recommended time between a second dose and the booster to 5 months or more (from 6 months). A third primary series dose is also now authorized for certain immunocompromised children between 5 and 11 years old. Full details are available in an FDA news release.
The CDC on Jan. 4 also backed the shortened time frame and a third primary series dose for some immunocompromised children 5-11 years old. But the CDC delayed a decision on a booster for 12- to 15-year-olds until it heard from its Advisory Committee on Immunization Practices on Jan. 5.
The decision came as school districts nationwide are wrestling with decisions of whether to keep schools open or revert to a virtual format as cases surge, and as pediatric COVID-19 cases and hospitalizations reach new highs.
The only dissenting vote came from Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University in Nashville, Tenn.
She said after the vote, “I am just fine with kids getting a booster. This is not me against all boosters. I just really want the U.S. to move forward with all kids.”
Dr. Talbot said earlier in the comment period, “If we divert our public health from the unvaccinated to the vaccinated, we are not going to make a big impact. Boosters are incredibly important but they won’t solve this problem of the crowded hospitals.”
She said vaccinating the unvaccinated must be the priority.
“If you are a parent out there who has not yet vaccinated your child because you have questions, please, please talk to a health care provider,” she said.
Among the 13 supporters of the recommendation was Oliver Brooks, MD, chief medical officer of Watts HealthCare Corporation in Los Angeles.
Dr. Brooks said extending the population for boosters is another tool in the toolbox.
“If it’s a hammer, we should hit that nail hard,” he said.
Sara Oliver, MD, ACIP’s lead for the COVID-19 work group, presented the case behind the recommendation.
She noted the soaring Omicron cases.
“As of Jan. 3, the 7-day average had reached an all-time high of nearly 500,000 cases,” Dr. Oliver noted.
Since this summer, she said, adolescents have had a higher rate of incidence than that of adults.
“The majority of COVID cases continue to occur among the unvaccinated,” she said, “with unvaccinated 12- to 17-year-olds having a 7-times-higher risk of testing positive for SARS-CoV-2 compared to vaccinated 12- to 17-year-olds. Unvaccinated 12- to 17-year-olds have around 11 times higher risk of hospitalization than vaccinated 12- to 17-year-olds.
“Vaccine effectiveness in adolescents 12-15 years old remains high,” Dr. Oliver said, but evidence shows there may be “some waning over time.”
Discussion of risk centered on myocarditis.
Dr. Oliver said myocarditis rates reported after the Pfizer vaccine in Israel across all populations as of Dec. 15 show that “the rates of myocarditis after a third dose are lower than what is seen after the second dose.”
A version of this article first appeared on WebMD.com.
The CDC had already said 16- and 17-year-olds “may” receive a Pfizer booster but the new recommendation adds the 12- to 15-year-old group and strengthens the “may” to “should” for 16- and 17-year-olds.
The committee voted 13-1 to recommend the booster for ages 12-17. CDC Director Rochelle Walensky, MD, must still approve the recommendation for it to take effect.
The vote comes after the FDA on Jan. 3 authorized the Pfizer vaccine booster dose for 12- to 15-year-olds.
The FDA action updated the authorization for the Pfizer vaccine, and the agency also shortened the recommended time between a second dose and the booster to 5 months or more (from 6 months). A third primary series dose is also now authorized for certain immunocompromised children between 5 and 11 years old. Full details are available in an FDA news release.
The CDC on Jan. 4 also backed the shortened time frame and a third primary series dose for some immunocompromised children 5-11 years old. But the CDC delayed a decision on a booster for 12- to 15-year-olds until it heard from its Advisory Committee on Immunization Practices on Jan. 5.
The decision came as school districts nationwide are wrestling with decisions of whether to keep schools open or revert to a virtual format as cases surge, and as pediatric COVID-19 cases and hospitalizations reach new highs.
The only dissenting vote came from Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University in Nashville, Tenn.
She said after the vote, “I am just fine with kids getting a booster. This is not me against all boosters. I just really want the U.S. to move forward with all kids.”
Dr. Talbot said earlier in the comment period, “If we divert our public health from the unvaccinated to the vaccinated, we are not going to make a big impact. Boosters are incredibly important but they won’t solve this problem of the crowded hospitals.”
She said vaccinating the unvaccinated must be the priority.
“If you are a parent out there who has not yet vaccinated your child because you have questions, please, please talk to a health care provider,” she said.
Among the 13 supporters of the recommendation was Oliver Brooks, MD, chief medical officer of Watts HealthCare Corporation in Los Angeles.
Dr. Brooks said extending the population for boosters is another tool in the toolbox.
“If it’s a hammer, we should hit that nail hard,” he said.
Sara Oliver, MD, ACIP’s lead for the COVID-19 work group, presented the case behind the recommendation.
She noted the soaring Omicron cases.
“As of Jan. 3, the 7-day average had reached an all-time high of nearly 500,000 cases,” Dr. Oliver noted.
Since this summer, she said, adolescents have had a higher rate of incidence than that of adults.
“The majority of COVID cases continue to occur among the unvaccinated,” she said, “with unvaccinated 12- to 17-year-olds having a 7-times-higher risk of testing positive for SARS-CoV-2 compared to vaccinated 12- to 17-year-olds. Unvaccinated 12- to 17-year-olds have around 11 times higher risk of hospitalization than vaccinated 12- to 17-year-olds.
“Vaccine effectiveness in adolescents 12-15 years old remains high,” Dr. Oliver said, but evidence shows there may be “some waning over time.”
Discussion of risk centered on myocarditis.
Dr. Oliver said myocarditis rates reported after the Pfizer vaccine in Israel across all populations as of Dec. 15 show that “the rates of myocarditis after a third dose are lower than what is seen after the second dose.”
A version of this article first appeared on WebMD.com.








